Summary
Background
Women have a better melanoma prognosis, and fairer skin/hair colour. The presence of inherited MC1R variants has been associated with a better melanoma prognosis, but its interaction with sex is unknown.
Objectives
To evaluate the relationship between germline MC1R status and survival, and determine any association with sex.
Methods
This was a cohort study including 1341 patients with melanoma from the Melanoma Unit of the Hospital Clinic of Barcelona, between January 1996 and April 2018. We examined known sex‐related prognosis factors as they relate to features of melanoma and evaluated the sex‐specific role of MC1R in overall and melanoma‐specific survival. Hazard ratios (HRs) were calculated using univariate and multivariate Cox logistic regression.
Results
Men showed lower overall survival than women (P < 0·001) and the presence of inherited MC1R variants was not associated with better survival in our cohort. However, in women the presence of MC1R variants was associated with better overall survival in the multivariate analysis [HR 0·57, 95% confidence interval (CI) 0·38–0·85; P = 0·006] but not in men [HR 1·26, 95% CI 0·89–1·79; P = 0·185 (P‐value for interaction 0·004)]. Analysis performed for melanoma‐specific survival showed the same level of significance.
Conclusions
Inherited MC1R variants are associated with improved overall survival in women with melanoma but not in men. Intrinsic sex‐dependent features can modify the role of specific genes in melanoma prognosis. We believe that survival studies of patients with melanoma should include analysis by sex and MC1R genotype.
What's already known about this topic?
Inherited MC1R variants have been associated with a better melanoma prognosis, but their interaction with sex is unknown.
What does this study add?
MC1R variants are related to better overall survival and melanoma‐specific survival in women but not in men.
What is the translational message?
These differences between the sexes could imply future changes in melanoma follow‐up and treatment strategies.
This provides a basis for understanding the interaction between sex‐related genes and germline variants in cancer.
Short abstract
https://www.bjdonline.com/article/
Linked Editorial: https://doi.org/10.1111/bjd.18555
Melanoma is the most aggressive of the common skin malignancies and is responsible for 75% of deaths from skin cancer.1 The American Joint Committee of Cancer staging system gives a clear prognostic value for patients with melanoma but cannot explain individual differences in survival.2
Femaleness has been demonstrated to be an independent, favourable prognostic factor in cutaneous malignant melanoma across nearly all tumour stages, including thin invasive tumours and advanced melanomas.3, 4, 5, 6, 7 Furthermore, males exhibit worse prognostic features, as they are more likely to have thick or ulcerated melanomas, and melanoma located on the trunk.7, 8, 9 Moreover, recent data highlight differences in body mass index and interferon regulation between men and women that may differently affect antitumour responses to immunotherapy.10, 11
Women have a higher prevalence of lighter hair colour and fairer skin than men.12, 13 The interaction between sex and genetic variants associated with tanning facility has been reported recently.14
MC1R is a highly polymorphic gene in the white population and one of the most important regulator genes in human skin and hair pigmentation.15, 16, 17 MC1R variants have been classified as ‘r’ variants when they have a low association with red hair colour phenotype (RHC) and ‘R’ variants when they are highly associated with RHC.18, 19, 20 Interestingly, R variants have been associated with fairer skin phenotypes in women.21 Finally, inherited MC1R variants increase the risk of developing a primary melanoma,22 yet appear to provide a survival advantage to these individuals.23, 24, 25
We hypothesized that inherited MC1R variants could play a differential role in survival in women and men.
Materials and methods
Cohort description
We used a cohort study design to analyse the prognostic value of germline MC1R status depending on sex in patients with melanoma. The cohort included 1341 consecutive patients with melanoma who were prospectively followed‐up in the Melanoma Unit of Hospital Clinic of Barcelona, between January 1996 and April 2018. All patients gave their written informed consent according to the Declaration of Helsinki, and the study was approved by the Research Ethics Committee of the Hospital Clinic of Barcelona (HCB/2015/0298 and HCB/2015/1032).
Clinical and phenotypic data from the patients and melanoma characteristics were collected in the Melanoma Unit database. For this study we extracted the basal demographic characteristics of the patients, clinical and pathological melanoma features, and survival outcome.
As inclusion criteria, only patients with a histopathological diagnosis of an invasive primary melanoma were considered for the study. For patients with multiple melanomas, the most invasive melanoma tumour by Breslow thickness was included, as it is the one that drives the prognosis.
Sentinel lymph node biopsy and follow‐up protocols were carried out according to the institution's guidelines.26, 27 Overall survival (OS) time was calculated from the date of diagnosis of the primary melanoma to the date of death by any cause or last follow‐up visit. Melanoma‐specific survival (MSS) was calculated from the time of diagnosis of the primary melanoma to the time of death by melanoma or the last follow‐up visit.
