Abstract
Several animal and emerging human studies suggest an association between an early exposure to general anesthesia (GA) and long-lasting problems with complex social and emotional behaviors such as inattentiveness, impulsivity, anxiogenic tendencies, as well as difficulties engaging in proper social intercourse, with significant increase in attention deficit and hyperactivity-type behaviors. To further investigate these behaviors, and to examine the potential of presently available rodent behavioral models to guide future assessments of long-term socio-emotional impairments in humans, we examined the long-term effects of GA on anxiety/fear and social behaviors. We exposed male and female Sprague-Dawley infant rats at the peak of their synaptogenesis to either GA containing midazolam (9 mg/kg, i.p.), 70% nitrous oxide (N2O) and 0.75% isoflurane (Iso) administered in 29–30% oxygen [experimental] or air (with 30% oxygen) plus the vehicle, 0.1% dimethyl sulfoxide [Sham] for 6 hrs. Behavioral experiments were conducted at adolescence (the open-field test) and young adulthood (the open-field test, the elevated plus-maze and the social novelty test). We report that an early exposure to GA during critical stages of brain development results in long-lasting increase in risk-taking tendencies and significant changes in the anxiety-related behaviors when tested in young adult rats. In addition, we noted novelty-seeking tendencies/less guarded behavior with changes in social discrimination. We conclude that early exposure to anesthesia may have lasting influences on emotional and social development. Importantly, our results show that currently used rodent behavioral models could be a good correlate to assess long-term socio-emotional GA-induced impairments observed in humans.
Keywords: developmental neurotoxicity, thigmotaxic behavior, social interactions, rodents, sex differences
Introduction
A growing number of studies in animals [1,2,3] and humans [4,5,6] suggests an association between early exposure to general anesthesia (GA) and long-term impairments in cognitive behavior. Of particular interest for this study is the rapidly growing armamentarium of evidence, which takes this correlation a step further to include association between early exposure to GA and long-lasting problems with social and emotional development not only in rodents [7] and non-human primates [8] but in humans as well [9,10,11]. The impairments in emotional development and social interactions have been described as inattentiveness, impulsivity, anxiogenic behaviors and difficulties engaging in proper social intercourse with significant increase in attention deficit and hyperactivity-type behaviors [ ]. In a rodent study, it was noted that anesthesia-exposed animals exhibited long-lasting deficits in contextual and cued fear conditioning, as well as abnormal social interactions [7].
Although the social interaction and emotional behaviors are less complex in rodents than in higher mammals, it is important to validate currently available rodent behavioral tests so that further mechanistic and observational studies can be performed using more economical and easy-to-modulate rodent models. As we continue to grasp the potentially detrimental effects of a class of drugs that is crucial to modern medicine and frequently used in daily pediatric care, it is important to recognize all aspects of these GA-induced detrimental effects on normal behavioral development – from cognitive to complex social and emotional behaviors.
Hence, we set out to study the long-term effects of early exposure to GA on the socio-emotional development of adolescent and young adult rats using presently available behavioral tests suitable for the assessment of fear and anxiety, risk-taking behavior and social interactions. We show that the GA-induced socio-emotional behaviors in rats are comparable to GA-induced socio-emotional behaviors described in non-human primates and humans confirming two important facts: 1) GA exposure during critical stages of brain synaptogenesis causes not only cognitive impairments as previously described, but also potentially significant impairments in anxiety and fear behaviors with modified risk-taking response and alterations in social preference; 2) presently available rodent behavioral models used for the assessment of complex socio-emotional behavioral development could be useful in guiding future assessments of potential long-term socio-emotional GA-induced impairments in humans.
Materials and Methods
I. Anesthesia Delivery
A total of 8 litters from 8 rat dams were used in this study. At post-natal day 7 (P7), both male and female Sprague-Dawley rats (Envigo, Indianapolis, IN, USA), were randomly assigned to either the experimental (anesthesia) or Sham control group. The anesthesia group was exposed to 6 h of a clinically-relevant triple combination consisting of midazolam, 9 mg/kg intraperitoneally (i.p.), 70% N2O and 0.75% isoflurane (Iso) administered in 29–30% oxygen in a temperature-controlled anesthesia chamber. Sham controls were littermates separated from their dams and exposed to 6 h of mock anesthesia in an air-filled temperature-controlled chamber (with 30% oxygen) immediately post i.p. injection of 0.1% dimethyl sulfoxide (DMSO), the vehicle used to dissolve midazolam in the experimental group. An agent-specific vaporizer was used to deliver a set percentage of Iso with a mixture of O2 and N2O gases. The temperature-controlled chambers were preset to maintain ambient temperature at 33–34 °C. The composition of gases was analyzed using real-time feedback (Datex Capnomac Ultima) to assess the levels of N2O, Iso, CO2, and O2. Both experimental and Sham control pups were separated from their dams during the exposure and were reunited immediately thereafter.
II. Behavioral experiments
All animals were weaned at 3 weeks of age. After weaning, rats of the same sex were housed in individually-ventilated cages in groups of five and had free access to the standard rodent diet (Teklad, Envigo) and water. All handling and testing took place during the light phase of the diurnal cycle. Animals were tested at two time points using three well-established behavioral paradigms: 1) the open-field, 2) the elevated plus-maze and, 3) the social novelty tests. The timeline of the behavioral tests is presented in Figure 1. The body weight of each animal was measured after the last behavioral test, and we found no difference between the control and treated groups (Sham females: 251.9 ± 3.9 g vs. GA females: 258.2 ± 4.4 g; t33=1.06, p=0.298; Sham males: 412.9 ± 11.2 g vs. GA males: 427.9 ± 8.5 g; t40=1.08, p=0.285). The experiments were approved by the Animal Use and Care Committee of the University of Virginia, Charlottesville, VA. Treatment of rats adhered to the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable data. Test sessions were recorded and analyzed using ANY-maze Video Tracking System software (Stoelting Co., USA).
