Abstract
Background: The Asia-Pacific region has made significant progress against malaria, reducing cases and deaths by over 50% between 2010 and 2015. These gains have been facilitated in part, by strong political and financial commitment of governments and donors. However, funding gaps and persistent health system challenges threaten further progress. Achieving the regional goal of malaria elimination by 2030 will require an intensification of efforts and a plan for sustainable financing. This article presents an investment case for malaria elimination to facilitate these efforts.
Methods: A transmission model was developed to project rates of decline of Plasmodium falciparum and Plasmodium vivax malaria and the output was used to determine the cost of the interventions that would be needed for elimination by 2030. In total, 80 scenarios were modelled under various assumptions of resistance and intervention coverage. The mortality and morbidity averted were estimated and health benefits were monetized by calculating the averted cost to the health system, individual households, and society. The full-income approach was used to estimate the economic impact of lost productivity due to premature death and illness, and a return on investment was computed.
Results: The study estimated that malaria elimination in the region by 2030 could be achieved at a cost of USD 29.02 billion (range: USD 23.65-36.23 billion) between 2017 and 2030. Elimination would save over 400,000 lives and avert 123 million malaria cases, translating to almost USD 90 billion in economic benefits. Discontinuing vector control interventions and reducing treatment coverage rates to 50% will result in an additional 845 million cases, 3.5 million deaths, and excess costs of USD 7 billion. Malaria elimination provides a 6:1 return on investment.
Conclusion: This investment case provides compelling evidence for the benefits of continued prioritization of funding for malaria and can be used to develop an advocacy strategy.
Keywords: malaria, elimination, financing, costs, government, donor, resource mobilization
Abbreviations
ACT: Artemisinin-based Combination Therapy; ADB: Asian Development Bank; APLMA: Asia-Pacific Leaders Malaria Alliance; ASEAN: Association of Southeast Asian Nations; GDP: Gross domestic product; Global Fund: Global Fund to Fight AIDS, Tuberculosis and Malaria; GMS: Greater Mekong Subregion; IMF: International Monetary Fund; IP: Inpatient; IRS: Indoor residual spraying; LIC: Low-income country; LLIN: Long-lasting insecticidal net; LMIC: Lower-middle-income country; MDA: Mass drug administration; MDB: Multilateral development bank; MOH: Ministry of Health; NMCP: National malaria control program; NSP: National strategic plan; OECD: Organization for Economic Cooperation and Development; OOP: Out-of-pocket; OP: Outpatient; PAR: Population at risk; PPP: Purchasing Power Parity; POR : Prevention of reintroduction; RDT: Rapid diagnostic test; ROI: Return on investment; STC: Sustainability, transition, and co-financing; UMIC: Upper-middle-income country; USD: United States dollar; VLY: Value of additional life year; WHO: World Health Organization.
Introduction
The Asia-Pacific region has achieved significant gains against malaria over the last decade. Malaria cases and deaths have declined by more than 50% between 2010 and 2015 in the region’s 22 malaria-endemic countries 1. Sri Lanka was declared malaria-free in 2016, becoming only the second country in Southeast Asia, after the Maldives, to successfully eliminate malaria. Apart from India, Indonesia, Myanmar, and Thailand, malaria-endemic countries have reported reductions in malaria incidence of more than 75% since 2000. In Bhutan, China, and Timor-Leste, cases have declined by almost 100%, with less than 200 cases being reported in 2016 1.
Progress in driving down malaria may be attributed to a number of factors. Strong political and financial support from governments and donors like the Global Fund to Fight AIDS, Tuberculosis and Malaria (the Global Fund) has enabled the scale-up of effective interventions to prevent, diagnose, and treat malaria. Financing for malaria in the Asia-Pacific region increased from less than USD 100 million in 2000 to about USD 415 million in 2016. Between 2006–2010, the Asia-Pacific region attracted between 12% and 21% of global malaria funding from the Global Fund to Fight AIDS, Tuberculosis and Malaria (hereafter Global Fund) 2. However, there has been a steady decline in external financing for malaria in several countries, particularly those that are middle-income and experience relatively lower malaria transmission 3. Although domestic financing for malaria has increased in many countries in the last decade, the need for malaria control and elimination far exceeds the available resources, particularly in the context of elimination where malaria is no longer perceived as a priority disease.
Despite the progress and opportunities for elimination, malaria remains a major cause of death and illness in the region, with an estimated 1.72 billion people at risk of the disease in 2016 4. The recent gains made are fragile and investments could be lost if malaria resurges. The case for malaria elimination has never been stronger, particularly with the growing threat of antimalarial drug resistance arising from the Greater Mekong Subregion (GMS) and the risk of it spreading to other regions. However, in order to achieve a malaria-free Asia-Pacific—a goal endorsed by leaders at the highest levels though the Asia-Pacific Leaders Malaria Alliance (APLMA) 2—financial resources will need to be sustained 5. Reduced funding or political commitment has historically been linked to 75 resurgences of malaria in 61 countries since the 1930s 6.
Countries and partners need better estimates of the resources required to eliminate malaria in the long term, as well as evidence on the financial and economic benefits of investing in its elimination in order to advocate for more resources. The objectives of this study were to estimate the cost to achieve malaria elimination in the Asia-Pacific region by 2030; generate an investment case for malaria by estimating the economic benefits of malaria elimination and prevention of reintroduction (POR); and identify the funding gaps and explore the potential opportunities for generating additional financial resources for achieving malaria elimination goals.
Financing for malaria in the Asia-Pacific region
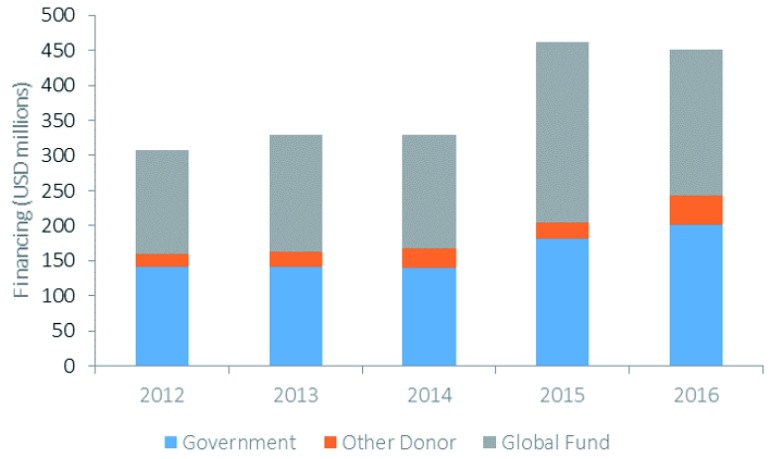
The main sources of financing for malaria in Asia-Pacific are domestic government resources and external financing from donors. Although domestic financing for malaria has increased by over 40% in Asia-Pacific between 2015–2017 compared to 2012–2014 5, most national malaria control programs (NMCPs) in the region continue to be highly reliant on external financing, particularly from the Global Fund. As Figure 1 illustrates, almost 50% of the total funding for malaria in Asia-Pacific in 2016 was from the Global Fund. This dependence on external financing is projected to continue 7.
