Highlights
-
•
The relation between parenting and children’s brain networks connectivity was examined.
-
•
Higher-quality maternal behaviour during infancy predicted child DMN-SN connectivity.
-
•
Maternal behaviour may affect brain maturation via experience-dependent processes.
-
•
Experiences provided by positive maternal behaviour may promote DMN-SN connectivity.
Keywords: Parenting, Mind-mindedness, Autonomy support, Cortico-cortical networks, Resting state, Functional magnetic resonance imaging
Abstract
Infants’ experiences are considered to determine to a large degree the strength and effectiveness of neural connections and fine tune the development of brain networks. As one of the most pervasive and potent relational experiences of infancy, parent-child relationships appear to be prime candidates to account for experience-driven differences in children’s brain development. Yet, studies linking parenting and functional connectivity are surprisingly scarce, and restricted to the connectivity of limbic structures. Accordingly, this longitudinal study explored whether normative variation in the quality of early maternal behaviour predicts the functional connectivity of large-scale brain networks in late childhood. Maternal mind-mindedness and autonomy support were assessed with 28 children when they were 13 and 15 months old respectively. When children were 10 years of age, children underwent a resting-state functional MRI exam. Functional connectivity was assessed between key regions of the default mode network (DMN), salience network (SN), and frontal-parietal central executive network (CEN). Results revealed that higher mind-mindedness and autonomy support predicted stronger negative connectivity between DMN and SN regions. These findings are the first to provide preliminary evidence suggestive of a long-lasting impact of variation within the normative range of early maternal behaviour on functional connectivity between large-scale brain networks.
1. Introduction
Brain development is driven by genetic factors, but also occurs as a function of environmental influences, in such a way that many brain maturation processes are determined by complex interactions between children and their environment (Fox et al., 2010; Greenough et al., 1987; Huttenlocher, 2002). As such, early relational experiences are thought to have a critical impact on child brain development, and are posited to influence the structure and functioning of the brain well beyond the first years of life (Belsky and De Haan, 2011; Cicchetti, 2016). However, much of the empirical knowledge about the impact of early relational experience on brain development is based on the study of extremely adverse experiences, suggesting that neglect, maltreatment, and caregiving deprivation can lead to abnormal brain maturation that may profoundly affect child cognitive and socio-emotional development (Cicchetti, 2016; De Bellis and Zisk, 2014; Hart and Rubia, 2012; Tottenham, 2014). The generalisability of these findings is, however, limited to clinical or otherwise high-risk populations, and it remains unclear whether normative variations in relational experience influence child brain development (Belsky and De Haan, 2011).
Parent-child relationships are among the most pervasive and potent relational experiences of childhood. The quality of early caregiving relationships forecasts diverse child outcomes such as social and emotional adjustment (Thompson, 2016), cognitive development (Bernier et al., 2012; Tamis-Lemonda et al., 2001), and academic achievement (Raby et al., 2015). Some have argued that such consequences are likely attributable to the intermediate effect of early caregiving relationships on the brain structures that underlie socio-emotional and cognitive processes (Belsky and De Haan, 2011; Gunnar et al., 2006; Tottenham, 2014). Yet, empirical work demonstrating the beneficial effects of positive parenting on children’s brain development is surprisingly scarce. To date, only a few studies have provided evidence for prospective links between normative variation in the quality of early parenting behaviour and child brain morphology or function. Positive parenting in early childhood predicts structural brain development in later childhood, as indicated by larger total, grey matter, and hippocampal volumes (Kok et al., 2015; Luby et al., 2012). Functionally, higher levels of positive maternal behaviour when children are 5 months of age are associated with higher frontal resting neural activity, measured via electroencephalography at 10 and 24 months of age, suggesting more advanced functional brain development (Bernier et al., 2016).
Infants’ day-to-day experiences are considered to determine to a large degree which synaptic connections persist and are strengthened by frequent use, and which are eliminated due to under-activity (Huttenlocher, 1979; Kolb et al., 2014; Singer, 1995). Consequently, caregiving relationships should influence the strength and effectiveness of neural connections and thus fine tune the development of brain networks. The development of the connectivity between the amygdala and the prefrontal cortex (PFC) is affected by extreme caregiving adversity (Gee et al., 2013), but also by normative variations in caregiving (Thijssen et al., 2017). In addition, early life stress, even at moderate levels (e.g., stress caused by non-physical conflict between parents), may induce long-lasting changes in the development of functional brain networks (Graham et al., 2015a, 2015b). Yet, current knowledge on the effect of the quality of parenting behaviour on brain functional connectivity in typically developing children is restricted to connectivity with limbic structures (Rifkin-Graboi et al., 2015; Thijssen et al., 2017), and putative caregiving influences have not been explored in other large-scale networks.
There exist a number of indicators of the quality of parenting behaviour during parent-infant interaction. Two in particular have been shown to promote multiple facets of child cognitive and socio-emotional adjustment: (1) mind-mindedness, representing parents’ tendency to consider their child as someone who has his/her own mental states, and to comment appropriately on the child’s ongoing mental activity (Meins, 1997), and (2) autonomy support, consisting of behaviours that encourage children’s independent problem solving, choice, and participation in decisions (Grolnick and Ryan, 1989). Mind-mindedness and autonomy support have both been shown to contribute to children’s executive functioning (Bernier et al., 2010; Meuwissen and Carlson, 2015), socio-emotional competence (Matte-Gagné et al., 2015), social adaptation (Joussemet et al., 2005), theory of mind (Kirk et al., 2015; Meins et al., 2013), and empathy (Centifanti et al., 2016), and are therefore crucial aspects of parenting related to child cognitive and social development.
Given their involvement in specific cognitive and socio-emotional processes shown to be predicted by mind-mindedness and autonomy support in previous work (i.e., executive functioning, socio-emotional competence, social adaptation, and theory of mind), three brain networks are of particular interest in relation to these parental behaviours: (1) the default mode network (DMN), comprised of the posterior cingulate cortex (PCC), the ventromedial prefrontal cortex (vmPFC) and the angular gyri, is involved in introspective processing, social cognition (e.g., theory of mind, moral cognition), and affective cognition (Andrews-Hanna et al., 2010; Buckner et al., 2008); (2) the frontal-parietal central executive network (CEN), anchored in the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PP), plays an important role in executive functions (Menon, 2011; Seeley et al., 2007); and (3) the salience network (SN), including the anterior insula (AI) and the dorsal anterior cingulate cortex (dACC), is involved in the detection of salient stimuli and the initiation of cognitive control by influencing activation of the CEN and the DMN (Menon, 2011; Menon and Uddin, 2010; Seeley et al., 2007). In both adults and children, these three networks work simultaneously during executive tasks (Bressler and Menon, 2010; Takeuchi et al., 2013), social tasks (Chiong et al., 2013; Eggebrecht et al., 2017; Rilling et al., 2008; Xiao et al., 2016), and cognitive control tasks (Dwyer et al., 2014; Kelly et al., 2008). Thus, we hypothesized that parental mind-mindedness and autonomy support would be specifically associated with connectivity of these networks.
