Abstract
Reactions that efficiently construct medium-sized lactones are significant, as they overcome the unfavorable entropic factor and transannular interactions for ring closure, and the lactones produced are common structural motifs recurring in many biologically active compounds. Herein, we describe a valuable strategy for medium-sized lactone synthesis by accomplishing site-selective C–H bond functionalization via a palladium carbene migratory insertion enabled 1,4-palladium shift. The overall process achieves the formal dimerization of two readily available benzaldehyde derivatives, providing value-added products medium-sized lactones. Our method is amenable to late-stage modification of approved drugs and other complex molecules. Mechanistic studies including deuterium-labeling experiments and DFT calculation shed light on the reaction pathways.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Synthetic chemistry methodology
Transannular construction of medium-sized lactones is entropically unfavoured. Herein, the authors describe a strategy for valuable medium-sized lactone synthesis from available benzaldehydes by selective C-H bond functionalization via palladium carbene migratory insertion-enabled 1,4-palladium shift.
Introduction
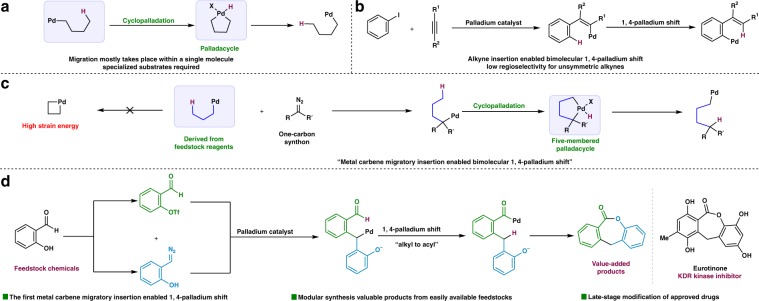
Palladium/hydride shift1–3 is an attractive strategy to achieve selective inert C–H bond activation4–12, while without the requirement of installing a directing group (Fig. 1a). This migration event provides a convenient way to activate a specific C–H bond, which might be difficult to achieve by conventional methods. However, for such a fundamental transformation, most studies are focused on migration proceeded within a single molecule13–27, which means the reactants involved have to be carefully designed to possess a fitted geometry to undergo the desired migration. Elegant exceptions were reported by Larock and coworkers almost 20 years ago (Fig. 1b)28–30, in which the migration of the palladium moiety took place after an intermolecular reaction of two readily available reactants. The insertion of alkyne was crucial to construct the vinylpalladium intermediate that was ready to undergo corresponding 1,4-palladium migration.
Fig. 1. Reaction modes for palladium-catalyzed C–H bond functionalization.
a Reaction mode for 1,4-palladium shift. b Alkyne insertion-enabled 1,4-palladium shift. c Proposed metal carbene migratory insertion-enabled 1,4-palladium shift. d Working hypothesis for seven-membered lactone synthesis.
Many feedstock reagents do not contain any directing groups. For related reactions of these compounds, the palladium metal center is too close to the reacting site. The unfavored strain energy makes it is inaccessible to form a palladacycle via direct C–H bond palladation31,32. Inspired by the seminal work from Larock28–30, we propose that whether we can use a modular one-carbon synthon to facilitate the formation of a thermodynamically stable five-membered palladacycle, which in turn realizes the selective C–H bond activation (Fig. 1c). We believe that the bridging of such a unit to simple reactants may open an avenue for functionalization of inert C–H bonds.
In line with our research interests in diazo compounds involved C–H bond activation33–35, we assumed that a palladium carbene-enabled acylation34 for dibenzo-fused seven-membered lactone synthesis could be an ideal probe to test our assumption (Fig. 1d). In this designed reaction, both reactants could be prepared in multi-gram scale from readily available salicylaldehyde analogs. Studies on palladium carbene participated cross-coupling reactions36–40 support the feasibility of our hypothesis. According to recent discovery, tricylic ring systems possessing a dibenzo structure joined to a central seven-membered lactone show the potential for preventing or treating malignant diseases41. Eurotinone, an effective KDR kinase inhibitor, possesses the exact tricylic backbone. Moreover, modular synthesis of these lactones based on traditional methods, such as Baeyer-Villiger (BV) oxidation42 and macrolactonization43, is challenging. As for the BV reaction, the regioselectivity is low when unsymmetric cyclic ketones are employed, and for the latter, multi-step manipulation is required for the synthesis of the particular reactants. As a common feature of intramolecular reactions, the diversity of the products is limited by the ease of synthesis of the reactants. Thus, it is hard to offer large number of samples that are required for massive screening and evaluation by these traditional methods. Here, we report a palladium carbene migratory insertion-enabled medium-sized lactone synthesis44. In this reaction, diazo compounds generated in situ are employed as modular dockable building blocks to promote the C–H bond activation. Moreover, this methodology was found to be efficient for late-stage functionalization of complex molecules, which could be useful for fragment-based drug discovery45.
Results
Reaction development
To validate our hypothesis, N-tosylhydrazone 2a was selected as the precursor of bifunctional diazo compound to react with 2-bromobenzaldehyde 1a. When the reaction was carried out in THF (tetrahydrofuran) at 80 °C for 10 h, using Pd2(dba)3•CHCl3 (2.5 mol%) as palladium source, dppm (bis(diphenylphosphanyl)methane, 7.5 mol%) as ligand, K3PO4 as base, the expected seven-membered lactone 3 was indeed formed in 5% nuclear magnetic resonance (NMR) yield (Table 1, entry 1). Initial examination of different bidentated phosphine ligands (Table 1, entries 1–10) found that Xantphos ((9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane)) performed the best, affording 3 with 80% NMR yield (Table 1, entry 10). After a brief survey of other palladium sources (Table 1, entries 11–13) (for a detailed optimization study, see Supplementary Information Table S1 to Table S4), we found Pd(OAc)2 (5 mol%) was ideal. The reaction could complete in a relatively short time, and the corresponding lactone 3 was obtained in 76% isolated yield (Table 1, entry 11). o-Iodobenzaldehyde 1b could participated in the palladium-catalyzed lactonization as well, the seven-membered lactone 3 was produced in slightly lower NMR yield (entry 14).
