Abstract
There are currently no FDA-approved medications to reduce cocaine relapse. The majority of preclinical studies aimed at identifying the neurobiology underlying relapse involve the self-administration of cocaine alone, whereas many, if not a majority, of cocaine users engage in polysubstance use. Here we developed a rat model of sequential cocaine and alcohol self-administration to test the hypothesis that this combination produces distinct neuroadaptations relative to those produced by cocaine alone. Male rats underwent intravenous cocaine self-administration (2 h/day) followed by 6 h access to unsweetened alcohol (20% v/v) for 12 days. After extinction training, we assessed surface expression of the glutamate transporter GLT-1 and glutamate efflux in the nucleus accumbens (NA) core during the reinstatement of cocaine-seeking. We also tested the ability of ceftriaxone to attenuate the reinstatement of cocaine-seeking and assessed reinstatement-induced Fos expression in several regions critical for reinstatement. Alcohol consumption did not alter cocaine intake, nor did access to cocaine alter alcohol consumption. However, we noted significant changes in glutamate homeostasis in the NA core of cocaine + alcohol rats relative to rats consuming cocaine alone, such as increased surface GLT-1 expression and a lack of increase in glutamate efflux during reinstatement of cocaine-seeking. A history of cocaine + alcohol also altered patterns of reinstatement-induced Fos expression. These changes likely account for the inability of ceftriaxone to attenuate cocaine relapse in cocaine + alcohol rats, while it does so in rats consuming only cocaine. As such glutamate neuroadaptations are targeted by medications to reduce cocaine relapse, preclinical models should consider polysubstance use.
Subject terms: Addiction, Addiction
Introduction
Approximately 1 million Americans meet criteria for cocaine use disorder (CUD) [1]. An estimated 50–90% of cocaine users also report using alcohol [2–7]. Individuals comorbid for CUD and alcohol use disorder have increased severity of dependence and worse treatment outcomes relative to CUD alone [8, 9]. Medications that reduce cocaine-seeking may not be effective in users also consuming alcohol; for example, modafinil decreases cocaine use only in subjects without alcohol-dependence [10].
The translational pipeline for the development of medications to reduce cocaine intake relies on rodent models to identify neuroadaptations produced by cocaine self-administration. However, if human polysubstance use patterns are not modeled, the correct neuroadaptations may not be identified. The few preclinical studies that assess neurobiology following the self-administration of a second drug in addition to cocaine find that cocaine + heroin alters dopaminergic neurotransmission in the nucleus accumbens (NA) in a different manner than cocaine alone [11–14]. In the case of cocaine and alcohol polysubstance use, there is also the potential for novel neuroadaptations to be produced by the psychoactive metabolite cocaethylene (CE) formed only when both cocaine and alcohol are in the liver.
Here we developed a rat model of sequential cocaine and alcohol consumption to investigate whether alcohol modulates cocaine-induced neuroadaptations in the glutamate system that drive relapse to cocaine-seeking. The reinstatement of cocaine-seeking is accompanied by glutamate efflux in the NA core, which is necessary for reinstatement to occur [15–22]. GLT-1 expression in the NA core has consistently been found to be decreased 2–3 weeks after the conclusion of cocaine self-administration [21, 23–26]. GLT-1 is also of interest here because the antibiotic ceftriaxone increases GLT-1 expression in the NA core and attenuates the reinstatement of cocaine-seeking in a GLT-1-dependent manner [23, 24, 27, 28]. While ceftriaxone also reduces both alcohol consumption [29] and the reinstatement of alcohol-seeking [29, 30], its efficacy to reduce cocaine-seeking in a polysubstance use model is unknown, and was a major goal of the present set of experiments. Despite the abundance of data on the prevalence of cocaine + alcohol polysubstance use, little is known regarding its granular temporal dynamics. The two studies that have collected such data find evidence of simultaneous intake as well as both possible patterns of sequential intake (alcohol prior to cocaine and vice versa), with sequential cocaine/alcohol use occurring within 3 h of one another [31, 32]. Here we provided alcohol access after cocaine self-administration to avoid potential effects of alcohol on learning the response necessary to obtain cocaine.
Methods
Animals
Rats (n = 114; Charles Rivers Laboratories, Raleigh, NC) were housed in a temperature- and humidity-controlled vivarium. Consistent with studies examining the same dependent measures (e.g., [21, 33]), adult male, Sprague–Dawley rats were used and received 20 g/day chow and water ad libitum throughout each study. Experiment 1 was conducted at the Medical University of South Carolina (Charleston, SC) while Experiments 2–4 were conducted at the University of Florida (Gainesville, FL). All procedures were approved by each institution’s Institutional Animal Care and Use Committees and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine hydrochloride (Research Triangle Institute, NC, USA) was dissolved in 0.9% physiological saline for intravenous self-administration (1.05 mg/kg/infusion) and intraperitoneal (IP) injection (10 mg/kg). Ceftriaxone (Sigma–Aldrich, St. Louis, MO) was prepared in 0.9% physiological saline and administered at a dose of 100 or 200 mg/kg (1 mg/mL, IP). These doses attenuate the reinstatement of both cocaine- and alcohol-seeking [23, 27, 30]. Vehicle was 0.9% physiological saline (0.3 mL IP).
Surgery
Rats were anesthetized with ketamine (87.5 mg/kg, IP) and xylazine (5 mg/kg, IP). Catheters and unilateral guide cannulae aimed at the NA core were implanted as described previously [34] and in Supplemental Methods. Catheters were flushed with heparinized saline (100 U/mL) daily. Catheter patency was tested periodically with methohexital sodium (10 mg/mL; Eli Lilly, Indianapolis, IN, USA).
