Abstract
Glaucoma is the second leading cause of blindness worldwide. Even though significant advances have been made in its management, currently available antiglaucoma therapies suffer from considerable drawbacks. Typically, the success and efficacy of glaucoma medications are undermined by their limited bioavailability to target tissues and the inadequate adherence demonstrated by patients with glaucoma. The latter is due to a gradual decrease in tolerability of lifelong topical therapies and the significant burden to patients of prescribed stepwise antiglaucoma regimens with frequent dosing which impact quality of life. On the other hand, glaucoma surgery is restricted by the inability of antifibrotic agents to efficiently control the wound healing process without causing severe collateral damage and long-term complications. Evolution of the treatment paradigm for patients with glaucoma will ideally include prevention of retinal ganglion cell degeneration by the successful delivery of neurotrophic factors, anti-inflammatory drugs, and gene therapies. Nanotechnology-based treatments may surpass the limitations of currently available glaucoma therapies through optimized targeted drug delivery, increased bioavailability, and controlled release. This review addresses the recent advances in glaucoma treatment strategies employing nanotechnology, including medical and surgical management, neuroregeneration, and neuroprotection.
Keywords: Glaucoma, Nanomedicine, Nanoparticles, Nanosystems, Nanotechnology, Ocular drug delivery, Ophthamlology
Key Summary Points
| This article offers a comprehensive review of nanotechnology-based treatments for patients with glaucoma. |
| Nanotechnology-based drugs will probably be incorporated into the arsenal of glaucoma specialists in the near future, allowing benefits such as reduced side effects, and less frequent dosing, among others. |
| Toxicity issues related to nanotechnology-based treatments have yet to be addressed to test their safety. |
| Further human studies in nanomedicine for glaucoma treatment are needed until this promising pharmacological innovation becomes available in the ophthalmologist’s daily therapeutic practice. |
Introduction
Glaucoma is the second leading cause of blindness worldwide [1, 2] and the most frequent cause of irreversible blindness. Indeed, the World Health Organization estimates that 5.2 million cases of blindness are a result of glaucoma (15% of the total burden of world blindness) [3]. Glaucoma is often characterized by elevated intraocular pressure (IOP), caused predominantly by blockage of the outflow system leading to the progressive death of retinal ganglion cells (RGCs) and resulting in optic neuropathy [4].
The main goal of virtually all glaucoma therapies today is to reduce IOP by either suppressing aqueous synthesis or by enhancing trabecular meshwork (TM) and uveoscleral outflow of aqueous humor [5–7]. Other proposed modalities for the treatment of glaucoma include laser [8], surgery [9], and non-IOP-dependent therapies such as neuroprotection [10].
Recently, research has focused on nanoparticles and their unique properties, which can provide a platform to surpass the limitations of traditional glaucoma therapies. Although some reviews have been published [11–15], we aimed to expand available evidence and provide a comprehensive summary of recent advances in nanotechnology-based antiglaucoma therapies with a focus on the following topics: hypotensive drugs, wound healing modulation, neuroprotection, and neuroregeneration.
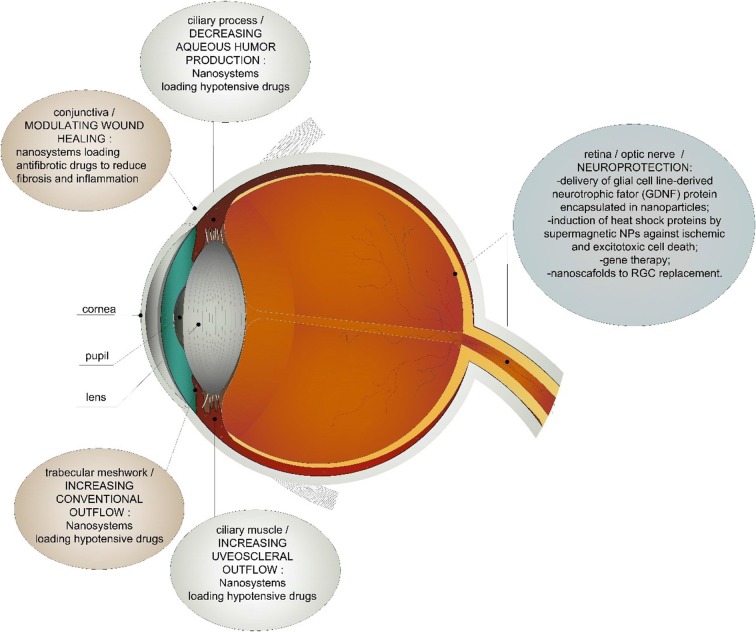
Figure 1 summarizes the main therapeutic targets in glaucoma treatment according to their anatomical location. Importantly, all of them could benefit from nanotechnology-based strategies.
Fig. 1.
Glaucoma therapeutic strategies and nanotechnology-based potential applications
Targeting Intraocular Pressure Reduction
Elevated IOP is considered the key risk factor for optic nerve damage in glaucoma, and most patients benefit from IOP-lowering therapies [16]. Despite the evidence that factors other than IOP are involved in the pathogenesis of glaucoma [17, 18], current treatment algorithms are based on decreasing IOP. First-line glaucoma therapy typically starts with eye drops that lower IOP by two mechanisms: suppression of aqueous humor production (beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors) or increase of aqueous humor outflow though the trabecular or uveoscleral pathways (pilocarpine, epinephrine, or prostaglandin analogues) [19]. In patients where topical medications are not sufficient to controI IOP, laser or surgery can be indicated. We will discuss both treatment strategies in the scenario of recent advancements in nanomedicine. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Drug Delivery Routes, Features, and Limitations of Current Glaucoma Medical Therapy
Although medical therapy of glaucoma is effective, it relies on a delivery system that is generally inefficient and presents several drawbacks. First, eye drops have to be administered 1–3 times daily, which is frequently associated with suboptimal use and lack of adherence [20]. This is a more pronounced issue in the elderly population that is predominantly affected by glaucoma [21]. A previous report showed that 60% of patients expressed one or more problems with taking their glaucoma medications, which results in a decreased adherence to long-term therapy [22]. Furthermore, following instillation, the bioavailability of topical glaucoma medications in the anterior chamber is very low [23]. Typical aqueous humor bioavailability of a drug following the administration of eye drops ranges from 1% to 10% in animal [24] and human studies [25]. This is due to the various physiological barriers to drug penetration into the eye [26].
There are two routes that control and influence the absorption of topically administered medication: the corneal and the non-corneal routes. Small molecules with a hydroxyl group have high corneal permeability, favoring the corneal route [27]. The trajectory of the drug molecules starts with their passive diffusion via the corneal epithelium, through the stroma and endothelium into the anterior chamber, where the drug will carry out its pharmacological action [28] or will bind to melanin in the ciliary body and iris [29], or plasma proteins [30], which prolongs its half-life.
The remaining drug and drug metabolites will be removed via the trabecular meshwork through Schlemm’s canal into the systemic bloodstream (conventional route), or via the uveoscleral route (unconventional outflow). Both of these pathways lead to the systemic blood circulation [31]. The “unconventional” route that includes the ciliary muscle, supraciliary and suprachoroidal spaces, may drain aqueous humor and/or drugs and their metabolites through two possible pathways: a uveovortex pathway where they enter the choroid to drain through the vortex veins, and a uveoscleral pathway where they pass across the sclera to be resorbed by orbital vessels [32]. In addition to these two routes, there is a third route, the “uveolymphatic pathway”, defined by Yücel et al. to describe a novel pathway for aqueous humor drainage through lymphatic channels in the human ciliary body, which can become a novel target for glaucoma treatment [33–35]. Drug penetration in the iris and diffusion via the aqueous humor flow occurs with a small portion of the drug (depending on the molecular weight and lipophilicity). Drug concentrations in the vitreous will be 10–100 times lower than in the aqueous humor and cornea, respectively [27].
The so-called non-corneal absorption route is preferred by drugs with low corneal permeability, such as proteins and large molecules. These large compounds penetrate the eye via the conjunctiva and/or sclera [36, 37]. This route delivers drugs to the vitreous via passive diffusion and allows 20 times lower drug concentration in the anterior chamber compared to the corneal route [38]. However, the conjunctiva and underlying sclera are more permeable and have a higher surface of absorption for the bloodstream than the cornea [39]. Furthermore, some hypotensive topical drugs, such as timolol and betaxolol, have been shown to accumulate in Tenon’s capsule, periocular tissues, and sclera of normal cynomolgus monkeys, and of glaucomatous patients under long-term topical therapy [40, 41]. These findings indicate that periocular accumulation of certain medications could provide differentiated access to the posterior segment and proximal vasculature of the eye.
Systemic absorption of topical medications can lead to systemic side effects such as hypotension, bronchospasm, and bradycardia (beta-blockers), or dry mouth and fatigue (alpha-agonists) [19]. Prostaglandin analogues are prodrugs which have the advantage of being cleaved to their active forms inside the eye, thereby minimizing side effects in nontarget organs [42].
There are numerous ongoing studies aimed at offering neuroprotective or neuroregenerative treatments [16], but clinical trials have been unsuccessful in the past at demonstrating their effectiveness in preventing glaucoma progression [43–45]. One of the main challenges of this approach has been how to allow access of the drug to the back of the eye, where the optic nerve and RGCs reside. There are two pathways to allow drugs to reach these posterior structures: crossing the vitreous and the inner limiting membrane of the retina, or accessing the retina through the vascular system. Intravitreal injections allow direct access to the vitreous cavity and bypass this barrier, but increase the risk for serious ocular adverse events, such as endophthalmitis [46]. The intravenous route is accompanied by systemic side effects and is particularly problematic since drugs must cross the blood–retinal barrier to reach the target tissue [47].
To overcome the multiple limitations of current medical antiglaucoma treatments, the development of safer and more efficient drug delivery systems is imperative. These attributes may be reached through the use of nanomedicine, which may improve solubility, reduce tissue irritation, prolong shelf life, and provide dose accuracy, sustained release, targeted delivery, and bioavailability [12].
Even though nanoparticulate systems can potentially improve drug bioavailability of the active loaded ingredients, they can also be selectively removed more or less quickly from the target organ by conventional, uveoscleral, and uveolymphatic routes, depending on the direct uptake of the nanoparticle, and their physicochemical specificities. Therefore, further pharmacokinetic studies specific for each nanosystem employed for therapeutic purposes will be required.
Role and Value of Nanoparticles
Nanoparticles (NPs) are tiny structures most commonly spherical in shape with sizes in the nanometer range (1–100 nm). As a result of their minuscule size, nanoparticles can bypass biological barriers, providing drug access directly to the target cells [48]. Moreover, smaller nanoparticles have a higher drug-loading capacity than the larger ones, owing to their higher surface area [49].
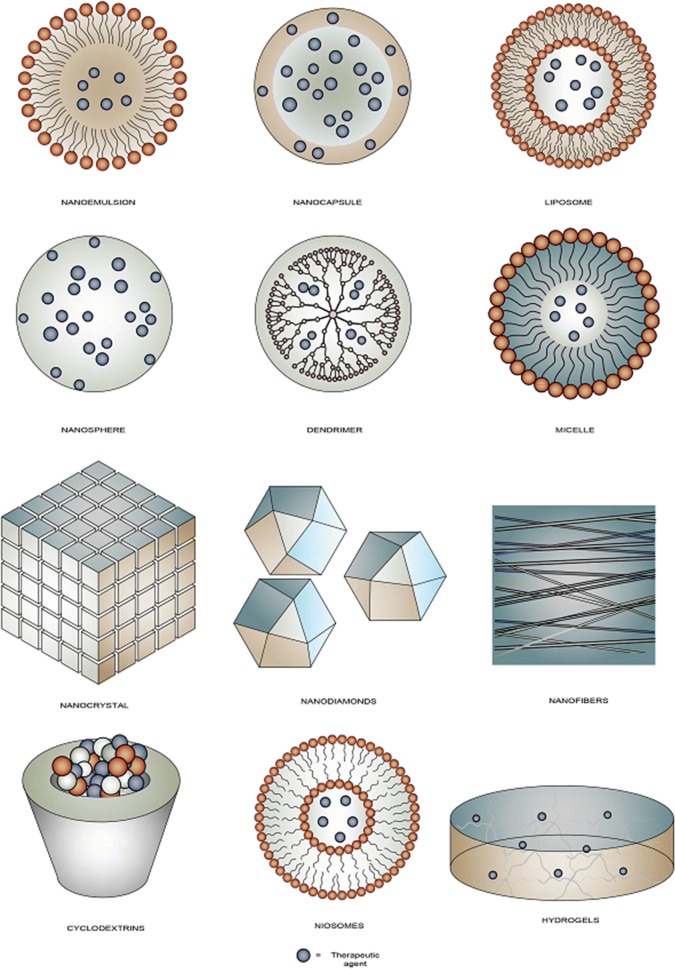
NPs are utilized in a wide range of shapes such as nanoemulsion [50], liposomes [51], dendrimers [52], nanospheres [53], hydrogels [54], nanocrystals [55], cyclodextrins [56], nanodiamonds [57], microspheres [58], niosomes [59], nanofibers [60], and nanocapsules [61]. Figure 2 shows a schematic representation of various nanoparticles mentioned throughout this article.
Fig. 2.
Schematic depiction of some nanoparticles
NPs can be designed to enhance active compound penetration [62] and drug targeting [63] and to promote controlled release [64]. Currently, NPs are generally made from sundry materials including polymers, lipids, and metals. Lipid and polymer NPs may be employed successfully to carry drugs [65], isolate their contents from degradation, and regulate their release [66]. Metallic NPs have specific physicochemical properties that serve both diagnostic [67] and therapeutic purposes [68, 69]. Injected nanoparticles accumulate in the liver and spleen and are mostly eliminated by the reticuloendothelial system soon after administration. It is, however, possible to employ a coating that can prevent opsonization and recognition of NPs by the macrophages. Indeed, NPs may be covered by extra layers to achieve special therapeutic properties, to reduce side effects, or to increase solubility [70]. Engineered NPs may be created to help the molecule to find its target. For instance, docetaxel and ketoconazole loaded in solid lipid nanoparticles had their surface modified with folic acid to improve brain targeting [71]. Another example is hyaluronic acid-modified lipid–polymer hybrid NPs, which were designed to improve the ocular bioavailability of hydrophilic moxifloxacin hydrochloride, in order to prolong precorneal retention, and enhance its corneal permeability and ocular bioavailability [72].
The selection of the appropriate system depends on the target tissue, route of administration, and the characteristics of the drug to be incorporated into the NPs (size, stability, hydrophobicity, etc.). However, there are common physicochemical properties that all nanosystems must possess, such as biocompatibility, biodegradability, absence of toxicity, and stability, which can provide effective and safe pharmacological action, better than those obtained by traditional drug formulations.
Emergence of Nanomedicine Formulations in Glaucoma
Several drugs traditionally employed in glaucoma treatment, e.g., timolol, carteolol, brimonidine, pilocarpine, brinzolamide, bimatoprost, travoprost, and latanoprost [73], have been investigated in nanomedicine formulations [11, 12, 15]. We emphasize here that all of these formulations are still under investigation and are not currently approved for clinical use. In the following pages, we describe nanosystems loaded with the most utilized drugs for the treatment of glaucoma.
Pilocarpine
Pilocarpine has by and large been avoided by ophthalmologists because of several undesirable adverse effects, most of which are due to its non-selective ocular and systemic action as a muscarinic receptor agonist: intense miosis, ocular irritation, myopic shift, bronchial mucus secretion, bronchospasm, vasodilation, bradycardia, excessive salivation, sweating, and diarrhea. Pilocarpine has been used in a nitrate or hydrochloride pharmaceutical formulation, usually at a 0.5–4% concentration administered four times daily because of the short duration of action. When administered as a solution, it has a reasonable water solubility, which shortens the residence time of aqueous solutions in the eye. Hence, the real obstacle with pilocarpine solutions has been its very low ocular bioavailability (0.1–3%) [74, 75] arising from its poor lipophilicity.
