Abstract
Early-life stress has pervasive, typically detrimental, effects on physical and mental health across the lifespan. In rats, maternal-separation stress results in premature expression of an adult-like profile of fear regulation that predisposes stressed rats to persistent fear, one of the hallmarks of clinical anxiety. Probiotic treatment attenuates the effects of maternal separation on fear regulation. However, the neural pathways underlying these behavioral changes are unknown. Here, we examined the neural correlates of stress-induced alterations in fear behavior and their reversal by probiotic treatment. Male Sprague-Dawley rats were exposed to either standard rearing conditions or maternal-separation stress (postnatal days [P] 2–14). Some maternally-separated (MS) animals were also exposed to probiotics (Lactobacillus rhamnosus and L. helveticus) via the maternal drinking water during the period of stress. Using immunohistochemistry, we demonstrated that stressed rat pups prematurely exhibit adult-like engagement of the medial prefrontal cortex during fear regulation, an effect that can be prevented using a probiotic treatment. The present results add to the cross-species evidence that early adversity hastens maturation in emotion-related brain circuits. Importantly, our results also demonstrate that the precocious neural maturation in stressed infants is prevented by a non-invasive probiotic treatment.
Keywords: Early-life stress, Probiotics, Neurodevelopment, Microbiota-gut-brain axis, Fear conditioning, Extinction
1. Introduction
Coping with real or perceived danger requires the ability to appropriately regulate fear. Across development, the processes of fear regulation change in accordance with the individual’s cognitive capacity and the changing demands of their social and physical environment. However, the typical developmental trajectory of fear regulation is altered by exposure to stress or adversity during early life (Callaghan et al., 2014; Callaghan and Tottenham, 2016a; Moriceau et al., 2009).
Preclinical studies have demonstrated that early-life stress accelerates the development of fear regulation behavior for both fear retention and fear inhibition. Using Pavlovian fear conditioning (i.e., pairing of an innocuous conditioned stimulus, CS, with an aversive unconditioned stimulus, US) and other training procedures, it has been repeatedly demonstrated that young animals typically exhibit rapid forgetting (infantile amnesia; Campbell and Spear, 1972; Josselyn and Frankland, 2012; Madsen and Kim, 2016). However, precocious long-term retention of fear memories is observed in infant rats exposed to early-life stress (Callaghan and Richardson, 2012; Cowan et al., 2013; Haroutunian and Riccio, 1979). Stressed infant rats also exhibit an adult-like phenotype of relapse-prone extinction (Callaghan and Richardson, 2011; Cowan et al., 2013). That is, older animals and infants exposed to early-life stress show a return of learned fear in various situations following extinction (the laboratory equivalent of exposure therapy, involving repeated presentation of the CS alone in order to reduce CS-elicited fear; Bouton, 2002). In contrast, unstressed infants do not show this return of fear (for review, see Kim and Richardson, 2010). In summary, the typically-developing infant exhibits a unique phenotype of fear regulation that is characterized by infantile amnesia and relapse-resistant extinction, but stressed infants exhibit extended fear retention and relapse-prone extinction, reflecting a more adult-like fear regulation phenotype. Such precocious maturation has been hypothesized to be adaptive over the short-term but also contribute to an elevated risk of enduring mental health problems (Callaghan and Tottenham, 2016b; Heim and Nemeroff, 2001; McEwen and Gianaros, 2010; Rincón-Cortés and Sullivan, 2014).
Given the broad range of negative long-term outcomes experienced by individuals with a history of early-life stress (Cowan et al., 2016a; Gluckman et al., 2008; Maccari et al., 2014; McLaughlin et al., 2012; Pohl et al., 2015), there is a clear need for research to identify ways of reducing vulnerability in such populations. One recent approach to this problem has been through targeting of the gut microbiota (the community of microorganisms that reside in the gastrointestinal tract; Callaghan, 2017; Gareau et al., 2007; O’Mahony et al., 2017). Growing evidence points to the role of the microbiota-gut-brain axis in modulating responses to stress across the lifespan (Cowan et al., 2018; Callaghan et al., in press; Foster et al., 2017; Gur et al., 2015; Jašarević et al., 2015; Moloney et al., 2014). In the case of early-life stress, there have been promising results with the use of probiotic treatments, which introduce high doses of beneficial bacteria to the microbiota (Callaghan et al., 2016; Cowan et al., 2016b; Desbonnet et al., 2010; Gareau et al., 2007; McVey Neufeld et al., 2017; Moya-Pérez et al., 2017). For example, administration of a commercially-available probiotic, Lacidofil®, during maternal separation has been found to normalize corticosterone release in stressed pups (Gareau et al., 2007), as well as restoring age-appropriate expression of infantile amnesia and relapse-resistant extinction (Callaghan et al., 2016; Cowan et al., 2016a). However, it remains unclear how exposure to stress or probiotic bacteria cause these changes in infants’ behavior. The current study explored the impact of these early-life experiences on the neural circuits underlying fear regulation during infancy.
There are several brain structures that contribute to emotion regulation. In particular, the amygdala and prefrontal cortex are considered critical for learning and extinction of conditioned fear (Maren and Quirk, 2004; Milad and Quirk, 2011), with the prelimbic (PL) and infralimbic (IL) subregions of the medial prefrontal cortex (mPFC) being critical for fear expression and fear inhibition, respectively (Herry and Johansen, 2014; Quirk and Mueller, 2008). Work from our laboratory, and others, has demonstrated that the neural signature of fear regulation changes across development (Gogolla et al., 2009; Kim and Richardson, 2010; Li et al., 2012; Moriceau et al., 2006; Pattwell et al., 2012; Raineki et al., 2010; Tsai et al., 2019). Although the amygdala is recruited during both fear expression and inhibition from a very young age (around postnatal day [P] 10 for fear expression and at least by P17 for fear inhibition; Kim et al., 2009; Moriceau et al., 2004), infant rats do not use or require the mPFC to express or inhibit conditioned fear (Kim et al., 2009; Li et al., 2012; also see Chan et al., 2011 for a similar finding in the expression of innate fear).
The simplified nature of the infant fear network is hypothesized to underlie the reduced flexibility and complexity of infant fear regulation (Kim et al., 2009; Madsen and Kim, 2016). Specifically, the less distributed neural network involved in infant fear expression is thought to destabilize infant fear memories, making them more vulnerable to forgetting (Madsen and Kim, 2016). For fear inhibition, the smaller neural network may reduce flexibility in infants’ behavioral responses after extinction, leading to low relapse rates (Kim et al., 2009). Interestingly, the developmental transition to an adult-like pattern of mPFC engagement occurs between the infancy and juvenile periods (i.e., between the ages of P17-23), coinciding with both the offset of infantile amnesia and the shift from relapse-resistant to relapse-prone extinction (Kim and Richardson, 2010; Li et al., 2012). Given that early-life stress accelerates the transition to an adult-like behavioral profile of fear regulation (Callaghan and Richardson, 2011, 2012), this hypothesis leads to the prediction that stressed infant rats would precociously engage the mPFC during fear regulation. It might also be predicted that the restoration of the normal developmental timing of fear regulation behavior by probiotics (Cowan et al., 2016b) would be accompanied by an age-appropriate, mPFC-independent neural signature in probiotic-exposed stressed infant rats.