Menon (2013) suggested that the DMN, CEN and SN are “the three most prominent networks to be examined from a development perspective” (Menon, 2013 p.631), and that analysis of the connectivity within and across these networks provides unique insight into the maturation of core neurocognitive systems. The progressive refinement of these three networks and their interplay throughout childhood leads to gradually more mature cognitive and socio-emotional functioning (Menon, 2013; Rubia, 2013). The reconfiguration (segregation and integration) of the DMN, CEN and SN during child development likely supports the maturation of flexible cognitive control processes (Dwyer et al., 2014; Menon, 2013; Uddin et al., 2011) and social abilities (Eggebrecht et al., 2017; Xiao et al., 2016).
The present longitudinal study examined whether maternal mind-mindedness and autonomy support during mother-infant interactions predicted the intrinsic (resting) functional connectivity within and between three core brain networks (DMN, CEN, SN) in late childhood. We hypothesized that higher-quality maternal behaviour during mother-infant interactions, as indexed by higher levels of mind-mindedness (at 13 months of age) and autonomy support (at 15 months), would predict more mature brain connectivity when children were 10 years old, as reflected by adult-like patterns of functional connectivity within and between the DMN, CEN and SN.
2. Material and methods
2.1. Participants
Participants included in the present study (N = 28) were followed annually as part of a larger longitudinal prospective cohort project investigating early relational predictors of several facets of child development (Bernier et al., 2010). Here, we report on the quality of maternal behaviour (mind-mindedness and autonomy support) assessed during infancy, and resting-state functional magnetic resonance imaging (rs-fMRI) data collected when children were 10 years of age. The study was approved by the local human research ethics committee and all families provided written informed consent for participation.
Families were recruited from birth lists randomly generated by the Ministry of Health and Social Services. Inclusion criteria were full-term pregnancy (i.e., at least 37 weeks of gestation) and the absence of any known disability or severe delay in the infant. When children were 10 years of age, they were invited to undergo a magnetic resonance imaging (MRI) exam including an rs-fMRI sequence. Inclusion criteria for the MRI study were the absence of neurological or psychiatric disorders, traumatic brain injury, psychoactive medication, and standard MRI contraindications. Of the 64 families approached for the current study, 39 (60.94%) agreed to participate. However, four (6.25%) of these children were not eligible due to standard MRI exclusion criteria (e.g., wearing braces); thus, 35 children underwent the MRI exam (54.69%). There were no sociodemographic differences between the 35 children who underwent the MRI exam and the 29 who did not in terms of family income, parental age, education, ethnicity, language, or child sex (see Table 1, all ps > .21).
Table 1.
Sociodemographic information of families whose child underwent the magnetic resonance imaging (MRI) exam (N = 35) and those who declined (N = 25) or were not eligible (N = 4) to participate in MRI protocol.
| Accepted MRI N = 35 |
Declined MRI or not eligible N = 29 |
t/chi2 | p | |
|---|---|---|---|---|
| Family income [> $60,000]. n (%) | 26 (74.28) | 23 (79.31) | 0.22 | .64 |
| Maternal age at recruitment. M (SD) | 31.63 (5.05) | 32.02 (3.50) | −0.36 | .73 |
| Paternal age at recruitment. M (SD) | 33.40 (5.29) | 34.07 (4.86) | −0.52 | .62 |
| Maternal education (years). M (SD) | 15.40 (2.23) | 15.26 (2.32) | 0.24 | .81 |
| Paternal education (years). M (SD) | 15.60 (1.94) | 14.97 (2.10) | 1.30 | .21 |
| Ethnicity mother [Caucasian]. n (%) | 28 (80) | 25 (86.21) | 1.02 | .32 |
| Ethnicity father [Caucasian]. n (%) | 26 (74.28) | 22 (75.86) | 0.21 | .64 |
| Language at home [French]. n (%) | 28 (80) | 24 (82.76) | 0.08 | .78 |
| Sex [girls]. n (%) | 21 (60) | 13 (44.83) | 1.47 | .23 |
| Maternal mind-mindedness. M (SD) | 2.30 (1.56) | 2.71 (1.40) | 0.76 | .45 |
| Maternal autonomy support. M (SD) | 3.49 (1.13) | 3.25 (1.03) | 0.88 | .38 |
Of the 35 families who took part in the MRI study, four were subsequently excluded because the child had a diagnosis of anxiety disorder (1) or attention deficit hyperactivity disorder and received psychoactive medication (3). In addition, the rs-fMRI data of three children were excluded due to significant motion (translation > 2.5 mm or rotation > 2.5 degrees). Therefore, data from 28 children (17 girls and 11 boys, χ2 (1) = 1.29; p = .26) and their mothers were used in the analyses. Note that there were no sociodemographic differences between the final sample (n = 28) and the other families in terms of family income, parental age, education, ethnicity, language, and child sex (all ps > .25).
2.2. Data collection
Children and their mothers took part in three assessment visits when children were approximately 13 months (T1, M = 13.09; SD = 1.39), 15 months (T2, M = 15.67; SD = 1.03) and 10 years old (T3, M = 10.57; SD = 0.46). The first two assessment time points (T1 and T2) were home visits during which mother-child interactive sequences were video-taped to assess the quality of maternal behaviour, specifically maternal mind-mindedness at T1 and maternal autonomy support at T2. The third visit (T3) consisted of the MRI acquisition.
