Supplemental Digital Content is available in the text.
Keywords: cytoplasm, fluorescence, inflammation, interleukin-6, venous thrombosis
Abstract
Objective:
Deep venous thrombosis (DVT), one of the most common venous thromboembolic disorders, is closely linked with pulmonary embolism and post-thrombotic syndrome, both of which have a high mortality. However, the factors that trigger DVT formation are still largely unknown. Elevated expression of IL (interleukin)-6—an important inflammatory cytokine—has been linked with DVT formation. However, the molecular mechanisms leading to the elevated IL-6 in DVT remain unclear. Here, we proposed that epigenetic modification of IL-6 at the post-transcriptional level may be a crucial trigger for IL-6 upregulation in DVT.
Approach and Results:
To explore the association between microRNAs and IL-6 in DVT, we performed microRNA microarray analysis and experiments both in vitro and in vivo. Microarray and quantitative real-time polymerase chain reaction results showed that IL-6 expression was increased while miR-338-5p level was decreased substantially in peripheral blood mononuclear cells of patients with DVT, and there was significant negative correlation between miR-338-5p and IL-6. Experiments in vitro showed that overexpressed miR-338-5p reduced IL-6 expression, while miR-338-5p knockdown increased IL-6 expression. Moreover, our in vivo study found that mice with anti–IL-6 antibody or agomiR-338-5p delivery resulted in decreased IL-6 expression and alleviated DVT formation, whereas antagomiR-338-5p acted inversely. Most of miR-338-5p was found located in cytoplasm by fluorescence in situ hybridization. Dual-luciferase reporter assay identified direct binding between miR-338-5p and IL-6.
Conclusions:
Our results suggest that decreased miR-338-5p promotes DVT formation by increasing IL-6 expression.
Highlights.
Increased IL (interleukin)-6 was identified to be involved in deep venous thrombosis, but the underlying mechanisms of upregulated IL-6 still remain unclear.
microRNA profile investigation found miR-338-5p was significantly decreased in the peripheral blood mononuclear cells of deep venous thrombosis patients.
Overexpressed miR-338-5p inhibited IL-6 expression at both mRNA and protein level both in vitro and in vivo while vice versa. Luciferase reporter assay confirmed that miR-338-5p regulated IL-6 expression directly by binding to its mRNA 3′ untranslated region.
The regulatory network involving miR-338-5p/IL-6 axis might highlight a better understanding for potential mechanism of pathogenesis and progression of deep venous thrombosis.
Deep vein thrombosis (DVT) is a type of blood clot that forms within the deep veins, usually of the leg, but DVT can occur in the arms and other parts of the body.1 The incidence of DVT is estimated at 465 715 cases per year in the European Union and 56 cases per 100 000 people in the United States.2 In China, 10 million DVT cases are diagnosed every year.3 In the acute stage of DVT, patients may develop pulmonary embolism, which is associated with significant mortality.4 In later stages, 23% to 60% of patients develop post-thrombotic syndrome, causing repeated or progressive limb swelling, stasis dermatitis, refractory skin ulceration, or limb necrosis, which seriously affect patient survival and quality of life.5 However, the pathogenesis of DVT is not fully understood. For over a century, Virchow’s triad has been considered as the 3 main factors that contribute to thrombus formation, including alterations in blood flow, endothelial injury, and hypercoagulability state.6 Although the triad has greatly contributed to our understanding of DVT, the underlying mechanisms still remain unclear.
Recent evidence has demonstrated that DVT is also closely related to inflammatory processes including cytokines, chemokines, and different types of leukocytes.7 Among these, increasing attention has been paid to the proinflammatory cytokine IL (interleukin)-6. IL-6 is a protein of ≈26 kDa, which is one of the major inflammatory cytokines and plays an important role in host defense against infections. Persistent dysregulated IL-6 will cause tissue injury.8–10 Increased IL-6 expression has been seen in the plasma of DVT patients, which may play a vital role in inflammatory injury of vascular endothelial cells, but the factors and signaling pathways that trigger the upregulation of IL-6 in DVT are still largely unknown.11,12
With the development of epigenetic studies, more and more microRNAs (miRNAs) have been identified. miRNAs are endogenous small noncoding single-stranded molecules about 21 to 24 nucleotides in length, which can repress the translation and cleave mRNA by base-pairing to the 3′ untranslated region (3′UTR) of a target gene.13–15 miRNAs extensively participate in physiological and pathological processes, including cell proliferation, differentiation, and apoptosis.16,17 Recent studies suggest that miRNAs are involved in the formation and development of DVT.18,19 Other studies found that miRNAs may contribute to DVT by promoting the apoptosis of endothelial cells or regulating the process such as proliferation and autophagy of endothelial progenitor cells.20–22 However, the regulatory effect of miRNAs on IL-6 in DVT formation has not been fully investigated.
In the present study, we observed increased IL-6 expression in the peripheral blood mononuclear cells (PBMCs) of patients with DVT and investigated the expression profile of miRNAs of patients with DVT using microarray. Subsequently, we explored the function and underlying molecular mechanism of miR-338-5p in DVT by targeting IL-6. The data showed that miR-338-5p was downregulated in patients with DVT and negatively correlated with IL-6 expression. Further functional and mechanistic investigation revealed that miR-338-5p could inhibit IL-6 expression by binding to its 3′UTR. Downregulated miR-338-5p will aggravate DVT formation by enhancing IL-6 expression, while upregulated miR-338-5p could alleviate DVT formation via inhibiting IL-6 expression effectively. Overall, our data suggest that miR-338-5p is involved in DVT formation via negatively regulating IL-6.
Materials and Methods
The authors declare that all supporting data are available within the article and its online-only Data Supplement.
Patients
Between January 2017 and July 2018, 36 patients admitted to our hospital or presented at the outpatient department (Affiliated Hospital of Shandong University of Traditional Chinese Medicine) with an objective verified DVT and symptom duration ≤21 days and 36 healthy control subjects were included in this study. The included DVT patients were confirmed by color Doppler ultrasound and lower extremity angiography and had no history of hypertension, diabetes mellitus, and other chronic diseases. All DVT patients and control subjects were matched by age, sex, and other risk factors (Table). The study was approved by the Ethics Review Committee of Shandong Academy of Medical Sciences. Written informed consent was obtained from each participant.
Table.
Baseline Characteristics of DVT Patients and Healthy Controls
Specimen Collection
Venous blood samples were obtained after overnight fasting at study inclusion. Samples were processed within 4 hours of collection. PBMCs were isolated by Ficoll density-gradient centrifugation. Samples were coded for blind analysis.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instruction. RNA was reverse transcribed with the miRNA 1st-Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) for miRNA or the PrimeScript RT Reagent Kit (Toyobo, Osaka, Japan) for mRNA per the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction using SYBR Green (Invitrogen) was performed on Applied Biosystems 7500 instrument (Applied Biosystems, Foster). For miRNA and mRNA analysis, the polymerase chain reaction primer sequences are shown in Tables I and II in the online-only Data Supplement, respectively. For each sample, the amplification reaction was performed in triplicate. Relative RNA quantification was performed via the comparative 2−ΔΔCt method. The relative expression levels of miRNA were normalized to that of internal control U6, whereas the relative expression of genes was normalized to the level of GAPDH expression in each sample.
