Abstract
Aim
To examine the adjunctive effect of a Lactobacillus reuteri probiotic (ATCC PTA 5289 & DSM 17938) on the re‐instrumentation of residual pockets.
Materials and Methods
This randomized, double‐blind, placebo‐controlled study included 39 previously non‐surgically treated periodontitis patients. A re‐instrumentation was carried out, and probiotic and/or placebo drops were applied according to the study protocoll. Patients afterwards received lozenges to use 2×/day for 12 weeks. Probing pocket depth (PPD), recession, bleeding on probing and plaque levels were analysed, next to the microbiological impact.
Results
No effects of the probiotic drops could be found. However, after 24 weeks, the overall PPD in the probiotic lozenges group (2.64 ± 0.33 mm) was significantly lower compared to the control lozenges (2.92 ± 0.42 mm). This difference was even more pronounced in moderate (4–6 mm) and deep (≥7 mm) pockets. In the probiotic lozenges group, there were also significantly more pockets converting from ≥4 mm at baseline to ≤3 mm at 24 weeks (67 ± 18% versus 54 ± 17%) and less sites in need for surgery (4 ± 4% versus 8 ± 6%). However, the probiotic products did not influence the microbiological counts of the periodontopathogens.
Conclusion
The adjunctive consumption of L. reuteri lozenges after re‐instrumentation improved the PPD reduction, without an impact on pocket colonization with periodontopathogens.
Keywords: Lactobacilli reuteri, periodontitis, probiotics, re‐instrumentation, residual pockets
Clinical Relevance.
Scientific rationale for the study: After scaling and root planing, residual pockets often remain. These pose a risk for further periodontal disease progression and tooth loss. This study investigated the use of a Lactobacillus reuteri probiotic adjunctive to the re‐instrumentation of these residual pockets.
Principle findings: Supplementing re‐instrumentation with the use of L. reuteri containing probiotic lozenges leads to more pocket depth reduction, more pocket closure and less sites in need for surgery, without affecting the microbiological parameters.
Practical implications: The adjunctive use of a dual‐strain L. reuteri containing lozenge after re‐instrumentation is a valuable treatment option for residual pockets.
1. INTRODUCTION
The objective of the initial, cause‐related therapy of periodontitis is removing the subgingival biofilm. This is traditionally performed by a combination of scaling, root planing and debridement (Jenkins, Said, Radvar, & Kinane, 2000; Smiley et al., 2015). However, even after the most meticulous mechanical instrumentation, it is a clinical reality that in many patients residual bleeding pockets of at least 5mm remain (Serino, Rosling, Ramberg, Socransky, & Lindhe, 2001b). These residual pockets are associated with a higher risk for disease progression and therefore require further treatment (Claffey & Egelberg, 1995; Matuliene et al., 2008). Periodontal surgery and re‐instrumentation are the two most common approaches to resolve these residual pockets (Becker et al., 2001; Heitz‐Mayfield, 2005; Konig et al., 2008). Of these, surgical treatment leads to the best pocket reduction, and this positive effect increases with the probing depth (Becker et al., 2001; Heitz‐Mayfield, 2005). In shallow and medium pockets, there is however more attachment gain with repeated instrumentation (Heitz‐Mayfield, 2005). Several (chemical) additives are proposed to improve the treatment results of re‐instrumentation, including antibiotics, essential oils and photodynamic therapy, however, with ambiguous results (Campos et al., 2013; Cappuyns, Cionca, Wick, Giannopoulou, & Mombelli, 2012; Carvalho et al., 2015; Feng et al., 2011; Laleman et al., 2017; Salvi et al., 2002; Serino, Rosling, Ramberg, Hellstrom, et al., 2001a).
Over the last decade, there has been an increased interest in the use of probiotics for enhancing periodontal health. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). In the periodontal field, a recent systematic review showed a positive effect of a combination probiotic of two Lactobacilli reuteri strains as additive to scaling and root planing (Martin‐Cabezas, Davideau, Tenenbaum, & Huck, 2016). The included randomized clinical trials unambiguously showed that in periodontitis patients, this probiotic leads to more pocket probing depth reduction after non‐surgical mechanical therapy (Ince et al., 2015; Tekce et al., 2015; Teughels et al., 2013; Vivekananda, Vandana, & Bhat, 2010). A positive influence of the probiotic at the microbiological and immunological level was also reported. Vivekananda and co‐workers showed that Lactobacilli reuteri leads to a significant decrease in Aggregatibacter Actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia counts (Vivekananda et al., 2010). Additionally, it was shown that the usage of these lozenges led to a statistically significantly greater decrease in P. gingivalis (Teughels et al., 2013) and in proportions of obligate anaerobes up to 180 days (Tekce et al., 2015). Additionally, this probiotic reduced specific inflammation‐associated parameters, such as MMP‐8 levels in gingival crevicular fluid (Ince et al., 2015). These studies are in line with previous research showing a positive effect of probiotics on plaque and gingivitis indices, bleeding on probing and pocket probing depth (Della Riccia et al., 2007; Harini & Anegundi, 2010; Krasse et al., 2005; Schlagenhauf et al., 2016; Vicario, Santos, Violant, Nart, & Giner, 2013). However, other studies failed to show positive effects of probiotics on periodontal parameters (Hallstrom et al., 2013; Iniesta et al., 2012; Shimauchi et al., 2008).
However, up to now, the effect of probiotics on the re‐instrumentation of residual pockets after initial non‐surgical therapy is not yet investigated. The aim of this study was therefore to examine the adjunctive effect of probiotics in this specific indication. Our hypothesis was that the supplementary use of a dual‐strain lactobacilli probiotics to the mechanical debridement of residual pockets would lead to better clinical and microbiological results.
2. MATERIALS AND METHODS
Patients visiting the Department of Oral Health Sciences (University Hospitals Leuven, Belgium) were asked to participate in this study. To be eligible, scaling and root planing for moderate to severe chronic periodontitis (according to the American Academy of Periodontology classification of 1999; Armitage, 1999) should have been carried out at least 3 months and maximum 6 months ago and residual pockets should still be present. In this study, residual pockets were defined as pockets ≥6 mm or pockets of 5 mm with bleeding on probing (Mendonca et al., 2012). There had to be at least one residual pocket in two contra‐lateral quadrants. Patients treated for aggressive periodontitis were excluded. Also, smokers, patients with diabetes and patients who were taking bisphosphonate medication were pregnant/lactating, had other systemic conditions likely to influence periodontal health or who took systemic antibiotics 3 months prior to treatment could not participate in the study. All patients fulfilling the eligibility criteria were informed about the study protocol and the potential benefits and risks. Those willing to participate were asked to sign the informed consent form.