The current series of experiments was designed to test these predictions. The first aim was to establish whether stress alters functional maturation of the mPFC by comparing standard-reared (SR) and maternally-separated (MS) infants. Thus, levels of phosphorylated mitogen activated protein kinase (pMAPK) were measured in the PL and IL of P17 SR and MS rats after fear expression and fear inhibition. In addition, the effect of probiotic treatment on pMAPK expression in the PL and IL was examined for MS infants. Phosphorylation of MAPK is a critical step in the MAPK/ERK signaling cascade (which modulates synaptic strength in various brain regions, including the mPFC, in an activity-dependent manner), and has previously been shown to be necessary for memory consolidation of both aversive and appetitive learning (Ribeiro et al., 2005; Schafe et al., 2000). Furthermore, pMAPK was the marker used in the previous experiments that demonstrated developmental dissociations in mPFC involvement in both fear memory and extinction (Kim et al., 2009, 2012; Li et al., 2012).
It was hypothesized that early-life maternal separation stress would accelerate the functional maturation of the mPFC, in parallel with the accelerated maturation of fear regulation observed in these individuals at the behavioral level (Callaghan and Richardson, 2011, 2012; Cowan et al., 2016b). Specifically, it was predicted that MS infants would exhibit higher levels of pMAPK expression in the PL following fear expression and in the IL after extinction when compared to SR infants. It was further hypothesized that probiotics would prevent any MS-induced changes to mPFC involvement in fear expression and inhibition during infancy.
2. Materials and methods
2.1. Experimental animals
Experimentally naïve Sprague Dawley-derived rats were bred and housed in the School of Psychology at UNSW Sydney (original stock obtained from Animal Resources Centre, Western Australia). Rats were housed with their dam and littermates (culled to 8 pups per litter within the first few days after birth) and maintained on a 12-h light/dark cycle (lights on at 0700) with food and water available ad libitum. The day of birth was designated P0. Only male pups were used, with no more than one rat from each litter allocated to any given experimental group. All animals were treated in accordance with The Australian Code of Practice for the Care and Use of Animals for Scientific Purposes 7th Edition (2004), and all procedures were approved by the Animal Care and Ethics Committee at UNSW.
2.2. Maternal separation
Maternal separation was conducted daily from P2-P14 for 3 h commencing between 0800 and 1200. All pups from a given litter were transferred together from the home cage to an incubator, where a heat pad was used to maintain the ambient temperature at ˜27 °C in order to approximate the warmth of the nest. In addition, 3 cm of bedding was provided so pups could behaviorally thermoregulate as needed.
2.3. Probiotic treatment
The probiotic treatment was a commercially available powder formulation, Lacidofil® (Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0052; Lallemand Health Solutions, Montreal, QC, Canada). Powdered Lacidofil was rehydrated in distilled water at a concentration of 109 CFU/mL and provided in dams’ drinking water from P2-14 (i.e., the same period as maternal separation).
2.4. Apparatus
Two distinct sets of experimental chambers were used to provide different contexts for behavioral testing. The first set, Context A, consisted of rectangular chambers (13.5 cm long x 9 cm wide x 9 cm high) with the front wall, rear wall, and ceiling constructed of clear Plexiglas, while the floor and side walls were stainless steel rods (3 mm diameter, 7 mm apart). The floor was connected to a custom-built constant-current shock generator and two high-frequency speakers were fitted on either side of the chamber. The Context B chambers were larger (30 cm x 30 cm x 23 cm), with Plexiglas walls and ceiling and a stainless steel rod floor (2 mm diameter, 5 mm apart). Two side walls were covered by vertical black and white stripes (25 mm wide) and two high-frequency speakers were mounted on the ceiling. All experimental chambers were individually housed in sound attenuating wood cabinets fitted with a white light-emitting diode (LED), an infrared LED, and an infrared camera. The infrared LED provided the sole source of illumination in Context A while both white and infrared LEDs were used in Context B. Ventilation fans in each cabinet produced a constant low-level (≈55dB) background noise. Chambers were wiped clean with tap water after each experimental session.
2.5. Behavioral procedures
2.5.1. Conditioning
On P17 ± 1, rats were placed in Context A for a 2-min adaptation period. This was followed by 6 presentations of a white noise CS (8 dB above background, 10 s) and 6 presentations of a shock US (0.6 mA, 1 s). In the Paired condition, the CS and US were presented together, with the US administered in the final second of the CS, and an inter-trial interval (ITI) ranging from 85 to 135 s (mean of 110 s). In the Unpaired condition, the CS and US were presented independently of each other across the same time period, with the ITIs ranging from 25 to 100 s (mean of 49 s). Rats were returned to their home cages 30–60 s after the final stimulus presentation.
2.5.2. Test
In Experiment 1 rats were tested in the training context (Context A) one day after conditioning. No test session was conducted in Experiment 2. In Experiment 3, rats were tested in the extinction context (Context B) the day after extinction. Test consisted of a 1-min adaptation period (baseline) followed by a 2-min continuous presentation of the CS.
2.5.3. Extinction
In Experiments 2–3, rats were given extinction training in Context B the day after conditioning. Extinction consisted of a 2-min adaptation period followed by 30 non-reinforced presentations of the 10 s CS with a 10 s ITI.
2.5.4. Scoring
Freezing was used to measure levels of learned fear and was defined as the absence of all movement except that required for respiration (Fanselow, 1980). A time sampling procedure was used whereby each rat was scored every 3 s as freezing or not freezing. These observations were then converted into a percentage score to indicate the proportion of total observations scored as freezing. A second scorer, unaware of the experimental condition of each rat, scored a random sample (30%) of all rats tested. The inter-rater reliability was high across all experiments, rs = .94 – .98.
2.6. Tissue processing
Rats were deeply anaesthetized by intraperitoneal injection with sodium pentobarbital (112 mg/kg) one hour after test (Experiments 1, 3) or 90 min after extinction (Experiment 2). A longer interval between behavior and perfusion was used in Experiment 2 based on previously published results (Kim et al., 2009, 2012) and unpublished pilot studies which indicated that the optimal time to observe pMAPK expression is later after extinction sessions than after conditioning or test sessions.
Transcardial perfusion was performed using a pre-wash solution (0.9% saline with 1% sodium nitrate and 5000 international units/mL heparin) followed by 150 mL of fixative (4% paraformaldehyde in 0.1 M phosphate buffer [PB], pH 7.4). Brains were removed and post-fixed for 1 h at 4 °C in the same fixative, washed for 2 h in 0.1 M phosphate buffered saline (PBS), then placed in cryoprotectant (20% sucrose in 0.1 M PBS, pH 7.2) overnight at 4 °C. A Leica CM1950 cryostat was used to cut 40 μm slices in four serially adjacent sets, which were stored in 0.1% sodium azide. One series from each rat was used in the immunohistochemical analysis for phospho-p44/42 mitogen-activated protein kinase (pMAPK).
