Abstract
Maintaining effective analgesia during invasive procedures performed under general anesthesia is important for minimizing postoperative complications and ensuring satisfactory patient wellbeing and recovery. While patients under deep sedation may demonstrate an apparent lack of response to noxious stimulation, areas of the brain related to pain perception may still be activated. Thus, these patients may still experience pain during invasive procedures. The current study used anesthetized or sedated cynomolgus macaques and functional magnetic resonance imaging (fMRI) to assess the activation of the parts of the brain involved in pain perception during the application of peripheral noxious stimuli. Noxious pressure applied to the foot resulted in the bilateral activation of secondary somatosensory cortex (SII) and insular cortex (Ins), which are both involved in pain perception, in macaques under either propofol or pentobarbital sedation. No activation of SII/Ins was observed in macaques treated with either isoflurane or a combination of medetomidine, midazolam, and butorphanol. No movement or other reflexes were observed in response to noxious pressure during stimulation under anesthesia or sedation. The current findings show that despite the lack of visible behavioral symptoms of pain during anesthesia or sedation, brain activation suggests the presence of pain depending on the anesthetic agent used. These data suggest that fMRI could be used to noninvasively assess pain and to confirm the analgesic efficacy of currently used anesthetics. By assessing analgesic efficacy, researchers may refine their experiments, and design protocols that improve analgesia under anesthesia.
Abbreviations and Acronyms: fMRI, functional magnetic resonance imaging; MMB, medetomidine, midazolam, and butorphanol; SII/Ins, secondary somatosensory cortex/insula
Minimizing pain and distress are fundamental concepts in the ethical use of laboratory animals, particularly during procedures that may be painful, even if the patient is unconscious. Symptoms of pain and distress may be inferred in awake nonhuman animals by the expression of pain-related behaviors such as guarding, vocalization and changes in species-specific behaviors such as social interaction.43,60 Pain may also be inferred noninvasively while a research animal is under anesthesia by changes in autonomic functioning, such as increased heart rate and arterial blood pressure.76 Increased heart rate and blood pressure have been observed following the application of noxious stimuli to either skin or viscera in both anesthetized and awake nonhuman animals.24,83,89 Conversely, decreased changes in autonomic function after a noxious stimulus suggests pain is minimized–analgesic administration has reduced stimulus-evoked increases in heart rate and blood pressure.
However, this relationship is controversial. The literature does not suggest a consistent relationship between pain and changes in autonomic functioning.37 Analgesics, even at doses that do not reduce nociception in both anesthetized and conscious animals, have been shown to reduce heart rate and blood pressure.25,75 Some anesthetics themselves, such as halothane, reduce both heart rate and blood pressure at low concentrations without significantly reducing nociception.89 Beyond analgesics and anesthetics, premedications, such as antihypertensives, and preexisting medical conditions that affect hemodynamics will suggest the absence or presence of pain when neither is truly the case.76
Direct, quantitative assessment of pain is possible through the measurement of neural activity with in vivo electrophysiological methods by observing the response of CNS neurons to peripherally applied noxious stimuli in anesthetized animals.12,13,15 Some cortical and subcortical nuclei involved in pain perception have been identified in this manner. The disadvantages of most preclinical electrophysiological methods are their invasiveness and that they are usually terminal procedures. Electroencephalography (EEG), which can be used in either awake or anesthetized patients, has been suggested as a relatively noninvasive method of quantifying nociception based on cortical electrical activity.44 However, the effects of various agents on EEG have led to surprising conclusions. Based on complete abolition of stimulus-evoked potentials with anesthetic doses of pentobarbital in cats, the authors concluded that pentobarbital would be a better anesthetic than ketamine, which, at anesthetic doses, increased stimulus-evoked potentials.88 In humans, propofol significantly reduced noxious stimulus-evoked potentials, suggesting that propofol has intrinsic analgesic activity.66,90 Conflicting effects of analgesics on EEG have also been observed.17 Opioid analgesics, such as fentanyl, significantly reduce evoked potentials, but nonopioid analgesics, such as cyclooxygenase inhibitors, do not. This would suggest that cyclooxygenase inhibitors would not be useful for perioperative pain control when, in fact, they are.39,71 Pregabalin, approved for the management of neuropathic pain, does not dose-dependently reduce brain electrical activity as measured by EEG in rats, yet it appears to modestly reduce postoperative pain when administered perioperatively during specific kinds of surgeries.22,44 While EEG may be useful in demonstrating nociception in anesthetized patients, findings thus far suggest that EEG may not be entirely suitable for predicting analgesia. A key, additional disadvantage of EEG is its lack of spatial resolution, in that it cannot distinguish or identify the numerous subcortical brain nuclei involved in pain perception.44
Alternatively, in vivo neuroimaging, such as functional magnetic resonance imaging (fMRI) has been used as a noninvasive approach in identifying brain nuclei that are activated following peripherally applied noxious stimuli in both anesthetized and conscious subjects.2,18,19,58 Preclinical and clinical studies have also demonstrated that different classes of analgesics significantly attenuated noxious stimulus-evoked activation of brain nuclei associated with pain perception.38,57,58,91 Furthermore, drugs that do not reduce nociception in awake animals do not affect stimulus-evoked activation of brain nuclei associated with pain perception, indicating that drugs could be categorized by their effect on brain activation.31,81 Although anesthetics are known to disrupt both cerebral electrical activity and blood flow, which are related to brain activity, the effects of anesthetic and sedative agents commonly used in preclinical research on brain nuclei involved in pain perception have yet to be explored with fMRI in NHP.35 The current study differentiated anesthetic and sedative agents by their ability to reduce stimulus-evoked brain activation as visualized with fMRI in NHP.
Materials and Methods
Animals.
Procedures involving macaques were reviewed and approved by the Hamamatsu Pharma Research Animal Care and Use Committee. A total of 8 male Macaca fascicularis (3.7 to 4.5 kg, 3 to 4 y old, Eve Bioscience, Wakayama, Japan) were used in this study. Prior to arrival, macaques were tested and were negative for Salmonella spp., Shigella spp., Yersinia spp., M. tuberculosis and Macacine alpha herpesvirus 1 (B virus). Macaques underwent a 2-wk period of quarantine and acclimation to the facility before experimental use. Macaques were observed daily during this period for signs of illness that may have precluded their use in the current study. Macaques were housed in stable groups of 2 or 3, each in stainless steel cages (W 195.0 cm × D 65.0 cm × H 80.0 cm, floor area: 12,675 cm2) following the quarantine and acclimation period. Stainless steel mirrors and other toys were placed in cages as environmental enrichment. To provide social enrichment and facilitate acclimation to humans, staff hand-fed treats to the macaques at least once per week. Macaques were fed about 100 g/animal per day of standard nonhuman primate chow (Oriental Yeast, Tokyo, Japan), which was supplemented weekly with either fresh fruits or vegetables. Macaques had free access to tap water by using an automatic watering system.
Environmental conditions of the holding rooms were under automatic control and followed standards described in the Guide for the Care and Use of Laboratory Animals: Eighth Edition.40 A 12-h light-dark cycle, with lights on at 0700, was used in the animal housing room. The room temperature and relative humidity were maintained at 20 to 26 °C and 30% to 70%, respectively. The facility where the study was conducted is fully accredited by AAALAC International.
Study outline.
Macaques that displayed normal species-specific behaviors, were without visible signs of systemic disease and displayed normal respiration on physical examination were used in the current study. Subjects could be classified as ASA I (that is “normal, healthy patients”).
Brain imaging was performed between 0830 and 1600. Macaques were fasted for approximately 12 h before undergoing brain imaging. Macaques were sedated with ketamine (10 mg/kg, IM) and transported to the MRI (Center for Molecular Imaging, Hamamatsu University School of Medicine (Hamamatsu, Japan)) in a dedicated NHP transport vehicle in portable squeeze cages.