2.1. Study protocol
This study was designed in accordance with the Declaration of Helsinki, and before the start, approval from the Ethics Committee Research UZ/KU Leuven was received (s57667); the study was registered at clinicaltrials.gov (NCT02490618). A single‐centre, double‐blind, randomized (1:1 ratio), placebo‐controlled design was used. The sample size was calculated using α = .05, a power of 85% and an expected difference of 1mm pocket probing depth (and SD = 1mm), leading to 20 patients per group. Taking into account a dropout rate of 10%, 22 patients were included in each treatment group. The clinical treatment and follow‐up as well as the sampling were done by the same examiner, an experienced periodontist (IL). These were done at baseline, after 12 and 24 weeks. This examiner was calibrated showing an intra‐examiner reproducibility of 96% for duplicate measurement of probing pocket depth (PPD) with a maximum difference of 1 mm in 5 patients.
2.2. Allocation to the study groups and masking
The randomization of the study protocols was performed by a staff member who was not further involved in this study. This was done based on a computer‐generated table (http://www.randomization.com) that linked each patient to one of the treatment groups. The same staff member was responsible for blinding the study products. This was done by labelling the packaging of these products with a letter indicating the treatment group. Additionally, these pots, containing the drops, and jars, containing the lozenges, were identical in appearance and non‐transparent. When a patient was included in the study, she handed out the study medication to the researcher according to group to which that patient was assigned based on the patient number and the randomization list. The similarity of the packaging, and the identical appearance, texture and taste of the study products (both the drops as the lozenges) made the double‐blinding of the researcher and patient possible. More information can be found in the online appendix.
2.3. Treatment protocol
During the baseline visit, all patients underwent a whole‐mouth scaling and the residual pockets were subgingivally debrided. This was carried out ultrasonically with the Satelec P5 Newtron XS BLED (Acteon) with specific tips (1S, H3, P2L, P2R) followed with hand instrumentation. Local anaesthesia was used for the comfort of the patients. All patients received customized oral hygiene instructions. Before the participants left the office, the study drops were applied with a syringe and blunt needle in all residual pockets. In the control group, there were control drops for all residual pockets in the whole mouth. In the probiotic group, this was done split‐mouth wise: in one half of the mouth, the control drops were applied, and in the other half of the mouth, the probiotic drops (a minimum of 2 x 108 colony‐forming units L. reuteri Prodentis/5 drops, BioGaia AB) were applied. The patients were advised not to drink, eat or rinse for 30 min afterwards.
Study lozenges were given to all patients to consume at home. The patients were instructed to dissolve these on their tongue twice a day, preferably after brushing, for 12 weeks. The patients of the probiotic group received probiotic lozenges containing Lactobacillus reuteri DSM 17938 and Lactobacillus reuteri ATCC PTA 5289 (a minimum of 2 × 108 colony‐forming units L. reuteri Prodentis/lozenge, BioGaia AB). The patients of the control group received control lozenges without live bacteria. Furthermore, the probiotic and control lozenges were identical in taste, texture and appearance. At the 12‐week consultation, the participants were asked to return the empty packages of the study medication to examine the adherence. At that time, side effects were also questioned by the examiner by means of an open question.
2.4. Outcome measures of interest
The primary outcome of interest was PPD. The secondary outcomes of interest were gingival recession (REC), clinical attachment level (CAL), Full‐Mouth Plaque Scores (FMPS), Full‐Mouth Bleeding Scores (FMBS), “risk for disease progression” and “need for surgery.”
During each visit, the full‐mouth PPD and REC were noted at six sites per tooth. PPD was defined as the distance between the gingival margin and the bottom of the pocket measured in millimetre (mm) with a Merrit‐B probe, REC as the distance between the cementoenamel junction and the gingival margin. The sum of these was defined as the CAL. Additionally, the FMPS and FMBS (20s after probing) were noted at six sites/tooth dichotomously as the present (1) or absent (0) and expressed as a percentage of examined sites within each subject. Based on these data, the “risk for disease progression” and “need for surgery” were calculated as described earlier (Laleman et al., 2015; Teughels et al., 2013). “Risk for disease progression” was defined at patient level as low (≤4 sites with PPD ≥5 mm), moderate (5–8 sites with PPD ≥5 mm) or high (≥9 sites with PPD ≥5 mm). A site was considered as “in need for surgery” if the PPD was ≥6 mm, or 5 mm and BOP positive.
Two teeth with residual pockets, one in each contra‐lateral quadrant, were selected for microbiological sampling. These were taken supragingivally with a scaler (H6/H7) and subgingivally with 8 paper points/ pocket and subsequently placed in 1ml of reduced transport fluid (RTF). Additionally, samples of the saliva and tongue were taken. For the latter, a distal area was swiped for 10 s with a sterile cotton swab (Nuova Aptaca), and the tip of this cotton swap was transferred to an Eppendorf tube with 1 ml RTF. Unstimulated saliva was collected, from which 100 μl was dispersed in 900 μl RTF. The presence of P. gingivalis, P. intermedia, Fusobacterium nucleatum and A. actinomycetemcomitans in these samples was detected by quantitative PCR assay (qPCR) as described by Teughels and co‐workers in 2013 (Teughels et al., 2013).
2.5. Statistical methods
For the comparisons of the drops, a linear mixed model was fit for the patients with the treatment lozenge and drop and time as crossed fixed factors and patient as random factor. A normal quantile plot from the residual values and residual dot plot showed that the residual values were normally distributed with equal variance. Likewise, a linear mixed model was fit with lozenge and time as crossed fixed factors and patient as random factor for comparing the treatments between the probiotic and control lozenge group. Data that were missing at 24 weeks were forward filled from the data obtained at 12 weeks. Comparisons between treatment and time groups were each time calculated, and p‐values were corrected for simultaneous hypothesis testing according to Sidak.
For the comparisons of the drops, data were log‐transformed before analysis. Data below quantification limit were considered as censored (below the quantification limit), and a frailty model was fit for the patients with the treatment lozenges, drops and time as crossed fixed factors and patient as random factor. Likewise, a frailty model was fit with lozenge and time as crossed fixed factors and patient as random factor for comparing the treatments between the probiotic and control lozenge group. An intention‐to‐treat analysis was carried out following the “last observation carried forward” principle, including all the patients that at least attended the 12‐week appointment without violating the inclusion criteria. Comparisons between treatment and time groups were calculated, and p‐values were corrected for simultaneous hypothesis testing according to Sidak. All data were analysed in S‐Plus 8.0 for Linux.
3. RESULTS
For this study, all patients were included between January 2016 and June 2018, and the last follow‐up consultation took place in December 2018. Forty‐four patients were recruited, from which the data of 5 patients could not be used since they dropped out before the 12‐week consultation (Figure 1). The data of 39 participants between 34 and 83 years old were thus used for the statistical analysis. These included 20 participants of which 11 were male in the probiotic group and 19 participants of which 16 were male in the control group. The mean age was, respectively, 58 ± 12 years and 58 ± 13 years.
Figure 1.