Research efforts have been devoted to optimizing the activity and tolerability of pilocarpine [76]. Many nanosystems have been tried in order to improve pilocarpine’s bioavailability, solubility, and stability and to extend its pharmacological activity [77]. Table 1 shows examples of pilocarpine nanoparticulate drug delivery systems.
Table 1.
Experimental evidence with pilocarpine-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Complex of pilocarpine prodrug, O,O′-dipropionyl-(1,4-xylylene) bispilocarpate, with various β-cyclodextrin derivatives | Cyclodextrin | Experimental/pigmented rabbits | Ocular irritation caused by pilocarpine prodrug is eliminated with viscous SBE7-β-CyD solution without compromising the ocular absorption of the prodrug | [78] |
| Poly(amidoamine) (PAMAM) dendrimers (with primary amino (G2, G4), hydroxyl (G2(OH), G4(OH)), and carboxylate (G1.5, G3.5) surface groups | Dendrimers | Experimental/New Zealand albino rabbits | Intensity of pilocarpine’s pharmacological activity associated with G1.5 and G4(OH) dendrimer solutions was reported to be superior | [79] |
| Pilocarpine HCl encapsulated neutral and negatively charged multilamellar vesicles (MLVs) | Liposomes | Experimental/pigmented rabbits |
Neutral MLVs lowered the IOP from 20.7 to 15 mmHg for 4–5 h Negatively charged MLVs decreased the IOP for a shorter period of time (~ 1 h), which was similar to the free drug |
[80] |
| Pilocarpine nitrate–cyclodextrin complex | Cyclodextrin | In vitro permeability study using isolated excised corneas of albino rabbits | HP-β-CD promoted an increase in the corneal permeability of pilocarpine nitrate (permeability coefficient = 6.38 × 10−5 ± 2.82 × 10−7 cm/s, compared to 1.67 × 10−5 ± 2.12 × 10−7 cm/s with neat pilocarpine nitrate) | [81] |
| Pilocarpine-loaded poly(ε-caprolactone) (PCL) nanocapsules (PILO-PCL NCs) and pilocarpine-loaded PCL nanospheres (PILO-PCL NSs) | Nanocapsules and nanospheres | In vitro pilocarpine cumulative release studies, and in vivo studies (glaucomatous rabbit eyes intracamerally injected with PILO-PCL NSs or PILO-PCL NCs) | Pilocarpine loading capacity of PCL NCs was nearly 3 times greater than that in PILO-PCL NSs. PILO-PCL NC-treated group reduced IOP for the entire period of the study (42 days), exhibiting a sustained drug release profile | [82] |
| Pilocarpine-loaded hydrogels (three different hydrogels containing l-valine residues, HVa) | Hydrogels |
In vitro pilocarpine release and its effect on cell proliferation Cytotoxicity experiments with mouse immortalized fibroblast NIH3T3 cells Cell viability, after 24 h of incubation with the native and pilocarpine-loaded hydrogel |
Pilocarpine-loaded in HVa hydrogels showed a 3 times greater releasing profile | [83] |
| Hydrogels were non-toxic to the mouse fibroblast NIH3T3 cells | ||||
| Cell proliferation was increased with the presence of pilocarpine, even after 2 days | ||||
| Pilocarpine nitrate-loaded liquid crystal nanoparticles (PN-loaded LCNPs) | Liquid crystal nanoparticles | In vitro release profile of PN | Maximum decrease in IOP was 41.93 ± 16.79% with the commercial drug (peak effect at 2 h), versus 59.21 ± 7.04% (peak after 5 h) with PN-LCNPs | [84] |
| Ex vivo penetration study (freshly excised albino rabbit cornea) | Ex vivo drug penetration study demonstrated that the amount of PN penetration across the cornea at 1 h was 0.54 ± 0.20 µg with PN-LCNPs and 0.12 ± 0.06 µg with PN solution | |||
| Pilocarpine hydrochloride-loaded nanocomposite formulations (cellulose nanocrystals and triblock poloxamer copolymer) | Hydrogel/nanocrystals | In vivo IOP measurements using an indentation tonometer | In vitro release of pilocarpine hydrochloride from the nanocomposite hydrogels was extended compared to the pure poloxamer gel | [85] |
| In vitro drug release analysis and toxicity assay of in vitro gel | The greater the concentration of cellulose nanocrystals, the higher the sustained drug release property of the nanocomposite hydrogels | |||
| Nanocomposite formulation exhibited non-toxic behavior | ||||
| Pilocarpine-loaded chitosan/Carbopol nanoparticle ophthalmic Formulation | Hydrogel | In vitro release profile | In vitro analysis showed that nanoparticles displayed the best sustained release profile for 24 h compared to the other 3 formulations | [86] |
| In vivo (albino rabbits) miotic effect of pilocarpine chitosan/Carbopol nanoparticles was compared with various pilocarpine formulations (traditional eye drops, gels, and liposomes) | In vivo study showed extended miosis of pilocarpine-loaded chitosan/Carbopol nanoparticles, which was superior to those of liposomes, pilocarpine gel, and pilocarpine drops |
Timolol Maleate
Timolol maleate is a non-selective beta-adrenergic blocker that acts by inhibiting the beta-adrenergic receptors in the ciliary body epithelium, promoting the reduction of aqueous humor production and resulting in decreased IOP [87]. The hydrophilic nature of timolol maleate leads to slow corneal diffusion and low bioavailability [88]. Timolol maleate absorption from the eye into the systemic circulation, via nasolacrimal drainage, causes systemic adrenergic beta-blockage and high incidence of respiratory and cardiovascular side effects [89]. To improve the pharmacological features of timolol maleate, it is necessary to enhance its corneal penetration and reduce systemic absorption, which may be attained by the use of nanocarriers. Table 2 shows examples of timolol maleate-loaded nanoparticulate drug delivery systems.
Table 2.
Experimental evidence with timolol maleate-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Timolol-loaded gold nanoparticles (GNPs) | Gold nanoparticles | In vitro release study | Maximum timolol concentration for blank contact lenses at 1 h was 581 µg/ml, while in the GNP-laden CLs it was 1685 µg/ml | [90] |
| In vivo drug release and pharmacodynamic studies (New Zealand Albino Rabbits) | In the GNP-laden CLs group, IOP decreased by 4 mmHg after 1 h, which progressively increased after 72 h. In the marketed eye drop group, maximum IOP decrease was 3 mmHg after 3 h, which returned to baseline IOP after 12 h | |||
| Ocular tissue distribution study | Analysis of the rabbit tear fluid showed higher timolol concentrations with the GNP-CLs at all time points | |||
| GNPs were incorporated into contact lenses (CLs) | After 24 h of GNP-laden CL wear, a twofold and a fourfold increase in the timolol concentration in the conjunctiva and in the iris-ciliary muscle occurred, respectively | |||
| Timolol maleate/gelatin nanoparticle conjugate | Hydrogel | Experimental/New Zealand albino rabbits | The average 24-h IOP reduction was 52%, compared to 31% provided by regular timolol eye drops | [91] |
| Timolol-loaded nanoparticles into a poly(hydroxyl ethyl methacrylate) (p-HEMA) matrix | Hydrogel | In vitro drug release experiments | The higher the amount of nanoparticles in the HEMA, the higher the rate of drug release (particle to HEMA ratio 25:75 provided a total release at 95 °C of 283.08 ± 4.48 µg; particle to HEMA ratio 100:0 provided a total release at 95 °C of 743.27 ± 25.40 µg, and also increased the release duration) | [92] |
| Timolol-loaded multilamellar vesicles (MLVs) | Liposomes | Experimental/New Zealand albino rabbits | Positively charged MLVs of multilamellar liposomes provided a sustained IOP reduction for more than 24 h (which extended for about 1 week), whereas the free drug lowered IOP for 4 h | [93] |
| Chitosan (REVTMbio1) or Carbopol (REVTMbio2 and 3) coated niosomal timolol maleate (0.25%) | Niosomes | In vitro release pattern of niosomal preparations | Peak effect of IOP reduction with marketed formulation was at 2 h, compared to 3 h with all the REVTMbio formulations. However, chitosan-coated vesicles (REVTMbio1) showed an effect sustained for up to 8 h, compared to the marketed formulation, whose effect lasted for 5 h. REVTMbio1 formulation containing 0.25% timolol maleate showed similar effect compared to 0.5% marketed gel formulation | [94] |
| Timolol maleate entrapped in PVA or PCL nanofibers | Biodegradable polymeric nanofibers | In vitro drug release studies and in vivo studies (New Zealand albino rabbits) | Nanofibers were capable of controlled drug delivery for up to 24 h | [95] |
| Nanofiber formulation showed a significant IOP reduction for 72 h, while the marketed formulation kept the IOP reduced for 4 h | ||||
| Chitosan-coated timolol maleate (TM) mucoadhesive film | Hydrogel | In vitro drug release studies and in vivo studies (New Zealand albino rabbits) | In vitro study showed that 85% of timolol was released from the mucoadhesive films in 2 weeks, and the total content was released within 4 weeks | [96] |
| IOP-lowering efficacy of the 0.5% TM commercial ophthalmic solution was similar to the films. However, animals that received TM-loaded chitosan films kept their IOP at lower levels over a 10-week period, while the effect of the timolol commercial eye drops group was maintained for 12 days | ||||
| Nanoencapsulation of timolol in neat chitosan (CS) and N-alkylated chitosans [chitosan derivatives with succinic anhydride (CSUC) and 2-carboxybenzaldehyde (CBCS)] | Hydrogel | In vitro drug release study | In the majority of nanoparticles formulations, timolol is entrapped in amorphous form, but the present study, using different diameters of nanoparticles and CS and two N-acylated derivatives of CS as carriers, proved that drug entrapment efficiency was higher in CBCS derivative. Different timolol release rates among the tested formulations ratified that they vary according to specific carrier, nanoparticle size, and drug loading | [97] |
| Timolol maleate encapsulated in PLGA/PLA microspheres | Microspheres | In vitro drug release study | PLGA 502H:PLA blended microspheres released 30% of their timolol maleate content after 1 day. However, the remaining drug was released in a sustained manner over 107 days | [98] |
| Timolol-loaded chitosan–sodium alginate (CS-SA blend) nanoparticles | Hydrogel | In vitro drug release study | In vitro release from the CS-SA nanoparticles showed a burst of about 20% within the first hour and 35% of timolol was released after a period of 5 h, demonstrating that it may be released from synthesized particles in a sustained mode | [99] |
| Timolol maleate chitosan coated liposomes (TM-CHL) | Liposomes | In vitro drug release study | TM-CHL produced a 3.18-fold increase in the corneal permeation coefficient | [100] |
| In vivo transcorneal permeation studies (New Zealand rabbits) | TM-CHL was more effective in lowering IOP than timolol eye drops (final IOP = 19.67 ± 1.14 mmHg versus 23.80 ± 1.49 mmHg, respectively) | |||
| Timolol liposomes (TLP), and TLP with 0.02% Trancutol P (TLPG) | Liposomal hydrogel |
In vitro transcorneal permeation study In vivo pharmacodynamics (New Zealand and pigmented glaucomatous rabbits) |
Timolol–liposome system showed a transcorneal penetration 1.50-fold higher than that of the marketed eye drop, while the addition of Trancutol P enhanced it 2.19 times Timolol liposomal gel showed superior IOP reduction compared with Timolol eye drops at each time point |
[101] |
| Timolol maleate-loaded galactosylated chitosan nanoparticles | Hydrogel | In vitro release study | Timolol maleate-loaded galactosylated chitosan nanoparticles showed an initial burst release of 91% in 8 h and had a sustained release compared with the commercial timolol eye drops | [102] |
| In vitro transcorneal permeation study | Preocular retention of timolol maleate-loaded galactosylated chitosan nanoparticles was longer than that of timolol eye drops | |||
| Preocular retention study | IOP-lowering effect of timolol maleate-loaded galactosylated chitosan nanoparticles reached 10.5 ± 0.51 mmHg 4 h after instillation, while the peak effect of the commercial eye drops was 6.8 ± 0.35 mmHg at 3 h | |||
| In vivo pharmacodynamics study (albino rabbits) | IOP-lowering effect of timolol maleate-loaded galactosylated chitosan nanoparticles continued for up to 12 h, while the effect of commercial eye drops ceased after 8 h | |||
| Timolol-loaded liposome incorporated ion-sensitive in situ gels | Liposomes | In vitro release studies | Timolol-loaded liposome formulations showed a 1.93-fold increase in permeability coefficients | [103] |
| Deacetylated gellan gum | Pharmacodynamics study (measurement of intraocular pressure in normal albino rabbits and in ocular hypotensive rabbits) | Timolol-loaded liposome formulations reduced IOP from 30 to 300 min after instillation (minimum IOP = 11.96 ± 0.74 mmHg at 1 h), while the IOP decreased from 30 to 180 min (minimum IOP = 13.61 ± 0.95 mmHg at 2 h) with timolol eye drops |
Carbonic Anhydrase Inhibitors
Carbonic anhydrase (CA) is a generic denomination to designate several isoenzymes distributed in diverse proportions in the various tissues responsible for catalyzing the reversible hydration of carbon dioxide [104]. In the eye, CA is a key enzyme in aqueous humor production. Nonpigmented ciliary epithelial cells are the site of aqueous humor secretion, where two CA isoenzymes (CA-II and CA-IV) are the predominant forms. The inhibition of CA activity in the ciliary processes promotes decreased aqueous humor secretion and consequently leads to lowering of the IOP [105]. CA-II is considered the most relevant intracytoplasmic isoenzyme responsible for aqueous humor secretion [106]. Therefore, selective pharmacological inhibition of CA-II to achieve an ocular hypotensive effect is the objective in the treatment of glaucoma [106].
Three different CA inhibitors (CAI) molecules, all of them sulfonamide derivatives, are currently used in clinical practice to reduce elevated IOP in glaucoma. Acetazolamide is administered orally, and dorzolamide and brinzolamide are both administered topically [107]. Acetazolamide is a compound that indiscriminately inhibits most of the CA isozymes (CA-I, CA-II, CA-IV, CA-VA, CA-VB, CA-VII, CA-XIII, and CA-XIV) present in other organs than the eye [108–110], and although systemically administered acetazolamide is very effective as an intraocular hypotensive agent, the non-selective inhibition of the enzyme results in a myriad of undesirable side effects [108–110]. Dorzolamide and brinzolamide are potent nanomolar inhibitors of human CA-II isozyme [106, 111–113], with less activity against CA-IV, CA-XII, and CA-I [114].
Acetazolamide
Acetazolamide (ACZ) is a CAI used systemically to obtain a substantial and immediate reduction of IOP in glaucoma management. It is currently considered one of the most effective available IOP-lowering agents [115]. Limitations of orally administered ACZ include several systemic adverse events (e.g., metabolic acidosis, paresthesias, fatigue, diuresis, malaise, anorexia, loss of libido, renal function impediment, etc.) [116]. As a rule, large oral doses of ACZ are necessary to obtain the desired IOP reduction, probably because of its low bioavailability and relative instability [117]. Topical administration of ACZ would have been preferred over systemic administration, but as a result of the pharmacological limitations of topical ACZ (e.g., poor permeability via the corneal epithelium, short residence time, frequent instillations, loss of drug through nasolacrimal drainage, poor penetration coefficient (only 1–6% of the active compound actually reaches intraocular tissues) [118], poor biphasic solubility, and poor corneal bioavailability) [119], many researchers have tried to develop optimized ACZ topical drug delivery systems, some employing nanotechnology-based approaches [115, 117, 120–124]. Table 3 shows examples of ACZ-loaded nanoparticulate systems.
Table 3.
