Abstract
Objectives:
To compare survival after nodal assessment using a sentinel lymph node (SLN) algorithm versus comprehensive pelvic and paraaortic lymphadenectomy (LND) in serous or clear cell endometrial carcinoma, and to compare survival in node-negative cases.
Methods:
Three-year recurrence-free survival (RFS) and overall survival were compared between one institution using comprehensive LND to the renal veins and a second institution using an SLN algorithm with ultra-staging with inverse-probability of treatment weighting (IPTW) derived from propensity scores to adjust for covariate imbalance between cohorts.
Results:
214 patients were identified (118 SLN cohort, 96 LND cohort). Adjuvant therapy differed between the cohorts; 84% and 40% in the SLN and LND cohorts, respectively, received chemotherapy ± radiation therapy. The IPTW-adjusted 3-year RFS rates were 69% and 80%, respectively. The IPTW-adjusted 3-year OS rates were 88% and 77%, respectively. The IPTW-adjusted hazard ratio (HR) for the association of surgical approach (SLN vs LND) with progression and death was 1.46 (95%CI: 0.70-3.04) and 0.44 (95%CI: 0.19-1.02), respectively. In the 168 node-negative cases, the IPTW-adjusted 3-year RFS rates were 73% and 91%, respectively. The IPTW-adjusted 3-year OS rates were 88% and 86%, respectively. In this subgroup, IPTW-adjusted HR for the association of surgical approach (SLN vs LND) with progression and death was 3.12 (95%CI: 1.02-9.57) and 0.69 (95%CI: 0.24-1.95), respectively.
Conclusion:
OS was not compromised with the SLN algorithm. SLN may be associated with a decreased RFS but similar OS in node-negative cases despite the majority receiving chemotherapy. This may be due to differences in surveillance.
Keywords: endometrial cancer, serous endometrial carcinoma, clear cell endometrial carcinoma, sentinel lymph node, lymphadenectomy
Background
Serous and clear cell carcinomas of the endometrium are rare tumors associated with poor prognoses, even when diagnosed at early stages [1]. Although the use of lymphadenectomy (LND) in low-risk endometrial cancer is somewhat controversial, it is broadly accepted as part of the surgical staging algorithm in cases with serous or clear cell histology, given their propensity for early spread despite minimal myometrial invasion [2–4]. In a series of 50 patients with presumed stage I-II serous uterine carcinoma who had undergone surgical staging, Goff et al found no association between depth of invasion and metastatic disease. Specifically, lymph node metastases were identified in 36% of patients with no myometrial invasion, 50% with <50% invasion, and 40% with >50% invasion [2]. Omission of LND based on uterine features is therefore not appropriate in these patients.
Sentinel lymph node (SLN) assessment has emerged as a technique with the potential to reduce morbidity compared with full LND to stage patients with endometrial cancer. The growing evidence base on the safety and efficacy of SLN assessment, particularly in low-risk endometrial cancer, has led to the addition of an SLN algorithm to the National Comprehensive Cancer Network (NCCN) guidelines and a Society of Gynecologic Oncology (SGO) consensus recommendation that nodal assessment using the algorithm is an appropriate method for surgical staging in low-risk endometrial cancer [5, 6].
Preliminary data with regard to the efficacy and safety of an SLN algorithm in serous and clear cell endometrial cancer are limited and inconclusive. We compared the outcomes of patients with apparently uterine-confined serous or clear cell endometrial carcinoma who had undergone surgical staging with an SLN algorithm versus comprehensive pelvic and paraaortic LND. We also evaluated the outcomes of patients with negative or non-assessed lymph nodes. As the patients in this retrospective study were not randomly assigned to the type of surgical staging, we used propensity score methodology to balance the two surgical staging cohorts on measured baseline covariates and potentially obtain less biased outcomes comparisons between the cohorts [7].
Methods
Patients with newly diagnosed, apparently uterine-confined serous or clear cell endometrial carcinoma with any degree of myometrial invasion were identified at the Mayo Clinic and Memorial Sloan Kettering Cancer Center using institutional databases. The Memorial Sloan Kettering Cancer Center database review encompassed the years 2006 through 2013 (SLN cohort), and the Mayo Clinic database review encompassed the years 2004 through 2008 (LND cohort). During these time periods, the surgical approach for endometrial cancer staging at these institutions differed only by method of lymph node assessment. Both cohorts underwent hysterectomy, bilateral salpingo-oophorectomy, intra-abdominal survey, biopsy of any suspicious lesions, and peritoneal cytology. The surgical approach at the Mayo Clinic included comprehensive pelvic and paraaortic LND to the renal veins. Patients in the Memorial Sloan Kettering Cancer Center cohort underwent evaluation with an SLN algorithm per institutional protocol, as previously described [8]. Briefly, lymphatic mapping was performed using an intracervical injection of either blue dye or indocyanine green dye. A total of 4 mL of dye was injected into the cervix, 1 mL each deep (1 cm) and superficially at the 3 and 9 o’clock positions. The retroperitoneum was examined, and any enlarged or suspicious lymph nodes were excised along with any identified SLNs. A full LND was performed in any non-mapping hemipelvis.
