Summary
Both artificial photosystems and natural photosynthesis have not reached their full potential for the sustainable conversion of solar energy into specific chemicals. A promising approach is hybrid photosynthesis combining efficient, non-toxic, and low-cost abiotic photocatalysts capable of water splitting with metabolically versatile non-photosynthetic microbes. Here, we report the development of a water-splitting enzymatic photocatalyst made of graphitic carbon nitride (g-C3N4) coupled with H2O2-degrading catalase and its utilization for hybrid photosynthesis with the non-photosynthetic bacterium Ralstonia eutropha for bioplastic production. The g-C3N4-catalase system has an excellent solar-to-hydrogen efficiency of 3.4% with a H2 evolution rate up to 55.72 μmol h−1 while evolving O2 stoichiometrically. The hybrid photosynthesis system built with the water-spitting g-C3N4-catalase photocatalyst doubles the production of the bioplastic polyhydroxybutyrate by R. eutropha from CO2 and increases it by 1.84-fold from fructose. These results illustrate how synergy between abiotic non-metallic photocatalyst, enzyme, and bacteria can augment solar-to-multicarbon chemical conversion.
Subject Areas: Catalysis, Microbial Biotechnology, Energy Materials
Graphical Abstract
Highlights
-
•
H2O2-degrading enzymes from R. eutropha enable visible-light water splitting by C3N4
-
•
C3N4 coupled with bovine catalase has a solar-to-hydrogen efficiency of 3.4%
-
•
C3N4-catalase increases CO2 conversion into bioplastic under light by R. eutropha
-
•
Heterotrophic bioplastic production by R. eutropha is also improved by C3N4-catalase
Catalysis; Microbial Biotechnology; Energy Materials
Introduction
Multiple technologies are being developed to convert and store solar energy, which is not only ubiquitous, sustainable, and clean but also intermittent. Artificial water-splitting photosystems have attracted interest mainly for the production of storable H2 from sunlight (Chen et al., 2017). The three main types of abiotic photosystems for H2 production are photovoltaic cell coupled with electrolyzer (PV-E), photoelectrochemical cell (PEC), and particulate photocatalyst (PC) (Chen et al., 2017). PV-E has shown the highest solar-to-hydrogen (STH) conversion efficiency, but H2 production cost by PV-E is not competitive with already established industrial processes. Between PEC and PC, techno-economical assessments conclude that PC would have a lower H2 production cost, as little as $1.60 kg−1 when similar STH efficiencies are considered (Pinaud et al., 2013). Compared with PV-E and PEC, PC is a simpler system more suitable for low-cost scaling up. However, STH efficiencies reported until now for PC and PEC are lower than those reported for PV-E (Chen et al., 2017, Prévot and Sivula, 2013). Another important challenge is that, with scarce exceptions, the most efficient PC systems are made with expensive rare-earth elements (Liu et al., 2015a).
A promising solar technology is hybrid photosynthesis where an abiotic photosystem converts sunlight into chemical energy and transfers it to microbes for the production of multicarbon chemicals (Zhang and Tremblay, 2017). In these systems, chemical energy from the abiotic photosystem can drive or accelerate either autotrophic or heterotrophic bioproduction processes (Xu et al., 2019, Zhang et al., 2018, Liu et al., 2015b, Liu et al., 2016, Sakimoto et al., 2016a, Sakimoto et al., 2016b, Guo et al., 2018, Nichols et al., 2015, Ye et al., 2019). In its autotrophic configuration where the greenhouse gas CO2 is the carbon source, hybrid photosynthesis will mirror natural photosynthesis carried out by living organisms if electrons come from water splitting. Hybrid photosynthesis is being pursued owing to the abiotic photosystems that are significantly more efficient at converting solar energy into chemical energy than natural photosynthesis. Thus, coupling abiotic photosystems with productive microbial catalysts may outcompete natural photosynthesis in terms of solar-to-specific chemicals efficiency (Zhang, 2015, Tremblay et al., 2017, Claassens et al., 2016).
Graphitic carbon nitride (g-C3N4) is a metal-free low-cost PC, easy to fabricate, and made of earth-abundant elements with a band gap of 2.7 eV that can convert solar energy into H2 (Liu et al., 2015a, Wang et al., 2009, Yan et al., 2010, Rao et al., 2019). Other applications of g-C3N4 include light-driven pollutant remediation and CO2 reduction into other C1 compounds (Hu et al., 2014, Ye et al., 2015, Zhang et al., 2015, Mishra et al., 2019a, Reddy et al., 2019). Recently, a hybrid photosynthesis system was developed with g-C3N4 where the production of the bioplastic polyhydroxybutyrate (PHB) from fructose by Ralstonia eutropha H16 was enhanced under light (Xu et al., 2019). R. eutropha is an aerobic bacterium also known as Cupriviadus necator that can grow heterotrophically or autotrophically with CO2 as carbon source and H2 or an electrode as electron donor (Liu et al., 2016, Tanaka et al., 1995, Li et al., 2012, Volodina et al., 2016). One of the main appeals of R. eutropha as a microbial factory is that it stores intracellularly large quantity of PHB when carbon is in excess (Tanaka et al., 1995). R. eutropha can also be genetically engineered to redirect metabolic fluxes toward the synthesis of a wide range of valuable chemicals (Li et al., 2012, Bi et al., 2013, Chakravarty and Brigham, 2018, Grousseau et al., 2014, Lu et al., 2012, Müller et al., 2013).
Most pristine g-C3N4 materials without modifications are unable to evolve H2 in the absence of sacrificial electron donors (SED) such as triethanolamine (TEOA), methanol, or lactic acid (Wang et al., 2017). Photocatalytic water splitting by g-C3N4 and resulting H2 and O2 evolution is theoretically possible but is hindered by sluggish kinetics (Zhang et al., 2016, Mishra et al., 2019b). Recently, a study by Fang et al. described a g-C3N4 photocatalyst prepared by an alternative method from a cocrystal precursor capable of using H2O as electron donor for H2 evolution (Fang et al., 2019). The authors attributed the water-splitting capacity of their PC to improved generation and mobility of the photoexcited charge carriers. One of the main obstacles for light-driven water splitting by g-C3N4 involves the O2 evolution reaction, which necessitates the transfer of four electrons and four protons for the formation of a double bond between two oxygen molecules (2H2O → 4H+ + 4e− + O2, 1.23 eV). With g-C3N4, the four-electron reaction for O2 evolution has been shown to be outcompeted kinetically by a two-electron reaction wherein H2O is oxidized into H2O2 (2H2O → 2H+ + 2e− + H2O2, 1.78 eV) (Liu et al., 2012). This phenomenon inhibits O2 and H2 evolution reactions because H2O2 deactivates g-C3N4 by staying attached to it and by occupying active surface sites. A solution reported in the past to overcome H2O2 poisoning and complete O2 and H2 evolution is to modify g-C3N4 PC with carbon nanodots (CDots), which have good catalytic activity for H2O2 degradation (Liu et al., 2015a).
