Significance
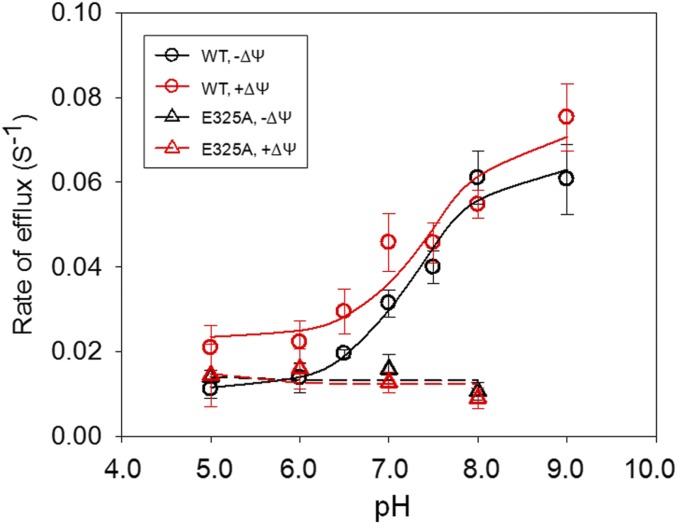
Protonation and deprotonation of Glu325 with a pKa of 10.5 is required for symport. Moreover, the H+ electrochemical gradient () accelerates deprotonation on the intracellular side with a 50- to 100-fold decrease in the Km. To probe the pK on the cytoplasmic side of the membrane, rates of lactose/H+ efflux were determined from pH 5.0 to 9.0 without or with a membrane potential (ΔΨ, interior positive) in right-side-out membrane vesicles. WT lactose efflux has an apparent pK of ∼7.2 that is unaffected by ΔΨ, mutant E325A is defective, and pH or ΔΨ (interior positive) has no effect. The effect of ΔΨ (interior positive) on the Km for efflux with WT LacY is insignificant relative to the marked effect on influx.
Keywords: membranes, transport, permease, membrane proteins, efflux
Abstract
LacY catalyzes accumulation of galactosides against a concentration gradient by coupling galactoside and H+ transport (i.e., symport). While alternating access of sugar- and H+-binding sites to either side of the membrane is driven by binding and dissociation of sugar, the electrochemical H+ gradient () functions kinetically by decreasing the Km for influx 50- to 100-fold with no change in Kd. The affinity of protonated LacY for sugar has an apparent pK (pKapp) of ∼10.5, due specifically to the pKa of Glu325, a residue that plays an irreplaceable role in coupling. In this study, rates of lactose/H+ efflux were measured from pH 5.0 to 9.0 in the absence or presence of a membrane potential (ΔΨ, interior positive), and the effect of the imposed ΔΨ on the kinetics of efflux was also studied in right-side-out membrane vesicles. The findings reveal that induces an asymmetry in the transport cycle based on the following observations: 1) the efflux rate of WT LacY exhibits a pKapp of ∼7.2 that is unaffected by the imposed ΔΨ; 2) ΔΨ increases the rate of efflux at all tested pH values, but enhancement is almost 2 orders of magnitude less than observed for influx; 3) mutant Glu325 ˗ Ala does little or no efflux in the absence or presence of ΔΨ, and ambient pH has no effect; and 4) the effect of ΔΨ (interior positive) on the Km for efflux is almost insignificant relative to the 50- to 100-fold decrease in the Km for influx driven by ΔΨ (interior negative).
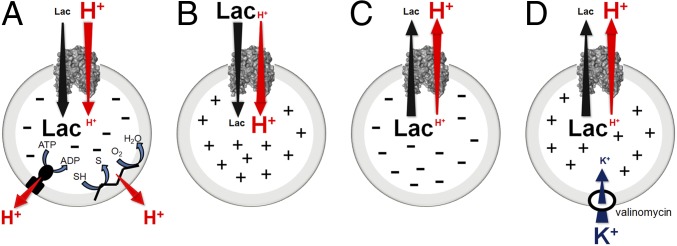
Escherichia coli lactose permease (LacY), the prototype of the major facilitator superfamily (MFS), catalyzes coupled transport of lactose and an H+ (lactose/H+ symport). Thus, in the presence of an electrochemical H+ gradient (, interior negative and/or alkaline), LacY utilizes free energy generated by the energetically downhill flux of H+ to drive uphill accumulation of lactose against a concentration gradient (aka active transport; Fig. 1A). Furthermore, in the absence of , downhill transport of lactose in response to a concentration gradient drives uphill flux of H+ with generation of , the polarity of which depends on the direction of the lactose gradient (Fig. 1B, influx ˗ interior positive and acid; Fig. 1C, efflux ˗ interior negative and alkaline) (reviewed in ref. 1).