Excised SLNs were evaluated with “pathologic ultra-staging”, as previously described [9]. This includes standard lymph node assessment consisting of sectioning the SLN longitudinally and staining it with hematoxylin and eosin (H&E) to assess for the presence of tumor cells. If tumor cells are identified, the lymph node is considered positive and no further assessment is performed. If no tumor cells are identified, the SLN is serially sectioned and stained with both H&E and immunohistochemistry for cytokeratin AE1:AE3 to identify low-volume metastases. Macrometastasis, defined as any nodal metastasis ≥2 mm, micrometastasis, defined as any nodal metastasis >0.2 mm but <2 mm, and isolated tumor cells, defined as metastasis ≤0.2 mm as seen on H&E, were considered node-positive.
Clinical, pathologic, and surgical characteristics were recorded for all patients. Date of last follow-up, date of recurrence, and disease status at last follow-up were also recorded. Adjuvant therapy was administered per each institution’s guidelines and disease surveillance was performed per institutional protocols, with routine imaging performed at Memorial Sloan Kettering Cancer Center and follow-up imaging left to the treating physician’s discretion at the Mayo Clinic. All stage IV cases were excluded.
Statistical analyses were performed using the SAS version 9.4 software package (SAS Institute, Inc.; Cary, NC). Separate analyses were performed for all patients and for the subgroup of node-negative patients. All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant. Baseline characteristics were compared between the SLN and LND cohorts using the two-sample t test for age and body mass index (BMI), the Wilcoxon rank-sum test for the number of nodes, and chi square or Fisher’s exact test for categorical variables. Considering the different time periods encompassed by the two cohorts, follow-up was restricted to the first 3 years after surgery to analyze each time-to-event outcome (OS and RFS). Propensity score methodology, specifically the use of inverse-probability of treatment weighting (IPTW), was used to account for differences in measured baseline covariates upon comparing the outcomes between the SLN and LND cohorts [10, 11]. IPTW-adjusted Kaplan-Meier curves were estimated. The association between cohort (SLN vs LND) and death and progression, respectively, was evaluated based on fitting IPTW-adjusted Cox proportional hazards models using robust sandwich covariance estimates to account for the use of estimated weights. The propensity score was defined as the estimated probability of a patient being in the LND cohort (vs the SLN cohort) given a set of measured baseline patient covariates and was derived from a multivariable logistic regression model that included the following covariates: age, BMI, myometrial invasion, lymphovascular space invasion (LVSI), cervical stroma invasion, peritoneal cytology, presence of positive pelvic nodes, presence of positive paraaortic nodes, International Federation of Gynecology and Obstetrics (FIGO) stage (2009), and adjuvant therapy. The weights were derived as 1/propensity score for patients in the LND cohort and 1/(1-propensity score) for patients in the SLN cohort. Extreme weights were trimmed at the 95th percentile, and the weights were stabilized according to the proportion of patients in the two cohorts. Covariate imbalance between the two cohorts was assessed by calculating standardized differences for each covariate, separately for the original unadjusted cohorts and the IPTW-adjusted cohorts. The standardized difference for a continuous covariate is defined as the absolute difference in group means divided by an estimate of the pooled standard deviation. The derivation is similar for nominal covariates. The desired standardized difference for the covariates in the IPTW-adjusted cohort was <0.10, denoting negligible imbalance between groups, or at most <0.25.
Lastly, each of the clinicopathologic characteristics along with cohort type (SLN vs LND) were evaluated for an association with OS and RFS, respectively, using the combined unadjusted cohorts. Univariate Cox proportional hazards models were fitted along with multivariable models using stepwise and backward variable selection methods. For the node-negative subgroup, the analysis for death was restricted to univariate models due to the small number of deaths. Associations were summarized by reporting hazard ratio (HR) and corresponding 95% confidence intervals (CIs).
Results
Overall Cohort
Review of institutional databases identified 214 cases—118 in the SLN cohort and 96 in the LND cohort. Clinical and pathologic characteristics are shown in Table 1. Fifty-six patients (47.5%) in the SLN cohort and 29 (30%) in the LND cohort had no myometrial invasion. Thirty-four (29%) in the SLN cohort and 44 (46%) in the LND cohort had <50% invasion (P=0.02). Adjuvant therapy differed between the two cohorts; 84% (99/118) in the SLN cohort and 40% (38/96) in the LND cohort received chemotherapy ± some form of radiation therapy (P<0.001). The remaining characteristics were well balanced among the two cohorts (Table 1), and reasonable balance overall was attained after applying IPTW (Supplemental Figure 1).
Table 1:
Clinical and pathologic characteristics
| Characteristic | SLN Cohort (n=118) | LND Cohort (n=96) | P† |
|---|---|---|---|
| Age (years), mean (SD) | 66.2 (±8.2) | 66.3 (±11.6) | 0.93 |
| BMI (kg/m2), mean (SD) | 29.8 (±6.6) | 30.8 (±8.2) | 0.33 |
| FIGO grade 3, n (%) | 118 (100.0%) | 96 (100.0%) | -- |
| FIGO stage (2009), n (%) | 0.38 | ||
| I | 80 (67.8%) | 70 (72.9%) | |
| II | 7 (5.9%) | 2 (2.1%) | |
| III | 31 (26.3%) | 24 (25.0%) | |
| Myometrial invasion, n (%) | 0.02 | ||
| None | 56 (47.5%) | 29 (30.2%) | |
| <50% | 34 (28.8%) | 44 (45.8%) | |
| ≥50% | 28 (23.7%) | 23 (24.0%) | |
| LVSI, n (%) | 44 (37.3%) | 25 (26.0%) | 0.08 |
| Cervical stromal invasion, n (%) | 11 (9.3%) | 4 (4.2%) | 0.14 |
| Malignant peritoneal cytology, n (%)‡ | 19/115 (16.5%) | 16/87 (18.4%) | 0.73 |
| Adjuvant therapy, n (%) | <0.001 | ||
| None | 10 (8.5%) | 22 (22.9%) | |
| IVRT | 7 (5.9%) | 27 (28.1%) | |
| EBRT ± IVRT | 1 (0.8%) | 2 (2.1%) | |
| Chemotherapy ± IVRT | 85 (72.0%) | 31 (32.3%) | |
| Chemotherapy and EBRT ± IVRT | 14 (11.9%) | 7 (7.3%) | |
| Unknown | 1 (0.8%) | 7 (7.3%) | |
SD=standard deviation, BMI=body mass index, FIGO=International Federation of Gynecology and Obstetrics, LVSI=lymphovascular space invasion, IVRT=intravaginal brachytherapy, EBRT=external beam radiation therapy.