2.2.1. Mind-mindedness at 13 months (T1)
Maternal mind-mindedness was assessed when children were 13 months old via a 10-minute free-play sequence between mother and infant. Videotaped interactions were later rated by trained coders using Meins et al.’s (2001) coding system. Five categories of comments were assessed: (1) infant’s mental states, such as thoughts, desires, knowledge (e.g. “You like this toy”, “You know this game”); (2) mental processes (e.g. “Where do you think the block goes?”, “You find this game difficult”); (3) infant’s emotional engagement (e.g. “You don't seem willing to play”, “You’ve had enough”); (4) infant’s attempts to manipulate other people’s thoughts (e.g. “You’re making fun of me”); and (5) mother speaking for the infant (e.g. mother saying “Hello dad, this is your son on the phone”, while her infant is playing with a toy phone). Each comment was then coded as appropriate or non-attuned according to Meins et al.’s (2001) guidelines. However, because non-attuned mind-related comments were extremely rare in this low-risk sample (over 90% of mothers made no such comments at all), this type of comment was not examined further. The number of appropriate comments in each category was summed into a total score, which was used in all subsequent analyses. Previous studies report that frequency scores and proportional scores (controlling for maternal verbosity) yield similar results (Meins et al., 2013, 2003). About half (48.4%) of the videotapes were randomly selected to be independently coded by a second trained rater. Inter-rater reliability on the total number of appropriate mind-related comments (intraclass correlation [ICC]) was excellent, ICC = .87.
2.2.2. Autonomy support at 15 months (T2)
At T2, mothers were asked to help their infant complete three puzzles that were designed to be slightly too difficult for the infants, such that they would require some adult assistance to complete them. Following Whipple et al.’s (2011) rating system, maternal behaviour was rated on four Likert scales assessing the extent (1–5) to which the mother (1) intervenes according to the child’s needs and adapts the task to create an optimal challenge; (2) encourages her child in the pursuit of the task and gives useful hints and suggestions; (3) takes her child’s perspective and demonstrates flexibility in her attempts to keep the child on task; (4) provides the child with the opportunity to make choices and ensures that the child plays an active role in completing the task. A high autonomy support score indicates that mothers adjust their behaviour according to child needs, abilities, rhythm, and emotional state. The mean of the four scales for each puzzle were averaged into a total autonomy support score (α = .89), which was used in all subsequent analyses. A randomly selected 56.5% of videotapes were independently coded by a second trained rater. Interrater reliability was excellent, ICC = .86.
2.2.3. MRI acquisition at 10 years (T3)
Neuroimaging data were collected using a 32-channel head coil on a Siemens 3 T scanner (MAGNETOM Trio, Siemens, Erlangen, Germany). Structural data were acquired using two high resolution anatomic sequences: (1) a three-dimensional T1-weighted 4-echo magnetization-prepared rapid gradient-echo sequence (3D-T1-4echo-MPRAGE sagittal; repetition time (TR): 2530 ms; 4 echo times (TE): 1.64/3.5/5.36/7.22 ms; echo spacing ΔTE: 1.86 ms; flip angle: 7°; 176 slices; slice thickness: 1 mm; no gap; matrix: 256 × 256; field of view (FoV): 256 mm; in-plane resolution: 1 x 1 mm; duration: 363 s), and (2) a two-dimensional T2-weighted turbo spin echo sequence (2D-T2-turbo-SE sagittal; TR: 9000 ms; TE: 106 ms; flip angle: 120°; 128 slices; slice thickness: 1.20 mm; gap: 1.20 mm; matrix: 192 × 160; FoV: 230 mm; in-plane resolution: 1.20 × 1.20 mm; duration : 191 s). Then, resting-state functional data were acquired with a 2D T2-star echo planar image sequence (2D-T2*-EPI axial; TR: 2430 ms; TE: 30 ms; flip angle: 70°; 40 slices; slice thickness: 3.5 mm; gap: 3.5 mm; matrix: 64 × 64; FoV: 224 mm; in-plane resolution: 3.50 × 3.50 mm; 120 volumes; duration: 298 s). During the 5-minute resting-state fMRI sequence, children were asked to fix a white dot on a black screen. This short 5-minute sequence allowed for a suitable balance between feasibility and reliability of resting state acquisition in children. Short acquisition times such as this are likely to reduce the risk of motion artefacts (Van Dijk et al., 2012), which are especially salient in paediatric imaging (Power et al., 2012). The three networks we focussed on (i.e., DMN, CEN and SN) remain stable with acquisition times as short as 3 min (White et al., 2014).
2.3. Resting-state fMRI pre-processing
After all images were visually inspected for motion artefacts and image quality, data pre-processing was performed with SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) and the CONN Functional Connectivity SPM Toolbox version 17a (Whitfield-Gabrieli and Nieto-Castanon, 2012) running on MATLAB version R2016a (MathWorks, Inc., Natick, MA, USA). Using the SPM platform: (1) the echo planar imaging (EPI) volumes were corrected for slice timing and realigned to the first volume to correct for head motions; (2) the mean EPI (calculated during realignment), the T2 and the T1 images were co-registered; (3) tissues (grey matter [GM], white matter [WM] and cerebrospinal fluid [CSF]) were segmented and normalized using T1 and T2 images and an age-appropriate stereotaxic template (NIHPD 7.5–13.5 asymmetric: www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj1; Fonov et al., 2011); (4) resulting parameters were applied to normalize the co-registered T1 image and EPI volumes with a voxel size of 2 × 2 x 2 mm; and (5) the normalized EPI images were smoothed at 6 mm full width at half-maximum (FWHM). Finally, the noise reduction step was performed to remove unwanted motion, physiological and other artefactual effects from the blood-oxygen-level dependent (BOLD) signal using the anatomical principal component-based noise-correction ‘aCompCor’ strategy (Behzadi et al., 2007; Muschelli et al., 2014; Whitfield-Gabrieli and Nieto-Castanon, 2012) implemented in the CONN toolbox. The aCompCor strategy accounts for motion, physiological and other artefactual effects based on principal components of the signals from the white matter and the cerebrospinal fluid voxels, along with the six motion parameters estimated during realignment. Then, a band‐pass filter between 0.009 and 0.08 Hz was applied.
2.4. Statistical analyses
2.4.1. Imputation of missing values for maternal behaviour
Due to technical problems with the recording equipment, maternal behaviour data were missing for two children on mind-mindedness and for one child on autonomy support. In line with recommendations for best practices for handling missing data, multiple imputation was employed to estimate these three missing values (Enders, 2010) using the Markov Chain Monte Carlo procedure (Geyer, 1992) in SPSS software version 24.0 (IBM Corp., Armonk, NY). As per recommendations, 10 imputations were used and then averaged to maximise the precision of imputed data (Enders, 2010; Graham, 2009). The imputations were performed based on the original 64 families using child sex and age at each visit as well as parental age and education at the time of recruitment as predictors.