Conscious, restrained macaques were treated with one of the 4 agents and were released from restraint when they demonstrated loss of movement and response to stimulation. Macaques demonstrated a loss of palpebral reflex and did not respond to interdigital pinching after administration of the anesthetic, prior to insertion in the MRI. Heart rate and respiration were measured after induction of anesthesia, before brain imaging, after completion of brain imaging, and before recovery from anesthesia. MRI-compatible respiration and blood pressure monitoring equipment were not available, so continuous monitoring during brain imaging was not possible. During the scan period, a veterinarian was present to provide appropriate and prompt treatment.
Macaques were transported back to Hamamatsu Pharma Research following brain imaging, returned to their home cages and monitored for recovery from sedation (if still sedated). Macaques were treated with 20 mg/kg/hr propofol followed by an additional 30 mg/kg/hr within the same day (Table 1). Macaques underwent a washout period of at least 1 d after treatment with either isoflurane or MMB. Macaques treated with pentobarbital underwent a washout period of at least 1 wk. The animals were treated with a different agent on each imaging session. Each macaque underwent no more than 4 different treatments.
Table 1.
Duration of fMRI brain imaging following induction using the agents examined in the current study.
| Time from induction (min) | ||
| Agent | Start | End |
| Propofol, 20 mg/kg/hr | 10-35 | 25-50 |
| Propofol, 30 mg/kg/hr | 40-60 | 55-75 |
| Pentobarbital | 18-24 | 33-39 |
| Isoflurane | 21-33 | 36-48 |
| MMB | 19-38 | 34-53 |
Times (min.) for initiation of brain imaging, after induction, and the end of brain imaging. Five macaques were anesthetized with a dose of 20 mg/kg/hr. propofol and brain imaging was performed beginning 10-35 min. after induction of sedation. At the end of brain imaging (25-50 min. after induction of propofol sedation), 3 macaques were then dosed with 30 mg/kg/hr. propofol. The end of propofol brain imaging was 55-75 min after induction of sedation with 20 mg/kg/hr. propofol. Brain imaging with the 2 doses of propofol was performed on the same day. Brain imaging with the other agents was performed in separate imaging sessions.
MMB, medetomidine, midazolam, and butorphanol.
Drug treatments.
All drugs used in the current study were pharmaceutical grade. The level of sedation required for the current study was such that no artifacts from either head movement or respiration were observed in the brain images. The doses of isoflurane, pentobarbital, and the combination of medetomidine, midazolam, and butorphanol (MMB) needed to induce either sedation or anesthesia were obtained from the literature.4,26,27,34,64
Data from rodent studies suggest that sedative doses of propofol, both 36 and 90 mg/kg/hr., are antinociceptive.74,94 It is possible that propofol's analgesic effect in NHP could be observed at doses that are higher than that needed for stable sedation, which would be, in macaques, between 12 to 36 mg/kg/hr.34 The current study tested the effects of 20 and 30 mg/kg/hr propofol on noxious stimulus-evoked brain activation. Conscious macaques were restrained in a transport squeeze cage and a 22 gauge cannula was inserted into the femoral vein. Propofol (Maruishi Pharmaceutical, Osaka, Japan) was administered by continuous intravenous infusion. Five macaques were treated with 20 mg/kg/hr propofol and underwent brain imaging. Three randomly selected macaques from this group of 5 were then treated with another 30 mg/kg/hr dose of propofol and underwent a second brain scan.
For the pentobarbital cohort, conscious macaques were restrained in the transport squeeze cage and pentobarbital (20 mg/kg; Somnopentyl, Kyoritsu Seiyaku, Tokyo, Japan) was administered as a single IV bolus.
To test the effects of inhalant anesthesia, macaques were restrained in a primate chair and isoflurane (2.0% to 2.5% in O2; Pfizer Japan, Tokyo, Japan) was delivered through a face mask. Sedation was observed within 1 to 2 min. of placement of the face mask over the nose and mouth. No other sedative was administered prior to isoflurane administration as the goal of the current study was to examine the effect of isoflurane anesthesia alone on stimulus-evoked brain activation.
Awake macaques were restrained in the transport squeeze cage for IM administration of the combination of MMB (medetomidine, 0.012 mg/kg, Meiji Seika Pharma, Tokyo, Japan; midazolam, 0.16 mg/kg, Sandoz K.K., Tokyo, Japan; butorphanol, 0.2 mg/kg; Vetorphale, Meiji Seika Pharma, Tokyo, Japan). Atipamezole (0.25 mg/kg; Mepatia, Meiji Seika Pharma, Tokyo, Japan) was administered IM to reverse the effects of medetomidine at the end of the brain imaging session. In 3 macaques, a dose of 0.6 mg/kg was given to reverse either cardiac arrhythmia or respiratory depression that emerged following MMB treatment.
The syringe pump (TOP, Tokyo, Japan) and the anesthesia vaporizer (Muromachi Kikai, Tokyo, Japan) used to deliver the anesthetics were not MRI compatible and so were placed outside of the MRI room.
Acute noxious stimulus.
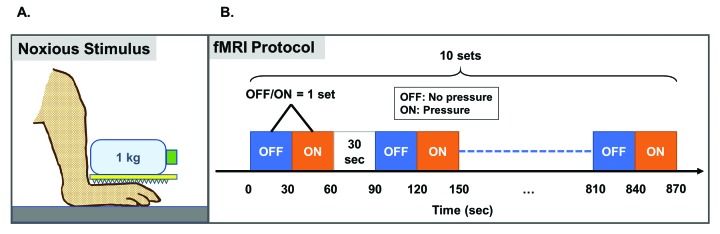
A plastic kitchen grater with dentate projections (1.2 mm in height at 3.5 mm intervals) was placed on the dorsum of the right foot and a weight of 1 kg was applied to the grater (Figure 1 A) to induce noxious pressure. A 1 L PET bottle was emptied of its contents (Pocari Sweat, Otsuka Pharmaceutical, Tokyo, Japan) and filled with approximately 1 L of water, which was placed on the grater, exerting a weight of 1 kg. A macaque previously habituated to restraint in a primate restraint chair was selected to determine if the grater and weight evoked a pain-related response (for example, tension of the muscles of the face and back of the head or withdrawal of the foot from the grater). The height of the chair was adjusted such that the feet rested flat on the floor. A pain-related response was observed within a few seconds of placement of the device on the foot. Placement of either the grater or water-filled PET bottle alone on the foot did not evoke a pain-related response. Application of the grater and filled PET bottle on the dorsum of the foot was painful in humans. Application of either apparatus alone to the dorsum of the foot did not evoke pain in humans. On each test session day, prior to application of the PET bottle to the foot, the weight of the water-filled PET bottle was checked with an electronic balance (HV-200KGV-K, A and D Company, Tokyo, Japan) and confirmed to be 1 kg in weight.
Figure 1.
Pressure-induced pain stimulation during MRI. A) Diagram shows placement of the water-filled bottle and grater on the dorsum of the macaque's foot. B) Block design stimulation applied to the right foot of anesthetized macaques during brain imaging. Ten sets of OFF/ON stimulation were preformed, separated by 30 s of no stimulation. One set consisted of 30 s without application of the grater and weight (“OFF”) and 30 s application of the grater and weight (“ON”).
fMRI data acquisition.