In a previous study, we showed that no SED was required for unmodified g-C3N4 to augment heterotrophic PHB production by R. eutropha under light (Xu et al., 2019). Possible explanations are that R. eutropha modifies the growth medium surrounding g-C3N4 or its surface in a way that improves g-C3N4's photocatalytic performance and/or enables water splitting. To understand the mechanisms involved, we investigated the photocatalytic performance and behavior of g-C3N4 suspended in cell-free spent medium from R. eutropha. The results indicated that H2O2-degrading enzymes present in the spent medium were degrading the H2O2 generated by g-C3N4 under light, and thus enabled photocatalytic water splitting. Based on these observations, we developed a novel PC system coupling g-C3N4 with pure bovine liver catalase, which is an enzyme specialized in the degradation of H2O2 into O2 and H2O. This novel g-C3N4-catalase PC system was employed to build a hybrid photosynthesis system where the production of PHB from either CO2 or fructose by R. eutropha was driven by water-splitting photocatalysis.
Results
H2O2-Degrading Enzymes from the Spent Medium of R. eutropha and Faster Hydrogen Evolution by Unmodified g-C3N4
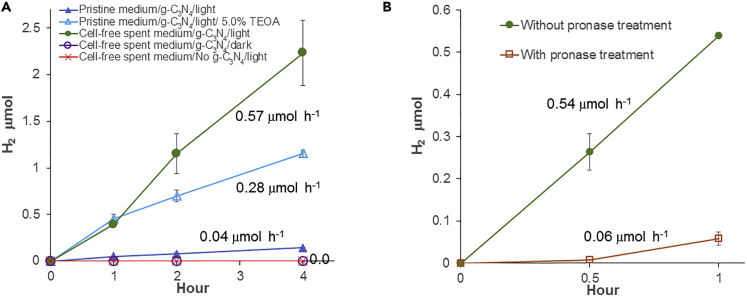
To establish how g-C3N4 was active under light when coupled with R. eutropha for PHB production in the absence of SEDs (Xu et al., 2019), fructose-grown R. eutropha cells were removed from the growth medium by centrifugation, g-C3N4 (Figures S1 and S2) was added to the cell-free spent medium, and light-driven H2 evolution was monitored under light-emitting diode (LED) light (Figure 1A). g-C3N4 in the cell-free spent medium had a H2 evolution rate (HER) of 0.57 ± 0.05 μmol h−1 (57.4 ± 4.7 μmol g−1 h−1), which was 16.4 times higher than that of pristine growth medium and 2.1 times higher than that of pristine growth medium with 0.5% TEOA. Next, cell-free spent medium was treated with the protease mixture pronase to establish if the compound responsible for improved HER was a protein or a group of proteins (Figure 1B). With pronase-treated cell-free spent medium, HER was significantly reduced to 0.06 ± 0.01 μmol h−1 (5.8 ± 1.2 μmol g−1 h−1), which is comparable to HER observed with pristine medium. Thus, the compound(s) in cell-free spent medium accelerating light-driven HER by g-C3N4 is proteinaceous.
Figure 1.
Light-Driven H2 Evolution by g-C3N4 in R. eutropha Spent Medium
(A) H2 evolution by g-C3N4 in pristine medium and cell-free spent medium under light. Where indicated, 5.0% TEOA was added.
(B) H2 evolution by g-C3N4 in cell-free spent medium treated with/without pronase under light. Each curve is the mean and standard deviation of at least three replicates.
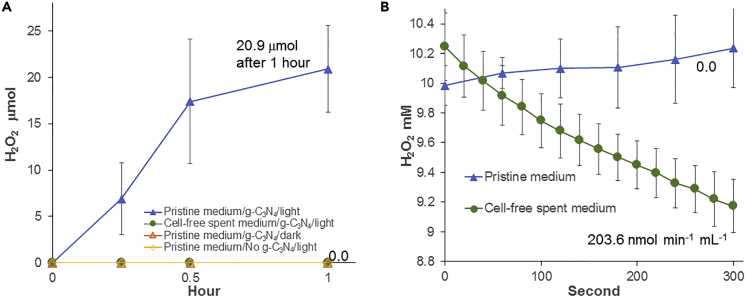
Besides HER, concentrations of the O2 evolution inhibitor H2O2 were also monitored when g-C3N4 was exposed to light in pristine growth medium and cell-free spent medium (Figure 2A). In pristine medium containing g-C3N4, H2O2 accumulated in a light-dependent manner up to 20.9 ± 4.7 μmol after 1 h. In comparison, no H2O2 accumulated with cell-free spent medium, which probably explained why HER was significantly higher under this condition.
Figure 2.
H2O2 Generation by g-C3N4 and Degradation by R. eutropha Spent Medium
(A) H2O2 generation by g-C3N4.
(B) H2O2-degrading activity in R. eutropha spent medium amended with 10 mM H2O2. Each curve is the mean and standard deviation of at least three replicates.
Many organisms including bacteria have an enzymatic mechanism to defend themselves against H2O2, which can cause extensive oxidative damages (Mishra and Imlay, 2012). Two enzymes ubiquitous in aerobic organisms are responsible for biological H2O2 degradation: the catalase and the peroxidase. Catalases degrade H2O2 according to the following equation: 2 H2O2 → 2 H2O + O2 (Mishra and Imlay, 2012). Peroxidases require a physiological electron donor such as thioredoxins, glutathione NAD(P)H, or cytochrome c to reduce H2O2 according to the following equation: RH2 + H2O2 → R + 2H2O. The genome of R. eutropha H16 comprises at least nine genes coding for catalases and peroxidases (Table S1) (Pohlmann et al., 2006). Results presented here strongly suggest that some of these enzymes are located in the cell-free spent medium of R. eutropha, either because of excretion or cell lysis, where they degraded H2O2 and increased HER by g-C3N4. In fact, when exogenous H2O2 was added to cell-free spent medium, it was degraded at a rate of 203.6 ± 16.7 nmol min−1 mL−1 or 9.1 ± 0.3 μmol min−1 per mg protein in the spent medium, whereas no degradation was observed in pristine medium (Figure 2B).
