Summary
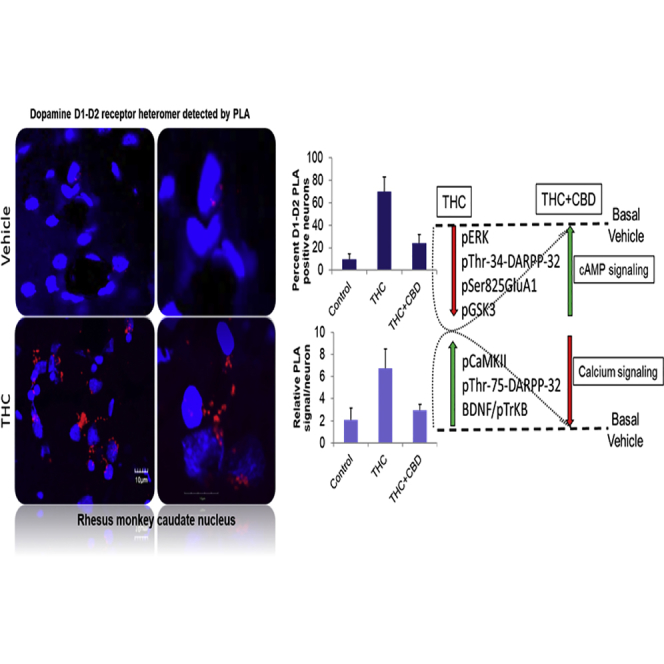
Long-term cannabis users manifest deficits in dopaminergic functions, reflecting Δ9-tetrahydrocannabinol (THC)-induced neuroadaptive dysfunctional dopamine signaling, similar to those observed upon dopamine D1-D2 heteromer activation. The molecular mechanisms remain largely unknown. We show evolutionary and regional differences in D1-D2 heteromer abundance in mammalian striatum. Importantly, chronic THC increased the number of D1-D2 heteromer-expressing neurons, and the number of heteromers within individual neurons in adult monkey striatum. The majority of these neurons displayed a phenotype co-expressing the characteristic markers of both striatonigral and striatopallidal neurons. Furthermore, THC increased D1-D2-linked calcium signaling markers (pCaMKIIα, pThr75-DARPP-32, BDNF/pTrkB) and inhibited cyclic AMP signaling (pThr34-DARPP-32, pERK1/2, pS845-GluA1, pGSK3). Cannabidiol attenuated most but not all of these THC-induced neuroadaptations. Targeted pathway analyses linked these changes to neurological and psychological disorders. These data underline the importance of the D1-D2 receptor heteromer in cannabis use-related disorders, with THC-induced changes likely responsible for the reported adverse effects observed in heavy long-term users.
Subject Areas: Drugs, Neuroscience, Cellular Neuroscience
Graphical Abstract
Highlights
-
•
Dopamine D1 and D2 receptors form complexes in the striatum of mammalian species
-
•
Evolutionary differences in D1-D2 expression: human > primate > rat > mouse
-
•
Chronic THC increased D1-D2-expressing neuron numbers in nonhuman primate striatum
-
•
Chronic THC altered primate striatal neuron phenotype and signaling reversed by CBD
Drugs; Neuroscience; Cellular Neuroscience
Introduction
Cannabis is the most widely consumed illicit substance globally, with use reaching more than 180 million people aged 15–64 years (United Nations Office on Drugs and Crimes, World Drug Report, 2013). In North America, the prevalence of cannabis use is higher than the global average, as the movement to medicalize, legalize, and normalize its use continues unabated in the United States and Canada. A number of deleterious effects linked to the use of cannabis have been described, with consequences related to early age of initiation, frequency, and duration of use (Volkow et al., 2014, Volkow et al., 2016). Cannabis use is associated with higher risk of depression, anxiety, addiction, and psychosis (Bechtold et al., 2016, Patton et al., 2002; reviewed in Casadio et al., 2011, Hasin et al., 2016, Han et al., 2019, De Aquino et al., 2018), all of which are linked to the pharmacological actions of Δ9-tetrahydrocannabinol (THC) (Gaoni and Mechoulam, 1964, Huestis et al., 2001). The cannabis plant produces over 100 different cannabinoids, including the structurally distinct principal constituents, THC and cannabidiol (CBD) (Gaoni and Mechoulam, 1964, Huestis et al., 2001). The composition of retail cannabis is unregulated; over the past two decades, it has undergone dramatic increases in THC concentrations, and corresponding decreases in CBD levels (De Aquino et al., 2018). The current levels of THC and ratio of THC:CBD in some street samples, 80:1, are unprecedented (ElSohly et al., 2016), and a source of increasing concern (United Nations Office on Drugs and Crimes, World Drug Report, 2015, Englund et al., 2017, Boggs et al., 2018).
Both factors, THC levels and THC:CBD ratios, are germane to establishing safety standards for all categories of cannabis, as THC and CBD engender markedly different or even antagonistic molecular, pharmacological, and neuropsychiatric effects (Englund et al., 2017, Boggs et al., 2018). Smokable high-potency street cannabis (∼20% THC; ∼0.9 mg/kg) delivers THC at levels approximately 20 times the US Food and Drug Administration-approved starting doses of oral THC (∼0.04 mg/kg). CBD is approved for treating rare, severe forms of epilepsy. Acutely, THC can elicit intoxication, psychosis, anxiolysis, and cognitive impairment, including factors that limit the therapeutic potential of cannabis. With early initiation and long-term exposure, cannabis use can engender anxiety, elevate the risk for developing schizophrenia and other psychoses, and evolve into a cannabis use disorder (The National Academies of Sciences, 2017, Moore et al., 2007). High concentrations of THC and high ratios of THC:CBD in cannabis are associated with more robust euphoria, anxiety, and psychotic symptoms in otherwise normal people. High CBD:THC ratios produce opposite effects. CBD attenuates anxiety, cognitive deficits, and psychosis in heavy cannabis users (Casadio et al., 2011, Englund et al., 2017, Morgan and Curran, 2008, Schubart et al., 2011; Colizzi and Bhattacharyya, 2017, Fusar-Poli et al., 2009). Functional magnetic resonance imaging research also differentiates brain activity patterns elicited by THC or CBD (Bhattacharyya et al., 2010). The molecular mechanisms underlying CBD's apparent mitigation of specific adverse effects of THC are poorly understood (Boggs et al., 2018). There is a compelling need to clarify the biological effects of various THC doses and THC:CBD ratios on the brain, particularly with the view of identifying novel therapeutic targets to conceivably reverse the adverse consequences of long-term cannabis use and abuse.
As dopamine signaling systems are implicated in addiction, psychosis, anxiety, and cognition, this system is an ongoing focus of cannabis research. Acute or chronic THC exposure produces complex and possibly durable adaptive changes in dopamine signaling pathways. In preclinical and clinical studies, an acute dose of THC modestly elevates dopamine release in the ventral striatum (Bossong et al., 2009, Gardner, 2005, Lupica and Riegel, 2005, Bloomfield et al., 2016). Heavy, long-term cannabis users manifest reduced dopamine release following methamphetamine challenge and deficits in dopaminergic-related functions, such as poor working memory, negative emotionality, and attenuated rewarding effects (Volkow et al., 2014, Bloomfield et al., 2016). Heavy cannabis use is also associated with impaired executive function, apathy, and lack of motivation (Volkow et al., 2014, Bloomfield et al., 2016). Conceivably, these adverse cognitive and behavioral consequences reflect THC-induced neuroadaptive changes in dopamine signaling, but the nexus between chronic THC exposure, dysfunctional dopamine systems, and functional consequences remains largely unknown (Volkow et al., 2016, Bloomfield et al., 2016).
Recent evidence points to the involvement of a mechanism by which the dopamine system modulates depression-like, anxiety-like, and amotivational behaviors in animals. This mechanism involves the dopamine D1-D2 receptor heteromer, the existence of which was recently confirmed in the striatum of rat using in situ proximity ligation assay (PLA), co-immunoprecipitation, and in situ fluorescence resonance energy transfer (FRET) (Hasbi et al., 2009, Hasbi et al., 2018, Perreault et al., 2016) and in the striatum of nonhuman primate using PLA (Hasbi et al., 2018, Rico et al., 2017). Activation of the heteromer led to anxiety-like (Shen et al., 2015a) and depression-like (Shen et al., 2015a, Hasbi et al., 2014) phenotypes in rodents, blocked the development of addiction-like behaviors, and prevented the development of drug-induced sensitization and ΔFosB accumulation (Hasbi et al., 2018). Specific disruption or blockade of the D1-D2 heteromer reversed the above-mentioned effects and revealed tonic inhibitory suppression of the hedonic value of psychostimulant and natural rewards (Perreault et al., 2016, Hasbi et al., 2018, Shen et al., 2015a, Shen et al., 2015b).
Based on the observation that chronic use of cannabis and D1-D2 heteromer activation induce similar consequences, i.e., dopamine function decrease, depression, anxiety, apathy, and lack of motivation, the present study was designed to investigate the relationship that may exist between chronic THC use and D1-D2 heteromer density and functionality in nonhuman primate striatum, as well as the potential protective role of CBD to ameliorate THC-induced effects.
Results
PLA Probe Validation and Evidence for D1-D2 Heteromer in Different Species
Antibodies against dopamine D1 and D2 receptors used for in situ PLA have been previously validated by different methodologies, including by immunocytochemistry in HEK cells expressing each of the five dopamine receptors, with no cross-reactivity (Lee et al., 2004), and by immunohistochemistry in D1−/− and D2−/− knockout (KO) mice (Perreault et al., 2010, Perreault et al., 2016). The antibodies have also been used to detect the D1-D2 heteromer by in situ PLA in macaque (Rico et al., 2017) and rat (Hasbi et al., 2018, Perreault et al., 2016) striatum. To avoid any non-specific labeling that may be due to the use of secondary antibodies, the D1 and D2 receptor antibodies were directly conjugated to the Plus and Minus oligonucleotides to generate the PLA probes. The D1-D2 heteromer was evaluated by in situ PLA in the striatum of different species, including mouse, rat, African green monkey, rhesus monkey, and human (Figures 1, S1, and S2), as well as in striatal sections from wild-type, D1−/−, D2−/−, and D5−/− receptor gene deleted mice.