Brain activation during foot stimulation was assessed using a 3.0 T MRI system (Signa HDxt; GE Healthcare, Milwaukee, WI). The anatomic MRI protocol consisted of a T1-weighted fast spoiled gradient-recalled (FSPGR) sequence (repetition time (TR)/echo time (TE), 15.8/7.0 ms; number of averages, 1; flip angle, 12°; field of view, 150 mm × 150 mm; matrix, 256 × 224; slice thickness/interval, 1.0/0.5 mm; number of slices, 168). Functional scan sequences consisted of field-echo, echo-planar imaging (TR/TE, 3000/35 ms; flip angle, 90°; field of view, 140 mm ×140 mm; matrix, 64 × 64; slice thickness, 2.4 mm; no. of slices, 30).
Under anesthesia, the macaque's head was fixed within an MRI compatible acrylic head holder (Matsui, Aichi, Japan). A block design was used to deliver a noxious stimulus to the right foot during imaging (Figure 1 B). A stimulation set consisted of an “OFF” stimulus, wherein the foot received no stimulus for 30 s, followed by an “ON” stimulus, wherein the plastic grater and water bottle were applied to the foot for 30 s. A 30 s interval separated each block of OFF-ON stimulation. During one imaging session, a macaque underwent a total of 10 sets of OFF-ON stimulations. Ten whole-brain scans of 3 s duration each were acquired during each ON and each OFF stimulation. One imaging session for each animal was about 15 min. Animals were kept warm during brain imaging with heat packs (Hakugen-Earth, Tokyo, Japan) and loosely wrapped in a wool blanket.
The times from induction to completion of brain imaging for each agent are shown in Table 1. No observable movement or reflex to noxious stimulation of the foot during brain imaging were observed. Upon awakening from sedation in their home cage, no abnormality was noted in general condition and behavior, and no tissue damage was observed in the stimulated foot.
fMRI data analysis.
Analyses of brain images were conducted with SPM12 software (Wellcome Department of Cognitive Neurology, London, UK). The images were realigned and resliced onto the mean echo-planar imaging (EPI) image to correct for head motion. The EPI images were coregistered to the corresponding T1-weighted anatomic image and normalized to a macaque brain template.6 The resulting image was smoothed with a 4 mm × 4 mm × 4 mm full-width at half-maximum Gaussian kernel. Voxel-wise statistical analysis was based on a general linear model. A fixed-effect model was used for group analysis of data from the 5 macaques. Contrast was defined as (pressure stimulation–no stimulation) to isolate regions responsive to noxious pressure in the entire brain. Peak voxels were considered significant at Z value greater than 1.96 (P < 0.05, uncorrected for multiple comparisons, one tailed t test) or Z value greater than 2.3 (P < 0.01).
Results
General observations.
Five macaques received isoflurane and 5 received pentobarbital. No adverse side effects were observed with either treatment during brain imaging. Recovery from isoflurane and pentobarbital anesthesia was uneventful.
Five macaques received 20 mg/kg/hr propofol and of these 3 were treated with a subsequent dose of 30 mg/kg/hr propofol. No adverse side effects were observed at either dose and subsequent recovery from anesthesia was uneventful.
The combination of MMB (medetomidine 0.012 mg/kg, midazolam 0.16 mg/kg and butorphanol 0.2 mg/kg) was administered to a total of 8 macaques. Additional MMB (medetomidine 0.006 mg/kg, midazolam 0.08 mg/kg and butorphanol 0.1 mg/kg) was given to 3 of these, which were not sufficiently sedated to minimize head movement. In the 3 macaques treated with additional MMB, 2 were sedated to an appropriate level. One macaque, however, displayed cardiac arrhythmia. Unstable respiration was observed in 2 macaques treated with a single dose of MMB. The 3 MMB-treated macaques that showed symptoms of physiologic distress were treated with 0.6 mg/kg atipamezole, which reverses medetomidine-induced sedation and respiratory depression, and monitored for recovery from sedation.10,92,93 The data from these 3 macaques were excluded from the analysis.
Excluding the 3 MMB-treated macaques, all other subjects demonstrated an appropriate level of sedation. While not directly measured during brain imaging, respiratory rate and heartbeat were observed to be stable. Immediately after brain imaging, but before recovery from sedation animals showed no reflexive response to interdigital pinch.
The heart rate of animals during treatment, before and after brain imaging, were between 60 to 152 bpm and 66 to 180 bpm, respectively. Respiration rates of animals under treatment, before and after brain imaging, were between 16 to 36 and 16 to 72, respectively.
Effect of agents on noxious stimulation-evoked activation of SII/Ins.
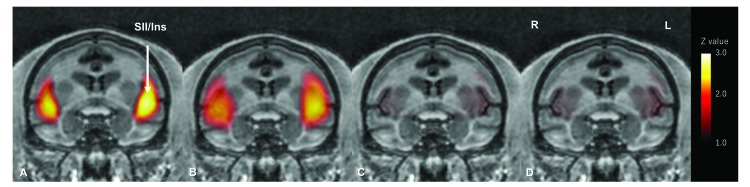
Significant bilateral activation of SII/Ins was observed under propofol and pentobarbital anesthesia but not under isoflurane and MMB anesthesia (Figure 2, Table 2).
Figure 2.
Noxious pressure-induced activation of secondary somatosensory cortex (SII) and insula (Ins) in cynomolgus macaques under various types of anesthetic or sedative agents. Macaques were treated with either A) propofol, B) pentobarbital, C) isoflurane or D) MMB. The average of 3 coronal brain activation maps under propofol sedation (30 mg/kg/hr.) is shown. Coronal section brain activation maps averaged from 5 macaques treated with either pentobarbital, isoflurane or medetomidine/midazolam/butorphanol (MMB) are shown. Activation of the somatosensory cortex SII and Ins was observed with noxious pressure applied to the right foot under either propofol or pentobarbital sedation. However, no activation was observed with either isoflurane or MMB treatment. L, anatomic left; R, anatomic right.
Table 2.
Noxious stimulation-evoked activation of secondary somatosensory cortex and insula in sedated macaques.
| Coordinates (mm) | |||||||
| Agent | Area | Hemisphere | Z value | P value | x | y | z |
| Propofol | SII/Ins | Left | 2.71 | 0.0067 | 14 | 24 | 0 |
| Right | 2.59 | 0.0096 | −16 | 22 | 2 | ||
| Pentobarbital | SII/Ins | Left | 2.81 | 0.0050 | 16 | 20 | 2 |
| Right | 2.63 | 0.0085 | −16 | 22 | 2 | ||
| Isoflurane | SII/Ins | Left | 1.24 | 0.2150 | 14 | 18 | 2 |
| Right | 1.19 | 0.2340 | −16 | 20 | 2 | ||
| MMB | SII/Ins | Left | 1.46 | 0.1443 | 16 | 20 | 2 |
| Right | 1.29 | 0.1971 | −16 | 22 | 2 | ||
A noxious stimulus was applied to the right foot and activation of secondary somatosensory cortex and insula (SII/Ins) was observed with fMRI under treatment with each agent. Z values for 30 mg/kg/hr. propofol are shown. Z values of peak voxels are shown. Stereotaxic coordinates (x, y, z) are according to Horsely-Clarke's stereotaxic coordinates. Peak voxels were considered significant at Z value > 1.96 (P < 0.0500) and Z value > 2.58 (P < 0.0100); peak voxel Z values below 1.96 are considered nonsignificant. MMB, medetomidine/midazolam/butorphanol.
Significant bilateral noxious pressure-induced activation of secondary somatosensory cortex (SII) and insular cortex (Ins) was observed in macaques treated with 20 mg/kg/hr propofol—Z values were greater than 1.96, the minimum value needed for statistical significance at P < 0.05 (data not shown). Significant bilateral SII/Ins activation was also observed with 30 mg/kg/hr propofol. Z values for 30 mg/kg/hr propofol were 2.71 (P = 0.0067; left hemisphere) and 2.59 (P = 0.0096; right hemisphere). Significant noxious pressure-induced activation of SII/Ins was also observed under pentobarbital anesthesia. Z values for pentobarbital were 2.81 (P = 0.0050; left hemisphere) and 2.63 (P = 0.0085; right hemisphere).
