Abstract
Despite historically known as “junk” DNA, nowadays non-coding RNA transcripts (ncRNAs) are considered as fundamental players in various physiological and pathological conditions. Nonetheless, any alteration in their expression level has been reported to be directly associated with the incidence and aggressiveness of several diseases. MicroRNAs (miRNAs) are the well-studied members of the ncRNAs family. Several reports have highlighted their crucial roles in the post-transcriptional manipulation of several signaling pathways in different pathological conditions. In this review, our main focus is the multifaceted microRNA-486 (miR-486). miR-486-5p and miR-486-3p have been reported to have central roles in several types oncological and non-oncological conditions such as lung, liver, breast cancers and autism, intervertebral disc degeneration and metabolic syndrome, respectively. Moreover, we spotted the light onto the pleiotropic role of miR-486-5p in acting as competing endogenous RNA with other members of ncRNAs family such as long non-coding RNAs.
Keywords: miR-486-5p, miR-486-3p, long non-coding RNAs, Xist, Lung cancer, Breast cancer, Liver cancer
Abbreviations
- AGO
Argonaute
- ARID1B
AT-rich interaction domain 1B
- BC
Breast Cancer
- BP
Blood pressure
- CAD
Coronary Artery Disease
- CRC
Colorectal cancer
- CV
Cardiovascular
- DDR1
Discoidin domain receptor-1
- DLGAP1-AS1
DLGAP1 antisense RNA 1
- DGCR8
Drosha-DiGeorge syndrome-critical region gene 8
- DOCK1
Dedicator of cytokinesis 1
- DSM-5
Disorders Diagnostic and Statistical Manual 5
- ECM1
Extracellular matrix protein 1
- EMT
Epithelial-mesenchymal transition
- eRNAs
Enhancer RNAs
- ESCC
Esophageal squamous cell carcinoma
- FGF9
Fibroblast growth factor 9
- FBN1
Fibrillin-1
- FLNA
Filamin A
- FOXO1
Forkhead box O1
- GC
Gastric carcinoma
- HbF
Fetal hemoglobin
- HCC
Hepatocellular Carcinoma
- HDL
High-density lipoprotein
- IDD
Intervertebral Disc Degeneration
- IR
Insulin resistance
- lncRNA
Long non-coding RNA
- LUSC
Lung Squamous cell Carcinoma
- Mets
Metabolic syndrome
- MMP
Matrix metalloproteinase
- miRNA
MicroRNA
- micRNA
mRNA-interfering complementary RNA
- ncRNAs
Non-Coding RNAs
- NP
Nucleus pulposus
- NSCLC
Non-Small cell Lung Cancer
- NFATc1
Nuclear factor activated T-cell cytoplasmic 1
- OSCC
Oral squamous cell
- PTC
Papillary Thyroid Cancer
- PD
Parkinson's Disease
- PLGA
Pegylated poly lactic-co-glycolic acid
- PIK3RI
Phosphoinositide-3-Kinase Regulatory Subunit 1
- piRNAs
Piwi-associated RNAs
- PLAGL2
Pleiomorphic adenoma gene-like 2
- Pri-miRNAs
Primary transcripts
- RCC
Renal cell carcinoma
- RISC
RNA-induced silencing complicated
- rRNA
ribosomal RNA
- siRNAs
small interfering RNAs
- SIRT2
Sirtuin 2
- ShRNA
Short hairpin RNA
- sncRNA
Short non-coding RNA
- snoRNAs
Small nucleolar RNAs
- snRNA
Small nuclear RNA
- TGF-β activated kinase 1
Transforming growth factor-beta 1
- TRBP
TAR RNA-binding protein
- tRNA
Transfer RNA
- TSP
Thrombospondin
- XIST
X-inactive specific transcript
- LARC
Locally advanced rectal cancer
1. The story behind non-coding RNAs (ncRNAs)
Many distinctive ncRNA sequences are found in human cells [1]. Approximately 30 years ago in the 1980s, ncRNAs' misconception of being ‘junk’ transcriptional products has been changed to be considered as functional regulatory molecules [1,2]. The first discovered regulatory ncRNA molecule was part of the MicF gene [1]. MicF RNA includes a stretched sequence that is complementary to the 5′ end region of the ompF mRNA (OmpF is a major component of the external layer protein of Escherichia coli) and was demonstrated to be a noteworthy player in bacterial cell physiology [3,4]. MicF RNA was then found to have the potential to regulate various targets by means of various RNA/RNA pairing [3]. Yet, the first discovery of the regulatory mechanism of the eukaryotic gene expression by ncRNAs was in the 1990s [1]. In 1993, the discovery of the first microRNA (miRNA), lin-4 in Caenorhabditis elegans by the Ambros and Ruvkun group has revolutionized the field of molecular biology [5]. While the first mammalian miRNA, let-7, was revealed 7 years later [6]. Since then ncRNAs and especially miRNAs were the main focus of several researchers until now.
ncRNAs were reported to participate in different cellular processes such as transcription, post-transcriptional modifications, and signal transduction networking [2]. ncRNAs are divided into house-keeping and regulatory molecules [7]. Firstly, the housekeeping ncRNA molecules includes the ribosomal (rRNA), transfer (tRNA), small nuclear (snRNA), and small nucleolar RNAs (snoRNAs) [7]. Secondly, the regulatory ncRNAs that can regulate the expression pattern of other coding transcripts includes long non-coding RNAs (lncRNAs) ≥200 nucleotides and short non-coding RNAs (sncRNAs) < 200 nucleotides as shown in Table 1 [[7], [8], [9]]. SncRNAs are divided into microRNAs (miRNAs), small interfering RNAs (siRNAs) and piwi-associated RNAs (piRNAs) [7]. However, antisense RNAs and enhancer RNAs (eRNAs) are intermediate-sized ncRNA molecules lying at the bay between both classes of lncRNAs and sncRNAs [7]. Furthermore, circular RNAs (circRNAs) have lately been identified as a promising class of ncRNAs involved in various biological processes and acts as an sponge for several miRNAs, hindering the miRNAs’ effects on their target transcripts [10].
Table 1.
Types of non-coding RNA molecules.
| Type of ncRNAs |
Functions | |
|---|---|---|
| Housekeeping ncRNAs | Regulatory ncRNAs | |
| rRNAs | Involved in mRNA translation | |
| tRNAs | Involved in mRNA translation | |
| snoRNAs | Involved in the modification of rRNAs | |
| snRNAs | Involved in splicing | |
| sncRNAs | mRNA translation regulation or stability | |
| lncRNAs | Chromatin remodeling and transcriptional regulation | |
2. MicroRNAs (miRNAs): Master-Maestro of the genome
As believed to be the heart of several signaling pathways, miRNAs are single-stranded RNA molecules, subclass from sncRNAs as previously mentioned (~22 nucleotides). miRNAs are branded to be the “Master-Maestro” of the genome where they were reported to post-transcriptionally modulate the expression of approximately 60% of the whole genome in animals, viruses, and plants taking over different metabolic and cellular trails [[11], [12], [13], [14]]. Moreover, intracellular miRNAs are ranked as crucial regulators of cellular development and are incorporated in a diversity of biological fates [5,15]. Extracellular miRNAs as well have been widely discussed as possible biomarkers for several diseases including malignancies and also serve as triggers for cell-cell interaction in several contexts [5]. Altered expression levels of miRNAs are accompanied with solid and humoral malignancies [15], cardiac illnesses such as hypertrophy and ischemia [16] and mental disorders such as schizophrenia or severe depression disorders [17]. Hundreds of targets could be regulated by a single miRNA, signifying the multifaceted and combinatorial role of miRNA in regulating mRNA expression [14,15]. The field of miRNAs is rapidly escalating providing more information about the functional characterization of each miRNA in each disease, thus partially solving the puzzle of multiple diseases‘ molecular pathophysiology.
2.1. miRNAs’ biogenesis
The biogenesis of miRNAs is divided into canonical and non-canonical pathways [5]. It starts with the processing of RNA polymerase II/III transcripts [18]. Approximately half of all presently known miRNAs are intragenic and mostly processed from introns and comparatively few exons of protein coding genes. While the rest minority of the miRNAs are intergenic, transcribed separately of a host gene and governed by their own promoters [5]. Often miRNAs are transcribed as a long transcript called clusters that may have comparable seed regions and are considered a family in that case [19].