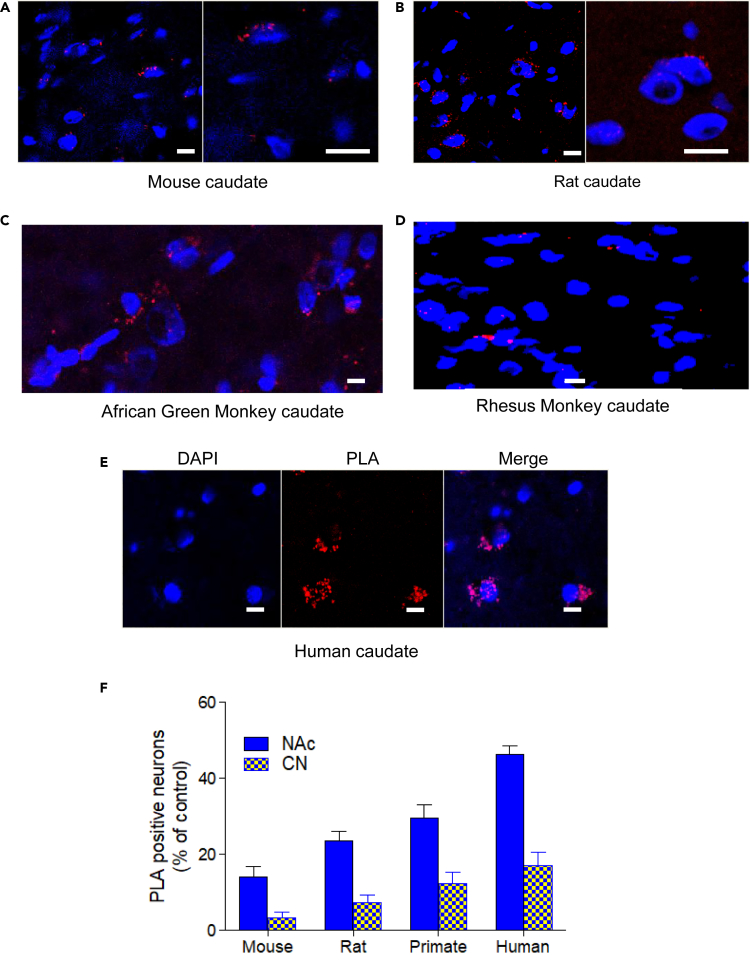
Figure 1.
Evidence for D1-D2 Heteromer Existence in Different Species
(A–F) Dopamine D1-D2 heteromers were revealed by PLA using specific D1 and D2 receptor antibodies directly conjugated to the Minus and Plus probes. PLA signal (red spots) was observed in striatal slices from mouse (A), rat (B), monkey (C and D), and humans (E). Quantification of the percentage of neurons (nuclei stained by DAPI) showing PLA signals in the dorsal striatum (caudate nucleus, CPu) and the ventral striatum (nucleus accumbens, NAc) in different species. Analyses included male only and showed regional and species differences (F). Two-way ANOVA test (p < 0.0001). Scale bars, 10 μm.
A positive D1-D2 PLA signal was observed in 14% and 16% of neurons from nucleus accumbens (NAc) of wild-type (Figures 1A and 1F) and D5−/− KO mice (Figure S1), respectively, and in 3%–4% of neurons in the caudate putamen (CPu or CN) (Figure 1F). In contrast, D1-D2 PLA signal was negative in both D1−/− and D2−/− KO mouse striata (Figure S1). In rat, a positive D1-D2 PLA signal, estimated from striatal sections from three male rats, was present in 24% ± 2% NAc neurons and 7% ± 2% CPu neurons (Figures 1B and 1F). Reversing the order of oligonucleotides linked to generate the probes (i.e., D1-Minus, D2-Plus instead of D1-Plus, D2-Minus) did not change the PLA results in rat NAc (24% ± 2% versus 26% ± 5%). D1-D2 PLA was negative in sections from the same rats when control experiments were conducted in parallel as follows: absence of one of the probes or of an enzyme responsible for the signal, e.g., the ligase or the polymerase (Figure S1). In African green monkey the PLA signal was positive in 30% ± 4% of NAc neurons and in 16% ± 2.5% of CPu neurons (Figures 1C and 1F). Similarly, in rhesus monkey PLA was positive in 35% ± 4% of neurons in sections from NAc and 11% ± 3% of CPu neurons (Figures 1D and 1F). PLA signal was negative in the controls (absence of one probe or the other) using sections from rhesus monkey in parallel (Figure S1). In human striatum, D1-D2 PLA signal was detected in 45% ± 4% NAc neurons and in 17% ± 5% CN neurons (Figures 1E and 1F).
Effects on D1-D2 PLA were examined in sections from the ventral (NAc) and dorsal striatum (CPu) of rats injected centrally with a peptide (TAT-D1) capable of specifically and selectively disrupting the D1-D2 heteromer without affecting other receptor complexes (Hasbi et al., 2014, Hasbi et al., 2018) or its scrambled control peptide (TAT-sc) (each 300 pmol, intracerebroventricularly). PLA was positive in 23% and 22% of neurons in NAc of rats injected with the control peptide (TAT-sc) or vehicle, respectively, whereas PLA signal was not detected in sections from rats injected with TAT-D1 peptide either in the NAc or CPu (Figure S1).
The dopamine D1-D2 receptor heteromer was detected in the NAc of mouse, rat, nonhuman primates, and human (Figure 1F). These results indicate clear species differences in the number of neurons expressing the D1-D2 heteromer in the ventral striatum (i.e., NAc), with estimates ranging as follows: human (42%–45%) > primate (28%–36%) > rat (20%–25%) > mouse (14%–16%). In all species, we noted a difference between the ventral and dorsal striatum, with the neurons expressing heteromer more abundant in the ventral than in the dorsal region. The heteromer is also expressed in the CPu of these species with a rank order corresponding to that observed in the NAc: human (17%–20%) > monkey (10%–12%) > rat (5%–7%) > mouse (1.5%–3%) (Figure 1F). z Stack analyses showed that the PLA signal was distributed in the perimeter of neuronal cell bodies, suggesting that D1-D2 heteromers were likely distributed on the cell surface. No PLA signal was detected within cell nuclei (Figure S2). These data were corroborated by statistical analyzes using a two-way ANOVA test, which showed that there was a species difference (F = 102.2; df = 3; p < 0.0001), a regional difference (F = 367.2; df = 1; p < 0.0001), and an interaction (region X species, F = 16.15; df = 3; p < 0.0001).
THC or THC and CBD Effects on D1-D2 Heteromer in Monkey Nucleus Accumbens
In the NAc of control rhesus monkeys (Figure 2), D1-D2 PLA signal was detected in 28% ± 0.6% of neurons (n = 1,799). In monkeys repeatedly administered THC, the PLA signal was detected in 78.2% ± 0.7% of NAc neurons (n = 3,390), indicating a dramatic and significant increase in the number of neurons expressing the D1-D2 heteromer in NAc (one-way ANOVA, F(2,2) = 997; p < 0.0001). Analysis of the number of PLA-positive signals per neuron showed that the density of the D1-D2 heteromer per neuron was increased in animals treated repeatedly with THC. The average positive signal increased approximately 3-fold compared with vehicle-treated animals, from 2.3% ± 0.2% to 6.3% ± 0.4% fluorescent signals per neuron (one-way ANOVA, F(2,2) = 169.6; p < 0.0001). However, the number of PLA signals detected per neuron after THC varied widely, with the majority expressing four or more fluorescent signals per neuron; some were as high as 17 signals per neuron, and others expressed two to four signals per neuron (Figure S2).
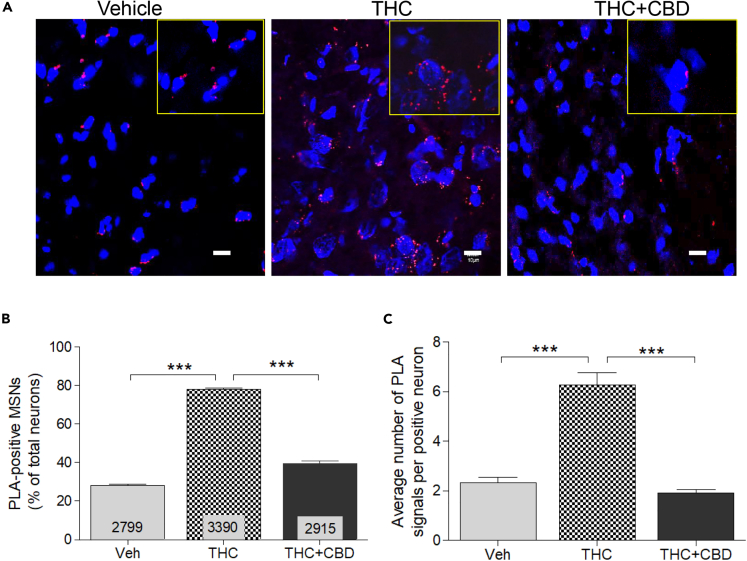
Figure 2.
Effect of Chronic THC and CBD on Dopamine D1-D2 Heteromer Expression in Monkey Nucleus Accumbens
(A–C) D1-D2 heteromer expression was analyzed by PLA in the NAc of monkeys treated chronically with vehicle, THC, or THC + CBD (A). Quantification of the PLA signals (B) from the three groups of monkeys (N = 3 per group) revealed that THC dramatically increased the number of neurons expressing the D1-D2 heteromer, an effect blocked in the presence of CBD. The relative average number of heteromer signals per neuron was also increased (C). Results are mean ± SEM from the analysis of different slices with the number of neurons analyzed indicated. Analysis was performed using one-way ANOVA, followed by Bonferroni correction, ***p < 0.0001. Scale bars, 10 μm.
In contrast to the effects of repeated THC administration, THC combined with CBD (1:3 ratio) attenuated the up-regulation of D1-D2 PLA signal (one-way ANOVA, F(2,2) = 997; p < 0.0001, with Bonferroni post-tests showing a difference with THC-treated (p < 0.001) and with vehicle-treated animals (p < 0.01)). The signal was detected in 39.6% ± 1.1% of neurons in NAc (n = 2,915), in contrast to 78.2% signal found in THC-only-treated animals and 28% in controls. PLA signal density in the NAc neurons of monkeys receiving THC and CBD revealed an average of 1.91% ± 0.13% fluorescent signal per neuron, suggesting that CBD administration inhibited THC-induced increase in the number of neurons expressing the D1-D2 heteromer and heteromer density per neuron.
THC or THC and CBD Effects on D1-D2 Heteromer in Monkey Caudate Nucleus
In the CPu of control monkeys (Figures 3A and 3B), D1-D2 PLA signal was detected in 10.9% ± 0.1% neurons (n = 2,342). In the CPu of animals repeatedly administered THC, the D1-D2 PLA signal was detected in 81.2% ± 0.2% neurons (n = 3,090), indicating a dramatic and significant increase in the number of neurons expressing the D1-D2 heteromer in this dopamine-rich brain region (one-way ANOVA, F(2,2) = 764.8, p < 0.0001; Bonferroni post-test control versus THC, p < 0.0001). The density of D1-D2 heteromers per neuron (Figure 3C, PLA signal) also increased an average of 3-fold in animals repeatedly administered THC, compared with vehicle-treated animals, from 2.1% ± 0.02% to 6.7% ± 0.03% signals per neuron (one-way ANOVA, F(2,2) = 308.3; p < 0.0001).
