Abstract
Background
Thanatin is the smallest member of Beta-hairpin class of cationic peptide derived from insects with vast activities against various pathogens.
Objective
In this study, the antimicrobial activity of this peptide against some species of human bacterial pathogens as well as its toxicity on NIH cells were evaluated.
Methods
Thanatin DNA sequence was cloned into pcDNA3.1+ vector and transformed into a DH5α bacterial strain. Then the recombinant plasmids were transfected into HEK-293 cells by calcium phosphate co-precipitation. After applying antibiotic treatment, the supernatant medium containing thanatin was collected. The peptide quantity was estimated by SDS-PAGE and GelQuant software. The antimicrobial activity of this peptide was performed with Minimum Inhibitory Concentration (MIC) method. In addition, its toxicity on NIH cells were evaluated by MTT assay.
Results
The peptide quantity was estimated approximately 164.21 μmolL-1. The antibacterial activity of thanatin was estimated between 0.99 and 31.58 μmolL-1 using MIC method. The result of cytotoxicity test on NIH cell line showed that the peptide toxicity up to the concentration of 394.10 μmolL-1 and for 48 hours, was not statistically significant from negative control cells (P>0.05). The antimicrobial assay demonstrated that thanatin had an antibacterial effect on some tested microorganisms. The results obtained in this study also showed that thanatin had no toxicity on mammalian cell lines including HEK293 and NIH.
Conclusion
Antimicrobial peptides such as thanatin are considered to be appropriate alternatives to conventional antibiotics in treating various human pathological diseases bacteria.
Keywords: Antimicrobial peptides, Recombinant, Antibiotic resistant, Bacteria, Thanatin, Pathogens
1. INTRODUCTION
The rapid emergence of antibiotic resistant bacteria might be problematic in most of bacterial infection treatments and has become the biggest challenge in modern medicine [1, 2]. Repeated and improper applications of antibiotics could be the primary factor of the increasing number of drug-resistant bacteria [3]. Although it is important to consider different factors to develop any new therapeutics, researchers are looking for natural alternatives to common antibiotics with fewer side effects [4].
Antimicrobial Peptides (AMPs), as an excellent alternative to traditional antibiotics, were discovered in 1939. These peptides are one of the major groups of natural antibiotics containing several (between 12 to 100) amino acids isolated from a wide range of prokaryotes (bacteria) and eukaryotes (insects, plants, protozoans, fungi, and mammals) [4-7]. Based on AMPs multiple biological functions against various types of microorganisms such as viruses and parasites, they have recently attracted much attention. The antimicrobial peptides are divided into several groups. First, Cationic peptides such as Cecropins, Defensins, Thionins, Amino acid-enriched class, Histone derived compounds, Beta-hairpin and other natural structural and functional proteins. Second, Anionic peptides including Neuropeptide derived molecules, Aspartic-acid-rich mole-cules, Aromatic dipeptides, Oxygen-binding proteins [4].
Thanatin is the smallest member of Beta-hairpin class of cationic peptides and has a strong net cationic charge (+6) with low hemolytic activity, which was extracted from Podisus maculiventris insect (belonging to hemiptera family) after immune system simulation. Interestingly, it is the first antimicrobial peptide derived from insect with vast activity against various pathogens such as Gram-negative and Gram-positive bacteria and fungi [4, 8, 9]. This peptide has a disulfide bridge between two cysteines at amino acids 11 and 18, that is crucial for peptide’s biological function and has an important role in antimicrobial activity of the bacteria [10]. This peptide fights against the bacteria by agglutinating through binding to the outer membrane of lipopolysaccharide or cell wall peptidoglycan [11]. This peptide has no sequence homology with other insect defense molecules except frog skin secretions [2, 12]. It was reported that thanatin peptide might improve antibacterial activity of some antibiotics such as chloramphenicol, norfloxacin against multidrug-resistant E. aerogenes through energy-dependent efflux mechanism and persuaded a remarkable betterment of antibiotic susceptibility in contrast to sensitive cells [13]. Moreover, it was demonstrated that thanatin itself did not show a better antibiotic effect against Pseudomonas aeruginosa infections compared to conventional treatments, although it may be introduced as an alternative in combination with antibiotics [14].
In the present study, recombinant thanatin peptide was produced in HEK293 (Human Embryonic Kidney) cell line. Then its antimicrobial activities against some human bacterial pathogens as well as its toxicity on HEK293 and NIH cell lines were evaluated.
2. MATERIALS AND METHODS
2.1. Designing, Codon Optimization and Chemical Synthesis
The coding sequence of thanatin, after codon optimization for a proper expression in HEK293 cells, was chemically synthesized by GenScript Corporation (Piscataway, NJ, USA). A single Ig Kappa chain from Mus musculus (Igk) secretion leader was used (Figure 1). This signal peptide induces translocation of the protein into Endoplasmic Reticulum (ER) for secretion or membrane integration [15].
Figure 1.