Table 1.
Optimization of reaction conditions for seven-membered lactone synthesis.
| Entrya | [Pd], x mol% | Ligand, 7.5 mol% | Base, z equiv. | Yield/%b |
|---|---|---|---|---|
| 1 | Pd2(dba)3•CHCl3, 2.5 | dppm | K3PO4, 4.0 | 5 |
| 2 | Pd2(dba)3•CHCl3, 2.5 | dppe | K3PO4, 4.0 | 52 |
| 3 | Pd2(dba)3•CHCl3, 2.5 | dppp | K3PO4, 4.0 | 67 |
| 4 | Pd2(dba)3•CHCl3, 2.5 | dppb | K3PO4, 4.0 | 57 |
| 5 | Pd2(dba)3•CHCl3, 2.5 | dpppe | K3PO4, 4.0 | 35 |
| 6 | Pd2(dba)3•CHCl3, 2.5 | dppf | K3PO4, 4.0 | 49 |
| 7 | Pd2(dba)3•CHCl3, 2.5 | DPEphos | K3PO4, 4.0 | 5 |
| 8 | Pd2(dba)3•CHCl3, 2.5 | Xantphos | K3PO4, 4.0 | 77(76)c |
| 9 | Pd2(dba)3•CHCl3, 2.5 | Xantphos | K3PO4, 4.0 | 77d |
| 10 | Pd2(dba)3•CHCl3, 2.5 | Xantphos | K2CO3, 4.0 | 70(74)c |
| 11 | Pd(OAc)2, 5 | Xantphos | K2CO3, 3.0 | 76(76)c,e |
| 12 | PdCl2, 5 | Xantphos | K2CO3, 3.0 | 73e |
| 13 | [(η-C3H6)PdCl]2, 2.5 | Xantphos | K2CO3, 3.0 | 75e |
| 14 | Pd(OAc)2, 5 | Xantphos | K2CO3, 3.0 | 64f |
 | ||||
aReaction condition: 1a (0.2 mmol), 2a (0.4 mmol), [Pd] (5 mol%), Ligand (7.5 mol%), base (z equiv.) in THF (2.0 mL), stirring under atmosphere of Argon at 80 °C for 24 h
bNMR yields were determined using mesitylene as internal standard
cIsolated yield
dDioxane
e12 h
f1b was employed instead of 1a
Substrate scope
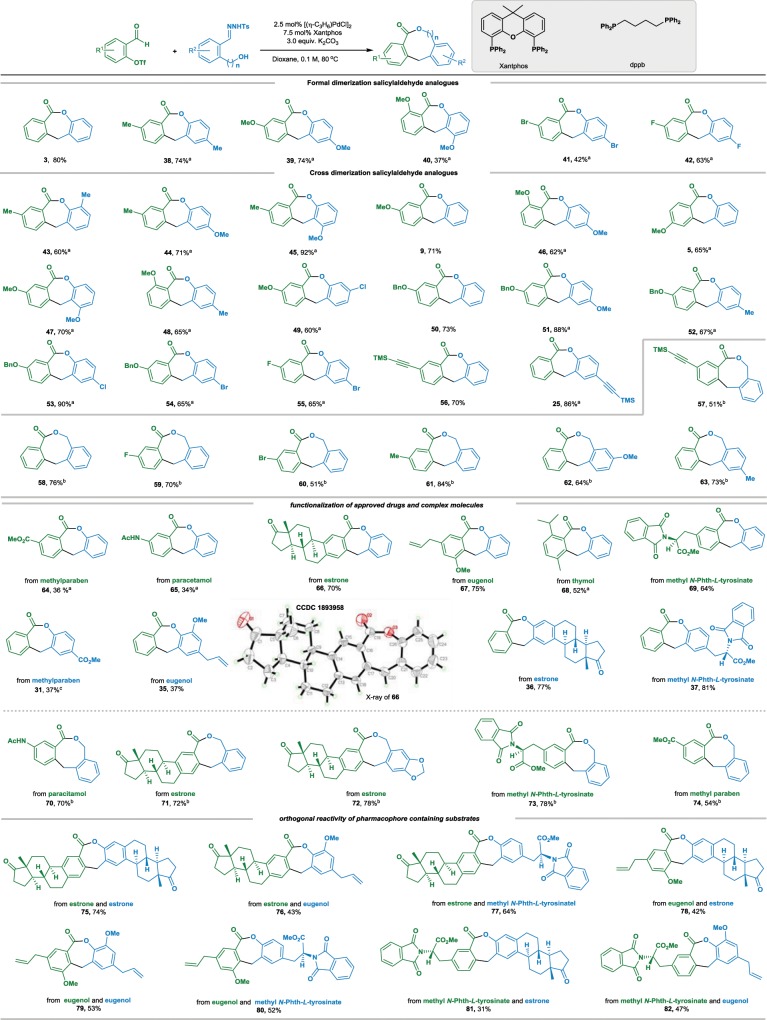
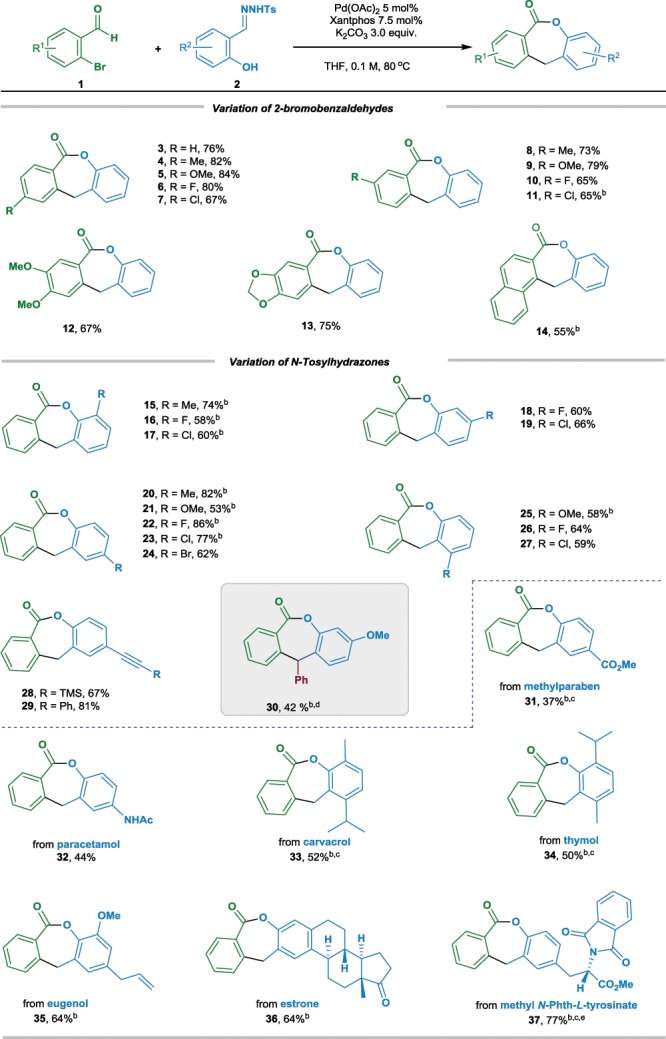
With the optimized reaction conditions in hand (Table 1, entry 11), we sought to explore the substrate scope with respect to different o-bromobenzaldehydes and N-tosylhydrazones bearing hydroxy tether (Table 2). As depicted, a variety of substituted o-bromoarylaldehydes 1 could participate in current lactonization reaction regardless of the electronic nature of substituents incorporated on the phenyl ring. The corresponding lactones were obtained in moderate to high yields (products 3–13, 65%–84% yields). Sterically hindered 1-bromo-2-naphthaldehyde was also a viable substrate for the reaction, leading to the corresponding product 14 in 55% isolated yield.
Table 2.
Substrate scope with respect to o-bromoarylaldehydes 1 and N-tosylhydrazones 2a.
aReaction condition: 1 (0.2 mmol), 2 (0.4 mmol), Pd(OAc)2 (5 mol%), Xantphos (7.5 mol%), K2CO3 (3 equiv) in THF (2.0 mL), stirring under atmosphere of Argon at 80 °C
bPd(OAc)2 10 mol%, Xantphos 15 mol%
c90 °C
d100 °C
eThe reaction was carried out in dioxane