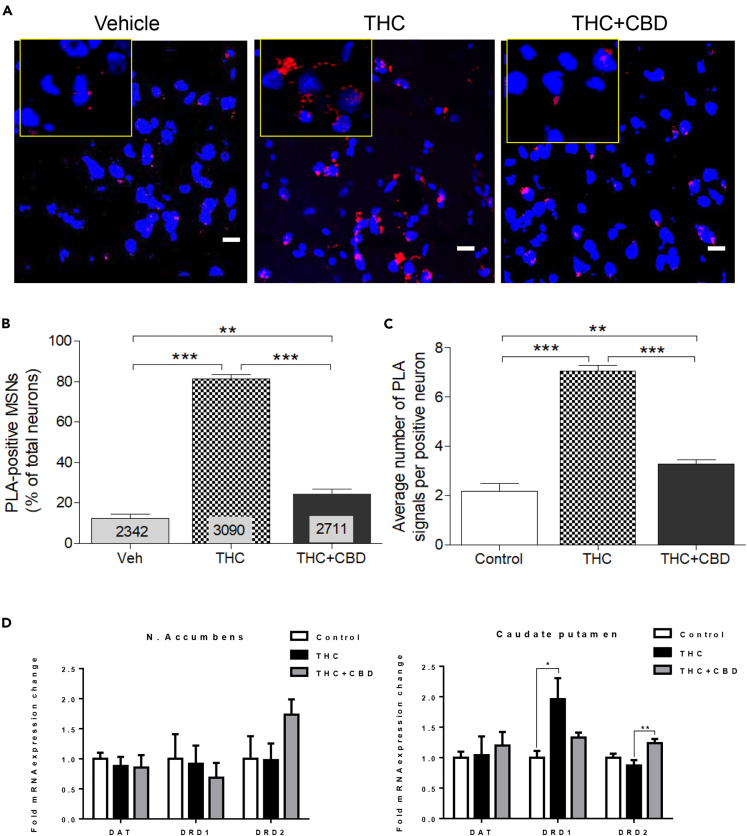
Figure 3.
Effect of Chronic THC and CBD on Dopamine D1-D2 Heteromer Expression in Monkey Caudate Nucleus
(A–C) D1-D2 heteromer expression was analyzed by PLA in the CPu of monkeys treated chronically with vehicle, THC, or THC + CBD (A). Quantification of the PLA signals (B) from the three groups of monkeys (N = 3 per group) revealed that THC increased dramatically the number of neurons expressing the D1-D2 heteromer, an effect blocked in the presence of CBD. The relative average number of heteromers per neuron was also increased (C). Results are mean ± SEM from the analysis of different slices with the number of neurons analyzed indicated. One-way ANOVA, followed by Bonferroni correction, **p < 0.01, ***p < 0.0001. Scale bars, 10 μm.
(D) The mRNA expression of D1 receptor, D2 receptor, and DAT was analyzed in the three groups of monkeys. No changes were noticed in the NAc (left). In the CPu (right), DAT mRNA did not change, whereas THC increased D1 mRNA expression. CBD with THC blocked the increase in D1 mRNA expression and increased D2 mRNA expression. Tukey's multiple test (*p < 0.05, **p < 0.01).
In the CPu of monkeys receiving THC and CBD repeatedly (Figures 3A and 3B), PLA signal was detected in 24.0% ± 0.2% of neurons (n = 2,711), indicating that CBD significantly attenuated THC-induced up-regulation of the number of PLA-positive neurons (one-way ANOVA, F(2,2) = 764.8, p < 0.0001; Bonferroni post-test THC versus THC + CBD, p < 0.0001). Similarly, PLA fluorescent signal density per neuron (Figure 3C) averaged 2.1% ± 0.02% in the CPu (one-way ANOVA, F(2,2) = 308.3; p < 0.0001). In the CPu, repeated CBD inhibited the THC-induced up-regulation of both the number of neurons expressing the D1-D2 heteromer and the number of heteromers per neuron.
THC or THC and CBD Effects on D1 and D2 Dopamine Receptor mRNA Expression
To explore if the THC-induced increase in D1-D2 heteromerization was related to a change in D1 or D2 receptor levels or ratios, or the dopamine transporter, RT-PCR was performed with extracts from CPu and NAc to measure mRNA expression of D1 and D2 receptors in the same experimental animals. In the CPu, repeated THC administration up-regulated D1 receptor mRNA expression, but had no significant effect on mRNA expression of the D2 receptor or the dopamine transporter, DAT (Figure 3D). Co-administered with THC, CBD blocked THC-induced D1 receptor mRNA up-regulation (Tukey's multiple comparisons test (D1: THC versus THC + CBD, p < 0.05)) and also increased D2 receptor mRNA compared with THC-treated animals (Tukey's multiple comparisons test [D2: THC versus THC + CBD, p < 0.01]). In sharp contrast with the CPu, mRNA expression of D1 and D2 receptors or DAT in the NAc remained unchanged, although the drug combination led to a nonsignificant rise in D2 receptor mRNA. mRNA expression of the dopamine transporter was unchanged in both brain regions and in animals treated with either drug regimen, compared with controls.
Characterization of Neurons Expressing the D1-D2 Heteromer
As the number of striatal medium spiny neurons (MSNs) expressing the D1-D2 heteromer was up-regulated by repeated THC administration, the origin and phenotype of these neurons was investigated using established markers for D1- or D2-expressing neurons. According to the classical segregation of the striatonigral and striatopallidal pathways, there are mainly two types of neurons, MSNs enriched in D1 receptor and co-expressing dynorphin and MSNs co-expressing D2 receptor with enkephalin, respectively, in the dorsal striatum (Gerfen et al., 1990, Le Moine and Bloch, 1995), although this distinction is not completely valid in the ventral striatum and some reappraisals should be taken into account (Calabresi et al., 2014, Soares-Cunha et al., 2016). After chronic treatment with THC, the expression (measured by immunofluorescence) of enkephalin (in D2-MSNs) and dynorphin (in D1-MSNs) was lower (Figure S3A), although a higher incidence of colocalization between the two neuropeptides was noted after THC repeated treatment. We also noted a higher colocalization between the D1-D2 PLA and either dynorphin or enkephalin reaching 67% and 69%, respectively (Figures S3B and S3C). However, due to low fluorescence signals from the neuropeptides, especially from enkephalin, these neuropeptides were considered insufficient to characterize the MSNs expressing the heteromer. Substance P (SP, D1-MSNs) and adenosine A2A receptor (A2AR, D2-MSNs) are also effective markers for MSNs expressing D1 receptor or D2 receptor within the striatonigral and striatopallidal pathways. Immunofluorescence generated by antibodies against these two markers showed that colocalization of SP and A2AR in neurons was increased after chronic THC, a response inhibited by CBD co-treatment. Moreover, most of A2AR-positive neurons (69%) also expressed the D1-D2 PLA signal (Figures S3D and S3F). Similarly, most neurons expressing SP (71%) showed a D1-D2 PLA signal (Figures S3E and S3F), suggesting that most of the heteromers were expressed in MSNs co-expressing SP and A2AR. Conceivably, a proportion of MSNs had assumed a phenotype that co-expressed D1 receptors, D2 receptors, SP, and A2AR.
Signaling Pathway Changes Elicited by THC or THC Combined with CBD
As a major proportion of striatal MSNs (70%–80%) expressed the D1-D2 heteromer after chronic THC, the resulting effect on the signaling pathway(s) was investigated. Our initial leads were derived from our previous observations that dopamine activation of the D1-D2 heteromer in stably transfected cells (Lee et al., 2004, Rashid et al., 2007) as well as in cultured striatal neurons (Hasbi et al., 2009) induced a dose-dependent Gq-mediated calcium signal, leading to CaMKIIα activation and brain-derived neurotrophic factor (BDNF) accumulation that resulted in enhanced neuronal maturation (Hasbi et al., 2009). The CPu and NAc were investigated for changes in these and other signaling proteins.
Phospho-CaMKIIα
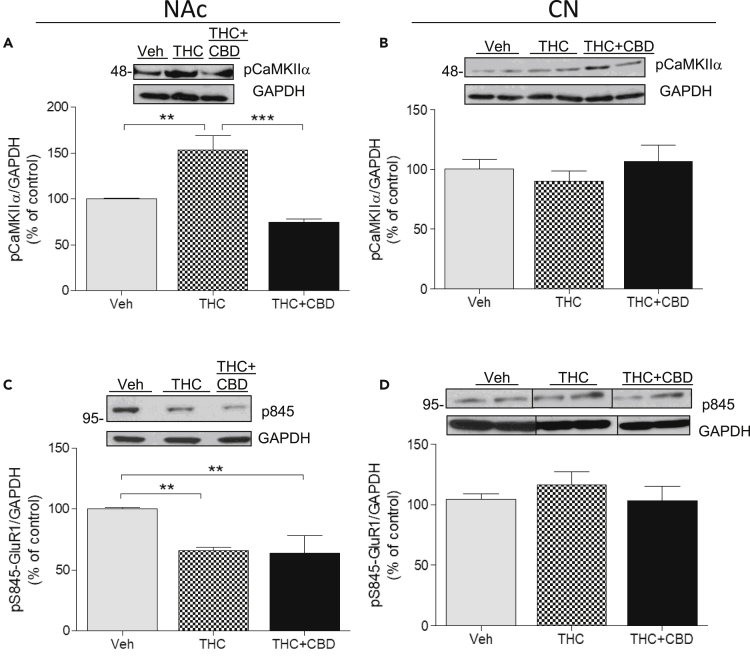
Repeated THC administration significantly increased pCaMKIIα in NAc by more than 50% over basal phosphorylated levels in vehicle-treated monkeys, as shown in western blot (153.4% ± 15.8%) (Figure 4A; one-way ANOVA; F(2,2) = 55.44; p = 0.0001; Bonferroni post-test control versus THC p < 0.01). This effect was abolished when CBD was co-administered with THC (74.96% ± 6.4%; Bonferroni post-test THC versus THC + CBD, p < 0.0001). In the CPu, phosphorylation of CaMKIIα did not significantly change (one-way ANOVA, F(2,2) = 1.98; p = 0.218) (Figure 4B) in the three groups (100.3% ± 7.8% versus 90.34% ± 3.1% versus 116.64% ± 13.44%), indicative of a regional difference.
Figure 4.
Molecular Mechanisms Involved in the Effects of Chronic THC and CBD: CaMKIIα and AMPA-GluA1
(A–D) Monkeys (N = 3 per group) were treated chronically with vehicle, THC, or THC + CBD. Homogenates from the NAc (A) or CPu (B) were subjected to western blot with antibodies for phospho-CaMKIIα (pCaMKIIα, A and B) or phosphoSerine845-GluA1 (p845, C and D). GAPDH was used as loading control. Representative images (top) and quantification of the density (bottom) are shown. Data are mean ± SD. One-way ANOVA followed by Bonferroni correction was used to compare statistical differences. (**p < 0.01; ***p < 0.001).
Phospho-Ser845-GluA1 AMPA Receptor
The D1 receptor/cyclic AMP (cAMP)/cAMP-dependent protein kinase A cascade is a canonical signaling pathway activated by elevated dopamine release consequent to administration of substances with addictive potential, including THC. cAMP-dependent protein kinase A (PKA) potentiates AMPA receptor activity by phosphorylating the glutamate receptor subunit GluA1 at serine 845 (Ser845-GluA1), and this phosphorylated target serves as a marker for the cAMP/PKA cascade. Western blot analysis showed that repeated THC administration significantly decreased Ser845-GluA1 phosphorylation in the NAc compared with control monkeys (65.98% ± 2.8%) (Figure 4C; one-way ANOVA F(2,2) = 16.45, p = 0.0037), suggesting that THC inhibited the cAMP/PKA pathway. Co-administration of THC and CBD resulted in a similar decrease in pSer845-GluA1 (63.63% ± 14.7%), indicating that CBD did not attenuate THC effects on this pathway (Bonferroni post-test THC versus THC + CBD p > 0.05; t = 0.33). In the CPu of monkeys receiving repeated THC or THC combined with CBD, we did not observe any significant effect (Figure 4D) on pSer845-GluA1 (104.3% ± 4.6%, 116.1% ± 11.23%, respectively) compared with vehicle-treated animals (one-way ANOVA F(2,2) = 1.61, p = 0.27).
