Abstract
The human DNA tumor viruses Epstein–Barr virus (EBV), Kaposi’s sarcoma-associated herpesvirus (KSHV), and human papillomavirus (HPV) share the common property of persisting as multicopy episomes in the nuclei of rapidly dividing host cells. These episomes form the molecular basis for viral latency and are etiologically linked to virus-associated cancers. Episome maintenance requires epigenetic programming to ensure the proper control of viral gene expression, DNA replication, and genome copy number. For these viruses, episome maintenance requires a dedicated virus-encoded episome maintenance protein (EMP), namely LANA (KSHV), EBNA1 (EBV), and E2 (HPV). Here, we review common features of these viral EMPs and discuss recent advances in understanding how they contribute to the epigenetic control of viral episome maintenance during latency.
Keywords: Gammaherpesvirus, KSHV, EBV, HPV, latency, episome, maintenance, epigenetics, chromatin, LANA, EBNA1, E2, oligomerization, 3D organization, segregation
Viral Episome Maintenance Proteins (EMPs): Binding and Tethering Genomes
The human DNA tumor viruses Epstein-Barr virus (EBV), Kaposi’s sarcoma-associated herpesvirus (KSHV), and human papillomavirus (HPV) persist as multicopy episomes (see Glossary) in latently infected cells (reviewed in [1–4]). These viral episomes are covalently closed circular genomes that are assembled into chromatin with histone and DNA modifications similar to host genomes. Epigenetic programming of the viral episome is important for all aspects of the viral life cycle, including the proper regulation of viral transcription during latency, cell-cycle-regulated DNA replication during latency, protection and repair of the viral genome, and the ability to switch to the lytic replication cycle in response to cell stress or differentiation signals. Each of these DNA tumor viruses encodes a dedicated episome maintenance protein (EMP) that performs a central role in viral episome maintenance and epigenetic programming. EBV EBNA1, KSHV LANA, and HPV E2 are structural and functional orthologs that provide essential functions, including initiation of DNA replication, regulation of viral gene expression, modulation of chromatin architecture, and tethering of viral episomes to host chromosomes during mitosis. In this review, we discuss the structural similarities of these EMPs and how this may serve as the molecular basis for epigenetic programming and episome maintenance during latent infection.
EMP DNA-Binding Domains (DBDs)
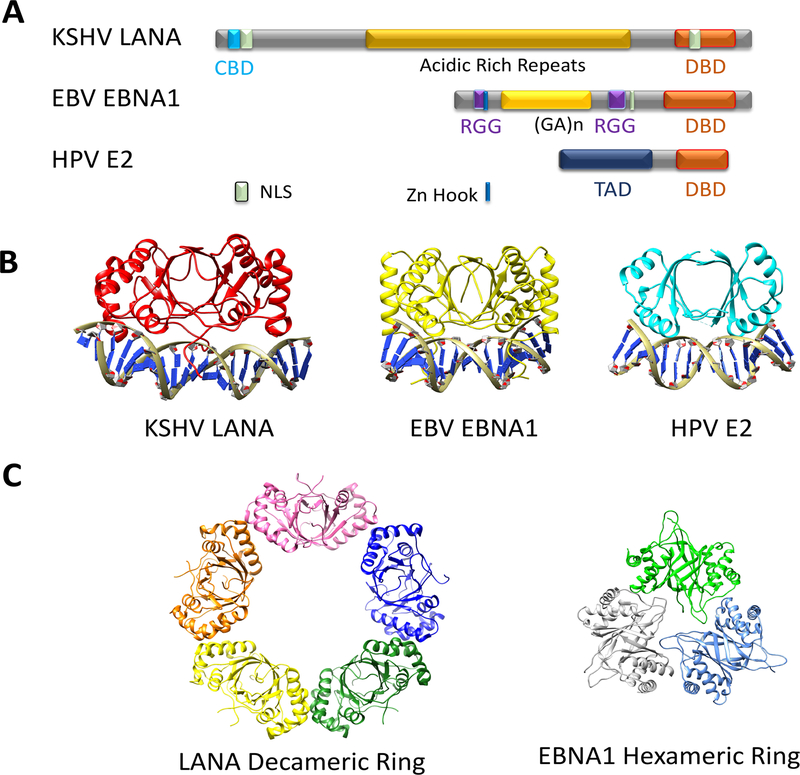
EMPs share several structural similarities, but the defining feature for this group is the structural homology between their DBDs (Figure 1). Each EMP contains a carboxy-terminal DBD and an amino-terminal chromosome-tethering domain (CTD). EBNA1 contains a repetitive glycine-alanine copolymeric stretch separating two CTD elements, while LANA has acidic repeats separating the DBD from the CTD, and E2 has a flexible hinge separating the DBD from its CTD. The DBD of each EMP directs sequence-specific binding to essential genetic elements in their respective genomes. The DBDs share a structural fold unique to these viral proteins, but not to any other known cellular protein (Figure 1A). The DBDs dimerize to form a core eight-stranded β-barrel surrounded by six α-helices with each monomer contributing four β-strands and three α-helices. DNA contacts are made primarily through amino acids in the α-helices. Additional contacts with regions extending beyond the conserved core structures also contribute to sequence-specific DNA binding. There are a number of important structural features and modes of DNA binding that distinguish each family member. One striking difference in the X-ray structures of the DNA-bound forms is that KSHV LANA binds asymmetrically to a GC-rich DNA binding site, while each monomer of the EBNA1 and E2 dimer binds symmetrically to two inverted DNA repeats [5].
Figure 1.
Episome Maintenance Proteins (EMPs). (A) Schematic structure showing C terminal DNA-binding domain (DBD) in red, and the various N terminal domains involved in chromosome tethering, DNA replication, and transcription-activation domains (TADs). (B) Co-crystal structures of EMPs KSHV LANA (red) (PDB 4UZBP), EBV EBNA1 (yellow) (PDB 1B3T), or HPV E2 (cyan) (PDB 1JJ4) bound to cognate DNA elements. (C) Oligomeric structures of EMPs including LANA decameric ring (PDB 4KJ2) and EBNA1 hexameric ring (PDB 5WMF). Abbreviations: KSHV, Kaposi’s sarcoma-associated herpesvirus; EBV, Epstein–Barr virus; HPV, human papillomavirus.
