Abstract
Despite the effectiveness of current medications to treat opioid use disorder, there is still a high rate of relapse following detoxification. Thus, there is critical need for innovative studies aimed at identifying novel neurobiological mechanisms that could be targeted to treat opioid use disorder. A growing body of preclinical evidence indicates that glucagon-like peptide-1 (GLP-1) receptor agonists reduce drug reinforcement. However, the efficacy of GLP-1 receptor agonists in attenuating opioid-mediated behaviors has not been thoroughly investigated. Using recently established models of opioid-taking and -seeking behaviors, we showed that systemic administration of the GLP-1 receptor agonist exendin-4 reduced oxycodone self-administration and the reinstatement of oxycodone-seeking behavior in rats. We also identified behaviorally selective doses of exendin-4 that reduced opioid-taking and -seeking behaviors and did not produce adverse feeding effects in oxycodone-experienced rats. To identify a central site of action, we showed that systemic exendin-4 penetrated the brain and bound putative GLP-1 receptors on dopamine D1 receptor- and dopamine D2 receptor-expressing medium spiny neurons in the nucleus accumbens shell. Consistent with our systemic studies, infusions of exendin-4 directly into the accumbens shell attenuated oxycodone self-administration and the reinstatement of oxycodone-seeking behavior without affecting ad libitum food intake. Finally, exendin-4 did not alter the analgesic effects of oxycodone, suggesting that activation of GLP-1 receptors attenuated opioid reinforcement without reducing the thermal antinociceptive effects of oxycodone. Taken together, these findings suggest that GLP-1 receptors could serve as potential molecular targets for pharmacotherapies aimed at reducing opioid use disorder.
Subject terms: Reward, Addiction
Introduction
Prescription opioid misuse and abuse are significant public health concerns, costing over US$78.5 billion annually in lost productivity, healthcare costs, and criminal justice costs [1]. Since the late 1990s, the prevalence of prescription opioid use disorder has escalated rapidly in the United States. In 2017, ~1.7 million Americans 12 years of age and older had a prescription opioid use disorder, and the number of recent initiates of prescription opioid misuse (~2.0 million) was the second highest among the illicit drugs [2]. Unfortunately, there is evidence indicating a relationship between increased abuse of prescription opioid analgesics and development of heroin use disorder [2]. Sadly, an estimated 47,600 Americans died from opioid overdose in 2017, representing a 12% increase from 2016 [3]. Despite the effectiveness of current medications to treat opioid use disorder [4], there is still a high rate of relapse following detoxification [5, 6]. Thus, there is a critical need for research investigating the neurobiological mechanisms contributing to opioid use disorder that could lead to the development of novel pharmacotherapies to treat this disease [7].
Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced peripherally from L cells of the small intestine and centrally by preproglucagon neurons in the nucleus tractus solitarius of the hindbrain [8, 9]. Recent preclinical studies have demonstrated an important role for GLP-1 receptors in addiction-like behaviors [10, 11]. Specifically, systemic administration of the GLP-1 receptor agonist exendin-4 has been shown to attenuate cocaine self-administration in mice [12] and cocaine priming-induced reinstatement of drug-seeking behavior in rats [13]. Systemic infusions of exendin-4 also reduce the rewarding effects of alcohol [14], nicotine [15, 16], cocaine [17, 18], and amphetamine [18] in mice. While these results suggest that GLP-1 receptor agonists, which are FDA-approved for treating type II diabetes and obesity, could be re-purposed to treat substance use disorders, no studies to date have examined the efficacy of GLP-1 receptor agonists to reduce opioid-taking and -seeking behaviors.
Oxycodone is a widely prescribed semisynthetic opioid analgesic and one of the most commonly abused drugs today [2, 19]. Recently, we established rodent models of oxycodone taking and seeking in order to study the neurobiological mechanisms underlying these behavioral responses and screen the efficacy of novel pharmacotherapies to treat opioid use disorder. The present study had four main goals: (1) to assess the ability of the GLP-1 receptor agonist exendin-4 to attenuate oxycodone self-administration and reinstatement; (2) to identify behaviorally selective doses of exendin-4 that reduce oxycodone reinforcement and do not produce adverse feeding effects; (3) to characterize the effects of exendin-4 on oxycodone-induced analgesic responses; and (4) to identify a central mechanism of action by which exendin-4 reduces oxycodone-taking and -seeking behaviors. Our findings indicate that both systemic and intra-accumbens shell infusions of exendin-4 attenuate oxycodone taking and seeking without altering the thermal antinociceptive effects of oxycodone. We also provide the first evidence indicating site- and cell type-specific actions of central GLP-1 receptors in reducing opioid-mediated behaviors. Taken together, the present findings support further studies examining the efficacy of GLP-1 receptor agonists to reduce opioid-mediated behaviors and the central mechanisms regulating these effects.
Materials and methods
Details regarding all drugs used, surgeries, oxycodone self-administration/reinstatement, ad libitum food intake, sucrose self-administration, verification of cannula placements, and immunohistochemical analyses are available in the Supplement.
Animals and housing
Male Sprague–Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY, USA). Transgenic rats expressing Cre recombinase under the rat GAD1 promoter (LE-Tg(Gad1-iCre)3Ottc), Drd1a promoter (LE-Tg(Drd1a-iCre)3Ottc), or the rat Drd2 promoter (LE-Tg(Drd2-iCre)1Ottc) were purchased from the Rat Resource and Research Center (RRRC P40OD011062; University of Missouri, Columbia, MO). Rats were housed individually with food and water available ad libitum in their home cages. A 12/12 h light/dark cycle was used with the lights on at 1900 h. All experimental procedures were performed during the dark cycle. The experimental protocols were consistent with the guidelines issued by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Oxycodone self-administration
Initially, rats were placed in operant conditioning chambers and allowed to lever press for intravenous infusions of oxycodone (0.06 mg/kg/59 µl saline, infused over 5 s) under a fixed-ratio 1 (FR1) schedule of reinforcement. The training dose of oxycodone was based on our pilot studies (Fig. S1) as well as previous studies of oxycodone self-administration in rats [20–22]. Once a rat achieved >15 infusions of oxycodone in three consecutive self-administration sessions under the FR1 schedule, the subject was switched to a fixed-ratio 3 (FR3) schedule of reinforcement. When a rat achieved >15 infusions of oxycodone in three consecutive sessions under the FR3 schedule, the subject was subsequently switched to and maintained on a fixed-ratio 5 (FR5) schedule of reinforcement. All self-administration sessions were 3 h in duration [23, 24] and were conducted 5 days per week. Rats continued to respond for oxycodone on a FR5 schedule for ~14 additional days prior to behavioral testing (i.e., a total of 21–28 days of oxycodone self-administration sessions). Each oxycodone infusion was paired with a 20 s contingent light cue illuminated directly above the active lever (i.e., drug-paired lever). For all FR schedules, a 20-s time-out period followed each oxycodone infusion, during which time active lever responses were tabulated, but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the self-administration sessions.
Effects of systemic exendin-4 on oxycodone self-administration
The effects of systemic exendin-4 were examined in rats that acquired stable oxycodone self-administration on a FR5 schedule as described above. A between-session, within-subjects design was used to screen the efficacy of exendin-4 in reducing oxycodone taking. Each test day was separated by 1–2 days of oxycodone self-administration to ensure that drug taking had stabilized between test sessions. In all experiments, rats were pretreated with vehicle or exendin-4 (0.3 or 3.0 µg/kg, intraperitoneally (i.p.)) 10 min prior to the beginning of the operant session. All doses were counterbalanced and based on previous studies identifying behaviorally relevant doses of exendin-4 that reduce drug-taking and -seeking behaviors in rats [13, 25].