Fig. 1.
Transport reactions of LacY. (A) -driven influx (i.e., active transport): free energy released from the downhill translocation of H+ in response to (interior negative and/or alkaline) generated by the respiratory chain or F1/Fo ATPase drives energetically uphill translocation of galactoside. (B) Influx: galactoside influx down a concentration gradient drives uphill H+ translocation with generation of (interior positive and acidic). (C) Efflux: galactoside efflux down a concentration gradient with generation of (interior negative and alkaline). (D) Imposition of ΔΨ (interior positive) during galactoside efflux: generated by influx of K+ down a concentration gradient in the presence of valinomycin.
LacY is structurally and functionally a monomer (2) with 12 transmembrane α-helices, many of which are shaped irregularly, arranged into N- and C-terminal 6-helix bundles with the N and C termini on the cytoplasmic side of the membrane (3). Two 3-helix inverted repeats are also observed within each 6-helix bundle (4), and there is a relatively long cytoplasmic loop between helix VI and VII that tethers the 2 6-helix pseudosymmetrical domains. Ten structures of LacY have been obtained by X-ray crystallography (3, 5–10). The first, a conformationally restricted mutant C154G, and the WT are inward (cytoplasmic)-open, apo conformers with a spacious, central aqueous cavity open on the cytoplasmic side and tightly sealed on the periplasmic side (3). This conformer appears to be the resting state of LacY in the membrane (11, 12). A second conformer obtained with double-Trp mutant G46W/G262W (LacYww) (13) is a partially outward (periplasmic)-open, occluded conformer with either of 2 bound lactose homologs in the middle of the molecule and a sealed cytoplasmic side (9, 10). An additional apo conformer was also obtained with LacYww in complex with a nanobody (Nb) bound to the periplasmic side (8).
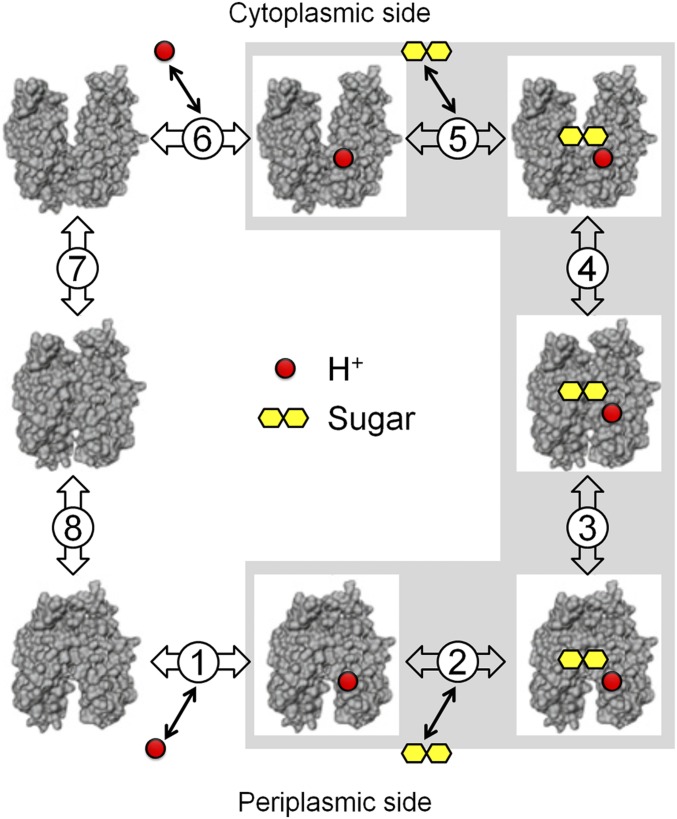
More than a half dozen independent biochemical/spectroscopic methods combined with the X-ray crystal structures of LacY provide virtually unequivocal evidence that conformational transitions between inward- and outward-facing forms result in sugar and H+ transport across the membrane (14). In this manner, cytoplasmic and periplasmic cavities open/close reciprocally, thereby allowing alternating exposure of galactoside- and H+-binding sites to either side of the membrane (Fig. 2) (15, 16). Transmembrane exchange reactions (i.e., equilibrium exchange and counterflow), which reflect alternating access, occur without deprotonation, and has no effect on these reactions. Therefore, the driving force for the conformational change(s) responsible for alternating access is not but binding and dissociation of galactoside (17).
