Abstract
Background:
Clinical and endoscopic remission are treatment targets in ulcerative colitis (UC). The value of histologic healing in altering clinical outcomes among patients with complete endoscopic healing is not well established.
Aims:
The aim of this study was to quantify the association between histology activity and clinical relapse among patients with UC who were in complete endoscopic remission.
Methods:
This study included patients with UC from a prospective registry who were in complete endoscopic remission. Histologic activity was quantified by a senior gastrointestinal pathologist. Histologic activity was defined as lack of normalization (Geboes > 0) as well as histologically active disease (Geboes ≥ 2.1 and ≥ 3.1). The primary outcome was clinical relapse within 2 years. Multivariable regression adjusting for potential confounders examined the independent predictive value of histologic changes.
Results:
The study included 83 patients (51% women) (median age 44 years; median disease duration 11 years). Forty-one patients (49%) had complete histologic normalization. Within two years, 26 patients (31%) experienced clinical relapse. Patients with complete histologic normalization were less likely to experience relapse (5/41, 12%) compared to those without normalization (21/42, 50%, p < 0.001) (multivariable OR 7.22, 95% confidence interval (CI) 2.48–24.70) by the Geboes score. The individual components of the Geboes score predictive of relapse were architectural changes (p=0.03) and increased chronic inflammatory infiltrate (p < 0.001).
Conclusions:
Complete histologic healing using the Geboes score was associated with reduced rates of clinical relapse among patients with ulcerative colitis in endoscopic remission.
Keywords: Histology, Ulcerative Colitis, Clinical Relapse, Geboes score
INTRODUCTION
Ulcerative colitis (UC) is a chronic immune mediated gastrointestinal disease that affects nearly 1 million Americans.1,2 Traditionally, the aim of medical therapy of UC has been relief from disease-related symptoms of rectal bleeding, urgency, and diarrhea2–5. However, it is increasingly recognized that endoscopic resolution of inflammation is a more robust outcome.4–6 Endoscopic healing is associated with reduced need for corticosteroids, clinical relapse, risk of hospitalizations, surgery, as well as colorectal neoplasia.7–10 While the exact definition of endoscopic healing has varied, the treatment target most often recommended has been attainment of a Mayo endoscopic sub-score of 0 or 14,5. However, an emerging body of evidence has suggested that this definition may be too broad and that short-term and long-term outcomes are superior with a Mayo endoscopic score of 0 (completely normal mucosa) compared to a score of 1, leading to the former being termed endoscopic remission and a score of 0 or 1 being termed endoscopic improvement.11,12 Furthermore, therapeutic intervention among those with a Mayo score of 1 has been associated with reduced risk of relapse, supporting a more stringent endoscopic target.13
Despite endoscopically normal appearing mucosa, it is recognized that a sizeable proportion of patients with UC will continue to have histologic activity.14–21 There are several studies that have examined whether persistent histologic changes modify long-term prognosis15,17,22,23 but such studies have several limitations that preclude robust interpretation of findings. First, many prior studies included patients both with an endoscopic score of 0 or 117,23. As histologic activity correlates with endoscopic severity, findings from such studies cannot be extrapolated to define the impact of histologic activity on prognosis of patients with completely normal mucosal appearance. Second, studies have retrospectively stratified histologic activity using various definitions and/or did not utilize a validated histologic activity score17,19. Thus, whether histologic normalization or persistence of inflammatory infiltrate modifies short- and medium-term outcomes in patients with UC with a completely normal endoscopic appearance has not been robustly established.
Therefore, in patients with UC with endoscopically normal mucosa (endoscopic subscore of 0), we aimed to examine if histologic normalization or persistence of inflammatory activity quantified using a validated scale of histologic activity was associated with (a) risk of disease relapse, need for UC-related surgery, or hospitalization over the subsequent 2 years; and (b) concurrent symptoms related to UC.
METHODS
Study Cohort
This was a single center study based at a tertiary referral IBD center serving the population of Boston and the surrounding New England area. To be eligible for inclusion, patients were required to meet the following criteria: (1) established diagnosis of UC according to standard diagnosis criteria; (2) enrollment in the prospective institutional registry; (3) a colonoscopy with complete endoscopic remission defined as a Baron score of 0 (normal mucosa with no erythema or friability)24; and (4) clinical follow-up of 2 years. Of all eligible patients, four were excluded (2 with no follow-up information and 2 with a cecal patch or mild right colonic activity). We did not require clinical remission as recent studies have demonstrated that persistent alteration in bowel frequency may remain even with endoscopic and histologic remission and is likely from non-inflammatory changes25.
Clinical Covariates and Outcome of Interest
The primary outcome was clinical relapse within two years from the time of the index colonoscopy. This was defined as any change in UC therapy (i.e dose escalation, class change, and/or need for systemic corticosteroids), UC-related hospitalization, or UC-related surgery within two years. Only dose change or medication alterations for symptomatic exacerbations of disease with clinical suspicion for active UC were characterized as relapse. Dose or therapy change for insurance related reasons or patient preference was not considered relapse. Patients in whom the colonoscopy was performed explicitly with the intention of de-escalating or ceasing therapy were excluded. All charts were independently reviewed for the outcome of interest.