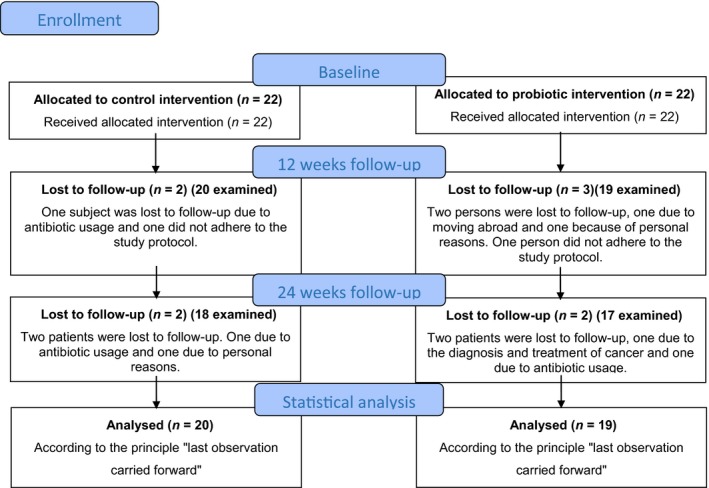
Course of the study
All, except two, participants reported a good adherence to the study protocol. Eight patients did not return the study medication, and the other 31 participants took on average 1.7 lozenges/day (control: 1.7, test: 1.6).
3.1. Side effects
No serious adverse events were experienced. However, when questioning these in detail, four patients reported altered sensations of the oral cavity. These patients were evenly distributed over the control and probiotic group. In the control group, one patient complained about a dry mouth and feeling thirstier than normal, the other complaint was about a bad taste in the oral cavity just after waking up. In the test group, one patient complained about a dry mouth and one experienced sometimes a different feeling in the mouth after usage of the lozenge.
3.2. Clinical measurements
Since no statistical significant differences were found for any of the studied clinical parameters in the probiotic lozenge group between the quadrants where the control drops were applied versus the quadrants where the probiotic drops were applied (online figure A), the data of the sites receiving the probiotic drops were pooled with the data of the sites receiving the placebo drops.
The mean PPD, CAL, FMBS and FMPS were significantly lower after the usage of the lozenge (12 weeks) and at the end of the study period (24 weeks) compared to the baseline values, both for the probiotic as the control group. Concerning the inter‐group differences, at the end of the study, the mean PPD was significantly lower in the probiotic group compared to the control group (p = .034). This difference was even more pronounced when looking solely to the moderate (4‐6 mm) (p = .015) and deep pockets (>6 mm) (p = .025). More detailed information about the PPD, CAL, REC, FMBS and FMPS and the associated p‐values can be found in Table 1.
Table 1.
Clinical characteristics of the group assigned to the probiotic lozenges versus the group assigned to the control lozenges displayed as mean or delta (∆) (difference with baseline value) and standard deviation (SD)
| Variable | Time point | Treatment group | p‐value | ||||
|---|---|---|---|---|---|---|---|
| Probiotic (n = 19) | Control (n = 20) | For mean | For delta | ||||
| Mean ± SD | ∆ ± SD | Mean ± SD | ∆ ± SD | ||||
| PPD (mm) | |||||||
| Overall | Baseline | 3.09 ± 0.32 | 3.28 ± 0.39 | .176 | |||
| 12 weeks | 2.66 ± 0.21* | −0.43 ± 0.23 | 2.84 ± 0.40* | −0.44 ± 0.28 | .199 | .995 | |
| 24 weeks | 2.64 ± 0.33* | −0.45 ± 0.20 | 2.92 ± 0.42* | −0.36 ± 0.26 | .034 | .375 | |
| Moderate pockets (4−6 mm) | Baseline | 4.56 ± 0.19 | 4.68 ± 0.22 | .524 | |||
| 12 weeks | 3.36 ± 0.20* | −1.22 ± 0.22 | 3.55 ± 0.51* | −1.13 ± 0.55 | .151 | .735 | |
| 24 weeks | 3.35 ± 0.38* | −1.21 ± 0.31 | 3.67 ± 0.42* | −1.01 ± 0.46 | .015 | .160 | |
| Deep pockets (≥7 mm) | Baseline | 7.29 ± 0.35 | 7.43 ± 0.42 | .876 | |||
| 12 weeks | 5.03 ± 0.80* | −2.26 ± 0.75 | 5.73 ± 1.02* | −1.70 ± 0.96 | .040 | .127 | |
| 24 weeks | 4.94 ± 1.08* | −2.32 ± 1.04 | 5.73 ± 1.06* | −1.70 ± 0.96 | .025 | .043 | |
| CAL (mm) | |||||||
| Overall | Baseline | 3.58 ± 0.69 | 3.67 ± 0.69 | .938 | |||
| 12 weeks | 3.02 ± 0.98* | −0.56 ± 0.9 | 3.36 ± 0.88* | −0.31 ± 0.28 | .331 | .265 | |
| 24 weeks | 3.04 ± 1.01* | −0.54 ± 0.91 | 3.49 ± 0.86 | −0.18 ± 0.24 | .120 | .075 | |
| Moderate pockets (4−6 mm) | Baseline | 5.04 ± 0.58 | 5.01 ± 0.47 | .992 | |||
| 12 weeks | 3.66 ± 1.08* | −1.38 ± 1.05 | 4.05 ± 0.89* | −0.96 ± 0.57 | .266 | .133 | |
| 24 weeks | 3.73 ± 1.14* | −1.31 ± 1.08 | 4.21 ± 0.83* | −0.81 ± 0.5 | .182 | .045 | |
| Deep pockets (≥7 mm) | Baseline | 7.85 ± 1.06 | 7.88 ± 0.73 | .999 | |||
| 12 weeks | 5.68 ± 1.42* | −2.17 ± 0.74 | 6.21 ± 1.47* | −1.66 ± 1.10 | .401 | .238 | |
| 24 weeks | 5.70 ± 1.46* | −2.16 ± 1.07 | 6.32 ± 1.52* | −1.56 ± 1.17 | .313 | .100 | |
| REC (mm) | |||||||
| Overall | Baseline | 0.50 ± 0.54 | 0.39 ± 0.53 | .685 | |||
| 12 weeks | 0.52 ± 0.59 | 0.07 ± 0.15 | 0.52 ± 0.64* | 0.13 ± 0.26 | .977 | .460 | |
| 24 weeks | 0.59 ± 0.59 | 0.09 ± 0.15 | 0.57 ± 0.64 | 0.18 ± 0.27 | .993 | .250 | |
| BOP (%) | |||||||
| Overall | Baseline | 34 ± 33 | 38 ± 14 | .443 | |||
| 12 weeks | 20 ± 18* | −14 ± 11 | 25 ± 12* | −13 ± 1 | .314 | .957 | |
| 24 weeks | 20 ± 20* | −16 ± 8 | 27 ± 12* | −11 ± 10 | .096 | .500 | |
| PI (%) | |||||||
| Overall | Baseline | 36 ± 14 | 50 ± 25 | .023 | |||
| 12 weeks | 27 ± 10 | −9 ± 11 | 31 ± 11* | −19 ± 22 | .732 | .195 | |
| 24 weeks | 25 ± 12* | −11 ± 18 | 33 ± 15* | −17 ± 22 | .275 | .451 | |
| Percentage of pockets at a certain time point (%) | |||||||
| ≥4 mm | Baseline | 23 ± 9 | 26 ± 11 | .483 | |||
| 12 weeks | 10 ± 4* | −13 ± 7 | 15 ± 9* | −11 ± 6 | .197 | .616 | |
| 24 weeks | 10 ± 7* | −13 ± 6 | 16 ± 10* | −10 ± 6 | .083 | .213 | |
| ≥5 mm | Baseline | 13 ± 7 | 17 ± 9 | .121 | |||
| 12 weeks | 4 ± 3* | −9 ± 5 | 10 ± 7* | −7 ± 6 | .033 | .633 | |
| 24 weeks | 5 ± 5* | −7 ± 4 | 10 ± 7* | −6 ± 6 | .045 | .774 | |
| ≥6 mm | Baseline | 4 ± 3 | 7 ± 5 | .007 | |||
| 12 weeks | 2 ± 2* | −3 ± 2 | 4 ± 3* | −3 ± 4 | .054 | .571 | |
| 24 weeks | 2 ± 2* | −2 ± 2 | 5 ± 4* | −3 ± 4 | .021 | .828 | |
| ≥7 mm | Baseline | 2 ± 2 | 5 ± 3 | .012 | |||
| 12 weeks | 1 ± 1* | −2 ± 2 | 3 ± 2* | −2 ± 3 | .031 | .917 | |
| 24 weeks | 1 ± 2* | −1 ± 1 | 3 ± 3* | −2 ± 3 | .032 | .878 | |
| Percentage of pockets converting from ≥4 mm at baseline to ≤3 mm after R/ (%) | |||||||
| 12 weeks | 64 ± 13% | 56 ± 20% | .046 | ||||
| 24 weeks | 67 ± 18% | 54 ± 17% | .030 | ||||
Significant intra‐group difference compared to the baseline value, Bold: significant inter‐group difference.