A Water-Splitting Photocatalytic System Coupling g-C3N4 and Pure Catalase
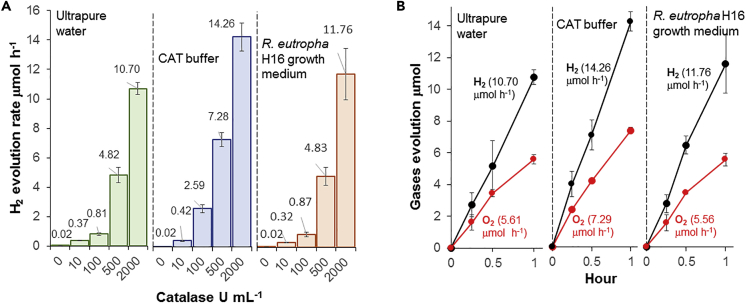
To investigate further the impact of H2O2-degrading enzymes on the photocatalytic activity of g-C3N4, the performance of g-C3N4 loaded with different quantity of bovine liver catalase was evaluated in ultrapure water, in catalase buffer (CAT buffer; 50 mM KH2PO4 pH 7.4), and in pristine R. eutropha growth medium (Figures 3 and S3–S5). Under LED light, increasing the quantity of catalase within the three types of suspension resulted in significant H2 production by g-C3N4 up to 14.26 ± 1.00 μmol h−1 (1,426.4 ± 95.9 μmol g−1 h−1) with 2,000 U mL−1 catalase in CAT buffer. H2 production was accompanied by nearly stoichiometric O2 evolution of 7.29 ± 0.19 μmol h−1 (729.5 ± 18.5 μmol g−1 h−1) demonstrating light-driven water splitting by the enzymatic PC system (Figure 3B). In the absence of catalase, O2 evolution was not detected in ultrapure water, CAT buffer, or growth medium, indicating that the presence of the enzyme not only accelerated H2 evolution but also enabled O2 evolution. Besides O2 and H2 evolution, H2O2 accumulation was also monitored with the PC system coupling g-C3N4 with 2,000 U ml−1 catalase under light. With all three different suspensions, no H2O2 was detected, showing that the catalase degraded its substrate and prevented the buildup of this reactive oxygen species.
Figure 3.
Light-Driven Water-Splitting Activity of the g-C3N4-Catalase System
(A) Impact of bovine liver catalase concentration on H2 evolution rate by g-C3N4 suspended in ultrapure water, CAT buffer, or R. eutropha growth medium under 4,200-lux LED light.
(B) H2 and O2 evolution under 4,200-lux LED light over time by g-C3N4 with 2,000 U mL−1 catalase. Each curve and bar is the average of at least three replicates with standard deviation.
The addition of 100 U ml−1 superoxide dismutase (SOD) to g-C3N4 instead of catalase did not improve H2 production with a rate of 0.01 ± 0.00 μmol h−1 (1.5 ± 0.2 and 1.2 ± 0.2 μmol g−1 h−1) in both ultrapure water and growth medium (Figure S6). Under similar light condition, pure g-C3N4 had a H2 production rate of 0.02 μmol h−1 (2.0 μmol g−1 h−1) in both ultrapure water and growth medium (Figure 3A). At the same concentration of 100 U ml−1, catalase already significantly improved H2 evolution by g-C3N4 to 0.81 ± 0.09 and 0.87 ± 0.15 μmol h−1 (81.2 ± 8.6 and 87.1 ± 14.5 μmol g−1 h−1) in ultrapure water and growth medium, respectively. Like the catalase, SOD is an enzyme participating in living cells' defense against oxidative stress (Johnson and Hug, 2019). SOD catalyzes the conversion of superoxide into O2 and H2O2. These results clearly suggest that the increased H2 production observed with the g-C3N4-catalase PC system was caused by the enzymatic activity of the catalase and not by an unspecific effect related to the addition of enzymes or proteins independently of their capacity to degrade H2O2.
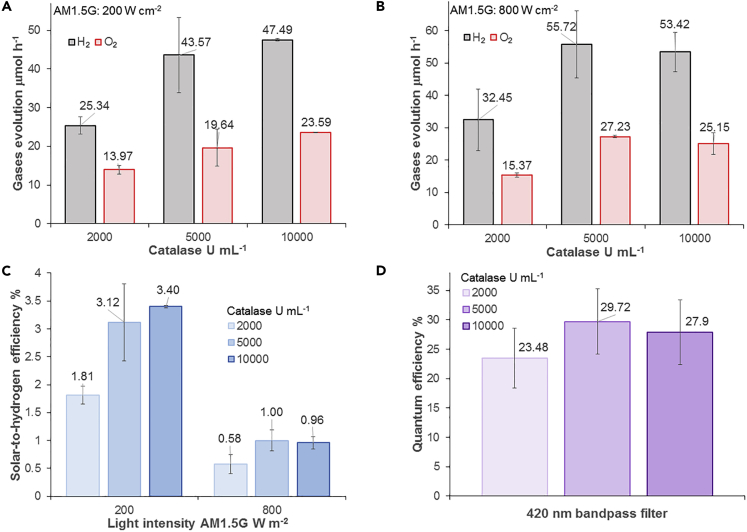
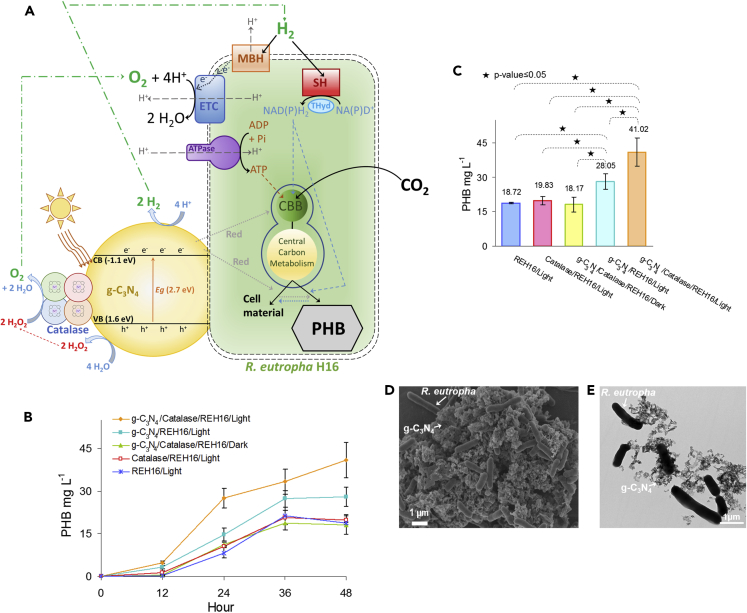
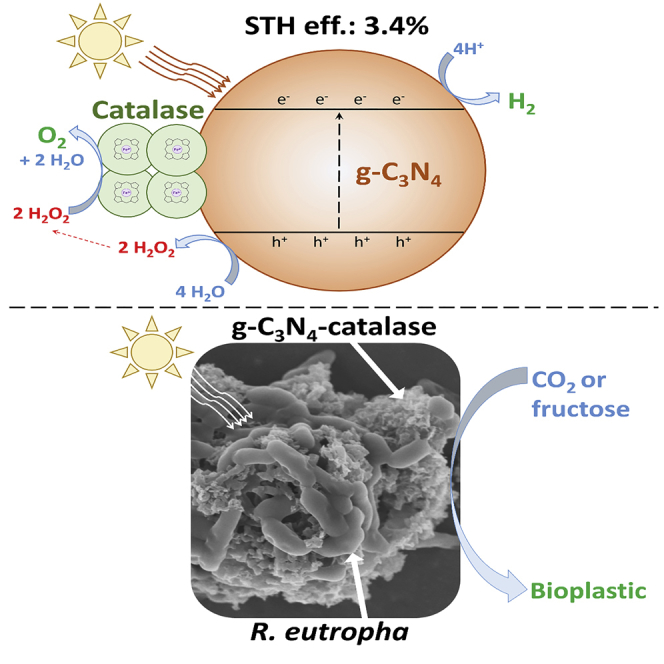
The energetic efficiency of the novel g-C3N4-catalase system was evaluated with 2,000, 5,000, and 10,000 U mL−1 bovine liver catalase in CAT buffer under an AM 1.5G solar simulator with a light intensity of 200 or 800 W m−2 (ca. 0.2 or 0.8 sun) (Figure 4). The highest STH efficiency of 3.40% ± 0.2% was recorded with 10,000 U mL−1 catalase at a light intensity of 200 W m−2. For this experiment, the irradiated surface was 4.6 cm2 for a total input energy in 1 h of 331.2 J. H2 production was 47.49 ± 0.32 μmol, which amounted to 11.26 J. At 5,000 U mL−1 catalase, the STH efficiency was statistically similar at 3.12% ± 0.69%, but it became 1.88 times lower when catalase concentration was reduced to 2,000 U