Abstract
The Zika endemic established by imported and local transmission is of significant concern and effective anti-ZIKV drugs remain an urgent unmet need. As andrographolide was identified to be an inhibitor of DENV and CHIKV and the importance of quinoline structure against infectious diseases was considered, we are interested in studying its andrographolide derivatives with quinoline moiety against Zika virus infection. In addition to screening eight in-house derivatives of andrographolide, sixteen new derivatives were designed, synthesized and tested against Zika virus infection. Among these compounds, two most potent anti-Zika compounds of 19-acetylated 14α-(5’,7’-dichloro-8’-quinolyloxy) derivative 17b and 14β-(8’-quinolyloxy)-3,19-diol derivative 3 with the highest selectivity were discovered. The SAR analysis indicates that rational and optimal combined modification/s at 3-, 14-, or 19-positions can make derivatives less toxic and more potent against Zika infection, and both of 3 and 17b are suitable as leads for designing new generation of andrographolide derivatives with quinoline or its structure- and property-related moieties against Zika virus and other arboviruses.
Keywords: Zika virus, antiviral, andrographolide and derivative, quinolyloxy, 14α-(8’-quinolyloxy)
Graphical Abstract
1. Introduction
Zika virus (ZIKV), one of arboviruses and belonging to Flaviviridae family, first emerged more than 70 years ago [1] but ZIKV infection at first was not considered as a threatening infectious disease, instead it was originally taken to be a mild self-resolving infection and frequently in that era misdiagnosed and treated as dengue virus (DENV) and chikungunya virus (CHIKV) infection due to clinical similarity and serological cross-reactivity with closely related viruses [2–5]. ZIKV, like other related arboviruses, has a monopartite, linear, positive single-stranded RNA genome with about 10794 nucleotides encoding 3419 amino acids [6] as a single polyprotein with multiple transmembrane domains that is cleaved into structural and non-structural proteins (NS) by both host and viral proteases. ZIKV is mainly spread via the bite of mosquitoes of the Aedes type, can also be sexually transmitted and potentially spread by blood transfusions, and infections in pregnant women can spread to the baby [7–9]. World Health Organization (WHO) declared a Public Health Emergency of International Concern in February 2016 [10] for the recent epidemic in the Americas in 2016 and at last in 84 countries as of March 2017. It is now recognized that ZIKV can cause significant neurological defects in both neonates as pediatric microcephaly[11–12] and adults as Guillain–Barré syndrome [13], which make ZIKV quite different from other arboviruses. So far, great efforts have been input in research of prevention and therapy development, but currently there is still no approved vaccine or specific antiviral against ZIKV [13–16], while clinical treatment of ZIKV-infected patients consists of only supportive care while the therapeutic approach to infected pregnant women is limited to the use of antipyretics and analgesics [17]. Avoiding mosquito bites is the best way to cut off the primary spreading of ZIKV and to prevent ZIKV infection. However, the international air travel is increased in the contemporary world by which a virus could be imported from one country into other countries in the tropical and subtropical regions [18]. Thus, the endemic established by imported and local transmission is of significant concern and mosquito transmitted diseases remain an ongoing global threat [19–21]. To search for effective antivirals to ZIKV is very urgent and of great medical significance.
Andrographis paniculata [Burm. F.] Nees is an herb known as a “natural antibiotic”. It and its compositions are commonly used in China, India and Southeast Asia for the treatment of a large variety of illnesses by especially reducing inflammation and “heat-clearing and detoxifying” defined in Chinese medicines [22–27]. Andrographolide (1, Fig. 1) is a bicyclic diterpenoid lactone and one of major components isolated from Andrographis paniculata. Importantly, some active derivatives of andrographolide of “Chuanhuning”, “Yanhuning”, and “Lianbizhi” have been used in China to treat bacterial and viral infections for many years [28–31]. In addition, andrographolide was revealed to possess many activities against microbial infection [28, 32–34], e.g. Candida albicans [35], Pseudomonas aeruginosa [36–37], Enterococcus faecali [38], HIV [39], herpes simplex virus type 1 [40], HBV [41], HCV [42–43], influenza virus [44–45]. Recently, andrographolide was reported to be an anti-viral agent against DENV [46–47] and (CHIKV) [48] but does not have a direct virucidal activity [46–47]. Attracted by its pharmacological activity, numerous andrographolide derivatives have been reported from time to time to improve its physiochemical properties and pharmaceutical features [28, 49–53]. We previously discovered that some 14-aryloxy analogues of andrographolide work as immunosuppressant agents [54] and antibacterial with immunosuppressant activity [38]. In this study, we pursued potent anti-Zika virus agents derived from the “natural antibiotic” lead of andrographolide, and envisioned that modification of 14-substituents can reduce the cytotoxicities of analogues and make some modified 14-aryloxy analogues be active against ZIKV infection.
Fig. 1.
Structures of andrographolide (1) and its previously reported derivatives (2 to 9) [38] from our in-house library.
2, Results and discussion
2.1. Design
As andrographolide can work as an inhibitor of DENV and CHIKV [46–48] and the importance of quinoline moiety against infectious diseases [38, 55–57] was also considered, we were interested in testing anti-Zika activity of eight derivatives of andrographolide from our in-house library with 14-quinolinyloxy group and related 14-pyridinyloxy group (Fig. 1), which were previously discovered as antibacterial agents [38]. It was discovered in this study one active anti-Zika 14-(8’-quinolyloxy) compound 3 (EC50 value is 1.3 μM) but its cytotoxicity (CC50 values are 22.7 μM in SNB-19 cell line and 20.9 μM in Vero cell line) is relatively high. In order to improve the antiviral activity and reduce the toxicity, modification of 14-(8’-quinolyloxy) group was explored that sixteen modified derivatives by the introduction of methyl group at 2-position or chloro group at 5,7-positions into 8-quinolinyloxy moiety were subsequently designed, synthesized and some more selective anti-Zika compounds were discovered.
2.2. Synthesis of titled compounds
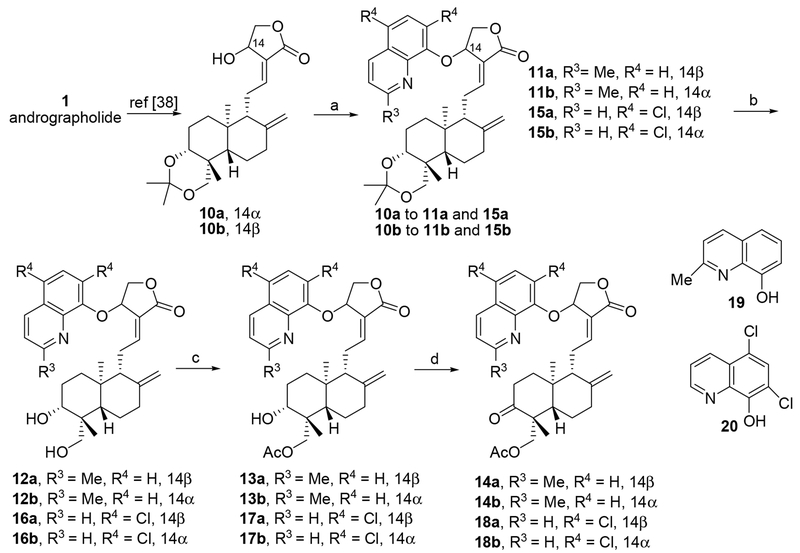
Compounds of 2 to 9 (Fig. 1) were synthesized previously from 10a and 10b [38]. Synthesis of sixteen new compounds of 11a to 18a and 11b to 18b was conducted according to the previously reported synthetic method [38], which started from 10a (14α-OH) and 10b (14β-OH), respectively, as shown in Scheme 1. Briefly, Mitsunobu reactions of 10a or 10b with 2-methyl-8-quinolinol (19) (Scheme 1) or 5,7-dichloro-8-quinolinol (20) (Scheme 1) were carried out in general from 0 °C to room temperature in anhydrous THF to afford corresponding 14β-(2’-methyl-quinolyl-8’-oxy) andrographolide 11a and 14β(5’,7’-dichloro-quinolyl-8’-oxy) andrographolide 15a, or 14α-(2’-methyl-quinolyl-8’-oxy) andrographolide 11b and 14a-(5’,7’-dichloro-quinolyl-8’-oxy) andrographolide 15b, respectively, in mild to moderate isolated yields. Deprotection of 3,19-acetonylidene from 11a, 11b, 15a, and 15b afforded the corresponding 3,19-diols of 12a, 12b, 16a, and 16b. Furthermore, mono-acetylation at 19-positions of 12a, 12b, 16a, and 16b produced corresponding 13a, 13b, 17a, and 17b, which were subsequently oxidized into 3-ketones of 14a, 14b, 18a, and 18b by Dess-Martin periodinane (DMP).
Scheme 1.
Reagents and conditions: (a) anhydrous THF, 10a or 10b (1.0 eq.), 19 or 20 (1.5 eq.), DIAD (1.5 eq.), PPh3 (1.5 eq.), 0 °C to room temperature; (b) MeOH/H2O (4/1), TsOH·H2O, 20 °C; (c) AcCl, TEA, 0 °C; (d) DCM, Dess–Martin periodinane, rt.
2.3. Anti-Zika activity and SAR analysis
The inhibitory activity against Zika virus infection was conducted by a ZIKV titer assay (in Vero cells for 48 h) [58–59] (Supplementary data) to measure ZIKV production in compound-treated human glioblastoma cells (SNB-19 cell line) (incubation for 24 h) infected by PRVABC59 strain at multiplicity of infection (MOI) of 1. Niclosamide, one of our previously identified active compounds against Zika virus infection [58–59], was used as a positive and quality control in each antiviral assay. The cytotoxicity of each compound in this study was assessed to SNB-19 cells for 24 h and Vero cells for 48 h corresponding to the incubation time in anti-Zika screening and listed in Table S1 (Supplementary data).