2.4.2. ROI-to-ROI resting-state fMRI analyses
ROI-to-ROI functional connectivity analyses were performed using the CONN toolbox. Eleven ROIs corresponding to the key nodes of DMN, CEN and SN consistently described in the literature (Buckner et al., 2008; Menon, 2011; Seeley et al., 2007; Uddin, 2015; Uddin et al., 2011) were selected from the predefined network atlases implemented in the CONN toolbox. These network atlases were derived from CONN’s independent component analysis of 497 young healthy adults from the Human Connectome Project dataset. Thereby, individual connectivity maps were created between and within regions of the DMN, CEN and SN (see Fig. 1 and Table 2 for detail on seed regions and coordinates). Spherical ROIs were defined with a radius of 10 mm.
Fig. 1.
Default mode network (magenta), salience network (orange) and fronto-parietal central executive network (green). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Abbreviations: AI, anterior insula; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; PCC, posterior cingulate cortex; PP, posterior parietal cortex; vmPFC, ventromedial prefrontal cortex.
*These seeds are medial.
Table 2.
Seed region coordinates in the default mode network (DMN), the salience network (SN), and the fronto-parietal central executive network (CEN) selected from the predefined networks atlas implemented in the CONN toolbox.
| Region | Abbreviation | MNI coordinates |
Network | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Ventromedial prefrontal cortex | vmPFC | 1 | 55 | −3 | DMN |
| Left angular gyrus | l angular | −39 | −77 | 33 | DMN |
| Right angular gyrus | r angular | 47 | −67 | 29 | DMN |
| Posterior cingulate cortex | PCC | 1 | −61 | 38 | DMN |
| Dorsal anterior cingulate cortex | dACC | 0 | 22 | 35 | SN |
| Left anterior insula cortex | l AI | −44 | 13 | 1 | SN |
| Right anterior insula cortex | r AI | 47 | 14 | 0 | SN |
| Left dorsolateral prefrontal cortex | l dlPFC | −43 | 33 | 28 | CEN |
| Right dorsolateral prefrontal cortex | r dlPFC | 41 | 38 | 30 | CEN |
| Left posterior parietal cortex | l PP | −46 | −58 | 49 | CEN |
| Right posterior parietal cortex | r PP | 52 | −52 | 45 | CEN |
Note. MNI = Montreal Neurological Institute.
At the first level of analysis, bivariate Pearson’s correlations were calculated between the mean BOLD signal time-courses of each pair of ROIs. This provided a ROI-to-ROI functional connectivity map (11 × 11 connectivity matrix) for each participant, in which positive and negative correlation coefficients defined positive and negative functional connectivity, respectively. The six motion parameters (3 translations, 3 rotations) estimated during the realignment step of pre-processing were added as nuisance regressors. Then, Fisher's r-to-z transformation was applied to allow for parametric testing.
For the second-level analysis, multiple regression was performed between ROI-to-ROI functional connectivity maps and each indicator of maternal behaviour (mind-mindedness and autonomy support scores separately, to guard against Type-II error in the context of the modest sample size) controlling for child age and sex as well as maternal education. Analyses were masked using a GM mask based on the means of the GM, WM and CSF normalized images (meanGM, meanWM and meanCSF) and calculated with the following formula:
GM mask = (meanGM > meanWM) ⋂ (meanGM > meanCSF) ⋂ (meanGM > 0.3)
The statistical threshold was set at a false discovery rate (FDR; Chumbley et al., 2010) corrected p < .05 at seed level to guard against Type-I error.
3. Results
3.1. Predicting functional connectivity from mind-mindedness
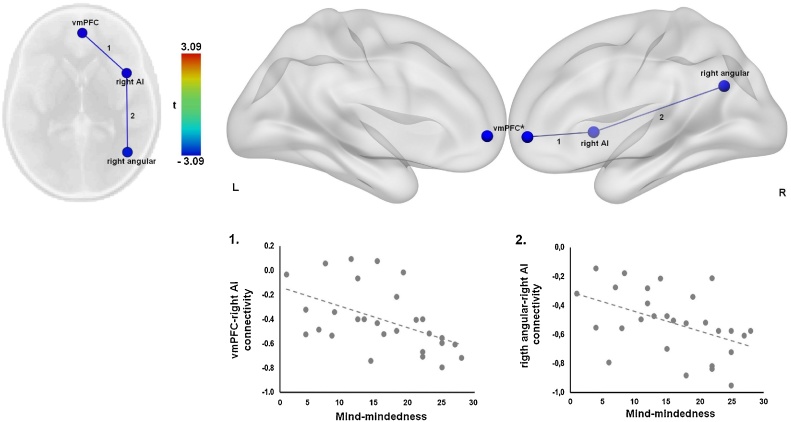
In the first ROI-to-ROI multiple regression analysis we examined whether mind-mindedness at 13 months of age predicts functional connectivity between DMN, SN and CEN nodes at 10 years (Fig. 2). The analysis revealed that higher mind-mindedness was associated with significantly stronger negative connectivity (anti-correlation) between the right AI (SN) and two seeds of the DMN: vmPFC (t(23) = -2.88, FDR-corrected p = .04) and the right angular gyrus (t(23) = -3.09, FDR-corrected p = .04). No significant relation was observed between mind-mindedness and connectivity of CEN seeds, dACC, PCC, left AI and left angular gyrus (FDR-corrected ps > .05). Consideration of the blood oxygenation level dependent (BOLD) signal time courses for the seed regions confirms that the right AI is anticorrelated with vmPFC and the right angular gyrus (Fig. 3, plots 1 and 2). Overall, these results suggest that higher maternal mind-mindedness at 13 months of age predicts stronger negative connectivity between the right AI (SN seed) and DMN regions (vmPFC and right angular gyrus) at 10 years of age.
Fig. 2.
Mind-mindedness at 13 months predicts stronger negative functional connectivity between regions of the default mode network and salience network. Connectivity between vmPFC and right AI (1), and right angular and right AI (2).
Abbreviations: AI, anterior insula; vmPFC, ventromedial prefrontal cortex.
*These seeds are medial.
Fig. 3.