Fig. 2.
Kinetic scheme for galactoside/H+ symport, exchange, and counterflow. Symport starts with protonation of LacY (step 1 or 6 for influx or efflux, respectively), which is required for high-affinity binding of lactose. Sugar binding to protonated LacY (step 2 or 5) causes a conformational change to an occluded state (step 3 or 4), which can relax to either side where sugar dissociates first (step 2 or 5), followed by deprotonation (step 1 or 6) and return of unloaded LacY via an apo occluded intermediate (steps 7 and 8). Exchange or counterflow involves only steps 2 to 5 (gray shaded area). Since LacY catalyzes symport in both directions, when symport is in the influx direction—step 1, protonation—the pK is very alkaline (∼10.5), and step 6—deprotonation—must have a much lower pK for deprotonation to occur. However, in the efflux direction, the pK values of these steps are reversed.
The affinity of LacY for galactosides varies with pH, and the apparent pK (pKapp) for galactoside binding is unexpectedly alkaline at ∼10.5 (18–21). Direct measurements of Glu325 in situ by surface enhanced infrared absorption spectroscopy demonstrate that the side chain has a pKa of 10.5 ± 0.1 (21), which concurs with the pKapp for galactoside affinity (18, 19). These and previous findings (reviewed in refs. 1 and 17) clearly indicate that LacY (i.e., Glu325) is protonated over the physiological pH range. Indeed, sugar binding to purified LacY in detergent does not induce a change in ambient pH under conditions where binding or release of 1 H+/LacY can be measured (20).
Coupling of galactoside with H+ translocation is clearly central to the mechanism of galactoside/H+ symport. Site-directed and Cys-scanning mutagenesis of the 417 residues in LacY reveal 9 irreplaceable aminoacyl side chains with respect to -driven lactose/H+ symport (22). Among the 9 residues, Glu325 is essential for H+ translocation since neutral replacement mutants at this position do not catalyze any reaction involving H+ translocation but bind galactosides with normal affinity and catalyze transmembrane sugar exchange reactions at least as well as WT LacY (23, 24). Strikingly, sugar binding becomes pH independent over a wide pH range in the mutant. This behavior is unique and indicates that Glu325 is directly involved in H+ binding and coupled H+ transport (17).
The mechanism of lactose/H+ symport with LacY may not be symmetrical. For instance, generation of (interior negative and/or alkaline) dramatically stimulates the initial rate of lactose influx by decreasing Km 50- to 100-fold (25, 26). However, a ∆Ψ (interior positive or negative) or a ∆pH (interior acid or alkaline) does not affect the Km for efflux dramatically (25). Recent stopped-flow experiments also demonstrate that the cytoplasmic side of LacY is able to open spontaneously when the periplasmic side is locked by a disulfide bond, indicating that an asymmetric conformational change can occur during the transport cycle (27). Nevertheless, there is strong evidence (reviewed in refs. 1 and 28) for a symmetrical ordered kinetic mechanism in which protonation precedes galactoside binding on one side of the membrane and follows sugar dissociation on the other side. A similar ordered mechanism, which also insures against futile H+ cycles, may be common to other members of the MFS (29–33). In an effort to study the problem more extensively, the present study was undertaken. However, because of the difficulty obtaining quantitative transport data from inverted vesicles due to their small internal volume (24), efflux from right-side-out (RSO) vesicles was utilized.
Initial rates of lactose efflux were measured from pH 5.0 to 9.0 in the absence or presence of a membrane potential (ΔΨ, interior positive) generated by K+ influx in the presence of valinomycin, a well-known K+ ionophore. Rates of efflux are pH dependent and exhibit a pKapp of ∼7.2. Imposition of ΔΨ (interior positive) increases the initial rate of efflux by only 2-fold or less, while a ΔΨ (interior negative) causes a 50- to 100-fold decrease in Km. Mutant E325A catalyzes efflux very slowly, and an imposed ΔΨ has no significant effect. Moreover, the Km for efflux remains unchanged when a ΔΨ (interior positive) is imposed. The results demonstrate that the pKapp for efflux also involves Glu325 but is hardly affected by , which imposes a strong asymmetry on the symport cycle.
Results
WT LacY.