No noxious pressure-evoked activation of SII/Ins was observed during isoflurane anesthesia (Figure 2, Table 2). The Z values of SII/Ins from isoflurane-treated macaques were 1.24 (P = 0.2150; left hemisphere) and 1.19 (P = 0.2340; right hemisphere), which are below the minimum (1.96) needed for statistical significance. Noxious pressure-induced brain activation was also absent with MMB treatment. The Z values of SII/Ins from MMB-treated macaques were 1.46 (P = 0.1443; left hemisphere) and 1.29 (P = 0.1971; right hemisphere), also below the minimum (1.96) needed for statistical significance.
Discussion
The current study differentiated anesthetic and sedative drugs commonly used in NHP studies by their effects on noxious stimulus-evoked activation of brain nuclei known to be involved in pain perception. Robust activation of SII and Ins was observed with noxious pressure under either propofol or pentobarbital anesthesia, whereas no activation of SII/Ins was observed after isoflurane or MMB treatment. The effects of the drugs on SII/Ins is in general agreement with the known antinociceptive properties of each drug. The current findings suggest that activity of brain nuclei involved in pain perception, such as the SII/Ins, could be used to indicate either pain or analgesia in patients who are unable to respond to noxious stimulation. Pharmacological modulation of pain-related brain nuclei could also be used to expand our understanding of the mechanism of action of both anesthetics and sedatives and possibly aid in the development of anesthetics or treatment protocols that improve antinociception and enhance postprocedural recovery.
Bilateral activation of SII/Ins with unilateral application of noxious pressure observed in macaques in the current study parallels findings in awake humans.20,51 Various noxious stimuli, in addition to noxious pressure, have been shown to activate SII/Ins in both humans and macaques.13,96 Conversely, lesions of SII/Ins due to cerebral infarction appear to decrease sensitivity to noxious stimuli, which suggests that inactivation of the SII/Ins leads to antinociception.96 The SII/Ins is likely involved in pathologic pain states as well as in acute pain perception. Nonnoxious stimulation does not normally activate SII/Ins, but under pathologic conditions such as peripheral neuropathy, nonnoxious stimulation can activate SII/Ins.58 Activation of SII/Ins in pathologic pain states is reduced with drugs that reduce nociception but not by those without antinociceptive efficacy.31,81 In the current study, activation of SII/Ins following an acute noxious stimulus could serve as a nonbehavioral measure for acute pain perception and SII/Ins inactivation could suggest antinociception.
The most likely mechanism by which MMB reduces noxious-stimulus evoked SII/Ins activation is direct inhibition of SII/Ins neurons. The antinociceptive effect of the combination sedative MMB could be through several mechanisms, independently or in concert, including α2-adrenoceptor activation, enhancing GABAA receptor activation, and partial agonist activity at the µ- and κ-opioid receptors. Significant levels of GABA binding sites are found within the macaque cerebral cortex, including SII/Ins.33,50 Activation of GABAA receptors with microinjection of the GABAA receptor agonist muscimol into SII/Ins is antinociceptive.58 Abundant µ- and κ-opioid receptor binding sites are present in macaque SII/Ins.79,82 While the effect of direct microinjection of an opioid into the macaque insula has not been reported, microinjection of opioid, including a naturally occurring κ-opioid receptor agonist into the insula in awake rats has been reported to be antinociceptive.9,16 Complete mapping of α2-adrenoceptors in the macaque brain has not been reported but the receptor has been identified in human SII/Ins.59 Intracerebroventricular injection of clonidine in macaques is antinociceptive, but the effect of direct microinjection into the SII/Ins has yet to be reported.49 Interaction between adrenoceptor, opioid, GABAergic receptors leads to an antinociception that is greater than either of the constituents alone, as demonstrated in rats.32,67 It is possible that such an interaction occurs, with MMB, in macaques as well, leading to the suppression of noxious stimulus-evoked SII/Ins activation. Further pharmacological studies are needed to confirm that an interaction between the various receptors within SII/Ins mediates the pharmacologic effect of MMB in the macaque.
Several molecular mechanisms have been suggested to mediate isoflurane's analgesic effects, including activation of CNS GABAA receptors and blockade of glutamate receptors and voltage-gated cation channels. These lead, in turn, to both decreased presynaptic neurotransmitter release and enhanced postsynaptic inhibition with an overall inhibition of synaptic neural transmission and transmission of pain signaling within the pain matrix.85 The brain may not be isoflurane's primary site of action as administration of isoflurane directly to the brain led to increased pain perception.7 Others have suggested, based on findings in rodents, that suppression of noxious transmission at the level of the spinal cord dorsal horn as isoflurane's primary mechanism of action.42,62 Isoflurane's mechanism of action and site of action in NHP has yet to be fully elaborated. If inhibition of pain transmission from the periphery to the CNS at the spinal dorsal horn is the key mechanism, then suppression of SII/Ins by isoflurane observed in the current study could be the result of suppressed supraspinally projecting nociceptive neurons that originated in the spinal dorsal horn.1 Nociceptive neurons originating in the spinal dorsal horn project to the contralateral thalamus. Postsynaptic thalamic neurons, in turn, project and terminate in SII/Ins.8 Isoflurane's apparent inhibitory effect on SII/Ins could potentially be an example of indirect suppression.
Isoflurane can increase cerebral blood flow, which can lead to increased blood oxygenation level dependent (BOLD) signaling, which is used in fMRI as an indicator of neural activation.46 An understanding of the effect of an anesthetic on cerebral blood flow is necessary to properly interpret analgesic drug effects on brain activation, while the patient is under anesthesia, based on blood flow. An isoflurane concentration of about 1% is adequate to sedate macaques, retard head movement and suppress movement in response to noxious stimulation.2,46 A higher concentration of isoflurane (2%) increases cerebral blood flow.47 Whether blood flow in specific cortical nuclei, such as SII/Ins, is increased has not been reported. Li and colleagues46,47 did not examine the effect of noxious stimulation during increased cerebral blood flow. Under a high concentration of isoflurane, assessments that increase neural activity, including noxious somatic stimulation, could appear exaggerated. In the current study, however, no SII/Ins activation was observed after the application of noxious pressure under isoflurane anesthesia. The findings suggest that while cerebral blood flow is increased at a concentration of isoflurane that is more than sufficient for sedation, this did not lead to enhanced pain perception (“hyperalgesia”). To avoid misinterpretation of drug or stimulus effects on brain activity, however, the anesthetic protocol should be optimized to the particular study objectives.2
Propofol and pentobarbital appear to share at least one common mechanism—modulation of GABA binding to the GABAA receptor and enhancing the opening of the GABAA receptor's chloride ion channel, thereby reducing neurotransmitter release and postsynaptic neurotransmission.27,65 Pentobarbital is generally thought of as having no intrinsic analgesic activity, but antinociception in a specific macaque pain model has been observed, albeit at a sedating dose.21,30 Defined spinal cord mechanisms of propofol and pentobarbital antinociception have been suggested for both drugs, and limited clinical data suggests reduced postoperative pain following intraoperative propofol treatment, compared with sevoflurane.11,62,70,74,86,94 Preclinical rodent findings suggest that both propofol and pentobarbital possess significant, intrinsic antinociceptive efficacy. However, the current findings in NHP demonstrated that sedating doses of either propofol or pentobarbital did not suppress noxious pressure activation of SII/Ins. Other findings suggest pentobarbital anesthesia leads to hyperalgesia in rats, and, under propofol anesthesia, in humans.28,56 The lack of effect of either of these anesthetics on SII/Ins activation could be due to increased baseline activity or sensitivity of SII/Ins neurons induced by these anesthetics. The mechanism of potential propofol- and pentobarbital-induced hyperalgesia proposed from the current findings is not entirely known, but the current findings underscore the need for appropriate analgesic treatment during painful procedures using these agents. At the same time, however, if the purpose of the study is to observe brain activation after noxious stimulation, then the use of either of these agents would be suitable. Other considerations for selecting a particular anesthetic, including compatibility with study objectives, include safety of the anesthetic to the patient and staff and intensity of the follow-up care after the termination of anesthesia.3,64,68
One other key consideration during the planning of studies that require significant sedation or anesthesia is the duration of the effect. Recovery from intravenous or inhaled agents, such as propofol and isoflurane, respectively, is rapid after discontinuation.3,27 Recovery from pentobarbital anesthesia occurs over a longer period of time compared with, for example, propofol.52,97 The short-lasting sedation after acute propofol treatment suggests rapid clearance of propofol from the target tissue.54 Measurable levels of propofol have been observed in plasma for up to 3 h after a single bolus dose, despite that sedation lasted for approximately 14 min. and macaques demonstrated ambulation by 25 min.54 Levels of propofol in the brain, the primary site of action, were not measured, but given the lack of behavioral effects, levels were likely negligible at 25 min after dosing. Significant sedative effects of MMB can be observed in macaques within 15 min after intramuscular administration and depending on the dose of each constituent, can last from 47 min to 190 min.64 The half-life of intramuscular medetomidine in dogs is only a “few minutes” and the half-life of IM midazolam is about 1 h.41,77,78 Of the 3 drugs that make up the combination of MMB, butorphanol appears to have the longest half-life (about 1.5 h in dogs).72 The likelihood of a carryover effect at 24 h after dosing with MMB in the current study is low.