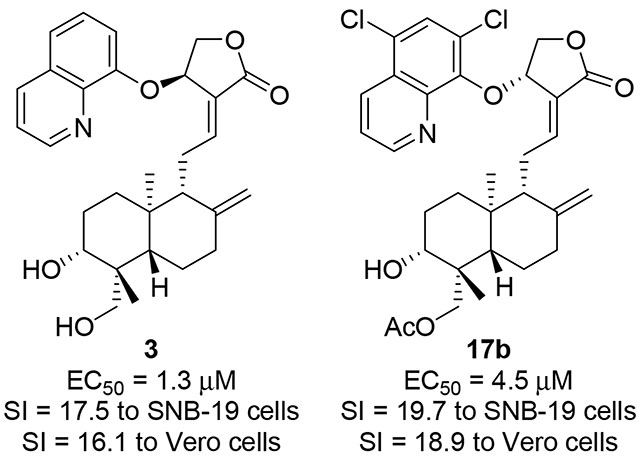
In our screening streamline, eight previously reported 14-aryloxy andrographolide derivatives (Fig. 1) of four 8’-quinolyloxy compounds of 2 to 5, two 4’-quinolyloxy compounds of 6 and 7, and two 2’-nitro-pyridinyl compounds of 8 and 9 were first tested. The results of active derivatives of 3, 7 and 9 are listed in Fig. 2 and all results were included in Table S1. As shown in Fig. 2 and Table S1, compounds of 2 to 9 are relatively cytotoxic and CC50 values of 8’-quinolyloxy derivatives (2 to 5) are around 20 μM. It was found that 14β-(8’-quinolyloxy) 3,19-diol analog 3 is very effective against Zika virus infection with EC50 value of 1.3 μM and its safety/selectivity index (SI) >16 (CC50 values of 22.7 and 20.9 μM in SNB-19 and Vero cell lines, respectively). However, the antiviral activity and cytotoxicity of 14β-(8’-quinolyloxy) 3,19-acetonylidene compound 2 are similar in values (CC50 = 21.1 μM in SNB-19 cells and 22.0 μM in Vero cells but EC50 = 25.9 μM), and 14β-8’-quinolyloxy 19-acetyl compounds of 3-alcohol 4 and 3-keto 5 are not active. These results imply that more polar 14β-(8’-quinolyloxy) anadrographolide derivative 3 is more active and selective against Zika virus infection than its more hydrophobic derivatives of 2, 4 and 5. Among two 14-(4’-quinolyloxy)-3,19-diol andrographolide derivatives 6 and 7, only does 14α-isomer of 7 show mild anti-Zika activity (EC50 = 11.0 μM, CC50 = 60.1 μM in SNB-19 cells and CC50 = 22.3 μM in Vero cells) but 14β-isomer of 6 is inactive. Anti-Zika activity difference between two 3,19-diol andrographolide derivatives of 3 bearing 14β-(8’-quinolyloxy) and 6 bearing 14β-(4’-quinolyloxy) suggests here that 14-(8’-quinolyloxy) pharmacophore is superior to 14-(4’-quinolyloxy) pharmacophore. Meanwhile, 14α-(2’-nitro-pyridinyl-3’-oxy)-3,19-diol andrographolide derivative 9 was discovered to be a mild anti-Zika agent (EC50 = 12.5 μM, CC50 = 67.7 μM in SNB-19 cells and CC50 = 37.7 μM in Vero cells) while 14α-(2’-nitro-pyridinyl-3’-oxy)-3,19-acetonylidene andrographolide (8) is not active. From the current data for eight compounds of 2 to 9 (Figs. 1 and 2), it is concluded that both of 14α- and 14β-isomers, and modifications at 3-, 19-, or 3,19-positions could contribute to the anti-Zika activity. 14β-(8’-quinolyloxy) andrographolide compound 3 is the most potent antiviral derivative among these compounds and its SI values are 17.5 to SNB-19 cell line and 16.1 to Vero cell line, respectively, but its cytotoxicity is not ideal.
Fig. 2.
Anti-Zika active derivatives of andrographolide. Compounds of 3 and 17b (in red rectangular boxes) are the most active and selective compounds. (SNB-19: SNB-19 cell line; Vero: Vero cell line)
In pursuit of improving the antiviral activity and reduce the cytotoxicity of lead compound 3, the first exploration is to modify 14-(8’-quinolyloxy) group. Considering that the cytotoxicity of 3 may derive from 1’-N and 8’-O of 14-(8’-quinolyloxy) group, it is envisaged that introduction of steric hindrance or electrostatic group/s at 2’-position or 7’-position could block or restrict some side interactions with 1’-N or 8’-O to reduce the cytotoxicity and possibly improve anti-Zika activity. Based on the available starting materials of 2-methyl-8-quinolinol (19, Scheme 1) and 5,7-dichloro-8-quinolinol (20, Scheme 1), eight 14-(2’-methyl 8’-quinolyloxy) derivatives (four 14β-siomers of 11a to 14a and four 14α-siomers of 11b to 14b) and eight 14-(5’,7’-dichloro 8’-quinolyloxy) derivatives (four 14β-siomers of 15a to 18a and four 14α-siomers of 15b to 18b) were designed, synthesized and assayed against Zika virus infection.
With compounds in hand, 2’-methyl-8’-quinolinol derivatives were first assayed and the results are shown in Fig. 2 and Table S1. As expected, eight 14-(2’-methyl 8’-quinolyloxy) andrographolide derivatives are generally less cytotoxic than 14-(8’-quinolyloxy) andrographolide derivatives. Both 11a (14β) and 11b (14α) exhibit lower cytotoxicities (CC50 values are 78.4 and 66.5 μM in SNB-19 cell line, 55.9 and 65.3 μM in Vero cell line, respectively) than compound 3 but their anti-Zika activities (EC50 values are 8.5 and 16.6 μM, respectively) and SIs (9.2 and 6.6 to SNB-19 cell line, 4.0 and 3.9 to Vero cell line, respectively) are much lower than those of 3. Compound 12a (14β) is much less active (EC50 = 25.8 μM) against Zika virus infection with lower cytotoxicities (CC50 > 100 μM to SNB-19 cells or 99.8 μM to Vero cells) than compounds of 3, 11a and 11b; whereas, compounds of 13a (14β), 14a (14β), and 12b (14α) to 14b (14α) are not active against Zika virus infection. The above results indicated that 14-(2’-methyl-8’-quinolyloxy) group can reduce the cytotoxicity but does not improve the antiviral activity; modifications at 3- or 19-position affect the cytotoxicity and antiviral activity. Compound 11a possesses the most potent anti-Zika activity and the highest SI values among (2’-methyl-8’-quinolyloxy) derivatives. In addition, inactive compounds of 2’-methyl-8’-quinolinol derivatives are also less cytotoxic (Table S1) than 8’-quinolyloxy compounds (2 to 5). Overall, introduction of 2’-methyl to 14-(8’-quinolyloxy) reduces the cytotoxicity but also obviously decreases the anti-Zika activity.
Then, 5’,7’-dichloro-8’-quinolinol derivatives were assayed (Fig. 2 and Table S1). As shown in Table S1, all of them possess relatively less cytotoxicities than 8’-quinolyloxy compounds (2 to 5). Two 3,19-acetonylidene-protected 5’,7’-dichloro-8’-quinolinol andrographolide derivatives 15a (14β) and 15b (14α) do not show anti-Zika activity even though their corresponding 3,19-acetonylidene-protected 2’-methyl-8’-quinolinol derivatives of 11a and 11b are active. Whereas, both of 3,19-diols of 16a (14β) and 16b (14α) are active against Zika virus infection that 16a (EC50 = 13.3 μM) is less active than 16b (EC50 = 7.8 μM) but 16a (CC50 values are higher than 100 μM in both cell lines) is much less toxic than 16b (CC50s = 85.2 and 58.6 μM in SNB-19 and Vero cell lines, respectively), indicating that the stereochemistry of 14α and 14β affects the inhibitory activity and also the cytotoxicity. 19-Acetylated 14β-derivative 17a is not active but 19-acetylated 14α-derivative 17b is the second most potent anti-Zika agent (SIs are 19.7 to SNB-19 cell line and 18.9 to Vero cell line, respectively) with EC50 value of 4.5 μM and CC50 values of 88.7 μM in SNB-19 cells and 85.0 μM in Vero cells. In contrast, 19-acetylated 3-keto-14β-derivative 18a is a weak inhibitor to Zika virus infection (EC50 = 24.6 μM, CC50s = 72.0 and 57.4 μM in SNB-19 and Vero cell lines, respectively) while 19-acetylated 3-keto-14α-derivative 18b is not active against Zika virus infection.
As a whole, anti-Zika activity of 14α-isomers of 16b, 17b and 18b or 14β-isomers of 16a, 17a and 18a varies with distinct modifications at 3-position or/and 19-position and the lipophilicity formed by these modifications (the upward order of lipophilicity from 3,19-diol, 3-alcohol with 19-actylation, to 3,19-acetonylidene or 3-keto with 19-acetlation). 3,19-Diol 16b is active against Zika virus infection and 19-acetylation makes 17b (3-OH-19-OAc) be more active against Zika virus and less toxic to SNB-19 and Vero cell lines than 16b; meanwhile 3-keto-19-acetylated product 18b is not active. These observations suggest that 3-alcohol with 19-acetylation and 14α in 17b are slightly synergistic to the antiviral activity, which may be partly derived from the increase of lipophilicity compared to 16b; however, too hydrophobic property of 18b with 19-acetlation and 3-keto makes 18b lost antiviral activity compared to 16b and 17b. On the other hand, 3,19-diol 16a is active against Zika virus infection with much less cytotoxicities to SNB-19 and Vero cell lines but 19-acetylated 17a (3-OH-19-OAc) becomes inactive and 3-keto-19-acetylated product 18a regains part of anti-Zika activity and the cytotoxicity is slightly higher than 16a. These results suggest that 3-alcohol, 19-acetylation and 14β in 17a are not a good combination for the antiviral activity while the combination of 14β, 19-acetylation and 3-keto in 18a exhibits better antiviral activity than that of 19-acetylation and 14β in 17a but less than that of 3,19-diol and 14β in 16a. These combined data indicate that modification of 14-(8’-quinolyloxy) by 5’,7’-dichloro reduces the cytotoxicity and some derivatives (e.g. 16b and 17b) possess comparable anti-Zika activity to 3.
Collectively, 14-(2’-methyl 8’-quinolyloxy) and 14-(5’,7’-dicloro 8’-quinolyloxy) derivatives are less cytotoxic than 14-(8’-quinolyloxy) derivatives; and in general, 14-(5’,7’-dichloro-8’-quinolyloxy) derivatives are more potent against Zika infection than 14-(2’-methyl-8’-quinolyloxy) derivatives. Two configurations of 14α and 14β could make distinct contribution to the anti-Zika activity, and optimal combination of modifications at 2’- or 5’,7’-positions of 14-(8’-quinolyloxy) and 3- or/and 19-positions improves the antiviral activity and the selectivity. Altogether, current results at least partially imply that optimal lipophilicity (hydrophobicity) by ideal combination of modifications is important for the action of a drug. It is noted that compound 17b possesses comparable anti-Zika activity to and less toxicity than compound 3.