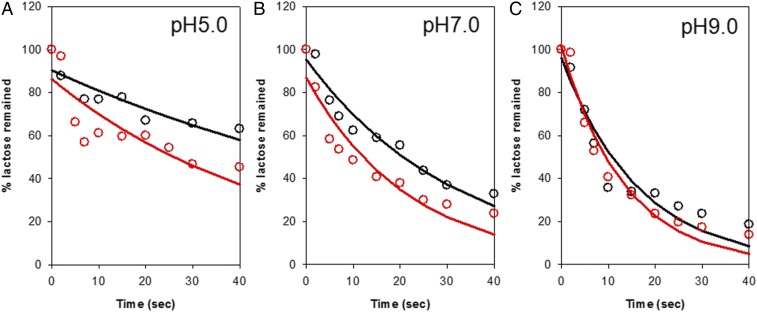
RSO membrane vesicles expressing WT LacY prepared in KPi (for efflux without an imposed ΔΨ) or NaPi and valinomycin (for efflux /with an interior positive ΔΨ) were preequilibrated with [14C]lactose at a given pH and rapidly diluted 200-fold into KPi at the same pH, and samples were rapidly filtered over the initial 40 s in order to measure initial rates of efflux (Fig. 3 and SI Appendix, Fig. S2). In the absence of an imposed ΔΨ, efflux is relatively slow at acidic pH and becomes more rapid with increasing pH. Thus, the half-time for efflux (t1/2) decreases from ∼62 s at pH 5.0 to ∼22 s at pH 7.0 to ∼11 s at pH 9.0 (Fig. 3 and SI Appendix, Fig. S2 and Table S1), as indicated previously (34).
Fig. 3.
Effect of pH on the rate of efflux in the absence or presence of ∆Ψ (interior positive) by WT LacY. Typical examples of efflux experiments with WT LacY were carried out by equilibrating RSO membrane vesicles with 10 mM [14C]lactose at given values of pH followed by a rapid 200-fold dilution (Materials and Methods) in the absence (black line) or presence (red line) of ∆Ψ (interior positive). ∆Ψ (interior positive) was generated by diluting [14C]lactose-loaded RSO membrane vesicles in 100 mM NaPi into 100 mM KPi in the presence of 25 μM valinomycin at given pH values. Efflux data of WT LacY obtained at pH 5.0, pH 7.0, and pH 9.0 are shown in A, B, and C, respectively. No effect was observed upon omission of valinomycin or dilution of vesicles loaded with KPi into equimolar KPi in the presence of the ionophore. Lines represent an exponential fit of the data.
Rapid dilution of RSO membrane vesicles preloaded with NaPi into equimolar KPi in the presence of valinomycin generates a membrane potential (ΔΨ, interior positive) that can be monitored by the fluorescence change in bis(1,3-diethylbarbituric acid) [DiBAC4 (3)] (Fig. 1D). The positive ΔΨ causes an increase in the fluorescence of DiBAC4 (3), which is maintained for at least 1.0 min (SI Appendix, Fig. S1). Under these conditions, the rate of efflux also increases with pH, and t1/2 decreases (Table 1). Compared to the rates in the absence of ΔΨ, imposition of ΔΨ (interior positive) clearly enhances the rate of efflux particularly below pH 7.0. For example, at pH 5.0, t1/2 decreases from ∼62 to ∼33 s; at pH 7.0, from ∼22 to ∼15 s; and at pH 9.0, from ∼11 to ∼9 s (Fig. 3, Table 1, and SI Appendix, Fig. S2). Enhancement of the rate is 2-fold at pH < 7.0 and decreases as pH increases to 9.0.
Table 1.
t1/2 of efflux of WT LacY in the absence or presence of ∆Ψ (interior positive) at given pH
| t1/2(s), pH | −ΔΨ | +ΔΨ |
| 5 | 62.4 ± 9.9 | 33.2 ± 6.7 |
| 6 | 50.2 ± 10.2 | 31.2 ± 5.8 |
| 6.5 | 35.5 ± 1.7 | 23.6 ± 3.6 |
| 7 | 22.1 ± 2.0 | 15.2 ± 2.0 |
| 7.5 | 17.4 ± 1.5 | 15.2 ± 1.4 |
| 8 | 11.4 ± 1.1 | 12.7 ± 0.7 |
| 9 | 11.4 ± 1.4 | 9.2 ± 0.9 |
Rate constant of efflux was obtained by fitting experimental data with exponential decay as described in Materials and Methods, and t1/2 of efflux was calculated by ln2/rate constant.