Cocaine and Alcohol Self-Administration
Rats assigned to receive access to alcohol after cocaine sessions were first provided intermittent access to unsweetened alcohol (20% v/v) on alternating days for five 24-h sessions prior to surgery (see [33, 35], Supplemental Methods). Rats were trained to self-administer cocaine (or receive yoked-saline infusions) in a two-lever operant chamber (Med Associates, St. Albans, VT), whereby one press on the active lever resulted in cocaine and cue-presentation (2900 Hz tone and stimulus light). Immediately following each 2-h self-administration session, rats received 6-h access to water alone or both water and unsweetened alcohol (20% v/v) in the homecage. Self-administration continued for 10 (Experiment 1) or 12 days (Experiments 2–4), after which rats experienced daily 2-h extinction training, during which presses on the previously active lever did not yield drug or cues. No alcohol was available during this time. After a minimum of 12 extinction sessions, rats were killed for western blotting (Experiment 1) or underwent reinstatement testing (Experiments 2–4).
Experiment 1
To assess the effects of cocaine and alcohol on the consumption of one another, 17 rats underwent cocaine self-administration while 16 rats received yoked-saline infusions for 10 days. Eight cocaine and eight yoked-saline rats were given access to only water in the homecage, while nine cocaine and eight yoked-saline rats experienced 2-bottle choice. Following 14–17 days of extinction training, rats were rapidly decapitated without anesthesia. The NA core was dissected on ice and surface proteins biotinylated as reported previously [33, 36, 37]. Western blotting was conducted for GLT-1, as described in the Supplemental Methods and [23]. Band density was quantified with Image J (NIH).
Experiment 2
In order to assess the effects of ceftriaxone and/or a history of alcohol consumption on the glutamate efflux that mediates the reinstatement of cocaine-seeking [20], rats underwent microdialysis during a cue + cocaine-primed reinstatement test. Rats (n = 45) underwent self-administration as described above. On the last day of self-administration, rats in the cocaine + alcohol condition underwent blood collection between 30–120 min after alcohol presentation (following cocaine self-administration as usual). A second set of rats was only sampled after 120 min access. Alcohol content was determined using the alcohol dehydrogenase assay [38]. For cocaine and cocaethylene detection, plasma proteins were precipitated from blood samples [39] and were analyzed via reverse phase gradient C18 HPLC/(+)ESI-MS and –MS/MS. For details see the Supplemental Methods.
After 12 days of self-administration, rats underwent extinction training as described above. For 5–7 days prior to reinstatement testing, rats received ceftriaxone (200 mg/kg IP) or vehicle immediately after the extinction session. Once rats met extinction criteria (≤20 active lever presses across 3 days), a reinstatement test occurred while rats underwent microdialysis procedures. Probes were implanted and rats spent the night in their homecage adjacent to operant chambers. The next morning, rats were placed into the operant chambers. After the collection of nine 10-min baseline samples, rats received a cocaine injection (10 mg/kg IP) and levers were extended for a 90 min cue + cocaine-primed reinstatement test, during which presses on the active lever yielded cues but not drug. Glutamate concentrations were determined via HPLC [24, 34]. See Supplemental Methods for details.
Experiment 3
In order to assess reinstatement-induced Fos expression, immediately upon conclusion of the reinstatement/microdialysis session in Experiment 2, rats were euthanized and transcardially perfused with 4% paraformaldehyde. Yoked-saline + water rats (n = 6) were placed back into the operant chamber with the levers extended prior to perfusion. Brains were extracted, post-fixed, and stored at −80°. Coronal sections were collected to confirm probe placement and for Fos immunohistochemistry (see Supplemental Methods).
Experiment 4
In order to assess the ability of ceftriaxone to attenuate the reinstatement of cocaine-seeking primed by cues or cocaine alone, rats (n = 30) underwent cocaine and alcohol self-administration as described above, followed by extinction training. During the 5–7 days prior to reinstatement testing, rats were administered ceftriaxone (100 or 200 mg/kg IP) or vehicle immediately after the extinction session. Rats were tested for cue-primed reinstatement during a 2-h test, wherein presses on the active lever produced only the cues associated with drug delivery. Rats then underwent extinction for a minimum of 3 days until criteria was met; ceftriaxone/vehicle injections continued during this time. For cocaine-primed reinstatement, rats were administered 10 mg/kg cocaine (IP) then immediately placed into the operant chamber; lever presses did not yield drug or cues.
Statistical analyses
Data were analyzed using SPSS (IBM, Amorak, NY) and GraphPad Prism (version 7, GraphPad Software, La Jolla, CA, USA). Behavioral measures and glutamate concentrations were compared using (RM) Analysis of Variance (ANOVA)s with Time as the within-subject factor and Treatment (ceftriaxone/vehicle) and Liquid (alcohol/water) as between-subject factors. AUC glutamate was also calculated according to [40], and AUC during test was divided by that during baseline to yield a “percent baseline AUC value.” The integrated optical density of GLT-1 bands was normalized to the yoked-saline-vehicle group, yielding “percent control values” that were compared with Infusion × Liquid ANOVAs, as were the percent baseline AUC values. One-way ANOVAs were used to compare Fos expression between groups. Significant main effects and/or interactions were followed by Sidak’s post-hoc analyses. Pearson r tests explored relationships between active lever presses during reinstatement tests, total alcohol consumed (g/kg), total cocaine consumption (mg/kg), and percent baseline AUC glutamate. Values more than two standard deviations from the mean were considered outliers [41].