Cell Culture and Transfection
293T cells, HeLa (Henrietta Lacks) cells, and human umbilical vein endothelial cells (HUVECs) were obtained from Procell Life Science and Technology, Co, Ltd (Wuhan, China), and maintained in DMEM (Bioind, Kibbuiz, Israel). PBMCs were cultured in RPMI Medium 1640 (Bioind). All culture medium was supplemented with 10% fetal bovine serum (Bioind) and 1% penicillin/streptomycin. Cells were cultured in a humidified incubator at 37°C and 5% CO2. To investigate the regulatory effect of miR-338-5p on IL-6, chemosynthetic miR-338-5p mimics, inhibitor, or respective negative control (NC; GenePharma, Shanghai, China) were transfected into cells at a final oligonucleotide concentration of 100 nmol/L with Lipofectamine 2000 (Invitrogen). siRNA for IL-6 was also transfected into HUVECs by HiperFect at the concentration of 50 nmol/L. Then, the cells were incubated at 37°C in a 5% CO2 atmosphere for 24 hours. The sequences of the mimics and inhibitor used for miR-338-5p overexpression and inhibition and siRNA used for IL-6 knockdown are listed in Table III in the online-only Data Supplement.
Enzyme-Linked Immunosorbent Assay
Human blood plasma samples, culture supernatant of HeLa cells and HUVECs, and supernatants from grinding mouse vessels were collected. The protein expression of IL-6, CCL (C-C motif chemokine ligand) 2, CCL3, ICAM-1 (intercellular cell adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1), SELP (P-selectin), eNOS (endothelial NO synthase), and ET-1 (endothelin-1) was detected by ELISA assay following the manufacturer’s instructions with human/mice IL-6 ELISA Kits (MultiSciences, Hangzhou, China), following the manufacturer’s instructions. The absorbance of 450 and 630 nm was measured using a microplate reader. Protein concentration was calculated based on the difference between 450 and 630 nm.
Dual-Luciferase Reporter Assay
The wild-type (IL-6, CCL2, CCL3, ICAM-1, VCAM-1, SELP, TNFα[tumor necrosis factor-alpha], and IL-17A) and mutant (IL-6) human mRNA 3′UTR luciferase reporter vectors were constructed by amplifying human wild-type or mutant mRNA 3′UTR and cloning into pGL3-3M-Luc vector (Promega, Madison), respectively. 293T cells were cotransfected with luciferase reporter plasmid and the miR-338-5p mimics or NC with final concentration of 100 nmol/L. After growing 24 hours, the cells were collected for application in the Dual-Luciferase Reporter Assay System (Promega, Madison) using a GloMax 20/20 Luminometer (Promega, Winooski) under recommended condition. Ratios of firefly luciferase luminescence relative to renilla luciferase luminescence were calculated.
Fluorescence In Situ Hybridization
Multiplexed miRNA fluorescence in situ hybridization is an advanced method for visualizing differentially expressed miRNAs together with other reference RNAs in cells and vascular tissues. Fluorescence in situ hybridization assay was used to observe the location and expression of miR-338-5p in mouse vascular tissues.
The mouse vascular tissues were fixed with 4% paraformaldehyde at room temperature and made into paraffin slices at 5-μm thickness. Then, paraffin slices were hybridized with Cy3-labeled miR-338-5p probes (Cy3-5′-GCCAATATTTCTGTGCTGCTA-3′; GenePharma) at 37°C overnight. 4,6-diamidino-2-phenylindole (DAPI) was used for cell nucleus counterstain, and the images were acquired with laser scanning confocal microscopy (FV3000; Olympus, Japan). All procedures were conducted according to the manufacturer’s protocol (Genepharma).
Immunofluorescence
HUVECs were plated in 24-well culture plates at a concentration of 5×104 cells per well for 24 hours and subsequently transfected with reagents for an additional 24 hours in serum-free DMEM. Thereafter, the treated cells were 4% fixed with paraformaldehyde at room temperature for 30 minutes and then incubated in 10% normal mice serum for 30 minutes. The cells were then incubated with rabbit polyclonal IL-6 antibody (3.5 ug/mL; Proteintech, Wuhan, China) overnight at 4°C. The secondary antibody FITC mouse anti-rabbit IgG (BOSTER, Wuhan, China) was used at a dose of 20 ug/mL for 1 hour. Sections were counterstained with DAPI to stain the nucleus and analyzed with a fluorescence microscope (IX73; Olympus, Japan).
DVT Mice Model and Treatment
Eight-week-old male C57BL/6J mice were purchased from the SPF Biotechnology Company (Beijing, China). The animal experiments were performed in accordance with the guidelines for the Care and Utilization of Laboratory Animals (Shandong Academy of Medical Sciences, China) and were approved by the Institutional Animal Care and Use Committee of Shandong Academy of Medical Sciences. The stenosis of the inferior vena cava (IVC) in DVT model was implemented as described previously.23 All mice that were observed to have bleeding during the surgery were excluded from further analysis.
Mice were randomly divided into 7 groups: (1) control group, no treatment and no surgical intervention; (2) sham group, sham operation without treatment; (3) DVT group, each mouse received 200 μL of saline via tail vein injection and underwent the surgical operation for DVT described above; (4) DVT agomir NC group; (5) DVT agomiR-338-5p group; (6) DVT antagomir NC group; (7) DVT antagomiR-338-5p group. Agomir NC, agomiR-338-5p, antagomir NC, or antagomiR-338-5p (Ribobio, Guangzhou, China) were directly injected into the tail vein at the dose of 5, 5, 10, and 10 nmol per mouse in 200 μL of saline, respectively. All mice were given the treatments 30 minutes before undergoing the surgery, and mice were sacrificed 48 hours after the operation, as described in a previous study.24 Vascular Doppler ultrasounds were performed 48 hours after surgery, and blood was collected by retro-orbital bleeding. At the time of euthanasia, fresh thrombi were collected to measure the weight. Sections of the specimens 2 mm below the IVC ligation were fixed with 4% paraformaldehyde for fluorescence in situ hybridization and hematoxylin and eosin (H&E) analysis.
For anti–IL-6 antibody administration experiments, mice were randomly divided into 6 groups: (1) DVT+IgG1 group, (2) DVT+anti–IL-6 antibody group, (3) DVT agomiR-338-5p+IgG1 group, (4) DVT agomiR-338-5p+anti–IL-6 antibody group, (5) DVT antagomiR-338-5p+IgG1 group, and (6) DVT antagomiR-338-5p+anti–IL-6 antibody group. Each mouse received 5 μg/g of mouse IL-6 antibody (R&D Systems, Minneapolis, MN) via tail vein injection, freshly dissolved in 200 μL PBS. Control mice received an injection of nonspecific rat IgG1 (R&D Systems) at the same dose. Anti–IL-6 antibody or rat IgG1 were given the treatments 30 minutes before agomiR-338-5p or antagomiR-338-5p injection, as described in a previous study.25–27 After 48 hours, euthanasia was performed, and blood was collected by retro-orbital bleeding. Sections of the specimens 2 mm below the IVC ligation were fixed with 4% paraformaldehyde for H&E analysis.
Murine Doppler Ultrasound
Doppler ultrasound was performed as described previously.28,29 Mice were anesthetized with isoflurane-oxygen mixture and placed in the supine position. The region of interest was shaved and covered with ultrasound gel. Images of the IVC and surrounding structures and vessels were obtained using the VisualSonics Vevo 2100 system with a cardiovascular scan.
Hematoxylin and Eosin
The fixed specimens were dehydrated and embedded in paraffin. Serial cross sections (4 μm) of the IVC with thrombus were cut to analyze thrombus formation. Tissue sections were stained with H&E following standard procedures. All histological images were acquired using an optical microscope (E100; Nikon, Japan) and analyzed by Image-ToupView software.
