Abstract
Background and Purpose
Gamma-glutamyl transferase (GGT) is reported to be associated with stroke independently of the conventional risk factors. However, the underlying mechanism remains to be identified. This study focused on atrial fibrillation (AF), which also reportedly has a close association with GGT.
Methods
Acute ischemic stroke patients who were admitted to the Seoul National University Hospital within 7 days of stroke onset were analyzed. Multinomial logistic regression was performed to assess the relationship between GGT and cardioembolic stroke. Mediation analysis based on binary logistic regression was used to determine whether AF mediates the relationship between GGT and cardioembolic stroke.
Results
AF was found in 132 (15.0%) of 880 eligible patients with acute ischemic stroke, and 270 (30.7%) patients were categorized as cardioembolic stroke. High GGT levels in acute ischemic stroke patients was associated with cardioembolic stroke [odds ratio (OR)=3.42, 95% CI=1.59–7.37], but not with large-artery atherosclerosis stroke (OR=1.10, 95% CI=0.54–2.23). Approximately half (53.9%) of the total effect of GGT levels on cardioembolic stroke was mediated by AF.
Conclusions
The GGT level was significantly associated with cardioembolic stroke via AF. The results obtained in the present study may explain why GGT is associated with stroke.
Keywords: stroke, gamma-glutamyl transferase, biomarkers, atrial fibrillation, cardioembolic stroke
INTRODUCTION
Gamma-glutamyl transferase (GGT), which is best known as a marker of liver disease, has recently attracted attention as a biomarker of various vascular diseases. Serial studies of the association between GGT and stroke have also highlighted the potential of GGT as a novel biomarker for predicting stroke.1,2,3,4,5,6,7 Nevertheless, it remains unclear why GGT is associated with stroke. The proper use GGT as a biomarker of stroke requires clarification of the mechanism via which GGT is associated with stroke. In a recent study we found that GGT may be more consistently associated with ischemic stroke than with hemorrhagic stroke.8 That study raised the question of which subtype of ischemic stroke is most closely associated with GGT. Several studies have recently found an independent relationship between GGT and atrial fibrillation (AF).9,10,11,12 Considering that AF is one of the most important causes of ischemic stroke, we hypothesized that AF mediates the association between GGT and ischemic stroke.
METHODS
Study population
We reviewed acute ischemic stroke patients who were admitted to Seoul National University Hospital and had their GGT measured within 7 days of onset between October 2002 and May 2017. Among 1,102 identified patients, those with missing laboratory data (n=144) were excluded. Seventy-eight patients were categorized as “other determined” using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification, and roughly half of them were cancer patients (Supplementary Table 1 in the online-only Data Supplement). The cancer patients, especially those with tumors involving the hepatobiliary tract or pancreas, tended to have very high GGT levels (Supplementary Table 2 in the online-only Data Supplement), possibly due to an association between GGT levels and liver disease or the cancer itself.13,14 Considering the effect that this might have on the outcome, we excluded all of the “other determined” patients. This study was approved by the Institutional Review Board at the Seoul National University Hospital (IRB No. H-1706-081-859). The need to obtain informed consent was waived.
Data collection
Data were collected at admission on sex, age, height, weight, initial systolic blood pressure, fasting blood glucose, total cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT, and the histories of previous stroke, hypertension, diabetes, hyperlipidemia, liver disease (e.g., hepatitis or fatty liver disease), and smoking (current or past). Body mass index (BMI) was calculated from the height and weight. The presence of AF was determined based on the results of electrocardiography, echocardiography, or Holter monitoring during admission. Baseline neurologic deficits were evaluated by at least two experienced neurologists using the National Institutes of Health Stroke Scale score at admission. The subtype was assessed using the TOAST classification after the stroke evaluation.
Mediation analysis
The following three paths should be analyzed when evaluating the mediation effect of the mediator variable (AF) on the relationship between the independent variable (GGT) and the dependent variable (cardioembolic stroke): 1) the association between the independent variable and the mediator, 2) the association between the mediator and the dependent variable, and 3) the association between the independent variable and the dependent variable. Mediation cannot be established if one of these associations is not significant. If there are significant associations between paths a through c, the next step is to assess the association between the independent variable and the dependent variable while controlling for the mediator (path c′, a direct effect). The mediator exerts a full mediation effect if the independent variable is no longer significant when controlling the mediator, and a partial mediation effect if the independent variable remains significant but with a decreased strength.