Figure 1. Financing for malaria in the Asia-Pacific region by source: unpublished data from the global fund.
Methods
Cost estimates and model framework
We used outputs from a mathematical transmission model to estimate the costs and benefits of malaria elimination. The model is a dynamic compartmental model that incorporates the transmission dynamics of both P. falciparum and P. vivax malaria and interactions between the two species of malaria. A full description of the model framework is available elsewhere 9.
For the investment case, a spatially dependent version of this multi-species model was applied at a national level to the 22 countries in the Asia-Pacific. This facilitated the estimation of the relative contribution of interventions in a spatially heterogeneous transmission setting. The region was partitioned into a number of interconnected patches with each patch representing a country having its own transmission intensity. Spatial dependence between the patches was incorporated such that the risk of infection of an individual in one patch from an individual in another patch is negatively correlated with the distance between the centroids of the patches 9.
The model was validated separately against the estimated burden of disease for P. falciparum and P. vivax (presented elsewhere 10) and accumulated case mortality. Several indicators (such as the estimated incidence of all malaria species and reported fatalities) were modelled for each country between 2016 and 2030, under scenario-specific assumptions. A total of 80 scenarios were simulated, based on 10 different sets of packages of interventions. These ranged from discontinuing most malaria activities to a very substantial scale-up of interventions, which could be supplemented by mass drug administration (MDA) or an increase in the coverage of long-lasting insecticide-treated nets (LLINs), at either a stable or increasing trajectory of drug resistance 11. The last component for each scenario computing the costs of interventions per country, year and component and developing an investment case.
While the reported coverage of interventions (particularly LLINs and indoor residual spraying (IRS)) were included in the model to inform changes in incidence, there was little available data on coverage of other interventions between 2000 and 2015, such as the introduction of community health workers). These coverage statistics were therefore imputed based on observed changes in reported incidence. The mortality predicted by the model was validated against reported deaths. A full description of the model is available elsewhere 11.
The model estimated the impact of several intervention scenarios on the transmission of P. falciparum and P. vivax malaria from 2016 to 2030 in each of the 22 countries. Data used to calibrate and validate the model were sourced from World Malaria Reports (2001–2016) 12– 19 and peer reviewed literature 20.
Elimination scenarios
A total of 80 scenarios were generated. Four counterfactual scenarios (No. 1–4 in Table 1) including one “business as usual” scenario was modelled in which coverage remained the same as for 2016 (the last data point for which covariate rates were available for all 22 countries), and three reverse scenarios that simulated the potential impact of scaling down the malaria program. The six elimination scenarios (No. 5–10 in Table 1 were modelled sequentially to increase in complexity and in the number of interventions included.
Table 1. Modelled scenarios used in the transmission model.
| Scenario | Description | |
|---|---|---|
| 1 | Business as usual | • Continue all interventions at 2015 levels from 2016 through 2030 |
| 2 | Reverse scenario 1 | • Business as usual
• IRS activities ceased |
| 3 | Reverse scenario 2 | • Reverse scenario 1
• Distribution of new LLINs ceased |
| 4 | Reverse scenario 3 | • Reverse scenario 2
• Treatment rates reduced by 50% |
| 5 | Universal coverage | • Business as usual
• Coverage of population at risk with test and treat increased from 2017 onwards in a linear fashion over eight years to 80% by 2025 • Quinine is switched to injectable artesunate for management of severe disease in 2017 |
| 6 | IRS | • Universal coverage
• IRS coverage in 2017 doubled in a linear fashion over eight years |
| 7 | Effective usage | • Universal coverage
• Effectiveness of LLINs increased • Surveillance increased |
| 8 | New P. vivax treatment | • Effective usage
• Replace primaquine with a new P. vivax treatment |
| 9 | New LLINs | • New
P. vivax treatment
• Life of LLINs doubled |
| 10 | New
P. falciparum
treatment |
• New LLINs
• First-line Artemisin based Combiantion Therapy (ACT) replaced with new candidate for P. falciparum treatment |
| Assumption | Description | |
| A | Artemisinin and ACT
resistance |
5% probability of treatment failure from ACTs across all countries is constant until 2018 and then
increased to 30% through 2025 |
| B | MDA | Five annual rounds of MDA at 50% coverage from 2018 starting four months before the peak of the
transmission season targeted at both species |
| C | LLINs | Scaling up LLINs to 80% effective coverage deployed in a 3-year cycle (50%, 25% and 25%) |
For each country, the minimum package of interventions that would achieve malaria elimination was determined, defined here as one year with less than one reported clinical case. This was taken to be the minimum elimination scenario for that particular country. Since the model did not distinguish between indigenous and imported cases, we assumed that certain thresholds of cases are imported, which we subtracted from the model outputs. In addition, we simulated the effect of improved targeting of malaria interventions on both costs and epidemiological outputs. We did this by reducing intervention coverage by 30% among the population at risk (PAR) for all scenarios, with and without the resistance and MDA assumptions.
The outputs of averted mortality and morbidity under the elimination scenarios were expressed as reported cases and deaths (projected from reported cases) and estimated cases and deaths projected from a range of estimates. Averted cases and deaths were then used to estimate the cost, benefits, and returns on investment (ROIs).
Additional assumptions
We applied additional assumptions to simulate various possible outcomes across all 10 scenarios: (i) The first was around the occurrence of artemisinin and ACT partner drug resistance; across all scenarios, a baseline treatment failure rate of 5% was applied across all countries from 2016–2030. Under the resistance assumption, the probability of treatment failure was kept constant at 5% through 2018 and increased to 30% between 2018 and 2025. (ii) The second assumption concerned the use of MDA. MDA was simulated as five annual rounds of dihydroartemisinin-piperaquine at 50% coverage of the population at risk from 2018 onwards, starting four months before the peak of the malaria transmission season. (iii) In a third set of simulations, LLIN scale-up was added to all the elimination scenarios in accordance with WHO guidelines for vector control, if malaria elimination was not predicted by 2030. LLIN scale-up was defined as LLIN coverage of up to 80% coverage achieved through three-year distribution cycles from 2017 to 2026. These additional rates of decline were projected separately.