Our study was performed at a well‐known referral centre for advanced cases of melanoma, but bias may exist as it was a single‐centre study.
MC1R molecular screening
Genomic DNA was extracted from peripheral blood lymphocytes or saliva by standard methods. The whole coding region of MC1R was sequenced as reported elsewhere.28
Nonsynonymous MC1R variants were classified as RHC (‘R’) or non‐RHC (‘r’) according to previously reported criteria.20, 23, 28 Therefore, the p.D84E, p.R142H, p.R151C, p.I155T, p.R160W and p.D294H MC1R variants were classified as ‘R’, while all the other nonsynonymous MC1R variants were classified as ‘r’. Synonymous variants were considered as wild‐type (WT) MC1R alleles (‘w’). According to this classification we determined the following genotypes: R/R, R/r, R/w, r/r, r/w, w/w.
Statistical analyses
Pearson's χ2‐test, trend test for ordinal variables and the Kruskal–Wallis rank sum test were used to compare categorical, ordinal and continuous variables, respectively. Survival curves based on Kaplan–Meier methods were used to calculate survival outcomes and the log‐rank test was used for curve comparison.
We considered OS and MSS as the main variables. In order to better manage possible statistical multiplicity when considering two primary variables (OS and MSS), P‐values were considered statistically significant if P ≤ 0·025, following Bonferroni correction. If the described model produced probability values that reached significance, the following hierarchical stepwise process to reject the null hypotheses was followed: the interaction effect with sex was evaluated and, if this was significant at P < 0·10 (the usual cut‐off for interaction effects), the analysis was stratified by sex.29, 30
Furthermore, in the stratified analysis, univariate and multivariate Cox logistic regression analysis were performed to assess the influence of germline MC1R status in OS and MSS depending on sex, and to estimate the hazard ratio (HR) for each of the survival outcomes.
Statistical analyses were performed using the computing environment R and RStudio.31, 32
Results
A total of 3999 patients with a histopathological diagnosis of an invasive primary melanoma were identified. However, after applying the inclusion and exclusion criteria, 2658 patients were excluded, leaving 1341 eligible for inclusion (Fig. 1). Total person‐months were 105 775 with an incidence rate of relapse of 2·72 cases per 1000 person‐months. Median follow‐up time was 63·5 months (interquartile range 31·5–115·9).
Figure 1.
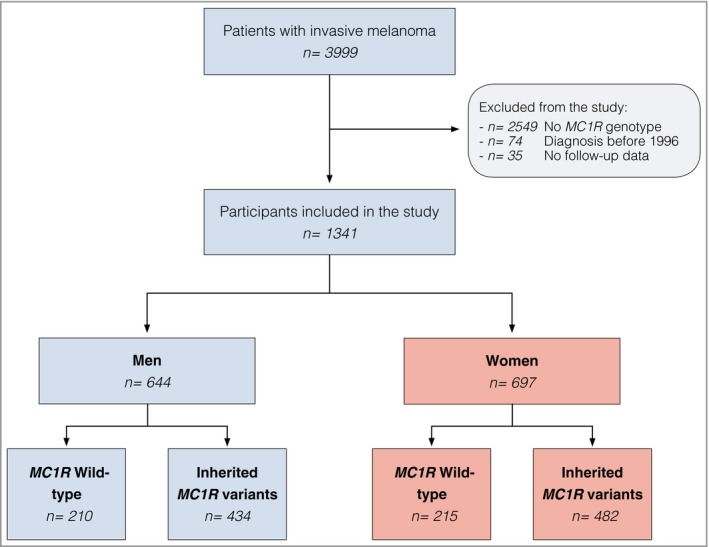
Flowchart of the study cohort.
The demographic, clinical and genetic characteristics of all included individuals are summarized in Table 1, and the clinicopathological characteristics of all tumours are given in Table 2. The average age of onset was 52·2 years in women and 56·7 years in men, and the mean Breslow thickness was 2·1 mm and 2·5 mm, respectively. Phenotypic characteristics showed a predominance of white skin, phototypes II and III, brown eyes and brown hair. Women had thinner tumours, which were less often ulcerated than those in men. Women presented more often with superficial spreading melanomas and tumours on the lower extremities. Men presented more often with nodular melanomas and tumours on the trunk.
Table 1.
