Highlights
-
•
Maladaptation to acute stress constitutes an important vulnerability and maintenance factor for psychiatric disorders.
-
•
We show transdiagnostic and disorder specific aspects of the brain's acute stress response.
-
•
Our results mainly suggest shared, transdiagnostic aspects of a maladaptive stress response.
-
•
Patients show relatively decreased frontoparietal network connectivity under stress.
-
•
Absence of default mode network downregulation marks a maladaptive stress response across disorders.
Keywords: Stress, fMRI, Network, Salience, Default, Frontoparietal
Abstract
Clinically, it is well-established that vulnerability to stress is a common feature across a broad spectrum of psychiatric disorders. However, this link has been mechanistically studied almost exclusively in patients with so-called stress-related disorders such as depression and anxiety. To probe transdiagnostic mechanisms, we set out to study the acute stress response across a broader range of psychiatric disorders taking a large-scale brain network perspective. We investigated the brain's response to a mild, experimentally well-controlled psychological stressor in the form of an aversive movie. We studied 168 patients with stress-related and/or neurodevelopmental disorders (including comorbidity) and 46 control subjects. We focused on three networks that have a central role in the brain's stress response and are affected in a wide range of psychiatric disorders: the salience network (SN), default mode network (DMN) and frontoparietal network (FPN). Our results support an increased vulnerability to stress across all patients, indicated by a higher subjective stress level at baseline and follow-up compared to matched controls. At the brain systems level, the stress response was characterized by a relatively decreased FPN connectivity and an absence of a decrease in the within DMN connectivity across all disorders compared to controls. At the neurocognitive level, these findings may reflect a diminished top-down control and a tendency to more pronounced (negative) self-referential processing. Besides these shared aspects of the maladaptive stress response, we also discuss indications for disorder-specific aspects. Taken together, our results emphasize the importance of investigating the mechanistic underpinnings of psychiatric disorders transdiagnostically as recently done in neurogenetics.
1. Introduction
Impaired coping with stress is a common feature across a broad range of psychiatric disorders (Ingram and Luxton, 2005; Shaw et al., 2014; White et al., 2014). It serves as the starting point for established stress-vulnerability models, that explain psychopathology in terms of stressors that interact with individual vulnerabilities (Ingram and Luxton, 2005). However, how this works mechanistically at the brain level is still unclear. We want to contribute to bridging this knowledge gap by investigating transdiagnostic mechanisms of a maladaptive stress response. In this study we investigate the brain's stress response to an aversive movie, containing extreme male to male violence, from an eye-witness perspective. This reflects real world threatening events, that induce a state of fearful arousal, leading to an emotion focused stress response and coping (Folkman and Lazarus, 1988; Hermans et al., 2011; Qin et al., 2009). By investigating the brain's acute stress response in patients we aim to get more insight into potential maladaptation to acute stress, since this may constitute an important vulnerability and maintenance factor for psychiatric disorders (Ingram and Luxton, 2005).
Functional brain networks have emerged as the fundamental, dynamically organized elements of human brain function (Beckmann et al., 2005; Smith et al., 2009), consisting of patterns of synchronized activity across distributed brain regions (Biswal et al., 1995). Three networks in particular are affected in a broad range of psychiatric disorders, i.e. the salience network (SN), the default mode network (DMN) and the frontoparietal network (FPN) (Menon, 2011). The SN is hypothesized to orient attention towards salient information (Hermans et al., 2014, 2011; Menon, 2011). The DMN is involved in spontaneous and self-referential thought (Buckner et al., 2008), whereas the FPN facilitates execution of higher-order, cognitive demanding tasks (Corbetta and Shulman, 2002). Changes in these networks are found in both stress-related (Mulders et al., 2015) and neurodevelopmental disorders (Kernbach et al., 2018) and are thought to underlie neurocognitive impairments in these disorders. Intriguingly, these same three networks play a central role in the brain's stress response (van Oort et al., 2017).
In health, the stress response comprises dynamic shifts in the three networks that facilitate adaptive coping with the stressor (Hermans et al., 2014; van Oort et al., 2017). Preliminary evidence, based on small samples of psychiatric patients with specific stress-related disorders (e.g. Dedovic et al., 2014; Lanius et al., 2005, 2004; Li et al., 2005), suggests that the three networks are differently affected by stress, which probably affects their ability to adaptively cope with stress. For example, patients with post-traumatic stress disorder and depression showed stress-induced increases in functional connectivity of the SN and increased and decreased activity patterns in specific DMN regions (Lanius et al., 2004, 2002; Ming et al., 2017), while patients with social phobia exhibited decreased activity within the FPN (Koric et al., 2012).
In this study, we set out to investigate the response to a mild psychological stressor from a functional network perspective in a cohort of patients with disorders across two diagnostic groups: stress-related disorders (mood and anxiety disorders) and neurodevelopmental disorders (attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD)). For the sake of this study, we consider the different stress-related disorders as one group, as they share a common underlying dimension (Kotov et al., 2017), with a maladaptive stress response as its central feature (de Kloet et al., 2005; Sharma et al., 2016). At the other end, ASD and ADHD are investigated as one group of neurodevelopmental disorders, since both are lifelong disorders, that start in early childhood and have a relatively stable, trait-like course and shared heritability (Franke et al., 2018; Rommelse et al., 2011). There are clear indications for increased stress sensitivity in neurodevelopmental disorders as well, such as arousal and emotion regulation problems (Kerns et al., 2015; Richey et al., 2015; Shaw et al., 2014). Although the mechanistic underpinnings for the increased stress sensitivity in this group are unclear, the high comorbidity rates with stress-related disorders (from 25% in ADHD to 60% in ASD (Rommelse et al., 2011)) suggests an overlap in underlying mechanisms across disorders.
Our setup allows us to investigate psychopathology at multiple levels. First, we aim to investigate transdiagnostic aspects of the maladaptive stress response by comparing the combined patient group with stress-related and neurodevelopmental disorders (including comorbidity) to controls. Second, we investigate whether there are (dis)similar patterns in broadly defined diagnostic groups by contrasting the controls with the group with stress-related disorders, the group with neurodevelopmental disorders and the comorbidity group separately. Within this setup, we primarily focus on stress-induced connectivity changes within our networks of interest, i.e. the SN, DMN and FPN, and expand this to changes in connectivity patterns of these networks across the entire brain.
2. Methods and materials
2.1. Subjects
Our study is part of the ongoing MIND-Set study (Measuring Integrated Novel Dimensions in Neurodevelopmental and Stress-related Mental Disorders) executed at the Department of Psychiatry of the Radboud University Medical Center and the Donders Institute. MIND-Set includes adult outpatients with a neurodevelopmental (ASD and/or ADHD) and/or stress-related disorder (mood and/or anxiety disorder). A psychiatrically healthy control group is also included in MIND-Set.