The pKapp for efflux by WT LacY was determined by plotting the rate of efflux at each pH shown in the absence (Fig. 4, solid black curve) or presence of the imposed ΔΨ (interior positive) (Fig. 4, solid red curve). The imposed ΔΨ (interior positive) has no significant effect on the pKapp for efflux, which exhibits a value of ∼7.2 in the absence or presence of ΔΨ (interior positive).
Fig. 4.
Effect of ΔΨ (interior positive) on the pH dependence of efflux. Rates of efflux are plotted as a function of pH. The error bar on each pH value indicates the SE of the rate constant.
Mutant E325A LacY.
Although mutant E325A binds galactosides and catalyzes alternating access (i.e., equilibrium exchange and counterflow) at least as well as WT LacY, the mutant is defective in net downhill efflux (17, 23, 24). When rates of lactose efflux by mutant E325A LacY are measured at various pH values in the same manner as described for WT LacY, it is apparent that mutant E325A catalyzes relatively little efflux at any pH tested. Furthermore, imposition of ΔΨ (interior positive) has no effect (Fig. 4 and SI Appendix, Fig. S3).
Kinetics of Efflux.
RSO vesicles expressing WT LacY were preequilibrated with [14C]lactose at given concentrations, and efflux was measured in the absence or presence of an imposed ΔΨ (interior positive) at pH 7.5 (SI Appendix, Fig. S4). The amount of lactose released in the first 10 s was used to estimate the velocity of efflux at each lactose concentration, and Km and Vmax for lactose efflux were determined with the Michaelis–Menten equation (SI Appendix, Fig. S5). The observed Km in the absence or presence of ΔΨ (interior positive) is about 8.4 or 6.8 mM, respectively. Therefore, ΔΨ (interior positive) has only a borderline significant effect on the Km for lactose efflux. Vmax for lactose efflux is also similar in the absence or presence of the imposed ΔΨ (interior positive), 72 or 68 nmol·min−1·mg protein−1, respectively.
Discussion
LacY catalyzes lactose/H+ symport across the cytoplasmic membrane chemiosmotically (17). However, rather than driving the conformational change in LacY responsible for alternating access, drives active transport by changing the rate-limiting step for turnover from deprotonation to reorientation of apo LacY (reviewed in refs. 1 and 28). Thus, causes a kinetic asymmetry because of 1) a change in rate-determining step and 2) a decrease in the Km from the outside by ∼50- to 100-fold with little effect on Km from the inside. The differences produce the kinetic asymmetry.
Thus, lactose/H+ symport catalyzed by LacY exhibits a kinetic asymmetry in the presence of . Importantly, a recent study (27) demonstrates that the cavity can open on the cytoplasmic side to provide access to the galactoside-binding site when the periplasmic side is locked by cross-linking, thereby indicating that conformational changes on one side of LacY can occur independent of structural changes on the other side (27).
Transmembrane exchange does not involve indicating that binding and dissociation of sugar, not H+ turnover, drives the alternating access conformational change (17). Since the driving force for accumulation against a concentration gradient is and E. coli has a stable internal pH of 7.6 (35–37), a pKa of 10.5 for binding (18, 21) suggests that decreasing the pKa to ∼pH 7.2 would deprotonate only ∼50% of the LacY molecules. Without decreasing the H+ concentration of the cytoplasm, how can deprotonation be increased? Neutral replacement mutants for Arg302 (helix IX), which is relatively close to Glu325 (∼6 Å), are unable to catalyze active transport but exhibit transmembrane exchange (38), and evidence has been presented (39, 40) in support of the idea that H+ may be extracted from Glu325 by spatial fluctuations in the position of Arg302 that expel H+ by moving near Glu325 or vice versa.
However, of course, LacY must also deprotonate for turnover to occur. One possibility is that the pKa of Glu325 (helix X) in a hydrophobic pocket between helices IX and X (21) may decrease by becoming more accessible to water. In this regard, galactosidic sugars are hydrated in aqueous solution. Although current structures do not diffract at a resolution sufficient to visualize water, it is likely that galactosides become dehydrated when they interact with side chains in the binding site. Since helices IX and X are next to each other in the tertiary structure, Arg302 (helix IX) is irreplaceable with respect to active transport and mutants R302A or R302S exhibit properties similar to mutants with neutral replacements for Glu325, positively charged Arg302 (helix IX) may be important with regard to deprotonation (41). Evidence supporting this idea has been presented (41, 42). Most recently, side chains in the vicinity of Glu325 were mutated with the rationale that interaction with Glu325 should alter its pKa. Remarkably, only mutant R302K of the sites tested causes the pKa to decrease and by over 2 pH units, thereby lending further support to the idea that H+ is displaced from Glu325 by spatial fluctuations in the position of Arg302 moving near Glu325 or vice versa (40). The possibility will be tested with a suppressor t-RNA approach using arginine homologs with side chains of varied lengths (43).