Comparisons between groups were evaluated using the two-sample t test for age and BMI, and the chi-square or Fisher’s exact test for all other categorical characteristics.
12 patients (3 in the SLN cohort and 9 in the LND cohort) with unknown peritoneal cytology sampling were ignored from the univariate analysis due to no peritoneal cytology sampling.
Three patients (2.5%) in the SLN cohort and 13 (13.5%) in the LND cohort did not have a pelvic lymphadenectomy (P=0.002, Table 2). The median number of pelvic lymph nodes removed in the SLN cohort was 11 (interquartile range [IQR]: 5, 16) compared with 30 (IQR: 26, 41) in the LND cohort (P<0.001). In patients who had pelvic lymph nodes assessed, positive lymph nodes were identified in 22% (25/115) in the SLN cohort and 20% (17/83) in the LND cohort (P=0.83). More patients in the LND cohort compared with the SLN cohort had paraaortic lymph nodes removed (81% vs 47%, P<0.001). The median number of paraaortic lymph nodes removed was 4 (IQR: 2, 9) in the SLN cohort compared with 17 (IQR: 11, 23) in the LND cohort (P<0.001). The detection of metastatic paraaortic lymph nodes in the group as a whole was similar; 7.6% of patients in the SLN cohort compared with 11.5% in the LND cohort had positive paraaortic lymph nodes (P=0.34). This remained true when evaluating only patients with paraaortic lymph nodes removed; 16% (9/55) of these patients in the SLN cohort and 14% (11/78) in the LND cohort had positive paraaortic lymph nodes (P=0.72).
Table 2:
Lymphadenectomy characteristics
| Characteristic | SLN Cohort (n=118) | LND Cohort (n=96) | P† |
|---|---|---|---|
| Pelvic LND, n (%) | 0.002 | ||
| No | 3 (2.5%) | 13 (13.5%) | |
| Yes | 115 (97.5%) | 83 (86.5%) | |
| Number of pelvic lymph nodes removed, median (IQR)* | 11 (5, 16) | 30 (26, 41) | <0.001 |
| Positive pelvic lymph nodes, n (%) | 0.52 | ||
| No or pelvic LND not performed | 93 (78.8%) | 79 (82.3%) | |
| Yes | 25 (21.2%) | 17 (17.7%) | |
| Positive pelvic lymph nodes, n (%)* | 0.83 | ||
| No | 90 (78.3%) | 66 (79.5%) | |
| Yes | 25 (21.7%) | 17 (20.5%) | |
| Paraaortic LND, n (%) | <0.001 | ||
| No | 63 (53.4%) | 18 (18.8%) | |
| Yes | 55 (46.6%) | 78 (81.3%) | |
| Number of paraaortic lymph nodes removed, median (IQR)* | 4 (2, 9) | 17 (11, 23) | <0.001 |
| Positive paraaortic lymph nodes, n (%) | 0.34 | ||
| No or paraaortic LND not performed | 109 (92.4%) | 85 (88.5%) | |
| Yes | 9 (7.6%) | 11 (11.5%) | |
| Positive paraaortic lymph nodes, n (%)* | 0.72 | ||
| No | 46 (83.6%) | 67 (85.9%) | |
| Yes | 9 (16.4%) | 11 (14.1%) | |
IQR=interquartile range, LND=lymphadenectomy.
Limited to patients with specified lymphadenectomy.
Comparisons between groups were evaluated using the chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for the number of nodes.
