Abstract
Carbon dioxide has been used in traps for more than six decades to monitor mosquito populations and help make informed vector management decisions. CO2 is sensed by gustatory receptors (GRs) housed in neurons in the maxillary palps. CO2-sensitive GRs have been identified from the vinegar fly and mosquitoes, but it remains to be resolved whether these receptors respond to CO2 or bicarbonate. As opposed to the vinegar fly, mosquitoes have three GR subunits, but it is assumed that subunits GR1 and GR3 form functional receptors. In our attempt to identify the chemical species that bind these receptors, we discovered that GR2 and GR3 are essential for receptor function and that GR1 appears to function as a modulator. While Xenopus oocytes coexpressing Culex quinquefasciatus subunits CquiGR1/3 and CquiGR1/2 were not activated, CquiGR2/3 gave robust responses to sodium bicarbonate. Interestingly, CquiGR1/2/3-coexpressing oocytes gave significantly lower responses. That the ternary combination is markedly less sensitive than the GR2/GR3 combination was also observed with orthologs from the yellow fever and the malaria mosquito. By comparing responses of CquiGR2/CquiGR3-co-expressing oocytes to sodium bicarbonate samples (with or without acidification) and measuring the concentration of aqueous CO2, we showed that there is a direct correlation between dissolved CO2 and receptor response. We then concluded that subunits GR2 and GR3 are essential for these carbon dioxide-sensitive receptors and that they are activated by CO2 per se, not bicarbonate.
Keywords: Culex quinquefasciatus, Anopheles gambiae, Aedes aegypti, CquiGR1, CquiGR2, CquiGR3
1. Introduction
Carbon dioxide is the oldest known and the most powerful mosquito attractant (Van Thiel and Weurman, 1947). It has been widely used for decades for trapping host-seeking female mosquitoes (Reeves, 1951, 1953) to monitoring populations and to assist in integrated vector management programs. In addition to being an attractant sensu stricto, CO2 gates mosquito perception of other sensory modalities (Gillies, 1980; Liu and Vosshall, 2019; McMeniman et al., 2014). In the vinegar fly, Drosophila melanogaster, CO2 elicits through different pathways attraction and aversion behaviors (van Breugel et al., 2018). Mosquitoes sense CO2 with olfactory receptor neurons (ORNs) (Grant et al., 1995; Majeed et al., 2017; Syed and Leal, 2007) housed in peg sensilla ( = capitate pegs) in the maxillary palps (McIver and Charlton, 1970). It has been unambiguously demonstrated that CO2 is sensed by the gustatory receptors DmelGR21a and DmelGR63a, housed in antennal neuron ab1C of the vinegar fly (Jones et al., 2007; Kwon et al., 2007; Suh et al., 2004), but it remains to be resolved whether carbon dioxide receptors are activated by CO2 per se or bicarbonate (Jones et al., 2007; Kwon et al., 2007; Ning et al., 2016; Xu and Anderson, 2015).
Mosquitoes have three closely related homologs of DmelGR21a and DmelGR63a (Robertson and Kent, 2009), GR1, GR2, and GR3. Because they were identified before the new nomenclature for these GR genes was proposed (Robertson and Kent, 2009), GR1–3 in the malaria mosquito, Anopheles gambiae, are still named AgamGR22, AgamGR23, and AgamGR24, respectively (Hill et al., 2002). While it has been clearly demonstrated that GR3 is essential for CO2 reception in the yellow fever mosquito, Aedes aegypti (McMeniman et al., 2014), it is not yet known whether GR1 and GR2 are functionally redundant (McMeniman et al., 2014). While no response to CO2 was recorded when both AgamGR22 and AgamGR24 (one copy of each) were coexpressed in the empty neuron system of the vinegar fly (Hallem et al., 2004), by increasing the dosage of both transgenes by two-fold led to significant response (Lu et al., 2007). Additionally, flies carrying one copy of each of the three subunits showed a significant response to CO2, albeit not as strong as responses recorded from the flies carrying two-fold of AgamGR22 and 24 (Lu et al., 2007). Thus, AgamGR23 was implicated in CO2 reception in the malaria mosquito, but its role was not clarified. On the other hand, by using RNAi combined with behavioral measurements, it has been suggested that AaegGR2 had no role in CO2 reception by the yellow fever mosquito (Erdelyan et al., 2012). In summary, it remains to be determined whether all three GR subunits are functionally required for CO2 detection in mosquitoes or whether GR1 and GR2 are functionally redundant (McMeniman et al., 2014).
We envisioned that having a functional carbon dioxide-detecting system in an aqueous environment as in the Xenopus oocyte recording system would allow us to address with the Le Chatelier’s principle the long-standing question whether carbon dioxide receptors are activated by CO2 per se or by bicarbonate (Jones et al., 2007; Kwon et al., 2007; Ning et al., 2016; Xu and Anderson, 2015). Because dissolved CO2 forms an equilibrium with bicarbonate in water , a shift of the equilibrium towards dissolved CO2 would increase a receptor response if CO2 per se binds carbon dioxide receptors, whereas a reduced response would indicate activation by bicarbonate. Here, we show that CO2 per se, not bicarbonate, activates the receptors. While preparing to address this question, we discovered that the gustatory receptor CquiGR2 from the southern house mosquito, Culex quinquefasciatus, is essential for function, whereas CquiGR1 appears to be a modulator. Additionally, we show a similar GR1 effect in the Ae. aegypti and An. gambiae CO2-sensing system. Specifically, CO2 elicited weaker responses in the ternary receptor systems than in the respective binary counterparts devoid of GR1 (GR22 in the case of the malaria mosquito receptors).