These additional scenarios produced a total of 80 scenarios: with and without resistance; with and without MDA; and with and without LLIN scale up to 80%.
PAR
For all the scenarios, a declining PAR was assumed in the model. PAR values used to estimate costs in the model were adjusted to reflect the decreases in incidence predicted from the implementation of elimination-focused interventions. Historical incidence and PAR data were analysed statistically to infer a predicted change in PAR for a given change in incidence. This relationship was applied to the 2015 PAR data and updated every year until 2030 as interventions were applied in the modelled scenarios. This method has limitations, including a non-standardized definition of PAR between countries.
Cost projections
We built a cost estimation model aligned with the outputs of the transmission model to estimate the total costs associated with implementing each of the scenarios above. Program costs included the costs of testing and treating uncomplicated or outpatient (OP) and severe or inpatient (IP) malaria cases; vector control (i.e., LLIN distribution and IRS); supply chains; surveillance through community health workers; information, education, communication; training; MDA; new treatments (e.g., tafenoquine for P. vivax); and rollout of new LLINs. Unit costs for each activity were obtained using a combination of empirical data collected in various Asia-Pacific countries by the authors, literature reviews, and proxies when the previous options were unavailable (Table E1, available as extended data 21).
In addition, we simulated the effect of improved targeting of malaria interventions on both costs and epidemiological outputs on cost. We did this by assuming a focal deployment of interventions where the target population comprises the high risk and only 70% of the low risk population.
The total cost of the elimination scenarios was used to build this investment case. We calculated the costs to reach elimination separately for each country and then summed them to obtain the total cost for elimination in the Asia-Pacific region. To calculate the incremental or additional costs of malaria elimination (which were used to calculate ROIs), we subtracted the estimated costs of the business as usual and reverse scenarios from the elimination scenario. All monetary figures are expressed in 2015 constant USD.
Economic benefits estimation
Using outputs from the model, we estimated the mortality and morbidity averted from malaria elimination by subtracting the estimated cases and deaths of the elimination scenario from the corresponding outputs of the “business as usual” and “reverse” scenarios. We then monetized these health benefits by looking at the averted cost to the health system, averted cost to individual households, and averted cost to society:
-
1.
Cost averted to the health system includes costs associated with diagnosis and treatment costs of IPs and OPs;
-
2.
Cost averted to the individual households is out-of-pocket (OOP) expenditures for seeking care; and
-
3.
Cost averted to the society due to patients’ lost productivity due to premature death and morbidity and caregivers’ reduced economic output.
The same cost inputs used in the cost estimation were used for calculating the economic benefits. Unit costs for case management included costs for OP visits, diagnostic tests, and drug treatments for OP malaria cases, as well as hospital hotel costs and drug treatments for IP malaria cases. OOP expenditures were estimated by applying country-specific OOP expenditure per capita separately for OP and IP cases. We calculated productivity losses among patients and caretakers by multiplying an estimate of daily productivity by the number of days lost due to illness or care seeking.
We used the full-income approach to estimate the economic impact of lost productivity due to premature death from malaria. We multiplied the number of averted deaths for each country by the country-specific values of additional life years (VLYs) and life expectancies at age 40 among males and females, which was the assumed average age of death due to malaria. One VLY was estimated to be 2.2 times the gross domestic product (GDP) per capita for each of the countries in South East Asia and the Pacific and 2.8 times the GDP per capita for each of the countries in South Asia, as suggested by the Lancet Commission on Investing in Health 22.
All costs and economic benefits were discounted at 3%.
Return on investment
The ROI was calculated by subtracting the incremental cost of elimination from the economic benefits and dividing the resulting figure by the incremental cost of elimination. The ROI is interpreted as the economic return from every additional dollar spent on malaria elimination.
We performed the ROI analysis for 2016–2030 by comparing the elimination scenario with the business as usual and reverse scenarios under the stable and increasing resistance assumptions.
Uncertainty analysis
We performed stochastic sensitivity analysis on the epidemiological and cost outputs of the malaria transmission model. The minimum, median, and maximum malaria cases and deaths predicted by the model for each scenario were used to calculate the minimum, median, and maximum economic benefits. For the costs, we assigned an uncertainty interval of ±25% on the value of the input costs used. A total of 300 random samples were drawn, which generated a range of costs. From the range of costs generated, we determined the minimum, maximum, median, mean, and other percentiles.
Gap analysis and opportunities for resource mobilization
Using available malaria financing data in the region (donor and domestic), between 2017 and 2020, we estimated the potential gap in financing assuming the total funding envelope would remain as projected. We also assessed potential opportunities for resource mobilization to fill financing gaps by mapping private sector investors and analysing the domestic funding landscape.
Results
Projected declines in transmission
The transmission model predicted that malaria elimination can be achieved by all the countries in the Asia-Pacific region by 2030 by implementing a variety of scenarios. Table 2 illustrates the predicted output of the transmission model under an assumption of increasing artemisinin resistance and identifies the minimum elimination scenario defined as the scenario under which the country can achieve elimination on or before 2030 with the least amount of effort.
Table 2. Scenarios and predicted elimination dates.
| Country | Minimum elimination
scenario and interventions |
MDA | LLIN | Elimination date
(predicted range) |
National
elimination goal |
|---|---|---|---|---|---|
| Afghanistan | Effective usage | Yes | Yes | 2025 (2025,2027) | None |
| Bangladesh | Effective usage | No | No | 2025 (2024,2029) | 2035 |
| Bhutan | Effective usage | No | No | 2024 (2023, 2025) | 2018 |
| Cambodia | New LLINs | Yes | No | 2023 (2022, 2030) | 2025 |
| China | Business as usual (already
eliminated by 2017) |
No | No | 2017 | 2020 |
| DPRK | New P. vivax drug | No | Yes | 2028 (2027, 2030) | 2025 |
| India | New LLINs | No | Yes | 2028 (2026, 2030) | 2030 |
| Indonesia | Effective usage | Yes | No | 2025 (2022,2028) | None |
| Lao PDR | New P. falciparum drug | Yes | Yes | 2025 (2022,>2030) | |
| Malaysia | IRS | No | No
No |
2023 (2019, 2029) | 2020 |
| Myanmar | New P. falciparum drug | Yes | Yes | 2025 (2024,>2030) | None |
| Nepal | Effective usage | No | No | 2022 (2017, 2026) | 2026 |
| Pakistan | Effective usage | Yes | Yes | 2022 (2021, 2030) | None |
| PNG | Effective usage | Yes | No | 2025 (2025,2028) | |
| Philippines | Effective usage | No | No | 2021 (2017,2023) | 2030 |
| ROK | Business as usual | No | No | 2017 (2017,2019) | 2017 |
| Solomon Islands | New LLINs | Yes | No | 2028(2026, 2029) | |
| Sri Lanka | Business as usual (already
eliminated by 2017) |
No | No | Already eliminated
in 2013 |
2012 |
| Thailand | New P. vivax drug | No | No | 2026 (2025, 2029) | 2024 |
| Timor-Leste | Universal coverage | No | No | 2019 (2017,2024) | |
| Vanuatu | Effective usage | Yes | No | 2021 (2021, 2024) | 2025 |
| Viet Nam | Effective usage | No | No | 2024 (2022, 2027) | 2030 |
The model predicted that it is possible for all 22 countries to achieve elimination of P. falciparum and P. vivax by 2030. China, ROK, and Sri Lanka 3 are the only countries predicted to achieve elimination without scaling up current interventions. Elimination was predicted to be possible in Cambodia, DPRK, India, Lao PDR, Myanmar, Solomon Islands, and Thailand by 2030 using new tools and technological innovation. Elimination was predicted to be possible by 2030 through the addition of MDA in Afghanistan, Cambodia, Indonesia, Lao, Myanmar, Pakistan, PNG, Solomon Islands, and Vanuatu. In all other countries, elimination is possible with the scale up of existing interventions.
