Abstract
Objective:
To compare the prevalence of patient-reported lower-extremity lymphedema (LEL) with sentinel lymph node (SLN) mapping versus comprehensive lymph node dissection (LND) for the surgical management of newly diagnosed endometrial carcinoma.
Methods:
Patients who underwent primary surgery for endometrial cancer from 01/2006–12/2012 were mailed a survey that included a validated 13-item LEL screening questionnaire in 08/2016. Patients diagnosed with LEL prior to surgery and those who answered ≤6 survey items were excluded.
Results:
Of 1,275 potential participants, 623 (49%) responded to the survey and 599 were evaluable (180 SLN, 352 LND, 67 hysterectomy alone). Median BMI was similar among cohorts (P=0.99). External-beam radiation therapy (EBRT) was used in 10/180 (5.5%) SLN and 35/352 (10%) LND patients (P=0.1). Self-reported LEL prevalence was 27% (49/180) and 41% (144/352), respectively (OR, 1.85; 95%CI, 1.25–2.74; P=0.002). LEL prevalence was 51% (23/45) in patients who received EBRT and 35% (170/487) in those who did not (OR, 1.95; 95%CI, 1.06–3.6; P=0.03). High BMI was associated with increased prevalence of LEL (OR, 1.04; 95%CI, 1.02–1.06; P=0.001). After controlling for EBRT and BMI, LND retained independent association with an increased prevalence of LEL over SLN (OR, 1.8; 95%CI, 1.22–2.69; P=0.003). Patients with self-reported LEL had significantly worse QOL compared to those without self-reported LEL.
Conclusions:
This is the first study to assess patient-reported LEL after SLN mapping for endometrial cancer. SLN mapping was independently associated with a significantly lower prevalence of patient-reported LEL. High BMI and adjuvant EBRT were associated with an increased prevalence of patient-reported LEL.
Keywords: endometrial cancer, sentinel lymph node, sentinel lymph node mapping, lymphadenectomy, lymphedema, patient-reported outcomes
Introduction
Pelvic and para-aortic lymphadenectomy (LND) has been considered standard of care for patients with newly diagnosed endometrial carcinoma.1 The role of comprehensive LND, however, is debatable. In 2 randomized trials, pelvic LND did not result in improved survival,2,3 but it was associated with the identification of nodal disease and more accurate staging, which many clinicians consider necessary to guide adjuvant treatment. Despite the potential therapeutic value of LND, the procedure is associated with an increased risk of lower-extremity lymphedema (LEL).4
Most lymphedema patient-reported outcome (PRO) assessment tools have been designed for the upper extremity, in the context of breast cancer. There are now at least 2 validated LEL PRO tools. Investigators at the Mayo Clinic developed and validated one of these tools,5 and showed that 23% of women who underwent a comprehensive LND compared to hysterectomy alone reported LEL attributable to the LND.6 Those who reported LEL also had significantly diminished quality of life (QOL) as assessed by validated QOL tools.6
Sentinel lymph node (SLN) mapping has emerged as an acceptable alternative to comprehensive LND in the staging of patients with endometrial cancer. The National Comprehensive Cancer Network (NCCN) guidelines now allow for SLN mapping for the surgical staging of endometrial carcinomas.7 Prospective trials have shown low false-negative predictive values with SLN mapping in the detection of nodal disease in these patients, including those with “high-risk” endometrial carcinoma.8,9 The therapeutic superiority of LND over SLN mapping alone, especially in high-risk cases and those with SLN metastasis, is still highly debatable. Retrospective analyses, however, have suggested that using SLN mapping over LND does not compromise oncologic outcome in such cases.10,11 Furthermore, SLN mapping compared with LND is associated with a much lower risk of LEL development in patients with vulvar or endometrial cancer.12,13
LEL assessment methods have varied in prior studies, ranging from physician assessment to the use of leg measurements, but no study has used LEL PRO tools to compare SLN mapping with LND. Here, we used a validated LEL PRO tool to assess the prevalence of LEL among patients who underwent either SLN mapping or LND during surgery for newly diagnosed endometrial cancer. We also assessed whether patient-reported LEL was associated with QOL.
Methods and Materials
After Institutional Review Board (IRB) approval, we identified all patients who had undergone primary surgery for newly diagnosed endometrial cancer at our institution (Memorial Sloan Kettering Cancer Center [MSK]) between 1/1/06 and 12/31/12. We excluded patients who had died or had a “do not contact” notation in the electronic medical record (EMR). The included patients were mailed a questionnaire that included a validated 13-item LEL screening survey and validated QOL assessment tools (Appendix 1) in August 2016—a minimum of 44 months after surgery. The original questionnaire6 was modified and used with permission.