Patients were diagnosed and classified by a trained clinician according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) using semi-structured interviews. The Structured Clinical interview for DSM-IV Axis I Disorders (SCID-I) (First et al., 1996) was used to diagnose mood and anxiety disorders. The Diagnostic Interview for ADHD in Adults (Kooij and Francken, 2010; Ramos-Quiroga et al., 2016) and the Dutch Interview for ASD in Adults (Vuijk, 2014) were used to diagnose ADHD and ASD respectively (see also the supplement for a more detailed description of the methods).
2.2. Subject groups
The combined patient group consists of three subgroups:
-
1
stress-related group: patients with a current depressive episode (unipolar or bipolar depression), (hypo)mania, dysthymia and/or anxiety disorder, but no ADHD or ASD.
-
2
neurodevelopmental group: patients with ASD and/or ADHD, but no stress-related disorder.
-
3
comorbidity group: patients with both a stress-related and a neurodevelopmental disorder.
The control group was matched on age, sex and level of education, as these factors might be potential confounders (Dubois et al., 2018; Everaerd et al., 2017; Lighthall et al., 2012).
2.3. Procedure
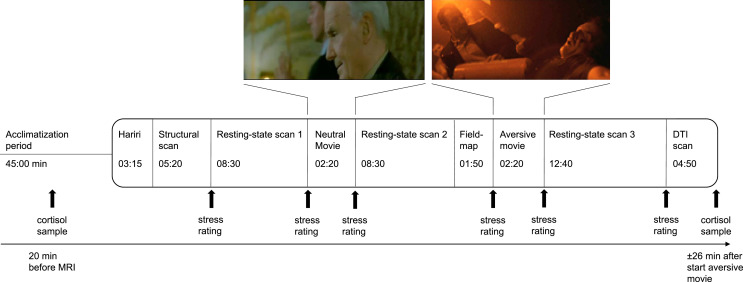
We investigated the brain's response to a mild psychological stressor using an experimentally well-controlled paradigm. Psychological stress was induced by watching an aversive movie clip, after an introductory text had put the subject in an eye-witness perspective (Hermans et al., 2011; Qin et al., 2009). A neutral movie clip served as a control condition (see supplement). The full functional magnetic resonance imaging (fMRI) session consisted of multiple scans (Fig. 1), of which we used the T1 structural scan and the three resting-state scans for the present study.
Fig. 1.
Experimental design. Subjects entered the scanner after a 45 min acclimatization period outside the scanner. The whole protocol consists of a series of scans of which we used the structural scan and the three resting-state scans for our present study. We selected the first 8:30 min of the third resting-state scan in order to match for length with the other resting-state scans. Stress induction took place with a highly aversive movie with a self-referential instruction. The neutral movie served as a control condition. Salivary cortisol sampling took place 20 min before scanning and ±26 min after the start of the aversive movie. Subjective stress levels were assessed at several moments during scanning with an 11-point rating scale. Heart rate was measured continuously during scanning. Other scans of our protocol that we do not use for our present study are the Hariri (an emotional face matching task), the fieldmap and a diffusion tensor imaging (DTI) scan.
The mean heart rate (in beats per minute) was measured continuously during scanning in order to assess autonomic responses to the psychological stressor. Subjective stress was assessed at six time-points, using an eleven-point rating scale (0 = no stress, 10 = maximal stress). For both these measures the effects of our intervention were assessed using a mixed model repeated measures ANOVA (alpha = 0.05). To evaluate the cortisol response, two saliva samples were collected: one 20 min before scanning and one approximately 26 min after the onset of the aversive movie. A Wilcoxon signed-rank test (non-normal distribution) was performed to test for cortisol changes (alpha = 0.05).
2.4. fMRI data acquisition
All images were collected using a 3T Siemens Magnetom Prisma MRI scanner (Erlangen, Germany) with a 32-channel head coil. T2*-weighted EPI BOLD-fMRI images were acquired for the resting-state scans, using a multi-band 6 protocol with an interleaved slice acquisition sequence (number of slices = 66, TR = 1000 ms, TE = 34 ms, flip angle = 60°, voxel size = 2.0 × 2.0 × 2.0 mm, slice gap = 0 mm, FOV = 210 mm). High-resolution structural images (1.0 × 1.0 × 1.0 mm) were acquired using a T1-weighted MP-RAGE sequence (TE/TR = 3.03/2300 ms, flip angle = 8°, FOV = 256 × 256 × 192 mm, GRAPPA acceleration factor 2).
2.5. fMRI preprocessing
Preprocessing and statistical analyses were performed on the three resting-state scans (each 500 vol) using FSL 5.0.11 (FMRIB, Oxford, UK). These scans were preprocessed using the FMRI Expert Analysis Tool (FEAT), which is part of the FMRIB Software Library (FSL) (Jenkinson et al., 2012). To allow for T2* equilibration effects, the first five images of each resting-state scan were discarded. Furthermore, the preprocessing steps included brain extraction, motion correction, bias field correction, high-pass temporal filtering with a cut-off of 100 s, spatial smoothing with a 4 mm full width at half maximum (FWHM) Gaussian kernel, registration of functional images to high-resolution T1 using boundary-based registration and nonlinear registration to standard space (MNI152). The final voxel size for group analysis was 2 mm isotropic. We used ICA-based Advanced Removal of Motion Artefacts (ICA-AROMA) for further single-subject denoising (Pruim et al., 2015). Subjects were excluded from analyses if motion resulted in more than 2 mm sudden relative mean displacement or translation. The preprocessed MRI data is available on request in line with the institutional ethics guidelines.
2.6. fMRI analyses
Differences in stress-induced connectivity changes were investigated between the combined patient group and controls and between the controls and the separate patient subgroups (i.e. the stress-related, neurodevelopmental and comorbidity group). We also investigated the effects of stress within the different groups by comparing the stress (resting-state 3) with the neutral condition (resting-state 2). Connectivity changes for our networks of interest (i.e. SN, DMN, right FPN and left FPN) were addressed at two levels: 1) within network connectivity and 2) whole brain connectivity. As a supplemental analysis we also compared the comorbidity group directly with the stress related group and with the neurodevelopmental group in order to investigate if the larger variety of symptoms in the comorbidity group leads to differences in the stress response at the brain level (see supplemental results and discussion).