Experimental evidence derived from acetazolamide-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Eudragit nanoparticles (NPs) of ACZ incorporated into an ocular insert | Eudragit nanoparticles |
In vitro drug diffusion study Ex vivo transcorneal permeability study (excised fresh goat corneas) In vivo ocular tolerability and IOP reduction study (albino rabbits): animals received drinking water (40 ml/kg of body weight of rabbit) to increase IOP 3 groups: (1) ACZ reference solution, (2) Eudragit NPs dispersion, and (3) ocular insert of Eudragit NPs |
Ex vivo transcorneal permeation study showed the following results. Flow across corneal tissue (µg/min): drug suspension. 0.671 ± 0.020; NPs suspension. 2.460 ± 0.028; ocular insert, 2.402 ± 0.032, which means that ACZ-loaded Eudragit NPs displayed better permeability and flow across corneal tissue than the drug suspension In vivo studies with optimized ACZ loaded Eudragit NPs and ocular insert demonstrated substantial IOP lowering and improved ocular tolerability when compared to ACZ suspension |
[125] |
| ACZ-loaded nanoparticulate in situ gels (NP-ISG) | Polymeric nanoparticles with Eudragit RL100, Eudragit RS100, or poly(lactide-co-glycolide) 75:25 (PLGA) | Ex vivo transcorneal permeability study (fresh goat cornea) | Ex vivo transcorneal permeation study displayed higher ACZ permeation at 8 h with NP10 (93.5 ± 2.25 mg/cm2) and with NP-ISG5 (74.50 ± 2.20 mg/cm2) than with ACZ eye drops (20.08 ± 3.12 mg/cm2) and ACZ suspension (16.03 ± 2.14 mg/cm2) | [126] |
| Ex vivo corneal toxicity study (fresh goat cornea) | NP-ISG did not display harmful effects on any corneal layer | |||
| In vivo pharmacodynamic activity study (normotensive rabbit) | 1% ACZ nanoparticulate in situ gel exhibited greater IOP-lowering effect 1 h after administration, which was sustained for up to 8 h, while 1% ACZ eye drops only sustained its action for approximately 2 h | |||
| ACZ-loaded water-soluble mucoadhesive carbosilane dendrimers | C–Si backbone (carbosilane) cationic dendrimers | In vitro (cytotoxicity and cell viability) investigation (using telomerase-immortalized, human corneal-limbal epithelial cell line, HCLE) | Generations 1 and 2 of the cationic dendrimers and all 3 generations of the anionic dendrimers were well tolerated at 10 μM, with higher than 80% cell survival for all of them, except for the G3-C (from the 3rd generation of carbosilane cationic dendrimers) | [127] |
| In vivo (ocular tolerability) study (normotensive New Zealand rabbits): specular microscopy, slit lamp examination, and IOP measurements | Formulation containing ACZ 0.07% (289.4 mOsm; 5.6 pH; 41.7 mN/m) and G3 cationic carbosilane dendrimers (5 μM) demonstrated the best IOP-lowering effect. It obtained a rapid (1 h post-instillation) and sustained (up to 7 h) hypotensive effect, reaching a peak 22.6% IOP reduction | |||
| ACZ-loaded hyper branched poly(propylene imine) (PPI) dendrimers | PPI dendrimers |
In vitro drug release studies Ex vivo studies (effect on the morphology of human erythrocytes) In vivo studies (normotensive New Zealand rabbits): determination of ocular irritation index, ocular residence time, IOP reduction (25 µL of dendrimer formulation was administered into the lower cul-de-sac of the eye) |
Hemolytic toxicity study showed a slightly higher hemolysis rate of dendrimer formulations (D1 = 4.8%, D2 = 5.6%, D3 = 7.2%) when compared to plain drug (3.3%) and plain 5.0G PPI dendrimer (7.9%) Plain ACZ solution produced IOP reductions up to 2 h after instillation, whereas the dendrimer-based formulation lowered IOP for longer (4 h) |
[128] |
| ACZ-loaded ion-activated nanoemulsion-based in situ gelling systems using gellan gum polymer alone and in combination with other polymers (xanthan gum, hydroxymethylcellulose, or Carbopol) |
Nanoemulsion Gellan gum (in various concentrations: 0.1%, 0.2%, 0.3%, 0.4%, 0.5%, and 0.6%) |
In vitro release studies | Optimized formula of ion-induced nanoemulsion-based in situ gels demonstrated a significantly sustained drug release profile | [129] |
| In vivo studies: ocular irritation and pharmacodynamic studies in adult male New Zealand albino rabbits |
Ocular irritation study revealed no damage to the ocular surface and other parts of the eye Area under the curve of percentage decrease in IOP versus time from 0 to 10 h: nanoemulsion formula = 189.15 ± 10.18; Azopt® = 82.51 ± 7.53, and Cidamex® = 79.77 ± 7.58 |
|||
| ACZ-loaded Eudragit RL100 nanoparticle suspension (ACZ-E-NPs) | Polymeric nanoparticles with Eudragit RL100 |
In vitro drug release study In vivo studies (albino rabbits): Group 1 received 0.5% ACZ solution Group 2 received formulation E3 (drug polymer ratio 1:10; organic aqueous phase ratio 1:4; organic phase acetone) Group 3 received formulation E8 (drug polymer ratio 1:10; organic aqueous phase ratio 1:3; organic phase acetone and ethanol) |
Plain solution of ACZ was able to lower the IOP for about 3 h after instillation, whereas ACZ-E-NP solutions showed a greater efficacy (IOP lowering for up to 8 h after instillation). The peak IOP reduction by plain ACZ solution was 2.98 ± 0.11 mmHg at 2 h after topical administration, whereas best ACZ-E-NP formulations (E3 and E8) progressively reduced IOP, peaking at 8 h after instillation (F3 = 5.32 ± 0.07 mmHg; E8 = 5.19 ± 0.06 mmHg; mean ± SD) | [130] |
Dorzolamide
Dorzolamide (DRZ) is a CAI used extensively in the treatment of glaucoma. Inhibition of carbonic anhydrase results in decrease of aqueous humor production and IOP reduction. The first commercially available topical CAI was 2% DRZ hydrochloride solution (Trusopt®) [131], available since 1995. When topical 2% DRZ is used as monotherapy, it reduces IOP by up to 23% from baseline [132]. However, as a result of its low pH and high viscosity, 2% DRZ causes significant ocular irritation, stinging, or burning [133]. Moreover, topical 2% DRZ requires 2–3 daily doses, which hinders long-term adherence to treatment [134]. Therefore, there is a clear clinical demand to enhance the delivery efficiency of DRZ, and nano-based drug systems appear to have the potential to fulfil this goal. Table 4 demonstrates examples of dorzolamide-loaded nanoparticulate systems.
Table 4.
Examples of dorzolamide-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Chitosan (CS) and water-soluble 6-O-carboxymethyl (OCM-CS) derivative of CS nanoparticles (NPs) loaded with DRZ | CS or OCM-CS nanoparticles | In vitro studies (drug release and mucoadhesion of NPs) | IOP peak effect and duration of action were | [135] |
| In vivo studies (in normotensive albino rabbits): three groups (OCM-CS NPs, CS NPs, and marketed eye drops) received a single dose followed by IOP measurements (after 30 min of drug administration and then every 1 h over a period of 8 h) |
With the commercial formulation: 2.9 mmHg 2 h after instillation, and the effect disappeared after 4 h With the DRZ-loaded OCM-CS NPs: peak effect was seen at 4 h, with an IOP reduction of 2.2 mmHg, and the effect was sustained for 8 h With DRZ-loaded CS NPs: peak effect seen at 3 h; IOP reduction was 1.9 mmHg, and the effect was sustained for 6 h |
|||
| DRZ/γ-cyclodextrin (γ-CD) (18% w/v) complexes stabilized by hydroxypropyl methylcellulose (HPMC) (0.5% w/v) | Cyclodextrins | In vitro mucoadhesive and permeation studies | DRZ distribution 24 h after topical instillation indicated that it penetrates effectively into the eye, including the posterior segment, while serum DRZ concentrations were very low | [136] |
| In vivo (using pigmented rabbits): DRZ microparticle suspension was administered to both eyes. After administration the animals were anesthetized, and a sample of aqueous humor was collected once. Blood samples were also collected at each time point (2, 4, 8, and 24 h) | DRZ-γ-CD suspension provided sustained delivery of DRZ in the aqueous humor for up to 24 h. Commercial DRZ led to concentrations in the aqueous humor near zero 8 h after topical administration. The DRZ concentration in the aqueous humor of eyes treated with 3% (w/v) dorzolamide was 45-fold higher than those obtained after instillation of the other three formulations | |||
| DRZ-loaded microparticles | Microparticles |
In vitro drug release studies In vivo studies (employing normotensive Dutch belted rabbits) |
Subconjunctival injection of DRZ-PEG3-PSA microparticles lowered IOP by up to 4.0 ± 1.5 mmHg, when compared to untreated fellow eyes for 35 days | [137] |
| DRZ-loaded microparticles or blank microparticles were administered into the subconjunctival space of the superotemporal region of each eye using a 27-gauge needle | Fluorescein-labeled PEG3-PSA microparticles were detected several days after the injection (at least 42 days), indicating a very long in vivo nanoparticle degradation period | |||
| IOP measurements were made after the injection | 2% DRZ eye drops reduced IOP for < 6 h | |||
| DRZ-loaded chitosan nanoparticles | In situ gelling polymeric (chitosan) nanoparticles |
In vitro release study Ex vivo transcorneal permeability studies (on freshly excised goat cornea) Mucoadhesion study of drug loaded in situ gel In vivo studies (using albino rabbits): ocular tolerance test, and gamma scintigraphy study to access precorneal retention time |
Optimized formulation (C2S4) demonstrated, in the ex vivo permeability study, a drug permeation of 35.80% within 2 h, compared to 75.30% with the commercial formulation (DRZ 2% ophthalmic solution) Optimized formulation C2S4 showed appropriate gelling feature with 98.1% entrapment efficiency Gamma scintigraphy study showed long retention time of the proposed formulation in the eye, indicating an improved bioavailability of DRZ |
[138] |
| DRZ-loaded nanoliposome | Liposomes |
DRZ-loaded nanoliposome was administered to 20 patients with ocular hypertension or primary open-angle glaucoma (in both eyes) and were followed up for 28 days IOP (days 0, 14, and 28) was compared between the group that received DRZ-loaded nanoliposome and the group that received the marketed DRZ solution |
IOP reduction in the DRZ-loaded nanoliposome group was significantly greater than that seen with the commercially available DRZ formulation group (p < 0.05) IOP lowering recorded at 2 weeks was 23.26 ± 9.24%, and 9.25 ± 5.76% for the DRZ-loaded and the control group, respectively IOP lowering at 4 weeks was 32.60 ± 7.90% and 17.48 ± 7.62% for the DRZ-loaded and the control group, respectively |
[139] |
| DRZ γ-cyclodextrin (γ-CD) nanoparticle | Cyclodextrins | Self-aggregating γ-CD nanoparticle eye drops containing 3% DRZ were instilled once a day in human eyes, compared with the marketed DRZ instilled three times a day, in a prospective randomized single-masked crossover trial | DRZ nanoparticle eye drops once a day and commercial formulation of dorzolamide 2% three times a day did not show statistically significant differences in terms of efficacy at all time points. Nanoparticle eye drops caused less burning sensation than the marketed solution | [140] |
Brinzolamide
Brinzolamide is a water-insoluble CAI, which acts on the ciliary processes and decreases aqueous humor secretion. This medication is employed widely in stepwise glaucoma therapy most often in combination with prostaglandin analogues or in conjunction with brimonidine and beta-blockers [141]. Brinzolamide is formulated commercially as a 1% aqueous suspension, because lower concentrations resulted in insufficient therapeutic effect [114]. As a result of the solubilization demand of particles on the ocular surface and because of its high lipophilicity, brinzolamide aqueous suspension has a low bioavailability [142], which may elicit blurred vision after administration [143] and foreign body sensation, often leading to discomfort after instillation [144]. For these reasons, novel topical ocular delivery systems for this drug must surpass current problems. Table 5 shows examples of brinzolamide-loaded nanoparticulate systems.
Table 5.
Examples of brinzolamide-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Brinzolamide (BZL)-loaded liquid crystalline nanoparticles (BZL LCNPs) | Liquid crystalline nanoparticles |
In vitro release study to measure the release of BZL from LCNPs Ex vivo corneal penetration study (employing New Zealand rabbits) Efficacy study (instillation of one drop of marketed BZL, 1% BZL solution, and BZL LCNPs) |
Ex vivo permeability coefficient of BZL LCNPs showed a 3.47-fold increase compared with commercial BZL Two hours after instillation, peak IOP decrease was 47.67 ± 3.58% by BZL LCNPs, and 33.75 ± 4.35% by commercial BZL |
[145] |
| BZL nanocrystal suspensions (BZL-Npsa) | Nanocrystal | Cellular toxicity assay using human corneal epithelial cells (standard cell viability method) | BZL-Npsa pH 4.5 lowered IOP after 60 min (71.4 ± 5.0%) more efficiently than BZL-Npsa pH 7.4 Polysorbate 80 formulation (51.0 ± 26.3%), and commercial BZL (49.6 ± 16.5%) | [146] |
| In vivo studies: to verify IOP reduction following BZL-Npsa in glaucomatous Wistar rats | All the tested formulations and commercial BZL showed mild or no toxicity to the human corneal epithelial cells | |||
| Trimethyl lock (TMLo) BZL prodrug nanoparticles | Nanocrystals | Instillation of nano eye drops of BZL prodrug in ocular normotensive Sprague–Dawley rats | TMLo BZL prodrug nano eye drops showed similar efficacy as commercial BZL at 1/5 the molar concentration (5.67 mM TMLo BZL prodrug nano eye drops were as effective as 26.1 mM Azopt™), with no toxic effects to the cornea | [147] |
|
BZL nanoemulsions (BZL NEs) Seven primary BZL NE combinations were used [variations of four nonionic surfactants (Tyloxapol, Labrasol, Cremophor (RH40), and Brij 35), two oils (Capryol 90 and triacetin), and one co-surfactant (Transcutol P)] The amount of BZL was 0.4% in all formulations |
Nanoemulsions |
In vitro drug release studies In vivo therapeutic studies (ocular normotensive New Zealand albino rabbits): BZL NEs were instilled, followed by IOP measurements at 30, 60, 120, 180, 240, 300, and 360 min after instillation |
BZL NEs displayed a sustained release profile with proper physicochemical characteristics, facilitating BZL penetration into the corneal tissue with lower drug concentrations (0.4% vs 1% with commercial BZL) | [148] |
| BZL-hydroxypropyl-cyclodextrin (BZL-HP-β-CD) inclusion complex | Cyclodextrins |
In vitro corneal permeability and release studies In vivo study (New Zealand normotensive rabbits): Group A: BZL-HP-β-CD 0.2% inclusion complex; Group B: BZL-HP-β-CD 0.5% inclusion complex; Group C: commercial BZL (1%) IOP measured 30, 60, 120, 150, 180, 240, and 300 min after instillation |
In vitro corneal accumulation and permeability of the BZL-HP-β-CD inclusion complex was increased 2.91-fold compared to commercial BZL Solubility of BZL increased 10-fold with the (BZL-HP-β-CD) inclusion complex (BZL-HP-β-CD) inclusion complex (0.5% BZL) showed IOP-lowering efficacy comparable to commercial BZL in vivo |
[56] |
|
Brinzolamide (BZL)-hydropropyl-β-cyclodextrin (HP-β-CD) inclusion complex (HP-β-CD/BZL) into nanoliposomes “HP-β-CD/BZL-loaded nanoliposomes” (BCL) |
Liposomes Cyclodextrins |
In vitro BZL release study Transcorneal permeability study In vivo IOP measurement |
BZL showed a moderate sustained release period of 9 h (1–10 h) BCL showed a 9.36-fold increase in the permeability coefficient compared with commercially available BZL BCL formulation reduced IOP in less than 1 h, reached peak efficacy (− 32.3%) at 2 h and showed a sustained effect for 12 h BZL suspension lowered IOP at 30 min and reached its peak efficacy at 1 h. From 2 to 12 h after instillation, BCL resulted in significantly lower IOPs compared with BZL suspension |
[149] |
| Brinzolamide (BZL)-loaded PLGA nanoparticles | Poly(lactic-co-glycolic acid) (PLGA) nanoparticles |
A single subconjunctival injection of BZL-PLGA nanoparticles In vitro drug release studies In vivo IOP measurements (on normotensive albino rabbits) |
Two formulations of BZL-loaded PLGA nanoparticles displayed excellent release efficiency values: A19 released about 70% of the drug in 6 months, while B11 released about 90% of the drug in 6 months After subconjunctival administration peak IOP lowering were 78.4 ± 3.4% for A19 BZL nanoparticles; 71.6 ± 2.0% for B11 BZL nanoparticles; and 56.8 ± 6.3% for BZL aqueous suspension |
[150] |
Brimonidine
Brimonidine (BRD) is an α2-adrenergic agonist which acts through two mechanisms: it promotes an acute reduction in aqueous production, associated with an increase in uveoscleral outflow [151]. Nevertheless, BRD exhibits low bioavailability through the corneal stroma (1–7%) [152] and shows a short duration of action that leads to the necessity of three instillations during the day [153]. Consequently, BRD demonstrates a relatively reduced adherence, as evidenced by the rate of adherence, which ranges from 31% to 67% at 12 months [20]. The reluctance of patients to instill their eye drops many times a day on a regular basis justifies the need to develop new BRD formulations. Table 6 shows examples of BRD-loaded nanoparticulate systems.