Blood oxygenation level dependent (BOLD) signal time courses for each pair of seed regions for which functional connectivity is predicted by mind-mindedness or autonomy support (1. vmPFC and right AI, 2. right angular and right AI, 3. vmPFC and left AI, and 4. vmPFC and dACC). The default mode network regions (vmPFC and right angular) are represented by the magenta lines and the salience network regions (dACC and bilateral AI) by the orange lines. Abbreviations: AI, anterior insula; BOLD, blood-oxygen-level dependent; dACC, dorsal anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
3.2. Predicting functional connectivity from autonomy support
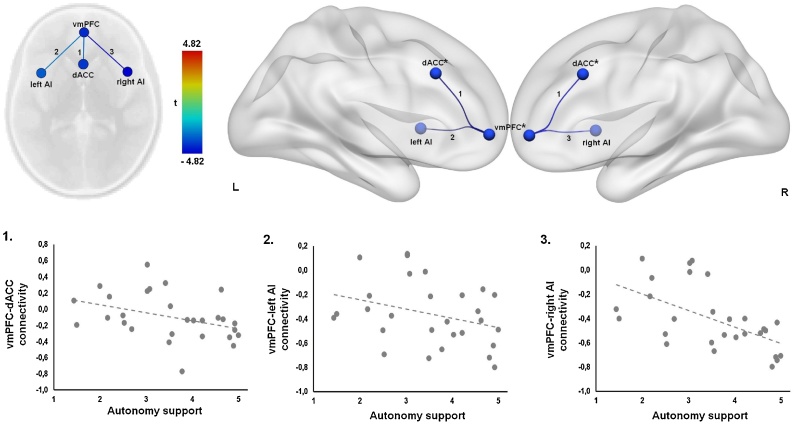
In the second ROI-to-ROI multiple regression analysis we examined whether autonomy support at 15 months of age predicts functional connectivity between DMN, SN and CEN nodes at 10 years (Fig. 4). This analysis revealed that higher autonomy support was associated with significantly stronger negative connectivity (anti-correlation) between the vmPFC (DMN seed) and the entire SN (dACC: t(23) = -3.25, FDR-corrected p = .02; left AI: t(23) = -2.97, FDR-corrected p = .02; right AI: t(23) = -4.82, FDR-corrected p < .001). No significant relation was observed between autonomy support and connectivity of the CEN seeds, PCC, and bilateral angular gyrus (FDR-corrected ps > .05). Time courses for the seed regions confirm that the vmPFC is anticorrelated with the dACC and bilateral AI (Fig. 3, plots 1, 3 and 4). Overall, these results suggest that higher maternal autonomy support at 15 months predicts stronger negative connectivity between the vmPFC and the SN regions at 10 years of age.
Fig. 4.
Autonomy support at 15 months predicts stronger negative functional connectivity between vmPFC and the salience network. Connectivity between vmPFC and dACC (1), vmPFC and left AI (2), and vmPFC and right AI (3).
Abbreviations: AI, anterior insula; dACC, dorsal anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
*These seeds are medial.
4. Discussion
The goal of this longitudinal study was to investigate whether normative variation in the quality of early maternal behaviour, namely mothers’ mind-mindedness and autonomy support during mother-infant interactions, predicts intrinsic functional connectivity within and between the DMN, SN and CEN in late childhood. Although our findings require replication in a larger sample to be generalised, we observed that better quality of maternal behaviour during infancy, as indexed by higher levels of mind-mindedness and autonomy support, predicted stronger negative connectivity (anti-correlation) between regions of DMN (vmPFC and right angular gyrus) and SN (AI and dACC) at 10 years of age. These findings are the first to provide preliminary evidence coherent with the notion that variation within the normative range of maternal behaviour may have a long-lasting impact on the functional connectivity between large-scale brain networks.
4.1. Stronger DMN-SN anti-correlation reflects more mature (adult-like) functional connectivity
Large scale brain networks, including the DMN and SN, can be readily identified in infancy, but are incomplete and fragmented at that time (Gao et al., 2013). They undergo protracted maturation throughout childhood and adolescence characterized by significant reorganization and strengthening of their connections, which spans both within- and across-network links (Fair et al., 2009; Gao et al., 2013; Solé-Padullés et al., 2016; Uddin et al., 2011). Children have weaker functional connectivity within and between the DMN and the SN than adults (Uddin et al., 2011). Across development, the default network and task-positive networks – sets of regions activated during attention-demanding cognitive tasks, including SN and CEN regions (Fox et al., 2005) – become increasingly more inversely coupled (Barber et al., 2013; Chai et al., 2014; Sherman et al., 2014). Chai and colleagues (2014) found positive correlations between DMN and task-positive brain regions in children, which then become somewhat negatively correlated in adolescents, and more strongly anti-correlated in adults (Chai et al., 2014). Based on these developmental trends, our results, although preliminary, appear to indicate that brain connectivity is more mature in children whose mothers were mind-minded and autonomy supportive during infancy. Hence, positive early maternal behaviour may promote DMN-SN connectivity development, reflected by stronger DMN-SN anti-correlation in late childhood.
4.2. Positive maternal behaviour provides social experiences that may promote DMN-SN connectivity development
Positive maternal behaviour may provide children with social experiences and situations that promote the development of socio-emotional abilities together with DMN-SN connectivity maturation. Mind-minded caregivers promote children’s socio-cognitive development by presenting children with appropriate alternative perspectives on reality in such a way that they can be readily assimilated (Fernyhough, 2008). Autonomy support promotes exploratory behaviour and self-regulatory skills that can represent precursors to child social adaptation (Frodi et al., 1985; Grolnick and Farkas, 2002; Meuwissen and Carlson, 2015; Whipple et al., 2011). The strength of connectivity between the AI, ACC and vmPFC was predicted by mind-mindedness and autonomy support in the present study. These regions are involved in social processes such as emotional perspective taking, prosocial behaviour, theory of mind, and empathy (Beauchamp and Anderson, 2010; Bernhardt and Singer, 2012; Blakemore, 2008). Furthermore, studies in adults using Granger causality analysis to characterize the dynamics and directionality of functional connectivity indicate that regions of the SN causally influence regions of the DMN during social interactions (Rilling et al., 2008) and moral reasoning (Chiong et al., 2013). A recent study with 2 year-old children reported significant concurrent associations between DMN-SN connectivity and joint attention, a socio-cognitive construct which is understood to lay the foundation for mentalizing processes such as theory of mind (Eggebrecht et al., 2017).
The exact mechanisms that underpin the link between early maternal behaviour and subsequent brain connectivity are not known. Although speculative, it is possible that this occurs via experience-dependent mechanisms that regulate synaptogenesis and synaptic pruning (i.e., micro mechanisms that underlie the maturation of brain networks). Through experience-dependent processes, synaptogenesis and synaptic pruning appear to occur especially in brain regions involved in the processing of information arising from events experienced by the individual (Cicchetti, 2016; Greenough et al., 1987). Children whose mothers provide higher mind-mindedness and autonomy support are likely to be more frequently engaged in stimulating emotionally significant social interactions, which may result in co-activation of brain regions involved in socio-emotional processing, in turn strengthening their connectivity. As such, we hypothesize that mind-mindedness and autonomy support could promote the development of DMN-SN connectivity via increased frequency of exposure to positive social experiences in emotionally salient contexts, such as those often characterizing parent-infant interactions.