Results
Experiment 1: sequential alcohol-cocaine self-administration does not influence intake of either drug but increases GLT-1 surface expression
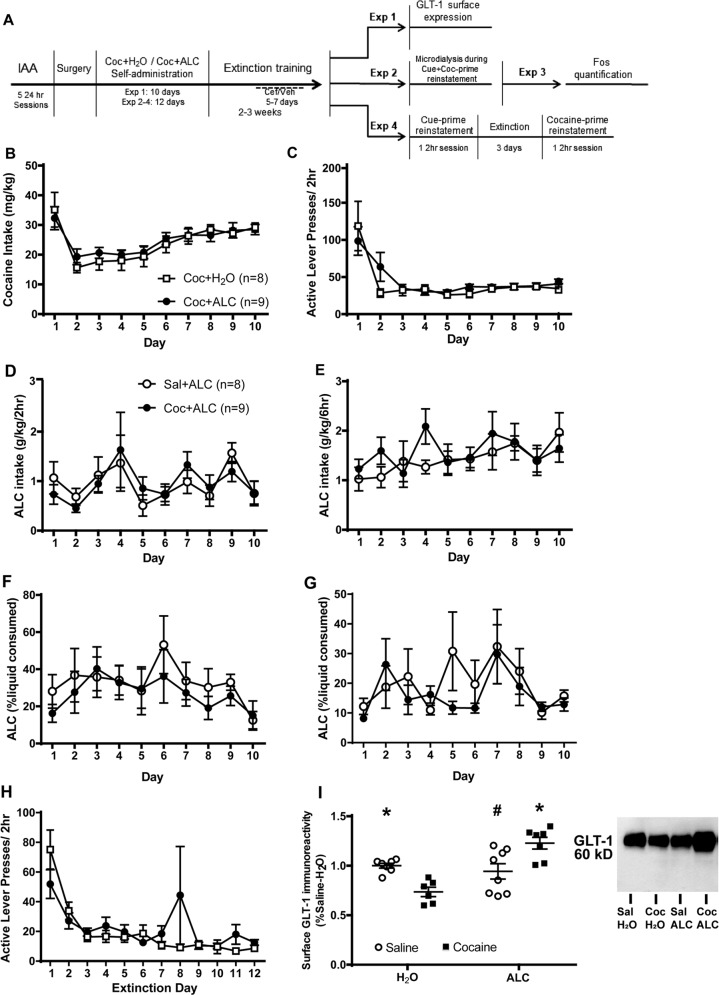
Alcohol intake prior to self-administration did not differ between groups (cocaine: 11.25 ± 1.386; yoked-saline: 9.190 ± 0.935 g/kg). Cocaine intake (Fig. 1b) and infusion number (not shown) did not differ between alcohol- and water-consuming groups. There were no group differences in active (Fig. 1c) or inactive lever presses (not shown). There were no differences in alcohol intake during the first 2 h (Fig. 1d) and entire 6 h of alcohol access after operant sessions (Fig. 1e). The percent total liquid intake that was alcohol did not differ between groups (Fig. 1f, g). Thus, neither of drug influenced the mean daily intake of the other.
Fig. 1.
Access to alcohol immediately following cocaine or saline self-administration sessions did not alter intake of either cocaine or alcohol. a Timeline of methods for all experiments. b Mean cocaine intake (mg/kg) attained during 2 h intravenous cocaine self-administration sessions did not differ between rats afforded 2-bottle choice between water and alcohol (ALC) and those given only water. c Mean number of active lever presses during self-administration also did not differ between groups. d, e The amount of alcohol intake during the 2 and 6 h access to alcohol did not differ between groups that self-administered cocaine or saline. f, g The percent liquid consumed that was alcohol did not differ between groups when assessed at 2 and 6 h after presentation of bottles. h Active lever presses during extinction did not differ between groups. i Surface GLT-1 expression was reduced in the NA core of Coc-H2O rats relative to SAL-H2O rats. Coc-ALC rats displayed greater expression relative to Coc-H2O and Sal-ALC rats. * = p < 0.05 vs. Coc-H2O; # = p < 0.05 vs. Coc-ALC
During extinction, there were no differences in active lever presses (Fig. 1h) but inactive lever presses differed by group [F(11,165) = 1.915, p = 0.0407]. Post-hoc tests failed to find any significant contrasts. A significant Infusion × Liquid interaction was detected for surface GLT-1 [F(1,24) = 21.71, p < 0.0001; Fig. 1i]. The cocaine + water group had reduced surface GLT-1 expression relative to saline-water rats, and cocaine + alcohol rats displayed greater expression relative to cocaine + water and saline + alcohol rats.
Experiment 2: sequential alcohol + cocaine self-administration alters the ability of ceftriaxone to attenuate reinstatement of cocaine-seeking and NA core glutamate content
Rats were eliminated from analyses due to catheter failure (n = 5), failure to acquire (n = 3), and extinguish cocaine-seeking (n = 1). Two rats (Coc + ALC + Veh: 1; Coc + H2O + Cef: 1) were eliminated from analysis due to reinstatement active lever pressing above two standard deviations away from the mean. One rat (Coc + ALC + Cef) displayed outlier glutamate levels for nine of 19 HPLC samples and was eliminated from analysis of both glutamate and Fos expression, as elevated glutamate may have confounded the Fos analysis. Thus, the analysis of behavior for this study is conducted on 33 rats. HPLC data are not present for five rats due to complications during microdialysis (e.g., broken probe).
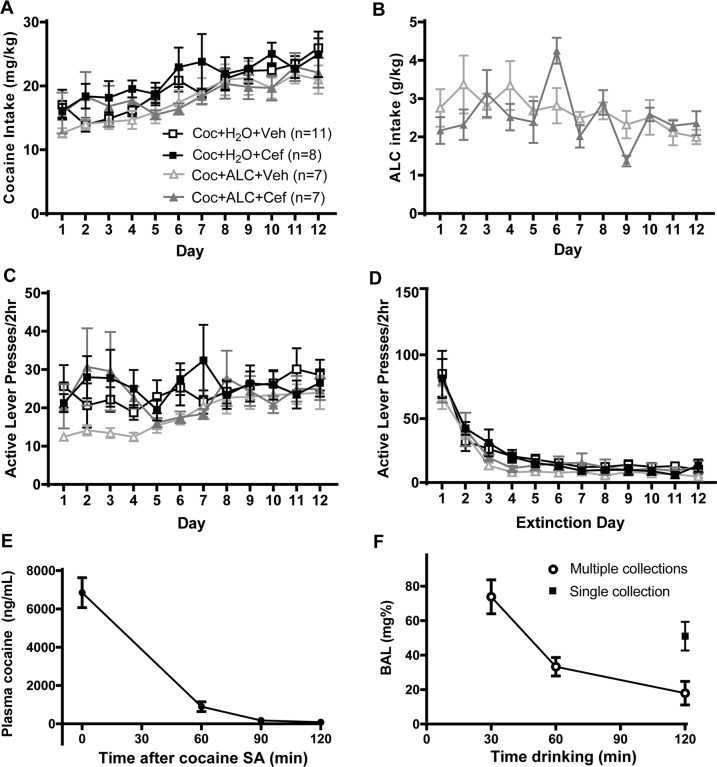
Prior to cocaine self-administration, mean alcohol intake did not differ between groups (Cef: 5.897 ± 0.3662; Veh: 5.9656 ± 0.8402 g/kg). Cocaine intake (Fig. 2a), mean infusions (not shown), active (Fig. 2c) and inactive lever (Fig. S1A) presses during self-administration did not differ between groups. There was a significant Group × Time interaction for the amount of alcohol consumed [F(11,132) = 2.1233, p = 0.0227; Fig. 2b]. However, post-hoc tests did not reveal significant contrasts and the total amount of alcohol consumed did not differ between groups.