Additional covariates included in the study cohort included age at diagnosis, age at visit, disease duration, disease extent, medication use at the time of the study visit, clinical disease activity as measured by the simple clinical colitis activity index (SCCAI)26, anxiety and depression scores as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires. Current immune modulator use was recorded for either thiopurine (azathioprine, 6-mercaptopurine) or methotrexate therapy. Current biologic use was recorded for either tumor necrosis factor α antagonists (infliximab, adalimumab, golimumab) or anti-integrin (vedolizumab) therapy.
Assessment of Histologic Activity
All histologic specimens from the index colonoscopy were reviewed by a single senior gastrointestinal pathologist (V.D.) who was blinded to the outcome of interest. The site with the most histologically active disease was scored according the Geboes score27. The individual components of the Geboes score were recorded for each patient: structural (architectural change) (Grade 0), chronic inflammatory infiltrate (Grade 1), lamina propria eosinophils (Grade 2A), lamina propria neutrophils (Grade 2B), neutrophils in the epithelium (Grade 3), crypt destruction (Grade 4), and erosion or ulceration (Grade 5).27 The expected lymphoplasmacytic infiltrate in the colonic segment chosen for review was accounted for when scoring the grade “chronic inflammatory infiltrate”. We modeled histologic activity as a predictor variable using three definitions.28 First, patients were classified as having histologically active disease if the Geboes was ≥ 3.1. According to the second definition, histologically active disease was defined as Geboes was ≥ 2.1. Finally, histologic normalization was defined as the complete absence of any histologic inflammatory activity, no increased chronic inflammatory infiltrate, no neutrophils, and no structural and architectural change (Geboes score= 0).
While the primary histologic assessment was using the Geboes score, we also examined the predictive value of the Robarts Histologic Index (RHI)29 and the Nancy Index30 in determining risk of relapse. To do so, features from the Geboes score were extrapolated to the two other indices and total RHI and Nancy scores derived as previously defined25. We compared the risk of relapse across different strata of the Nancy histologic score. For the RHI, we examined the predictive value of each of the separate cut-offs for histologic remission that have been proposed (≤ 131, ≤ 331, and ≤ 632) in addition to comparing the total RHI between relapsing and non-relapsing patients.
Statistical Analysis
The study was approved by the Institutional Review Board of Partners Healthcare. All patients provided informed consent. Categorical variables are presented as counts and percentages. Comparisons between categorical variables were performed using the chi square test or Fisher exact test, as appropriate. Continuous variables are presented as means +/− standard deviations or medians +/− interquartile ranges based on normal distribution of the variable. Comparisons between continuous variables were performed with the student’s t test or Wilcoxon rank sum test, as appropriate. First, univariate regression was performed against the outcome of relapse to identify potentially influential factors. Covariates significant in this analysis at p < 0.05 or those that had been previously determined to be important in predicting relapse were included in a final multivariable model that examined the independent effect of histologic changes. A two-sided p-value < 0.05 indicated independent statistical significance. A receiver operating curve (ROC) was constructed for the final model and the area under the curve (AUC) was calculated. We repeated the analysis using Cox regression models and plotted a Kaplan-Meier curve of time to relapse stratifying by histologic normalization or activity. Analysis were performed in R (version 3.5.3) using RStudio, version 1.1.456, and Stata 13.2 (StataCorp, College Station, TX).33–35 The “yardstick” package was used for ROC construction and AUC calculation.36
RESULTS
Study Cohort
There were a total of 811 patients with UC in the prospective institutional registry. There was equal distribution of men and women (50% men, 50% women) with a median age of diagnosis of 27 years. Most patients had pancolitis (55%) with 66% exposed to immune modulators and 39% exposed to anti-TNF therapy. The included study cohort was comparable to the full registry cohort in terms of study demographics and disease extent (26 years vs 27 years; 49% male vs 50% male; 55% with pancolitis vs 55% with pancolitis) but had less frequent use of immune modulators (41% vs 66%) and anti-TNFs (30% vs 39%). In the final study cohort, there were a total of 83 participants with UC who met the specified inclusion criteria. The median disease duration was 11 years. About half the patients with complete endoscopic remission (endoscopic sub-score of 0) demonstrated complete histologic normalization by the Geboes score (n=41, 49%). A total of 20 patients (24%) and 27 patients (33%) met the criteria for histologically active disease defined using a Geboes score of ≥ 3.1 and ≥ 2.1a respectively. Interestingly, fewer patients on biologic therapy had histologically active disease, as defined by a Geboes score ≥ 3.1 (11% vs 31%, p=0.08); though this did not reach statistical significance.
Over a follow-up of 2 years, the primary study outcome (disease relapse) occurred in 26 patients (31%). Among the patients who relapsed, 1 required surgery, 3 required hospitalization for a flare of their UC, 16 were initiated on systemic steroids, 11 were started on topical therapy, 4 required new initiation of biologic therapy, 3 changed their biologic agent due to loss of response, and 2 additionally had a dose increase of their thiopurine. Only two patients de-escalated therapy after the index colonoscopy – one stopped mesalamine and the other stopped an anti-TNF. Both relapsed of which one had complete histologic normalization at baseline while the other had histologically active disease. Exclusion of these two patients from the analysis did not alter our findings. Clinical symptoms, defined as an SCCAI ≥ 2, were present in 19 of 81 patients assessed at the time of colonoscopy (23%) despite complete endoscopic remission. Patients who experienced clinical relapse within two years of histologic evaluation were similar in demographic characteristics compared to those who did not relapse (Table 1). Age at diagnosis (27 vs 25 years, p=0.78), disease duration (14 vs 10 years, p=0.11), gender (p=0.34), and race/ethnicity (p=0.59) were not significantly different between those who relapsed compared to those who did not. There was also no difference in current use of immune modulators (p=1.00) or biologics (p=0.52) between the two groups.