Motivation to self-administer oxycodone was tested in separate groups of rats responding on a progressive-ratio (PR) schedule of reinforcement. Once rats had acquired stable oxycodone self-administration on a FR5 schedule of reinforcement, they were switched to a PR schedule. Under a PR schedule the response requirement for each subsequent infusion increased exponentially until the rat failed to meet a requirement in 60 min [26, 27]. The response requirement for the ith reinforcement was given by R(i) = [5e0.2i − 5]. The breakpoint was operationally defined as the last response requirement completed before the termination of the PR test session. To investigate the effects of exendin-4 on motivation to self-administer oxycodone, rats were pretreated with vehicle or exendin-4 (0.3 or 3.0 µg/kg, i.p.) 10 min prior to PR test sessions using a within-subjects, counterbalanced design. Between PR test sessions, oxycodone self-administration was maintained on a FR5 schedule to ensure stable responding.
Effects of intra-accumbens shell exendin-4 on oxycodone self-administration
To determine the effects of GLP-1 receptor activation in the nucleus accumbens shell on oxycodone-taking behavior, rats were infused with vehicle or exendin-4 (0.005 and 0.05 µg) bilaterally into the shell 10 min prior to oxycodone self-administration test sessions. Separate groups of rats were used to study the effects of intra-accumbens shell exendin-4 on oxycodone self-administration maintained on FR5 and PR schedules of reinforcement. Initially, obturators were removed from the guide cannulas and 33-gauge stainless-steel microinjectors (Plastics One) were inserted. Bilateral infusions into the shell were performed in a total volume of 500 nl with 1.0 µl/min flow rate. Following infusion, microinjectors were left in place for an additional 1 min in order to allow for diffusion of the drug solution away from the tips of the microinjectors. Using a within-subjects design, each rat served as its own control. To control for potential rank-order effects of drug and vehicle administrations, all treatments were counterbalanced across FR5 and PR test sessions. Doses and time course of administration were based on previous studies of intra-cranial exendin-4 on drug-mediated behaviors [13, 25, 27].
Effects of exendin-4 on the reinstatement of oxycodone-seeking behavior
Oxycodone self-administration was extinguished, and the reinstatement of drug-seeking behavior was assessed in separate groups of rats as described in the Supplement. The effects of systemic vehicle and exendin-4 (0.3 and 3.0 µg/kg, i.p.) on drug- and cue-primed reinstatement of oxycodone-seeking behavior were tested using a within-subjects, counterbalanced design. A separate cohort of rats was used to assess the effects of intra-accumbens shell exendin-4 on the reinstatement of oxycodone-seeking behavior. Using a within-subjects design, rats were pretreated with vehicle or exendin-4 (0.005 or 0.05 µg) 10 min prior to an acute priming injection of oxycodone (0.25 mg/kg, i.p.). Rats were then placed immediately into the operant conditioning chambers and allowed to respond for cue lights as described above. Doses of exendin-4 were counterbalanced to avoid rank-order effects of drug treatment and were based on previous studies of systemic and intra-cranial exendin-4 on drug-mediated behaviors [13, 25, 27]. Reinstatement of oxycodone-seeking behavior elicited by cues alone was assessed in a separate group of rats as described in the Supplement.
Thermal nociceptive tests
The effects of exendin-4 on oxycodone-induced analgesic responses were measured using the tail immersion test according to previously described protocols [28–30]. Briefly, each rat was mildly restrained in a clear plastic restrainer that allowed free movement of the tail while limiting movement of the rest of the body. Rats were habituated to the restrainers prior to thermal nociceptive tests. Tail-flick/withdrawal responses to a noxious thermal stimulus were measured before exendin-4 pretreatment (baseline latency) and immediately following oxycodone self-administration and reinstatement test sessions (post-session latencies). The distal 2.5 cm tail was rapidly immersed in a water bath maintained at 51 ± 0.5 °C. Using a hand stopwatch, tail-flick latency was recorded from the time of submersion to the first tail-flick or withdrawal response. The mean of three consecutive latencies, taken at 10 s intervals, was recorded as the tail-flick latency. A cutoff time of 12 s was used to avoid excessive tissue injury following multiple tests. Tail-flick/withdrawal responses were not affected by repeated testing on the same day (Fig. S2). Oxycodone-induced analgesia was calculated by converting the tail-flick latency to percentage of Maximum Possible Effect (%MPE) using the following formula: %MPE = [(post-session latency − baseline latency)/(cutoff latency − baseline latency)] × 100%.
Statistics
For all oxycodone self-administration and reinstatement experiments, total active and inactive lever responses were analyzed with repeated-measures (RM) two-way analyses of variance (ANOVAs). Analyses of total infusions, breakpoints, and total oxycodone infused were performed using RM one-way ANOVAs. For feeding behavior, cumulative chow intake was analyzed with RM two-way ANOVAs, while body weight changes and water intake were analyzed with RM one-way ANOVAs. For hot water immersion tests, tail-flick latencies were analyzed with RM two-way ANOVAs and %MPE were analyzed with RM one-way ANOVAs. Pairwise comparisons were made with Bonferroni post hoc tests (p < 0.05). All data are presented as mean ± SEM.
Results
Systemic administration of exendin-4 dose-dependently attenuates oxycodone self-administration
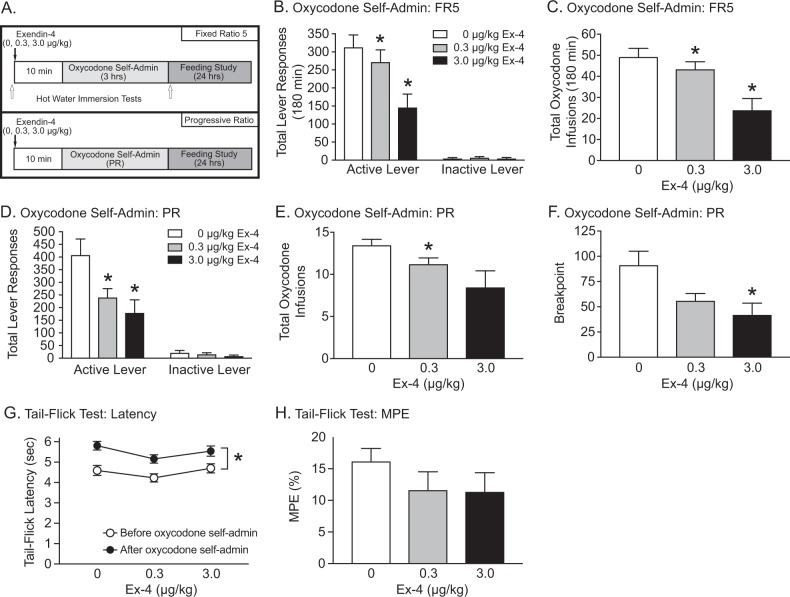
Initially, we established a rodent model of oxycodone self-administration in order to screen the efficacy of novel pharmacotherapies to treat opioid use disorder (Fig. S1). Using this model, we determined the efficacy of exendin-4 to reduce opioid taking in rats (n = 20/treatment) self-administering oxycodone on a FR5 schedule of reinforcement (Fig. 1a). Total lever responses in Fig. 1b were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,38) = 16.87, p < 0.0001]. Total oxycodone infusions in Fig. 1c were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,38) = 15.68, p < 0.001]. Subsequent pairwise analyses indicated that total active lever responses and total oxycodone infusions were significantly decreased in rats pretreated with 0.3 and 3.0 µg/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.05).
