Abstract
Background and Purpose
Obesity, an important risk factor for developing chronic kidney disease (CKD), affects the kidneys by two main molecular signalling pathways: the endocannabinoid/CB1 receptor system, whose activation in obesity promotes renal inflammation, fibrosis, and injury, and the inducible NOS (iNOS), which generates ROS resulting in oxidative stress. Hence, a compound that inhibits both peripheral CB1 receptors and iNOS may serve as an effective therapeutic agent against obesity‐induced CKD.
Experimental Approach
Here, we describe the effect of a novel peripherally restricted, orally bioavailable dual CB1 receptor/iNOS antagonist, MRI‐1867 (3 mg·kg−1), in ameliorating obesity‐induced CKD, and compared its metabolic and renal efficacies to a stand‐alone peripheral CB1 receptor antagonist (JD5037; 3 mg·kg−1), iNOS antagonist (1400W; 10 mg·kg−1), and pair feeding. Mice with high‐fat diet‐induced obesity were treated orally with these compounds or vehicle (Veh) for 28 days. Standard diet‐fed mice treated with Veh served as controls.
Key Results
Enhanced expression of CB1 receptors and iNOS in renal tubules was found in human kidney patients with obesity and other CKDs. The hybrid inhibitor ameliorated obesity‐induced kidney morphological and functional changes via decreasing kidney inflammation, fibrosis, oxidative stress, and renal injury. Some of these features were independent of the improved metabolic profile mediated via inhibition of CB1 receptors. An additional interesting finding is that these beneficial effects on the kidney were partially associated with modulating renal adiponectin signalling.
Conclusions and Implications
Collectively, our results highlight the therapeutic relevance of blocking CB1 receptors and iNOS in ameliorating obesity‐induced CKD.
What is already known
Obesity promotes development of chronic kidney disease with or without other aetiological factors.
Activation of CB1 receptors and/or iNOS induces renal oxidative stress, inflammation, fibrosis, and kidney dysfunction.
What this study adds
A novel, peripherally restricted, orally bioavailable, dual CB1 receptor/iNOS antagonist ameliorated obesity‐induced chronic kidney disease.
Adiponectin signalling was also involved in the therapeutic effects of the dual CB1 receptor/iNOS antagonist.
What is the clinical significance
Our results support clinical evaluation of peripherally restricted CB1 receptor antagonists in treating obesity and its co‐morbidities.
Simultaneous action on two targets, CB1 receptors and iNOS, has clear therapeutic benefits.
Abbreviations
- 4‐HNE
4‐hydroxynonenal
- ACC
acetyl‐CoA carboxylase
- BUN
blood urea nitrogen
- CCr
creatinine clearance
- CKD
chronic kidney disease
- DAB
3,3′‐diaminobenzidine
- DIO
diet‐induced obesity
- eCB
endocannabinoid
- FSGS
focal segmental glomerulosclerosis
- HFD
high‐fat diet
- iNOS
inducible NOS
- KIM‐1
kidney injury marker 1
- LKB1
liver kinase B1
- ORG
obesity‐related glomerulopathy
- PDGFa
PDGF subunit A
- RPTCs
renal proximal tubule cells
- TIMP‐1
tissue inhibitor of metalloproteinase 1
- Veh
vehicle
1. INTRODUCTION
The prevalence of global obesity has risen significantly over the past three decades (Ng et al., 2014). Among its various complications, obesity has been shown to directly affect kidney function with or without other aetiological factors (Mathew, Okada, & Sharma, 2011). In fact, obesity is considered a risk factor not only for developing chronic kidney disease (CKD) but also for accelerating the progression of an existing kidney disease to a greater extent in obese, compared with non‐obese, subjects (Othman, Kawar, & El Nahas, 2009). Additionally, a higher body mass index in individuals with end‐stage renal disease remains a strong independent risk factor for its development after adjusting for 42 possible confounding parameters such as age, smoking status, history of myocardial infarction, serum cholesterol level, hypertension, and diabetes (Hsu, McCulloch, Iribarren, Darbinian, & Go, 2006). Therefore, there is a crucial need to explore new potential therapeutic pathways to prevent and/or reverse the deleterious effects of obesity on the kidney.
At the molecular level, obesity‐induced CKD may involve two signalling pathways that have been shown to be overactivated in the kidney during obesity: the endocannabinoid (eCB)/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 receptor system (Udi et al., 2017) and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250 (iNOS; Lee et al., 2012). The former, best known for its role in regulating energy homeostasis and metabolism (Bermudez‐Silva, Cardinal, & Cota, 2012), involves the endogenous cannabinoids, their synthesizing and breakdown enzymes, and the cannabinoid receptors. eCBs, acting via CB1 receptors in the kidney (Larrinaga et al., 2010; Lecru et al., 2015), induce renal dysfunction and fibrosis, whereas genetically deleting CB1 receptors or globally inhibiting them induces anti‐inflammatory and anti‐fibrogenic effects (Lecru et al., 2015; Steinberg & Cannon, 2007; Tam et al., 2010; Tam et al., 2018). In a previous study, we reported that a specific deletion of CB1 receptors in the renal proximal tubule cells (RPTCs) markedly attenuated the obesity‐induced lipid accumulation in the kidney, as well as renal dysfunction, injury, inflammation, and fibrosis via regulating the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2212/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=255#1263 signalling pathway (Udi et al., 2017). These deleterious effects in RPTCs, mediated via activating CB1 receptors, also modify the shape of the mitochondria, leading to reduced oxygen consumption, ATP production, and mitochondrial dysfunction (Drori et al., 2019). An additional signalling pathway that may promote renal dysfunction and fibrosis is the activation of iNOS, an enzyme catalysing the production of the free radical NO. A substantial up‐regulation of iNOS, commonly considered the main perpetrator of autotoxicity under oxidative stress such as obesity (Bogdan, 1998), has been shown to promote kidney fibrosis (Ozbek et al., 2009). However, iNOS inhibitors lack oral bioavailability (Lopez‐Sanchez et al., 2010), and currently, there is no effective antifibrotic therapy approved for clinical use.
A molecule that may link CB1 receptors and iNOS, regarding their deleterious effect on the kidney in obesity, is https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3726, as activation or blockade of either CB1 receptors or iNOS in adipose tissue regulates adiponectin expression, synthesis, and subsequent release (Bensaid et al., 2003; Dallaire et al., 2008; Jeon et al., 2012), and may also affect the signalling pathways of adiponectin via its receptors in the liver (Kabir et al., 2015; Tam et al., 2014). In fact, adiponectin and its receptors, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=649 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=650, are expressed in RPTCs (Perri et al., 2013), and down‐regulating the adiponectin signalling pathway, specifically in these cells, may induce obesity‐induced kidney injury. Interestingly, although adiponectin knockout mice have normal glucose tolerance, body weight, and blood pressure (Ma et al., 2002), they develop microalbuminuria at double the normal range at an early age and display elevated oxidative stress (Ohashi et al., 2007).
Here, we examined the effect of blocking the CB1 receptor and iNOS signalling pathways in ameliorating obesity‐induced CKD by using a novel peripherally restricted, orally bioavailable dual CB1 receptor/iNOS antagonist, MRI‐1867 (Cinar et al., 2016). The results indicate that the beneficial effects of this compound on the kidney is partly associated with modulating adiponectin signalling in RPTCs.
2. METHODS
2.1. Animals and experimental protocol
All animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Hebrew University (AAALAC Accreditation #1285; Ethical Approval Number MD‐17‐15070‐3). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, Altman & Group, 2010) and with the recommendations made by the British Journal of Pharmacology. The current experiment is based on the rule of the replacement, refinement, or reduction. All the animals used in this study were housed under specific pathogen‐free conditions, up to five per cage, in standard plastic cages with natural soft sawdust as bedding. The animals were maintained under controlled temperature of 22–24°C, humidity at 55 ± 5%, and alternating 12‐hr light/dark cycles (lights were on between 7:00 a.m. and 7:00 p.m.) and provided with food and water ad libitum. To generate diet‐induced obesity (DIO; body weight >50 g), 6‐week‐old male C57Bl/6J mice (The Jackson Laboratory, Cat# 000664, RRID:IMSR_JAX:000664) were fed with either a high‐fat diet (HFD; 60% of calories from fat, 20% from protein, and 20% from carbohydrates; Research Diet, Cat# D12492) or a standard diet (14% fat, 24% protein, and 62% carbohydrates; Cat#, NIH‐31 rodent diet) for 18 weeks. Then, obese mice were randomly divided into the following experimental groups and treated orally for 28 consecutive days with either vehicle (Veh; 1% Tween 80, 4% DMSO, and 95% saline), the peripherally restricted dual CB1 receptor/iNOS antagonist, MRI‐1867 (3 mg·kg−1), a peripherally restricted CB1 receptor antagonist, JD5037 (3 mg·kg−1), or the iNOS inhibitor, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=5102 (10 mg·kg−1). A parallel HFD group treated with Veh was pair fed to the MRI‐1867 group (Figure S1). The entire study included 58 animals in total (eight standard diet/Veh‐, 14 HFD/Veh‐, 14 HFD/MRI‐1867‐, eight HFD/JD5037‐, seven HFD/1400W‐treated mice and seven HFD/pair‐fed animals).
During the treatment, body weight and food intake were monitored every day. Mice were also subjected to a complete metabolic and renal analysis. Twenty‐four‐hour urine was collected 1 week before euthanasia using mouse metabolic cages (CCS2000 Chiller System, Hatteras Instruments, NC, USA). At week 22, mice were euthanized by a cervical dislocation under anaesthesia, the kidneys, brain, liver, fat pads, and muscles were removed and weighed, and samples were either snap frozen or fixed in buffered 4% formalin. Trunk blood was collected for determining the biochemical parameters.
2.2. Human kidney samples
This study of human tissue samples was approved by the Rabin Medical Center Institutional Ethics Committee. Formalin‐fixed paraffin‐embedded renal biopsy materials were obtained from the pathological archives of the Department of Pathology at Rabin Medical Center, Israel. Kidney samples were obtained from unused portions of diagnostic kidney biopsies of patients with diabetic nephropathy (n = 8), obesity‐related glomerulopathy (ORG; n = 10), and focal segmental glomerulosclerosis (FSGS; n = 1). The control biopsies (normal; n = 6) were renal biopsies that appeared normal by histological, immunofluorescence, and electron microscopic examination. ORG was defined morphologically as FSGS and glomerulomegaly occurring in obese patients with a body mass index higher than 30 kg·m−2 (Wang et al., 2016).