EMP Function at Viral Origins of DNA Replication
A common function of EMPs is their ability to establish an origin of DNA replication when bound to specific sites in their respective viral genomes. EBNA1 binding to the dyad symmetry (DS) element of oriP constitutes a minimal origin of plasmid DNA replication [6]. A dimer–dimer interface, important for DNA replication, is formed only when EBNA1 dimers are bound with 3 bp separating each dimer at the DS [7]. EBNA1 also induces a DNA bend angle at the DS that may contribute to DNA replication activity [8]. For KSHV LANA, three tandem binding sites in the terminal repeat (TR) DNA constitute a minimal origin of DNA replication [9]. One high-affinity site nucleates the cooperative binding of the other two sites, and a positioning of 22 bp from center to center between sites 1 and 2 is necessary for DNA replication [9]. LANA also induces a strong bend in the TR DNA [10], and the bend angle is consistent with binding to the oligomeric forms of LANA that were observed in X-ray crystal studies [11]. HPV E2 also binds to multiple sites at the viral origin of replication and bends DNA [12,13]. However, unlike EBV and KSHV, E2 recruits the virus-encoded E1 helicase to initiate DNA replication during the HPV productive replication. E2 can interact with the host replication machinery, including the origin recognition complex (ORC), but it is not clear if E2 regulates origin function during the HPV latent phase [14]. Interactions with host replication proteins, including ORC, minichromosome maintenance (MCM)s, replication protein A (RPA), and proliferating cell nuclear antigen PCNA have been demonstrated for each EMP. However, the requirement for replication initiation within these viral genetic elements bound by EMPs has been called into question. Genetic studies show that EBV episomes can be established with recombinant virus where the DS element has been deleted [15], and single-molecule analysis of replicating DNA (SMARD) demonstrated that replication can initiate at sites other than the DS in EBV [16] and the TR in KSHV [17]. Earlier genetic studies showed that the DS is required for the establishment of OriP-containing plasmids, but can be deleted after this establishment without affecting replication of the viral plasmid [18]. These studies suggest that the replication initiation function of EMPs may be critical during the early establishment phase, but may not be an essential function of EMPs in the long-term maintenance of viral episomes (Box 1).
Box 1. Episomes in Natural Infection and Cancer.
Episomal forms of KSHV, EBV, and HPV are found in most of the corresponding virus-associated cancers (reviewed for KSHV [118–120], EBV [121], and HPV [122]). In normal carriers, EBV episomes persist in long-lived memory B cells [123,124], and HPV episomes persist in basal epithelial cells [125]. While integrated viral DNA can be found in the host genomes of cell lines and tumors, these are almost always aberrant genomes that fail to produce infectious virus upon reactivation [126,127]. Aberrant integration can lead to deregulated expression of viral and cellular oncogenes, and this is a common oncogenic mechanism for Merkel cell carcinoma and a subset of HPV-associated tumors [128,129]. Long-term genome maintenance may also require sporadic productive infection [130]. In most model systems, the efficiency for the establishment of a stable viral episome is very low and requires positive selection [130–132]. Therefore, these viruses must provide a selective advantage to the cells and tumors that maintain stable episomes. Evidence suggests that epigenetic events in the cell and on the viral genome must occur to establish and maintain a stable episome [133,134].
Epigenetic and transcriptomic analyses of tumor tissue reveal important differences compared with cell culture models. The epigenetic landscape of KSHV in KS tumor tissue was found mostly similar to KSHV in latently infected PEL and SLK cell lines [62]. The overall enrichment of H3K27me3 was similar, but histone acetylation was restricted to the latency locus, including the LANA promoter and the K12 transcripts, with no significant acetylation in the TR or vIRF3 locus in tumor-associated KSHV genome [62]. RNA-seq studies from KS tissue revealed some differences from cell culture models [135,136]. In one study, viral gene expression was found to be a mix of both latent and lytic transcripts, suggesting a heterogeneous population with some small percentage of cells in the lytic cycle [136]. In a second study, with more samples and focusing primarily on viral transcripts, viral transcript patterns in the latency-control region were significantly different than those reported in latently infected PEL and SLK cells, with unusually low levels of transcripts initiating at the LANA promoter and high levels of transcripts initiating from a downstream promoter driving viral miRNAs and Kaposin A [135]. Another recent study of naturally occurring infections revealed that KSHV genomes have different subtypes, and these undergo extensive recombination [137].
Cooperative DNA Binding and Oligomerization of EMPs
Another shared feature between EMPs is their ability to bind cooperatively to multiple DNA sites at their viral origins of replication. The cooperative binding of LANA and EBNA1 can be partly attributed to stabilizing interactions and oligomerization potential of their DNA-binding domains. Cooperative DNA binding of LANA at TRs is critical for its stable binding in vitro and in vivo [5,19]. The LANA DBD oligomerization interface is flexible and allows for several different states, including a pentameric ring of dimers, a noncircular cluster of four dimers, or a continuous helical spiral [5,11,19] (reviewed in [1]). By contrast, the MHV68 LANA DBD forms a rigid, linear conformation, suggesting that various EMP structures can support episome maintenance [19]. The EBNA1 DBD can form a hexameric ring with an oligomeric interface that contributes to episome maintenance, but not to DNA replication function [20]. The EBNA1 hexamer interface was distinct from the dimer–dimer interface observed at the DS, suggesting that EBNA1 can exist in at least two functionally distinct oligomeric states. Cooperative DNA binding of E2 is also critical for HPV replication [21].
For each EMP, the corresponding DBD contains some unique structural features. EBNA1 has extended PG loops and N terminal arms that are critical for DNA binding and replication. The LANA DBD contains a unique patch of basic amino acids on the surface dorsal to the sequence-specific DNA-binding interface. This basic patch lines the interior of the pentameric ring observed in the LANA X-ray structures [5,11,22]. This lysine cluster contributes to nonspecific DNA binding, and mutations in this region reduced several protein–protein interactions and impaired episome maintenance [22].
Chromosome Tethering of EMPs
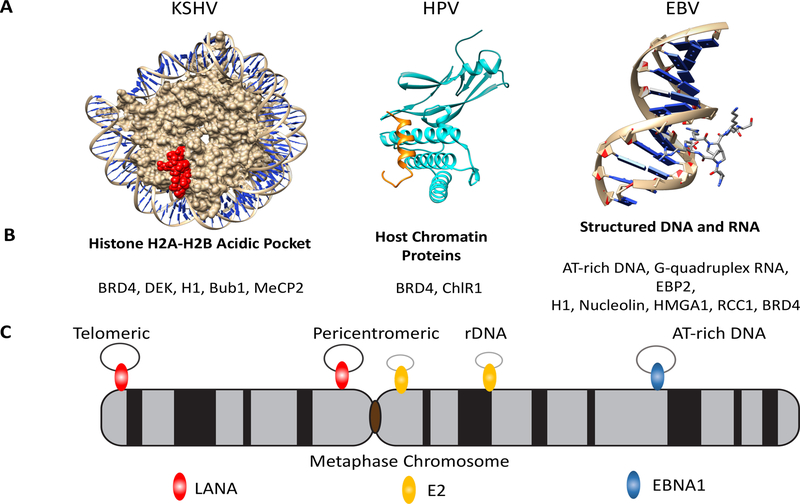
EMP attachment (or tethering) to metaphase chromosomes is necessary for episome maintenance and for DNA replication ([23,24] and reviewed in [1]), suggesting that these functions are mechanistically linked. Each EMP contains multiple domains with metaphase chromosome binding activity (Figure 1A). While the unifying function of this attachment is to tether the viral genomes to cellular metaphase chromosomes, the primary interaction targets for each EMP and each tethering domain appear to be different (Figure 2).
Figure 2.