Statistical Analyses
Unless otherwise stated, all experiments were performed at least 3 independent times. Values are presented as the mean±SEM. The 2-tailed Student t test was used to compare the data between any 2 groups through the normality and equal variance tests. If data for either normality or variance tests failed, nonparametric Mann-Whitney U test was used. For multiple comparisons, 1-way ANOVA on ranks with Bonferroni post hoc test was used. If data did not pass either test, then nonparametric Kruskal Wallis test with Dunn post hoc test was used. Data were plotted using GraphPad Prism 6.0 software (GraphPad Software, Inc, CA). Statistical analyses were performed with SPSS software (version 16.0). Correlations were analyzed by Pearson correlation. The diagnostic value was evaluated using the receiver operating characteristic curve. P≤0.05 was considered significant.
Results
IL-6 Was Upregulated and Negatively Correlated With miR-338-5p in Patients With DVT
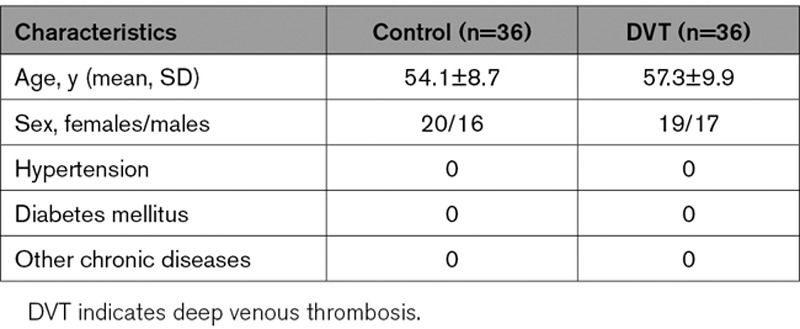
Quantitative real-time polymerase chain reaction results showed that compared with healthy control group, IL-6 mRNA expression in the PBMCs of patients with DVT was robustly increased. Moreover, IL-6 protein expression was significantly upregulated in the plasma of patients with DVT (Figure 1A), which was consistent with a previous study.30
Figure 1.
The expression of IL (interleukin)-6 and miR-338-5p in patients with deep venous thrombosis (DVT) and healthy controls (Ctrls). A, Relative expression levels of IL-6 mRNA in the peripheral blood mononuclear cells (PBMCs) and IL-6 protein in the plasma from 30 DVT patients and 30 Ctrls were determined by quantitative real-time polymerase chain reaction (qRT-PCR) and ELISA. B, The volcano plot was constructed using fold-change values and P. The vertical lines correspond to 2-fold up and down, respectively, and the horizontal line represents a P of 0.05. The red point in the plot represents the significantly upregulated and the blue point represents the significantly downregulated microRNAs (miRNAs). C, Heat map showing the profiling data of downregulated miRNAs in the blood of 6 DVT patients compared with that of 6 Ctrls determined by microarray analysis. Red indicates increased relative expression, while green indicates decreased relative expression. D, The Venn diagram shows miR-338-5p predicted by Targetscan, miRDB, and Chip. E and F, Levels of miR-338-5p in PBMCs from 6 patients and 6 Ctrls by microarray return samples and DVT patients (n=30) compared with Ctrls subjects (n=30) were measured by qRT-PCR. G and H, The correlation between miR-338-5p and IL-6 was analyzed using Pearson correlation analysis (n=30). I, Diagnostic value of miR-338-5p for DVT was evaluated by receiver operating characteristic curve. AUC indicates area under the curve. *P<0.05, ***P<0.001.
To further explore the miRNAs potentially involved in DVT, we examined the global miRNA expression profiles in the PBMCs of patients with DVT (n=6) compared with that of healthy control subjects (n=6) by miRNA microarray assay. The microarray analysis was performed by OE Biotech, Co, Ltd (Shanghai, China). A total of 59 dysregulated miRNAs were detected by at least 2-fold with P<0.05 in the patients with DVT, including 29 upregulated and 30 downregulated, respectively (Figure 1B and 1C). Of interest, in silico prediction of the miRNA-target gene interaction using TargetScan, miRDB database, and Chip revealed a high probability of interaction between miR-338-5p and IL-6 (Figure 1D). Quantitative real-time polymerase chain reaction was performed to verify the expression of miR-338-5p by samples for microarray assay (Figure 1E) and expanded samples (Figure 1F), which showed that miR-338-5p expression was dramatically decreased in patients with DVT. Furthermore, significantly negative correlation was found between miR-338-5p and IL-6 expression (Figure 1G and 1H). Receiver operating characteristic analysis revealed that miR-338-5p in PBMCs could sensitively discriminate DVT from healthy donor (Figure 1I), with an area under the curve of 0.797 (95% CI, 0.685–0.908). These data indicate that decreased miR-338-5p may inhibit IL-6 expression in DVT and encourage us to verify the functional relationship between miR-338-5p and IL-6 in DVT formation.
miR-338-5p Influences Endothelial Function by Regulating IL-6 In Vitro
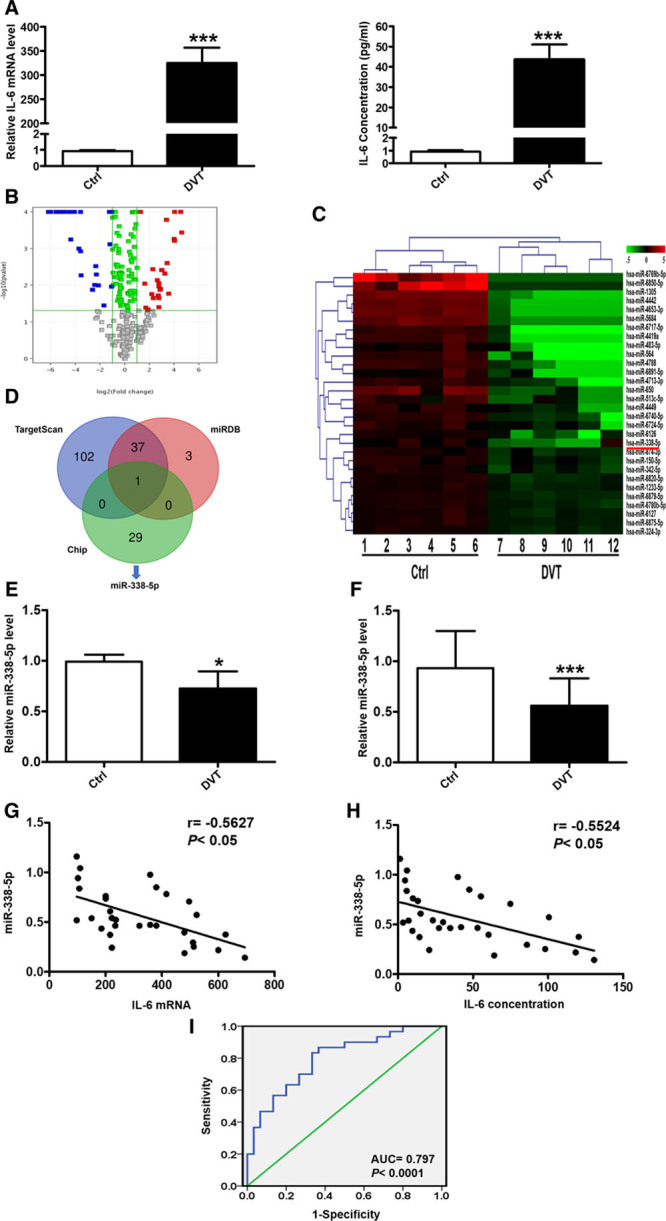
To determine the effect of miR-338-5p on IL-6 expression, we altered miR-338-5p expression and detected both the mRNA and protein levels of IL-6 in HeLa cells, HUVECs, and PBMCs in vitro. miR-338-5p mimics and inhibitor were used to increase and reduce the expression of miR-338-5p, respectively. The miR-338-5p mimics enhanced, while the inhibitor suppressed miR-338-5p expression effectively in HeLa cells, HUVECs, and PBMCs (Figure 2A; Figure IIA and IIB in the online-only Data Supplement). Moreover, the mRNA and protein levels of IL-6 were decreased after miR-338-5p mimics transfection compared with that of the NC (Figure 2B and 2C; Figure IIC and IIE in the online-only Data Supplement). On the contrary, miR-338-5p knockdown by inhibitor transfection increased IL-6 mRNA and protein levels (Figure 2B and 2C; Figure IID and IIF in the online-only Data Supplement). In addition, immunofluorescence analysis revealed that overexpression of miR-338-5p markedly decreased the IL-6 expression of HUVECs, whereas knockdown of miR-338-5p displayed an opposite effect (Figure IIG in the online-only Data Supplement).