In the present study, paths a through c were tested with binary logistic regression to assess whether the association between GGT and cardioembolic stroke (compared with other stroke subtypes) is mediated by AF. The coefficients needed to first be standardized in order to allow comparisons of those derived from different logistic regression models for each path. Multiplying each coefficient by the SD of the predictor per SD of outcome resulted in the calculation of comparable coefficients. The percentage of the effect mediated by the mediator was determined by dividing the comparable coefficient of the indirect effect by the total effect. The Sobel test was applied to confirm the significance of the mediation effect.15,16
Statistical methods
Patients were divided into sex-specific GGT quartiles (males: ≤20, 21–29, 30–53, and ≥54 IU/L; females: ≤14, 15–21, 22–34, and ≥35 IU/L). Baseline clinical data across GGT quartiles were analyzed using the chi-square test or Kruskal-Wallis test. Multinomial logistic regression was performed to determine whether the relationship between GGT and ischemic stroke is affected by the subtype. Adjustments were first made for age and sex, and then for the following covariates: BMI, systolic blood pressure, fasting blood glucose, total cholesterol, AST, ALT, previous stroke, hypertension, diabetes, hyperlipidemia, liver disease, and smoking. Additional adjustment for AF was made when analyzing the relationship between GGT and cardioembolic stroke. The presence of multicollinearity among the variables was determined by calculating the generalized variance-inflation factor (GVIF). In order to standardize GVIF values for different dimensions and allow them to be compared, we took the 1/(2×df) power of the GVIF, where df is the degrees of freedom. A GVIF1/(2×df) value of above 2 means that multicollinearity may be present in the model.17 The p value for the trend across the GGT quartiles was calculated using the Wald test, and the GGT quartiles were regarded as continuous when testing for trends.
The results are presented as odds ratios with their 95% CIs. Two-sided probability values of <0.05 were considered statistically significant. All of the statistical analyses were performed using R statistical software (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
This study included 880 eligible acute ischemic stroke patients. Those with higher GGT levels were more likely to be obese and have higher glucose, AST, and ALT levels. The 880 patients included 132 (15.0%) with AF and 270 (30.7%) classified as cardioembolic according to the TOAST criteria (Table 1). AF was present in 116 (43.0%) of the 270 patients with cardioembolic stroke.
Table 1. Baseline characteristics according to GGT quartiles.
| GGT quartile | Q1 (n=195) | Q2 (n=237) | Q3 (n=224) | Q4 (n=224) | p |
|---|---|---|---|---|---|
| Sex | 0.41 | ||||
| Male | 123 (63.1) | 132 (55.7) | 138 (61.6) | 132 (58.9) | |
| Female | 72 (36.9) | 105 (44.3) | 86 (38.4) | 92 (41.1) | |
| Age, years | 72.0 [64.0–78.0] | 71.0 [61.0–77.0] | 68.0 [60.0–75.0] | 67.0 [59.0–75.0] | 0.001 |
| BMI, kg/m2 | 22.6 [20.7–24.5] | 23.1 [21.2–25.3] | 23.8 [22.0–26.0] | 23.7 [21.7–26.1] | <0.001 |
| Initial systolic blood pressure, mm Hg | 157.0 [133.0–179.5] | 153.0 [138.0–174.0] | 150.0 [130.5–171.0] | 150.0 [130.0–175.0] | 0.33 |
| Fasting blood glucose, mg/dL | 98.0 [85.0–117.0] | 98.0 [88.0–122.0] | 100.0 [88.0–124.5] | 107.0 [90.5–132.0] | 0.002 |
| Total cholesterol, mg/dL | 170.0 [141.5–200.5] | 171.0 [147.0–198.0] | 175.0 [146.0–198.5] | 176.0 [147.0–205.0] | 0.52 |
| AST, IU/L | 19.0 [15.5–23.0] | 21.0 [18.0–27.0] | 24.0 [19.0–31.0] | 30.0 [22.0–43.5] | <0.001 |
| ALT, IU/L | 14.0 [10.0–18.0] | 16.0 [13.0–23.0] | 20.0 [15.0–30.0] | 27.5 [17.0–45.5] | <0.001 |
| GGT, IU/L | 13.0 [11.0–17.0] | 21.0 [17.0–24.0] | 32.0 [29.0–37.5] | 68.0 [52.5–99.5] | <0.001 |
| Previous stroke | 34 (17.4) | 58 (24.5) | 45 (20.1) | 36 (16.1) | 0.12 |
| Hypertension | 126 (64.6) | 168 (70.9) | 161 (71.9) | 151 (67.4) | 0.35 |
| Diabetes | 67 (34.4) | 79 (33.3) | 74 (33.0) | 85 (37.9) | 0.68 |
| Hyperlipidemia | 56 (28.7) | 71 (30.0) | 86 (38.4) | 79 (35.3) | 0.11 |
| Liver disease | 17 (8.7) | 35 (14.8) | 33 (14.7) | 53 (23.7) | <0.001 |
| Smoking history | 65 (33.3) | 85 (35.9) | 86 (38.4) | 89 (39.7) | 0.54 |
| AF | 19 (9.7) | 34 (14.3) | 35 (15.6) | 44 (19.6) | 0.04 |
| NIHSS score at admission | 4 [2–8] | 4 [2–10] | 4 [2–9] | 5 [2–11] | 0.17 |
| TOAST classification | 0.01 | ||||
| LAA | 78 (40.0) | 82 (34.6) | 88 (39.3) | 68 (30.4) | |
| SVO | 34 (17.4) | 42 (17.7) | 27 (12.1) | 27 (12.1) | |
| CE | 40 (20.5) | 74 (31.2) | 71 (31.7) | 85 (37.9) | |
| UD | 43 (22.1) | 39 (16.5) | 38 (17.0) | 44 (19.6) |
Data are n (%) or median [interquartile range] values.