DARPP-32
The 32-kDa dopamine and cAMP-regulated phosphoprotein (DARPP-32) is a significant modulator of cAMP signaling in striatal neurons. Two major phosphorylation sites, Thr34 and Thr75, exist on DARPP-32, which when activated can engender either a kinase or a phosphatase activity, respectively (Greengard et al., 1999, Greengard, 2001, Nairn et al., 2004). Administration of single doses of drugs with addictive potential (e.g., THC or cocaine) leads to D1/cAMP/PKA-catalyzed phosphorylation of Thr34-DARPP-32, whereas repeated administration of drugs leads to Cdk5-catalyzed phosphorylation of Thr75-DARPP-32 (Bibb et al., 2001, reviewed in Nairn et al., 2004). Activation of the D1-D2 heteromer activates this Cdk5-mediated phosphorylation, which seems to block the D1/cAMP/PKA pathway (Hasbi et al., 2018).
Phosphorylation of Thr34-DARPP-32
Western blot analysis showed that repeated THC injections had no significant effect on Thr34-DARPP-32 phosphorylation compared with basal phosphorylation in the NAc and CPu of vehicle-treated monkeys (92% ± 15.3% versus 101% ± 12.9%, and 100% ± 8.7% versus 126.7% ± 7.61%, respectively) (Figure S4). Monkeys co-injected with THC and CBD also had no significant effect on Thr34-DARPP-32 phosphorylation in NAc or CPu (112.5% ± 10.7% and 128.69% ± 10.5% of vehicle monkeys, respectively) compared with vehicle or with THC alone (one-way ANOVA, F(2,2) = 0.60; p = 0.5766 for NAc; one-way ANOVA, F(2,2) = 3.13; p = 0.117 for CPu). Accordingly, the Thr34 site of DARPP-32 was not activated by chronic THC administration with or without the presence of CBD.
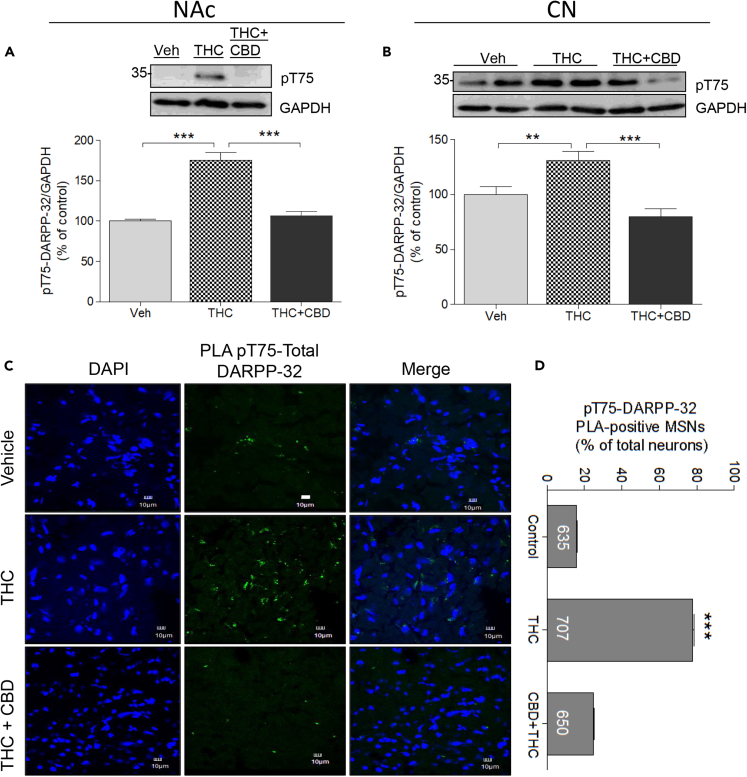
Phosphorylation of Thr75-DARPP32
In contrast, repeated THC injections significantly increased Thr75-DARPP-32 phosphorylation in NAc by 75% over the basal phosphorylation in vehicle-treated animals (175.27% ± 16.98%; Figure 5A). CBD co-treatment with THC abolished this response (106.2% ± 10.23%; Figure 5A). These results were corroborated by a one-way ANOVA analysis (F(2,2) = 115.4; p < 0.0001) followed by Bonferroni post-tests (control versus THC, p < 0.0001; THC versus THC + CBD; p < 0.0001). In the CPu, repeated THC increased pThr75-DARPP-32 by approximately 30% (131.1% ± 8.41% of vehicle; Figure 5B), whereas co-injection of CBD with THC blocked the effect of THC (79.7% ± 7.5% of vehicle monkeys). Statistical analyses corroborated these observations (one-way ANOVA; F(2,2) = 33.80; p < 0.0005), with Bonferroni post-test correction showing a significant difference between control and THC-treated animals (p < 0.001) and between THC and THC + CBD-treated animals (p < 0.0001) and no difference between control and THC + CBD-treated animals (p > 0.05; t = 3.22). Accordingly, repeated THC promotes DARPP-32 activation at the Thr75 site in the NAc and CPu of primate, a response blocked by CBD.
Figure 5.
Molecular Mechanisms Involved in the Effects of Chronic THC and CBD: DARPP-32
Monkeys (N = 3 per group) were treated chronically with vehicle, THC, or THC + CBD.
(A and B) Western blot analysis of homogenates from NAc (A) or CPu (B) using an antibody raised against phospho-threonine-75-DARPP-32 (pT75-DARPP-32) was conducted. Representative images (top) and quantification of the density (bottom) are shown. Data are mean ± SD.
(C and D) Analysis of pT75-DARPP-32 using PLA from monkeys treated as in (A). PLA was performed using anti-DARPP-32 and anti-pT75-DARPP-32, with the PLA recognizing DARPP-32 only when pT75 was activated. Representative images from each group of monkeys are shown in (C). (D) Graph summarizing the quantification of PLA-pT75-DARPP-32 using Duolink software. Number of neurons analyzed from each group is indicated. Data are mean ± SEM. One-way ANOVA followed by Bonferroni correction was used to compare statistical differences. (**p < 0.01; ***p < 0.001). Scale bars, 10 μm.
PLA of phosphoThr75-DARPP-32/Total DARPP-32
The effect of THC on pThr75-DARPP-32 was further investigated using in situ unimolecular PLA (Figures 5C and 5D). PLA assays visualized total DARPP-32 and pThr75 within striatal neurons. Both in the NAc and CPu of vehicle-treated monkeys, the PLA signal was detected in fewer than 20% neurons (n = 635). Following THC administration, the PLA signal between total DARPP-32 and pThr75-DARPP-32 was detected in approximately 80% neurons (n = 707), indicating a dramatic and significant increase in the number of neurons expressing the Thr-75 phosphorylated form of DARPP-32 (one-way ANOVA, F(2,2) = 29.83; p = 0.0008 for NAc and F(2,2) = 38.34; p = 0.0004 for CPu). Monkeys repeatedly co-injected with THC and CBD showed a much lower positive signal for pThr75-DARPP-32 PLA, in less than 25% neurons in CPu compared with THC-treated animals (n = 650). Thus, repeated exposure to THC activated Thr75-DARPP-32, but THC administered together with CBD prevented the increase.
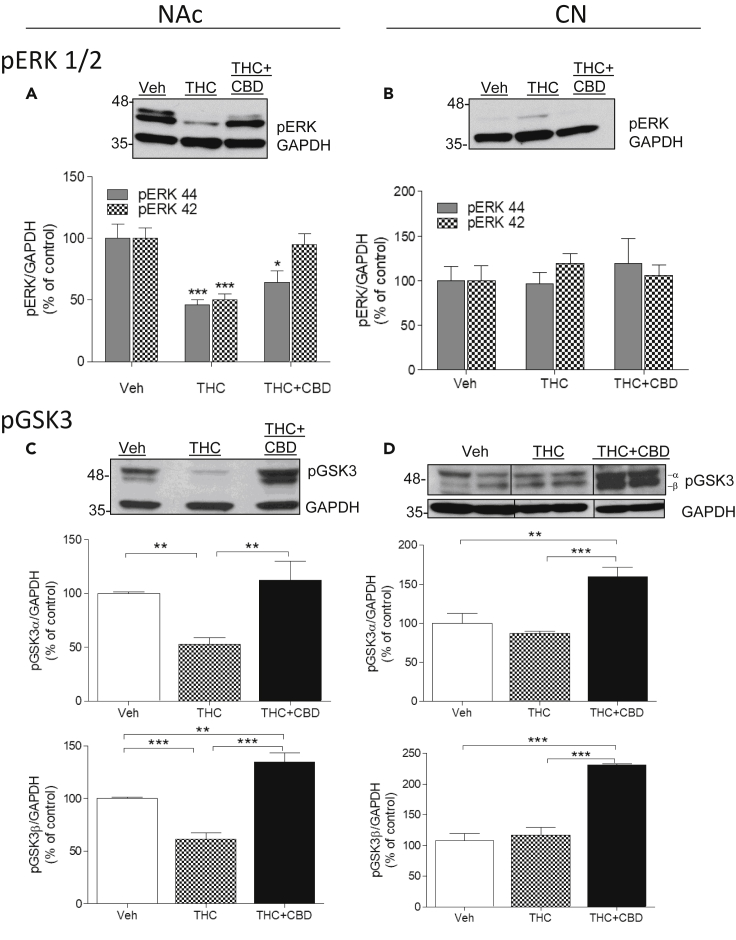
Phospho-ERK
Extracellular regulated kinase (ERK) is significantly involved in the signaling cascade of dopamine. Previously, it was reported that an acute dose of THC (1 mg/kg) activated pERK1/2 in mouse NAc and dorsal striatum, primarily via dopamine D1 receptors and partly via the D2 receptor (Valjent et al., 2001, Valjent et al., 2004). NAc of monkeys treated repeatedly with THC showed dramatic decreases in phosphorylation of both subunits of pERK1/2 compared with vehicle (46.10% ± 4.1% and 50.0% ± 4.8%, respectively; Figure 6A). A two-way ANOVA analysis using subunits (pERK1 or pERK2) and treatment as factors of variation showed a significant effect of treatment (F(2,2) = 59.99; p < 0.0001), of subunits (F(2,2) = 9.00; p = 0.011), and an interaction (treatment X subunits; F(2,2) = 6.30; p = 0.013). Bonferroni post-tests showed that the subunit factor variation was due to the differential effect of co-injection of THC + CBD on the two subunits (p < 0.01). That is, co-injection of CBD with THC restored pERK2 phosphorylation state (95.2% ± 8.4% of control) and only partly reversed THC effects on pERK1 (64% ± 9.6%). Thus, repeated doses of THC inhibit the pERK pathway, but co-administration with CBD attenuates the THC effect in the NAc, notably on pERK2.
Figure 6.