2.7. Immunohistochemistry
Free-floating sections were consecutively washed in 0.1 M PB, 50% ethanol, and 50% ethanol with 3% hydrogen peroxide for 30 min per wash. Sections were then blocked in 5% normal horse serum (NHS) in PB for 30 min before 48-h incubation at 4 °C in rabbit antiserum against pMAPK (phospho-p44/42 mitogen-activated protein kinase [Erk1/2] [Thr202/Tyr204] [D13.14.4E] XP® Rabbit mAb, Cell Signaling Technology, diluted 1:1000 in PBT-X [2% NHS and 0.2% Triton X-100 in 0.1 M PB]). After washing off unbound primary antibody (3 x 20 min washes in 0.1 M PB), sections were incubated overnight in secondary antibody (biotinylated anti-rabbit IgG, Jackson Immunoresearch Laboratories, diluted 1:2000 in PBT-X). Sections were then washed (3 x 20 min in 0.1 M PB) and incubated for 2 h in ABC reagent (Vector Elite kit: 6 μL/mL avidin and 6 μL/mL biotin; Vector Laboratories). Following further washes (2 x 20 min in 0.1 M PB, 1 x 20 min 0.1 M acetate buffer, pH 6.0), a nickel-intensified diaminobenzidine (DAB) reaction was performed to allow visualization of immunoreactive cytoplasm labelled for pMAPK. Sections were incubated for 15 min in the DAB solution (2% nickel sulfate, 0.025% 3,3 DAB, 0.04% ammonium chloride, and 0.02% d-glucose in 0.1 M acetate buffer). The peroxidase reaction was started by adding glucose oxidase (0.2 μL/mL) to the reaction vials and stopped using acetate buffer. After washing (3 x 20 min in 0.1 M PB), sections were mounted immediately or stored in 0.1% sodium azide until mounting. Sections were mounted onto gelatin-coated slides then dehydrated, cleared in histolene, and cover-slipped with Entellen (Biolab, Victoria, Australia).
Immunoreactive neurons were quantified by manual blind counts at 10x magnification using an Olympus BX53 light microscope equipped with a DP72 digital camera. Predefined boundaries based on a rat brain atlas (Paxinos and Watson, 2009) were used to delineate the PL (1.0 x 1.0 mm, with the top of the counting grid aligned to the top of the corpus callosum) and IL (1.0 x 0.6 mm, with the top of the counting grid aligned to the bottom of the PL). For each subregion, the left hemisphere was examined for 3 consecutive sections (160 μm apart, 3.20 mm – 2.88 mm anterior to bregma).
2.8. Exclusions and statistics
Statistical outliers from the behavioral tests (>3 SD from the mean during 3 out of 5 blocks of extinction or during test) were excluded from all analyses. This led to 3 exclusions from Experiment 3: 1 rat each from groups ‘MS Paired’, ‘MS-Pro Paired’, and ‘MS-Pro Unpaired’. In addition, statistical outliers in the immunohistochemical analysis (>3 SD from the mean) were excluded from the specific regional analysis (see Table 1).
Table 1.
Statistical Outliers Excluded from the Immunohistochemistry Analyses.
| Outliers Excluded (>3 SD from group mean) |
||
|---|---|---|
| Experiment | PL | IL |
| Experiment 1a | – | 1 MS Paired 1 SR Paired |
| Experiment 1b | 1 MS-Pro Paired | 1 MS Paired |
| Experiment 2 | 1 MS-Pro Paired 1 Unpaired | 1 MS Paired |
| Experiment 3 | 1 MS Paireda 1 MS-Pro Paired 1 SR Paireda 1 Unpaired |
1 MS Paireda 1 MS-Pro Paireda |
Indicates data point was not excluded in a secondary analysis using an outlier criterion of 3.5SD (see S1. Supplemental Results).
The statistical analysis was performed using R (version 3.4.0; R Core Team, 2017). Normality was assessed using the Shapiro-Wilks test. Overall differences between groups were assessed using one-way ANOVA (normally-distributed data) or the Kruskal-Wallis H test (non-normal distributions). Bonferroni-adjusted planned contrasts were used to assess specific group differences based on our hypotheses. For the behavioral data, the planned contrasts compared 1) Paired and Unpaired groups; and 2) Differences between rearing conditions for the Paired groups. For the immunohistochemistry data, the planned contrasts compared 1) MS Paired with all other groups; and 2) SR Paired and/or MS-Pro Paired with the Unpaired group. In the case of non-normal data, contrasts were conducted using rank-transformed data but raw confidence intervals. Whenever a mixed-design ANOVA was used, Greenhouse-Geisser adjusted p values and nominal df are reported if the assumption of sphericity was violated. For all analyses, p values less than .05 were considered statistically significant.
3. Results
3.1. Prefrontal cortex activation following learned fear expression in infant rats
Infant rats engage a simplified neural circuit when expressing learned fear, which may explain why memories formed during infancy are more susceptible to forgetting (i.e., infantile amnesia; Madsen and Kim, 2016). If this is the case, then it raises the question of whether infant fear memories are represented differently following early-life stress, which has been shown to lead to early offset of infantile amnesia (Callaghan and Richardson, 2012; Cowan et al., 2016b). Here, we first assessed whether the precocious behavioral maturation observed in MS infants is accompanied by precocious maturation of the neural circuits underpinning fear expression, as measured by levels of pMAPK expression (Experiment 1a). Then, in Experiment 1b, we examined pMAPK expression in MS infants that received a probiotic treatment shown to reverse MS-induced changes in infant fear retention (Cowan et al., 2016b).
All rats were conditioned during infancy (P17 ± 1) using either a Paired or Unpaired training procedure. Test was conducted in the same chamber the following day and brain tissue was subsequently analyzed for pMAPK immunohistochemistry in the PL and IL regions of the mPFC.
3.1.1. Experiment 1a
There was a significant effect of group on baseline freezing, χ2(2) = 9.21, p = .01 (see Table 2). Thus, results for the behavioral test data are presented and analyzed as difference scores (percent freezing during the CS minus the percent pre-CS freezing; Fig. 1A). Note, however, that the results were the same if the raw percent freezing scores to the CS were analyzed.
Table 2.
Baseline Freezing across Experiments.
| Pre-CS Freezing (± SEM) |
||||
|---|---|---|---|---|
| Experiment | Group | n | Extinction | Test |
| 1a: MS vs. SR fear expression* | MS Paired | 8 | – | 11.88 (7.73) |
| SR Paired | 8 | – | 31.25 (6.46) | |
| Unpaired | 8 | – | 4.38 (3.20) | |
| 1b: MS vs. MS-Pro fear expression | MS Paired | 8 | – | 25.00 (11.46) |
| MS-Pro Paired | 7 | – | 19.01 (13.62) | |
| Unpaired | 8 | – | 11.25 (7.43) | |
| 2: Fear extinction | MS Paired | 8 | 9.69 (1.97) | – |
| MS-Pro Paired | 8 | 9.69 (3.55) | – | |
| SR Paired | 8 | 16.88 (9.41) | – | |
| Unpaired | 10 | 9.75 (4.04) | – | |
| 3: Extinction retention | MS Paired | 12 | 8.33 (2.03) | 2.50 (1.15) |
| MS-Pro Paired | 9 | 2.78 (1.14) | 0.56 (0.56) | |
| SR Paired | 12 | 12.29 (3.39) | 1.67 (1.12) | |
| Unpaired | 15 | 6.50 (2.18) | 0.67 (0.45) | |
significant differences (p < .05) in baseline freezing at test.