Table 6.
Examples of brimonidine-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Nanovesicles of BRD | Liposomes and niosomes |
In vitro and ex in vitro drug release studies In vivo IOP-lowering activity in albino rabbits Group 1: marketed formulation (BRD 0.02%) Group 2: liposomes Group 3: niosomes |
In vitro and ex in vitro drug release profiles of all the nanovesicule formulations showed a more extended drug release compared to the currently available commercial solution Efficacy was greater compared to the commercial product, whose activity was not sustained beyond 60 min |
[154] |
| Eudragit-based brimonidine tartrate nanoparticle formulations (BRD-loaded ERS– ERL nanoparticles) | Eudragit nanoparticles |
In vitro drug release studies In vivo pharmacodynamic studies (employing glaucomatous New Zealand rabbits): IOP-lowering efficacy studies performed by instillation of aqueous dispersion of nanoparticles and conventional eye drop solution |
All the selected BRD-loaded ERS–ERL nanoparticles showed an extension of the drug release in vitro Results of in vivo pharmacodynamic efficacy studies of selected BRD-loaded ERS–ERL nanoparticle formulations and marketed BRD eye drops showed a similar peak IOP reduction, but prolonged IOP efficacy (marketed eye drops duration of 6 h compared to 36–72 h with the nanoparticles formulations) |
[155] |
| BRD-loaded microspheres using poly(lactic acid) (PLA) | Microspheres |
In vitro release kinetics of BRD In vivo experimental treatment groups (employing New Zealand rabbits): Single microneedle injection of BRD-loaded microspheres into the supraciliary space BRD (0.15%) commercial eye drops (administered three times a day to the upper conjunctival sac, for a week) |
After topical delivery of BRD, a consistent IOP reduction of 2–4 mmHg was detected, but the IOP quickly returned to baseline after the interruption of the drops BRD-loaded microspheres high dose group (30% BRD-loaded microspheres) showed IOP reduction of the treated eye for 33 days, after a single injection into the supraciliary space |
[156] |
| Optimized BRD-loaded chitosan (CS) and sodium alginate (ALG) nanoparticles | CS and ALG nanoparticles |
In vitro studies (drug release and cytotoxicity assays) In vivo studies (mice strains BXD29 and BXD96): a single dose of the test formulations or commercial BRD eye drops (BRD 0.15%) were instilled into the inferior conjunctival sac |
In vitro toxicity studies did not show significant differences between nano-based formulations and commercial BRD eye drops All nano-based formulations showed a greater sustained IOP-lowering effect compared to the commercial BRD. Time required for IOP to return to baseline ranged from 17.2 to 25.2 h for nano-based formulations, compared to 7–7.4 h for commercial BRD |
[157] |
| BRD-loaded nanostructured lipid carriers | Nanostructured (NLC) and solid (SLN) lipid nanoparticles |
In vitro drug release study Ex vivo permeability study In vivo studies (a single dose (50 µL) of lipid nanoparticles was instilled into the lower conjunctival sac of a normotensive albino rabbit). Ocular tolerance analysis, IOP measurements, and ocular histology were performed |
Both NLCs and SLNs showed a biphasic release pattern. SLNs showed 61.74 ± 2.56% drug released after 2 h and 74.34 ± 0.14% after 6 h, while NLCs showed 66.89 ± 3.4% drug released after 2 h and 95.8 ± 2.31% after 6 h. Commercial BRD showed a unique burst release of 88.76 ± 1.78% within the first hour NLCs demonstrated a permeability coefficient 1.23-fold higher than that of SLNs Peak IOP lowering with NLCs, SLNs, and commercial BRD eye drops was 13.14 ± 1.28, 10.03 ± 0.32, and 7.84 ± 1.04 mmHg, respectively The peak IOP effect occurred after 6 h for NLCs, after 4 h for commercial BRD, and after 2 h for SLNs NLCs and SLNs were found in the anterior chamber of treated eyes, indicating that lipid nanoparticles penetrate through the cornea |
[158] |
| BRD-loaded microspheres/carrier system (M/CS) | Microspheres poly(d,l-lactic-co-glycolic acid) (PLGA) |
In vitro release studies In vivo studies (IOP measurements after BRD-loaded M/CS subconjunctival implantation in normal and glaucomatous eyes) |
After a single dose of the BRD-loaded M/CS, an IOP reduction of 20 mmHg was achieved after 1 day, and was sustained over a period of 55 days M/CS structure remained intact for easy removal after BRD was fully released, even as long as 70 days after implantation |
[159] |
Prostaglandin Analogues
Topically instilled prostaglandin F2α (PGF2α) analogues (latanoprost, travoprost, bimatoprost, and tafluprost) have been the fist-line choice in the medical management of glaucoma over the last decade, owing to their favorable safety profile, efficacy, and patient compliance [160, 161]. All PGF2α analogues lead to common side effects, such as eyelash growth, darkening of the iris and periocular pigmentation, conjunctival hyperemia, and rare adverse reactions (e.g., damage to the blood–aqueous barrier, cystoid macular edema, and prostaglandin-associated periorbitopathy) [162]. These side effects reduce patient compliance. To overcome the side effects, to reduce dosing, to optimize the efficacy and safety of this class of drugs, and to get a reliable dosing technology delivered continuously over a prolonged time (weeks or more), novel nanoformulations of PGF2α analogues may prove greatly advantageous. Table 7 shows examples of prostaglandin analogue-loaded nanoparticulate systems.
Table 7.
Experimental evidence obtained with prostaglandin analogue-loaded nanoparticulate systems
| Hypotensive drug/nanosystem | Pharmaceutical form | Study design/model | Results | References |
|---|---|---|---|---|
| Latanoprost-loaded liposomes (large unilamellar vesicles) | Liposomes | In vivo human study: a single subconjunctival injection of liposomal latanoprost was administered to one eye of 6 subjects with ocular hypertension or primary open-angle glaucoma | Baseline IOP was 27.55 ± 3.25 mmHg | [163] |
| Mean IOP decreased within 1 h to 14.52 ± 3.31 mmHg (range 10–18 mmHg) | ||||
| A minimum 20% IOP reduction was observed through 3 months after injection | ||||
| No adverse events were reported | ||||
| Latanoprost acid (LA)-loaded poly(lactide)/monomethoxy-poly(ethylene glycol) (PLA-PEG) nanoparticles (NPs) | PLA (40) PEG (5) nanoparticles |
In vivo studies: subconjunctival injections were administered into the subconjunctival space of three groups of normotensive albino rabbits: (A) LA-loaded NPs (equivalent to 8.5 mg LA); (B) A free LA solution of the same drug content; (C) blank NPs IOP was monitored for 8 consecutive days In vitro studies analyzed drug entrapment efficiency, and release of LA Aqueous humor (AH) levels of LA were also measured 6 days post-administration |
IOP was lower in the LA-loaded PLA-PEG NPs group compared to the other 3 study groups (free drug, blank NP, and control group) The drug entrapment efficiency was 18.3% AH levels of LA were initially higher in the free LA group than the nanoparticle group, but they decreased over time In the nanoparticle group, AH levels of LA increased with time, becoming higher on the 6th day than in the free LA group |
[164] |
| Latanoprost-loaded egg-phosphatidylcholine (EggPC) liposomes | Liposomes | In vivo studies: after a single subconjunctival injection of the latanoprost-loaded formulation, rabbit eyes were clinically monitored and the IOP recorded | Latanoprost-loaded EggPC liposomes were able to provide a sustained IOP lowering and greater effect compared with daily instillations of topical latanoprost for up to 90 days (4.8 ± 1.5 vs 2.5 + 0.9 mmHg; P = 0.001), with no signs of inflammation | [165] |
| Latanoprost-loaded thermosensitive chitosan-based hydrogel (as a topical eye drop formulation) | Hydrogel |
In vitro drug release and biocompatibility study of the latanoprost-loaded hydrogel (cell viability assays, hemolysis analysis, and ocular irritation test) In vivo release study (aqueous humor levels of the drug) Latanoprost-loaded hydrogel was administered weekly into the lower lid of an experimental glaucoma model (rabbit). IOP was assessed |
No difference was found in cell viability between latanoprost-loaded hydrogel group and the controls No cytotoxic effects were detected on rabbit corneal epithelial cells Latanoprost-loaded hydrogel instilled once a week showed a similar IOP-lowering effect of commercial latanoprost instilled once daily |
[166] |
| Latanoprost-loaded liposomes, thymoquinone-loaded liposomes, and latanoprost/thymoquinone-loaded liposomes | Liposomes |
In vitro drug release In vivo studies: glaucomatous white albino rabbits were treated with latanoprost eye drops and diverse liposome formulations for a period of 6 weeks |
Latanoprost/thymoquinone-loaded liposomes and latanoprost-loaded liposomes were able to provide a significant IOP lowering that lasted 8 h Effect of the free latanoprost did not persist for more than 24 h |
[167] |
| Latanoprost-propylamino-β-cyclodextrin (CD) | Cyclodextrins |
In vitro stability and phase solubility analyses Ex vivo corneal permeation studies In vivo ocular tolerability evaluation Histology study |
Latanoprost-propylamino-β-CD demonstrated a significant improvement in its solubility and stability Clinical evaluations during 14 days showed that ocular irritation was 15.5% with the latanoprost marketed formulation, 9.5% with the latanoprost- propylamino-β-CD formulation, and 7.1% with the vehicle of the formulations Histological evaluation of ocular tissues demonstrated that Xalatan® induced higher inflammatory cell infiltrates than latanoprost- propylamino-β-CD formulation and the vehicle |
[168] |
| Chitosan bimatoprost (BIM)-loaded inserts | Hydrogel |
In vitro drug release studies Biodistribution studies (free and entrapped BIM, radiolabeled with technetium-99m) In vivo studies (glaucoma induced in Wistar rats): Group 1: BIM-loaded inserts were administered once into the conjunctival sac Group 2: marketed BIM drops Group 3: placebo inserts |
Biodistribution studies showed that a higher amount of 99mTc-BIM remained in the eye after chitosan insert implantation compared to eye drop instillation BIM-loaded inserts were able to lower IOP for 4 weeks after one application, whereas marketed eye drop could only lower IOP for 1 day |
[169] |
| A drug-agnostic intraocularly implantable device was used loaded with bimatoprost. The device was called nanofluidic Vitreal System for Therapeutic Administration (nViSTA) | Implantable intraocular device using a nanochannel membrane |
The nViSTA implantable device was designed for sustained and controlled drug delivery and based on a nanochannel membrane (which measures from 2.5 to 250 nm), without the need for actuation, pumps, or repeated clinical intervention This device was tested within a 3-dimensional anatomically similar in silico human eye model to obtain information on the intraocular pharmacokinetic profile |
Results from in vitro testing demonstrated a burst of approximately 40 µg of bimatoprost during the first 2 days, followed by a sustained release of bimatoprost from the nViSTA over the subsequent 18 days | [170] |
| Bimatoprost-loaded nanosponges (NS); travoprost-loaded nanosponges (NS) |
Nanosponge One travoprost nanosponge formulation (50-nm), and 3 bimatoprost nanosponge (NS) formulations were tested: (a) 400-nm NS; (b) 700-nm NS with amorphous (A-NS) cross-linkers, and (c) 700-nm NS with amorphous/crystalline (AC-NS) cross-linkers |
Ocular hypertensive C57 mice received NS loaded with 2 prostaglandin analogue hypotensive drugs (travoprost or bimatoprost) by a single intravitreal injection IOP was monitored for 7 weeks To evaluate the possibility of retinal deposition and retinal ganglion cell uptake of NS, 50-nm NS loaded with Neuro-DiO was injected intravitreally |
Travoprost NS formulation lowered IOP 19–29% for up to 4 days compared to saline injection Outcomes of the 3 bimatoprost NS formulations were: 400 nm NS lowered IOP 24–33% for up to 17 days compared to saline 700 nm A-NS lowered IOP 22–32% for up to 32 days 700 nm AC-NS lowered IOP 18–26% for up to 32 days Confocal microscopy and orthogonal projections suggested internalization of Neuro-DiO by retinal ganglion cells, which means NS may be effective at targeting these cells |
[171] |
Glaucoma Drainage and Nanodevices
Glaucoma drainage implants are often employed in glaucoma care when medical therapy, laser procedures, and standard filtration surgery fail to sufficiently control IOP [172]. The main types of glaucoma drainage devices available commercially are the Molteno, the Baerveldt, and the Ahmed, all of them designed to create an alternate aqueous pathway by channeling aqueous humor from the anterior chamber to the collection plate positioned under the conjunctiva [173, 174].
The long-term success of glaucoma drainage implants, however, is reduced as a result of the fibrous reaction developing around the end-plate, which leads to encapsulation and reduction of aqueous humor absorption. Epstein [175] was the first author to consider that aqueous humor in the subconjunctival space, in itself, would be a stimulus for the fibrovascular proliferation in the episcleral tissue. The intensity of the fibrotic reaction was attributed to a multifactorial origin, although not all pathogenetic factors are well understood. Some elements, such as the type of biomaterial, design and/or size of the end-plate, and the patient’s immune response, certainly influence the formation of fibrous tissue around the valve plate [176]. Therefore, refinement of biomaterials, as well as the associated use of antifibrotic agents with more advanced drug delivery nanosystems may meaningfully improve the long-term success of glaucoma drainage devices.
A novel double-layered porous coating for Ahmed glaucoma valves based on biodegradable poly(lactic-co-glycolic acid) (PLGA) was developed by Ponnusamy et al. [177] to achieve continuous drug release of antifibrotic agents [mitomycin C (MMC) and/or 5-fluorouracil (5-FU)] to the subconjunctival space. This novel poriferous coating for the Ahmed glaucoma drainage implant may have the advantage of modifying the degradation pattern of the polymer, and the drug release features. The purpose of this double-layered film is to provide a burst of MMC release followed by a slow release of 5-FU, which would prevent fibroblast proliferation over the most active period of postoperative wound healing (0–28 days). Results showed a very rapid release of MMC within 1 day, followed by the initial release of 5-FU within the first 3–5 days, which lasted for 3 to 4 weeks. Cytotoxicity assays have demonstrated significant cytotoxic activity from day 1 which persisted until the PLGA film was almost totally degraded.
Pan et al. [178] were able to manufacture a nano-drainage device fabricated through microelectromechanical systems (MEMS; miniaturized devices containing submillimeter features). This nano-drainage implant is made of a nanoporous membrane (mimicking the drainage function of the trabecular meshwork) connected to an integrated polymeric shaft inserted through the sclera into the anterior chamber, thus enabling a bypass route for the aqueous humor outflow. The nano-filtration membrane provides the designed flow resistance to the aqueous humor flow, but clogging of proteins inside the nanopores from plasma perfusion significantly increased the resistance. Further efforts will be necessary to apply modifications to the nanoporous membrane to reach the ideal aqueous outflow.