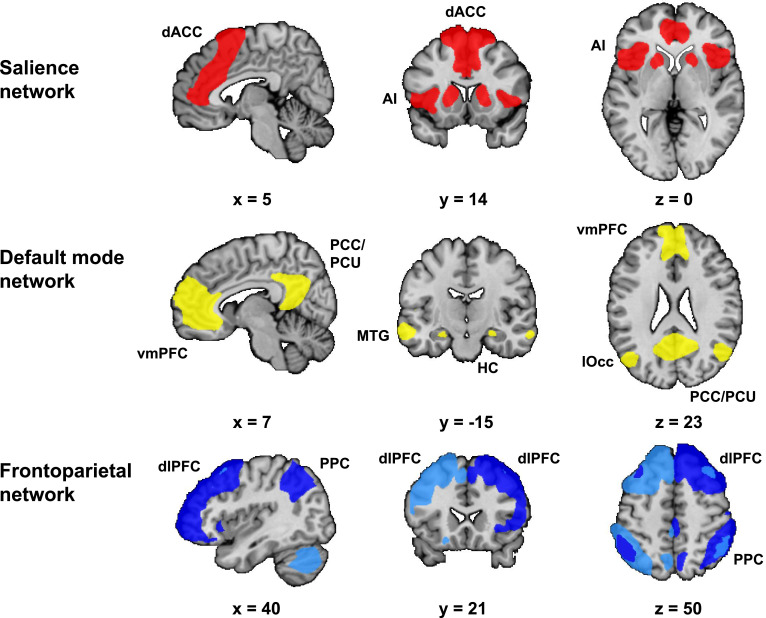
We identified our networks of interest (see supplement) in the first resting-state scan using group independent component analysis (ICA) (Beckmann et al., 2005). This allowed us to select these networks without biasing the results towards either the neutral or stress condition (Fig. 2). Next, we used these spatial network maps as masks to extract the mean within network connectivity strength in both resting-state scan 2 (neutral condition) and resting-state scan 3 (stress condition). This approach results in one value per subject and network, which represents an aggregate measure of mean within network connectivity. The effect of our intervention was investigated with a paired comparison between the connectivity strength in resting-state scan 2 and resting-state scan 3, using a Wilcoxon signed-rank test (non-normal distribution). The differences between the controls and different patient groups were calculated using the Mann-Whitney U test (non-normal distribution) on the difference scores (stress - neutral) (alpha = 0.05).
Fig. 2.
Networks of interest. Networks of interest identified with group independent component analysis (ICA) in resting-state 1 scan, i.e. the salience network (SN), default mode network (DMN) and frontoparietal network (FPN). These network maps are group maps that combine the individual template maps of all subjects. We identified two FPNs: the left FPN (light blue) and right FPN (dark blue). The selected networks included all areas that are typically considered as core regions in these networks. The SN included the bilateral anterior insula (AI) and dorsal anterior cingulate cortex (dACC). The DMN included the ventromedial prefrontal cortex (vmPFC), hippocampus (HC), posterior cingulate cortex (PCC) and precuneus (PCU). Additionally, the DMN included the middle temporal gyrus (MTG) and lateral occipital cortex (lOcc). Both FPNs included the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC).
Dual regression was used to investigate the whole brain connectivity changes of our networks of interest. The spatial maps resulting from dual regression were subtracted (resting-state 3 minus resting-state 2) to investigate the effects of stress induction in the different subject groups and the differences between patients and controls. Inference testing was implemented using permutation tests via randomise (10,000 permutations) (Winkler et al., 2014). The results from these tests were considered significant using a threshold-free cluster enhancement corrected p-value of 0.05 (Smith and Nichols, 2009) and a minimum cluster size of 5 voxels.
Finally, we performed exploratory correlational analyses in the different groups between stress-induced changes of connectivity within the networks and changes in the behavioral measures (subjective stress, heart rate and cortisol) (Spearman's correlation, alpha = 0.05). We investigated if the correlation coefficients differed between controls and the different patient groups, by comparing the standardized correlation coefficients (Fisher's r to z transform), using an ANOVA for summary data (alpha = 0.05).
3. Results
3.1. Study population
Of the 192 patients that participated in the fMRI study, 168 were included in the present analyses (stress-related group (n = 63), neurodevelopmental group (n = 52) and comorbidity group (n = 53)) (see supplement) (Table 1). In the combined patient group, the median age (range) was 34 (18–74) years and 58.9% was male. We selected 46 control subjects from the MIND-Set database, that were matched with the combined patient group on age (U = 3697, p = 0.654), sex (χ2(1) = 1.18, p = 0.278) and level of education (χ2(3) = 3.15, p = 0.369). When the controls were compared to the three patient subgroups, the only difference found was a lower level of education in the comorbidity group (χ2(3) = 8.14, p = 0.043).
Table 1.
Demographic and clinical characteristics.
| Controls (n = 46) | Combined patient group (n = 168) | Comparison between combined patient group and controls: statistics (χ2/t/U), p-value | SR (n = 63) | Comparison between controls and SR: statistics (χ2/t/U), p-value | ND (n = 52) | Comparison between controls and ND: statistics (χ2/t/U), p-value | CM (n = 53) | Comparison between controls and CM: statistics (χ2/t/U), p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Age (years), median (range) | 33 (20–75) | 34 (18–74) | U = 3697, p = 0.654 | 40 (19–73) | U = 1221, p = 0.162 | 33 (19–74) | U = 1152, p = 0.754 | 31 (18–64) | U = 1202, p = 0.905 |
| Sex,%male (M/F) | 50% (23/23) | 58.9% (99/69) | χ2 = 1.18, p = 0.278 | 54% (34/29) | χ2 = 0.17, p = 0.682 | 63.5% (33/19) | χ2 = 1.81, p = 0.179 | 60.4% (32/21) | χ2 = 1.07, p = 0.300 |
| Level of education (4 Levels) | |||||||||
| No (n = ..) | 0 | 6 | χ2 = 3.15, p = 0.369 | 2 | χ2 = 10.21, p = 0.147 | 2 | χ2 = 2.81, p = 0.421 | 2 | χ2 = 8.14, p = 0.043* |
| Low (n = ..) | 3 | 19 | 9 | 5 | 5 | ||||
| Middle (n = ..) | 22 | 80 | 19 | 26 | 35 | ||||
| High (n = ..) | 21 | 62 | 33 | 18 | 11 | ||||
| Time of day of cortisol sampling | |||||||||