2. Material and methods
2.1. RNA extraction, DNA synthesis, and cloning
Three hundred pairs of palps from 4- to 6-d-old female Culex mosquitoes were dissected under a stereomicroscope (Zeiss, Stemi DR 1663) and collected in 75% (vol/vol) ethanol diluted in diethylpyrocarbonate (DEPC)-treated water on ice. The samples were centrifuged, and ethanol was removed before total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized from 1 μg of total RNA using GoScript™ Reverse Transcription kit according to the manufacturer’s instructions (Promega). For An. gambiae and Ae. aegypti, gene sequences of AgamGR22, 23, 24 and AaegGR1, 2, 3 from Genbank were synthesized by GenScript USA Inc. (Piscataway, NJ). Full-length gene-specific cloning primers were designed based on the sequences from Genbank and added 15-bp In-Fusion cloning adapters.
CqGR1-Fw GATCAATTCCCCGGGaccATGATTCACAGTCAGATGGAGGACG
CqGR1-Rv CAAGCTTGCTCTAGACTATTGTGGGTTCGTTTTGGCCG.
CqGR2-Fw GATCAATTCCCCGGGaccATGGTCATCAAGGACAGTGACTTTGAC
CqGR2-Rv CAAGCTTGCTCTAGATTAGTGAGCGTGAGCTTTCTGTAAATTCTC.
CqGR3-Fw GATCAATTCCCCGGGaccATGAGCATATTTCCGGATACTCTGCG
CqGR3-Rv CAAGCTTGCTCTAGATCAATGCGCTGCCGTCG.
AgGR22-Fw GATCAATTCCCCGGGaccATGATTCACACACAGATGGAAG
AgGR22-Rv CAAGCTTGCTCTAGATTAGGTGTTCACTTTGTCTGC.
AgGR23-Fw GATCAATTCCCCGGGaccATGGTTATCAAGGAAAGTGAGTTC
AgGR23-Rv CAAGCTTGCTCTAGATTACTGTTTGTGTAGCAGCTTAACA.
AgGR24-Fw GATCAATTCCCCGGGaccATGAGTCTCTACTTCAACGCGG.
AgGR24-Rv CAAGCTTGCTCTAGACTAAGAATGAGACGAATTACTGTGC.
AaGR1-Fw GATCAATTCCCCGGGaccATGATTCACAGCCAGATGGAAG
AaGR1-Rv CAAGCTTGCTCTAGACTAGTTCTCCTTCAGCTTAGTTAGA.
AaGR2-Fw GATCAATTCCCCGGGaccATGGTCATCAAAGACAGTGAGT
AaGR2-Rv CAAGCTTGCTCTAGACTATCCCTTATGACTGTGCTTGATT.
AaGR3-Fw GATCAATTCCCCGGGaccATGAATCTCAACCAAGACCCCA
AaGR3-Rv CAAGCTTGCTCTAGACTACTCGCGATATGAACCCGTCATA.
Adapter sequences are underlined, and lower case acc is Kozak consensus. PCR products were purified by Monarch® DNA Gel Extraction Kit (NEBioLab) and directly inserted to linearized destination vector-pGEMHE by In-Fusion reaction (Clontech). The colonies were picked, and vectors were extracted using Monarch® Plasmid Miniprep Kit. Inserts were submitted to DNA Sequencing Facility, UC Berkeley for verification of sequences.
2.2. Oocytes preparations and two-electrode voltage clamp recordings
The vectors carrying GR genes were linearized with restriction endonuclease NheI, except for AgamGR22, which was cut by SphI. The linearized vectors with gene inserts were used as templates to transcribe capped cRNAs with poly(A) using an mMESSAGE mMACHINE T7 kit (Ambion) following the manufacturer’s protocol. The cRNAs were dissolved in RNase-free water and adjusted at a concentration of 200 μg/mL by UV spectrophotometry (NanoDrop™ Lite Spectrophotometer, ThermoFisher). 9.2 nl of each cRNA samples were microinjected into stage V or VI Xenopus oocytes (purchased from EcoCyte Bioscience, Austin, TX). Then injected oocytes were incubated at 18 °C for 3–7 days in modified Barth’s solution [in mM: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, 10 HEPES, pH 7.4] supplemented with 10 μg/mL of gentamycin, 10 μg/mL of streptomycin, and 1.8 mM sodium pyruvate. For two-electrode voltage clamp (TEVC) recordings, as previously done (Xu et al., 2019), oocytes were placed in a perfusion chamber and challenged with test compounds. Compound-induced currents were amplified with an OC-725C amplifier (Warner Instruments, Hamden, CT), the voltage held at −80 mV, low-pass filtered at 50 Hz and digitized at 1 kHz. Data acquisition and analysis were carried out with Digidata 1440 A and pClamp10 software (Molecular Devices, LLC, Sunnyvale, CA).