The 13-item LEL PRO survey (Items 9–21 of Appendix 1), validated by investigators from the Mayo Clinic,5 results in a score of 0–52, with a total score ≥5 indicative of LEL (primary endpoint). The tool’s sensitivity and specificity for detecting LEL is 95.5% and 86.5%, respectively, in all patients, and 94.8% and 76.5%, respectively, in obese patients.5 The mailed questionnaire also included validated QOL assessment tools—EORTC QLQ-C30 (Items 22–49 of Appendix 1) and EORTC QLQ-EN24 (Items 50–75 of Appendix 1).14–16 Item 8 was included to identify patients who had LEL prior to surgery; these patients were subsequently excluded.
We used a highly proven 2-phase mail-first recruitment design to yield higher coverage and garner a higher response rate at a lower cost compared to phone-first design.17,18 After the first mailing, a second mailing went out to non-respondents one month later. A month after that, remaining non-respondents were called and reminded to complete the questionnaire using an IRB-approved phone script. Potential participants were called a maximum of 2 times. Questionnaire responses and clinicopathologic data were abstracted from the EMR and entered into the Web-based Research Electronic Data Capture (REDCap) platform. Those who reported preoperative LEL, had answered 6 or fewer of the 13 items on the LEL PRO survey, or reported having undergone a radical orthopedic resection of the pelvis and/or extremities since their hysterectomy were excluded.
The primary endpoint was the prevalence of patient-reported LEL among those who had undergone hysterectomy with SLN mapping alone (SLN cohort) and those who had undergone hysterectomy with standard LND, with or without SLN mapping (LND cohort). We also assessed the prevalence of patient-reported LEL in those who had undergone hysterectomy alone (HYST cohort). The HYST cohort included patients who had undergone hysterectomy alone with or without bilateral salpingo-oophorectomy, as well as those in whom 1 or 2 “enlarged/suspicious” lymph nodes were removed without intent for LND or SLN mapping. The SLN cohort included those in whom only SLN mapping was performed and SLNs excised, with at least one SLN identified both clinically and pathologically. Those who had a unilateral side-specific LND of an unmapped hemi-pelvis were included in the SLN cohort, as per our algorithm. The LND cohort included those in whom a bilateral LND was performed alone or as a “backup” after SLN mapping, and in those who had a failed bilateral SLN mapping.
The statistical design assumed a two-sided type I error of 5% and power of 95% with an expected sample size of 413 LND and 260 SLN patients in order to detect a 10% difference in the rate of LEL between the LND and SLN cohorts of 5 to 15%. The final sample size was 352 LND and 180 SLN patients.
The rate of LEL in each cohort and the 95% confidence interval (CI) was estimated assuming binomial distribution. A two-sample binomial proportions test was used to compare LEL prevalence between the 2 groups. As a secondary analysis, time to development of LEL was analyzed as a time-to-event variable from surgery date to questionnaire date while considering the interval censored data (LEL exact event date is not known). A type I interval censoring method was applied to compare LEL incidence between the cohorts.19
Descriptive statistics were provided for all baseline variables for the entire cohort and subgroups (i.e., SLN/LND/HYST or LEL/No LEL). The Fisher exact test and Wilcoxon rank sum test were used to compare the distribution of prespecified covariates between the groups. Univariate logistic regression was used to investigate the effect of baseline covariates on the presence of patient-reported LEL. A multivariate logistic model was built based on significant variables (P<0.05) in univariate setting, except the number of lymph nodes was excluded as a covariate since it was highly correlated with whether LND was performed or not.
QOL questionnaire scoring was calculated according to the EORTC QLQ-C30 and EORTC QLQ-EN24 scoring manuals.15,16 The QLQ C-30 summary score is calculated from the mean of 13 of the 15 QLQ-C30 scales.20 The Wilcoxon rank sum test is applied to compare the scores’ distribution between patients who developed LEL and those who did not. Multiple comparisons adjustment is applied to the QOL analysis using Bonferroni correction.