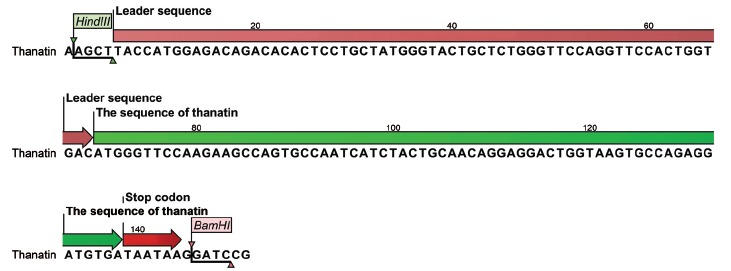
Schematic representation of the coding sequence of thanatin including IgK signal peptide (pink), thanatin sequence (green), and stop codon (red). (The color version of the figure is available in the electronic copy of the article).
2.2. Gene Cloning
The thanatin sequence in plasmid vector (pGHvector+thanatin) was artificially ordered. First, pGH and pcDNA3.1+ vectors were digested separately with HindIII restriction enzymes (Thermo Fisher Scientific, USA) and then purified by gel extraction kit (Thermo Fisher Scientific, USA). Then, the purified products were again digested with BamHI restriction enzyme (Thermo Fisher Scientific, USA) and purified. The next step in constructing a recombinant plasmid was to ligate the thanatin DNA sequence into a compatibly digested vector backbone by fast ligation kit (Thermo Fisher Scientific, USA). Subsequently, the recombinant plasmid was transformed into the E. coli strain DH5α (Invitrogen, USA). In order to identify DH5α containing recombinant plasmids, the bacteria were cultured on antibiotic culture medium. Only the bacteria containing the recombinant plasmid could be colonized on the culture medium containing antibiotics. Colony PCR was performed by universal pcDNA3.1+ primers for T7 promoter (forward: TAATACGACTCACTATAGGG) and BGH (reverse: TAGAAGGCACAGTCGAGG). Afterwards, colonies were harvested and plasmid elicitation was performed by HiPure Plasmid Isolation kit (Roche, Germany). Finally, mapping and sequencing were carried out.
2.3. Transfection and Expression
HEK293 cells (ATCC® CRL-1573™) were cultured in Dulbecco’s modified Eagle’s medium (DMEM/F12) supplemented with 10% Fetal Bovine Serum (FBS) and 1% amphotericin b (Invitrogen, USA) and were then grown in an incubator at 37°C with 5% CO2. For transfection, the cells were reseeded in a 35 mm culture plate and were then allowed to grow to 50-80% confluency. Moreover, calcium phosphate co-precipitation procedure was performed in DMEM/F12 medium supplemented with 10% FBS [16]. Next, the recombinant and self pCDNA3.1+ (with and without thanatin fragment) were transfected separately in cell lines, and transfected cells were selected after antibiotic therapy with 400 mgmL-1 Geneticin (Sigma-Aldrich, Germany) for two weeks. The supernatant containing 21–residue peptide was collected and was stored at 4°C for SDS-PAGE analysis.
2.4. Tricine SDS-PAGE Analysis
In this study, recombinant thanatin peptide was secreted into the culture media without any tags; thus, SDS-PAGE was used for size determination. Besides, 30 µL of the cultured supernatant (transfected cells by recombinant and self-ligated pcNDA3.1+) was electrophoresed on SDS-PAGE in Tris/glycine/SDS buffer using 16% polyacrylamide gels. The gel was stained with coomassie Brilliant Blue. The quantification of peptide band was carried out by GelQuant software v 1.8.2 (www.biochemlabsolutions.com).
2.5. Minimal Inhibitory Concentration Test on Some Strains of Bacteria
Methicillin resistant staphylococcus aureus (MRSA) ATCC3359, Acinetobacter baumannii ATCC 13304, Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 700603, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212 were obtained from American Type Culture Collection (USA). Pseudomonas aeruginosa VIM+, Vancomycin resistant Enterococci (VRE) Salmonella typhi, Salmonella paratyphi C, Listeria monocytogenes, Shigella dysantriae were obtained from Mashhad School of Medicine Collection, Mashhad, Iran.
Minimum Inhibitory Concentrations (MIC) was used based on a microbroth dilution method by 96-well microtiter with four replications [17].
The bacteria were cultured overnight on nutrient agar and was suspended to approximately 1 × 106 CFU mL−1, then 100 μL volumes were placed into a 96-well microtiter plate. A 100μL medium containing thanatin peptide from stock solution was used to prepare a serial dilution; 100 μL from each dilution was added to each 96-well microtiter plate. The plate was incubated at 37 °C for 24 h without shaking. In this study, the lowest concentration of thanatin that could inhibit the bacterial growth was used as MIC for different bacteria.