Figure 1.
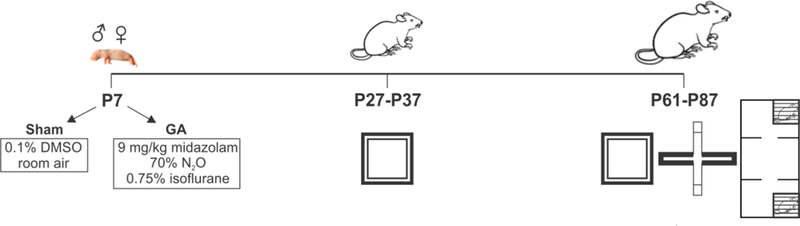
The summary of experimental protocol and timeline of behavioral experiments in rat pups (P7), adolescent (P27-P37) and young adult (P61-P87) rats.
-
1)
Open-field
Rats were brought into the testing room in their home cage at least 30 min prior to the start of testing. For the testing, each rat was placed in the square arena (91.4 cm × 91.4 cm × 45.7 cm) and was allowed to freely explore the environment during a period of 10 min, always starting from the same position close to one of the borders of the arena. The animal activity in the open-field arena was recorded using ANY-maze software. The open-field arena was virtually divided in two zones: the border zone (within 36% of the total field area closest to the wall) and the inner zone (the remaining central 64% of the floor surface). The total distance travelled was evaluated as a measure of locomotor activity. The distance travelled in the border zone and the number of entries of the animals’s head to the inner zone were used to assess the level of thigmotaxic behavior. The floor and walls of the open-field arena were cleaned with diluted ethanol after each trial to eliminate the odors of previously tested rats.
-
2)
Elevated plus-maze
The elevated plus-maze apparatus consisted of two open (50 cm × 10 cm) and two enclosed arms(50 cm × 10 cm × 40 cm), connected by a junction area (10 cm × 10 cm). At the start of the test, each animal was placed in the middle area facing the open arm, away from the experimenter and was allowed to explore the maze for 5 min. The total distance travelled was evaluated as a measure of locomotor activity, while the percentage of time spent in the open arms and the number of entries to the distal part of the open arms was used to assess the level of anxiety. The distal part was virtually defined as a square that includes approximately 40% of the open arm with an open area surrounding it (see the diagram in Figure 3).
-
3)
Social novelty test
The social novelty test was used to analyze the behavior of an animal exploring an apparatus made of three communicating chambers (total: 91.4 cm × 45.7 cm × 45.7 cm; three communicating chambers: 30.5 cm × 45.7 cm × 45.7 cm each; elevation from the floor: 31.8 cm; opening between the chambers: 9.5 cm). In the first stage (habituation), the test subject was placed in the middle chamber and was allowed to explore only this chamber during a period of 5 min. In the second stage (sociability), the test subject was again placed in the middle chamber, but was allowed to explore all three chambers. One of the chambers contained a stimulus animal (Stranger 1) protected by a wire-cage, which permitted only minimal contact interaction initiated by the tested subject, while the opposite chamber contained only an empty wire-cage similar to the one in the first chamber. In the last stage (social novelty), Stranger 1 was left in the same place, and another stimulus animal (Stranger 2) was added inside the previously empty wire-cage. The test animal repeated the experiment with a choice between exploring the novel (Stranger 2) or the familiar animal (Stranger 1), both of which were sex- and age-matched with the test animals. Sociability and social novelty stages lasted 10 min each. Between the sessions, the apparatus was cleaned with diluted ethanol and allowed to dry.
Figure 3. Anxiety-related behavior of Sham and GA-exposed rats in the elevated plus-maze.
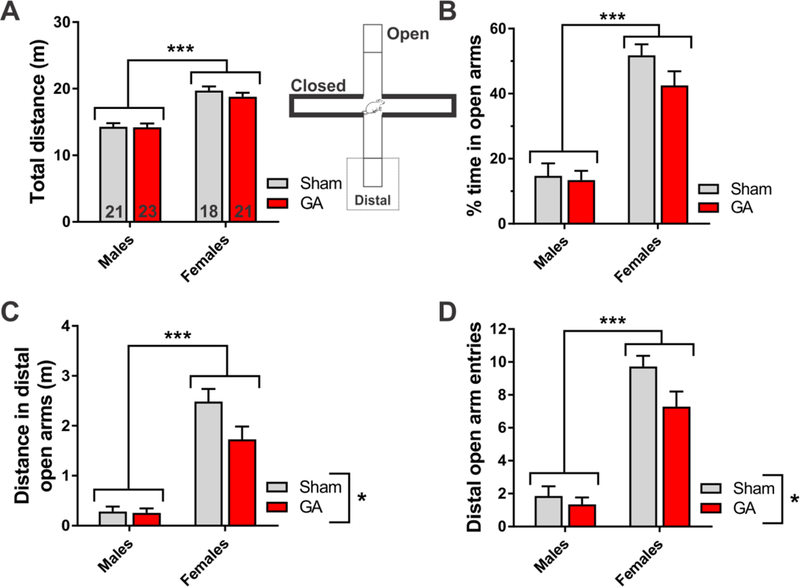
A. Total distance traveled in the elevated plus-maze during 5 min, measured in young adult rats. Female rats, irrespective of treatment, traveled longer distances than male littermates at both time points, as assessed by two-way ANOVA (factor sex: F1,79=73.50, p<0.001; interaction and factor treatment not significant). The number of animals in each group is indicated at the bottom of each bar. B. The percent of time young adult rats spent in open arms of the elevated plus-maze. Female rats, regardless of treatment, showed lower anxiety than male littermates (factor sex: F1,79=81.44, p<0.001; interaction and factor treatment not significant). C. The distance traveled in the distal zone of the open arms measured in young adult rats. Female rats, regardless of treatment, had longer distances in the distal parts of the open arms than male littermates (factor sex: F1,79=98.35, p<0.001; interaction not significant). Both male and female young adult rats exposed to GA at P7 traveled shorter distances in the distal zone, relative to Sham controls (factor treatment: F1,79=4.46, p=0.038). D. The number of entries to the distal zone of the open arms, measured in young adult rats. Female rats, regardless of treatment, explored the distal parts of the open arms more freely than male littermates (factor sex: F1,79=108.00, p<0.001; interaction not significant). Both male and female young adult rats exposed to GA at P7 had fewer entries to the distal zone than Sham controls (factor treatment: F1,79=4.92, p=0.030). *p<0.05 and ***p<0.001, two-way ANOVA. Each bar represents the mean + SEM.