Additional experiments revealed that the current protocol for seven-membered lactone synthesis was efficient, as a wide array of N-tosylhydrazones derived from salicylaldehyde analogs could also react well with 2-bromobenzaldehyde 1a (products 15–37). Electron-donating (methyl and methoxy, products 15, 20, 21, and 25) and electron-withdrawing (chloro, fluoro, even bromo, products 16–19, 22–24, and 26–27) groups decorated on the aryl ring were tolerated, regardless of the position and steric effects. The tolerance of chloro and bromo groups has offered convenient handle for further transition-metal catalyzed cross-coupling reactions. Functional moieties, such as carbon–carbon triple bond and labile silyl group, stayed intact, and corresponding lactones 28 and 29 were isolated in 67% and 81% yields, respectively. It is worthwhile to mention that N-tosylhydrazone derived from corresponding hydroxylketone was also a viable bifunctional carbene precursor, giving the substituted lactone in moderated yield under a slightly modified condition (product 30). The establishment of a chiral carbon center shows the synthetic potential of current method in catalytic asymmetric synthesis, which could be our future research objective. The current lactonization shows potential application on the area of fragment-based drug discovery. As o-hydoxy-N-tosylhydrazones derived from bio-relevant molecules, such as methylparaben, paracetamol, carvacrol, thymol, eugenol, estrone and methyl N-Phth-L-tyrosinate, were competent diazo precursors, and the potentially bioactive ε-lactones 31–37 were obtained in moderate to good yields.
To further demonstrate the synthetic potential, we proceeded to examine the applicability of our designed chemistry for o-pseudo-halo benzaldehyde substrates instead of 2-bromobenzaldehyde. In principle, both reactants could be availed by exploiting 2-hydroxy benzaldehyde as the same starting material. Treatment of 2-formylphenyl trifluoromethanesulfonate 1c and 2 under the previous optimized conditions (Table 1, entry 11) produced 3 with 53% of GC yield. Either changing Xantphos to other biphosphine ligands or using other bases instead of K2CO3 did not improve the results. The best result (80% of isolated yield) was obtained when Pd(OAc)2 was replaced by [Pd(η-C3H6)Cl]2 (2.5 mol%) (for a detailed condition experiments, see Supplementary Information Tables S5 to Table S8).