3. Conclusions
In summary, eight previously reported and sixteen new synthesized derivatives of andrographolide were tested for anti-Zika virus activity. As a result, andrographolide scaffold mounted with quinoline moiety is a potential anti-Zika strategy. By combining these data, we found that rational/optimal modification/s at 3-, 14-, or 19-positions increase derivative selectivity via lowering cell toxicity and increasing antiviral potency. In general, andrographolide derivatives of 14-(2’-methyl 8’-quinolyloxy) and 14-(5’,7’-dicloro 8’-quinolyloxy) are relatively less cytotoxic than those of 14-(8’-quinolyloxy) and interestingly, 5’,7’-dichloro derivatives are more effective than 2’-methyl derivatives against Zika infection from the current data in this study. It is noteworthy that with their distinctive pharmacological property including rational lipophilicity (hydrophobicity), 19-acetylated 14α-(5’7’-dichloro-8’-quinolyloxy) derivative 17b and 14β-(8’-quinolyloxy)-3,19-diol derivative 3 are the most potent compounds against Zika virus infection and possess the similar SI values but the cytotoxicity of 17b is less than that of 3. Therefore, both of 3 and 17b are suitably taken as leads to design new generation of andrographolide derivatives against Zika virus and other arboviruses (for example, DENV, CHIKV, West Nile virus (WNV) and Japanese encephalitis virus (JEV)), we can envisage that it is promising to discover more potent and less toxic andrographolide derivatives with quinoline or its structure- and property-related moieties to effectively treat the infection by Zika virus and other arboviruses.
4. Experimental
4.1. Chemistry
1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer at 400 and 100 MHz, respectively, in CDCl3, CD3OD, (CD3)2SO and C6D6 as indicated. Coupling constants (J) are expressed in hertz (Hz). Chemical shifts (δ) of NMR are reported in parts per million (ppm) units relative to the solvent. The high resolution of ESI-MS was recorded on an Applied Biosystems Q-STAR Elite ESI-LC-MS/MS mass spectrometer, respectively. Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. Anhydrous tetrahydrofuran (THF) was distilled from sodium-treated THF under nitrogen atmosphere and anhydrous dichloromethane (DCM) was distilled from calcium hydride-treated DCM under nitrogen atmosphere. Melting points were measured using an YRT-3 melting point apparatus (Shanghai, China) and were uncorrected. HPLC analysis condition: Sunfire C18 column (4.6 × 250 mm, 5 μm), elution by 90% methanol with 10% purified water (for compounds of 2, 3, 5, 8, 11a, 11b, 14a, 14b, 15a, 15b, 18a, 18b) or 80% methanol with 20% purified water (for compounds of 4, 6, 7, 9, 12a, 12b, 13a, 13b, 16a, 16b, 17a, 17b), rate = 1.0 ml/min, detection wavelength of 230 nm. The purity of all compounds by HPLC analysis is not lower than 95% (Supplementary data).
4.1.1. Preparation of 14β-isomers of 11a and 15a from 10a (14α), and 14α-isomers of 11b and 15b from 10b (14β).
The synthesis is conducted according to ref [38].
(14β)-(Quinolyl-2’-methyl-8’-oxy)-3,19-acetonylidene andrographolide (11a): white solid; m.p. 146.1–148.4 °C; 75% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.26 (d, J = 8.4 Hz, 1H, 4’-H), 7.57 (dd, J = 8.2, 1.2 Hz, 1H, 5’-H), 7.51 – 7.43 (m, 2H, 6’-H and 7’-H), 7.24 – 7.11 (m, 2H, 3’-H and 12’-H), 6.05 (d, J = 5.4 Hz, 1H ,14-H), 4.87 (s, 1H ,17-H), 4.81 (m, 1H, 15-H), 4.69 (s, 1H ,17-H), 4.46 (m, 1H, 15-H), 3.67 (d, J = 11.6 Hz, 1H, 19-H), 2.94 (d, J = 11.6 Hz, 1H, 19-H), 2.67 (s, 3H, 2’-CH3), 2.65 – 2.61 (m, 1H, 3-H), 2.39 – 2.24 (m, 3H, 7-CH2 and 11-H), 2.16 (d, J = 10.2 Hz, 1H, 6-H), 2.00 (m, 1H, 9-H), 1.55 (d, J = 12.7 Hz, 1H, 11-H), 1.28 (m, 1H, 6-H), 1.19 (s, 3H, acetonylidene–CH3), 1.14 (m, 5H, acetonylidene-CH3, 2-H and 1-H), 0.78 (s, 3H, 20-CH3), 0.75 (s, 1H, 2-H), 0.66 (s, 3H, 18-CH3), 0.61 (m, 2H, 1-H, 5-H); 13C NMR (101 MHz, DMSO-d6) δ 169.6 (16-C), 157.8 (8’-C), 151.8 (2’-C), 150.18 (12-C), 148.0 (8-C), 139.6 (9’-C), 136.4 (4’-C), 127.9 (13-C), 126.7 (10’-C), 125.9 (6’-C), 122.9 (3’-C), 121.0 (5’-C), 111.8 (acetonylidene-C), 108.1 (17-C), 98.3 (7’-C), 75.4 (3-C), 71.7 (15-C), 71.5 (14-C), 62.8 (19-C), 56.1 (9-C), 50.5 (5-C), 38.2 (4-C), 37.1 (10-C), 37.1 (7-C), 33.0 (1-C), 27.2 (2-C), 25.31 (6-C), 25.26 (2C, acetonylidene-2C), 25.2 (quinolyl-2’-CH3), 24.1 (11-C), 22.8 (20-C), 15.7 (18-C); ESI-HRMS: m/z found 532.3060, [M+H]+, calcd for C33H42NO5, 532.3063.
(14α)-(Quinolyl-2’-methyl-8’-oxy)-3,19-acetonylidene andrographolide (11b): white solid; m.p. 105.1-107.7 °C; 78% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.24 (d, J = 8.4 Hz, 1H, 4’-H), 7.61 (dd, J = 8.2, 1.2 Hz, 1H, 5’-H), 7.44 (m, 2H, 6’-H and 7’-H), 7.18 (dd, J = 7.7, 1.2 Hz, 1H, 3’-H), 6.98 – 6.73 (m, 1H, 12-H), 6.04 (d, J = 5.5 Hz, 1H, 14H), 4.80 (s, 1H, 17-H), 4.76 (m, 1H, 15-H), 4.58 (s, 1H, 17-H), 4.53 (m, 1H, 15-H), 3.74 (d, J = 11.8 Hz, 1H, 19-H), 3.23 (d, J = 13.4 Hz, 1H, 19-H), 3.01 (d, J = 11.6 Hz, 1H, 3-H), 2.65 (s, 3H, 2’-CH3), 2.26 (d, J = 12.5 Hz, 1H, 7-H), 2.20 (m, 2H, 7-H and 11-H), 1.90 – 1.80 (m, 2H, 6-H and 9-H), 1.76 – 1.64 (m, 1H, 11-H), 1.57 (d, J = 10.7 Hz, 1H, 6-H), 1.45 (m, 1H, 2-H), 1.25 (s, 3H, acetonylidene-CH3), 1.20 (s, 3H, acetonylidene-CH3), 1.17 – 1.06 (m, 3H, 2-H and 1-CH2), 1.03 (s, 3H, 20-CH3), 0.97 (m, 1H, 5-H), 0.53 (s, 3H, 20-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.7 (16-C), 158.0 (8’-C), 151.9 (2’-C), 149.9 (12-C), 147.5 (8-C), 140.3 (9’-C), 136.5 (4’-C), 127.9 (13-C), 125.9 (10’-C), 125.7 (6’-C), 122.8 (3-C’), 122.3 (5’-C), 115.7 (acetonylidene-C), 109.0 (17-C), 98.2 (7’-C), 76.1 (3-C), 73.7 (15-C), 71.7 (4-C), 62.8 (19-C), 54.8 (9-C), 51.6 (5-C), 37.9 (4-C), 37.1 (10-C), 37.1 (7-C), 33.9 (1-C), 27.8 (2-C), 25.9 (6-C), 25.4 (acetonylidene-CH3), 25.2 (acetonylidene-CH3), 25.0 (quinolyl-2’-CH3), 24.9 (11-C), 22.8 (20-C), 15.4 (18-C); ESI-HRMS: m/z found 532.3052, [M+H]+, calcd for C33H42NO5, 532.3063.
(14β)-(Quinolyl-5’,7’-dichloro-8’-oxy)-3,19-acetonylidene andrographolide (15a): white solid; m.p. 80.5-82.5 °C; 73% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.12 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.63 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.05 (s, 1H, 6’-H), 7.84 (dd, J = 8.6, 4.2 Hz, 1H, 3’-H), 6.71 – 6.51 (m, 1H, 12-H), 6.44 (d, J = 4.5 Hz, 1H, 14-H), 4.75 – 4.66 (m, 2H, 17-H and 15-H), 4.61 (m, 1H, 15-H), 4.18 (s, 1H, 17-H), 3.75 (d, J = 11.6 Hz, 1H, 19-H), 3.04 (d, J = 11.5 Hz, 1H, 19-H), 2.22 (d, J = 11.7 Hz, 1H, 3-H), 1.79 (m, 3H, 3-H and 7-CH2), 1.66 (d, J = 11.5 Hz, 1H, 6-H), 1.62 – 1.47 (m, 2H, 9-H and 11-H), 1.31 (s, 3H, acetonylidene–CH3), 1.24 (s, 3H, acetonylidene –CH3), 1.19 (d, J = 6.2 Hz, 1H, 6-H), 1.08 (m, 5H, 20-CH3, 2-H and 1-H), 0.93 – 0.77 (m, 2H, 2-H and 1-H), 0.72 (m, 1H, 5-H), 0.38 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.4 (16-C), 151.5 (8’-C), 149.1 (2’-C)), 147.4 (12-C), 147.2 (8-C), 143.0 (9’-C), 133.3 (4’-C), 127.6 (13-C), 127.2 (6’-C), 126.5 (10’-C), 125.87 (3’-C) 125.89 (5’-C), 123.4 (acetonylidene-C), 108.3 (7’-C), 98.0 (17-C), 77.0 (3-C), 75.8 (15-C), 71.7 (14-C), 62.6 (19-C), 54.3 (9-C), 51.4 (5-C), 37.6 (4-C), 36.9 (10-C), 36.8 (7-C), 33.5 (1-C), 27.6 (2-C), 25.8 (6-C), 25.4 (acetonylidene-CH3), 25.1 (acetonylidene-CH3), 24.9 (11-C), 22.6 (20-C), 15.1 (18-C); ESI-HRMS: m/z found 586.2120, [M+H]+, calcd for C32H38Cl2NO5, 586.2127.