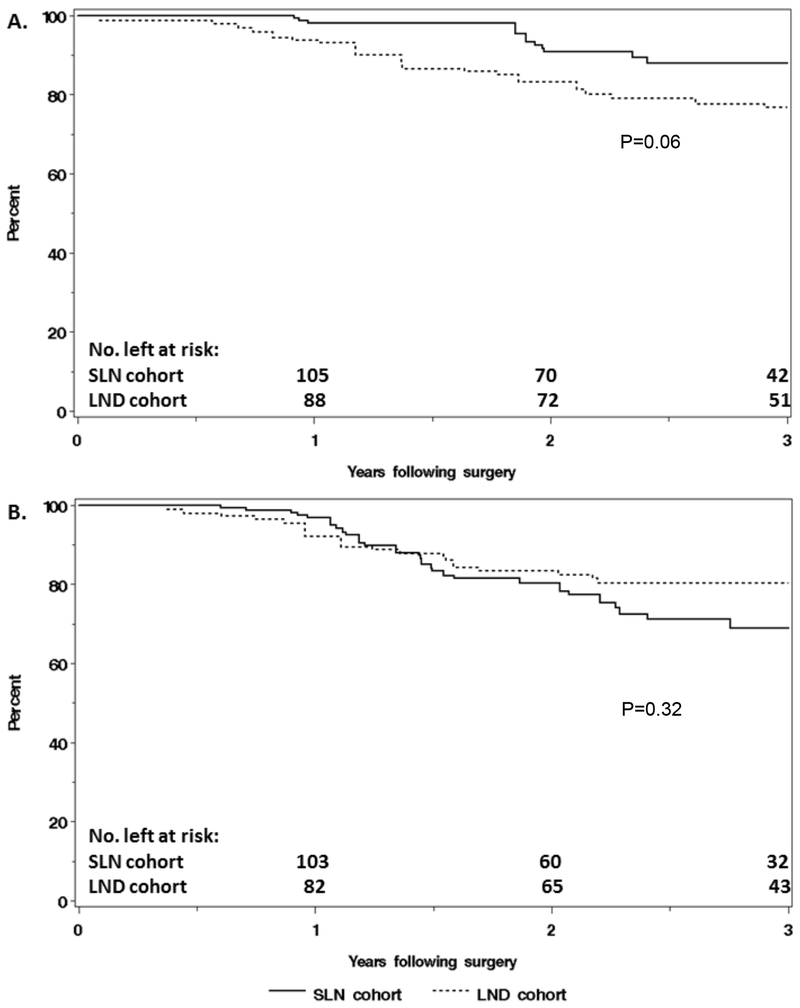
The overall median follow-up time, without restricting to the first 3 years following surgery, was 2.3 years (IQR: 1.6, 3.3) in the SLN cohort and 3.2 years (IQR: 2.0, 4.5) in the LND cohort. The IPTW-adjusted HR for the association of surgical approach (SLN vs LND) with death due to any cause was 0.44 (95% CI: 0.19, 1.02), with IPTW-adjusted 3-year OS rates of 88% and 77%, respectively (Table 3, Figure 1A). There was no statistically significant association between surgical approach and progression. The IPTW-adjusted HR was 1.46 (95% CI: 0.70,3.04), with IPTW-adjusted 3-year RFS rates of 69% in the SLN cohort and 80% in the LND cohort (Table 3, Figure 1B). In the SLN cohort, there were 13 hematogenous and/or peritoneal, 1 vaginal, 6 lymphatic, and 7 hematogenous and/or peritoneal and lymphatic progressions (n=27). In the LND cohort, there were 4 hematogenous and/or peritoneal, 2 vaginal, 1 hematogenous and/or peritoneal and vaginal, 3 lymphatic, 4 hematogenous and/or peritoneal and lymphatic, and 2 unknown site progressions (n=16).
Table 3.
Comparison of outcomes of the two different surgical approaches within the first 3 years following surgery for all patients and the subgroup of node-negative patients, respectively
| Outcome | No. of events within 3 years | IPTW-adjusted 3-year Kaplan-Meier rates, % (95% CI) |
IPTW-adjusted HR for SLN vs. LND |
||
|---|---|---|---|---|---|
| SLN cohort | LND cohort | HR (95% CI) | P | ||
| All patients | |||||
| Death | 29 | 87.9 (79.9, 96.7) | 76.8 (67.1, 87.9) | 0.44 (0.19, 1.02) | 0.06 |
| Recurrence | 43 | 68.9 (57.8, 82.2) | 80.3 (70.9, 90.9) | 1.46 (0.70, 3.04) | 0.32 |
| Node-negative patients | |||||
| Death | 17 | 88.5 (79.7, 98.2) | 86.4 (77.9, 95.9) | 0.69 (0.24, 1.95) | 0.48 |
| Recurrence | 25 | 72.9 (61.2, 86.8) | 91.4 (84.3, 99.3) | 3.12 (1.02, 9.57) | 0.05 |
CI=confidence interval, HR=hazard ratio, IPTW=inverse probability of treatment weighting.
Figure 1.
Comparison of (A) overall survival and (B) recurrence-free survival, adjusted using inverse-probability of treatment weighting, between patients in the sentinel lymph node (SLN) cohort (n=118) and the lymphadenectomy (LND) cohort (n=96).
A multivariable analysis was performed using the combined unadjusted cohorts. On multivariable analysis, age (adjusted HR: 1.51; 95% CI: 1.04, 2.18), myometrial invasion ≥50% (adjusted HR: 2.76; 95% CI: 1.32, 5.76), and malignant peritoneal cytology (adjusted HR: 3.02; 95% CI: 1.40, 6.51) were associated with death. Malignant peritoneal cytology remained associated with RFS on multivariable analysis (adjusted HR: 2.54; 95% CI: 1.31, 4.93), as were the presence of LVSI (adjusted HR: 2.38; 95% CI: 1.23, 4.61), and FIGO stage III disease (vs. stage I; adjusted HR: 2.46; 95% CI: 1.23, 4.94).
Node-Negative Subgroup
Ninety-two patients in the SLN cohort and 76 in the LND cohort were either found to have lymph nodes negative for metastasis or had no lymphadenectomy performed. The differences in the clinical and pathologic features between the cohorts in this subgroup were similar to the group as a whole (Table 4). More patients in the SLN cohort had no myometrial invasion on final pathology (53% vs 33%), and fewer patients in the SLN cohort had <50% myometrial invasion (27% vs 49%) (P=0.01). In the SLN cohort, 10% (9/92) of patients received no adjuvant therapy compared with 26% (20/76) in the LND cohort (P<0.001). More patients in the SLN cohort had pelvic lymph nodes removed (97% vs 84%, P=0.005), but fewer patients had paraaortic lymph nodes removed (41% vs 76%, P<0.001). The balance between the cohorts attained after applying IPTW was improved; however, for one covariate, the magnitude of the standardized difference was still above 0.25 (Supplemental Figure 2).