Fig. 1.
Systemic administration of exendin-4 attenuates oxycodone self-administration in rats and does not alter the thermal antinociceptive effects of oxycodone. Rats were pretreated with systemic infusions of vehicle or exendin-4 prior to oxycodone self-administration test sessions maintained on FR5 or PR schedules. Food intake, water consumption, and body weight were measured up to 24 h post infusion and thermal nociception was measured before exendin-4 pretreatment and after oxycodone self-administration test sessions (a). In FR5 test sessions, total active lever responses (b) and total oxycodone infusions (c) were significantly decreased in rats (n = 20/treatment) pretreated with 0.3 and 3.0 µg/kg exendin-4 versus vehicle (*p < 0.05, Bonferroni). In PR test sessions, total active lever responses (d) in rats (n = 8/treatment) pretreated with 0.3 and 3.0 µg/kg exendin-4 were significantly decreased compared with vehicle (*p < 0.05, Bonferroni). Total oxycodone infusions (e) in rats pretreated with 0.3 µg/kg exendin-4, and breakpoints (f) in rats pretreated with 3.0 µg/kg exendin-4 were decreased compared with vehicle (*p < 0.05, Bonferroni). Tail-flick latencies were significantly increased after oxycodone self-administration test sessions compared to baseline measurements (g), indicating a thermal analgesic response (*p < 0.05; n = 9/treatment). Percentage maximum possible effect (%MPE) was not significantly different between rats pretreated with exendin-4 and those pretreated with vehicle, indicating no effect of exendin-4 on oxycodone-induced analgesic responses in this model (h)
Next, the efficacy of exendin-4 in reducing motivation to self-administer oxycodone was determined in rats (n = 8/treatment) self-administering oxycodone on a PR schedule of reinforcement (Fig. 1a). Training data for all self-administration/reinstatement experiments are shown in Fig. S3. Total lever responses are shown in Fig. 1d and were analyzed with a two-way RM ANOVA, which revealed a significant drug × lever interaction [F(2,14) = 7.77, p < 0.01]. Subsequent pairwise analyses showed that total active lever responses were decreased in rats pretreated with 0.3 and 3.0 µg/kg exendin-4 versus vehicle-treated controls (Bonferroni, p < 0.05). Separate RM one-way ANOVAs showed significant main effects of exendin-4 on total number of oxycodone infusions [F(2,14) = 5.47, p < 0.05] (Fig. 1e) and breakpoints [F(2,14) = 8.47, p < 0.01] (Fig. 1f). Subsequent post hoc tests revealed a significant decrease in total oxycodone infusions in rats pretreated with 0.3 µg/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.05). In addition, there was a significant decrease in breakpoints in rats pretreated with 3.0 µg/kg exendin-4 versus vehicle-treated controls (Bonferroni, p < 0.05).
Systemic administration of GLP-1 receptor agonists reduces food intake and body weight in both humans and animal models [31]. To control for potential adverse feeding effects, we examined the effects of systemic exendin-4 on ad libitum chow intake, water intake, and body weight in some of the oxycodone-experienced rats (n = 9/treatment) used in the FR5 study above. Cumulative chow intake was analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(2,16) = 5.97, p < 0.01] and time [F(3,24) = 363.2, p < 0.0001]. Subsequent pairwise analyses indicated that food intake was significantly decreased 1 and 3 h post session in rats pretreated with 0.3 and 3.0 µg/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.05). The effects of 0.3 µg/kg exendin-4 on food intake were transient as no significant effects were observed 6 and 24 h post session (Fig. S4A). No significant effects of systemic exendin-4 on 24-h water intake (Fig. S4B) or body weight (Fig. S4C) were noted.
To examine potential nonspecific effects of exendin-4 on operant responding, a separate cohort of rats (n = 12/treatment) was pretreated with vehicle and exendin-4 prior to sucrose self-administration test sessions. No effects of exendin-4 were found on sucrose self-administration (Fig. S5). These results combined with our ad libitum food and water studies, indicate that the suppressive effects of exendin-4 on oxycodone self-administration are not due to deficits in operant responding, locomotor suppression or nonspecific sedative effects.
Systemic exendin-4 does not alter the thermal antinociceptive effects of oxycodone
Responsivity to thermal nociceptive stimuli was measured in rats self-administering oxycodone to determine if exendin-4 pretreatment affects opioid-induced analgesic responses. Thermal nociception was measured in a subset of rats (n = 9/treatment) stably self-administering oxycodone on a FR5 schedule. Tail-flick latencies (Fig. 1g) were analyzed with a RM two-way ANOVA, which revealed a significant main effect of oxycodone self-administration [F(1,8) = 39.63, p < 0.0001] but no effect of exendin-4 [F(2,16) = 2.94, p = 0.09]. Consistent with these findings, a RM one-way ANOVA showed no effect of exendin-4 on %MPE [F(2,16) = 1.610, p = 0.23] (Fig. 1h). Collectively, these data indicate that systemic exendin-4 does not alter the thermal antinociceptive effects of oxycodone at doses that significantly attenuated oxycodone self-administration.
Systemic administration of exendin-4 dose-dependently attenuates the reinstatement of oxycodone-seeking behavior
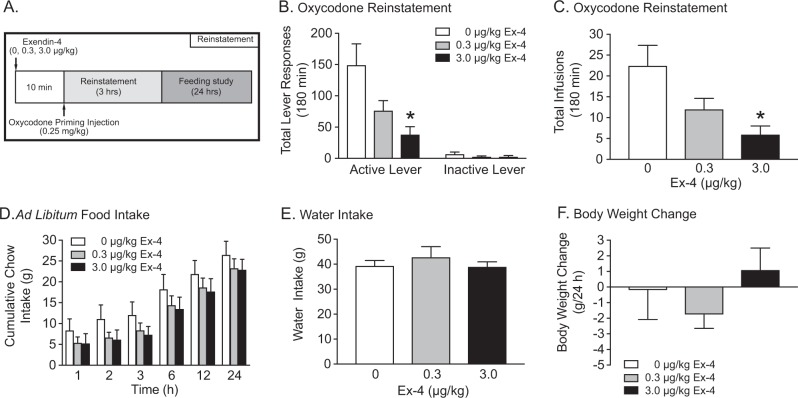
To screen the efficacy of systemic exendin-4 to reduce oxycodone-seeking behavior during abstinence, rats (n = 12/treatment) were pretreated with exendin-4 prior to reinstatement test sessions (Fig. 2a). Total lever responses were analyzed with a RM two-way ANOVA, which revealed a significant drug × lever interaction [F(2,22) = 8.42, p < 0.01] (Fig. 2b). A RM one-way ANOVA revealed a significant main effect of treatment on total infusions during reinstatement test sessions [F(2,22) = 9.32, p < 0.01] (Fig. 2c). Subsequent post hoc analyses indicated that both total active lever responses and total infusions were significantly different between rats pretreated with vehicle and 3.0 µg/kg exendin-4 (Bonferroni, p < 0.05). The effects of systemic exendin-4 on food intake (Fig. 2d), water intake (Fig. 2e), and body weight (Fig. 2f) were also assessed following reinstatement test sessions (n = 6/treatment). No significant effects of exendin-4 were found on any of these measures. We also investigated the ability of exendin-4 to attenuate the reinstatement of oxycodone-seeking behavior elicited by re-exposure to cues alone. Consistent with the effects above, 3.0 µg/kg exendin-4 pretreatment significantly reduced cue-induced reinstatement of oxycodone-seeking behavior (Fig. S6). In addition, we showed that exendin-4 itself did not reinstate oxycodone-seeking behavior in the absence of reinstating stimuli (Fig. S7). Taken together, these findings indicate that systemic exendin-4 attenuates oxycodone-seeking behavior at doses that do not produce adverse feeding or drinking effects.
