Abstract
Despite common notion that the correlation of socioeconomic status with child cognitive performance may be driven by both environmentally– and genetically–mediated transactional pathways, there is a lack of longitudinal and genetically informed research that examines these postulated associations. The present study addresses whether family income predicts associative memory growth and hippocampal development in middle childhood and tests whether these associations persist when controlling for DNA–based polygenic scores of educational attainment. Participants were 142 6–to–7–year–old children, of which 127 returned when they were 8–to–9 years old. Longitudinal analyses indicated that the association of family income with children’s memory performance and hippocampal volume remained stable over this age range and did not predict change. On average, children from economically disadvantaged background showed lower memory performance and had a smaller hippocampal volume. There was no evidence to suggest that differences in memory performance were mediated by differences in hippocampal volume. Further exploratory results suggested that the relationship of income with hippocampal volume and memory in middle childhood is not primarily driven by genetic variance captured by polygenic scores of educational attainment, despite the fact that polygenic scores significantly predicted family income.
Keywords: Socioeconomic status, Memory, Hippocampus, Childhood, Longitudinal, Genetics
1. Introduction
Longitudinal research investigating the relationship of socioeconomic status (SES; income, education, occupation) and children’s cognitive development is recently amassing. Children from socioeconomically disadvantaged background tend to have lower levels and slopes in general cognitive ability as well as multiple cognitive and achievement domains (Hackman et al., 2015; Lawson and Farah, 2017; von Stumm and Plomin, 2015; Wang et al., 2017). For instance, children growing up at–risk of poverty in the US perform nearly 1 SD below children not at–risk of poverty on achievement measures of verbal comprehension and math ability throughout middle childhood and early adolescence (Raffington et al., 2018a). Achievement disparities are rooted in differences in psychological characteristics, including self–control and motivation (Belsky et al., 2018; Malanchini et al., 2017), as well as more basic cognitive processes such as executive functions (Lawson et al., 2017), and episodic memory (Akshoomoff et al., 2014; Noble et al., 2007), which show moderately sized linear association with SES indicators in middle childhood.
A few studies have examined the repeated, time–lagged relationship of income and cognition with structural equation models to strengthen inferences on bivariate relationships (Hamaker et al., 2015). Indeed, longitudinal changes in income predict child cognition in early childhood (Dearing et al., 2001) as well as in later childhood and early adolescence (Raffington et al., 2018a), but only for children growing up at–risk of poverty. This could suggest that income losses are especially detrimental to child cognitive development at the lower end of the income spectrum.
An increasing number of neuroscientific studies further suggest that SES indicators are positively correlated with children’s hippocampal volume (Brody et al., 2017; Ellwood-Lowe et al., 2018; Hair et al., 2015; Hanson et al., 2011; Jednoróg et al., 2012; Luby et al., 2013; Merz et al., 2019; Noble et al., 2015, 2012; Raffington et al., 2018b; Yu et al., 2017), with the association between SES and hippocampal volume growing from ages 5–to–25–years (McDermott et al., 2019). Based on work in the animal literature, it is commonly theorized that smaller hippocampal volume in socioeconomically disadvantaged individuals may partially reflect differences in stimulating experiences and exposure to stress (Luby et al., 2013; Lupien et al., 2009). It is also known that hippocampal volume is partially heritable, thus SES–hippocampus volume associations could derive from passive gene–environment correlations (Sullivan et al., 2001). In either case, it is plausible to assume that SES disparities in hippocampal volume mediate SES disparities in memory performance, since learning and memory critically relies on the hippocampus and connected regions (Shing et al., 2010). However, evidence to suggest that hippocampal volume mediates SES–memory correlations is currently lacking.
More generally, the developmental relationship of hippocampal volume and memory functioning is not well–understood. For instance, a meta–analysis suggests that hippocampal volume has a negative association with memory in children and adults (Van Petten, 2004). Recent evidence suggests that structural hippocampal development continues beyond middle childhood, is non–linear in some subfield regions, and is complexly linked to different memory functions (Daugherty et al., 2016; Keresztes et al., 2017; J. K. Lee et al., 2014). However, the overwhelmingly cross–sectional nature of these studies has been shown to obscure true longitudinal developmental patterns (Kievit et al., 2013). Therefore, the coupling of hippocampal volume and memory is likely to differ along developmental time and remains largely obscure. Middle childhood is of particular interest given marked improvements in episodic memory performance (Ghetti and Bunge, 2012; Shing et al., 2010).
Individual differences in cognitive development that are commonly found to correlate with SES are known to be both environmentally and genetically transmitted (Belsky et al., 2018; Plomin and von Stumm, 2018; Tucker-Drob and Briley, 2014). Recently, the use of genome–wide polygenic scores (PGS) has been validated as a way to account for a small proportion of the variance in cognitive functioning that is due to genetic factors (Belsky et al., 2018; Plomin and von Stumm, 2018). PGS aggregate the effects of thousands of genetic variants based on weights calculated from very large genome–wide association studies (GWAS) and can be applied in samples several orders smaller than necessary for GWAS (i.e., of 100 participants; Plomin and von Stumm, 2018). For example, PGS of educational attainment have been found to predict educational attainment, but also cognitive, psychological, and socioeconomic characteristics over the life course (Belsky et al., 2018). Indeed, the genetic factors associated with income and education may be very similar (Davies et al., 2015; Hill et al., 2016). It remains unexplored whether PGS of educational attainment accounts for some of the covariance between SES and brain structure in children.
The present study consists of (1) a preregistered confirmatory section that addresses whether family income predicts memory growth and hippocampal development in childhood to elucidate longitudinal dynamics and (2) an exploratory section that explores whether these associations persist when adding children’s PGS of educational attainment as a control variable to examine whether the association is driven by a gene–environment correlation. Although parental education is a better predictor of children’s general cognitive ability than income and wealth (Rindermann and Ceci, 2018), income is the most volatile SES indicator over time (Duncan et al., 2010) and change in income may be an important predictor of cognitive development (Raffington et al., 2018). Therefore, we focus on family income, due to its dynamic nature over time.
In the first section, we examine our preregistered hypotheses (https://osf.io/7f42h) with one major deviation: Upon further consideration of statistical power necessary for interactions (Fan, 2003; McClelland and Judd, 1993), the present sample size, and expected effect sizes no larger than a standardized parameter estimate of 0.20 (Raffington et al., 2018), we did not test for the initially hypothesized moderating effects of poverty grouping. Rather, we followed a continuous approach and hypothesized that a higher income score at wave 1 predicts a larger gain from wave–1–to–wave–2 in associative memory and hippocampal volume. We further expected hippocampal volume to mediate the income–memory association. In addition, we performed non–preregistered exploratory analyses that add a PGS of educational attainment as a control variable and hypothesized that this would attenuate the income–memory and income–hippocampus associations, by having the PGS accounting for some of the variance that underlies these associations.