Table 4.
Clinical and pathologic characteristics of patients with either negative lymph nodes or no lymphadenectomy performed
| Characteristic | SLN Cohort (n=92) | LND Cohort (n=76) | P† |
|---|---|---|---|
| Age (years), mean (SD) | 66.0 (±8.6) | 66.6 (±12.2) | 0.69 |
| BMI (kg/m2), mean (SD) | 29.8 (±6.6) | 30.5 (±7.8) | 0.57 |
| FIGO grade 3, n (%) | 92 (100.0%) | 76 (100.0%) | -- |
| FIGO stage (2009), n (%) | 0.45 | ||
| I | 80 (87.0%) | 70 (92.1%) | |
| II | 7 (7.6%) | 2 (2.6%) | |
| III | 5 (5.4%) | 4 (5.3%) | |
| Myometrial invasion, n (%) | 0.01 | ||
| None | 49 (53.3%) | 25 (32.9%) | |
| <50% | 25 (27.2%) | 37 (48.7%) | |
| ≥50% | 18 (19.6%) | 14 (18.4%) | |
| LVSI, n (%) | 26 (28.3%) | 15 (19.7%) | 0.20 |
| Cervical stromal invasion, n (%) | 8 (8.7%) | 2 (2.6%) | 0.11 |
| Malignant peritoneal cytology, n (%)‡ | 12/89 (13.5%) | 9/67 (13.2%) | 0.96 |
| Pelvic LND, n (%) | 0.005 | ||
| No | 3 (3.3%) | 12 (15.8%) | |
| Yes | 89 (96.7%) | 64 (84.2%) | |
| Paraaortic LND, n (%) | <0.001 | ||
| No | 54 (58.7%) | 18 (23.7%) | |
| Yes | 38 (41.3%) | 58 (76.3%) | |
| Adjuvant therapy, n (%) | <0.001 | ||
| None | 9 (9.8%) | 20 (26.3%) | |
| IVRT | 7 (7.6%) | 27 (35.5%) | |
| EBRT ± IVRT | 1 (1.1%) | 1 (1.3%) | |
| Chemotherapy ± IVRT | 69 (75.0%) | 21 (27.6%) | |
| Chemotherapy and EBRT ± IVRT | 6 (6.5%) | 1 (1.3%) | |
| Unknown | 0 (0%) | 6 (7.9%) | |
SD=standard deviation, BMI=body mass index, LVSI=lymphovascular space invasion, LND=lymphadenectomy.
Comparisons between groups were evaluated using the two-sample t-test for age and BMI, and the chi-square or Fisher’s exact test for all other categorical characteristics.
11 patients (3 in the SLN cohort and 8 in the LND cohort) with unknown peritoneal cytology sampling were ignored from the univariate analysis due to no peritoneal cytology sampling.
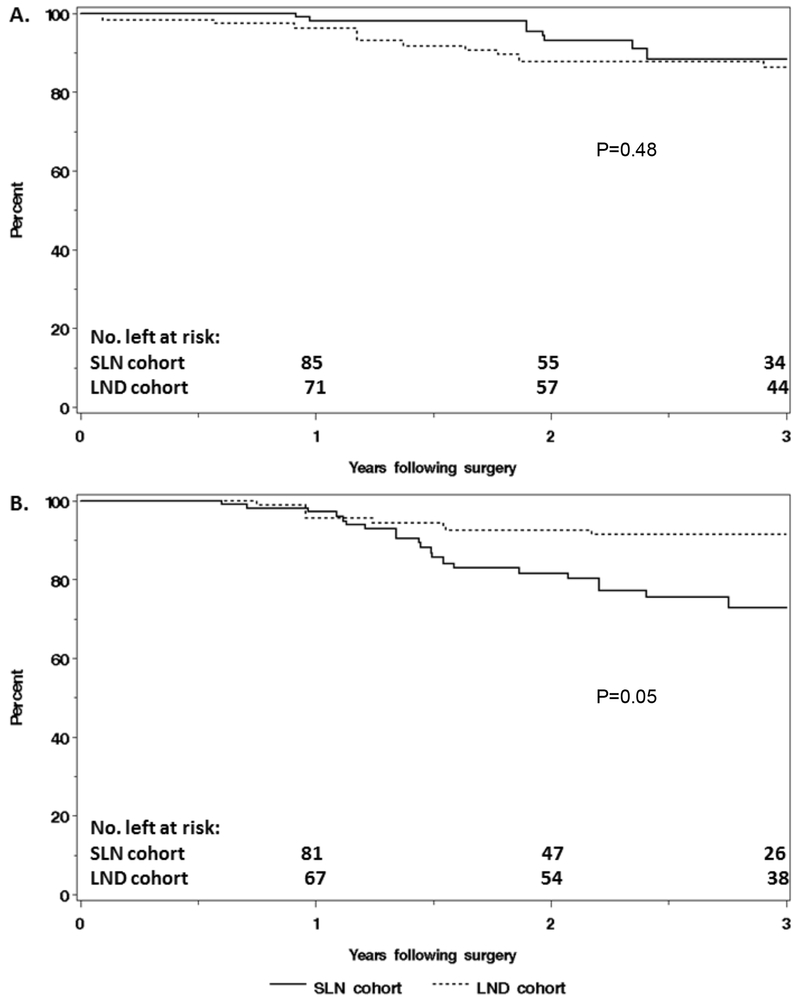
The overall median follow-up time, without restricting to the first 3 years following surgery, for the subgroup with either negative lymph nodes or no lymphadenectomy performed was 2.4 years (IQR: 1.6, 3.4) for the SLN cohort and 3.3 years (IQR: 2.1, 4.5) for the LND cohort. The IPTW-adjusted HR for the association of surgical approach (SLN vs LND) with death was 0.69 (95% CI: 0.24, 1.95), with IPTW-adjusted 3-year OS rates of 88% and 86%, for the SLN and LND cohorts, respectively (Table 3, Figure 2A). For progression, the IPTW-adjusted HR was 3.12 (95% CI: 1.02, 9.57), with IPTW-adjusted 3-year RFS rates of 73% and 91% in the SLN and LND cohorts, respectively (Table 3, Figure 2B).