Demographic, clinical and genetic characteristics of patients included at baseline
| Women (n = 697) | Men (n = 644) | Total (n = 1341) | P‐value | |
|---|---|---|---|---|
| Mean ± SD age of onset (y) | 52·22 ± 18·12 | 56·68 ± 16·58 | 54·37 ± 17·53 | < 0·001 |
| Eye colour | < 0·001 | |||
| Brown | 355 (56·5) | 335 (58·6) | 690 (57·5) | |
| Green | 160 (25·5) | 81 (14·2) | 241 (20·1) | |
| Blue | 98 (15·6) | 138 (24·1) | 236 (19·7) | |
| Black | 13 (2·1) | 11 (1·9) | 24 (2·0) | |
| Other | 2 (0·3) | 7 (1·2) | 9 (0·8) | |
| Missing | 69 | 72 | 141 | |
| Hair colour | < 0·001 | |||
| Brown | 419 (66·9) | 328 (57·5) | 747 (62·5) | |
| Black | 29 (4·6) | 64 (11·2) | 93 (7·8) | |
| Blonde | 138 (22·0) | 150 (26·3) | 288 (24·1) | |
| Red | 40 (6·4) | 28 (4·9) | 68 (5·7) | |
| Missing | 71 | 74 | 145 | |
| Ethnicity | 0·620 | |||
| White | 629 (99·5) | 574 (99·3) | 1203 (99·4) | |
| Latin | 1 (0·2) | 2 (0·3) | 3 (0·2) | |
| Asian | 0 (0·0) | 1 (0·2) | 1 (0·1) | |
| Black | 0 (0·0) | 0 (0·0) | 0 (0·0) | |
| Other | 2 (0·3) | 1 (0·2) | 3 (0·2) | |
| Missing | 65 | 66 | 131 | |
| Phototype | 0·760 | |||
| I | 42 (6·6) | 40 (6·8) | 82 (6·7) | |
| II | 328 (51·3) | 301 (51·5) | 629 (51·4) | |
| III | 228 (35·7) | 210 (35·9) | 438 (35·8) | |
| IV | 40 (6·3) | 33 (5·6) | 73 (6·0) | |
| V | 1 (0·2) | 1 (0·2) | 2 (0·2) | |
| Missing | 58 | 59 | 117 | |
| Naevus count | 0·919 | |||
| 0–50 | 307 (54·0) | 282 (53·6) | 589 (53·8) | |
| 51–100 | 149 (26·2) | 143 (27·2) | 292 (26·7) | |
| ≥ 101 | 113 (19·9) | 101 (19·2) | 214 (19·5) | |
| Missing | 128 | 118 | 246 | |
| Number of melanomas | 0·036 | |||
| Single | 549 (78·8) | 476 (73·9) | 1025 (76·4) | |
| Multiple | 148 (21·2) | 168 (26·1) | 316 (23·6) | |
| MC1R status | 0·488 | |||
| Wild‐type | 215 (30·8) | 210 (32·6) | 425 (31·7) | |
| Variants | 482 (69·2) | 434 (67·4) | 916 (68·3) | |
| MC1R genotype | 0·245 | |||
| RR | 34 (4·9) | 31 (4·8) | 65 (4·8) | |
| Rr | 80 (11·5) | 51 (7·9) | 131 (9·8) | |
| Rw | 116 (16·6) | 121 (18·8) | 237 (17·7) | |
| rr | 58 (8·3) | 44 (6·8) | 102 (7·6) | |
| rw | 194 (27·8) | 187 (29·0) | 381 (28·4) | |
| ww | 215 (30·8) | 210 (32·6) | 425 (31·7) |
Data are n (%) unless otherwise indicated.
Table 2.
Clinicopathological characteristics of the included tumours
| Women (n = 697) | Men (n = 644) | Total (n = 1341) | P‐value | |
|---|---|---|---|---|
| Mean ± SD Breslow index | 2·10 ± 3·27 | 2·47 ± 2·81 | 2·28 ± 3·06 | < 0·001 |
| Ulceration | < 0·001 | |||
| Absent | 508 (80·6) | 437 (72·2) | 945 (76·5) | |
| Present | 122 (19·4) | 168 (27·8) | 290 (23·5) | |
| Missing | 67 | 39 | 106 | |
| Mean ± SD mitotic index | 2·18 ± 3·95 | 2·53 ± 4·59 | 2·35 ± 4·28 | 0·122 |
| Missing | 133 | 106 | 239 | |
| Location | < 0·001 | |||
| Trunk | 248 (38·2) | 330 (54·1) | 578 (45·9) | |
| Lower limbs | 184 (28·3) | 78 (12·8) | 262 (20·8) | |
| Head and neck | 69 (10·6) | 95 (15·6) | 164 (13·0) | |
| Upper limbs | 78 (12·0) | 54 (8·9) | 132 (10·5) | |
| Acral | 50 (7·7) | 48 (7·9) | 98 (7·8) | |
| Mucosa | 18 (2·8) | 5 (0·8) | 23 (1·8) | |
| Other | 3 (0·5) | 0 (0·0) | 3 (0·2) | |
| Missing | 47 | 34 | 81 | |
| Histological subtype | 0·003 | |||
| Superficial spreading | 459 (66·8) | 397 (62·5) | 856 (64·8) | |
| Nodular | 99 (14·4) | 105 (16·5) | 204 (15·4) | |
| Acral lentiginous | 33 (4·8) | 45 (7·1) | 78 (5·9) | |
| Lentiginous malignant | 27 (3·9) | 46 (7·2) | 73 (5·5) | |
| Other | 69 (10·0) | 42 (6·6) | 111 (8·4) | |
| Missing | 10 | 9 | 19 |
Data are n (%) unless otherwise indicated.