A feature for current transformation by using o-pseudo-halo aryl aldehydes as the electrophiles is the implementation of formal dimerization of abundant salicylaldehyde analogs to a range of functionalized seven-membered lactones. Under the optimized conditions we found that both reactants derived from 2-hydroxy benzaldehydes containing a variety of substituents on the phenyl ring could be formally dimerized, giving corresponding lactones in moderate to high yields (Table 3, products 3, and 38–42). Notable examples are those reactants possessing potentially reactive bromo group, could participate in current formal dimerization smoothly (product 41). Similarly, the formal cross-dimerization of salicylaldehyde analogs also exhibits very general substrate scope, diverse substituents including electron-donating and electron-withdrawing groups were tolerated, and displayed good orthogonal reactivities of the triflates and N-tosylhydrazones (Table 3, products 5, 9 and 43 to 55). Alkynyl group and labile trimethylsilyl (TMS) moiety stayed intact under standard reaction conditions (products 56 and 28).
Table 3.
Substrate scope for formal dimerization of salicyaldehyde analogs.a
a5 mol% [(η-C3H6)PdCl]2 and 15 mol% Xantphos were employed
b5 mol% [(η-C3H6)PdCl]2 and 7.5 mol% dppb were employed
cThe reaction was carried out at 100 °C
Current strategy was found applicable for more challenging eight-membered lactone synthesis. Under the optimal conditions for the seven-membered lactone synthesis, we indeed observed the formation of desired lactone 58 (n = 1) from the reaction mixture, albeit in 15% NMR yield. After identification of the effects of phosphine ligands, palladium sources and bases (for a detailed optimization study, see Supplementary Information Tables S9–S13), the best result with 76% of isolated yield of 58 was obtained with dppb (1,4-bis(diphenylphosphanyl)butane) at 5 mol% Pd loading. It is worthwhile to mention that, for this specific reaction, we have observed the formation of dihydroisobenzofuran as side-product in small amount (5% isolated yield under optimized conditions). This outcome is noteworthy, as the formation of eight-membered lactone 58, which is generally considered to be energetically less favorable, could override the seemingly favorable pathway for the formation of five-membered compound. This unusual chemo selectivity turned out to be quite general under current conditions for a range of eight-membered lactone synthesis. As the examples shown in Table 3 (products from 57 to 63), lactone formation was not significantly affected by the presence of electron-donating or -withdrawing groups on the phenyl ring of the triflates. As exemplified by the products with strongly electron-withdrawing group (product 59) and with weakly electron-withdrawing group (product 60) were isolated in 70% and 51% yields, respectively. The reactions for the substrates bearing weakly electron-donating group (products 61 and 63) and with strongly electron-donating group (product 62) proceeded well. Similarly, functional groups, such as TMS and alkynyl groups (product 57) were compatible.
The abundance of phenolic-derivatives and well-established procedure of ortho-formylation of phenols46 offered us the opportunity to enrich the substrate scope with lactones having different functionalities. As depicted, the triflates bearing various pharmacophore fragments, such as methylparaben, paracitamol, estrone, eugenol, thymol and methyl N-Phth-L-tyrosinate, could react with salicylaldehyde-derived N-tosylhydrazone under standard conditions, giving the corresponding functionalized bio-relevant seven-membered lactones in 34% to 75% isolated yields (products from 64 to 69). For comparison, N-tosylhydrazones derived from methylparaben, eugenol estrone and N-Phth-L-tyrosinate were also tested as the precursors of diazo compounds to react with triflate 1c. Again, the corresponding lactones could be prepared efficiently (Table 3, products from 31 to 37, up to 81% isolated yield after column chromatography on silica gel). These potentially bio-relevant reactants were competent substrates for eight-membered homo-analogs synthesis as well. The corresponding lactones containing paracetamol (product 70), estrone (products 71 and 72), N-Phth-L-tyrosine (product 73) and methylparaben (product 74) motifs were prepared in moderate to high yields (54–78%). Unambiguous proof of structure and absolute configuration of the bio-relevant lactone 66 was achieved by single-crystal X-ray analysis.
Notably, this fragment-based technology was found to be applicable to couple two pharmacophore fragments, giving a variety of complex molecules in a modular fashion (Table 3, products from 75–82). The proof-of-concept was firstly expressed by the formal dimerization of estrone and eugenol derivatives under the standard conditions, giving corresponding dimers (75 and 79) with seven-membered lactone linkers in 74% and 53% isolated yields, respectively. Subsequently, we examined the orthogonal reactivity of both reactants containing pharmacophore structural motifs. Triflate derived from estrone could react with the corresponding N-tosylhydrones prepared from eugenol and methyl N-Phth-L-tyrosinate, and the cross dimerized lactones 76 and 77 were obtained in 43% and 64% isolated yields after chromatography. Following the similar reaction design, highly complex molecules 78, 80, 81, and 82 containing seven-membered lactone scaffolds could be obtained conveniently.