Figure 2.
miR-338-5p negatively regulates IL (interleukin)-6 expression in human umbilical vein endothelial cells. A, The expression level of miR-338-5p after negative control (NC), miR-338-5p mimics, inhibitor NC (INC), and miR-338-5p inhibitor transfection as detected by quantitative real-time polymerase chain reaction (qRT-PCR). B, The expression level of IL-6 mRNA after NC, miR-338-5p mimics, INC, and miR-338-5p inhibitor transfection as detected by qRT-PCR. C, The expression level of IL-6 protein was detected by ELISA. *P<0.05, **P<0.01, ***P<0.001.
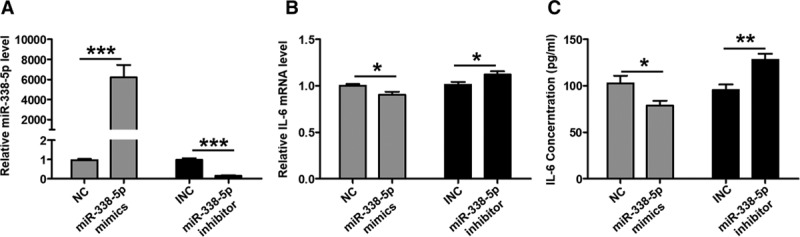
It was well known that adhesion molecules such as ICAM-1, VCAM-1, as well as CCL2, CCL3, and SELP, which could be induced by IL-6, were markers of vascular function.31–33 Interestingly, CCL2, CCL3, ICAM-1, VCAM-1, and SELP protein expression was increased in the plasma of patients with DVT. All of the upregulation of these 5 molecules was positively correlated with IL-6 protein level, which indicates that inflammatory state mediated by elevated IL-6 was probably involved in vascular dysfunction and thus contributes to DVT formation (Figure I in the online-only Data Supplement).
To directly check the regulatory effects of IL-6 on these vascular functional markers, we knocked down IL-6 mRNA expression with siRNA in HUVECs and found that both the mRNA and protein levels of CCL2, CCL3, ICAM-1, VCAM-1, and SELP were reduced (Figure III in the online-only Data Supplement). To investigate whether the influence of miR-338-5p on endothelial function was potentially mediated by IL-6 targeting, the protein expression of CCL2, CCL3, ICAM-1, VCAM-1, and SELP was detected in HUVECs. We found that the overexpressed miR-338-5p inhibited CCL2, CCL3, ICAM-1, VCAM-1, and SELP expression at both mRNA and protein level while vice versa (Figure 3A through 3D). All of these results suggest that miR-338-5p inhibits IL-6 and thereby influences endothelial function.
Figure 3.
miR-338-5p is correlated with vascular markers of endothelial function. A and B, CCL (C-C motif chemokine ligand) 2, CCL3, ICAM-1 (intercellular cell adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1), and SELP (p-selectin) mRNA expression in HUVECs from each treatment group was determined via quantitative real-time polymerase chain reaction analysis. C and D, CCL2, CCL3, ICAM-1, VCAM-1, and SELP protein expression in HUVECs from each treatment group was determined by ELISA. INC indicates inhibitor negative control; and NC, negative control. *P<0.05, **P<0.01, ***P<0.001.
IL-6 Is the Direct Target Gene of miR-338-5p
To validate the post-transcriptional suppressive effect of miR-338-5p on IL-6, luciferase reporter assay was used with cotransfection of miR-338-5p mimics/inhibitor and plasmids encoding wild-type and mutant 3′UTR of IL-6 (Figure 4A). The miR-338-5p mimics markedly inhibited the activity of the luciferase encoded with wild-type IL-6 mRNA 3′UTR. Conversely, this suppressive effect of miR-338-5p was not observed for the luciferase reporter activity of mutant IL-6 mRNA 3′UTR (Figure 4B). Meanwhile, miR-338-5p inhibitor significantly enhanced the luciferase reporter activity of wild-type IL-6 mRNA 3′UTR but not that with mutant IL-6 3′UTR (Figure 4C). To exclude the possibility that except for IL-6, miR-338-5p might also target on other inflammatory factors closely related to DVT, such as TNFα, IL-17A, or vascular functional markers, we constructed plasmids of 3′UTR for these genes and performed luciferase reporter analysis. It was found that miR-338-5p mimics could not inhibit the luciferase activity of wild-type mRNA 3′UTR of CCL2, CCL3, ICAM-1, VCAM-1, SELP, TNFα, and IL-17A (Figures IVA, IVB, and VIIG in the online-only Data Supplement). Besides, agomir and antagomir of miR-338-5p did not affect the mRNA expression of TNFα and IL-17A in the PBMCs of mice (Figure IVC and IVD in the online-only Data Supplement). Collectively, it was demonstrated that IL-6 was the direct target of miR-338-5p and downregulation of miR-338-5p attributed to IL-6 elevation in DVT.
Figure 4.
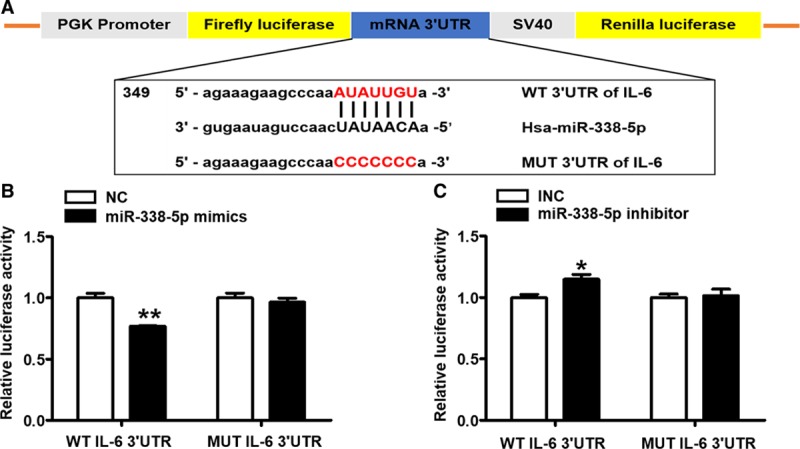
miR-338-5p targets IL (interleukin)-6 mRNA 3′ untranslated region (3′UTR) directly. A, Schematic representation of IL-6 mRNA 3′UTR demonstrating putative microRNA target site, luciferase activities of WT (wild-type) and MUT (mutant) constructs. B and C, The luciferase activity was determined by cotransfecting the vectors (IL-6 3′UTR-WT and MUT) combined with negative control (NC), miR-338-5p mimics, inhibitor negative control (INC), or miR-338-5p inhibitor into 293T cells. *P<0.05, **P<0.01.