Table 1.
Characteristics of Study participants based on relapse status within 2 years of index examination
| Clinical Relapse (n=26) | No Clinical Relapse (n=57) | p value | |
|---|---|---|---|
| Age at Diagnosis | 27.5 +/− 15.3 | 25 +/− 20 | 0.78 |
| Age at Visit | 44.5 +/− 19.3 | 43 +/− 23 | 0.57 |
| Disease Duration | 14.5 +/− 12 | 10 +/− 9 | 0.11 |
| Gender | 0.34 | ||
| Male | 10 (38.4%) | 30 (52.6%) | |
| Female | 16 (61.5%) | 27 (47.4%) | |
| Race | 0.59* | ||
| White | 24 (92.3%) | 55 (96.5%) | |
| Non-white | 2 (7.7%) | 2 (3.5%) | |
| Pancolitis | 1.00 | ||
| Yes | 14 (53.8%) | 32 (56.1%) | |
| No | 12 (46.2%) | 25 (43.9%) | |
| Current Immune Modulator | 1.00 | ||
| Yes | 11 (42.3%) | 23 (40.4%) | |
| No | 15 (57.7%) | 34 (59.6%) | |
| Current Biologic | 0.52 | ||
| Yes | 7 (26.9%) | 21 (36.8%) | |
| No | 19 (73.1%) | 36 (63.2%) | |
| Current Steroid | 0.59* | ||
| Yes | 2 (7.7%) | 2 (3.5%) | |
| No | 24 (92.3%) | 55 (96.5%) |
Fisher’s Exact test
Histologic changes and relapse
Patients with complete histologic normalization by the Geboes score were less likely to experience relapse (12%) compared to those without (50%, p < 0.001) (Figure 1). In contrast, histologic activity as assessed by a Geboes score of ≥ 2.1a (44% vs 25%, p= 0.12), ≥ 2.1b (38% vs 29%, p= 0.62) and ≥ 3.1 (40% vs 28%, p= 0.49) were not significantly associated with risk of relapse (Table 2). The percentage of patients with clinical relapse was higher in all groups with higher Geboes scores, however. (Figure 2). Two subcomponents of the Geboes score demonstrated significant associations with risk of clinical relapse within two years. (Table 3) The strongest association was observed with the presence of increased numbers of chronic inflammatory cells (p<0.001) with more patients who had clinical relapse having a mild (65% vs 23%) or moderate increase (15% vs 5%). Most patients with no clinical relapse within two years had no increase in chronic inflammatory infiltrate (65%). The second significant association with clinical relapse occurred with the presence of architectural changes (p=0.03). More patients who experienced clinical relapse within two years had a mild abnormality (31% vs 10%) or mild-moderate diffuse or multifocal abnormalities (8% vs 3%). Most patients in the group who did not experience clinical relapse within two years had no abnormality (86%). The presence of lamina propria neutrophils (p=0.69) and/or eosinophils (p=0.08) were not statistically significant between those who relapsed and those who did not. There was also no difference between groups in the presence of neutrophils in the epithelium (p=0.35) or crypt destruction (p=0.59). A Kaplan-Meier survival curve describing time to relapse similarly demonstrated a lower frequency of and longer time to relapse among those achieving complete histologic normalization (log-rank p=0.0002) but not when stratifying by histologic activity at a cut-off of Geboes 3.1 (p=0.52) (Figure 3).
Figure 1.
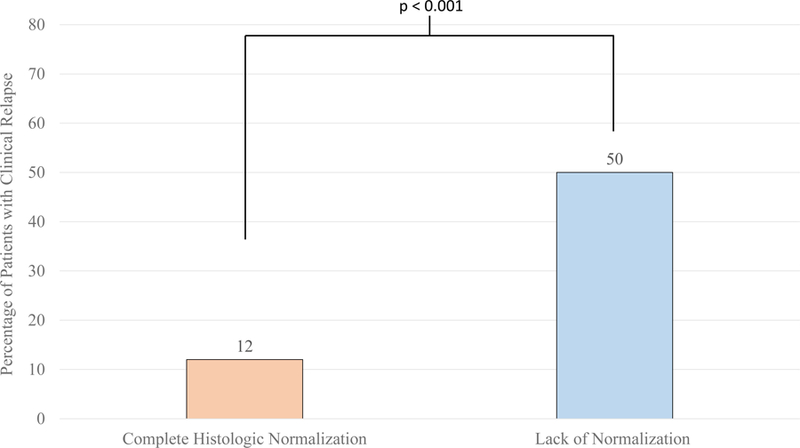
Percentage of Patients with Clinical Relapse within Two Years of Endoscopy by Histologic Normalization (Geboes score 0: 5/41, 12%, Geboes Score >0: (21/42, 50%)
* Clinical Relapse: Defined as Need for Therapy Escalation, Surgery, and/or Hospitalization
Table 2.