2.3. Cell culture
Wild‐type or CB1 receptor deleted HK‐2 cells (see below) are human immortalized RPTCs (ATCC Cat# CRL‐2190, RRID:CVCL_0302) and were cultured in REGM BulletKit medium (Lonza, USA) at 37°C in a humidified atmosphere of 5% CO2/95% air. Cell experiments under lipotoxic conditions were conducted at >80% confluence in six‐well plates as described previously (Udi et al., 2017). Briefly, lipotoxic conditions were established by incubating the cells with medium containing 1% fatty acid‐free BSA (Amresco, Cat# 0332‐TAM), 0.5‐mM sodium oleate (Sigma‐Aldrich, Cat# O7501), and sodium palmitate (Sigma‐Aldrich, Cat# P9767) in a ratio of 2:1 and dissolved in 11% fatty acid‐free BSA (Sigma‐Aldrich, Cat# A7030) and ultra‐pure DDW (Biological Industries, Cat# 01‐866‐1A). MRI‐1867 (100 ng·ml−1 dissolved in DMSO), JD5037 (100 ng·ml−1 dissolved in DMSO), and 1400W (100 μg·ml−1 dissolved in saline) were added to the medium for 24 hr prior to collecting the cells for further evaluation. Cells were harvested using Bio‐Tri RNA lysis buffer (Bio‐Lab, Israel).
2.4. Genetic deletion of CB1 receptors in RPTCs
HK‐2 cells were transfected with CRISPR‐CAS9 vector containing an sgRNA sequence to target all human isoforms of CNR1 (Genecopeia™, Cat# CS‐HCP263432‐CG01‐01‐B) using Lipofectamine 3000 (Invitrogen, Cat# L3000‐001) and selected with hygromycin (200‐μM; Sigma‐Aldrich, Cat# H3274). Antibiotic‐resistant cells were then seeded at single‐cell density and allowed to grow. Single‐cell clones were analysed by DNA sequencing and mRNA analysis to confirm the deletion of CB1 receptors as reported previously (Drori et al., 2019); these cells are referred to as CB1R−/− HK‐2 cells.
2.5. ATP measurement
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713 content in HK‐2 cells was quantified using an ATP assay kit (Abcam, Cat# ab83355) following the manufacturer's instructions and as described previously (Udi et al., 2017).
2.6. ROS measurement
ROS content in the medium of wild‐type or CB1R−/−HK‐2 cells was quantified using DCFDA—Cellular ROS Detection Assay Kit (Abcam, Cat# ab113851) following the manufacturer's instructions.
2.7. Multi‐parameter metabolic assessment
Metabolic and activity profiles of the mice were assessed by using the Promethion High‐Definition Behavioral Phenotyping System (Sable Instruments, Inc., Las Vegas, NV, USA) as described previously (Knani et al., 2016; Udi et al., 2017).
2.8. Blood and urine biochemistry
Serum levels of creatinine and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4539 as well as urine levels of creatinine were determined by using the Cobas C‐111 chemistry analyzer (Roche, Switzerland). Blood urea nitrogen (BUN) was calculated by serum urea levels (BUN mg·dl−1 = Urea mM × 2.801). Creatinine clearance was calculated using urine and serum creatinine levels (CCr ml·hr−1 = Urine creatinine mg·dl−1 × Urine volume∕Serum creatinine mg·dl−1 × 24 hr). Urine levels of albumin, kidney injury marker 1 (KIM‐1), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5309) were measured by elisa kits (albumin, Bethyl Laboratories, TX, USA; KIM‐1 and TIMP‐1, R&D Systems, MN, USA).
2.9. Histopathological analyses
Three‐micrometre paraffin‐embedded kidney sections from 7 to 14 animals per group were stained with periodic acid–Schiff, followed by haematoxylin staining. In addition, kidney sections were stained for collagen types I and III deposition by using Sirius Red (Abcam, Cat# ab150681) and Masson's trichrome (Abcam, Cat# ab150686) staining according to the manufacturer's procedures. Kidney images were captured with a Zeiss AxioCam ICc5 colour camera mounted on a Zeiss Axio Scope.A1 light microscope and taken from 10 random 40× fields of each animal. Mesangial expansion, glomerular, and Bowman's space cross‐sectional areas were quantified using ZEN imaging software (Zeiss, Germany). The Sirius Red‐ and Masson's trichrome‐positive areas were quantified in a blinded manner using ImageJ software with a minimum of 10 random kidney sections per mouse.
2.10. Immunohistochemistry
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology, (Alexander et al. 2018 ). Kidney tissue was fixed in buffered 4% formalin for 48 hr and then embedded in paraffin. Sections were deparaffinized and hydrated. Heat‐mediated antigen was retrieved with 10‐mM citrate buffer pH 6.0 (Thermo Scientific, IL, USA). Endogenous peroxide was inhibited by incubating with a freshly prepared 3% H2O2 solution in MeOH. Non‐specific antigens were blocked by incubating sections for 1 hr with 2.5% horse serum (Vector Laboratories, Cat# VE‐S‐2000). For assessment of tubulointerstitial fibrosis, 3‐μm mouse kidney sections were stained with rabbit anti‐mouse collagen‐1 (1:1,500; Abcam, Cat# ab34710, RRID:AB_731684), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 (MCP‐1; 1:5,000; Abcam, Cat# ab25124, RRID:AB_448636), iNOS (1:10,000; Abcam, Cat# ab3523, RRID:AB_303872), TIMP‐1 (1:10,000; Proteintech, Cat# 10753–1‐AP, RRID:AB_2204547), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6274; 1:2,000; Abcam, Cat# ab46545, RRID:AB_722490) antibodies, followed by a goat anti‐rabbit IgG HRP conjugate (Vector Laboratories, Cat# MP‐7401, RRID:AB_2336529). Human kidney biopsies were stained with rabbit anti‐human CB1 receptor (1:100; Alomone, Cat# ACR‐001, RRID:AB_2039795) and iNOS (1:100; Abcam, Cat# ab3523, RRID:AB_303872), followed by an HRP One‐Step Polymer Detection System: anti‐Mouse–Rabbit–Rat ZyoChem. Colour was developed after an incubation with 3,3′‐diaminobenzidine (DAB) substrate (Vector Laboratories, ImmPACT DAB Peroxidase (HRP) Substrate, Cat# SK‐4105, RRID:AB_2336520), followed by haematoxylin counterstaining and mounting (Vector laboratories, Vecmount Cat# H‐5000, RRID:AB_2336786). Stained sections were photographed as described above. The positive (stained) area for each marker was calculated using colour thresholding and by measuring area fractions with ImageJ software (NIH Public Domain, RRID:SCR_003070), with a minimum of 10 random kidney sections per mouse. Images are presented in the figures, showing the animal with the median value for each group.
2.11. Western blotting
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). Cells were harvested using Bio‐Tri RNA lysis buffer (Bio‐Lab, Israel), and proteins were extracted by isopropanol, 0.3‐M guanidine hydrochloride, and 0.1% SDS. Protein concentrations were measured with the Pierce™ BCA Protein Assay Kit (Thermo Scientific, IL, USA). Samples were resolved by SDS‐PAGE (4–15% acrylamide, 150V) and transferred to PVDF membranes using the Trans‐Blot® Turbo™ Transfer System (Bio‐Rad, CA). Membranes were then incubated for 1 hr in 5% milk (in 1× TBS‐T) to block unspecific binding. Blots were incubated overnight with antibody against 4‐HNE (1:700; Abcam, Cat# ab46545, RRID:AB_722490) and adiponectin (1:500; Abcam, Cat# ab22554, RRID:AB_447152) at 4°C. Anti‐mouse (1:5,000) or rabbit (1:2,500) HRP‐conjugated secondary antibodies were used for 1 hr at room temperature, followed by chemiluminescence detection using the Clarity™ Western ECL Blotting Substrate (Bio‐Rad, CA). Densitometry was quantified using ImageJ (NIH Public Domain, RRID:SCR_003070). Quantification was normalized to anti‐β actin antibody (1:30,000; Abcam, Cat# ab49900, RRID:AB_867494).
2.12. Real‐time PCR
Total mRNA from mouse kidney and HK‐2 cells was extracted using Bio‐Tri RNA lysis buffer (Bio‐Lab, Israel) followed by DNase I treatment (Thermo Scientific, IL, USA) and reverse transcribed using the iScript cDNA kit (Bio‐Rad, CA). Real‐time PCR was performed using iTaq Universal SYBR Green Supermix (Bio‐Rad, CA) and the CFX connect ST system (Bio‐Rad, CA). The following Mus musculus genes were detected: Col1a1, Mcp1, Nos2, Timp‐1, Cnr1, Adipoq, Adipor1, and Adipor 2. All genes were normalized to Mus musculus β‐actin (primer information is described in Tables S1 and S2).
The expression of the following human genes was analysed: ADIPOQ, ADIPOR1, ADIPOR2, PDGF subunit A (PDGFa), FGF2, MitoDNA, and β2M. All genes were normalized to human RPLP0 (primer information is described in Table S1 and S2).
2.13. Total DNA extraction and mitochondrial DNA/genomic DNA measurement
Cells were harvested using trypsin, and DNA was extracted with the DNeasy Blood & Tissue Kit (Qiagen, Cat# 69056) according to the manufacturer's protocol. Isolated DNA served as a template for qPCR reaction using primer sets for β2 microglobulin to amplify genomic DNA and D‐loop region to amplify mitochondrial DNA. Primer information is described in Tables S1.
2.14. Materials
MRI‐1867 was kindly provided by Dr George Kunos of the NIH and synthesized as described previously (Cinar et al., 2016). JD5037 was synthesized as described previously (Chorvat, Berbaum, Seriacki, & McElroy, 2012) and purchased from Haoyuan Chemexpress Co., Ltd. 1400W was purchased from Cayman Chemical.
2.15. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Randomization was used to assign samples to the experimental groups and treatment conditions for all in vivo studies. Data collection and acquisition of all in vivo and in vitro experimental paradigms were performed in a blinded manner. Data are expressed as the mean ± SEM. Statistical significance among groups and time‐dependent variables was compared by ANOVA, followed by a Bonferroni post hoc analysis using GraphPad Prism v6 for Windows (San Diego, CA; RRID:SCR_002798). Post hoc tests were conducted only if F was significant, and there was no variance inhomogeneity. Significance was set at P < .05.
2.16. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017).
3. RESULTS
3.1. Elevated expression of CB1 receptors and iNOS in CKD human patients
To evaluate the contribution of CB1 receptors and iNOS to the development of CKD, we assessed their protein levels by immunohistochemistry in kidney biopsies from healthy humans (normal) and patients with FSGS, diabetic nephropathy, and ORG and found that both proteins were dramatically increased (Figure 1a). Interestingly, elevated protein levels were found in the tubular areas under all disease conditions. Whereas CB1 receptors were highly expressed in the proximal tubules, iNOS was mainly expressed in the distal tubules (Figure 1b,c).