III. Data analysis
The results are presented as means ± SEM. Statistical analysis was performed using two-way ANOVA (sex and treatment as main factors) and significance was accepted as p<0.05. Where applicable, Sidak’s post hoc test for multiple comparisons was used. The issues of normality and heterogeneity of variation were evaluated to determine the adequacy of the ANOVA models and whether additional analyses were warranted. Statistical and graphical analyses were performed using GraphPad Prism 7.02 software (GraphPad Software, La Jolla, CA).
Results
Young adult rats exposed to general anesthesia at P7 exhibit abnormal open-field behavior.
To assess the long-term effects of an early exposure to anesthesia (at P7) on exploration and locomotor activity, as well as the thigmotaxic behavior, we utilized the open-field test. As depicted in Fig. 1, we used this paradigm in two age groups: P27-P37 (adolescent) and P61-P65 (young adults). First, we analyzed the total distance traveled in the open-field arena during a 10 min-interval. Two-way ANOVA revealed that female rats, regardless of treatment protocol, traveled significantly longer distances [in meters (m)] than males in both age groups (Fig. 2A and B) (***, p<0.001). For example, Sham male rats traveled an average 51.5 ± 2.1 m (adolescents) and 49.6 ± 1.7 m (young adults) whereas Sham female rats traveled an average of 60.1 ± 2.0 m (adolescents) and 60.7 ± 2.7 m (young adults). However, there were no anesthesia-induced effects on locomotor activity in either males or females compared to their respective Sham controls.
Figure 2. Open-field behavior of rats exposed to general anesthesia at P7.
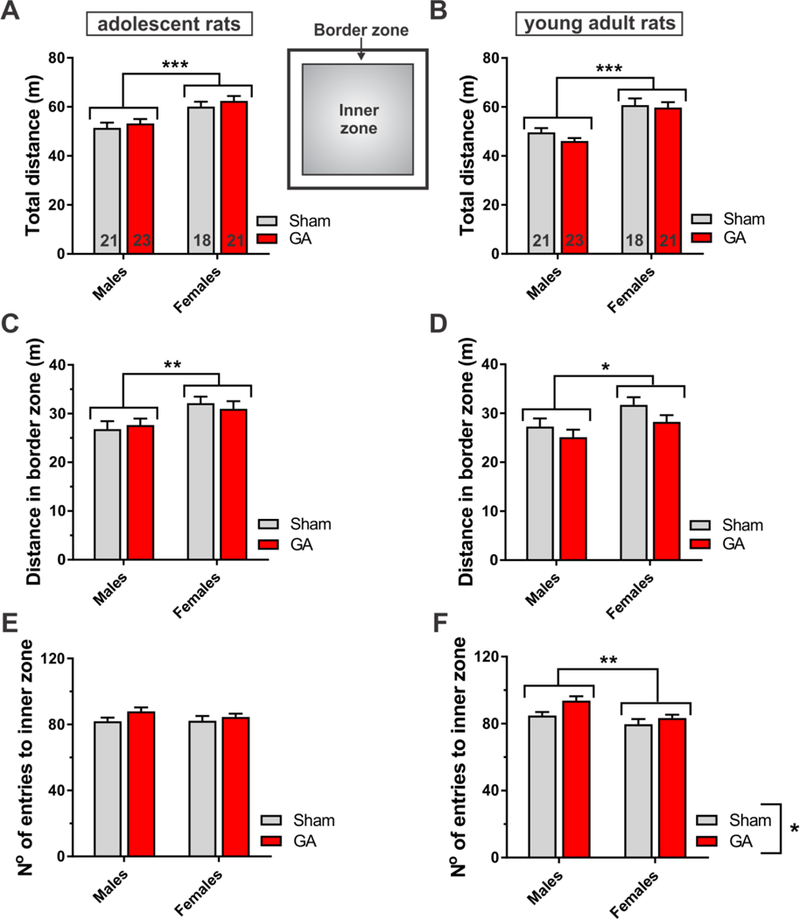
A-B. Total distance traveled in the open-field arena during 10 min, measured in adolescent (A) and young adult rats (B). Female rats traveled longer distances than male littermates at both time points, as assessed by two-way ANOVA (adolescent – factor sex: F1,79=20.58, p<0.001; young adult – factor sex: F1,79=39.58, p<0.001; interaction and factor treatment not significant for both age groups). The number of animals in each group is indicated at the bottom of each bar. C-D. The distance traveled in the border zone (36% of the entire arena), measured in adolescent (C) and young adult rats (D). Female rats traveled longer distances in the border zone than male littermates at both time points (adolescent – factor sex: F1,79=8.49, p=0.005; young adult – factor sex: F1,79=6.03, p=0.016; interaction and factor treatment not significant for both age groups). E-F. The number of entries to the inner zone, measured in adolescent (E) and young adult rats (F). Both male and female young adult rats exposed to GA at P7 had more entries to the inner zone of the open-field arena (64% of the entire arena), as compared to the Sham group (factor treatment: F1,79=6.58, p=0.012; interaction not significant). Female rats had overall fewer entries to the inner zone (factor sex: F1,79=9.88, p=0.002). *p<0.05, **p<0.01 and ***p<0.001, two-way ANOVA. Each bar represents the mean + SEM.