Metaphase Chromosome Attachment Mechanism. (A) Crystal structures of episome maintenance protein (EMP) interactions with targets in metaphase chromosome attachments. LANA (red) N terminal peptide bound to histone H2A-H2B pocket of the nucleosome (PDB 1ZLA), HPV E2 peptide bound to BRD4 (cyan) (PDB 2NNU), and an AT-rich minor-groove binding molecule netropsin with DNA representative of the EBNA1-AT hook interactions with metaphase chromosomes (PDB 1Z8V). (B) Partial list of metaphase tethering targets for EMPs. LANA interacts with BRD4, DEK, H1, Bub1, MeCP2; E2 interacts with BRD4 and ChIR1; and EBNA1 interacts with AT-rich DNA, RNA-G quadruplex, and protein-target (EBP2, nucleolin, HMGA1, H1). (C) Depiction of the viral episome (small black rings) bound to metaphase chromosome positions by LANA (red) at peritelomeric and pericentromeric regions, E2 (yellow) bound to pericentromeric and rDNA regions, and EBNA1 (blue) bound to AT-rich DNA elements. Abbreviations: KSHV, Kaposi’s sarcoma-associated herpesvirus; EBV, Epstein–Barr virus; HPV, human papillomavirus.
N Terminal CTD
The predominant CTD of KSHV LANA is found in the N terminus and forms an arginine-rich hairpin structure. The LANA N terminal hairpin binds to nucleosomes through an acidic pocket formed by histones H2A/H2B [25] (Figure 2). This acidic pocket is a site frequently targeted by other cellular and viral proteins, such as cellular interleukin (IL)-33 [26], human cytomegalovirus (CMV) IE1 [27], and some retroviral GAG proteins [28–30], suggesting that the H2A/H2B pocket is an attractive and frequent target for virus association with host chromosomes [31]. Cell-cycle phosphorylation of the LANA histone-binding domain alters its affinity for the H2A/H2B pocket [31], suggesting that this interaction can be subject to regulation. Surprisingly, neither EBNA1 nor E2 appear to interact with histones through this mechanism.
EBNA1 metaphase attachment occurs through two related arginine-glycine (RG) domains, each of which can bind to AT-rich DNA through direct interactions with the minor groove, similar to AT-hook proteins [32], or with G-quadruplex RNA [33] (reviewed in [3,34]). Furthermore, each RG domain can bind to several cellular proteins that can serve as anchors to metaphase chromosomes, including EBP2 [35], nucleolin [36], histone H1 [37], HMGA1 [38], and RCC1 [39]. Substitution of the EBNA1 N terminus with histone H1 or HMG1A was sufficient to reconstitute episome maintenance in a short-term plasmid-based assay [37], but substitution of HMG1, which lacks an AT-hook domain and does not bind to metaphase chromosomes, was not able to maintain viral plasmids [40], highlighting the importance of AT-hook domains and metaphase chromosome tethering for viral episome maintenance.
E2 can also associate with metaphase chromosomes by various mechanisms [41]. BRD4, a member of the BET family of proteins, can mediate interactions with metaphase chromosomes [42]. Other protein-binding partners have also been implicated in metaphase chromosome attachment, such as the helicase ChlR1 [43] and the cohesin-like proteins SMC5/6 [44].
Other Chromosome-Binding Domains
In addition to the N terminal histone H2A/H2B interaction domain, other regions of LANA contribute to metaphase chromosome binding and episome maintenance, including the central repeats and DBD [45] (reviewed in [1]). Deletion of the central repeats reduces episome maintenance, but not viral DNA replication [45,46]. Interactions with nonhistone proteins, such as centromeric factors CENPF and Bub1, also contribute to episome maintenance [47,48]. Interestingly, the KSHV LANA N terminal histone-binding domain is not conserved in MHV68 LANA, suggesting that MHV68 LANA uses an alternative mechanism to achieve metaphase chromosome attachment and episome maintenance during latency [19].
In addition to tethering to metaphase chromosomes, EMPs can also bind to host chromosomes through sequence-specific interactions mediated by the DBDs. EBNA1 was found to interact with ~1000 specific sites on the host chromosome [49,50]. Only a few of these could be shown to have functional impact on cellular gene expression, suggesting that the majority of binding sites have unknown function. Similar observations were made for KSHV LANA, which binds to GC-rich sequences similar to that found in KSHV TR [51–53]. LANA was found to bind and upregulate some cellular genes, including SENP6, which encodes a SUMO protease that binds and modulates LANA function during the establishment of latency [54]. While the regulation of a small cohort of cellular target genes bound by EMPs may contribute to viral latency and oncogenic transformation, it is also possible that the many cellular binding sites have other functions, such as facilitating viral genome attachment to host chromosomes. (Box 2).
Box 2. Dynamic Changes in EMPs.
Variant forms of EMPs have also been identified. A splice variant containing the E2 DBD, termed E8^E2, can function as a negative regulator of HPV transcription and replication [138]. Smaller forms of LANA have been shown to function in the cytoplasm [139,140]. Smaller isoforms of LANA have been identified in the cytoplasm, and interact with and downregulate the stimulator of interferon genes (STING)-cyclic GMP-AMP synthase (cGAS) pathway of innate immune signaling [2,140]. Another potential function of the cytoplasmic forms of LANA could be to protect the viral genomic material during nuclear membrane breakdown in mitosis.
Epigenetic Programming of Viral Episomes
Viral episomes maintained by EMPs assemble into chromatin that is epigenetically modified and structurally organized similar to the cellular chromosome. The chromatin structure and epigenetic programming are necessary for the proper control of viral gene expression and stable maintenance of viral DNA. LANA, EBNA1, and E2 have all been implicated in epigenetic programming, including regulation of replication origin function, transcriptional repression of viral lytic cycle genes, and recruitment of epigenetic modifiers [55–58].
Histone Modifications and Epigenetic Patterning
The epigenetic landscape of the EBV and KSHV genomes has been examined in significant detail [59–61]. Histone tail modifications associated with transcriptional activation (acetylated H3K9/K14 and H3K4me3) and repression (H3K9me3 and H3K27me3) can be found at various positions along the EBV and KSHV genomes during latency. Latent KSHV genomes are broadly associated with repressive H3K27me3, with the exception of the regions encompassing the major latency transcripts and the TR. A bivalent histone modification pattern with both H3K27me3 and H3K4me3 was identified at the transcriptional regulatory regions for the ORF50/Rta immediate early gene, indicating that they are poised for rapid response during viral reactivation. Similar epigenetic patterning has been detected in KS tumor biopsies [62]. In models of EBV latency, transcriptionally active promoters were enriched with H3K4me3 and H3K9Ac, but the patterns of repressive histone marks were not as broadly distributed as that found in KSHV [59]. EBV can also adopt different latency types with different extents of transcriptional repression that may be preferentially regulated by DNA methylation rather than histone modification (reviewed in [63]).