AF: atrial fibrillation, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, CE: cardioembolism, GGT: gamma-glutamyl transferase, LAA: large-artery atherosclerosis, NIHSS: National Institutes of Health Stroke Scale, SVO: small-vessel occlusion, TOAST: Trial of ORG 10172 in Acute Stroke Treatment, UD: undetermined.
The median GGT level was higher in patients with cardioembolic stroke than in those with other stroke subtypes (Supplementary Table 3 in the online-only Data Supplement). Higher GGT levels were significantly associated with cardioembolic stroke after adjusting for age and sex (model 1), for vascular diseases and laboratory data (model 2), and additionally for AF. However, GGT was not associated with large-artery atherosclerosis (LAA) stroke (Table 2). No multicollinearity was found among the variables (Supplementary Table 4 in the online-only Data Supplement).
Table 2. ORs and 95% CIs for the subtypes of ischemic stroke according to GGT based on multinomial logistic regression.
| GGT quartile | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| CE (reference=SVO) | |||||
| Unadjusted | 1 | 1.50 (0.83–2.71) | 2.24 (1.18–4.23) | 2.68 (1.43–5.02) | 0.001 |
| Model 1* | 1 | 1.50 (0.82–2.73) | 2.43 (1.27–4.62) | 3.00 (1.58–5.70) | <0.001 |
| Model 2† | 1 | 1.61 (0.84–3.06) | 2.79 (1.37–5.70) | 3.42 (1.59–7.37) | <0.001 |
| Model 2+AF | 1 | 1.52 (0.75–3.08) | 2.37 (1.10–5.10) | 2.60 (1.14–5.96) | 0.01 |
| LAA (reference=SVO) | |||||
| Unadjusted | 1 | 0.85 (0.49–1.47) | 1.42 (0.79–2.56) | 1.10 (0.60–2.00) | 0.38 |
| Model 1 | 1 | 0.87 (0.50–1.51) | 1.47 (0.81–2.66) | 1.16 (0.63–2.13) | 0.29 |
| Model 2 | 1 | 0.81 (0.46–1.43) | 1.36 (0.72–2.58) | 1.10 (0.54–2.23) | 0.45 |
| UD (reference=SVO) | |||||
| Unadjusted | 1 | 0.73 (0.39–1.37) | 1.11 (0.57–2.17) | 1.29 (0.67–2.49) | 0.26 |
| Model 1 | 1 | 0.74 (0.39–1.38) | 1.10 (0.56–2.15) | 1.27 (0.65–2.46) | 0.29 |
| Model 2 | 1 | 0.73 (0.38–1.42) | 1.10 (0.53–2.29) | 1.25 (0.57–2.75) | 0.39 |
Data are OR (95% CI) values.
*Adjusted for sex and age, †Adjusted for sex, age, body mass index, systolic blood pressure, fasting blood glucose, total cholesterol, aspartate aminotransferase, alanine aminotransferase, previous stroke, hypertension, diabetes, hyperlipidemia, liver disease, and smoking.
AF: atrial fibrillation, CE: cardioembolism, GGT: gamma-glutamyl transferase, LAA: large-artery atherosclerosis, OR: odds ratio, SVO: small-vessel occlusion, UD: undetermined.