(14α)-(Quinolyl-5’,7’-dichloro-8’-oxy)-3,19-acetonylidene andrographolide (15b): white solid; m.p. 83.5-85.3 °C; 77% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.08 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.61 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.00 (s, 1H, 6’-H), 7.81 (dd, J = 8.6, 4.2 Hz, 1H, 3’-H), 6.70 (dd, J = 4.5 Hz, 1H, 12-H), 6.32 (d, J = 4.5 Hz, 1H, 14-H), 4.72 – 4.63 (m, 2H, 17-H and 15-H), 4.54 (m, 1H, 15-H), 4.10 (s, 1H, 17-H), 3.74 (d, J = 11.7 Hz, 1H, 19-H), 3.28 (m, 1H, 19-H), 3.01 (d, J = 11.6 Hz, 1H, 3-H), 2.22 (m, 1H, 7-H), 1.92 – 1.67 (m, 3H, 7-H, 11-H and 6-H), 1.62 (d, J = 11.5 Hz, 1H, 9-H), 1.52 (m, 3H, 11-H, 6-H and 2-H), 1.30 (s, 3H, acetonylidene–CH3), 1.22 (m, 4H, acetonylidene–CH3), 1.17 (d, J = 6.2 Hz, 1H, 1-H), 1.07 (m, 2H, 2-H and 1-H), 1.04 (s, 3H, 20–CH3), 0.89 (m, 1H, 5-H), 0.43 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.8 (16-C), 151.7 (8’-C), 148.7 (2’-C), 147.5 (12-C), 147.4 (8-C), 143.1 (9’-C), 133.6 (4’-C), 127.7 (13-C), 127.4 (6’-C), 126.6 (10’-C), 126.2 (3’-C), 126.1 (5’-C), 123.6 (acetonylidene-C), 108.1 (7’-C), 98.3 (17-C), 77.0 (3-C), 75.9 (15-C), 71.8 (14-C), 62.8 (19-C), 54.5 (9-C), 51.5 (5-C), 37.9 (4-C), 37.2 (10-C), 37.0 (7-C), 33.9 (1-C), 27.7 (2-C), 25.9 (acetonylidene-CH3), 25.5 (acetonylidene-CH3), 24.9 (16-C), 24.8 (11-C), 22.8 (20-C), 15.3 (8-C); ESI-HRMS: m/z found 586.2127, [M+H]+, calcd for C32H38Cl2NO5, 586.2127.
4.1.2. Preparation of 14β-isomers of 12a and 16a, and 14α-isomers of 12b and 16b.
The synthesis is conducted according to ref [38].
(14β)-(Quinolyl-2’-methyl-8’-oxy) andrographolide (12a): white solid; m.p. 98.0–101.0 °C; 92% yield; 1H NMR (400 MHz,DMSO-d6) δ 8.24 (d, J = 8.4 Hz, 1H, 4’-H), 7.57 (dd, J = 8.3, 1.1 Hz, 1H, 5’-H), 7.46 (dd, J = 8.3, 6.9 Hz, 2H, 6’-H and 7’-H), 7.15 (dd, J = 7.7, 1.2 Hz, 1H, 3’-H), 7.10 (m, 1H, 12-H), 6.05 (d, J = 5.3 Hz, 1H, 14-H), 4.81 (s, 1H, 17-H), 4.77 (m, 2H, 3-OH and 15-H), 4.58 (s, 1H, 17-H), 4.46 (m, 1H, 15-H), 4.03 (d, J = 7.1 Hz, 1H, 19-OH), 3.64 (m, 1H, 19-H), 3.08 (m, 1H, 19-H), 2.67 (s, 3H, 2’-CH3), 2.32 – 2.15 (m, 4H, 3-H, 7-CH2 and 11-H), 2.04 (d, J = 10.8 Hz, 1H, 6-H), 1.94 (m, 1H, 9-H), 1.61 (d, J = 13.1 Hz, 2H, 11-H and 6-H), 1.33 – 1.22 (m, 2H, 2-H and 1-H), 1.10 – 0.96 (m, 2H, 2-H and 1-H), 0.82 (s, 3H, 20-CH3), 0.72 (m, 1H, 5-H), 0.41 (s, 3H, 19- CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.5 (16-C), 157.7 (8’-C), 151.8 (2’-C), 150.2 (12-C), 147.9 (8-C), 139.6 (9’-C), 136.3 (4’-C), 127.8 (13-C), 126.5 (10’-C), 125.8 (6’-C), 122.8 (3’-C), 121.1 (5’-C), 112.3 (17-C), 107.6 (7’-C), 78.1 (3-C), 72.0 (15-C), 71.4 (14-C), 62.5 (19-C), 56.0 (9-C), 53.5 (15-C), 41.9 (4-C), 38.7 (10-C), 37.4 (7-C), 35.6 (1-C), 27.7 (2-C), 25.2 (6-C), 25.0 (quinolyl-2’-CH3), 23.9 (11-C), 22.7 (20-C), 14.5 (18-C); ESI-HRMS: m/z found 492.2746, [M+H]+, calcd for C30H38NO5, 492.2750.
(14α)-(Quinolyl-2’-methyl-8’-oxy) andrographolide (12b): white solid; m.p. 106.2–107.8 °C; 90% yield; 1H NMR (400 MHz,DMSO-d6) δ 8.25 (d, J = 8.5 Hz, 1H, 4’-H), 7.64 – 7.56 (m, 1H, 5’-H), 7.45 (m, J = 8.2 Hz, 2H, 6’-H and 7’-H), 7.18 (dd, J = 7.7, 1.2 Hz, 1H, 3’-H), 6.89 – 6.79 (m, 1H, 12-H), 6.05 (d, J = 5.5 Hz, 1H, 14-H), 4.99 (d, J = 4.8 Hz, 1H, 3-OH), 4.77 (m, 2H, 17-H and 15-H), 4.59 – 4.52 (m, 2H, 17-H and 15-H), 4.11 – 4.03 (m, 1H, 19-OH), 3.71 (d, J = 10.9 Hz, 1H, 19-H), 3.18 – 3.12 (m, 1H, 19-H), 3.06 (m, 1H, 3-H), 2.66 (s, 3H, 2’-CH3), 2.24 (m, 1H, 7-H), 2.19 – 2.09 (m, 2H, 7-H and 11-H), 1.82 (m, 3H, 6-H, 9-H and 11-H), 1.65 (d, J = 17.7 Hz, 2H, 6-H and 2-H), 1.39 (d, J = 4.7 Hz, 1H, 1-H), 1.21 – 1.18 (m, 2H, 2-H and 1-H), 1.00 (s, 3H, 20-CH3), 0.96 – 0.89 (m, 1H, 5-H), 0.33 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.5 (16-C), 157.8 (8’-C), 151.8 (2’-C), 149.7 (12-C), 147.3 (8-C), 140.2 (9’-C), 136.4 (4’-C), 127.8 (13-C), 125.7 (10’-C), 125.6 (6’-C), 122.6 (3’-C), 122.2 (5’-C), 115.8 (17-C), 108.4 (7’-C), 78.3 (3-C), 73.6 (15-C), 71.6 (14-C), 62.5 (19-C), 54.9 (9-C), 54.2 (5-C), 42.2 (4-C), 38.3 (10-C), 37.3 (7-C), 36.1 (1-C), 27.8 (2-C), 25.1 (6-C), 24.6 (quinolyl-2’-CH3), 23.9 (11-C), 23.0 (20-C), 14.4 (18-C); ESI-HRMS: m/z found 492.2745, [M+H]+, calcd for C30H38NO5, 492.2750.
(14β)-(Quinolyl-5’,7’-dichloro-8’-oxy)andrographolide(16a): white solid; m.p. 161.5– 163.0 °C; 89% yield; 1H NMR (400 MHz,DMSO-d6) δ 9.11 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.62 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.04 (s, 1H, 6’-H), 7.83 (m, 1H, 3’-H), 6.58 (m, 1H, 12’-H), 6.52 – 6.33 (m, 1H, 14-H), 5.03 (d, J = 4.8 Hz, 1H, 3-OH), 4.71 (d, J = 10.9 Hz, 1H, 17-H), 4.67 – 4.54 (m, 2H, 15-H and 17-H), 4.07 (m, 2H, 15-H and 19-OH), 3.68 (m, 1H, 19-H), 3.10 (m, 2H, 19-H and 3-H), 2.21 – 2.11 (m, 1H, 7-H), 1.76 (m, 1H, 7-H), 1.65 – 1.52 (m, 3H, 11-H, 6-H and 9-H), 1.52 – 1.36 (m, 2H, 11-H and 6-H), 1.24 – 1.17 (m, 1H, 2-H), 1.14 – 1.06 (m, 1H, 1-H), 1.00 (s, 3H, 20-CH3), 0.96 (m, 1H, 2-H), 0.72 – 0.57 (m, 2H, 1-H and 5-H), 0.10 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.4 (16-C), 151.6 (8’-C), 149.2 (2’-C), 147.5 (12-C), 147.2 (8-C), 143.1 (9’-C), 133.4 (4’-C), 127.6 (13-C), 127.4 (6’-C), 126.6 (10’-C), 125.9 (3’-C), 125.8 (5’-C), 123.5 (7’-C), 107.9 (17-C), 78.1 (3-C), 77.0 (15-C), 71.7 (14-C), 62.5 (19-C), 54.4 (9-C), 54.0 (5-C), 42.1 (4-C), 38.0 (10-C), 37.2 (7-C), 35.9 (1-C), 27.8 (2-C), 25.1 (6-C), 23.9 (11-C), 23.0 (20-C), 14.2 (18-C); ESI-HRMS: m/z found 546.1809, [M+H]+, calcd for C29H34Cl2NO5, 546.1814.