mL−1. Increasing light intensity to 800 W m−2 augmented HER while reducing STH efficiency. The best PC system at 800 W m−2 had a catalase concentration of 5,000 U mL−1 with an STH efficiency of 1.00% ± 0.19% and a normalized HER of 55.72 ± 10.40 μmol h−1 (1,857.16 ± 346.72 μmol g−1 h−1). O2 evolution for all tested conditions was nearly stoichiometric (Figures 4A and 4B). Quantum efficiency at a wavelength of 420 nm, which is in the optimal absorption spectrum for g-C3N4, was 23.48% ± 5.06% with 2,000 U mL−1 catalase, 29.72% ± 5.53% with 5,000 U mL−1 catalase, and 27.90% ± 5.50% with 10,000 U mL−1 catalase (Figure 4D). STH efficiencies at low light intensity for the g-C3N4-catalase PC system described here are higher than CDots-C3N4 PC, which is, with an efficiency of 2%, one of the best reported water-splitting photocatalysts (Table S2) (Liu et al., 2015a). In addition, the g-C3N4-based PC system was stable when tested with 10,000 U mL−1 catalase under a light intensity of 200 W m−2 (Figure S7). After three cycles, H2 and O2 evolution rates were comparable to the initial rates.
Figure 4.
H2 Evolution and O2 Evolution by g-C3N4-Catalase under an AM1.5G
(A) Gases evolution at a light intensity of 200 W m-2. (B) Gases evolution at a light intensity of 800 W m-2.
(C and D) (C) Solar-to-hydrogen efficiency and (D) quantum efficiency at 420 nm for the g-C3N4-catalase PC system. Each curve and bar is the average of at least three replicates with standard deviation.
Hybrid Photosynthesis with g-C3N4-Catalase and R. eutropha for CO2 Reduction
Autotrophic hybrid photosynthesis apparatuses developed until now rely on metallic inorganic PC harvesting visible light to drive biological CO2 reduction by non-photosynthetic bacteria (Table S3) (Zhang and Tremblay, 2017, Zhang et al., 2018, Sakimoto et al., 2016a, Ye et al., 2019). Most of these systems photogenerated the reducing equivalents ultimately transferred to the autotrophic microbe by the oxidation of an SED such as cysteine. In these circumstances, the requirement for an SED diminishes significantly the sustainability prospect of PC-based bioprocesses. Only one hybrid photosynthesis system reported until now relies on water splitting and not on an SED (Sakimoto et al., 2016b). This tandem “Z-scheme” platform included potentially toxic TiO2 combined with the cocatalyst Mn(II) phthalocyanine photo-oxidizing water and transferring the electrons indirectly to CdS via a cysteine shuttle. CdS then photogenerated reducing equivalents, which were carried out to the acetogen Moorella thermoacetica for the reduction of CO2 into acetate.
To establish if the water-splitting g-C3N4-catalase PC system developed here could provide energy for autotrophic bioproduction process, it was coupled with R. eutropha growing autotrophically under a N2:H2:CO2:O2 atmosphere and synthesis of PHB was monitored (Figure 5A). With g-C3N4-catalase, PHB production from CO2 under light reached 41.02 ± 6.22 mg L−1 after 48 h compared with 18.72 ± 0.31mg L−1 for a light-exposed R. eutropha culture grown without the PC system (Figures 5B and 5C). Both scanning electron microscopy and transmission electron microscopy images showed the formation of large aggregates under autotrophic conditions with direct physical contact between g-C3N4 particles and R. eutropha cells (Figures 5D, 5E, and S8). Bovine liver catalase was not required for g-C3N4 to improve autotrophic PHB production, but the addition of the enzyme still had a significant positive effect. g-C3N4 without catalase augmented PHB production from CO2 by R. eutropha to 28.05 ± 3.37 mg L−1 under light. This can be explained by native H2O2-degrading enzymes possibly released by R. eutropha cells, which enables water splitting by g-C3N4, but not to the same level as with bovine liver catalase.