| Cortisol sample 1, median (range) | 14:48 (11:37 – 19:46) | 14:55 (11:41 – 18:51) | U = 3450, p = 0.626 | 14:47 (11:41 – 18:09) | U = 1291, p = 0.808 | 14:59 (12:18 – 18:51) | U = 934, p = 0.155 | 14:49 (11:55 – 17:20) | U = 1152, p = 0.894 |
| Cortisol sample 2, median (range) | 16:48 (13:30 – 21:55) | 16:53 (13:45 – 20:41) | U = 3608, p = 0.967 | 16:57 (13:45 – 20:20) | U = 1273, p = 0.721 | 16:54 (14:20 – 20:41) | U = 1012, p = 0.398 | 16:44 (13:48 – 19:21) | U = 1126, p = 0.748 |
| Current diagnosis | |||||||||
| Stress-related disorders | |||||||||
| Depressive episode (n = ..) | – | 77 | 50 | – | 27 | ||||
| Dysthymia (n = ..) | – | 20 | 10 | – | 10 | ||||
| Panic disorder (n = ..) | – | 13 | 8 | – | 5 | ||||
| Agoraphobia (n = ..) | – | 1 | 1 | – | 0 | ||||
| Social phobia (n = ..) | – | 18 | 9 | – | 9 | ||||
| Specific phobia (n = ..) | – | 5 | 2 | – | 3 | ||||
| OCD (n = ..) | – | 8 | 2 | – | 6 | ||||
| PTSD (n = ..) | – | 4 | 2 | – | 2 | ||||
| GAD (n = ..) | – | 10 | 6 | – | 4 | ||||
| Anxiety disorder NOS (n = ..) | – | 6 | 1 | – | 5 | ||||
| (hypo)mania (n = ..) | – | 2 | 1 | – | 1 | ||||
| Neurodevelopmental disorders | |||||||||
| ADHD (n = ..) | – | 67 | – | 36 | 31 | ||||
| ASD (n = ..) | – | 57 | – | 24 | 33 | ||||
| Current medication use + | |||||||||
| Antidepressant (n = ..) | 0 | 57 | 28 | 9 | 20 | ||||
| Mood stabilizer (n = ..) | 0 | 4 | 3 | 0 | 1 | ||||
| Antipsychotic (n = ..) | 0 | 16 | 11 | 1 | 4 | ||||
| Stimulantium (n = ..) | 0 | 21 | 3 | 9 | 9 | ||||
| Benzodiazepines (daily use) (n = ..) | 0 | 16 | 10 | 2 | 4 | ||||
| Other psychopharmaca (n = …) ‡ | 0 | 4 | 2 | 1 | 1 | ||||
Abbreviations: CM: comorbidity group, F: female, M: male, ND: neurodevelopmental group, SR: stress-related group.
χ2:: Pearson's Chi square (2-tailed), t = independent t-test (2-tailed), U: Mann-Whitney-U test (2-tailed).
p<0.05, + number of subjects using the type of medication stated below, ‡ other psychopharmaca include: melatonin and pregabalin.
3.2. Behavioral and physiological measurements
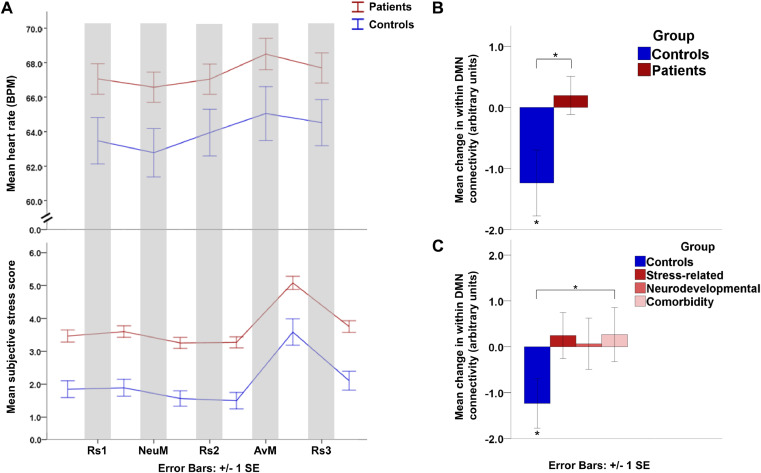
Heart rate: the analysis of the controls and combined patient group together showed a main effect of time (F(2.6, 516.5) = 20.28, p < 0.001). To resolve this time-effect, post-hoc tests showed that compared to baseline the heart rate decreased during the neutral movie (p = 0.003) and increased during the aversive movie (p < 0.001) and third resting-state scan (p < 0.001) (Fig. 3). Note however the absence of a significant main effect of group (F(1, 201) = 2.45, p = 0.119) or a significant group by time interaction (F(2.6, 516.5) = 0.97, p = 0.400). Similar patterns were observed for the patient subgroups (see supplement).
Fig. 3.
Behavioral and physiological stress measures. A. Longitudinal course of mean heart rate (in beats per minute) and subjective stress (on an 11-point rating scale: 0 = no stress, 10 = maximal stress) in the controls and combined patient group. B. Mean change in within default mode network (DMN) functional connectivity (stress – neutral condition) in control group and combined patient group. The control group showed a significant decrease in within DMN connectivity in the stress compared to the neutral condition. This decrease in within network connectivity in the control group differed significantly from the combined patient group. C. Mean change in within DMN functional connectivity (stress – neutral condition) in control group and patient subgroups. The difference in the stress induced change in DMN connectivity was most pronounced between the controls and comorbidity group. Abbreviations: AvM: aversive movie, BPM: beats per minute, DMN: default mode network, NeuM: neutral movie, Rs: resting-state scan, SE: standard error, * p<0.05.
Subjective stress: analysis of the control and combined patient group together showed a main effect of group (F(1, 212) = 24.71, p < 0.001) and time (F(3.3, 709.4) = 63.39, p < 0.001), but no significant interaction effect (F(3.3, 709.4) = 0.27, p = 0.867). Post-hoc tests showed that, compared to baseline, subjective stress decreased after the neutral movie (p = 0.013) and before the aversive movie (p = 0.021), and increased after the aversive movie (p < 0.001) and third resting-state scan (p = 0.047). This reflects a higher baseline stress level in patients than in controls and an increase after the aversive movie in the two groups together. Similar patterns were observed for the patient subgroups.
Cortisol: both the control (T = 186, p < 0.001) and combined patient group (T = 2609.5, p < 0.001) showed a decrease in cortisol levels. There was no significant difference between the decreases in these two groups (U = 3525, p = 0.783). A post-hoc test showed there was no significant difference for the baseline cortisol levels (U = 3478, p = 0.683), nor were there differences for the time of day that the cortisol sampling took place (cortisol sample 1: U = 3450, p = 0.626, sample 2: U = 3608, p = 0.967) (see Table 1). Again, similar patterns were observed in the three patient subgroups. These results suggest that the effects of diurnal fluctuations in cortisol levels and anticipation anxiety before scanning were stronger than the influence of our mild psychological stressor (in line with Everaerd et al., 2017).