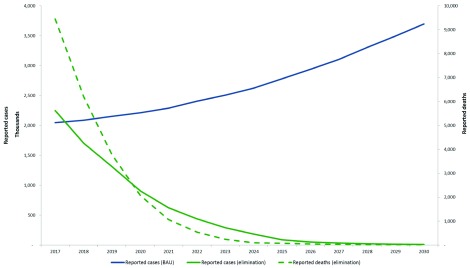
Figure 2 illustrates the median reported cases and deaths between 2016–30 under the “business as usual” scenario and minimum elimination scenarios for the region. These are predictions projected from the reported cases in 2015.
Figure 2. Median predicted reported cases and deaths in the Asia-Pacific region, 2017–2030 under the business as usual scenario (BAU) and elimination scenario.
These are predictions projected from the reported cases in 2015.
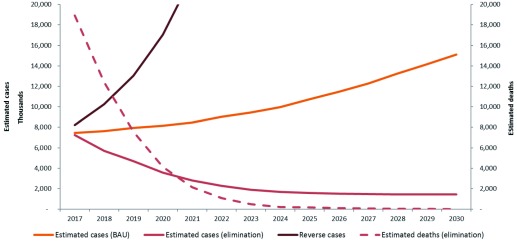
Figure 3 Illustrates the median estimated cases and deaths between 2016 and 2030 under the “business as usual” scenario and minimum elimination scenarios for the region. These are predictions projected from the estimated cases in 2015.
Figure 3. Median predicted estimated cases and deaths in the Asia-Pacific region, 2017–2030 under the “business as usual” scenario. minimum elimination and reverse scenarios.
In the business as usual scenario for all countries in the region, clinical cases rose from an estimated 7 million in 2016 to 15 million in 2030. Implementing the elimination scenario in each country will avert a total of over 123 million clinical cases and approximately 3.5 million deaths in the region over 14 years. In a “reverse” or worst case scenario, where interventions are halted and reduced (reverse scenario), cases increase to about 180 million by 2030. There would be about 1 billion additional cases and 3.5 million additional deaths, costing an excess of USD 7 billion between 2016–2030.
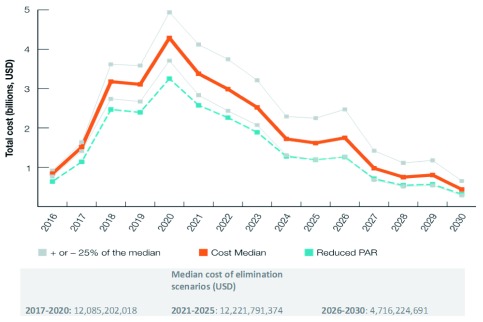
Cost of malaria elimination through 2030
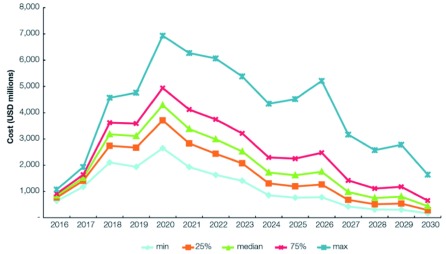
The cost of malaria elimination is shown in Figure 4 and Table 3 The total cost to achieve malaria elimination in the Asia-Pacific between 2017 and 2030 was estimated to be USD 29.024 billion (range: USD 23.65–36.23 billion). The median cost in 2017 for the elimination scenarios was USD 1.51 billion (range: USD 1.41–1.64 billion). Costs peak in 2020 at USD 4.29 billion (range: USD 3.71–4.94 billion), then decrease to less than USD 1 billion in 2027 (range: USD 0.68–1.42 billion) and less than USD 450 million (range: USD 0.29–0.65 billion) in 2030 when elimination is expected to be achieved in all 22 countries. Lower costs incurred are expected to continue after the elimination date as POR of malaria interventions continue. The reverse scenario would cost an excess of USD 7 billion between 2017 and 2030. If interventions were only applied to 70% of the PAR in the low-transmission areas (a crude proxy for the effect of improved targeting of interventions), the total cost would be about USD 22.49 billion.
Figure 4. Modeled costs of the elimination scenario, 2017–2030.
Median and median +/- 25% costs of the modelled minimum elimination scenarios as well as an estimate of the median cost under a scenario of reduced PAR between 2017–2030.
Table 3. Summary of costs and benefits, 2017–2030.
| Scenarios compared | Total cost (USD) | Estimated clinical
cases averted |
Deaths averted | Economic benefits
(USD) |
Incremental
cost (USD) |
ROI |
|---|---|---|---|---|---|---|
| Business as usual
vs. elimination (with resistance assumption) |
29.024 billion
(range: 23.64–36.23) |
123.14 million
(estimated) 4 16.54 million (reported) 5 |
386,167
(estimated) 193,084 (reported) |
87.73 billion
(range: 26.3–347.14) |
14.05 billion | 6:1 |
| Business as usual vs.
elimination (baseline) |
28.953 billion
(range: 23.38–35.72) |
92.23 million
(estimated clinical) 11.68 million (reported) |
264,322
(estimated clinical) 132,161 (reported) |
72.90 billion | 13.79 billion | 5:1 |
| Reverse vs. elimination
(with resistance assumption) |
NA | 845.73 million | 3.487 million | N/A | 6.693 billion | N/A |
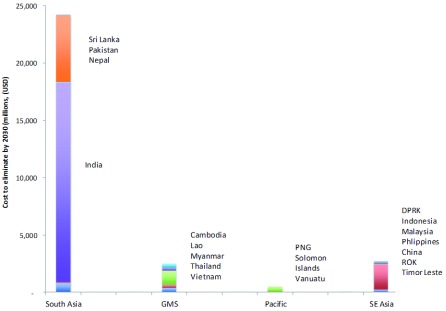
Figure 5 illustrates how the relative costs are skewed by sub region with over 80% of the costs predicted to be incurred in South Asia, most notably, India.