Figure 5.
Autotrophic Hybrid Photosynthesis with g-C3N4-Catalase and R. eutropha
(A) Schematic diagram of hybrid photosynthesis with g-C3N4-catalase and R. eutropha producing PHB from CO2. Water-splitting g-C3N4-catalase could augment PHB production either by increasing the quantity of H2 and/or O2 available for bacterial metabolism or by transferring unknown reducing equivalents (red) to R. eutropha. CB, conduction band; VB, valence band; ETC, electron transport chain; MBH, membrane-bound hydrogenase; SH, soluble hydrogenase; Thyd, NAD(P)+ transhydrogenase; CBB, Calvin-Benson-Bassham cycle (Brigham et al., 2013).
(B and C) (B) PHB production from CO2 over time and (C) PHB concentration after 48 h. REH16, R. eutropha H16.
(D and E) (D) Scanning and (E) transmission electron micrographs of R. eutropha grown with g-C3N4-catalase under autotrophic condition. R. eutropha cultures were grown at 30 ⁰C in minimal medium under a N2:H2:O2:CO2 (49:37:7:7) atmosphere in the presence or absence 0.5 g g-C3N4 and/or 5,000 U mL−1 bovine liver catalase. Where indicated, cultures were incubated under 4,200-lux LED light. Each curve and bar is the mean of at least three replicates with standard deviation.
Production improvement observed with g-C3N4-catalase was light-dependent. R. eutropha with g-C3N4-catalase in the dark produced only 18.17 ± 3.29 mg L−1 PHB after 48 h (Figures 5B and 5C). The addition of 5,000 U mL−1 catalase alone without g-C3N4 did not improve PHB production, which demonstrates that R. eutropha cells could not use the enzyme as substrate for their metabolism.
The increase of PHB production observed with the photocatalytic system during autotrophic growth of R. eutropha may have three possible explanations (Figure 5A). First, H2 initially provided in the gas phase to drive bacterial growth may become scarcer after biological oxidation. g-C3N4-catalase generates supplementary H2 used by R. eutropha to keep its metabolism active, which results in additional PHB production. Second, O2 may also become rarer in the gas phase after bacterial reduction during growth. While generating reducing equivalents, g-C3N4-catalase also evolves additional O2 by water splitting that can be used by the bacterial cells as electron acceptor. Third, the photocatalytic system may transfer electrons directly to R. eutropha or generate reducing equivalents under a different form than H2 that are preferentially used by the bacteria.
Heterotrophic Production of PHB from Fructose with g-C3N4-Catalase
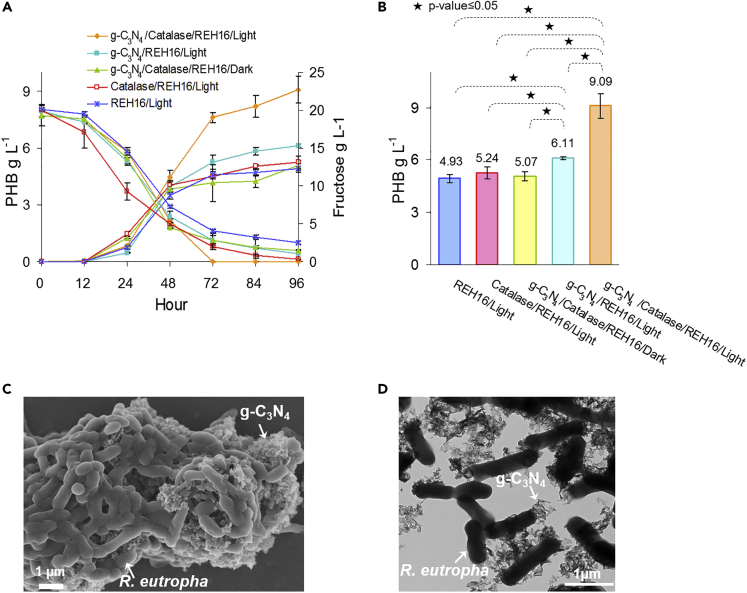
The impact of the g-C3N4-catalase system on heterotrophic production of PHB from fructose by R. eutropha was also evaluated (Figure S9). As reported previously (Xu et al., 2019), the addition of g-C3N4 alone is sufficient to improve PHB production from fructose under light with a 124% increase compared with an R. eutropha culture without g-C3N4 (Figures 6A, 6B, and S10). The only other example where heterotrophic bioproduction was enhanced by a light-harvesting photocatalyst coupled the rare element indium phosphide with a Saccharomyces cerevisiae strain producing shikimate from glucose (Guo et al., 2018). When the g-C3N4-catalase system developed here was combined with R. eutropha, PHB production reached 9.09 ± 0.71 g L−1 after 96 h of growth under light, which was 1.84-fold higher compared with heterotrophic R. eutropha without the PC system. Large aggregates with direct physical contact between g-C3N4 and R. eutropha cells were also observed in the heterotrophic mode (Figures 6C and 6D). Fructose-grown R. eutropha control with catalase only and no g-C3N4 under light as well as a control grown in the dark with the g-C3N4-catalase system had PHB production comparable to R. eutropha without g-C3N4 and catalase (Figures 6A and 6B).
Figure 6.
Heterotrophic Hybrid Photosynthesis with g-C3N4-Catalase and R. eutropha
(A and B) (A) PHB production and fructose consumption over time and (B) PHB concentration after 96 h.
(C and D) (C) Scanning and (D) transmission electron micrographs of R. eutropha grown with g-C3N4-catalase under heterotrophic conditions. R. eutropha cultures were grown at 30 ⁰C in minimal medium with 20 g L−1 fructose in the presence or absence 0.5 g g-C3N4 and/or 5,000 U mL−1 bovine liver catalase. Where indicated, cultures were incubated under 4,200-lux LED light. Each curve and bar is the mean of at least three replicates with standard deviation.