Mechanistic investigation
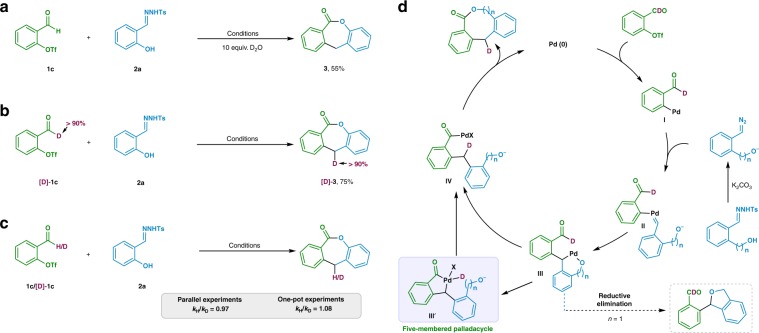
In order to gain insights about the reaction mechanism, representative isotopic labeling experiments were carried out. Under otherwise identical conditions, triflate 1c reacted with 2-hydroxy N-tosylhydrazone 2a in presence of 10 equiv. of D2O, 3 without any incorporation of deuterium atom was isolated in 55% yield (Fig. 2a). Whereas, when compound [D]-1c treated with hydrazone 2a under the standard condition, [D]-3 was produced (Fig. 2b). Finally, the relatively lower value of kH/kD indicated C–H bond cleavage in the aldehyde moiety was not involved in the rate-determining step47 (Fig. 2c).
Fig. 2. Mechanistic experiments.
a Reaction proceeded in presence of 10 equiv. of D2O. b Deuterium-labeling experiment as a probe for 1,4-palladium migration. c Kinetic isotopic effect experiment for C–H bond cleavage in aldehyde 1c. d Proposed catalytic cycle for palladium-catalyzed medium-sized lactone synthesis. X stands for possible counter anion.
Taken all the data together, we have proposed a reasonable mechanism for current medium-sized lactone synthesis (Fig. 2d). The reaction is initiated by oxidative addition of palladium(0) catalyst to o-pseudo-halo benzaldehyde to generate palladium(II) intermediate I. I could be considered as a receptor to react with bifunctional diazo compound, giving palladium-carbene intermediate II. Migratory insertion of II would produce intermediate III, which possesses the exact geometry that is ready to undergo a 1,4-palladium/hydride shift, thus achieving a selective C–H bond activation of the aldehyde moiety48. We anticipate such a reaction mode may not be limited to C–H bond activation of an aldehyde moiety. Other functional moieties possessing similar geometry could be applicable for related metal migration. The step for 1,4-palladium migration proceeds in an irreversible manner, since we did not observe any deuterium atom scrambling during the isotopic labeling experiments (Fig. 2b, c). The fact that no deuterium labeling of 3 occurred when the reaction was carried out in presence of 10 equiv. D2O (Fig. 2a) indicates that H-D exchange between of the potential palladium intermediates and the reaction media is relatively slow. Ring closure of IV generates a high-value medium-sized lactone, where two readily available aldehyde derivatives have been formally dimerized. In the case of eight-membered lactone synthesis, reductive elimination of intermediate III (n = 1) would give the side-product dihydroisobenzofuran.
Density functional theory (DFT) calculations
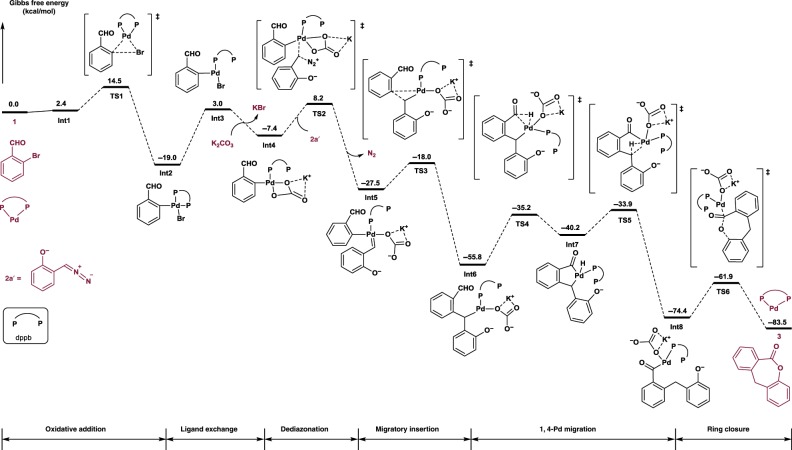
To get more details on the mechanism, a theoretical study of the energy surface of the proposed pathway for the reaction of 1a and 2a catalyzed by a palladium catalyst generated from Pd(OAc)2 and dppb was carried out by DFT calculations. As shown in Fig. 3, a weak interaction between Pd complex and reactant 1a is virtually thermoneutral (ΔG = 2.4 kcal/mol). Oxidative addition of 1a to form Int2 is thermodynamically downhill by 21.4 kcal/mol and requires an energy barrier of 12.1 kcal/mol (TS1). To provide a vacant site for further reaction, dissociation of one site of the bidentated ligand is endothermic of 22.0 kcal/mol, yielding Int3. Ligand exchange of bromide by K2CO3 to form Int4 is an exothermic process. According to our study, the dissociation of dinitrogen is not that facile. Compared to Int2, the reaction of 2a’ with Int4 has to overcome a barrier of 27.2 kcal/mol (TS2) in energy to generate palladium carbene species Int5. A subsequent migratory insertion takes place, giving Int6 via a three-membered transition state TS3, and encountering a low barrier of 9.5 kcal/mol. The C–H activation process starts from Int6. Palladium atom inserts into the C–H bond of the aldehyde moiety, and produces Int7, which possesses a five-membered palladacycle with a hydride species sitting on the palladium center. This step presents a barrier of 20.6 kcal/mol (TS4). Hydride transfer from the palladium atom to dibenzylic position has a small barrier of 6.3 kcal/mol. These two steps in whole could be considered as 1,4-palladium/hydride shift, which was consistent with experimental observation of deuterium experiments (Fig. 2b). For ring closure of Int8, an outersphere displacement of the palladium moiety by the tethered phenolic anion leads to the formation of final product 3, which suffers a barrier of 12.5 kcal/mol. Our calculation shows the rate-determining step is dediazonation to form the metal carbene species (from Int2 to Int5). Other possible pathways were detailed and discussed in Supplementary Information (See Supplementary Information Fig. S3).