Fig. 2.
Systemic administration of exendin-4 attenuates oxycodone and cue priming-induced reinstatement of drug-seeking behavior. Rats were pretreated with systemic infusions of vehicle or exendin-4 prior to oxycodone and cue-primed reinstatement test sessions. Food intake, water consumption, and body weight were measured up to 24 h post infusion and reinstatement test sessions (a). Total active lever responses (b) and total infusions (c) were attenuated in rats (n = 12/treatment) pretreated with 3.0 µg/kg exendin-4 versus vehicle (*p < 0.05, Bonferroni). Cumulative chow intake (d), water intake (e), and body weight (f) were not altered in a subset of oxycodone-experienced rats (n = 6/treatment) pretreated with systemic exendin-4 during withdrawal
GLP-1 receptors are expressed on D1R- and D2R-expressing MSNs in the nucleus accumbens shell
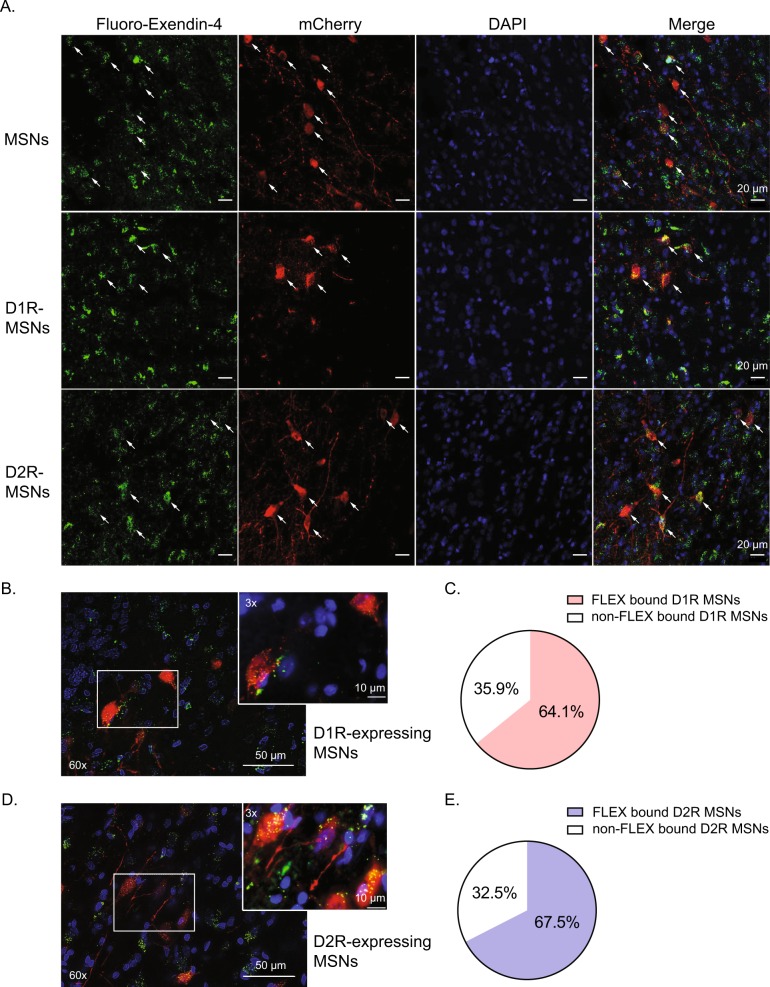
GLP-1 receptors are expressed throughout the brain including the nucleus accumbens [32], a brain region known to play an important role in opioid reinforcement [33–36]. Our results indicate that systemic fluoro-Ex-4 crosses the blood–brain barrier and binds putative GLP-1 receptors expressed on GABAergic MSNs in the accumbens shell (Fig. 3a). Further characterization revealed that GLP-1 receptors are expressed on both dopamine D1 receptor (D1R)- and D2R-expressing MSNs in the shell (Fig. 3a), indicating that GLP-1 receptors are expressed on the two main output pathways of the ventral striatum. Moreover, fluoro-Ex-4 was internalized in D1R- and D2R-expressing MSNs, an event that requires binding of an intact peptide and activation of the cognate receptor (Fig. 3b, d). Our results also indicate that ~64.1% of D1R-expressing MSNs and ~67.5% of D2R-expressing MSNs were bound by fluoro-Ex-4 (Fig. 3c, e).
Fig. 3.
Systemic fluoro-exendin-4 crosses the blood–brain barrier, distributes to the nucleus accumbens shell, and binds putative GLP-1 receptors expressed on D1R- and D2R-expressing MSNs. Transgenic rats expressing Cre recombinase under the GAD1 promoter, Drd1a promoter, or Drd2 promoter were infused with AAV2-hSyn-DIO-mCherry into the nucleus accumbens shell. Three weeks following viral infusion, rats were pretreated with fluoro-Ex-4 (3.0 µg/kg, i.p.) and perfused 3 h later. White arrows highlight neurons colabeled with Cre recombinase (mCherry; red) and fluoro-exendin-4 (green fluorescence). DAPI-labeled cell nuclei are indicated by blue fluorescence. Fluoro-Ex-4 was internalized by D1R-expressing MSNs (b) and D2R-expressing MSNs (d) 3 h post infusion. Approximately 64.1% of D1R-expressing MSNs (c) and 67.5% of D2R-expressing MSNs (e) in the accumbens shell were bound by fluoro-Ex-4 (n = 3/group) (FLEX=fluoro-Ex-4)
Activation of GLP-1 receptors in the nucleus accumbens shell decreases oxycodone self-administration
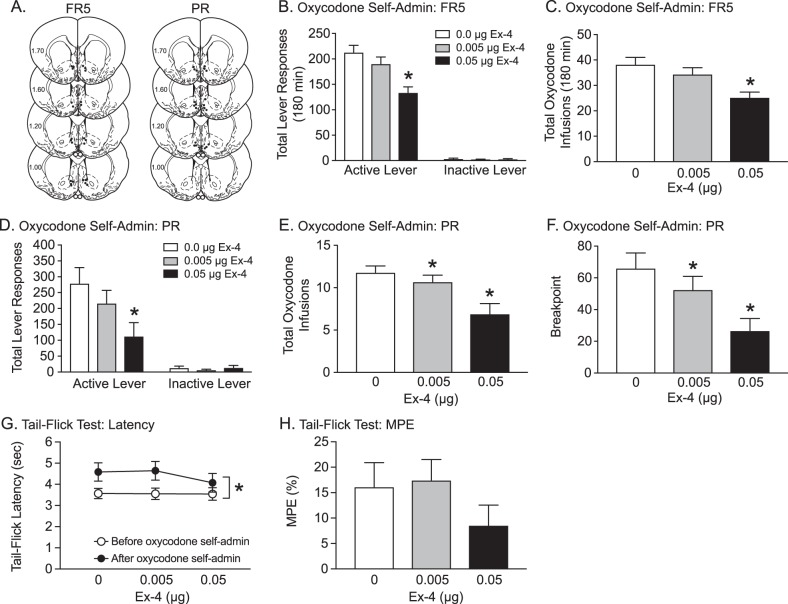
To investigate the role of accumbens shell GLP-1 receptors in oxycodone taking, rats (n = 12/treatment) were pretreated with vehicle or exendin-4 directly into the shell prior to oxycodone self-administration test sessions maintained on FR5. Intra-accumbens infusion sites are shown in Fig. 4a. Total lever responses (Fig. 4b) were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,22) = 18.52, p < 0.0001]. Total oxycodone infusions (Fig. 4c) were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,22) = 18.86, p < 0.0001]. Subsequent pairwise analyses indicated that total active lever responses and total oxycodone infusions were significantly decreased in rats pretreated with 0.05 µg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.05).