Molecular Mechanisms Involved in the Effects of Chronic THC and CBD: ERK1/2 and GSK-3
(A–D) Monkeys (N = 3 per group) were treated chronically with vehicle, THC, or THC + CBD. Analysis by western blot of homogenates from the NAc (left panels) or CPu (right panels) using antibodies to phospho-ERK (pERK, A and B) or phospho-GSK3 (pGSK, C and D). GAPDH was used as a loading control. Representative images (top) and quantification of the density (bottom) are shown. Data are mean ± SD. Two-way ANOVA followed by Bonferroni correction was used to compare statistical differences. (*p < 0.05; **p < 0.01; ***p < 0.001).
In contrast to the NAc, THC did not affect pERK1 in the CPu (96.2% ± 13.45%; Figure 6B) and only slightly increased pERK2 without reaching significance (119.52% ± 10.83%). Co-administered CBD did not affect phosphorylation of either of the subunits of pERK1/2 in CPu (119% ± 28.1% and 105.5% ± 12.3%, respectively), suggesting that THC regulation of ERK1/2 is region specific (two-way ANOVA; treatment (F = 0.80; p = 0.47); subunits (F = 0.14; p = 0.70); interaction (treatment X subunits); [F = 1.77; p = 0.21]).
Phospho-GSK3
The Akt-GSK3 pathway was then investigated as a signaling pathway that previous studies showed to be modulated by cannabinoid receptor activity (Renard et al., 2016). The phosphorylated state of GSK3 reduces its activity, whereas dephosphorylation increases its activity.
In the NAc, repeated administration of THC to monkeys decreased phosphorylation of both subunits of GSK3 (pGSK3α: 52.55% ± 6%; pGSK3β: 61.21% ± 17% of vehicle values; Figure 6C). CBD not only blocked the effects of THC but also increased GSK3 phosphorylation above values detected in vehicle-treated animals, notably at the β-subunit (pGSK3α: 122% ± 6%; pGSK3β: 134.5% ± 12%). These results were corroborated by a one-way ANOVA analysis followed by Bonferroni post-tests for each subunit. For pGSK3α: (F(2,2) = 25.11; p = 0.0012; Bonferroni post-tests: vehicle (Veh) versus THC (p < 0.01), THC versus THC + CBD (p < 0.01), Veh versus THC + CBD (p > 0.05)). For pGSK3β: (F(2,2) = 102.4; p < 0.0001; Bonferroni post-tests: Veh versus THC (p < 0.0001), THC versus THC + CBD (p < 0.001), Veh versus THC + CBD (p < 0.0001)).
In the CPu, THC did not affect pGSK3 (pGSKα: 87.2% ± 13%; pGSKβ: 117.1% ± 11%; Figure 6D), but co-administration of THC and CBD resulted in a 59% increase in pGSK3 over basal values for pGSKα (159% ± 12% of vehicle) and a 130% increase over basal values for pGSKβ (230.9% ± 11% of vehicle treatment). These results were corroborated by a one-way ANOVA analysis followed by Bonferroni post-tests for each subunit. For pGSK3α: (F(2,2) = 40.20; p = 0.0003; Bonferroni post-tests: Veh versus THC (p > 0.05; t = 1.44), THC versus THC + CBD (p < 0.001), Veh versus THC + CBD (p < 0.0001)). For pGSK3β: (F(2,2) = 145.6; p < 0.0001; Bonferroni post-tests: Veh versus THC (p > 0.05; t = 1.12), THC versus THC + CBD (p < 0.0001), Veh versus THC + CBD (p < 0.0001)). Once again, modulation of GSK3 by chronic THC is region specific with an effect in the ventral but not in the dorsal striatum. In both subregions, however, CBD combined with THC increased GSK3 phosphorylation, a reduced activity state for this kinase.
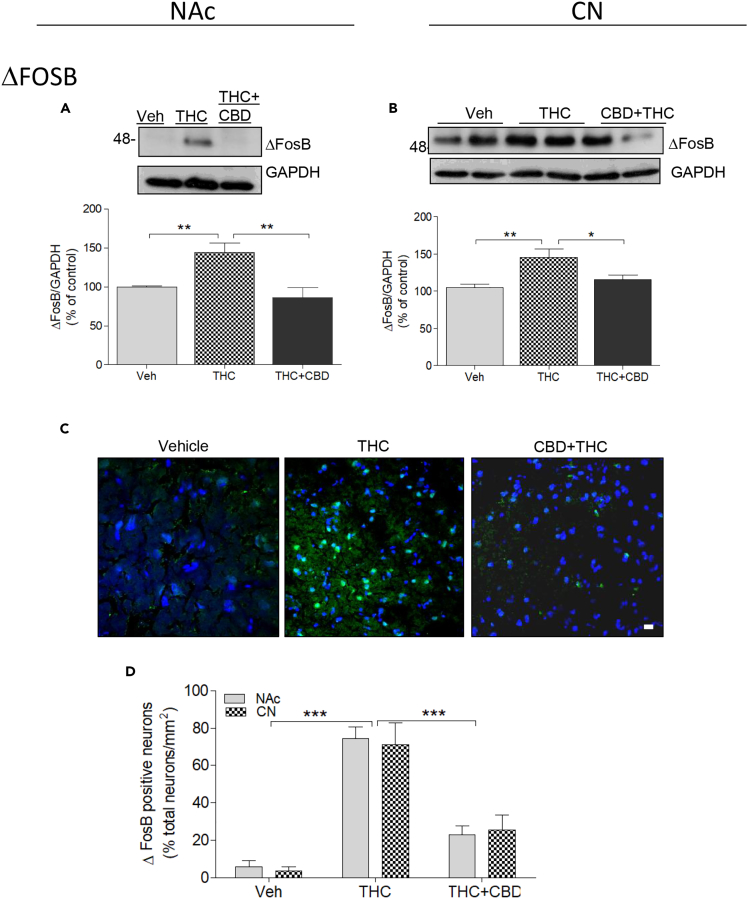
ΔFosB
After repeated administration, many drugs with addictive potential increase ΔFosB, a process thought to be key to triggering neuroadaptive mechanisms (reviewed in Nestler, 2008). In monkey NAc, repeated THC injections significantly increased ΔFosB by 45% over vehicle treatment (144.5% ± 11.3%; Figure 7A). This effect was abolished when THC was co-injected with CBD (86.24% ± 11.59%). One-way ANOVA followed by Bonferroni post-tests corroborated these findings (F(2,2) = 26.58; p = 0.001; post-tests: Veh versus THC (p < 0.001); Veh versus THC + CBD (p > 0.05; t = 1.64); THC versus THC + CBD (p < 0.001)).
Figure 7.
Molecular Mechanisms Involved in the Effects of Chronic THC and CBD: ΔFosB
(A and B) Monkeys (N = 3 per group) were treated chronically with vehicle, THC, or THC + CBD. Analysis by western blot of homogenates from the NAc (A) or CPu (B) using an antibody to ΔFosB. GAPDH was used as a loading control. Representative images (top) and quantification of the density (bottom) are shown. Data are mean ± SD.
(C and D) Analysis of ΔFosB by immunohistochemistry, with the secondary antibody conjugated to Alexa 488 (green). Scale bar, 10 μm. (D) Quantification of ΔFosB-positive neurons performed by Duolink with DAPI-stained nuclei representing the total number of neurons. The number of neurons analyzed from each group is indicated. Data are mean ± SEM. One-way ANOVA followed by Bonferroni correction was used to compare statistical differences. (*p < 0.05; **p < 0.01; ***p < 0.001).
In the CPu similar responses were elicited by THC and CBD. In this brain region, repeated THC increased ΔFosB accumulation (145.14% ± 11.8% of vehicle values; Figure 7B), whereas co-injection of THC with CBD blocked the THC effect (115.39% ± 6.7%, compared with vehicle controls). One-way ANOVA followed by Bonferroni post-tests corroborated these findings (F(2,2) = 19.27; p = 0.0024; post-tests: Veh versus THC (p < 0.001); Veh versus THC + CBD (p > 0.05; t = 1.58); THC versus THC + CBD (p < 0.01)). Furthermore, immunohistochemical analysis showed that the THC-induced rise in ΔFosB was observed in more than 70% of NAc and CPu neurons (Figures 7C and 7D). These results may suggest that the D1-D2 PLA-expressing MSNs are expressing ΔFosB after the repeated THC regimen.
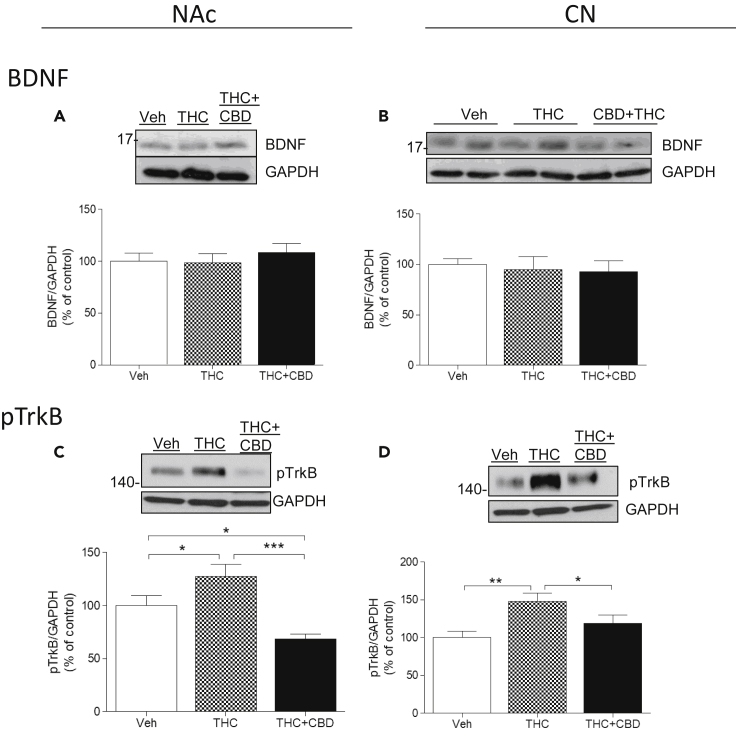
BDNF and phosphoTrkB
BDNF is one of the most widely studied neurotrophins because of its linkage to neuropsychiatric disease and its fundamental role in synaptic plasticity (Notaras et al., 2015). In comparison with basal levels of BDNF in the NAc of vehicle-treated monkeys, repeated THC injections had no significant effect on BDNF accumulation, whether injected alone or co-injected with CBD (100% ± 7.7%; 98.62% ± 8.6%; 108% ± 8.8%, respectively; Figure 8A). No significant effect was observed in the CPu on BDNF levels in the three groups of monkeys (100% ± 1.6%; 94.82% ± 3.8%; 92.51% ± 11.26%; Figure 8B). One-way ANOVA followed by Bonferroni post-tests analysis for each region corroborated these data. In NAc: (F(2,2) = 1.16; p = 0.07; post-tests: Veh versus THC (p > 0.05; t = 0.20); Veh versus THC + CBD (p > 0.05; t = 1.20); THC versus THC + CBD (p > 0.05; t = 1.40)). In the CPu: (F(2,2) = 0.41; p = 0.67; post-tests: Veh versus THC (p > 0.05; t = 0.61); Veh versus THC + CBD (p > 0.05; t = 0.89); THC versus THC + CBD (p > 0.05; t = 0.27)).
Figure 8.