Materials and Methods
Materials.
[d-glucose-14C-(U)]Lactose was purchased from Moravek Biochemicals. Valinomycin was purchased from Calbiochem-Novabiochem Corporation. Isopropyl-β-d-1-thiogalactopyranoside (IPTG) was obtained from Gold Biotechnology. Bis(1,3-dibutylbarbituric acid) trimethine oxonol was obtained from Sigma-Aldrich. All other materials were reagent grade and obtained from commercial sources. The genes of WT and mutant E325A were sequenced in their entirety.
Preparation of RSO Membrane Vesicles.
E. coli T184 [lacI+O+ Z− Y− (A) rpsL met− thr− recA hsdM hsdR/F′lacIq O+ ZD118 (Y+ A+)] cells transformed with plasmid pT7-5 encoding a cassette lacY gene with WT LacY or mutant E325A with a 10-His tag at the C terminus were grown in 1.0 L of LB broth at 37 °C. After 2 h induction with 1 mM IPTG, cells were harvested by centrifugation, and RSO membrane vesicles were prepared as described (44, 45). The vesicles were resuspended in 100 mM sodium or potassium phosphate (NaPi or KPi as indicated; pH 7.5)/10 mM MgSO4 at a protein concentration of 10 to 15 mg/mL. Aliquots were flash frozen in liquid nitrogen and stored at −80 °C until use.
Measurement of ΔΨ (Interior Positive).
The imposed ΔΨ (interior positive) generated by influx of K+ in the presence of valinomycin was estimated by measuring the fluorescence increase of Bis(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4 (3)] at 25 °C. RSO membrane vesicles (2 mg/mL total protein, in 100 mM NaPi at a given pH) were incubated with 4 μM DiBAC4 (3) at 25 °C for 30 min. An aliquot (50 μL) of RSO vesicles was harvested at 14,000 rpm for 10 min. The pellet was immediately resuspended in 50 μL 100 mM NaPi and diluted into 2 mL of 100 mM phosphate buffer at a given Na+/K+ ratio and the same pH in a quartz cuvette. Fluorescence of DiBAC4 (3) was monitored in a SLM-Aminco 8100 spectrofluorimeter (modified by OLIS, Inc.) at excitation and emission wavelengths of 490 and 516 nm, respectively. After 0.5 and 1.5 min, valinomycin and CCCP were added to a final concentration of 25 μM, respectively, and measurement of fluorescence was continued for a total of 5 min.
Efflux.
Efflux was carried out with RSO membrane vesicles as described (34, 46) with minor modifications. RSO membrane vesicles expressing WT LacY or mutant E325A were harvested by centrifugation, resuspended to a protein concentration of 25 mg/mL in 100 mM KPi with 0.5% DMSO at a given pH, and equilibrated with 10 mM [14C]lactose (5 mCi/mmol) at 25 °C for 3 h. Aliquots (2 μL) were rapidly diluted 200-fold into 100 mM KPi at the same pH. For efflux with ΔΨ (interior positive), RSO membrane vesicles were harvested by centrifugation, washed twice with 100 mM sodium phosphate (NaPi) at a given pH (15-min incubation each time), and resuspended in the same NaPi buffer to a protein concentration of 25 mg/mL. After 3-h equilibration with 10 mM [14C]lactose (5 mCi/mmol) in the presence of 25 μM valinomycin (dissolved in 0.5% DMSO final concentration) at 25 °C, an aliquot of 2 μL was rapidly diluted 200-fold into 100 mM KPi at the appropriate pH. Reactions were terminated by addition of 3 mL of termination buffer (100 mM KPi/100 mM LiCl, pH 5.5) and rapid filtration at given times. Radioactivity of the samples was determined by liquid scintillation spectrometry, and rate constants for efflux were obtained by fitting data with the equation for exponential decay (y = ae−bx).
Kinetics of Efflux.