Figure 5. Modeled costs by country and region of the elimination scenario from 2016 to 2030.
Economic benefits estimation
Compared to a business as usual scenario, interrupting local transmission can save over 400,000 lives and avert 123 million malaria cases, translating to almost USD 90 billion (range: USD 26.3–347.14 billion) in economic benefits. The economic benefits included costs averted for diagnosis and treatment costs as inpatients and outpatients, costs averted to individual and households and the monetized value of lost productivity due to premature death and morbidity and caretaker’s reduced economic output as a result of taking care of patients.
Discontinuing vector control interventions and reducing treatment coverage rates to 50% will reverse the gains made, resulting in an additional 845 million cases, 3.5 million deaths, and excess costs of USD 7 billion.
Return on investment
The cost of malaria elimination was weighed against the epidemiological and economic costs of inaction. When the net benefits of elimination compared to the cases and costs averted in the business as usual scenario of the transmission model for the period of 2017 to 2030, the median ROI for each additional dollar invested in malaria elimination was calculated to be over 6:1. This increases to 7:1 if interventions are better targeted in low-risk areas.
Financial gap
A median resource envelope of about USD 3 billion is needed annually to achieve elimination between 2018–2020. Total financing for the region is projected to be USD 0.5 billion annually for 2018–2020. Therefore, the anticipated gap is likely to be over 80% of the resources required for elimination between 2018–2020.
Sensitivity analysis
Figure 6 illustrates the sensitivity of the total cost to the individual cost inputs. At the peak in 2020, costs vary from USD 2.5 billion to USD 7 billion.
Figure 6. Sensitivity of the total cost to the individual cost inputs (2016–2030).
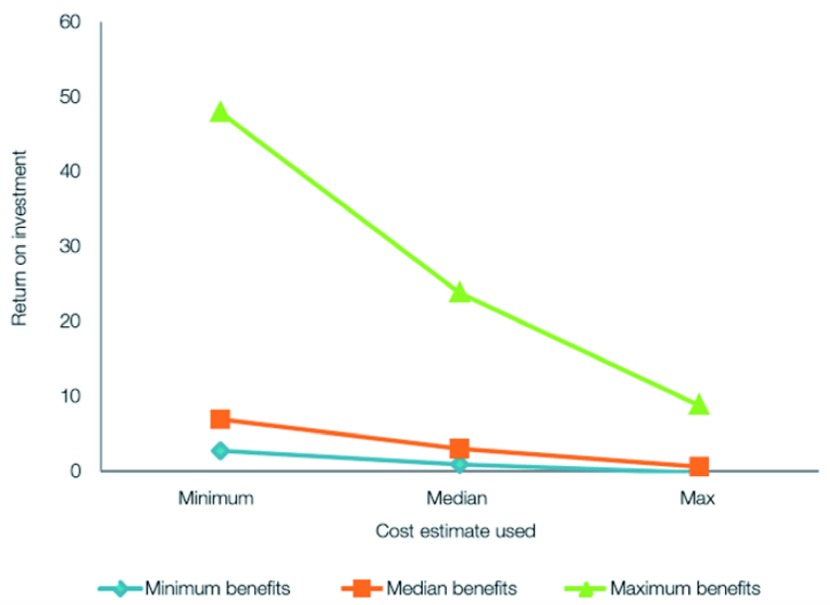
Figure 7 illustrates that using minimum values of the benefits will still produce a positive ROI.
Figure 7. Sensitivity of the ROI to varied costs and benefits.
Discussion
This analysis compared the monetized value of expected benefits from malaria elimination to the investment costs over a 14-year investment period (2017–2030) in the 22 countries of the Asia-Pacific, demonstrating a robust median return of about six times the incremental investment.
The study found that by employing a variety of existing and new interventions, all countries in the Asia-Pacific could eliminate malaria by 2028—two years before the 2030 APLMA regional goal. The health, social, and economic returns are potentially formidable. Malaria elimination will save over 400,000 lives and avert over 123 million cases, translating to economic benefits of almost USD 90 billion.
Successfully achieving elimination, however, will require sustained financial resources. Our model estimates that the total cost of achieving elimination and POR is about USD 29.02 billion (range: USD 23.64–36.23 billion) over 14 years or USD 12 billion between 2017–2020. Total financing for malaria in the Asia-Pacific in 2016 was estimated at USD 415 million. Using co-financing data from Global Fund concept notes, total financing for malaria was projected at USD 1.4 billion between 2018–2020, leaving an annual gap of about USD 2.5 billion or 80% of the estimated cost of elimination.
Numerous countries in the region continue to rely on Global Fund resources to provide up to 50% of their total financing for malaria elimination. However, the allocation methodology adopted by the Global Fund in 2012, utilizes a combination of disease burden and gross national income (GNI) per capita to determine the financing that countries will receive. By definition, malaria-eliminating countries have lower disease burdens, have higher incomes and are therefore a lessor priority for donors. Country-specific funding from the Global Fund to the sub-set of countries attempting to eliminate malaria has declined by over 30% 2. Further declines in allocations have been noted under a subsequently revised model adopted in November 2016 23. Given the downward trend in malaria burden and the region’s rising economic status, this level of support is likely to be even more diminished in subsequent years.
Many malaria-eliminating countries are middle-income countries (MICs) as defined by the World Bank 24. The International Monetary Fund (IMF) projects average annual GDP growth rates of 3–10%, which means that economies in Asia will double or triple in size in the next decade. By 2020, four countries in Asia that are currently lower-middle-income countries (LMICs); Bhutan, Indonesia, the Philippines, Sri Lanka, will surpass the World Bank threshold for MICs of USD 4,125 GDP per capita. This means that while there is increased potential for domestic financing, more countries will also start to graduate out of aid eligibility. Of the 22 countries in the Asia-Pacific region, three are currently LICs, 15 are LMICs, and three are UMICs and one is an UIC. There are 18 currently eligible for Global Fund financing 25, out of which an additional two countries will be receiving the final transitional grants in the next two years (the Philippines and Sri Lanka). Political and policy changes in other donor constituencies also pose similar risks.