Next, we investigated the thigmotaxic behavior in these animals by measuring the time spent (data not shown) and the distance traveled in the border zone (Fig. 2C and D). In adolescent rats, there was no significant influence of treatment in the time spent nor the distance traveled in the border zone (Fig. 2C). In young adults, however, both males and females in the GA group traveled shorter distances, with no change in the time spent close to the wall of the arena, as compared to the Sham littermates (Fig. 2D). For example, GA-treated females traveled 28.3 ± 1.4 m, compared to 31.7 ± 1.6 m in the control females (p=0.073). Female rats in both age groups, regardless of the treatment, preferred to stay in the border zone, as compared to their male littermates. Since the time spent in the border zone was not affected either by sex or treatment in both age groups, we conclude that females moved faster than males in the open-field arena.
To further analyze the rats’ thigmotaxic behavior we assessed the number of entries to the inner zone of the open-field arena (Fig. 2E and F). This parameter remained unchanged in adolescent rats, although a statistical trend was detected (factor Treatment: p=0.084; Fig. 2E). The difference between Sham and GA animals in this parameter reached significance in young adulthood, suggesting enhanced risk-taking behavior and less guarded/more impulsive approach in the GA group (*, p<0.05, Fig. 2F). Finally, young adult, but not adolescent, female rats in both treatment groups had fewer entries to the inner zone than the corresponding male littermates. Based on these results, and other parameters not shown here, we infer that an early exposure to GA may alter risk-taking behavior in young adulthood, but not in adolescence.
Young adult rats exposed to general anesthesia at P7 exhibit changes in anxiety-related behavior in the elevated plus-maze.
To investigate the anxiety-related behavior of rats exposed to GA at P7, we subjected them to an elevated plus-maze task at P75-P80 (young adults), a widely used rodent model for testing anxiety [12,13]. Similar to our observation in the open-field test, young female rats compared to males showed an increase in general activity, as assessed by longer distance traveled in the maze during a 5 min-interval, irrespective of the treatment conditions (Fig. 3A). Female rats were also more prone to investigate the open arms of the maze, which is indicative of less cautions/more risk-taking behavior (Fig. 3B). Interestingly, females in the GA group showed a slight decrease in the percent time spent in the open arms (42.5 ± 4.3% vs. 51.8 ± 3.4% for females in the Sham group). When we analyzed the animal behavior in the distant part of the open arms by assessing the distance and the number of entries to this area, we found that GA-exposed animals of both sexes traveled shorter distances (Fig. 3C; *, p<0.05) and had fewer entries to the distal parts of the open arms (Fig. 3D; *, p<0.05), as compared to their Sham controls. It appears, however, that females were more affected than males. Namely, the interaction of two analyzed factors approached significance (F1,79=3. 87, p=0.053), thus we used Sidak’s multiple comparison test to analyze the treatment effect within males and females for the distance traveled. This post hoc analysis revealed a significant effect of GA exposure in females (p=0.013), but not in males (p=0.993). Taken together, these results suggest a GA-induced increase in anxiety/fear-related behavior in young adult rats, with females being more susceptible than male littermates.
Young adult rats exposed to general anesthesia at P7 show increased sociability and social novelty discrimination.
To examine social discrimination, sociability and social novelty preference deficits we first analyzed the general activity of young adult rats (P83-P87) exposed to the Sham condition or GA at P7 during three test stages of the social novelty paradigm. We used the social novelty test as an established behavioral paradigm for assessing social discrimination [14,15], as well as sociability and social novelty preference deficits in rodents [16].
In the habituation stage, both Sham and GA-treated female rats showed increased locomotor activity (Fig. 4A), an effect similar to that observed in the open-field test, as well as the elevated plus-maze. During the sociability stage, however, only females in the GA group showed significantly increased activity while exploring the three chambers (Fig. 4B; 35.2 ± 1.2 m in the GA group vs. 30.0 ± 1.7 m in the Sham group; **, p<0.01). The effect of sex on the total distance traveled in the third, social novelty stage was comparable to the one observed in the habituation stage (Fig. 4C). Namely, although there was no effect from treatment, there was a sex difference, with females exhibiting significantly higher activity compared to males (***, p<0.001).
Figure 4. General activity in the three stages of the social novelty test.