2.3. Sample preparations and CO2 measurement
Sodium chloride (Fisher Scientific, > 99%) and sodium bicarbonate (Sigma-Aldrich, 99.7%) were used to prepare fresh samples by dissolving the appropriate amounts of these salts in perfusion Ringer buffer (NaCl 96 mM, KCl 2 mM, CaCl2 1.8 mM, MgCl2 1 mM, HEPES 5 mM, pH 7.6) to make 0.5 M solutions. Then, the desired concentrations were prepared by diluting with perfusion Ringer buffer. CO2 samples were prepared by bubbling MediPure™ CO2 (Praxair, Danbury, CT) directly into perfusion buffer for 5 s, and subsequently adjusting the pH.
The concentration of dissolved CO2, ie, CO2 (aq), was calculated by equation (1):
| (1) |
Koverall, which is sometimes referred to as Ka, is a constant, which incorporates the constant of hydrolysis of CO2 and the first dissociation constant of carbonic acid, Ka1, ie, Koverall = Kh × Ka1. pKa is 6.3 (https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-bicarbonate#section=pKa). The molar concentration of dissolved CO2 is, therefore, related to the pH of the solution and the nominal concentration of sodium bicarbonate. Thus, the concentration of dissolved CO2 in a 10 mM NaHCO3 solution at pH 7.73 is approximately 0.358 mM or 15.76 ppm. At pH 7.32, the calculated concentration of dissolved CO2 is 0.872 mM or 38.36 ppm.
pH and carbon dioxide were measured with an Orion Star™ A214 pH/ISE Benchtop Meter (ThermoFisher Scientific, Waltham, MA 02451). A carbon dioxide electrode was used for CO2 measurements. The internal filling solution of this electrode is separated from the sample solution with a gas-permeable membrane. CO2 dissolved in a sample solution diffuses through this membrane until an equilibrium between CO2 in the electrode filling and the sample solution is reached. The influx of CO2 causes a change in hydrogen ion concentration in the electrode filling solution, which is measured by a sensing element behind the membrane. The system was calibrated before measurements with freshly prepared 22, 55 and 110 ppm solutions. Standard solutions were sealed with parafilm before measurements. To minimize losses of carbon dioxide during measurements, we used 50 mL Falcon tubes to house sample solutions. A hole was opened in the tube cap, and the electrode was inserted and kept in place with two O-ring seals below and two O-ring seals on the top of the cap. Then the cap was sealed with Teflon tapes and finally covered with electrical tape. Fifty milliliter Falcon tubes housing test samples were attached to the electrode and fastened tightly. The concentrations of dissolved CO2 in bicarbonate solutions were measured by preparing 10, 25, 50, 100, 150, 200, 250, and 300 mM solutions in perfusion Ringer buffer, with four replicates for each sample. To compare the concentrations of dissolved CO2 without adjustment and after acidification, 7 mL aliquots of 10 mM sodium bicarbonate buffers were prepared in pairs. One of the two aliquots in a pair was used to determine CO2 concentration without pH change. To the other pair, 150 μl of 0.1 N HCl was added and immediately used for CO2 measurement, and then the pH was recorded. To compare oocyte response, samples were similarly prepared by using smaller aliquots (700 μl) in V-vials. In this case, 0.1 N HCl was injected through the Teflon liner of the open-top caps. After that, an aliquot was collected to be applied to the Xenopus oocyte recording system, and the remainder was used for pH measurement.
2.4. Statistical analysis and graphical preparations