Rodents are the most frequently used species for preclinical studies, and findings from rodent pain studies have served as a cornerstone of current clinical understanding of pain mechanism and therapy.73,95 The current fMRI findings in macaques appear to differ from fMRI studies performed in rodents. As noted earlier, propofol appears to be antinociceptive in rats at doses that “prevents movement in 50% of the rats” and, furthermore, prevents the onset of “wind up”, the phenomenon of persistent neural hyperactivity following brief, intense peripheral stimulation, in spinal dorsal horn neurons.62 Pretreatment with propofol before noxious chemical stimulation of the hind paw suppressed the emergence of wind up-type pain.63,74 A “small area of [contralateral] sensorimotor cortex”, which presumably included the rat homolog of NHP SII/Ins, responding to acute noxious stimulation was reduced with propofol in rats, as observed with fMRI.45 Lahti and colleagues44 further showed that the propofol-induced reduction was significant compared with rats that underwent fMRI without anesthesia, suggesting that propofol (48 mg/kg/hr) is antinociceptive in rats. By contrast, a previous fMRI study in humans demonstrated the persistence of noxious stimulus-evoked activation of SII/Ins in propofol-treated subjects under deep sedation, which parallels the current finding in macaques.36,48 Behavioral studies suggest that barbiturates, such as pentobarbital, are not antinociceptive in rodents, but could be antinociceptive under a specific condition in macaques.23,30,63,80 A direct comparison between species on the antinociceptive effects of anesthetics or analgesics using fMRI, using similar stimulation procedures, could reveal crucial neurophysiologic differences between species and identify species-specific mechanism of action.
Findings in rodents suggest marked sex-based sensitivities to noxious stimuli and analgesics.55 Also, human females appear to be more sensitive to pressure pain, demonstrating a lower threshold to pain than males, and greater sensitivity to butorphanol analgesia than males.14,53,87 Human sex-based differences in nociception may have a biologic basis, but sex-based differences could also be cultural-environmental in origin.29 Controlling for environmental factors and genetic background to examine potential genetic mediation of behavior is more manageable in rodents compared with primates. Likewise, findings in NHP of sex differences in nociception and analgesic efficacy are not as robust as those demonstrated by rodent studies. For example, female macaques may be more sensitive to the antinociceptive effect of κ-opioid agonist, whereas male rats appear to be more sensitive to the antinociceptive effect of a κ-opioid agonist.5,61 To avoid sex-based differences, the current study exclusively used male macaques to demonstrate the effects of anesthetics on noxious pain-evoked SII/Ins activation. The human and rodent findings suggest that there may be a sex-based difference in SII/Ins response to anesthetics or sedatives, so future studies could use female macaques to uncover a possible sex difference in pain perception and the effects of anesthetics on pain perception.
Some of the agents used in the current study share some common molecular mechanisms, such as positive modulation of GABA binding to the GABAA receptor, yet significantly differ in their antinociceptive efficacy. The current study points out the utility of in vivo neuroimaging as a tool for further elaboration of the mechanism of action of similar agents, particularly the effect of the agent on brain nuclei with known involvement in pain perception. The SII/Ins showed significant stimulus-evoked activation during propofol and pentobarbital sedation, suggesting the lack of intrinsic antinociceptive properties at doses that suppressed behavioral responding to noxious stimulation. The current study also demonstrated that isoflurane and MMB suppressed SII/Ins activation, supporting previous preclinical findings of intrinsic antinociceptive properties of these agents. The actual pathway mediating the suppression of SII/Ins, whether the anesthetic acts directly or indirectly on SII/Ins neurons, or via other CNS nuclei involved in pain perception that synapse with SII/Ins, has yet to be determined. Functional connectivity mapping could be used to elaborate on the functional relationship between brain nuclei and the effect of analgesics on this functional relationship.69
A general limitation of fMRI is the need for dedicated housing, maintenance, and safety protocols. Moreover, current imaging protocols will need improvement to increase temporal resolution compared with that which can be obtained with EEG.84 Despite these limitations, fMRI may be best used as preclinical tool to characterize the antinociceptive efficacy of anesthetics and potentially aid in the development of novel treatments that not only suppress nociception during painful procedures but also prevent the emergence of persistent, postprocedural pain and pain that evolves from other types of tissue injury.
Acknowledgments
This study was sponsored by Hamamatsu Pharma Research, Inc. Expert care of the HPR Animal Care Group was highly appreciated.
All authors are either current or previous employees of HPR at the time of this study and declare a competing interest.