Association Between Histologic Activity and Clinical Relapse, By Geboes Score Classification
| Clinical Relapse (n=26) | No Clinical Relapse (n=57) | p value | |
|---|---|---|---|
| Lack of normalization (Geboes > 0) | <0.001 | ||
| Yes | 21 (50%) | 21 (50%) | |
| No | 5 (12%) | 36 (88%) | |
| Geboes ≥ 2.1a | 0.12 | ||
| Yes | 12 (44%) | 15 (56%) | |
| No | 14 (25%) | 42 (75%) | |
| Geboes ≥ 2.1b | 0.62 | ||
| Yes | 8 (38%) | 13 (62%) | |
| No | 18 (29%) | 44 (71%) | |
| Geboes ≥ 3.1 | 0.49 | ||
| Yes | 8 (40%) | 12 (60%) | |
| No | 18 (28%) | 45 (72%) |
Figure 2.
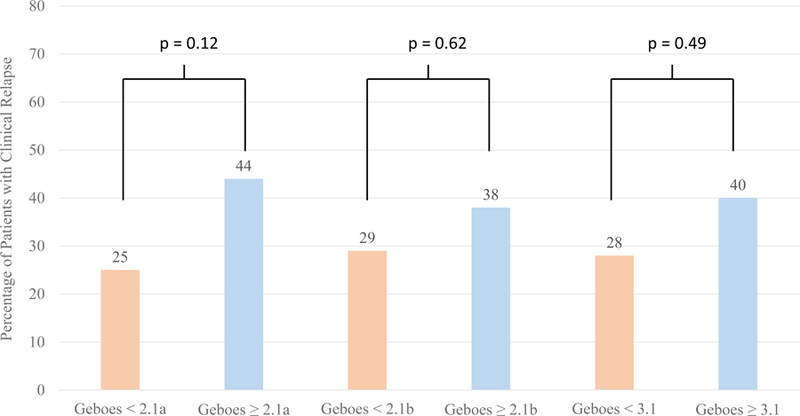
Percentage of Patients Who Have Clinical Relapse by Geboes Score Threshold Within 2 Years of Endoscopy
Table 3.
Components of the Geboes Score by Clinical Relapse Within Two Years of Colonoscopic evaluation among Patients with Ulcerative Colitis
| Clinical Relapse (n=26) | No Clinical Relapse (n=57) | p value | |
|---|---|---|---|
| Structural (Architectural Change) | 0.03 | ||
| No abnormality | 16 (61.5%) | 49 (86%) | |
| Mild Abnormality | 8 (30.8%) | 6 (10.5%) | |
| Mild or moderate diffuse or multifocal abnormalities | 2 (7.7%) | 2 (3.5%) | |
| Severe diffuse or multifocal abnormalities | 0 | 0 | |
| Chronic inflammatory infiltrate | <0.001* | ||
| No increase | 5 (19.2%) | 37 (64.9%) | |
| Mild but unequivocal increase | 17 (65.4%) | 13 (22.8%) | |
| Moderate increase | 4 (15.4%) | 3 (5.3%) | |
| Marked increase | 0 | 4 (7%) | |
| Lamina Propria neutrophils and eosinophils | |||
| 2 A: Eosinophils | 0.08* | ||
| No increase | 16 (61.5%) | 47 (82.4%) | |
| Mild but unequivocal increase | 7 (26.9%) | 9 (15.8%) | |
| Moderate increase | 1 (3.8%) | 0 | |
| Marked increase | 2 (7.7%) | 1 (1.8%) | |
| 2B: Neutrophils | 0.69* | ||
| None | 26 (100%) | 53 (93%) | |
| Mild but unequivocal increase | 0 | 3 (5.3%) | |
| Moderate increase | 0 | 1 (1.7%) | |
| Marked increase | 0 | 0 | |
| Neutrophils in the epithelium | 0.35* | ||
| None | 18 (69.2%) | 45 (78.9%) | |
| <5% crypts involved | 6 (23.1%) | 6 (10.5%) | |
| <50% crypts involved | 1 (3.8%) | 5 (8.8%) | |
| >50% crypts involved | 1 (3.8%) | 1 (1.8%) | |
| Crypt destruction | 0.59* | ||
| None | 24 (92.3%) | 55 (96.5%) | |
| Probable – local excess of neutrophils in part of crypt | 2 (7.7%) | 2 (3.5%) | |
| Probably - marked attenuation | 0 | 0 | |
| Unequivocal crypt destruction | 0 | 0 | |
| Erosion or Ulceration | N/A | ||
| No erosion, ulceration, or granulation tissue | 26 (100%) | 57 (100%) | |
| Recovering epithelium + adjacent inflammation | 0 | 0 | |
| Probable erosion – focally stripped | 0 | 0 | |
| Unequivocal erosion | 0 | 0 | |