Fig. 4.
Administration of exendin-4 into the nucleus accumbens shell attenuates oxycodone self-administration and does not alter the thermal antinociceptive effects of oxycodone. Microinjection sites in the ventral striatum for oxycodone self-administration experiments in which rats were pretreated with vehicle or exendin-4 (a). In FR5 test sessions, total active lever responses (b) and total oxycodone infusions (c) were significantly attenuated in rats (n = 12/treatment) pretreated with 0.05 µg exendin-4 versus vehicle in the shell (*p < 0.05, Bonferroni). In PR test sessions, total active lever responses (d) were significantly decreased in rats (n = 10/treatment) pretreated with 0.05 µg exendin-4 versus vehicle in the shell (*p < 0.05, Bonferroni). Total oxycodone infusions (e) and breakpoints (f) were decreased in rats pretreated with 0.005 and 0.05 µg exendin-4 versus vehicle in the shell (*p < 0.05, Bonferroni). g Tail-flick latencies were significantly increased following oxycodone self-administration test sessions compared to baseline measurements (*p < 0.05), indicating a thermal analgesic response. h No effects of intra-accumbens shell exendin-4 were found on the percentage of maximum possible effect (%MPE), indicating that activation of GLP-1 receptors in the shell does not alter oxycodone-induced analgesic responses in this model (n = 7/treatment)
To determine the effects of accumbens GLP-1 receptor activation on motivation to self-administer oxycodone, exendin-4 was infused directly into the shell of rats (n = 10/treatment) self-administering oxycodone on a PR schedule. Intra-accumbens infusion sites are shown in Fig. 4a. Total lever responses (Fig. 4d) were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,18) = 13.05, p < 0.001]. Subsequent pairwise analyses revealed a significant decrease in active lever responses in rats pretreated with 0.05 µg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.05). Total oxycodone infusions (Fig. 4e) and breakpoints (Fig. 4f) were analyzed with separate RM one-way ANOVAs, which revealed significant main effects of treatment ([F(2,18) = 18.27, p < 0.0001] and [F(2,18) = 18.35, p < 0.001], respectively). Post hoc analyses showed that both 0.005 and 0.05 µg exendin-4 significantly reduced total oxycodone infusions and breakpoints compared to vehicle.
With regard to food intake in oxycodone-experienced rats (n = 10/treatment) pretreated with intra-accumbens shell exendin-4, a RM two-way ANOVA revealed a significant dose × time interaction [F(10,90) = 2.68, p < 0.01] (Fig. S8A). Subsequent pairwise analyses showed that 0.05 µg exendin-4 significantly decreased chow intake at all time points compared to controls (Bonferroni, p < 0.05). Water intake was analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,18) = 21.06, p < 0.0001] (Fig. S8B). Post hoc analyses revealed a significant decrease in water intake in rats pretreated with 0.05 µg exendin-4 versus vehicle (Bonferroni, p < 0.05). Body weight was analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,18) = 6.45, p < 0.01] (Fig. S8C). However, post hoc analyses did not reveal significant differences in body weight between treatments.
Activation of GLP-1 receptors in the nucleus accumbens shell does not alter the thermal antinociceptive effects of oxycodone
To determine whether GLP-1 receptor activation in the shell alters oxycodone-induced analgesic responses, thermal nociception was measured before exendin-4 treatments and after oxycodone self-administration test sessions (n = 7/treatment). Tail-flick latencies (Fig. 4g) were analyzed with a RM two-way ANOVA, which revealed a significant main effect of oxycodone self-administration [F(1,6) = 36.26, p < 0.001], but no effect of exendin-4 [F(2,12) = 0.76, p = 0.49]. Consistent with these findings, no effect of exendin-4 was found on %MPE [F(2,12) = 1.27, p = 0.32] (Fig. 4h). Collectively, these findings indicate that activation of GLP-1 receptors in the shell decreases oxycodone reinforcement without altering oxycodone-induced analgesia.
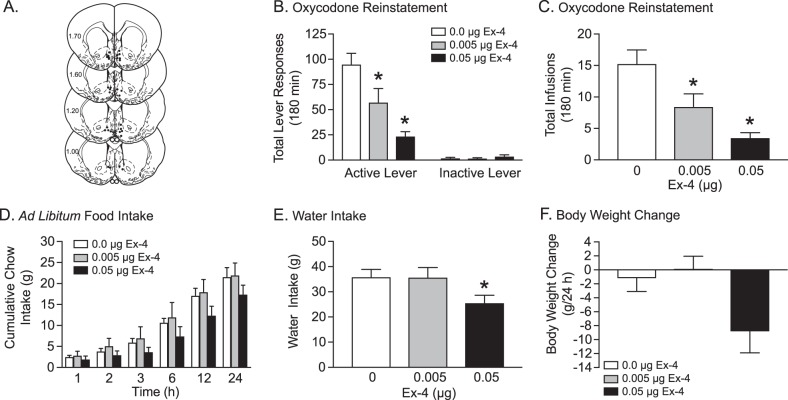
Activation of GLP-1 receptors in the accumbens shell attenuates the reinstatement of oxycodone-seeking behavior
Rats (n = 16/treatment) were pretreated with intra-shell exendin-4 prior to reinstatement test sessions to determine if activation of GLP-1 receptors in the shell reduces oxycodone-seeking behavior during abstinence. Intra-accumbens shell infusion sites are shown in Fig. 5a. Total lever responses (Fig. 5b) were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,30) = 27.21, p < 0.0001]. Subsequent pairwise analyses showed that both 0.005 and 0.05 µg exendin-4 treatments significantly reduced active lever response compared to vehicle-treated controls (Bonferroni, p < 0.05). Total infusions (Fig. 5c) during reinstatement test sessions were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,30) = 23.93, p < 0.0001]. Post hoc analyses showed that total infusions were significantly decreased in rats pretreated with 0.005 and 0.05 µg exendin-4 compared to vehicle-treated controls (Bonferroni, p < 0.005).
Fig. 5.