Results
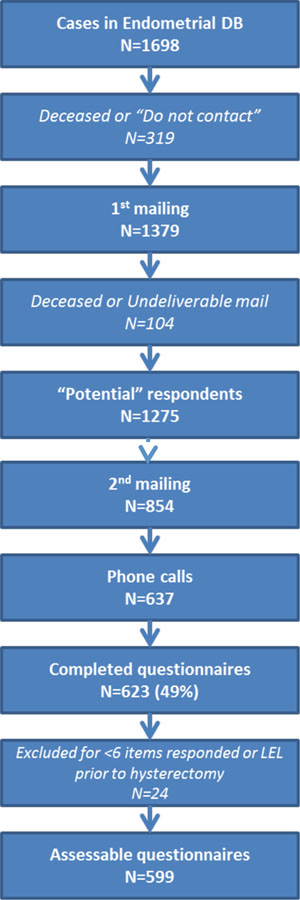
Of 1275 potential participants, 623 (49%) responded to the survey, an acceptable response rate for our study design. Twenty-four were excluded for either having answered 6 or fewer of the 13 items on the LEL PRO survey (n=11) or for indicating preoperative LEL (n=13). There were 599 evaluable patients (180 SLN, 352 LND, 67 HYST) (Figure 1). The median time from date of surgery to date of filling out the questionnaire was 63.2 months (range, 44.3–101.2 months) in the SLN cohort, 93.1 months (range, 44.4–131.3 months) in the LND cohort, and 84.5 months (range, 45.1–127.9 months) in the HYST cohort (P<0.001 for SLN vs LND). Clinicopathologic characteristics for the entire cohort and each sub-cohort are listed in Table 1. Median age and body mass index (BMI) did not differ between the SLN and LND cohorts. The differences noted in International Federation of Gynecology and Obstetrics (FIGO) stage, grade, and histology reflect the evolution of patient selection for SLN mapping during the selected time period.
Figure 1.
Study recruitment flow
Table 1.
Select clinicopathologic characteristics. P value refers to the comparison between SLN and LND groups only.
| Characteristic | Whole Cohort | SLN | LND | HYST | P value for SLN vs LND only |
|---|---|---|---|---|---|
| N | 599 | 180 | 352 | 67 | |
| Age at surgery (years) | 0.37 | ||||
| Median | 61 | 61 | 61 | 61 | |
| Range | 27–85 | 34–85 | 27–83 | 31–85 | |
| BMI (kg/m2) | 0.99 | ||||
| Median | 29 | 29.1 | 29.0 | 33.0 | |
| Range | 17.9–68.6 | 17.9–67.6 | 18.2–59.1 | 19.5–68.6 | |
| FIGO stage | 0.01 | ||||
| I | 492 (82.3) | 159 (88.3) | 271 (77) | 62 (93.9) | |
| II | 15 (2.5) | 2 (1.1) | 12 (3.4) | 1 (1.5) | |
| III | 78 (13) | 18 (1.0) | 59 (16.8) | 1 (1.5) | |
| IV | 13 (2.2) | 1 (0.6) | 10 (2.8) | 2 (3) | |
| FIGO tumor grade | <0.001 | ||||
| 1 | 305 (51) | 122 (67.8) | 135 (38.4) | 48 (72.7) | |
| 2 | 132 (22.1) | 34 (18.9) | 88 (25) | 10 (15.2) | |
| 3 | 161 (26.9) | 24 (13.3) | 129 (36.6) | 8 (12.1) | |
| Histology | <0.001 | ||||
| Endometrioid | 472 (78.8) | 162 (90) | 256 (72.7) | 54 (80.6) | |
| Non-endometrioid | 60 (10) | 8 (4.4) | 47 (13.4) | 5 (7.5) | |
| Carcinosarcoma | 25 (4.2) | 2 (1.1) | 23 (6.5) | 0 (0) | |
| Sarcoma | 8 (1.3) | 1 (0.6) | 2 (0.6) | 5 (7.5) | |
| Other* | 34 (5.7) | 7 (3.9) | 24 (6.8) | 3 (4.5) | |
| Hypertension | 0.17 | ||||
| No | 281 (46.9) | 96 (53.3) | 165 (46.9) | 20 (29.9) | |
| Yes | 318 (53.1) | 84 (46.7) | 187 (53.1) | 47 (70.1) | |
| Diabetes | 0.01 | ||||
| No | 495 (82.6) | 161 (89.4) | 285 (81) | 49 (73.1) | |
| Yes | 104 (17.4) | 19 (10.6) | 67 (19) | 18 (26.9) | |
| CHF | 0.27 | ||||
| No | 592 (98.8) | 178 (98.9) | 351 (99.7) | 63 (94) | |
| Yes | 7 (1.2) | 2 (1.1) | 1 (0.3) | 4 (6) | |
| Renal disease | 1.0 | ||||
| No | 588 (98.2) | 178 (98.9) | 347 (98.6) | 63 (94) | |
| Yes | 11 (1.8) | 2 (1.1) | 5 (1.4) | 4 (6) | |
| EBRT | 0.1 | ||||
| No | 550 (91.8) | 170 (94.4) | 317 (90.1) | 63 (94) | |
| Yes | 49 (8.2) | 10 (5.6) | 35 (9.9) | 4 (6) | |