Figure 2.
Comparison of (A) overall survival and (B) recurrence-free survival, adjusted using inverse-probability of treatment weighting, between node-negative patients in the sentinel lymph node (SLN) cohort (n=92) and the lymphadenectomy (LND) cohort (n=76).
On univariate analysis, myometrial invasion ≥50% (HR: 3.69; 95% CI: 1.43, 9.58) and LVSI (HR: 3.54; 95% CI: 1.37, 9.18) were each associated with death, but neither was statistically significant when included together in a multivariable model. On multivariable analysis, cohort (SLN referent, adjusted HR: 0.38; 95% CI: 0.15, 0.97) and LVSI (adjusted HR: 4.34; 95% CI: 1.95, 9.64) were associated with progression.
Discussion
Patients with serous and clear cell endometrial carcinoma are at an increased risk of nodal metastasis compared to patients with endometrioid endometrial carcinoma, regardless of depth of invasion [2]. Therefore, the decision to perform a lymphadenectomy in these tumors should not be based on uterine features. We demonstrated no adverse effect on OS with the use of an SLN algorithm compared to a complete LND in patients with apparent uterine-confined serous and clear cell endometrial carcinoma. This remained true when only the cohort of patients with negative lymph nodes was assessed. There did appear to be a difference in RFS in the node-negative subgroup, with patients in the SLN cohort having a worse RFS compared to the LND cohort. This is likely due in part to differing surveillance strategies between the two cohorts; patients in the SLN cohort received scheduled surveillance with computed tomography while patients in the LND cohort were followed by imaging at the treating physician’s discretion. OS was essentially the same in this group, however, and there appeared to be a trend toward improved OS in the study population as whole in the SLN cohort (P=0.06). These data are hypothesis generating, and more data are needed; however, when considered with other currently available data, use of an SLN algorithm in non-endometrioid endometrial carcinomas does not appear to adversely affect outcomes.
Multiple studies have demonstrated a low false-negative detection rate using an SLN algorithm in high-risk endometrial carcinoma, including serous and clear cell carcinomas [12–15]. Soliman et al enrolled patients with high-risk endometrial carcinoma (grade 3 endometrioid, serous, clear cell, or carcinosarcoma) in a prospective study evaluating the false-negative rate associated with an SLN algorithm. In the 101 evaluable patients, the sensitivity of SLN detection was 95% and the false-negative rate was 4.3% when the SLN algorithm was applied [13]. Similarly, Ehrisman et al evaluated patients with presumed uterine-confined grade 3 endometrioid adenocarcinoma, clear cell carcinoma, serous carcinoma, and carcinosarcoma who underwent SLN injection and biopsy followed by completion LND. When the SLN algorithm was applied, the false-negative rate was 0% and the negative predictive value was 100% [12]. Ducie et al reported no difference in the stage IIIC detection rate between a United States cohort of serous and clear cell endometrial carcinoma patients who underwent staging with an SLN algorithm versus an international cohort of patients who underwent staging with a complete pelvic and paraaortic LND (P=0.3) [16]. These data support the adequate detection of metastatic lymphatic disease using an SLN algorithm in high-risk endometrial carcinoma.
Some researchers have argued that LND is not only diagnostic but therapeutic as well, particularly in “high-risk” disease. In a retrospective analysis of 649 patients, Kilgore et al reported that those with multi-site pelvic lymph node sampling had improved OS compared to those with no lymph node sampling (P=0.0002). This survival advantage remained when patients with either grade 3 disease or deep myometrial invasion were treated with postoperative radiation therapy (P=0.0009) [17]. Mahdi et al performed a review of the Surveillance, Epidemiology, and End Results (SEER) data of 4178 women with serous endometrial cancer and found that any LND, as well as a more extensive LND, were associated with improved 5-year OS, even in patients with negative lymph nodes [18]. The SEPAL trial compared survival following surgical staging with pelvic nodal assessment versus pelvic and paraaortic nodal assessment in a retrospective fashion. In that study, all stages of serous or clear cell endometrial cancer were considered to be either intermediate- or high-risk. The authors reported improved overall, disease-specific, and recurrence-free survival in the intermediate- and high-risk groups with the addition of paraaortic LND. Of note, more patients in the paraaortic LND group received adjuvant chemotherapy, and serous and clear cell histology accounted for only 13% of the entire cohort [19]. The results of these retrospective reports are in contrast to the results of a prospective randomized trial. The MRC ASTEC trial did not demonstrate a survival difference between patients who underwent a systematic LND compared to no LND; however, serous and clear cell histology accounted for only 11 % of the study population, and adjuvant radiation therapy was randomized as opposed to being based on high-risk disease characteristics [20]. None of these studies included SLN detection, which identifies and removes the lymph nodes at the highest risk for disease spread. Schiavone et al compared PFS using an SLN algorithm in serous uterine carcinoma to a historical cohort of full LND at one institution and found no difference in the 2-year PFS rates between the two cohorts (77% vs 71%, respectively; P=0.3). This study is particularly important, as the two cohorts had similar rates of adjuvant treatment and similar mechanisms of follow-up, negating some of the confounding factors present in the current study [21]. In another multi-institutional retrospective study, Multinu et al evaluated survival outcomes in patients with deeply invasive endometrioid endometrial adenocarcinoma and non-endometrioid histologies with any invasion with non-bulky positive lymph nodes identified by SLN technique versus full LND. Approximately 35% of the patients in each cohort had a non-endometrioid histology. Though a small study, cohort (SLN versus LND) was not associated with PFS, and specifically, there was no difference in nodal recurrences between the two groups [Francesco Multinu, MD; Personal Communication; July 3, 2019]. These data suggest that the value of LND in these patients is in identifying nodal disease instead of removing all potentially involved lymph nodes.