3.3. Functional MRI
The fMRI results are ordered by network of interest. First, the influence of stress induction within the separate groups will be described for the within network connectivity changes. Followed by the differences between controls and patients. Next, the whole brain results will be reported, following the same structure.
3.3.1. Salience network
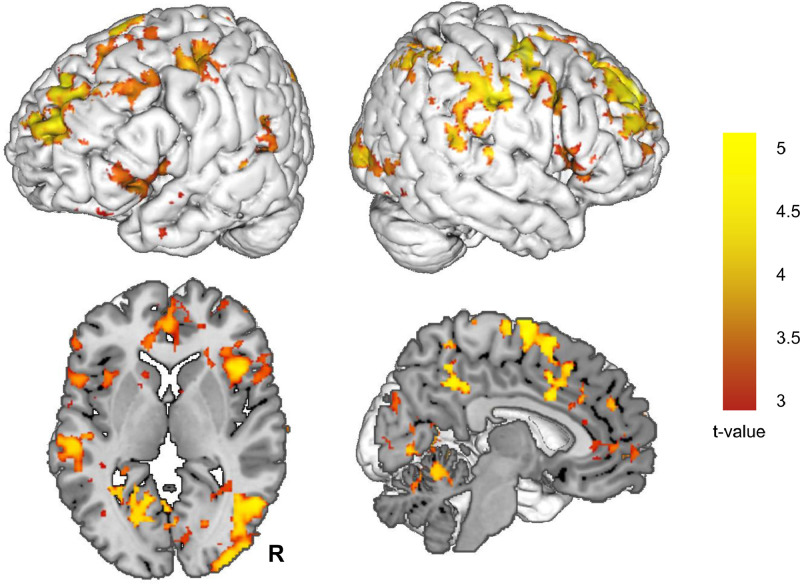
While we did find a stress-induced increase in within SN connectivity in the combined patient group (T = 9313, p = 0.000), we did not find this effect in the control group. The increase in within SN connectivity in patients was most pronounced in the neurodevelopmental group (T = 1004, p = 0.004) and less pronounced in the stress-related (T = 1264, p = 0.080) and comorbidity group (T = 853, p = 0.224). The changes in within SN connectivity did not significantly differ between patients and controls. The whole brain connectivity analysis did not show a significant effect of stress induction in the control group. However, the combined patient group did show a stress-induced increase of SN connectivity with widespread areas across the brain (including core regions of the DMN and FPN) (Fig. 4, Table S1). The neurodevelopmental group showed a similar pattern of a stress induced increase of SN connectivity with widespread areas across the brain (Figure S1, Table S2). The comparison between controls and patients revealed only one difference, with the neurodevelopmental group showing a relatively increased connectivity of the SN with the lateral occipital cortex (Fig. 5, Table 2).
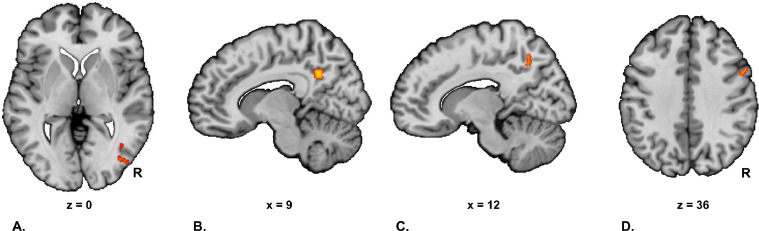
Fig. 4.
Stress induced changes in salience network connectivity in the combined patient group. This figure displays the stress induced change (stress – neutral condition) in salience network functional connectivity in the combined patient group. Abbreviations: R: right.
Fig. 5.
Whole brain analyses of networks of interest. These figures display differences in stress induced changes (stress – neutral contrast) between patients and controls. A. There is an increased functional connectivity of the salience network (SN) with the lateral occipital cortex in the neurodevelopmental group compared to the control group, B. Increased default mode network (DMN) connectivity with precuneus cluster (within DMN) in the stress-related group compared to the control group, C. Increased FC of the right frontoparietal network (FPN) with the precuneus in the control group compared to the combined patient group. D. Increased connectivity of the Left FPN with the right middle frontal gyrus in the control group compared to the comorbidity group. Abbreviation: R = right.
Table 2.
Differences in stress-induced functional connectivity changes between controls and patients for the whole brain analyses.
| Brain area | Hemisphere | Peak voxels (MNI coordinates) | Cluster size (# of voxels) | Significance level (p = ..) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Increased FC of the SN in the ND group (stress – neutral) compared to the control group (stress – neutral) with: | ||||||
| Lateral Occipital Cortex, inferior division | Ri | 40 | −76 | 0 | 8 | 0.045* |
| Increased FC of the DMN in the SR group (stress – neutral) compared to the control group (stress – neutral) with: | ||||||
| PCC/Precuneus | Ri | 10 | −54 | 32 | 47 | 0.018* |
| Increased FC of the Ri FPN in the control group (stress – neutral) compared to the combined patient group (stress – neutral) with: | ||||||
| Precuneus | Ri | 12 | −60 | 48 | 5 | 0.046* |
| Increased FC of the Le FPN in the control group (stress – neutral) compared to the CM group (stress – neutral) with: | ||||||
| Middle Frontal Gyrus | Ri | 56 | 14 | 40 | 14 | 0.033* |
Abbreviations: CM: comorbidity, DMN: default mode network, FC: functional connectivity, FPN: frontoparietal network, Le: left, MNI: Montreal Neurological Institute, ND: neurodevelopmental, PCC: posterior cingulate cortex, Ri: right, SN: salience network, SR: stress-related.
p < 0.05.
3.3.2. Default mode network
While the control group showed a stress-induced decrease in the within DMN connectivity (T = 350, p = 0.037), this effect was not found in patients (T = 7431, p = 0.598). Comparison between controls and patients showed that this decrease in within network connectivity in the control group differed significantly from the combined patient group (U = 3047, p = 0.028). This effect was most pronounced in the comorbidity group (U = 927, p = 0.041) and less pronounced in the stress-related (U = 1159, p = 0.075) and neurodevelopmental group (U = 961, p = 0.094) (Fig. 3).
The whole brain analysis showed a relatively higher stress-induced connectivity of the PCC/precuneus with the rest of the DMN in the stress-related group compared to controls (Fig. 5, Table 2).
3.3.3. Right and left frontoparietal network
While there were no significant results for the within network connectivity analyses for the right and left FPN, the whole brain analysis did show multiple significant results. The control group showed a stress-induced increase in connectivity of the left FPN with the PCC/precuneus (within DMN) (Table 3). Moreover, compared to the combined patient group the controls showed a relatively higher connectivity of the right FPN with the precuneus under stress. Additionally, compared to the comorbidity group the controls showed higher connectivity of the left FPN with the right middle frontal gyrus (Fig. 3, Table 2).