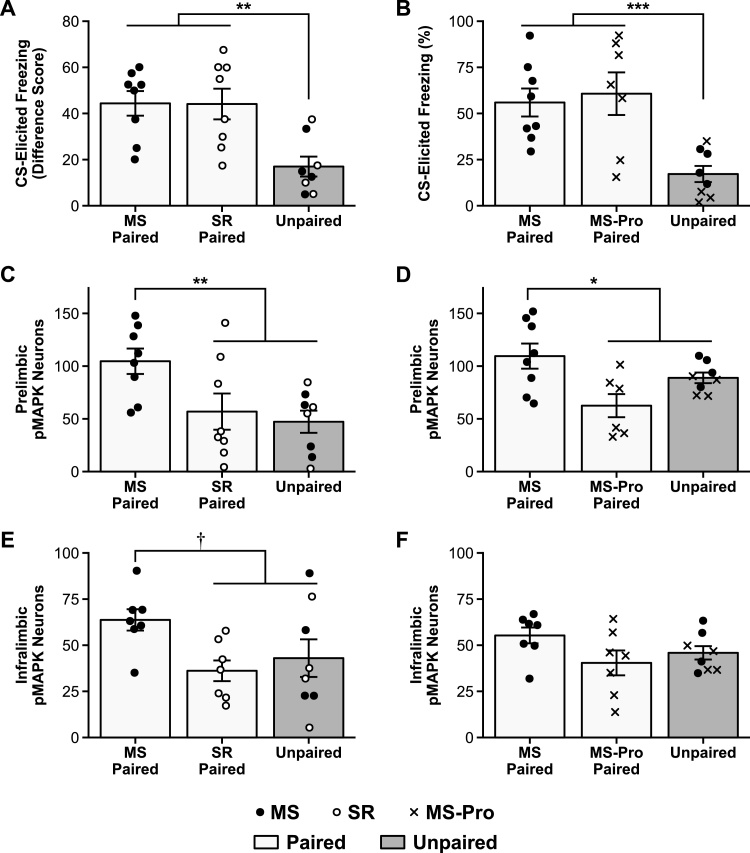
Fig. 1.
Conditioned fear and pMAPK expression in infant rats.
A, C, E: Results of Experiment 1a, comparing standard-reared (SR) and maternally-separated (MS) infant rats that received either Paired or Unpaired presentations of the CS and US at training. B, D, F: Results of Experiment 1b, comparing untreated MS and probiotic-exposed MS (MS-Pro) infant rats. A, B: Mean (± SEM) CS-elicited freezing at test. Rats in the Paired groups froze more than rats in the Unpaired groups. C, D: Mean (± SEM) number of pMAPK stained neurons in the PL and E, F: the IL following test. Only MS rats showed increased pMAPK activation following Paired conditioning and this effect was specific to the PL. † p = .050, * p < .05, ** p < .01, *** p < .001.
As expected, animals in the Paired condition exhibited higher levels of CS-elicited freezing compared to animals in the Unpaired condition, F1,21 = 16.24, p = .001, 95% confidence interval (CI) [10.93–43.58]. The SR Paired and MS Paired groups did not differ from each other, F < 1, replicating previous findings that SR and MS infants exhibit similar levels of fear expression after a short retention interval (though after a long retention interval [i.e., 1 week or more], MS infants exhibit increased fear expression compared to SR infants; Callaghan and Richardson, 2012).
Despite the similarities in the behavioral responses of the SR and MS infants, differences in levels of pMAPK following fear expression were observed in the PL (Fig. 1C; see also Fig. 2A-C for representative photomicrographs). Specifically, MS infants in the Paired condition exhibited higher levels of pMAPK compared to animals in the SR-Paired and Unpaired groups, F1,21 = 10.04, p = .009, 95% CI [12.52–92.61]. SR rats in the Paired condition exhibited levels of pMAPK comparable to Unpaired animals, F < 1, replicating previous findings that SR infant rats do not recruit the mPFC during expression of learned fear (Li et al., 2012). In the IL, there was a similar but less striking pattern observed (Fig. 1E). There was a trend towards higher pMAPK levels in the MS-Paired group, F1,19 = 5.91, p = .050, 95% CI [-.03-48.31], and the SR-Paired and Unpaired groups did not differ, F < 1.
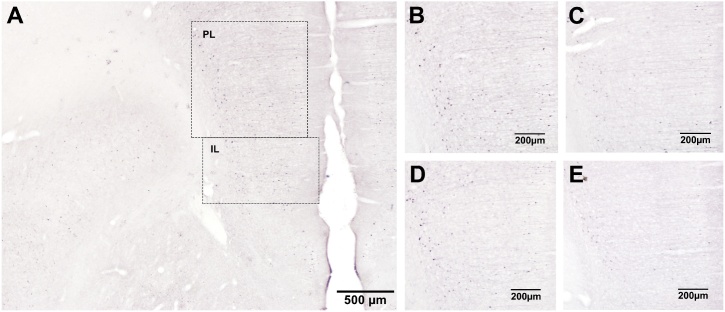
Fig. 2.
Representative photomicrographs of pMAPK immunoreactivity following conditioned fear expression in Experiment 1.
pMAPK-labelled cells in the medial prefrontal cortex (mPFC) of infant rats in the Paired condition 1 h after fear expression in A-C: Experiment 1a and D-E: Experiment 1b. A: mPFC of an untreated maternally-separated (MS) infant, showing the prelimbic (PL) and infralimbic (IL) regions as defined for neuronal counting. B-D: PL of B,D: MS infants, C: a standard-reared (SR) infant, and E: a probiotic-exposed maternally-separated (MS-Pro) infant. Photomicrographs were selected from animals with the median level of staining in each group.
3.1.2. Experiment 1b
This experiment was designed to replicate the finding that MS infants exhibit elevated pMAPK in the mPFC following fear expression, and to test the effects of a probiotic treatment on mPFC recruitment in stressed infants. The results demonstrate that exposure to probiotics counteracts the effects of early-life stress on the neural circuitry of fear expression. Although the behavior of MS-Pro and MS infants was similar (Fig. 1B), F < 1, Paired animals exhibited higher levels of freezing compared to Unpaired animals, F1,20 = 17.83, p < .001, 95% CI [17.53–64.75]. Further, pMAPK in the PL following fear expression was elevated only in untreated stressed infant rats (Fig. 1D; see also Fig. 2D-E for representative photomicrographs), F1,19 = 8.08, p = .02, 95% CI [4.87–62.75]. The level of pMAPK expression observed in the MS-Pro Paired group did not differ from the Unpaired group (F1,19 = 3.34, p = .16) and was comparable to unstressed infants in Experiment 1a. In the IL, low levels of pMAPK expression were observed across all groups (Fig. 1F; largest F1,19 = 3.79, p = .13) and were similar to the levels observed in Experiment 1a.