Administration of exendin-4 into the nucleus accumbens shell attenuates oxycodone and cue priming-induced reinstatement of drug-seeking behavior. Microinjection sites in rats pretreated with vehicle and exendin-4 in the shell prior to reinstatement test sessions (a). Total active lever responses (b) and total infusions (c) were attenuated in rats (n = 16/treatment) pretreated with 0.005 and 0.05 µg exendin-4 versus vehicle in the shell (*p < 0.05, Bonferroni). Exendin-4 infusions in the shell had no effect on cumulative chow intake (d) or body weight change (f) in rats during abstinence (n = 11/treatment). In contrast, 24-h water intake (e) was significantly decreased in rats pretreated with 0.05 µg exendin-4 versus vehicle in the shell (*p < 0.05, Bonferroni)
Food intake, water consumption, and body weight were also assessed in rats (n = 11/treatment) during the reinstatement phase. No effects of exendin-4 were found on cumulative chow intake (Fig. 5d). In contrast, a RM one-way ANOVA revealed a significant main effect of treatment on water intake [F(2,20) = 6.36, p < 0.01] (Fig. 5e). Subsequent pairwise analyses revealed that water intake was significantly decreased in rats pretreated with 0.05 µg exendin-4 in the shell compared to vehicle-treated controls (Bonferroni, p < 0.005). No effects of exendin-4 were found on body weight (Fig. 5f).
Discussion
In the current study, oxycodone maintained robust self-administration in rats and this behavioral response was attenuated by buprenorphine, similar to previously published reports [20–22, 37]. Using this preclinical model of opioid use disorder, we showed that activation of GLP-1 receptors attenuated oxycodone-taking and -seeking behaviors in rats. Specifically, we identified systemic doses of the GLP-1 receptor agonist exendin-4 that selectively reduced oxycodone self-administration and reinstatement in rats and did not produce adverse feeding effects commonly associated with these doses in drug-naive rodents [38, 39]. Moreover, oxycodone-induced thermal antinociceptive responses were maintained in rats pretreated with exendin-4, suggesting that GLP-1 receptor activation reduces opioid reinforcement without affecting opioid-induced analgesic responses. We also discovered that systemic exendin-4 penetrates the brain and binds putative GLP-1 receptors expressed on both D1R- and D2R-expressing MSNs in the nucleus accumbens shell, providing the first insights into the central mechanisms and circuits mediating the effects of systemic exendin-4 on oxycodone-taking and -seeking behaviors. Consistent with our systemic studies, direct infusions of exendin-4 into the accumbens shell reduced oxycodone self-administration and reinstatement at doses that did not affect ad libitum food intake [40, 41]. Collectively, these findings highlight a novel role for GLP-1 receptors in opioid-mediated behaviors and suggest that central GLP-1 receptors could serve as targets for novel medications aimed at treating opioid use disorder.
GLP-1 receptors and opioid-mediated behaviors
Here, we showed that the net effect of systemic exendin-4 pretreatment in rats self-administering oxycodone was an overall attenuation of responding. Specifically, systemic exendin-4 decreased oxycodone consumption in rats responding on FR5 and PR schedules of reinforcement. Taken together, these findings suggest that GLP-1 receptor activation reduces the reinforcing efficacy of oxycodone and motivation to self-administer oxycodone in rats [42]. However, the current studies examined the effects of exendin-4 on only one unit dose of oxycodone, making it difficult to draw firm conclusions about how exactly GLP-1 receptor agonists shift the oxycodone dose–response curve (e.g., downward versus leftward shifts) [43]. Regardless, the present findings are consistent with a growing literature showing that GLP-1 receptor activation reduces the reinforcing effects of licit and illicit drugs [10, 11].
One potential limitation to re-purposing GLP-1 receptor agonists for substance use disorders is their ability to suppress food intake and produce malaise-like effects in rodents and humans [38, 39]. Previous studies in drug-naive rats found that acute administration of exendin-4 at doses higher than 0.25 µg/kg reduced cumulative chow intake for at least 24 h [38, 39]. Interestingly, both doses of exendin-4 (0.3 and 3.0 µg/kg) that reduced oxycodone self-administration in the present study only produced transient decreases in ad libitum food intake in oxycodone-experienced rats. Despite a previous study that showed reduced 24-h water intake in drug-naive rats treated with 3.0 µg/kg exendin-4 [44], neither dose of exendin-4 reduced water consumption in our study. Moreover, neither dose of exendin-4 altered 24-h body weight in oxycodone-experienced rats, consistent with previous findings in drug-naive rats [38, 45]. Taken together, these results highlight behaviorally selective doses of systemic exendin-4 that reduce oxycodone reinforcement and do not affect food intake or body weight.
Systemic exendin-4 also attenuated the reinstatement of oxycodone-seeking behavior at doses that did not affect food intake, water consumption, and body weight during abstinence. Moreover, exendin-4 reduced oxycodone-seeking behavior elicited by both a priming infusion of oxycodone and conditioned light cues as well as re-exposure to cues alone. These findings add to an emerging literature identifying behaviorally selective doses of exendin-4 that reduce both drug- and cue-mediated drug-seeking behaviors [13]. It will be interesting to extend these findings and determine if GLP-1 receptor agonists also reduce somatic and affective withdrawal behaviors during abstinence [46, 47].
The suppressive effects of exendin-4 on oxycodone self-administration and reinstatement are not likely due to motor impairments or deficits in operant responding. We identified systemic (0.3 and 3.0 µg/kg) and intra-cranial (0.005 and 0.05 µg) doses of exendin-4 that attenuated oxycodone self-administration and did not affect 24-h ad libitum food and water intake in opioid-dependent rats. We also showed that systemic exendin-4 (0.3, 1.0, and 3.0 µg/kg) did not affect sucrose self-administration in drug-naive rats. While not statistically significant, there was a trend (p = 0.06) towards decreased sucrose self-administration in rats pretreated with 3.0 µg/kg exendin-4. These results are consistent with previous studies showing that systemic doses of exendin-4 >1.2 µg/kg are required to reduce self-administration of palatable foods (i.e., sweetened vegetable shortening and sucrose pellets) on FR and PR schedules in rats [48, 49]. While doses of exendin-4 higher than 0.6 µg/kg reduced locomotor activity, this effect did not preclude rats from self-administering food (i.e., there was no effect on food self-administration) [48, 49]. Moreover, doses of systemic exendin-4 as high as 2.4 µg/kg did not affect latency to first lever press in a food self-administration paradigm [49]. With regard to intra-cranial infusions of exendin-4, we showed previously that infusions of 0.05 µg exendin-4 directly into the VTA and nucleus accumbens did not affect sucrose self-administration and reinstatement [25, 27]. These effects are consistent with a previous study that showed no effect of intracerebroventricular and intra-accumbens shell 0.03 µg exendin-4 on sucrose self-administration [48]. Moreover, intra-cranial infusions of 0.03 µg exendin-4 did not alter locomotion in rats [48]. Taken together, these findings indicate that exendin-4 attenuates oxycodone self-administration and reinstatement at doses that do not affect operant responding for nondrug rewards and are subthreshold for inhibiting locomotor activity and producing nonspecific sedative effects.
While our study identified systemic doses of exendin-4 that attenuated oxycodone self-administration and reinstatement in rats, a recent paper showed no effects of systemic exendin-4 on remifentanil self-administration in mice [50]. These discordant findings are not easily explained, but may be due to differences in experimental designs. For example, operant responding for remifentanil in Bornebusch et al. [50] was facilitated by prior food training and varying the dose of remifentanil every 3–4 days during the acquisition phase in mice responding on a FR1 schedule of reinforcement. Moreover, mice did not titrate their responding to maintain intake levels when the response requirement was increased and failed to self-administer more remifentanil than saline during subsequent PR tests [50]. Together with a small sample size (n = 4–7/treatment), these limitations produced “strong variability” in responding that make drawing firm conclusions regarding the efficacy of exendin-4 in reducing opioid-mediated behaviors in mice difficult at best. While the pharmacokinetics of exendin-4 are well described in rats [51], there is a paucity of published data describing these parameters in mice. It is also possible that these discordant findings may be due to potential species differences in the pharmacokinetics (i.e., renal clearance, half-life, etc.) of exendin-4 [52]. Thus, more studies are needed to determine the exact role of GLP-1 receptors in opioid-mediated behaviors.