2. Method
2.1. Participants
142 children (66 girls) and their parents from 136 unique families (1 non–twin sibling pair, 4 dizygotic twin pairs, 1 monozygotic twin pair) participated in wave 1 of this longitudinal study (see Raffington et al. (2018b) for more details on sample). The children were identified by their parents as being of European (88%), European–African (4%), or European–Asian (6%) geographical ancestry (2% missing). 15% of this sample (13% at wave 2) were at-risk of poverty (monthly family net income at or below the Berlin state poverty line of that year, adjusted for family size and composition; Statistische Ämter des Bundes und der Länder, 2018). This is slightly less than the 19.2% of Berliners who were at-risk of poverty in 2017 (Statistische Ämter des Bundes und der Länder, 2018).
127 children (59 girls) from 121 unique families returned approximately two years later for wave 2 (see Table 1 for descriptive statistics). Inclusion criteria at wave 1 included the child attending first or second grade, no psychiatric, developmental and physical health disorders, no prolonged steroid medication use, no parent–reported maltreatment or severe illness, at least 37 weeks gestation, and at least one fluent German–speaking parent. There were no exclusion criteria for wave 2. Nine children had a definite or probable medical diagnosis at wave 2 (e.g., ADHD, autism spectrum disorder). Excluding these children did not affect the results, thus they were retained. At wave 1, a subsample (n = 90) of randomly selected children balanced by gender and willing to participate in MRI was invited to scanning. At wave 2, all children were invited to scanning and 104 accepted. At wave 1, all participants were invited to participate in genome–wide DNA extraction for polygenic scoring and 118 contributed data. The study was approved by the ‘Deutsche Gesellschaft für Psychologie’ ethics committee (YLS_012015).
Table 1.
Descriptive statistics and correlations across time (wave 1 and 2) of measures of interest.
| Mean (SD) n | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Income 1a (Euros) | 3634 (2087) n = 137 | 0.81* | 0.24* | 0.07 | 0.21 | 0.14 | 0.02 | −0.06 | 0.24* |
| 2 | Income 2 (Euros) | 4089 (2128) n = 124 | – | 0.10 | 0.06 | 0.24* | 0.21* | −0.02 | −0.03 | 0.14 |
| 3 | Memory 1 (proportion correct) | 0.41 (0.15) n = 142 | – | 0.34* | −0.08 | −0.15 | 0.04 | −0.03 | −0.04 | |
| 4 | Memory 2 (proportion correct) | 0.5 (0.15) n = 127 | – | 0.02 | 0.07 | 0.17 | 0.15 | 0.02 | ||
| 5 | Hippo 1 (mm3) | 7888 (709) n = 82 | – | 0.90* | 0.34* | 0.28* | 0.12 | |||
| 6 | Hippo 2 (mm3) | 8130 (8130) n = 99 | – | 0.28* | 0.19 | 0.08 | ||||
| 7 | Age 1 (years) | 7.19 (0.46) n = 142 | – | 0.93* | 0.13 | |||||
| 8 | Age 2 (years) | 9.25 (0.45) n = 127 | – | 0.08 | ||||||
| 9 | Polygenic Score | 1.94 (2.86) n = 118 | – |
Pearson’s correlation p < 0.05.
2.2. Procedure
At both waves, parents provided informed written consent and children verbal assent. While children completed the memory and other cognitive tasks not reported here, parents filled out a digitized questionnaire battery pertaining to SES and covariates. Children willing to participate in MRI were invited to scanning within 3 weeks.
2.3. Measures
2.3.1. Household income
Parents self–reported their total combined monthly household income after taxes (see Table 1 for descriptive statistics). There were no outliers over 5 SDs above or below the mean.
2.3.2. Memory
Participants completed exactly the same item–association memory task at both waves. They had to remember at what location on a computer screen they had seen a black–colored sketched item (e.g., a shoe, lemon; adapted from Kessels et al., 2007). The targets were randomly selected from the stimuli pool and targets versus new items were screened to not be categorically or semantically closely related. For encoding, they were instructed to name the item and memorize at what location in a grid of 36 gray boxes they saw it. All children saw the same 15 pictures shown consecutively for 3 s at the respective same location with an interstimulus interval of 1 s. The experimenter then distracted the child for 60 s by asking them to name their favorite animals, foods, or toys. During retrieval, the child saw 30 items consecutively, of which 15 had been previously seen. They verbally responded whether they had seen the picture or not and, if yes, they pointed to the corresponding location. Prior to the task, participants completed a practice version with 3 items, which was repeated until they correctly located 2 of 3 items. A correct item–location matching was scored as 1 and an incorrect one as 0. The outcome variable was the proportion of correct locations from 15 trials. There were no outliers over 5 SDs above or below the mean at either wave.
2.3.3. Hippocampal volume
Structural MRI images were acquired on a Siemens Magnetom TrioTim syngo 3 T scanner with a 12-channel head coil (Siemens Medical AG, Erlangen, Germany) using a 3D T1–weighted MPRAGE sequence (192 slices; field of view =256 mm; voxel size = 1 mm3; TR =2500 ms; TE =3.69 ms; flip angle = 7°; TI = 1100 ms).
Volumetric segmentation was performed with the Freesurfer 6.0.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu/) described elsewhere (Fischl, 2012). Previous studies suggest that software tools based on adult brain templates provide inaccurate segmentation for pediatric samples, which can be improved through the use of study–specific template brains (Phan, Smeets, Talcott, & Vandermosten, 2017; Schoemaker et al., 2016). We created two study-specific template brains (one for each wave) using Freesurfer’s “make_average_subject” command (https://surfer.nmr.mgh.harvard.edu/fswiki/make_average_subject). This pipeline utilizes the default adult template brain registrations of the “recon–all–all” command to average surfaces, curvatures, and volumes from all subjects into a study–specific template brain. All subjects were then re–registered to this study–specific template brain to improve segmentation accuracy. Segmented images were inspected for accuracy and 8 cases at wave 1 and 5 cases at wave 2 were excluded for inaccurate or failed registration due to excessive motion. The use of study–specific template brains was not preregistered. There were no outliers over 5 SDs above or below the mean.