| Total LNs removed | <0.001 | ||||
| Median | 11 | 4 | 19 | 0 | |
| Range | 0–80 | 1–21 | 1–80 | 0–1 |
Values are N(%) except where noted otherwise
SLN=sentinel lymph node mapping cohort; LND=lymphadenectomy cohort; HYST=hysterectomy alone cohort BMI=body mass index; FIGO=International Federation of Gynecology and Obstetrics; CHF=congestive heart failure; EBRT=external beam radiotherapy (postoperative); LN=lymph nodes
other histology includes: adenocarcinoma NOS, carcinoma NOS, atypical hyperplasia, mixed histologies, squamous cell carcinoma, undifferentiated carcinoma, yolk sac tumor
Overall, 220 (37%) of 599 patients were noted to have LEL based on the 13-item LEL PRO questionnaire. Forty-nine (27.2%; 95% CI, 20.7–33.7%) of 180 patients in the SLN cohort screened positive for self-reported LEL compared with 144 (40.9%; 95% CI, 35.8–46.1%) of 352 patients in the LND cohort (P=0.002 using two-sample binomial proportion test and P=0.039 using interval censoring method), representing an absolute difference of approximately 14%, which we interpret to mean that LND contributed to the development of LEL in 14% of women compared to SLN mapping alone. Patient-reported LEL was also noted in 27 (40.3%; 95% CI, 28.6–52.0%) of the 67 patients in the HYST cohort.
The pre-trial statistical design assumed a two-sided type I error of 5% and power of 94% with an expected sample size of 413 LND and 260 SLN patients in order to detect a 10% absolute difference in the rate of LEL between the LND (20%) and SLN (10%) cohorts. The post-hoc power calculation confirms that the study has 88% power to detect a difference in LEL rate from 27% (SLN cohort) to 41% (LND cohort) in the two arms with n=532 (352 LND+180 SLN) (two-sided Type I error=0.05).
Table 2 describes the association of patient-reported LEL with various factors such as BMI, hypertension, diabetes, and use of external-beam radiation therapy (EBRT). Three patients had congestive heart failure and were not included in our univariate analysis. We did not include FIGO stage, grade or histology, as these were likely to be correlated with the need for additional therapies. Furthermore, at earlier time points, tumor grade and histology would have been correlated with the decision to perform an LND. In addition to LND, increasing BMI and the use of EBRT were also associated with patient-reported LEL on univariate analysis. The distribution of total lymph node counts was skewed to the right, so we performed the log transformation, which resulted in a significant association with patient-reported LEL. Limiting analysis to only the SLN cohort, the median number of nodes removed was 4 (range, 1–14) in those without LEL and 4 (range, 1–21) in those with LEL (P=0.6). The total number of lymph nodes removed was also not associated with the risk of LEL on univariate logistic regression (P=0.3). However, only 8 (4.4%) of the 180 patients in the SLN cohort had more than 10 nodes removed, limiting the interpretation of this specific analysis.
Table 2.