RSO membrane vesicles expressing WT LacY were resuspended to a protein concentration of 25 mg/mL in 100 mM KPi (pH 7.5) with 0.5% DMSO and equilibrated with given concentrations of [14C]lactose overnight at 4 °C. An aliquot of 2 μL was rapidly diluted 200-fold into 100 mM KPi (pH 7.5). Reactions were terminated at given times by addition of termination buffer and rapidly filtered. For measurements in the presence of ΔΨ (interior positive), RSO vesicles were washed twice with 100 mM NaPi (pH 7.5) and concentrated to 25 mg protein/mL in the same buffer. After equilibrating with given concentrations of [14C]lactose in the presence of 25 μM valinomycin (dissolved in DMSO; final concentration of DMSO was 0.5%) overnight at 4 °C, an aliquot of 2 μL was rapidly diluted 200-fold into 100 mM KPi (pH 7.5). Efflux was terminated by addition of termination buffer and rapid filtration at given times. The amount of lactose lost in the first 10 s was calculated for each efflux reaction, and the Km and Vmax of efflux were determined by fitting the data with the Michaelis–Menten equation.
Data Availability.
All of the data are included in the manuscript.
Supplementary Material
Acknowledgments
We are deeply indebted to Irina Smirnova and Vladimir Kasho for multiple discussions, and we thank Maria Luisa Garcia and Gregory Kaczorowski for insightful editorial advice. We also thank Wen Chi Huang and Bryan Chau for technical support. This work was supported by the National Science Foundation (Eager Grant MCB-1547801 to H.R.K.), National Institutes of Health (Grant 1R01 GM120043 to H.R.K.), and a grant from Ruth and Bucky Stein (to H.R.K.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916563117/-/DCSupplemental.
References
- 1.Guan L., Kaback H. R., Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 35, 67–91 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin-Tóth M., Lawrence M. C., Kaback H. R., Properties of permease dimer, a fusion protein containing two lactose permease molecules from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 91, 5421–5425 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson J., et al. , Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–615 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Radestock S., Forrest L. R., The alternating-access mechanism of MFS transporters arises from inverted-topology repeats. J. Mol. Biol. 407, 698–715 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Mirza O., Guan L., Verner G., Iwata S., Kaback H. R., Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 25, 1177–1183 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaptal V., et al. , Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc. Natl. Acad. Sci. U.S.A. 108, 9361–9366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan L., Mirza O., Verner G., Iwata S., Kaback H. R., Structural determination of wild-type lactose permease. Proc. Natl. Acad. Sci. U.S.A. 104, 15294–15298 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X., et al. , Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation. Proc. Natl. Acad. Sci. U.S.A. 113, 12420–12425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar H., Finer-Moore J. S., Kaback H. R., Stroud R. M., Structure of LacY with an α-substituted galactoside: Connecting the binding site to the protonation site. Proc. Natl. Acad. Sci. U.S.A. 112, 9004–9009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H., et al. , Structure of sugar-bound LacY. Proc. Natl. Acad. Sci. U.S.A. 111, 1784–1788 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Y., Kaback H. R., Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc. Natl. Acad. Sci. U.S.A. 107, 9903–9908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X., et al. , Evidence for an intermediate conformational state of LacY. Proc. Natl. Acad. Sci. U.S.A. 109, E698–E704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smirnova I., Kasho V., Sugihara J., Kaback H. R., Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation. Proc. Natl. Acad. Sci. U.S.A. 110, 8876–8881 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova I., Kasho V., Kaback H. R., Lactose permease and the alternating access mechanism. Biochemistry 50, 9684–9693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jardetzky O., Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966). [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P., Translocations through natural membranes. Adv. Enzymol. Relat. Areas Mol. Biol. 29, 33–87 (1967). [DOI] [PubMed] [Google Scholar]