Harake et al. [179] designed an anti-biofouling micro-device which provides an innovative platform, also using MEMS, for IOP reduction in patients with glaucoma. This novel device has an array of parallel micro-channels built inside to yield controlled aqueous outflow resistance. Polyethylene glycol (PEG), a synthetic hydrogel, was chosen as the primary device material owing to its favorable biocompatibility and its antifouling properties, aiming to reduce protein clogging of the micro-channels [180]. PEG 214 and PEG 4000 were jointly used to avoid channel swelling. In vitro, continuous testing under artificial flow conditions showed that the device design containing 23 channels provided a pressure drop of about 10 mmHg.
Ferrofluids are the colloidal mixtures of magnetic nanoparticles (ferri- or ferromagnetic particles) suspended in a non-magnetic, inert fluid, such as water or an organic solvent [181]. As a result of their nanosize (tipically 10–100 nm in diameter), the nanoparticles are subject to Brownian motion. Brownian motion or pedesis is the random movement of microscopic particles in suspension mixed in a fluid, and it results from the averaged impacts of fast-moving molecules of the surrounding medium [182]. Magnetite (Fe3O4) particles are composed of a single magnetic domain and show superparamagnetic properties, i.e., a drag force along field gradients. If a static magnetic field exists, the interfacial force causes the ferrofluid to conform to its boundaries. Accordingly, a ferrofluid in a capillary tube can operate as an on/off valve to flow through capillary blockade. A permanent magnet may be utilized to keep the ferrofluid in the capillary and a secondary magnet may be utilized to regulate the force necessary to form the capillary barrier. Whenever the pressure exerted on the ferrofluid by the liquid surpasses the magnetic force between the ferrofluid and the secondary magnet, flow can start after the barrier is broken. Paschalis et al. [183] designed a novel, replaceable, ferromagnetic valve tube able to afford pressure regulation without the requirement of subconjunctival encapsulation. Preliminary in vivo results in rabbits showed that during the 2 weeks of follow-up, there were no signs of inflammation or infection at the surgical site. The mean IOP value in the valve implanted eye (11.8 ± 2 mmHg) was significantly lower than the contralateral control eye (14 ± 3 mmHg), and a continuous flow at the outlet tip of the valve was observed until the last follow-up.
Biomaterial improvement provided by nanoparticle-coated glaucoma drainage devices, associated with design changes and innovative drug delivery systems may potentially reduce fibrous reaction, and improve their efficiency.
Nanoparticle-Based Antimetabolites and Wound Healing Modulation
Currently, trabeculectomy is still the gold standard surgical treatment for glaucoma. Postoperative wound healing produces a hypercellular response in the subconjunctival tissues, which may gradually close the filtration site, limiting its long-term success [184]. Subconjunctival antifibrotics, such as MMC and 5-FU, have been used to reduce the generation of scar tissue, by inhibiting fibroblast proliferation [184–187].
In order to improve the efficiency of these antifibrotics drugs, nanoformulations have been recently employed. Hou et al. [188] have developed a new method to prepare MMC-loaded polylactic acid (PLA) nanoparticles, employing soybean phosphatidylcholine to improve the liposolubility of MMC. Results showed refined formulation characteristics and longer sustained drug release. Gomes dos Santos et al. [189] designed nanosized complexes of antisense TGFβ2 phosphorothioate oligonucleotides (PS-ODN) with polyethylenimine (PEI), and naked PS-ODN encapsulated into poly(lactide-co-glycolide) microspheres, based on the hypothesis that the encapsulation of antisense TGFβ2 ODN-PEI nanosized complexes in different types of porous particles could be an efficacious system for oligonucleotide delivery in vivo. Results showed that the MS-AsPS-ODN-PEI nanosized complex significantly improved the bleb survival rate following trabeculectomy (100% at 42 days) in rabbits compared to controls (0% of survival at day 21). The improved efficiency of the complexes released from microspheres is attributed to a superior intracellular delivery in conjunctival cells, along with the inhibition of TGFβ2.
Mitomycin C is considered the most effective antifibrotic agent used in glaucoma filtering surgery [190]. It is used in more than 85% of the trabeculectomies, but severe adverse effects, such as conjunctival thinning, bleb leaks, hypotony, and infection, limit its safety [190]. Our group has been working on the development of a safer and effective alternative nanoparticle-based antifibrotic agent. Paclitaxel associated with lipid nanoemulsions (LDE-PTX) was tested on rabbits undergoing trabeculectomy [191]. Subconjunctival injection of LDE-PTX was as effective as MMC in preventing scarring after trabeculectomy, but with substantially less toxicity to the conjunctiva and ciliary body. After these promising results in the animal model, we have recently started the first clinical trial to investigate the effectiveness of LDE-PTX in patients with primary open-angle glaucoma (POAG) undergoing trabeculectomy.
Duan et al. [192] investigated the effects of IkappaB kinase subunit beta (IKKβ) inhibition using RNA interference (RNAi) technology on the proliferation of human Tenon’s capsule fibroblasts (HTFs), which have a fundamental role in the scarring process. Ye et al. [193] developed a small interfering RNA (siRNA) targeting IKKβ (IKKβ-siRNA) associated with a ternary cationic nano-copolymer called CS-g-(PEI-b-mPEG) as the vehicle delivered into HTFs. They found that the expression of IKKβ was downregulated, the activation of nuclear factor kappa B (NF-κB) in the HTFs was inhibited, and the proliferation of HTFs was suppressed through the blocking of the NF-κB pathway, effects that improved the surgical outcome in a non-human primate model of trabeculectomy.
There is increasing interest in the use of angiogenesis-inhibiting compounds, especially those that act against vascular endothelial growth factor (VEGF), aiming to modulate the effects of VEGF on the migration and proliferation of human fibroblasts in Tenon’s capsule [194]. VEGF is upregulated in the aqueous humor of the glaucoma rabbit model as well as in patients with glaucoma, and it stimulates fibroblast proliferation in vitro [195]. Bevacizumab is a full-length humanized non-selective monoclonal antibody directed against all isoforms of VEGFA, which has been reported to inhibit proliferation of fibroblasts in vitro and to reduce angiogenesis and collagen deposition in eyes undergoing trabeculectomy [194–197]. Han et al. [198] investigated the use of bevacizumab combined with PEG-PCL-PEG (PECE) hydrogel, by intracameral injection, after experimental glaucoma filtration surgery (GFS). Results demonstrated that the antifibrotic effect of bevacizumab-loaded PECE hydrogel was superior to bevacizumab alone in the prevention of postoperative scarring after GFS in the rabbits.
Neuroprotection and Neuroregeneration and Neurotrophic Factors
Although IOP is considered the main risk factor for the development and progression of glaucoma, RGC loss may continue in spite of IOP reduction in some patients with glaucoma [199, 200]. The physiopathology behind RGC death is complex and has been attributed to various factors, including oxidative stress, intermittent ischemia, defective axon transport, excitotoxicity, loss of electrical activity, and trophic factor withdrawal [16], which justifies the development of neuroprotection/neuroregeneration strategies.
Glial cell-derived neurotrophic factor (GDNF) is an important neurotrophic factor implicated in the growth, differentiation, maintenance, and regeneration of specific neuronal populations in the adult brain, as well as in peripheral neurons [201].
In vitro and in vivo studies have shown that GDNF may rescue photoreceptor and RGC functions during retinal degeneration [202]. Previous studies have demonstrated that exogenous GDNF is an interesting therapeutic approach for neurodegenerative diseases affecting the retina, including glaucoma [203]. Checa-Casalengua et al. [203] described a novel protein microencapsulation method to ensure protection of the biological factor during this procedure, allowing the controlled release of proteins to keep their bioactivity for prolonged periods of time. GDNF in combination with vitamin E released from the microspheres was active for at least 3 months after a single intravitreal injection in an animal model of glaucoma. There was a significant increase of RGC survival compared with GDNF alone, vitamin E alone, or blank microspheres, which indicates that this novel formulation may be clinically applicable as a neuroprotective tool in the treatment of glaucomatous optic neuropathy. Ward et al. [204] demonstrated long-term RGC survival in a spontaneous glaucoma animal model by injecting slow-release PLGA microspheres containing GDNF into the vitreous. Early treatment resulted in 3.5 times greater RGC density than untreated mice after 15 months.
In a rat glaucoma model, animals were treated with GDNF injected into their vitreous cavity. 10% GDNF-microsphere emulsion resulted in a significant reduction of optic nerve head excavation, and increased RGC survival, compared with 2% GDNF-microspheres, blank microspheres, and saline solution [205]. In order to evaluate the pharmacokinetics of GDNF, García-Caballero et al. [206] analyzed its levels after a single intravitreal injection of GDNF/vitamin E microspheres in rabbits. This method allowed a sustained controlled release of GDNF for up to 6 months.
Ciliary neurotrophic factor (CNTF) has also been shown to be neuroprotective on motor neurons following injury to the adult central nervous system [207], and also for neurons in other regions in degenerative diseases [208–210]. Effective neuroprotection could be reached if sustained and local delivery without adverse side effects were achievable, but sustained CNTF delivery has proven arduous to accomplish and control. Nkansah et al. [211] examined the effects of CNTF nanospheres on neural stem cells (NSC) and also on the factors that influence controlled CNTF delivery from microspheres and nanospheres. Protein bioactivity after encapsulation was studied treating neural stem cells with CNTF released from nanospheres and compared to those treated with unencapsulated CNTF. Results showed that encapsulated CNTF provided NSC differentiation with no loss of potency compared to the unencapsulated growth factor.
In addition, Pease et al. [212] demonstrated the neuroprotective effect of virally mediated overexpression of CTNF and brain-derived neurotrophic factor (BDNF) in experimental glaucoma. Injection of adeno-associated viral vectors carrying either BDNF, CTNF, or both, in laser-induced glaucoma eyes, was performed. Combined CNTF-BDNF and BDNF overexpression alone had no noticeable improvement in RGC axon survival, but CNTF alone showed a significant protective effect, with 15% less RGC death.
The induction of heat shock proteins (HSPs) has been investigated as a modality for the protection of optic nerve damage in glaucoma [213–215]. Caprioli et al. [214] conducted the first demonstration of the neuroprotective effects of 72-kDa HSPs in cultured RGCs and Müller cells after hyperthermia and sublethal hypoxia. Since then, many researchers have been attempting to apply the induction of HSPs as a neuroprotective strategy in patients with glaucoma.
The calcium (Ca2+)-binding protein oncomodulin is a potent macrophage-derived growth factor for RGCs and other neurons. Yin et al. [216] demonstrated that, in vivo, oncomodulin released from nanoparticles (microspheres) promotes regeneration in the mature rat optic nerve.
More recently, Jeun et al. [217] investigated a new approach to use localized HSPs—a promising treatment modality for the ocular neuroprotection—without the unwanted side effects typically occurring with the current clinical approaches to induce HSPs in RGCs. These authors proposed the induction of HSPs by local magnetic hyperthermia utilizing engineered superparamagnetic Mn0.5Zn0.5Fe2O4 nanoparticle agents (EMZF-SPNPAs). The results showed that the EMZF-SPNPAs possess all the ideal characteristics as an in vivo localized HSP induction agent. Moreover, the authors also used an innovative infusion technique, which delivers the silica-coated EMZF-SPNPAs through the vitreous body to the retina for neuroprotection.
Anti-inflammatory Drugs
Inflammation is a common physiological protective response to injury. Inflammation is generally beneficial to the organism, promoting tissue survival, repair, and recovery, provided that it occurs for a limited period of time. Otherwise, it may be detrimental when extensive, prolonged, or unregulated [218]. In the central nervous system, the activation of astrocytes and microglia, which is indicative of inflammation, occurs in patients with Parkinson’s, Alzheimer’s, Huntington’s diseases, multiple sclerosis, and amyotrophic lateral sclerosis [219–221]. Hence, in the nervous system, prolonged inflammation may have a dual role, increasing neuronal loss or impeding regeneration. This justifies why anti-inflammatory drugs might also have a neuroprotective activity.
In glaucoma, the neuroinflammatory responses during RGC degeneration have not been elucidated. However, microglia are pleiotrophic immune cells and seem to take part in both neuroprotection and neurodegeneration of RGCs [222]. Thus, it is plausible that low-grade inflammation may occur in glaucoma in response to hypoxia, ischemia, vascular dysfunction, and elevated IOP. In fact, genes associated with cyclooxygenase 2 (COX-2)/PGE2 [223, 224], cytokines [225], tumor necrosis factor alpha (TNFα) in glial cells [226], and hypoxia inducible factor 1-alpha (HIFα) [227] are all upregulated in glaucomatous RGCs.
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a polyphenol extracted from Curcuma longa [228]. It has been reported that this compound can beneficially modulate several biochemical processes involved in neurodegenerative diseases. For example, Dong et al. [229] showed that long-term (12-week) curcumin-supplemented diet increased hippocampal neurogenesis and cognitive function in aged rats. Similarly, Kim et al. [230] demonstrated the beneficial effects of low dose curcumin on mouse multi-potent neural progenitor cells, which means it can stimulate neural plasticity and repair. Furthermore, Belviranlı et al. [231] concluded that curcumin supplementation improves cognitive function in aged female rats by reducing the lipid peroxidation in brain tissue, which demonstrates its protective effect against neural oxidative stress.
Curcumin has been reported to protect RGCs and the microvasculature against ischemic damage via inhibition of NF-κB, signal transducer and activator of transcription 3 (STAT3), and monocyte chemotactic protein 1 (MCP-1; also known as C-C motif chemokine 2) overexpression [232]. Wang et al. conducted a study to investigate the ability of curcumin to inhibit retinal ischemia/reperfusion injury. Pretreatment with curcumin inhibited ischemia/reperfusion-induced cell loss in the ganglion cell layer. Also, 0.05% curcumin administered 2 days after the injury showed a vasoprotective effect [232]. On the basis of the hypothesis that local and systemic oxidative stress participate in the pathogenesis of glaucoma, Yue et al. [233] evaluated the antioxidant effects of curcumin both in vitro (BV-2 microglia cell line) and in vivo and found that curcumin may improve cell viability and decrease intracellular reactive oxygen species and apoptosis of RGCs.
Although the therapeutic potential of curcumin in ophthalmology is real, its poor water solubility [234], and low bioavailability [228, 235] are important limiting factors for clinical applicability. To surpass these limitations, Davis et al. [236] used a nanotechnology approach to provide a hydrophobic environment for a poorly soluble molecule such as curcumin, and to improve its bioavailability through the development of a nanocarrier suitable for utilization as a topical formulation. This nanoformulation was able to increase the solubility of curcumin by a factor of 400,000, which is more than enough to overcome natural ocular barriers. Topical application of curcumin-loaded nanocarriers twice-daily for 3 weeks, in in vivo models of ocular hypertension and partial optic nerve transection, significantly reduced RGC loss. These results suggest that topical curcumin nanocarriers have potential as a neuroprotective therapy in glaucoma.
Ketorolac is a synthetic pyrrolizine carboxylic acid derivative that belongs to the group of NSAIDs. Ketorolac is a non-selective inhibitor of the enzymes COX-1 and COX-2. The inhibition of COX-2, upregulated at sites of inflammation, prevents conversion of arachidonic acid to pro-inflammatory prostaglandins [237]. Cyclooxygenases are expressed by RGCs in the rodent retina [238] and are upregulated in the retina after optic nerve injury [239] and ischemia [240]. Nadal-Nicolás et al. [241] first described the neuroprotective effects of ketorolac on RGCs after optic nerve axotomy in rats. Two treatments were evaluated: intravitreal administration of ketorolac tromethamine solution and/or ketorolac-loaded PLGA microspheres, 1 week before the optic nerve lesion and intravitreal administration right after the optic nerve crush. In all treated groups there was a significant increase in the number of RGCs compared to all control groups, which confirmed that an NSAID can delay neuronal death in an acute model of axonal injury. Pretreatment (ketorolac solution administered pre-optic nerve lesion) resulted in 63% survival of RGCs, while simultaneous administration of ketorolac solution provided a 53% survival. However, in this study, microspheres did not show an additional effect, contrary to other investigations where intravitreal administration of the microparticle formulation was found to be advantageous [242].