These changing polices have major implications for the financing and delivery of health services, for malaria elimination. Malaria financing will therefore need to depend on larger contributions from government budgets. Indeed, the expectation of the economic and health financing transition suggests that as countries develop they will spend more on health than they did before. Although domestic financing for malaria has increased by over 40% in the Asia-Pacific between 2015 and 2017 compared to 2012–2014 7, the resources required far exceed the amounts available.
The potential consequences of funding gaps at this critical juncture can be serious. This analysis estimates that scaling back interventions in the Asia-Pacific could lead to an additional 3.5 million deaths, almost 1 billion cases, and economic costs of almost USD 7 billion. Emerging artemisinin resistance further threatens the gains made against malaria and regional health security with estimates of 9,560 excess deaths and USD 51 million in productivity losses annually 26.
To ensure uninterrupted availability of key malaria interventions, mechanisms to augment and prioritize domestic funding and improve efficiencies in the existing malaria envelope will need to be explored. The Addis Ababa Action Agenda calls on a number of resource mobilization efforts encompassing aid, domestic public resources, and support from the private sector 27.
Many national governments are considering raising health budgets by improving the capacity to raise tax revenue including the implementation of Pigovian or sin taxes. In the Philippines, increased taxes on tobacco and alcohol generated USD 2.3 billion within just 2 years, increasing the Department of Health budget by 63% in 2015 28. This revenue has freed up resources, which would have otherwise been used for social protection of the poor. Indonesia and Vietnam have similarly implemented such revenue generating structures.
The diversification of Asia-Pacific countries’ economies presents a unique opportunity to engage the private sector in malaria elimination 29. Private Asian companies such as AirAsia, Samsung, the Tata group, and Alibaba have become internationally recognizable brands. Government incentives for the private sector engagement could include tax relief or tax credit schemes and policies that promote expansion or diversification of programs. For example, the Cambodian Ministry of Health has developed a policy framework for public-private partnerships in the health sector. Similarly, an airline levy such as the UNITAID model could raise more than USD 300 million per year 30.
Multilateral Development Banks (MDBs) and partners can provide new financing opportunities to governments and the private sector, including cross-sectoral financing for health programs, incentivizing companies to invest in health interventions 31. Countries can seek out additional grants and soft-loans from MDBs to help frontload the costs of elimination. Several MDBs are currently engaged in innovative models including ADB, the Inter-American Development Bank, the Islamic Development Bank, and others in collaboration with the Bill & Melinda Gates Foundation, the Global Fund and other partners 32– 34.
Innovative financing options can also fill the gap between needs and resources until government budgets catch up with the financing transition. These may include health bonds, debt swaps, and blended financing mechanisms. Social impact bonds and development impact bonds are other types of instruments that have been implemented in selected settings. One example is the Mozambique Malaria Performance Bond, which is being used to raise funding from investors interested in both financial and social returns 35– 37. Such innovative instruments have been used to raise financing for health and other sectors, such as education and environment.
In addition to increasing available health revenue and allocating additional resources, improved efficiencies can generate cost-savings, freeing up resources to cover financing gaps. Assessing and identifying current inefficacies and drivers of inefficiency can increase utilization of current funds.
Mathematical modelling suggests that the optimal mix of interventions will vary depending on the national or subnational setting 11. Reviewing efficiency of the malaria program on an annual basis, including an efficiency assessment as a pre-requisite for donor funding and linking disbursements to efficiency indictors will mitigate future inefficiencies. In-country mathematical and economic modelling could support this process and thus efforts are being made to build national modelling capacity.
In the coming years, many countries will rise in income status and will graduate from donor financing. Malaria programs, given the low disease burden, may lose eligibility before then. In addition to pursuing additional domestic financing and meeting current co-financing requirements of existing grants, countries should appropriately plan the transition from donor to domestic funding sources 3–5 years in advance of the actual transition.
A number of unknown factors and limitations impact the findings of this report. The costs of medicines and other interventions have been estimated based on available data and proxies were used when data were unavailable. The cost of new interventions, such as new LLINs, and new treatments such as tafenoquine, were based on historical estimates of the cost of new tools when they were first adopted rather than actual costs. In particular, separating out the cost of interventions in integrated systems is challenging and the analysts have relied on country-level partners to apportion the amounts spent on each intervention to arrive at disaggregated costs.
The cost estimates produced are highly dependent on the output of the transmission model, which was designed with a single homogeneous patch for the whole of each country, using national level data on incidence and intervention coverage. Treating the whole country as a single unit in this way is likely to lead to over-estimates in costs of elimination. Furthermore, spatial heterogeneity within each country was not modelled. These estimates are therefore subject to error, particularly in countries with heterogeneous transmission patterns. Elimination often requires targeted interventions to risk areas or populations, rather than ubiquitous coverage to an entire country. Without subnational estimates of incidence and coverage, targeted interventions are difficult to estimate and cost.
Due to the lack of suitable data, population movement was included in a rudimentary way. The transmission rate of malaria in each country was influenced by the malaria prevalence in other countries with the level of influence between countries reflecting a simple gravity model assumption. This assumption is likely to have reduced the predicted costs.
We were unable to predict the impact that economic development and housing improvements may have on malaria transmission or how the costs of commodities or interventions may change at the global or national levels. While we modelled for a declining PAR based on historical changes in PAR compared to changes in incidence, this method has limitations including a non-standardized definition of PAR.
While we have tried to estimate the effect that drug and insecticide resistance would have on cost, it is impossible at this stage to predict accurately the future extent and effect of drug and insecticide resistance and the actual interventions that would be implemented to address these. In addition, the impact and cost of known tools in the innovation pipeline have been modelled, however, the impact of new tools and approaches not yet developed is unknown and will be likely to decrease costs in the long term given that the cost of new tools is greatest at the time of adoption with economies of scale and competition driving costs down over time. It is also difficult to predict how the costs of interventions may change at the regional or national levels over time.
Lastly, current assessments of reported malaria incidence have limitations. Research suggests that there may be significant under-reporting in the scale of global malaria incidence and mortality due to the weakness of health reporting and information management systems as well as widespread and undocumented use of the private sector in many endemic countries. For example, the Institute for Health Metrics and Evaluation estimated a figure of 1.2 million malaria deaths in 2010—almost double the WHO’s figure of 655,000 38. Similarly, a widely quoted study in The Lancet estimated that in India, 205,000 deaths per year could be attributed directly to malaria, which differed by more than ten times the numbers reported by the malaria program in the same year 39.