Charge Transfer at the Interface in the Hybrid Photosynthesis System
To gain additional insights into the mechanisms involved in the photocatalytic activity of the autotrophic hybrid photosynthesis systems described here, the photogenerated charge transfer process was evaluated via electrochemical impedance spectroscopy (EIS) (Figure 7). In Nyquist plots obtained from EIS study the semicircle at high frequency is related to the electron transfer-limiting process and its radius corresponds to the charge transfer resistance (Fu et al., 2019, Liu et al., 2020, Han et al., 2018). Thus, a Nyquist plot with a smaller radius indicates lower charge transfer impedance. Here, EIS under light showed that semicircles for g-C3N4-catalase (5,000 U mL−1) with R. eutropha and pure g-C3N4 with R. eutropha had smaller radiuses compared with R. eutropha alone and R. eutropha with catalase, indicating that adding g-C3N4 to the bacterial culture lowered the charge transfer impedance and promoted interfacial charge transfer. When catalase was added to either pure g-C3N4 or g-C3N4 with R. eutropha, both Nyquist plots exhibited the smallest semicircle radiuses, which demonstrated that combining g-C3N4 with catalase can effectively lower charge transfer resistance and facilitate photogenerated charge transfer at the interface, thus improving H2 or PHB generation. This is in accordance with the observation that the catalase prevents H2O2 poisoning at the surface of g-C3N4 and thus promotes charger transfer for proton reduction.
Figure 7.
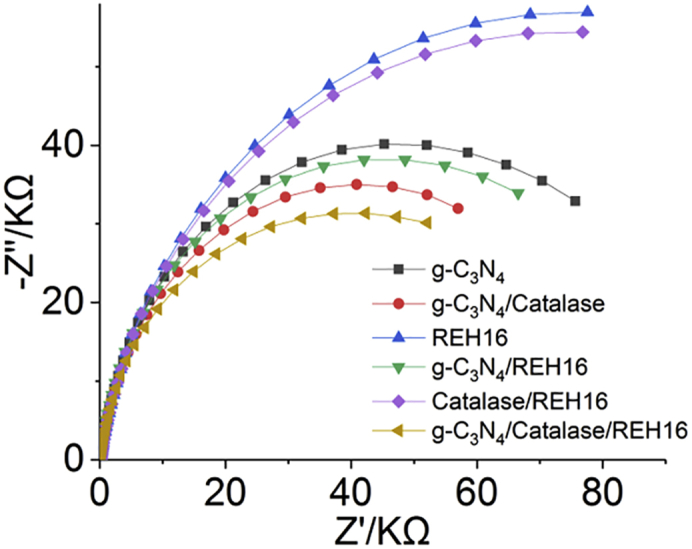
Electrochemical Impedance Spectroscopy of the Autotrophic Hybrid Photosynthesis System under Light
Discussion
The discovery that H2O2-degrading enzymes present in the spent medium of R. eutropha complete the reaction series required for light-driven water splitting by g-C3N4 opens up possibilities for the development of efficient enzymatic PC systems for H2 production. This new avenue for PC development began to be explored here with the coupling of bovine liver catalase with g-C3N4. Although the STH efficiency reported here is promising, it is below 5%–10%, which is considered to be the baseline for economically viable production of H2 via PC (Chen et al., 2017, Pinaud et al., 2013). It is likely that the water-splitting performance of g-C3N4-H2O2-degrading enzyme PC can be further improved. Pure g-C3N4 has other drawbacks besides H2O2 poisoning for optimal photocatalytic activity such as narrow light absorption range below 470 nm, slow surface kinetics, and charge carrier transport as well as important activation energy requirement (Zhang et al., 2017a). Several studies described approaches relying on earth-abundant co-catalysts and on new structural designs to overcome these limitations (Zhang et al., 2017a, Niu et al., 2014). Other groups have improved the photocatalytic activity of g-C3N4 by fine-tuning its morphology, by increasing its crystallinity, or by optimizing its chemical arrangement and electronic properties (Lin et al., 2019, Li et al., 2019, Zhang et al., 2017b, Wu et al., 2019, Niu et al., 2012, Guo et al., 2016). Similar strategies could be employed to improve g-C3N4-catalase PC. In addition, other catalases or different H2O2-degrading enzymes such as peroxidases could be better suited for interacting with g-C3N4.
In its lower-cost configuration design, the g-C3N4-H2O2-degrading enzyme system can split water by coupling g-C3N4 with waste medium from microbial cultures. This strategy could create value and store energy under the form of H2 by harnessing enzyme-rich wastes from bio-industrial processes. For this purpose, water-splitting performance of g-C3N4 coupled with biotechnological wastes from different sources containing unique profiles of H2O2-degrading enzymes should also be evaluated.
When immersed in an R. eutropha culture, g-C3N4 splits water and converts light energy into reducing equivalents, which are transferred to bacteria for PHB production. The addition of catalase to this hybrid photosynthesis system significantly improves autotrophic and heterotrophic productivity by curbing H2O2 poisoning of g-C3N4 and by accelerating water splitting. Simultaneously with H2O2 conversion into H2O and O2, the catalase may improve photocatalysis by g-C3N4 by other means. For instance, it has recently been shown that CDots enable water splitting by g-C3N4 and accelerate H2 production by serving as a catalyst for H2O2 decomposition and at the same time as a co-catalyst for H2 evolution (Qu et al., 2018). Further investigation is required to achieve a complete understanding of the functional interactions between g-C3N4 and the catalase enzyme.
The results presented here also show that coupling g-C3N4 with a bacterial catalyst significantly expands the photocatalytic products of the abiotic PC beyond H2 including the conversion of CO2 into carbon-chain polymers. However, to outcompete autotrophic bioproduction relying on natural photosynthesis, microbial CO2 reduction driven by g-C3N4-catalase needs further improvement. Besides upgrading g-C3N4-catalase PC, this can be achieved by optimizing the metabolism of the microbial catalyst and by finding the best compromise in terms of growth conditions and bioreactor configuration to maximize microbial cell and PC activities.
In both the autotrophic and heterotrophic modes, g-C3N4-catalase doubled or nearly doubled PHB production by R. eutropha, which suggests great potential for CO2 reduction by hybrid photosynthesis as well as for photoaugmentation of white biotechnologies designed for the conversion of organic substrate into valuable chemicals. In conclusion, the results demonstrate that g-C3N4 can harness the activity of H2O2-degrading enzymes such as the catalase to split water efficiently under sunlight and generate reducing equivalents for microbial production processes.
Limitations of the Study
A comprehensive study of the mechanisms involved in the transfer of electrons from g-C3N4 to R. eutropha is necessary to have a complete understanding of the hybrid photosynthesis system described here and be able to improve its performance in the future. A major optimization effort is also needed for practical applications.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the Chinese Thousand Talents Plan Program and Wuhan University of Technology.
Author Contributions
P.-L.T. and T.Z. conceived the study. M.X. carried out the enzymatic photocatalyst experiments and the hybrid photosynthesis experiments. P.-L.T. completed H2O2-degrading enzyme activity assay. Y.C. carried out scanning electron microscopy experiments. P.-L.T., M.X., and T.Z. interpreted the data and wrote the manuscript. All the authors reviewed and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100784.