In this table, also the percentage of sites with a PPD of a specific threshold can be found. For all thresholds (≥4, ≥5, ≥6 and ≥7 mm), there was a significant reduction between the baseline values and those after 12 and 24 weeks, both for the probiotic and control groups. After 24 weeks, a statistically significant inter‐group difference could also be noted. In the probiotic group, there was a lower percentage of pockets ≥5, ≥6 and ≥7 mm compared to the placebo group. However, this difference was also present at baseline concerning ≥6 and ≥7 mm as threshold levels. Additionally, a statistically significant better pocket closure (pockets that were ≥4 mm at baseline and ≤3 mm at follow‐up) was noticed at the 12‐ and 24‐week follow‐up.
Information about clinical relevant outcomes for the patients, “risk for disease progression” and “need for surgery,” can be found in Table 2. These were more favourable in the probiotic group. Twelve and 24 weeks after the re‐instrumentation, the risk for disease progression is consistently lower in the probiotic group compared to the control group. At the end of the study, 14 patients in the control group were classified as high risk for disease progression compared to only 8 in the probiotic group. These differences however never reached statistical significance. On the site level, the need for surgery was significantly lower in the probiotic group compared to the control group at the follow‐up visits. At the 24‐week visit, on average 4% of all sites in the probiotic group were in need for surgery and 8% of the sites of the control patients. At the patient level, 3 patients from the probiotic group had no need for surgery at the end of the study compared to one in the control group. However, for the patients that still needed surgery, the extent was much less (on the site and tooth level) in the probiotic than the control group. This difference was only statistically significant at the site level.
Table 2.
Patient‐centred outcomes: risk for disease progression and need for surgery (statistically significant differences are shown in bold)
| Risk for disease progression | |||
|---|---|---|---|
| Time point | Treatment group | p‐value | |
| Probiotic (n = 19) | Control (n = 20) | ||
| Baseline | |||
| Low (≤4 sites) | 1 | 0 | .826 |
| Medium (5–8 sites) | 3 | 3 | |
| High (≥9 sites) | 15 | 17 | |
| 12 weeks | |||
| Low (≤4 sites) | 7 | 3 | .217 |
| Medium (5–8 sites) | 6 | 6 | |
| High (≥9 sites) | 6 | 11 | |
| 24 weeks | |||
| Low (≤4 sites) | 8 | 3 | .133 |
| Medium (5–8 sites) | 3 | 3 | |
| High (≥9 sites) | 8 | 14 | |
| Need for surgery | |||
|---|---|---|---|
| Sites in need for surgery (%) | (n = 2,712) | (n = 2,682) | |
| Baseline | 11 ± 6% | 15 ± 8% | .110 |
| 12 weeks | 3 ± 3%* | 8 ± 6%* | .032 |
| 24 weeks | 4 ± 4%* | 8 ± 6%* | .048 |
| Teeth in need for surgery (%) | (n = 452) | (n = 447) | |
|---|---|---|---|
| Baseline | 29 ± 14% | 37 ± 16% | .299 |
| 12 weeks | 13 ± 8%* | 23 ± 16%* | .060 |
| 24 weeks | 15 ± 14%* | 24 ± 16%* | .080 |
| Patients in need for surgery (%) | (n = 19) | (n = 20) | |
|---|---|---|---|
| 12 weeks | |||
| 0 sites | 2 (11%) | 3 (15%) | .072 |
| 1–2 sites | 5 (26%) | 0 (0%) | |
| ≥ 3 sites | 12 (63%) | 17 (85%) | |
| 24 weeks | |||
| 0 sites | 3 (16%) | 1 (5%) | .312 |
| 1–2 sites | 4 (21%) | 2 (10%) | |
| ≥ 3 sites | 12 (63%) | 17 (85%) | |
3.3. Microbiological data
As for the clinical data, no intra‐group, nor inter‐group differences could be found between the quadrants treated with the probiotic versus the control drops in the probiotic lozenge group (online table B). Therefore, the probiotic lozenge group was further compared with the control lozenge group (Table 3).
Table 3.