Figure 1.
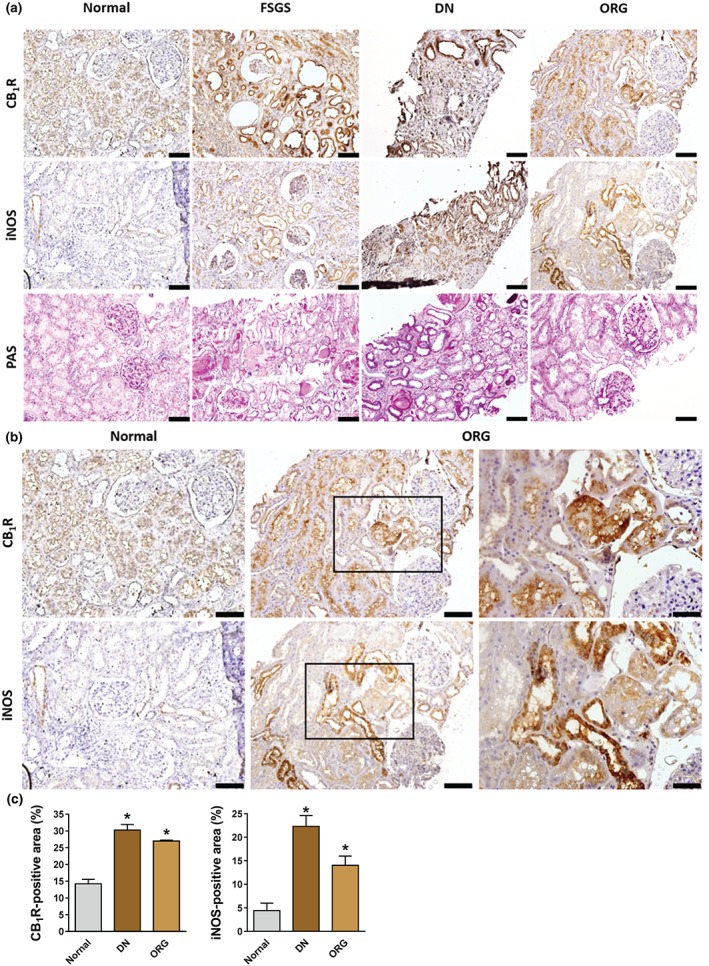
Elevated renal CB1 receptor (CB1R) and inducible NOS (iNOS) protein expression in humans with chronic kidney disease. (a) Elevated protein expression levels of CB1 receptors and iNOS were detected in 25 kidney biopsies from focal segmental glomerulosclerosis (FSGS), diabetic nephropathy (DN), and obesity‐related glomerulopathy (ORG) patients. (b, c) CB1 receptors and iNOS are highly expressed in the kidney tubules in DN and ORG. Scale bar, 100 μm in all images except for the right panels in (b), where the scale is 50 μm. Data represent the mean ± SEM from six normal, eight DN, and 10 ORG kidney samples. * P < .05, significantly different from normal kidney; one‐way ANOVA, followed by a Bonferroni post hoc test
3.2. Dual blockade of CB1 receptors and iNOS induces a metabolic shift in diet‐induced obese mice
To test the metabolic and renal effects of dual inhibition of CB1 receptors and iNOS in DIO mice, C57Bl/6J animals were maintained on an HFD for 18 weeks and then treated with MRI‐1867 (3 mg·kg−1, p.o.) or Veh for 28 days. A substantial body weight reduction (Figure 2a,b) and food intake inhibition (Figure 2c,d), with no effect on water intake (Figure 2e), were found in DIO animals treated with MRI‐1867. Additionally, these mice exhibited a decreased respiratory quotient (Figure 2f,g), an increased total energy expenditure (Figure 2h,i), and fat oxidation (Figure 2j,k), without a significant difference in carbohydrate oxidation (Figure 2l,m), suggesting higher fat utilization. The improved metabolic profile of DIO mice treated with MRI‐1867 was not associated with changes in ambulatory (Figure S2a,b) or voluntary (Figure S2c,d) activities as well as restoring the decreased ability of Veh‐treated DIO mice to run on a wheel (Figure S2e,f). Collectively, these metabolic effects are identical to those achieved by both central (Christopoulou & Kiortsis, 2011) and peripheral (Tam et al., 2012) CB1 receptor blockade, indicating that the contribution of iNOS inhibition to these metabolic effects is relatively small.
Figure 2.
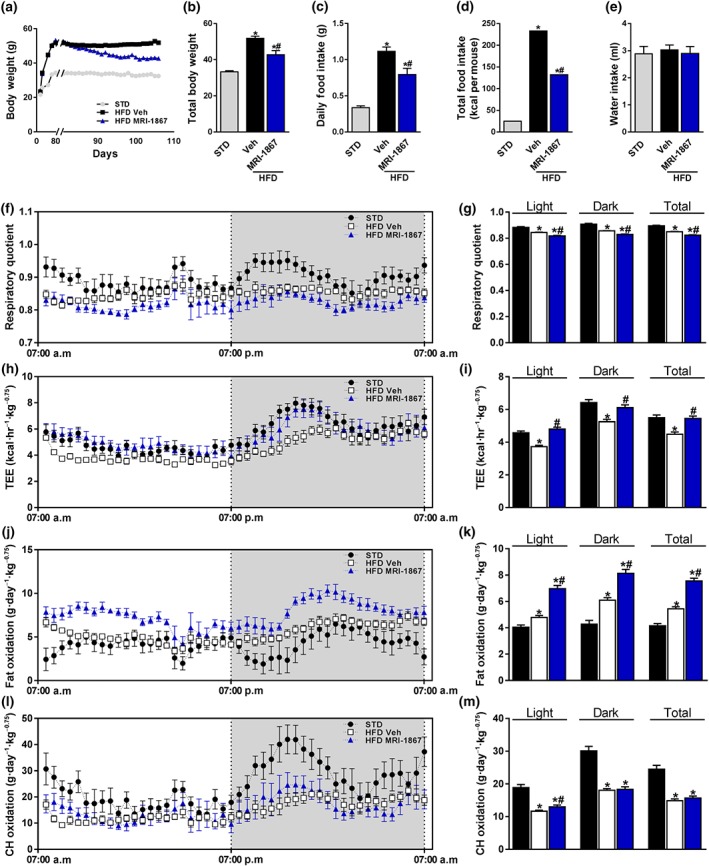
MRI‐1867 improves the metabolic profile in diet‐induced obese mice. Male mice on standard diet (STD) or high‐fat diet (HFD) for 18 weeks were treated with vehicle (Veh) or MRI‐1867 (3 mg·kg−1) orally for 28 days. MRI‐1867 reduced (a, b) the body weight, (c) daily, and (d) the total food intake but not (e) the water intake. Metabolically, MRI‐1867 decreased (f, g) the respiratory quotient, which indicates a higher fat utilization, and increased (h, i) total energy expenditure (TEE) and (j, k) fat oxidation, without affecting (l, m) carbohydrate oxidation. Data represent the mean ± SEM from 8 to 14 mice per group. * P < .05, significantly different from animals on STD; # P < .05, significantly different from animals on the same diet; one‐way ANOVA, followed by a Bonferroni post hoc test
3.3. Dual blockade of CB1 receptors and iNOS improves renal morphological and functional parameters in diet‐induced obese mice
Progressive morphological malformations and a decline in renal function, assessed by increased albuminuria, kidney injury markers, and changes in GFR, are common features associated with obesity‐related CKD in both humans and animals (Chagnac et al., 2008; Deji et al., 2009; Tobar et al., 2013; Udi et al., 2017). Indeed, Veh‐treated DIO mice displayed a glomerular enlargement (Figure 3a,b), increased Bowman's space area (Figure 3a,c), and mesangial expansion (Figure 3a,d). However, all of these changes were completely normalized by chronic MRI‐1867 treatment. Functionally, the significantly elevated urinary albumin (Figure 3e) and creatinine (Figure 3f) levels, also reflected by an up‐regulated albumin‐to‐creatinine ratio (Figure 3g) in DIO mice, were markedly attenuated by MRI‐1867. Similarly, chronic dual inhibition of CB1 receptors and iNOS was able to reverse the decrease in the kidney‐to‐body weight ratio (Figure 3h) and to ameliorate the elevation in the BUN levels (Figure 3i), as well as the urinary secretion levels of KIM‐1 (Figure 3j) and the TIMP‐1 (Figure 3k). Notably, treatment with MRI‐1867 insignificantly ameliorated the elevated creatinine clearance (CCr) noted in Veh‐treated DIO mice (Figure 3l).
Figure 3.