When evaluating all patients with positive and negative lymph nodes in our study, cohort (SLN vs LND) was not a significant factor for RFS or OS on univariate or multivariable analysis. When only patients with negative lymph nodes or unassessed lymph nodes were considered, the SLN cohort was associated with a decreased RFS in adjusted analyses, but no difference was found in OS between the cohorts. Of note, this difference in RFS was identified despite the fact that more patients in the node-negative SLN cohort received adjuvant therapy and the median follow-up time was longer for the LND cohort. This is due to differing practice patterns between the two institutions in this study during the time periods evaluated. Previously published data regarding the benefits of adjuvant treatment in stage I uterine serous carcinoma are conflicting. In a multi-institutional retrospective study, Fader et al reported a lower risk of recurrence (11.2%) in patients treated with chemotherapy +/− radiation therapy compared to those treated with radiation alone (25%, P=0.146) or observation (30.3%, P=0.016) [22]. Another multi-institutional retrospective report by Huh et al demonstrated no difference in disease-free survival (DFS) or OS between stage I uterine serous carcinoma patients who received adjuvant radiation therapy (5-year DFS rate, 60%; 5-year OS rate, 59%) versus observation (5-year DFS rate, 65%; 5-year OS rate, 66%) [23]. The recently published PORTEC-3 randomized trial evaluated radiation therapy alone versus chemotherapy with radiation in high-risk endometrial cancer, with serous and clear cell carcinoma accounting for 25% of their population. They demonstrated no difference in OS but improved DFS in the chemotherapy plus radiation group [24]. GOG-258 evaluated chemotherapy with radiation therapy followed by additional chemotherapy compared to chemotherapy alone in early stage (I or II) clear cell or serous endometrial cancer and advanced-stage (stage III or IVA) endometrioid endometrial cancer with <2 cm of residual disease. Similar to PORTEC-3, the serous and clear cell endometrial cancers accounted for approximately 20% of the study population. This trial demonstrated no difference in relapse-free survival between the two groups; however, the risk of regional recurrence was higher in the chemotherapy-only group [25]. Continued investigation is needed to evaluate the role of adjuvant treatment in surgically staged stage I uterine serous and clear cell carcinoma.
The primary strength of our study is the comparison of two groups of patients with serous or clear cell carcinoma of the endometrium in whom the only difference in their surgical staging was the lymph node evaluation technique. Limitations to our study include all the limitations inherent to a retrospective cohort, but particularly, differences between the cohorts with regards to adjuvant treatment and surveillance. In this relatively small study, we did not demonstrate a difference in OS between the two cohorts; however, more patients in the SLN cohort received adjuvant treatment. Given the retrospective nature of this study, we cannot comment on whether omitting adjuvant treatment in these patients would have affected their survival. We did evaluate the effect of adjuvant treatment by multivariable analysis and it was not found to be associated with RFS or OS in our study as a whole or in the node-negative subgroup. Our study also had a relatively limited follow-up period, which could cause some recurrences in either group to be missed; however, most recurrences occur within the first 2-3 years after primary treatment, and therefore, the majority should be represented in our cohort. Another potential confounder is the learning curve associated with the SLN technique in the early years of our data collection. This potentially led to more pelvic and paraaortic LNDs early in the SLN cohort, which decreased over time as surgeons became more comfortable with the technique. We are unable to comment on the rate of isolated paraaortic lymph node metastases; however, the rate of paraaortic nodal positivity is similar between the two groups and there was no increased rate of nodal recurrences in the SLN cohort. In the literature, the rate of isolated paraaortic lymph nodes is approximately 1% [26]. We also did not assess perioperative or long-term adverse effects of either intervention or the morbidity associated with treatment as a whole. We utilized propensity score methodology to minimize observed confounding and potentially obtain less biased outcomes comparisons between the cohorts. However, residual and unmeasured confounding may still exist between the two cohorts.
In conclusion, OS is not compromised with the use of an SLN algorithm in serous or clear cell endometrial cancer. In the cohort of patients with negative lymph nodes, OS was similar but RFS shorter with the use of the algorithm, despite more patients receiving adjuvant chemotherapy. This may be due to differences in surveillance between the two cohorts; however, more investigation is needed into this subgroup.
Supplementary Material
Research Highlights.