2.1.1. Canonical pathway
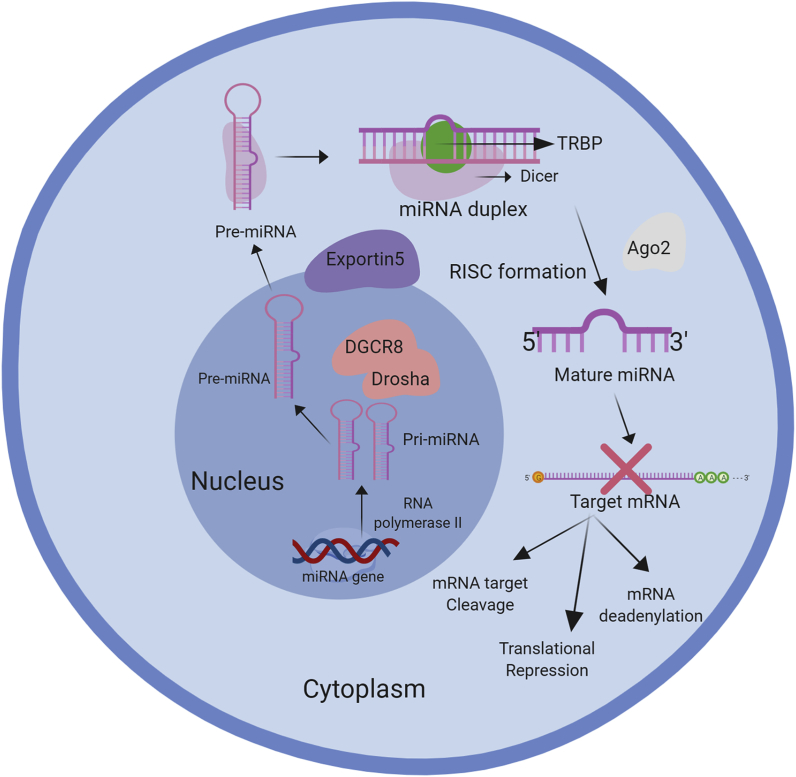
The canonical pathway of the miRNAs biogenesis starts with their transcription to primary miRNA (pri-miRNA) by RNA polymerase II in the nucleus [[17], [18], [19], [20]]. These primary transcripts are then processed by the Drosha & DiGeorge syndrome-critical region gene 8 (DGCR8), which produces the precursor miRNA (pre-miRNA, ~70 nucleotide) [20]. More specifically, DGCR8 recognizes a pri-miRNA N6-methyladenylated GGAC, whereas Drosha cut the pri-miRNA duplex at the base of the pri-miRNA hairpin structure [21]. The pre-miRNA has a brief stem, which is recognized by the nuclear exportin-5 that facilitates its exportation to the cytoplasm as shown in Fig. 1 [[17], [18], [19]]. In the cytoplasm, Dicer RNase III induces the processing of miRNA duplexes [[17], [18], [19]]. During the cytoplasmic processing of the miRNA, TAR RNA-binding protein (TRBP) and Argonaute (AGO) 1–4 co-operatively resolve the pre-miRNA processing and RNA-induced silencing complicated (RISC) assembly [[17], [18], [19]]. After miRNA duplex formation, one strand of the miRNA duplex is separated from this complex, leading to a single-stranded miRNA associated with RISC. But the question now is which strand is loaded to the RISC to form the functional regulatory miRNA-RISC complex?
Fig. 1.
Biogenesis of miRNA's Canonical Pathway and mechanism of action.
The biogenesis of Canonical miRNA is the chief path by which miRNAs are processed. miRNA gene is transcribed in the nucleus to produce primary microRNA (pri-miRNA) that goes through nuclear cleavage to generate a precursor (pre-miRNA). Pre-miRNA is transported to the cytoplasm to be cleaved resulting in miRNA duplex formation. Formerly, the unwinding of miRNA duplex takes place followed by binding to the RISC complex to form a mature miRNA. Each base-pair of a mature miRNA binds to its target mRNA leading to a direct gene silencing through inhibiting translation or mRNA cleavage or mRNA deadenylation.
The -5p or -3p strand choice is partly based on the thermodynamic stability at the 5′ ends of the miRNA duplex or 5′- untranslated region (UTR) at nucleotide position 1 [22]. The unloaded strand is named the passenger strand, which unwind from the guide strand (loaded strand) by different mechanisms dependent on complementarity [5]. However, recently some miRNAs violate this rule such as miR-486 where both miR-486-5p and miR-486-3p associate with RISC and act as functional regulatory miRNAs.
2.1.2. Non-canonical pathway
The non-canonical pathway of miRNAs’ biogenesis is divided into Drosha/DGCR8-independent or D;icer-independent routes [5]. Drosha/DGCR8-independent pathway includes splicing of mRNA introns and their conversion into mirtrons transcripts. Then Drosha/DGCR8 processing step is skipped, where those transcripts are directly transported to the cytoplasm via exportin-1 [5]. Alternatively, Dicer-independent miRNAs are handled by Drosha/DGCR8 from endogenous short hairpin RNA (shRNA) transcripts [23]. Since those transcripts are not long enough to act as Dicer substrates, in this case AGO2 takes the lead in their cytoplasmic maturation steps [24].
2.2. Mechanism of action
Functional miRNAs bind to its target mRNA, where the “seed” sequence which is 2–7 nucleotides from the 5′ end of the miRNA complements to target mRNA sequences, known as miRNA response elements (MREs) [[25], [26], [27]]. miRNAs could bind to those MREs in 3′ UTR, 5′UTR, open reading frame (ORF) or its promoter region according to complementary base pairing rules [28]. Binding of the miRNA to its target genes' 3′-UTR could be perfect or imperfect complementarity and results in post-transcription silencing through mRNA destabilization, deadenylation,k de-capping and translational repression [5,16,17,25,29]. Binding of the miRNA to its target gene ORF needs almost ideal complementarity and results in mRNA degradation or cleavage [28]. However, the binding of miRNA to its target's promoter region induces transcription, while binding to 5′ UTR leads to either silencing or activation of target gene expression [29]. Reports suggest that each miRNA can control various mRNAs and that several miRNAs can target a single mRNA [30].
2.2.1. Silencing mode
The induced silencing mode of the miRNAs to their targets mainly depends on the degree of MRE complementarity. It specifies whether target mRNA will be degraded or AGO2-dependent splicing of target mRNA will occur. A fully complementary interaction activates AGO2 endonuclease to cleave target mRNA [31]. The development of a miRISC silencing complex begins with the recruitment by miRISC for the GW182 family of proteins. GW182 structure helps in recruiting numerous effector proteins such as poly (A)-binding protein C which helps in the deadenylation process. Eventually, decapping occurs by the help of decapping protein 2 (DCP2) and derived proteins shadowed by 5′–3′ degradation [5].
2.2.2. Activation mode
It has been reported that miRNAs could also induce the expression level of its target mRNAs not only inhibiting their translation [5]. Translation activation mediated by miRNA includes AGO2 and Fragile-x-mental retardation related protein 1 (FXR1) rather than GW182. Several miRNAs, have been found to be connected with AGO2 and FXR1 to enable the transcription in the cell cycle arrest mode, yet they suppress transcription in proliferating cells [32]. For instance, miR-486-5p was found to induce one of its targets (Insulin-like growth factor-1, IGF-1) in non-cancerous natural killer cells [33], yet it was found to repress the same target in cancerous liver cancer cells [34]. Also as previously discussed, other forms of miRNA-induced gene expression activation involves binding to the 5′ UTR of mRNAs throughout amino acid starvation [35].
3. miRNAs as prominent players in the battle against malignancies
Being a multifactorial player simultaneously targeting several coding genes, this ranks the miRNAs as dominant players in the field of oncology [36,37]. When a miRNA is able to target/repress several signaling mediators in the oncogenic signaling circuits, this nominates such miRNA to act as a tumor suppressor mediator [[38], [39], [40], [41]]. On the contrary, if a miRNA targets the chief tumor suppressor proteins and/or the cell-cycle checkpoint proteins, this nominates such miRNA as an oncogenic miRNA or an oncomiR as summarized in Table 2 [42]. The very first study that linked miRNA to cancer was in chronic lymphocytic leukemia patients [43]. Where miR-15 and miR-16 were found to be down-regulated [43]. One of the top miRNAs that were found to have a paradoxical role, acting as tumor suppressor miRNA or an oncomiR depending on the cellular context, disease condition, expression level of its upstream ncRNAs (lncRNAs or circRNAs) is microRNA-486. Commonly, miR-486 is refereed to miR-486-5p. However, it was reported in literature that miR-486-3p is also acting as a functional regulatory miRNA with aberrant expression in several pathological conditions. In terms of oncology, miR-486-5p was extensively studied in comparison to miR-486-3p. miR-486-5p was found to have a significantly aberrant expression in several solid malignancies such as hepatocellular cancer (HCC), non-small cell lung cancer (NSCLC), breast cancer (BC), esophageal squamous cell carcinoma (ESCC) and pancreatic cancer (PC) [44].
Table 2.