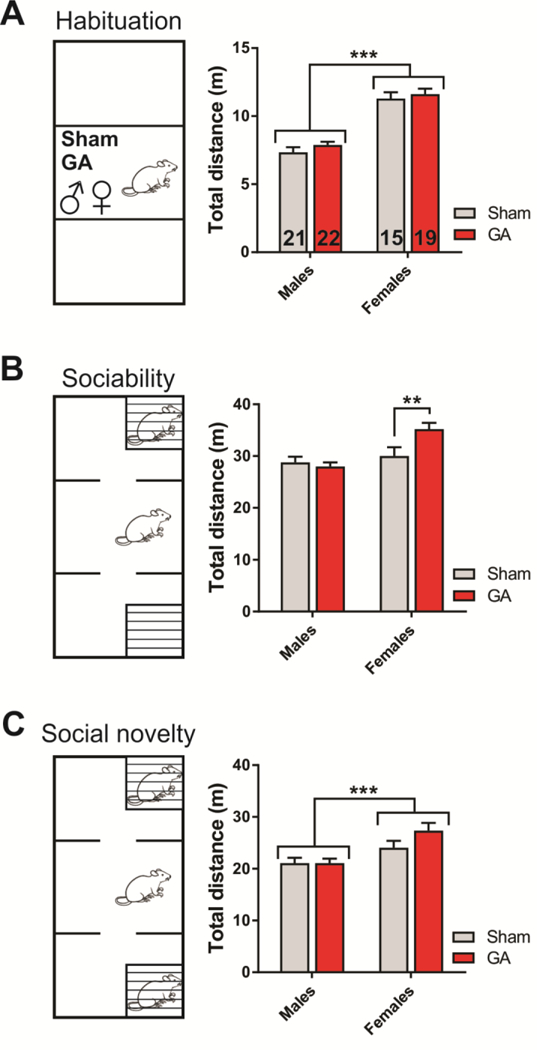
A. Total distance traveled during 5 min of habituation, measured in Sham and GA-treated young adult rats. Female rats, irrespective of treatment, traveled longer distances than male littermates, as assessed by two-way ANOVA (factor sex: F1,73=109.70, p<0.001; interaction and factor treatment not significant). The number of animals in each group is indicated at the bottom of each bar. B. Total distance traveled during 10 min of the sociability phase, measured in Sham and GA-treated young adult rats. Only female rats in the GA group traveled longer distances than Sham controls (interaction: F1,73=6.37, p=0.014; Sidak’s multiple comparisons test: p=0.009). C. Total distance traveled during 10 min of the social novelty phase, measured in Sham and GA-treated young adult rats. Female rats, irrespective of treatment, traveled longer distances than male littermates (factor sex: F1,73=15.07, p<0.001; interaction and factor treatment not significant). **p<0.01 and ***p<0.001, two-way ANOVA or Sidak’s test. Each bar represents the mean + SEM.
To assess the sociability of Sham and GA-treated rats we first measured the time and number of explorations of either an unfamiliar rat (Stranger 1) or an empty wire cage (Fig. 5A and B, respectively). Based on these parameters, we noticed that both males and females, regardless of the treatment protocol, preferred a stranger rat to an empty cage, suggesting a normal level of sociability commonly observed in rodents [15,16]. For example, males in the GA group spent an average of 166.6 ± 7.1 s exploring the stranger rat, compared to 110.5 ± 5.3 s exploring an empty cage. Female rats in both Sham and GA groups had fewer explorations of the stranger rat and spent more time exploring an empty cage, as compared to males. When we analyzed the time and number of entries to the chambers containing a stranger rat (Chamber 1) or an empty cage (Chamber 2) we found that the rats of both sexes spent more time in Chamber 1 than Chamber 2 (Fig. 5C and D), with females spending more time in the chamber containing the empty cage compared to males. Interestingly, GA females were more active than their Sham counterparts, as evidenced by a significant increase in the number of entries to both chambers (*, p<0.05). The results from the sociability phase indicate a normal level of exploration and preference for an unfamiliar conspecific. However, the total distance traveled (Fig. 4B) and the number of entries to both chambers of the apparatus (lower panels, Fig. 5C and D) revealed an increased locomotor activity of GA females compared to Sham ones, suggesting an altered behavior when stimulated by the environment containing either an unfamiliar conspecific or a physical object.
Figure 5. Rat behavior during the sociability stage of the social novelty test.
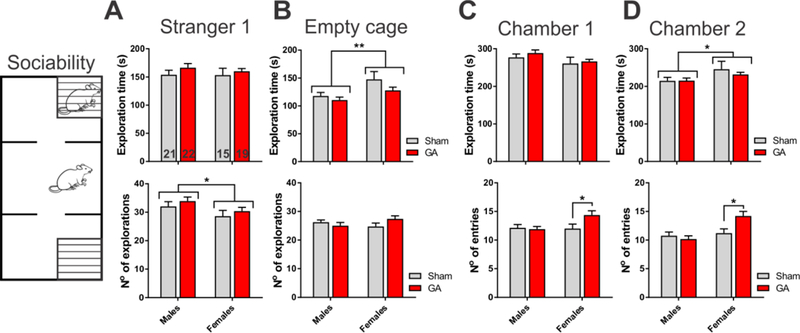
A. Time spent exploring (upper panel) and number of explorations (lower panel) of an unfamiliar conspecific (Stranger 1), measured in Sham and GA-treated young adult rats. The number of animals in each group is indicated at the bottom of each bar. Female rats, irrespective of treatment, had fewer exploration episodes than male littermates, as assessed by two-way ANOVA (factor sex: F1,73=9.16, p=0.003; interaction and factor treatment not significant). B. Time spent exploring (upper panel) and number of explorations of an empty wire cage (lower panel), measured in Sham and GA-treated young adult rats. Female rats, irrespective of treatment, spent more time exploring an empty cage than male littermates (factor sex: F1,73=4.61, p=0.035; interaction and factor treatment not significant). C. Time spent exploring (upper panel) and number of entries (lower panel) to the chamber containing an unfamiliar conspecific (Chamber 1), measured in Sham and GA-treated young adult rats. Female rats in the GA group had more entries to Chamber 1 than Sham controls (interaction: F1,73=4.09, p=0.047; Sidak’s multiple comparisons test: p=0.033). D. Time spent exploring (upper panel) and number of entries (lower panel) to the chamber containing an empty wire cage (Chamber 2), measured in Sham and GA-treated young adult rats. Female rats, irrespective of treatment, spent more time exploring Chamber 2 than male littermates (factor sex: F1,73=4.77, p=0.032; interaction and factor treatment not significant). Female rats in the GA group had more entries to Chamber 2 than Sham controls (interaction: F1,73=6.67, p=0.012; Sidak’s multiple comparisons test: p=0.010). *p<0.05 and **p<0.01, two-way ANOVA or Sidak’s test. Each bar represents the mean + SEM.