| Ulcer or granulation tissue | 0 | 0 |
Fisher’s Exact test
Figure 3:
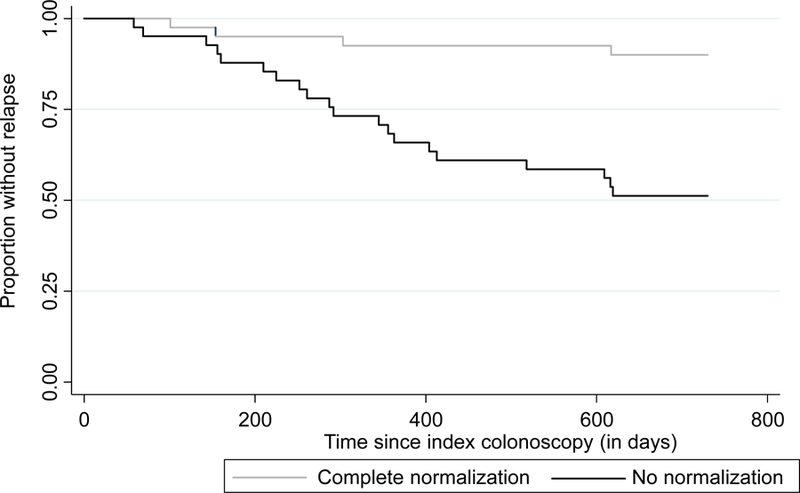
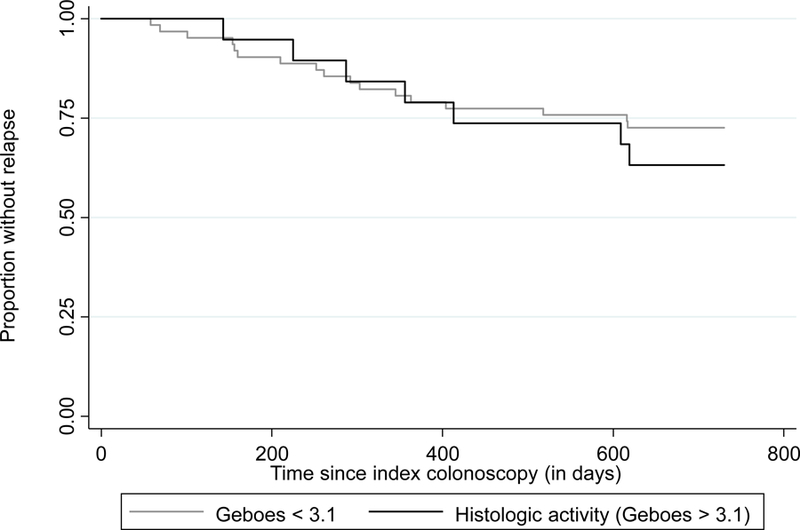
Kaplan Meier curve of time to relapse in patients with ulcerative colitis in endoscopic remission stratified by (a) complete histologic normalization (Geboes=0; n=41); and (b) histologic activity (Geboes ≥ 3.1; n=20)
Multivariable predictors of relapse
Lack of complete histologic normalization by the Geboes score was predictive of clinical relapse at two years (OR 7.22, 95% CI 2.48–24.70) when controlling for disease duration, current biologic use, and age at time of the visit (Table 4). Age at visit (OR 0.99, 95% CI 0.96–1.04), , disease duration (OR 1.03, 95% CI 0.97–1.08), and biologic use (OR 0.75, 95% CI 0.24–2.28) were not predictive of clinical relapse within two years. The ROC curve demonstrated good predictive ability of the Geboes score to discriminate the outcome of interest, clinical relapse, with an AUC of 0.78 (Figure 4).
Table 4.
Multivariable Logistic Regression Evaluating Predictors of Clinical Relapse at Two Years
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Lack of Complete Normalization** | 7.22 | 2.48–24.7 | <0.001 |
| Disease Duration | 1.03 | 0.97–1.08 | 0.38 |
| Current Biologic Use | 0.75 | 0.24–2.28 | 0.68 |
| Age at Visit | 0.99 | 0.96–1.04 | 0.93 |
Complete histologic normalization indicates a Geboes 0
Figure 4.
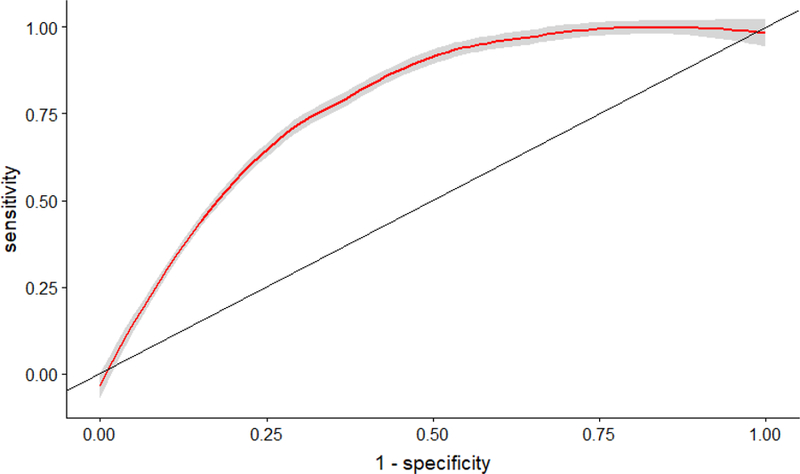
Accuracy of histologic normalization in predicting Clinical Relapse in patients with ulcerative colitis in endoscopic remission (Area Under the Curve = 0.78)
Predictive models using the Robarts Histologic Index and Nancy Scores
We examined whether the RHI or Nancy score while in endoscopic remission predicted relapse. The distribution of the Nancy index in the cohort is presented in Supplemental Table 1. There was no statistically significant difference in rates of relapse across the Nancy histologic scores, with relapse rates ranging from 25% (2 out of 8 patients) with a Nancy score of 3 and 50% (1 out of 2 patients) with a Nancy score of 1. There were no patients with a Nancy score of 4. The rates of relapse in those with a Nancy score of 0 (28%) was similar to those with a score ≥ 1 (39%, p=0.34). The RHI in our cohort ranged from 0 to 13 (median 1, IQR 0–4). The mean RHI in patients who relapsed (2.23±2.57) was similar to those who did not relapse (1.72±3.18) (p=0.47). Dichotomizing histologic remission using the cut-offs of ≤ 131 (39% vs 28%, p=0.34), ≤ 331 (38% vs 29%, p=0.44), and ≤ 632 (25% vs 32%, p=0.69) did not reveal any significant associations between RHI and risk of relapse.