The mechanisms that determine histone modification patterns and gene promoter selection on a newly established episome are not completely understood. EMPs contribute to this programming, but many other factors, including prepackaged viral tegument proteins and cellular factors, regulate the earliest events leading to the establishment of the viral epigenome [64]. EMPs can recruit histone- and DNA-modifying enzymes that may contribute to epigenetic patterning (reviewed in [65]). LANA interacts with BET proteins BRD2 and BRD4 [22,66] that can interact with acetylated lysines, histone H3.3 chaperone DAXX [67,68], DNA methyltransferase DNMT3a [69,70], and recruits histone acetyltransferases CBP and HBO1 to KSHV TR [71]. EBNA1 can also interact with BRD4 [72], and recruit histone H3K4 methyltransferase MLL through HCF1 to OriP to facilitate replication and episome maintenance [49]. E2 interacts with BET protein BRD4 [73], histone H3K4 demethylase SMCX, and histone acetyltransferase TIP60 [74] to regulate viral transcription. These many interactions of EMPs underscore their complex role in orchestrating various stages of epigenetic programming with episome maintenance.
EMP Autoregulation
Autoregulatory mechanisms for EMP gene expression and copy number control have been identified for each of these viruses. LANA interacts with the components of the Polycomb repressor complex (PRC2) required for generating the repressive H3K27me3 modification across the majority of the latent episome [75]. However, the LANA promoter and latency locus is spared, consistent with its continuous transcription during latency. The LANA promoter (LANAp) has remarkably simple features of a constitutively active RNA polymerase II core initiator (INR) element with no obvious essential transcription factors or enhancer elements[76]. ChIP-Seq data indicated that LANA can interact with its own promoter region [51–53,62]. Chromosome conformation capture (3C) analysis has identified a DNA interaction loop between the TR and the region upstream of LANAp [77]. This interaction was dependent on LANA oligomerization and was required for autoregulation of the LANA transcript and protein [77]. LANA can also interact with histone H3K9 methyltransferase SUV39H and recruit heterochromatin protein 1 (HP1) to the TR, resulting in transcriptional repression of neighboring genes [78]. Since LANA binding sites are found at TR and upstream of LANAp, it is presumed that LANA mediates these DNA loop interactions to autoregulate its own expression (Figure 3).
Figure 3.
Architectures of Viral Epigenomes. Episome maintenance proteins (EMPs) mediating autoregulatory interactions to control epigenome architecture and regulation of gene expression during latency. Kaposi’s sarcoma-associated herpesvirus (KSHV) (top left), Epstein– Barr virus (EBV) (top right), human papillomavirus (HPV) (lower left), and protein key (lower right). Abbreviations: DS, dyad symmetry; ORC, origin recognition complex; PRC, Polycomb repressor complex; Qp, promoter.
Related transcriptional control is observed for the EBV EBNA1 gene that can be constitutively expressed from a dedicated promoter, termed Qp. Qp utilizes a simple initiator (INR) element, and is subject to negative autoregulation by EBNA1 that binds sequence-specifically to a pair of sites overlapping this INR [79,80]. EBNA1 forms a DNA loop between DS and FR at oriP [81] and can also mediate interactions between oriP and Qp [82], suggesting a direct communication between these regulatory elements. Both LANAp and Qp are subject to autorepression [76,79], and are bound by the cellular chromatin boundary factor CTCF, which plays an important role in preventing epigenetic silencing of these critical promoters. For HPV, E2 is known to autoregulate expression of early viral transcripts for E6 and E7, as well as E2 and E1 [74]. Thus, EMPs function in autoregulation through DNA binding and DNA-looping mechanisms (Figure 3). (Box 3).
Box 3. Potential Interactions between EMPs.
Coinfections of DNA tumor viruses can occur in some tumor types, and the potential interactions between viral proteins is important to consider. EBV and KSHV are frequently found as coinfections in pleural effusion lymphoma (PEL). In dually infected PEL cells, KSHV episome maintenance was found to be codependent on EBNA1 and EBV episome maintenance [141]. Although each viral episome was found as distinct and separate foci by IF, a CRISPR deletion of EBNA1 led to a corresponding loss of both EBV and KSHV episomes. EBNA1 was sufficient to enhance KSHV episome maintenance, potentially through an indirect effect on the host cell environment. Thus, EMPs may influence the functionality and cell permissivity for other EMP family members.
Genomic Architecture
Cellular chromatin organizing factors, such as CTCF, YY1, and cohesins, are found enriched at specific sites in these viral genomes and contribute to various aspects of viral gene regulation, including EMP autoregulation and episome maintenance. CTCF and cohesins are involved in numerous cellular chromatin functions, including boundary element, insulator, transcriptional repressor, regulator of RNA processing, and DNA loop interactions [83]. Their role in viral episome maintenance is multifunctional and complex, as it is for the cellular epigenome. For HPV, CTCF and YY1 regulate viral gene expression through a DNA loop [84], and cohesin subunit SMC1 has been implicated in chromosome tethering and DNA damage repair during replication [85]. For EBV and KSHV, CTCF and cohesins have been implicated in transcription control, suppression of epigenetic drift and 3D architecture, including loop formation between DNA regulatory elements (reviewed in [63]). Disruption of loops mediated by CTCF and poly (ADP-ribose) polymerase 1 (PARP1) in EBV leads to a switch between latency type I and III [86], while in KSHV depletion of cohesin subunits RAD21, SMC1, or SMC3 activates lytic cycle gene expression in latently infected plural effusion lymphoma (PEL) cells [87,88]. Thus, viral DNA loops and chromosome architecture are important for maintaining stable gene expression programs in latency.
Genome-wide DNA interaction assays, such as HiC, revealed an extensive web of interactions for KSHV, with strong clustering of lytic cycle gene promoters with the PAN gene locus during lytic cycle reactivation [89]. The findings suggest that RNA polymerase remains poised at the PAN promoter during latency and is involved in extensive remodeling of the KSHV genome during reactivation. KSHV genomes colocalize with RNA polymerase-associated factories during lytic cycle reactivation [90]. HiC analysis of the EBV genome revealed an association of the viral genome with heterochromatic domains during latency, and a shift towards euchromatic regions during reactivation [91].
Higher-Ordered Episomal Structures in Nuclear Bodies
EMPs and viral episomes can form various structures in the host cell nucleus as visualized by fluorescent-light microscopy. For each virus, the formation, localization, and mobilization of these structures appear different. These structures are almost always associated with metaphase chromosomes but may have additional colocalizations during interphase. Here, we consider aspects of EMP function in forming higher-ordered structures and how this contributes to episome maintenance and protection.
Episome Nuclear Bodies Resist Promyelocytic Leukemia (PML) Nuclear Bodies
There is complex interplay between nuclear viral genomes and the host nuclear intrinsic antiviral resistance structures formed by PML-nuclear bodies (PML-NBs). Latent episomes of EBV are associated with metaphase and interphase chromatin, and do not colocalize with PML-NBs [92]. However, EBNA1 can interact with PML and disrupt its function during the lytic phase [93]. LANA, in association with viral episomes, forms large discrete structures, termed LANA nuclear bodies, that colocalize with metaphase and interphase chromosomes [94]. DAXX, a typical component of PML-NBs, interacts with LANA and colocalizes with LANA bodies by IF imaging [67,68]. Interestingly, LANA bodies do not colocalize with PML-NBs, indicating that they are distinct structures from PML-NBs [77,95]. LANA has been shown to redistribute SP100, another PML-NB component, into different subcellular compartments [67]. LANA bodies partly colocalize with ORC and are enriched for H3K27me3 and PRC components [75,77]. HPV E2 was also shown to colocalize with PML-NBs [96]. Subsequent studies have implicated PML in HPV transcription activation [97], and SP100 in restricting viral transcription and replication [98].