Univariate analysis of the association of various clinicopathologic characteristics with patient-reported lower-extremity lymphedema. OR: odds ratio for developing LEL
| Characteristic | No patient-reported LEL | Patient-reported LEL | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Surgery Cohort | |||||
| LND | 208 (59.1) | 144 (40.9) | 1.85 | 1.25–2.74 | 0.002 |
| SLN | 131 (72.7) | 49 (27.2) | |||
| BMI (kg/m2) | |||||
| One unit increase | - | - | 1.04 | 1.02–1.06 | 0.001 |
| Hypertension | |||||
| Yes | 174 (64.2) | 97 (35.8) | 0.96 | 0.67–1.36 | 0.8 |
| No | 165 (63.2) | 96 (36.8) | |||
| Diabetes | |||||
| Yes | 48 (55.8) | 38 (44.2) | 1.49 | 0.93–2.37 | 0.1 |
| No | 291 (65.2) | 155 (34.8) | |||
| Renal disease | |||||
| Yes | 4 (57.1) | 3 (42.9) | 1.32 | 0.29–5.97 | 0.7 |
| No | 335 (63.8) | 190 (36.2) | |||
| EBRT | |||||
| Yes | 22 (48.9) | 23 (51.1) | 1.95 | 1.06–3.6 | 0.03 |
| No | 317 (65.1) | 170 (34.9) | |||
| Number LNs removed | |||||
| Total LNs | - | - | 1.01 | 0.997–1.03 | 0.1 |
| Log (total LNs)* | - | - | 1.25 | 1.04–1.52 | 0.02 |
% is for the total in row
LEL=lower-extremity lymphedema; SLN=sentinel lymph node mapping cohort; LND=lymphadenectomy cohort; EBRT=external beam radiotherapy (postoperative); LN=lymph nodes
Log transformation also shown as the distribution of lymph nodes removed was skewed
LND retained an independent association with patient-reported LEL compared to SLN after adjusting for BMI and EBRT (Table 3). Increasing BMI was also independently associated with patient-reported LEL. Independent statistical significance was not achieved for the use of EBRT, but the cohort that received EBRT was small. Number of lymph nodes removed was not included in the multivariate model, as it is directly related to whether LND was performed or not. Total and global QOL scores were significantly worse in patients with patient-reported LEL, and these patients had worse scores on all subscales.
Table 3.
Multivariate model assessing independent association with patient-reported lower-extremity lymphedam. OR: odds ratio for developing LEL
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Surgery cohort: LND vs SLN | 1.81 | 1.22–2.69 | 0.003 |
| EBRT: Yes vs No | 1.85 | 0.99–3.46 | 0.05 |
| BMI: one unit increase | 1.04 | 1.02–1.06 | <0.001 |
Total N of cases included in model=532
LEL=lower-extremity lymphedema; SLN=sentinel lymph node mapping cohort; LND=lymphadenectomy cohort; EBRT=external beam radiotherapy (postoperative); BMI, body mass index
Discussion and Conclusions
To our knowledge, there are no other published reports using LEL PRO tools comparing SLN mapping to LND. We are reassured this tool is valid and reflects a true correlation, since we also found an association of patient-reported LEL with both BMI and EBRT. Of note, our 27% LEL prevalence rate in the SLN cohort may seem high; however, age and the associated comorbidities of age are also associated with LEL development. As the median age of our SLN cohort was 61 years, with an upper range of 85 years, a 27% prevalence rate gives further credence to the validity of our LEL PRO instrument.
Our findings are consistent with those of the Mayo Clinic, in which the same LEL PRO tool showed an LEL prevalence rate of 52% in patients who underwent an LND compared with 37% in those who underwent a hysterectomy alone.6 Despite the differences in individual rates between our study and theirs, the absolute difference was similar (14% and 15%, respectively). This may indicate that SLN mapping does not contribute to the development of LEL beyond the hysterectomy itself and/or aging.