Association between histologic changes and disease symptoms
Sixty-two patients (77%) were in clinical remission. No difference was observed in total SCCAI score between those who had histologic activity as defined by a Geboes ≥ 3.1 (p=0.17) or lack of complete histologic normalization (p=0.60). There was also no difference in individual symptoms by histologic activity. Specifically, when histologic activity was defined as a Geboes score of ≥ 3.1, there was no difference in fecal urgency (20% vs 35%, p=0.27), general well-being (15% vs 27%, p=0.37), blood in the stool (15% vs 8%, p=0.39), daily bowel frequency (0% vs 10%, p=−0.33), or nocturnal bowel frequency (0% vs 2%, p =1.00) between the two groups. (Supplementary Table 2) Similarly, there was no difference in any of these symptoms between those who had complete histologic normalization and those who did not. (Supplementary Table 3)
DISCUSSION
The treatment target in UC has evolved from symptomatic remission to endoscopic remission with the recognition that persistent endoscopic inflammatory activity even in the absence of symptoms is associated with worse long-term outcomes4–6. However, the recognition that histologic activity persists even in a significant subset of patients with complete endoscopic normalization has led to consideration of histologic healing as the optimal therapeutic target5,6,37. However, studies of histologic activity have been either cross-sectional in examining associations with symptoms25, used non-validated retrospective assessments of histologic activity17, or did not examine longer-term outcomes. Further, few studies have attempted to define the optimal histologic target necessary for modifying future disease course and whether this differs from that used for assessing therapeutic response to pharmacologic intervention38,39. In this study, using blinded expert histologic assessments in patients with UC in complete endoscopic remission, we demonstrate that complete histologic normalization with absence of structural / architectural features rather than resolution of active inflammatory infiltrate was associated with reduced future risk of relapse. These results may suggest incremental benefit in attaining histologic remission in UC and further suggest that features that discriminate therapeutic effect may be different than those required to alter disease course. However, attainment of histologic remission is still infrequent with the existing therapies, and while our study may provide prognostic information to clinicians when faced with a discrepancy between histologic activity and endoscopic remission, there are still insufficient data to recommend histologic healing as a necessary therapeutic target in all patients.
A few prior studies have evaluated the impact of histologic disease activity on clinical outcomes in UC. A large retrospective cohort by Christensen et al. demonstrated that histologic normalization was beneficial even in those with endoscopic remission, but histologic activity was not assessed using validated scoring systems17. Of those that have used histologic scores, most have relied on the Geboes score. Several have used a Geboes score with a threshold of ≥ 3.1 to define histologic activity.15,21,40,41 Bessissow et al. retrospectively evaluated the role of histologic disease activity on clinical relapse within 12 months among 75 patients with UC who had Mayo 0 endoscopic disease.15 The authors found that a Geboes ≥ 3.1 and basal plasmacytosis were associated with relapse on univariate analysis but only basal plasmacytosis was associated with relapse on multivariate analysis. Zenlea et al. evaluated the role of histology as a predictor of clinical relapse in UC among patients who were in clinical remission.21 The authors found that Geboes ≥ 3.1 was associated with clinical relapse on both univariate and multivariate analysis. Our study expands upon previous findings by showing that histologic activity was associated with clinical relapse among a larger group of patients in complete endoscopic remission and expands the histological analysis to include derived RHI and Nancy histology indices.”
A systematic review and meta-analysis performed in 2016 investigated the available data in this area to determine the association between histologic healing and relapse.14 The authors found that histologic remission was associated with reduced risk of relapse (relative risk 0.48). Furthermore, absence of neutrophils in the epithelium, neutrophils or eosinophils in the lamina propria, crypt abscesses, and chronic inflammatory cell infiltrates were all associated with lower risk of clinical relapse. One explanation of the heterogeneity in previous findings including association between histologic remission and relapse in prior studies is inclusion of patients with endoscopic score 0 or 1. However, one limitation of this analysis is pooling together of patients across a spectrum of endoscopic activity. Patients with endoscopic subscore of 1 are more likely to have histologic disease activity and also more likely to relapse, raising the possibility of confounding in the prior studies. With increasing acceptance of endoscopic subscore 0 as the treatment target for endoscopic remission and strong correlation between histologic and endoscopic activity, it is important to robustly examine the additive benefit of histologic inflammation or normalization in this subpopulation rather than in a more heterogenous group of patients with broadly varying endoscopic activity. In our study, larger than these prior two and with a longer duration of follow up, we did not detect an association between histologically active disease and clinical relapse using various Geboes thresholds, the RHI, or Nancy index. However, interestingly, resolution of chronic architectural distortion was one of the two features in the Geboes score that was most predictive of relapse and this characteristic is not a part of the RHI or Nancy index. This finding intriguingly suggests that perhaps histologic changes that modify disease course (like relapse) are different from those that can measure therapeutic response to an intervention (like RHI or Nancy score). This merits further examination in future prospective studies.