2.3.4. Polygenic score for educational attainment
Genotyping was performed using Illumina GSA chips following the manufacturer’s protocol. After genotyping, we performed a stringent quality control using PLINK (https://www.cog-genomics.org/plink2, Chang et al., 2015) and removed any SNPs presenting with a call rate<98%, a minor allele frequency below 1%, or a p-value for Hardy-Weinberg-Equilibrium below 1 × 10−05. We calculated the identical-by-descent matrix (with a fraction of shared genotypes of at least 12.5%) and excluded the sibling sample with a lower call-rate from each sibling pair. We performed a MDS-analysis on the pruned genotypes (using the PLINK parameters --indep-pairwise 200 100 0.2) and removed any samples and the respective sib-pair identified as outliers (defined as presenting with a position on any of the first ten MDS-components, which deviated with at least 4 SDs from the respective mean of this component). Furthermore, we removed samples which presented with a heterozygosity rate deviating by at least 4 SDs from the mean heterozygosity over all samples.
Imputation was performed using shapeit2 (https://mathgen.stats.ox.ac.uk/genetics_software/shapeit/shapeit.html, O’Connell et al., 2014) and impute2 (https://mathgen.stats.ox.ac.uk/impute/impute_v2.html, Howie et al., 2009). After imputation, we only kept SNPs presenting with an info score metric of at least 0.6. This resulted in a dataset containing 9,629,396 imputed SNP genotypes and 118 samples.
PGS were calculated using PLINK and were based on the summary statistics of a GWAS of educational attainment by Lee et al. (J. J. Lee et al., 2018).
At first, we used LD-clumping on the best-guessed SNP genotypes based on these summary statistics and derived 464,967 independent SNPs. Afterwards, imputed genotype probabilities of these SNPs were extracted and PGS calculations were performed on these probabilities with the p-value threshold for inclusion of SNP being p = 1 and using the effect-estimates reported by Lee et al. as weights.
2.3.5. Missingness
At wave 1, logistic regression analyses showed that the final MRI subsample (n = 82) did not differ from the full sample in income or memory (p’s > 0.14), but they were slightly older (mean difference 85 days, t = -3.12, p < 0.05). Those providing income data at wave 1 did not differ from families that did not in terms of their children's wave 1 memory performance, hippocampal volume, or PGS (p’s > 0.79). Missingness in PGS was not predicted by age, sex, income, memory performance, or hippocampal volume at wave 1 (p’s > 0.26).
Longitudinally, missingness in income at wave 2 was not predicted by age, sex, income, memory, hippocampus, or PGS at wave 1 (p’s > 0.33). Similarly, missingness in memory at wave 2 was not predicted by age, sex, income, memory, hippocampus, or PGS at wave 1 (p’s > 0. 33). Lastly, missingness in hippocampus at wave 2 was not predicted by age, sex, income, memory, hippocampus, or PGS (p’s > 0.14).
2.4. Data analysis
2.4.1. Confirmatory analyses
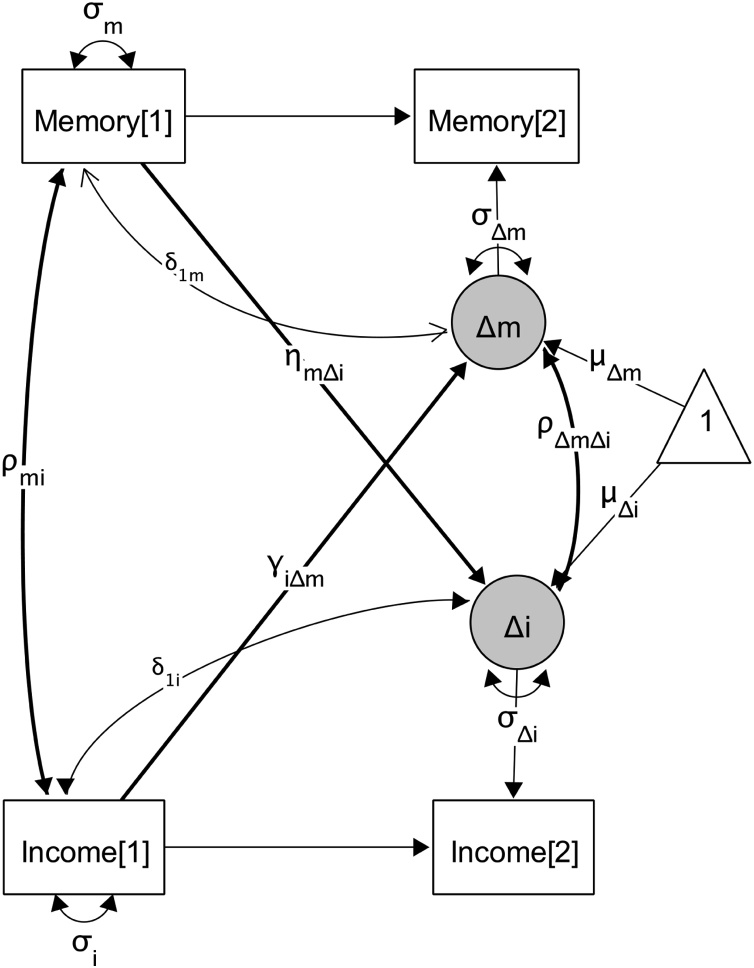
First, univariate latent difference score (LDS) structural equation models (SEM) of income, memory and hippocampus were compiled (Ferrer and McArdle, 2010; Kievit et al., 2018). Aside from being a useful tool for longitudinal analyses, these SEMs also allowed the estimation of measurement error in hippocampal volume by building a latent hippocampal volume factor indicated by right and left volume. Individual growth is described by wave 1 (i.e., the intercept exemplified by Income[1] in Fig. 1) and a person’s change over time, which is directly modeled as the unobserved difference between the initial observation and subsequent observations (Δi). The random intercept is modeled with variance (σi) to indicate between–person differences in intercepts. Similarly, the random latent change component is modeled with variance (σΔi) to indicate between–person differences in change. The overall effect of change may be positive or negative depending on parameter estimates and the previously observed score. A group average trajectory is estimated by the mean latent change parameter (μΔi). Models included gender and age as covariates of memory and hippocampus intercepts. All variables were standardized to the measure of the first wave, hence the mean intercept is 0 and wave 2 measures represent the deviation from wave 1 (Raffington et al., 2018a; Small et al., 2013).
Fig. 1.