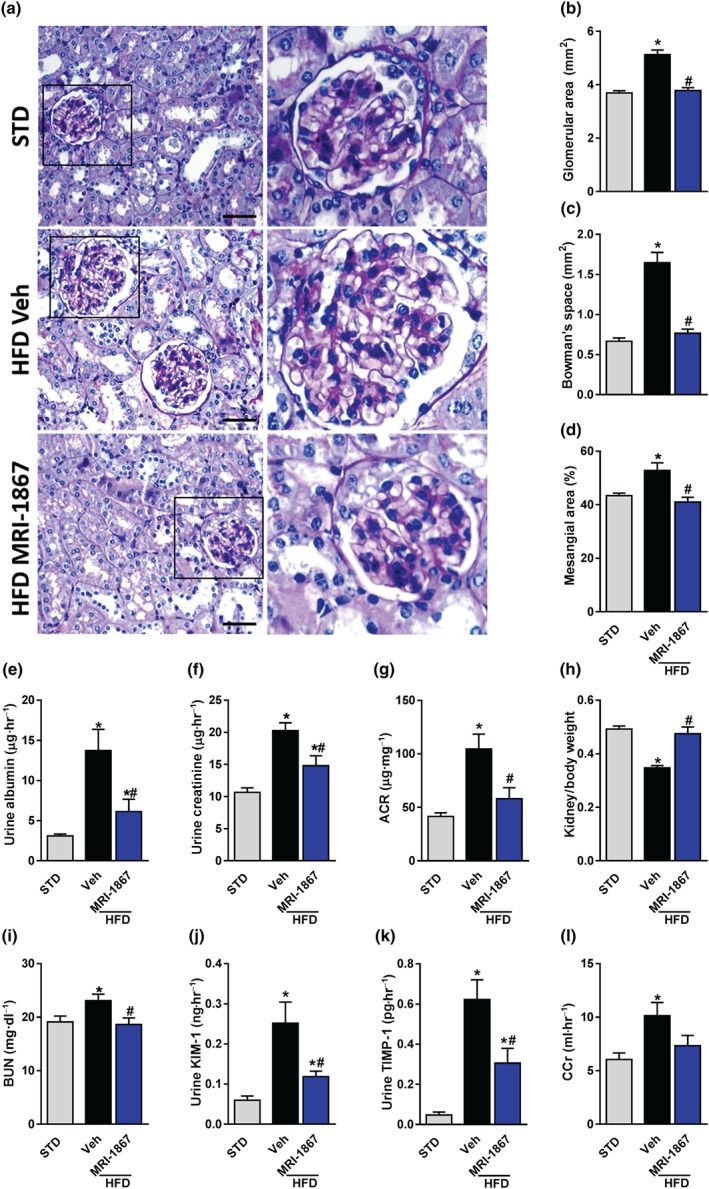
MRI‐1867 improves kidney morphology and function in diet‐induced obese mice. Mice on standard diet (STD) or high‐fat diet (HFD) for 18 weeks were treated with vehicle (Veh) or MRI‐1867 (3 mg·kg−1) orally for 28 days. Chronic MRI‐1867 treatment protected diet‐induced obese (DIO) mice from obesity‐induced morphological changes, such as (a, b) glomerular enlargement, (a, c) increased Bowman's space area, and (a, d) mesangial expansion. Moreover, MRI‐1867 treatment improved kidney function, indicated by reduced urinary (e) albumin and (f) creatinine levels, as well as (g) the albumin‐to‐creatinine ratio (ACR). (h) Reduced kidney‐to‐body weight ratio, (i) elevated blood urea nitrogen (BUN), and (j) urinary secretion levels of kidney injury marker 1 (KIM‐1), and (k) tissue inhibitor of metalloproteinase 1 (TIMP‐1) were normalized in MRI‐1867‐treated DIO mice. (l) A tendency towards reduction in creatinine clearance (CCr) was also observed in DIO mice treated with MRI‐1867. Scale bar, 20 μm. Data represent the mean ± SEM from 8 to 14 mice per group. * P < .05, significantly different from animals on STD; # P < .05, significantly different from animals on the same diet; one‐way ANOVA, followed by a Bonferroni post hoc test
As MRI‐1867 inhibits both CB1 receptors and iNOS , we further evaluated its renal efficacy concomitantly with the orally bioavailable peripherally restricted CB1 receptor inverse agonist, JD5037 (3 mg·kg−1), and the orally bioavailable iNOS antagonist, 1400W (10 mg·kg−1). A parallel obese group was pair fed to the HFD‐treated group with MRI‐1867 in order to determine whether the positive renal effects of MRI‐1867 could be solely due to its effect on weight and energy homeostasis (Figure 2). Metabolically, both MRI‐1867 and JD5037 resulted in reduced body weight and food intake, although the latter was found to be even more effective in reducing weight (Figure 4a–d). Morphologically, both MRI‐1867 and JD5037 significantly reduced the HFD‐induced enlargement of glomerular and Bowman's space areas as well as reduced mesangial expansion (Figure 4e–h), and both were able to reduce circulating BUN and increase the kidney‐to‐body weight ratio (Figure 5a,b). However, only MRI‐1867 improved the HFD‐induced kidney dysfunction (Figure 5c–g). Interestingly, neither the iNOS inhibition nor pair feeding resulted in any metabolic or renal improvements (Figures 4 and 5). Taken together, these findings support the pivotal roles of CB1 receptors and iNOS in mediating obesity‐induced renal injury and dysfunction as well as metabolic homeostasis.
Figure 4.
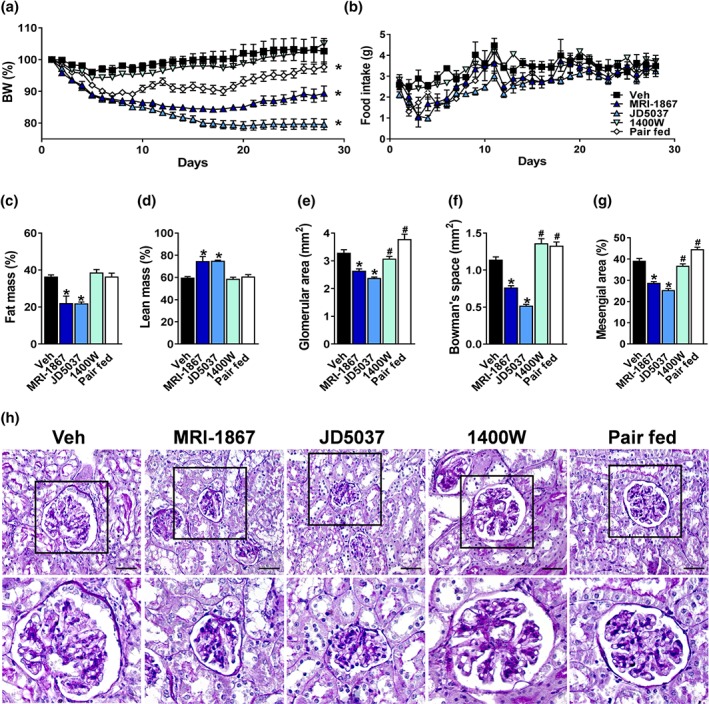
MRI‐1867 and peripheral CB1 receptor blockade effectively restore metabolic and kidney morphological changes in diet‐induced obese (DIO) mice in comparison with inducible NOS inhibition or pair feeding. Six‐week‐old male C57Bl/6J mice were fed with high‐fat diet (HFD) for 18 weeks and then treated chronically by oral gavage with either vehicle (Veh; 1% Tween 80, 4% DMSO, and 95% saline), MRI‐1867 (3 mg·kg−1), peripherally restricted CB1 receptor antagonist, JD5037 (3 mg·kg−1), and inducible NOS inhibitor, 1400W (10 mg·kg−1). A parallel group of mice, which was pair fed similarly to the MRI‐1867 group, was treated with Veh. Treatment was given for 28 consecutive days. MRI‐1867 and JD5037 reduced (a) body weight (BW) and (b) food intake. Both drugs reduced (c) body fat mass and increased (d) lean mass. Histologically, MRI‐1867 and JD5037 reduced (e, h) HFD‐induced glomerular enlargement, increased (f, h) Bowman's space area, and (g, h) mesangial expansion. Scale bar, 20 μm. Data represent the mean ± SEM from 7 to 14 mice per group. * P < .05, significantly different from diet‐induced obese animals treated with Veh; # P < .05, significantly different from diet‐induced obese mice treated with MRI‐1867; one‐way ANOVA, followed by a Bonferroni post hoc test
Figure 5.
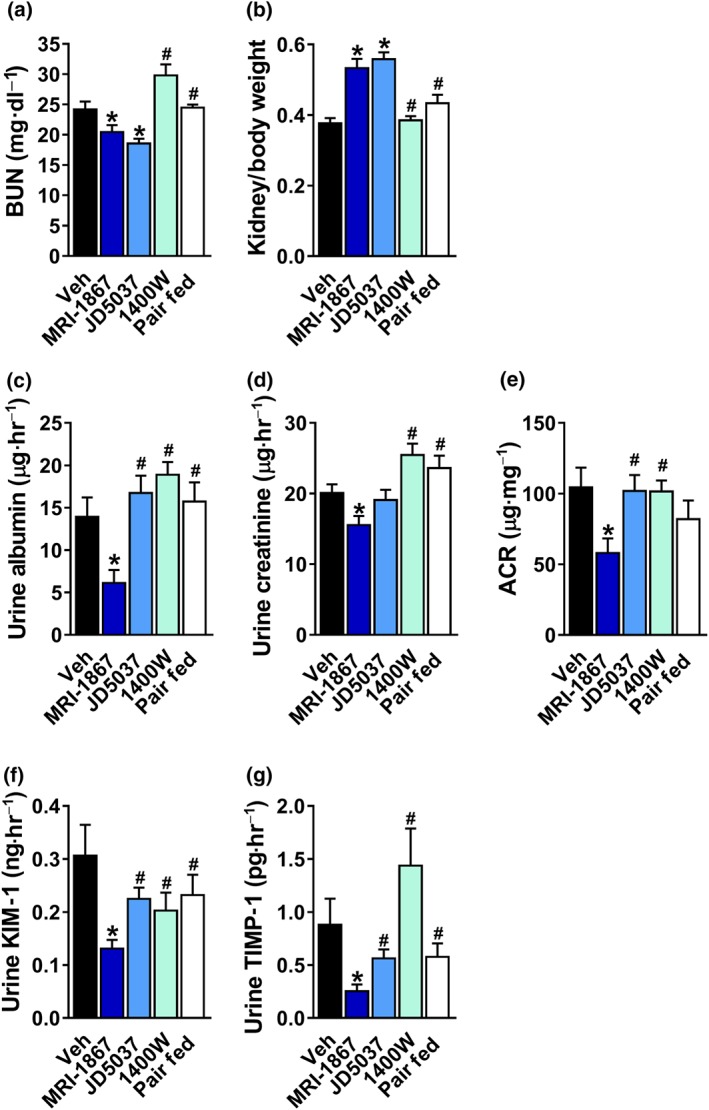
A greater efficacy of MRI‐1867 in restoring kidney function in diet‐induced obese mice in comparison with peripheral CB1 receptor blockade, inducible NOS inhibition, or pair feeding. MRI‐1867 or JD5037 treatment reduced (a) BUN levels and (b) the kidney‐to‐body weight ratio. Only MRI‐1867 was effective in reducing urinary (c) albumin, (d) creatinine, and (e) the albumin‐to‐creatinine (ACR) ratio, as well as the urinary secretion levels of (f) kidney injury marker 1 (KIM‐1) and (g) tissue inhibitor of metalloproteinase 1 (TIMP‐1). Data represent the mean ± SEM from 7 to 14 mice per group. * P < .05, significantly different from diet‐induced obese animals treated with Veh; # P < .05, significantly different from diet‐induced obese mice treated with MRI‐1867; one‐way ANOVA, followed by a Bonferroni post hoc test
3.4. Dual blockade of CB1 receptors and iNOS decreases kidney inflammation and fibrosis in diet‐induced obese mice
Previous work by Cinar and colleagues emphasized the anti‐fibrogenic role of MRI‐1867, in both the liver (Cinar et al., 2016) and the lungs (Cinar et al., 2017). Therefore, we also investigated its anti‐fibrogenic and anti‐inflammatory roles in the kidney. As shown in Figure 6, Veh‐treated DIO mice displayed up‐regulated mRNA and protein expression levels of collagen‐1 (Figure 6a–c), the chemokine CCL2 (Figure 6a,b,d), iNOS (Figure 6a,b,e), and TIMP‐1 (Figure 6a,b,f). Except for Nos2 gene expression, the mRNA and protein levels of all of these markers were completely normalized in the MRI‐1867‐treated DIO mice. Furthermore, the elevated collagen deposition, evident in Veh‐treated DIO mice, was significantly reversed by MRI‐1867 (Figure 6g–j), highlighting the anti‐fibrogenic role of MRI‐1867 also in the kidney. As previously reported by us, HFD increased renal CB1 receptors (Udi et al., 2017), and as expected from a compound capable of blocking CB1 receptors, MRI‐1867 completely down‐regulated the renal expression of CB1 receptors (Figure S3).