(14α)-(Quinolyl-5’,7’-dichloro-8’-oxy)andrographolide(16b): white solid; m.p. 120.8–121.9 °C; 86% yield; 1H NMR (400 MHz, DMSO-d6) δ9.10 (dd, J = 4.1, 1.6 Hz, 1H, 2’-H), 8.62 (dd, J = 8.7, 1.6 Hz, 1H, 4’-H), 8.02 (s, 1H, 6’-H), 7.83 (dd, J = 8.6, 4.2 Hz, 1H, 3’-H), 6.73 (m, 1H, 12-H), 6.32 (d, J = 4.5 Hz, 1H, 14-H), 5.00 (d, J = 4.9 Hz, 1H, 3-OH), 4.67 (d, J = 10.3 Hz, 2H, 17-H and 15-H), 4.56 (m, 1H, 19-OH), 4.10 (m, 2H, 17-H and 15-H), 3.71 (m, 1H, 19-H), 3.17 (m, 1H, 19-H), 3.07 (m, 1H, 3-H), 2.26 – 2.16 (m, 1H, 7-H), 1.93 – 1.72 (m, 2H, 7-H and 11-H), 1.61 (m, 2H, 6-H and 9-H), 1.48 (m, 3H, 11-H, 6-H and 2-H), 1.30 – 1.12 (m, 3H, 1-H, 2-H and 1-H), 1.00 (s, 3H, 20-CH3), 0.82 (m, 1H, 5-H), 0.25 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 169.5 (16-C), 151.5 (8’-C), 148.5 (2’-C), 147.4 (12-C), 147.3 (8-C), 142.9 (9’-C), 133.5 (4’-C), 127.6 (13-C), 127.2 (6’-C), 126.4 (10’-C), 126.1 (3’-C), 125.9 (5’-C), 123.4 (7’-C), 107.4 (17-C), 78.2 (3-C), 76.9 (15-C), 71.6 (14-C), 62.5 (19-C), 54.5 (9-C), 54.1 (5-C), 42.2 (4-C), 38.3 (10-C), 37.3 (7-C), 36.2 (1-C), 27.7 (2-C), 24.5 (6’-C), 23.9 (11-C), 23.0 (20-C), 14.2 (18-C); ESI-HRMS: m/z found 546.1810, [M+H]+, calcd for C29H34Cl2NO5, 546.1814.
4.1.3. Preparation of 14β-isomers of 13a and 17a, and 14α-isomers of 13b and 17b.
The synthesis is conducted according to ref [38].
(14β)-(Quinolyl-2’-methyl-8’-oxy)-19-acetoxyandrographolide (13a): white solid; m. p. 79.9–81.5 °C; 83% yield; 1H NMR (400 MHz, DMSO-d6) δ8.24 (d, J = 8.5 Hz, 1H, 4’-H), 7.57 (d, J = 8.1 Hz, 1H, 3’-H), 7.46 (m, 2H, 6’-H and 7’-H), 7.15 (d, J = 7.6 Hz, 1H, 3’-H), 7.11 (m, 1H, 12-H), 6.06 (d, J = 5.3 Hz, 1H, 14-H), 4.83 (s, 1H, 17-H), 4.79 (m, 2H, 3-OH and 15-H), 4.59 (s, 1H, 17-H), 4.46 (d, J = 10.9 Hz, 1H, 15-H), 4.40 (d, J = 4.6 Hz, 1H, 19-H), 4.09 (m, 1H, 19-H), 3.14 (m, 1H, 3-H), 2.67 (s, 3H, 2’-CH3), 2.34 – 2.28 (m, 2H, 7-CH2), 2.20 (m, 2H, 11-H and 6-H), 2.10 – 2.02 (m, 1H, 9-H), 1.95 (s, 3H, 19-acetoxy-CH3), 1.80 – 1.63 (m, 2H, 11-H and 6-H), 1.46 (m, 2H, 2-H and 1-H), 1.01 (d, J = 15.2 Hz, 1H, 2-H,), 0.96 – 0.88 (m, 1H, 1-H), 0.77 (s, 3H, 20-CH3), 0.54 (m, 1H, 5-H), 0.43 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 170.8 (19-acetoxy-C), 169.8 (16-C), 158.0 (8’-C), 152.2 (2’-C), 150.5 (12-C), 148.3 (8-C), 140.1 (9’-C), 136.8 (4’-C), 128.2 (13-C), 126.9 (10’-C), 126.2 (6’-C), 123.2 (3’-C), 121.6 (5’-C), 112.9 (17-C), 108.0 (7’-C), 76.6 (3-C), 72.5 (15-C), 71.8 (14-C), 65.4 (19-C), 56.4 (9-C), 53.5 (5-C), 41.7 (4-C), 39.2 (10-C), 37.9 (7-C), 36.1 (1-C), 27.7 (2-C), 25.6 (6-C), 25.3 (2’-CH3), 25.2 (11-C), 22.9 (20-C), 21.4 (19-acetoxy-CH3), 14.2 (18-C); ESI-HRMS: m/z found 534.2851, [M+H]+, calcd for C32H40NO6, 534.2855.
(14α)-(Quinolyl-2’-methyl-8’-oxy)-19-acetoxyandrographolide (13b): white solid; m. p. 76.8–77.9 °C; 80% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.25 (d, J = 8.5 Hz, 1H, 4’-H), 7.62 (dd, J = 8.2, 1.2 Hz, 1H, 3’-H), 7.45 (m, 2H, 6’-H and 7’-H), 7.17 (dd, J = 7.7, 1.2 Hz, 1H, 3’-H), 6.85 (m, 1H, 12-H), 6.06 (d, J = 5.7 Hz, 1H, 14-H), 4.79 – 4.75 (m, 2H, 3’-OH and 17-H), 4.67 (d, J = 4.7 Hz, 1H, 15-H), 4.56 – 4.51 (m, 2H, 15-H and 17-H), 4.06 – 3.99 (m, 2H, 19-H), 3.00 (m, 1H, 3-H), 2.66 (s, 3H, 2’-CH3), 2.26 (m, 2H, 7-CH2), 2.21 – 2.10 (m, 2H, 11-H and 6-H), 1.95 (m, 1H, 9-H), 1.91 (s, 3H, 19-acetoxy-CH3), 1.83 (m, 2H, 9-H and 11-H), 1.71 (m, 2H, 6-H and 2-H), 1.34 (m, 2H, 1-H and 2-H), 1.06 – 0.99 (m, 2H, 1-H and 5-H), 0.94 (s, 3H, 20-CH3), 0.34 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 170.4 (19-acetoxy-C), 169.5 (16-C), 157.8 (8’-C), 151.8 (2’-C), 149.7 (12-C), 147.3 (8-C), 140.3 (9’-C), 136.4 (4’-C), 127.8 (13-C), 125.7 (10’-C), 125.7 (6’-C), 122.6 (3’-C), 122.2 (5’-C), 116.0 (17-C), 108.4 (7’-C), 76.4 (3-C), 73.6 (15-C), 71.6 (14-C), 65.0 (19-C), 54.9 (9-C), 53.8 (5-C), 41.6 (4-C), 38.5 (10-C), 37.5 (7-C), 36.2 (1-C), 27.4 (2-C), 25.1 (6-C), 24.7 (2’-CH3), 24.5 (11-C), 22.7 (20-C), 21.0 (19-acetoxy-CH3), 13.6 (18-C); ESI-HRMS: m/z found 534.2856, [M+H]+, calcd for C32H40NO6, 534.2855.
(14β)-(Quinolyl-5’,7’-dichloro-8’-oxy)-19-acetoxyandrographolide (17a): white solid; m. p. 86.1–87.4 °C; 78% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.12 (dd, J = 4.3, 1.6 Hz, 1H, 2’-H), 8.63 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.05 (s, 1H, 6’-H), 7.84 (dd, J = 8.6, 4.2 Hz, 1H, 3’-H), 6.60 (d, J = 8.1 Hz, 1H, 12-H), 6.44 (d, J = 4.5 Hz, 1H, 14-H), 4.74 – 4.65 (m, 3H, 3-OH and 17-H2), 4.61 (m, 1H, 15-H), 4.10 – 4.03 (m, 2H, 15-H and 19-H), 3.94 (d, J = 11.7 Hz, 1H, 19-H), 3.03 (m, 1H, 3-H), 2.19 (d, J = 12.6 Hz, 1H, 7-H), 1.92 (s, 3H, 19-acetoxy-CH3), 1.74 (m, 2H, 7-H and 11-H), 1.63 – 1.53 (m, 2H, 6-H and 9-H), 1.44 – 1.22 (m, 3H, 11-H, 6-H and 2-H), 1.14 – 1.06 (m, 1H, 1-H), 1.05 – 0.98 (m, 1H, 2-H), 0.96 (s, 3H, 20-CH3), 0.71 (m, 2H, 1-H and 5-H), 0.12 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 170.5 (19-acetoxy-C), 169.5 (16-C), 151.6 (8’-C), 149.3 (2’-C), 147.5 (12-C), 147.2 (8-C), 143.1 (9’-C), 133.5 (4’-C), 127.6 (13-C), 127.5 (10’-C), 126.7 (6’-C), 126.0 (3’-C), 125.9 (5’-C), 123.5 (17-C), 107.9 (7’-C), 77.1 (3-C), 76.3 (15-C), 71.7 (14-C), 65.0 (19-C), 54.4 (9-C), 53.6 (5-C), 41.5 (4-C), 38.2 (10-C), 37.4 (7-C), 36.0 (1-C), 27.5 (2-C), 25.0 (6-C), 24.6 (11-C), 22.7 (20-C), 20.9 (19-acetoxy-CH3), 13.6 (18-C); m/z found 588.1913, [M+H]+, calcd for C31H36Cl2NO6, 588.1920.