Oncogenic, Ttumor suppressor and Controversial miRNAs in different malignant Phenotypes.
| miRNAs | Oncogenic/Tumor Suppressor | Type of cancer | Reference |
|---|---|---|---|
| miR-17-92 cluster | Oncogenic | Lung cancer | [24] |
| miR-132 | Oncogenic | Pancreatic adenocarcinoma | [25] |
| miR-212 | |||
| miR-195 | Oncogenic | Melanoma adenoma Pituitary adenoma |
[26,27] |
| miR-128a | |||
| miR-155 | |||
| miR-516a-3p | |||
| miR-372 | |||
| miR-504 | Oncogenic | Osteosarcoma Lung cancer Neuroblastoma Colorectal carcinoma Hepatoblastoma Breast cancer |
[[28], [29], [30], [31]] |
| miR-25 | |||
| miR-30d | |||
| miR-125b | |||
| miR-1285 | |||
| miR-24 | Oncogenic | Human diploid fibroblasts Cervical carcinoma Mouse embryonic fibroblasts |
[32,33] |
| miR-31 | |||
| miR-21 | Oncogenic | Prostate cancer Gastric cancer |
[34,35] |
| miR 221/222 cluster | |||
| miR-25 | |||
| miR-10b | Oncogenic | Breast Cancer | [36,37] |
| miR-373 | |||
| miR-520c | |||
| miR-223 | Oncogenic | Gastric Cancer | [38] |
| miR-31 | Oncogenic | Lung cancer cells | [39,40] |
| miR-411 | |||
| miR-217 | Tumor Suppressor | Pancreatic ductal adenocarcinoma | [41] |
| miR-335 | Tumor Suppressor | Breast Cancer | [37,42] |
| miR-101 | |||
| miR-34a | Tumor Suppressor | Pancreatic cancer | [43] |
| miR-143 | Tumor Suppressor | Colon cancer | [44] |
| miR-145 | |||
| miR-15a | Tumor Suppressor | Leukemia | [45,46] |
| miR 16-1 | |||
| miR-495 | |||
| miR-203 | Tumor Suppressor | Lung cancer | [47,48] |
| miR-218 | |||
| miR-451 | Tumor Suppressor | Non-small cell lung cancer | [49] |
| miR-96 | Tumor Suppressor | Pancreatic cancer | [50] |
| miR-150 | Tumor Suppressor | Malignant lymphoma | [51] |
| miR‐122 | Tumor Suppressor | Hepatocellular carcinoma | [52] |
| miR-143 | Tumor Suppressor | Bladder Cancer | [53,54] |
| miR-99a-5p | |||
| miR-7 | Controversial | Lung cancers | [[55], [56], [57]] |
| miR-486 | Controversial | Breast Cancer Prostate Cancer |
[58,59] |
4. miR-486: chromosomal location, behavior and functions
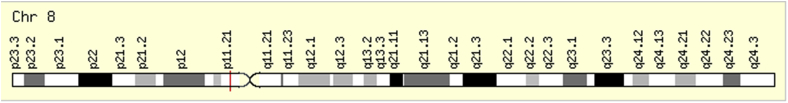
miR-486 is encoded by the MIR486-1 gene in the human genome. Its genomic location is on chromosome 8 (8p11.21) as shown in Fig. 2. This region in the genome is called a deletion region having the most potent tumor suppressor genes [34]. As previously described, there are 2 mature miRNA strands originating from opposite arms of the same pre-miR-486 and are denoted with a -3p or -5p suffix as shown in Table 3 miR-486-5p and miR-486-3p shows a controversial role in various pathological conditions [45,46]. In many forms of cancers, they could act as a tumor-suppressive miRNA through inhibiting the migration and invasion capacities of several cancers [47,48]. On the contrary, miR-486-5p showed its oncogenic properties in other forms of cancers such as PC [46]. Moreover, miR-486-5p and miR-486-3p were found to have a high potential to act as diagnostic and prognostic marker in several diseases such as cancers and metabolic syndromes (MetS). On the national level, miR-486-5p was reported to act as a promising prognostic factor for major public health issues such as insulin resistance (IR) and elevated blood pressure (BP) among the Egyptian males [49]. Therefore this review focuses on the multifaceted miR-486-5p and miR-486-3p and its differential expression and mechanism of actions in several cancers and pathological conditions.
Fig. 2.
Chromosomal location of miR-486-5p.
Schematic representation of miR-486-5p gene locus; chromosomal mapping showed that miR-486 gene is located on somatic chromosome 8, long arm, region 11, band 21 (8p11.21).
Table 3.
The difference in sequence of miR-486-5p and miR-486-3p.
| miR-486-5p Sequence | miR-486-3p sequence |
|---|---|
| 0- UCCUGUACUGAGCUGCCCCGAG -22 | 42- CGGGGCAGCUCAGUACAGGAUA -64 |
5. miR-486-5p role in different oncological phenotypes
5.1. Non-small-cell lung cancer (NSCLC)
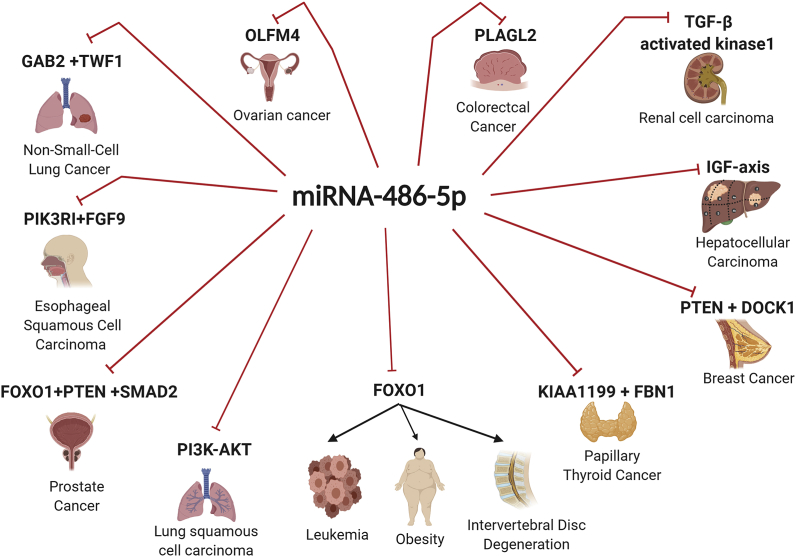
NSCLC is considered one of the most prevalent types of lung cancer [50]. In NSCLC, miR-486-5p was reported to be downregulated in NSCLC patients and cell lines (A549 cells) [51]. This was believed to be related to the abnormal methylation of ANK1 promoter that harbors the same chromosomal location of miR-486-5p (8p11.21) [52]. miR-486-5p gene is located in the last intron of ANK1 [52]. Furthermore, miR-486-5p levels in NSCLC were directly correlated with the prognosis of the diseases where patients with low levels of miR-486-5p showed poor prognosis. NSCLC treatment protocol mainly includes Cisplatin as a potent chemotherapeutic agent [51]. However, the rise of Cisplatin resistance has become one of the main obstacles for effective treatment of NSCLC patients. miR-486-5p is believed to have a role in chemo-resistance of several cancers [51]. This was explained by twinfilin actin-binding protein 1 (TWF1) which is a direct target gene for miR-486-5p in HEK-293T cells and plays an undisputable role in tumor invasion and chemotherapy resistance [48,49,51]. TWF1 suppression by miR-486-5p leads to increase NSCLC cell line (A549 cells) susceptibility to Cisplatin [51]. Moreover, miR-486-5p's role in managing Cisplatin resistance was investigated in vivo, revealing that miR-486-5p expression has increased the NSCLC tumor sensitivity to cisplatin treatment by reducing NSCLC tumor size in nude mice [51]. Hence miR-486-5p is considered to be an effective therapeutic agent in managing Cisplatin resistant NSCLC [51]. Grb2-Associated Binding Protein 2 (GAB2) is another direct target gene for miR-486-5p and it is usually up-regulated in numerous human malignancies [53]. GAB2 functions as a mediator for vital cellular processes containing proliferation and migration [52]. Forced expression of miR-486-5p in SPC-A1 and A549 cell lines leads to inhibition of GAB2, thus preventing the metastasis and progression of NSCLC [53]. Therefore, this shows the potential therapeutic and prognostic roles of miR-486-5p in NSCLC patients.
5.2. Lung squamous cell carcinoma (LUSC)
LUSC is the 2nd most common cancer among the world, yet until now it lacks a specific, reliable and detective diagnostic biomarker [54]. miRNAs are fortunately produced in extracellular fluids, which highly appoints miRNAs as easily detectable biomarkers for various diseases [5]. miR-486-5p is down-regulated in LUSC patients compared to healthy controls and it was found to be also down-regulated in LUSC cell lines such as NCI–H520, human bronchial epithelial cells and B(a)P malignantly transformed HBE cells which supports its ability to act as a diagnostic marker for LUSC [54]. Functionally, miR-486-5p regulates the aggressive oncogenic signaling pathway; PI3K/AKT/mTOR causing the hindrance of LUSC development [54].