Finally, we tested the rats’ ability to recognize the familiar rat from a previous stage (Stranger 1), and show preference for social interaction with a novel conspecific (Stranger 2). Indeed, all animals spent more time and had more interactions with Stranger 2, as compared to Stranger 1 (Fig. 6A and B). For example, males in the GA group spent on average 167.1 ± 11.2 s exploring Stranger 2, compared to only 101.1 ± 6.1 s spent with Stranger 1 (upper panels). However, it is noteworthy that both males and females in the GA group spent significantly more time with and exhibit higher number of explorations of Stranger 2 than Sham controls, suggesting even more pronounced preference for a novel conspecific and less guarded approach to novel situations. This effect was also observed when we compared the time spent exploring the chamber containing Stranger 2 (Fig. 6D, upper panel). Finally, both Sham and GA females showed significantly more activity exploring the Chamber 1 (Fig. 6C, lower panel), but only GA females had more entries to Chamber 2 (Fig. 6D, lower panel). These results suggest that GA-treated animals may exhibit less guarded behavior in social novelty test, with GA females also showing an increased activity.
Figure 6. Social novelty discrimination of rats exposed to general anesthesia at P7.
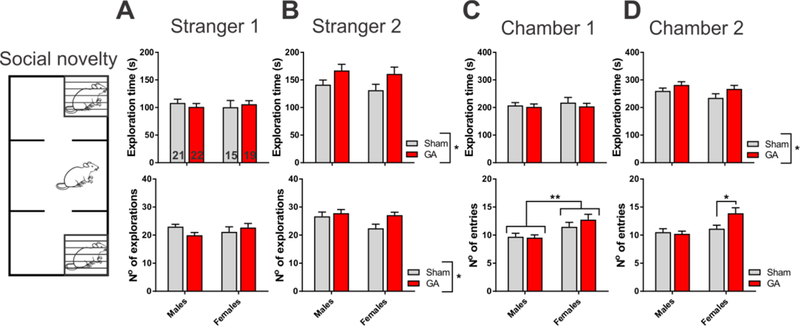
A. Time spent exploring (upper panel) and number of explorations (lower panel) of a familiar conspecific (Stranger 1), measured in Sham and GA-treated young adult rats. The number of animals in each group is indicated at the bottom of each bar. B. Time spent exploring (upper panel) and number of explorations (lower panel) of a novel conspecific (Stranger 2). Both male and female rats in the GA group spent more time (factor treatment: F1,73=6.43, p=0.013) and had more exploration episodes than Sham controls (factor treatment: F1,73=4.47, p=0.038; interaction and factor sex not significant for both parameters measured). C. Time spent exploring (upper panel) and number of entries (lower panel) to the chamber containing a familiar conspecific (Chamber 1). Female rats, irrespective of treatment, had more entries to Chamber 1 than male littermates (factor sex: F1,73=11.43, p=0.001; interaction and factor treatment not significant). D. Time spent exploring (upper panel) and number of entries (lower panel) to the chamber containing a novel conspecific (Chamber 2), measured in Sham and GA-treated young adult rats. Both male and female rats in the GA group spent more time exploring Chamber 2 than Sham controls (factor treatment: F1,73=4.76, p=0.032; interaction and factor sex not significant for both parameters measured). Female rats in the GA group had more entries to Chamber 2 than Sham controls (interaction: F1,73=4.75, p=0.033; Sidak’s multiple comparisons test: p=0.021). *p<0.05 and **p<0.01, two-way ANOVA or Sidak’s test. Each bar represents the mean + SEM.
Discussion
In this study, we show that early exposure to GA during critical stages of brain synaptogenesis [1–3, 7] results in long-lasting increases in risk-taking tendencies and significant changes in anxiety-related behaviors when tested in young adult rats. Furthermore, the GA-treated animals exhibit novelty-seeking tendencies/less guarded behavior with changes in social discrimination, suggesting that early exposure to anesthesia could have lasting influences on emotional and social development.
Interestingly, our report presented herein shares similarities with the recent reports in humans [4,9,10,11], which have suggested that children exposed to anesthesia during early stages of their development exhibit behavioral and emotional impairments later in life by being prone to anxiogenic behaviors, inattentiveness, impulsivity and difficulties engaging in proper social intercourse. For example, a cohort study by Bakri et al. (2015) compared children who had repeated exposures to GA before the age of 5 years to non-exposed children of the same age and concluded that GA-exposed children were at higher risk to become anxious or depressed, and to suffer from attention deficit disorder [17]. This confirms earlier findings by Sprung and his research group (2012), which suggested a higher incidence of attention deficit, hyperactivity disorder diagnoses in children exposed multiple times to GA [11]. Interestingly, in non-human primate studies, it was confirmed that multiple exposures to GA during infancy result in scratching, yawning and excessive grooming behaviors indicative of increased emotional reactivity and heightened fear [8]. Although the complexity of social and emotional behavioral development in rodents is substantially lower than in monkeys or humans, it is reassuring that the currently available testing paradigms we chose to examine herein can capture the impairments in rodents that are similar to the ones described in higher mammalian species.