2.6. Cytotoxicity Assays on Human Cells
To determine thanatin toxicity on mammalian cells, MTT assay was used. NIH/3T3 (ATCC® CRL-1658™) cells were cultured by Minimum Essential Medium with fetal bovine serum. Cells were seeded into 96-well plate at a concentration of 1 × 105 cells per well and then incubated in a 5% CO2 incubator for 24 h at 37°C. A day after the initial plating, the cultured cells with 3 different dilutions of thanatin were stored in triplicates for 48 h under similar conditions. Subsequently, the cells were treated with10 μL of MTT working solution (5 mgmL-1) (Sigma Chemical Co., St. Louis, MO) and incubated for 4 h. The supernatant of medium was elicited, and the remaining formazan crystals were dissolved in 100 μL of DMSO per well for 30 min at 37°C in a CO2 incubator. Finally, the absorbance was measured at 570 nm by Epoch Microplate Spectrophotometer (BioTek, USA). All statistical analysis was performed using R software version 3.0.
3. RESULTS
3.1. Cloning and Expression of Thanatin Peptide Gene
The results showed that the restriction digestion (Figure 2A) and colony PCR amplification (Figure 2B) of thanatin peptide was successful. The sequencing revealed that the fragment was inserted into the correct frame without any mutation in pcDNA3.1+ vector.
Figure 2.
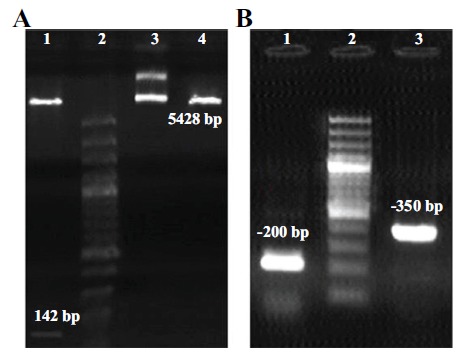
A) Restriction digestion with HindIII and BamHI enzymes lane 1. Leader sequence+thanatin (142 bp fragment) lane 2. DNA size marker 100 bp lane 3. Circular pcDNA3.1+ inserted sequence lane 4. Linear pcDNA3.1+ B) Colony PCR amplification with universal pcDNA3.1+ primers lane 1. The amplified sequence of self-ligated pcDNA3.1+ (without thanatin insert) lane 2. Marker 100 bp lane 3. The amplified sequence of recombinant pcDNA3.1+ with thanatin.
3.2. Tricine–SDS–PAGE
The expression of thanatin in HEK293 cells was evaluated by Tricine-SDS-PAGE (Figure 3). A protein band with a size about 2.4 KDa was observed on the gel, which suggested that the thanatin peptide was properly expressed in HEK293 cells and then secreted into the culture medium. The concentration of thanatin in culture medium was approximately estimated to be 164.21 μmolL-1.
Figure 3.
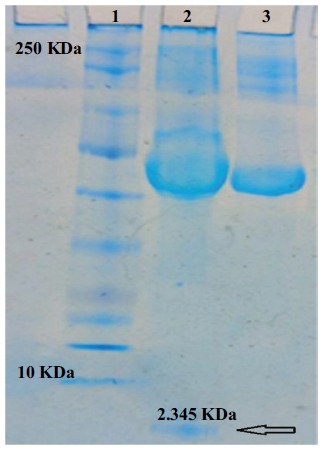
SDS-PAGE analysis of secreted thanatin from HEK293 cells in culture medium. lane 1. Protein marker 10-250 KDa lane 2. 2.345 KDa thanatin peptide (Shown by an arrow) lane 3. Control cells transformed with self-ligated vector without thanatin sequence.
3.3. MIC Assays of Thanatin Peptide
The results of MIC assay against typical gram-positive and gram-negative bacteria are summarized in Table 1. According to the results, thanatin showed a strong activity against Staphylococcus aureus ATCC 12923 and Staphylococcus aureus ATCC 25923 with the least value of MIC (2.57 μmolL-1). In contrast, Escherichia coli ATCC 25922 showed the highest value of MIC (82.10 μmolL-1). In this study thanatin did not show any activity against drug-resistant bacteria, except for Methicillin resistant Staphylococcus aureus (MRSA) ATCC 33591. In addition, the comparison of MIC obtained from thanatin peptide with Gentamycin, Vancomycin, Ampicillin, Ceftazidime antibiotics is shown in Table 1. The results showed that this peptide has an acceptable antibacterial activity compared to some antibiotics.
Table 1. Comparative Minimum Inhibitory Concentration (MIC) of the recombinant Thanatin with antibiotics suggested by Clinical and Laboratory Standard Institute (CLSI).