5.3. Colorectal cancer (CRC)
One of the main reasons for cancer-related deaths is CRC [55,56]. The early detection of CRC using biomarkers could help to decrease the death rate through preventing the disease progression [55,57]. Circulating exosomal miRNAs could act as CRC biomarkers [58,59]. Some studies reported that the circulating exosomal miR-486-5p levels were highly low in locally advanced rectal cancer (LARC) cell line (LoVo cells) with a high metastatic grade. Additionally, other studies reported that miR-486-5p CRC tissues’ expression was also highly repressed in early stage CRC patients [57,60]. Collectively, exosomal miR-486-5p is believed to act as a circulating marker for high-risk LARC [57]. In an explanation for the reduced miR-486-5p expression in CRC patients and cell lines and similar to NSCLC, miR-486-5p expression was found to be lowered in CRC tissues and cell lines (HT29 and SW480 cells) due to hyper-methylation of miR-486-5p promoter [56]. On the functional level, forcing miR-486-5p expression resulted in suppression of CRC invasion both in vitro and in vivo by directly targeting pleiomorphic adenoma gene-like 2 (PLAGL2) which eventually represses 2 of the most vital oncogenic pathways β-catenin and Insulin Growth Factor 2 signaling pathways [56]. Collectively, miR-486-5p could act as a potential therapeutic and diagnostic biomarker for CRC patients [56].
5.4. Breast cancer (BC)
BC is considered to be one of the most widely spread cancers between women worldwide [37]. miR-486-5p is one of the well-studied miRNAs in BC. It was found to be strongly down-regulated in BC patients and BC cell lines (MDA-MB231, MCF-7, SK-BR-3, and T47D cells) [61,62]. Functionally, ectopic expression of miR-486-5p causes the repression of the oncogenes PIM1 and PTEN which eventually results in total abrogation of BC proliferation [61,62]. Moreover, miR-486-5p also directly targets dedicator of cytokinesis 1 (DOCK1) which is directly fueling BC motility and invasion capacities through activating the Dock1/NF-ƙB/Snail pathway [63]. Thus miR-486-5p is found to repress BC progression, proliferation, migration and invasion potentials through targeting several oncogenes and affecting multiple signaling pathways simultaneously [63]. Nevertheless, miR-486-5p role in BC is not only restricted to the malignant phenotype of the disease. However, miR-486-5p also affects the tumor microenvironment conditions and the immunogenic profile of BC patients. It was found that miR-486-5p directly targets the immune-suppressive Interleukin-22 (IL-22), thus alleviating the immune suppressive nature of the BC tumor microenvironment [63]. Moreover, our research group has recently highlighted the potential role of miR-486-5p in inducing the activating immune ligands MICA, MICB and CD155 in order to increase the recognition ability of BC cells to the cytotoxic T lymphoctyes and natural killer cells in BC patients (unpublished data). Nonetheless, another study revealed the significance of miR-486-5p to serve as a useful marker for documenting BC high-risk patients [62]. Collectively, miR-486-5p could serve as a diagnostic marker for BC patients, potential therapeutic approach revealing the malignant phenotypic behavior of BC cells, alleviating the immune-suppressive tumor microenvironment and boosting the immunogenic recognition of BC cells by the immune system.
5.5. Hepatocellular carcinoma (HCC)
HCC is the most common primary liver malignancy and is a prominent contributor to cancer mortalities worldwide [64]. Our research group has found that miR-486-5p was down-regulated in HCC liver tissues and cell lines (Huh-7 cells), where its ectopic expression was found to block one of the most aggressive deregulated oncogenic signaling pathway in HCC patients. Moreover, miR-486-5p was also found to repress IGF-1R downstream signaling mediators known as mTOR, STAT3 and c-Myc [34,61]. Moreover, miR-486-5p was also found to boost the chief l2player of the innate immune system which is the natural killer cells of HCC patients mainly through augmenting its killing capacity against HCC cell lines through inducing the expression levels of perforins [33]. Therefore, this shows the potential role of miR-486-5p in HCC where it dually acts as a tumor suppressor miRNA and an enhancer immune surveillance capabilities of HCC patients through directly increasing the cytotoxic potential of natural killer cells.
5.6. Renal cell carcinoma (RCC)
RCC is a mutual kidney tumor in humans [65]. Surgical resection is a preferred choice in the early stage of RCC [66]. However, therapy resistance is the major obstacle in the late stage of RCC [67]. Therefore, it is important to find new targets in RCC treatment [68]. miR-486-5p expression is found to be lowered in RCC patients and cell lines (786-O, GRC-1, A-498, ACHN, Caki-2, 786-P, SK-RC-42, and Ketr3 cells) [47]. Forcing its expression leads to the inhibition of RCC cell proliferation and the increase of cell apoptosis through suppressing Transforming growth factor-beta 1 (TGF-β activated kinase 1) [47] thus crystallizes the tumor suppressor potential of miR-486-5p in RCC patients.
5.7. Gastric cancer (GC)/Esophageal squamous cell carcinoma (ESCC)
The highest incidence rates for GC/ESCC were found to be in eastern Asia, eastern and southern Africa. The late stage of GC/ESCC always has restricted treatment options. A recent study proved that miR-486-5p was chiefly presented in the cytoplasm of cells and its expression was reduced in the majority of ESCC and GC [47]. Phosphoinositide-3-Kinase Regulatory Subunit 1 (PIK3RI) and fibroblast growth factor 9 (FGF9) were found as direct targets for miR-486-5p in GC [47,69,70]. As previously described, a frequent characteristic in plentiful of human malignancies is the deregulation of the PI3K/Akt pathway leading to cancer progression [71]. FGF9 is secreted from cancer-associated fibroblasts that stimulate GC cells invasiveness [72]. Thus this nominates miR-486-5p as a tumor suppressor miRNA through repressing PI3KR1 and FGF9 in GC and ESCC cells [47].
5.8. Papillary thyroid cancer (PTC)
The most severe malignancy of the endocrine system is thyroid cancer [73]. About 80% of the world's diagnosed thyroid cancers are PTC [74]. New studies have shown that KIAA1199 also plays a key role in the development of PTC inducing its invasion and metastatic potentials [74]. Moreover, KIAA1199 switched on several signaling pathways such as the Wnt/β-catenin and induces the enzymatic activities of matrix metalloproteinase (MMP), which relate to the epithelial-mesenchymal transition (EMT) [74]. It was found that KIAA1199 is expressively up-regulated in PTC tissues with a negative correlation with miR-486-5p expression [74]. This study revealed that KIAA1199 is a direct target of miR-486-5p with and thus ectopic expression of miR-486-5p resulted in a marked repression of KIAA1199 mRNA and protein expression helping in averting PTC invasion and metastasis [74]. Another study unraveled another onogenic target of miR-486-5p which is Fibrillin-1 (FBN1) [75] and thus also confirming the tumor suppressor activity of miR-486-5p in PTC patients.
5.9. Ovarian cancer
Ovarian cancer is considered to be one of the utmost common gynecological malignant tumors [76]. Estrogen and progesterone are being secreted by the ovaries, where estrogen plays a dominant role in ovarian tumor growth and metastasis [76]. It has been found that the hyper-activation of estrogen receptor alpha (ERα) causes changes on the genetic and epigenetic levels in ovarian cancer cells [76]. ER signaling regulates the expression of Olfactomedin 4 (OLFM4) in ovarian serous adenocarcinoma. It has been proven that gynecological tumors especially serous adenocarcinoma tissues have aberrant OLFM4 expression [76]. Impairment of the expression of ERα-mediated OLFM4 facilitates malignant development of ovarian serous adenocarcinoma [77]. In ovarian serous adenocarcinoma, the down-regulated miR-486-5p potentially targets OLFM4 in SKOV3 ovarian cancer cell lines, repressing its expression level and activity and thus repressing ovarian cancer progression [76].
5.10. Leukemia
Leukemia is known for its atypical blood precursor cells proliferation of myeloid or lymphoid nature [78]. Lately, studies have shown that miRNAs and lncRNAs could act as prognostic and diagnostic biomarkers for leukemic patients [79,80]. miR-486-5p was found to be down-regulated in leukemic cell lines (K562, Kasumi-1, and THP-1 cells) [81]. miR-486-5p showed an interaction with the 3′-UTR of forkhead box O1(FOXO1) [81]. FOXO1 existence during oxidative stress leads to apoptotic cell injury [82]. Thus miR-486-5p ectopic-expression leads to an increase in the apoptosis of leukemia cells by targeting FOXO1 [81].
5.11. Prostate cancer (PC)
The most common malignancy among men is PC [83]. Human PC gene expression profiling has shown several miRNAs involvements where miR-486-5p was on top of the list. On the contrary to other solid malignancies mentioned above and summarized in Fig. 3, miR-486-5p was found to be over-expressed in PC tissues and cell lines (DU145, PC-3, and LnCap) [83]. miR-486-5p overexpression stimulates different oncogenic pathways through suppressing FOXO1, PTEN and SMAD2 [83]. Where PTEN and FOXO1 act as negative regulators for PI3K/AKT signaling straightly suppressing the proliferation [83]. Besides, SMAD2 acts as a tumor suppressor by taking part in the stem cell compartment of prostate cancer [83]. In vivo, it was proven that miR-486-5p acts as a oncomiR in PC, as it plays an important role in PC pathogenicity [83].