Toxicity of Nanomaterials
Nanoparticles may generate cellular toxicity through oxidative stress, interaction with the cell membrane, and inflammation [243]. In contrast to chemical toxicity, where compound purity and drug concentration are the essential determinants, in nanotoxicity other characteristics should be taken into account, such as nanoparticle size, surface shape, and ionic charge [244]. In addition, there are a few toxicity forms that may be independent of the drug delivered, namely particle toxicity, excipient toxicity, contaminant toxicity, and inflammatory toxicity [243]. Each one must be independently investigated in vitro and in vivo.
Specific literature about the toxicity of therapeutic nanoparticles in the eye remains scarce [243]. Nanotoxicity is a subject of key importance to be determined in conjunction with the therapeutic potential of each nanoparticle [245, 246], particularly for those that require repeat-dose regimens [247]. All nanoparticles proposed to be utilized for therapeutic purposes must be thoroughly investigated in terms of local and systemic toxicity, before obtaining the full regulatory approval.
Conclusions and Perspectives
Glaucoma is a complex and multifactorial disease and truly effective treatments need to be developed and specifically targeted to the pathophysiological pathways that have been identified to date. We believe that, in the future, glaucoma treatment will include the use of hypotensive drugs associated with neuroprotective agents directed to the preservation of RGCs and the optic nerve.
Nanotechnology has been shown to be a robust and valuable modality for the treatment of ocular diseases, including glaucoma. In this review, we focused on the recent advances in the development of nanosystems for glaucoma treatment. Bearing in mind the desired therapeutic targets in glaucoma therapy, nanotechnology has the ability to meaningfully improve the efficacy of current treatment approaches. By improving the pharmacological characteristics of drugs, or the features of surgical devices, nanotechnology has remarkable potential in future glaucoma management. There is convincing experimental evidence that many nanoparticles may enhance the solubility of lipophilic drugs, which improves bioavailability, and promote sustained delivery owing to their optimized biodegradability and physicochemical characteristics. Nanomedicine formulations designed to lower IOP may not require frequent dosing and may reduce drug-related side effects, which ultimately will result in optimizing adherence. In addition, nanoformulated drugs have been shown to improve wound healing modulation following filtering surgery and at the same time reduce the toxicity to the conjunctiva and ciliary body. Nanoparticle-coated filtering drainage devices may also reduce the fibrotic reaction around the plate, increasing their long-term success.
Although promising, possible toxic effects of nanoparticles have to be thoroughly analyzed during preclinical evaluation. The detailed investigation of every possible biological repercussion is requisite to the future introduction of nanomaterials. Importantly, the vast majority of studies on the use of nanoparticles in glaucoma have been performed in animal models. Human studies are needed not only for the detailed determination of the actual cytotoxicity of each nanoparticle but also to investigate their tolerability and efficacy.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Marcelo Luís Occhiutto, and Raul Cavalcante Maranhão have nothing to disclose. Vital Paulino Costa has received research funding from Allergan, Alcon, Novartis; honoraria from Allergan, Alcon, Novartis, Aerie, Genom, Ofta, Iridex; and congress expenses from Allergan, Alcon and Novartis. Anastasios-Georgios Konstas has received research funding from Allergan, Bayer and Santen; honoraria from Allergan, Mundipharma and Santen; and congress expenses from Vianex. Anastasios-Georgios Konstas is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10265768.
References
- 1.Thylefors B, Négrel AD. The global impact of glaucoma. Bull World Health Organ. 1994;72(3):323–326. [PMC free article] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. The global impact of glaucoma. Bull World Health Organ. 1994;72(3):323–6. http://www.who.int/blindness/publications/glaucoma/en/. Accessed 7 Dec 2019. [PMC free article] [PubMed]
- 4.Stein JD, Lee PP. Screening for glaucoma. In: Yanoff M, Duker JS, editors. Ophthalmology. Amsterdan, The Netherlands: Elsevier Sanders; 2009. p. 1007. [Google Scholar]
- 5.Heiji A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros FA, Weinreb RN. Medical backgrounders: glaucoma. Drugs Today (Barc) 2002;38(8):563–570. doi: 10.1358/dot.2002.38.8.704676. [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Mansoori T, Warjri GB, et al. Lasers in glaucoma. Indian J Ophthalmol. 2018;66(11):1539–1553. doi: 10.4103/ijo.IJO_555_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams KL. Medical and surgical management of the glaucoma patient. Clin Tech Small Anim Pract. 2001;16(1):71–76. doi: 10.1053/svms.2001.22809. [DOI] [PubMed] [Google Scholar]
- 10.Levin LA, Crowe ME, Quigley HA. Lasker/IRRF initiative on astrocytes and glaucomatous neurodegeneration participants. Neuroprotection for glaucoma: requirements for clinical translation. Exp Eye Res. 2017;157:34–37. doi: 10.1016/j.exer.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cetinel S, Montemagno C. Nanotechnology applications for glaucoma. Asia Pac J Ophthalmol (Phila) 2016;5(1):70–78. doi: 10.1097/APO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 12.Goyal G, Garg T, Rath G, et al. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 2014;31(5):365–405. doi: 10.1615/critrevtherdrugcarriersyst.2014010123. [DOI] [PubMed] [Google Scholar]
- 13.Cardigos J, Ferreira Q, Crisóstomo S, et al. Nanotechnology-ocular devices for glaucoma treatment: a literature review. Curr Eye Res. 2019;44(2):111–117. doi: 10.1080/02713683.2018.1536218. [DOI] [PubMed] [Google Scholar]
- 14.Foldvari M. Noninvasive ocular drug delivery: potential transcorneal and other alternative delivery routes for therapeutic molecules in glaucoma. J Glaucoma. 2014;23(8 Suppl 1):S80–S82. doi: 10.1097/IJG.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 15.Kim NJ, Harris A, Gerber A, et al. Nanotechnology and glaucoma: a review of the potential implications of glaucoma nanomedicine. Br J Ophthalmol. 2014;98(4):427–431. doi: 10.1136/bjophthalmol-2013-304028. [DOI] [PubMed] [Google Scholar]
- 16.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Gupta SK, Agarwal P, et al. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57(4):257–266. doi: 10.4103/0301-4738.53049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 20.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463. doi: 10.2147/PPA.S23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83:711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. doi: 10.1016/j.ophtha.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Loch C, Zakelj S, Kristl A, et al. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur J Pharm Sci. 2012;47:131–138. doi: 10.1016/j.ejps.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CH, Schoenwald RD. Ocular pharmacokinetic models of clonidine-3H hydrochloride. J Pharmacokinet Biopharm. 1986;14(2):175–211. doi: 10.1007/BF01065260. [DOI] [PubMed] [Google Scholar]
- 25.Schoenwald RD. Ocular pharmacokinetics/pharmacodynamics. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2. New York: Dekker; 2003. [Google Scholar]
- 26.Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23(5):279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 27.Del Amo EM, Rimpela AK, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–185. doi: 10.1016/j.preteyeres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Shikamura Y, Yamazaki Y, Matsunaga T, et al. Hydrogel ring for topical drug delivery to the ocular posterior segment. Curr Eye Res. 2016;41:653–661. doi: 10.3109/02713683.2015.1050738. [DOI] [PubMed] [Google Scholar]
- 29.Agrahari V, Mandal A, Agrahari V, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6:735–754. doi: 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelkonen L, Tengvall-Unadike U, Ruponen M, et al. Melanin binding study of clinical drugs with cassette dosing and rapid equilibrium dialysis inserts. Eur J Pharm Sci. 2017;109:162–168. doi: 10.1016/j.ejps.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Carreon TA, Edwards G, Wang H, et al. Segmental outflow of aqueous humor in mouse and human. Exp Eye Res. 2017;158:59–66. doi: 10.1016/j.exer.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: a review. Exp Eye Res. 2017;158:94–111. doi: 10.1016/j.exer.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yücel YH. Discovery of lymphatics in the human eye and implications. Can J Ophthalmol. 2010;45(2):115–117. doi: 10.3129/i10-005. [DOI] [PubMed] [Google Scholar]
- 34.Tomczyk-Socha M, Turno-Kręcicka A. A novel uveolymphatic drainage pathway-possible new target for glaucoma treatment. Lymphat Res Biol. 2017;15(4):360–363. doi: 10.1089/lrb.2017.0019. [DOI] [PubMed] [Google Scholar]
- 35.Yücel Y, Gupta N. Lymphatic drainage from the eye: a new target for therapy. Prog Brain Res. 2015;220:185–198. doi: 10.1016/bs.pbr.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Kim SJ, Kim ES, et al. Trans-scleral permeability of Oregon Green 488. J Ocul Pharmacol Ther. 2008;24:579–586. doi: 10.1089/jop.2008.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: clinical predictability and quality of the published data. Exp Eye Res. 2015;137:111–124. doi: 10.1016/j.exer.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–587. [PubMed] [Google Scholar]
- 39.Hamalainen KM, Kananen K, Auriola S, et al. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- 40.Sponsel WE, Terry S, Khuu HD, Lam KW, Frenzel H. Periocular accumulation of timolol and betaxolol in patients with glaucoma under long-term therapy. Surv Ophthalmol. 1999;43(suppl 1):S210–S213. doi: 10.1016/s0039-6257(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 41.Holló G, Whitson JT, Faulkner R, et al. Concentrations of betaxolol in ocular tissues of patients with glaucoma and normal monkeys after 1 month of topical ocular administration. Invest Ophthalmol Vis Sci. 2006;47(1):235–240. doi: 10.1167/iovs.05-0945. [DOI] [PubMed] [Google Scholar]
- 42.Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye (Lond) 2011;25:578–586. doi: 10.1038/eye.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sena DF, Ramchand K, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. 2010;(2):CD006539. [DOI] [PMC free article] [PubMed]
- 44.Krupin T, Liebmann JM, Greenfield DS, et al. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–681. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol. 2012;23:144–154. doi: 10.1097/ICU.0b013e32834ff490. [DOI] [PubMed] [Google Scholar]
- 46.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 47.Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: cellular basis and development. Vision Res. 2017;139:123–137. doi: 10.1016/j.visres.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Molas M, Grossmann GA, et al. Biological properties of poly-l-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276(37):34379–34387. doi: 10.1074/jbc.M105250200. [DOI] [PubMed] [Google Scholar]
- 49.Nagarwal RC, Kant S, Singh PN, et al. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;28(252):28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 52.Akbarzadeh A, Khalilov R, Mostafavi E, et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Exp Oncol. 2018;40(3):178–183. [PubMed] [Google Scholar]
- 53.Cheng B, He H, Huang T, et al. Gold nanosphere gated mesoporous silica nanoparticle responsive to near-infrared light and redox potential as a theranostic platform for cancer therapy. J Biomed Nanotechnol. 2016;12(3):435–449. doi: 10.1166/jbn.2016.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao W, Zhang Y, Zhang Q, et al. Nanoparticle–hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44(6):2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y, Qi J, Dong X, et al. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22(4):744–750. doi: 10.1016/j.drudis.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Ren K, He Z, et al. Development of inclusion complex of brinzolamide with hydroxypropyl-β-cyclodextrin. Carbohydr Polym. 2013;98(1):638–643. doi: 10.1016/j.carbpol.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhary HM, Duttagupta AS, Jadhav KR, et al. Nanodiamonds as a new horizon for pharmaceutical and biomedical applications. Curr Drug Deliv. 2015;12(3):271–281. doi: 10.2174/1567201812666141229104304. [DOI] [PubMed] [Google Scholar]
- 58.Prajapati VD, Jani GK, Kapadia JR. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin Drug Deliv. 2015;12(8):1283–1299. doi: 10.1517/17425247.2015.1015985. [DOI] [PubMed] [Google Scholar]
- 59.Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;10(185):22–36. doi: 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Haidar MK, Eroglu H. Nanofibers: new insights for drug delivery and tissue engineering. Curr Top Med Chem. 2017;17(13):1564–1579. doi: 10.2174/1568026616666161222102641. [DOI] [PubMed] [Google Scholar]
- 61.Talevi A, Gantner ME, Ruiz ME. Applications of nanosystems to anticancer drug therapy (Part I. Nanogels, nanospheres, nanocapsules) Recent Pat Anticancer Drug Discov. 2014;9(1):83–98. doi: 10.2174/1574891x113089990035. [DOI] [PubMed] [Google Scholar]
- 62.Priwitaningrum DL, Blonde JG, Sridhar A, et al. Tumor stroma-containing 3D spheroid arrays: a tool to study nanoparticle penetration. J Control Release. 2016;244(Pt B):257–268. doi: 10.1016/j.jconrel.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Hornung A, Poettler M, Friedrich RP, et al. Treatment efficiency of free and nanoparticle-loaded mitoxantrone for magnetic drug targeting in multicellular tumor spheroids. Molecules. 2015;20(10):18016–18030. doi: 10.3390/molecules201018016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, Yin T, Liu Y, et al. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016;46:177–190. doi: 10.1016/j.actbio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl. 1):S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 66.Tamboli V, Mishra GP, Mitrat AK. Polymeric vectors for ocular gene delivery. Ther Deliv. 2011;2:523–536. doi: 10.4155/tde.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Huang Y, David AE, et al. Magnetic nanoparticles for MRI of brain tumors. Curr Pharm Biotechnol. 2012;13(12):2403–2416. doi: 10.2174/138920112803341824. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 69.Salem HF, Ahmed SM, Omar MM. Liposomal flucytosine capped with gold nanoparticle formulations for improved ocular delivery. Drug Des Devel Ther. 2016;13(10):277–295. doi: 10.2147/DDDT.S91730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gref R, Domb A, Quellec P, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 1995;19:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venishetty VK, Komuravelli R, Kuncha M, et al. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine. 2013;9(1):111–121. doi: 10.1016/j.nano.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Liu D, Lian Y, Fang Q, et al. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int J Biol Macromol. 2018;17(116):1026–1036. doi: 10.1016/j.ijbiomac.2018.05.113. [DOI] [PubMed] [Google Scholar]
- 73.Jonas JB, Aung T, Boune RR, et al. Glaucoma. Lancet. 2017;390:2083–2093. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 74.Chrai SS, Robinson JR. Corneal permeation of topical pilocarpine nitrate in the rabbit. Am J Ophthalmol. 1974;77(5):735–739. doi: 10.1016/0002-9394(74)90541-8. [DOI] [PubMed] [Google Scholar]
- 75.Lazare R, Horlington M. Pilocarpine levels in the eyes of rabbits following topical application. Exp Eye Res. 1975;21(3):281–287. doi: 10.1016/0014-4835(75)90099-8. [DOI] [PubMed] [Google Scholar]
- 76.Plazoneet J, Grove M, Durr C, et al. Recent advances in pilocarpine delivery. In: Saettone MF, Bucci M, Speiser P, et al., editors. Ophthalmic drug delivery: biopharmaceutical, technological and clinical aspects. Padova: Springer & Liviana; 1987. p. 122. [Google Scholar]