Molecular Mechanisms Involved in the Effects of Chronic THC and CBD: BDNF and TrkB
(A–D) Monkeys (N = 3 per group) were treated chronically with vehicle, THC, or THC + CBD. Analysis by western blot of homogenates from the NAc (left panels) or CPu (right panels) using antibodies to BDNF (A and B) or phospho-TrkB (pTrkB, C and D). GAPDH was used as a loading control. Representative images (top) and quantification of the density (bottom) are shown. Data are mean ± SD. One-way ANOVA followed by Bonferroni correction was used to compare statistical differences (*p < 0.05; **p < 0.01; ***p < 0.001).
Although no change in BDNF was observed, a significant increase in the phosphorylation (activation) of its receptor tropomyosin kinase B (pTrkB) was observed after chronic THC in both the NAc (127% ± 8%; Figure 8C) and the CPu (145% ± 9%; Figure 8D), a response reduced by co-administration of CBD (68% ± 4% and 127% ± 11%, respectively). One-way ANOVA followed by Bonferroni post-tests analysis for each region corroborated these data. In NAc: (F(2,2) = 33.40; p = 0.0006; post-tests: Veh versus THC (p < 0.05; t = 3.8); Veh versus THC + CBD (p < 0.05; t = 4.36); THC versus THC + CBD (p < 0.001; t = 8.16)). In the CPu: (F(2,2) = 16.69; p = 0.0035; post-tests: Veh versus THC (p < 0.001; t = 5.73); Veh versus THC + CBD (p > 0.05; t = 2.24); THC versus THC + CBD (p < 0.05; t = 3.49)).
Ingenuity Pathway Analysis
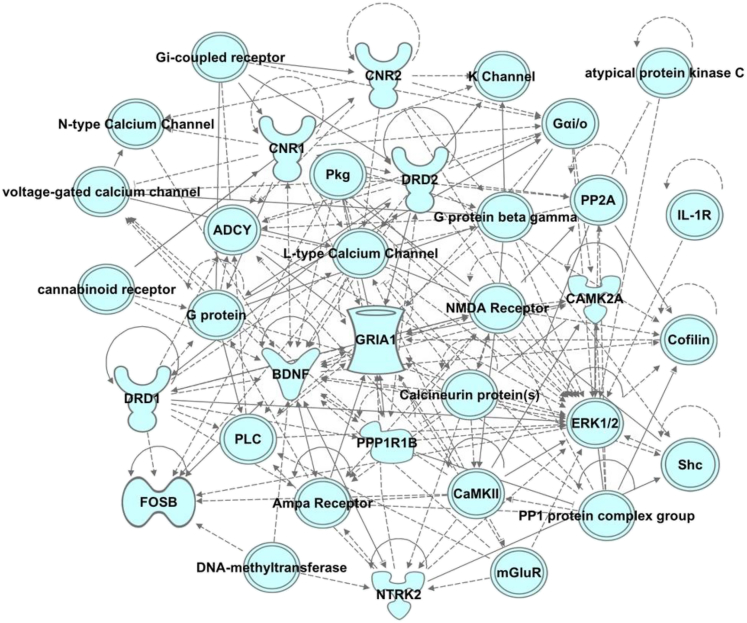
Pathway analyses can reveal three categories of physiological and pathophysiological states: diseases and disorders, molecular and cellular functions, and physiological system development and function. Ingenuity pathway analysis (IPA) was used to identify gene networks and generate a network score and provide a probability that the set of genes occurring in this network could be explained by chance alone. Networks with a score ≥2 have ≥99% confidence that they are not generated by chance. The genes of interest used in the IPA analysis were generated from western blot analyses of proteins regulated by THC or THC + CBD, and included BDNF, D1 and D2 dopamine receptors, ΔFosB, cannabinoid 1 and 2 receptors, CAMKII, GluA1, DARPP-32, ERK-1, ERK-2, GSK3-alpha, GSK3-beta and TrkB. We assumed that these proteins were altered not by drug-induced changes in protein turnover, but by regulated transcription/translation. Based on IPA, we found that treatment with THC or THC in combination with CBD regulated gene networks that include, but are not limited to, those involved in emotional, cognitive, and conditioned behaviors; learning and memory processes; nervous system development; function; and organ development. The most robust statistical probabilities were related to neurological diseases (p value range: 1.09 × 10−3–2.97 × 10−8) and psychological disorders (p value range: 1.09 × 10−3–2.97 × 10−8), organ injury and abnormalities (p value range: 1.09 × 10−3–8.06 × 10−8), cell-to-cell signaling (p value range: 9.08 × 10−4–2.47 × 10−8), and nervous system development (p value range: 1.09 × 10−3–7.67 × 10−8). An example of the IPA gene network results is shown in Figure 9.
Figure 9.
Ingenuity Pathway Analysis (IPA): Predicted Gene Network
Representative gene network. Gene networks of candidate markers were explored using IPA core analysis. IPA scores mapped gene networks, and predicted the likelihood that the gene network occurred by chance. Networks with a score ≥2 have ≥99% confidence that they are not generated by chance.
Discussion
This study highlights intriguing discoveries relevant to dopamine receptor signaling and cannabinoid-induced neuroadaptive changes in primate basal ganglia. (1) We provide abundant morphological evidence that dopamine D1 and D2 receptors form complexes in the dorsal striatum and NAc of mammalian species, including mouse, rat, nonhuman primate and human. Evolutionary differences were noted in the expression of dopamine D1-D2 heteromers, with heteromer abundance in the order human > primate > rat > mouse. In all these species, a higher number of MSNs expressing the D1-D2 heteromer was observed in the ventral striatum (i.e., NAc) than in the dorsal striatum. (2) THC increased the number of neurons expressing the D1-D2 heteromer in both regions of the striatum of nonhuman primate brain after chronic administration, from 35% or less, to approximately 80%, together with a 3-fold increase of heteromer density within individual neurons. (3) The chronic low-dose THC regimen led to a phenotypic change in MSNs indicating a reprogramming of these MSNs to co-express the characteristic markers of both striatonigral and striatopallidal neurons together with co-expression of D1 and D2 receptors. (4) The chronic THC regimen appeared to increase calcium signaling while inhibiting cAMP signaling. Hence, chronic THC increased pCaMKIIα, pThr75-DARPP-32, and BDNF/TrkB signaling, whereas it decreased pERK1/2 and pS845-GluA1 and had no effect on PKA-mediated pThr34-DARPP-32. (5) CBD, administered together with THC, markedly attenuated most but not all neuroadaptive responses mediated by THC alone: (a) CBD + THC showed minimal change from vehicle treatment in the number of neurons expressing the D1-D2 heteromer and the density of the receptor heteromer within neurons. CBD + THC abolished chronic THC-induced (b) elevated pCaMKIIα, (c) increased Thr75-DARPP-32 phosphorylation, (d) increased ΔFosB, (e) phosphorylation (activation) of BDNF receptor pTrkB, (f) decreased phosphorylation of pERK1/2, and (g) reduced phosphorylation of both subunits of GSK3.
An association between the species evolutionary rank and expression level of the D1-D2 heteromer was observed, with higher-order species demonstrating greater D1-D2 heteromer expression levels in the striatum. These findings conceivably reflect an evolutionary adaptive advantage to dopamine signaling modulation by heteromer formation. In all analyzed species, a higher number of MSNs expressing the D1-D2 heteromer was observed in the ventral striatum (i.e., NAc) than in the dorsal striatum (caudate and putamen). In mouse NAc the number of neurons with D1-D2 PLA signal (14%–16%) was consistent with the rate of colocalization between D1 receptor and D2 receptor in the ventral striatum, notably in the NAc-shell subregion, recently shown in the double BAC Drd1a-TdTomato/Drd2-GFP transgenic mice (Gagnon et al., 2017). Similarly, the low PLA signal in the dorsal striatum of mouse was in agreement with the low percentage of MSNs co-expressing D1 and D2 receptors in the mouse CPu (Gagnon et al., 2017). In rat, the PLA results (20%–25% in NAc and <10% in CPu) were consistent with previous estimates of D1-D2 heteromer expression using in situ confocal FRET or in situ PLA (Hasbi et al., 2009, Hasbi et al., 2018, Perreault et al., 2010, Perreault et al., 2016). In rhesus monkey, our PLA data (∼28% in the caudate nucleus and ∼36% in NAc) were equivalent to those reported for the macaque monkey (23%–25% in CPu and 36% in NAc-core) (Rico et al., 2017).
Our primary goal was to investigate whether repeated administration of relatively average doses of THC or THC combined with CBD (1:3 ratio) affected the expression of the dopamine D1-D2 receptor heteromer, and whether other neuroadaptive mechanisms associated with dopamine or cannabinoid signaling were equally affected by the two dosing regimens. The number of neurons expressing the D1-D2 heteromer in the NAc and the CPu of nonhuman primate increased dramatically after chronic THC administration, together with a 3-fold increase in heteromer expression levels within individual neurons, effects that were markedly attenuated by CBD co-injection. A critical question to be investigated was the origin of the neurons in which THC induced D1-D2 heteromer expression, whether this had occurred in the D1 receptor (direct pathway MSNs) or the D2 receptor (indirect pathway MSNs) neuronal subpopulations. Attempts were made to clarify the nature of these MSNs using the established conventional markers for D1- or D2-expressing neurons. According to the segregation of the two receptors, essentially based on observations in the dorsal striatum, two classical phenotypes of MSNs are thought to exist, where D1 receptor is co-expressed with dynorphin, whereas D2 receptor is co-expressed with enkephalin (Gerfen et al., 1990, Le Moine and Bloch, 1995). After chronic treatment with THC, analysis of the expression of the neuropeptide markers revealed a higher colocalization between enkephalin and dynorphin, although it was noted that the MSN content of both dynorphin and especially of enkephalin was very low after THC. The neurons expressing these neuropeptides also expressed the D1-D2 heteromer after the THC regimen. Two other markers for MSNs expressing D1 receptor or D2 receptor within the striatonigral and striatopallidal pathways are Substance P (in D1-MSNs) and adenosine A2A receptor (in D2-MSNs). Our data showed a high colocalization between D1-D2 PLA and either SP or A2AR after chronic THC. These results indicate that a significant percentage of MSNs had adopted a phenotype co-expressing D1 receptor/D2 receptor/SP/A2A receptor. Substantial evidence from mouse studies showed that ΔFosB was primarily expressed in D1 receptor MSNs after drugs of abuse (reviewed in Nestler, 2008). Our data indicate that chronic THC increased the number of ΔFosB-expressing neurons to approximately 75%–80% in the NAc and CPu, which represents more than the number of MSNs expressing D1 receptor only. These data suggest again that a proportion of neurons have adopted a phenotypic profile co-expressing the D1-D2 heteromer and were capable of expressing ΔFosB after chronic THC treatment. Therefore, it may reasonably indicate that a proportion of each MSN subtype (D1/SP or D2/A2AR) may have changed into the phenotype of MSN expressing the D1-D2 heteromer, and also co-expressing SP and A2AR. The present data showed that chronic THC had long-lasting effects directly on dopamine receptor expression, significantly altering the expression pattern of D1 and D2 receptors and neuropeptides within adult striatal MSNs. These results clearly indicate that the phenotype of MSNs in the adult brain does not remain static but can dynamically change, and specifically after chronic treatment with THC. These findings provide evidence for dramatic changes in the adult striatal neuronal population and the probable functional consequences generated by long-term use of an average dose of THC are intriguing. Only one other recent article described that MSNs in adult mouse striatum were reengineered into dopamine-like neurons (Niu et al., 2018). In that study, phenotypic reprogramming of MSNs was achieved through injections of a stem cell factor, three dopaminergic neuron-enriched transcription regulators, and valproic acid. In the present study, the MSNs seem to return to a stage where most of the neurons co-express D1 and D2 receptors and the different neuropeptides. This phenotype is reminiscent of the profile of MSNs in culture collected from fetal, neonatal, and 2- to 3-week-old rats, where higher colocalization of D1 and D2 receptors (60%–100%) was observed, when compared with the number of neurons in control adult rat ventral (17%–25%) or dorsal (2%–7%) striatum (Hasbi et al., 2009, Aizman et al., 2000, Iwatsubo et al., 2007).