Prism 8.2.0 from GraphPad Software (La Joya, CA) was used for both statistical analysis and graphical preparations. A dataset that passed the Shapiro-Wilk normality test was analyzed by t-test; otherwise, data were analyzed by Wilcoxon matched-pairs signed-rank test. All data are presented as mean ± SEM.
3. Results and discussion
3.1. Cx. quinquefasciatus gustatory receptor GR2/GR3 is sensitive to carbon dioxide
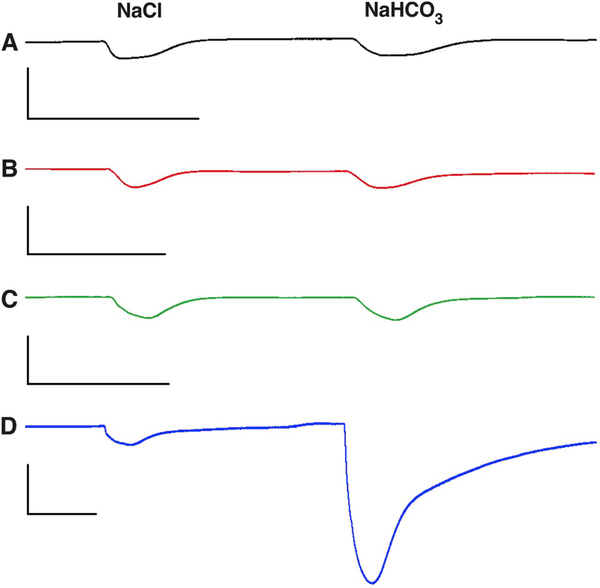
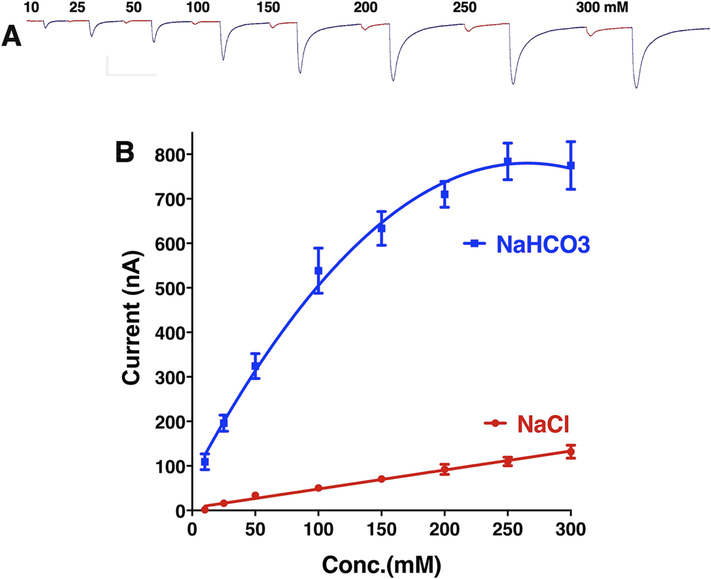
We cloned three gustatory receptors from the southern house mosquito, specifically CquiGR1 (VectorBase, CPIJ006622), CquiGR2 (GenBak, MN428502), and CquiGR3 (MN428503), which are likely to be involved in CO2 reception. Initially, we coexpressed combinations of these three GRs in the Xenopus laevis oocytes and recorded their responses to sodium bicarbonate. Of note, the application of sodium bicarbonate increases the sodium concentration in the perfusion Ringer, thus causing inward currents through endogenous oocyte channels due to an increase in the extracellular concentration of Na+ (Fig. 1A, Fig. S1). We, therefore, applied equivalent doses of NaCl and NaHCO3 in all experiments to control for the Na+ response. Equal responses to NaCl and NaHCO3 at the same dose, as in the case of naked oocytes (Fig. 1A), indicate that the currents were generated by Na+ ions. Surprisingly, oocytes coexpressing CquiGR1 and CquiGR3 did not respond to bicarbonate as the response to sodium bicarbonate did not differ from the response to sodium chloride (Fig. 1B, Fig. S1). A lack of specific response to sodium bicarbonate was also observed with CquiGR1/CquiGR2-coexpressing oocytes (Fig. 1C, Fig. S1). By contrast, CquiGR2/CquiGR3-expressing oocytes gave robust dose-dependent responses to sodium bicarbonate (Figs. 1D and 2, Fig. S1). Although it is not surprising that injections of sodium chloride generated dose-dependent currents, they were significantly smaller (t-test, P = 0.0008) than those generated by sodium bicarbonate at the same dose (Fig. 2).
Fig. 1. Responses of Oocytes to Sodium Chloride and Sodium Bicarbonate.
Representative traces obtained with the following types of oocytes from the same batch and age:
(A) Intact (naked) Xenopus oocytes. (B) Oocyte coexpressing CquiGR1 and CquiGR3 (C) Oocyte coexpressing CquiGR1 and CquiGR2 (D) Oocyte coexpressing CquiGR2 and CquiGR3. All scales are 200 nA and 0.5 min. Oocytes were first challenged with 200 mM NaCl and then 200 mM NaHCO3.
Fig. 2. Responses of CquiGR2 + CquiGR3-coexpressing oocytes to sodium chloride and sodium bicarbonate.
(A) Representative trace from a single oocyte preparation stimulated with increasing doses (10–300 mM) of the two compounds. For clarity, responses to sodium chloride and sodium bicarbonate were colored red and blue, respectively. (B) Concentration-dependent curves obtained with five different oocytes coexpressing CquiGR2 and CquiGR3. Data are presented as mean ± SEM. Some error bars for NaCl curve do not appear, because they are shorter than the size of the symbol. From left to right: 0.81, 2.59, 3.24, 5.35, 6.92, 11.24, 9.59, and 14.42. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
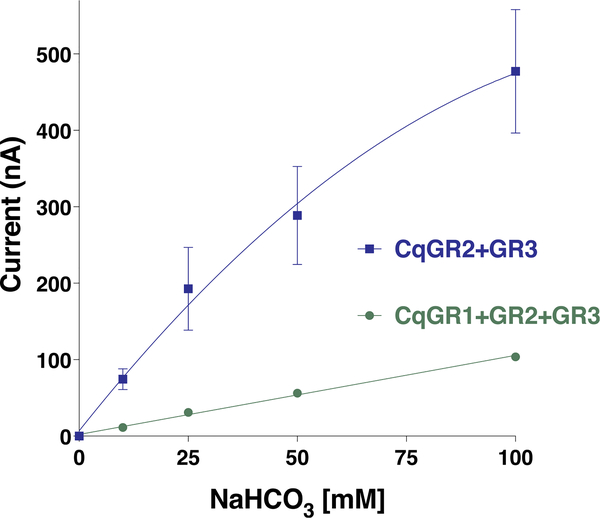
We then compared CquiGR2/CquiGR3 and CquiGR1/CquiGR2/CquiGR3 (Fig. 3). The ternary receptor system showed a remarkably lower (t-test, P = 0.0320) response to sodium bicarbonate than the binary receptor (Fig. 3).
Fig. 3. Comparative concentration-dependent curves for oocytes coexpressing either two or three functional subunits.