(14α)-(Quinolyl-5’,7’-dichloro-8’-oxy)-19-acetoxyandrographolide (17b): white solid; m.p. 84.9–96.1 °C; 81% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.10 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.63 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.03 (s, 1H, 6’-H), 7.85 – 7.80 (m, 1H, 3’-H), 6.72 (dd, J = 8.7, 5.1 Hz, 1H, 12-H), 6.33 (d, J = 4.6 Hz, 1H, 14-H), 4.71 – 4.65 (m, 3H, 3-OH and 17–2H), 4.55 (m, 1H, 15-H), 4.12 – 4.05 (m, 2H, 15-H and 19-H), 3.96 (d, J = 11.7 Hz, 1H, 19-H), 3.03 (m, 1H, 3-H), 2.26 – 2.19 (m, 1H, 7-H), 1.93 (s, 3H, 19-acetoxy-CH3), 1.75 (m, 2H, 7-H and 11-H), 1.62 (d, J = 11.5 Hz, 1H, 6-H), 1.51 (m, J = 16.1, 2H, 9-H and 11-H), 1.40 – 1.31 (m, 2H, 6-H and 2-H), 1.08 – 0.99 (m, 2H, 1-H and 2-H), 0.96 (s, 3H, 20-CH3), 0.93 – 0.82 (m, 2H, 1-H and 5-H), 0.27 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 170.3 (19-acetoxy-C), 169.5 (16-C), 151.5 (8’-C), 148.4 (2’-C), 147.3 (12-C), 147.3 (8-C), 142.9 (9’-C), 133.5 (4’-C), 127.6 (13-C), 127.1 (10’-C), 126.4 (6’-C), 126.1 (3’-C), 125.9 (5’-C), 123.3 (17-C), 107.4 (7’-C), 76.8 (3-C), 76.3 (15-C), 71.6 (14-C), 64.9 (19-C), 54.5 (9-C), 53.7 (5-C), 41.5 (4-C), 38.5 (10-C), 37.4 (7-C), 36.2 (1-C), 27.4 (2-C), 24.6 (6-C), 24.4 (11-C), 22.7 (20-C), 20.9 (19-acetoxy-CH3), 13.4 (18-C); m/z found 588.1922, [M+H]+, calcd for C31H36Cl2NO6, 588.1920.
4.1.4. Preparation of 14β-isomers of 14a and 18a, and 14α-isomers of 14b and 18b.
The synthesis is conducted according to ref [38].
(14β)-(Quinolyl-2’-methyl-8’-oxy)-3-keto-19-acetoxy andrographolide (14a): white solid; m.p. 72.6–73.1 °C; 90% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 8.4 Hz, 1H, 4’-H), 7.63 – 7.53 (m, 1H, 5’-H), 7.45 (dd, J = 8.3, 7.0 Hz, 2H, 6’-H and 7’-H), 7.26 – 7.10 (m, 1H, 3’-H), 7.03 (t, J = 7.1 Hz, 1H, 12-H), 6.11 (d, J = 5.5 Hz, 1H, 14-H), 4.90 (s, 1H, 17-H), 4.79 (m,, 1H, 15-H), 4.63 (s, 1H, 17-H), 4.47 (d, J = 11.3 Hz, 1H, 15-H), 4.33 (d, J = 11.3 Hz, 1H, 19-H), 3.78 (d, J = 11.4 Hz, 1H, 19-H), 2.64 (s, 3H, 2’-CH3), 2.32 (d, J = 11.0 Hz, 3H, 7-CH2 and 11-H), 2.11 (d, J = 10.1 Hz, 1H, 6-H), 2.03 – 1.89 (m, 2H, 9-H and 11-H), 1.86 (s, 3H, 19-acetoxy-CH3), 1.63 (m, 2H, 6-H and 2-H), 1.52 (m, 1H, 1-H), 1.18 (m, 1H, 2-H), 1.07 – 0.90 (m, 2H, 1-H and 5-H), 0.83 (s, 3H, 20-CH3), 0.66 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 211.2 (3-C), 170.0 (19-acetoxy-C), 169.3 (16-C), 157.7 (8’-C), 151.6 (2’-C), 149.5 (12-C), 146.9 (8-C), 139.6 (9’-C), 136.4 (4’-C), 127.8 (13-C), 126.5 (10’-C), 125.7 (6’-C), 122.7 (3’-C), 121.5 (5’-C), 113.3 (17’-C), 108.6 (7’-C), 72.4 (15-C), 71.4 (14-C), 65.3 (19-C), 55.0 (9-C), 54.6 (5-C), 51.1 (4-C), 38.6 (10-C), 36.8 (7-C), 36.4 (1-C), 34.5 (2-C), 25.2 (6-C), 25.1 (2’-CH3), 24.2 (11-C), 20.5 (20-C), 20.1 (19-acetoxy-CH3), 13.8 (18-C); m/z found532.2695, [M+H]+, calcd for C32H38NO6, 532.2699.
(14α)-(Quinolyl-2’-methyl-8’-oxy)-3-keto-19-acetoxy andrographolide (14b): white solid; m.p. 62.8–63.6 °C; 86% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.25 (d, J = 8.4 Hz, 1H, 4’-H), 7.62 (d, J = 8.1 Hz, 1H, 5’-H), 7.45 (t, J = 7.7 Hz, 2H, 6’-H and 7’-H), 7.19 (d, J = 7.6 Hz, 1H, 3’-H), 6.87 (t, J = 6.5 Hz, 1H, 12-H), 6.06 (d, J = 5.5 Hz, 1H, 14-H), 4.87 (s, 1H, 17-H), 4.78 (m, 1H, 15-H), 4.69 (s, 1H, 17-H), 4.55 (m, 1H, 15-H), 4.39 (d, J = 11.3 Hz, 1H, 19-H), 3.82 (d, J = 11.4 Hz, 1H, 19-H), 2.66 (s, 3H, 2’-CH3), 2.59 (m, 1H, 7-H), 2.36 – 2.22 (m, 3H, 7-H, 11-H and 6-H), 1.88 (s, 3H, 19-acetoxy-CH3), 1.70 – 1.63 (m, 1H, 9-H), 1.58 (m, 1H, 11-H), 1.52 – 1.44 (m, 1H, 6-H), 1.39 (m, 3H, 2-CH2 and 1-H), 1.32 (m, 1H, 1-H), 0.98 (s, 3H, 20-CH3), 0.85 (d, J = 4.9 Hz, 1H, 5-H), 0.60 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 211.8 (3-C), 170.1 (19-acetoxy-C), 169.4 (16-C), 157.8 (8’-C), 151.9 (2’-C), 149.4 (12-C), 146.5 (8-C), 140.2 (9’-C), 136.4 (4’-C), 127.8 (13-C), 125.8 (10’-C), 125.7 (6’-C), 122.7 (3’-C), 122.1 (5’-C), 115.6 (17’-C), 109.5 (7’-C), 73.6 (15-C), 71.5 (14-C), 65.4 (19-C), 55.4 (9-C), 53.9 (5-C), 51.3 (4-C), 38.3 (10-C), 36.8 (7-C), 36.7 (1-C), 34.8 (2-C), 25.1 (6-C), 24.7 (2’-CH3), 24.2 (11-C), 20.5 (20-C), 20.3 (19-acetoxy-CH3), 13.8(18-C); m/z found532.2693, [M+H]+, calcd for C32H38NO6, 532.2699.
(14β)-(Quinolyl-5’,7’-dichloro-8’-oxy)-3-keto-19-acetoxy andrographolide (18a): white solid; m.p. 77.5–78.2 °C; 86% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.13 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.62 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.03 (s, 1H, 6’-H), 7.83 (dd, J = 8.6, 4.2 Hz, 1H, 3’-H), 6.62 (m, 1H, 12-H), 6.45 (d, J = 4.5 Hz, 1H, 14-H), 4.76 – 4.66 (m, 2H, 15-H and 17-H), 4.60 (m, 1H, 15-H), 4.38 (d, J = 11.3 Hz, 1H, 19-H), 4.20 (s, 1H, 17-H), 3.81 (d, J = 11.3 Hz, 1H, 19-H), 2.60 (m, 1H, 7-H), 2.24 (m, 1H, 7-H), 2.15 – 2.05 (m, 1H, 11-H), 1.90 (s, 3H,19-acetoxy-CH3), 1.88 – 1.81 (m, 1H, 6-H), 1.81 – 1.71 (m, 2H, 9-H and 11-H), 1.66 (m, 1H, 6-H), 1.54 (m, 1H, 2-H), 1.27 (m, J = 23.0, 13.7, 4.4 Hz, 2H, 1-H and 2-H), 1.17 – 1.03 (m, 2H, 1-H and 5-H), 0.99 (s, 3H, 20-CH3), 0.40 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 211.6 (3-C), 170.1 (19-acetoxy-C), 169.4 (16-C), 151.6 (8’-C), 149.0 (2’-C), 147.2 (12-C), 146.6 (8-C), 143.1 (9’-C), 133.4 (4’-C), 127.7 (13-C), 127.5 (10’-C), 126.6 (6’-C), 126.0 (3’-C), 125.9 (5’-C), 123.5 (17-C), 108.9 (7’-C), 77.1 (15-C), 71.6 (14-C), 65.4 (19-C), 55.1 (9-C), 53.2 (5-C), 51.2 (4-C), 38.0 (10-C), 36.6 (7-C), 36.5 (1-C), 34.8 (2-C), 25.2 (6-C), 24.1 (11-C), 20.5 (20-C), 20.4 (19-acetoxy-CH3), 13.7 (18-C); m/z found 586.1758, [M+H]+, calcd for C31H34Cl2NO6, 586.1763.