Chronic THC administration led also to a decrease in signaling via the cAMP-PKA-ERK pathway and increased calcium signaling. These chronic THC-induced effects contrast with the acute effects of THC on these same pathways. For example, acute THC (1 mg/kg) in mice led to the activation of pERK1/2 in the NAc and in the dorsal striatum (Valjent et al., 2001, Valjent et al., 2004) through a mechanism involving dopamine D1 receptor and partially involving D2 receptor. This effect was not observed with a higher dose of THC (5 mg/kg; Valjent et al., 2001), and was even inhibited in the dorsal striatum at a dose of 10 mg/kg. In vitro data showed that acute CB1 receptor activation led to the activation of pERK and PI3K/Akt pathways (Ozaita et al., 2007). The acute THC-induced activation of the PI3K/Akt pathway, was shown to be responsible for the increased phosphorylation of GSK3, thus inhibiting its function (Renard et al., 2016). In the NAc of the primates injected chronically with THC in our study, there was instead an increase in calcium signaling and an inhibition in cAMP signaling in a manner similar to what occurs after activation of the D1-D2 heteromer (Rashid et al., 2007, Hasbi et al., 2009, Hasbi et al., 2018). Also, and in contrast to the acute effects of THC in rat or mouse, where THC induced an increase in the phosphorylation (inhibition) of GSK3 (Renard et al., 2016), our data showed that chronic THC in nonhuman primate led to a dramatic decrease in pGSK3, synonymous with increased GSK3 activation. An increase in GSK3 activity in the striatum was required for the development of cocaine conditioned reward in mouse (Miller et al., 2014), and was also shown to play key roles in mental diseases (Li and Jope, 2010). For example, higher GSK3 activity in the NAc was associated with social defeat stress, whereas GSK3 blockade in this brain region produces antidepressant effects in behavioral models of depression (Li and Jope, 2010, Wilkinson et al., 2011). In addition, increased levels of activated GSK3 in major depressive disorder or bipolar disorder were reported in postmortem studies (Li and Jope, 2010 and references herein).
The mechanisms leading to the changes in MSN phenotype are presently not known. However, this array of adaptive responses conceivably originates via multiple mechanisms, likely triggered initially via activation of CB1 cannabinoid receptors by THC. It is known that the cannabinoid and dopamine systems interact at different levels. For example, CB1 receptors are expressed in striatal GABA MSNs of the direct pathway (dMSNs) and MSNs of the indirect pathway (iMSNs) (Hohmann and Herkenham, 2000, Pickel et al., 2004). Dopamine transmission is modulated by endogenous cannabinoids (eCBs) via CB1 receptor on presynaptic glutamatergic and GABAergic terminals (Lovinger and Mathur, 2012). There is also evidence for a direct interaction between the two systems at the receptor level, notably between D2R and CB1R (Carriba et al., 2007, Navarro et al., 2008). Taking into account that addiction vulnerability involves genetic and epigenetic mechanisms (reviewed in Egervari et al., 2018), our hypothesis is that repeated activation of CB1R by long-term THC treatment led to some epigenetic modifications through activation and/or inhibition of different transcription factors. These modifications resulted in alterations in the expression patterns of the receptors and neuropeptides, which by consequence resulted in a change in the MSN phenotype, blurring the distinctive characteristics of the D1 and D2 MSNs. This hypothesis warrants further experiments to decipher the different steps that led to these dramatic changes.
Another interesting observation was that the change in the MSN phenotype was much lower or prevented when CBD was co-injected with THC, indicating the reversibility of the changes and underlining a very important role for CBD in abolishing some of the long-term effects of THC. The contrasting effects of THC alone versus when combined with CBD warrant further comparisons of the pharmacological and pathological consequences of high or low THC:CBD ratios and whether CBD can attenuate the effects of a range of THC doses, especially after prolonged use. In pilot studies reported in abstract form, THC given repeatedly to nonhuman primates increased expression of the dcc gene, whereas CBD blocked THC-induced up-regulation of dcc mRNA expression in frontal cortex (Madras et al., 2017). Evidence of an association between the dcc gene and major psychiatric disorders is increasing (Grant et al., 2012, Grant et al., 2014, Luo et al., 2018, Manitt et al., 2013, Ward et al., 2017), along with reports of the DCC receptor as an essential guide for dopamine circuit formation in frontal cortex during adolescent brain development (Manitt et al., 2013, Reynolds et al., 2015). The molecular mechanisms underlying CBD's apparent mitigation of some pharmacological effects of THC are poorly understood (Boggs et al., 2018) and still have to be fully investigated, keeping in mind that the pharmacological targets for CBD are numerous and not fully established, some being controversial. Furthermore, the mechanisms by which THC and CBD exert their effects are different and are more complicated when delivered in combination (Russo, 2011), rendering the attribution of the actions of the THC + CBD combination to one proposed molecular mechanism more difficult (Hudson et al., 2019, Bassir Nia and Hurd, 2018). Whichever of the diverse mechanisms is involved, it seems imperative that CBD should be integrated in any cannabis preparation and its ratio to THC should be taken into account, due to the multiple beneficial effects of CBD in moderating or completely inhibiting THC effects, as we showed here.
A single dose of THC promotes release of dopamine in human striatum, as detected by brain imaging. In long-term human marijuana abusers, dopamine signaling is altered and manifest by blunted reward (less “high”) and heightened negative responses (anxiety, depression, amotivation), correlating objectively with attenuated dopamine release and impaired dopamine function (Volkow et al., 2014). These effects involve, in part at least, the striatal MSNs, which are involved in very important physiological functions, such as reward, decision-making, and reinforcement of goal-directed and habitual learning. It is our hypothesis that the induction of a dramatic change in the phenotype of striatal neuronal populations by chronic low doses of THC may be responsible for the reported reduced reward sensitivity and negative emotionality (Volkow et al., 2014), apathy, lack of motivation, and cognitive impairments usually associated with heavy cannabis use (reviewed in Volkow et al., 2014, Bloomfield et al., 2016). It is also tempting to extend these modifications to the higher incidence of depression and anxiety in adolescent heavy users (Patton et al., 2002), or even to the reported modifications in adolescent brain structure (Rais et al., 2008, Yucel et al., 2008). Indeed, our targeted pathway analyses showed that the most robust statistical probabilities resulting from our observed changes were related to pathways involved in neurological diseases, psychological disorders, organ injury/abnormalities, cell-to-cell signaling, and nervous system development. More discretely, the results implicated regulated gene networks that include, but are not limited to, those involved in emotional, cognitive, and conditioned behaviors and learning and memory processes.
Taking into account all these considerations, a focus on the D1-D2 receptor heteromer as a target for pharmacological manipulation should be considered in the development of therapeutic strategies to combat cannabis use disorder. This is important in regard to the increasing availability of potent synthetic cannabinoids and especially in the context of the recent accelerated legalization of cannabis not only for medicinal use but also for recreational use in many countries, which without appropriate policies may harm the adult and adolescent population irreparably.
Limitations of the Study
For ethical reasons, it is understandable that like any study involving living nonhuman primates the present study was limited to the use of only three animals per group for a total of nine subjects. As 8/9 primate subjects were repurposed from previous drug self-administration research with nicotine or cocaine, prior drug exposure may have contributed to observed effects. However: (1) All animals were drug-free for at least two months before this study began and the majority were drug-free for longer periods; (2) All control animals had been exposed to nicotine or cocaine; (3) one THC-treated animal had no prior exposure to any drug, yet responses were the same as the other two animals in the group; (4) Baseline behavioural recordings of subjects were similar; (5) THC administration in rats have generated similar effects (preliminary results). However, the findings evaluating the D1-D2 heteromer in thousands of neurons per condition and the signaling pathways investigated were clearly different between the vehicle-treated versus THC-treated animals. For that reason, we are confident that these findings represent a broader effect of THC and CBD and are confident of our conclusions. For the same ethical reasons, our study did not extend to examine if a sex difference may exist at the basal level and also after the different treatments. We, however, used a combination of male and female subjects, evenly distributed within the groups.
More experiments using rats as a model are underway in our laboratory with higher numbers of animals per group, to investigate further the conclusions of the present article and to examine the differences that may exist between male and female.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This project was funded by grants from National Institutes of Health NIDA DA042178 (to B.K.M. and S.R.G.) and Canadian Institutes of Health Research CIHR PJT-148633 (to S.R.G.).
Author Contributions
A.H., B.K.M., and S.R.G. designed the experiments; A.H., J.B., S.K., Z.L., and S.L.W. performed the experiments; A.H., B.K.M., and S.R.G. wrote the paper.
Declaration of Interests
The authors declare no conflict of interest.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100794.