Overall survival in non-endometrioid endometrial carcinoma appears uncompromised using a sentinel lymph node algorithm
With negative nodes, recurrence-free survival is shorter with a sentinel node algorithm, but overall survival is similar
Lymphatic recurrences do not appear increased with sentinel lymph node assessment in non-endometrioid endometrial carcinoma
Acknowledgments
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748 (Drs. Nadeem R. Abu-Rustum and Mario M. Leitao Jr)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Outside the submitted work, Dr. Abu-Rustum reports grants from Stryker/Novadaq, Olympus, and GRAIL. Outside the submitted work, Dr. Leitao is an ad hoc speaker for Intuitive Surgical, Inc. The other authors have no potential conflicts to disclose.
References
- [1].Boruta II DM, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: A Society of Gynecologic Oncology (SGO) review. Gynecologic Oncology. 2009;115:142–53. [DOI] [PubMed] [Google Scholar]
- [2].Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine Papillary Serous Carcinoma: Patterns of Metastatic Spread. Gynecologic Oncology. 1994;54:264–8. [DOI] [PubMed] [Google Scholar]
- [3].Gehrig PA, Groben PA, Fowler WC Jr., Walton LA, Van Le L. Noninvasive Papillary Serous Carcinoma of the Endometrium. Obstetrics and Gynecology. 2001;97:153–7. [DOI] [PubMed] [Google Scholar]
- [4].Chan JK, Loizzi V, Youssef M, Osann K, Rutgers J, Vasilev SA, et al. Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecologic Oncology. 2003;90:181–5. [DOI] [PubMed] [Google Scholar]
- [5].Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Lowery WJ, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecologic Oncology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koh W-J, Greer BE, Abu-Rustum NR. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Uterine Neoplasms: National Comprehensive Cancer Network; 2017. [Google Scholar]
- [7].D’Agostino RB Jr. Propensity scores in cardiovascular research. Circulation. 2007;115(17):2340–3. [DOI] [PubMed] [Google Scholar]
- [8].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. The improtance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecologic Oncology. 2012;125:531–5. [DOI] [PubMed] [Google Scholar]
- [9].Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic Ultrastaging Improves Micrometastasis Detection in Sentinel Lymph Nodes During Endometrial Cancer Staging. International Journal of Gynecological Cancer. 2013;23:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–8. [DOI] [PubMed] [Google Scholar]
- [11].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ehrisman J, Secord AA, Berchuck A, Lee PS, Di Santo N, Lopez-Acevedo M, et al. Performance of sentinel lymph node biopsy in high-risk endometrial cancer. Gynecologic Oncology Reports. 2016;17:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecologic Oncology. 2017;146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Touhami O, Gregoire J, Marie-Claude R, Sebastianelli A, Plante M. Performance of sentinel lymph node (SLN) mapping in high-risk endometrial cancer. Gynecologic Oncology. 2017;147. [DOI] [PubMed] [Google Scholar]
- [15].Persson J, Salehi S, Bollino M, Lonnerfors C, Falconer H, Geppert B. Pelvic sentinel lymph node detection in high-risk endometrial cancer (SHREC-trial)- the final step towards a paradigm shift in surgical staging. European Journal of Cancer. 2019;116:77–85. [DOI] [PubMed] [Google Scholar]
- [16].Ducie JA, Eriksson AGZ, Mosgaard BJ, Nedergaard L, Lajer H, Abu-Rustum NR, et al. An international comparison of surgically staged patients with serous and clear cell endometrial carcinoma. Gynecologic Oncology. 2015;137:33. [Google Scholar]
- [17].Kilgore LC, Partridge EE, Alvarez RD, Austin JM, Shingleton HM, Noojin III F, et al. Adenocarcinoma of the Endometrium: Survival Comparisons of Patients with and without Pelvic Node Sampling. Gynecologic Oncology. 1995:29–33. [DOI] [PubMed] [Google Scholar]
- [18].Mahdi H, Kumar S, Al-Wahab Z, Ali-Fehmi R, Munkarah AR. Prognostic impact of lymphadenectomy in uterine serous cancer. BJOG. 2013;120:384–91. [DOI] [PubMed] [Google Scholar]
- [19].Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of paraaortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010:1165–72. [DOI] [PubMed] [Google Scholar]
- [20].ASTEC sg. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schiavone MB, Scelzo C, Straight C, Zhou Q, Alektiar KM, Makker V, et al. Survival of Patients with Serous Uterine Carcinoma Undergoing Sentinel Lymph Node Mapping. Ann Surg Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fader AN, Drake RD, O’Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, et al. Platinum/Taxane-based Chemotherapy With or Without Radiation Therapy Favorably Impacts Survival Outcomes in Stage I Uterine Papillary Serous Carcinoma. American Cancer Society. 2009;115:2119–27. [DOI] [PubMed] [Google Scholar]
- [23].Huh WK, Powell MA, Leath III CA, Straughn M Jr., Cohn DE, Gold MA, et al. Uterine papillary serous carcinoma: comparisons of outcomes in surgical Stage I patients with and without adjuvant therapy. Gynecologic Oncology. 2003;91:470–5. [DOI] [PubMed] [Google Scholar]
- [24].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncology. 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matei D, Filiaci VL, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. The New England Journal of Medicine. 2019;380:2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abu-Rustum NR, Gomez JD, Alektiar KM, Soslow RA, Hensley ML, Leitao MM Jr, Gardner GJ, Sonoda Y, Chi DS, Barakat RR. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;115(2):236–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.