Considering that Breslow thickness and ulceration are two of the main prognostic factors in melanoma, we evaluated the association of MC1R genotype with these variables in our cohort. We saw no significant differences between MC1R WT individuals and MC1R variant carriers regarding Breslow index (P = 0·908). Similarly, no significant differences were detected between MC1R WT and MC1R variant carriers when stratified by ulceration (P = 0·309) (Table S1; see Supporting Information). Interestingly, the analysis was not statistically significant when stratified for female sex (Breslow index P = 0·895; ulceration P = 0·925).
Kaplan–Meier curves showed that men had lower OS rates than women (P < 0·001; Fig. 2). This worse prognosis in men was further confirmed in the analysis using the multivariate Cox logistic regression model [HR 1·48, 95% confidence interval (CI) 1·15–1·91; P = 0·002].
Figure 2.
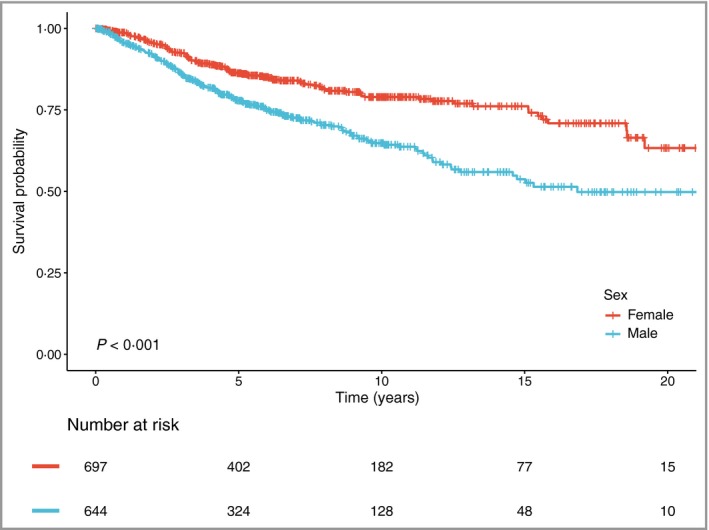
Overall survival by sex.
Analysis stratified by sex and MC1R genotype
Kaplan–Meier curves were modelled for patients with MC1R status. We did not detect statistically significant differences in OS between MC1R WT patients and carriers of any MC1R variant (P = 0·24; Fig. 3). Multivariate Cox regression analysis adjusted by sex, age, Breslow index and ulceration showed that MC1R genotype was not associated with better prognosis in this cohort (HR 0·88, 95% CI 0·68–1·14; P = 0·349).
Figure 3.
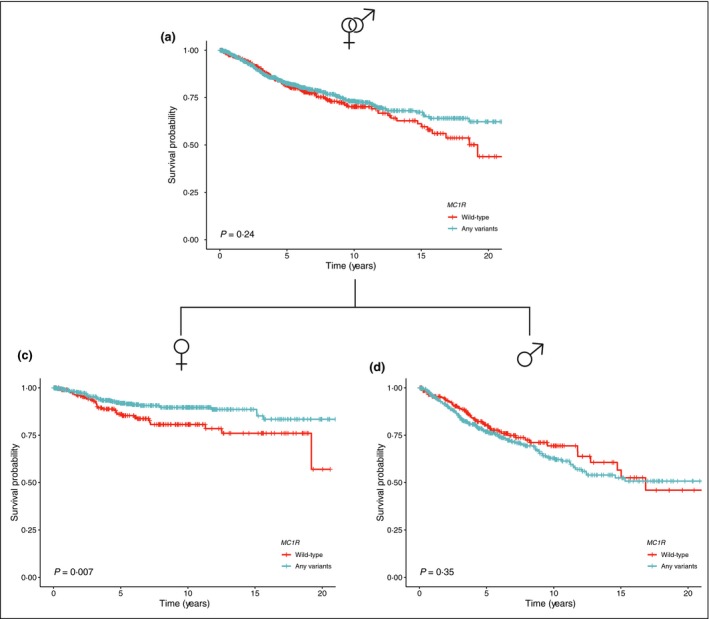
Overall survival by MC1R status depending on sex.
We subsequently analysed the influence of sex on the effect of MC1R variants in survival. Kaplan–Meier curves showed that women with inherited MC1R variants had a better melanoma prognosis than those with MC1R WT (P < 0·007). In contrast, the protective effect of inherited MC1R variants was not found in men (P = 0·35; Fig. 3).