Supplemental Information
References
- Aizman O., Brismar H., Uhlén P., Zettergren E., Levey A.I., Forssberg H., Greengard P., Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat. Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Bassir Nia A., Hurd Y.L. Cannabidiol, an adjunct player in the antipsychosis arsenal. Am. J. Psychiatry. 2018;175:197–198. doi: 10.1176/appi.ajp.2017.17121295. [DOI] [PubMed] [Google Scholar]
- Bechtold J., Hipwell A., Lewis D.A., Loeber R., Pardini D. Concurrent and sustained cumulative effects of adolescent marijuana use on subclinical psychotic symptoms. Am. J. Psychiatry. 2016;173:781–789. doi: 10.1176/appi.ajp.2016.15070878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Nosarti C., O'Carroll C.M., Seal M., Allen P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J.A., Chen J., Taylor J.R., Svenningsson P., Nishi A., Snyder G.L., Yan Z., Sagawa Z.K., Ouimet C.C., Nairn A.C. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bloomfield M.A., Ashok A.H., Volkow N.D., Howes O.D. The effects of Δ(9)-tetrahydrocannabinol on the dopamine system. Nature. 2016;539:369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D.L., Nguyen J.D., Morgenson D., Taffe M.A., Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2018;43:142–154. doi: 10.1038/npp.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong M.G., van Berckel B.N., Boellaard R., Zuurman L., Schuit R.C., Windhorst A.D., van Gerven J.M., Ramsey N.F., Lammertsma A.A., Kahn R.S. Delta 9-tetra-hydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Carriba P., Ortiz O., Patkar K., Justinova Z., Stroik J., Themann A., Müller C., Woods A.S., Hope B.T., Ciruela F. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Casadio P., Fernandes C., Murray R.M., Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci. Biobehav. Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Colizzi M., Bhattacharyya S. Does cannabis composition matter? Differential effects of delta-9 tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017;4:62–74. doi: 10.1007/s40429-017-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aquino J.P., Sherif M., Radhakrishnan R., Cahill J.D., Ranganathan M., D'Souza D.C. The psychiatric consequences of cannabinoids. Clin. Ther. 2018;40:1448–1456. doi: 10.1016/j.clinthera.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Egervari G., Ciccocioppo R., Jentsch J.D., Hurd Y.L. Shaping vulnerability to addiction - the contribution of behavior, neural circuits and molecular mechanisms. Neurosci. Biobehav. Rev. 2018;85:117–125. doi: 10.1016/j.neubiorev.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly M.A., Mehmedic Z., Foster S., Gon C., Chandra S., Church J.C. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol. Psychiatry. 2016;79:613–619. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A., Freeman T.P., Murray R.M., McGuire P. Can we make cannabis safer? Lancet Psychiatry. 2017;4:643–648. doi: 10.1016/S2215-0366(17)30075-5. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Crippa J.A., Bhattacharyya S., Borgwardt S.J., Allen P., Martin-Santos R., Seal M., Surguladze S.A., O'Carrol C., Atakan Z. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Gagnon D., Petryszyn S., Sanchez M.G., Bories C., Beaulieu J.M., De Koninck Y., Parent A., Parent M. Striatal Neurons Expressing D(1) and D(2) Receptors are morphologically distinct and differently affected by dopamine denervation in Mice. Sci. Rep. 2017;7:41432. doi: 10.1038/srep41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [Google Scholar]
- Gardner E.L. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol. Biochem. Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Engber T.M., Mahan L.C., Susel Z., Chase T.N., Monsma F.J., Jr., Sibley D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Grant A., Fathalli F., Rouleau G., Joober R., Flores C. Association between schizophrenia and genetic variation in DCC: a case-control study. Schizophr Res. 2012;137:26–31. doi: 10.1016/j.schres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Grant A., Manitt C., Flores C. Haloperidol treatment downregulates DCC expression in the ventral tegmental area. Neurosci. Lett. 2014;575:58–62. doi: 10.1016/j.neulet.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of dopamine signaling. Biosci. Rep. 2001;21:247–269. doi: 10.1023/a:1013205230142. [DOI] [PubMed] [Google Scholar]
- Greengard P., Allen P.B., Nairn A.C. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Han B., Compton W.M., Blanco C., Jones C.M. Time since first cannabis use and 12-month prevalence of cannabis use disorder among youth and emerging adults in the United States. Addiction. 2019;114:698–707. doi: 10.1111/add.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M.L., O'Dowd B.F., George S.R. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A., Perreault M.L., Shen M.Y., Zhang L., To R., Fan T., Nguyen T., Ji X., O'Dowd B.F., George S.R. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo: effective selective antagonism. FASEB J. 2014;28:4806–4820. doi: 10.1096/fj.14-254037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A., Perreault M.L., Shen M.Y.F., Fan T., Nguyen T., Alijaniaram M., Banasikowski T.J., Grace A.A., O'Dowd B.F., Fletcher P.J., George S.R. Activation of dopamine D1-D2 receptor complex attenuates cocaine reward and reinstatement of cocaine-seeking through inhibition of DARPP-32, ERK, and ΔFosB. Front. Pharmacol. 2018;8:924. doi: 10.3389/fphar.2017.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D.S., Kerridge B.T., Saha T.D., Huang B., Pickering R., Smith S.M., Jung J., Zhang H., Grant B.F. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: findings from the National epidemiologic survey on alcohol and related conditions-III. Am. J. Psychiatry. 2016;173:588–599. doi: 10.1176/appi.ajp.2015.15070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A.G., Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hudson R., Renard J., Norris C., Rushlow W.J., Laviolette S.R. Cannabidiol counteracts the psychotropic side-effects of Δ-9-tetrahydrocannabinol in the ventral Hippocampus through bi-directional control of ERK1-2 phosphorylation. J. Neurosci. 2019;39:8762–8777. doi: 10.1523/JNEUROSCI.0708-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis M.A., Gorelick D.A., Heishman S.J., Preston K.L., Nelson R.A., Moolchan E.T., Frank R.A. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Iwatsubo K., Suzuki S., Li C., Tsunematsu T., Nakamura F., Okumura S., Sato M., Minamisawa S., Toya Y., Umemura S., Ishikawa Y. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am. J. Physiol. Cell Physiol. 2007;5:C1498–C1508. doi: 10.1152/ajpcell.00088.2007. [DOI] [PubMed] [Google Scholar]
- Le Moine C., Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Lee S.P., So C.H., Rashid A.J., Varghese G., Cheng R., Lança A.J., O'Dowd B.F., George S.R. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Li X., Jope R.S. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D.M., Mathur B.N. Endocannabinoids in striatal plasticity. Parkinsonism Relat. Disord. 2012;18(Suppl 1):S132–S134. doi: 10.1016/S1353-8020(11)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N., Sui J., Chen J., Zhang F., Tian L., Lin D., Song M., Calhoun V.D., Cui Y., Vergara V.M. A schizophrenia-related genetic-brain-cognition pathway revealed in a large Chinese population. EBioMedicine. 2018;37:471–482. doi: 10.1016/j.ebiom.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica C.R., Riegel A.C. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Madras B., Bergman J., Kohut S., George S., Hurd Y., Lin Z. THC of marijuana upregulates DCC mRNA expression in prefrontal cortex: THC + cannabidiol does not. ACNP annual meeting abstract. 2017 [Google Scholar]
- Manitt C., Eng C., Pokinko M., Ryan R.T., Torres-Berrío A., Lopez J.P., Yogendran S.V., Daubaras M.J., Grant A., Schmidt E.R. dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl. Psychiatry. 2013;3:e338. doi: 10.1038/tp.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.S., Barr J.L., Harpe L.J., Poole R.L., Gould T.J., Unterwald E.M. The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PLoS One. 2014;9:e88026. doi: 10.1371/journal.pone.0088026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T.H., Zammit S., Lingford-Hughes A., Barnes T.R., Jones P.B., Burke M., Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morgan C.J., Curran H.V. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br. J. Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- Nairn A.C., Svenningsson P., Nishi A., Fisone G., Girault J.A., Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Navarro G., Carriba P., Gandía J., Ciruela F., Casadó V., Cortés A., Mallol J., Canela E.I., Lluis C., Franco R. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. ScientificWorldJournal. 2008;8:1088–1097. doi: 10.1100/tsw.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W., Zang T., Wang L.L., Zou Y., Zhang C.L. Phenotypic reprogramming of striatal neurons into dopaminergic neuron-like cells in the adult mouse brain. Stem Cell Reports. 2018;11:1156–1170. doi: 10.1016/j.stemcr.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M., Hill R., van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci. Biobehav. Rev. 2015;51:15–30. doi: 10.1016/j.neubiorev.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Ozaita A., Puighermanal E., Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J. Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Patton G.C., Coffey C., Carlin J.B., Degenhardt L., Lynskey M., Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M.L., Hasbi A., Alijaniaram M., Fan T., Varghese G., Fletcher P.J., Seeman P., O'Dowd B.F., George S.R. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M.L., Hasbi A., Shen M.Y., Fan T., Navarro G., Fletcher P.J., Franco R., Lanciego J.L., George S.R. Disruption of a dopamine receptor complex amplifies the actions of cocaine. Eur. Neuropsychopharmacol. 2016;26:1366–1377. doi: 10.1016/j.euroneuro.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Pickel V.M., Chan J., Kash T.L., Rodríguez J.J., MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Rais M., Cahn W., Van H.N., Schnack H., Caspers E., Hulshoff P.H., Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am. J. Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- Rashid A.J., So C.H., Kong M.M., Furtak T., El-Ghundi M., Cheng R., O’Dowd B.F., George S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J., Loureiro M., Rosen L.G., Zunder J., de Oliveira C., Schmid S., Rushlow W.J., Laviolette S.R. Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J. Neurosci. 2016;36:5160–5169. doi: 10.1523/JNEUROSCI.3387-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L.M., Makowski C.S., Yogendran S.V., Kiessling S., Cermakian N., Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology. 2015;40:1101–1112. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A.J., Dopeso-Reyes I.G., Martínez-Pinilla E., Sucunza D., Pignataro D., Roda E., Marín-Ramos D., Labandeira-García J.L., George S.R., Franco R., Lanciego J.L. Neurochemical evidence supporting dopamine D1-D2 receptor heteromers in the striatum of the long-tailed macaque: changes following dopaminergic manipulation. Brain Struct. Funct. 2017;222:1767–1784. doi: 10.1007/s00429-016-1306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E.B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart C.D., Sommer I.E., van Gastel W.A., Goetgebuer R.L., Kahn R.S., Boks M.P. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130:216–221. doi: 10.1016/j.schres.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Shen M.Y., Perreault M.L., Bambico F.R., Jones-Tabah J., Cheung M., Fan T., Nobrega J.N., George S.R. Rapid anti-depressant and anxiolytic actions following dopamine D1-D2 receptor heteromer inactivation. Eur. Neuropsychopharmacol. 2015;25:2437–2448. doi: 10.1016/j.euroneuro.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Shen M.Y., Perreault M.L., Fan T., George S.R. The dopamine D1-D2 receptor heteromer exerts a tonic inhibitory effect on the expression of amphetamine-induced locomotor sensitization. Pharmacol. Biochem. Behav. 2015;128:33–40. doi: 10.1016/j.pbb.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C., Coimbra B., Sousa N., Rodrigues A.J. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci. Biobehav. Rev. 2016;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021. [DOI] [PubMed] [Google Scholar]
- The National Academies of Sciences, Engineering, Medicine, 2017. http://nationalacademies.org/hmd/reports/2017/health-effects-of-cannabis-and-cannabinoids.aspx. [DOI] [PubMed]
- United Nations Office on Drugs and Crimes, World Drug Report 2013. https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- United Nations Office on Drugs and Crimes, World Drug Report 2015. https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- Valjent E., Pagès C., Hervé D., Girault J.A., Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E., Pagès C., Rogard M., Besson M.J., Maldonado R., Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends ondopaminergic transmission. Eur. J. Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Swanson J.M., Evins A.E., DeLisi L.E., Meier M.H., Gonzalez R., Bloomfield M.A., Curran H.V., Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73:292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Telang F., Fowler J.S., Alexoff D., Logan J., Jayne M., Wong C., Tomasi D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl. Acad. Sci. U S A. 2014;111:E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J., Strawbridge R.J., Bailey M.E.S., Graham N., Ferguson A., Lyall D.M., Cullen B., Pidgeon L.M., Cavanagh J., Mackay D.F. Genome-wide analysis in UK Biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl. Psychiatry. 2017;7:1264. doi: 10.1038/s41398-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M.B., Dias C., Magida J., Mazei-Robison M., Lobo M., Kennedy P., Dietz D., Covington H., 3rd, Russo S., Neve R. A novel role of the WNT-dishevelled-GSK3β signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J. Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M., Solowij N., Respondek C., Whittle S., Fornito A., Pantelis C., Lubman D.I. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.