- 17.Kaback H. R., A chemiosmotic mechanism of symport. Proc. Natl. Acad. Sci. U.S.A. 112, 1259–1264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnova I. N., Kasho V., Kaback H. R., Protonation and sugar binding to LacY. Proc. Natl. Acad. Sci. U.S.A. 105, 8896–8901 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirnova I., Kasho V., Sugihara J., Choe J. Y., Kaback H. R., Residues in the H+ translocation site define the pKa for sugar binding to LacY. Biochemistry 48, 8852–8860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smirnova I., Kasho V., Sugihara J., Vázquez-Ibar J. L., Kaback H. R., Role of protons in sugar binding to LacY. Proc. Natl. Acad. Sci. U.S.A. 109, 16835–16840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grytsyk N., Sugihara J., Kaback H. R., Hellwig P., pKa of Glu325 in LacY. Proc. Natl. Acad. Sci. U.S.A. 114, 1530–1535 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frillingos S., Sahin-Tóth M., Wu J., Kaback H. R., Cys-scanning mutagenesis: A novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 12, 1281–1299 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Carrasco N., Antes L. M., Poonian M. S., Kaback H. R., Lac permease of Escherichia coli: Histidine-322 and glutamic acid-325 may be components of a charge-relay system. Biochemistry 25, 4486–4488 (1986). [DOI] [PubMed] [Google Scholar]
- 24.Carrasco N., et al. , Characterization of site-directed mutants in the lac permease of Escherichia coli. 2. Glutamate-325 replacements. Biochemistry 28, 2533–2539 (1989). [DOI] [PubMed] [Google Scholar]
- 25.Kaczorowski G. J., Robertson D. E., Kaback H. R., Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delata psi, delta pH, and Delta mu H+. Biochemistry 18, 3697–3704 (1979). [DOI] [PubMed] [Google Scholar]
- 26.Robertson D. E., Kaczorowski G. J., Garcia M. L., Kaback H. R., Active transport in membrane vesicles from Escherichia coli: The electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry 19, 5692–5702 (1980). [DOI] [PubMed] [Google Scholar]
- 27.Smirnova I., Kasho V., Jiang X., Kaback H. R., An asymmetric conformational change in LacY. Biochemistry 56, 1943–1950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madej M. G., Kaback H. R., “The life and times of Lac permease: Crystals ain’t enough, but they certainly do help” in Membrane Transporter Function: To Structure and Beyond, Ziegler C., Kraemer R., Eds. (Series in Biophysics: Transporters, Springer, 2014), vol. 17, pp. 121–158. [Google Scholar]
- 29.Dang S., et al. , Structure of a fucose transporter in an outward-open conformation. Nature 467, 734–738 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Sun L., et al. , Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490, 361–366 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Iancu C. V., Zamoon J., Woo S. B., Aleshin A., Choe J. Y., Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc. Natl. Acad. Sci. U.S.A. 110, 17862–17867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan L., Jakkula S. V., Hodkoff A. A., Su Y., Role of Gly117 in the cation/melibiose symport of MelB of Salmonella typhimurium. Biochemistry 51, 2950–2957 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ethayathulla A. S., et al. , Structure-based mechanism for Na(+)/melibiose symport by MelB. Nat. Commun. 5, 3009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaczorowski G. J., Kaback H. R., Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry 18, 3691–3697 (1979). [DOI] [PubMed] [Google Scholar]
- 35.Padan E., Zilberstein D., Rottenberg H., The proton electrochemical gradient in Escherichia coli cells. Eur. J. Biochem. 63, 533–541 (1976). [DOI] [PubMed] [Google Scholar]
- 36.Zilberstein D., Schuldiner S., Padan E., Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry 18, 669–673 (1979). [DOI] [PubMed] [Google Scholar]
- 37.Ramos S., Schuldiner S., Kaback H. R., The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 73, 1892–1896 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahin-Tóth M., Lawrence M. C., Nishio T., Kaback H. R., The C-4 hydroxyl group of galactopyranosides is the major determinant for ligand recognition by the lactose permease of Escherichia coli. Biochemistry 40, 13015–13019 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Weinglass A. B., Sondej M., Kaback H. R., Manipulating conformational equilibria in the lactose permease of Escherichia coli. J. Mol. Biol. 315, 561–571 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Grytsyk N., Santos Seiça A. F., Sugihara J., Kaback H. R., Hellwig P., Arg302 governs the pKa of Glu325 in LacY. Proc. Natl. Acad. Sci. U.S.A. 116, 4934–4939 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinglass A. B., Smirnova I. N., Kaback H. R., Engineering conformational flexibility in the lactose permease of Escherichia coli: Use of glycine-scanning mutagenesis to rescue mutant Glu325–>Asp. Biochemistry 40, 769–776 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Andersson M., et al. , Proton-coupled dynamics in lactose permease. Structure 20, 1893–1904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Öjemalm K., et al. , Energetics of side-chain snorkeling in transmembrane helices probed by nonproteinogenic amino acids. Proc. Natl. Acad. Sci. U.S.A. 113, 10559–10564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Short S. A., Kaback H. R., Kohn L. D., Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J. Biol. Chem. 250, 4291–4296 (1975). [PubMed] [Google Scholar]
- 45.Kaback H. R., “Bacterial membranes” in Methods in Enzymology, Kaplan N. P., Jakoby W. B., Colowick N. P., Eds. (Elsevier, New York, 1971), vol. XXII, pp. 99–120. [Google Scholar]
- 46.Guan L., Kaback H. R., Properties of a LacY efflux mutant. Biochemistry 48, 9250–9255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data are included in the manuscript.