There have been various attempts at quantifying the true burden of malaria and more recent publications of the World Malaria Reports contain data on reported cases to health facilities as well as estimated cases based on a number of assumptions. This report utilized reported cases from the World Malaria Reports as well as estimated cases for the Asia-Pacific countries derived by Mahidol-Oxford Tropical Medicine Research Unit in collaboration with a number of partners including the WHO 10. These estimates were obtained by combining and triangulating data from a variety of data sources. Both reported and estimated cases are depicted in the graphs. Nevertheless, the wide variation in estimates of burden makes it harder to be sure of the resources required to eliminate the disease. Without an informed and complete understanding of the current cartography of malaria risk and prevalence, future projections of the cost of eliminating malaria face an overwhelming uncertainty.
We believe that the estimated benefits of elimination are conservative. Beyond the benefits of achieving malaria elimination as explained in this report, other benefits are likely, but are harder to quantify as there are no reliable quantitative estimates on how malaria may impact these. As a by-product of national elimination, other positive externalities are increased tourism, a strengthened health system, better cognitive development, and improved regional health security. In addition, elimination may bring significant benefits to other regional public goods including opportunities to create stronger cross-border disease coordination. These estimates can therefore be considered conservative.
Because of these uncertainties, estimated costs can only provide an indicative guide or baseline to help determine financing needs. It is therefore important that economic estimates are constantly reviewed in the light of new information, through to 2030. Importantly, due to the diversity of the region, further analysis is required to adapt the model to individual country settings and develop country-level estimates based on the national context. This, however, makes it even more important that funds can be put in place quickly to match currently expected costs.
Despite limitations above, this investment case provides robust evidence of the benefits of continued prioritization of funding for malaria. The ROIs remain robust, comparable to those obtained for other high impact investments such as immunization programs and cardiovascular disease research 40. Although the short-term investment needed may seem substantial, these must be considered in the context of other major public health, developmental projects and other national level expenditures. For example, the new High-Speed II railway system in the United Kingdom is expected to cost more than USD 70 billion, more than twice the amount for eliminating malaria from the Asia-Pacific 41. Similarly, in 2015, US military spending amounted to almost USD 600 billion 42 and spending on costumes, candy and decorations on Halloween is expected to amount to over USD 8 billion this year 43. On the contrary, the savings from malaria elimination will allow the treatment of 11 million outpatients for 10 years or allow the construction of over 100,000 km of roads in Asia.
Focused advocacy at all levels is needed to reach key decision-makers in order to highlight the social and economic benefits of investing in malaria elimination and the risks of not doing so. In particular, emphasis on the threat of drug resistance in undermining success, increasing costs and posing a risk of regional health security is needed. Continued engagement is needed with governments to focus attention on increased domestic budgets to reach the regional goal of a malaria-free Asia-Pacific by 2030.
Conclusion
Global progress against malaria has been dramatic over the past decade. These gains, however, have been driven by substantial political and financial commitments that must be sustained to avoid a resurgence of malaria. There are several critical reasons why malaria elimination should receive a special focus for financing. Malaria is a major ongoing cost driver burdening national health systems and eliminating the disease will confer public health benefits as well as major cost savings to national health systems. The investments needed are time-limited as costs taper off significantly as more countries eliminate the disease. Secondly, there is a strong correlation between the decline in malaria burden and financing. Declining financing for malaria is an imminent threat to malaria elimination, the spread of drug resistance, and regional health security in the Asia-Pacific region. This investment case provides compelling evidence for the benefits of continued prioritization of funding for malaria, and can be used to develop an advocacy strategy for increased domestic and external funding for the region to reach its goal to be malaria-free by 2030.
Data availability
Underlying data
Data used to calibrate and validate the model were sourced from World Malaria Reports (2008–2016) and peer-reviewed literature 9, 12– 17, 20. Where there were gaps in the data, these were imputed based on observed changes in reported incidence.
Extended data
Zenodo: sheetalsilal/METCAP: METCAP Model. https://doi.org/10.5281/zenodo.2575474 21. The following extended data are available:
Extended Cost Database.docx (Table E1. Cost estimations used in calculations)
Extended data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgments
We sincerely thank Allison Phillips, Erika Larson and Roly Gosling, for reviewing and providing valuable comments on the manuscript. In addition, we would like to thank Tom Drake from MORU for assistance with cost data. A previous version of this was used for part of a PhD dissertation.
Funding Statement
RS, SS, AA, KF, RB and BZ were funded by a grant from the Asian Development Bank and the Bill and Melinda Gates Foundation (BMGF) (OPP1089413). OJC was supported by the BMGF (OPP1110500). LJW was supported by the BMGF (OPP1110500) and the Wellcome-Trust Major Overseas Programme in SE Asia (106698). RJM and CEM were funded by the BMGF (OPP1110500), Asian Development Bank (TA-8763 and TA-8656), Australian Department of Foreign Affairs and Trade (71215) and the Wellcome-Trust Major Overseas Programme in SE Asia (106698/Z/14/Z).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved, 1 approved with reservations]
Notes
1 The Asia-Pacific region in this report encompasses the 22 malaria-endemic countries as defined by APLMA. Sri Lanka has since been declared as malaria free but still implements prevention of reintroduction activities. Countries include: Afghanistan, Bangladesh, Bhutan, Cambodia, Democratic People’s Republic of Korea (DPRK), India, Indonesia, Lao People’s Democratic Republic (Lao PDR), Malaysia, Myanmar, Nepal, Pakistan, Papua New Guinea (PNG), People’s republic of China, Philippines, Republic of Korea (ROK), Solomon Islands, Sri Lanka, Thailand, Timor Leste, Vanuatu and Vietnam.
2 At the 2013 East Asia Summit (EAS), the Asia-Pacific Leaders Malaria Alliance (APLMA) was established to accelerate progress towards a reduction in malaria cases and deaths. In 2014 at the ninth EAS, the APLMA Co- Chairs (the Prime Ministers of Viet Nam and Australia) tabled a recommendation for the Asia-Pacific region to become free of malaria by 2030. EAS Heads of Government agreed to the goal, and tasked APLMA Co- Chairs to present a plan to reach malaria elimination through a “Leaders’ Malaria Elimination Roadmap” 8. The APLMA roadmap was presented to Heads of Government during the 10th EAS Meeting in 2015.