There are several strengths of this study. First, all patients had extensive data available for review given that they were followed as part of a prospective registry. This allowed us to examine associations between symptoms at the time of the endoscopy and biopsy collection as well as have up to date information on current medication use at the time of endoscopy. Second, histologic specimens were reviewed by a senior gastrointestinal pathologist who was blinded to the outcome of interest. This limited the potential for observer bias. Third, we used the Geboes score for primary histologic assessment but also included RHI and Robarts. To our knowledge, ours is the first study to simultaneously examine all three scoring systems to predicting relapse. The Geboes score is a comprehensive histologic scoring system which evaluates both acute and chronic inflammatory changes in addition to architectural distortion. This score has been shown to have good concordance with other histologic scoring systems in inflammatory bowel disease including the Robarts score and the Nancy Index.28 Further, the comprehensiveness of the Geboes score allowed us to evaluate several thresholds to score histologic activity as well as transpose to derive RHI and Nancy histology indices. Finally, we isolated the impact of histologic activity on the outcome of clinical relapse by defining endoscopic remission using a strict threshold, which was a Baron score of 0. By excluding a Baron score of 1, we could evaluate an extremely well controlled group of patients and isolate the impact of histology on relapse in this group.
We readily acknowledge some limitations of this work. First, there was a relatively small sample size which may have limited power for statistical analyses. Despite this, ours remains one of the largest to examine the value of histologic activity on medium-term natural history in patients with UC in complete endoscopic remission. We did not have sufficient numbers to examine the predictive value of histology within subgroups of patients by disease extent or existing treatments. One such consideration would be the influence of steroids prior to colonoscopy which could bias towards increased histologic healing. However, if there were a transient steroid exposure (due to a recent flare) resulting in histologic healing, this would be expected bias the population with healing towards a higher rate of relapse in the future (and lack of significance between groups) which we did not see. Second, histologic assessment was performed by one expert gastrointestinal pathologist. A recent study by Romkens, et al highlighted a concern for reproducibility in histologic scoring but this was most notable between general and expert gastrointestinal pathologists, which was not the case for this study which utilized expert review only.42 However, future studies would benefit from incorporation of more than one expert GI pathologist to arrive at consensus histologic assessments as well as repeat assessments by an individual pathologist to look at intra-rater consistency in scoring. Third, these data showed no association between active inflammatory infiltrate and subsequent risk of relapse which has been shown in previous studies. We hypothesize that this was most likely secondary to the small numbers of those with active inflammatory infiltrate at the time of colonoscopy (less than a third of the patient cohort) limiting statistical power. Fourth, we used clinically meaningful endpoints including relapse, hospitalization, and surgery. However, structured disease activity assessments using validated scores were not available systematically following the index examination. Future studies should be prospective with a systematic algorithm for evaluation and confirmation of symptomatic flares. Overall, the small sample size, small event rate in the Geboes 0 group, and retrospective nature of the design are important limitations of this study. However, this work offers the most comprehensive evaluation of the association between histologic healing and medium-term ulcerative colitis outcomes to date.
In conclusion, histologic normalization, but not resolution of inflammatory infiltrate, was associated with a reduced risk of clinical relapse within two years of endoscopic remission among patients with ulcerative colitis. The value of histologic remission as a therapeutic target in IBD continues to evolve. While mucosal and clinical remission remain as dual endpoints in therapeutic-decision making, the ability to achieve deeper remission with complete histologic healing may not be achievable in all patients.38,43,44 Our findings highlight the importance of consensus definitions across studies regarding the definition of histologic remission and establishment of relevant cut offs not just for therapeutic response but also to modify long-term history of disease to optimize patient outcomes.
Supplementary Material
Acknowledgments
Sources of Funding: Ananthakrishnan is funded by the Crohn’s and Colitis Foundation, National Institutes of Health (R03 DK112909), and the Chleck Family Foundation. This work is supported by the National Institutes of Health (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases.
Footnotes
Statement of Interests:
Kelly Cushing has no conflicts of interests to declare.
William Tan: No conflicts of interest
David Alpers: Consulting for Pfizer, GSK, Otsuka North America, Takeda North America
Vikram Deshpande: Consulting for Agios; Reseach support ACD
Ashwin Ananthakrishnan has served on scientific advisory boards for Abbvie, Gilead, Takeda, and Merck.