References
- 1.Antognini JF, Atherley R, Carstens E. 2003. Isoflurane action in spinal cord indirectly depresses cortical activity associated with electrical stimulation of the reticular formation. Anesth Analg 96:999–1003. [DOI] [PubMed] [Google Scholar]
- 2.Asad AB, Seah S, Baumgartner R, Feng D, Jensen A, Manigbas E, Henry B, Houghton A, Evelhoch JL, Derbyshire SW, Chin CL. 2016. Distinct BOLD fMRI responses of capsaicin-induced thermal Sensation reveal pain-related brain activation in nonhuman primates. PLoS One 11:1–22. 10.1371/journal.pone.0156805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Authier S, Chaurand F, Legaspi M, Breault C, Troncy E. 2006. Comparison of 3 anesthetic protocols for intraduodenal drug administration using endoscopy in rhesus monkeys (Macaca mulatta). J Am Assoc Lab Anim Sci 45:73–79. [PubMed] [Google Scholar]
- 4.Barletta M, Quandt J, Hofmeister E. 2016. Determination of minimum alveolar concentration of isoflurane in dogs and cats using the up-and-down method. A preliminary study. Res Vet Sci 106:81–83. 10.1016/j.rvsc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. 2002. Sex and rat strain determine sensitivity to k opioid-induced antinociception. Psychopharmacology (Berl) 160:170–181. 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- 6.Black KJ, Koller JM, Snyder AZ, Perlmutter JS. 2004. Atlas template images for nonhuman primate neuroimaging: baboon and macaque. Methods Enzymol 385:91–102. 10.1016/S0076-6879(04)85006-7. [DOI] [PubMed] [Google Scholar]
- 7.Borges M, Antognini JF. 1994. Does the brain influence somatic responses to noxious stimuli during isoflurane anesthesia? Anesthesiology 81:1511–1515. 10.1097/00000542-199412000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Brooks J, Tracey I. 2005. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat 207:19–33. 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkey AR, Carstens E, Wenniger JJ, Tang J, Jasmin L. 1996. An opioidergic cortical antinociception triggering site in the agranular insular cortex of the rat that contributes to morphine antinociception. J Neurosci 16:6612–6623. 10.1523/JNEUROSCI.16-20-06612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butelman ER, Woods JH. 1993. Effects of clonidine, dexmedetomidine and xylazine on thermal antinociception in rhesus monkeys. J Pharmacol Exp Ther 264:762–769. [PubMed] [Google Scholar]
- 11.Carlsson KH, Jurna I. 1986. Interaction of pentobarbital and morphine in the tail-flick test performed on rats: synergism at the spinal and antagonism at the supraspinal level. Neurosci Lett 71:356–360. 10.1016/0304-3940(86)90647-6. [DOI] [PubMed] [Google Scholar]
- 12.Chao TH, Chen JH, Yen CT. 2018. Plasticity changes in forebrain activity and functional connectivity during neuropathic pain development in rats with sciatic spared nerve injury. Mol Brain 11:1–16. 10.1186/s13041-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LM. 2018. Cortical representation of pain and touch: evidence from combined functional neuroimaging and electrophysiology in nonhuman primates. Neurosci Bull 34:165–177. 10.1007/s12264-017-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. 2003. Gender differences in pressure pain threshold in healthy humans. Pain 101:259–266. 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 15.Chudler EH, Dong WK, Kawakami Y. 1986. Cortical nociceptive responses and behavioral correlates in the monkey. Brain Res 397:47–60. 10.1016/0006-8993(86)91368-5. [DOI] [PubMed] [Google Scholar]
- 16.Coffeen U, Canseco-Alba A, Simon-Arceo K, Almanza A, Mercado F, Leon-Olea M, Pellicer F. 2017. Salvinorin A reduces neuropathic nociception in the insular cortex of the rat. Eur J Pain 22:311–318. 10.1002/ejp.1120. [DOI] [PubMed] [Google Scholar]
- 17.Constant I, Sabourdin N. 2012. The EEG signal: a window on the cortical brain activity. Paediatr Anaesth 22:539–552. 10.1111/j.1460-9592.2012.03883.x. [DOI] [PubMed] [Google Scholar]
- 18.Créac'h C, Henry P, Caillé JM, Allard M. 2000. Functional MR imaging analysis of pain-related brain activation after acute mechanical stimulation. AJNR Am J Neuroradiol 21:1402–1406. [PMC free article] [PubMed] [Google Scholar]
- 19.Davis KD, Kwan CL, Crawley AP, Mikulis DJ. 1998. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80:1533–1546. 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- 20.Davis MT, Daniel TA, Witte TK, Beyers RJ, Willis JZ, Wang Y, Denney TS, Jr, Katz JS, Salibi N, Deshpande G. 2016. Demonstration and validation of a new pressure-based MRI-safe pain tolerance device. J Neurosci Methods 271:160–168. 10.1016/j.jneumeth.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Dykstra LA, Woods JH. 1986. A tail withdrawal procedure for assessing analgesic activity in rhesus monkeys. J Pharmacol Methods 15:263–269. 10.1016/0160-5402(86)90056-2. [DOI] [PubMed] [Google Scholar]
- 22.Eipe N, Penning J, Yazdi F, Mallick R, Turner L, Ahmadzai N, Ansari MT. 2015. Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain 156:1284–1300. 10.1097/j.pain.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 23.Endoh T, Tajima A, Izumimoto N, Suzuki T, Saitoh A, Suzuki T, Narita M, Kamei J, Tseng LF, Mizoguchi H, Nagase H. 2001. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol 85:282–290. 10.1254/jjp.85.282. [DOI] [PubMed] [Google Scholar]
- 24.Field KJ, White WJ, Lang CM. 1993. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim 27:258–269. 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- 25.Flacke JW, Flacke WE, Bloor BC, Olewine S. 1983. Effects of fentanyl, naloxone, and clonidine on hemodynamics and plasma catecholamine levels in dogs. Anesth Analg 62:305–313. 10.1213/00000539-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Flecknell P. 2009. Anesthesia of common laboratory species: special considerations. Chapter 6. p 181–241. In: Flecknell P, Laboratory animal anesthesia. Waltham (MA): Academic Press; 10.1016/B978-0-12-369376-1.00006-X [DOI] [Google Scholar]
- 27.Fowler KA, Huerkamp MJ, Pullium JK, Subramanian T. 2001. Anesthetic protocol: propofol use in Rhesus macaques (Macaca mulatta) during magnetic resonance imaging with stereotactic head frame application. Brain Res Brain Res Protoc 7:87–93. 10.1016/S1385-299X(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 28.Frölich MA, Price DD, Robinson ME, Shuster JJ, Theriaque DW, Heft MW. 2005. The effect of propofol on thermal pain perception. Anesth Analg 100:481–486. 10.1213/01.ANE.0000142125.61206.7A. [DOI] [PubMed] [Google Scholar]
- 29.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJConsensus Working Group of the Sex, Gender, and Pain SIG of the IASP. 2007. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132 Suppl 1:S26–S45. 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha H, Wu RS, Contreras RA, Tan EC. 1978. Measurement of pain threshold by electrical stimulation of tooth pulp afferents in the monkey. Exp Neurol 61:260–269. 10.1016/0014-4886(78)90245-5. [DOI] [PubMed] [Google Scholar]
- 31.Hama A, Natsume T, Ogawa SY, Awaga Y, Hayashi I, Matsuda A, Takamatsu H. 2018. Pain-related behavior and brain activation in a cynomolgus macaque model of postoperative pain. CNS Neurol Disord Drug Targets 17:348–360. 10.2174/1871527317666180515121350. [DOI] [PubMed] [Google Scholar]
- 32.Hara K, Saito Y, Kirihara Y, Yamada Y, Sakura S, Kosaka Y. 1999. The interaction of antinociceptive effects of morphine and GABA receptor agonists within the rat spinal cord. Anesth Analg 89:422–427. [DOI] [PubMed] [Google Scholar]
- 33.Hartvig P, Eckernäs SA, Lindberg BS, Lundqvist H, Antoni G, Rimland A, Långström B. 1990. Regional distribution of the opioid receptor agonist N-(methyl-11C)pethidine in the brain of the rhesus monkey studied with positron emission tomography. Pharmacol Toxicol 66:37–40. 10.1111/j.1600-0773.1990.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 34.Hawk CT, Leary SL, Morris TH. 2005. Formulary for laboratory animals. Ames (IA): Blackwell Publishing. [Google Scholar]