Figure 6.
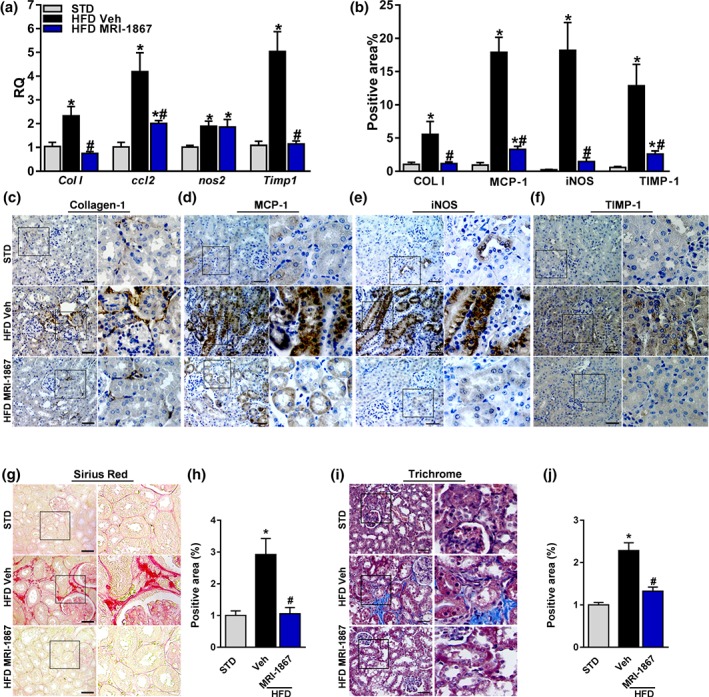
MRI‐1867 improves renal inflammation and fibrosis in diet‐induced obese mice. Mice on standard diet (STD) or high‐fat diet (HFD) for 18 weeks were treated with vehicle (Veh) or MRI‐1867 (3 mg·kg−1) orally for 28 days. The HFD‐induced up‐regulation in the renal mRNA and/or the protein expression levels of (a–c) collagen‐1, (a, b, d) CCL2, (a, b, e) inducible NOS (iNOS), and (a, b, f) tissue inhibitor of metalloproteinase 1 (TIMP‐1) were attenuated or normalized in the MRI‐1867‐treated DIO mice. Similarly, the elevated collagen deposition in Veh‐treated DIO mice, measured by (g, h) Sirius Red staining and (i, j) Masson's trichrome staining, was reversed by chronic MRI‐1867 treatment. Scale bar, 20 μm. RQ, relative quantitation. Data represent the mean ± SEM from 8 to 14 mice per group. * P < .05, significantly different from animals on STD; # P < .05, significantly different from animals on the same diet; one‐way ANOVA, followed by a Bonferroni post hoc test
3.5. Dual blockade of CB1 receptors and iNOS ameliorates oxidative stress in vivo and in vitro
Obesity‐induced oxidative stress is manifested by elevated levels of lipid oxidation (Marseglia et al., 2014) and decreased mitochondrial content and biogenesis (Jeon et al., 2012). We next tried to dissociate the inhibitory effects of CB1 receptors from iNOS in modulating mitochondrial function and oxidative stress by comparing the in vitro effects of MRI‐1867 with those of the peripherally restricted CB1 receptor blocker, JD5037, in the presence of a 0.5‐mM mixture of oleate and palmitate (O:P, 2:1, respectively). Importantly, both MRI‐1867 and JD5037 were effective in restoring ATP levels (Figure 7a) and mitochondrial DNA content (Figure 7b). However, under the same conditions, only MRI‐1867 resulted in reduced cellular ROS production (Figure 7c) and down‐regulated the O:P‐associated increase in the CB1 receptor‐independent fibrogenic expression markers, PDGFa (Figure 6d) and FGF2 (Figure 7e).
Figure 7.
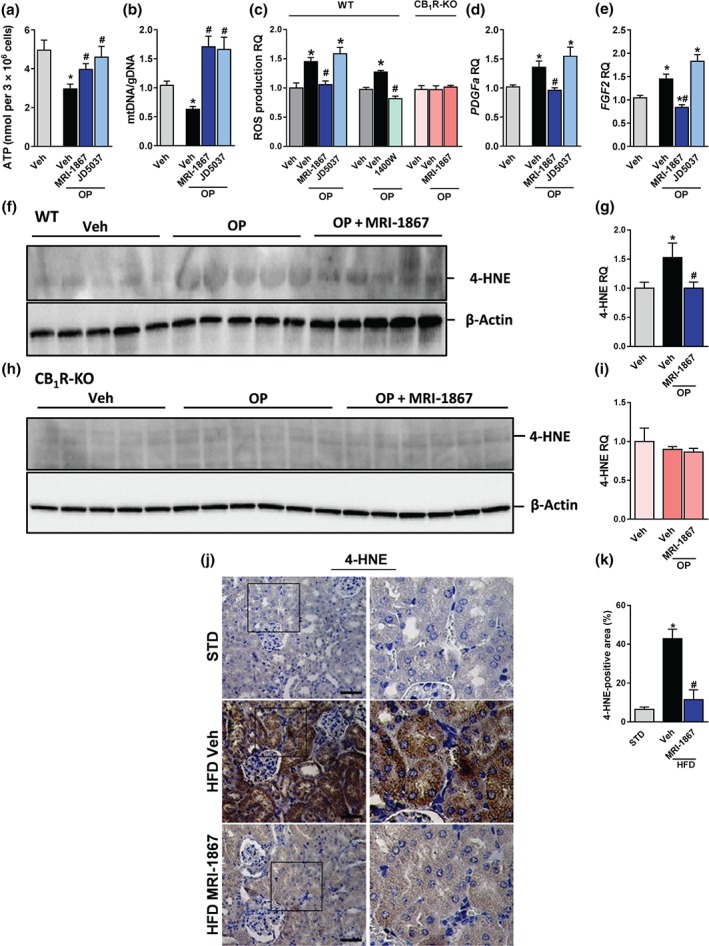
MRI‐1867 improves renal inflammation and fibrosis in diet‐induced obese mice. Mice on standard diet (STD) or high‐fat diet (HFD) for 18 weeks were treated with vehicle (Veh) or MRI‐1867 (3 mg·kg−1) orally for 28 days. The HFD‐induced up‐regulation in the renal mRNA and/or the protein expression levels of (a–c) collagen‐1, (a, b, d) CCL2, (a, b, e) inducible NOS (iNOS), and (a, b, f) tissue inhibitor of metalloproteinase 1 (TIMP‐1) were attenuated or normalized in the MRI‐1867‐treated DIO mice. Similarly, the elevated collagen deposition in Veh‐treated DIO mice, measured by (g, h) Sirius Red staining and (i, j) Masson's trichrome staining, was reversed by chronic MRI‐1867 treatment. Scale bar, 20 μm. RQ, relative quantitation. Data represent the mean ± SEM from 8 to 14 mice per group. * P < .05, significantly different from animals on STD; # P < .05, significantly different from animals on the same diet; one‐way ANOVA, followed by a Bonferroni post hoc test
We further tested the effect of MRI‐1867 in reducing O:P‐induced lipid oxidation, measured by protein‐bound 4‐HNE (Uchida et al., 1999), and oxidative stress in wild‐type and CB1R−/− HK‐2 cells. Interestingly, the null cells were completely protected from the O:P‐induced up‐regulation of 4‐HNE expression (Figure 7f–i) and ROS production (Figure 7c) and, therefore, MRI‐1867 lost its effects. On the other hand, reduced ROS levels in wild‐type HK‐2 cells exposed to O:P were achieved by 100 μg·ml−1 of 1400W (Figure 7c). Assessing lipid oxidation and consequently tissue damage in our in vivo obese mouse model, by measuring 4‐HNE expression levels, revealed a marked up‐regulation in its expression in RPTCs of Veh‐treated DIO mice (Figure 7j,k), an effect that was completely normalized by chronic MRI‐1867 treatment. Taken together, these findings emphasize the obligatory role of CB1 receptors in inducing oxidative stress under obese conditions and also the unique role of MRI‐1867 in inhibiting iNOS and reducing oxidative stress in the kidney independently of inhibiting CB1 receptors.
3.6. Dual blockade of CB1 receptors and iNOS ameliorates the obesity‐induced down‐regulation of adiponectin signalling in RPTCs
Similarly to adipocytes, RPTCs are known to express adiponectin and its receptors, Adipo1 and Adipo2, (Martinez Cantarin, Keith, Waldman, & Falkner, 2014; Perri et al., 2013). As activation or blockade of CB1 receptors and iNOS ameliorated adiponectin expression and synthesis in adipocytes (Bensaid et al., 2003; Dallaire et al., 2008; Jeon et al., 2012), we sought to determine the in vivo and in vitro effect of MRI‐1867 on adiponectin signalling in RPTCs. Importantly, chronic treatment with MRI‐1867 restored the HFD‐induced reduction in renal mRNA expression of adiponectin and the Adipo2 receptor, (Figure 8a–c). In vitro, exposing RPTCs to O:P resulted in a similar reduction in the mRNA and protein expression levels of adiponectin (Figure 8d–f) as well as Adipo1 receptors (Figure 8g) and AdipoR2 (Figure 8h). Pretreatment with MRI‐1867 (100 ng·ml−1) completely restored these levels, suggesting that modulating adiponectin signalling by dual inhibition of CB1 receptors and iNOS could contribute to protecting the kidney during obesity.
Figure 8.