Graphical illustration of bivariate income–memory latent difference score model. Observed variables are depicted as squares, regressions as one–headed arrows, and (co–) variances (σ) as two–headed arrows. Unmarked paths were fixed at 1. Figure compiled using Onyx 1.0 (http://onyx.brandmaier.de).
Second, bivariate models of income–memory (see Fig. 1 for graphical depiction) and income–hippocampus were compiled. We also assembled a bivariate hippocampus–memory model (this step was not explicitly preregistered, but is a necessary step to explore mediation). To test whether intercepts covaried, their covariance was estimated (ρmi). To examine whether income score at wave 1 predicts memory change from wave 1 to wave 2, change in memory was regressed on income at wave 1 (γiΔm). In reverse, to examine whether memory score at wave 1 predicts income change from wave 1 to wave 2, change in income was regressed on memory at wave 1 (ηmΔi). Thus, evaluation of score–onto–change coupling allow for inferences to be made between income at wave 1 being a leading indicator in time of wave–1–to–wave–2 changes in memory and vice versa (Ferrer and McArdle, 2004). These models also allowed for residual covariation between income and memory latent change variables (ρΔmΔi). The income–hippocampus and hippocampus–memory models were assembled in the same manner.
Third, the preregistered trivariate mediation model, including an indirect path of income onto hippocampus onto memory, was not tested, because such links were not indicated in the bivariate models.
All models were implemented in Mplus 8.2 and fitted using full information maximum likelihood (FIML) estimation to accommodate missing at random data. Models corrected standard errors for nesting of individuals within families (using the TYPE = COMPLEX feature in Mplus). Model fit was evaluated using the comparative fit index (CFI), root mean square error of approximation (Ea), and Chi–Squared (χ²) likelihood ratio test, where CFI values > .95 and Ea < 0.08 generally constitute good fit. Univariate and bivariate models showed good fit to the data (see Results Tables). Given that statistical tests were preregistered, no adjustments for multiple comparisons were made. We report standardized parameter estimates as effect size estimates.
2.4.2. Exploratory analyses
PGS of educational attainment was included as a predictor of intercepts and latent change in each domain in the bivariate models of income–memory and income–hippocampus. To test whether the covariance of income–memory was attenuated while controlling for PGS, a model fit comparison evaluated whether fit was significantly affected when the covariance parameter was free versus fixed to the parameter estimate from a model where the polygenic score paths were fixed to 0 (a 1 df Chi–square test). PGS of educational attainment were regressed on sex, age, parent–reported geographical ancestry (European, European–African or European–Asian), and the first 10 MDS-components of the principal components analysis to control for population stratification.
3. Confirmatory results
3.1. Univariate models
Income, memory performance and hippocampal volume showed average increasing trajectories over time (see Table 2 for fit indices and parameter estimates). Nevertheless, there were some decreasing individual trajectories in all domains (income: 22%, memory: 35%, hippocampus: 10%). Correspondingly, there was significant variability in intercepts and change in all domains.
Table 2.
Parameter estimates from three separate univariate models.
| Income | Memory | Hippocampusc | ||
|---|---|---|---|---|
| Model Fit | χ² (df) | 0 (1) | 2.17 (3) | 13.18 (15) |
| CFI | 1 | 1 | 1 | |
| Ea (CI) | 0 (0-0) | 0 (0.13) | 0 (0-0.07) | |
| SRMR | 0 | 0.04 | 0.07 | |
| Mean change μΔ | 0.19* (0.06) | 0.57* (0.09) | 0.36* (0.05) | |
| Intercept variance σ b | 1* (0.14) | 1* (0.11) | 0.33* (0.05) | |
| Change variance σΔb | 0.40* (0.13) | 1.28* (0.16) | 0.09* (0.03) | |
| Correlated intercept-change δ | −0.33* (0.09) | −0.59* (0.06) | −0.18 (0.14) | |
| Age onto Intercept | – | 0.10 (0.07) | 0.19* (0.09) | |
| Girla onto Intercept | – | −0.02 (0.11) | −0.16 (0.22) | |
| ICV onto Intercept | – | – | 0.67* (0.11) |
Standardized regression estimates and bivariate correlations, unstandardized variance estimates. Standard errors in parentheses.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
Residual variances and residual correlations of left and right hippocampus as well as ICV variance and ICV correlations with gender are not shown.
3.2. Bivariate income and memory
Lower family income intercepts were associated with lower memory performance intercepts (see Table 3 for fit indices and parameter estimates). Accordingly, a 1 SD increase in family income (i.e. 2087 Euros/month; mean = 3634, range = 500–10000) was associated with 0.23 SD better performance in memory. Contrary to our first hypothesis, income score at wave 1 did not predict changes in memory or vice versa. Thus, while the association of income and memory remained stable over time, there was no dynamic longitudinal association between the two (see Fig. 2a).
Table 3.
Bivariate income–memory parameter estimates.
| Income | Memory | |
|---|---|---|
| Model Fit | χ² = 2.96, df = 10, CFI = 1, Ea = 0, CI = 0-0, SRMR = 0.03 | |
| Mean change μΔ | 0.19* (0.06) | 0.57* (0.09) |
| Intercept variance σ | 1* (0.14) | 1* (0.11) |
| Change variance σΔ | 0.39* (0.12) | 1.26* (0.16) |
| Correlated intercept–change δ | −0.29* (0.09) | −0.57* (0.06) |
| Age onto Intercept | – | 0.10 (0.07) |
| Girla onto Intercept | – | −0.01 (0.11) |
| Bivariate Couplings | ||
| Intercept correlation ρmi | 0.23* (0.09) | – |
| Income onto memory change γiΔm | −0.12 (0.08) | – |
| Memory onto income change ηmΔi | −0.17 (0.10) | – |
| Change–change correlation ρΔmΔi | −0.01 (0.06) | – |
Standardized regression estimates and bivariate correlations, unstandardized variance estimates. Standard errors in parentheses.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
Fig. 2.
Individual raw monthly post–tax income in Euros (a), memory performance in proportion correct (b), and bilateral hippocampal volume in mm3(c) plotted over time. Average trajectories are plotted for families earning +1 SD above mean income (blue line) and -1 SD below mean income (red line), where income was averaged over wave 1 and 2 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3. Bivariate income and hippocampus
Lower family income intercepts were associated with smaller hippocampal volume intercepts (see Table 4 for fit indices and parameter estimates). Accordingly, a 1 SD increase in family income (i.e. 2087 Euros/month) was associated with 0.29 SD larger hippocampal volume, or 205 mm3. Contrary to our second hypothesis, income score at wave 1 did not predict changes in hippocampus or vice versa. Thus, the association of income and hippocampal volume remained stable over time (see Figure 4a).