Results were obtained with four oocytes coexpressing CquiGR2 and CquiGR3 and four oocytes coexpressing the three GRs. For simplicity, in figures, we use two-letter symbols for proteins as opposed to four-letter symbols (eg, Cq = Cqui) used elsewhere. Data are presented as mean ± SEM. Error bars for the green curve do not appear because they are shorter than the size of the symbol. From left to right: 0, 0.63, 3.24, 1.65, and 3.84. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
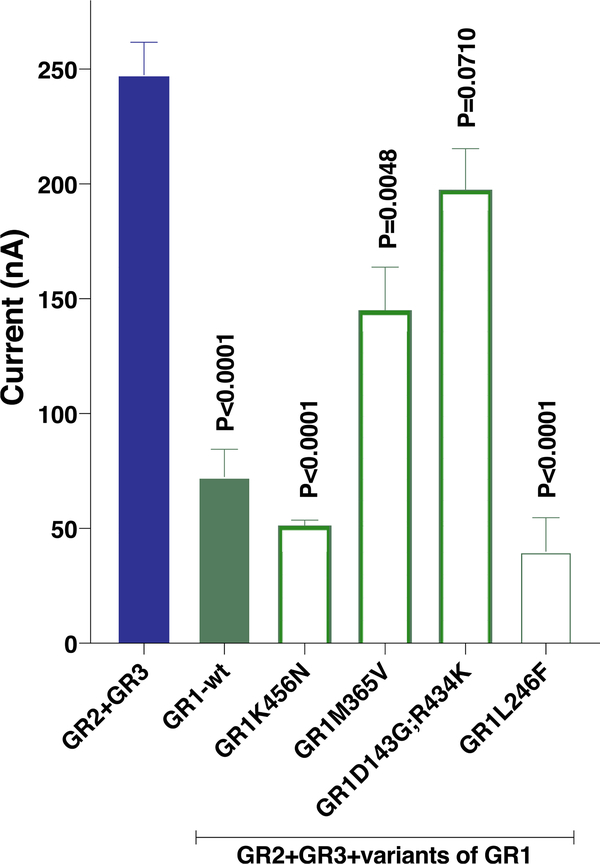
Next, we examined whether variants of CquiGR1 would have different effects. We cloned five CquiGR1 variants, one of them showed an amino acid sequence identical to the sequence appearing in VectorBase (CPIJ 006622). We named this variant the wild type form. Three other variants differed in one amino acid residue each, specifically CquiGR1K456 N (GenBank, MN418391), CquiGR1M365 V (MN418394), and CquiGR1L246 F (MN418393). Lastly, one variant differed in two amino acid residues, ie, CquiGR1D143G; R434K (MN418392). We then expressed variants along with CquiGR2 + CquiGR3 in the Xenopus oocyte recording system and compared their responses to those obtained with CquiGR2/CquiGR3-coexpressing oocytes when stimulated with the same dose of sodium bicarbonate (Fig. S2). None of the five ternary receptor systems showed a stronger response than those recorded from CquiGR2/CquiGR3-coexpressing oocytes (Fig. 4). Responses recorded from CquiGR1D143G; R434K were not significantly different from those measured from the binary receptor system (n = 4, unpaired t-test, P = 0.0710). All other variants, including the wild type, generated significantly lower responses than those recorded from CquiGR2/CquiGR3-coexpressing oocytes (Fig. 4). These findings suggest that CquiGR1 may modulate the receptor system response to sodium bicarbonate.
Fig. 4. Effect of different variants of CquiGR1 on the response of CquiGR2 + CquiGR3-coexpressing oocytes.
All experiments were performed with four replicates with oocytes from the same batch coexpressing either CquiGR2 + CquiGR3 or CquiGR2 + CquiGR3 plus one of the CquiGR1 variants (K456N, M365V, D143G; R434K, and L246F). The clone with sequence identical to that in VectorBase (CJPI 006622) was named wild type (wt). Responses recorded from CquiGR2 + CquiGR3-coexpressing oocytes and those recorded from oocytes coexpressing CquiGR2 + CquiGR3 plus one CquiGR1 variant after stimulus with 200 mM sodium bicarbonate (n = 4) were compared by unpaired t-test. P values are presented on the top of each variant column. Data are presented as mean ± SEM.