Supplemental Information
References
- Bi C., Su P., Müller J., Yeh Y.-C., Chhabra S.R., Beller H.R., Singer S.W., Hillson N.J. Development of a broad-host synthetic biology toolbox for Ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microb. Cell Fact. 2013;12:107. doi: 10.1186/1475-2859-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham C.J., Gai C.S., Lu J., Speth D.R., Worden R.M., Sinskey A.J. Engineering Ralstonia eutropha for production of isobutanol from CO2, H2, and O2. In: Lee J.W., editor. Advanced Biofuels and Bioproducts. Springer New York; 2013. pp. 1065–1090. [Google Scholar]
- Chakravarty J., Brigham C.J. Solvent production by engineered Ralstonia eutropha: channeling carbon to biofuel. Appl. Microbiol. Biotechnol. 2018;102:5021–5031. doi: 10.1007/s00253-018-9026-1. [DOI] [PubMed] [Google Scholar]
- Chen S., Takata T., Domen K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017;2:17050. [Google Scholar]
- Claassens N.J., Sousa D.Z., dos Santos V.A.P.M., de Vos W.M., van der Oost J. Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol. 2016;14:692–706. doi: 10.1038/nrmicro.2016.130. [DOI] [PubMed] [Google Scholar]
- Fang X., Gao R., Yang Y., Yan D. A cocrystal precursor strategy for carbon-rich graphitic carbon nitride toward high-efficiency photocatalytic overall water splitting. iScience. 2019;16:22–30. doi: 10.1016/j.isci.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Xu Q., Low J., Jiang C., Yu J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B. 2019;243:556–565. [Google Scholar]
- Grousseau E., Lu J., Gorret N., Guillouet S.E., Sinskey A.J. Isopropanol production with engineered Cupriavidus necator as bioproduction platform. Appl. Microbiol. Biotechnol. 2014;98:4277–4290. doi: 10.1007/s00253-014-5591-0. [DOI] [PubMed] [Google Scholar]
- Guo Y., Li J., Yuan Y., Li L., Zhang M., Zhou C., Lin Z. A Rapid microwave-assisted thermolysis route to highly crystalline carbon nitrides for efficient hydrogen generation. Angew. Chem. Int. Ed. 2016;55:14693–14697. doi: 10.1002/anie.201608453. [DOI] [PubMed] [Google Scholar]
- Guo J., Suástegui M., Sakimoto K.K., Moody V.M., Xiao G., Nocera D.G., Joshi N.S. Light-driven fine chemical production in yeast biohybrids. Science. 2018;362:813–816. doi: 10.1126/science.aat9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Meng P., Waclawik E.R., Zhang C., Li X.-H., Yang H., Antonietti M., Xu J. Palladium/graphitic carbon nitride (g-C3N4) stabilized emulsion microreactor as a store for hydrogen from ammonia borane for use in alkene hydrogenation. Angew. Chem. Int. Ed. 2018;57:14857–14861. doi: 10.1002/anie.201809882. [DOI] [PubMed] [Google Scholar]
- Hu X., Ji H., Chang F., Luo Y. Simultaneous photocatalytic Cr(VI) reduction and 2,4,6-TCP oxidation over g-C3N4 under visible light irradiation. Catal. Today. 2014;224:34–40. [Google Scholar]
- Johnson L.A., Hug L.A. Distribution of reactive oxygen species defense mechanisms across domain bacteria. Free Radic. Biol. Med. 2019;140:93–102. doi: 10.1016/j.freeradbiomed.2019.03.032. [DOI] [PubMed] [Google Scholar]
- Li H., Opgenorth P.H., Wernick D.G., Rogers S., Wu T.-Y., Higashide W., Malati P., Huo Y.-X., Cho K.M., Liao J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science. 2012;335:1596. doi: 10.1126/science.1217643. [DOI] [PubMed] [Google Scholar]
- Li J., Wu D., Iocozzia J., Du H., Liu X., Yuan Y., Zhou W., Li Z., Xue Z., Lin Z. Achieving efficient incorporation of π-electrons into graphitic carbon nitride for markedly improved hydrogen generation. Angew. Chem. Int. Ed. 2019;58:1985–1989. doi: 10.1002/anie.201813117. [DOI] [PubMed] [Google Scholar]
- Lin L., Yu Z., Wang X. Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew. Chem. Int. Ed. 2019;58:6164–6175. doi: 10.1002/anie.201809897. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang Y., Lu L., Wu G., Chen W. Self-regenerated solar-driven photocatalytic water-splitting by urea derived graphitic carbon nitride with platinum nanoparticles. Chem. Commun. 2012;48:8826–8828. doi: 10.1039/c2cc33644h. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Y., Liu N., Han Y., Zhang X., Huang H., Lifshitz Y., Lee S.-T., Zhong J., Kang Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science. 2015;347:970–974. doi: 10.1126/science.aaa3145. [DOI] [PubMed] [Google Scholar]
- Liu C., Gallagher J.J., Sakimoto K.K., Nichols E.M., Chang C.J., Chang M.C.Y., Yang P. Nanowire–bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 2015;15:3634–3639. doi: 10.1021/acs.nanolett.5b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Colón B.C., Ziesack M., Silver P.A., Nocera D.G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science. 2016;352:1210–1213. doi: 10.1126/science.aaf5039. [DOI] [PubMed] [Google Scholar]
- Liu Y., He M., Guo R., Fang Z., Kang S., Ma Z., Dong M., Wang W., Cui L. Ultrastable metal-free near-infrared-driven photocatalysts for H2 production based on protonated 2D g-C3N4 sensitized with chlorin e6. Appl. Catal. B. 2020;260:118137. [Google Scholar]
- Lu J., Brigham C.J., Gai C.S., Sinskey A.J. Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2012;96:283–297. doi: 10.1007/s00253-012-4320-9. [DOI] [PubMed] [Google Scholar]
- Mishra S., Imlay J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch. Biochem. Biophys. 2012;525:145–160. doi: 10.1016/j.abb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Basu S., Shetti N.P., Reddy K.R., Aminabhavi T.M. Chapter 27-Photocatalysis of graphene and carbon nitride-based functional carbon quantum dots. In: Thomas S., Pasquini D., Leu S.-Y., Gopakumar D.A., editors. Nanoscale Materials in Water Purification Micro and Nano Technologies. Elsevier; 2019. pp. 759–781. [Google Scholar]
- Mishra A., Mehta A., Basu S., Shetti N.P., Reddy K.R., Aminabhavi T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: a review. Carbon. 2019;149:693–721. [Google Scholar]