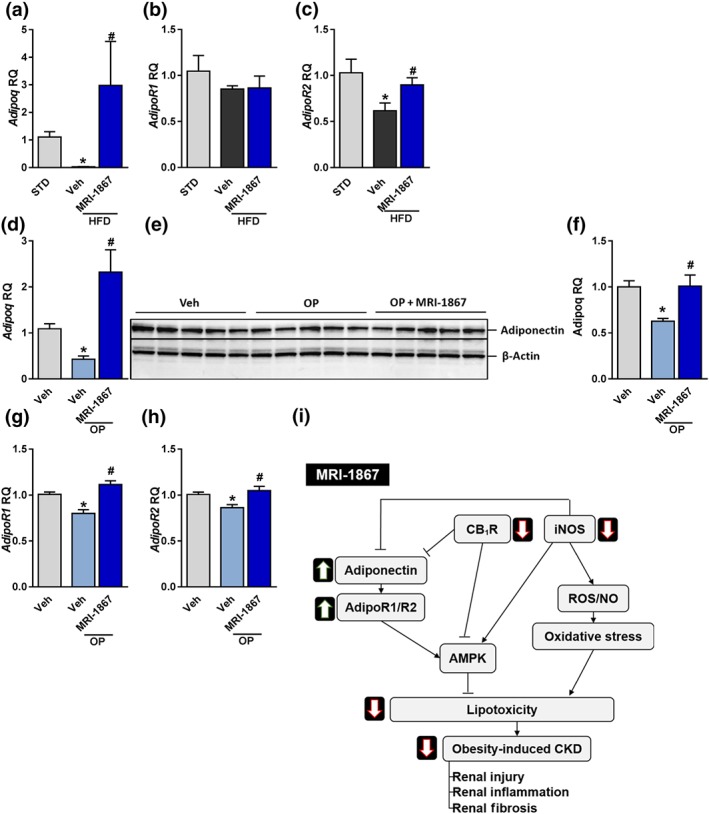
MRI‐1867 reverses the fatty acid‐induced reduction in adiponectin signalling. Mice on standard diet (STD) or high‐fat diet (HFD) for 18 weeks were treated with vehicle (Veh) or MRI‐1867 (3 mg·kg−1) orally for 28 days. (a–c) MRI‐1867 restored the HFD‐induced reduction in the mRNA renal expression of adiponectin, and Adipo2 but not Adipo1 receptors. Similarly, exposing HK‐2 cells to O:P (0.5 mM, 2:1, respectively) resulted in reduced (d–f) mRNA and protein expression of adiponectin as well as reduced mRNA levels of (g) Adipo1 and (h) Adipo2 receptors. Pretreatment of the cells with MRI‐1867 (100 ng·ml−1) completely normalized these changes. (i) A proposed mechanism for the dual blockade of CB1 receptors and inducible NOS by MRI‐1867 in reversing obesity‐induced chronic kidney disease (CKD) is shown. RQ, relative quantitation. In vivo data represent the mean ± SEM from 8 to 14 mice per group. * P < .05, significantly different from animals on STD; # P < .05, significantly different from animals on the same diet. In vitro data represent the mean ± SEM from five independent experiments. * P < .05, significantly different from Veh‐treated cells under normal conditions; # P < .05, significantly different from Veh‐treated cells under O:P conditions. Data were analysed by one‐way ANOVA, followed by a Bonferroni post hoc test
4. DISCUSSION
Obesity is perceived as both an initiator and accelerator of CKD (Othman, Kawar, & El Nahas, 2009). Interventions to prevent the development and/or progression of CKD to end‐stage renal disease have great potential to save lives as well as decrease health care‐associated costs, specifically with the increased prevalence of obesity and its co‐morbidities (Zandi‐Nejad & Brenner, 2005). Nonetheless, existing data suggest that the incidence of CKD differs significantly across different global regions, which have different prevalence of obesity, indicating that obesity is an important risk factor for CKD (Chalmers, Kaskel, & Bamgbola, 2006; Hallan et al., 2006). Consequently, the search for novel therapeutic targets/agents against obesity‐induced CKD has a crucial significance.
Obesity may cause deleterious effects on the kidney via inducing two main molecular signalling pathways. The first modulator, which was identified by us as a crucial player in renal lipotoxicity, is the CB1 receptor (Drori et al., 2019; Udi et al., 2017). Its overactivation in the kidney during obesity may lead to chronic inflammatory responses and alter the normal functioning parenchyma to exhibit more fibrogenic characteristics. These alternations impair RPTC function and, consequently, may lead to kidney dysfunction. Interestingly, this cascade of events is attributed to a halted beta‐oxidation signalling pathway governed by the LKB1/AMPK/ACC signalling pathway (Udi et al., 2017). Moreover, activating CB1 receptors in RPTCs results in a parallel signalling pathway that involves recruiting the canonical fission protein, dynamin‐related protein 1, to the mitochondria to induce mitochondrial fragmentation, reduced oxygen consumption and ATP production, elevated ROS and cellular lactate levels, and a decline in mitochondrial biogenesis (Drori et al., 2019), contributing to renal tubular injury associated with lipotoxicity.
The second obesity‐related regulator that may affect the kidney is the iNOS signalling pathway, which is well known to promote renal dysfunction and fibrosis (Correia‐Costa et al., 2016) via producing the free radical NO by iNOS (Ruggiero, Ehrenshaft, Cleland, & Stadler, 2011). In fact, obesity provokes elevated oxidative stress by up‐regulating beta‐oxidation (Marseglia et al., 2014) and decreasing mitochondrial content (Jeon et al., 2012). Moreover, this oxidative stress is mainly derived from the production of NO by iNOS, which makes this enzyme to be considered as a fundamental perpetrator of autotoxicity (Bogdan, 1998).
An up‐regulation in the expression of both regulators in the kidney under diabetic conditions was reported previously (Barutta et al., 2010; Barutta et al., 2014; Chang et al., 2004; Cosenzi et al., 2002; Jenkin, McAinch, Zhang, Kelly, & Hryciw, 2015; Jourdan et al., 2014; Lim et al., 2011; Lim & Park, 2012; Nam et al., 2012; Slyvka et al., 2016; Veelken et al., 2000), and our current findings support these observations. However, we report here, for the first time, a strong up‐regulation of both CB1 receptors and iNOS in kidney samples from patients with FSGS and ORG, suggesting their pathogenic role in these CKDs. These findings in ORG patients are supported by our recent study demonstrating increased eCB “tone” in DIO mice (Udi et al., 2017) and by the study by Kang and colleagues (Kang et al., 2015) indicating that expression of two genes involved in anandamide degradation (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1402) are significantly reduced in renal samples collected from CKD patients, whereas https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3084, a key enzyme implicated in the biosynthesis of anandamide (Liu et al., 2006), is increased. Taken together, these changes could result in a marked CKD‐associated increase in eCB “tone” in humans as well.
Orally available peripherally restricted CB1 receptor antagonists , which are potentially able to ameliorate the deleterious effects of obesity, via the first signalling mechanism previously mentioned, have been preclinically tested (Tam et al., 2010; Tam et al., 2012) and have been further developed into the clinic (Chorvat, 2013). However, most iNOS inhibitors, targeting the second signalling mechanism, lack oral bioavailability. Therefore, obesity‐induced kidney fibrosis, oxidative stress, and dysfunction currently have no effective antifibrotic therapy approved for clinical use. By using a novel, orally bioavailable, dual‐targeted drug, MRI‐1867, which can simultaneously inhibit CB1 receptors and iNOS in peripheral organs (Cinar et al., 2016), we demonstrated here, for the first time, its beneficial effect in mitigating obesity‐induced CKD. This molecule does not penetrate the brain and shows negligible occupancy of CB1 receptors in the CNS, as shown by PET analysis, leading to no central CB1 receptor‐associated side effects (Cinar et al., 2016). In fact, this novel molecule holds promise in blocking both CB1 receptor and iNOS signalling in the kidney. For instance, with regard to CB1 receptors, its peripherally restricted inhibition creates a “positive” metabolic shift in DIO mice by decreasing body mass and food intake, as well as enhancing total energy expenditure and fat oxidation. These general metabolic effects are in accordance with a previous work published by Tam et al. (2012), which investigated the efficacy of the well‐characterized, peripherally restricted CB1 receptor inverse agonist, JD5037, whose structure is quite similar to MRI‐1867, and which was also generated, based on the parent compound SLV‐319 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9234; Cinar et al., 2016). The positive effect of MRI‐1867 was further demonstrated here in restoring renal morphological and functional parameters in DIO mice. Whereas the morphological improvements in glomerulus size, Bowman's space area, and mesangial expansion, as well as the reversal of the kidney‐to‐body weight ratio and BUN, are indistinguishable from the positive effect observed in DIO mice lacking CB1 receptors in the RPTCs (Udi et al., 2017) or in obese animals treated chronically with JD5037 tested here, the ability of MRI‐1867 to restore kidney function, assessed by measuring the urinary levels of albumin, creatinine, KIM‐1, and TIMP‐1, is most likely mediated via its dual action to block both CB1 receptors and iNOS. The renal effects of MRI‐1867 were not replicated by pair feeding, which rules out the role of a transient reduction in food intake by MRI‐1867 as their cause. This, of course, further suggests that renal CB1 receptors and iNOS are key modulators of nephropathy during obesity.
Notably, these improvements in DIO mice were associated with decreased inflammation and fibrosis in renal parenchyma, characterized by reduced levels of fibrogenic and proinflammatory markers such as collagen‐1, CCL2, and TIMP‐1. These modalities of MRI‐1867 are in accordance with previous studies done by Cinar and colleagues underscoring the anti‐fibrogenic role of MRI‐1867 in mitigating both liver (Cinar et al., 2016) and lung (Cinar et al., 2017) fibrosis. As MRI‐1867 induces weight loss and improves metabolic features, we could not exclude the possibility that the reversal of obesity‐induced CKD by this compound may be related to an extrinsic effect that modulates renal function indirectly and not via CB1 receptors and/or iNOS. Nevertheless, the additive effect of reversing obesity‐induced renal oxidative stress in DIO mice and more specifically in RPTCs can be attributed to the iNOS inhibitory modality of MRI‐1867, as its chronic administration was able to completely reduce the levels of the oxidative stress marker 4‐HNE. In addition, the up‐regulation of iNOS protein expression in the kidney of DIO mice and its reversal by MRI‐1867 strongly suggest that one should consider its involvement in obesity‐induced nephropathy. In fact, its ability to normalize the fatty acid flux‐induced up‐regulation in 4‐HNE, ROS production, and PDGFa and FGF2 expression emphasizes the distinctive role of MRI‐1867 in iNOS inhibition and resolution of renal oxidative stress, independently of CB1 receptor inhibition. Nevertheless, we could not completely rule out the obligatory role of CB1 receptors in modulating renal oxidative stress as well, because knocking it out in RPTCs protected the cells from O:P‐induced lipid oxidation and oxidative stress. These results support our previous findings in mice lacking CB1 receptors specifically in RPTCs, which are completely protected from the deleterious effects of HFD feeding and fatty acid flux‐induced renal dysfunction, inflammation, mitochondrial damage, oxidative stress, and fibrosis (Drori et al., 2019; Udi et al., 2017).