3.2. Gustatory receptors are activated by carbon dioxide per se, not bicarbonate
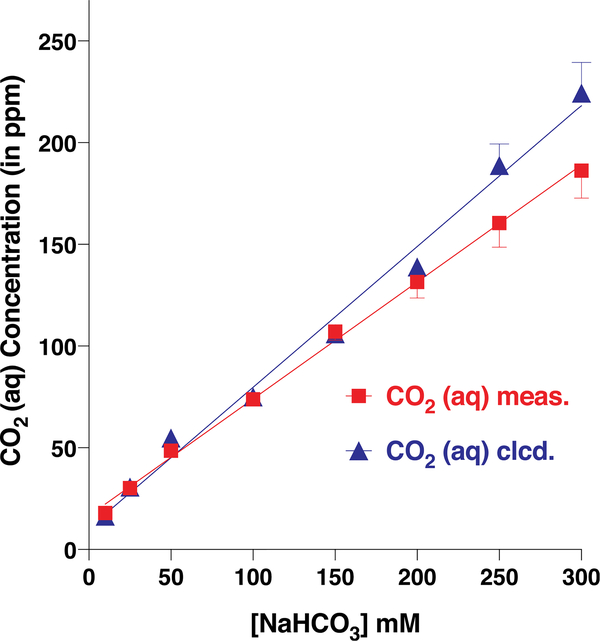
To address this question, we used a carbon dioxide electrode, which was designed to measure the total amount of dissolved carbon dioxide in solution. The solution in the internal filling of the electrode is separated from the analytical sample by a CO2 permeable membrane. Typically, a carbon dioxide buffer is added to the analytical sample to lower the pH to 4.8–5.2 thus shifting the CO2/bicarbonate equilibrium towards free CO2. Carbon dioxide diffuses through the membrane, and an equilibrium is reached between the concentration of CO2 in the analyte and the internal filling. The increase of CO2 in the internal filling causes an increase in hydrogen ion concentration, which is ultimately measured by the pH electrode. To measure the actual (equilibrium concentration) rather than the total concentration of carbon dioxide, we used this electrode in a sealed system and without the carbon dioxide buffer. To calibrate our measurement system, we compared the calculated concentrations of CO2 in sodium bicarbonate buffers with those obtained by direct measurements with the carbon dioxide electrode (Fig. 5). The results showed a faithful relationship (two-tailed t-test, P = 0.1037) between calculated and measured CO2 concentrations (Fig. 5). We then used this system to measure the concentrations of dissolved CO2 in samples obtained by bubbling CO2 in perfusion Ringer buffer. The higher the concentration of CO2, the larger the currents recorded from CquiGR2/CquiGR3-coexpressing oocytes (Fig. S3). Of note, currents recorded from sodium bicarbonate is the summation of carbon dioxide and Na+ responses. Although we adjusted the pH of the tested bubbled CO2 samples (Fig. S3) to rule out the possible effect of hydrogen ion concentrations on oocyte responses, these recordings did not unambiguously determine whether the receptor system is responding to carbon dioxide per se or bicarbonate.
Fig. 5. CO2 concentrations in solutions of sodium bicarbonate prepared in Ringer buffer.
The calculated concentrations based on the final pH of each preparation (n = 4) are shown in red. The actual concentration of CO2 in each solution was measured with a CO2 Ion Selective Electrode. Data are presented as mean ± SEM. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The next experiment further examined the possible role of pH on recorded currents. We injected Ringer and 50 mM sodium chloride samples with their pH adjusted to that of the perfusion Ringer buffer, as well as to 7, 6.5, 6, and 5.5. To make certain that the CquiGR2/CquiGR3-coexpressing oocyte system was functional throughout the tests, we injected Ringer, sodium chloride, and sodium bicarbonate at the beginning and the end of each run (Fig. S4). These experiments (n = 6) further demonstrated that hydrogen ion concentration at the pH range of 7.94 to 5.5 had little or no effect on sodium bicarbonate-elicited response (Fig. S4).
Having developed tools to measure the amount of free carbon dioxide in solution, CO2 (aq), and demonstrated that pH change had minimal or no effect on oocyte responses, we next tested whether sifting the equilibrium of CO2/bicarbonate buffers would cause an increase or a decrease in the receptor response. We prepared in duplicates aliquots of 10 mM sodium bicarbonate buffers and placed them in sealed V-vials with Teflon liner, open-top caps. One of the experimenters took a sample from one of a pair of vials, applied to a CquiGR2/CquiGR3-coexpressing oocyte, and recorded the elicited current. Meanwhile, the other experimenter injected an aliquot of 0.1 N HCl to the second pair of the same sample, vortexed, and provided the sample to the other experimenter to apply immediately to the oocyte preparation (Fig. S5). The pairs of unadjusted and pH adjusted samples were kept sealed until their pH values were recorded. As expected, the initial pH was nearly the same (pH = 7.73) because they were aliquots from the same 10 mM sodium bicarbonate sample. The final pH of the tested samples was 7.39 ± 0.05.
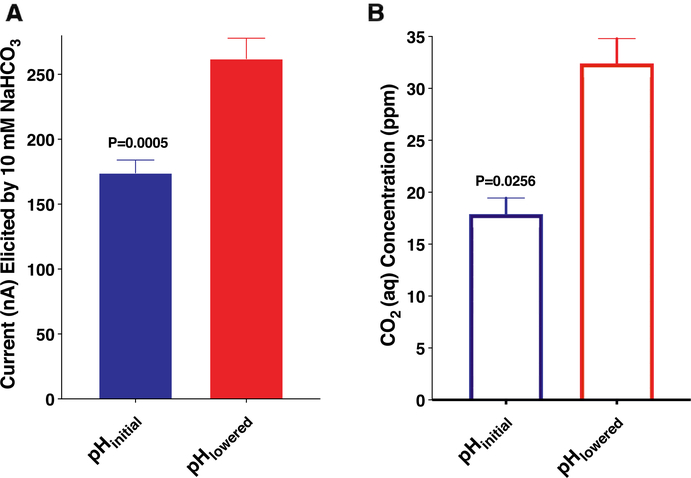
Quantification of the recorded currents from paired samples showed a significant increase (two-tailed t-test, n = 12, p = 0.0005) in the lower pH samples (Fig. 6A). Similar experiments were performed to measure the actual concentration of dissolved CO2 in paired samples with initial and lowered pH. The initial and final pH values of these samples (n = 4) were 7.73 and 7.32 ± 0.03, respectively. As expected, the quantification of CO2 (aq) showed an increase in dissolved CO2 at lower pH (Fig. 6B). The measured CO2 concentrations in the samples without adjustment (pHinitial) and with pH adjusted (pHlowered) were 17.90 ± 1.55 ppm and 32.40 ± 2.40 ppm, whereas the calculated CO2 concentrations were approximately 15.8 and 38.4 ppm, respectively. An increase in dissolved CO2 concentration by lowering pH is predicted by Le Chatelier’s Principle, given that an increase in H + concentration shifts the equilibrium towards CO2 (aq). Because the increased oocyte response (Fig. 6A) resulted from the increase of in situ CO2 concentration (Fig. 6B), we concluded that CO2 per se, not bicarbonate, activates the Cx. quinquefasciatus gustatory receptor system when expressed in Xenopus oocytes. It has also been reported that the BAG neurons of the nematode Caenorhabditis elegans are activated by molecular CO2, although they can be activated by acid stimuli (Smith et al., 2013). We found no evidence for the activation of the Culex mosquito GR system by hydrogen ions.