3 Sri Lanka saw its last indigenous case in 2012 and obtained WHO certification in 2016.
4 Projected from estimated cases
5 Projected from reported cases
References
- 1. WHO: World Malaria Report. 2016. Geneva, Switzerland: World Health Organization.2016. Reference Source [Google Scholar]
- 2. Zelman B, Melgar M, Larson E, et al. : Global fund financing to the 34 malaria-eliminating countries under the new funding model 2014-2017: an analysis of national allocations and regional grants. Malar J. 2016;15:118. 10.1186/s12936-016-1171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shretta R, Zelman B, Birger M, et al. : Tracking development assistance and government health expenditures: 1990-2017. Malar J. 2017;16(1):251. 10.1186/s12936-017-1890-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO: World Malaria Report 2016. Geneva, Switzerland: World Health Organization.2017. [Google Scholar]
- 5. East Asia Summit: Chairman's Statement of 9 th East Asia Summit (9 th EAS). 13 November 2014. Nay Pyi Taw, Myanmar.2014. Reference Source [Google Scholar]
- 6. Cohen JM, Smith DL, Cotter C, et al. : Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. 10.1186/1475-2875-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Global Fund: Unpublished data. Geneva, Switzerland: Global Fund for HIV/TB and Malaria.2017. [Google Scholar]
- 8. APLMA: APLMA malaria elimination roadmap. 2015; published online Oct 26. Asia Pacific Leaders’ Malaria Alliance.2015. Reference Source [Google Scholar]
- 9. WHO: World Malaria Report 2018. Geneva, Switzerland: World Health Organization.2008. Reference Source [Google Scholar]
- 10. Maude RJ, Mercado CEG, Rowley J, et al. : Estimating malaria disease burden in the Asia-Pacific [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2019;4:59 10.12688/wellcomeopenres.15164.1 [DOI] [Google Scholar]
- 11. Silal SP, Shretta R, Celhay OJ, et al. : Malaria elimination transmission and costing in the Asia-Pacific: a multi-species dynamic transmission model [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2019;4:62 10.12688/wellcomeopenres.14771.1 [DOI] [Google Scholar]
- 12. WHO: World Malaria Report 2010. Geneva, Switzerland: World Health Organization.2010. Reference Source [Google Scholar]
- 13. WHO: World Malaria Report 2011. Geneva, Switzerland: World Health Organization.2011. Reference Source [Google Scholar]
- 14. WHO: World Malaria Report 2012. Geneva, Switzerland: World Health Organization.2012. Reference Source [Google Scholar]
- 15. WHO: World Malaria Report 2013. Geneva, Switzerland: World Health Organization.2013. Reference Source [Google Scholar]
- 16. WHO: World Malaria Report 2014. Geneva, Switzerland: World Health Organization.2014. Reference Source [Google Scholar]
- 17. WHO: World Malaria Report 2015. Geneva, Switzerland: World Health Organization.2015. Reference Source [Google Scholar]
- 18. Howes RE, Piel FD, Patil AP, et al. : G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. Seidlein von L, editor. PLoS Med. 2012;9(11):e1001339. 10.1371/journal.pmed.1001339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Earth System Research Laboratory website for El Niño Southern Oscillation time series. Reference Source [Google Scholar]
- 20. Mercado CE, Ekapirat N, Dondorp AM, et al. : An assessment of national surveillance systems for malaria elimination in the Asia Pacific. Malar J. 2017;16(1):127. 10.1186/s12936-017-1774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silal S: sheetalsilal/METCAP: METCAP Model (Version v1.0.2). Zenodo. 2019. 10.5281/zenodo.2575474 [DOI] [Google Scholar]
- 22. Jamison DT, Summers LH, Alleyne G, et al. : Global health 2035: a world converging within a generation. Lancet. 2013;382(9908):1898–1955. 10.1016/S0140-6736(13)62105-4 [DOI] [PubMed] [Google Scholar]
- 23. Global Fund for HIV/TB and Malaria.2018. Reference Source [Google Scholar]
- 24. World Bank: Gross Domestic Product Ranking.2016; Accessed June 2017. Reference Source [Google Scholar]
- 25. Global Fund for HIV/TB and Malaria.2018. Reference Source [Google Scholar]
- 26. Lubell Y, Dondorp A, Guérin PJ, et al. : Artemisinin resistance--modelling the potential human and economic costs. Malar J. 2014;13:452. 10.1186/1475-2875-13-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. UNGA: Addis Ababa Action Agenda of the Third International Conference on Financing for Development (Addis Ababa Action Agenda). United Nations General Assembly, New York, NY: United Nations.2015. Reference Source [Google Scholar]
- 28. Paul J, Jr: Philippines sin tax reform: lessons for financing malaria elimination. 2015 in UCSF/MEI. 2017. A Survey of Innovative Financing Mechanisms and Instruments: Opportunities for Malaria Elimination Financing. 2017. [Google Scholar]
- 29. Mulakala A, Surie MK: Asia’s Private Sector Driving Region’s Social and Economic Development. Asia Foundation,2016. Reference Source [Google Scholar]
- 30. UNESCAP: Economic and Social Survey of Asia and the Pacific. United Nations Economic and Social Commission for Asia and the Pacific.2016. Reference Source [Google Scholar]
- 31. Asian Development Bank: Strengthened ADB Support for Regional Health Security. Asian Development Fund. ADF 12 Replenishment Meeting. 24–27 February 2016 Kathmandu, Nepal.2016. Reference Source [Google Scholar]
- 32. UCSF/MEI: A Survey of Innovative Financing Mechanisms and Instruments: Opportunities for Malaria Elimination Financing.2017. Reference Source [Google Scholar]
- 33. BMGF.2018. Reference Source [Google Scholar]
- 34. BMGF.2017. Reference Source [Google Scholar]
- 35. Devex Impact: Goodbye Malaria: Mozambique Malaria Performance Bond.2016; Accessed June 29, 2016. Reference Source [Google Scholar]
- 36. Kumar K, Pigazzini A, Stenson B: Financing for Malaria Elimination.2013; Accessed September 8, 2015. Reference Source [Google Scholar]
- 37. Murray S: ‘Malaria bond’ set to play innovative role in fundraising. FT.com.2016; Accessed June 29, 2016. Reference Source [Google Scholar]
- 38. Murray CJ, Ortblad KF, Guinovart C, et al. : Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dhingra N, Jha P, Sharma VP, et al. : Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010;376(9754):1768–1774. 10.1016/S0140-6736(10)60831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozawa S, Clark S, Portnoy A, et al. : Return On Investment From Childhood Immunization In Low- And Middle-Income Countries, 2011-20. Health Aff (Millwood). 2016;35(2):199–207. 10.1377/hlthaff.2015.1086 [DOI] [PubMed] [Google Scholar]
- 41. Anonymous: What is HS2 and how much will it cost?The guardian. Reference Source [Google Scholar]
- 42. National Priorities Project: Military Spending in the United States. Institute for policy studies, Northampton. MA. Reference Source [Google Scholar]
- 43. Akhtar A: Here's How Much Americans Spend on Every Big Holiday, Ranked. 2017. Reference Source [Google Scholar]