Using the open-field test as an unconditioned behavioral paradigm designed to assess exploration and locomotor activity, as well as anxiety-related behavior [18,19], we detected a substantial sex difference in the locomotor activity and risk-taking behavior. In particular, we found that female rats, both in the adolescent and the young adult stages, show higher locomotor activity when compared to their male counterparts, which confirms previously reported finidings [20,21]. For example, an older study that focused on detailed analysis of locomotor activity and circling behavior noted that a significant difference in locomotor activity, but not circling, in females when compared to their age-matched male counterparts starting from the age of 8 weeks. A very significant gap was noted after the age of 11 weeks, with sex-specific differences not being as profound after the 16 weeks of age [22]. Although our data do not show such a substantial change in locomotor activity from adolescence to young adulthood, it is noteworthy that females indeed had a tendency to travel longer distance than males in both age groups. The precise etiology for sex differences remains unclear; however, there is an old school of thought suggesting that higher distance travelled during their reproductive age enables female rats to find a male mate with higher frequency [23].
We also report that GA-exposed rats display a lower degree of thigmotaxis (i.e. GA-exposed animals were more prone to leave the safety of the border wall), which indicates an increase in risk-taking behavior, considering their natural tendency is to view the open areas as threatening and to seek safety closer to the walls. Although risk-taking behavior has been described in many instances, in particular during adolescence, its etiology remains unclear. Some recent studies suggest an association between the epigenetic changes noted in prefrontal cortical neurons and an increase in risk-taking behavior in rodents [24,25]. Of particular interest is the report suggesting an association between the modulation of histone methylation status and risk-taking tendencies [24], which correlates with our recently reported findings that show an early exposure to GA results in substantial histone hypoacetylation in the promotors of the target genes [26]. Although it is an interesting possibility that GA-induced epigenetic modifications we have previously reported may play an important role in GA-induced increases in risk-taking and less guarded behavior presented here, this association will have to be critically examined in future studies.
We report herein that early exposure to GA promotes the development of anxiety-like behavior in young adult rats, as assessed by the elevated plus-maze study. Interestingly, this change in anxiety seems to be more pronounced in GA-treated females when compared to their controls (most likely because control males showed higher levels of baseline anxiety hence making the GA-induced increase less prominent). It is well established that adult male rats generally avoid exploring the open arms of the elevated plus-maze [27], which may be the reason why this effect was easier to detect in female rats. Interestingly, a similar lack of effect of early exposure to isoflurane on anxiety in male mice was previously reported in the same behavioral paradigm [28]. Somewhat contradicting are the reports suggesting that an inhaled anesthetic, sevoflurane when administered to mice at P4-P6 induced anxiety-like behavior with males being more susceptible than females in young adulthood [29]. Finally, it is important to note that the two seemingly opposite characteristics, such are higher impulsivity and increased anxiety, may coexist in rats [30], as well as humans [31]. Taken together, these findings suggest that early exposure to GA may play an important role in the modulation of the perceived anxiety and fear exhibited later in life and that the outcomes can be complex and heterogeneous.
We performed analysis in the elevated plus-maze that included a detailed assessment of the entries to the distal parts of its arms, which proved to be a very useful approach in detecting subtle, but significant increases in anxiety in the GA-treated animals, with female rats being more sensitive to GA effects than age-matched male counterparts. This relatively new type of analysis, which in the classic sense was not a part of the plus-maze behavior [32], was introduced in the early 2000s [33,34] and is believed to be a good assessment of anxiety and fear behavior [35]. Indeed, a detailed analysis of distal open arm entries enabled us to capture the GA-induced fear-related behaviors in young adult females that otherwise would have gone unrecognized.
An elegant study by Satomoto et al. (2009) was the first to provide an extensive analysis of social behavior in mice exposed to sevoflurane [7], an inhaled anesthetic most commonly used in modern anesthesia practice. The authors confirmed that early exposure to sevoflurane resulted in significant impairments in cognitive development. Importantly, this study also introduced a concept of sevoflurane-induced abnormal social behaviors and significant deficits in fear conditioning later in life. Their findings suggested that GA-treated mice did not develop social memory and showed ‘decreased interactions with a social target compared with controls in the social interaction test’. This is somewhat different from what we report herein with our tests of social interactions, which suggest more pronounced preference for a novel conspecific and less guarded approach to novel situations in GA-treated rats compared to Sham controls. The difference in social interactions between mice and rats notwithstanding, it seems reasonable to propose that neonatal exposure to GA results in a significant modification of social and emotional development, although the specifics of the observed impairments may be different in nature.
In summary, our study shows that early exposure to GA leads to lasting modulation of emotional and social behaviors in young adult rats with sex-specific tendencies toward higher locomotor activity and risk-taking/less guarded behaviors.
Acknowledgments:
Supported in part by funds from the Department of Anesthesiology at the University of Colorado Anschutz Medical campus, R0144517, R0144517-S, R01 GM118197, R01 GM118197-S, R21 HD080281, and March of Dimes National Award, USA (to VJT) and Harold Carron and CU Medicine Endowments (to VJT).