Fig. 6. Effect of pH on the oocyte response and the concentrations of CO2 in solution.
(A) Responses of CquiGR2 + CquiGR3-expressing oocytes to 10 mM of sodium bicarbonate without acidification and after acidification (n = 12). The final pH (pHlowered) of the sodium bicarbonate buffer was 7.39 ± 0.05. (B) Quantification of CO2 in sodium bicarbonate samples (n = 4) without and after pH adjustment (acidification) as measured with a CO2 Ion Selective Electrode. After acidification (pHlowered), the pH of the sodium bicarbonate samples was 7.32 ± 0.03. Data are presented as mean ± SEM.
3.3. CquiGR1 orthologs in An. gambiae and Ae. aegypti are putative modulators
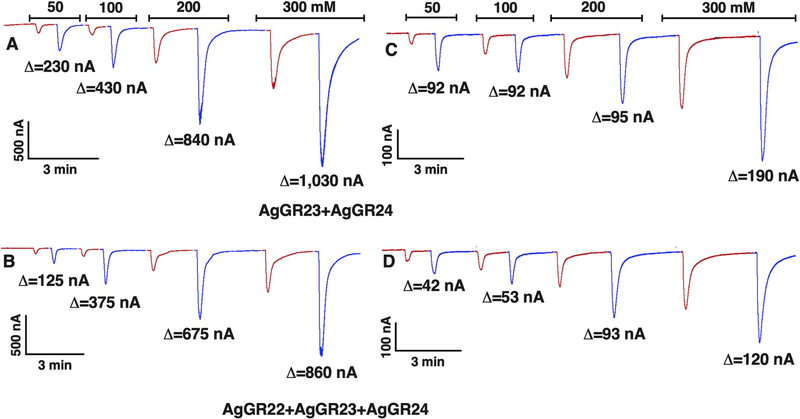
Given the unexpected results that CquiGR1 was not necessary for receptor function, but rather attenuated the responses of the CquiGR2/CquiGR3 receptor system to CO2, we asked whether this would be a common feature of mosquito biology. We prepared oocytes to coexpress the three GR subunits from the malaria mosquito, specifically AgamGR22, AgamGR23, and AgamGR24. Using the same batch of oocytes, we prepared a binary combination of these receptors devoid of AgamGR22, ie, AgamGR23/AgamGR24-expressing oocytes. Then we compared the responses of these oocytes to the same concentrations of sodium chloride and sodium bicarbonate. Specifically, we challenged each oocyte preparation of the same age and the same batch of oocytes with 50, 100, 200, and 300 mM solutions of NaCl and NaHCO3. AgamGR23/AgamGR24-coexpressing oocytes gave stronger, concentration-dependent responses to sodium bicarbonate (Fig. 7A) than the oocytes coexpressing a ternary receptor system, AgamGR22/AgamGR23/AgamGR24 (Fig. 7B). These experiments were replicated with another batch of oocytes (Fig. 7C and D). These results suggest that the CquiGR1 ortholog from the malaria mosquito, ie, AgamGR22, functions as a modulator.
Fig. 7. Responses of An. gambiae GRs-expressing oocytes to sodium chloride and sodium bicarbonate.
(A) Trace obtained with an oocyte coexpressing AgamGR23 and AgamGR24. (B) Responses of another oocyte (from the same batch) co-expressing the three GRs from An. gambiae, GR22, GR23, and GR24. (C) Replication of experiment in (A), but using a different batch of oocytes. (D) Replication of the experiment in (B) with the same batch of oocytes used in (C). Δ values represent responses to sodium bicarbonate subtracted from responses to sodium chloride at the same concentration. In a third replication with a different batch of oocytes the Δ values recorded from a GR23 + GR24-coexpressing oocyte stimulated with 50, 100, 200, and 300 mM were 120, 225, 250, and 780 nA, whereas the respective values recorded from an oocyte from the same batch and coexpressing GR22 + GR23 + GR24 were 23, 89, 193, and 270 nA.
Next, we prepared oocytes coexpressing the equivalent binary and ternary receptor systems with GRs from the yellow fever mosquito. Specifically, we compared the responses of AaegGR2/AaegGR3-coexpressing oocytes to oocytes of the same batch and the same age that coexpressed AaegGR1, AaegGR2, and AaegGR3. Again, the receptor system devoid of AaegGR1 generated stronger, dose-dependent responses to bicarbonate (Fig. S6A) than the ternary system (Fig. S6B), thus implicating AaegGR1 in a modulatory role.