Fig. 2.
Self-administration behavior in rats that later underwent microdialysis during a reinstatement test. a Cocaine intake did not differ between groups later assigned to vehicle or ceftriaxone, nor did it differ between groups permitted access to alcohol vs. water. b Alcohol consumed immediately following cocaine self-administration did not differ between groups later assigned to receive ceftriaxone or vehicle. Active lever presses during both self-administration (c) and extinction (d) did not differ between groupd later assigned to receive ceftriaxone or vehicle. e Plasma cocaine levels following removal from the last cocaine self-administration session. f Blood alcohol levels in two cohorts of rats; one in which multiple collections were sampled from the catheter during a 2 h drinking period that began at Time 0 and the second in which a single sample was collected at the conclusion of a 2 h period of alcohol access
When rats were sampled throughout the 2-h drinking period, BAL’s peaked at the first sample (Fig. 2f), then declined, likely due to decreased drinking upon repeated sampling. Mean (±SEM) alcohol consumption was 0.9205 ± 0.1182 g/kg at 30 min. While cumulative intake was recorded at 60 (1.605 ± 0.0911 g/kg) and 120 min (2.297 ± 0.1635 g/kg) for these rats, intake did not correlate with BAL, potentially due to alcohol spillage upon repeated cage top openings. When a separate cohort of rats was sampled only after 120 min of alcohol access, BAL was 51.1 ± 8.3 mg%, mean alcohol consumption was 2.132 ± 0.1074 g/kg, and alcohol intake correlated with BAL (r = 0.9092, p = 0.0017). Cocaine levels peaked after conclusion of self-administration (Time 0; Fig. 2e) and declined according to its half-life of ~15 min [42]. Despite rats attaining a mean BAL of 73.8 mg% after 30 min of alcohol access, CE was below the limit of detection (10 ng/mL) in all samples.
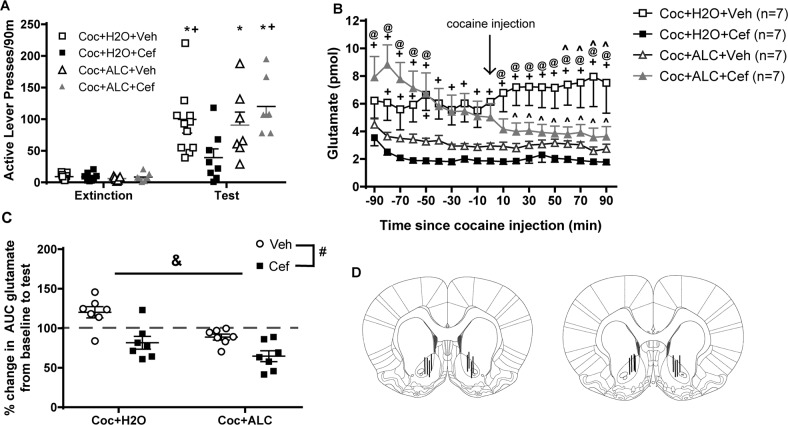
Active (Fig. 2d) and inactive lever presses (Fig. S2B) during extinction did not differ between groups. Comparing active lever pressing during extinction to test, a significant Time × Liquid × Treatment interaction was found [F(11,319) = 4.481, p = 0.043; Fig. 3a], indicating that alcohol consumption influenced the effects of ceftriaxone on reinstatement lever pressing. Post-hoc comparisons determined that active lever presses during the test were greater than those during extinction for all groups except for the Coc + H2O + Cef group. Between-group comparisons found that the Coc + H2O + Cef group displayed less presses on the previously active lever during the test than did both the Coc + H2O + Veh and Coc + ALC + Cef groups. No main effects or interaction was observed for inactive lever presses during extinction and testing (Fig. S1C).
Fig. 3.
During cue + cocaine-primed reinstatement of cocaine-seeking, both active lever pressing and NA core glutamate efflux were influenced by prior exposure to alcohol and ceftriaxone. a Ceftriaxone prevented the reinstatement of cocaine-seeking in rats that consumed water but not those that consumed alcohol. b Glutamate efflux both prior to and during the reinstatement test differed between groups. c Computing area under curve of glutamate content during the reinstatement test relative to baseline revealed significant effects of both Liquid (ALC vs. H2O) and Treatment (Cef vs. Veh). d Representative dialysis probe placements from 16 rats. * = p < 0.05 compared to extinction; + = p < 0.05 vs. Coc + H2O + Cef; @ = p < 0.05 vs. Coc + ALC + Veh; ^ = p < 0.05 vs. respective baseline; # = p < 0.05 comparing Veh to Cef; & = p < 0.05 comparing ALC to H2O
A significant Time × Liquid × Treatment interaction was found for glutamate content [F(18,432) = 1.260, p = 0.03]. Post-hoc analyses revealed several significant between-group and within-group comparisons (Fig. 3b). The most notable is that cocaine + water rats displayed increased glutamate efflux during reinstatement relative to baseline and to other groups during reinstatement; this pattern was prevented by subchronic ceftriaxone. Ceftriaxone had a different effect in cocaine + alcohol rats, producing increased baseline glutamate levels that declined over the course of collection. During the last 30 min of the baseline period, glutamate concentrations did not vary by more than 4% of the mean for all groups. Main effects of Liquid [F(1,24) = 22.19, p < 0.0001] and Treatment [F(1,24) = 13.14, p = 0.0013; Fig. 3c] were found for the percent change in AUC glutamate from baseline to test and total alcohol consumption negatively correlated with these values (r = −0.4644, p = 0.0128). Placement of microdialysis probes from 16 representative rats is depicted in Fig. 3d.