Subsequently, we evaluated the interaction effect of sex and MC1R status for OS (P‐value for interaction 0·004) and for MSS (P‐value for interaction 0·052), which were in both cases statistically significant at alpha (P < 0·10), the usual measure of statistical significance for interaction effect. Consequently, we observed that the survival benefit derived from the MC1R mutational status depends on the sex of the patients.
Univariate Cox logistic regression analysis of OS and MSS in women showed that later age at onset, thicker tumours, high mitotic index and tumour ulceration were associated with a worse prognosis. By contrast, total naevus count > 50 and the presence of inherited MC1R variants were protective. Multivariate Cox logistic regression analysis including 630 women confirmed that the presence of inherited MC1R variants was a protective factor for OS (HR 0·57, 95% CI 0·38–0·85; P = 0·006) and MSS (HR 0·49, 95% CI 0·29–0·81; P = 0·005) (Table 3). After adjustment for other baseline characteristics (mitotic index, histological subtype and body site of the primary melanoma) the presence of any MC1R variation consistently showed a sizeable and statistical significance in HR estimation for OS and MSS.
Table 3.
Univariate and multivariate Cox regression analysis of overall survival (OS) and melanoma‐specific survival (MSS) in women
| OS | MSS | |||
|---|---|---|---|---|
| Univariable [HR (95% CI, P‐value)] | Multivariable [HR (95% CI, P‐value)]a | Univariable [HR (95% CI, P‐value)] | Multivariable [HR (95% CI, P‐value)]b | |
| Inherited MC1R variants (yes) | 0·57 (0·39–0·83, 0·003) | 0·57 (0·38–0·85, 0·006) | 0·53 (0·34–0·85, 0·008) | 0·49 (0·29–0·81, 0·005) |
| Age (continuous) | 1·06 (1·05–1·08, < 0·001) | 1·06 (1·05–1·08, < 0·001) | 1·04 (1·03–1·06, < 0·001) | 1·04 (1·02–1·05, < 0·001) |
| Breslow index (continuous) | 1·06 (1·04–1·08, < 0·001) | 1·04 (1·01–1·06, 0·006) | 1·06 (1·04–1·08, < 0·001) | 1·03 (1·00–1·07, 0·027) |
| Ulceration (present) | 3·71 (2·48–5·56, < 0·001) | 1·96 (1·27–3·01, 0·002) | 4·83 (2·92–8·00, < 0·001) | 3·02 (1·76–5·18, < 0·001) |
| Mitotic index (continuous) | 1·09 (1·06–1·13, < 0·001) | – | 1·12 (1·08–1·16, < 0·001) | – |
| Naevus total count (< 50) | – | – | – | – |
| 51–100 | 0·52 (0·29–0·92, 0·026) | – | 0·73 (0·38–1·39, 0·335) | – |
| ≥ 101 | 0·41 (0·21–0·81, 0·010) | – | 0·47 (0·21–1·07, 0·072) | – |
HR, hazard ratio; CI, confidence interval. aMultivariable analysis based on 630 women and 101 events (C‐index = 0·784); bMultivariable analysis based on 630 women and 63 events (C‐index = 0·777)
Similarly, univariate Cox logistic regression analysis in men showed that later age at onset, thicker tumours, the presence of ulceration and a high mitotic index were mortal risk factors, and a naevus total count > 50 was a protective factor. Contrary to women, the presence of inherited MC1R variants was not associated with improved survival in men. Multivariate Cox logistic regression analysis including 605 men showed no effects on OS (HR 1·26, 95% CI 0·89–1·79; P = 0·185) or MSS (HR 1·09, 95% CI 0·71–1·67; P = 0·685) (Table 4).
Table 4.