Microbiological (log‐transferred) outcome measures: mean and standard deviation values at baseline and the differences (∆) after 12 and 24 weeks
| Variable | Time point | Treatment group | p‐value | ||||
|---|---|---|---|---|---|---|---|
| Probiotic (n = 19) | Control (n = 20) | ||||||
| Mean ± SD | ∆ ± SD | Mean ± SD | ∆ ± SD | For mean | For delta | ||
| Tongue | |||||||
| A. actinomycetemcomitans | Baseline | 0.22 ± 0.95 | 0.46 ± 1.43 | .999 | |||
| 12 weeks | 0.48 ± 1.14 | 0.26 ± 1.56 | 0.64 ± 1.56 | 0.17 ± 0.82 | .999 | .999 | |
| 24 weeks | 0.66 ± 1.33 | 0.44 ± 1.12 | 0.22 ± 1.01 | −0.24 ± 0.98 | .891 | .744 | |
| F. nucleatum | Baseline | 6.72 ± 1.03 | 6.30 ± 1.73 | .997 | |||
| 12 weeks | 6.66 ± 0.89 | −0.06 ± 0.85 | 6.44 ± 0.72 | 0.14 ± 1.67 | .928 | .990 | |
| 24 weeks | 6.98 ± 0.84 | 0.26 ± 0.65 | 6.52 ± 0.84 | 0.22 ± 1.69 | .850 | .928 | |
| P. gingivalis | Baseline | 3.52 ± 2.59 | 3.06 ± 2.73 | .999 | |||
| 12 weeks | 3.40 ± 2.56 | −0.12 ± 0.54 | 3.48 ± 2.71 | 0.41 ± 1.10 | .999 | .867 | |
| 24 weeks | 3.17 ± 2.59 | −0.35 ± 1.41 | 3.51 ± 2.74 | 0.45 ± 1.14 | .999 | .602 | |
| P. intermedia | Baseline | 2.02 ± 2.50 | 1.32 ± 2.47 | .971 | |||
| 12 weeks | 2.06 ± 2.43 | 0.17 ± 0.90 | 1.43 ± 2.35 | 0.11 ± 0.88 | .925 | .999 | |
| 24 weeks | 1.91 ± 2.38 | −0.11 ± 1.67 | 1.57 ± 2.33 | 0.26 ± 0.99 | .980 | .999 | |
| Saliva | |||||||
| A. actinomycetemcomitans | Baseline | 0.56 ± 1.36 | 0.64 ± 1.63 | .994 | |||
| 12 weeks | 0.75 ± 1.51 | 0.20 ± 0.76 | 0.84 ± 1.76 | 0.20 ± 1.29 | .999 | .994 | |
| 24 weeks | 0.40 ± 1.24 | −0.16 ± 1.20 | 0.24 ± 1.09* | −0.40 ± 1.12 | .766 | .104 | |
| F. nucleatum | Baseline | 6.36 ± 0.69 | 6.27 ± 0.63 | .999 | |||
| 12 weeks | 6.46 ± 0.64 | 0.10 ± 0.61 | 6.19 ± 0.63 | −0.07 ± 0.69 | .904 | .949 | |
| 24 weeks | 6.37 ± 0.70 | 0.01 ± 0.65 | 6.26 ± 0.57 | −0.01 ± 0.83 | .999 | .999 | |
| P. gingivalis | Baseline | 4.26 ± 3.12 | 4.22 ± 3.02 | .999 | |||
| 12 weeks | 4.20 ± 3.34 | −0.06 ± 0.85 | 4.19 ± 3.27 | −0.03 ± 0.98 | .999 | .999 | |
| 24 weeks | 4.23 ± 3.14 | −0.03 ± 2.20 | 4.20 ± 3.24 | −0.03 ± 0.99 | .999 | .997 | |
| P. intermedia | Baseline | 2.94 ± 2.42 | 2.60 ± 2.32 | .999 | |||
| 12 weeks | 2.26 ± 2.38 | −0.69 ± 2.15 | 2.79 ± 2.23 | 0.19 ± 1.71 | .999 | .969 | |
| 24 weeks | 1.73 ± 2.03 | −1.21 ± 2.42 | 2.23 ± 2.35 | −0.37 ± 1.93 | .995 | .808 | |
| Supragingival | |||||||
| A. actinomycetemcomitans | Baseline | 0.58 ± 1.3 | 0.44 ± 1.3 | .999 | |||
| 12 weeks | 0.37 ± 1.07 | −0.24 ± 1.44 | 1.03 ± 1.82 | 0.57 ± 1.45 | .662 | .468 | |
| 24 weeks | 0.91 ± 1.58 | 0.33 ± 1.92 | 0.50 ± 1.21 | 0.05 ± 1.51 | .999 | .999 | |
| F. nucleatum | Baseline | 6.16 ± 1.17 | 5.97 ± 1.08 | .999 | |||
| 12 weeks | 5.57 ± 1.50 | −0.60 ± 1.35 | 5.70 ± 1.09 | −0.36 ± 1.32 | .946 | .233 | |
| 24 weeks | 5.72 ± 1.23 | −0.43 ± 1.13 | 5.68 ± 1.40 | −0.29 ± 1.60 | .991 | .999 | |
| P. gingivalis | Baseline | 2.99 ± 3.03 | 2.95 ± 2.9 | .999 | |||
| 12 weeks | 2.32 ± 2.84 | −0.83 ± 2.60 | 3.39 ± 2.97 | 0.28 ± 1.22 | .999 | .873 | |
| 24 weeks | 2.54 ± 2.90 | −0.45 ± 2.71 | 2.97 ± 2.93 | 0.02 ± 1.82 | .999 | .999 | |
| P. intermedia | Baseline | 1.36 ± 2.15 | 2.33 ± 2.26 | .985 | |||
| 12 weeks | 1.61 ± 2.30 | 0.44 ± 2.12 | 2.27 ± 2.2 | −0.18 ± 2.1 | .999 | .999 | |
| 24 weeks | 1.83 ± 2.23 | 0.48 ± 2.09 | 1.79 ± 2.20 | −0.53 ± 2.11 | .999 | .930 | |
| Subgingival | |||||||
| A. actinomycetemcomitans | Baseline | 0.89 ± 1.55 | 1.30 ± 1.85 | NC | |||
| 12 weeks | 0.36 ± 1.07 | −0.58 ± 1.17 | 0.72 ± 1.75 | −0.59 ± 2.06 | NC | NC | |
| 24 weeks | 0.56 ± 1.37 | −0.33 ± 1.45 | 1.03 ± 2.17 | −0.27 ± 2.42 | NC | NC | |
| F. nucleatum | Baseline | 6.92 ± 1.09 | 7.21 ± 0.83 | .996 | |||
| 12 weeks | 6.38 ± 1.41 | −0.51 ± 0.97 | 6.56 ± 1.30 | −0.65 ± 1.34 | .999 | .999 | |
| 24 weeks | 5.37 ± 2.27 | −1.55 ± 2.39 | 6.78 ± 1.30 | −0.43 ± 1.34 | .508 | .985 | |
| P. gingivalis | Baseline | 4.21 ± 3.57 | 4.05 ± 3.59 | .999 | |||
| 12 weeks | 3.81 ± 3.37 | −0.63 ± 1.29 | 3.89 ± 3.19 | −0.17 ± 1.65 | .999 | .999 | |
| 24 weeks | 3.11 ± 3.23 | −1.10 ± 2.26 | 3.73 ± 3.38 | −0.32 ± 1.59 | ,999 | .917 | |
| P. intermedia | Baseline | 2.9 ± 2.37 | 2.53 ± 2.46 | .999 | |||
| 12 weeks | 1.90 ± 2.37 | −0.95 ± 1.76 | 2.34 ± 2.08 | −0.19 ± 2.24 | .998 | .932 | |
| 24 weeks | 2.23 ± 2.29 | −0.67 ± 1.76 | 2.098 ± 2.77 | −0.43 ± 2.24 | .999 | .999 | |
Significant intra‐group difference compared to the baseline value, Bold: significant inter‐group difference.
This study protocol did not show any microbiological impact on the four studied microorganisms (P. gingivalis, P. intermedia, F. nucleatum and A. actinomycetemcomitans). No statistically significant differences could be found between these bacteria at baseline and at the 12‐ and 24‐week control. Additionally, no statistically significant inter‐group differences could be found between the probiotic and the control groups for the counts of these bacteria at any time point (baseline, 12‐ and 24‐week control).