Together, the results of Experiments 1a and 1b suggest that mPFC recruitment during fear expression in infancy is altered by early-life experience. The pattern of these changes is such that a stressful early rearing environment accelerates the involvement of the prelimbic region in the neural circuitry of fear expression, while probiotic treatment prevents this stress-induced alteration in the developmental trajectory. That is, levels of pMAPK were elevated in the mPFC, and specifically in the prelimbic region of this structure, for MS infants following expression of learned fear. This pattern of PL involvement in fear expression is generally only observed in older animals (Li et al., 2012). In contrast, probiotic-exposed MS infants were more similar to unstressed SR animals of the same age, exhibiting low levels of pMAPK in the mPFC after fear expression.
It has been proposed that the development of a more complex, mature circuit (i.e., one that includes the PL) underpins the transition from infantile amnesia to more stable, adult-like long-term fear memories (Li et al., 2012; Madsen and Kim, 2016). The present results fit nicely with this hypothesis as the patterns of pMAPK expression in untreated and probiotic-exposed MS infants reflect the changes observed in the behavioral expression of infantile amnesia across these groups. That is, the normal developmental phenotype of rapid forgetting observed in SR (Akers et al., 2014; Callaghan and Richardson, 2012; Campbell and Spear, 1972) and probiotic-exposed MS infants (Cowan et al., 2016b) is accompanied by a lack of evidence for mPFC engagement during fear expression. In contrast, animals that exhibit early offset of infantile amnesia (i.e., MS infants; Callaghan and Richardson, 2012) also exhibit precocious involvement of the mPFC in fear expression. This supports the hypothesis that recruitment of the PL into the neural fear circuitry is key to the development of long-term fear retention.
3.2. Prefrontal cortex activation following fear extinction in infant rats
Experiment 1 demonstrated that early-life stress results in precocious recruitment of the mPFC during infant fear expression, an effect that was reversed by exposure to a probiotic treatment. The mPFC has also been shown to have a differential role in fear inhibition across development. From the juvenile period into adulthood, animals recruit the infralimbic region (IL) of the mPFC during inhibition of learned fear responses (Kim et al., 2009; Quirk and Mueller, 2008). However, this is not the case for typically-developing infant rats (Kim et al., 2009; Kim and Richardson, 2010). The aim of Experiment 2 was to determine whether mPFC involvement in infant fear extinction is altered by early-life stress and probiotic exposure, as was the case for infant fear expression in Experiment 1. Conditioning was conducted as in Experiment 1. Extinction took place the following day in a novel context and animals were perfused 90 min. later.
Consistent with Experiment 1 and our previous findings for within-session extinction in stressed and probiotic-treated infants (Callaghan and Richardson, 2011; Cowan et al., 2016b), behavior was similar across all rearing conditions. There was no effect of group on baseline freezing prior to extinction, F < 1 (see Table 2). During extinction (Fig. 3A), there were significant effects of block, F4,120 = 23.81, p < .001, group, F3,30 = 8.39, p < .001, as well as a significant Block x Group interaction, F12,120 = 5.17, p < .001. All three Paired groups exhibited similar levels of CS-elicited freezing, Fs < 1, that were significantly higher than animals in the Unpaired group at the start of extinction, F1,30 = 33.56, p < .001, 95% CI [29.52–68.63]. Freezing in the Paired groups diminished across blocks, reaching low levels equivalent to the Unpaired group by the final block of extinction, χ2(3) = 5.21, p = .16.
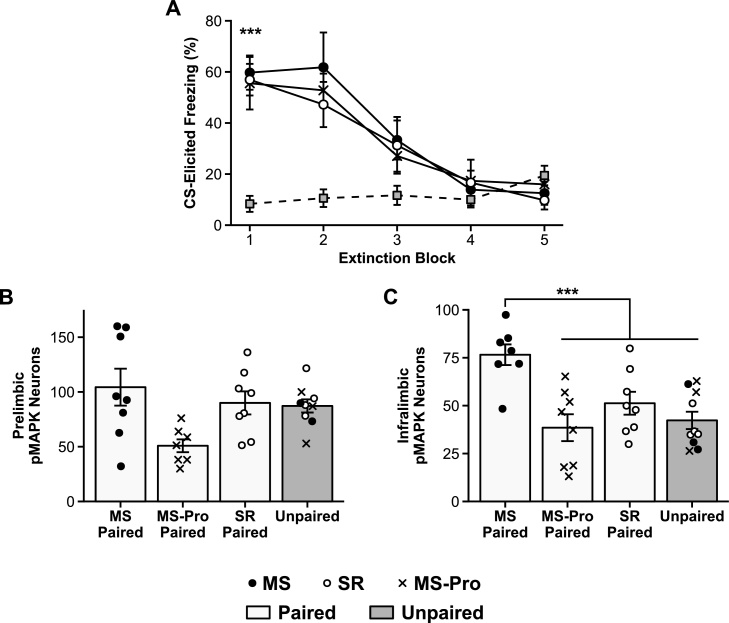
Fig. 3.
Fear extinction and pMAPK expression in infant rats.
A: Mean (± SEM) CS-elicited freezing during extinction training. B, C: Mean (± SEM) number of pMAPK stained neurons in B: the prelimbic region (PL) and C: the infralimbic region (IL) of the medial prefrontal cortex (mPFC) of infant rats following extinction training. *** p < .001.
Between-group differences in pMAPK expression following extinction were observed in the mPFC, most notably in the IL. In the PL (Fig. 3B), the planned contrast analysis did not reveal any significant differences between groups, largest F1,28 = 5.47, p = .08. In the IL (Fig. 3C), untreated MS infants in the Paired condition exhibited significantly higher levels of pMAPK compared to the other groups (i.e., SR Paired, MS-Pro Paired, and Unpaired), F1,29 = 21.22, p < .001, 95% CI [14.60–50.51]. SR and probiotic-exposed MS infants in the Paired condition, however, exhibited pMAPK levels that were comparable to Unpaired animals, largest F1,29 = 1.30, p = .79.
Overall, these results demonstrate that, relative to unstressed animals, untreated MS infants exhibit elevated expression of pMAPK in the IL following fear extinction, as is the case in older animals (Kim et al., 2009). However, this precocious involvement of the IL was attenuated by exposure to probiotics. As was the case for fear expression, these results parallel previous behavioral evidence that MS infants display an adult-like, relapse-prone extinction profile that is reverted to an age-appropriate, relapse-resistant extinction profile by probiotic treatment (Callaghan and Richardson, 2011; Cowan et al., 2016b).
3.3. Prefrontal cortex activation following extinction recall in infant rats
In Experiment 1, precocious maturation of the neural circuitry underlying fear expression was observed in rats with a history of early-life stress. Experiment 2 showed a similar pattern of stress-induced precocious maturation in the neural circuitry underlying fear extinction. In both cases, adult-like engagement of the mPFC in MS infant rats was attenuated by probiotic treatment. In Experiment 3, levels of pMAPK expression were examined after a test of extinction retention. Based on the results of the previous two experiments, it was hypothesized that only untreated MS infants would exhibit activation of the IL during this test of fear inhibition.