Mediation analysis
Binary logistic regression was used for the mediation analysis (Table 3, Supplementary Table 5 in the online-only Data Supplement). Significant relationships were confirmed between GGT and AF (path a: coefficient β=0.24, 95% CI=0.07–0.42, p=0.005), between AF and cardioembolic stroke (path b: coefficient β=3.33, 95% CI=2.78–3.88, p<0.001), and between GGT and cardioembolic stroke (path c: coefficient β=0.25, 95% CI=0.11–0.38, p<0.001). GGT had a significant but weaker effect on cardioembolic stroke when AF was controlled for (path c′: coefficient β=0.20, 95% CI= 0.05–0.36, p=0.01). These results indicated that AF exerted a partial mediation effect on the association between GGT and cardioembolic stroke.
Table 3. ORs and 95% CIs for atrial fibrillation according to GGT in ischemic stroke patients.
| GGT quartile | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| Unadjusted | 1 | 1.55 (0.85–2.82) | 1.72 (0.95–3.11) | 2.26 (1.27–4.03) | 0.005 |
| Model 1* | 1 | 1.60 (0.87–2.95) | 2.06 (1.12–3.80) | 2.83 (1.56–5.15) | <0.001 |
| Model 2† | 1 | 1.76 (0.94–3.32) | 2.47 (1.29–4.73) | 3.31 (1.70–6.46) | <0.001 |
Data are OR (95% CI) values.
*Adjusted for sex and age, †Adjusted for sex, age, body mass index, systolic blood pressure, fasting blood glucose, total cholesterol, aspartate aminotransferase, alanine aminotransferase, previous stroke, hypertension, diabetes, hyperlipidemia, liver disease, and smoking.
GGT: gamma-glutamyl transferase, OR: odds ratio.
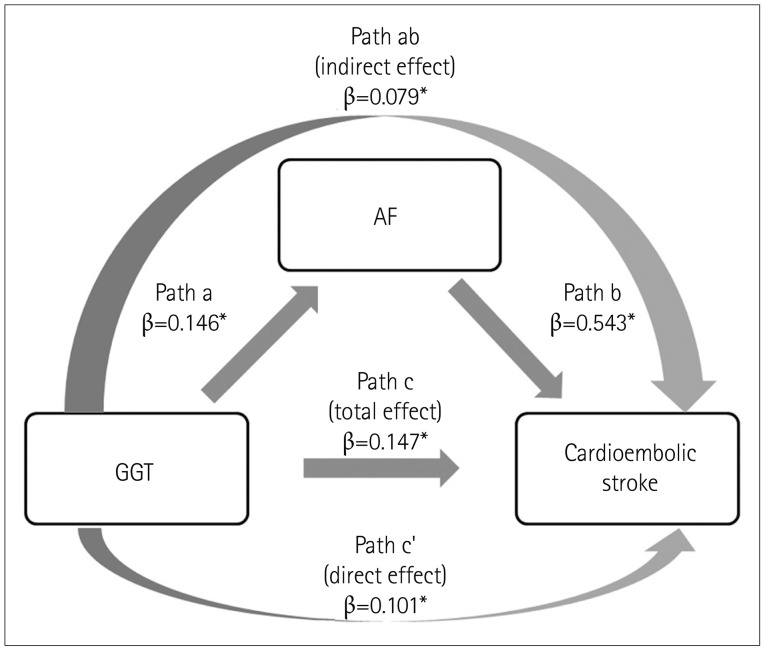
Comparable coefficients for each path are shown in Fig. 1. The total effect of GGT on cardioembolic stroke had a strength of 0.147, while that for the indirect effect via AF was 0.079. In other words, 53.9% of the total effect of GGT on cardioembolic stroke was mediated by AF. The Sobel test indicated that the mediation effect was statistically significant (Z=2.70, p=0.007).
Fig. 1. Mediating role of AF on the effect of GGT on cardioembolic stroke. The effect of GGT on cardioembolic stroke was partially mediated by AF. Path values are standardized regression coefficients. *p<0.05. AF: atrial fibrillation, GGT: gamma-glutamyl transferase.
DISCUSSION
The present study found that acute ischemic stroke patients with higher GGT levels are likely to have an increased risk of cardioembolic stroke. Around half of the association between GGT and cardioembolic stroke was mediated by AF. However, there was no significant relationship between GGT and LAA stroke in the population analyzed in this study.