(14α)-(Quinolyl-5’,7’-dichloro-8’-oxy)-3-keto-19-acetoxy andrographolide (18b): white solid; m.p. 81.2–83.0 °C; 85% yield; 1H NMR (400 MHz, DMSO-d6) δ 9.11 (dd, J = 4.2, 1.6 Hz, 1H, 2’-H), 8.64 (dd, J = 8.6, 1.6 Hz, 1H, 4’-H), 8.04 (s, 1H, 6’-H), 7.91 – 7.73 (m, 1H, 3’-H), 6.74 (m, 1H, 12-H), 6.36 (d, J = 4.5 Hz, 1H, 14-H), 4.77 (d, J = 1.6 Hz, 1H, 17-H), 4.66 (d, J = 11.0 Hz, 1H, 15-H), 4.53 (m, 1H, 15-H), 4.41 (d, J = 11.3 Hz, 1H, 19-H), 4.20 (s, 1H, 17-H), 3.83 (d, J = 11.4 Hz, 1H, 19-H), 2.62 (m, 1H, 7-H), 2.33 – 2.23 (m, 1H, 7-H), 2.09 (m, 1H, 11-H), 1.97 – 1.91 (m, 1H, 6-H), 1.90 (s, 3H, 19-acetoxy-CH3), 1.86 (m, 1H, 9-H), 1.80 (d, J = 11.6 Hz, 1H, 11-H), 1.68 (m, 2H, 6-H and 2-H), 1.59 (m, 1H, 1-H), 1.36 – 1.20 (m, 3H, 2-H, 1-H and 5-H), 0.99 (s, 3H, 20-CH3), 0.53 (s, 3H, 18-CH3); 13C NMR (101 MHz, DMSO-d6) δ 211.7 (3-C), 170.1 (19-acetoxy-C), 169.5 (16-C), 151.6 (8’-C), 148.3 (2’-C), 147.3 (12-C), 146.5 (8-C), 142.9 (9’-C), 133.5 (4’-C), 127.6 (13-C), 127.2 (10’-C), 126.4 (6’-C), 126.2 (3’-C), 125.9 (5’-C), 123.4 (17-C), 108.5 (7’-C), 76.9 (15-C), 71.6 (14-C), 65.4 (19-C), 55.2 (4-C), 53.4 (5-C), 51.3 (4-C), 38.3 (10-C), 36.8 (7-C), 36.7 (1-C), 34.7 (2-C), 24.7 (6-C), 24.2 (11-C), 20.5 (20-C), 20.3 (19-acetoxy-CH3), 13.6 (18-C); m/z found 586.1761, [M+H]+, calcd for C31H34Cl2NO6, 586.1763.
4.2. Biology
The screening procedure was adopted from the references [58–59]. All compounds were dissolved in DMSO before diluted with medium into stock solutions and the final concentration of DMSO in each well is 0.1%. Niclosamide of a previously identified active compound against Zika virus infection in Nature Medicine (2016) [59] was included in viral titer assays each time as a reference and quality control compound.
Cell culture
The glioblastoma SNB-19 cell line was maintained at 37 °C in 5% CO2 in RPMI-1640 medium, 1× penicillin–streptomycin-amphotericin B (PSA), and 10% fetal bovine serum (FBS) (Thermo Fisher Scientific). The monkey kidney Vero cell line was maintained at 37 °C in 5% CO2 in DMEM, 1× penicillin-streptomycin, and 10% FBS.
The Aedes albopictus C6/36 cell line was maintained at 28 °C in 5% CO2 in EMEM media (ATCC), penicillin-streptomycin, and 10% FBS.
ZIKV culture, ZIKV infection of SNB-19 and drug addition
Zika virus stocks were generated in Aedes albopictus clone C6/36 cells from PRVABC59-ZIKV strain stocks (ATCC). To amplify viral stocks, a T-75 flask of C6/36 cells (90–95% confluency) was inoculated with 1 X 106 Zika virions in low volume (3 ml) for 1 hour, with rocking to disperse media every 15 minutes. After 1 hour, 17ml of media was added and C6/36 cells were maintained at 28°C in 5% CO2 for 7 days. At day 7 and day 8 post viral inoculation, supernatants were harvested, filtered, and stored at −80°C. ZIKV titer was determined by focus forming unit assay.
SNB-19 cells were seeded into 96-well plates 1 day prior to viral infection, and then SNB-19 cells were incubated with medium (control) or gradient concentrations of each compound for 1 h prior to addition of C6/36 derived-ZIKV at MOI = 1. SNB-19 cell supernatants were then harvested at 24 hours post infection from the 96-well plates and either frozen at −80°C or used immediately for focus forming unit assay.
ZIKV titer assay
Vero cells were seeded overnight into 96-well plates to achieve a confluent monolayer the next day. Supernatant from drug-treated ZIKV-infected SNB-19 cells was diluted in sterile 96-well plates and subsequently used to inoculate the naive Vero cell plate, which was then incubated for 2 hours prior to removal of supernatant and replacement with a methyl-cellulose overlay. After 48 hours further incubation, the methyl-cellulose overlay was removed by aspiration, wells gently washed with phosphate buffered saline (PBS), and wells fixed with 100μl 4% paraformaldehyde for 15 minutes. Then, paraformaldehyde was removed, wells gently washed with 200μ1 PBS per well three times, and cells blocked with 100μl PBTG (PBS containing 0.1% Triton X-100, 10% normal goat serum, and 1% bovine serum albumin) for 1 hour at room temperature while rocking. Blocking solution was removed and wells were incubated with 50μl primary antibody solution (4G2, 1:500 in PBTG) at 4°C overnight while rocking. The next day, cells were washed gently with 200μl per well PBS three times, and then incubated with 50μl secondary antibody solution (goat anti-mouse-IgG HRP, 1:500 in PBTG) for 1 hour at room temperature. Cells were then washed with 200μl per well PBS three times, and incubated with 50μl freshly prepared DAB solution per well for 10 minutes, prior to rinsing with deionized water (DAB prepared with nickel according to manufacturer’s instructions) and colonies quantified.
Cytotoxicity of testing compounds to SNB-19 cells and Vero cells.
SNB-19 cells or Vero cells were plated in a 96-well plate at a concentration of 1.0 ×104 cells per well overnight to allow cell attachment. Working solutions of the testing compounds, or the 0.1% DMSO vehicle as a control were dispensed appropriately into the partitioned 96-well plates, which were then incubated for another 24 h for SNB-19 cells or 48 h for Vero cells. Then, the medium was discarded and the cells were incubated for 4 h at 37 °C in MTT solution (final concentration 0.5 mg/mL). The solution was then replaced with 100 mL DMSO to dissolve the violet formazan crystals in the intact cells. Cell growth was assessed by MTT according to the manufacturer’s protocol. The absorbance was measured at 570 nm as the reference wavelength. The cytotoxicity as the CC50 value (concentration of 50% cell growth/viability inhibition) was calculated based on the percentage of cell viability data compared to the control group. Each concentration was repeated 3 times independently.
Supplementary Material
Highlights.
24 Analogs of 14-aryloxy andrographolide were tested for anti-Zika virus activity.
Optimal modification/s at 3-, 14-, or 19-positions can make derivatives less toxic.
14β-(8’-Quinolyloxy)-3,19-diol derivative 3 exhibits the most potent activity.
14α-(5’,7’-Dichloro-8’-quinolyloxy)-19-OAc analog 17b is the most selective agent.
Acknowledgements
The work was partially supported by the National Natural Science Foundation of China to GCZ (30973621 and U0632001) and NIH grant U19 AI131130 to HT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data of NMR spectra of new compounds to this article can be found online at http://dx.doi.Org/10.1016/j.ejmech
Declaration of interests
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].Dick GWA, Kitchen SF, Haddow AJ, Zika virus. I. Isolations and serological specificity, T ROY SOC TROP MED H, 46 (1952) 509. [DOI] [PubMed] [Google Scholar]
- [2].Cardoso CW, Paploski IAD, Kikuti M, Rodrigues MS, Silva MMO, Campos GS, Sardi SI, Kitron U, Reis MG, Ribeiro GS, Outbreak of Exanthematous Illness Associated with Zika, Chikungunya, and Dengue Viruses, Salvador, Brazil, EMERG INFECT DIS, 21 (2015) 2274–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Campos GS, Bandeira AC, Sardi SI, Zika Virus Outbreak, Bahia, Brazil, EMERG INFECT DIS, 21 (2015) 1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].(a) Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D, An update on Zika virus infection, The Lancet, 390 (2017) 2099–2109. [DOI] [PubMed] [Google Scholar]; (b) Musso D, Gubler DJ, Zika virus, Clin Microbiol Rev, 29 (2016) 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cao-Lormeau V, Musso D, Emerging arboviruses in the Pacific, The Lancet, 384 (2014) 1571–1572. [DOI] [PubMed] [Google Scholar]
- [6].Kuno G, Chang GJJ, Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses, ARCH VIROL, 152 (2007) 687–696. [DOI] [PubMed] [Google Scholar]
- [7].Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR, Zika Virus and Birth Defects — Reviewing the Evidence for Causality, NEW ENGL J MED, 374 (2016) 1981–1987. [DOI] [PubMed] [Google Scholar]
- [8].Osuna CE, Lim S, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EFC, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB, Zika viral dynamics and shedding in rhesus and cynomolgus macaques, NAT MED, 22 (2016) 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC, Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage, PLOS NEGLECT TROP D, 6 (2012) e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].WHO, Zika situation report, Archived from the original on 25 May 2017. [Google Scholar]
- [11].Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendoza MCL, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RMR, Tanuri A, de Filippis AMB, Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study, The Lancet Infectious Diseases, 16 (2016) 653–660. [DOI] [PubMed] [Google Scholar]
- [12].Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pižem J, Petrovec M, Avšič Županc T, Zika Virus Associated with Microcephaly, NEW ENGL J MED, 374 (2016) 951–958. [DOI] [PubMed] [Google Scholar]
- [13].Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonça MCL, Nogueira RMR, de Filippis AMB, Solomon T, Guillain-Barré syndrome associated with Zika virus infection, The Lancet, 387 (2016) 1482. [DOI] [PubMed] [Google Scholar]
- [14].Wahid B, Ali A, Rafique S, Idrees M, Current status of therapeutic and vaccine approaches against Zika virus, EUR J INTERN MED, 44 (2017) 12–18. [DOI] [PubMed] [Google Scholar]