Table 4.
Bivariate income–hippocampal volume parameter estimates.
| Income | Hippocampus | |
|---|---|---|
| Model Fit | χ² = 14.48, df = 27, CFI = 1, Ea = 0, CI = 0-0, SRMR = 0.04 | |
| Mean change μΔ | 0.20* (0.05) | 0.37* (0.05) |
| Intercept variance σ | 1* (0.14) | 0.33* (0.05) |
| Change variance σΔ | 0.39* (0.13) | 0.09* (0.03) |
| Correlated intercept–change δ | −0.35* (0.08) | −0.13 (0.14) |
| Age onto Intercept | – | 0.17 (0.09) |
| Girla onto Intercept | – | −0.16 (0.14) |
| ICV onto Intercept | – | 0.66* (0.08) |
| Bivariate Couplings | ||
| Intercept correlation ρmi | 0.29* (0.09) | – |
| Income onto hippocampus change γiΔm | −0.15 (0.15) | – |
| Hippocampus onto income change ηmΔi | 0.12 (0.09) | – |
| Change–change correlation ρΔmΔi | −0.02 (0.15) | – |
Standardized regression estimates and correlations, unstandardized variance estimates. Standard errors in parentheses.
cResidual variances and residual correlations of left and right hippocampal volume as well as ICV variance and ICV correlation with gender are not shown.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
3.4. Bivariate hippocampus and memory
There were no bivariate relationships between hippocampal volume and memory performance, therefore hippocampal volume did not mediate memory differences (see Table 5 for fit indices and parameter estimates).
Table 5.
Bivariate hippocampus–memory parameter estimates.
| Hippocampus | Memory | |
|---|---|---|
| Model Fit | χ² = 22.34, df = 24, CFI = 1, Ea = 0, CI = 0-0.06, SRMR = 0.07 | |
| Mean change μΔ | 0.37* (0.05) | 0.57* (0.09) |
| Intercept variance σ | 0.33* (0.06) | 0.99* (0.11) |
| Change variance σΔ | 0.08* (0.03) | 1.25* (0.16) |
| Correlated intercept–change δ | −0.19 (0.17) | −0.59* (0.06) |
| Age onto Intercept | 0.18* (0.09) | 0.09 (0.08) |
| Girla onto Intercept | −0.15 (0.23) | 0.02 (0.11) |
| ICV onto Intercept | 0.67* (0.11) | – |
| Bivariate Couplings | ||
| Intercept correlation ρmi | 0 (0.11) | – |
| Hippocampus onto memory change γiΔm | 0.11 (0.09) | – |
| Memory onto hippocampus change ηmΔi | −0.31 (0.17) | – |
| Change–change correlation ρΔmΔi | 0.01 (0.09) | – |
Standardized regression estimates and correlations, unstandardized variance estimates. Standard errors in parentheses.
cResidual variances and residual correlation of left and right hippocampal volume as well as ICV variance and ICV correlation with gender are not shown.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
4. Exploratory results
Contrary to our fourth hypothesis, adding children’s PGS of educational attainment did not attenuate the income–memory association (correlation with PGS control = 0.25 (0.09), p < 0.05 versus correlation without PGS control = 0.23 (0.09), p < 0.05, difference in correlation Chi–square (1) = 0.06, ns). In addition, PGS significantly predicted family income intercepts, but not income change, memory intercepts or memory change (see Table 6 for parameter estimates). Accordingly, a 1 SD increase in the children’s genetic predisposition to higher educational attainment was associated with living in a family that earned 536 Euros (0.26 SD’s) more per month. The association of family income intercepts and PGS persisted when constrained to participants of European descent (0.23 (0.09), p < 0.05).
Table 6.
Income–memory parameter estimates for whole sample with polygenic scores for educational attainment.
| Income | Memory | |
|---|---|---|
| Model Fit | χ² = 84, df = 131, CFI = 1, Ea = 0, CI = 0-0, SRMR = 0.07 | |
| Mean change μΔ | 0.19* (0.05) | 0.57* (0.09) |
| Intercept variance σ | 0.94* (0.14) | 1* (0.11) |
| Change variance σΔ | 0.38* (0.12) | 1.24* (0.17) |
| Correlated intercept–change δ | −0.27* (0.09) | −0.56* (0.06) |
| Age onto Intercept | – | 0.11 (0.07) |
| Girla onto Intercept | – | −0.02 (0.11) |
| Bivariate Couplings | ||
| Intercept correlation ρmi | 0.25* (0.09) | – |
| Income onto memory change γiΔm | −0.14 (0.08) | – |
| Memory onto income change ηmΔi | −0.17 (0.09) | – |
| Change–change correlation ρΔmΔi | −0.01 (0.06) | – |
| Polygenic Scores | ||
| Polygenic scores on income intercept | 0.23* (0.08) | – |
| Polygenic scores on income change | −0.14 (0.07) | – |
| Polygenic scores on memory intercept | −0.06 (0.09) | – |
| Polygenic scores on memory change | 0.10 (0.10) | – |
| Girl on polygenic scores | 0.10 (0.20) | – |
| Age on polygenic scores | 0.13 (0.08) | – |
| Geographical ancestry on polygenic scoresb | 0.15 (0.15) | – |
Standardized regression estimates and correlations, unstandardized variance estimates. Standard errors in parentheses.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
Principal components correcting for population stratification onto polygenic scores are not shown for brevity.
Contrary to our fifth hypothesis, adding children’s PGS of educational attainment did not attenuate the income–hippocampus association (correlation with PGS control = 0.29 (0.10), p < 0.05 versus correlation without PGS control = 0.29 (0.00), p < 0.05, difference in correlation Chi-square (1) = 0.11, ns). In addition, PGS did not significantly predict hippocampus intercepts or change (see Table 7 for parameter estimates).
Table 7.