| Strain | Antibacterial Activity (μgml-1) | |||||
|---|---|---|---|---|---|---|
| Thanatin Peptide | Gentamycin | Vancomycin | Ampicillin | Ceftazidime | ||
| Gram positive bacteria | ||||||
| Listeria monocytegenes | 76.92 | 31.58 | ≥32 | ND | ≥32 | ND |
| Staphylococcus aureus ATCC 25923 | 2.40 | 0.99 | ≥16 | ≥16 | 0.5-2 | ND |
| Staphylococcus aureus ATCC 29213 | 2.40 | 0.99 | ≥16 | ≥16 | 0.5-2 | ND |
| Resistant strain (Gram positive bacteria) | ||||||
| Methicillin resistant Staphylococcus aureus (MRSA) ATCC 33591 | 9.61 | 3.95 | ≥16 | ≥16 | ND | ND |
| Vancomycin resistant Enterococci (VRE) a | Resistant | ≥16 | ≥32 | ND | ND | |
| Gram negative bacteria | ||||||
| Shigella dysenteriae a | 38.46 | 15.79 | ≥16 | ND | ≥32 | ND |
| Escherichia coli ATCC 25922 | 76.92 | 31.58 | ≥16 | ND | 2-8 | ≥16 |
| Salmonella typhi a | 4.80 | 1.97 | ≥16 | ND | ≥32 | ≥16 |
| Salmonella paratyphi C a | 19.23 | 7.89 | ≥16 | ND | ≥32 | ≥16 |
| Resistant strain (Gram negative bacteria) | ||||||
| Pseudomonas aeruginosa VIM+ a | Resistant | ≥16 | ND | ≥16 | ≥32 | |
| Klebsiella pneumonia ATCC 700603 | Resistant | ≥16 | ND | ≥128 | ≥16 | |
| Acinetobacter baumannii ATCC 13304 | Resistant | ≥16 | ND | ND | ≥32 | |
Data is collected as MICs according to CLSI (www.clsi.org), ND: Not determined.
aFrom Mashhad School of Medicine Collection, Mashhad, Iran.
3.4. Cytotoxicity Assays on Human Cells
In the present study, the potential cytotoxicity of thanatin at different concentrations was evaluated by MTT assay. The result of cytotoxicity test on NIH cells (up to 394.10 μmolL-1 for 48 hours) showed no significant differences between various concentrations of thanatin and control group (P > 0.05). Also no adverse effects were observed on HEK293 cells producing thanatin peptide.
4. DISCUSSION
In the present study, we have used HEK expression system for recombinant production of thanatin peptide. Using expressive E. coli BL21 system is common because of its low cost, clear genetic background, and its simple culture, but several studies have shown that this system is not suitable for small size and high expression AMPs since it kills the bacteria [18]. Furthermore, it was shown that the quantity of thanatin expression in HEK293 cells was higher than that of Pichia Pastoris [19]. This peptide was previously expressed in Pichia and prokaryotic expression systems and showed acceptable activity towards some bacterial pathogens [19, 20].
In this study, thanatin did not show any antimicrobial activity against drug-resistant bacteria, except Methicillin resistant Staphylococcus aureus (MRSA) ATCC 33591. It might be related to higher concentrations of thanatin which is required in growth prevention of these bacteria. It has been proven that AMPs could have a synergism activity with other chemicals such as antibiotics, essential oils [21, 22].
The main mechanism of many AMPs is the membrane disruption, but some antimicrobial peptides like single peptides could not create a pore in a lipid membrane. The tendency to form intra-molecular connections is also important in peptide’s function, which is concentration-dependent. During peptides encounter with the pathogens, they might use two methods to disrupt the membrane, they either accumulate on the surface or once they reach a critical concentration, they penetrate into the membrane [23]. A single peptide could have multiple cellular targets which simultaneously leads to the death of microorganisms [24]. Some peptides have different mechanism against microorganisms including indolicidin (membrane depolarization and DNA synthesis inhibition), Nisin (by binding to lipid II, forms membrane pores and lipids III and IV cause autolysin function), PR-39 (blocks bacterial DNA and protein synthesis and it also inhibits the degradation of inhibitor of nuclear factor κB). However, the intracellular targets of thanatin have not been reported yet [10].
Some features of antibacterial activity of cationic peptide are as follows: amino acid profile, sequence direction, structural conformation [25], and membrane lipid composition [23]. In other words, AMPs destroy the bacteria using different modes including pore formation in plasma membrane or through barrel-stave pore model, toroidal pore model or an overall detergent-like action [26-28]. It was demonstrated that the antimicrobial activity of thanatin was independent from multi-drug resistance spectrum of bacteria which could be due to the different membrane composition of this bacteria [29, 30]. Also we showed that thanatin is more active against Salmonella typhi than other Gram negative species. According to Richards, 2010 S. typhi was more sensitive to AMPs than S. typhimurium, which might be due to gene regulations involved in modification of lipid A. Lipid A gives a negative charge to the bacterial surface which may attract some AMPs; thus, make bacteria more sensitive to AMPs [31]. Mutations in lipid A modifying enzymes may exhibit an increase in sensitivity to AMP [29].
Unlike pore forming AMPs, some AMPs such as thanatin could kill the bacteria through cell agglutination. On the other hand, they directly interact with the components within the bacterial membranes such as Lipopolysaccharide (LPS) of gram negative or cell wall peptidoglycans of gram positive bacteria, which leads to the inhibition of cell wall synthesis [11, 32]. Unfortunately, the use of some antimicrobial peptides such as pore forming AMPs, as alternative to chemical antibiotics, has been limited due to hemolytic toxicity and other types of cytotoxicity [33]. In contrast, thanatin can agglutinate bacteria and then can be efficiently removed by immune system without releasing toxic substances [11, 34]. The MIC of thanatin against gram-negative and positive bacteria and fungi is mostly reported under 2.5 μM (6.089μgml-1) [35, 36]. Experiments on the effect of S-thanatin on Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Enterobacter aerogenes, Klebsiella ornithinolytica and Klebsiella oxytoca showed that MICs were between 4 and 16 μgml-1, and in gram positive bacteria, MIC was above 128 μgml-1 [30, 37].