4. DISCUSSION
Since residual pockets present risks for periodontal disease progression and tooth loss, there is a need for additional therapies to reduce these (Claffey & Egelberg, 1995; Matuliene et al., 2008). This can be done surgically or non‐surgically through re‐instrumentation. To the best of our knowledge, this was the first trial that focused on the adjunctive effect of a dual‐strain L. reuteri probiotic on re‐instrumentation of residual pockets. Our results, in accordance with different authors in the past (Konig et al., 2008; Mendonca et al., 2012), confirmed the usefulness of re‐instrumentation. This trial also showed an additional beneficial clinical effect of the usage of L. reuteri probiotics. The usage of these L. reuteri lozenges led to statistically significantly lower PPD than the control lozenges after 24 weeks, and this difference was even more pronounced in moderate and deep pockets. Moreover, the PPD in the probiotic group still improved between weeks 12 and 24, contrastingly to the PPD in the control groups that even deteriorated between 12 and 24 weeks. The probiotic also positively influenced the patient clinical relevant outcomes, leading to better pocket closure, fewer pockets in need for surgery and a lower risk for disease progression compared to the control group.
Re‐instrumentation decreased the whole‐mouth PPD with 0.36 mm after 24 weeks, when adding a probiotic to this treatment a 0.45 mm reduction was seen. Looking to moderate and deep pockets, this difference was even more pronounced with 1.21 and 2.32 mm PPD reduction, respectively, in the probiotic group and 1.01 and 1.70 mm PPD reduction, respectively, in the control group. These clinical results are in line with earlier research about re‐instrumentation. For example, Wennström and co‐workers reported a 0.4 mm additional reduction of PPD after re‐instrumentation (Wennstrom, Tomasi, Bertelle, & Dellasega, 2005). When they analysed the data for only the sites subjected to re‐treatment, they measured an additional pocket probing depth reduction of 1.0 mm when ultrasonic instruments were used and 0.8 mm when hand instruments were used. When supplementing re‐instrumentation with local antibiotics, Salvi and co‐workers reported a 0.25–0.33 mm PPD reduction at all experimental sites (Salvi et al., 2002). However, in this study, a negative control group where solely re‐instrumentation was carried out was not included. Few years later, Tomasi and co‐workers failed to show improved healing outcomes of re‐instrumentation supplemented with locally delivered doxycycline compared to re‐instrumentation (Tomasi, Koutouzis, & Wennstrom, 2008).
Unlike the positive clinical results found in this trial, this study failed to find any significant suppressive effects on four well‐known periodontal pathogens. The possible explanations for this are twofold. Firstly, it could be that there was really no effect on the microbiological level. These are all patients that previously undergone scaling and root planing, which probably already caused a shift as hypothesized by Salvi et al. (2002). This could imply that the positive effects of probiotics on oral health are based on a different mechanism than a direct suppressing effect on periodontopathogens and are rather due to immunological mechanisms as previously stated (Hallstrom, Lindgren, Widen, Renvert, & Twetman, 2016; Schlagenhauf et al., 2016). A significant decrease in the levels of pro‐inflammatory markers such as TNF‐α, IL‐1β, IL‐8 and MMP‐8 was already shown after the usage of L. reuteri probiotics (Ince et al., 2015; Szkaradkiewicz, Stopa, & Karpinski, 2014; Twetman et al., 2009), next to an increase in anti‐inflammatory markers as TIMP‐1 (Ince et al., 2015). Future research should therefore, in addition to clinical parameters, also investigate certain immunological markers in the gingival crevicular fluid (such as IL‐1β, IL‐6, IL‐8, IL‐10, IL‐17 and TNF‐α). Secondly, it can be that there was an effect on the oral microbiome, but that we did not detect this since only four periodontopathogens were examined based on qPCR. Other current (but more expensive and intensive) techniques could provide a more complete picture of all changes in the total oral microbiome during and after re‐instrumentation and probiotic therapy.
The idea behind the topical application of the drops was to apply it as close as possible to the site where an effect was desired. However, in contrast to the effect of the probiotic lozenge, no clinical effects of the application of the probiotic drops could be found. A possible explanation is based on the washout effect of the gingival crevicular fluid in the sulcus, because of this the contact time of the probiotic drops with the periodontal inflamed sites was possibly too short to have any effect.
A frequently heard comment about re‐instrumentation is that when the same instrument is used as the initial therapy, the effectiveness of the root debridement is not necessarily increased (Konig et al., 2008). We tried to overcome this by the application of specific ultrasonic tips that were not used during the initial instrumentation (at the initial instrumentation solely 1S was used, at re‐instrumentation this was 1S supplemented with the use of tips H3, P2L, P2R), to access sites that were possibly not reached during the primary treatment. This confirmed the results of König and co‐workers that a carefully executed re‐instrumentation (with a combination of instruments and with special periodontal tips) increases the effectiveness of the initial instrumentation (Konig et al., 2008).
Based on the returned lozenges, the adherence to the study protocol (with on average 6% of the study medication missed) seemed comparable to the medication adherence rate reported in clinical trials in medicine (Shiovitz et al., 2016). This was also comparable to the adherence rate reported by Schlagenhauf and co‐workers (Schlagenhauf et al., 2016). These authors reported a consumption of 2.45 lozenges/day in the test group and 2.55 lozenges/day in the control, while 2 lozenges/day were recommended. The only differences between this study and the current one were that these subjects took 0.45/0.55 lozenges per day more than recommended, while in our study, they took 0.4/0.3 lozenges less than recommended. The adherence in this study was however worse than the 100% adherence rate of Vicario and co‐workers (Vicario et al., 2013). A possible explanation for this could be the shorter study duration of their study compared to our study (30 days versus 3 months).
Few side effects were noticed by the patients; however, since these were similar in nature and number in the probiotic as control group, these are not expected to be due to the probiotic additive. It can be suspected that these altered sensations of the oral cavity were rather the result of increased attention for the mouth based on study participation rather than to the use of the study products. This attention bias is already mentioned in previous probiotic trials (Vicario et al., 2013). Improved reporting of adverse outcomes could be done by specifically questioning certain side effects instead of using an open question.
The use of probiotics as additional therapy to re‐instrumentation is therefore certainly a field that requires further investigation. However, the recent introduction of a new classification of periodontal diseases will make it difficult to compare between past and future studies. With the updated classification system, all the patients in this study would be diagnosed as having generalized stage III or IV periodontitis, grade B (Caton et al., 2018; Tonetti, Greenwell, & Kornman, 2018).