All rats underwent fear conditioning (either Paired or Unpaired), extinction, and test in Contexts A, B, and B, respectively, on consecutive days in infancy, starting on P17 ± 1. Sixty minutes after the test of extinction recall, animals were perfused and brain tissue was collected.
Behavioral responses were similar across all rearing conditions, replicating our previous findings (Callaghan and Richardson, 2011; Cowan et al., 2016b). There was no effect of group on baseline freezing prior to extinction, χ2(3) = 5.13, p = .16 (see Table 2). During extinction (Fig. 4A), there were significant effects of block, F4,176 = 31.87, p < .001, group, F3,44 = 7.61, p < .001, and a significant Block x Group interaction, F12,176 = 4.35, p < .001. The three Paired groups exhibited significantly higher levels of CS-elicited freezing compared to the Unpaired group during the first block of extinction, F1,44 = 39.55, p < .001, 95% CI [21.75–52.51], but the Paired groups did not differ from each other, largest F1,44 = 2.78, p = .41. Freezing in the Paired groups declined across extinction such that, by the final block, there were no significant differences between groups, χ2(3) = .50, p = .92.
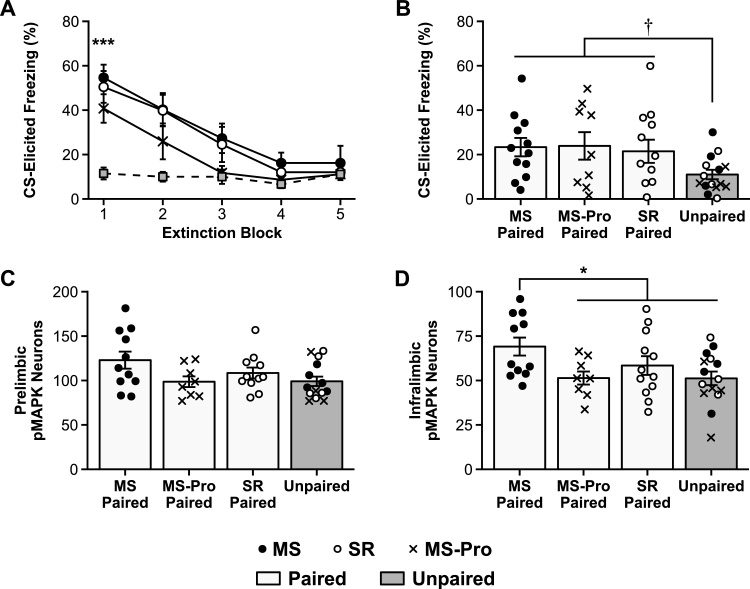
Fig. 4.
Extinction retention and pMAPK expression in infant rats.
Mean (± SEM) CS-elicited freezing during A: extinction training and B: test for standard-reared (SR), maternally-separated (MS), and maternally-separated, probiotic treated (MS-Pro) infant rats that received either Paired or Unpaired CS-US presentations at training. Mean (± SEM) number of pMAPK stained neurons in C: the prelimbic region (PL) and D: the infralimbic region (IL) of the medial prefrontal cortex (mPFC) of infant rats following the extinction retention test. Only MS-Paired rats showed increased pMAPK activation in the IL. † p = .053, * p < .05, *** p < .001.
At test, there were no differences between groups on baseline freezing, χ2(3) = 2.53, p = .47 (Table 2). All groups also exhibited very low levels of CS-elicited freezing during the extinction retention test (Fig. 4B). There was a trend towards higher CS-elicited freezing in the Paired groups compared to the Unpaired group, F1,44 = 6.67, p = .053, but the Paired groups did not differ, F < 1. It is important to note here that although maternal separation induces an adult-like profile of fear relapse following a change of context (i.e., fear renewal) or a stressful reminder foot-shock (i.e., fear reinstatement), even stressed infants typically exhibit low levels of freezing under the conditions used in the present experiment (i.e., when tested in the extinction context within 24 h; Callaghan and Richardson, 2011; Cowan et al., 2013, 2016b), as do adult animals (e.g., Kim et al., 2011). This is an important feature of the experimental design as it allows comparison of neuronal signatures in the context of equivalent behavior.
As was the case following within session extinction, between-group differences in pMAPK expression following extinction recall were observed in the mPFC and more specifically in the IL. In the PL (Fig. 4C), the planned contrast analysis did not reveal any significant differences between groups, largest F1,40 = 3.45, p = .21. In the IL (Fig. 4D), untreated MS infants in the Paired condition exhibited significantly higher levels of pMAPK compared to the other groups (i.e., SR Paired, MS-Pro Paired, and Unpaired), F1,42 = 7.72, p = .02, 95% CI [1.58–29.28]. SR and probiotic-exposed MS infants in the Paired condition, however, exhibited pMAPK levels that were comparable to Unpaired animals, largest F1,42 = 1.37, p = .75. As there were a number of outliers in this experiment (6 exclusions across both regions, see Table 1), the statistical analysis was repeated using a more stringent exclusion criterion (≥ 3.5 rather than ≥ 3.0 SD from the mean) with the same outcomes (see S1. Supplemental Results).
The results of this experiment are consistent with the findings of Experiment 2 and provide further support for the hypothesis that MS infant rats, but not SR or MS-Pro infants, recruit the mPFC during fear inhibition. For the SR and MS-Pro groups, the findings are consistent with work showing that infant rats do not recruit the mPFC during extinction training (Experiment 2; Kim et al., 2009) or during fear expression (Experiment 1; Kim et al., 2012; Li et al., 2012). In contrast, the elevated pMAPK expression in the mPFC during extinction recall in MS infants is reminiscent of findings in adult animals (e.g., Knapska and Maren, 2009; Milad and Quirk, 2002). For example, adult rats exhibit elevated levels of c-Fos, a marker of neuronal activity, in the IL following extinction retrieval (Knapska and Maren, 2009). This suggests that MS infants exhibit a more adult-like neural signature of fear inhibition, which is reversed by probiotic treatment.
4. Discussion
This study provides evidence that the neural circuits supporting emotional learning develop differently depending on the early rearing environment. Replicating previous results (Chan et al., 2011; Kim et al., 2009, 2012; Li et al., 2012), standard-reared (SR) infant rats did not engage the medial prefrontal cortex (mPFC) during either fear expression or fear inhibition. In contrast, infant rats with a history of early-life stress exhibited a more mature pattern of mPFC engagement. Following a test of fear expression, maternally-separated (MS) infant rats exhibited elevated levels of pMAPK in the prelimbic region (PL) of the mPFC, a neural response that is normally only observed in older animals (Li et al., 2012). These results are consistent with findings that stressed infants exhibit accelerated development of fear behavior, including early offset of infantile amnesia (Callaghan and Richardson, 2012; Cowan et al., 2013), as well as precocious maturation of other fear-related neural structures (i.e., the amygdala and hippocampus; Bath et al., 2016; Moriceau et al., 2006). These results also map remarkably well onto recent findings in humans. Specifically, children exposed to early-life stress in the form of childhood institutionalization exhibit more mature patterns of PFC-amygdala connectivity in response to emotional stimuli (fearful faces) and stronger connectivity in the amygdala-PFC-hippocampus network during Pavlovian fear conditioning (Gee et al., 2013; Silvers et al., 2016).