Current treatment of antibiotic resistant microorganisms is an important challenge. The number of antibiotic-resistant bacteria has been increasing significantly, especially resistance to beta-lactam antibiotics. Generally, the mechanisms of resistance to beta-lactam antibiotics are as follows: mutations and changes in the target molecule; altering the enzymes and turning them into a family of hydrolyzing and inactive beta-lactam called beta-lactamases; removing purine proteins in the bacterial cell wall; activating the Efflux pump in order to exhaust antibiotics from the bacteria; altering the cell wall of the bacterium to minimize its antibiotic access [38] and reducing the activity of Autolytic enzymes [17]. AMPs seem to be a proper solution [39]. Thanatin is an AMP with antibacterial activities against drug-resistant bacteria [9, 40] such as β-lactamase-producing Escherichia coli with minor hemolytic toxicity [41]. S-thanatin, as an analog of thanatin, could be a promising candidate against some antibiotic resistant bacteria [42]. It was reported that shorter derivative peptide (R-thanatin) had higher antibacterial features against coagulase-negative staphylococci, including S. epidermidis, S. haemolyticus, and S. hominis at concentrations of 2 to 64 μgml-1. Additionally, this peptide inhibited the growth and biofilm formation of methicillin-resistant S. epidermidis (MRSE) in vitro and in vivo conditions [33].
In this study, thanatin had no cytotoxicity on NIH cells, which makes this peptide a proper candidate in treating human diseases. In case of eukaryotes and prokaryotes, different cell membranes have different composition and structure. Eukaryotic cell membranes have more cholesterol compared to bacterial cells, which seems to be related to this process. The presence of this neutral lipid in the membranes of animal cells inhibited the fragmentation of lipid vesicles by cationic, linear Magainin derived AMPs [43, 44]. Although, the mechanism of thanatin peptide against cells has not been fully explained, three anticellular mechanisms have been reported for AMPs: cell plasma membrane disruption (extracellular mechanism), inhibition of angiogenesis and activation of apoptosis (intracellular mechanism) [45]. Since membrane disruption is not the only mechanism of action of antimicrobial peptides, the intracellular targets of thanatin have not been yet reported due to the low permeability of thanatin [10]; therefore, thanatin may not effectively inhibit cell growth through intracellular mechanisms.
Investigating the cytotoxicity of R-thanatin on human in vitro umbilical vein endothelial cells revealed that R-thanatin, up to concentration of 1.024 μgml-1, had little toxicity in mammalian cells (P> 0.05) [33]. In another study, it was shown that cyclic thanatin (C-thanatin) and linear thanatin (L-thanatin) had toxicity against human Red Blood Cells (hRBCs) and Human Umbilical Vein Endothelial Cells (HUVECs) at concentrations as high as 256 μgml-1 [10].
CONCLUSION
In conclusion, the results obtained in this study confirmed the feasibility of mammalian cell lines for heterologous expression of thanatin. Leader and secretion sequence were placed in gene cassette to promote the expression and to facilitate the harvest of recombinant protein. We included a leader sequence (Igk) to the N terminus of thanatin peptide sequence, which allowed peptide secretion to culture medium. An important finding here was the low cytotoxicity of thanatin for mammalian cells. The antimicrobial assay conducted in the present study showed that the recombinant thanatin had an antibacterial effect on some tested species. Synergy of thanatin with other components such as antibiotics, essential oils or even other AMPs might be another option to consider in future research to overcome antibiotic resistant pathogens.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Clinical and Laboratory Standard Institute (CLSI) at www.clsi.org.