Role of miR-338-5p in DVT
To examine the role of miR-338-5p in DVT formation, we first performed fluorescence in situ hybridization to detect the localization and expression of miR-338-5p in mouse vascular tissues. The majority of miR-338-5p mRNA (red) was found located in the cytoplasm, and the expression of miR-338-5p in the DVT model was significantly lower than that in normal control mice (Figure VA in the online-only Data Supplement). Specifically, we found that miR-338-5p expression was decreased by 2.26- and 2.09-folds in vascular tissue and PBMCs, respectively, in DVT mice compared with normal control mice (Figure VIA in the online-only Data Supplement). Meanwhile, the levels of IL-6 mRNA and protein in vascular tissue and PBMCs of DVT mice were significantly increased (Figure VIB and VIC in the online-only Data Supplement). To directly check the regulatory effects of DVT on these vascular functional markers, the expression of CCL2, CCL3, ICAM-1, VCAM-1, and SELP mRNA in both vascular tissue and PBMCs of DVT mice was significantly increased compared with that in normal control mice (Figure VIIA in the online-only Data Supplement). In addition, the expression of eNOS protein in the plasma of DVT mice was significantly decreased, whereas the expression of ET-1 protein was dramatically increased (Figure VIIIA and VIIIB in the online-only Data Supplement). All these data pointed that aberrantly decreased miR-338-5p was involved in DVT formation.
Neutralizing IL-6 Reduced the Formation of DVT In Vivo
To further identify the pathogenic role of IL-6 in the formation and development of DVT, we injected anti–IL-6 antibody into mice to neutralize the function of IL-6. We found that the thrombosis rate and the weight of thrombus were significantly decreased after the treatment of anti–IL-6 antibody (Figure 5B; Table IV in the online-only Data Supplement, Figure IXA through IXC in the online-only Data Supplement). Meanwhile, the levels of IL-6 protein in plasma of DVT mice were significantly decreased after injection of IL-6 neutralizing antibody (Figure IXD through IXF in the online-only Data Supplement). Additionally, we found that inhibition of IL-6 alone similarly affects the mRNA expression of CCL2, CCL3, ICAM-1, VCAM-1, and SELP as miR-338-5p overexpression (Figure IXG in the online-only Data Supplement). The results demonstrate that IL-6 plays a crucial role in the formation and development of DVT.
Figure 5.
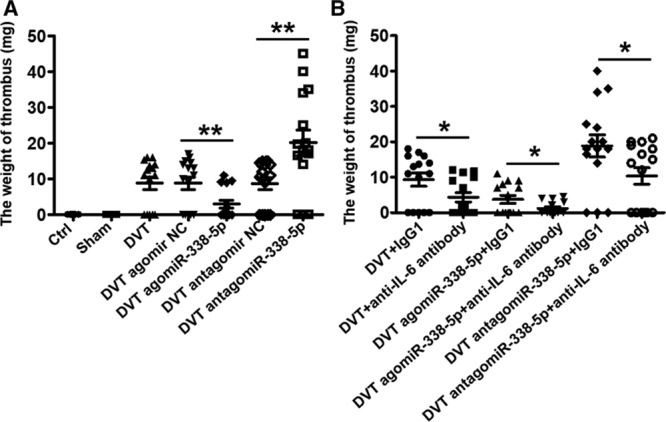
The role of miR-338-5p in deep venous thrombosis (DVT) formation by targeting IL (interleukin)-6 in vivo. A, Thrombus weights at 48 h post-operation were measured in the different treatment groups (n=15). B, Effect of anti–IL-6 antibody on thrombosis in different treatment groups (n=15). Ctrl indicates control; INC, inhibitor negative control; and NC, negative control. *P<0.05, **P<0.01.
Knockdown of miR-338-5p Is Associated With Upregulation of IL-6 and Increase in DVT
To further investigate whether miR-338-5p affects thrombus formation in vivo, antagomiR-338-5p or its control vehicle were injected via tail vein before the DVT operation. Indeed, compared with the DVT antagomir NC group, the expression of miR-338-5p was significantly decreased in vascular tissue and PBMCs of DVT antagomiR-338-5p group (Figure VID in the online-only Data Supplement). Meanwhile, we found that the levels of IL-6 mRNA and protein increased in both vascular tissue and PBMCs of mice treated with antagomiR-338-5p (Figure VIE and VIF in the online-only Data Supplement). The thrombus weight greatly increased in the DVT antagomiR-338-5p group compared with those in the DVT or DVT antagomir NC group (Figure 5A). In addition, Doppler and H&E staining showed that compared with DVT antagomir NC group, mice in the antagomiR-338-5p group showed obvious thrombosis in IVC at 48 hours after the ligation (Figure VB and VC in the online-only Data Supplement). No thrombosis occurred in the normal control or sham groups. There was no significant difference between the DVT group and DVT antagomir NC group. But if the mice were pretreated with antagomiR-338-5p, which means the miR-338-5p was preinhibited in vivo, more blood clots were observed compared with the NC group.
Moreover, expressions of CCL2, CCL3, ICAM-1, VCAM-1, and SELP remarkably increased in both the vascular tissue and PBMCs of mice treated with antagomiR-338-5p (Figure VIIB through VIIF in the online-only Data Supplement). Inhibition of miR-338-5p dramatically decreased eNOS expression, whereas increased ET-1 expression at protein level in the plasma (Figure VIIIC and VIIID in the online-only Data Supplement). The results demonstrate that inhibited expression of miR-338-5p could promote thrombosis formation by enhancing the expression of IL-6 and thereby leading to the vascular endothelial dysfunction.
Overexpression of miR-338-5p Is Associated With Decreased IL-6 Expression and DVT
To extend the study of the role of miR-338-5p in DVT formation, we introduced agomiR-338-5p to imitate the effect of miR-338-5p in vivo. As expected, the expression of miR-338-5p was significantly increased in both vascular tissue and PBMCs of the DVT agomiR-338-5p group compared with the DVT agomir NC group (Figure VID in the online-only Data Supplement). Furthermore, we found that IL-6 expression decreased synchronously in mice treated with agomiR-338-5p (Figure VIE and VIF in the online-only Data Supplement), indicating that miR-338-5p could negatively regulate IL-6 expression. However, the thrombus weight greatly decreased in the DVT agomiR-338-5p group compared with those in the DVT or DVT agomir NC group (Figure 5A). Similarly, Doppler and H&E staining showed that alleviated blood clots were observed compared with the DVT agomir NC group (Figure VB and VC in the online-only Data Supplement).
Moreover, expressions of CCL2, CCL3, ICAM-1, VCAM-1, and SELP were decreased significantly in both vascular tissue and PBMCs of mice treated with agomiR-338-5p (Figure VIIB through VIIF in the online-only Data Supplement). Overexpression of miR-338-5p markedly increased eNOS expression while inhibited ET-1 expression at protein level (Figure VIIIC and VIIID in the online-only Data Supplement). Taken together, these findings suggest that enhanced miR-338-5p could inhibit IL-6 expression, thereby alleviating inflammation and protecting the function of vascular endothelial cells.