Fig. 3. DFT calculations.
Reaction energy profiles calculated at M06/def2-TZVP//B3LYP/6-31G(d)(LANL2DZ) level.
Synthetic applications
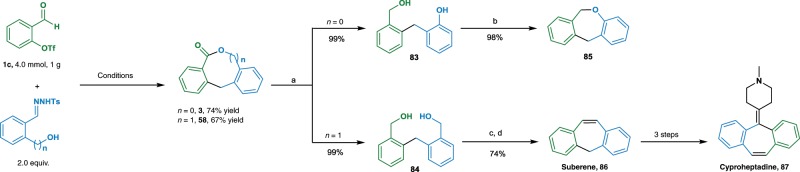
The catalytic methods for the synthesis of medium-sized lactones were found to be efficient for large scale of reactions (Fig. 4). Thus, when 4 mmol (1 g) of 1c was treated with the bifunctional diazo precursors under optimal conditions, lactones 3 and 58 were obtained in 74% and 67% of isolated yields, respectively. These lactones could be reduced efficiently by LAH, giving the corresponding diols 83 and 84 in quantitative yields. Intramolecular cyclization of 83 would give dibenzo-oxepine 85. Diol 84 was converted to suberene 86 by using a consecutive DMP oxidation and TiCl4-mediated McMurry reaction protocol. To our delight, we found compound 86 has significant utility in preparing miscellaneous medicinally important molecules49–51. For example, cyproheptadine 87, an antihistaminic and antiserotonergic agent, could be prepared by following reported procedures in three steps52,53.
Fig. 4. Synthetic manipulations.
Reagents and conditions: a LiAlH4, THF, 0 °C, 3 h; b Ph3P, diethyl diazene-1,2-dicarboxylate, toluene, 70 °C, 6 h; c DMP, tBuOH, DCM, RT, 5 h; d TiCl4, Zn, pyridine, THF, 0–80 °C, 6 h.
Discussion
In summary, we present a rapid approach to dibenzo-fused seven- and eight-membered lactones. The current palladium-catalyzed medium-sized lactonization is efficient, featuring broad substrate scope, good functional group compatibility, and benefitted by utilization of easily available feedstocks as reactants. From a mechanistic viewpoint, the migratory insertion of palladium carbene is critical, as it afforded the exact palladium(II) intermediate III (Fig. 2d) possessing a fitted geometry to undergo 1,4-palladium shift. This migration process was further supported by DFT calculations. We believe that such a metal/hydride migration could be a general reaction mode to achieve site-selective C–H bond activation. Further studies following this designed principle are ongoing in our laboratories.
Methods
General procedure
An oven-dried Schlenk tube was cooled to room temperature and filled with argon. To this tube was added Pd(OAc)2 (5 mol%), Xantphos (7.5 mol%), K2CO3 (3.0 equiv.), N-tosylhydrazone 2a (0.4 mmol). After the tube was evacuated and refilled with argon three times, 1a (0.2 mmol) and anhydrous THF (2.0 mL) were added. The mixture was stirred at 80 °C. After the reaction was complete (monitored by thin-layer chromatography), the crude mixture was cooled to room temperature, filtered through a short pad of celite and eluented with EtOAc. The resulting solution was concentrated by rotary evaporation. Then, the residual was purified by column chromatography on silica gel to give the pure product 3.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We acknowledge financial support by the NSFC (21573237, 21603227, 21871259, 21901244), NSF of Fujian province (2017J05032), the Hundred-Talent Program, and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000). Professors Armido Studer from Westfälische Wilhelms-Universität Münster, Nuno Maulide from University of Vienna and Hideki Yorimitsu from Kyoto University are greatly acknowledged for their helpful discussion and comments on this work.
Author contributions
X.H. conceived and directed the project; Y.Y. and X.H. designed the experiments; Y.Y., P.C., and L.Z. performed the experiments; J.S. and C.L. performed the theoretical studies; X.H., Y.Y., and P.C. analyzed all the results and prepared the manuscript.
Data availability
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files, or from the corresponding author upon request. X-ray structural data of compound 66 are available free of charge from the Cambridge Crystallographic Data Center under the deposition number CCDC 1893958. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks De-Cai Fang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yinghua Yu, Pushkin Chakraborty, Jinshuai Song.
Contributor Information
Chunsen Li, Email: chunsen.li@fjirsm.ac.cn.
Xueliang Huang, Email: huangxl@fjirsm.ac.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-14101-5.