FUNDING
This study was supported by grant number 2/47173 from Ferdowsi University of Mashhad.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Butler M.S., Blaskovich M.A., Cooper M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. (Tokyo) 2013;66(10):571–591. doi: 10.1038/ja.2013.86. [http://dx.doi.org/10.1038/ja.2013.86]. [PMID: 24002361]. [DOI] [PubMed] [Google Scholar]
- 2.Simmaco M., Mignogna G., Barra D., Bossa F. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J. Biol. Chem. 1994;269(16):11956–11961. [PMID: 8163497]. [PubMed] [Google Scholar]
- 3.Laxminarayan R., Brown G.M. Economics of antibiotic resistance: a theory of optimal use. J. Environ. Econ. Manage. 2001;42(2):183–206. [Google Scholar]
- 4.Vizioli J., Salzet M. Antimicrobial peptides from animals: focus on invertebrates. Trends Pharmacol. Sci. 2002;23(11):494–496. doi: 10.1016/s0165-6147(02)02105-3. [http://dx.doi.org/10.1016/S0165-6147(02)02105-3]. [PMID: 12413797]. [DOI] [PubMed] [Google Scholar]
- 5.Bahar A.A., Ren D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013;6(12):1543–1575. doi: 10.3390/ph6121543. [http://dx.doi.org/10.3390/ph6121543]. [PMID: 24287494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenssen H., Hamill P., Hancock R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [http://dx.doi.org/10.1128/CMR.00056-05]. [PMID: 16847082]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Luo J., Xu C., Ren F., Peng C., Wu G., Zhao J. Purification, characterization, and molecular cloning of the gene of a seed-specific antimicrobial protein from pokeweed. Plant Physiol. 2000;122(4):1015–1024. doi: 10.1104/pp.122.4.1015. [http://dx.doi.org/10.1104/pp.122.4.1015]. [PMID: 10759497]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimarcq J.L., Bulet P., Hetru C., Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers. 1998;47(6):465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [http://dx.doi.org/10.1002/(SICI)1097-0282(1998)47:6<465:AID-BIP5>3.0.CO;2-#]. [PMID: 10333738]. [DOI] [PubMed] [Google Scholar]
- 9.Fehlbaum P., Bulet P., Chernysh S., Briand J.P., Roussel J.P., Letellier L., Hetru C., Hoffmann J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 1996;93(3):1221–1225. doi: 10.1073/pnas.93.3.1221. [http://dx.doi.org/10.1073/pnas.93.3.1221]. [PMID: 8577744]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma B., Niu C., Zhou Y., Xue X., Meng J., Luo X., Hou Z. The disulfide bond of the peptide thanatin is dispensable for its antimicrobial activity in vivo and in vitro. Antimicrob. Agents Chemother. 2016;60(7):4283–4289. doi: 10.1128/AAC.00041-16. [http://dx.doi.org/10.1128/AAC.00041-16]. [PMID: 27161645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha S., Zheng L., Mu Y., Ng W.J., Bhattacharjya S. Structure and interactions of a host defense antimicrobial peptide thanatin in lipopolysaccharide micelles reveal mechanism of bacterial cell agglutination. Sci. Rep. 2017;7(1):17795. doi: 10.1038/s41598-017-18102-6. [http://dx.doi.org/10.1038/s41598-017-18102-6]. [PMID: 29259246]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morikawa N., Hagiwara K., Nakajima T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992;189(1):184–190. doi: 10.1016/0006-291x(92)91542-x. [http://dx.doi.org/10.1016/0006-291X(92)91542-X]. [PMID: 1449472]. [DOI] [PubMed] [Google Scholar]
- 13.Pagès J.M., Dimarcq J.L., Quenin S., Hetru C. Thanatin activity on multidrug resistant clinical isolates of Enterobacter aerogenes and Klebsiella pneumoniae. Int. J. Antimicrob. Agents. 2003;22(3):265–269. doi: 10.1016/s0924-8579(03)00201-2. [http://dx.doi.org/10.1016/S0924-8579(03)00201-2]. [PMID: 13678832]. [DOI] [PubMed] [Google Scholar]
- 14.Cirioni O., Wu G., Li L., Orlando F., Silvestri C., Ghiselli R., Shen Z., Gabrielli E., Brescini L., Lezoche G., Provinciali M., Guerrieri M., Giacometti A. S-thanatin in vitro prevents colistin resistance and improves its efficacy in an animal model of Pseudomonas aeruginosa sepsis. Peptides. 2011;32(4):697–701. doi: 10.1016/j.peptides.2011.01.016. [http://dx.doi.org/10.1016/j.peptides.2011.01.016]. [PMID: 21262298]. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez R., Jennings L.L., Knuth M., Orth A.P., Klock H.E., Ou W., Feuerhelm J., Hull M.V., Koesema E., Wang Y., Zhang J., Wu C., Cho C.Y., Su A.I., Batalov S., Chen H., Johnson K., Laffitte B., Nguyen D.G., Snyder E.Y., Schultz P.G., Harris J.L., Lesley S.A. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc. Natl. Acad. Sci. USA. 2010;107(8):3552–3557. doi: 10.1073/pnas.0914019107. [http://dx.doi.org/10.1073/pnas.0914019107]. [PMID: 20133595]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan M., Schallhorn A., Wurm F.M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24(4):596–601. doi: 10.1093/nar/24.4.596. [http://dx.doi.org/10.1093/nar/24.4.596]. [PMID: 8604299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomanen E., Durack D.T., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob. Agents Chemother. 1986;30(4):521–527. doi: 10.1128/aac.30.4.521. [http://dx.doi.org/10.1128/AAC.30.4.521]. [PMID: 3539006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryksa B.C., MacDonald L.D., Patrzykat A., Douglas S.E., Mattatall N.R. A C-terminal glycine suppresses production of pleurocidin as a fusion peptide in Escherichia coli. Protein Expr. Purif. 2006;45(1):88–98. doi: 10.1016/j.pep.2005.04.010. [http://dx.doi.org/10.1016/j.pep.2005.04.010]. [PMID: 15935695]. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Zhu M., Chen X., Yang G., Yang T., Yu L., Wang X. Expression and antibacterial activity of hybrid antimicrobial peptide cecropinA-thanatin in Pichia pastoris. Front. Lab. Med. 2018;2(1):23–29. [http://dx.doi.org/10.1016/j.flm.2018.04.001]. [Google Scholar]
- 20.Wang L.N., Yu B., Han G.Q., He J., Chen D.W. Design, expression and characterization of recombinant hybrid peptide Attacin-Thanatin in Escherichia coli. Mol. Biol. Rep. 2010;37(7):3495–3501. doi: 10.1007/s11033-009-9942-3. [http://dx.doi.org/10.1007/s11033-009-9942-3]. [PMID: 19967452]. [DOI] [PubMed] [Google Scholar]
- 21.Shurko J.F., Galega R.S., Li C., Lee G.C. Evaluation of LL-37 antimicrobial peptide derivatives alone and in combination with vancomycin against S. aureus. J. Antibiot. (Tokyo) 2018;71(11):971–974. doi: 10.1038/s41429-018-0090-7. [http://dx.doi.org/10.1038/s41429-018-0090-7]. [PMID: 30120393]. [DOI] [PubMed] [Google Scholar]
- 22.Zouhir A., Jridi T., Nefzi A., Ben Hamida J., Sebei K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm. Biol. 2016;54(12):3136–3150. doi: 10.1080/13880209.2016.1190763. [http://dx.doi.org/10.1080/13880209.2016.1190763]. [PMID: 27246787]. [DOI] [PubMed] [Google Scholar]
- 23.Sani M.A., Separovic F. How membrane-active peptides get into lipid membranes. Acc. Chem. Res. 2016;49(6):1130–1138. doi: 10.1021/acs.accounts.6b00074. [http://dx.doi.org/10.1021/acs.accounts.6b00074]. [PMID: 27187572]. [DOI] [PubMed] [Google Scholar]
- 24.Guilhelmelli F., Vilela N., Albuquerque P. Derengowski, Lda.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013;4:353. doi: 10.3389/fmicb.2013.00353. [http://dx.doi.org/10.3389/fmicb.2013.00353]. [PMID: 24367355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 1997;156(3):197–211. doi: 10.1007/s002329900201. [http://dx.doi.org/10.1007/s002329900201]. [PMID: 9096062]. [DOI] [PubMed] [Google Scholar]
- 26.Choi H., Chakraborty S., Liu R., Gellman S.H., Weisshaar J.C. Single-cell, time-resolved antimicrobial effects of a highly cationic, random nylon-3 copolymer on live Escherichia coli. ACS Chem. Biol. 2016;11(1):113–120. doi: 10.1021/acschembio.5b00547. [http://dx.doi.org/10.1021/acschembio.5b00547]. [PMID: 26493221]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66(4):236–248. doi: 10.1002/bip.10260. [http://dx.doi.org/10.1002/bip.10260]. [PMID: 12491537]. [DOI] [PubMed] [Google Scholar]
- 28.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5(10):905–917. doi: 10.1021/cb1001558. [http://dx.doi.org/10.1021/cb1001558]. [PMID: 20698568]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Band V.I., Weiss D.S. Mechanisms of antimicrobial peptide resistance in gram-negative bacteria. Antibiotics (Basel) 2015;4(1):18–41. doi: 10.3390/antibiotics4010018. [http://dx.doi.org/10.3390/antibiotics4010018]. [PMID: 25927010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu G., Li X., Fan X., Wu H., Wang S., Shen Z., Xi T. The activity of antimicrobial peptide S-thanatin is independent on multidrug-resistant spectrum of bacteria. Peptides. 2011;32(6):1139–1145. doi: 10.1016/j.peptides.2011.03.019. [http://dx.doi.org/10.1016/j.peptides.2011.03.019]. [PMID: 21453736]. [DOI] [PubMed] [Google Scholar]
- 31.Richards S.M. PhoPQ- and PmrAB-mediated Lipopolysaccharide Modification and Cationic Antimicrobial Peptide Resistance in Salmonella enterica Serovars Typhimurium and Typhi. Ph.D. Desertion. The Ohio State University; 2010. [Google Scholar]