Episome Bodies during Mitosis
Several studies have addressed the localization of EMPs and viral episomes to specific regions of the metaphase chromosome. For HPV, most E2 proteins, with the exception of the alpha family members, remain associated with ribosomal DNA and pericentromeric regions of metaphase chromosomes throughout mitosis [41,99]. For KSHV, LANA foci on metaphase chromosomes were found to be mostly random, with some enrichments at pericentromeres, peritelomeres, and furrows between cohering sister chromatids [100]. LANA interacts with centromere regulatory protein Bub1 to block phosphorylation of H2A [47], and can regulate centromere protein biology, suggesting that tethering at these sites may involve pirating centromeric functions. For EBV, EBNA1 foci on metaphase chromosomes appear to be randomly distributed but symmetrically dividing during chromatid separation [101]. At least one study found an interaction between EBNA1 and the kinetochore component Survivin, suggesting that a coordination with centromere regulatory factors may also occur with EBV EBNA1 [102].
Live-cell imaging studies of KSHV LANA have been controversial. In one study, LANA bodies formed stable structures throughout mitosis. They underwent considerable movement and reorientation during chromosome congregation, and segregated with near equal distribution to each daughter cell to form a pattern nearly identical to that of the parent cells [77]. A different imaging system utilizing the LACi repressor system found that KSHV genomes did not segregate faithfully, but rather formed large clusters that segregated asymmetrically with large copy number increases in one of the two daughter cells [103]. These discrepancies may be due to different experimental models, but may also reflect multiple mechanisms of episome segregation for the same virus and EMP.
Super-resolution microscopy of LANA bound to 2xTR plasmids revealed important structural information on how LANA interacts with the TR [104]. This study revealed that the TR chromatin conforms to a euchromatic active state with the ordered tethering domain and the internal repeats forming a coiled-coil. The LANA N termini appear to be oriented in a way that facilitates exploration of the surrounding environment to promote further tethering.
Episome Maintenance and Phase Separation
There has been recent interest in self-organizing structures, such as viral replication compartments, and phase-separation biochemistry [105]. Whether stable episomes, such as those associated with LANA-NBs, result from phase separation is not yet known. RNA polymerase transcription factories and super-enhancers have been associated with phase transitions [106,107]. The formation of RNA pol II factories during KSHV reactivation [90], EBNA2-induced super-enhancers [108], and LANA-NBs may also be different forms of phase transition regulating structure and function of viral episomes [77].
Concluding Remarks
Episome maintenance is an essential part of the life cycle of the persistent DNA tumor viruses KSHV, EBV, and HPV. Disruption of episome maintenance can deregulate many aspects of the viral life cycle, including gene expression, DNA replication, and genome segregation. Several efforts to inhibit EMPs have been explored, including inhibitor assays of the LANA histone interaction domain [109], small-molecule inhibitors of the EBNA1 DNA-binding domain [110–112], and peptide inhibitors of E2 functions [113,114]. CRISPR/CAS9 genome editing of KSHV LANA [115], or EBV OriP [116,117] can reduce or eliminate viral episomes in cell culture models. Further understanding of the structure, function, and biology of these EMPs will provide important new insights for improving these potential therapeutic strategies (see Outstanding Questions).
Outstanding Questions.
How do oligomeric forms of EMPs relate to higher-ordered viral chromosome structure and functional organization? Are the different geometric forms, such as hexamers and pentamers, related to different functions in the viral life cycle?
How do episome maintenance proteins function to establish origins of DNA replication and centromeric segregation functions?
Do EMPs form a physical shell that protects the viral genomes and facilitates their mobilization through nuclear compartments and during chromosome segregation?
Are viral episome bodies formed through biophysical forces of liquid-phase change or some other forces of biomolecular self-assembly?
Will viral EMPs serve as good targets for antiviral therapies?
Highlights.
Viruses have evolved elaborate mechanisms to maintain their genomes as passengers in host cells.
The oncogenic human herpesviruses, EBV and KSHV, and the small DNA tumor virus, HPV, persist as multicopy episomes.
EBV, KSHV, and HPV have dedicated EMPs that bind their viral genomes and tether to cellular metaphase chromosomes.
EMPs contribute to the epigenetic programming of latent viral genomes.
Acknowledgments
This work was funded in part from National Institutes of Health (NIH) National Cancer Institute (NCI) grants PO1 CA174439, RO1 CA186775, and RO1 CA117830 to P.M.L., NCI T32 Training Grant CA009171 to A.C., and the Wistar Institute NCI Cancer Center grant P30 CA0101815-48.
Glossary
- AT-hook
a protein domain, such as that found in HMGA1 and EBNA1 tethering domains, that bind to the minor groove of AT-rich DNA.
- Epigenetic programming
epigenetics describes the information that can be transmitted between generations above the primary DNA sequence. Latent episomal viruses contain epigenetic information for regulating transcription, DNA replication, segregation, and genome integrity. EMPs are thought to help establish epigenetic programming during viral latency.
- Episome
although originally named for mobile DNA elements that can both integrate and form circular DNA molecules, the term is commonly used in the Gammaherpesvirus field to describe the covalently closed circular form of EBV and KSHV genomes that remains unintegrated and competent for viral transcription and replication during latency.
- INR
initiator element involved in directing RNA polymerase to start transcription. The INR is one of several genetic elements that determines transcriptional start sites. They are found at the start sites of the KSHV LANA and EBV EBNA1 genes.
- Metaphase chromosome tethering
episomal viral genomes attach to metaphase chromosomes to persist in dividing cells. Failure to attach results in loss of episomes. The EMPs mediate interactions between the viral genome and host metaphase chromosome. This tethering mechanism appears to be varied and different for each virus.
- Origin of DNA replication
herpesviruses, such as EBV and KSHV, have genetic elements that confer the capacity to initiate DNA replication during latency. The oriP for EBV and the terminal repeats (TRs) of KSHV can function as origins of DNA replication during latency. In addition to these latency-associated origins, the viruses have a separate and dedicated origin of lytic replication (oriLyt) that requires virus-encoded DNA replication enzymes and produces many viral genomes through a rolling-circle mechanism. Papillomaviruses also have latent and lytic replication cycles but utilize the genetic element for these different replication mechanisms, both mediated by E2.
- PML-NB
promyelocytic leukemia (PML)-nuclear bodies (NBs) are punctate nuclear structures that form in response to many viral infections. They restrict viral transcription and DNA replication through chromatin-based mechanisms. Many viruses encode factors that destroy PML-NBs or hijack their functions for viral purposes, such as establishment of latency.