References
- 1.Ma S, Gu Z. 1,4-migration of rhodium and palladium in catalytic organometallic reactions. Angew. Chem. Int. Ed. 2005;44:7512–7517. doi: 10.1002/anie.200501298. [DOI] [PubMed] [Google Scholar]
- 2.Shi F, Larock RC. Remote C–H activation via through-space palladium and rhodium migrations. Top. Curr. Chem. 2010;292:123–164. doi: 10.1007/128_2008_46. [DOI] [PubMed] [Google Scholar]
- 3.Rahim A, Feng J, Gu Z. 1,4-Migration of transition metals in organic synthesis. Chin. J. Chem. 2019;37:929–945. doi: 10.1002/cjoc.201900180. [DOI] [Google Scholar]
- 4.Dupont J, Consorti CS, Spencer J. The potential of palladacycles: more than just precatalysts. Chem. Rev. 2005;105:2527–2571. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]
- 5.Godula K, Sames D. C–H bond functionalization in complex organic synthesis. Science. 2006;312:67–72. doi: 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Engle KM, Wang DH, Yu JQ. Palladium(II)-catalysed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons TW, Sanford M. S. palladium-catalysed ligand-directed C−H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies HML, Du Bois J, Yu J-Q. C–H functionalization in organic synthesis. Chem. Soc. Rev. 2011;40:1855–1856. doi: 10.1039/c1cs90010b. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi J, Yamaguchi AD, Itami K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 10.He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Palladium-catalyzed transformations of alkyl C-H bonds. Chem. Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton CG, Wang SG, Oliveira CC, Cramer N. Catalytic enantioselective transformations involving C-H bond cleavage by transition-metal complexes. Chem. Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- 12.Baudoin O. Ring construction by palladium(0)-catalyzed C(sp3)-H activation. Acc. Chem. Res. 2017;50:1114–1123. doi: 10.1021/acs.accounts.7b00099. [DOI] [PubMed] [Google Scholar]
- 13.Campo MA, Larock RC. Novel 1,4-palladium migration in organopalladium intermediates derived from o-iodobiaryls. J. Am. Chem. Soc. 2002;124:14326–14327. doi: 10.1021/ja027548l. [DOI] [PubMed] [Google Scholar]
- 14.Karig, G., Moon, M. -T., Thasana, N. & Gallagher, T. C−H activation and palladium migration within biaryls under Heck reaction conditions. Org. Lett. 4, 3115–3118 (2002). [DOI] [PubMed]
- 15.Campo MA, Huang Q, Yao T, Tian Q, Larock RC. 1,4-palladium migration via C-H activation, followed by arylation: synthesis of fused polycycles. J. Am. Chem. Soc. 2003;125:11506–11507. doi: 10.1021/ja035121o. [DOI] [PubMed] [Google Scholar]
- 16.Huang Q, Fazio A, Dai G, Campo MA, Larock RC. Pd-catalyzed alkyl to aryl migration and cyclization: an efficient synthesis of fused polycycles via multiple C-H activation. J. Am. Chem. Soc. 2004;126:7460–7461. doi: 10.1021/ja047980y. [DOI] [PubMed] [Google Scholar]
- 17.Barder TE, Walker SD, Martinelli JR, Buchwald SL. Catalysts for Suzuki-Miyaura coupling processes: scope and studies of the effect of ligand structure. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 18.Campo MA, et al. Aryl to aryl palladium migration in the Heck and Suzuki coupling of o-halobiaryls. J. Am. Chem. Soc. 2007;129:6298–6307. doi: 10.1021/ja069238z. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Yue D, Campo MA, Larock RC. An aryl to imidoyl palladium migration process involving intramolecular C-H activation. J. Am. Chem. Soc. 2007;129:5288–5295. doi: 10.1021/ja070657l. [DOI] [PubMed] [Google Scholar]
- 20.Kesharwani T, Verma AK, Emrich D, Ward JA, Larock RC. Studies in acyl C-H activation via aryl and alkyl to acyl “through space” migration of palladium. Org. Lett. 2009;11:2591–2593. doi: 10.1021/ol900940k. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Bercedo P, Flores-Gaspar A, Correa A, Martin R. Pd-catalysed intramolecular acylation of aryl bromides via C-H functionalization: a highly efficient synthesis of benzocyclobutenones. J. Am. Chem. Soc. 2010;132:466–467. doi: 10.1021/ja909811t. [DOI] [PubMed] [Google Scholar]
- 22.Pan J, Su M, Buchwald SL. Palladium(0)-catalysed intermolecular amination of unactivated C(sp3)-H bonds. Angew. Chem. Int. Ed. 2011;50:8647–8651. doi: 10.1002/anie.201102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores-Gaspar A, Gutierrez-Bonet A, Martin R. N-Heterocyclic carbene dichotomy in Pd-catalyzed acylation of aryl chlorides via C-H bond functionalization. Org. Lett. 2012;14:5234–5237. doi: 10.1021/ol3023819. [DOI] [PubMed] [Google Scholar]
- 24.Piou T, Bunescu A, Wang Q, Neuville L, Zhu J. Palladium-catalysed through-space C(sp3)-H and C(sp2)-H bond activation by 1,4-palladium migration: efficient synthesis of [3,4]-fused oxindoles. Angew. Chem. Int. Ed. 2013;52:12385–12389. doi: 10.1002/anie.201306532. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Zhang X, Zhuang YX, Xu YH, Loh TP. Pd-catalysed intramolecular C-N bond cleavage, 1,4-migration, sp3 C-H activation, and Heck reaction: four controllable diverse pathways depending on the judicious choice of the base and ligand. J. Am. Chem. Soc. 2015;137:1341–1347. doi: 10.1021/ja512212x. [DOI] [PubMed] [Google Scholar]
- 26.Hu TJ, Zhang G, Chen YH, Feng CG, Lin GQ. Borylation of olefin C-H bond via aryl to vinyl palladium 1,4-migration. J. Am. Chem. Soc. 2016;138:2897–2900. doi: 10.1021/jacs.5b11990. [DOI] [PubMed] [Google Scholar]