- 77.Pepic I, Jalsenjak N, Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int J Pharm. 2004;272:57–64. doi: 10.1016/j.ijpharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 78.Jarho P, Järvinen K, Urtti A, et al. Modified beta-cyclodextrin (SBE7-beta-CyD) with viscous vehicle improves the ocular delivery and tolerability of pilocarpine prodrug in rabbits. J Pharm Pharmacol. 1996;48(3):263–269. doi: 10.1111/j.2042-7158.1996.tb05914.x. [DOI] [PubMed] [Google Scholar]
- 79.Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharm. 2000;198:29–38. doi: 10.1016/s0378-5173(99)00348-8. [DOI] [PubMed] [Google Scholar]
- 81.Aktaş Y, Unlü N, Orhan M, et al. Influence of hydroxypropyl beta-cyclodextrin on the corneal permeation of pilocarpine. Drug Dev Ind Pharm. 2003;29(2):223–230. doi: 10.1081/ddc-120016730. [DOI] [PubMed] [Google Scholar]
- 82.Lee CH, Li YJ, Huang CC, et al. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: single dose for long-term glaucoma treatment. Nanoscale. 2017;9(32):11754–11764. doi: 10.1039/c7nr03221h. [DOI] [PubMed] [Google Scholar]
- 83.Casolaro M, Casolaro I, Lamponi S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. Eur J Pharm Biopharm. 2012;80(3):553–561. doi: 10.1016/j.ejpb.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Wu L, Wu W, et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int J Pharm. 2013;455(1–2):75–84. doi: 10.1016/j.ijpharm.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 85.Orasugh JT, Sarkar G, Saha NR, et al. Effect of cellulose nanocrystals on the performance of drug loaded in situ gelling thermo-responsive ophthalmic formulations. Int J Biol Macromol. 2019;1(124):235–245. doi: 10.1016/j.ijbiomac.2018.11.217. [DOI] [PubMed] [Google Scholar]
- 86.Kao HJ, Lin HR, Lo YL, et al. Characterization of pilocarpine-loaded chitosan/Carbopol nanoparticles. J Pharm Pharmacol. 2006;58(2):179–186. doi: 10.1211/jpp.58.2.0004. [DOI] [PubMed] [Google Scholar]
- 87.Heel RC, Brogden RN, Speight TM, Avery GF. Timolol: a review of its therapeutic efficacy in the topical treatment of glaucoma. Drugs. 1979;17(1):38–55. doi: 10.2165/00003495-197917010-00002. [DOI] [PubMed] [Google Scholar]
- 88.Fukuda M, Sasaki H. The transcorneal penetration of commercial ophthalmic formulations containing timolol maleate in rabbit eyes. J Ocul Pharmacol Ther. 2015;31(1):57–60. doi: 10.1089/jop.2014.0015. [DOI] [PubMed] [Google Scholar]
- 89.Van Buskirk EM. Adverse reactions from timolol administration. Ophthalmology. 1980;87(5):447–450. doi: 10.1016/s0161-6420(80)35215-9. [DOI] [PubMed] [Google Scholar]
- 90.Maulvi FA, Patil RJ, Desai AR, et al. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation. Acta Biomater. 2019;1(86):350–362. doi: 10.1016/j.actbio.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 91.Shokry M, Hathout RM, Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for timolol maleate: augmented in vivo efficacy and safe histological profile. Int J Pharm. 2018;545(1–2):229–239. doi: 10.1016/j.ijpharm.2018.04.059. [DOI] [PubMed] [Google Scholar]
- 92.Jung HJ, Chauhan A. Extended release of timolol from nanoparticle-loaded fornix insert for glaucoma therapy. J Ocular Pharmacol Ther. 2013;29(2):229–235. doi: 10.1089/jop.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shafaa MW, Sabra NM, Fouad RA. The extended ocular hypotensive effect of positive liposomal cholesterol bound timolol maleate in glaucomatous rabbits. Biopharm Drug Dispos. 2011;32(9):507–517. doi: 10.1002/bdd.778. [DOI] [PubMed] [Google Scholar]
- 94.Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290(1):155–159. doi: 10.1016/j.ijpharm.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 95.Gagandeep, Garg T, Malik B, Goyal AK. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 2014;53:10–16. doi: 10.1016/j.ejps.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 96.Fulgêncio Gde O, Viana FA, Ribeiro RR, et al. New mucoadhesive chitosan film for ophthalmic drug delivery of timolol maleate: in vivo evaluation. J Ocul Pharmacol Ther. 2012;28(4):350–358. doi: 10.1089/jop.2011.0174. [DOI] [PubMed] [Google Scholar]
- 97.Siafaka PI, Titopoulou A, Koukaras EN, et al. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int J Pharm. 2015;495(1):249–264. doi: 10.1016/j.ijpharm.2015.08.100. [DOI] [PubMed] [Google Scholar]
- 98.Bertram JP, Saluja SS, McKain J, et al. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J Microencapsul. 2009;26:18–26. doi: 10.1080/02652040802095250. [DOI] [PubMed] [Google Scholar]
- 99.Ilka R, Mohseni M, Kianirad M, et al. Nanogel-based natural polymers as smart carriers for the controlled delivery of timolol maleate through the cornea for glaucoma. Int J Biol Macromol. 2018;1(109):955–962. doi: 10.1016/j.ijbiomac.2017.11.090. [DOI] [PubMed] [Google Scholar]
- 100.Tan G, Yu S, Pan H, et al. Bioadhesive chitosan-loaded liposomes: a more efficient and higher permeable ocular delivery platform for timolol maleate. Int J Biol Macromol. 2017;94(Pt A):355–363. doi: 10.1016/j.ijbiomac.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 101.Zhang HH, Luo QH, Yang ZJ, et al. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Deliv. 2011;18(7):502–510. doi: 10.3109/10717544.2011.595839. [DOI] [PubMed] [Google Scholar]
- 102.Zhao R, Li J, Wang J, et al. Development of timolol-loaded galactosylated chitosan nanoparticles and evaluation of their potential for ocular drug delivery. AAPS PharmSciTech. 2017;18(4):997–1008. doi: 10.1208/s12249-016-0669-x. [DOI] [PubMed] [Google Scholar]
- 103.Yu S, Wang QM, Wang X, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1–2):128–136. doi: 10.1016/j.ijpharm.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 104.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74(1):1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 105.Holló G. Carbonic anhydrase inhibitors. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG, editors. Glaucoma. 2nd ed. Philadelphia: Elsevier Saunders; 2015. p. 559.
- 106.Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res. 2000;19(1):87–112. doi: 10.1016/s1350-9462(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 107.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 108.Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008–2012) Expert Opin Ther Pat. 2012;22(7):747–758. doi: 10.1517/13543776.2012.698264. [DOI] [PubMed] [Google Scholar]
- 109.Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600. [Google Scholar]
- 110.Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 111.Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem. 2014;75:349–359. doi: 10.1007/978-94-007-7359-2_17. [DOI] [PubMed] [Google Scholar]
- 112.Ilies M, Supuran CT, Scozzafava A, et al. Carbonic anhydrase inhibitors: sulfonamides incorporating furan-, thiophene- and pyrrole-carboxamido groups possess strong topical intraocular pressure lowering properties as aqueous suspensions. Bioorg Med Chem. 2000;8(8):2145–2155. doi: 10.1016/s0968-0896(00)00143-7. [DOI] [PubMed] [Google Scholar]
- 113.Winum JY, Casini A, Mincione F, et al. Carbonic anhydrase inhibitors: N-(p-sulfamoylphenyl)-alpha-d-glycopyranosylamines as topically acting antiglaucoma agents in hypertensive rabbits. Bioorg Med Chem Lett. 2004;14(1):225–229. doi: 10.1016/j.bmcl.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 114.DeSantis L. Preclinical overview of brinzolamide. Surv Ophthalmol. 2000;44(Suppl 2):S119–S129. doi: 10.1016/s0039-6257(99)00108-3. [DOI] [PubMed] [Google Scholar]
- 115.Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199(2):119–127. doi: 10.1016/s0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 116.Epstein DL, Grant WM. Carbonic anhydrase inhibitor side effects. Serum chemical analysis. Arch Ophthalmol. 1977;95(8):1378–1382. doi: 10.1001/archopht.1977.04450080088009. [DOI] [PubMed] [Google Scholar]
- 117.Kaur IP, Kapil M, Smitha R, et al. Development of topically effective formulations of acetazolamide using HP-beta-CD-polymer co-complexes. Curr Drug Deliv. 2004;1(1):65–72. doi: 10.2174/1567201043480054. [DOI] [PubMed] [Google Scholar]
- 118.Sasaki H, Yamamura K, Mukai T, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst. 1999;16(1):85–146. [PubMed] [Google Scholar]
- 119.Kaur IP, Smitha R, Aggarwal D, et al. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248(1–2):1–14. doi: 10.1016/s0378-5173(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 120.Friedman Z, Allen RC, Raph SM. Topical acetazolamide and methazolamide delivered by contact lenses. Arch Ophthalmol. 1985;103(7):963–966. doi: 10.1001/archopht.1985.01050070089036. [DOI] [PubMed] [Google Scholar]
- 121.Loftsson T, Frithriksdóttir H, Stefánsson E, et al. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J Pharm Pharmacol. 1994;46(6):503–504. doi: 10.1111/j.2042-7158.1994.tb03835.x. [DOI] [PubMed] [Google Scholar]
- 122.El-Gazaierly O, Hikal AH. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int J Pharm. 1997;158(2):121–127. [Google Scholar]
- 123.Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm. 2007;338(1–2):21–26. doi: 10.1016/j.ijpharm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 124.Duarte AR, Roy C, Vega-González A, Duarte CM, Subra-Paternault P. Preparation of acetazolamide composite microparticles by supercritical anti-solvent techniques. Int J Pharm. 2007;332(1–2):132–139. doi: 10.1016/j.ijpharm.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 125.Rathod LV, Kapadia R, Sawant KK. A novel nanoparticle impregnated ocular insert for enhanced bioavailability to posterior segment of eye: in vitro, in vivo and stability studies. Mater Sci Eng C Mater Biol Appl. 2017;1(71):529–540. doi: 10.1016/j.msec.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 126.Singh J, Chhabra G, Pathak K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. Ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm. 2014;40(9):1223–1232. doi: 10.3109/03639045.2013.814061. [DOI] [PubMed] [Google Scholar]
- 127.Bravo-Osuna I, Vicario-de-la-Torre M, Andrés-Guerrero V, et al. Novel water-soluble mucoadhesive carbosilane dendrimers for ocular administration. Mol Pharm. 2016;13(9):2966–2976. doi: 10.1021/acs.molpharmaceut.6b00182. [DOI] [PubMed] [Google Scholar]
- 128.Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461(1–2):380–390. doi: 10.1016/j.ijpharm.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 129.Morsi N, Ibrahim M, Refai H, et al. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;15(104):302–314. doi: 10.1016/j.ejps.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 130.Verma P, Gupta RN, Jha AK, et al. Development, in vitro and in vivo characterization of Eudragit RL 100 nanoparticles for improved ocular bioavailability of acetazolamide. Drug Deliv. 2013;20(7):269–276. doi: 10.3109/10717544.2013.834417. [DOI] [PubMed] [Google Scholar]
- 131.Pfeiffer N. Dorzolamide: development and clinical application of a topical carbonic anhydrase inhibitor. Surv Ophthalmol. 1997;42(2):137–151. doi: 10.1016/s0039-6257(97)00053-2. [DOI] [PubMed] [Google Scholar]
- 132.Strahlman E, Tipping R, Vogel R. A double-masked, randomized 1-year study comparing dorzolamide (Trusopt), timolol, and betaxolol. International Dorzolamide Study Group. Arch Ophthalmol. 1995;113(8):1009–1016. doi: 10.1001/archopht.1995.01100080061030. [DOI] [PubMed] [Google Scholar]
- 133.Martens-Lobenhoffer J, Banditt P. Clinical pharmacokinetics of dorzolamide. Clin Pharmacokinet. 2002;41(3):197–205. doi: 10.2165/00003088-200241030-00004. [DOI] [PubMed] [Google Scholar]
- 134.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl 1):S57–S58. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Shinde U, Ahmed MH, Singh K. Development of dorzolamide loaded 6-O-carboxymethyl chitosan nanoparticles for open angle glaucoma. J Drug Deliv. 2013;2013:562727. doi: 10.1155/2013/562727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jansook P, Stefánsson E, Thorsteinsdóttir M, et al. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm. 2010;76(2):208–214. doi: 10.1016/j.ejpb.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 137.Fu J, Sun F, Liu W, et al. Subconjunctival delivery of dorzolamide-loaded poly(ether-anhydride) microparticles produces sustained lowering of intraocular pressure in rabbits. Mol Pharm. 2016;13(9):2987–2995. doi: 10.1021/acs.molpharmaceut.6b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katiyar S, Pandit J, Mondal RS, et al. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym. 2014;15(102):117–124. doi: 10.1016/j.carbpol.2013.10.079. [DOI] [PubMed] [Google Scholar]
- 139.Kouchak M, Malekahmadi M, Bavarsad N, et al. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev Ind Pharm. 2018;44(8):1239–1242. doi: 10.1080/03639045.2017.1386196. [DOI] [PubMed] [Google Scholar]
- 140.Gudmundsdottir BS, Petursdottir D, Asgrimsdottir GM, et al. γ-Cyclodextrin nanoparticle eye drops with dorzolamide: effect on intraocular pressure in man. J Ocul Pharmacol Ther. 2014;30(1):35–41. doi: 10.1089/jop.2013.0060. [DOI] [PubMed] [Google Scholar]
- 141.Iester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol. 2008;2(3):517–523. doi: 10.2147/opth.s3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kadam RS, Jadhav G, Ogidigben M, et al. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: implications for effects on ocular blood flow. Drug Metab Dispos. 2011;39(9):1529–1537. doi: 10.1124/dmd.111.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silver LH, the Brinzolamide Comfort Study Group Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution. Results from two multicenter comfort studies. Surv Ophthalmol. 2000;44:141–145. doi: 10.1016/s0039-6257(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 144.Tsukamoto H, Noma H, Mukai S, et al. The efficacy and ocular discomfort of substituting brinzolamide for dorzolamide in combination therapy with latanoprost, timolol, and dorzolamide. J Ocul Pharmacol Ther. 2005;21(5):395–399. doi: 10.1089/jop.2005.21.395. [DOI] [PubMed] [Google Scholar]
- 145.Wu W, Li J, Wu L, et al. Ophthalmic delivery of brinzolamide by liquid crystalline nanoparticles: in vitro and in vivo evaluation. AAPS Pharm Sci Tech. 2013;14(3):1063–1071. doi: 10.1208/s12249-013-9997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tuomela A, Liu P, Puranen J, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1–2):34–41. doi: 10.1016/j.ijpharm.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 147.Ikuta Y, Aoyagi S, Tanaka Y, et al. Creation of nano eye-drops and effective drug delivery to the interior of the eye. Sci Rep. 2017;14(7):44229. doi: 10.1038/srep44229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mahboobian MM, Seyfoddin A, Rupenthal ID, et al. Formulation development and evaluation of the therapeutic efficacy of brinzolamide containing nanoemulsions. Iran J Pharm Res. 2017;16(3):847–857. [PMC free article] [PubMed] [Google Scholar]
- 149.Wang F, Bao X, Fang A, et al. Nanoliposome-encapsulated brinzolamide-hydropropyl-β-cyclodextrin inclusion complex: a potential therapeutic ocular drug-delivery system. Front Pharmacol. 2018;13(9):91. doi: 10.3389/fphar.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salama HA, Ghorab M, Mahmoud AA, et al. PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech. 2017;18(7):2517–2528. doi: 10.1208/s12249-017-0710-8. [DOI] [PubMed] [Google Scholar]
- 151.Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128(1):8–14. doi: 10.1016/s0002-9394(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 152.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3(2):275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 153.Konstas AG, Stewart WC, Topouzis F, et al. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% in primary open-angle glaucoma. Am J Ophthalmol. 2001;131(6):729–733. doi: 10.1016/s0002-9394(01)00834-0. [DOI] [PubMed] [Google Scholar]
- 154.Prabhu P, Nitish KR, Koland M, et al. Preparation and evaluation of nano-vesicles of brimonidine tartrate as an ocular drug delivery system. J Young Pharm. 2010;2(4):356–361. doi: 10.4103/0975-1483.71623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhagav P, Upadhyay H, Chandran S. Brimonidine tartrate-Eudragit long-acting nanoparticles: formulation, optimization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2011;12(4):1087–1101. doi: 10.1208/s12249-011-9675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiang B, Kim YC, Doty AC, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;28(228):48–57. doi: 10.1016/j.jconrel.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ibrahim MM, Abd-Elgawad AH, Soliman OA, et al. Natural bioadhesive biodegradable nanoparticle-based topical ophthalmic formulations for management of glaucoma. Transl Vis Sci Technol. 2015;4(3):12. doi: 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.El-Salamouni NS, Farid RM, El-Kamel AH, et al. Nanostructured lipid carriers for intraocular brimonidine localisation: development, in vitro and in vivo evaluation. J Microencapsul. 2018;35(1):102–113. doi: 10.1080/02652048.2018.1425753. [DOI] [PubMed] [Google Scholar]