3.4. Overall conclusions
Our research plans to address a long-standing question regarding CO2 reception led to unexpected results. Because it has been well-established in the literature that the reception of CO2 in the vinegar fly antennae is mediated by DmelGR21a and DmelGR63a (Jones et al., 2007; Kwon et al., 2007; Suh et al., 2004), it has been assumed that CO2 is detected by GR1+GR3 in mosquitoes. This notion has been supported by RNAi-based experimental data suggesting that GR2 was not involved in CO2 reception in Ae. aegypti (and Cx. quinquefasciatus) (Erdelyan et al., 2012). On the other hand, AgamGR23, the CquiGR2 ortholog from the malaria mosquito An. gambiae, was implicated in CO2 response (Lu et al., 2007), but the role of AgamGR22 is yet to be unraveled. Our findings suggest that the three subunits might be involved, with GR1 (GR22 in the case of An. gambiae) being a putative modulator. Future studies will determine whether GR2 (or GR23) knock out mosquitoes are indeed insensitive to CO2, whereas GR1 (or GR22) lines will allow us to get a better understanding of the mechanism(s) of modulation. It is conceivable that only the reception of CO2 at high doses might be different in GR1 (or GR22) knock out mosquitoes. With a functional carbon dioxide-detecting receptor system in an aqueous environment, we applied a fundamental principle in chemistry to interrogate which of two species in equilibrium (CO2 or HCO3−) might activate the receptors. We compared receptor responses as well as CO2 concentrations in paired samples without acidification and after acidification and showed that an increase in the concentrations of dissolved CO2 was associated with an increase in receptor response. We then concluded that CO2 per se, neither HCO3− nor protons, activates the carbon dioxide-detecting system in mosquitoes.
Supplementary Material
Acknowledgments
We thank Dr. Su Liu for comments on a draft version of the manuscript.
Funding
X.W. was supported by the Chinese Scholarship Council. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award number R01AI095514. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing financial interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ibmb.2019.103284.
References
- Erdelyan CNG, Mahood TH, Bader TSY, Whyard S, 2012. Functional validation of the carbon dioxide receptor genes in Aedes aegypti mosquitoes using RNA interference. Insect Mol. Biol. 21, 119–127. [DOI] [PubMed] [Google Scholar]
- Gillies MT, 1980. The role of carbon-dioxide in host-finding by mosquitos (Diptera, Culicidae) - a Review. Bull. Entomol. Res. 70, 525–532. [Google Scholar]
- Grant AJ, Wigton BE, Aghajanian JG, O’Connell RJ, 1995. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 177, 389–396. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR, 2004. The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979. [DOI] [PubMed] [Google Scholar]
- Hill C, Fox A, Pitts R, Kent L, Tan P, Chrystal M, Cravchik A, Collins F, Robertson H, Zwiebel L, 2002. G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178. [DOI] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB, 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR, 2007. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 104, 3574–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MZ, Vosshall LB, 2019. General visual and contingent thermal cues interact to elicit attraction in female Aedes aegypti mosquitoes. Curr. Biol. 29, 2250–2257 e2254. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, Zwiebel LJ, 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed S, Hill SR, Dekker T, Ignell R, 2017. Detection and perception of generic host volatiles by mosquitoes: responses to CO2 constrain host-seeking behaviour. Roy. Soc. Open Sci. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver S, Charlton C, 1970. Studies on the sense organs on the palps of selected culicine mosquitoes. Can. J. Zool. 48, 293–295. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB, 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning C, Yang K, Xu M, Huang LQ, Wang CZ, 2016. Functional validation of the carbon dioxide receptor in labial palps of Helicoverpa armigera moths. Insect Biochem. Molec. 73, 12–19. [DOI] [PubMed] [Google Scholar]
- Reeves WC, 1951. Field studies on carbon dioxide as a possible host simulant to mosquitoes. Proc. Soc. Exp. Biol. Med. 77, 64–66. [DOI] [PubMed] [Google Scholar]
- Reeves WC, 1953. Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. Am. J. Trop. Med. Hyg. 2, 325–331. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Kent LB, 2009. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J. Insect Sci. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Martinez-Velazquez L, Ringstad N, 2013. A chemoreceptor that detects molecular carbon dioxide. J. Biol. Chem. 288, 37071–37081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ, 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–859. [DOI] [PubMed] [Google Scholar]
- Syed Z, Leal WS, 2007. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem. Senses 32, 727–738. [DOI] [PubMed] [Google Scholar]
- van Breugel F, Huda A, Dickinson MH, 2018. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature 564, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Thiel PH, Weurman C, 1947. Attraction exerted on Anopheles maculipennis atroparvus by carbon dioxide. Acta Leiden. 1 (18), 219–228. [PubMed] [Google Scholar]
- Xu P, Choo YM, Chen Z, Zeng F, Tan K, Chen TY, Cornel AJ, Liu N, Leal WS, 2019. Odorant inhibition in mosquito olfaction. iScience 19, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Anderson A, 2015. Carbon dioxide receptor genes in cotton bollworm Helicoverpa armigera. Sci. Nat-Heidelberg 102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.