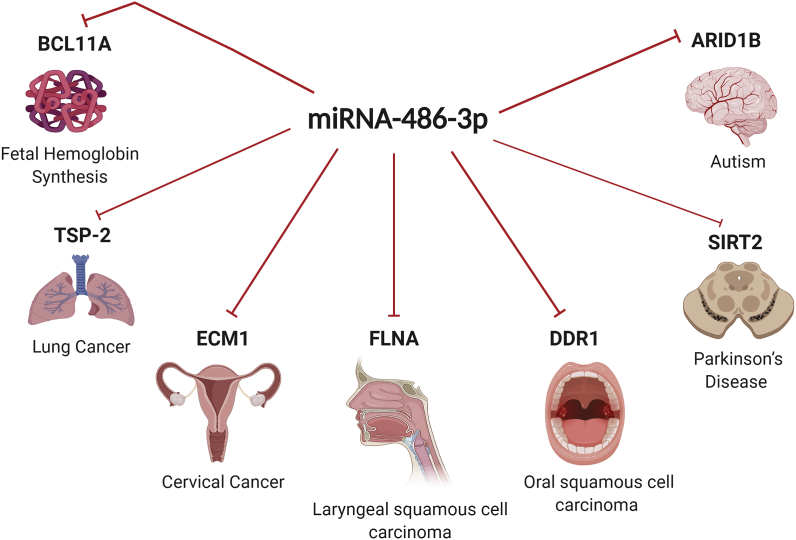
Fig. 3.
Multi-functional Role of miR-486-3p in simultaneously targeting several targets in different cellular contexts.
miR-486-3p has several targets in different malignant and non-malignant conditions. It modulates the expression of several target proteins such as BCL11A, TSP-2, ECM1, FLNA, DDR1, SIRT2 and ARID1B in different body compartments as indicated in the diagram.
6. miR-486-3p role in different oncological phenotypes
6.1. Oral squamous cell carcinoma (OSCC)
OSCC, is one of the world's deadly cancers, with a significant increase in incidence and mortality in the last century [84]. Betel quid chewing was acknowledged as a significant contributor to the incidence and mortality rate of oral cancer [85]. miR-486-3p expression was found to be down-regulated in OSCC [86]. Discoidin domain receptor-1(DDR1) is a tyrosine kinase and was reported to be up-regulated in OSCC tissues and cell lines (OEC-M1 and TW2-6) leading to tumor progression [86]. Fortunately, DDR1 was found to be a direct gene target for tumor suppressor miR-486-3p [86]. As it was mentioned above that ANK1 irregular epigenetic modifications could lead to suppression of miR-486-5p in NSCLC [86]. Accordingly, hyper-methylation of ANK1 promoter by DNMT3B which is found in arecoline (a major component of betel quid chewing) leads to a reduction of ANK1 and miR-486-3p expression subsequently leading to DDR1 expression [86]. Therefore restoring the expression of miR-486-3p in OSCC patients might lead to apoptosis of OSCC cancer cells and therapeutically alleviating OSCC progression [86].
6.2. Lung cancer
As mentioned earlier, lung cancer is the most common malignancy worldwide. It is also the major cause of cancer-related death among males, and the world's 2nd leading cause of cancer-related deaths among females [87]. The matricellular glycoprotein thrombospondin-2 (TSP-2) is found to regulate numerous biological processes and play a dominant role in the development and metastasis of lung cancer [88]. Additionally, TSP-2 expression is strongly associated with lung cancer stage [88]. It was recently discovered that TSP-2 also stimulates osteoclastogenesis through the RANKL-dependent pathway and that TSP-2-mediated osteoclastogenesis encompasses the transactivation of the Nuclear factor activated T-cell cytoplasmic 1 (NFATc1) through repression of miR-486-3p expression [88]. Therefore, restoring the expression of miR-486-3p leads to the abrogation of TSP-2 effects and inhibition of osteolytic metastasis in vivo [88].
6.3. Cervical cancer (CC)
Cervical cancer (CC) is the 4th common gynecological malignancy and the 2nd most common cancer in developing countries [89]. miR-486-3p expression is found to be reduced in CC besides being correlated with metastasis in those patients [90]. Extracellular matrix protein-1 (ECM-1) has been predicted and validated as a miR-486-3p target gene as shown in Fig. 4 [90]. Eventually, cell growth and metastasis were inhibited by miR-486-3p ectopic-expression [90]. miR-486-3p is a tumor suppressor miRNA and its induction is a possible strategy for inhibiting the development of CC [90].
Fig. 4.
The pleiotropic role of miR-486-5p in manipulating several target genes in different cellular contexts.
miR-486-5p has several targets in different malignant and non-malignant conditions. It modulates the expression of several target proteins such as TGF-β activated kinase1, PTEN, DOCK1,IGF- signaling pathway, KIAA1199, FBN1, FOXO1, PI3K-AKT,SMAD2, FGF9, PIK3RI, TWF1, GAB2, OLFM4 and PLAGL2.
6.4. Laryngeal squamous cell carcinoma (LSCC)
LSCC is the world's 2nd most widely diagnosed head and neck cancer [91]. Since Circular RNA (circRNA) is a vital regulator of miRNA activity [92]. circRNAs is believed to have fundamental biological roles in the migration and development of cancer [93]. Filamin A (FLNA) is essential for organogenesis during development, due to its capability to induce cell migration through its actin-binding properties [94]. In LSCC tissues and cell lines (Tu212, SCC-2 and SCC-40), circFLNAs were found to be up-regulated and sponge miR-486-3p [93]. The results also showed that miR-486-3p reduced LSCC cell migration by negative moderation of FLNA protein level and its level of expression was associated with lymph node metastasis [93]. These outcomes recommend that miR-486-3p have an important role in LSCC migration [93].
7. miR-486-5p role in non-oncological phenotypes
7.1. Metabolic syndrome (MetS)
MetS is a cluster of various interrelated cardiovascular (CV) and cerebrovascular disease risk factors including visceral obesity, insulin resistance (IR) and elevated blood pressure (BP) [95]. It was reported that the circulating levels of muscle-enriched miR-486-5p is declined in acute and chronic exercises due to metabolic changes throughout exercise and adaptation persuaded by training [96]. Another study reported the elevation of serum levels of miR-486-5p in metabolic syndrome Egyptian male patients draws a hypothesis that it could be used as an early biomarker for the diagnosis and prognosis of metabolic syndrome as previously mentioned [49]. miR-486-5p was found to be up-regulated in the plasma of patients having visceral obesity [49]. A high carbohydrate diet which is a cause for visceral obesity induces the expression of miR-486-5p [49]. On the molecular level, FOXO1 is an insulin mediator and was found to be targeted by miR-486-5p [81]. Thus upon connecting the lines, miR-486-5p could be used as a biomarker to screen obesity in children and thus reducing the risk of having diabetes in adulthood [49].
7.2. Intervertebral disc degeneration (IDD)
One of the most common causes of lower back pain is IDD, which contributes to disability and social burden [97]. Recently, IDD therapies have been mainly aimed at alleviating pain symptoms, which only give temporary advantages rather than permanent cure [97]. Some factors have been attributed to the pathogenesis of IDD, including apoptosis of nucleus pulposus (NP) cells (Inner core of vertebral disc), loss of extracellular matrix and miRNAs dysfunction [98]. miR-486-5p expression was found to be down-regulated and FOXO1 was up-regulated in NP cells [62]. FOXO1 stimulates inflammatory cytokines and extracellular matrix degradation [99]. Ectopic expression of miR-486-5p leads to FOXO1 inhibition [99]. Consequently, miR-486-5p could lead to prevention of inflammatory responses, suppression of matrix degradation and apoptosis of NP Cells and thus radically alleviating IDD [99].
7.3. Coronary artery disease (CAD)
Atherosclerosis is considered an inflammatory disease caused and/or worsened by lipid metabolic disorders [100]. The development of atherosclerotic plaque in the coronary artery wall results in CAD [101]. miR-486-5p expression was found to be highly up-regulated in CAD patients [101]. Also, miR-486-5p expression was found to be elevated in a similar pattern as HDL2 [101]. Thus this study suggests that measurement of miR-486-5p along with some lipid-related biomarkers might be a tool in differentiating between stable and vulnerable CAD patients [101].
7.4. Cystic fibrosis (CF)
CF is a genetic disease caused by mutations in the gene of cystic fibrosis conductance regulator (CFTR) [102,103]. The Caucasian population's most prominent lethal genetic disease is the CF [102]. A significant clinical problem remains the phenotypic heterogeneity of patients with the same CFTR genotype [103,104]. This has contributed to an ongoing research for new molecular drivers important to CF pathophysiology that could be an interest as biomarkers or therapeutic tools [105]. High throughput microarray was used to identify Extracellular miRNAs (EC miRNAs) in plasma of CF patients to be used as diagnostic markers [103]. miR-486-5p was the most deregulated EC miRNA differentially expressed in CF patients' plasma samples compared to healthy controls thus verifying its potential to act as a CF-associated biomarker [103].
7.5. Congenital heart diseases
It was found that miR-486-5p is elevated in children with cyanotic as well as acyanotic heart diseases and increases the differentiation of CD34 + cells (upstream of hematopoietic differentiation) [106,107]. Thus, increased expression of miR-486-5p is thought to be a compensatory mechanism by raising circulating hemocytes to compensate for chronic tissue hypoxia [106].