Importantly, our results also demonstrate that the precocious recruitment of the mPFC in stressed infants is prevented by a probiotic treatment. Specifically, probiotic-exposed MS infants exhibited a neural profile that was characterized by low levels of pMAPK expression in the mPFC, regardless of the training conditions or their behavioral fear response, as was the case for SR infants. This is in keeping with previous findings that probiotics prevent precocious expression of long-lasting fear retention and fear relapse in stressed infants (Cowan et al., 2016b). In other words, probiotic treatment normalizes developmental trajectories of behavioral and neural fear regulation in stressed infant rats (see Table 3 for a summary), strengthening the evidence for the role of the microbiota-gut-brain axis in mediating the effects of stress (e.g., Kelly et al., 2015).
Table 3.
Effects of Rearing Condition on Behavioral and Neural Features of Fear Expression and Fear Inhibition in Infant Rats.
| Adult | SR Infant | MS Infant | MS-Pro Infant | ||
|---|---|---|---|---|---|
| Fear expression | Behavior: Long-term fear retention | ✓ | ✗ | ✓ | ✗ |
| (Campbell and Spear, 1972) | (Campbell and Spear, 1972) | (Callaghan and Richardson, 2012) | (Cowan et al., 2016b) | ||
| Brain: Engagement of mPFC (PL) | ✓ | ✗ | ✓ | ✗ | |
| (Maren and Quirk, 2004) | (Li et al., 2012) | (Experiment 1) | (Experiment 1) | ||
| Fear inhibition | Behavior: Fear relapse after extinction | ✓ | ✗ | ✓ | ✗ |
| (Bouton, 2002) | (Kim and Richardson, 2010) | (Callaghan and Richardson, 2011) | (Cowan et al., 2016b) | ||
| Brain: Engagement of mPFC (IL) | ✓ | ✗ | ✓ | ✗ | |
| (Milad and Quirk, 2002) | (Kim et al., 2009) | (Experiments 2 & 3) | (Experiments 2 & 3) |
SR: standard-reared; MS: maternally-separated; MS-Pro: probiotic-exposed MS; mPFC: medial prefrontal cortex; PL: prelimbic region; IL: infralimbic region.
In the current experiments (and our previous work), the probiotic treatment was administered through the maternal drinking water. While this is an indirect treatment approach, we have shown that there is direct exposure of pups in the treatment group, with the specific bacterial strains being detected in pups’ stomach and colon contents (Callaghan et al., 2016; Cowan et al., 2016b). This is in keeping with clinical studies showing that Lactobacillus strains can be transferred to the breast milk following oral intake by pregnant or breastfeeding women (e.g., Arroyo et al., 2010; Dotterud et al., 2015; Jiménez et al., 2008). We do not observe changes in maternal pup retrieval or anxiety-like behavior following probiotic treatment (Callaghan et al., 2016; Cowan et al., 2016b), although further work should be done to investigate the possibility of more nuanced changes in maternal care that may contribute to the effects observed here.
Several questions remain unanswered in the present study, leaving open important lines of enquiry for future studies. First, it will be critical to determine the causal nature of the current findings. Here we have presented evidence that the mPFC is engaged in MS infants during tests of fear expression and fear inhibition. However, it is unknown whether mPFC engagement is necessary for stressed infants to express or inhibit fear, as is the case for older animals (Kim et al., 2009; Li et al., 2012; Sotres-Bayon and Quirk, 2010). Second, future studies should investigate the effects of probiotic treatment on fear behavior and its neural correlates during normal development (i.e., in unstressed, SR infants), or following other manipulations of the early rearing environment (e.g., early handling, which has also been shown to accelerate infant growth and alter stress responding in rodents; Daly, 1973; Meaney et al., 1991). Finally, the current study is limited by its focus on male subjects. While one might not necessarily expect to see sex differences at these ages (i.e., prior to puberty onset), there is evidence that rates of fear relapse are higher in female rats in the juvenile period (Park et al., 2017). Further, there is evidence that MS has some different effects in males and females, both in adulthood (e.g., Diehl et al., 2007; Kalinichev et al., 2002) and during development (e.g., puberty onset: Cowan and Richardson, 2019; and hippocampal neurogenesis in infancy: Loi et al., 2014). Clearly, exploring possible sex differences in terms of neural consequences of MS, and the impact of probiotic treatment on these effects, is an area worthy of future investigation.
4.1. Conclusions
The present results add to the cross-species evidence that early adversity hastens maturation in emotion-related brain circuits, in support of the stress acceleration hypothesis (Callaghan et al., 2014; Callaghan and Tottenham, 2016b). According to this hypothesis, deviations from the normative timeline of emotional development form part of an adaptive response to the absence of species-expected parental caregiving, preparing these individuals to survive independently of parents. However, the hypothesis also proposes that such developmental deviations are likely to have inherent costs. This idea has been raised in the critical period literature as well, where Hensch and Bilimoria (2012) proposed that the hierarchical sequencing of critical periods implies that changes in the timing of one critical period are likely to have flow-on effects that alter the timing of other critical periods or disrupt the synchronization of the system. Here, the successful restoration of age-appropriate neural fear circuitry in stressed infants by a simple probiotic treatment offers a promising, non-invasive method of preventing this type of ‘domino effect’ of critical period disruption. In turn, this suggests that targeting the gut-brain axis may be key to protecting against the psychological vulnerability commonly associated with early-life stress.
Conflict of interest statement
C.C. has been an invited speaker at a meeting organized by Hanson Wade. R.R. and A.S. report no potential conflicts of interest. Lallemand Health Solutions provided the Lacidofil® used in this work gratis, but was not involved in any aspect of the study.