Nodal assessment in patients with newly diagnosed endometrial carcinoma is an important aspect of the initial management of these patients. The therapeutic value of comprehensive LND, however, is debatable.2,3 Two randomized trials that showed no survival benefit have been highly criticized for the lack of para-aortic lymphadenectomy, the inclusion of mostly low-risk cases, and inconsistencies or a lack of adjuvant therapy in those with nodal disease. The addition of a para-aortic lymphadenectomy likely would not impact survival considering that the nodal chains do not end at the level of the renal vessels. Those with paraaortic metastases will likely have nodal disease above the renal vessels, and there are no data to support extending lymphadenectomy to the mediastinum and scalenes in endometrial cancer. The comprehensive removal of both clinically and pathologically normal lymph nodes, which is the case in the majority of patients with endometrial carcinoma, is not beneficial. Neither the number of lymph nodes removed nor the performance of a para-aortic lymphadenectomy were predictive of survival in a classification and regression tree (CART) analysis.21 The first branching point, meaning the most important factor, was stage of disease.21
The exclusion of any nodal assessment is also not recommended in our opinion, as this would lead to improper staging and under- or over-treatment, with adjuvant therapy decisions based on patient and uterine features alone. For example, adjuvant chemotherapy has been shown to provide a significant improvement in overall survival in patients with extrauterine disease, including nodal involvement. In a randomized trial, doxorubicin and cisplatin therapy compared with whole abdominal radiation resulted in significantly greater progression-free and overall survival in patients with FIGO stage III or IV endometrial carcinoma.22 The number of lymph nodes removed was not associated with survival outcomes in an ancillary analysis of the study.23 The NCCN guidelines recommend some form of adjuvant therapy for patients with FIGO stage III or IV disease, although the optimal regimen has not been determined.7
SLN mapping has evolved as a viable alternative to comprehensive LND since its introduction in endometrial cancer in 1996.24 The MSK SLN algorithm, which is endorsed by the NCCN, has a false-negative predictive value (FNPV) of 0.5%.25 In short, the algorithm requires the removal of any suspicious nodes, irrespective of dye uptake, as well as a side-specific lymph node dissection in hemi-pelvises that do not map. The FIRES trial demonstrated an FNPV of 0.4% in mapped SLNs in patients with clinical stage I endometrial cancer who underwent SLN mapping followed by an immediate LND.8 In another prospective trial, the FNPV was 1.4% in patients with high-risk endometrial carcinoma.9
Based on our study results and those of others, the benefit of SLN mapping over comprehensive LND lies in the reduction of lymphatic morbidity and subsequent improvement in QOL. The GROINSS V1 study in vulvar cancer reported an LEL rate of 25% in patients who had undergone SLN mapping followed by an inguinofemoral LND compared to only 2% in those who had undergone SLN mapping of the groin alone,26 although LEL diagnoses were based on physician assessment. In a prospective study of 188 patients with endometrial cancer, the incidence of LEL after SLN mapping alone was 1.3% compared with 18.1% after pelvic and para-aortic LND (P=0.0003). Lymphedema diagnoses in the study were based on the assessment of a physiotherapist using the Common Toxicity Criteria (CTC) version 3.0.13 Currently, there are no agreed upon standard guidelines for the diagnosis of LEL, and the use of PRO instruments in this setting is lacking.
The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) is another LEL PRO tool, which was modified from the Lymphedema Breast Cancer Questionnaire (LBCQ). The 20-item GCLQ has acceptable reported sensitivity and specificity (85.7% and 90%, respectively).27 We decided to use the Mayo Clinic LEL PRO tool for our study, because of the reduced patient burden of answering only 13 items as opposed to 20. However, both instruments are acceptable, and it would be interesting to see them assessed in a head-to-head study.
We recognize the limitations of our study. Varying cutoff points among studies may alter baseline rates of LEL. Recall bias is a concern in all studies of this design. Even though we feel that the survey response rate was acceptable, half of the potential respondents did not return the survey, which may impact the generalizability of our findings. We could only assess prevalence rates at the time patients received the questionnaires, and the time since surgery varied. We cannot assess the incidence rates over time as this was not a prospective study and the exact timing of LEL development is unknown. We would ideally like to conduct a study in a cohort of patients who present with newly diagnosed endometrial cancer and assess patient-reported LEL and QOL before surgery and then at timed intervals for some years after surgery in order to better capture the timing of LEL after surgery. We also recognize that the median time since surgery was different between the SLN and LND cohorts, which may impact the rate of patient-reported LEL, especially as patients continue to age. The minimum time from surgery was 44 months in both cohorts, which seems to be a reasonable amount of time to assess for the possible development of surgery-related, patient-reported LEL.
The noted range of 1–21 lymph nodes removed in the SLN cohort is due to multiple reasons. One of the reasons is related to the learning curve of surgeons as they adopted SLN mapping. Surgeons tend to remove more “SLNs” early on in their experience, and the number removed decreases with increased experience and understanding of true SLN mapping. Also, there may be a few nodes within a packet that are removed as the “SLN”. The other reasons are related to the use of our algorithm, which includes the removal of any “suspicious” nodes irrespective of mapping, performance of a para-aortic LND at the surgeon’s discretion, and the performance of a unilateral LND in cases with an unmapped hemi-pelvis. The number of cases with true unilateral LND of unmapped hemi-pelvis was low, limiting any meaningful analysis comparing those with only SLN mapping to those with unilateral LND. Additionally, the PRO LEL questionnaire cannot differentiate laterality of LEL.