8. miR-486-3p role in non-oncological phenotypes
8.1. Heart diseases
As previously described the high stability of miRNAs promotes its usage as potential diagnostic and prognostic tools in several contexts [108]. miR-486-3p was reported to act as a promising biomarker in acute coronary syndrome [109]. It could discriminate between patients with stable ischemic heart disease and patients with three months of ST elevated acute myocardium infarction [110].
8.2. Parkinson's disease (PD)
PD mainly affects the dopamine secreting neurons. Besides, it is characterized by the presence of Lewy bodies (accumulation of abnormal alpha-synuclein) [111]. miR-486-3p was found to be less expressed in PD patients, while Sirtuin 2 (SIRT2) mRNA was up-regulated in PD patients [112]. Where SIRT2 is a target gene for miR-486-3p, SIRT2 elevation was reported to deteriorate the motor injury and its inhibition reduces alpha-synuclein accumulation in U87 and SH-SY5Y cells [112]. Thus the overexpression of miR-486-3p inhibits SIRT2, consequently reducing alpha-synuclein induced neurotoxicity in PD [112].
8.3. Autism
Autism is a neurodevelopmental disorder characterized by 3 characteristics in the Mental Disorders Diagnostic and Statistical Manual 5 (DSM-5): Loss of social communication, Limited interest, and Repetitive behavior [113]. AT-rich interaction domain 1B (ARID1B) is a mutated gene that is an autism spectrum disorder, also codes a factor for chromatin remodeling [114]. It was believed that miR-486-3p has an important role in autism, where miR-486-3p inhibition leads to an increase in the mRNA and protein levels of ARID1B in SH-SY5 cell line [115]. Thus miR-486-3p/ARIDIB axis acts as a revolutionizing pathway alleviating a vicious circuit of behavior disorders experienced by the autistic patients [116].
8.4. Recurrent miscarriage (RM)
miR-486-3p expression was found to be reduced in patients experiencing RM [45]. However, in the peri-implantation period, miR-486-3p expression is significantly high directly correlated with the implantation capacity [45]. Thus upon miR-486-3p repression, implantation of embryos is affected and thus RM is directly associated with miR-486-3p abnormal expression [45].
8.5. Deficiencies in fetal hemoglobin synthesis
Many miRNAs have been involved in the developmental progression of globin gene expression and in the reactivation of fetal hemoglobin (HbF)-related genes [117]. For adults, variable HbF levels can continue without medical consequences; more elevated HbF levels have a major impact on the incidence of certain hemoglobin disorders, such as sickle cell anemia and β-thalassemia [118]. Modern genetic studies concentrated on natural variation of the level of HbF expression in the human population established BCL11A as a new regulator of hemoglobin switching developmental control and silencing expression of γ-globin in adults [119]. In general, it has been stated that miR-486-3p is abundantly expressed in erythroid cells in the adult hematopoietic system, where miR-486-3p could inhibit erythroid differentiation by regulating TAL1 (SCL), a transcription factor involved in regulating erythroid-specific gene expression [120]. It has been shown that miR-486-3p specifically targets BCL11A mRNA and shows that altering the expression miR-486-3p controls the levels of BCL11A protein modulating the expression of γ-globin [120]. These findings show that miR-486-3p contributes to HbF control during adult erythropoiesis through post-transcriptional inhibition of BCL11A expression as summarized in Fig. 4 [120].
9. Interplay between miR-486-5p/miR-486-3p and other ncRNA molecules
Recently the crosstalk between the ncRNAs has spotted the light onto the importance of investigating the upstream regulators of the upstream regulators (miRNAs) [36]. Competing endogenous RNAs (ceRNAs) is a new concept of networking and complex circuit that has been drawn connecting ncRNAs with each-others. Upon the discovery of other ncRNA molecules such as lncRNAs, circRNAs, pseudoRNAs, those ncRNAs have been positioned in an upstream regulatory role to the miRNAs acting through sponging the miRNAs and consequently de-repressing the miRNAs' targets [60,121,122]. Lately, miR-486-5p has been highlighted to play an essential part of different ceRNAs circuits. For instance, miR-486-5p has been reported to be a direct target for the oncogenic lncRNA, X-inactive specific transcript (XIST). where XIST acts as an oncogenic lncRNA in colorectal cancer cells through sponging the tumor suppressor miR-486-5p and de-repressing (or inducing) miR-486-5p pro-invasive protein target, neuopilin-2 [60]. Lately it has been reported that XIST is not the solo-player in modulating miR-486-5p levels as also the lncRNA-DLGAP1-AS1 has been reported to be an oncogenic lncRNA in HCC that is able of sequestering miR-486-5p in Huh7 cells [123]. On the other hand, it is important to note that our research group has recently highlighted a dominant role of miR-486-5p in trimming the oncogenic activity of the lncRNAs H19 and MALAT1 (unpublished data). Nonetheless, as reported earlier miR-486-3p could also be the prey of circRNAs. In LSCC, circFLNAs were found to act an oncogenic circRNA also through impounding miR-486-3p and suppress its anti-tumor activity [93]. Collectively, those few studies highlight a clear research gap in connecting the lines between different classes of ncRNAs and the assembly of the ceRNAs circuits that when dys-regulated lead to several malignant and non-malignant phenotypes.
10. Conclusion
Unraveling the factors underlying the development and the progression of several deadly or life-threatening diseases such cancer, heart diseases and metabolic disorders remains a research priority for all scientists across the globe. In this review, we highlighted very important players from our point of view which are the ncRNAs. As ncRNAs have the potential to simultaneously affect the disease development, progression, aggression, metastasis in case of malignant diseases and the resistance to the conventional therapeutic approaches in several pathological conditions. miR-486-5p and miR-486-3p have been crystallized as 2 dominant players in several malignant and non-malignant conditions. miR-486-5p and miR-486-3p have been positioned in a very high rank with regards to its diagnostic and prognostic values. Yet still more studies on larger cohort of patients are needed to verify such conclusions. On the other hand, the escalating number of studies focusing on miR-486-5p and/or miR-486-3p in oncology had put them under the spotlight and promotes their therapeutic potential as promising tumor suppressor miRNAs with a positive role on the immune recognition ability and the alleviating of the immune suppressive tumor microenvironment in BC and HCC. Extensive studies are now concerned about implementing different strategies for refilling the miRNAs with tumor suppressive function such as miR-486-5p and miR-486-3p inside the tumors in cancer patients [124]. Fortunately, therapeutic strategies to manipulate miRNAs have advanced from laboratory to bedside in the short time since the discovery of miRNAs, with some productive phase I trials and continuing phase II trials [6]. One of the major challenges in the design of miRNA-based therapies is finding the appropriate miRNAs candidates or miRNAs targets for each type of disease. Other obstacles include developing miRNA delivery vehicles that provide the therapeutic candidate with higher stability and tissue-specific targeting, and limiting possible toxicity and off-target effects [125]. But the bright side of the story is that a very recent study has proved the success of delivering anticancer miRNA-loaded pegylated poly lactic-co-glycolic acid (PLGA) nanoparticles to target regions in deep organs in large animal models [123]. Therefore, this review highlights the promising role of miR-486-5p as therapeutic tools for HCC and BC in particular and hope to inspire scientists to unravel more extensive research about the role of miR-486-3p in different oncological settings to be able to have the full picture and also highlights the importance to focus on the other ceRNAs that could affect the levels and the activity of miR-486-5p and miR-486-3p in the cells.
Contributor Information
R.A. Youness, Email: rana.ahmed-youness@guc.edu.eg, rana.youness21@gmail.com.
M.Z. Gad, Email: Mohamed.gad@guc.edu.eg.