Income–hippocampus parameter estimates for whole sample with polygenic scores for educational attainment.
| Income | Hippocampus | |
|---|---|---|
| Model Fit | χ² = 143, df = 184, CFI = 1, Ea = 0, CI = 0-0, SRMR = 0.09 | |
| Mean change μΔ | 0.20* (0.05) | 0.37* (0.05) |
| Intercept variance σ | 0.94* (0.13) | 0.32* (0.05) |
| Change variance σΔ | 0.38* (0.13) | 0.08* (0.03) |
| Correlated intercept–change δ | −0.33* (0.09) | −0.13 (0.13) |
| Age onto Intercept | – | 0.18* (0.09) |
| Girla onto Intercept | – | −0.16 (0.13) |
| ICV onto Intercept | – | 0.66* (0.08) |
| Bivariate Couplings | ||
| Intercept correlation ρmi | 0.29* (0.10) | – |
| Income onto hippocampus change γiΔm | −0.13 (0.15) | – |
| Hippocampus onto income change ηmΔi | 0.13 (0.09) | – |
| Change–change correlation ρΔmΔi | −0.03 (0.16) | – |
| Polygenic Scores | ||
| Polygenic scores on income intercept | 0.23* (0.08) | – |
| Polygenic scores on income change | −0.14 (0.08) | – |
| Polygenic scores on hippocampus intercept | 0 (0.09) | – |
| Polygenic scores on hippocampus change | −0.14 (0.16) | – |
| Girl on polygenic scores | 0.08 (0.20) | – |
| Age on polygenic scores | 0.13 (0.08) | – |
| Geographical ancestry on polygenic scoresd | 0.16 (0.15) | – |
Standardized regression estimates and correlations, unstandardized variance estimates. Standard errors in parentheses.
cResidual variances and residual correlations of left and right hippocampus as well as ICV variance and ICV correlation with gender are not shown.
Asterisks denote significance at the α level of 0.05.
Gender dummy coded as 1 = girls.
Principal components correcting for population stratification onto polygenic scores are not shown for brevity.
5. Discussion
Despite evidence that the association of SES and child cognitive performance is driven by both environmentally– and genetically– mediated transactional pathways, there has been little longitudinal and genetically informed research on this topic. Motivated by a lack of longitudinal research examining change and recent advances in using PGS derived from large GWAS, we applied longitudinal models to estimate dynamic associations of family income with children’s memory performance and hippocampal volume whilst controlling for genetic predispositions for educational attainment.
Our results indicate that the association of family income with children’s memory performance and hippocampal volume remains stable from ages 6 to 8 years. Contrary to hypotheses, change in hippocampal volume and associative memory was not predicted by family income. This null result could be due to sample composition, given the limited number of families living in poverty. On the other hand, widening socioeconomic differences over age across different cognitive and academic domains are often (Harden et al., 2019; Tucker-Drob, 2013), but not always found in middle childhood and adolescence (Hackman et al., 2015; Hair et al., 2015; Lawson and Farah, 2017; Raffington et al., 2018a; von Stumm and Plomin, 2015; Wang et al., 2017).
Yet, it should be noted that in studies with larger samples that allow an exploration of poverty moderation and more waves of data collection, it has been reported that changes in income predict child cognition in early childhood (Dearing et al., 2001) and later childhood and early adolescence (Raffington et al., 2018a), but only for children growing up at–risk of poverty. This could suggest threshold effects of the income–cognition association, such that being at–risk of poverty is a moderator in the longitudinal association of family income and children’s cognitive development. In the present study, we were not able to explore coupling effects of income changes onto cognitive development, for which at least three waves of data are necessary. We recommend future studies to consider a minimum of three data collection waves. Of note, intervention research has shown that positive outcomes in noncognitive domains (e.g., motivation, school achievement) may be present despite a lack of cognitive effects (Heckman, 2006). Hence, family income could have effects on change in noncognitive domains, even in children not at–risk of poverty.
Why do children from socioeconomically disadvantaged background show stably lower memory performance and a smaller hippocampal volume in middle childhood? One potential explanation is that shared genes predisposing the parents to make more earnings and the children to have a larger hippocampal volume and perform better on cognitive tasks account for their association, a phenomenon called gene–environment correlation (Plomin et al., 1977). Contrary to expectations, we found no evidence that genetic variance captured by PGS of educational attainment account for the correlation of income with children’s associative memory and hippocampal volume in middle childhood. This null result is surprising, given that those same genetic differences did predict family income, which previous studies suggest is partially, but not fully, mediated by parental education (Belsky et al., 2016). Put simply, children with a higher genetic predisposition to attain more education tend to have parents with a higher genetic predisposition to more education, and these parents are more highly educated, which results in higher family income (Belsky et al., 2018). Thus, our results provide no evidence for the notion that the correlation of family income with memory performance or hippocampal volume is driven by a gene–environment correlation captured by PGS of educational attainment.
We believe two other mechanisms are likely to be involved in the relationship of family income with children’s hippocampal volume and memory performance in middle childhood: First, genetic variance not captured by PGS of educational attainment, such as genetic variance of hippocampal structure, confer a gene–environment correlation and, second, socioeconomic disadvantage occurring earlier in development offsets a lower trajectory that results in a fairly stable difference in later childhood. For instance, socioeconomic–related stress in prenatal and early childhood development may initiate long–lasting maturational neural processes along a different course to maximize functioning in those environments, potentially at the cost of certain cognitive functions preferred in cognitive testing and academic contexts. Correspondingly, intervention efforts have a substantially larger impact on cognitive and school achievement when they target children in early compared to later childhood (Duncan et al., 1994, 1998; Heckman, 2006). Indeed, both genetically and environmentally–mediated effects transferred through family and neighborhood environments influence children’s cognitive development and academic attainment (Belsky et al., 2018; Engelhardt et al., 2019; Harden et al., 2019; Koellinger and Harden, 2018). Thus, PGS of educational attainment combine genetic effects mediated via the home environment and transactional gene-environment correlations (Cheesman et al., 2019). Interestingly, these transactional mechanisms may differ across the socioeconomic spectrum, for instance by school quality (Harden et al., 2019). Future research should investigate intervention or quasi–experimental effects in combination with PGS as a powerful way to explore the ways in which socioeconomic disadvantage and genetic predispositions contribute to individual differences in cognitive development.
Furthermore, there was no evidence to suggest that differences in memory performance were mediated by differences in hippocampal volume, since both intercepts and change over time were unrelated to each other. Another study reports null associations of changes in episodic memory allowed to correlate with changes in the gray matter volume of frontal and parietal cortex areas in 8–to–38–year–olds (Breukelaar et al., 2017). The lack of mediation may arise from partially non–linear linkages between hippocampal subfield structure and memory performance (Keresztes et al., 2017, 2018), or brain and cognition more generally (Wenger et al., 2017). Therefore, it is possible that longitudinal trajectories of subregions of the hippocampus are related to specific memory functions not captured in our memory task. Alternatively, hippocampal function may be more closely related to associative memory than its structure, and its functional engagement may be moderated by SES (Farah, 2017; Leonard et al., 2015; Sheridan et al., 2013).