Our results demonstrate that SLN mapping over LND is independently associated with a significantly lower prevalence of patient-reported LEL in patients who have undergone surgery for endometrial carcinoma. Our data also may inform discussions regarding the risks and benefits of adjuvant radiation therapy. These data provide additional support for SLN mapping in women with endometrial carcinoma. SLN mapping provides accurate surgical staging, as well as decreased morbidity and improved QOL.
Supplementary Material
Table 4.
EORTC QLQ-C30 and -EN24 scores between patients with and without patient-reported lower-extremity lymphedema
| No patient-reported LEL | Patient-reported LEL | P value† | |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| Overall Score | |||
| QLQ Total Score | 94.9/91.8 (27.6–100) | 84.7/79 (19.6–100) | <0.001 |
| Global QOL | 83.3/83.6 (0–100) | 66.7/66.8 (0–100) | <0.001 |
| Functional Scales | |||
| Physical functioning | 100/90.4 (0–100) | 86.7/75.8 (0–100) | <0.001 |
| Role functioning | 100/95 (0–100) | 83.3/80 (0–100) | <0.001 |
| Emotional functioning | 91.7/86.3 (0–100) | 75/73.2 (0–100) | <0.001 |
| Cognitive functioning | 100/89.4 (16.7–100) | 83.3/77.4 (0–100) | <0.001 |
| Social functioning | 100/93.8 (0–100) | 83.3/77.3 (0–100) | <0.001 |
| Symptom Scales | |||
| Fatigue | 0/12.9 (0–100) | 22.3/31.7 (0–100) | <0.001 |
| Nausea and vomiting | 0/2.3 (0–100) | 0/7.3 (0–100) | <0.001 |
| Pain | 0/7.7 (0–100) | 16.7/28.5 (0–100) | <0.001 |
| Dyspnea | 0/6.4 (0–100) | 0/17.5 (0–100) | <0.001 |
| Insomnia | 0/16.1 (0–100) | 33.3/31.1 (0–100) | <0.001 |
| Appetite loss | 0/3.3 (0–100) | 0/9.9 (0–100) | <0.001 |
| Constipation | 0/7.8 (0–100) | 0/17.8 (0–100) | <0.001 |
| Diarrhea | 0/5.4 (0–100) | 0/15.9 (0–100) | <0.001 |
| Financial difficulties | 0/5 (0–100) | 0/19.5 (0–100) | <0.001 |
| EORTC QLQ-EN24 | |||
| Functional Scales | |||
| Sexual interest* | 33.3/23.3 (0–100) | 0/19.5 (0–100) | 0.035 |
| Sexual activity** | 0/19.5 (0–100) | 0/12.7 (0–100) | 0.01 |
| Sexual enjoyment*** | 33.3/49.2 (0–100) | 33.3/33.3 (0–100) | <0.001 |
| Symptom Scales | |||
| Lymphedema | 0/3 (0–100) | 33.3/38.3 (0–100) | <0.001 |
| Urologic symptoms | 8.3/15.1 (0–75) | 25/29.9 (0–100) | <0.001 |
| Gastrointestinal symptoms | 6.7/7.8 (0–100) | 13.3/20.7 (0–86.7) | <0.001 |
| Poor body image | 0/9.1 (0–100) | 16.7/25.6 (0–100) | <0.001 |
| Sexual/vaginal problems**** | 22.2/35.3 (0–100) | 44.4/48.5 (0–100) | 0.019 |
| Pain in back and pelvis | 0/14.4 (0–100) | 33.3/36.4 (0–100) | <0.001 |
| Tingling/numbness | 0/17.8 (0–100) | 33.3/38.6 (0–100) | <0.001 |
| Muscular pain | 0/21.4 (0–100) | 33.3/43.2 (0–100) | <0.001 |
| Hair loss | 0/12.4 (0–100) | 0/25 (0–100) | <0.001 |
| Taste change | 0/4.4 (0–100) | 0/10.1 (0–100) | <0.011 |
LEL=lower-extremity lymphedema; QOL=quality of life
Data are reported as Median/Mean (range)
P-value obtained using Wilcoxon Rank Sum Test and all except “sexual interest” and “sexual activity” remain significant using Bonferroni correction for multiple comparisons adjustment
Data missing from 56
Data missing from 62
Data missing from 321
Data missing from 317
Research Highlights.