Experiment 3: a history of alcohol consumption alters the pattern of reinstatement-induced Fos expression in several brain regions
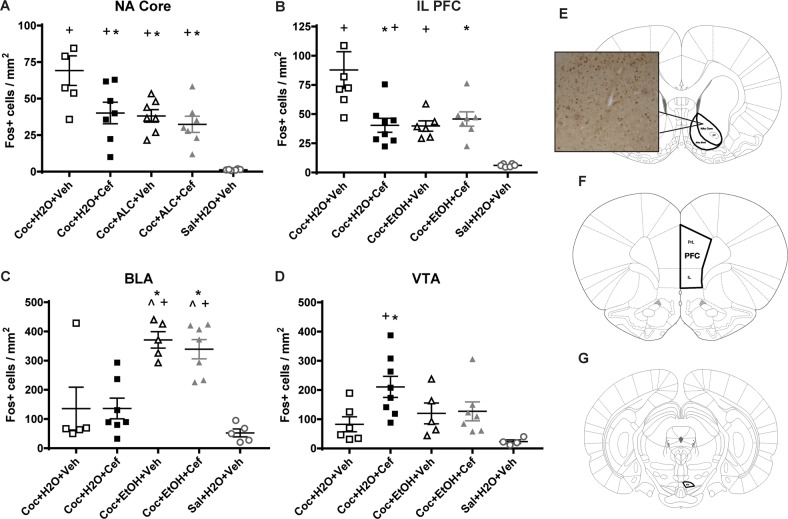
NA core [F(4,28) = 13.24, p < 0.0001; Fig. 4a, S3] and NA shell [F(4,28) = 9.768, p < 0.0001; not shown] Fos expression differed by group with all cocaine groups displaying more Fos expression than the Sal + H2O + Veh group. In the core only, the Coc + H2O + Veh group expressed more Fos than all other groups. NA shell Fos expression negatively correlated with total alcohol consumption (r = −0.3721, p = 0.03) while NA core expression did not (r = −0.4153, p = 0.05).
Fig. 4.
Reinstatement-induced Fos expression was influenced by prior exposure to alcohol and ceftriaxone. In the NA core (a) and Infralimbic (IL) PFC (b), all groups showed more Fos expression than the Sal + H2O + Veh group and the Coc + H2O + Veh group showed more Fos than all other cocaine groups. c In the BLA, both alcohol-consuming groups displayed greater Fos expression than both cocaine and water-consuming groups as well as the Sal + H2O + Veh group. d The Coc + H2O + Cef group displayed greater Fos expression in the VTA than the Coc + H2O + Veh and the Sal + H2O + Veh groups. * = p < 0.05 vs. Coc + H2O + Veh; + = p < 0.05 vs. Sal + H2O + Veh. ^ = p < 0.05 compared to Coc + Cef + Veh. The number of rats per group ranges from 5–8 due to damage to tissue during immunohistochemistry. e Fos analysis sites in the NA core and shell, PFC (f), and VTA (g)
The NA core receives glutamate projections from both the prelimbic (PrL) and infralimbic (IL) regions of the PFC, which have distinct and overlapping roles in mediating reinstatement of cocaine-seeking [43]. For both the PrL [F(4,30) = 10.23, p < 0.0001; not shown] and IL [F(4,29) = 11.15, p < 0.0001; Fig. 4b], Fos expression differed by group. For both regions, post-hoc analyses showed that all cocaine groups displayed higher Fos expression than the Sal + H2O + Veh group, and the Coc + H2O + Veh group displayed greater Fos expression than both alcohol-consuming groups. Neither PrL nor IL Fos expression correlated with alcohol consumption.
BLA Fos expression differed by group [F(4,24) = 11.64, p < 0.0001; Fig. 4c] and correlated with total alcohol consumption (r = 0.7037, p = 0.002). Both alcohol-consuming groups displayed greater Fos expression than all other groups. VTA Fos expression differed by group [F(4,25) = 4.278, p = 0.0090; Fig. 4d]. The Coc + H2O + Cef group displayed more expression than the Coc + H2O + Veh and the Sal + H2O + Veh group.