Table 3.
Stress-induced changes (stress – neutral) of the left frontoparietal network in the whole brain functional connectivity analyses in the control group.
| Brain area | Hemisphere | Peak voxels (MNI coordinates) | Cluster size (# of voxels) | Significance level (p = ..) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Stress induction (stress – neutral) in the control group resulted in increased FC of the Le FPN with: | ||||||
| PCC/precuneus | Le | −10 | −46 | 32 | 16 | 0.004⁎⁎ |
Abbreviations: FC: functional connectivity, FPN: frontoparietal network, Le: left, MNI: Montreal Neurological Institute, PCC: posterior cingulate cortex.
p < 0.01.
3.3.4. Correlations between stress-induced changes in connectivity and behavioral stress measures
See Table 4 for the results of the correlational analyses (all outcomes with a p < 0.100 are displayed). Importantly, the stress-induced change in within SN connectivity was positively correlated with the change in subjective stress in the combined patient group (Spearman's r = 0.179, p = 0.020) and comorbidity group (Spearman's r = 0.364, p = 0.007). This correlation was not significant in the control group. While this correlation differed between the control and comorbidity group (controls: zr (±SEzr) = −0.113 (±0.15), comorbidity group: zr (±SEzr) = 0.382 (±0.14), p = 0.020), it did not significantly differ between controls and the combined patient group (controls: zr (±SEzr) = −0.113 (±0.15), combined patient group: zr (±SEzr) = 0.181 (±0.08), p = 0.084).
Table 4.
Correlational analyses for relation between stress-induced changes in stress measures and within network functional connectivity.
| Correlations between Δ-within network FC and Δ-stress measures (Spearman's rho) | ||||
|---|---|---|---|---|
| Δ-subjective stress | Δ-heart rate | Δ-cortisol | ||
| Δ-within SN FC | rs, p-value | Pt: rs = 0.179, p = 0.020* | ||
| CM: rs = 0.364, p = 0.007⁎⁎ | Co: rs = −0.269, p = 0.074 | |||
| Δ-within DMN FC | rs, p-value | CM: rs = −0.342, p = 0.012* | ||
| Δ-within Le PFN FC | rs, p-value | Co: rs = −0.270, p = 0.080 | SR: rs = 0.323, p = 0.013* | |
| Δ-within Ri FPN FC | rs, p-value | Co: rs = 0.302, p = 0.049* | ||
| Differences in standardized correlation coefficients between controls and patients | ||||
| Δ-subjective stress | Δ-heart rate | Δ-cortisol | ||
| Δ-within SN FC | zr, p-value | Co versus Pt. Co: zr = −0.113, Pt: zr = 0.181, p = 0.084 | ||
| Co versus CM. Co: zr = −0.113, CM: zr = 0.382, p = 0.020* | Co versus SR. Co: zr = −0.276, SR: zr = 0.143, p = 0.043* | |||
| Co versus ND. Co: zr = −0.276, ND: zr = 0.083, p = 0.094 | ||||
| Δ-within DMN FC | zr, p-value | |||
| Δ-within Le PFN FC | zr, p-value | Co versus SR. Co: zr = −0.076, SR: zr = 0.335, p = 0.046* | ||
| Δ-within Ri FPN FC | zr, p-value | Co versus Pt. Co: zr = 0.312, Pt: zr = −0.068, p = 0.031* | ||
| Co versus SR. Co: zr = 0.312, SR: zr = −0.101, p = 0.046* | ||||
| Co versus CM. CO: zr = 0.312, CM: zr = −0.056, p = 0.088 | ||||
Abbreviations: Δ: stress induced change (stress - neutral), CM: comorbidity group, Co: control group, FC: functional connectivity, ND: neurodevelopmental group, Pt: combined patient group, rs: Spearman's correlation coefficient, SR: stress-related group. zr: standardized correlation coefficient.
p < 0.05.
p < 0.01.
4. Discussion
In this study we investigated common and disorder-specific connectivity changes in response to a mild psychological stressor on a large-scale network level across stress-related disorders, neurodevelopmental disorders and a comorbid group. In contrast with controls, all three patient groups failed to show a decrease in connectivity of the default mode network (DMN) in response to stress, while there was a relatively decreased connectivity of the frontoparietal network (FPN).
Our study shows that the DMN is differently affected by stress in all patients, which points to a maladaptive feature of their stress response. In the healthy controls, the mild experimental stressor induced a decrease in within DMN connectivity. This result nicely fits with a recent study that identified a decrease in within DMN connectivity as a neural marker of stress responsiveness in health (Zhang et al., 2019). Such a response may serve an adaptive allocation of attentional resources to the external world, since DMN regions consistently deactivate when outward attention is required (Raichle et al., 2001). This response is in line with the theory that stress leads to a dynamic reallocation of resources between brain networks in order to support a hypervigilant state, promoting threat detection and survival (Hermans et al., 2014).
A decrease in DMN connectivity in response to stress was markedly absent in the combined patient group, with most pronounced differences between controls and the comorbidity group. The absence of this response in patients may reflect an impaired ability to suppress attention to internal emotional states when faced with stress, interfering with the adaptive allocation of resources to the external world to eventually support allostatic regulation (Kaiser et al., 2015; Sheline et al., 2009). This same mechanism seems to underlie the failure to downregulate DMN activity in depression during emotion regulation (Sheline et al., 2009). The new and unique contributing factor from our study is that an absence of DMN downregulation indicates a transdiagnostic mechanism of a maladaptive stress response, which may serve as a biomarker of increased stress-vulnerability.
Previous studies have implicated the FPN in emotion regulation and executive functioning (Buhle et al., 2014; Kohn et al., 2014) and have shown an inverted u-shaped relationship between stress and FPN performance (Arnsten, 2009; van Oort et al., 2017). Impairments in this network are implicated in emotion regulation problems in psychiatric disorders, which may worsen under the influence of stress (Park et al., 2019). Our results of a relatively decreased FPN connectivity under stress in patients compared to controls, indicates a worse top-down regulation of stress in patients independent of the type of disorder. The differences in DMN and FPN connectivity may reflect distinct mechanisms of a maladaptive stress response, but could also be related to each other, since an anti-correlation is presumed to underlie the dynamics of these networks (Fox et al., 2005). Moreover, relative dominance of the DMN over the FPN has been implicated in maladaptive rumination in depression (Hamilton et al., 2011). Our results, with an absence of a decrease in within DMN connectivity and a relatively lower FPN connectivity in psychopathology, also point to an imbalance between these networks with a relative dominance of the DMN under conditions of stress.