- Viral latency
the state of infection where no infectious virus is produced. For the DNA viruses discussed here, limited viral transcription occurs during latency, and can be required for maintenance of the latent state in proliferating cells. Viral DNA can replicate using host replication machinery, but is not packaged into infectious virions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juillard F et al. (2016) Kaposi’s sarcoma Herpesvirus genome persistence. Front. Microbiol 7, 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidner-Glunde M et al. (2017) Kaposi’s sarcoma-associated Herpesvirus latency-associated nuclear antigen: replicating and shielding viral DNA during viral persistence. J. Virol 91, e01083–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu YF and Sugden B (2018) Plasmid partitioning by human tumor viruses. J. Virol 92, e02170–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda K (2018) KSHV genome replication and maintenance in latency. Adv. Exp. Med. Biol 1045, 299–320 [DOI] [PubMed] [Google Scholar]
- 5.Hellert J et al. (2015) The 3D structure of Kaposi sarcoma herpesvirus LANA C-terminal domain bound to DNA. Proc. Natl Acad. Sci. U. S. A 112, 6694–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates JL et al. (2000) The minimal replicator of Epstein–Barr virus oriP. J. Virol 74, 4512–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malecka KA et al. (2019) Structural basis for cooperative binding of EBNA1 to the Epstein–Barr virus dyad symmetry minimal origin of replication. J. Virol JVI.00487-19 [DOI] [PMC free article] [PubMed]
- 8.Bashaw JM and Yates JL (2001) Replication from oriP of Epstein–Barr virus requires exact spacing of two bound dimers of EBNA1 which bend DNA. J. Virol 75, 10603–10611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J and Renne R (2005) Characterization of the minimal replicator of Kaposi’s sarcoma-associated herpesvirus latent origin. J. Virol 79, 2637–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong LY and Wilson AC (2005) Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces a strong bend on binding to terminal repeat DNA. J. Virol 79, 13829–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domsic JF et al. (2013) Molecular basis for oligomeric-DNA binding and episome maintenance by KSHV LANA. PLoS Pathog 9, e1003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham SV (2016) Human Papillomavirus E2 protein: linking replication, transcription, and RNA processing. J. Virol 90, 8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBride AA (2013) The Papillomavirus E2 proteins. Virology 445, 57–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSmet M et al. (2016) The replicative consequences of Papillomavirus E2 protein binding to the origin replication factor ORC2. PLoS Pathog 12, e1005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ott E et al. (2011) The dyad symmetry element of Epstein–Barr virus is a dominant but dispensable replication origin. PLoS One 6, e18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norio P et al. (2000) Initiation of DNA replication within oriP is dispensable for stable replication of the latent Epstein–Barr virus chromosome after infection of established cell lines. J. Virol 74, 8563–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma SC et al. (2011) Single molecule analysis of replicated DNA reveals the usage of multiple KSHV genome regions for latent replication. PLoS Pathog 7, e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CY and Sugden B (2008) Identifying a property of origins of DNA synthesis required to support plasmids stably in human cells. Proc. Natl Acad. Sci. U. S. A 105, 9639–9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponnusamy R et al. (2015) KSHV but not MHV-68 LANA induces a strong bend upon binding to terminal repeat viral DNA. Nucleic Acids Res 43, 10039–10054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deakyne JS et al. (2017) Structural and functional basis for an EBNA1 hexameric ring in Epstein–Barr virus episome maintenance. J. Virol 91, e01046–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ustav M Jr. et al. (2015) Human Papillomavirus type 18 cis-elements crucial for segregation and latency. PLoS One 10, e0135770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellert J et al. (2013) A structural basis for BRD2/4-mediated host chromatin interaction and oligomer assembly of Kaposi sarcoma-associated herpesvirus and murine gammaherpesvirus LANA proteins. PLoS Pathog 9, e1003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbera AJ et al. (2004) The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol 78, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim C et al. (2004) Mitotic chromosome-binding activity of latency-associated nuclear antigen 1 is required for DNA replication from terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus. J. Virol 78, 7248–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbera AJ et al. (2006) The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science 311, 856–861 [DOI] [PubMed] [Google Scholar]
- 26.Roussel L et al. (2008) Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep 9, 1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucke K et al. (2014) Human cytomegalovirus major immediate early 1 protein targets host chromosomes by docking to the acidic pocket on the nucleosome surface. J. Virol 88, 1228–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesbats P et al. (2017) Structural basis for spumavirus GAG tethering to chromatin. Proc. Natl Acad. Sci. U. S. A 114, 5509–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesbats P et al. (2016) Retroviral DNA integration. Chem. Rev 116, 12730–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskell DP et al. (2015) Structural basis for retroviral integration into nucleosomes. Nature 523, 366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodard C et al. (2012) Phosphorylation of the chromatin binding domain of KSHV LANA. PLoS Pathog 8, e1002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravorty A and Sugden B (2015) The AT-hook DNA binding ability of the Epstein Barr virus EBNA1 protein is necessary for the maintenance of viral genomes in latently infected cells. Virology 484, 251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norseen J et al. (2009) Role for G-quadruplex RNA binding by Epstein–Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. J. Virol 83, 10336–10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frappier L (2015) Ebna1. Curr. Top. Microbiol. Immunol 391, 3–34 [DOI] [PubMed] [Google Scholar]
- 35.Nayyar VK et al. (2009) Mitotic chromosome interactions of Epstein–Barr nuclear antigen1 (EBNA1) and human EBNA1-binding protein 2 (EBP2). J. Cell Sci 122, 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YL et al. (2014) Nucleolin is important for Epstein–Barr virus nuclear antigen 1-mediated episome binding, maintenance, and transcription. Proc. Natl Acad. Sci. U. S. A 111, 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung SC et al. (2001) Maintenance of Epstein–Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proc. Natl Acad. Sci. U. S. A 98, 1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh G et al. (2009) Optimal transactivation by Epstein–Barr nuclear antigen 1 requires the UR1 and ATH1 domains. J. Virol 83, 4227–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deschamps T et al. (2017) Epstein–Barr virus nuclear antigen 1 interacts with regulator of chromosome condensation 1 dynamically throughout the cell cycle. J. Gen. Virol 98, 251–265 [DOI] [PubMed] [Google Scholar]
- 40.Sears J et al. (2003) Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein–Barr nuclear antigen 1. J. Virol 77, 11767–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira JG et al. (2006) Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc. Natl Acad. Sci. U. S. A 103, 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You J et al. (2004) Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117, 349–360 [DOI] [PubMed] [Google Scholar]
- 43.Parish JL et al. (2006) ChlR1 is required for loading papillomavirus E2 onto mitotic chromosomes and viral genome maintenance. Mol. Cell 24, 867–876 [DOI] [PubMed] [Google Scholar]