Discussion
Accumulating evidence suggests that the immune system is closely linked to the formation of DVT. Studies have demonstrated that the inflammatory injury of vascular endothelial cells caused by the imbalance of cytokine expression is involved in the occurrence and development of DVT.30,34 IL-6 is a soluble proinflammatory and immunoregulatory cytokine contributing to host defense through the stimulation of acute phase responses, hematopoiesis, and immune reactions. Although its expression is strictly controlled by transcriptional and post-transcriptional mechanisms, dysregulated continual synthesis of IL-6 plays a pathological effect on tissue injury and is involved in many diseases.35 Previous studies found increased IL-6 expression in both DVT patients and animal models.36–40 However, the upstream regulatory factors leading to increased IL-6 expression have not been fully elucidated. Consistent with previous studies, we found that IL-6 was significantly upregulated in both DVT patients and DVT mice. Although blocking IL-6 in mice with the neutralizing antibody inhibited the formation and development of thrombosis, large sample clinical studies of patients before and after the DVT formation are still needed to further verify the conclusions. Importantly, we provide the evidence that miR-338-5p plays an important role as a suppressor of IL-6 by directly targeting its 3′UTR. Furthermore, we showed that decreased miR-338-5p increased IL-6 expression and promoted DVT formation. Therefore, miR-338-5p may provide as a promising therapeutic modulator for DVT.
miRNAs are small noncoding RNAs that regulate gene expression by binding to the 3′UTR of their target in a sequence-specific manner, thereby decreasing expression of the target gene.41,42 miRNAs have been shown to play crucial roles in most physiological and pathological processes, including cell growth and differentiation, metabolism, immunity, vascular development, cancer, and autoimmune disorders.43–46 Because of their tremendous potential as physiological and pathological regulators, miRNAs are in the limelight as promising markers for diagnosis and targets for therapeutics. Recently, studies reported that the expression and degradation of IL-6 is regulated by several miRNAs such as let-7a-5p, miRNA-223, and miRNA-146.47–49 Meanwhile, altered miRNA expression has been reported to be involved in DVT. For instance, it was identified that let-7e-5p was downregulated in DVT patients and demonstrated that let-7e-5p could inhibit the migration and tube formation via targeting Fas ligand.50 Moreover, a study reported that the expression of miR-582, miR-195, and miR-532 was significantly increased in patients with DVT.51,52 Besides, miR-150 expression was found to be downregulated in patients with DVT, while upregulation of miR-150 promoted angiogenesis and proliferation of endothelial progenitor cells by targeting SRCIN1 (SRC kinase signaling inhibitor 1) in vitro and thrombus resolution in a DVT rat model.22 Moreover, it was demonstrated that miR-126 was decreased in the DVT rat model and its expression was negatively correlated to the apoptosis of HUVECs through targeting PI3K (phosphatidylinositol 3-kinase)/Akt (AKT serine/threonine kinase 1) signaling pathway.20 However, the role of miRNA in DVT by regulating the expression of IL-6 remains largely unclear.
miR-338-5p is a new 22-nucleotide member of endogenous noncoding single-stranded RNAs, which is transcribed from human chromosome 17 and mouse chromosome 11.53,54 Increased miR-338-5p was initially identified in patients with colorectal tumors and subsequently characterized in other cancers such as hepatocellular carcinoma.55,56 Moreover, downregulated miR-338-5p was reported in lung tissues from mice with bleomycin-induced pulmonary fibrosis.53 Recently, studies demonstrated that miR-338-5p was upregulated in patients with rheumatoid arthritis and identified that miR-338-5p could promote the viability, proliferation, and migration of rheumatoid arthritis fibroblast-like synoviocytes via targeting NFAT5 (nuclear factor of activated T cells 5), suggesting a potential role of miR-338-5p in immunoregulation.57
Here, we demonstrated the downregulated miR-338-5p in DVT patients, which was negatively related to the expression of IL-6. Moreover, in silico bioinformatics analysis predicted that there were complementary binding sites between miR-338-5p and IL-6 mRNA 3′UTR. Therefore, based on these findings above, we proposed that decreased miR-338-5p may be one of the critical triggers for the enhanced IL-6 expression in DVT. However, we are aware that the sample size in this study is not large enough, and well-designed investigations with larger sample size are required for future validation of our findings.
Specifically, we investigated the regulatory effect of miR-338-5p on IL-6 and the interaction mode between them. Interestingly, it was found that overexpression of miR-338-5p effectively inhibited IL-6 expression at both mRNA and protein level, while suppression of miR-338-5p functioned oppositely. Moreover, dual-luciferase assay confirmed the direct binding between miR-338-5p and IL-6 mRNA 3′UTR and the inhibitory effect of miR-338-5p on IL-6. Furthermore, our study demonstrated that overexpressed miR-338-5p could inhibit IL-6 expression and reduced thrombus size and weight effectively in mice. On the contrary, downregulated miR-338-5p could promote DVT formation by enhancing IL-6 expression.
In summary, our results suggest that miR-338-5p downregulated IL-6 expression by directly targeting its mRNA 3′UTR. We first demonstrated that the aberrant downregulation of miR-338-5p contributes to DVT by enhancing the expression of IL-6. The regulatory network involving miR-338-5p/IL-6 axis might highlight a better understanding for potential mechanism of pathogenesis and progression of DVT.
Acknowledgments
We thank Dr Yanan Zhao in the Affiliated Hospital of Shandong University of Traditional Chinese Medicine for helping to collect DVT clinical samples.
Sources of Funding
This work was supported by the Natural Science Foundation of China (81673981, 81873337, 81601442, and 81704116), the Primary Research and Development Plan of Shandong Province (2017GSF219118, 2017G006018, and 2016GSF202016), the Project of Transformation in High-Tech Achievements (2013ZHZX2A0405), the Natural Science Foundation of Shandong Province (ZR2019MH039, ZR2018PH042, and ZR2017PH008), Taishan Scholars (Tsqn201812125 and ts201712042), the Innovation Project of Shandong Academy of Medical Sciences, and the academic promotion program of Shandong First Medical University.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- 3′UTR
- 3′ untranslated region
- DAPI
- 4,6-diamidino-2-phenylindole
- DVT
- deep venous thrombosis
- eNOS
- endothelial NO synthase
- ET-1
- endothelin-1
- H&E
- hematoxylin and eosin
- HUVEC
- human umbilical vein endothelial cell
- ICAM-1
- intercellular cell adhesion molecule-1
- IL
- interleukin
- IVC
- inferior vena cava
- miRNA
- microRNA
- NC
- negative control
- NFAT5
- nuclear factor of activated T cells 5
- PBMC
- peripheral blood mononuclear cell
- SELP
- P-selectin
- SRCIN1
- SRC kinase signaling inhibitor 1
- TNFα
- tumor necrosis factor-alpha
- VCAM-1
- vascular cell adhesion molecule-1
For Sources of Funding and Disclosures, see pages 332 and 333.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.119.313137.