- 35.He B, Yang L, Wilke C, Yuan H. 2011. Electrophysiological imaging of brain activity and connectivity-challenges and opportunities. IEEE Trans Biomed Eng 58:1918–1931. 10.1109/TBME.2011.2139210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC. 2004. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology 100:386–394. 10.1097/00000542-200402000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Höglund OV, Dyall B, Grasman V, Edner A, Olsson U, Höglund K. 2018. Effect of nonsteroidal antiinflammatory drugs on postoperative respiratory and heart rate in cats subjected to ovariohysterectomy. J Feline Med Surg 20:980–984. 10.1177/1098612X17742290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooker BA, Tobon G, Baker SJ, Zhu C, Hesterman J, Schmidt K, Rajagovindan R, Chandran P, Joshi SK, Bannon AW, Hoppin J, Beaver J, Fox GB, Day M, Upadhyay J. 2013. Gabapentin-induced pharmacodynamic effects in the spinal nerve ligation model of neuropathic pain. Eur J Pain 18:223–237. 10.1002/j.1532-2149.2013.00364.x. [DOI] [PubMed] [Google Scholar]
- 39.Howard ML, Isaacs AN, Nisly SA. 2018. Continuous infusion nonsteroidal antiinflammatory drugs for perioperative pain management. J Pharm Pract 31:66–81. 10.1177/0897190016665539. [DOI] [PubMed] [Google Scholar]
- 40.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 41.Kallio-Kujala IJ, Raekallio MR, Honkavaara J, Bennett RC, Turunen H, Scheinin M, Hautajarvi H, Vainio O. 2018. Peripheral α2-adrenoceptor antagonism affects the absorption of intramuscularly coadministered drugs. Vet Anaesth Analg 45:405–413. 10.1016/j.vaa.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Davies MF, Clark JD, Kobilka BK, Maze M. 2002. Isoflurane and nociception: spinal α2A adrenoceptors mediate antinociception while supraspinal α1 adrenoceptors mediate pronociception. Anesthesiology 96:367–374. 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Saito Y, Takeuchi T. 2016. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp Anim 65:27–36. 10.1538/expanim.15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama S, LeBlanc BW, Smith KA, Roach C, Levitt J, Edhi MM, Michishita M, Komatsu T, Mashita O, Tanikawa A, Yoshikawa S, Saab CY. 2018. An electroencephalography bioassay for preclinical testing of analgesic efficacy. Sci Rep 8:1–9. 10.1038/s41598-018-34594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. 1999. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med 41:412–416. . [DOI] [PubMed] [Google Scholar]
- 46.Li CX, Patel S, Auerbach EJ, Zhang X. 2013. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 541:58–62. 10.1016/j.neulet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li CX, Patel S, Wang DJ, Zhang X. 2014. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging 32:956–960. 10.1016/j.mri.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichtner G, Auksztulewicz R, Kirilina E, Velten H, Mavrodis D, Scheel M, Blankenburg F, von Dincklage F. 2018. Effects of propofol anesthesia on the processing of noxious stimuli in the spinal cord and the brain. Neuroimage 172:642–653. 10.1016/j.neuroimage.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Lin MT, Lee JM, Cheng JT. 1987. Changes in central serotoninergic transmission affect clonidine analgesia in monkeys. Naunyn Schmiedebergs Arch Pharmacol 335:491–495. 10.1007/BF00169113. [DOI] [PubMed] [Google Scholar]
- 50.Lin SF, Bois F, Holden D, Nabulsi N, Pracitto R, Gao H, Kapinos M, Teng JK, Shirali A, Ropchan J, Carson RE, Elmore CS, Vasdev N, Huang Y. 2017. The search for a subtype-selective PET imaging agent for the GABAA receptor complex: evaluation of the radiotracer [11C]ADO in nonhuman primates. Mol Imaging 16:1–10. 10.1177/1536012117731258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda L, Ono M, Koyama T, Oshiro Y, Sumitani M, Mashimo T, Shibata M. 2011. Human brain activity associated with painful mechanical stimulation to muscle and bone. J Anesth 25:523–530. 10.1007/s00540-011-1173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mallory MD, Baxter AL, Kost SI, Pediatric Sedation Research Consortium 2009. Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: results from the Pediatric Sedation Research Consortium. Paediatr Anaesth 19:601–611. 10.1111/j.1460-9592.2009.03023.x. [DOI] [PubMed] [Google Scholar]
- 53.Miller PL, Ernst AA. 2004. Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. South Med J 97:35–41. 10.1097/01.SMJ.0000085743.68121.A9. [DOI] [PubMed] [Google Scholar]
- 54.Miyabe-Nishiwaki T, Masui K, Kaneko A, Nishiwaki K, Shimbo E, Kanazawa H. 2010. Hypnotic effects and pharmacokinetics of a single bolus dose of propofol in Japanese macaques (Macaca fuscata fuscata). [corrected] Vet Anaesth Analg 37:501–510. 10.1111/j.1467-2995.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 55.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. 2003. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA 100:4867–4872. 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montagne-Clavel J, Oliveras JL, Martin G. 1995. Single-unit recordings at dorsal raphe nucleus in the awake-anesthetized rat: spontaneous activity and responses to cutaneous innocuous and noxious stimulations. Pain 60:303–310. 10.1016/0304-3959(94)00129-3. [DOI] [PubMed] [Google Scholar]
- 57.Nagakubo D, Hamamoto Y, Hasegawa D, Kamata M, Iizuka T, Muta K, Fujita N, Nakagawa T, Nishimura R. 2017. Functional MRI-based identification of brain regions activated by mechanical noxious stimulation and modulatory effect of remifentanil in cats. Res Vet Sci 114:444–449. 10.1016/j.rvsc.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Nagasaka K, Yamanaka K, Ogawa S, Takamatsu H, Higo N. 2017. Brain activity changes in a macaque model of oxaliplatin-induced neuropathic cold hypersensitivity. Sci Rep 7:4305 10.1038/s41598-017-04677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahimi A, Jakobsen S, Munk OL, Vang K, Phan JA, Rodell A, Gjedde A. 2015. Mapping α2 adrenoceptors of the human brain with 11C-Yohimbine. J Nucl Med 56:392–398. 10.2967/jnumed.114.145565. [DOI] [PubMed] [Google Scholar]
- 60.National Research Council. 1992. Recognition and alleviation of pain and distress in laboratory animals. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 61.Negus SS, Mello NK. 1999. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290:1132–1140. [PubMed] [Google Scholar]
- 62.Ng KP, Antognini JF. 2006. Isoflurane and propofol have similar effects on spinal neuronal windup at concentrations that block movement. Anesth Analg 103:1453–1458. 10.1213/01.ane.0000247732.33602.f5. [DOI] [PubMed] [Google Scholar]
- 63.O'Connor TC, Abram SE. 1995. Inhibition of nociception-induced spinal sensitization by anesthetic agents. Anesthesiology 82:259–266. 10.1097/00000542-199501000-00031. [DOI] [PubMed] [Google Scholar]
- 64.Ochi T, Nishiura I, Tatsumi M, Hirano Y, Yahagi K, Sakurai Y, Matsuyama-Fujiwara K, Sudo Y, Nishina N, Koyama H. 2014. Anesthetic effect of a combination of medetomidine-midazolam-butorphanol in cynomolgus monkeys (Macaca fascicularis). J Vet Med Sci 76:917–921. 10.1292/jvms.13-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olsen RW. 2018. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology 136:10–22. 10.1016/j.neuropharm.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orth M, Barter L, Dominguez C, Atherley R, Carstens E, Antognini JF. 2005. Halothane and propofol differentially affect electroencephalographic responses to noxious stimulation. Br J Anaesth 95:477–484. 10.1093/bja/aei208. [DOI] [PubMed] [Google Scholar]