Acknowledgements
This work was supported by grants from the Australian Research Council [grant number DP150104835] and the National Health and Medical Research Council [grant number APP1031688] to R.R., a Petre Foundation scholarship and a UNSW Research Excellence Award to C.C., and an Australian Government Research Training Program Stipend Scholarship to A.S. The authors thank Dr. Thomas Tompkins and Jocelyn Belvis at Lallemand Health Solutions (Montreal, QC, Canada) who provided the Lacidofil® gratis.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100627.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Akers K.G., Martinez-Canabal A., Restivo L., Yiu A.P., De Cristofaro A., Hsiang H.-L. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Arroyo R., Martín V., Maldonado A., Jiménez E., Fernández L., Rodríguez J.M. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010;50:1551–1558. doi: 10.1086/652763. [DOI] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M.E. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L. Generational patterns of stress: Help from our microbes? Curr. Dir. Psychol. Sci. 2017;26:323–329. [Google Scholar]
- Callaghan B.L., Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav. Neurosci. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Richardson R. Adverse rearing environments and persistent memories in rats: removing the brakes on infant fear memory. Transl. Psychiatry. 2012;2:e138. doi: 10.1038/tp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology. 2016;41:163–176. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Sullivan R.M., Howell B., Tottenham N. The International Society for Developmental Psychobiology Sackler symposium: early adversity and the maturation of emotion circuits—a cross-species analysis. Dev. Psychobiol. 2014;56:1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Cowan C.S.M., Richardson R. Treating generational stress: effect of paternal stress on offspring memory and extinction development is rescued by probiotic treatment. Psychol. Sci. 2016;27:1171–1180. doi: 10.1177/0956797616653103. [DOI] [PubMed] [Google Scholar]
- Callaghan, B.L., Fields, A., Gee, D.G., Gabard-Durnam, L., Caldera, C., Humphreys, K.L., Goff, B., Flannery, J., Telzer, E.H., Shapiro, M., Tottenham, N., (in press). Mind and gut: associations between mood and gastrointestinal distress in children exposed to adversity. Dev. Psychopathol., 10.1017/S0954579419000087. [DOI] [PMC free article] [PubMed]
- Campbell B.A., Spear N.E. Ontogeny of memory. Psychol. Rev. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Chan T., Kyere K., Davis B.R., Shemyakin A., Kabitzke P.A., Shair H.N. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J. Neurosci. 2011;31:4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S.M., Richardson R. Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Dev. Psychobiol. 2019 doi: 10.1002/dev.21765. (in press) [DOI] [PubMed] [Google Scholar]
- Cowan C.S.M., Callaghan B.L., Richardson R. Acute early-life stress results in premature emergence of adult-like fear retention and extinction relapse in infant rats. Behav. Neurosci. 2013;127:703–711. doi: 10.1037/a0034118. [DOI] [PubMed] [Google Scholar]
- Cowan C.S.M., Callaghan B.L., Kan J.M., Richardson R. The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention. Genes Brain Behav. 2016;15:155–168. doi: 10.1111/gbb.12263. [DOI] [PubMed] [Google Scholar]
- Cowan C.S.M., Callaghan B.L., Richardson R. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl. Psychiatry. 2016;6:e823. doi: 10.1038/tp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S.M., Hoban A.E., Ventura-Silva A.P., Dinan T.G., Clarke G., Cryan J.F. Gutsy moves: the amygdala as a critical node in microbiota to brain signaling. BioEssays. 2018;40 doi: 10.1002/bies.201700172. [DOI] [PubMed] [Google Scholar]
- Daly M. Early stimulation of rodents: a critical review of present interpretations. Br. J. Psychol. 1973;64:435–460. doi: 10.1111/j.2044-8295.1973.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Diehl L.A., Silveira P.P., Leite M.C., Crema L.M., Portella A.K., Billodre M.N. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain Res. 2007;1144:107–116. doi: 10.1016/j.brainres.2007.01.084. [DOI] [PubMed] [Google Scholar]
- Dotterud C.K., Avershina E., Sekelja M., Simpson M.R., Rudi K., Storrø O. Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J. Pediatr. Gastroenterol. Nutr. 2015;61:200–207. doi: 10.1097/MPG.0000000000000781. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S. Conditional and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., Jury J., MacQueen G., Sherman P.M., Perdue M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008;2008(359):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N., Caroni P., Lüthi A., Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gur T.L., Worly B.L., Bailey M.T. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front. Psychiatry. 2015;6:5. doi: 10.3389/fpsyt.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V., Riccio D.C. Reduction of ontogenetic retention decrements in rats by pretraining stressful experiences. J. Comp. Physiol. Psychol. 1979;93:501–511. doi: 10.1037/h0077567. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hensch T.K., Bilimoria P.M. 2012. Re-Opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum: The Dana Forum on Brain Science. 2012, 11. [PMC free article] [PubMed] [Google Scholar]
- Herry C., Johansen J.P. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Jašarević E., Rodgers A.B., Bale T.L. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol. Stress. 2015;1:81–88. doi: 10.1016/j.ynstr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E., Fernández L., Maldonado A., Martín R., Olivares M., Xaus J., Rodríguez J.M. Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl. Environ. Microbiol. 2008;74:4650–4655. doi: 10.1128/AEM.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn S.A., Frankland P.W. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 2012;19:423–433. doi: 10.1101/lm.021311.110. [DOI] [PubMed] [Google Scholar]
- Kalinichev M., Easterling K.W., Plotsky P.M., Holtzman S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in long–Evans rats. Pharmacol. Biochem. Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol. Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Hamlin A.S., Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J. Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Li S., Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb. Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Li S., Hamlin A.S., McNally G.P., Richardson R. Phosphorylation of mitogen-activated protein kinase in the medial prefrontal cortex and the amygdala following memory retrieval or forgetting in developing rats. Neurobiol. Learn. Mem. 2012;97:59–68. doi: 10.1016/j.nlm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Knapska E., Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Kim J.H., Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav. Neurosci. 2012;126:217–225. doi: 10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- Loi M., Koricka S., Lucassen P., Joels M. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front. Endocrinol. 2014;5:13. doi: 10.3389/fendo.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S., Krugers H.J., Morley-Fletcher S., Szyf M., Brunton P.J. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J. Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- Madsen H.B., Kim J.H. Ontogeny of memory: an update on 40 years of work on infantile amnesia. Behav. Brain Res. 2016;298:4–14. doi: 10.1016/j.bbr.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld K.A., O’Mahony S.M., Hoban A.E., Waworuntu R.V., Berg B.M., Dinan T.G., Cryan J.F. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr. Neurosci. 2017:1–10. doi: 10.1080/1028415X.2017.1397875. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Mitchell J.B., Aitken D.H., Bhatnagar S., Bodnoff S.R., Iny L.J., Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2011;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney R.D., Desbonnet L., Clarke G., Dinan T.G., Cryan J.F. The microbiome: stress, health and disease. Mamm. Genome. 2014;25:49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Roth T.L., Okotoghaide T., Sullivan R.M. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R.M. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Pérez A., Perez-Villalba A., Benítez-Páez A., Campillo I., Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav. Immun. 2017;65:43–56. doi: 10.1016/j.bbi.2017.05.011. [DOI] [PubMed] [Google Scholar]
- O’Mahony S.M., Clarke G., Dinan T.G., Cryan J.F. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Park C.H.J., Ganella D.E., Kim J.H. Juvenile female rats, but not male rats, show renewal, reinstatement, and spontaneous recovery following extinction of conditioned fear. Learn. Mem. 2017;24:630–636. doi: 10.1101/lm.045831.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S., Duhoux S., Hartley C.A., Johnson D.C., Jing D., Elliott M.D. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. compact 6th ed. Academic Press; San Diego, CA: 2009. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pohl C.S., Medland J.E., Moeser A.J. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G927–G941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: a Language and Environment for Statistical Computing.https://www.R-project.org/ Retrieved from. [Google Scholar]
- Raineki C., Holman P.J., Debiec J., Bugg M., Beasley A., Sullivan R.M. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20:1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M.J., Schofield M.G., Kemenes I., O’Shea M., Kemenes G., Benjamin P.R. Vol. 12. Learning & memory Cold Spring Harbor; N.Y: 2005. pp. 538–545. (Activation of MAPK Is Necessary for Long-Term Memory Consolidation Following Food-Reward Conditioning). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M., Sullivan R.M. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front. Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 2016;36:6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Quirk G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.-C., Huang C.-C., Hsu K.-S. Infantile amnesia is related to developmental immaturity of the maintenance mechanisms for long-term potentiation. Mol. Neurobiol. 2019 doi: 10.1007/s12035-018-1119-4. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.