References
- 1.Delihas N. Discovery and characterization of the first non-coding RNA that regulates gene expression, micF RNA: a historical perspective. World J. Biol. Chem. 2015;6(4):272–280. doi: 10.4331/wjbc.v6.i4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18(1):5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno T., Chou M.Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA) Proc. Natl. Acad. Sci. U. S. A. 1984;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters L.S., Storz G. Regulatory RNAs in bacteria. Cell. 2009;136(4):615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien J. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 7.Losko M., Kotlinowski J., Jura J. Long noncoding RNAs in metabolic syndrome related disorders. Mediat. Inflamm. 2016;2016:5365209. doi: 10.1155/2016/5365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zampetaki A., Albrecht A., Steinhofel K. Long non-coding RNA structure and function: is there a link? Front. Physiol. 2018;9:1201. doi: 10.3389/fphys.2018.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L.L., Carmichael G.G. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell Biol. 2010;22(3):357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolha L., Ravnik-Glavac M., Glavac D. Circular RNAs: biogenesis, function, and a role as possible cancer biomarkers. Int J Genomics. 2017;2017:6218353. doi: 10.1155/2017/6218353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12(11):847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z., Xu R., Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr. Metab. 2018;15:68. doi: 10.1186/s12986-018-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skalsky R.L., Cullen B.R. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hombach S., Kretz M. Non-coding RNAs: classification, biology and functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 15.Hayder H. MicroRNAs: crucial regulators of placental development. Reproduction. 2018;155(6):R259–R271. doi: 10.1530/REP-17-0603. [DOI] [PubMed] [Google Scholar]
- 16.Liu N., Olson E.N. MicroRNA regulatory networks in cardiovascular development. Dev. Cell. 2010;18(4):510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issler O., Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015;16(4):201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 18.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 19.Tanzer A., Stadler P.F. Molecular evolution of a microRNA cluster. J. Mol. Biol. 2004;339(2):327–335. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 20.Abd El-Maqsoud N.M. Golgi phosphoprotein-3 and Y-Box-Binding protein-1 are novel markers correlating with poor prognosis in prostate cancer. Clin. Genitourin. Cancer. 2016;14(2):e143–e152. doi: 10.1016/j.clgc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Alarcon C.R. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khvorova A., Reynolds A., Jayasena S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115(2):209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 23.Yang J.S. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(34):15163–15168. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheloufi S. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisarello M.J. MicroRNAs in the cholangiopathies: pathogenesis, diagnosis, and treatment. J. Clin. Med. 2015;4(9):1688–1712. doi: 10.3390/jcm4091688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iorio M.V., Croce C.M. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenouda S.K., Alahari S.K. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28(3–4):369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 29.Dharap A. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillai R.S. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11(12):1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo M.H. Human Argonaute 2 has diverse reaction pathways on target RNAs. Mol. Cell. 2015;59(1):117–124. doi: 10.1016/j.molcel.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Vasudevan S., Steitz J.A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128(6):1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youness R.A. Contradicting interplay between insulin-like growth factor-1 and miR-486-5p in primary NK cells and hepatoma cell lines with a contemporary inhibitory impact on HCC tumor progression. Growth Factors. 2016;34(3–4):128–140. doi: 10.1080/08977194.2016.1200571. [DOI] [PubMed] [Google Scholar]
- 34.Youness R.A. MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol Lett. 2016;12(4):2567–2573. doi: 10.3892/ol.2016.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truesdell S.S. MicroRNA-mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci. Rep. 2012;2:842. doi: 10.1038/srep00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youness R.A., Gad M.Z. Long non-coding RNAs: functional regulatory players in breast cancer. Noncoding RNA Res. 2019;4(1):36–44. doi: 10.1016/j.ncrna.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemal A. Cancer statistics. CA A Cancer J. Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. 2009. [DOI] [PubMed] [Google Scholar]
- 38.Youness R.A. A methoxylated quercetin glycoside harnesses HCC tumor progression in a TP53/miR-15/miR-16 dependent manner. Nat. Prod. Res. 2018:1–6. doi: 10.1080/14786419.2018.1509326. [DOI] [PubMed] [Google Scholar]
- 39.Shaalan Y.M. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018;32(18):2217–2220. doi: 10.1080/14786419.2017.1366478. [DOI] [PubMed] [Google Scholar]
- 40.Rahmoon M.A. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35(2–3):76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 41.Youness R.A. A novel role of sONE/NOS3/NO signaling cascade in mediating hydrogen sulphide bilateral effects on triple negative breast cancer progression. Nitric Oxide. 2018;80:12–23. doi: 10.1016/j.niox.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Awad A.R. An acetylated derivative of vitexin halts MDA-MB-231 cellular progression and improves its immunogenic profile through tuning miR- 20a-MICA/B axis. Nat. Prod. Res. 2019:1–5. doi: 10.1080/14786419.2019.1686372. [DOI] [PubMed] [Google Scholar]
- 43.Calin G.A. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang M. MiR-486 as an effective biomarker in cancer diagnosis and prognosis: a systematic review and meta-analysis. Oncotarget. 2018;9(17):13948–13958. doi: 10.18632/oncotarget.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu Y. Aberrant placental villus expression of miR-486-3p and miR-3074-5p in recurrent miscarriage patients and uterine expression of these MicroRNAs during early pregnancy in mice. Gynecol. Obstet. Investig. 2015 doi: 10.1159/000435879. [DOI] [PubMed] [Google Scholar]
- 46.Peng X., Wei F., Hu X. Long noncoding RNA DLGAP1-AS1 promotes cell proliferation in hepatocellular carcinoma via sequestering miR-486-5p. J. Cell. Biochem. 2019 doi: 10.1002/jcb.29430. [DOI] [PubMed] [Google Scholar]
- 47.He Y. Role of miR-486-5p in regulating renal cell carcinoma cell proliferation and apoptosis via TGF-beta-activated kinase 1. J. Cell. Biochem. 2019;120(3):2954–2963. doi: 10.1002/jcb.26900. [DOI] [PubMed] [Google Scholar]
- 48.Li C. Serum miR-486-5p as a diagnostic marker in cervical cancer: with investigation of potential mechanisms. BMC Canc. 2018;18(1):61. doi: 10.1186/s12885-017-3753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakr Zaki M. Potential role of circulating microRNAs (486-5p, 497, 509-5p and 605) in metabolic syndrome Egyptian male patients. Diabetes Metab Syndr Obes. 2019;12:601–611. doi: 10.2147/DMSO.S187422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zappa C., Mousa S.A. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 2016;5(3):288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin X. MicroRNA-486-5p improves nonsmall-cell lung cancer chemotherapy sensitivity and inhibits epithelial-mesenchymal transition by targeting twinfilin actin binding protein 1. J. Int. Med. Res. 2019 doi: 10.1177/0300060519850739. 300060519850739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar M. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30(7):843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu W. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol. Cell. 2010;38(5):689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S. Expression of miR-486-5p and its signi fi cance in lung squamous cell carcinoma. J. Cell. Biochem. 2019;120(8):13912–13923. doi: 10.1002/jcb.28665. [DOI] [PubMed] [Google Scholar]
- 55.Xu P. Colorectal cancer characterization and therapeutic target prediction based on microRNA expression profile. Sci. Rep. 2016;6:20616. doi: 10.1038/srep20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X. DNA-methylation-mediated silencing of miR-486-5p promotes colorectal cancer proliferation and migration through activation of PLAGL2/IGF2/beta-catenin signal pathways. Cell Death Dis. 2018;9(10):1037. doi: 10.1038/s41419-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjornetro T. An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumour cells as circulating indicators of high-risk rectal cancer. J. Extracell. Vesicles. 2019;8(1):1567219. doi: 10.1080/20013078.2019.1567219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tovar-Camargo O.A., Toden S., Goel A. Exosomal microRNA biomarkers: emerging frontiers in colorectal and other human cancers. Expert Rev. Mol. Diagn. 2016;16(5):553–567. doi: 10.1586/14737159.2016.1156535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hon K.W. Exosomes as potential biomarkers and targeted therapy in colorectal cancer: a mini-review. Front. Pharmacol. 2017;8:583. doi: 10.3389/fphar.2017.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu A., Liu L., Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J. Cell. Physiol. 2019;234(8):13747–13761. doi: 10.1002/jcp.28054. [DOI] [PubMed] [Google Scholar]
- 61.Zhang G. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumour Biol. 2014;35(11):11137–11145. doi: 10.1007/s13277-014-2412-0. [DOI] [PubMed] [Google Scholar]
- 62.Rask L. Differential expression of miR-139, miR-486 and miR-21 in breast cancer patients sub-classified according to lymph node status. Cell. Oncol. 2014;37(3):215–227. doi: 10.1007/s13402-014-0176-6. [DOI] [PubMed] [Google Scholar]
- 63.Li H. MiR-486-5p inhibits IL-22-induced epithelial-mesenchymal transition of breast cancer cell by repressing Dock1. J. Cancer. 2019;10(19):4695–4706. doi: 10.7150/jca.30596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balogh J. Hepatocellular carcinoma: a review. J. Hepatocell. Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J. Clin. Investig. 2010;120(4):1298–1309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen D.Y., Uzzo R.G. Optimal management of localized renal cell carcinoma: surgery, ablation, or active surveillance. J. Natl. Compr. Cancer Netw. 2009;7(6):635–642. doi: 10.6004/jnccn.2009.0044. quiz 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jedeszko C. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent antitumor activity of metronomic oral topotecan with pazopanib. Sci. Transl. Med. 2015;7(282):282ra50. doi: 10.1126/scitranslmed.3010722. [DOI] [PubMed] [Google Scholar]