Univariate and multivariate Cox regression analysis of overall survival (OS) and melanoma‐specific survival (MSS) in men
| OS | MSS | |||
|---|---|---|---|---|
| Univariable [HR (95% CI, P‐value)] | Multivariable [HR (95% CI, P‐value)]a | Univariable [HR (95% CI, P‐value)] | Multivariable [HR (95% CI, P‐value)]b | |
| Inherited MC1R variants (yes) | 1·17 (0·84–1·63, 0·347) | 1·26 (0·89–1·79, 0·185) | 0·98 (0·65–1·47, 0·917) | 1·09 (0·71–1·67, 0·685) |
| Age (continuous) | 1·05 (1·04–1·06, < 0·001) | 1·04 (1·03–1·05, < 0·001) | 1·02 (1·01–1·03, 0·001) | 1·02 (1·00–1·03, 0·011) |
| Breslow index (continuous) | 1·18 (1·14–1·22, < 0·001) | 1·15 (1·10–1·20, < 0·001) | 1·21 (1·17–1·25, < 0·001) | 1·17 (1·12–1·23, < 0·001) |
| Ulceration (present) | 2·75 (2·02–3·75, < 0·001) | 1·41 (0·97–2·06, 0·074) | 3·55 (2·40–5·25, < 0·001) | 1·80 (1·13–2·88, 0·013) |
| Mitotic index (continuous) | 1·06 (1·03–1·08, < 0·001) | – | 1·08 (1·05–1·11, < 0·001) | – |
| Naevus total count (< 50) | – | – | – | – |
| 51–100 | 0·47 (0·28–0·77, 0·003) | – | 0·49 (0·26–0·93, 0·030) | – |
| ≥ 101 | 0·65 (0·40–1·06, 0·086) | – | 0·59 (0·30–1·15, 0·119) | – |
HR, hazard ratio; CI, confidence interval. aMultivariable analysis based on 605 men and 165 events (C‐index = 0·758). bMultivariable analysis based on 605 men and 103 events (C‐index = 0·758)
Discussion
The significance of sex difference on melanoma prognosis is well known. Better MSS in women has been attributed to both lifestyle and biological factors. Women perform self‐examination or tend to contact dermatologists for screening more often than men, allowing an earlier diagnosis of melanoma in women.33 Moreover, the survival prognosis is higher in women than in men throughout childbearing age, except during pregnancy. However, this survival benefit is not maintained after menopause. This suggests the importance of hormonal signalling in melanoma development.34, 35, 36
A BioGenoMEL collaborative study suggested that the presence of any inherited MC1R variants was associated with improved melanoma survival, adjusting for age and sex (HR 0·82, 95% CI 0·66–1·02; P = 0·08).23 The result only reached statistical significance with the addition of the Leeds cohort data (HR 0·77, 95% CI 0·64–0·93; P = 0·005).23 Taylor et al. also suggested that the presence of MC1R variants is associated with better MSS (but not OS) in individuals with a first incident primary melanoma.25 These analyses did not stratify by sex and, consequently, this putative protective effect in women was not evaluated.
In our study we identified that inherited MC1R variants are associated with better survival in women but not in men. Additionally, we did not observe more severe pathological tumour characteristics in women with MC1R variants. Therefore, our study indicates, for the first time, that the presence of inherited MC1R variants may be an independent protective factor in the survival of female patients with melanoma. Although the sex‐related biological mechanisms that underlie this finding are unknown, we hypothesize that the regulation of oxidative stress could offer a partial explanation. In melanocytes, the loss of melanocortin 1 receptor (MC1R) function reduces DNA repair capacity and decreases antioxidant response,37 causing genomic instability and increasing oxidative stress.38 This may lead melanocytes to undergo malignant transformation. Interestingly, oestrogen (the primary female sex hormone) induces the generation of reactive oxidative species (ROS).39 This fact is consistent with the higher incidence of melanoma in adult premenopausal women.40 The effect of ROS on malignant cell transformation is dose dependent. While certain levels of ROS promote malignant transformation, excessively high ROS levels cause detrimental oxidative stress that can lead to cell death.41 Therefore, we suggest that in women melanocytes harbouring MC1R variants could cause excessive oxidative damage that is detrimental to melanoma progression.
Previous studies have indicated that inherited MC1R variants contribute differently to pigmentation phenotype in men and women. Moreover, women carrying RHC MC1R variants tended to exhibit significantly fairer phototypes than men with the same MC1R genotypes.42 Differences between the sexes according to MC1R status do not only affect pigmentation phenotype. A recently published study has shown that the risk of developing melanoma associated with inherited MC1R variants is much higher in women than in men.43 In the same line, several studies have identified a sex‐differential effect of MC1R on analgesic response.21, 44 Specifically, women with two inherited MC1R variants show greater analgesia than men.44 Taken together, these findings highlight the fact that MC1R is a pleiotropic protein involved in the regulation of different biological processes and show that some of these MC1R effects are sex dependent.
In conclusion, our results show that the presence of inherited MC1R variants improves survival in women with melanoma. This could explain some of the prognostic differences between men and women. Future prospective studies should take into account sex differences in survival and could help to explain how the inherited MC1R variants may play a role in survival. This could imply an improvement in personalized melanoma follow‐up.
Supporting information
Table S1 Analysis of clinicopathological characteristics according to MC1R genotype.
Acknowledgments
We thank our patients and their families, who are the main reason for our studies. We also thank the nurses at the Melanoma Unit of the Hospital Clinic of Barcelona; Daniel Gabriel, Pablo Iglesias and Maria E. Moliner for helping to collect patient data; Judit Mateu from the ‘Melanoma: image, genetics and immunology’ group at IDIBAPS for her technical assistance; Paul Hetherington for his help with English editing and correction of the manuscript; and José Ríos for his help with biostatistics.