References
- 1.Jevtovic-todorovic V, Hartman RE, Izumi Y, et al. (2003) Early Exposure to Common Anesthetic Agents Causes Widespread Neurodegeneration in the Developing Rat Brain and Persistent Learning Deficits. 23:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loepke AW, Istaphanous GK, McAuliffe JJ, et al. (2009) The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108:90–104. 10.1213/ane.0b013e31818cdb29 [DOI] [PubMed] [Google Scholar]
- 3.Paule MG, Li M, Allen RR, et al. (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33:220–30. 10.1016/j.ntt.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder RT, Flick RP, Sprung J, et al. (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804. 10.1097/01.anes.0000344728.34332.5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flick RP, Katusic SK, Colligan RC, et al. (2011) Cognitive and Behavioral Outcomes After Early Exposure to Anesthesia and Surgery. Pediatrics 128:e1053–e1061. 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ing C, DiMaggio C, Whitehouse A, et al. (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–85. 10.1542/peds.2011-3822 [DOI] [PubMed] [Google Scholar]
- 7.Satomoto M, Satoh Y, Terui K, et al. (2009) Neonatal Exposure to Sevoflurane Induces Abnormal Social Behaviors and Deficits in Fear Conditioning in Mice. Anesthesiology 110:628–637. 10.1097/ALN.0b013e3181974fa2 [DOI] [PubMed] [Google Scholar]
- 8.Raper J, Alvarado MC, Murphy KL, Baxter MG (2015) Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology 123:1084–92. 10.1097/ALN.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMaggio C, Sun LS, Kakavouli A, et al. (2009) A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 21:286–91. 10.1097/ANA.0b013e3181a71f11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemaly M, El-Rajab MA, Ziade FM, Naja ZM (2014) Effect of one anesthetic exposure on long-term behavioral changes in children. J Clin Anesth 26:551–6. 10.1016/j.jclinane.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 11.Sprung J, Flick RP, Katusic SK, et al. (2012) Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87:120–9. 10.1016/j.mayocp.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson GR, Tricklebank MD (1995) Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16:33–6 [DOI] [PubMed] [Google Scholar]
- 13.Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322–8. 10.1038/nprot.2007.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Kooij MA, Sandi C (2012) Social memories in rodents: Methods, mechanisms and modulation by stress. Neurosci Biobehav Rev 36:1763–1772. https://doi.org/10.1016ZJ.NEUBI0REV.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 15.Moy SS, Nadler JJ, Perez A, et al. (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain Behav 3:287–302. 10.1111/j.1601-1848.2004.00076.x [DOI] [PubMed] [Google Scholar]
- 16.Cavigelli SA, Michael KC, West SG, Klein LC (2011) Behavioral responses to physical vs. social novelty in male and female laboratory rats. Behav Processes 88:56–9. 10.1016/j.beproc.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakri M, Ismail E, Ali M, et al. (2015) Behavioral and emotional effects of repeated general anesthesia in young children. Saudi J Anaesth 9:161 10.4103/1658-354X.152843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh RN, Cummins RA (1976) The Open-Field Test: a critical review. Psychol Bull 83:482–504 [PubMed] [Google Scholar]
- 19.Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- 20.Blizard DA, Lippman HR, Chen JJ (1975) Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav 14:601–8 [DOI] [PubMed] [Google Scholar]
- 21.Johnston AL, Filet SE, Johnston AL, And SE Sex Differences in Animal Tests of Anxiety [DOI] [PubMed] [Google Scholar]
- 22.Hyde JF, Jerussi TP (1983) Sexual dimorphism in rats with respect to locomotor activity and circling behavior. Pharmacol Biochem Behav 18:725–729. 10.1016/0091-3057(83)90014-X [DOI] [PubMed] [Google Scholar]
- 23.Russell PA (1977) Sex differences in rats’ stationary exploration as a function of stimulus and environmental novelty. Anim Learn Behav 5:297–302. 10.3758/BF03209243 [DOI] [Google Scholar]
- 24.Viola TW, Wearick-Silva LE, Creutzberg KC, et al. (2018) Postnatal impoverished housing impairs adolescent risk-assessment and increases risk-taking: A sex-specific effect associated with histone epigenetic regulation of Crfr1 in the medial prefrontal cortex. Psychoneuroendocrinology 99:8–19. 10.1016/j.psyneuen.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 25.de Vocht F, Suderman M, Tilling K, et al. (2018) DNA methylation from birth to late adolescence and development of multiple-risk behaviours. J Affect Disord 227:588–594. 10.1016/j.jad.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalla Massara L, Osuru HP, Oklopcic A, et al. (2016) General anesthesia causes epigenetic histone modulation of c-Fos and brain-derived neurotrophic factor, target genes important for neuronal development in the immature rat hippocampus. Anesthesiology 124:. 10.1097/ALN.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imhof JT, Coelho ZMI, Schmitt ML, et al. (1993) Influence of gender and age on performance of rats in the elevated plus maze apparatus [DOI] [PubMed] [Google Scholar]
- 28.Kodama M, Satoh Y, Otsubo Y, et al. (2011) Neonatal Desflurane Exposure Induces More Robust Neuroapoptosis than Do Isoflurane and Sevoflurane and Impairs Working Memory. Anesthesiology 115:979–991. 10.1097/ALN.0b013e318234228b [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Tan S, Zhang J, et al. (2015) Anesthesia with sevoflurane in neonatal rats: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl – importer antagonists HHS Public Access. Psychoneuroendocrinology 60:173–181. 10.1016/j.psyneuen.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mejia-Toiber J, Boutros N, Markou A, Semenova S (2014) Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res 266:19–28. 10.1016/J.BBR.2014.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakuszkowiak-Wojten K, Landowski J, Wiglusz MS, Cubala WJ (2015) Impulsivity in anxiety disorders. A critical review. Psychiatr Danub 27 Suppl 1:S452–5 [PubMed] [Google Scholar]
- 32.Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–67 [DOI] [PubMed] [Google Scholar]
- 33.Dere E, Topic B, De Souza Silva MA, et al. (2002) The graded anxiety test: a novel test of murine unconditioned anxiety based on the principles of the elevated plus-maze and light-dark test. J Neurosci Methods 122:65–73 [DOI] [PubMed] [Google Scholar]
- 34.File S, Gonzalez LE, Gallant R (1999) Role of the Dorsomedial Hypothalamus in Mediating the Response to Benzodiazepines on Trial 2 in the Elevated Plus-Maze Test of Anxiety. Neuropsychopharmacology 21:312–320. 10.1016/S0893-133X(99)00028-7 [DOI] [PubMed] [Google Scholar]
- 35.Cruz AP, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49:171–6 [DOI] [PubMed] [Google Scholar]