- 68.Zarrabi K., Fang C., Wu S. New treatment options for metastatic renal cell carcinoma with prior anti-angiogenesis therapy. J. Hematol. Oncol. 2017;10(1):38. doi: 10.1186/s13045-016-0374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H. Expression and prognostic value of miR-486-5p in patients with gastric adenocarcinoma. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang X.P. MicroRNA-486-5p, which is downregulated in hepatocellular carcinoma, suppresses tumor growth by targeting PIK3R1. FEBS J. 2015;282(3):579–594. doi: 10.1111/febs.13167. [DOI] [PubMed] [Google Scholar]
- 71.Fransson S. Stage-dependent expression of PI3K/Aktpathway genes in neuroblastoma. Int. J. Oncol. 2013;42(2):609–616. doi: 10.3892/ijo.2012.1732. [DOI] [PubMed] [Google Scholar]
- 72.Sun C. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Canc. 2015;15:333. doi: 10.1186/s12885-015-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegel R. Cancer statistics, 2014. CA A Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 74.Jiao X. KIAA1199, a target of MicoRNA-486-5p, promotes papillary thyroid cancer invasion by influencing epithelial-mesenchymal transition (EMT) Med. Sci. Monit. 2019;25:6788–6796. doi: 10.12659/MSM.918682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma X. miR-486-5p inhibits cell growth of papillary thyroid carcinoma by targeting fibrillin-1. Biomed. Pharmacother. 2016;80:220–226. doi: 10.1016/j.biopha.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 76.Ma H. Estrogen receptor-mediated miR-486-5p regulation of OLFM4 expression in ovarian cancer. Oncotarget. 2016;7(9):10594–10605. doi: 10.18632/oncotarget.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan C. Oestrogen receptor-mediated expression of Olfactomedin 4 regulates the progression of endometrial adenocarcinoma. J. Cell Mol. Med. 2014;18(5):863–874. doi: 10.1111/jcmm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lightfoot T. Aetiology of childhood leukemia. Bioelectromagnetics. 2005;(Suppl 7):S5–S11. doi: 10.1002/bem.20140. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H. Long non-coding RNA: a new player in cancer. J. Hematol. Oncol. 2013;6:37. doi: 10.1186/1756-8722-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hassan O. Recent updates on the role of microRNAs in prostate cancer. J. Hematol. Oncol. 2012;5:9. doi: 10.1186/1756-8722-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu H. MiR-486-5p inhibits the proliferation of leukemia cells and induces apoptosis through targeting FOXO1. Mol. Cell. Probes. 2019;44:37–43. doi: 10.1016/j.mcp.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura T., Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol. Cell. Endocrinol. 2008;281(1–2):47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y. The miR-486-5p plays a causative role in prostate cancer through negative regulation of multiple tumor suppressor pathways. Oncotarget. 2017;8(42):72835–72846. doi: 10.18632/oncotarget.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jemal A. Global cancer statistics. CA A Cancer J. Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 85.Shiu M.N. Risk factors for leukoplakia and malignant transformation to oral carcinoma: a leukoplakia cohort in Taiwan. Br. J. Canc. 2000;82(11):1871–1874. doi: 10.1054/bjoc.2000.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chou S.T. MicroRNA-486-3p functions as a tumor suppressor in oral cancer by targeting DDR1. J. Exp. Clin. Cancer Res. 2019;38(1):281. doi: 10.1186/s13046-019-1283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Torre L.A., Siegel R.L., Jemal A. Lung cancer statistics. Adv. Exp. Med. Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 88.Wang M. Thrombospondin enhances RANKL-dependent osteoclastogenesis and facilitates lung cancer bone metastasis. Biochem. Pharmacol. 2019;166:23–32. doi: 10.1016/j.bcp.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Torre L.A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol. Biomark. Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 90.Ye H. MiR-486-3p targeting ECM1 represses cell proliferation and metastasis in cervical cancer. Biomed. Pharmacother. 2016;80:109–114. doi: 10.1016/j.biopha.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 91.Steuer C.E. An update on larynx cancer. CA A Cancer J. Clin. 2017;67(1):31–50. doi: 10.3322/caac.21386. [DOI] [PubMed] [Google Scholar]
- 92.Hansen T.B., Kjems J., Damgaard C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73(18):5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 93.Wang J.X. Upregulation of circFLNA contributes to laryngeal squamous cell carcinoma migration by circFLNA-miR-486-3p-FLNA axis. Cancer Cell Int. 2019;19:196. doi: 10.1186/s12935-019-0924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Savoy R.M., Ghosh P.M. The dual role of filamin A in cancer: can't live with (too much of) it, can't live without it. Endocr. Relat. Cancer. 2013;20(6):R341–R356. doi: 10.1530/ERC-13-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernandez-Mendoza J. Impact of the metabolic syndrome on mortality is modified by objective short sleep duration. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.117.005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aoi W. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front. Physiol. 2013;4:80. doi: 10.3389/fphys.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guehring T. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration--an in vivo animal study. Spine. 2005;30(22):2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 98.Zhang W.L. Role of miR-155 in the regulation of MMP-16 expression in intervertebral disc degeneration. J. Orthop. Res. 2017;35(6):1323–1334. doi: 10.1002/jor.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chai X. miR-486-5p inhibits inflammatory response, matrix degradation and apoptosis of nucleus pulposus cells through directly targeting FOXO1 in intervertebral disc degeneration. Cell. Physiol. Biochem. 2019;52(1):109–118. doi: 10.33594/000000008. [DOI] [PubMed] [Google Scholar]
- 100.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 101.Niculescu L.S. MiR-486 and miR-92a identified in circulating HDL discriminate between stable and vulnerable coronary artery disease patients. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bardin P. Emerging microRNA therapeutic approaches for cystic fibrosis. Front. Pharmacol. 2018;9:1113. doi: 10.3389/fphar.2018.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ideozu J.E. Microarray profiling identifies extracellular circulating miRNAs dysregulated in cystic fibrosis. Sci. Rep. 2019;9(1):15483. doi: 10.1038/s41598-019-51890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terlizzi V. Clinical expression of cystic fibrosis in a large cohort of Italian siblings. BMC Pulm. Med. 2018;18(1):196. doi: 10.1186/s12890-018-0766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ideozu J.E. Transcriptome profiling and molecular therapeutic advances in cystic fibrosis: recent insights. Genes. 2019;10(3) doi: 10.3390/genes10030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mukai N. Potential contribution of erythrocyte microRNA to secondary erythrocytosis and thrombocytopenia in congenital heart disease. Pediatr. Res. 2018;83(4):866–873. doi: 10.1038/pr.2017.327. [DOI] [PubMed] [Google Scholar]
- 107.Wang L.S. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood. 2015;125(8):1302–1313. doi: 10.1182/blood-2014-06-581926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 109.Giles K.M. miRNA-7-5p inhibits melanoma cell migration and invasion. Biochem. Biophys. Res. Commun. 2013;430(2):706–710. doi: 10.1016/j.bbrc.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 110.Wei T. MicroRNA 486-3P as a stability marker in acute coronary syndrome. Biosci. Rep. 2016;36(3) doi: 10.1042/BSR20160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y. miR-486-3p influences the neurotoxicity of a-synuclein by targeting the SIRT2 gene and the polymorphisms at target sites contributing to Parkinson's disease. Cell. Physiol. Biochem. 2018;51(6):2732–2745. doi: 10.1159/000495963. [DOI] [PubMed] [Google Scholar]
- 113.Shibutani M. Arid1b haploinsufficiency causes abnormal brain gene expression and autism-related behaviors in mice. Int. J. Mol. Sci. 2017;18(9) doi: 10.3390/ijms18091872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D'Gama A.M. Targeted DNA sequencing from autism spectrum disorder brains implicates multiple genetic mechanisms. Neuron. 2015;88(5):910–917. doi: 10.1016/j.neuron.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu D. Serum miRNA expression profiling reveals miR-486-3p may play a significant role in the development of autism by targeting ARID1B. Neuroreport. 2018;29(17):1431–1436. doi: 10.1097/WNR.0000000000001107. [DOI] [PubMed] [Google Scholar]
- 116.Hoyer J. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am. J. Hum. Genet. 2012;90(3):565–572. doi: 10.1016/j.ajhg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Byon J.C., Papayannopoulou T. MicroRNAs: allies or foes in erythropoiesis? J. Cell. Physiol. 2012;227(1):7–13. doi: 10.1002/jcp.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bauer D.E., Kamran S.C., Orkin S.H. Reawakening fetal hemoglobin: prospects for new therapies for the beta-globin disorders. Blood. 2012;120(15):2945–2953. doi: 10.1182/blood-2012-06-292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sankaran V.G. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460(7259):1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lulli V. MicroRNA-486-3p regulates gamma-globin expression in human erythroid cells by directly modulating BCL11A. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han D. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget. 2016;7(16):22159–22173. doi: 10.18632/oncotarget.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Imig J. miR-CLIP capture of a miRNA targetome uncovers a lincRNA H19-miR-106a interaction. Nat. Chem. Biol. 2015;11(2):107–114. doi: 10.1038/nchembio.1713. [DOI] [PubMed] [Google Scholar]
- 123.Di Ianni T. Ultrasound/microbubble-mediated targeted delivery of anticancer microRNA-loaded nanoparticles to deep tissues in pigs. J. Control. Release. 2019;309:1–10. doi: 10.1016/j.jconrel.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chipman L.B., Pasquinelli A.E. miRNA targeting: growing beyond the seed. Trends Genet. 2019;35(3):215–222. doi: 10.1016/j.tig.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z., Rana T.M. Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]