Central GLP-1 circuits and opioid-mediated behaviors
A growing body of evidence indicates that the nucleus accumbens shell is an important node in the neural circuits regulating opioid-mediated behaviors [34, 53, 54]. For example, opioid self-administration is associated with increased dopamine release in the shell, but not core, subregion of the nucleus accumbens [55–57]. Consistent with these effects, a recent study found that chemogenetic inhibition of VTA dopamine neurons that project to the medial shell attenuates heroin reinforcement in mice [34]. In addition, the nucleus accumbens shell plays an important role in the reinstatement of opioid-seeking behavior [53, 54, 58]. Here, we showed that infusions of exendin-4 directly into the accumbens shell reduces oxycodone self-administration and reinstatement. Consistent with our systemic studies, we identified a behaviorally selective dose of intra-accumbens shell exendin-4 (0.005 µg) that reduced opioid-taking and -seeking behaviors and did not affect ad libitum chow intake, water consumption, or body weight in oxycodone-experienced rats. Interestingly, the high dose of exendin-4 (0.05 µg) used in our study attenuated ad libitum chow intake in oxycodone-experienced rats, effects not observed in drug-naive rodents [40]. Collectively, these results indicate that the efficacy of GLP-1 receptor agonists in reducing food intake differs between drug-naive and opioid-experienced rats. Infusion of 0.05 µg exendin-4 into the shell decreased water intake in oxycodone-experienced rats during oxycodone self-administration and withdrawal, consistent with previous studies showing that intra-cerebroventricular infusions of doses of exendin-4 as low as 0.1 µg reduced water intake [44, 59].
The two primary output pathways of the nucleus accumbens consist of GABAergic medium spiny neurons (MSNs) expressing D1Rs (D1R-expressing MSNs) and D2Rs (D2R-expressing MSNs) [60]. Our study reveals that fluoro-Ex-4 binds to both D1R- and D2R-expressing MSNs in the nucleus accumbens shell, indicating that both cell populations express GLP-1 receptors. These two cell populations have opposing functional roles in reward-related behaviors [60, 61]. Activation of D1R-expressing MSNs promotes reward seeking, while activation of D2R-expressing MSNs produces aversive effects and decreases drug reinforcement [60, 61]. With regard to opioid-mediated behaviors, a recent study showed that repeated morphine exposure augments synaptic strength and AMPA receptor-mediated transmission exclusively in D1R-expressing MSNs in the accumbens shell [58]. In contrast, repeated morphine weakens excitatory input at D2R-expressing MSNs, indicating separate roles for these two MSN cell populations in opioid-mediated behaviors [58]. Acute morphine administration exclusively activates D1R-expressing MSNs in the nucleus accumbens, while withdrawal following chronic morphine exposure activates D2R-expressing MSNs [62], results that further support a divergent role for D1R- and D2R-expressing MSNs in opioid-mediated behaviors. Consistent with these effects, remifentanil self-administration desensitizes µ opioid receptor function in D1R-expressing MSNs, but not D2R-expressing MSNs, in the accumbens shell [63]. Taken together, these findings indicate that D1R-expressing MSNs play a greater role in opioid reinforcement and acquisition of opioid taking [62, 63], whereas D2R-expressing MSNs play a more prominent role in opioid-seeking behavior during withdrawal. Since GLP-1 receptors are expressed on both D1R- and D2R-expressing MSNs (present findings), it is possible that exendin-4 attenuates opioid taking by blocking opioid-induced activation of D1R-expressing MSNs and reduces opioid-seeking behavior by inhibiting activation of D2R-expressing MSNs during opioid withdrawal. To validate this hypothesis, future experiments should address how GLP-1 receptor activation regulates D1R- and D2R-expressing MSN function following opioid self-administration and withdrawal.
GLP-1 receptor activation and the antinociceptive effects of oxycodone
Previous studies showed that activating GLP-1 receptors produces analgesic responses in drug-naive rodents. For example, administration of a GLP-1 receptor agonist or exogenous GLP-1 blocked pain hypersensitivity in rats exposed to chronic, but not acute, pain [64, 65]. In agreement with these findings, our results indicate that exendin-4 does not alter the thermal antinociceptive effects of oxycodone, indicating that activation of GLP-1 receptors decreases opioid reinforcement while maintaining the antinociceptive effects of oxycodone. However, it is possible that activating GLP-1 receptors could augment oxycodone-induced analgesic responses in oxycodone-dependent rats exposed to chronic pain. One limitation of the current study is that the antinociceptive effects of exendin-4 were examined in only one pain model. Since pain is a heterogenous condition that differs widely based on the affected tissue(s) and the mechanism of injury [66], a more comprehensive analysis of the analgesic effects of GLP-1 receptor agonists in opioid-dependent rats using other pain models is required to further our understanding of how GLP-1 receptor activation may influence opioid-induced analgesic responses.
GLP-1 receptor expression is increased in spinal microglia, but not astrocytes and neurons, in rats exposed to chronic pain [64, 65]. Activating GLP-1 receptors on spinal microglia releases β-endorphin, which in turn activates opioid receptors on neurons to produce an analgesic response [64]. While it is not clear if similar mechanisms occur in brain nuclei known to regulate drug reinforcement and pain responsivity, it is intriguing to speculate that the efficacy of exendin-4 in reducing oxycodone taking and seeking may be due, in part, to activating GLP-1 receptors expressed on microglia. Indeed, microglial dysregulation has been found to impair reward-related behaviors. Microglial activation decreases reward-related behaviors and inhibition of microglial activation restores reward-related behaviors in opioid-dependent mice [67]. These findings suggest that the suppressive effects of exendin-4 on oxycodone-taking and -seeking behaviors may be mediated via activation of GLP-1 receptors on microglia and regulation of microglial function.
Conclusion
The present study identifies a novel role for GLP-1 receptors in opioid-taking and -seeking behaviors. The thermal antinociceptive effects of oxycodone were not altered by exendin-4, indicating that activation GLP-1 receptors reduced opioid reinforcement and drug seeking without affecting opioid-induced analgesic responses. While the present study focused on acute exendin-4 administration, future experiments examining repeated administration of exendin-4 are required to determine if tolerance develops to the suppressive effects of exendin-4 in opioid-dependent rats. In addition, the efficacy of GLP-1 receptor agonists with different pharmacokinetic profiles should also be evaluated. Together, these findings support further preclinical studies of the role of central GLP-1 signaling in opioid-mediated behaviors.
Funding and disclosure
This work was supported by the following grant from the National Institutes of Health: R01 DA037897 (HDS). HDS was also supported by the Dr. Dorothy Mereness Endowed Research Fund through a Faculty Grant Award in the School of Nursing. YZ is supported by the Jeane B. Kempner postdoctoral fellowship from the University of Texas Medical Branch. HDS and MWK were partially supported by a Louis H. Castor, MD, C’48 Undergraduate Research Grant from the Center for Undergraduate Research and Fellowships at the University of Pennsylvania. HDS and VRW were partially supported by a Mary L. And Matthew S. Santirocco College Alumni Society Undergraduate Research Grant and a Pincus-Magaziner Family Undergraduate Research Grant from the Center for Undergraduate Research and Fellowships at the University of Pennsylvania. NSH is a Howard Hughes Medical Institute Gilliam Fellow. HDS receives funding from Novo Nordisk that was not used to support these studies. The authors declare no other competing financial interests.