Experiment 4: ceftriaxone does not attenuate cue- and cocaine-primed reinstatement in cocaine + alcohol rats
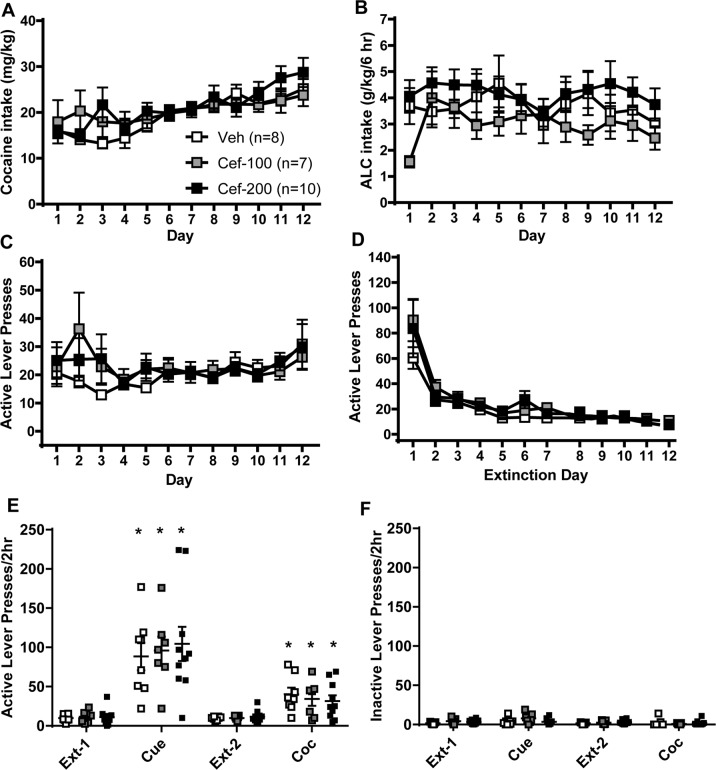
We then tested the ability of two doses of ceftriaxone (100 and 200 mg/kg) to attenuate the reinstatement of cocaine-seeking prompted by cues and cocaine separately. Four rats were excluded for catheter failure. Cocaine intake (Fig. 5a) and infusions (not shown) did not differ between groups, nor did the amount of alcohol consumed prior to surgery (Veh: 4.783 ± 0.4085; Cef-100: 4.934 ± 0.5316; Cef-200: 4.791 ± 0.3297 g/kg). The amount of alcohol consumed (Fig. 5b), and active (Fig. 5c) and inactive lever presses during self-administration sessions did not differ between groups. During extinction, the number of active (Fig. 5d) and inactive lever presses did not differ between groups. For both cue- and cocaine-primed reinstatement, no Group × Time interaction was detected. There was a main effect of Time for both cue- [F(1,22) = 56.89, p < 0.0001; Fig. 5e] and cocaine-primed reinstatement [F(1,22) = 28.35, p < 0.0001]. Post-hoc tests comparing extinction to test found that all groups, regardless of dose of ceftriaxone, reinstated cocaine-seeking. No Group × Time interaction was found for inactive lever presses (Fig. 5f).
Fig. 5.
Two doses of ceftriaxone that attenuate alcohol and cocaine-seeking when each drug is consumed alone do not attenuate cue- or cocaine-primed reinstatement of cocaine-seeking in rats with a history of both cocaine and alcohol use. a Cocaine intake (mg/kg) does not differ between groups later assigned to receive vehicle or ceftriaxone. b Alcohol (g/kg) consumed during self-administration did not differ between groups later assigned to receive ceftriaxone or vehicle. Lever presses on the previously active lever during self-administration (c) and extinction training (d) did not differ between groups later assigned to receive ceftriaxone or vehicle during self-administration or extinction training. All groups reinstated pressing on the previously active (e) but not inactive (f) lever during both cue-prime and cocaine-prime reinstatement tests. * = p < 0.05 comparing extinction to test
Discussion
Here we developed a model of cocaine and alcohol polysubstance use in which rats reliably self-administered cocaine and unsweetened alcohol. Rats consumed between 2 and 5 g/kg alcohol in 6 h, attaining BAL’s of ~51 mg% in the first 2 h. On average, rats that self-administered cocaine did not consume more alcohol than yoked-saline rats and, conversely, rats with access to alcohol did not consume more cocaine than rats with access to only water. This contrasts with our previous work showing that rats administered non-contingent intravenous cocaine (1 mg/kg once/day/20 days) increase consumption of a sweetened 8% alcohol solution relative to rats receiving saline infusions [44]. Notable differences between the studies include the contingency and cumulative intake of cocaine. Furthermore, in our previous work, rats were water-deprived and access to daily sweetened alcohol was limited to 30 min/day, while here rats had 6 h/day access to unsweetened alcohol.
In rhesus monkeys trained to self-administer intravenous cocaine in the absence of alcohol, daily alcohol access 4 h after the conclusion of cocaine self-administration increased the self-administration of lower doses of cocaine [11]. Intake of higher doses (0.1–1.0 mg/kg) was not affected by alcohol co-consumption, in agreement with the present data generated with a dose of 1.05 mg/kg/infusion. Alcohol intake in monkeys was not increased by cocaine access relative to pre-cocaine baseline consumption [11], also in agreement with the present results. Thus, the limited data available indicate that intake of higher doses of cocaine is unaffected while self-administration of lower doses of cocaine may be increased by alcohol consumption.
Alcohol intake results in increased surface GLT-1 expression after cocaine
Similar levels of cocaine intake between conditions allowed us to probe the neurobiology underlying reinstatement without the confound of altered cocaine intake. In agreement with previous work, cocaine reduced surface GLT-1 expression [23, 24, 28]. GLT-1 surface expression in yoked-saline rats was unaffected by alcohol, in agreement with previous assessment 24 h after cessation of drinking [33]. Interestingly, cocaine + alcohol rats displayed greater surface GLT-1 expression relative to cocaine + water rats, indicating a non-additive effect of cocaine and alcohol on GLT-1 expression. In continuously drinking alcohol-preferring “P” rats given daily cocaine injections (10 mg/kg IP for 7 days) and killed 5 days later, nucleus accumbens GLT-1 mRNA is increased relative to drug-naïve rats and rats that consumed only alcohol [45]. Thus, the increased GLT-1 observed here in the cocaine + alcohol condition may arise from increased transcription, despite no changes reported in GLT-1 mRNA after alcohol or cocaine alone [24, 45, 46]. We speculate that this may be a compensatory adaptation following daily acute increases in NA glutamate during cocaine self-administration and alcohol consumption, as has been previously demonstrated [47, 48].
Ceftriaxone attenuates cue + cocaine-primed reinstatement only in rats without a history of alcohol consumption
In rats that did not consume alcohol, cue + cocaine-primed cocaine-seeking was prevented by ceftriaxone, in agreement with its ability to attenuate cue- and cocaine-primed reinstatement [21, 24, 27, 28, 49]. Ceftriaxone does not affect consumption of chow, sucrose, or sweetened condensed milk, reinstatement of food-seeking, or spontaneous locomotion [23, 27, 30, 50]. Ceftriaxone unable to attenuate cue-, cocaine-, or cue + cocaine-primed reinstatement of cocaine-seeking in rats with a history of alcohol consumption. The failure of ceftriaxone to attenuate reinstatement in cocaine + alcohol rats is likely due to the fact that neither GLT-1 expression nor glutamate efflux displayed the same pattern as in the cocaine + water condition, as ceftriaxone targets those adaptations to attenuate reinstatement [21, 24].