Future research should focus on the underlying, immunomodulatory mechanism(s) of this positive clinical effect of probiotics on re‐instrumentation. It would also be interesting to compare probiotic supplementation of re‐instrumentation with surgery to examine the impact of both on residual pockets. Surgery is still a popular option to treat residual pockets; however, it is less beneficial cost‐benefit wise, with increased treatment time and costs compared to re‐instrumentation (Patel, Richards, Wang, & Inglehart, 2006). Moreover, fearful and anxious patients are also less reluctant to undergo surgical periodontal treatment (Patel et al., 2006). However, it is important to realize that in most cases, re‐instrumentation cannot replace surgery entirely. As demonstrated in this study, re‐instrumentation reduces the PPD and as a result the number of sites in need of surgery. However, the result on the patient level is less clear, only 4 out of 39 patients did no longer needed periodontal surgery at the end of the study. Thus, while it is a good treatment to limit the extent of surgery within the patient, most of them still are in need of surgery. A randomized controlled clinical trial directly comparing surgery and re‐instrumentation with probiotic supplementation is needed in this light focussing not only on periodontal outcomes, but also on cost‐effectiveness and patient experience/satisfaction.
CONFLICT OF INTEREST
Wim Teughels received fees for lecturing on probiotics from BioGaia.
Supporting information
ACKNOWLEDGEMENTS
The study products (both the probiotic as the placebo products) used in this clinical trial were kindly provided by BioGaia. We hereby thank our secretary, Andrea Van Obberghen, for her logistical help with the randomization and blinding the study medication.
Laleman I, Pauwels M, Quirynen M, Teughels W. A dual‐strain Lactobacilli reuteri probiotic improves the treatment of residual pockets: A randomized controlled clinical trial. J Clin Periodontol. 2020;47:43–53. 10.1111/jcpe.13198
Funding information
This study was partially financially supported by BioGaia, Sweden. Additional support came from grants from the KU Leuven (C24/17/086) and the FWO (G091218N).
REFERENCES
- Armitage, G. C. (1999). Development of a classification system for periodontal diseases and conditions. Annals of Periodontology, 4, 1–6. 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- Becker, W. , Becker, B. E. , Caffesse, R. , Kerry, G. , Ochsenbein, C. , Morrison, E. , & Prichard, J. (2001). A longitudinal study comparing scaling, osseous surgery, and modified Widman procedures: Results after 5 years. Journal of Periodontology, 72, 1675–1684. 10.1902/jop.2001.72.12.1675 [DOI] [PubMed] [Google Scholar]
- Campos, G. N. , Pimentel, S. P. , Ribeiro, F. V. , Casarin, R. C. , Cirano, F. R. , Saraceni, C. H. , & Casati, M. Z. (2013). The adjunctive effect of photodynamic therapy for residual pockets in single‐rooted teeth: A randomized controlled clinical trial. Lasers in Medical Science, 28, 317–324. 10.1007/s10103-012-1159-3 [DOI] [PubMed] [Google Scholar]
- Cappuyns, I. , Cionca, N. , Wick, P. , Giannopoulou, C. , & Mombelli, A. (2012). Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split‐mouth controlled clinical trial. Lasers in Medical Science, 27, 979–986. 10.1007/s10103-011-1027-6 [DOI] [PubMed] [Google Scholar]
- Carvalho, V. F. , Andrade, P. V. , Rodrigues, M. F. , Hirata, M. H. , Hirata, R. D. , Pannuti, C. M. , … Conde, M. C. (2015). Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. Journal of Clinical Periodontology, 42, 440–447. 10.1111/jcpe.12393 [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Armitage, G. , Berglundh, T. , Chapple, I. L. C. , Jepsen, S. , Kornman, K. S. , … Tonetti, M. S. (2018). A new classification scheme for periodontal and peri‐implant diseases and conditions – Introduction and key changes from the 1999 classification. Journal of Periodontology, 89(Suppl 1), S1–S8. 10.1002/jper.18-0157 [DOI] [PubMed] [Google Scholar]
- Claffey, N. , & Egelberg, J. (1995). Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. Journal of Clinical Periodontology, 22, 690–696. 10.1111/j.1600-051X.1995.tb00828.x [DOI] [PubMed] [Google Scholar]
- Della Riccia, D. N. , Bizzini, F. , Perilli, M. G. , Polimeni, A. , Trinchieri, V. , Amicosante, G. , & Cifone, M. G. (2007). Anti‐inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Diseases, 13, 376–385. 10.1111/j.1601-0825.2006.01291.x [DOI] [PubMed] [Google Scholar]
- Feng, H. S. , Bernardo, C. C. , Sonoda, L. L. , Hayashi, F. , Romito, G. A. , De Lima, L. A. , … Pannuti, C. M. (2011). Subgingival ultrasonic instrumentation of residual pockets irrigated with essential oils: A randomized controlled trial. Journal of Clinical Periodontology, 38, 637–643. 10.1111/j.1600-051X.2011.01725.x [DOI] [PubMed] [Google Scholar]
- Hallstrom, H. , Lindgren, S. , Widen, C. , Renvert, S. , & Twetman, S. (2016). Probiotic supplements and debridement of peri‐implant mucositis: A randomized controlled trial. Acta Odontologica Scandinavica, 74, 60–66. 10.3109/00016357.2015.1040065 [DOI] [PubMed] [Google Scholar]
- Hallstrom, H. , Lindgren, S. , Yucel‐Lindberg, T. , Dahlen, G. , Renvert, S. , & Twetman, S. (2013). Effect of probiotic lozenges on inflammatory reactions and oral biofilm during experimental gingivitis. Acta Odontologica Scandinavia, 71, 828–833. 10.3109/00016357.2012.734406 [DOI] [PubMed] [Google Scholar]
- Harini, P. M. , & Anegundi, R. T. (2010). Efficacy of a probiotic and chlorhexidine mouth rinses: A short‐term clinical study. Journal of Indian Society of Pedodontics and Preventive Dentistry, 28, 179–182. 10.4103/0970-4388.73799 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. (2005). How effective is surgical therapy compared with nonsurgical debridement? Periodontology 2000, 37, 72–87. 10.1111/j.1600-0757.2004.03797.x [DOI] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , Pot, B. , … Sanders, M. E. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11, 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Ince, G. , Gursoy, H. , Ipci, S. D. , Cakar, G. , Emekli‐Alturfan, E. , & Yilmaz, S. (2015). Clinical and biochemical evaluation of lozenges containing lactobacillus reuteri as an adjunct to non‐surgical periodontal therapy in chronic periodontitis. Journal of Periodontology, 86, 746–754. 10.1902/jop.2015.140612 [DOI] [PubMed] [Google Scholar]
- Iniesta, M. , Herrera, D. , Montero, E. , Zurbriggen, M. , Matos, A. R. , Marin, M. J. , … Sanz, M. (2012). Probiotic effects of orally administered Lactobacillus reuteri ‐containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. Journal of Clinical Periodontology, 39, 736–744. [DOI] [PubMed] [Google Scholar]