REFERENCES
- 1.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(6):857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal M, Colombel JF. Treat-to-Target in Inflammatory Bowel Diseases, What Is the Target and How Do We Treat? Gastrointest Endosc Clin N Am 2019;29(3):421–436. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. The American journal of gastroenterology. 2015;110(9):1324–1338. [DOI] [PubMed] [Google Scholar]
- 5.Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L. A Treat-to-Target Update in Ulcerative Colitis: A Systematic Review. The American journal of gastroenterology. 2019;114(6):874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert review of gastroenterology & hepatology. 2016;10(8):915–927. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–1201. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(6):483–489.e483. [DOI] [PubMed] [Google Scholar]
- 9.Flores BM, O’Connor A, Moss AC. Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2017;86(6):1006–1011.e1008. [DOI] [PubMed] [Google Scholar]
- 10.Meucci G, Fasoli R, Saibeni S, et al. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflammatory bowel diseases. 2012;18(6):1006–1010. [DOI] [PubMed] [Google Scholar]
- 11.Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the Risk of Relapse in Ulcerative Colitis According to the Degree of Mucosal Healing (Mayo 0 vs 1): A Longitudinal Cohort Study. Journal of Crohn’s & colitis. 2016;10(1):13–19. [DOI] [PubMed] [Google Scholar]
- 12.Manginot C, Baumann C, Peyrin-Biroulet L. An endoscopic Mayo score of 0 is associated with a lower risk of colectomy than a score of 1 in ulcerative colitis. Gut 2015;64(7):1181–1182. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda T, Naganuma M, Sugimoto S, et al. Efficacy of Therapeutic Intervention for Patients With an Ulcerative Colitis Mayo Endoscopic Score of 1. Inflammatory bowel diseases. 2019;25(4):782–788. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Abdi T, Gentry M, Laine L. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients With Ulcerative Colitis: Systematic Review and Meta-Analysis. The American journal of gastroenterology. 2016;111(12):1692–1701. [DOI] [PubMed] [Google Scholar]
- 15.Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. The American journal of gastroenterology. 2012;107(11):1684–1692. [DOI] [PubMed] [Google Scholar]
- 16.Calafat M, Lobaton T, Hernandez-Gallego A, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017;49(12):1327–1331. [DOI] [PubMed] [Google Scholar]
- 17.Christensen B, Hanauer SB, Erlich J, et al. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated With Improved Clinical Outcomes. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(10):1557–1564.e1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil DT, Moss AC, Odze RD. Role of Histologic Inflammation in the Natural History of Ulcerative Colitis. Gastrointest Endosc Clin N Am 2016;26(4):629–640. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg L, Nanda KS, Zenlea T, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(8):991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem MS, Melmed GY. The Role of Histology in Determining Disease Activity, Treatment, and Prognosis: Are We There yet? Gastrointest Endosc Clin N Am 2019;29(3):437–446. [DOI] [PubMed] [Google Scholar]
- 21.Zenlea T, Yee EU, Rosenberg L, et al. Histology Grade Is Independently Associated With Relapse Risk in Patients With Ulcerative Colitis in Clinical Remission: A Prospective Study. The American journal of gastroenterology. 2016;111(5):685–690. [DOI] [PubMed] [Google Scholar]
- 22.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32(2):174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki R, Kobayashi T, Okabayashi S, et al. Histological Risk Factors to Predict Clinical Relapse in Ulcerative Colitis With Endoscopically Normal Mucosa. Journal of Crohn’s & colitis. 2018;12(11):1288–1294. [DOI] [PubMed] [Google Scholar]
- 24.Baron JH, Connell AM, Lennard-Jones JE. VARIATION BETWEEN OBSERVERS IN DESCRIBING MUCOSAL APPEARANCES IN PROCTOCOLITIS. British medical journal. 1964;1(5375):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017;66(12):2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jairath V, Peyrin-Biroulet L, Zou G, et al. Responsiveness of histological disease activity indices in ulcerative colitis: a post hoc analysis using data from the TOUCHSTONE randomised controlled trial. Gut 2019;68(7):1162–1168. [DOI] [PubMed] [Google Scholar]
- 29.Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut 2017;66(1):50–58. [DOI] [PubMed] [Google Scholar]
- 30.Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut 2017;66(1):43–49. [DOI] [PubMed] [Google Scholar]
- 31.Pai RK, Khanna R, D’Haens GR, et al. Definitions of response and remission for the Robarts Histopathology Index. Gut 2018. [DOI] [PubMed]
- 32.Magro F, Lopes J, Borralho P, et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut 2019;68(4):594–603. [DOI] [PubMed] [Google Scholar]
- 33.RStudio Team (2016). RStudio: Integrated Development for R. RStudio I, Boston, MA: URL http://www.rstudio.com/. [Google Scholar]
- 34.StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- 35.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 36.Vaughan MKaD. yardstick: Tidy Characterizations of Model Performance. 2019.
- 37.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. The American journal of gastroenterology. 2015;110:1324. [DOI] [PubMed] [Google Scholar]
- 38.Battat R, Duijvestein M, Guizzetti L, et al. Histologic Healing Rates of Medical Therapies for Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. The American journal of gastroenterology. 2019;114(5):733–745. [DOI] [PubMed] [Google Scholar]
- 39.Ma C, Guizzetti L, Panaccione R, et al. Systematic review with meta-analysis: endoscopic and histologic placebo rates in induction and maintenance trials of ulcerative colitis. Alimentary pharmacology & therapeutics. 2018;47(12):1578–1596. [DOI] [PubMed] [Google Scholar]
- 40.Li CQ, Liu J, Ji R, Li Z, Xie XJ, Li YQ. Use of confocal laser endomicroscopy to predict relapse of ulcerative colitis. BMC gastroenterology. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zittan E, Kelly OB, Kirsch R, et al. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn’s Disease. Inflammatory bowel diseases. 2016;22(3):623–630. [DOI] [PubMed] [Google Scholar]
- 42.Romkens TEH, Kranenburg P, Tilburg AV, et al. Assessment of Histological Remission in Ulcerative Colitis: Discrepancies Between Daily Practice and Expert Opinion. Journal of Crohn’s & colitis. 2018;12(4):425–431. [DOI] [PubMed] [Google Scholar]
- 43.Long MD, Rubin DT. Histologic Remission in Ulcerative Colitis: Are We There Yet? The American journal of gastroenterology. 2019;114(5):713–715. [DOI] [PubMed] [Google Scholar]
- 44.Sands BE, Peyrin-Biroulet L, Loftus EV, et al. 416a – Vedolizumab Shows Superior Efficacy Versus Adalimumab: Results of Varsity—The First Head-To-Head Study of Biologic Therapy for Moderate-To-Severe Ulcerative Colitis. Gastroenterology. 2019;156(6):S-81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