The present study elaborates on earlier findings of the central role of the salience network (SN) in the acute stress response in health (Hermans et al., 2014, 2011), by now showing its role in psychopathology. The central role of the SN in patients is highlighted by an increase in connectivity within the SN, which correlated with changes in subjectively experienced stress. These connectivity changes within the network were accompanied by extensive changes in whole brain connectivity, pointing to its central role in allocating resources under stress. Interestingly, while we did not find this effect in the controls, there were almost no differences for the SN between patients and controls. This suggests a similar stress-induced pattern in both groups, which reaches significance in patients only. This may be related to the higher stress levels in patients in response to our mild psychological stressor.
Our results mainly suggest shared, transdiagnostic aspects of a maladaptive stress response, but also support some specific aspects in the distinct patient subgroups. These specific aspects are revealed by the differences between the comorbidity and neurodevelopmental group in the supplementary analysis and by a different contribution of the networks in the different subgroups. A maladaptive response of the DMN to stress seems to be most strongly present in subject groups that include patients with stress-related disorders. This is also reflected in the relatively increased connectivity of the posterior DMN with the rest of the DMN in the stress-related group specifically. This may be related to negative self-referential processing (Ming et al., 2017), which is common in this group. The involvement of both the DMN and FPN in the comorbidity group may result from these patients being affected in a larger variety of (symptom) domains.
The main strength of our study is that this is the first project using a well-investigated fMRI stress induction procedure to demonstrate stress-related changes at a large-scale network level across psychiatric disorders. However, our study has to be interpreted in the light of some limitations. As we had the chance to investigate the patients only once, the stress condition always followed the neutral condition, so that an order effect cannot be excluded. However, the main aim of this study is to investigate the difference between controls and patients and the order in both groups is the same. Furthermore, we did not find a significant increase in all our stress measures. The decrease in cortisol probably resulted from a stronger diurnal fluctuation in cortisol levels than the influence of our mild psychological stressor. However, this is a well investigated paradigm, which has shown effective induction of mild stress in several studies (e.g. Everaerd et al., 2017; Hermans et al., 2011; van Marle et al., 2010). In addition, the networks we identified in the first resting-state scan with ICA are a mixture of patients and controls. Because of the larger size of the patient group, there may be a potential bias in the network maps towards the patients. However, we think that every way of identifying the networks has its specific limitations. Using independent network templates from the literature has the major disadvantage that these maps are based on specific populations, especially healthy subjects (Shirer et al., 2012; Smith et al., 2009), which potentially leads to bias to our healthy control group. Since our study is the first study investigating the stress response transdiagnostically in a relatively large group of patients, we think that it is important that our network maps reflect the networks in the patients adequately and thus are generated using a control scan in our large heterogeneous group. Finally, since the stress response is a continuous process, it is not possible to make a strict distinction between the acute stress and recovery phase, and our results possibly reflect a mixture of both.
Taken together, our results emphasize the importance of our networks of interest (i.e. SN, DMN and FPN) in the stress response. General and specific aspects of the maladaptive stress response across stress-related and neurodevelopmental disorders can be characterized by describing aberrant responses of these networks. The specific aspects may be related to distinct vulnerabilities in the different patient groups. The shared aspects may on the one hand result from distinct vulnerabilities across disorders, which lead to the same maladaptive response, as a final common pathway. On the other hand, shared patterns may also arise from transdiagnostic vulnerabilities and symptom dimensions that are present across a broad spectrum of disorders. This is in line with the idea that the current clinical diagnostic groups reflect heterogeneous, partly overlapping categories that do not reflect distinct underlying pathophysiological processes. The deeply interconnected nature of psychiatric disorders is reflected by the high degree of genetic correlation among psychiatric disorders (Brainstorm Consortium, 2018) and a general psychopathology factor that represents the liability shared by all mental disorders (Kotov et al., 2017). We conclude that vulnerability to stress is an important transdiagnostic feature across stress-related and neurodevelopmental disorders. Individual vulnerabilities should be an important focus in daily clinical practice, also in disorders that are traditionally not regarded as stress-related disorders.
Our results provide initial insight in transdiagnostic core mechanisms of a maladaptive stress response, which could support the development of a neural circuit taxonomy of the stress response (Williams, 2016). Further research in larger samples should try to disentangle individual differences in the stress response by characterizing the (dynamic) interplay between the SN, DMN and FPN and relate this to symptom dimensions. Individual characterization of maladaptive features of the stress response may open up opportunities for personalized treatment in the future, as it has for example been shown that DMN hyperconnectivity together with FPN hypoconnectivity predicts the response to transcranial magnetic stimulation (Dichter et al., 2014). Neural-circuit guided personalized treatments could in this way lead to better therapies that cut across traditional diagnostic boundaries (Williams, 2016).
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors and the authors declare to have no conflicts of interest related to this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102176.
Appendix. Supplementary materials
References
- Arnsten A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brainstorm Consortium T. Analysis of shared heritability in common disorders of the brain. Science (80-.) 2018;360 doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The Brain's default network anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Duchesne A., Engert V., Lue S.D., Andrews J., Efanov S.I., Beaudry T., Pruessner J.C. Psychological, endocrine and neural responses to social evaluation in subclinical depression. Soc. Cogn. Affect. Neurosci. 2014;9:1632–1644. doi: 10.1093/scan/nst151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2014;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Galdi P., Paul L.K., Adolphs R. A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Philos. Trans. R. Soc. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2017.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., Voshaar R.O., Fernández G., Tendolkar I. Acute stress enhances emotional face processing in the aging brain. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2017;2:591–598. doi: 10.1016/j.bpsc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- First, M.B., Spitzer, R.L., Gibbon, M., Williams, J.B.W., 1996. Structured clinical interview for DSM-IV axis I disorders, CLINICIAN version (SCID-CV). Washington, DC.