4.3. Positive maternal behaviour provides successful emotion regulation that may promote DMN-SN connectivity development
Positive maternal behaviour may also encourage children to appropriately respond to and regulate emotions, thereby promoting DMN-SN connectivity development. The quality of mother-infant interaction is conceptualized as an important antecedent of the developing child’s ability to regulate his or her own emotions (Cole et al., 2004). When parents comment appropriately on their infant’s mental states (mind-mindedness) or provide a sense of autonomy to their child (autonomy support), they act as external regulators of their child’s affect and behaviour and this facilitates child self-regulation (Grolnick and Farkas, 2002; Grolnick and Ryan, 1989; McMahon and Bernier, 2017).
In adults, inter-subject variation in the strength of anti-correlation between task-positive (grouping SN and CEN regions) and task-negative (i.e., DMN) networks is significantly related to individual differences in cognitive control (Kelly et al., 2008), which is involved in cognitive affective regulation processes such as reappraisal (Ochsner et al., 2012; Ochsner and Gross, 2005). It has been suggested that the functional maturation of the SN is a critical process by which human brain networks reconfigure and mature during development to support more flexible cognitive control processes (Menon, 2013; Uddin et al., 2011). The protracted maturation of the prefrontal cortex and the process of fine-tuning neural connections in the brain may underlie children's improvements in emotion regulation over time (Kovacs et al., 2008). Thus, we speculate that mind-minded and autonomy supportive caregivers are likely to provide their child with frequent experiences of successful emotion regulation that in turn, can promote the development of connectivity between the DMN and the SN through experience-dependent processes.
4.4. Direct or indirect relation between early maternal behaviour and child brain connectivity?
The proposition that the relation between early maternal behaviour and subsequent brain connectivity may occur via experience-dependent processes, presented above, could reflect both direct and indirect associations. Indeed, experiences promoted by mind-minded and autonomy supportive mothers (e.g., social experience and stimulation, external regulation of child affect) may directly activate (or deactivate) regions of the DMN and SN and thus strengthen DMN-SN connectivity by frequent use. The relation between maternal behaviour and child brain development could also be indirect, via child behaviour. In this case, maternal behaviour may promote the development of child competencies such as social abilities and self-regulation, which in turn activate DMN and SN regions and thus strengthen their connectivity. These hypotheses should be tested by future longitudinal studies.
4.5. Implications for clinical conditions
Throughout childhood, weak cross-network signalling is a source of vulnerability for developmental psychopathologies (Menon, 2013). Alteration of the connectivity within and between the DMN and the SN has been implicated in various developmental and psychiatric conditions (Barkhof et al., 2014; Menon, 2011; Peters et al., 2016), such as depression (Gaffrey et al., 2012; Pannekoek et al., 2014), attention deficit hyperactivity disorder (Choi et al., 2013; Tomasi and Volkow, 2012), and autism spectrum disorders (Jann et al., 2015; Supekar et al., 2013), all of which have been shown to affect socio-emotional processes either primarily or secondarily (Bora and Pantelis, 2016; Cole et al., 2008; Leekam, 2016; Pfeifer and Peake, 2012). There is now ample evidence, including meta-analytic data (Bakermans-Kranenburg et al., 2003), that the quality of parenting behaviour can be improved using evidence-based intervention, and emerging findings are beginning to suggest that mind-mindedness (Schacht et al., 2017) and autonomy support (Joussemet et al., 2014) in particular can be effectively promoted using brief focused interventions. In turn, such parenting interventions show beneficial effects for child functioning (Guttentag et al., 2014). As such, the current results suggest that it may be beneficial to address poorer quality parenting behaviour early in development, given the potential for better quality caregiving relationships to translate to healthy brain development and optimal socio-emotional abilities.
4.6. Limitations
The current results must be interpreted in the context of some limitations. First, the links between maternal behaviour and child brain connectivity may be underestimated because of the limited statistical power related to the modest sample size and the use of several covariates. Hence, these results should be considered preliminary, and need to be examined in larger samples to test their generalizability and robustness. Second, although longitudinal, the non-experimental design of this study precludes causal inference and determination of directionality. As hypothesized by Bernier and colleagues (2016), it is probable that the links between parenting and child development are bidirectional, such that positive parenting promotes infant brain development, and infants whose brains are more mature trigger better quality of parenting. Finally, we did not assess father behaviour, which may also influence the developing brain given that paternal mind-mindedness and autonomy support make unique contributions to children’s social and cognitive development (Gagné et al., 2018; Meuwissen and Carlson, 2015).
5. Conclusion
This 9-year longitudinal study suggests that the quality of maternal behaviour during mother-infant interactions predicts stronger DMN-SN anti-correlation in late childhood, which is thought to reflect more mature functional brain connectivity. This is consistent with classic (Greenough et al., 1987) and recent propositions (Cicchetti, 2016), with animal studies (see Meaney, 2001), and with research on children who experience severely adverse conditions (see Belsky and De Haan, 2011), suggesting that early relational experiences may have a critical impact on child brain development. The findings of this study provide rare evidence that normative variation in parenting quality may contribute to the development of functional connectivity in typically developing young children. Future studies should aim to explore the neurophysiological mechanisms that may account for these parenting effects, and whether these effects, if they are replicated and found to be robust, translate into meaningful differences in child behaviour, particularly social competence and emotion regulation.
Conflict of Interest
None.
Acknowledgments
This work was supported by grants awarded to A. Bernier by the Canadian Institutes of Health Research [MOP-119390], the Social Sciences and Humanities Research Council of Canada [410-2010-1366], and the Fonds de Recherche du Québec - Société et Culture [2012-RP-144923]. The authors wish to acknowledge Natasha Ballen, Marie-Ève Bélanger, Stéphanie Bordeleau, Andrée-Anne Bouvette-Turcot, Catherine Cimon-Paquet, Isabelle Demers, Marie Deschênes, Christine Gagné, Sarah Hertz, Véronique Jarry-Boileau, Jessica Laranjo, Élodie Larose-Grégoire, Nadine Marzougui, Célia Matte-Gagné, Rachel Perrier, Émilie Rochette, Marie-Soleil Sirois, Émilie Tétreault, and Natasha Whipple for help with data collection. The authors want to express special gratitude to the participating families of the Grandir Ensemble project who generously opened their homes to us. The authors thank Andre van der Kouwe from the Massachusetts General Hospital for the use of the MPRAGE 4-echo sequence.