- 32.Jung S., Sönnichsen F.D., Hung C.W., Tholey A., Boidin-Wichlacz C., Haeusgen W., Gelhaus C., Desel C., Podschun R., Waetzig V., Tasiemski A., Leippe M., Grötzinger J. Macin family of antimicrobial proteins combines antimicrobial and nerve repair activities. J. Biol. Chem. 2012;287(17):14246–14258. doi: 10.1074/jbc.M111.336495. [http://dx.doi.org/10.1074/jbc.M111.336495]. [PMID: 22396551]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Z., Da F., Liu B., Xue X., Xu X., Zhou Y., Li M., Li Z., Ma X., Meng J., Jia M., Wang Y., Luo X. R-thanatin inhibits growth and biofilm formation of methicillin-resistant Staphylococcus epidermidis in vivo and in vitro. Antimicrob. Agents Chemother. 2013;57(10):5045–5052. doi: 10.1128/AAC.00504-13. [http://dx.doi.org/10.1128/AAC.00504-13]. [PMID: 23917310]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torrent M., Pulido D., Nogués M.V., Boix E. Exploring new biological functions of amyloids: bacteria cell agglutination mediated by host protein aggregation. PLoS Pathog. 2012;8(11):e1003005. doi: 10.1371/journal.ppat.1003005. [http://dx.doi.org/10.1371/journal.ppat.1003005]. [PMID: 23133388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulet P., Hetru C., Dimarcq J.L., Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999;23(4-5):329–344. doi: 10.1016/s0145-305x(99)00015-4. [http://dx.doi.org/10.1016/S0145-305X(99)00015-4]. [PMID: 10426426]. [DOI] [PubMed] [Google Scholar]
- 36.Javadmanesh A., Tanhaeian A., Mousavi S.Z., Azghandi M. Investigation of recombinant thanatin effects on the growth inhibition of e. coli mastitis in dairy cows; Proceedings of the 2nd International Congress on Biomedicine; Tehran, Iran. December 24-27, 2018. [Google Scholar]
- 37.Wu G.Q., Ding J.X., Li L.X., Wang H.L., Zhao R., Shen Z.L. Activity of the antimicrobial peptide and thanatin analog S-thanatin on clinical isolates of Klebsiella pneumoniae resistant to conventional antibiotics with different structures. Curr. Microbiol. 2009;59(2):147–153. doi: 10.1007/s00284-009-9410-2. [http://dx.doi.org/10.1007/s00284-009-9410-2]. [PMID: 19459007]. [DOI] [PubMed] [Google Scholar]
- 38.Fisher J.F., Meroueh S.O., Mobashery S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005;105(2):395–424. doi: 10.1021/cr030102i. [http://dx.doi.org/10.1021/cr030102i]. [PMID: 15700950]. [DOI] [PubMed] [Google Scholar]
- 39.Mansour S.C., Pena O.M., Hancock R.E. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35(9):443–450. doi: 10.1016/j.it.2014.07.004. [http://dx.doi.org/10.1016/j.it.2014.07.004]. [PMID: 25113635]. [DOI] [PubMed] [Google Scholar]
- 40.Robert É., Lefèvre T., Fillion M., Martial B., Dionne J., Auger M. Mimicking and understanding the agglutination effect of the antimicrobial peptide thanatin using model phospholipid vesicles. Biochemistry. 2015;54(25):3932–3941. doi: 10.1021/acs.biochem.5b00442. [http://dx.doi.org/10.1021/acs.biochem.5b00442]. [PMID: 26057537]. [DOI] [PubMed] [Google Scholar]
- 41.Hou Z., Lu J., Fang C., Zhou Y., Bai H., Zhang X., Xue X., Chen Y., Luo X. Underlying mechanism of in vivo and in vitro activity of C-terminal-amidated thanatin against clinical isolates of extended-spectrum β-lactamase-producing Escherichia coli. J. Infect. Dis. 2011;203(2):273–282. doi: 10.1093/infdis/jiq029. [http://dx.doi.org/10.1093/infdis/jiq029]. [PMID: 21288828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu G., Fan X., Li L., Wang H., Ding J., Hongbin W., Zhao R., Gou L., Shen Z., Xi T. Interaction of antimicrobial peptide s-thanatin with lipopolysaccharide in vitro and in an experimental mouse model of septic shock caused by a multidrug-resistant clinical isolate of Escherichia coli. Int. J. Antimicrob. Agents. 2010;35(3):250–254. doi: 10.1016/j.ijantimicag.2009.11.009. [http://dx.doi.org/10.1016/j.ijantimicag.2009.11.009]. [PMID: 20045294]. [DOI] [PubMed] [Google Scholar]
- 43.Lee D.K., Bhunia A., Kotler S.A., Ramamoorthy A. Detergent-type membrane fragmentation by MSI-78, MSI-367, MSI-594, and MSI-843 antimicrobial peptides and inhibition by cholesterol: a solid-state nuclear magnetic resonance study. Biochemistry. 2015;54(10):1897–1907. doi: 10.1021/bi501418m. [http://dx.doi.org/10.1021/bi501418m]. [PMID: 25715195]. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki K., Sugishita K., Fujii N., Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry. 1995;34(10):3423–3429. doi: 10.1021/bi00010a034. [http://dx.doi.org/10.1021/bi00010a034]. [PMID: 7533538]. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Xiang Q., Zhang Q., Huang Y., Su Z. Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides. 2012;37(2):207–215. doi: 10.1016/j.peptides.2012.07.001. [http://dx.doi.org/10.1016/j.peptides.2012.07.001]. [PMID: 22800692]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available in the Clinical and Laboratory Standard Institute (CLSI) at www.clsi.org.