- 44.Bentley P et al. (2018) The SMC5/6 complex interacts with the Papillomavirus E2 protein and influences maintenance of viral episomal DNA. J. Virol 92, pii: e00356-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez Ede L et al. (2013) Identification of Kaposi’s sarcoma-associated herpesvirus LANA regions important for episome segregation, replication, and persistence. J. Virol 87, 12270–12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkharsah KR and Schulz TF (2012) A role for the internal repeat of the Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen in the persistence of an episomal viral genome. J. Virol 86, 1883–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang F et al. (2018) Shugoshin 1 is dislocated by KSHV-encoded LANA inducing aneuploidy. PLoS Pathog 14, e1007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Z et al. (2015) Bub1 in complex with LANA recruits PCNA to regulate Kaposi’s sarcoma-associated herpesvirus latent replication and DNA translesion synthesis. J. Virol 89, 10206–10218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dheekollu J et al. (2016) HCF1 and OCT2 cooperate with EBNA1 to enhance OriP-dependent transcription and episome maintenance of latent Epstein–Barr virus. J. Virol 90, 5353–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu F et al. (2010) Genome-wide analysis of host-chromosome binding sites for Epstein–Barr virus nuclear antigen 1 (EBNA1). Virol. J 7, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercier A et al. (2014) Site-specific association with host and viral chromatin by Kaposi’s sarcoma-associated herpesvirus LANA and its reversal during lytic reactivation. J. Virol 88, 6762–6777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu F et al. (2012) Identification of host-chromosome binding sites and candidate gene targets for Kaposi’s sarcoma-associated herpesvirus LANA. J. Virol 86, 5752–5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J et al. (2014) LANA binds to multiple active viral and cellular promoters and associates with the H3K4methyltransferase hSET1 complex. PLoS Pathog 10, e1004240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin X et al. (2017) The Latency-associated nuclear antigen of Kaposi’s sarcoma-associated Herpesvirus inhibits expression of SUMO/Sentrin-specific peptidase 6 to facilitate establishment of latency. J. Virol 91, e00806–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tempera I et al. (2016) Identification of MEF2B, EBF1, and IL6R as direct gene targets of Epstein–Barr virus (EBV) nuclear antigen 1 critical for EBV-infected B-lymphocyte survival. J. Virol 90, 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu F et al. (2006) Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi’s sarcoma-associated herpesvirus lytic transcription. J. Virol 80, 5273–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouvard V et al. (1994) Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J 13, 5451–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen KW et al. (2009) Disruption of LANA in rhesus rhadinovirus generates a highly lytic recombinant virus. J. Virol 83, 9786–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arvey A et al. (2012) An atlas of the Epstein–Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 12, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toth Z et al. (2013) The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses 5, 1346–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunther T and Grundhoff A (2010) The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 6, e1000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun R et al. (2017) Epigenetic landscape of Kaposi’s sarcoma-associated Herpesvirus genome in classic Kaposi’s sarcoma tissues. PLoS Pathog 13, e1006167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tempera I and Lieberman PM (2014) Epigenetic regulation of EBV persistence and oncogenesis. Semin. Cancer Biol 26, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang H et al. (2016) Structural basis underlying viral hijacking of a histone chaperone complex. Nat. Commun 7, 12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunther T and Grundhoff A (2017) Epigenetic manipulation of host chromatin by Kaposi sarcoma-associated herpesvirus: a tumor-promoting factor? Curr. Opin. Virol 26, 104–111 [DOI] [PubMed] [Google Scholar]
- 66.Viejo-Borbolla A et al. (2005) Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol 79, 13618–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunther T et al. (2014) Influence of ND10 components on epigenetic determinants of early KSHV latency establishment. PLoS Pathog 10, e1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murakami Y et al. (2006) Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem 281, 28113–28121 [DOI] [PubMed] [Google Scholar]
- 69.Shamay M et al. (2006) Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi’s sarcoma-associated herpesvirus LANA. Proc. Natl Acad. Sci. U. S. A 103, 14554–14559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Journo G et al. (2018) Modulation of cellular CpG DNA methylation by Kaposi’s sarcoma-associated Herpesvirus. J. Virol 92, e00008–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stedman W et al. (2004) ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J. Virol 78, 12566–12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin A et al. (2008) The EBNA1 protein of Epstein–Barr virus functionally interacts with Brd4. J. Virol 82, 12009–12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iftner T et al. (2017) Involvement of Brd4 in different steps of the papillomavirus life cycle. Virus Res 231, 76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith JA et al. (2014) SMCX and components of the TIP60 complex contribute to E2 regulation of the HPV E6/E7 promoter. Virology 468–470, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toth Z et al. (2016) LANA-mediated recruitment of host polycomb repressive complexes onto the KSHV genome during de novo infection. PLoS Pathog 12, e1005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeong JH et al. (2004) Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Biol. Chem 279, 16822–16831 [DOI] [PubMed] [Google Scholar]
- 77.De Leo A et al. (2019) LANA oligomeric architecture is essential for KSHV nuclear body formation and viral genome maintenance during latency. PLoS Pathog 15, e1007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakakibara S et al. (2004) Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol 78, 7299–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshioka M et al. (2008) Autorepression of Epstein–Barr virus nuclear antigen 1 expression by inhibition of pre-mRNA processing. J. Virol 82, 1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nonkwelo C et al. (1997) The Epstein–Barr virus EBNA-1 promoter Qp requires an initiator-like element. J. Virol 71, 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldsmith K et al. (1993) Identification of EBNA1 amino acid sequences required for the interaction of the functional elements of the Epstein–Barr virus latent origin of DNA replication. J. Virol 67, 3418–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tempera I et al. (2011) EBV latency types adopt alternative chromatin conformations. PLoS Pathog 7, e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ong CT and Corces VG (2014) CTCF: an architectural protein bridging genome topology and function. Nat. Rev. Genet 15, 234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pentland I et al. (2018) Disruption of CTCF-YY1-dependent looping of the human papillomavirus genome activates differentiation-induced viral oncogene transcription. PLoS Biol 16, e2005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta K et al. (2015) Human papillomaviruses activate and recruit SMC1 cohesin proteins for the differentiation-dependent life cycle through association with CTCF insulators. PLoS Pathog 11, e1004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lupey-Green LN et al. (2018) PARP1 stabilizes CTCF binding and chromatin structure to maintain Epstein–Barr virus latency type. J. Virol 92, e00755–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Leo A et al. (2017) Deregulation of KSHV latency conformation by ER-stress and caspase-dependent RAD21-cleavage. PLoS Pathog 13, e1006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen HS et al. (2012) Cohesins repress Kaposi’s sarcoma-associated herpesvirus immediate early gene transcription during latency. J. Virol 86, 9454–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell M et al. (2018) KSHV episomes reveal dynamic chromatin loop formation with domain-specific gene regulation. Nat. Commun 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen CP et al. (2018) Functional imaging of viral transcription factories using 3D fluorescence microscopy. J. Vis. Exp 2018, e56832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moquin SA et al. (2018) The Epstein–Barr virus episome maneuvers between nuclear chromatin compartments during reactivation. J. Virol 92, e01413–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bell P et al. (2000) Lytic but not latent replication of Epstein–Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J. Virol 74, 11800–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frappier L (2011) Viral disruption of promyelocytic leukemia (PML) nuclear bodies by hijacking host PML regulators. Virulence 2, 58–62 [DOI] [PubMed] [Google Scholar]