Previous studies have shown that adiponectin has renoprotective effects in rodent experiments and that knocking it out in mice results in microalbuminuria and elevated oxidative stress (Ohashi et al., 2007). This led us to test the involvement of adiponectin signalling in the reversal of obesity‐induced CKD by MRI‐1867. Interestingly, activation of either CB1 receptors or iNOS, independently of each another, decreases adiponectin expression (Bensaid et al., 2003; Dallaire et al., 2008; Jeon et al., 2012) and mitigates adiponectin signalling via its receptors, Adipo1 and Adipo2 (Kabir et al., 2015; Tam et al., 2014). As reported previously (Perri et al., 2013), we were also able to identify adiponectin and its receptors within the kidney and more specifically in RPTCs. Interestingly, the dual blockade of CB1 receptors and iNOS, achieved by MRI‐1867, ameliorated obesity‐induced down‐regulation in renal adiponectin signalling both in vivo and in vitro. Others (Rutkowski et al., 2013; Sharma et al., 2008) have reported the causative relationship of adiponectin with albuminuria and oxidative stress in the kidney, most likely to be mediated via activating the energy sensor AMPK. Our previous findings, that CB1 receptors modulate AMPK activity to induce lipotoxicity in RPTCs and renal dysfunction (Udi et al., 2017), may link our two findings, suggesting that simultaneous targeting of CB1 receptor and iNOS signalling by MRI‐1867 could contribute to kidney protection during obesity via the induced activity of AMPK by increasing adiponectin signalling in RPTCs (Figure 8i).
In conclusion, this study presents the beneficial effects of a novel, orally bioavailable dual‐targeted molecule, MRI‐1867, which possesses the ability to inhibit both the CB1 receptor and iNOS signalling pathways, and it pharmacologically ameliorates obesity‐induced CKD. After the Food and Drug Administration recently approved testing clinically novel molecules that block CB1 receptors in peripheral organs for the treatment of obesity and its co‐morbidities, one should consider further emphasizing the therapeutic benefit obtained by simultaneously acting on more than one target.
CONFLICT OF INTEREST
R.C. and M.R.I. are listed as co‐inventors on a U.S. patent application covering MRI‐1867 and related compounds (Patent No. 2.PCT/US2013/069686). All other co‐authors declare that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
S.U., L.H., M.A., and A.D. conducted the experiments and analysed the data. R.C. provided the reagents and technical assistance. M.R.I. and R.C. provided the reagents and contributed in writing the manuscript. M.H.‐E. performed the human analysis study. S.U., L.H., and J.T. designed and supervised the experiments and wrote the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206 and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1. MRI‐1867 chemical structure and experimental protocol.
Figure S2. MRI‐1867 does not affect ambulatory and voluntary activities in diet‐induced obese mice.
Figure S3. MRI‐1867 reduces CB1 receptor expression in DIO mice.
Table S1. Primer information.
Table S2. Primer information.
ACKNOWLEDGEMENTS
We would like to thank Mr Asaad Gammal for his technical assistance. This work was supported by an European Research Council grant (676841) to J.T.
Udi S, Hinden L, Ahmad M, et al. Dual inhibition of cannabinoid CB1 receptors and inducible NOS attenuates obesity‐induced chronic kidney disease. Br J Pharmacol. 2020;177:110–127. 10.1111/bph.14849
REFERENCES
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(Suppl 1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta, F. , Corbelli, A. , Mastrocola, R. , Gambino, R. , Di Marzo, V. , Pinach, S. , … Gruden, G. (2010). Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes, 59, 1046–1054. 10.2337/db09-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta, F. , Grimaldi, S. , Franco, I. , Bellini, S. , Gambino, R. , Pinach, S. , … Gruden, G. (2014). Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin‐induced diabetic mice. Kidney International, 86, 979–990. 10.1038/ki.2014.165 [DOI] [PubMed] [Google Scholar]
- Bensaid, M. , Gary‐Bobo, M. , Esclangon, A. , Maffrand, J. P. , Le Fur, G. , Oury‐Donat, F. , & Soubrié, P. (2003). The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Molecular Pharmacology, 63, 908–914. 10.1124/mol.63.4.908 [DOI] [PubMed] [Google Scholar]
- Bermudez‐Silva, F. J. , Cardinal, P. , & Cota, D. (2012). The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. Journal of Psychopharmacology, 26, 114–124. 10.1177/0269881111408458 [DOI] [PubMed] [Google Scholar]
- Bogdan, C. (1998). The multiplex function of nitric oxide in (auto)immunity. The Journal of Experimental Medicine, 187, 1361–1365. 10.1084/jem.187.9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac, A. , Herman, M. , Zingerman, B. , Erman, A. , Rozen‐Zvi, B. , Hirsh, J. , & Gafter, U. (2008). Obesity‐induced glomerular hyperfiltration: Its involvement in the pathogenesis of tubular sodium reabsorption. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association ‐ European Renal Association, 23, 3946–3952. 10.1093/ndt/gfn379 [DOI] [PubMed] [Google Scholar]
- Chalmers, L. , Kaskel, F. J. , & Bamgbola, O. (2006). The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Advances in Chronic Kidney Disease, 13, 352–364. 10.1053/j.ackd.2006.07.010 [DOI] [PubMed] [Google Scholar]
- Chang, P. C. , Chen, T. H. , Chang, C. J. , Hou, C. C. , Chan, P. , & Lee, H. M. (2004). Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK‐dependent pathway. Kidney International, 65, 1664–1675. 10.1111/j.1523-1755.2004.00602.x [DOI] [PubMed] [Google Scholar]
- Chorvat, R. J. (2013). Peripherally restricted CB1 receptor blockers. Bioorganic & Medicinal Chemistry Letters, 23, 4751–4760. 10.1016/j.bmcl.2013.06.066 [DOI] [PubMed] [Google Scholar]
- Chorvat, R. J. , Berbaum, J. , Seriacki, K. , & McElroy, J. F. (2012). JD‐5006 and JD‐5037: Peripherally restricted (PR) cannabinoid‐1 receptor blockers related to SLV‐319 (ibipinabant) as metabolic disorder therapeutics devoid of CNS liabilities. Bioorganic & Medicinal Chemistry Letters, 22, 6173–6180. 10.1016/j.bmcl.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Christopoulou, F. D. , & Kiortsis, D. N. (2011). An overview of the metabolic effects of rimonabant in randomized controlled trials: Potential for other cannabinoid 1 receptor blockers in obesity. Journal of Clinical Pharmacy and Therapeutics, 36, 10–18. 10.1111/j.1365-2710.2010.01164.x [DOI] [PubMed] [Google Scholar]
- Cinar, R. , Gochuico, B. R. , Iyer, M. R. , Jourdan, T. , Yokoyama, T. , Park, J. K. , … Kunos, G. (2017). Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight, 2 10.1172/jci.insight.92281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar, R. , Iyer, M. R. , Liu, Z. , Cao, Z. , Jourdan, T. , Erdelyi, K. , … Kunos, G. (2016). Hybrid inhibitor of peripheral cannabinoid‐1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight, 1 10.1172/jci.insight.87336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia‐Costa, L. , Sousa, T. , Morato, M. , Cosme, D. , Afonso, J. , Areias, J. C. , … Albino‐Teixeira, A. (2016). Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. The British Journal of Nutrition, 116, 805–815. 10.1017/S0007114516002804 [DOI] [PubMed] [Google Scholar]
- Cosenzi, A. , Bernobich, E. , Bonavita, M. , Trevisan, R. , Bellini, G. , & Campanacci, L. (2002). Early effects of diabetes on inducible nitric oxide synthase in the kidney. Acta Diabetologica, 39, 91–96. 10.1007/s005920200019 [DOI] [PubMed] [Google Scholar]
- Dallaire, P. , Bellmann, K. , Laplante, M. , Gelinas, S. , Centeno‐Baez, C. , Penfornis, P. , … Marette, A. (2008). Obese mice lacking inducible nitric oxide synthase are sensitized to the metabolic actions of peroxisome proliferator‐activated receptor‐γ agonism. Diabetes, 57, 1999–2011. 10.2337/db08-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deji, N. , Kume, S. , Araki, S. , Soumura, M. , Sugimoto, T. , Isshiki, K. , … Uzu, T. (2009). Structural and functional changes in the kidneys of high‐fat diet‐induced obese mice. American Journal of Physiology Renal Physiology, 296, F118–F126. 10.1152/ajprenal.00110.2008 [DOI] [PubMed] [Google Scholar]
- Drori, A. , Permyakova, A. , Hadar, R. , Udi, S. , Nemirovski, A. , & Tam, J. (2019). Cannabinoid‐1 receptor regulates mitochondrial dynamics and function in renal proximal tubular cells. Diabetes, Obesity & Metabolism, 21, 146–159. 10.1111/dom.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallan, S. I. , Coresh, J. , Astor, B. C. , Asberg, A. , Powe, N. R. , Romundstad, S. , … Holmen, J. (2006). International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. Journal of the American Society of Nephrology, 17, 2275–2284. 10.1681/ASN.2005121273 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C. Y. , McCulloch, C. E. , Iribarren, C. , Darbinian, J. , & Go, A. S. (2006). Body mass index and risk for end‐stage renal disease. Annals of Internal Medicine, 144, 21–28. 10.7326/0003-4819-144-1-200601030-00006 [DOI] [PubMed] [Google Scholar]
- Jenkin, K. A. , McAinch, A. J. , Zhang, Y. , Kelly, D. J. , & Hryciw, D. H. (2015). Elevated cannabinoid receptor 1 and G protein‐coupled receptor 55 expression in proximal tubule cells and whole kidney exposed to diabetic conditions. Clinical and Experimental Pharmacology & Physiology, 42, 256–262. 10.1111/1440-1681.12355 [DOI] [PubMed] [Google Scholar]