Glutamate efflux in the NA core was altered by both a history of alcohol consumption and ceftriaxone
The reinstatement of cocaine-seeking is accompanied by glutamate efflux in the NA core when reinstatement is “primed” by cocaine itself [18, 20, 21, 34], cocaine-associated cues [51], and the cocaine self-administration context [24]. Here, cue + cocaine-primed reinstatement was accompanied by increased NA core glutamate efflux in rats with a history of cocaine self-administration alone. In contrast, rats with a history of both cocaine and alcohol consumption did not show elevated glutamate in the NA core during reinstatement of cocaine-seeking. These data are notable because no other factor has been demonstrated to change the role of NA core glutamate efflux in cocaine reinstatement (e.g., withdrawal period, amount of cocaine intake, extinction training vs. abstinence). Glutamate efflux during the test (AUC) negatively correlated with the total amount of alcohol consumed, further supporting a relationship between these variables.
In the NA core, “basal” glutamate levels are reduced 2–3 weeks after cocaine self-administration (relative to cocaine-naïve rats) and are restored by subchronic ceftriaxone [21]. In this previous work, basal glutamate was assessed via no-net-flux microdialysis outside the cocaine-taking environment. The “baseline” glutamate assessed here in the cocaine-taking context should not be compared to basal glutamate, but instead should be viewed as glutamate evoked by the cocaine-taking context, as has been demonstrated previously [17]. In cocaine + water rats that received ceftriaxone, glutamate levels were low throughout baseline and the reinstatement test, consistent with the lack of reinstatement in this group and previous literature [21]. Thus, the present data provide evidence that ceftriaxone reduces glutamate efflux both prior to and during a cocaine + cue-primed reinstatement test, likely a consequence of the increased GLT-1 expression and activity that we and others have previously reported in this condition [21, 23, 24, 27]. Consistent with this interpretation, vehicle-treated cocaine + alcohol rats displayed both increased GLT-1 surface expression and reduced glutamate efflux prior to and during reinstatement, relative to cocaine + water rats treated with vehicle. These results agree with the previously established role for GLT-1 in reducing glutamate efflux in the cocaine-associated environment [34]. Ceftriaxone-treated cocaine + alcohol rats displayed higher baseline glutamate relative to ceftriaxone-treated cocaine + water rats, and no increase in efflux from baseline to test. Future work will identify the molecular underpinnings of altered glutamate homeostasis after cocaine + alcohol self-administration, with a focus on proteins known to regulate basal and evoked glutamate, such as xCT and mGlu2/3 [52]. Another future goal will be to assess basal glutamate using no-net-flux microdialysis.
Fos expression patterns are altered by both a history of alcohol access and ceftriaxone treatment
In cocaine + water rats, NA core and PFC Fos expression was reduced following ceftriaxone. Reduced glutamate efflux in the NA during reinstatement likely resulted in less Fos production, as NMDA and metabotropic glutamate receptor signaling yield Fos [53, 54]. Inactivation of the dmPFC prevents glutamate efflux during cocaine-primed reinstatement [20]; thus a reduction in PFC activation may contribute to reduced glutamate efflux and reinstatement in ceftriaxone-treated rats. Ceftriaxone-treated cocaine + water rats also displayed increased Fos expression in the VTA. This was unexpected, and future work will determine the cell-type upregulated and whether it is required for ceftriaxone to attenuate reinstatement.
In the BLA, Fos expression was elevated in both cocaine + alcohol groups, relative to all other groups and expression positively correlated with alcohol intake. Pharmacological inactivation of the BLA inhibits cocaine + cue-primed reinstatement of cocaine-seeking [55] and optogenetic inactivation of the BLA and the BLA to NA core pathway inhibits cue-primed reinstatement of cocaine-seeking [56]. Thus, it is possible that this region is mediating the reinstatement of cocaine-seeking in cocaine + alcohol rats in a ceftriaxone-insensitive manner.
Conclusion
Sequential cocaine and alcohol consumption produced non-additive neuroadaptations unique from those produced by either drug alone. These differences were not caused by cocaethylene, as it was not formed here. The amount of alcohol consumed varied within groups and between experimental cohorts, and correlated both with BLA and NA shell Fos expression and with glutamate efflux during reinstatement, indicating that these neuroadaptations are dose-dependent. At present, the mechanism(s) underlying reinstatement of cocaine-seeking following cocaine + alcohol self-administration is unknown. Identifying such mechanisms are an important goal for future research in order to develop targeted medications for reducing relapse in cocaine + alcohol polysubstance users.
Funding and disclosure
This work was funded by National Institutes of Health grants DA033436 and DA045140 awarded to Lori Knackstedt (UF) and a pilot grant from P50 DA015369 awarded to Peter Kalivas (MUSC). The authors declare no competing interests.
Supplementary information
Acknowledgements
We would like to thank Lizhen Wu and Brooke Jackson for their technical assistance with these projects. BAS and LAK designed the studies. BAS and YP-H contributed to data collection. BAS and LAK analyzed the data and wrote the manuscript.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0452-2).