- 94.Ballestas ME and Kaye KM (2011) The latency-associated nuclear antigen, a multifunctional protein central to Kaposi’s sarcoma-associated herpesvirus latency. Future Microbiol 6, 1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szekely L et al. (1999) Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J. Gen. Virol 80, 2889–2900 [DOI] [PubMed] [Google Scholar]
- 96.Rivera-Molina YA et al. (2012) Nuclear domain 10-associated proteins recognize and segregate intranuclear DNA/protein complexes to negate gene expression. Virol. J 9, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guion L et al. (2019) PML nuclear body-residing proteins sequentially associate with HPV genome after infectious nuclear delivery. PLoS Pathog 15, e1007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stepp WH et al. (2017) Sp100 colocalizes with HPV replication foci and restricts the productive stage of the infectious cycle. PLoS Pathog 13, e1006660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poddar A et al. (2009) The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes. J. Virol 83, 640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahayu R et al. (2016) Localization of latency-associated nuclear antigen (LANA) on mitotic chromosomes. Virology 496, 51–58 [DOI] [PubMed] [Google Scholar]
- 101.Kanda T et al. (2007) Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. J. Cell Sci 120, 1529–1539 [DOI] [PubMed] [Google Scholar]
- 102.Dheekollu J et al. (2017) Carcinoma-risk variant of EBNA1 deregulates Epstein–Barr virus episomal latency. Oncotarget 8, 7248–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chiu YF et al. (2017) Kaposi’s sarcoma-associated herpesvirus stably clusters its genomes across generations to maintain itself extrachromosomally. J. Cell Biol 216, 2745–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grant MJ et al. (2018) Superresolution microscopy reveals structural mechanisms driving the nanoarchitecture of a viral chromatin tether. Proc. Natl Acad. Sci. U. S. A 115, 4992–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McSwiggen DT et al. (2019) Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 8, e47098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabari BR et al. (2018) Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hnisz D et al. (2017) A phase separation model for transcriptional control. Cell 169, 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang S et al. (2017) The Epstein–Barr virus regulome in lymphoblastoid cells. Cell Host Microbe 22, 561–573 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beauchemin C et al. (2014) Assay development and high-throughput screening for inhibitors of Kaposi’s sarcoma-associated herpesvirus N-terminal latency-associated nuclear antigen binding to nucleosomes. J. Biomol. Screen 19, 947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Messick TE et al. (2019) Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein–Barr virus latent infection and tumor growth. Sci. Transl. Med 11, eaau5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee EK et al. (2014) Small molecule inhibition of Epstein–Barr virus nuclear antigen-1 DNA binding activity interferes with replication and persistence of the viral genome. Antivir. Res 104, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang L et al. (2014) EBNA1-specific luminescent small molecules for the imaging and inhibition of latent EBV-infected tumor cells. Chem. Commun. (Camb) 50, 6517–6519 [DOI] [PubMed] [Google Scholar]
- 113.Kantang W et al. (2016) Design of peptides as inhibitors of human papillomavirus 16 transcriptional regulator E1-E2. Chem. Biol. Drug Des 88, 475–484 [DOI] [PubMed] [Google Scholar]
- 114.Deng SJ et al. (2004) Identification of peptides that inhibit the DNA binding, trans-activator, and DNA replication functions of the human papillomavirus type 11 E2 protein. J. Virol 78, 2637–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tso FY et al. (2019) Reduction of Kaposi’s sarcoma-associated Herpesvirus latency using CRISPR-Cas9 to edit the latency-associated nuclear antigen gene. J. Virol 93, e02183–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J and Quake SR (2014) RNA-guided endonuclease provides a therapeutic strategy to cure latent herpesviridae infection. Proc. Natl Acad. Sci. U. S. A 111, 13157–13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Diemen FR et al. (2016) CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog 12, e1005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dittmer DP and Damania B (2019) Kaposi’s sarcoma-associated Herpesvirus (KSHV)-associated disease in the AIDS patient: an update. Cancer Treat. Res 177, 63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cesarman E et al. (2019) Kaposi sarcoma. Nat. Rev. Dis. Primers 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schulz TF and Cesarman E (2015) Kaposi sarcoma-associated Herpesvirus: mechanisms of oncogenesis. Curr. Opin. Virol 14, 116–128 [DOI] [PubMed] [Google Scholar]
- 121.Young LS et al. (2016) Epstein–Barr virus: more than 50 years old and still providing surprises. Nat. Rev. Cancer 16, 789–802 [DOI] [PubMed] [Google Scholar]
- 122.Krump NA et al. (2018) Mechanisms of persistence by small DNA tumor viruses. Curr. Opin. Virol 32, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Babcock GJ et al. (2000) The expression pattern of Epstein–Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13, 497–506 [DOI] [PubMed] [Google Scholar]
- 124.Joseph AM et al. (2000) EBV persistence involves strict selection of latently infected B cells. J. Immunol 165, 2975–2981 [DOI] [PubMed] [Google Scholar]
- 125.Shanmugasundaram S and You J (2017) Targeting persistent human Papillomavirus infection. Viruses 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen X et al. (2019) A virome-wide clonal integration analysis platform for discovering cancer viral etiology. Genome Res 29, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu M et al. (2019) Genome-wide profiling of Epstein–Barr virus integration by targeted sequencing in Epstein–Barr virus associated malignancies. Theranostics 9, 1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan IM et al. (2017) Integration of human Papillomavirus genomes in head and neck cancer: is it time to consider a paradigm shift? Viruses 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harms PW et al. (2018) The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nat. Rev. Clin. Oncol 15, 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grundhoff A and Ganem D (2004) Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest 113, 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ueda K et al. (2006) Lack of a mechanism for faithful partition and maintenance of the KSHV genome. Virus Res 122, 85–94 [DOI] [PubMed] [Google Scholar]
- 132.Skalsky RL et al. (2007) Analysis of viral cis elements conferring Kaposi’s sarcoma-associated herpesvirus episome partitioning and maintenance. J. Virol 81, 9825–9837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakravorty A et al. (2019) An epigenetic journey: Epstein–Barr Virus transcribes chromatinized and subsequently unchromatinized templates during its lytic cycle. J. Virol 93, e02247–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leight ER and Sugden B (2001) Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol. Cell Biol 21, 4149–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rose TM et al. (2018) Quantitative RNAseq analysis of Ugandan KS tumors reveals KSHV gene expression dominated by transcription from the LTd downstream latency promoter. PLoS Pathog 14, e1007441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tso FY et al. (2018) RNA-Seq of Kaposi’s sarcoma reveals alterations in glucose and lipid metabolism. PLoS Pathog 14, e1006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sallah N et al. (2018) Genome-wide sequence analysis of Kaposi sarcoma-associated Herpesvirus shows diversification driven by recombination. J. Infect. Dis 218, 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dreer M et al. (2017) Control of viral replication and transcription by the papillomavirus E8^E2 protein. Virus Res 231, 96–102 [DOI] [PubMed] [Google Scholar]
- 139.Garrigues HJ et al. (2017) Full-length isoforms of Kaposi’s sarcoma-associated Herpesvirus latency-associated nuclear antigen accumulate in the cytoplasm of cells undergoing the lytic cycle of replication. J. Virol 91, e01532–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang G et al. (2016) Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc. Natl Acad. Sci. U. S. A 113, E1034–E1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bigi R et al. (2018) Epstein–Barr virus enhances genome maintenance of Kaposi sarcoma-associated herpesvirus. Proc. Natl Acad. Sci. U. S. A 115, E11379–E11387 [DOI] [PMC free article] [PubMed] [Google Scholar]