SLN mapping was independently associated with significantly lower rate of patient-reported lower extremity lymphedema (LEL)
Increasing BMI and use of adjuvant EBRT were associated with an increased prevalence of patient-reported LEL
SLN mapping in the surgical management of newly diagnosed endometrial cancer may spare these patients from LEL
Survival outcomes were similar between SLN mapping and comprehensive lymphadenectomy after endometrial cancer surgery
Funding
This study was supported in part by the National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748 (Drs. Leitao, Iasonos, Baser, Chi, Long Roche, Sonoda, Brown, Mueller, Gardner, Jewell, Broach, Zivanovic, and Abu-Rustum).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Outside the submitted work, Dr. Abu-Rustum reports grants from Stryker/Novadaq, Olympus, and GRAIL. Outside the submitted work, Dr. Leitao is an ad hoc speaker for Intuitive Surgical, Inc. Outside the submitted work, Dr. Chi reports personal fees from Bovie Medical Co., Verthermia Inc. (now Apyx Medical Corp.), C Surgeries, and Biom ‘Up, as well as other from Intuitive Surgical, Inc. and TransEnterix, Inc. Outside the submitted work, Dr. Jewell reports personal fees from Covidien/Medtronic. The other authors have no potential conflicts to disclose.
Presented at: Society of Gynecologic Oncology 49th Annual Meeting on Women’s Cancer 2018, New Orleans, LA
References
- 1.SGO Clinical practice Endometrial Cancer Working Group. Burke WM, Orr J, Leitao M, et al. ; Society of Gynecologic Oncology Clinical Practive Committee. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol. 2014;134:385–392. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. [DOI] [PubMed] [Google Scholar]
- 3.ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized trial. Lancet. 2009;373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol. 2006;103:714–718. [DOI] [PubMed] [Google Scholar]
- 5.Yost KJ, Cheville AL, Weaver AL, Al Hilli M, Dowdy SC. Development and validation of a self report lower-extremity lymphedema screening questionnaire in women. Phys Ther. 2013;93:694–703. [DOI] [PubMed] [Google Scholar]
- 6.Yost KJ, Cheville AL, Weaver AL, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Uterine Neoplasms (version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed May 21, 2019.
- 8.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicenter, prospective, cohort study. Lancet Oncol. 2017;18:384–392. [DOI] [PubMed] [Google Scholar]
- 9.Soliman PT, Westin SN, Dioun S, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol. 2017;146:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson AGZ, Ducie J, Ali N, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol Oncol. 2016;140:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlappe BA, Weaver AL, Ducie JA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: a sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol Oncol. 2018;151:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levenback CF, Ali S, Coleman RF, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol. 2012;30:3786–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geppert B, Lonnerfors C, Bollino M, Persson J. Sentinel lymph node biopsy in endometrial cancer – feasibility, safety and lymphatic complications. Gynecol Oncol. 2018;148:491–498. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 15.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Published by: European Organisation for Research and Treatment of Cancer, Brussels: 2001. [Google Scholar]
- 16.Greimel E, Nordin A, Lanceley A, et al. Psychometric validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Endometrial Cancer Module (EORTC QLQ-EN24). Eur J Cancer. 2011;47:183–190. [DOI] [PubMed] [Google Scholar]
- 17.Amaya A, Leclere F, Carris K, Liao Y. An evaluation of primary data-collection modes in an address-based sampling design. Public Opin Q. 2015;79:420–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montaquila JM, Brick JM, Williams D, Kim K, Han D. A study of two-phase mail survey data collection methods. J Survey Stats Methodol. 2013;1:66–87. [Google Scholar]
- 19.Zhang Z, Sun J. Interval censoring. Stat Methods Med Res. 2010:19:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88. [DOI] [PubMed] [Google Scholar]
- 21.Barlin JN, Zhou Q, St. Clair CM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: seeing the forest for the trees. Gynecol Oncol. 2013;130:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36–44. [DOI] [PubMed] [Google Scholar]
- 23.Tewari KS, Filiaci VL, Spirtos NM, et al. Association of number of positive nodes and cervical stroma invasion with outcome of advanced endometrial cancer treated with chemotherapy or whole abdominal irradiation: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:87–93. [DOI] [PubMed] [Google Scholar]
- 24.Burke TW, Levenback C, Tornos C, Morris M, Wharton JT, Gershenson DM. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study. Gynecol Oncol. 1996;62:169–173. [DOI] [PubMed] [Google Scholar]
- 25.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. [DOI] [PubMed] [Google Scholar]
- 26.Van der Zee AGJ, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. [DOI] [PubMed] [Google Scholar]
- 27.Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol. 2010;117:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.