We acknowledge further limitations of this study. First, our sample was somewhat biased in attracting parents that were more highly educated than the population average, and only included children that passed stringent exclusion criteria (see Raffington et al., 2018b). Second, the moderate sample size did not allow us to explore threshold effects of growing up in poverty. These limitations are likely to underestimate effects of SES and poverty on child development. Similarly, a lack of power could be a potential factor contributing to null results, since all tested factors only explain a low amount of the total variance. Third, our analyses were restricted to income–hippocampus–memory associations and may not generalize to parental education (Duncan and Magnuson, 2012) or other cognitive functions that the hippocampus is known to be involved in, such as emotion regulation (Lupien et al., 2009). Lastly, our analysis was restricted by only having two waves of data, which limits the reliability of change and understanding of longitudinal dynamics (Willett, 1989). Nevertheless, representing longitudinal assessments of change in each variable as an outcome of the other variable’s prior score or vice versa informs our understanding of bivariate relationships far beyond cross–sectional or longitudinal correlations.
In conclusion, we found the association of family income with children’s memory performance and hippocampal volume to be stable from ages 6–to–8–years without bivariate effects on change. Accordingly, children from economically disadvantaged background on average showed lower memory performance and had smaller hippocampal volumes. There was no evidence to suggest that differences in memory performance were mediated by differences in hippocampal volume. The relationship of income with hippocampal volume and memory in middle childhood was not driven by genetic variance captured by PGS of educational attainment, despite the fact that PGS significantly predicted family income. Furthermore, change in hippocampal volume and memory performance observed in middle childhood seems largely independent of family income, at least in samples of moderate SES variation. Their stable association may derive from socioeconomic disadvantage occurring in earlier childhood and genetic variance not captured by PGS of educational attainment. This study also highlights the utility of including DNA–based PGS as control variables to zero–in on explanatory mechanisms involved in the study of childhood adversity and development to promote positive development.
Declaration of Competing Interest
None.
Acknowledgments
Funding: This work was supported by the Jacobs Foundation [grant 2014–1151 to YLS and CH] and conducted at the Center for Lifespan Psychology, Max Planck Institute for Human Development. LR is supported by the German Research Foundation (DFG, RA 3208/1-1). We thank all members of the Jacobs study team for their vital contribution, and all participants and family members for taking part in the study.
References
- Akshoomoff N., Newman E., Thompson W.K., McCabe C., Bloss C.S., Chang L. The NIH Toolbox Cognition Battery: Results from a large normative developmental sample (PING) Neuropsychology. 2014;28(1):1–10. doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D.W., Domingue B.W., Wedow R., Arseneault L., Boardman J.D., Caspi A. Genetic analysis of social-class mobility in five longitudinal studies. Proc. Natl. Acad. Sci. 2018;115(31):E7275–E7284. doi: 10.1073/pnas.1801238115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukelaar I.A., Antees C., Grieve S.M., Foster S.L., Gomes L., Williams L.M., Korgaonkar M.S. Cognitive control network anatomy correlates with neurocognitive behavior: a longitudinal study. Hum. Brain Mapp. 2017;38(2):631–643. doi: 10.1002/hbm.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Gray J.C., Yu T., Barton A.W., Beach S.R.H., Galván A. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171(1):46. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman R., Hunjan A., Coleman J.R.I., Ahmadzadeh Y., Plomin R., McAdams T.A. Comparison of adopted and non-adopted individuals reveals gene-environment interplay for education in the UK Biobank. BioRxiv. 2019 doi: 10.1177/0956797620904450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A.M., Bender A.R., Raz N., Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26(2):220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.M., Hemani G., Timpson N.J., Windmeijer F., Davey Smith G. The role of common genetic variation in educational attainment and income: evidence from the National Child Development Study. Sci. Rep. 2015;5(1):16509. doi: 10.1038/srep16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing E., McCartney K., Taylor B.A. Change in family income-to-needs matters more for children with less. Child Dev. 2001;72(6):1779–1793. doi: 10.1111/1467-8624.00378. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Brooks-Gunn J., Klebanov P.K. Economic deprivation and early childhood development. Child Dev. 1994;65(2):296–318. [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Yeung W.J., Brooks-Gunn J., Smith J.R. How much does childhood poverty affect the life chances of children? Am. Sociol. Rev. 1998;63(3):406–423. [Google Scholar]
- Duncan G.J., Ziol-Guest K.M., Kalil A. Early-childhood poverty and adult attainment, behavior, and health. Child Dev. 2010;81(1):306–325. doi: 10.1111/j.1467-8624.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Humphreys K.L., Ordaz S.J., Camacho M.C., Sacchet M.D., Gotlib I.H. Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Dev. Cogn. Neurosci. 2018;30(December 2017):41–50. doi: 10.1016/j.dcn.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt L.E., Church J.A., Paige Harden K., Tucker‐Drob E.M. Accounting for the shared environment in cognitive abilities and academic achievement with measured socioecological contexts. Dev. Sci. 2019;22(1) doi: 10.1111/desc.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. Power of latent growth modeling for detecting group differences in linear growth trajectory parameters. Struct. Equ. Model. A Multidiscip. J. 2003;10(3):380–400. [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Ferrer E., McArdle J.J. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Dev. Psychol. 2004;40(6):935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- Ferrer E., McArdle J.J. Longitudinal modeling of developmental changes in psychological research. Curr. Dir. Psychol. Sci. 2010;19(3):149–154. [Google Scholar]