References
- 1.Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015;37. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.htm
- 2.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 3.Brookoff D, Rotondo MF, Shaw LM, Campbell EA, Fields L. Coacaethylene levels in patients who test positive for cocaine. Ann Emerg Med. 1996;27:316–320. doi: 10.1016/s0196-0644(96)70266-4. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- 5.Kedia S, Sell MA, Relyea G. Mono- versus polydrug abuse patterns among publicly funded clients. Subst Abus Treat Prev Policy. 2007;2:33. doi: 10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Williamson V, Setlow B, Cottler LB, Knackstedt LA. The importance of considering polysubstance use: lessons from cocaine research. Drug Alcohol Depend. 2018;192:16–28. doi: 10.1016/j.drugalcdep.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- 9.Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis. 1993;181:71–79. doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Anderson AL, Reid MS, Li S-H, Holmes T, Shemanski L, Slee A, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czoty PW. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2015;153:278–285. doi: 10.1016/j.drugalcdep.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattison LP, McIntosh S, Budygin EA, Hemby SE. Differential regulation of accumbal dopamine transmission in rats following cocaine, heroin and speedball self-administration. J Neurochem. 2012;122:138–146. doi: 10.1111/j.1471-4159.2012.07738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pattison LP, McIntosh S, Sexton T, Childers SR, Hemby SE. Changes in dopamine transporter binding in nucleus accumbens following chronic self-administration cocaine: heroin combinations. Synapse. 2014;68:437–444. doi: 10.1002/syn.21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker DA, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 16.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur Neuropsychopharmacol. 2016;26:186–194. doi: 10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutgen V, Kong L, Kau KS, Madayag A, Mantsch JR, Baker DA. Time course of cocaine-induced behavioral and neurochemical plasticity. Addict Biol. 2014;19:529–538. doi: 10.1111/j.1369-1600.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. Drug-induced plasticity contributing to heightened relapse susceptibility: neurochemical changes and augmented reinstatement in high-intake rats. J Neurosci. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCrosse AL, O’Donovan SM, Sepulveda-Orengo MT, McCullumsmith RE, Reissner KJ, Schwendt M, et al. Contrasting the role of xCT and GLT-1 upregulation in the ability of ceftriaxone to attenuate the cue-induced reinstatement of cocaine seeking and normalize AMPA receptor subunit expression. J Neurosci. 2017;37:5809–5821. doi: 10.1523/JNEUROSCI.3717-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepulveda-Orengo MT, Healey KL, Kim R, Auriemma AC, Rojas J, Woronoff N, et al. Riluzole impairs cocaine reinstatement and restores adaptations in intrinsic excitability and GLT-1 expression. Neuropsychopharmacology. 2018;43:1212–1223. doi: 10.1038/npp.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20:316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bechard AR, Hamor PU, Schwendt M, Knackstedt LA. The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology. 2018;235:837–848. doi: 10.1007/s00213-017-4802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stennett BA, Frankowski JC, Peris J, Knackstedt LA. Ceftriaxone reduces alcohol intake in outbred rats while upregulating xCT in the nucleus accumbens core. Pharmacol Biochem Behav. 2017;159:18–23. doi: 10.1016/j.pbb.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Weiland A, Garcia S, Knackstedt LA. Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol. 2015;6:44. doi: 10.3389/fphar.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald S, MacIntyre P, Joordens C, Stockwell T, Martin G. Factors related to simultaneous cocaine and alcohol use for clients in treatment. J Alcohol Drug Depend. 2015;2:100193. [Google Scholar]
- 32.Gossop M, Manning V, Ridge G. Concurrent use and order of use of cocaine and alcohol:behavioural differences between users of crack cocaineand cocaine powder. Addiction. 2006;101:1292–8. doi: 10.1111/j.1360-0443.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 33.Pati D, Kelly K, Stennett B, Frazier CJ, Knackstedt LA. Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur J Neurosci. 2016;44:1896–1905. doi: 10.1111/ejn.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logan CN, LaCrosse AL, Knackstedt LA. Nucleus accumbens GLT-1a overexpression reduces glutamate efflux during reinstatement of cocaine-seeking but is not sufficient to attenuate reinstatement. Neuropharmacology. 2018;135:297–307. doi: 10.1016/j.neuropharm.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knackstedt Lori A., Schwendt Marek. mGlu5 Receptors and Relapse to Cocaine-Seeking: The Role of Receptor Trafficking in Postrelapse Extinction Learning Deficits. Neural Plasticity. 2016;2016:1–10. doi: 10.1155/2016/9312508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peris J, Zharikova A, Li Z, Lingis M, MacNeill M, Wu MT, et al. Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav. 2006;85:562–568. doi: 10.1016/j.pbb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagerdeo E, Montgomery MA, Lebeau MA, Sibum M. An automated SPE/LC/MS/MS method for the analysis of cocaine and metabolites in whole blood. J Chromatogr B, Anal Technol Biomed Life Sci. 2008;874:15–20. doi: 10.1016/j.jchromb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict Biol. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman DW. A note on the influence of outliers on parametric and nonparametrictests. J Gen Psychol. 1994;121:391–401. [Google Scholar]
- 42.Pan WJ, Hedaya MA. Cocaine and alcohol interactions in the rat: effect on cocaine pharmacokinetics and pharmacodynamics. J Pharm Sci. 1999;88:459–467. doi: 10.1021/js980282p. [DOI] [PubMed] [Google Scholar]
- 43.McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J Neurosci. 2016;36:8700–8711. doi: 10.1523/JNEUROSCI.1291-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knackstedt LA, Ben-Shahar O, Ettenberg A. Alcohol consumption is preferred to water in rats pretreated with intravenous cocaine. Pharmacol Biochem Behav. 2006;85:281–286. doi: 10.1016/j.pbb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Hammad AM, Althobaiti YS, Das SC, Sari Y. Effects of repeated cocaine exposure and withdrawal on voluntary ethanol drinking, and the expression of glial glutamate transporters in mesocorticolimbic system of P rats. Mol. Cell. Neurosci. 2017;82:58–65. doi: 10.1016/j.mcn.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim R, Sepulveda-Orengo MT, Healey KL, Williams EA, Reissner KJ. Regulation of glutamate transporter 1 (GLT-1) gene expression by cocaine self-administration and withdrawal. Neuropharmacology. 2018;128:1–10. doi: 10.1016/j.neuropharm.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miguéns M, Del Olmo N, Higuera-Matas A, Torres I, García-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 48.Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–17. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer KD, Houston ACW, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33:9319–9327. [Google Scholar]
- 50.Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, et al. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci. 2017;37:742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang JQ. Regulation of immediate early gene c-fos and zif/268 mRNA expression in rat striatum by metabotropic glutamate receptor. Brain Res Mol Brain Res. 1998;57:46–53. doi: 10.1016/s0169-328x(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 55.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.