- [15].Saiz J, Martín-Acebes MA, The Race To Find Antivirals for Zika Virus, ANTIMICROB AGENTS CHEMOTHER, 61 (2017) e00411–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Richner JM, Diamond MS, Zika virus vaccines: immune response, current status, and future challenges, CURR OPIN IMMUNOL, 53 (2018) 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Duarte G, Moron A, Timerman A, Fernandes C, Mariani Neto C, Almeida Filho G, Werner Junior H, Espírito Santo H, Steibel J, Bortoletti Filho J, Andrade J, Burlá M, Silva De Sá M, Busso N, Giraldo P, Moreira De Sá R, Passini Junior R, Mattar R, Francisco R, Zika Virus Infection in Pregnant Women and Microcephaly, Revista Brasileira de Ginecologia e Obstetrícia / RBGO Gynecology and Obstetrics, 39 (2017) 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW, Dengue: a continuing global threat, NAT REV MICROBIOL, 8 (2010) S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, García-Sastre A, Krammer F, Lim JK, Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity, SCIENCE, 356 (2017) 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barrows NJ, Campos RK, Liao K, Prasanth KR, Soto-Acosta R, Yeh S, Schott-Lerner G, Pompon J, Sessions OM, Bradrick SS, Garcia-Blanco MA, Biochemistry and Molecular Biology of Flaviviruses, CHEM REV, 118 (2018) 4448–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Castanha PM, Nascimento EJM, Cynthia B, Cordeiro MT, de Carvalho OV, de Mendoza LR, Azevedo EA, França RF, Rafael D, Marques ET, Dengue virus (DENV)-specific antibodies enhance Brazilian Zika virus (ZIKV) infection, J INFECT DIS, 215 (2017) 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deng WL, Liu JY, Nie RJ, Pharmacological studies on 14-deoxy-11, 12-didehydroandrographolide-3, 19-disuccinate. I. Anti-inflammatory activity (article in Chinese), Yao Xue Xue Bao, 15 (1980) 590–597. [PubMed] [Google Scholar]
- [23].Liu X, Wang Y, Li G, Advances in pharmacological study of andrographolide and its derivatives (article in Chinese), Zhong yao cai (Journal of Chinese medicinal materials), 26 (2003) 135–138. [PubMed] [Google Scholar]
- [24].Cava MP, Chan WR, Haynes LJ, Johnson LF, Weinstein B, The structure of andrographolide, TETRAHEDRON, 18 (1962) 397–403. [Google Scholar]
- [25].Gorter MK, The bitter constituent of Andrographis paniculata, Recl. Trav. Chim. Pays-Bas, 30 (1911) 151–160. [Google Scholar]
- [26].Kleipool RJC, Constituents of Andrographis paniculata Nees, NATURE, 169 (1952) 33–34.14910682 [Google Scholar]
- [27].Zhang T, Advances in the study of Andrographis paniculata (Burm.f.) Nees (article in Chinese), Zhong Yao Cai., 23 (2000) 366–368. [PubMed] [Google Scholar]
- [28].Yan YY, Shi GX, Shao J, Wang TM, Wang CZ, Advance in studies on anti-infection of andrographolide and its derivatives in past 10 years (article in Chinese), Zhongguo Zhong Yao Za Zhi, (2013) 3819–3824. [PubMed] [Google Scholar]
- [29].Chinese Pharmacopoeia Commission, Chuanhuning (article in Chinese), Chinese Pharmacopoeia, Part 2 (2010) 619–620. [Google Scholar]
- [30].Basak A, Cooper S, Roberge AG, Banik UK, Chretien M, Seidah NG, Inhibition of proprotein convertases-1, −7 and furin by diterpines of Andrographis paniculata and their succinoyl esters, BIOCHEM J, 338 ( Pt 1) (1999) 107–113. [PMC free article] [PubMed] [Google Scholar]
- [31].Chang RS, Ding L, Chen GQ, Pan QC, Zhao ZL, Smith KM, Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus, Proc Soc Exp Biol Med, 197 (1991) 59–66. [DOI] [PubMed] [Google Scholar]
- [32].Gupta S, Mishra KP, Ganju L, Broad-spectrum antiviral properties of andrographolide, ARCH VIROL, 162 (2017) 611–623. [DOI] [PubMed] [Google Scholar]
- [33].Kishore V, Yarla N, Bishayee A, Putta S, Malla R, Neelapu N, Challa S, Das S, Shiralgi Y, Hegde G, Dhananjaya B, Multi-targeting Andrographolide and its Natural Analogs as Potential Therapeutic Agents, CURR TOP MED CHEM, 17 (2017) 845–857. [DOI] [PubMed] [Google Scholar]
- [34].Banerjee M, Parai D, Chattopadhyay S, Mukherjee SK, Andrographolide: antibacterial activity against common bacteria of human health concern and possible mechanism of action, FOLIA MICROBIOL, 62 (2017) 237–244. [DOI] [PubMed] [Google Scholar]
- [35].Shi GX, Yan YY, Shao J, Zhang MX, Lu KQ, Wang TM, Wang CZ, [Effect of andrographolide derivative Yanhuning on in vivo Candida albicans biofilms in rats], Zhongguo Zhong Yao Za Zhi, 39 (2014) 2924–2929. [PubMed] [Google Scholar]
- [36].Zeng X, Liu X, Bian J, Pei G, Dai H, Polyak SW, Song F, Ma L, Wang Y, Zhang L, Synergistic Effect of 14-Alpha-Lipoyl Andrographolide and Various Antibiotics on the Formation of Biofilms and Production of Exopolysaccharide and Pyocyanin by Pseudomonas aeruginosa, ANTIMICROB AGENTS CH, 55 (2011) 3015–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma L, Liu X, Liang H, Che Y, Chen C, Dai H, Yu K, Liu M, Ma L, Yang C, Song F, Wang Y, Zhang L, Effects of 14-Alpha-Lipoyl Andrographolide on Quorum Sensing in Pseudomonas aeruginosa, ANTIMICROB AGENTS CH, 56 (2012) 6088–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li F, Li X, Sheng D, Chen S, Nie X, Liu Z, Wang D, Zhao Q, Wang Y, Wang Y, Zhou G, Discovery and preliminary SAR of 14-aryloxy-andrographolide derivatives as antibacterial agents with immunosuppressant activity, RSC ADV, 8 (2018) 9440–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calabrese C, Berman SH, Babish JG, Ma X, Shinto L, Dorr M, Wells K, Wenner CA, Standish LJ, A phase I trial of andrographolide in HIV positive patients and normal volunteers, PHYTOTHER RES, 14 (2000) 333–338. [DOI] [PubMed] [Google Scholar]
- [40].Wiart C, Kumar K, Yusof MY, Hamimah H, Fauzi ZM, Sulaiman M, Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1, PHYTOTHER RES, 19 (2005) 1069–1070. [DOI] [PubMed] [Google Scholar]
- [41].Chen H, Ma Y, Huang X, Geng C, Zhao Y, Wang L, Guo R, Liang W, Zhang X, Chen J, Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents, BIOORG MED CHEM LETT, 24 (2014) 2353–2359. [DOI] [PubMed] [Google Scholar]
- [42].Chandramohan V, Kaphle A, Chekuri M, Gangarudraiah S, Bychapur Siddaiah G, Evaluating Andrographolide as a Potent Inhibitor of NS3–4A Protease and Its Drug-Resistant Mutants Using In Silico Approaches, Advances in Virology, 2015 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee J, Tseng C, Young K, Sun H, Wang S, Chen W, Lin C, Wu Y, Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells, BRIT J PHARMACOL, 171 (2014) 237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen JX, Xue HJ, Ye WC, Fang BH, Liu YH, Yuan SH, Yu P, Wang YQ, Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro, BIOL PHARM BULL, 32 (2009) 1385–1391. [DOI] [PubMed] [Google Scholar]
- [45].Yu B, Dai C, Jiang Z, Li E, Chen C, Wu X, Chen J, Liu Q, Zhao C, He J, Ju D, Chen X, Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway, CHIN J INTEGR MED, 20 (2014) 540–545. [DOI] [PubMed] [Google Scholar]
- [46].Edwin E, Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, Ponsankar A, Pradeepa V, Selin-Rani S, Kalaivani K, Hunter WB, Abdel-Megeed A, Duraipandiyan V, Al-Dhabi NA, Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae), ACTA TROP, 163 (2016) 167–178. [DOI] [PubMed] [Google Scholar]
- [47].Panraksa P, Ramphan S, Khongwichit S, Smith DR, Activity of andrographolide against dengue virus, ANTIVIR RES, 139 (2017) 69–78. [DOI] [PubMed] [Google Scholar]
- [48].Wintachai P, Kaur P, Lee RCH, Ramphan S, Kuadkitkan A, Wikan N, Ubol S, Roytrakul S, Chu JJH, Smith DR, Activity of andrographolide against chikungunya virus infection, SCI REP-UK, 5 (2015) 14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mishra SK, Tripathi S, Shukla A, Oh SH, Kim HM, Andrographolide and analogues in cancer prevention, Frontiers in bioscience (Elite edition), 7 (2015) 255. [DOI] [PubMed] [Google Scholar]
- [50].Aromdee C, Andrographolide: progression in its modifications and applications – a patent review (2012 – 2014), EXPERT OPIN THER PAT, 24 (2014) 1129–1138. [DOI] [PubMed] [Google Scholar]
- [51].Lim JCW, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong WF, Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer, CLIN EXP PHARMACOL P, 39 (2012) 300–310. [DOI] [PubMed] [Google Scholar]
- [52].Zhou B, Zhang D, Wu X, Biological Activities and Corresponding Sar Analysis of Andrographolide and its Derivatives, Mini Rev Med Chem, 2012. Apr 17. [Epub ahead of print]. [PubMed] [Google Scholar]
- [53].Aromdee C, Modifications of andrographolide to increase some biological activities: a patent review (2006 – 2011), EXPERT OPIN THER PAT, 22 (2012) 169–180. [DOI] [PubMed] [Google Scholar]
- [54].Nie X, Chen S, Wang K, Peng Y, Wang Y, Wang D, Wang Y, Zhou G, Attenuation of Innate Immunity by Andrographolide Derivatives Through NF-κΒ Signaling Pathway, SCI REP-UK, 7 (2017) 4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Delvecchio R, Higa L, Pezzuto P, Valadao A, Garcez P, Monteiro F, Loiola E, Dias A, Silva F, Aliota M, Caine E, Osorio J, Bellio M, O Connor D, Rehen S, de Aguiar R, Savarino A, Campanati L, Tanuri A, Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models, Viruses, 8 (2016) 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Han Y, Pham HT, Xu H, Quan Y, Mesplede T, Antimalarial drugs and their metabolites are potent Zika virus inhibitors, J MED VIROL, 91 (2019) 1182–1190. [DOI] [PubMed] [Google Scholar]
- [57].Kumar A, Liang B, Aarthy M, Singh SK, Garg N, Mysorekar IU, Giri R, Hydroxychloroquine Inhibits Zika Virus NS2B-NS3 Protease, ACS Omega, 3 (2018) 18132–18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee EM, Titus SA, Xu M, Tang H, Zheng W, High-Throughput Zika Viral Titer Assay for Rapid Screening of Antiviral Drugs, ASSAY DRUG DEV TECHN, 17 (2019) 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu M, Lee EM, Wen Z, Cheng Y, Huang W, Qian X, TCW J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming G, Zheng W, Song H, Tang H, Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen, NAT MED, 22 (2016) 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.