- 159.Pek YS, Wu H, Mohamed ST, et al. Long-term subconjunctival delivery of brimonidine tartrate for glaucoma treatment using a microspheres/carrier system. Adv Healthc Mater. 2016;5(21):2823–2831. doi: 10.1002/adhm.201600780. [DOI] [PubMed] [Google Scholar]
- 160.Bean GW, Camras CB. Commercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differences. Surv Ophthalmol. 2008;53(Suppl 1):S69–S84. doi: 10.1016/j.survophthal.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 161.Tanna AP, Lin AB. Medical therapy for glaucoma: what to add after a prostaglandin analogs? Curr Opin Ophthalmol. 2015;26(2):116–120. doi: 10.1097/ICU.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 162.Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6(1):45–52. doi: 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- 163.Wong TT, Novack GD, Natarajan JV, et al. Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res. 2014;4(4):303–309. doi: 10.1007/s13346-014-0196-9. [DOI] [PubMed] [Google Scholar]
- 164.Giarmoukakis A, Labiris G, Sideroudi H, et al. Biodegradable nanoparticles for controlled subconjunctival delivery of latanoprost acid: in vitro and in vivo evaluation. Preliminary results. Exp Eye Res. 2013;112:29–36. doi: 10.1016/j.exer.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 165.Natarajan JV, Ang M, Darwitan A, et al. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomed. 2012;7:123–131. doi: 10.2147/IJN.S25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheng YH, Tsai TH, Jhan YY, et al. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr Polym. 2016;25(144):390–399. doi: 10.1016/j.carbpol.2016.02.080. [DOI] [PubMed] [Google Scholar]
- 167.Fahmy HM, Saad EAES, Sabra NM, et al. Treatment merits of latanoprost/thymoquinone—encapsulated liposome for glaucomatus rabbits. Int J Pharm. 2018;548(1):597–608. doi: 10.1016/j.ijpharm.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 168.Rodriguez-Aller M, Guinchard S, Guillarme D, et al. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur J Pharm Biopharm. 2015;95(Pt B):203–214. doi: 10.1016/j.ejpb.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 169.Franca JR, Foureaux G, Fuscaldi LL, et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: in vitro and in vivo evaluation. PLoS One. 2014;9(4):e95461. doi: 10.1371/journal.pone.0095461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Di Trani N, Jain P, Chua CYX, et al. Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomedicine. 2019;16:1–9. doi: 10.1016/j.nano.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 171.Lambert WS, Carlson BJ, van der Ende AE, et al. Nanosponge-mediated drug delivery lowers intraocular pressure. Transl Vis Sci Technol. 2015;4(1):1. doi: 10.1167/tvst.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aref AA, Gedde SJ, Budenz DL. Glaucoma drainage implant surgery. Dev Ophthalmol. 2017;59:43–52. doi: 10.1159/000458485. [DOI] [PubMed] [Google Scholar]
- 173.Ayyala RS, Duarte JL, Sahiner N. Glaucoma drainage devices: state of the art. Expert Rev Med Devices. 2006;3(4):509–521. doi: 10.1586/17434440.3.4.509. [DOI] [PubMed] [Google Scholar]
- 174.Chaudhry M, Grover S, Baisakhiya S, et al. Artificial drainage devices for glaucoma surgery: an overview. Nepal J Ophthalmol. 2012;4(2):295–302. doi: 10.3126/nepjoph.v4i2.6547. [DOI] [PubMed] [Google Scholar]
- 175.Epstein E. Fibrosing response to aqueous. Its relation to glaucoma. Br J Ophthalmol. 1959;43:641–647. doi: 10.1136/bjo.43.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hong CH, Arosemena A, Zurakowski D, et al. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50(1):48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 177.Ponnusamy T, Yu H, John VT, et al. A novel antiproliferative drug coating for glaucoma drainage devices. J Glaucoma. 2014;23(8):526–534. doi: 10.1097/IJG.0b013e318294869b. [DOI] [PubMed] [Google Scholar]
- 178.Pan T, Brown JD, Ziaie B. An artificial nano-drainage implant (ANDI) for glaucoma treatment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3174–3177. doi: 10.1109/IEMBS.2006.260147. [DOI] [PubMed] [Google Scholar]
- 179.Harake RS, Ding Y, Brown JD, et al. Design, fabrication, and in vitro testing of an anti-biofouling glaucoma micro-shunt. Ann Biomed Eng. 2015;43(10):2394–2405. doi: 10.1007/s10439-015-1309-4. [DOI] [PubMed] [Google Scholar]
- 180.Popat KC, Desai TA. Poly(ethylene glycol) interfaces: an approach for enhanced performance of microfluidic systems. Biosens Bioelectron. 2004;19(9):1037–1044. doi: 10.1016/j.bios.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 181.Shokrollahi H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater Sci Eng C Mater Biol Appl. 2013;33(5):2476–2487. doi: 10.1016/j.msec.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 182.Feynman R. “The Brownian Movement”. 1964. In: The Feynman Lectures of Physics, Volume I. pp. 41–1. http://www.feynmanlectures.caltech.edu/I_41.html. Accessed 7 Dec 2019.
- 183.Paschalis EI, Chodosh J, Sperling RA, et al. A novel implantable glaucoma valve using ferrofluid. PLoS One. 2013;8(6):e67404. doi: 10.1371/journal.pone.0067404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–346. doi: 10.1016/s0039-6257(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 185.Costa VP, Spaeth GL, Eiferman RA, Orengo-Nania S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:152–170. [PubMed] [Google Scholar]
- 186.Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005;19(4):CD002897. doi: 10.1002/14651858.CD002897.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.The Fluorouracil Filtering Surgery Study Group. Three-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1993;115:82–92. [DOI] [PubMed]
- 188.Hou Z, Wei H, Wang Q, et al. New method to prepare mitomycin C loaded PLA-nanoparticles with high drug entrapment efficiency. Nanoscale Res Lett. 2009;4(7):732–737. doi: 10.1007/s11671-009-9312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gomes dos Santos AL, Bochot A, Doyle A, et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006;112(3):369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 190.Desai MA, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthal Surg Lasers Imaging. 2011;42:202–208. doi: 10.3928/15428877-20110224-04. [DOI] [PubMed] [Google Scholar]
- 191.Occhiutto ML, Freitas FR, Lima PP, Maranhão RC, Costa VP. Paclitaxel associated with lipid nanoparticles as a new antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2016;57(3):971–978. doi: 10.1167/iovs.15-18671. [DOI] [PubMed] [Google Scholar]
- 192.Duan Y, Guan X, Ge J, et al. Cationic nano-copolymers mediated IKKbeta targeting siRNA inhibit the proliferation of human Tenon’s capsule fibroblasts in vitro. Mol Vis. 2008;14:2616–2628. [PMC free article] [PubMed] [Google Scholar]
- 193.Ye H, Qian Y, Lin M, et al. Cationic nano-copolymers mediated IKKβ targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery. Mol Vis. 2010;26(16):2502–2510. [PMC free article] [PubMed] [Google Scholar]
- 194.Paula JS, Ribeiro VR, Chahud F, et al. Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery. J Ocul Pharmacol Ther. 2013;29(6):566–573. doi: 10.1089/jop.2012.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50(11):5217–5225. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- 196.Grewal DS, Jain R, Kumar H, Grewal SPS. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115(12):2141–2145. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 197.Vandewalle E, Abegão Pinto L, Van Bergen T, et al. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol. 2014;98(1):73–78. doi: 10.1136/bjophthalmol-2013-303966. [DOI] [PubMed] [Google Scholar]
- 198.Han Q, Wang Y, Li X, et al. Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery. J Mater Sci Mater Med. 2015;26(8):225. doi: 10.1007/s10856-015-5556-6. [DOI] [PubMed] [Google Scholar]
- 199.Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18(2):110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 200.Brubaker RF. Delayed functional loss in glaucoma. LII Edward Jackson memorial lecture. Am J Ophthalmol. 1996;121:473–483. doi: 10.1016/s0002-9394(14)75421-2. [DOI] [PubMed] [Google Scholar]
- 201.Allen SJ, Watson JJ, Shoemark DK, et al. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 202.Frasson M, Picaud S, Léveillard T, et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40(11):2724–2734. [PubMed] [Google Scholar]
- 203.Checa-Casalengua P, Jiang C, Bravo-Osuna I, et al. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J Control Release. 2011;156(1):92–100. doi: 10.1016/j.jconrel.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 204.Ward MS, Khoobehi A, Lavik EB, et al. Neuroprotection of retinal ganglion cells in DBA/2 J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007;96(3):558–568. doi: 10.1002/jps.20629. [DOI] [PubMed] [Google Scholar]
- 205.Jiang C, Moore MJ, Zhang X, et al. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;24(13):1783–1792. [PubMed] [Google Scholar]
- 206.García-Caballero C, Prieto-Calvo E, Checa-Casalengua P, et al. Six month delivery of GDNF from PLGA/vitamin E biodegradable microspheres after intravitreal injection in rabbits. Eur J Pharm Sci. 2017;30(103):19–26. doi: 10.1016/j.ejps.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 207.Clatterbuck RE. Cliliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc Natl Acad Sci USA. 1993;90:2222–2226. doi: 10.1073/pnas.90.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Emerich DF, Winn SR, Hantraye PM, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997;386(6623):395–399. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 209.Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci USA. 1993;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sendtner M, Schmalbruch H, Stockli KA, et al. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358(6386):502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- 211.Nkansah MK, Tzeng SY, Holdt AM, et al. Poly(lactic-co-glycolic acid) nanospheres and microspheres for short- and long-term delivery of bioactive ciliary neurotrophic factor. Biotechnol Bioeng. 2008;100(5):1010–1019. doi: 10.1002/bit.21822. [DOI] [PubMed] [Google Scholar]
- 212.Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(5):2194–2200. doi: 10.1167/iovs.08-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kitagawa K, Matsumoto M, Tagaya M, et al. Hyperthermia-induced neuronal protection against ischemic injury in gerbils. J Cereb Blood Flow Metab. 1991;11(3):449e52. doi: 10.1038/jcbfm.1991.86. [DOI] [PubMed] [Google Scholar]
- 214.Caprioli J, Kitano S, Morgan JE. Hyperthermia and hypoxia increase tolerance of retinal ganglion cells to anoxia and excitotoxicity. Invest Ophthalmol Vis Sci. 1996;37(12):2376e81. [PubMed] [Google Scholar]
- 215.Barbe MF, Tytell M, Gower DJ, et al. Hyperthermia protects against light damage in the rat retina. Science. 1988;241(4874):1817e20. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- 216.Yin Y, Henzl MT, Lorber B, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 217.Jeun M, Jeoung JW, Moon S, et al. Engineered superparamagnetic Mn0.5Zn0.5Fe2O4 nanoparticles as a heat shock protein induction agent for ocular neuroprotection in glaucoma. Biomaterials. 2011;32(2):387–394. doi: 10.1016/j.biomaterials.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 218.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 219.Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer’s disease: experimental approaches and clinical interventions. J Neurosci Res. 1998;54:1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 220.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 221.Julien JP. Amyotrophic lateral sclerosis: unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 222.Mac Nair CE, Nickells RW. Neuroinflammation in glaucoma and optic nerve damage. Prog Mol Biol Transl Sci. 2015;134:343–363. doi: 10.1016/bs.pmbts.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 223.Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 224.Kolko M, DeCoster MA, de Turco EB, et al. Synergy by secretory phospholipase A2 and glutamate on inducing cell death and sustained arachidonic acid metabolic changes in primary cortical neuronal cultures. J Biol Chem. 1996;271:32722–32728. doi: 10.1074/jbc.271.51.32722. [DOI] [PubMed] [Google Scholar]
- 225.Li G, Luna C, Liton PB, et al. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 226.Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ergorul C, Ray A, Huang W, et al. Hypoxia inducible factor1alpha (HIF-1alpha) and some HIF-1 target genes are elevated in experimental glaucoma. J Mol Neurosci. 2010;42:183–191. doi: 10.1007/s12031-010-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 229.Dong S, Zeng Q, Mitchell ES, et al. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7(2):e31211. doi: 10.1371/journal.pone.0031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim DS, Kim JY, Han Y. Curcuminoids in neurodegenerative diseases. CNS Drug Discov. 2012;7(3):184–204. doi: 10.2174/157488912803252032. [DOI] [PubMed] [Google Scholar]
- 231.Belviranlı M, Okudan N, Atalık KE, et al. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology. 2013;14(2):187–196. doi: 10.1007/s10522-013-9422-y. [DOI] [PubMed] [Google Scholar]
- 232.Wang L, Li C, Guo H, et al. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS One. 2011;6(8):e23194. doi: 10.1371/journal.pone.0023194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yue YK, Mo B, Zhao J, et al. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J Ocul Pharmacol Ther. 2014;30(8):657–664. doi: 10.1089/jop.2014.0022. [DOI] [PubMed] [Google Scholar]
- 234.Kaminaga Y, Nagatsu A, Akiyama T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555(2):311–316. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- 235.Gupta S, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Davis BM, Pahlitzsch M, Guo L, et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci Rep. 2018;8(1):11066. doi: 10.1038/s41598-018-29393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.National Cancer Institute Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/ketorolac. Accessed 7 Dec 2019.
- 238.Ju WK, Neufeld AH. Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J Comp Neurol. 2002;452:392–399. doi: 10.1002/cne.10400. [DOI] [PubMed] [Google Scholar]
- 239.Wang AG, Lee CM, Wang YC, et al. Up-regulation of cytochrome oxidase in the retina following optic nerve injury. Exp Eye Res. 2002;74:651–659. doi: 10.1006/exer.2002.1190. [DOI] [PubMed] [Google Scholar]
- 240.Ju WK, Kim KY, Neufeld AH. Increased activity of cyclooxygenase-2 signals early neurodegenerative events in the rat retina following transient ischemia. Exp Eye Res. 2003;77:137–145. doi: 10.1016/s0014-4835(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 241.Nadal-Nicolás FM, Rodriguez-Villagra E, Bravo-Osuna I, et al. Ketorolac administration attenuates retinal ganglion cell death after axonal injury. Invest Ophthalmol Vis Sci. 2016;57(3):1183–1192. doi: 10.1167/iovs.15-18213. [DOI] [PubMed] [Google Scholar]
- 242.Barcia E, Herrero-Vanrell R, Díez A, et al. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp Eye Res. 2009;89(2):238–245. doi: 10.1016/j.exer.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 243.Prow TW. Toxicity of nanomaterials to the eye. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):317–333. doi: 10.1002/wnan.65. [DOI] [PubMed] [Google Scholar]
- 244.Voigt N, Henrich-Noack P, Kockentiedt S, et al. Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res. 2014;16(6):2379. [DOI] [PMC free article] [PubMed]
- 245.De Matteis V, Rinaldi R. Toxicity assessment in the nanoparticle era. Adv Exp Med Biol. 2018;1048:1–19. doi: 10.1007/978-3-319-72041-8_1. [DOI] [PubMed] [Google Scholar]
- 246.El-Ansary A, Al-Daihan S, Bacha AB, et al. Toxicity of novel nanosized formulations used in medicine. Methods Mol Biol. 2013;1028:47–74. doi: 10.1007/978-1-62703-475-3_4. [DOI] [PubMed] [Google Scholar]
- 247.De Jong WH, Van Der Ven LT, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.