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van IJzendoorn M.H., Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol. Bull. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F., Haller S., Rombouts S.A.R.B. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272:29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.H., Anderson V. SOCIAL: an integrative framework for the development of social skills. Psychol. Bull. 2010;136:39–64. doi: 10.1037/a0017768. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J., De Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry Allied Discip. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. The neural basis of empathy. Annu. Rev. Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bernier A., Carlson S.M., Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. 2010;81:326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Bernier A., Carlson S.M., Deschênes M., Matte-Gagné C. Social factors in the development of early executive functioning: a closer look at the caregiving environment. Dev. Sci. 2012;15:12–24. doi: 10.1111/j.1467-7687.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- Bernier A., Calkins S.D., Bell M.A. Longitudinal associations between the quality of mother–infant interactions and brain development across infancy. Child Dev. 2016;87:1159–1174. doi: 10.1111/cdev.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bora E., Pantelis C. Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): comparison with healthy controls and autistic spectrum disorder. Psychol. Med. 2016;46:699–716. doi: 10.1017/S0033291715002573. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Centifanti L.C.M., Meins E., Fernyhough C. Callous-unemotional traits and impulsivity: distinct longitudinal relations with mind-mindedness and understanding of others. J. Child Psychol. Psychiatry. 2016;57:84–92. doi: 10.1111/jcpp.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W., Wilson S.M., D’Esposito M., Kayser A.S., Grossman S.N., Poorzand P., Seeley W.W., Miller B.L., Rankin K.P. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136:1929–1941. doi: 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Jeong B., Lee S.W., Go H.-J. Aberrant development of functional connectivity among resting state-related functional networks in medication-naïve ADHD children. PLoS One. 2013;8:e83516. doi: 10.1371/journal.pone.0083516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J., Worsley K., Flandin G., Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti, D. (Ed.), 2016. Developmental Psychopathology, Volume 2: Developmental Neuroscience, 3rd ed., John Wiley & Sons, Hoboken, New Jersey.
- Cole P.M., Martin S.E., Dennis T.A. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole P.M., Luby J., Sullivan M.W. Child Development Perspectives. 2008. Emotions and the development of childhood depression: bridging the gap; pp. 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Zisk A. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2014;23:185–222. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D.B., Harrison B.J., Yucel M., Whittle S., Zalesky A., Pantelis C., Allen N.B., Fornito A. Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci. 2014;34:14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht A.T., Elison J.T., Feczko E., Todorov A., Wolff J.J., Kandala S., Adams C.M., Snyder A.Z., Lewis J.D., Estes A.M., Zwaigenbaum L., Botteron K.N., McKinstry R.C., Constantino J.N., Evans A., Hazlett H.C., Dager S., Paterson S.J., Schultz R.T., Styner M.A., Gerig G., Das S., Kostopoulos P., Schlaggar B.L., Petersen S.E., Piven J., Pruett J.R. Joint attention and brain functional connectivity in infants and toddlers. Cereb. Cortex. 2017;27:1709–1720. doi: 10.1093/cercor/bhw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C.K. Guilford Press; New York, NY: 2010. Applied Missing Data Analysis. [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough C. Getting Vygotskian about theory of mind: mediation, dialogue, and the development of social understanding. Dev. Rev. 2008;28:225–262. [Google Scholar]
- Fonov V., Evans A.C., Botteron K.N., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodi A., Bridges L., Grolnick W. Correlates of mastery-related behavior: a short-term longitudinal study of infants in their second year. Child Dev. 1985;56:1291–1298. doi: 10.1111/j.1467-8624.1985.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Gaffrey M.S., Luby J.L., Botteron K.N., Repovš G., Barch D.M. Default mode network connectivity in children with a history of preschool onset depression. J. Child Psychol. Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné C., Bernier A., McMahon C. The role of paternal mind-mindedness in preschoolers’ self-regulated conduct. Infant Child Dev. 2018:e2081. [Google Scholar]
- Gao W., Gilmore J.H., Shen D., Smith J.K., Zhu H., Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb. Cortex. 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer C. Practical markov chain monte carlo. Stat. Sci. 1992;7:473–483. [Google Scholar]
- Graham J.W. Missing data analysis: making it work in the real world. Annu. Rev. Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Carpenter S., Fair D.A. Early life stress is associated with default system integrity and emotionality during infancy. J. Child Psychol. Psychiatry. 2015;56:1212–1222. doi: 10.1111/jcpp.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. Experience and brain development. Child Dev. 1987;58:539. [PubMed] [Google Scholar]
- Grolnick W.S., Farkas M. Parenting and the development of children’s selfregulation. In: Bornstein M.H., editor. Vol. 5. Lawrence Erlbaum Associates; Mahwah, NJ: 2002. pp. 89–110. (Handbook of Parenting: Practical Issues). [Google Scholar]
- Grolnick W.S., Ryan R.M. Parent styles associated with children’s self-regulation and competence in school. J. Educ. Psychol. 1989;81:143–154. [Google Scholar]
- Gunnar M.R., Fisher P.A., Early Experience, Stress, and Prevention Network Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev. Psychopathol. 2006;18:651–677. [PubMed] [Google Scholar]
- Guttentag C.L., Landry S.H., Williams J.M., Baggett K.M., Noria C.W., Borkowski J.G., Swank P.R., Farris J.R., Crawford A., Lanzi R.G., Carta J.J., Warren S.F., Ramey S.L. “My Baby & Me”: effects of an early, comprehensive parenting intervention on at-risk mothers and their children. Dev. Psychol. 2014;50:1482–1496. doi: 10.1037/a0035682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Harvard University Press; Cambridge, MA: 2002. Neural Plasticity: The Effects of Environment on the Development of the Cerebral Cortex. [Google Scholar]
- Jann K., Hernandez L.M., Beck-Pancer D., McCarron R., Smith R.X., Dapretto M., Wang D.J.J. Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav. 2015;5:e00358. doi: 10.1002/brb3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussemet M., Koestner R., Lekes N., Landry R. A longitudinal study of the relationship of maternal autonomy support to children’s adjustment and achievement in school. J. Pers. 2005;73:1215–1235. doi: 10.1111/j.1467-6494.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Joussemet M., Mageau G.A., Koestner R. Promoting optimal parenting and children’s mental health: a preliminary evaluation of the how-to parenting program. J. Child Fam. Stud. 2014;23:949–964. [Google Scholar]
- Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kirk E., Pine K., Wheatley L., Howlett N., Schulz J., Fletcher B.C. A longitudinal investigation of the relationship between maternal mind-mindedness and theory of mind. Br. J. Dev. Psychol. 2015;33:434–445. doi: 10.1111/bjdp.12104. [DOI] [PubMed] [Google Scholar]
- Kok R., Thijssen S., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Verhulst F.C., White T., van IJzendoorn M.H., Tiemeier H. Normal variation in early parental sensitivity predicts child structural brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:824–831. doi: 10.1016/j.jaac.2015.07.009. e1. [DOI] [PubMed] [Google Scholar]
- Kolb B., Mychasiuk R., Gibb R. Brain development, experience, and behavior. Pediatr. Blood Cancer. 2014;61:1720–1723. doi: 10.1002/pbc.24908. [DOI] [PubMed] [Google Scholar]
- Kovacs M., Joormann J., Gotlib I.H. Emotion (dys)regulation and links to depressive disorders. Child Dev. Perspect. 2008;2:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam S. Social cognitive impairment and autism: what are we trying to explain? Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150082. doi: 10.1098/rstb.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Barch D.M., Belden A., Gaffrey M.S., Tillman R., Babb C., Nishino T., Suzuki H., Botteron K.N. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matte-Gagné C., Harvey B., Stack D.M., Serbin L.A. Contextual specificity in the relationship between maternal autonomy support and children’s socio-emotional development: a longitudinal study from preschool to preadolescence. J. Youth Adolesc. 2015;44:1528–1541. doi: 10.1007/s10964-014-0247-z. [DOI] [PubMed] [Google Scholar]
- McMahon C.A., Bernier A. Twenty years of research on parental mind-mindedness: empirical findings, theoretical and methodological challenges, and new directions. Dev. Rev. 2017 [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meins E. Psychology Press; 1997. Security of Attachment and the Social Development of Cognition. [Google Scholar]
- Meins E., Fernyhough C., Fradley E., Tuckey M. Rethinking maternal sensitivity: mothers’ comments on infants’ mental processes predict security of attachment at 12 months. J. Child Psychol. Psychiatry. 2001;42:637–648. [PubMed] [Google Scholar]
- Meins E., Fernyhough C., Wainwright R., Clark-Carter D., Das Gupta M., Fradley E., Tuckey M. Pathways to understanding mind: construct validity and predictive validity of maternal mind-mindedness. Child Dev. 2003;74:1194–1211. doi: 10.1111/1467-8624.00601. [DOI] [PubMed] [Google Scholar]
- Meins E., Fernyhough C., Arnott B., Leekam S.R., de Rosnay M. Mind-mindedness and theory of mind: mediating roles of language and perspectival symbolic play. Child Dev. 2013;84:1777–1790. doi: 10.1111/cdev.12061. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17:627–640. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010:1–13. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen A.S., Carlson S.M. Fathers matter: the role of father parenting in preschoolers’ executive function development. J. Exp. Child Psychol. 2015;140:1–15. doi: 10.1016/j.jecp.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J., Nebel M.B., Caffo B.S., Barber A.D., Pekar J.J., Mostofsky S.H. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers Ja., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek J.N., van der Werff S.J.A., Meens P.H.F., van den Bulk B.G., Jolles D.D., Veer I.M., van Lang N.D.J., Rombouts S.A.R.B., van der Wee N.J.A., Vermeiren R.R.J.M. Aberrant resting-state functional connectivity in limbic and salience networks in treatment--naïve clinically depressed adolescents. J. Child Psychol. Psychiatry. 2014;55:1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Peters S.K., Dunlop K., Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Dev. Cogn. Neurosci. 2012;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby K.L., Roisman G.I., Fraley R.C., Simpson J.A. The enduring predictive significance of early maternal sensitivity: social and academic competence through age 32 years. Child Dev. 2015;86:695–708. doi: 10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F.P., Chen H., Wong E., Kwek K., Saw S.-M., Chong Y.-S., Gluckman P.D., Fortier M.V., Pederson D., Meaney M.J., Qiu A. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5:e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Dagenais J.E., Goldsmith D.R., Glenn A.L., Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. Neuroimage. 2008;41:1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht R., Meins E., Fernyhough C., Centifanti L.C.M., Bureau J.-F., Pawlby S. Proof of concept of a mind–mindedness intervention for mothers hospitalized for severe mental illness. Dev. Psychopathol. 2017;29:555–564. doi: 10.1017/S0954579417000177. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.E., Rudie J.D., Pfeifer J.H., Masten C.L., McNealy K., Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- Solé-Padullés C., Castro-Fornieles J., De La Serna E., Calvo R., Baeza I., Moya J., Lázaro L., Rosa M., Bargalló N., Sugranyes G. Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Dev. Cogn. Neurosci. 2016;17:35–44. doi: 10.1016/j.dcn.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Khouzam A., Phillips J., Gaillard W.D., Kenworthy L.E., Yerys B.E., Vaidya C.J., Menon V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5:738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Nouchi R., Hashizume H., Sekiguchi A., Kotozaki Y., Nakagawa S., Miyauchi C.M., Sassa Y., Kawashima R. Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex. 2013;49:2106–2125. doi: 10.1016/j.cortex.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Tamis-Lemonda C.S., Bornstein M.H., Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Dev. 2001;72:748–767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., White T., Van Ijzendoorn M.H. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Thompson A.R. Early attachment and later development: reframing the questions. In: Cassady J., Shaver P.R., editors. Handbook of Attachment: Theory, Research, and Clinical Applications. The Guilford Press; New York, NY: 2016. pp. 330–348. [Google Scholar]
- Tomasi D., Volkow N.D. Abnormal functional connectivity in children with Attention-Deficit/Hyperactivity disorder. BPS. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. Curr. Top. Behav. Neurosci. 2014;16:109–129. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple N., Bernier A., Mageau G.A. Broadening the study of infant security of attachment: maternal autonomy-support in the context of infant exploration. Soc. Dev. 2011;20:17–32. [Google Scholar]
- White T., Muetzel R., Schmidt M., Langeslag S.J.E., Jaddoe V., Hofman A., Calhoun V.D., Verhulst F.C., Tiemeier H. Time of acquisition and network stability in pediatric resting-state functional magnetic resonance imaging. Brain Connect. 2014;4:417–427. doi: 10.1089/brain.2013.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Zhai H., Friederici A.D., Jia F. The development of the intrinsic functional connectivity of default network subsystems from age 3 to 5. Brain Imaging Behav. 2016;10:50–59. doi: 10.1007/s11682-015-9362-z. [DOI] [PubMed] [Google Scholar]