References
- 1.Strijkers RH, Cate-Hoek AJ, Bukkems SF, Wittens CH. Management of deep vein thrombosis and prevention of post-thrombotic syndrome. BMJ. 2011;343:d5916. doi: 10.1136/bmj.d5916. doi: 10.1136/bmj.d5916. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Lei B, Zhang F, Niu L, Zhang H, Zhang M. Anti-inflammatory effects of simvastatin during the resolution phase of experimentally formed venous thrombi. J Investig Med. 2017;65:999–1007. doi: 10.1136/jim-2017-000442. doi: 10.1136/jim-2017-000442. [DOI] [PubMed] [Google Scholar]
- 3.Fu Y, Wang D. Emergency care and prevention of thromboembolic disease. Chinese Journal for Clinicians. 2006;34:51–53. [Google Scholar]
- 4.Giordano NJ, Jansson PS, Young MN, Hagan KA, Kabrhel C. Epidemiology, pathophysiology, stratification, and natural history of pulmonary embolism. Tech Vasc Interv Radiol. 2017;20:135–140. doi: 10.1053/j.tvir.2017.07.002. doi: 10.1053/j.tvir.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Appelen D, van Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev. 2017;9:CD004174. doi: 10.1002/14651858.CD004174.pub3. doi: 10.1002/14651858.CD004174.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365:1163–1174. doi: 10.1016/S0140-6736(05)71880-8. doi: 10.1016/S0140-6736(05)71880-8. [DOI] [PubMed] [Google Scholar]
- 7.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Narazaki M, Masuda K, Kishimoto T. Regulation of IL-6 in immunity and diseases. Adv Exp Med Biol. 2016;941:79–88. doi: 10.1007/978-94-024-0921-5_4. doi: 10.1007/978-94-024-0921-5_4. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10:a028456. doi: 10.1101/cshperspect.a028456. doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Singh K, Biswas A, Ranjan R, Kishor K, Pandey H, Kumar R, Mahapatra M, Oldenburg J, Saxena R. Impact of interleukin 6 promoter polymorphisms (-174 G > C, -572 G > C and -597 G > A) on plasma IL-6 levels and their influence on the development of DVT: a study from India. Hematology. 2018;23:833–838. doi: 10.1080/10245332.2018.1483546. doi: 10.1080/10245332.2018.1483546. [DOI] [PubMed] [Google Scholar]
- 12.Malaponte G, Polesel J, Candido S, Sambataro D, Bevelacqua V, Anzaldi M, Vella N, Fiore V, Militello L, Mazzarino MC, et al. IL-6-174 G > C and MMP-9-1562 C > T polymorphisms are associated with increased risk of deep vein thrombosis in cancer patients. Cytokine. 2013;62:64–69. doi: 10.1016/j.cyto.2013.02.017. doi: 10.1016/j.cyto.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Felekkis K, Touvana E, Stefanou Ch, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–240. [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu L, Tan EK, Zeng L. microRNAs and neurodegenerative diseases. Adv Exp Med Biol. 2015;888:85–105. doi: 10.1007/978-3-319-22671-2_6. doi: 10.1007/978-3-319-22671-2_6. [DOI] [PubMed] [Google Scholar]
- 15.Lozano C, Duroux-Richard I, Firat H, Schordan E, Apparailly F. MicroRNAs: key regulators to understand osteoclast differentiation? Front Immunol. 2019;10:375. doi: 10.3389/fimmu.2019.00375. doi: 10.3389/fimmu.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang N, Zhu S, Lv X, Qiao Y, Liu YJ, Chen J. MicroRNAs: pleiotropic regulators in the tumor microenvironment. Front Immunol. 2018;9:2491. doi: 10.3389/fimmu.2018.02491. doi: 10.3389/fimmu.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RH, Meng Q, Shi YP, Xu HS. Regulatory role of microRNA-320a in the proliferation, migration, invasion, and apoptosis of trophoblasts and endothelial cells by targeting estrogen-related receptor γ. J Cell Physiol. 2018;234:682–691. doi: 10.1002/jcp.26842. doi: 10.1002/jcp.26842. [DOI] [PubMed] [Google Scholar]
- 18.Zhang E, Wu Y. MicroRNAs: important modulators of oxLDL-mediated signaling in atherosclerosis. J Atheroscler Thromb. 2013;20:215–227. doi: 10.5551/jat.15180. doi: 10.5551/jat.15180. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Ma J, Wang Q, Wu F, Ping J, Ming L. Circulating microRNA expression and their target genes in deep vein thrombosis: a systematic review and bioinformatics analysis. Medicine (Baltimore) 2017;96:e9330. doi: 10.1097/MD.0000000000009330. doi: 10.1097/MD.0000000000009330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Wang J, Wang B, Yang J, Gong Z, Zhao X, Zhang C, Du K. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol. 2016;95:365–374. doi: 10.1007/s00277-015-2567-9. doi: 10.1007/s00277-015-2567-9. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Meng S, Liu B, Li MQ, Li Y, Fang L, Li YG. MicroRNA-130a regulates autophagy of endothelial progenitor cells through Runx3. Clin Exp Pharmacol Physiol. 2014;41:351–357. doi: 10.1111/1440-1681.12227. doi: 10.1111/1440-1681.12227. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Zhu X, Du X, Xu A, Yuan X, Zhan Y, Liu M, Wang S. MiR-150 promotes angiogensis and proliferation of endothelial progenitor cells in deep venous thrombosis by targeting SRCIN1. Microvasc Res. 2019;123:35–41. doi: 10.1016/j.mvr.2018.10.003. doi: 10.1016/j.mvr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Schönfelder T, Jäckel S, Wenzel P. Mouse models of deep vein thrombosis. Gefasschirurgie. 2017;22(suppl 1):28–33. doi: 10.1007/s00772-016-0227-6. doi: 10.1007/s00772-016-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geddings J, Aleman MM, Wolberg A, von Brühl ML, Massberg S, Mackman N. Strengths and weaknesses of a new mouse model of thrombosis induced by inferior vena cava stenosis: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:571–573. doi: 10.1111/jth.12510. doi: 10.1111/jth.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John B, Naczki C, Patel C, Ghoneum A, Qasem S, Salih Z, Said N. Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene. 2019;38:4366–4383. doi: 10.1038/s41388-019-0728-3. doi: 10.1038/s41388-019-0728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, Philipsen L, Weinert S, Ghosh A, Saalfeld FC, et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128:4359–4371. doi: 10.1172/JCI90312. doi: 10.1172/JCI90312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg. 2011;25:229–239. doi: 10.1016/j.avsg.2010.09.003. doi: 10.1016/j.avsg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Vitverova B, Blazickova K, Najmanova I, Vicen M, Hyšpler R, Dolezelova E, Nemeckova I, Tebbens JD, Bernabeu C, Pericacho M, et al. Soluble endoglin and hypercholesterolemia aggravate endothelial and vessel wall dysfunction in mouse aorta. Atherosclerosis. 2018;271:15–25. doi: 10.1016/j.atherosclerosis.2018.02.008. doi: 10.1016/j.atherosclerosis.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Kenwright DA, Wang S, Hossack JA, Hoskins PR. Fabrication of two flow phantoms for doppler ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:53–65. doi: 10.1109/TUFFC.2016.2634919. doi: 10.1109/TUFFC.2016.2634919. [DOI] [PubMed] [Google Scholar]
- 30.Roumen-Klappe EM, den Heijer M, van Uum SH, van der Ven-Jongekrijg J, van der Graaf F, Wollersheim H. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35:701–706. doi: 10.1067/mva.2002.121746. doi: 10.1067/mva.2002.121746. [DOI] [PubMed] [Google Scholar]