- Folkman S., Lazarus R.S. The relationship between coping and emotion: implications for theory and research. Soc. Sci. Med. 1988;26:309–317. doi: 10.1016/0277-9536(88)90395-4. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B., Michelini G., Asherson P., Banaschewski T., Bilbow A., Buitelaar J.K., Cormand B., Faraone S.V., Ginsberg Y., Haavik J., Kuntsi J., Larsson H., Lesch K., Ramos-quiroga J.A., Réthelyi J.M., Ribases M., Reif A. Live fast, die young ? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018;28:1059–1088. doi: 10.1016/j.euroneuro.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M.J., a G., Joëls M., Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Marle H.J.F., Van, Ossewaarde L., Henckens M.J.A.G., Qin S., Kesteren M.T.R., Van, Schoots V.C., Cousijn H., Rijpkema M., Oostenveld R., Fernández G., van Marle H.J.F., Ossewaarde L., Henckens M.J.A.G., Qin S., van Kesteren M.T.R., Schoots V.C., Cousijn H., Rijpkema M., Oostenveld R., Fernández G., Marle H.J.F., Van, Ossewaarde L., Henckens M.J.A.G., Qin S., Kesteren M.T.R., Van, Schoots V.C., Cousijn H., Rijpkema M., Oostenveld R., Fernández G. Stress-Related noradrenergic activity prompts large-scale neural network reconfiguration. Science (80-.) 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Ingram R.E., Luxton D.D. Chapter: Vulnerability-Stress Models. Sage; CA: 2005. Dvelopment of psychopathology: a vulnerability-stress perspective. [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-hanna J.R., Wager T.D., Pizzagalli D.A., Hospital M. Large-scale network dysfunction in major depressive disorder: meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbach, J.M., Satterthwaite, T.D., Bassett, D.S., Smallwood, J., Margulies, D., Krall, S., Shaw, P., Varoquaux, G., Thirion, B., Konrad, K., Bzdok, D., 2018. Shared endo-phenotypes of default mode dysfunction in attention deficit / hyperactivity disorder and autism spectrum disorder. Transl. Psychiatry. 10.1038/s41398-018-0179-6. [DOI] [PMC free article] [PubMed]
- Kerns C.M., Newschaffer C.J., Berkowitz S.J. Traumatic childhood events and autism spectrum disorder. J. Autism Dev. Disord. 2015;45:3475–3486. doi: 10.1007/s10803-015-2392-y. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation - An Ale meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij J.J., Francken M.H. DIVA Foundation, The Hague; The Netherlands: 2010. Diagnostic Interview for ADHD in Adults Version 2.0 (Dutch: Diagnostisch Interview Voor ADHD Bij Volwassenen Versie 2.0 (DIVA 2.0)) [Google Scholar]
- Koric L., Volle E., Seassau M., Bernard F.A., Mancini J., Dubois B., Pelissolo A., Levy R. How cognitive performance-induced stress can influence right vlpfc activation: an fMRI study in healthy subjects and in patients with social phobia. Hum. Brain Mapp. 2012;33:1973–1986. doi: 10.1002/hbm.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Krueger R.F., Watson D., Bagby M., Carpenter W.T., Caspi A. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 2017:1–48. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Bluhm R.L., Densmore M., Boksman K., Neufeld R.W.J., Gati J.S., Menon R.S. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol. Psychiatry. 2005;57:873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Lanius, R.A., Williamson, P.C., Boksman, K., Densmore, M., Gupta, M., Neufeld, R.W.J., Gati, J.S., Menon, R.S., 2002. Brain activation during script-driven imagery induced dissociative responses in PTSD : a functional magnetic resonance imaging investigation3223. [DOI] [PubMed]
- Lanius R.A., Williamson P.C., Densmore M., Sc B., Boksman K., Neufeld R.W., Ph D., Gati J.S., Sc M., Menon R.S., Ph D. The nature of traumatic memories : a 4-T fMRI functional connectivity analysis. Am. J. Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Li, C.R., Kosten, T.R., Sinha, R., 2005. Sex differences in brain activation during stress imagery in abstinent cocaine users : a functional magnetic resonance imaging study. Biol. Psychiatry.10.1016/j.biopsych.2004.11.048. [DOI] [PubMed]
- Lighthall N.R., Sakaki M., Vasunilashorn S., Nga L., Somayajula S., Chen E.Y., Samii N., Mather M. Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci. 2012;7:476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Ming Q., Ph D., Zhong X., Ph D., Zhang X., Ph D., Pu W., Ph D., Dong D. State-Independent and dependent neural responses to psychosocial stress in current and remitted depression. Am. J. Psychiatry. 2017:971–979. doi: 10.1176/appi.ajp.2017.16080974. [DOI] [PubMed] [Google Scholar]
- Mulders, P.C., van Eijndhoven, P.F., Schene, A.H., Beckmann, C.F., Tendolkar, I., 2015. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed]
- Park C., Rosenblat J.D., Lee Y., Pan Z., Cao B., Iacobucci M., McIntyre R.S. The neural systems of emotion regulation and abnormalities in major depressive disorder. Behav. Brain Res. 2019;367:181–188. doi: 10.1016/j.bbr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., Rooij D.V., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Qin S., Hermans E.J., van Marle H.J.F., Luo J., Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Macleod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Quiroga, J.A., Nasillo, V., Richarte, V., Corrales, M., Palma, F., Ibáñez, P., Michelsen, M., Van de Glind, G., Casas, M., Kooij, J.J.S., 2016. Criteria and concurrent validity of diva 2.0: a semi-structured diagnostic interview for adult ADHD. J. Atten. Disord. 10.1177/1087054716646451. [DOI] [PubMed]
- Richey J.A., Petty C., Bs B., Bizzell J., Ms B., Voyvodic J. Neural mechanisms of emotion regulation in autism spectrum disorder. J. Autism Dev. Disord. 2015;45:3409–3423. doi: 10.1007/s10803-015-2359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse N.N.J., Geurts H.M., Franke B., Buitelaar J.K., Hartman C.A. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit / hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci. Biobehav. Rev. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Sharma, S., Powers, A., Bradley, B., Ressler, K.J., 2016. Gene × environment determinants of stress- and anxiety-related disorders. 10.1146/annurev-psych-122414-033408. [DOI] [PMC free article] [PubMed]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatr. Rehabil. 2014:276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement : addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- van Marle H.J.F., Hermans E.J., Qin S., Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- van Oort J., Tendolkar I., Hermans E.J., Mulders P.C., Beckmann C.F., Schene A.H., Fernández G., van Eijndhoven P.F. How the brain connects in response to acute stress: a review at the human brain systems level. Neurosci. Biobehav. Rev. 2017;83:281–297. doi: 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Vuijk, R., 2014. Diagnostic interview for ASD in Adults (Dutch: Nederlands Interview ten behoeve van Diagnostiek Autismespectrumstoornis bij Volwassenen (NIDA)).
- White S.W., Mazefsky C.A., Dichter G.S., Chiu P.H., Richey J.A., Ollendick T.H. Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. Int. J. Dev. Neurosci. 2014;39:22–36. doi: 10.1016/j.ijdevneu.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016:1–9. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Hashemi, M.M., Kaldewaij, R., Koch, S.B.J., Klumpers, F., Roelofs, K., 2019. Acute stress alters the “default” brain processing. Neuroimage. 10.1016/j.neuroimage.2019.01.063. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.