Funding sources The study at the Melanoma Unit, Hospital Clinic, Barcelona, was supported, in part, by grants from Fondo de Investigaciones Sanitarias, Spain (P.I. 12/00840, PI15/00956 and PI15/00716); by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co‐funded by ‘Fondo Europeo de Desarrollo Regional (FEDER). Unión Europea. Una manera de hacer Europa’; by AGAUR 2014_SGR_603 and 2017_SGR_1134 of the Catalan Government, Spain; by a grant from ‘Fundació La Marató de TV3, 201331‐30’, Catalonia, Spain; by the European Commission under the 6th Framework Programme [contract no.: LSHC‐CT‐2006‐018702 (GenoMEL)]; by CERCA Programme/Generalitat de Catalunya and by a Research Grant from ‘Fundación Científica de la Asociación Española Contra el Cáncer’ GCB15152978SOEN, Spain; and by a grant from the European Academy of Dermatology and Venereology (PPRC‐2017/19). Part of the work was developed at the building Centro Esther Koplowitz, Barcelona. M.P. is the recipient of PhD Fellowship FI14/00231 (PFIS) from Instituto de Salud Carlos III, Spain. N.C.‐L. is the recipient of PhD Fellowship FPU17/05453 (FPU) from Ministerio de Educación, Cultura y Deportes, Spain. The sponsors had no role in the design or conduct of the study; in the collection, analysis and interpretation of data; nor in the preparation, review or approval of the manuscript.
Conflicts of interest None declared.
F.E.L. and S.P. contributed equally to this work.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Balch CM, Gershenwald JE, Soong SJ et al Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joosse A, De Vries E, Eckel R et al Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol 2011; 131:719–26. [DOI] [PubMed] [Google Scholar]
- 4. Joosse A, Collette S, Suciu S et al Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European organisation for research and treatment of cancer phase III trials. J Clin Oncol 2012; 30:2240–7. [DOI] [PubMed] [Google Scholar]
- 5. Joosse A, Collette S, Suciu S et al Sex is an independent prognostic indicator for survival and relapse/progression‐free survival in metastasized stage III to IV melanoma: a pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol 2013; 31:2337–46. [DOI] [PubMed] [Google Scholar]
- 6. Lideikait≐ A, Mozūraitien≐ J, Letautien≐ S. Analysis of prognostic factors for melanoma patients. Acta Med Litu 2017; 24:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khosrotehrani K, Dasgupta P, Byrom L et al Melanoma survival is superior in females across all tumour stages but is influenced by age. Arch Dermatol Res 2015; 307:731–40. [DOI] [PubMed] [Google Scholar]
- 8. Garbe C, Büttner P, Bertz J et al Primary cutaneous melanoma. Identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer 1995; 75:2484–91. [DOI] [PubMed] [Google Scholar]
- 9. Johnson TM, Smith JW, Nelson BR, Chang A. Current therapy for cutaneous melanoma. J Am Acad Dermatol 1995; 32:689–707. [DOI] [PubMed] [Google Scholar]
- 10. Capone I, Marchetti P, Ascierto PA et al Sexual dimorphism of immune responses: a new perspective in cancer immunotherapy. Front Immunol 2018; 9:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McQuade JL, Daniel CR, Hess KR et al Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018; 19:310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hysi PG, Valdes AM, Liu F et al Genome‐wide association meta‐analysis of individuals of European ancestry identifies new loci explaining a substantial fraction of hair color variation and heritability. Nat Genet 2018; 50:652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 2000; 39:57–106. [DOI] [PubMed] [Google Scholar]
- 14. Visconti A, Duffy DL, Liu F et al Genome‐wide association study in 176,678 Europeans reveals genetic loci for tanning response to sun exposure. Nat Commun 2018; 9:1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gudbjartsson DF, Sulem P, Stacey SN et al ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet 2008; 40:886–91. [DOI] [PubMed] [Google Scholar]
- 16. Potrony M, Badenas C, Aguilera P et al Update in genetic susceptibility in melanoma. Ann Transl Med 2015; 3:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Torre C, Garcia‐Casado Z, Martínez‐Escribano JA et al Influence of loss of function MC1R variants in genetic susceptibility of familial melanoma in Spain. Melanoma Res 2010; 20:342–8. [DOI] [PubMed] [Google Scholar]
- 18. Dessinioti C, Antoniou C, Katsambas A, Stratigos AJ. Melanocortin 1 receptor variants: functional role and pigmentary associations. Photochem Photobiol 2011; 87:978–87. [DOI] [PubMed] [Google Scholar]
- 19. Flanagan N, Healy E, Ray A et al Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet 2000; 9:2531–7. [DOI] [PubMed] [Google Scholar]