The previous studies that found a relationship between GGT and stroke did not adequately explain the underlying mechanism. Answering this question requires the association between GGT and stroke to be evaluated according to the subtype given the heterogeneous nature of stroke. A few studies separated ischemic stroke and hemorrhagic stroke when analyzing the association, but these studies produced arbitrary and inconclusive results.3,4,5,6,7 We recently reported an independent association between GGT and stroke in an analysis of 456,100 representative Koreans.8 That study found that ischemic stroke was more consistently associated with GGT than was hemorrhagic stroke. We suggested that AF or atherosclerosis could explain the relationship between GGT and stroke; however, we could not provide evidence because the database we used did not contain information about the subtype of ischemic stroke.
GGT is reportedly independently associated with AF. Consistent with the results of previous studies, GGT was associated with AF in acute ischemic stroke patients in the present study. A high GGT level was significantly associated with cardioembolic stroke, and this relationship was substantially mediated by AF. The results of this study suggest that AF is a critical component in the link between GGT and stroke. A recent study of 266,550 Korean patients without a history of AF found that the participants with a higher baseline GGT level had a higher incidence of AF.11 That result suggests that GGT elevation is likely to precede the development of AF rather than occur as a result of existing AF. Oxidative stress, low-grade inflammation, and metabolic syndrome have been suggested as possible explanations for the association between GGT and AF.9,10,11 When excessive oxidative stress is present, more antioxidants such as glutathione are necessary for its resolution. In this situation, the need for GGT might also be increased since it contributes to the reutilization of glutathione. However, there is no evidence of a direct role of GGT in the development of AF, which suggests that an alternative explanation is needed. GGT is currently thought to function as a biomarker of oxidative stress and cardiometabolic risk factors inducing AF rather than as a direct effector for AF. This means that it is appropriate to use GGT as a diagnostic biomarker, while it is currently questionable as to whether reducing GGT can exert a therapeutic effect. This interpretation might change if additional evidence is found in future studies.
In the present study, GGT was not significantly related to LAA stroke, unlike cardioembolic stroke. This result might be attributable to the point that the present study only included ischemic stroke patients rather than a general population. Since the relationship between GGT and LAA stroke itself could not be determined in the present study, further evaluations of this relationship in the general population are necessary.
To our knowledge, this is the first study to show that the association between GGT and ischemic stroke can be mainly explained by AF and resulting cardioembolic stroke. Despite considerable diagnostic efforts, there are still many cryptogenic stroke patients in whom a cause is not identified. Since paroxysmal AF is expected to be one of the main causes, more diligent diagnostic efforts to identify AF may be required for cryptogenic stroke patients with higher GGT levels. Further studies are needed to verify whether GGT can be used as an adjunctive diagnostic tool to identify hidden AF among cryptogenic stroke patients, focusing on the relationships between GGT, AF, and cardioembolic stroke.
Several limitations of this study should be mentioned. First, we did not consider the effect of alcohol consumption, which is an especially important factor contributing to GGT elevation. This limitation was due to the utilized database not providing adequate information regarding alcohol consumption by patients. The large impact of alcohol consumption on GGT elevation means that caution is required when interpreting the results of the present study. Second, there may have been temporal changes in the stroke care, classification of stroke mechanisms, and detection of AF during the study period. Furthermore, AF and cardioembolic stroke may have been underdetected early during the study period, and the previous stroke care might not have been optimal. Third, the current study included only a single center and Korean patients. Finally, information regarding the type of AF (i.e., paroxysmal, persistent, or permanent) was not included in the analyses performed in the present study.
The results obtained in this study suggest that GGT is more strongly associated with cardioembolic stroke among ischemic stroke patients, owing in large part to its relationship with AF. This may explain the association between the GGT level and stroke. The present results further indicate the value of evaluating the potential of GGT as an adjunctive biomarker for determining subtypes in stroke patients, as well as for the primary prevention of stroke. Thus, sustained interest in GGT is necessary.
Acknowledgements
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI17C0076), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2018R1A2A2A05022369).
Footnotes
- Conceptualization: Wookjin Yang, Dong-Wan Kang, Seung-Hoon Lee.
- Data curation: Wookjin Yang, Seung-Hoon Lee.
- Formal analysis: Wookjin Yang, Dong-Wan Kang, Seung-Hoon Lee.
- Software: Wookjin Yang, Dong-Wan Kang.
- Investigation: Wookjin Yang, Seung-Hoon Lee.
- Methodology: Wookjin Yang, Dong-Wan Kang, Seung-Hoon Lee.
- Validation: Wookjin Yang, Dong-Wan Kang, Seung-Hoon Lee.