- Fischl B. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Bunge S.A. Neural Changes Underlying Episodic Memory During Middle Childhood. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Gallop R., Evans G.W., Farah M.J. Socioeconomic status and executive function: developmental trajectories and mediation. Dev. Sci. 2015;18(5):686–702. doi: 10.1111/desc.12246. [DOI] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker E.L., Kuiper R.M., Grasman R.P.P.P. A critique of the cross-lagged panel model. Psychol. Methods. 2015;20(1):102–116. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden K.P., Domingue B.W., Belsky D.W., Boardman J.D., Crosnoe R., Malanchini M. Genetic associations with mathematics tracking and persistence in secondary school. BioRxiv. 2019 doi: 10.1038/s41539-020-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J.J. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hill W.D., Hagenaars S.P., Marioni R.E., Harris S.E., Liewald D.C.M., Davies G. Molecular genetic contributions to social deprivation and household income in UK Biobank. Curr. Biol. 2016;26(22):3083–3089. doi: 10.1016/j.cub.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B.N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6) doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A., Bender A.R., Bodammer N.C., Lindenberger U., Shing Y.L., Werkle-Bergner M. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc. Natl. Acad. Sci. 2017;114(34):9212–9217. doi: 10.1073/pnas.1710654114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A., Ngo C.T., Lindenberger U., Werkle-Bergner M., Newcombe N.S. Hippocampal maturation drives memory from generalization to specificity. Trends Cogn. Sci. (Regul. Ed.) 2018;22(8):676–686. doi: 10.1016/j.tics.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels R.P., Hobbel D., Postma A. Aging, context memory and binding: a comparison of “what, where and when” in young and older adults. Int. J. Neurosci. 2007;117(6):795–810. doi: 10.1080/00207450600910218. [DOI] [PubMed] [Google Scholar]
- Kievit R.A., Brandmaier A.M., Ziegler G., van Harmelen A.-L., de Mooij S.M.M., Moutoussis M. Developmental cognitive neuroscience using latent change score models: a tutorial and applications. Dev. Cogn. Neurosci. 2018;33(2017):99–117. doi: 10.1016/j.dcn.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit R.A., Frankenhuis W.E., Waldorp L.J., Borsboom D. Simpson’s paradox in psychological science: a practical guide. Front. Psychol. 2013;4(August):1–14. doi: 10.3389/fpsyg.2013.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellinger P.D., Harden K.P. Using nature to understand nurture. Science. 2018;359(6374):386–387. doi: 10.1126/science.aar6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G.M., Farah M.J. Executive function as a mediator between SES and academic achievement throughout childhood. Int. J. Behav. Dev. 2017;41(1):94–104. doi: 10.1177/0165025415603489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G.M., Hook C.J., Farah M.J. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Developmental Science, Advance on. 2017:e12529. doi: 10.1111/desc.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Wedow R., Okbay A., Kong E., Maghzian O., Zacher M. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 2018;50(8):1112–1121. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Leonard J.A., Mackey A.P., Finn A.S., Gabrieli J.D.E. Differential effects of socioeconomic status on working and procedural memory systems. Front. Hum. Neurosci. 2015;9:554. doi: 10.3389/fnhum.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A., Botteron K., Marrus N., Harms M.P., Babb C. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C.M. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Malanchini M., Wang Z., Voronin I., Schenker V.J., Plomin R., Petrill S.A., Kovas Y. Reading self-perceived ability, enjoyment and achievement: a genetically informative study of their reciprocal links over time. Dev. Psychol. 2017;53(4):698–712. doi: 10.1037/dev0000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland G.H., Judd C.M. Statistical difficulties of detecting interactions and moderator effects. Psychol. Bull. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- McDermott C.L., Seidlitz J., Nadig A., Liu S., Clasen L.S., Blumenthal J.D. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 2019;39(8):1365–1373. doi: 10.1523/JNEUROSCI.1808-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Desai P.M., Maskus E.A., Melvin S.A., Rehman R., Torres S.D. Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biol. Psychiatry. 2019 doi: 10.1016/j.biopsych.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- O’Connell J., Gurdasani D., Delaneau O., Pirastu N., Ulivi S., Cocca M. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 2014;10(4) doi: 10.1371/journal.pgen.1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., DeFries J.C., Loehlin J.C. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol. Bull. 1977;84(2):309. [PubMed] [Google Scholar]
- Plomin R., von Stumm S. The new genetics of intelligence. Nat. Rev. Genet. 2018;19(3):148–159. doi: 10.1038/nrg.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffington L., Prindle J.J., Shing Y.L. Income gains predict cognitive functioning longitudinally throughout later childhood in poor children. Developmental Psychology, Advance on. 2018 doi: 10.1037/dev0000529. [DOI] [PubMed] [Google Scholar]
- Raffington L., Prindle J., Keresztes A., Binder J., Heim C.M., Shing Y.L. Blunted cortisol stress reactivity in low–income children relates to lower memory function. Psychoneuroendocrinology. 2018;90:110–121. doi: 10.1016/j.psyneuen.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Rindermann H., Ceci S.J. Parents’ education is more important than their wealth in shaping their children’s intelligence: results of 19 samples in seven countries at different developmental levels. J. Educ. Gift. 2018;41(4):298–326. [Google Scholar]
- Sheridan M.A., How J., Araujo M., Schamber M.A., Nelson C.A. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev. Sci. 2013;5(16):665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Brehmer Y., Müller V., Li S.-C., Lindenberger U. Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci. Biobehav. Rev. 2010;34(7):1080–1091. doi: 10.1016/j.neubiorev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Small B.J., Dixon R.A., McArdle J.J., Grimm K.J. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria longitudinal Study. Neuropsychology. 2013;26(2):144–155. doi: 10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistische Ämter des Bundes und der Länder . 2018. Sozialberichterstattung Der Amtlichen Statistik 2017. Retrieved from http://www.amtliche-sozialberichterstattung.de/A2armutsgefaehrdungsschwellen.html. [Google Scholar]
- Sullivan E.V., Pfefferbaum A., Swan G.E., Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11(6):754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob E.M. How many pathways underlie socioeconomic differences in the development of cognition and achievement? Learn. Individ. Differ. 2013;25(5):12–20. doi: 10.1016/j.lindif.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob E.M., Briley D.A. Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol. Bull. 2014;140(4):949–979. doi: 10.1037/a0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- von Stumm S., Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence. 2015;48:30–36. doi: 10.1016/j.intell.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Soden B., Deater-Deckard K., Lukowski S.L., Schenker V.J., Willcutt E.G. Development in reading and math in children from different SES backgrounds: the moderating role of child temperament. Dev. Sci. 2017;20(3) doi: 10.1111/desc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger E., Brozzoli C., Lindenberger U., Lövdén M. Expansion and renormalization of human brain structure during skill acquisition. Trends Cogn. Sci. (Regul. Ed.) 2017;21(12):930–939. doi: 10.1016/j.tics.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett J.B. Some results on reliability for the longitudinal measurement of change: implications for the design of studies of individual growth. Educ. Psychol. Meas. 1989;49(3):587–602. [Google Scholar]
- Yu Q., Daugherty A.M., Anderson D.M., Nishimura M., Brush D., Hardwick A. Socioeconomic status and hippocampal volume in children and young adults. Dev. Sci. 2017:e12561. doi: 10.1111/desc.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]