- Jenkins, W. M. , Said, S. H. , Radvar, M. , & Kinane, D. F. (2000). Effect of subgingival scaling during supportive therapy. Journal of Clinical Periodontology, 27, 590–596. 10.1034/j.1600-051x.2000.027008590.x [DOI] [PubMed] [Google Scholar]
- Konig, J. , Schwahn, C. , Fanghanel, J. , Plotz, J. , Hoffmann, T. , & Kocher, T. (2008). Repeated scaling versus surgery in young adults with generalized advanced periodontitis. Journal of Periodontology, 79, 1006–1013. 10.1902/jop.2008.070380 [DOI] [PubMed] [Google Scholar]
- Krasse, P. , Carlsson, B. , Dahl, C. , Paulsson, A. , Nilsson, A. , & Sinkiewicz, G. (2005). Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swedish Dental Journal, 30, 55–60. [PubMed] [Google Scholar]
- Laleman, I. , Cortellini, S. , De Winter, S. , Rodriguez Herrero, E. , Dekeyser, C. , Quirynen, M. , & Teughels, W. (2017). Subgingival debridement: End point, methods and how often? Periodontol 2000, 75(1):189–204. 10.1111/prd.12204. Review. PMID:28758304. [DOI] [PubMed] [Google Scholar]
- Laleman, I. , Yilmaz, E. , Ozcelik, O. , Haytac, C. , Pauwels, M. , Herrero, E. R. , … Teughels, W. (2015). The effect of a streptococci containing probiotic in periodontal therapy: A randomized controlled trial. Journal of Clinical Periodontology, 42, 1032–1041. 10.1111/jcpe.12464 [DOI] [PubMed] [Google Scholar]
- Martin‐Cabezas, R. , Davideau, J. L. , Tenenbaum, H. , & Huck, O. (2016). Clinical efficacy of probiotics as an adjunctive therapy to non‐surgical periodontal treatment of chronic periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 43, 520–530. 10.1111/jcpe.12545 [DOI] [PubMed] [Google Scholar]
- Matuliene, G. , Pjetursson, B. E. , Salvi, G. E. , Schmidlin, K. , Bragger, U. , Zwahlen, M. , & Lang, N. P. (2008). Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. Journal of Clinical Periodontology, 35, 685–695. 10.1111/j.1600-051X.2008.01245.x [DOI] [PubMed] [Google Scholar]
- Mendonca, A. C. , Santos, V. R. , Ribeiro, F. V. , Lima, J. A. , Miranda, T. S. , Feres, M. , & Duarte, P. M. (2012). Surgical and non‐surgical therapy with systemic antimicrobials for residual pockets in type 2 diabetics with chronic periodontitis: A pilot study. Journal of Clinical Periodontology, 39, 368–376. 10.1111/j.1600-051X.2012.01860.x [DOI] [PubMed] [Google Scholar]
- Patel, A. M. , Richards, P. S. , Wang, H. L. , & Inglehart, M. R. (2006). Surgical or non‐surgical periodontal treatment: Factors affecting patient decision making. Journal of Periodontology, 77, 678–683. 10.1902/jop.2006.050206 [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Mombelli, A. , Mayfield, L. , Rutar, A. , Suvan, J. , Garrett, S. , & Lang, N. P. (2002). Local antimicrobial therapy after initial periodontal treatment. Journal of Clinical Periodontology, 29, 540–550. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf, U. , Jakob, L. , Eigenthaler, M. , Segerer, S. , Jockel‐Schneider, Y. , & Rehn, M. (2016). Regular consumption of Lactobacillus reuteri‐containing lozenges reduces pregnancy gingivitis: An RCT. Journal of Clinical Periodontology, 43, 948–954. 10.1111/jcpe.12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino, G. , Rosling, B. , Ramberg, P. , Hellstrom, M. K. , Socransky, S. S. , & Lindhe, J. (2001a). The effect of systemic antibiotics in the treatment of patients with recurrent periodontitis. Journal of Clinical Periodontology, 28, 411–418. 10.1034/j.1600-051x.2001.028005411.x [DOI] [PubMed] [Google Scholar]
- Serino, G. , Rosling, B. , Ramberg, P. , Socransky, S. S. , & Lindhe, J. (2001b). Initial outcome and long‐term effect of surgical and non‐surgical treatment of advanced periodontal disease. Journal of Clinical Periodontology, 28, 910–916. 10.1034/j.1600-051x.2001.028010910.x [DOI] [PubMed] [Google Scholar]
- Shimauchi, H. , Mayanagi, G. , Nakaya, S. , Minamibuchi, M. , Ito, Y. , Yamaki, K. , & Hirata, H. (2008). Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: A randomized, double‐blind, placebo‐controlled study. Journal of Clinical Periodontology, 35, 897–905. [DOI] [PubMed] [Google Scholar]
- Shiovitz, T. M. , Bain, E. E. , McCann, D. J. , Skolnick, P. , Laughren, T. , Hanina, A. , & Burch, D. (2016). Mitigating the effects of nonadherence in clinical trials. Journal of Clinical Pharmacology, 56, 1151–1164. 10.1002/jcph.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley, C. J. , Tracy, S. L. , Abt, E. , Michalowicz, B. S. , John, M. T. , Gunsolley, J. , … Hanson, N. (2015). Systematic review and meta‐analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. Journal of the American Dental Association, 146, 508–524.e505. 10.1016/j.adaj.2015.01.028 [DOI] [PubMed] [Google Scholar]
- Szkaradkiewicz, A. K. , Stopa, J. , & Karpinski, T. M. (2014). Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro‐inflammatory cytokine response in patients with chronic periodontitis. Archivum Immunolgiae Et Therapiae Experimentalis, 62, 495–500. 10.1007/s00005-014-0277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekce, M. , Ince, G. , Gursoy, H. , Dirikan Ipci, S. , Cakar, G. , Kadir, T. , & Yilmaz, S. (2015). Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1‐year follow‐up study. Journal of Clinical Periodontology, 42, 363–372. 10.1111/jcpe.12387 [DOI] [PubMed] [Google Scholar]
- Teughels, W. , Durukan, A. , Ozcelik, O. , Pauwels, M. , Quirynen, M. , & Haytac, M. C. (2013). Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo‐controlled study. Journal of Clinical Periodontology, 40, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, C. , Koutouzis, T. , & Wennstrom, J. L. (2008). Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets. Journal of Periodontology, 79, 431–439. 10.1902/jop.2008.070383 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45, S149–S161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Twetman, S. , Derawi, B. , Keller, M. , Ekstrand, K. , Yucel‐Lindberg, T. , & Stecksen‐Blicks, C. (2009). Short‐term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontologica Scandinavica, 67, 19–24. 10.1080/00016350802516170 [DOI] [PubMed] [Google Scholar]
- Vicario, M. , Santos, A. , Violant, D. , Nart, J. , & Giner, L. (2013). Clinical changes in periodontal subjects with the probiotic Lactobacillus reuteri Prodentis: A preliminary randomized clinical trial. Acta Odontologica Scandinavica, 71, 813–819. 10.3109/00016357.2012.734404 [DOI] [PubMed] [Google Scholar]
- Vivekananda, M. R. , Vandana, K. L. , & Bhat, K. G. (2010). Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. Journal of Oral Microbiology, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennstrom, J. L. , Tomasi, C. , Bertelle, A. , & Dellasega, E. (2005). Full‐mouth ultrasonic debridement versus quadrant scaling and root planing as an initial approach in the treatment of chronic periodontitis. Journal of Clinical Periodontology, 32, 851–859. 10.1111/j.1600-051X.2005.00776.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