- Writing—original draft: Wookjin Yang, Seung-Hoon Lee.
- Writing—review & editing: Wookjin Yang, Dong-Wan Kang, Seung-Hoon Lee.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.1.60.
Causes of stroke in patients classified as “other determined” stroke
GGT levels according to causes of stroke in “other determined” patients
GGT levels according to subtypes of ischemic stroke
GVIF of the covariates
OR for cardioembolic stroke compared with all other subtypes of ischemic stroke according to GGT
References
- 1.Wannamethee SG, Lennon L, Shaper AG. The value of gamma-glutamyltransferase in cardiovascular risk prediction in men without diagnosed cardiovascular disease or diabetes. Atherosclerosis. 2008;201:168–175. doi: 10.1016/j.atherosclerosis.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Strasak AM, Kelleher CC, Klenk J, Brant LJ, Ruttmann E, Rapp K, et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008;28:1857–1865. doi: 10.1161/ATVBAHA.108.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bots ML, Salonen JT, Elwood PC, Nikitin Y, Freire de Concalves A, Inzitari D, et al. Gamma-glutamyltransferase and risk of stroke: the EUROSTROKE project. J Epidemiol Community Health. 2002;56 Suppl 1:i25–i29. doi: 10.1136/jech.56.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahim S, Sung J, Song YM, Ferrer RL, Lawlor DA, Davey Smith G. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ. 2006;333:22. doi: 10.1136/bmj.38855.610324.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jousilahti P, Rastenyte D, Tuomilehto J. Serum gamma-glutamyl transferase, self-reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851–1855. doi: 10.1161/01.str.31.8.1851. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu Y, Imano H, Ohira T, Kitamura A, Kiyama M, Okada T, et al. Gamma-glutamyltranspeptidase and incident stroke among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS) Stroke. 2010;41:385–388. doi: 10.1161/STROKEAHA.109.569061. [DOI] [PubMed] [Google Scholar]
- 7.Weikert C, Drogan D, Di Giuseppe R, Fritsche A, Buijsse B, Nöthlings U, et al. Liver enzymes and stroke risk in middle-aged German adults. Atherosclerosis. 2013;228:508–514. doi: 10.1016/j.atherosclerosis.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Kim CK, Kim DY, Jeong HG, Lee SH. Gamma-glutamyl transferase predicts future stroke: a Korean nationwide study. Ann Neurol. 2018;83:375–386. doi: 10.1002/ana.25158. [DOI] [PubMed] [Google Scholar]
- 9.Alonso A, Misialek JR, Amiin MA, Hoogeveen RC, Chen LY, Agarwal SK, et al. Circulating levels of liver enzymes and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities cohort. Heart. 2014;100:1511–1516. doi: 10.1136/heartjnl-2014-305756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekin G, Tekin YK, Senarslan DA, Gocmen AY, Senarslan O, Erbay AR. Serum γ-glutamyltransferase activity in patients with nonvalvular atrial fibrillation. Angiology. 2013;64:157–160. doi: 10.1177/0003319712438956. [DOI] [PubMed] [Google Scholar]
- 11.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Association between γ-glutamyltransferase level and incidence of atrial fibrillation: a nationwide population-based study. Int J Cardiol. 2017;245:149–155. doi: 10.1016/j.ijcard.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 12.Kunutsor SK, Laukkanen JA, Bluemke DA, Butler J, Khan H. Baseline and long-term gamma-glutamyltransferase, heart failure and cardiac arrhythmias in middle-aged Finnish men: prospective study and pooled analysis of published evidence. Eur J Prev Cardiol. 2016;23:1354–1362. doi: 10.1177/2047487316644086. [DOI] [PubMed] [Google Scholar]
- 13.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 14.Kunutsor SK, Apekey TA, Van Hemelrijck M, Calori G, Perseghin G. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta-analysis. Int J Cancer. 2015;136:1162–1170. doi: 10.1002/ijc.29084. [DOI] [PubMed] [Google Scholar]
- 15.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- 16.Kenny DA. Mediation with dichotomous outcomes [Internet] Washington, DC: NRHPSYCH; 2013. [cited 2018 May 12]. Available from: http://www.nrhpsych.com/mediation/logmed.html. [Google Scholar]
- 17.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87:178–183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Causes of stroke in patients classified as “other determined” stroke
GGT levels according to causes of stroke in “other determined” patients
GGT levels according to subtypes of ischemic stroke
GVIF of the covariates
OR for cardioembolic stroke compared with all other subtypes of ischemic stroke according to GGT