- Müller J., MacEachran D., Burd H., Sathitsuksanoh N., Bi C., Yeh Y.-C., Lee T.S., Hillson N.J., Chhabra S.R., Singer S.W. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl. Environ. Microbiol. 2013;79:4433–4439. doi: 10.1128/AEM.00973-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E.M., Gallagher J.J., Liu C., Su Y., Resasco J., Yu Y., Sun Y., Yang P., Chang M.C.Y., Chang C.J. Hybrid bioinorganic approach to solar-to-chemical conversion. Proc. Natl. Acad. Sci. USA. 2015;112:11461–11466. doi: 10.1073/pnas.1508075112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu P., Zhang L., Liu G., Cheng H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012;22:4763–4770. [Google Scholar]
- Niu P., Yin L.-C., Yang Y.-Q., Liu G., Cheng H.-M. Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 2014;26:8046–8052. doi: 10.1002/adma.201404057. [DOI] [PubMed] [Google Scholar]
- Pinaud B.A., Benck J.D., Seitz L.C., Forman A.J., Chen Z., Deutsch T.G., James B.D., Baum K.N., Baum G.N., Ardo S. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013;6:1983–2002. [Google Scholar]
- Pohlmann A., Fricke W.F., Reinecke F., Kusian B., Liesegang H., Cramm R., Eitinger T., Ewering C., Pötter M., Schwartz E. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006;24:1257–1262. doi: 10.1038/nbt1244. [DOI] [PubMed] [Google Scholar]
- Prévot M.S., Sivula K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C. 2013;117:17879–17893. [Google Scholar]
- Qu D., Liu J., Miao X., Han M., Zhang H., Cui Z., Sun S., Kang Z., Fan H., Sun Z. Peering into water splitting mechanism of g-C3N4-carbon dots metal-free photocatalyst. Appl. Catal. B. 2018;227:418–424. [Google Scholar]
- Rao V.N., Reddy N.L., Kumari M.M., Cheralathan K.K., Ravi P., Sathish M., Neppolian B., Reddy K.R., Shetti N.P., Prathap P. Sustainable hydrogen production for the greener environment by quantum dots-based efficient photocatalysts: a review. J. Environ. Manage. 2019;248:109246. doi: 10.1016/j.jenvman.2019.07.017. [DOI] [PubMed] [Google Scholar]
- Reddy K.R., Reddy C.H.V., Nadagouda M.N., Shetti N.P., Jaesool S., Aminabhavi T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: synthesis methods, properties and photocatalytic applications. J. Environ. Manage. 2019;238:25–40. doi: 10.1016/j.jenvman.2019.02.075. [DOI] [PubMed] [Google Scholar]
- Sakimoto K.K., Wong A.B., Yang P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science. 2016;351:74–77. doi: 10.1126/science.aad3317. [DOI] [PubMed] [Google Scholar]
- Sakimoto K.K., Zhang S.J., Yang P. Cysteine–cystine photoregeneration for oxygenic photosynthesis of acetic acid from CO2 by a tandem inorganic–biological hybrid system. Nano Lett. 2016;16:5883–5887. doi: 10.1021/acs.nanolett.6b02740. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ishizaki A., Kanamaru T., Kawano T. Production of poly(D-3-hydroxybutyrate) from CO2, H2, and O2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995;45:268–275. doi: 10.1002/bit.260450312. [DOI] [PubMed] [Google Scholar]
- Tremblay P.-L., Angenent L.T., Zhang T. Extracellular electron uptake: among autotrophs and mediated by surfaces. Trends Biotechnol. 2017;35:360–371. doi: 10.1016/j.tibtech.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Volodina E., Raberg M., Steinbüchel A. Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit. Rev. Biotechnol. 2016;36:978–991. doi: 10.3109/07388551.2015.1079698. [DOI] [PubMed] [Google Scholar]
- Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J.M., Domen K., Antonietti M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009;8:76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- Wang M., Shen S., Li L., Tang Z., Yang J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017;52:5155–5164. [Google Scholar]
- Wu D., Hu S., Xue H., Hou X., Du H., Xu G., Yuan Y. Protonation and microwave-assisted heating induced excitation of lone-pair electrons in graphitic carbon nitride for increased photocatalytic hydrogen generation. J. Mater. Chem. A. 2019;7:20223–20228. [Google Scholar]
- Xu M., Tremblay P.-L., Jiang L., Zhang T. Stimulating bioplastic production with light energy by coupling Ralstonia eutropha with the photocatalyst graphitic carbon nitride. Green Chem. 2019;21:2392–2400. [Google Scholar]
- Yan S.C., Lv S.B., Li Z.S., Zou Z.G. Organic–inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans. 2010;39:1488–1491. doi: 10.1039/b914110c. [DOI] [PubMed] [Google Scholar]
- Ye S., Wang R., Wu M.-Z., Yuan Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015;358:15–27. [Google Scholar]
- Ye J., Yu J., Zhang Y., Chen M., Liu X., Zhou S., He Z. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Appl. Catal. B. 2019;257:117916. [Google Scholar]
- Zhang T. More efficient together. Science. 2015;350:738–739. doi: 10.1126/science.aad6452. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Q., Shi Q., Cai Z., Yang Z. Acid-treated g-C3N4 with improved photocatalytic performance in the reduction of aqueous Cr(VI) under visible-light. Sep. Purif. Technol. 2015;142:251–257. [Google Scholar]
- Zhang G., Lan Z.-A., Lin L., Lin S., Wang X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 2016;7:3062–3066. doi: 10.1039/c5sc04572j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Tremblay P.-L. Hybrid photosynthesis-powering biocatalysts with solar energy captured by inorganic devices. Biotechnol. Biofuels. 2017;10:249. doi: 10.1186/s13068-017-0943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Lan Z.-A., Wang X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 2017;8:5261–5274. doi: 10.1039/c7sc01747b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Savateev A., Zhao Y., Li L., Antonietti M. Advancing the n → π* electron transition of carbon nitride nanotubes for H2 photosynthesis. J. Mater. Chem. A. 2017;5:12723–12728. [Google Scholar]
- Zhang H., Liu H., Tian Z., Lu D., Yu Y., Cestellos-Blanco S., Sakimoto K.K., Yang P. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotechnol. 2018;13:900. doi: 10.1038/s41565-018-0267-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.