- 20. Raimondi S, Sera F, Gandini S et al MC1R variants, melanoma and red hair color phenotype: a meta‐analysis. Int J Cancer 2008; 122:2753–60. [DOI] [PubMed] [Google Scholar]
- 21. Arout CA, Caldwell M, Rossi G, Kest B. Spinal and supraspinal N‐methyl‐d‐aspartate and melanocortin‐1 receptors contribute to a qualitative sex difference in morphine‐induced hyperalgesia. Physiol Behav 2015; 147:364–72. [DOI] [PubMed] [Google Scholar]
- 22. Tagliabue E, Gandini S, Bellocco R et al MC1R variants as melanoma risk factors independent of at‐risk phenotypic characteristics: a pooled analysis from the M‐SKIP project. Cancer Manag Res 2018; 10:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies JR, Randerson‐Moor J, Kukalizch K et al Inherited variants in the MC1R gene and survival from cutaneous melanoma: a BioGenoMEL study. Pigment Cell Melanoma Res 2012; 25:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatvani Z, Brodszky V, Mazán M et al Genotype analysis in Hungarian patients with multiple primary melanoma. Exp Dermatol 2014; 23:361–4. [DOI] [PubMed] [Google Scholar]
- 25. Taylor NJ, Reiner AS, Begg CB et al Inherited variation at MC1R and ASIP and association with melanoma‐specific survival. Int J Cancer 2015; 136:2659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Podlipnik S, Moreno‐Ramírez D, Carrera C et al Cost‐effectiveness analysis of imaging strategy for an intensive follow‐up of patients with AJCC stage IIB, IIC and III malignant melanoma. Br J Dermatol 2019; 180:1190–7. [DOI] [PubMed] [Google Scholar]
- 27. Podlipnik S, Carrera C, Sánchez M et al Performance of diagnostic tests in an intensive follow‐up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: A prospective cohort study. J Am Acad Dermatol 2016; 75:516–24. [DOI] [PubMed] [Google Scholar]
- 28. Vallone MG, Tell‐Marti G, Potrony M et al Melanocortin 1 receptor (MC1R) polymorphisms’ influence on size and dermoscopic features of nevi. Pigment Cell Melanoma Res 2018; 31:39–50. [DOI] [PubMed] [Google Scholar]
- 29. Brookes ST, Whitely E, Egger M et al Subgroup analyses in randomized trials: risks of subgroup‐specific analyses; power and sample size for the interaction test. J Clin Epidemiol 2004; 57:229–36. [DOI] [PubMed] [Google Scholar]
- 30. Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010; 340:c117. [DOI] [PubMed] [Google Scholar]
- 31. RStudio Team . RStudio: Integrated Development Environment for R. RStudio: Boston, MA, 2016. [Google Scholar]
- 32. R Core Team . R: A Language and Environment for Statistical Computing. Foundation for Statistical Computing: Vienna, 2018. [Google Scholar]
- 33. Carli P, De Giorgi V, Palli D et al Self‐detected cutaneous melanomas in Italian patients. Clin Exp Dermatol 2004; 29:593–6. [DOI] [PubMed] [Google Scholar]
- 34. Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther 2010; 10:1811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt AN, Nanney LB, Boyd AS et al Oestrogen receptor‐β expression in melanocytic lesions. Exp Dermatol 2006; 15:971–80. [DOI] [PubMed] [Google Scholar]
- 36. de Giorgi V, Gori A, Gandini S et al Oestrogen receptor beta and melanoma: a comparative study. Br J Dermatol 2013; 168:513–19. [DOI] [PubMed] [Google Scholar]
- 37. Abdel‐Malek ZA, Knittel J, Kadekaro AL et al The melanocortin 1 receptor and the UV response of human melanocytes – a shift in paradigm. Photochem Photobiol 2008; 84:501–8. [DOI] [PubMed] [Google Scholar]
- 38. Robles‐Espinoza CD, Roberts ND, Chen S et al Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun 2016; 7:12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roy D, Cai Q, Felty Q, Narayan S. Estrogen‐Induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen‐dependent cancers. J Toxicol Environ Heal Part B 2007; 10:235–57. [DOI] [PubMed] [Google Scholar]
- 40. Roh MR, Eliades P, Gupta S et al Cutaneous melanoma in women. Int J Womens Dermatol 2017; 3:S11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11:85–95. [DOI] [PubMed] [Google Scholar]
- 42. Hernando B, Ibarrola‐Villava M, Peña‐Chilet M et al Sex and MC1R variants in human pigmentation: differences in tanning ability and sensitivity to sunlight between sexes. J Dermatol Sci 2016; 84:346–8. [DOI] [PubMed] [Google Scholar]
- 43. Wendt J, Mueller C, Rauscher S et al Contributions by MC1R variants to melanoma risk in males and females. JAMA Dermatol 2018; 154:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mogil JS, Wilson SG, Chesler EJ et al The melanocortin‐1 receptor gene mediates female‐specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A 2003; 100:4867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Analysis of clinicopathological characteristics according to MC1R genotype.