- 67.Ossipov MH, Lozito R, Messineo E, Green J, Harris S, Lloyd P. 1990. Spinal antinociceptive synergy between clonidine and morphine, U69593, and DPDPE: isobolographic analysis. Life Sci 47:PL71–PL76. 10.1016/0024-3205(90)90530-5. [DOI] [PubMed] [Google Scholar]
- 68.Paasonen J, Salo RA, Shatillo A, Forsberg MM, Narvainen J, Huttunen JK, Grohn O. 2016. Comparison of 7 different anesthesia protocols for nicotine pharmacologic magnetic resonance imaging in rat. Eur Neuropsychopharmacol 26:518–531. 10.1016/j.euroneuro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 69.Paasonen J, Stenroos P, Salo RA, Kiviniemi V, Gröhn O. 2018. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage 172:9–20. 10.1016/j.neuroimage.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Peng K, Liu HY, Wu SR, Liu H, Zhang ZC, Ji FH. 2016. Does propofol anesthesia lead to less postoperative pain compared with inhalational anesthesia?: A systematic review and meta-analysis. Anesth Analg 123:846–858. 10.1213/ANE.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 71.Peng YZ, Li XX, Wang YW. 2010. Effects of Parecoxib and Fentanyl on nociception-induced cortical activity. Mol Pain 6:1–12. 10.1186/1744-8069-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfeffer M, Smyth RD, Pittman KA, Nardella PA. 1980. Pharmacokinetics of subcutaneous and intramuscular butorphanol in dogs. J Pharm Sci 69:801–803. 10.1002/jps.2600690715. [DOI] [PubMed] [Google Scholar]
- 73.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, 't Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. 2014. Why primate models matter. Am J Primatol 76:801–827. 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu Q, Sun L, Wang XM, Lo ACY, Wong KL, Gu P, Wong SCS, Cheung CW. 2017. Propofol produces preventive analgesia via GluN2B-containing NMDA Receptor/ERK1/2 signaling pathway in a rat model of inflammatory pain. Mol Pain 13:1–13. 10.1177/1744806917737462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Randich A, Thurston CL, Ludwig PS, Timmerman MR, Gebhart GF. 1991. Antinociception and cardiovascular responses produced by intravenous morphine: the role of vagal afferents. Brain Res 543:256–270. 10.1016/0006-8993(91)90036-U. [DOI] [PubMed] [Google Scholar]
- 76.Rantanen M, Yli-Hankala A, van Gils M, Yppärilä-Wolters H, Takala P, Huiku M, Kymäläinen M, Seitsonen E, Korhonen I. 2006. Novel multiparameter approach for measurement of nociception at skin incision during general anaesthesia. Br J Anaesth 96:367–376. 10.1093/bja/ael005. [DOI] [PubMed] [Google Scholar]
- 77.Salonen JS. 1989. Pharmacokinetics of medetomidine. Acta Vet Scand Suppl 85:49–54. [PubMed] [Google Scholar]
- 78.Schwartz M, Muñana KR, Nettifee-Osborne JA, Messenger KM, Papich MG. 2012. The pharmacokinetics of midazolam after intravenous, intramuscular, and rectal administration in healthy dogs. J Vet Pharmacol Ther 36:471–477. 10.1111/jvp.12032. [DOI] [PubMed] [Google Scholar]
- 79.Seah S, Asad AB, Baumgartner R, Feng D, Williams DS, Manigbas E, Beaver JD, Reese T, Henry B, Evelhoch JL, Chin CL. 2014. Investigation of cross-species translatability of pharmacological MRI in awake nonhuman primate - a buprenorphine challenge study. PLoS One 9:1–11. 10.1371/journal.pone.0110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaw FZ, Chen RF, Yen CT. 2001. Dynamic changes of touch- and laser heat-evoked field potentials of primary somatosensory cortex in awake and pentobarbital-anesthetized rats. Brain Res 911:105–115. 10.1016/S0006-8993(01)02686-5. [DOI] [PubMed] [Google Scholar]
- 81.Shidahara Y, Natsume T, Awaga Y, Ogawa S, Yamoto K, Okamoto S, Hama A, Hayashi I, Takamatsu H, Magata Y. 2019. Distinguishing analgesic drugs from non-analgesic drugs based on brain activation in macaques with oxaliplatin-induced neuropathic pain. Neuropharmacology 149:204–211. 10.1016/j.neuropharm.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 82.Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. 1999. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience 94:651–662. 10.1016/S0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- 83.Sivarao DV, Langdon S, Bernard C, Lodge N. 2007. Colorectal distension-induced pseudoaffective changes as indices of nociception in the anesthetized female rat: morphine and strain effects on visceral sensitivity. J Pharmacol Toxicol Methods 56:43–50. 10.1016/j.vascn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, Borsook D, Edwards RR, Harris RE, Wager TD, Arendt-Nielsen L, Burke LB, Carr DB, Chappell A, Farrar JT, Freeman R, Gilron I, Goli V, Haeussler J, Jensen T, Katz NP, Kent J, Kopecky EA, Lee DA, Maixner W, Markman JD, McArthur JC, McDermott MP, Parvathenani L, Raja SN, Rappaport BA, Rice ASC, Rowbotham MC, Tobias JK, Wasan AD, Witter J. 2017. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain 18:757–777. 10.1016/j.jpain.2017.02.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stachnik J. 2006. Inhaled anesthetic agents. Am J Health Syst Pharm 63:623–634. 10.2146/ajhp050460. [DOI] [PubMed] [Google Scholar]
- 86.Stein C, Morgan MM, Liebeskind JC. 1987. Barbiturate-induced inhibition of a spinal nociceptive reflex: role of GABA mechanisms and descending modulation. Brain Res 407:307–311. 10.1016/0006-8993(87)91108-5. [DOI] [PubMed] [Google Scholar]
- 87.Takala EP. 1990. Pressure pain threshold on upper trapezius and levator scapulae muscles. Repeatability and relation to subjective symptoms in a working population. Scand J Rehabil Med 22:63–68. [PubMed] [Google Scholar]
- 88.Tamásy V, Korányi L, Tekeres M. 1975. E.E.G. and multiple unit activity during ketamine and barbiturate anaesthesia. Br J Anaesth 47:1247–1251. 10.1093/bja/47.12.1247. [DOI] [PubMed] [Google Scholar]
- 89.Taylor BK, Peterson MA, Basbaum AI. 1995. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci 15:7575–7584. 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Untergehrer G, Jordan D, Eyl S, Schneider G. 2013. Effects of propofol, sevoflurane, remifentanil, and (S)-ketamine in subanesthetic concentrations on visceral and somatosensory pain-evoked potentials. Anesthesiology 118:308–317. 10.1097/ALN.0b013e318279fb21. [DOI] [PubMed] [Google Scholar]
- 91.Upadhyay J, Baker SJ, Rajagovindan R, Hart M, Chandran P, Hooker BA, Cassar S, Mikusa JP, Tovcimak A, Wald MJ, Joshi SK, Bannon A, Medema JK, Beaver J, Honore P, Kamath RV, Fox GB, Day M. 2013. Pharmacological modulation of brain activity in a preclinical model of osteoarthritis. Neuroimage 64:341–355. 10.1016/j.neuroimage.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 92.Vainio O. 1990. Reversal of medetomidine-induced cardiovascular and respiratory changes with atipamezole in dogs. Vet Rec 127:447–450. [PubMed] [Google Scholar]
- 93.Vainio O, Vähä-Vahe T. 1990. Reversal of medetomidine sedation by atipamezole in dogs. J Vet Pharmacol Ther 13:15–22. 10.1111/j.1365-2885.1990.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 94.Wong SS, Sun L, Qiu Q, Gu P, Li Q, Wang XM, Cheung CW. 2019. Propofol attenuates postoperative hyperalgesia via regulating spinal GluN2B-p38MAPK/EPAC1 pathway in an animal model of postoperative pain. Eur J Pain 23:812–822. 10.1002/ejp.1349. [DOI] [PubMed] [Google Scholar]
- 95.Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. 1999. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA 96:7680–7686. 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang ZH, Dougherty PM, Oppenheimer SM. 1999. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience 94:351–360. 10.1016/S0306-4522(99)00339-5. [DOI] [PubMed] [Google Scholar]
- 97.Zola-Morgan S, Micheletti C. 1986. Respiration rate, heart rate, and body temperature values in cynomolgus monkeys (Macaca fascicularis) during barbiturate anesthesia. J Med Primatol 15:399–408. [PubMed] [Google Scholar]