- 27.Hu TJ, Li MY, Zhao Q, Feng CG, Lin GQ. Highly stereoselective synthesis of 1,3-dienes through an aryl to vinyl 1,4-Palladium migration/Heck sequence. Angew. Chem. Int. Ed. 2018;57:5871–5875. doi: 10.1002/anie.201801963. [DOI] [PubMed] [Google Scholar]
- 28.Tian Q, Larock RC. Synthesis of 9-alkylidene-9H-fluorenes by a novel palladium-catalysed rearrangement. Org. Lett. 2000;2:3329–3332. doi: 10.1021/ol000220h. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Campo M, Larock RC. Consecutive vinylic to aryl to allylic palladium migration and multiple C-H activation processes. Angew. Chem. Int. Ed. 2005;44:1873–1875. doi: 10.1002/anie.200462327. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Larock RC. Synthesis of substituted carbazoles by a vinylic to aryl palladium migration involving domino C-H activation processes. Org. Lett. 2005;7:701–704. doi: 10.1021/ol0474655. [DOI] [PubMed] [Google Scholar]
- 31.McNally A, Haffemayer B, Collins BSL, Gaunt MJ. Palladium-catalysed C−H activation of aliphatic amines to give strained nitrogen heterocycles. Nature. 2014;510:129–133. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]
- 32.Willcox D, et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science. 2016;354:851–857. doi: 10.1126/science.aaf9621. [DOI] [PubMed] [Google Scholar]
- 33.Davies HML, Manning JR. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu F, Xia Y, Ma C, Zhang Y, Wang J. C-H bond functionalization based on metal carbene migratory insertion. Chem. Commun. 2015;51:7986–7995. doi: 10.1039/C5CC00497G. [DOI] [PubMed] [Google Scholar]
- 35.Yu Y, Lu Q, Chen G, Li C, Huang X. Palladium-catalysed intermolecular acylation of aryl diazoesters with ortho-bromobenzaldehydes. Angew. Chem. Int. Ed. 2018;57:319–323. doi: 10.1002/anie.201710317. [DOI] [PubMed] [Google Scholar]
- 36.Barluenga J, Valdes C. Tosylhydrazones: new uses for classic reagents in palladium-catalysed cross-coupling and metal-free reactions. Angew. Chem. Int. Ed. 2011;50:7486–7500. doi: 10.1002/anie.201007961. [DOI] [PubMed] [Google Scholar]
- 37.Shao Z, Zhang H. N-tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012;41:560–572. doi: 10.1039/C1CS15127D. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Q, Zhang Y, Wang J. Diazo compounds and N-tosylhydrazones: novel cross-coupling partners in transition-metal-catalysed reactions. Acc. Chem. Res. 2013;46:236–347. doi: 10.1021/ar300101k. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Wang J. N-Tosylhydrazones: versatile synthons in the construction of cyclic compounds. Chem. Soc. Rev. 2017;46:2306–2362. doi: 10.1039/C6CS00737F. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Qiu D, Wang J. Transition-metal-catalysed cross-couplings through carbene migratory insertion. Chem. Rev. 2017;117:13810–13889. doi: 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- 41.Eder, C., Kogler, H. & Toti, L. Eurotinones, and Derivatives Thereof, Processes for Preparing Them, and Their Use. U. S. Patent 0125375A1 (2003).
- 42.Hashemi, M. M. & Beni, Y. A. Nickel(II) and iron(II) Dowex 50W: an effective catalyst for the Baeyer-Villiger oxidation of ketones using molecular oxygen and benzaldehyde. J. Chem. Res. (S)2000, 196–197 (2000).
- 43.Baker, W., Clark, D., Ollis, W. D. & Zealley, T. S. Eight- and higher-membered ring compounds. Part VIII. Anhydro-derivatives of 2-carboxy-2′-hydroxybenzophenone and of 2-carboxy-2′-hydroxydiphenylmethane. J. Chem. Soc. 1452–1457 (1952).
- 44.Rousseau G. Medium ring lactones. Tetrahedron. 1995;51:2777–2849. doi: 10.1016/0040-4020(94)01064-7. [DOI] [Google Scholar]
- 45.Murray CW, Rees DC. Opportunity knocks: organic chemistry for fragment based drug discovery (FBDD) Angew. Chem. Int. Ed. 2016;55:488–492. doi: 10.1002/anie.201506783. [DOI] [PubMed] [Google Scholar]
- 46.Hansen TV, Skattebøl L. Ortho-formylation of phenols; preparation of 3-bromosalicylaldehyde. Org. Syn. 2005;82:64–68. doi: 10.1002/0471264229.os082.10. [DOI] [Google Scholar]
- 47.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 48.Larock RC, Doty MJ, Cacchi S. Synthesis of indenones via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1993;58:4579–4583. doi: 10.1021/jo00069a017. [DOI] [Google Scholar]
- 49.Winthrop SO, et al. New psychotropic agents. Derivatives of dibenzo[a,d]-1,4-cycloheptadiene. J. Org. Chem. 1962;27:230–240. doi: 10.1021/jo01048a057. [DOI] [Google Scholar]
- 50.Eichstadt KE, et al. Novel analogs of tricyclic psychopharmacological agents. J. Med. Chem. 1976;19:47–51. doi: 10.1021/jm00223a010. [DOI] [PubMed] [Google Scholar]
- 51.Nampalli, S. S., Tatel, B., Tharial, P. X. & Kovi, M. Process for Preparation of 5h-dibenzo[a,d] Cycloheptene Derivatives. U. S. Patent 0139848A1 (2008).
- 52.Amiel Y, Ginsburg D. Alicyclic studies—XII. Tetrahedron. 1957;1:19–21. doi: 10.1016/0040-4020(57)85005-4. [DOI] [Google Scholar]
- 53.Hatano M, Ito O, Suzuki S, Ishihara K. Zinc(II)-catalyzed addition of Grignard reagents to ketones. J. Org. Chem. 2010;75:5008–5016. doi: 10.1021/jo100563p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the main data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files, or from the corresponding author upon request. X-ray structural data of compound 66 are available free of charge from the Cambridge Crystallographic Data Center under the deposition number CCDC 1893958. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.