Supplementary information
Acknowledgements
We would like to thank Christopher Turner, Amanda Moreno, and Suditi Rahematpura for their technical contributions to this project.
Authors contributions
YZ contributed to the acquisition and analyses of the data as well as drafted the manuscript. JAE, MWK, VRW, NSH, and LMS contributed to data collection. HDS was responsible for the study concept and design, supervised the acquisition of the data, and helped draft the manuscript. All authors reviewed content and approved the final version for publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at (10.1038/s41386-019-0531-4).
References
- 1.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54:901–6. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SAMHSA. 2017. https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report.
- 3.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. Morb Mortal Wkly Rep. 2018;67:1419–27. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis BB, Naji L, Bawor M, Bonner A, Varenbut M, Daiter J, et al. The effectiveness of opioid substitution treatments for patients with opioid dependence: a systematic review and multiple treatment comparison protocol. Syst Rev. 2014;3:105. doi: 10.1186/2046-4053-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes EV, Gordon M, Friedmann PD, Fishman MJ, Lee JD, Chen DT, et al. Relapse to opioid use disorder after inpatient treatment: protective effect of injection naltrexone. J Subst Abus Treat. 2018;85:49–55. doi: 10.1016/j.jsat.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalana H, Kundal T, Gupta V, Malhari AS. Predictors of relapse after inpatient opioid detoxification during 1-year follow-up. J Addict. 2016;2016:7620860. doi: 10.1155/2016/7620860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow Nora D., Jones Emily B., Einstein Emily B., Wargo Eric M. Prevention and Treatment of Opioid Misuse and Addiction. JAMA Psychiatry. 2019;76(2):208. doi: 10.1001/jamapsychiatry.2018.3126. [DOI] [PubMed] [Google Scholar]
- 8.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 10.Jerlhag Elisabet. Gut-brain axis and addictive disorders: A review with focus on alcohol and drugs of abuse. Pharmacology & Therapeutics. 2019;196:1–14. doi: 10.1016/j.pharmthera.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci. 2016;9:66–70. doi: 10.1016/j.cobeha.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–8. doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018;43:2000–8. doi: 10.1038/s41386-018-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013;38:1259–70. doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS ONE. 2013;8:e77284. doi: 10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20:708–16. doi: 10.1038/nn.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2013;18:961–2. doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS ONE. 2013;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:163–72. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- 20.Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS ONE. 2014;9:e101807. doi: 10.1371/journal.pone.0101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2015;40:421–8. doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 2017;234:977–87. doi: 10.1007/s00213-017-4536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, et al. Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci. 2017;8:1065–73. doi: 10.1021/acschemneuro.6b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You Zhi-Bing, Bi Guo-Hua, Galaj Ewa, Kumar Vivek, Cao Jianjing, Gadiano Alexandra, Rais Rana, Slusher Barbara S., Gardner Eliot L., Xi Zheng-Xiong, Newman Amy Hauck. Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology. 2018;44(8):1415–1424. doi: 10.1038/s41386-018-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD. Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addiction Biol. 2019;24:170–81. doi: 10.1111/adb.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology. 2012;37:2310–21. doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2016;41:1917–28. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loram LC, Mitchell D, Skosana M, Fick LG. Tramadol is more effective than morphine and amitriptyline against ischaemic pain but not thermal pain in rats. Pharm Res. 2007;56:80–5. doi: 10.1016/j.phrs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Bao Y, Zhang X, Zeng L, Wang L, Wang J, et al. Optimal interval for hot water immersion tail-flick test in rats. Acta Neuropsychiatr. 2014;26:218–22. doi: 10.1017/neu.2013.57. [DOI] [PubMed] [Google Scholar]
- 30.Haroutiunian S, Kagan L, Yifrach-Damari I, Davidson E, Ratz Y, Hoffman A. Enhanced antinociceptive efficacy of epidural compared with i.v. methadone in a rat model of thermal nociception. Br J Anaesth. 2014;112:150–8. doi: 10.1093/bja/aet234. [DOI] [PubMed] [Google Scholar]
- 31.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–10. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Navratilova E, Atcherley CW, Porreca F. Brain circuits encoding reward from pain relief. Trends Neurosci. 2015;38:741–50. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, et al. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife. 2018;7:e39945. doi: 10.7554/eLife.39945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–63. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–91. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 38.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity. 2011;19:1342–9. doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 39.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–27. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–58. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 43.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 44.McKay NJ, Kanoski SE, Hayes MR, Daniels D. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1755–64. doi: 10.1152/ajpregu.00472.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–12. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology (Berl) 2012;219:59–72. doi: 10.1007/s00213-011-2380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology (Berl) 2017;234:223–34. doi: 10.1007/s00213-016-4452-1. [DOI] [PubMed] [Google Scholar]
- 48.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–20. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernosky-Smith KA, Stanger DB, Trujillo AJ, Mitchell LR, Espana RA, Bass CE. The GLP-1 agonist exendin-4 attenuates self-administration of sweetened fat on fixed and progressive ratio schedules of reinforcement in rats. Pharmacol Biochem Behav. 2016;142:48–55. doi: 10.1016/j.pbb.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bornebusch Annika Billefeld, Fink-Jensen Anders, Wörtwein Gitta, Seeley Randy J., Thomsen Morgane. Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs. eneuro. 2019;6(2):ENEURO.0443-18.2019. doi: 10.1523/ENEURO.0443-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, et al. Pharmacokinetic actions of exendin-4 in the rat: Comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–67. [Google Scholar]
- 52.Chen T, Mager DE, Kagan L. Interspecies modeling and prediction of human exenatide pharmacokinetics. Pharm Res. 2013;30:751–60. doi: 10.1007/s11095-012-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bossert JM, Adhikary S,St, Laurent R, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2016;233:1991–2004. doi: 10.1007/s00213-015-4060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–8. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerrits MA, Petromilli P, Westenberg HG, Di Chiara G, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res. 2002;924:141–50. doi: 10.1016/s0006-8993(01)03105-5. [DOI] [PubMed] [Google Scholar]
- 57.Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194:103–16. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- 58.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKay NJ, Daniels D. Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. J Neuroendocrinol. 2013;25:929–38. doi: 10.1111/jne.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Enoksson T, Bertran-Gonzalez J, Christie MJ. Nucleus accumbens D2- and D1-receptor expressing medium spiny neurons are selectively activated by morphine withdrawal and acute morphine, respectively. Neuropharmacology. 2012;62:2463–71. doi: 10.1016/j.neuropharm.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 63.James AS, Chen JY, Cepeda C, Mittal N, Jentsch JD, Levine MS, et al. Opioid self-administration results in cell-type specific adaptations of striatal medium spiny neurons. Behav Brain Res. 2013;256:279–83. doi: 10.1016/j.bbr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong N, Xiao Q, Zhu B, Zhang CY, Wang YC, Fan H, et al. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci. 2014;34:5322–34. doi: 10.1523/JNEUROSCI.4703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan H, Gong N, Li TF, Ma AN, Wu XY, Wang MW, et al. The non-peptide GLP-1 receptor agonist WB4-24 blocks inflammatory nociception by stimulating β-endorphin release from spinal microglia. Br J Pharmacol. 2015;172:64–79. doi: 10.1111/bph.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14:1255–69. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, et al. Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology. 2016;41:949–59. doi: 10.1038/npp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.