- Jeon, M. J. , Leem, J. , Ko, M. S. , Jang, J. E. , Park, H. S. , Kim, H. S. , … Koh, E. H. (2012). Mitochondrial dysfunction and activation of iNOS are responsible for the palmitate‐induced decrease in adiponectin synthesis in 3T3L1 adipocytes. Experimental & Molecular Medicine, 44, 562–570. 10.3858/emm.2012.44.9.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, T. , Szanda, G. , Rosenberg, A. Z. , Tam, J. , Earley, B. J. , Godlewski, G. , … Kunos, G. (2014). Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proceedings of the National Academy of Sciences of the United States of America, 111, E5420–E5428. 10.1073/pnas.1419901111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir, M. , Iyer, M. S. , Richey, J. M. , Woolcott, O. O. , Asare Bediako, I. , Wu, Q. , … Bergman, R. N. (2015). CB1R antagonist increases hepatic insulin clearance in fat‐fed dogs likely via upregulation of liver adiponectin receptors. American Journal of Physiology. Endocrinology and Metabolism, 309, E747–E758. 10.1152/ajpendo.00196.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H. M. , Ahn, S. H. , Choi, P. , Ko, Y. A. , Han, S. H. , Chinga, F. , … Susztak, K. (2015). Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature Medicine, 21, 37–46. 10.1038/nm.3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & Group NCRRGW (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knani, I. , Earley, B. J. , Udi, S. , Nemirovski, A. , Hadar, R. , Gammal, A. , … Tam, J. (2016). Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader–Willi syndrome. Mol Metab, 5, 1187–1199. 10.1016/j.molmet.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrinaga, G. , Varona, A. , Perez, I. , Sanz, B. , Ugalde, A. , Candenas, M. L. , … López, J. I. (2010). Expression of cannabinoid receptors in human kidney. Histology and Histopathology, 25, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Lecru, L. , Desterke, C. , Grassin‐Delyle, S. , Chatziantoniou, C. , Vandermeersch, S. , Devocelle, A. , … François, H. (2015). Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney International, 88, 72–84. 10.1038/ki.2015.63 [DOI] [PubMed] [Google Scholar]
- Lee, W. , Eom, D. W. , Jung, Y. , Yamabe, N. , Lee, S. , Jeon, Y. , … Kim, S. N. (2012). Dendrobium moniliforme attenuates high‐fat diet‐induced renal damage in mice through the regulation of lipid‐induced oxidative stress. The American Journal of Chinese Medicine, 40, 1217–1228. 10.1142/S0192415X12500905 [DOI] [PubMed] [Google Scholar]
- Lim, J. C. , Lim, S. K. , Park, M. J. , Kim, G. Y. , Han, H. J. , & Park, S. H. (2011). Cannabinoid receptor 1 mediates high glucose‐induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. American Journal of Physiology Renal Physiology, 301, F179–F188. 10.1152/ajprenal.00032.2010 [DOI] [PubMed] [Google Scholar]
- Lim, S. K. , & Park, S. H. (2012). The high glucose‐induced stimulation of B1R and B2R expression via CB1R activation is involved in rat podocyte apoptosis. Life Sciences, 91, 895–906. 10.1016/j.lfs.2012.07.020 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Wang, L. , Harvey‐White, J. , Osei‐Hyiaman, D. , Razdan, R. , Gong, Q. , … Kunos, G. (2006). A biosynthetic pathway for anandamide. Proceedings of the National Academy of Sciences of the United States of America, 103, 13345–13350. 10.1073/pnas.0601832103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Sanchez, L. M. , Corrales, F. J. , Barcos, M. , Espejo, I. , Munoz‐Castaneda, J. R. , & Rodriguez‐Ariza, A. (2010). Inhibition of nitric oxide synthesis during induced cholestasis ameliorates hepatocellular injury by facilitating S‐nitrosothiol homeostasis. Laboratory Investigation, 90, 116–127. 10.1038/labinvest.2009.104 [DOI] [PubMed] [Google Scholar]
- Ma, K. , Cabrero, A. , Saha, P. K. , Kojima, H. , Li, L. , Chang, B. H. , … Chan, L. (2002). Increased β‐oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. The Journal of Biological Chemistry, 277, 34658–34661. 10.1074/jbc.C200362200 [DOI] [PubMed] [Google Scholar]
- Marseglia, L. , Manti, S. , D'Angelo, G. , Nicotera, A. , Parisi, E. , Di Rosa, G. , … Arrigo, T. (2014). Oxidative stress in obesity: A critical component in human diseases. International Journal of Molecular Sciences, 16, 378–400. 10.3390/ijms16010378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Cantarin, M. P. , Keith, S. W. , Waldman, S. A. , & Falkner, B. (2014). Adiponectin receptor and adiponectin signaling in human tissue among patients with end‐stage renal disease. Nephrology, Dialysis, Transplantation, 29, 2268–2277. 10.1093/ndt/gfu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, A. V. , Okada, S. , & Sharma, K. (2011). Obesity related kidney disease. Current Diabetes Reviews, 7, 41–49. 10.2174/157339911794273928 [DOI] [PubMed] [Google Scholar]
- Nam, D. H. , Lee, M. H. , Kim, J. E. , Song, H. K. , Kang, Y. S. , Lee, J. E. , … Cha, D. R. (2012). Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology, 153, 1387–1396. 10.1210/en.2011-1423 [DOI] [PubMed] [Google Scholar]
- Ng, M. , Fleming, T. , Robinson, M. , Thomson, B. , Graetz, N. , Margono, C. , … Gakidou, E. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 384, 766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, K. , Iwatani, H. , Kihara, S. , Nakagawa, Y. , Komura, N. , Fujita, K. , … Funahashi, T. (2007). Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin‐knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1910–1917. 10.1161/ATVBAHA.107.147645 [DOI] [PubMed] [Google Scholar]
- Othman, M. , Kawar, B. , & El Nahas, A. M. (2009). Influence of obesity on progression of non‐diabetic chronic kidney disease: A retrospective cohort study. Nephron. Clinical Practice, 113, c16–c23. 10.1159/000228071 [DOI] [PubMed] [Google Scholar]
- Ozbek, E. , Ilbey, Y. O. , Ozbek, M. , Simsek, A. , Cekmen, M. , & Somay, A. (2009). Melatonin attenuates unilateral ureteral obstruction‐induced renal injury by reducing oxidative stress, iNOS, MAPK, and NF‐κB expression. Journal of Endourology, 23, 1165–1173. 10.1089/end.2009.0035 [DOI] [PubMed] [Google Scholar]
- Perri, A. , Vizza, D. , Lofaro, D. , Gigliotti, P. , Leone, F. , Brunelli, E. , … Bonofiglio, R. (2013). Adiponectin is expressed and secreted by renal tubular epithelial cells. Journal of Nephrology, 26, 1049–1054. 10.5301/jn.5000269 [DOI] [PubMed] [Google Scholar]
- Ruggiero, C. , Ehrenshaft, M. , Cleland, E. , & Stadler, K. (2011). High‐fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. American Journal of Physiology. Endocrinology and Metabolism, 300, E1047–E1058. 10.1152/ajpendo.00666.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski, J. M. , Wang, Z. V. , Park, A. S. , Zhang, J. , Zhang, D. , Hu, M. C. , … Scherer, P. E. (2013). Adiponectin promotes functional recovery after podocyte ablation. Journal of the American Society of Nephrology, 24, 268–282. 10.1681/ASN.2012040414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, K. , Ramachandrarao, S. , Qiu, G. , Usui, H. K. , Zhu, Y. , Dunn, S. R. , … Goldstein, B. J. (2008). Adiponectin regulates albuminuria and podocyte function in mice. The Journal of Clinical Investigation, 118, 1645–1656. 10.1172/JCI32691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyvka, Y. , Malgor, R. , Inman, S. R. , Ding, J. , Heh, V. , & Nowak, F. V. (2016). Antioxidant diet and sex interact to regulate NOS isoform expression and glomerular mesangium proliferation in Zucker diabetic rat kidney. Acta Histochemica, 118, 183–193. 10.1016/j.acthis.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, B. A. , & Cannon, C. P. (2007). Cannabinoid‐1 receptor blockade in cardiometabolic risk reduction: Safety, tolerability, and therapeutic potential. The American Journal of Cardiology, 100, 27P–32P. [DOI] [PubMed] [Google Scholar]
- Tam, J. , Cinar, R. , Liu, J. , Godlewski, G. , Wesley, D. , Jourdan, T. , … Kunos, G. (2012). Peripheral cannabinoid‐1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metabolism, 16, 167–179. 10.1016/j.cmet.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J. , Godlewski, G. , Earley, B. J. , Zhou, L. , Jourdan, T. , Szanda, G. , … Kunos, G. (2014). Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet‐induced obesity. American Journal of Physiology. Endocrinology and Metabolism, 306, E457–E468. 10.1152/ajpendo.00489.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J. , Hinden, L. , Drori, A. , Udi, S. , Azar, S. , & Baraghithy, S. (2018). The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. European Journal of Internal Medicine, 49, 23–29. 10.1016/j.ejim.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Tam, J. , Vemuri, V. K. , Liu, J. , Bátkai, S. , Mukhopadhyay, B. , Godlewski, G. , … Kunos, G. (2010). Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. The Journal of Clinical Investigation, 120, 2953–2966. 10.1172/JCI42551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobar, A. , Ori, Y. , Benchetrit, S. , Milo, G. , Herman‐Edelstein, M. , Zingerman, B. , … Chagnac, A. (2013). Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS ONE, 8, e75547 10.1371/journal.pone.0075547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, K. , Shiraishi, M. , Naito, Y. , Torii, Y. , Nakamura, Y. , & Osawa, T. (1999). Activation of stress signaling pathways by the end product of lipid peroxidation. 4‐Hydroxy‐2‐nonenal is a potential inducer of intracellular peroxide production. The Journal of Biological Chemistry, 274, 2234–2242. 10.1074/jbc.274.4.2234 [DOI] [PubMed] [Google Scholar]
- Udi, S. , Hinden, L. , Earley, B. , Drori, A. , Reuveni, N. , Hadar, R. , … Tam, J. (2017). Proximal tubular cannabinoid‐1 receptor regulates obesity‐induced CKD. J Am Soc Nephrol, 28, 3518–3532. 10.1681/ASN.2016101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veelken, R. , Hilgers, K. F. , Hartner, A. , Haas, A. , Bohmer, K. P. , & Sterzel, R. B. (2000). Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. Journal of the American Society of Nephrology: JASN, 11, 71–79. [DOI] [PubMed] [Google Scholar]
- Wang, X. X. , Edelstein, M. H. , Gafter, U. , Qiu, L. , Luo, Y. , Dobrinskikh, E. , … Levi, M. (2016). G protein‐coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol, 27, 1362–1378. 10.1681/ASN.2014121271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi‐Nejad, K. , & Brenner, B. M. (2005). Strategies to retard the progression of chronic kidney disease. The Medical Clinics of North America, 89, 489–509. 10.1016/j.mcna.2004.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. MRI‐1867 chemical structure and experimental protocol.
Figure S2. MRI‐1867 does not affect ambulatory and voluntary activities in diet‐induced obese mice.
Figure S3. MRI‐1867 reduces CB1 receptor expression in DIO mice.
Table S1. Primer information.
Table S2. Primer information.