- 31.Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, Katz S, Fuentes-Duculan J, Cannizzaro MV, Jelic S, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39:787–798. doi: 10.1161/ATVBAHA.118.312246. doi: 10.1161/ATVBAHA.118.312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzzocrea S, De Sarro G, Costantino G, Ciliberto G, Mazzon E, De Sarro A, Caputi AP. IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J Leukoc Biol. 1999;66:471–480. doi: 10.1002/jlb.66.3.471. doi: 10.1002/jlb.66.3.471. [DOI] [PubMed] [Google Scholar]
- 33.Ravi AK, Khurana S, Lemon J, Plumb J, Booth G, Healy L, Catley M, Vestbo J, Singh D. Increased levels of soluble interleukin-6 receptor and CCL3 in COPD sputum. Respir Res. 2014;15:103. doi: 10.1186/s12931-014-0103-4. doi: 10.1186/s12931-014-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatò F. [Deep vein thrombosis - advances in diagnosis and treatment]. MMW Fortschr Med. 2014;156 Spec no 2:59–63. doi: 10.1007/s15006-014-3298-x. quiz 64. doi: 10.1007/s15006-014-3298-x. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matos MF, Lourenço DM, Orikaza CM, Bajerl JA, Noguti MA, Morelli VM. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6 -174GC, IL-8 -251AT and MCP-1 -2518AG in the risk of venous thromboembolism: a case-control study. Thromb Res. 2011;128:216–220. doi: 10.1016/j.thromres.2011.04.016. doi: 10.1016/j.thromres.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Vormittag R, Hsieh K, Kaider A, Minar E, Bialonczyk C, Hirschl M, Mannhalter C, Pabinger I. Interleukin-6 and interleukin-6 promoter polymorphism (-174) G > C in patients with spontaneous venous thromboembolism. Thromb Haemost. 2006;95:802–806. [PubMed] [Google Scholar]
- 38.Mahemuti A, Abudureheman K, Aihemaiti X, Hu XM, Xia YN, Tang BP, Upur H. Association of interleukin-6 and C-reactive protein genetic polymorphisms levels with venous thromboembolism. Chin Med J (Engl) 2012;125:3997–4002. [PubMed] [Google Scholar]
- 39.Wu J, Zhu H, Yang G, Wang Y, Wang Y, Zhao S, Zhao M, Peng S. IQCA-TAVV: To explore the effect of P-selectin, GPIIb/IIIa, IL-2, IL-6 and IL-8 on deep venous thrombosis. Oncotarget. 2017;8:91391–91401. doi: 10.18632/oncotarget.20588. doi: 10.18632/oncotarget.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nosaka M, Ishida Y, Kimura A, Hama M, Kawaguchi T, Yamamoto H, Kuninaka Y, Shimada E, Kondo T. Immunohistochemical detection of intrathrombotic IL-6 and its application to thrombus age estimation. Int J Legal Med. 2015;129:1021–1025. doi: 10.1007/s00414-015-1147-9. doi: 10.1007/s00414-015-1147-9. [DOI] [PubMed] [Google Scholar]
- 41.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 42.Thum T, Mayr M. Review focus on the role of microRNA in cardiovascular biology and disease. Cardiovasc Res. 2012;93:543–544. doi: 10.1093/cvr/cvs085. doi: 10.1093/cvr/cvs085. [DOI] [PubMed] [Google Scholar]
- 43.Kane NM, Thrasher AJ, Angelini GD, Emanueli C. Concise review: MicroRNAs as modulators of stem cells and angiogenesis. Stem Cells. 2014;32:1059–1066. doi: 10.1002/stem.1629. doi: 10.1002/stem.1629. [DOI] [PubMed] [Google Scholar]
- 44.Samidurai A, Kukreja RC, Das A. Emerging role of mTOR signaling- related miRNAs in cardiovascular diseases. Oxid Med Cell Longev. 2018;2018:6141902. doi: 10.1155/2018/6141902. doi: 10.1155/2018/6141902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosisio D, Gianello V, Salvi V, Sozzani S. Extracellular miRNAs as activators of innate immune receptors. Cancer Lett. 2019;452:59–65. doi: 10.1016/j.canlet.2019.03.021. doi: 10.1016/j.canlet.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Iswariya GT, Paital B, Padma PR, Nirmaladevi R. microRNAs: epigenetic players in cancer and aging. Front Biosci (Schol Ed) 2019;11:29–55. doi: 10.2741/S525. [DOI] [PubMed] [Google Scholar]
- 47.Barbagallo C, Passanisi R, Mirabella F, Cirnigliaro M, Costanzo A, Lauretta G, Barbagallo D, Bianchi C, Pagni F, Castorina S, et al. Upregulated microRNAs in membranous glomerulonephropathy are associated with significant downregulation of IL6 and MYC mRNAs. J Cell Physiol. 2019;234:12625–12636. doi: 10.1002/jcp.27851. doi: 10.1002/jcp.27851. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Li C, Wu H, Xie X, Sun Y, Dai M. Paeonol attenuated inflammatory response of endothelial cells via stimulating monocytes- derived exosomal microRNA-223. Front Pharmacol. 2018;9:1105. doi: 10.3389/fphar.2018.01105. doi: 10.3389/fphar.2018.01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao N, Dong L. MicroRNA-146 regulates the inflammatory cytokines expression in vascular endothelial cells during sepsis. Pharmazie. 2017;72:700–704. doi: 10.1691/ph.2017.7600. doi: 10.1691/ph.2017.7600. [DOI] [PubMed] [Google Scholar]
- 50.Kong L, Du X, Hu N, Li W, Wang W, Wei S, Zhuang H, Li X, Li C. Downregulation of let-7e-5p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis via targeting FASLG. Thromb Res. 2016;138:30–36. doi: 10.1016/j.thromres.2015.12.020. doi: 10.1016/j.thromres.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Qin J, Liang H, Shi D, Dai J, Xu Z, Chen D, Chen X, Jiang Q. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J Thromb Thrombolysis. 2015;39:215–221. doi: 10.1007/s11239-014-1131-0. doi: 10.1007/s11239-014-1131-0. [DOI] [PubMed] [Google Scholar]
- 52.Mo J, Zhang D, Yang R. MicroRNA-195 regulates proliferation, migration, angiogenesis and autophagy of endothelial progenitor cells by targeting GABARAPL1. Biosci Rep. 2016;36:e00396. doi: 10.1042/BSR20160139. doi: 10.1042/BSR20160139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo L, Liang T, Gu W, Xu Y, Bai Y, Lu Z. Cross-mapping events in miRNAs reveal potential miRNA-mimics and evolutionary implications. PLoS One. 2011;6:e20517. doi: 10.1371/journal.pone.0020517. doi: 10.1371/journal.pone.0020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Chen P, Zu L, Liu B, Wang M, Zhou Q. MicroRNA-338-3p suppresses metastasis of lung cancer cells by targeting the EMT regulator Sox4. Am J Cancer Res. 2016;6:127–140. [PMC free article] [PubMed] [Google Scholar]
- 55.Yong FL, Law CW, Wang CW. Potentiality of a triple microRNA classifier: miR-193a-3p, miR-23a and miR-338-5p for early detection of colorectal cancer. BMC Cancer. 2013;13:280. doi: 10.1186/1471-2407-13-280. doi: 10.1186/1471-2407-13-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang Y, Dai J, Wang Y, Zhang H, Li X, Wang C, Cao M, Liu Y, Ding J, Cai H, et al. MiR-338* targeting smoothened to inhibit pulmonary fibrosis by epithelial-mesenchymal transition. Am J Transl Res. 2016;8:3206–3213. [PMC free article] [PubMed] [Google Scholar]
- 57.Guo T, Ding H, Jiang H, Bao N, Zhou L, Zhao J. miR-338-5p Regulates the viability, proliferation, apoptosis and migration of rheumatoid arthritis fibroblast- like synoviocytes by targeting NFAT5. Cell Physiol Biochem. 2018;49:899–910. doi: 10.1159/000493222. doi: 10.1159/000493222. [DOI] [PubMed] [Google Scholar]


