Abstract
Background & Aims:
Dietary changes can modulate gut microbiota and interact with cirrhosis. Our prior study demonstrated that microbial diversity was higher in Turkish versus USA cirrhotics, which was associated with lower risk of 90-day hospitalizations. We aimed to define gut microbial functional and metabolomic changes to increase insight into benefits of the Mediterranean compared to Western diets.
Methods:
139 Turkish (46 controls/50 compensated/43 decompensated) and 157 American subjects (48 controls/59 compensated/50 decompensated) were studied. Turkish subjects consumed a modified Mediterranean diet with daily fermented milk intake while Americans consumed a Western diet. Predicted gut microbial functionalities and plasma metabolomics were compared between/within countries. Correlation network differences between microbiota and metabolites in cirrhotics from Turkey versus USA were evaluated.
Results:
Predicted microbial function showed lower amino acid, bioenergetics and lipid pathways, with functions related to vitamin B, glycan, xenobiotic metabolism, DNA/RNA synthesis, in Turkey compared to USA cirrhotics. Plasma metabolomics demonstrated higher relative lactate levels in Turkey versus USA. The metabolite changes in decompensated cirrhosis, compared to controls, showed similar trends in Turkey and USA, with reduced lipids and phosphocholines. Phosphocholines were significantly lower in patients hospitalized in 90 days (p=0.03). Correlation networks in cirrhotics demonstrated linkage differences between beneficial taxa, Blautia and Oscillispira, and lactate and unsaturated lipids, in Turkey compared to American patients.
Conclusions:
A modified Mediterranean diet was associated with altered plasma metabolomics and beneficially alters microbiota functionality and correlations compared to Western diet in cirrhosis. These altered diet-microbial interactions could potentially affect the 90-day hospitalization risk.
Keywords: cirrhosis, Mediterranean diet, Western diet, metabolomics, gut microbial function, fermented milk products
Lay summary:
We show that the different diets followed in Turkey and America contribute to alterations in gut microbial function and plasma metabolomics observed in patients with cirrhosis, which may explain the lower 90-hospitalization in Turkish patients. This should help in the design of dietary interventions across international cohorts of patients with cirrhosis.
INTRODUCTION
Patients with cirrhosis have an altered gut-liver axis, which can influence clinical and psychosocial outcomes1. Differences in microbial composition are important as potential biomarkers of disease progression in cirrhosis1. Indeed, prior studies have shown an increased relative abundance of gram-negative Proteobacteria and lowering of gram-positive Firmicutes as the disease progresses2. Changes in gut microbial composition and function are a major determinant of the gut-liver axis and differences in cultural, ethnic and dietary practices can modulate the gut microbiota composition even within associated disease3–5. These changes are affected by macronutrient and fermented food intake, which can drive beneficial changes in gut microbiota for those on whose diet typically involves more fermented, probiotic foods such as a Mediterranean diet, compared to typical Western diets in patients with cirrhosis6. Our previous results, comparing cirrhotics from USA and Turkey, demonstrated lower Shannon diversity in all American subjects compared to their Turkish counterparts, which reflects a poor dietary intake of probiotic constituents such as fermented milk products, and was associated with lower risk of 90-day hospitalizations6 . This study showed that despite having compensated and decompensated cirrhosis, the Shannon diversity of Turkish patients was similar to their respective controls. However, the role of gut microbial function and its impact on the differences between cirrhotic patients on a modified Mediterranean, compared to Western diets, is unclear. Therefore, the focus of this study was the functional analysis of the microbiota in cirrhosis, determined in the same Turkey-USA control/cirrhotic cohort6, using the bioinformatics tool, (PiCRUST: Phylogenetic Investigation of Communities by Reconstruction of Unobserved States7) and metabolomics analyses. Metabolomics can be used to analyze the systemic impact of dietary factors on gut microbiota function from bio-fluids using either mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy techniques8,9. Certain metabolites may be linked with a specific species or group of gut bacteria10. However, other metabolites, for example lactate and 3-hydroxybutyrate, may be related to a diet rich in acidified foods8 and can reflect interactions between disease states, diet and microbial function.
Our aim was to define predicted gut microbial functional changes and plasma NMR metabolomics associated with Western and modified Mediterranean diets in healthy and cirrhotic subjects from USA and Turkey to assess beneficial effects of either diet.
METHODS
Three groups of subjects, healthy controls, compensated cirrhotic and decompensated cirrhotic outpatients were recruited from centers in USA (Virginia Commonwealth University and McGuire VA Medical Center, Richmond, Virginia) and Turkey (Ankara University School of Medicine, Ankara, Turkey).
The diagnosis of cirrhosis was based on liver biopsy if available, ultrasound-based elastography or clinical, biochemical and radiological evidence of cirrhosis. Decompensation was defined as variceal bleeding in the immediate past, ascites under treatment, hepatic encephalopathy under treatment and those with jaundice11. Patients with alcohol misuse and/or illicit drug usage (defined by DSM-V criteria)12, inability to provide samples and those who were hospitalized within the last 30 days were excluded from the study. The healthy controls were free of chronic diseases, were not on any prescription medications and had normal liver function.
The subjects underwent a dietary evaluation over the previous week which inquired about their overall dietary preferences (vegan, vegetarian, or predominantly non-vegetarian) using a specifically designed food-frequency questionnaire. Specifically, we inquired about coffee, tea, caffeinated/decaffeinated carbonated drinks, fermented foods (yogurt, ayran/kefir, curds), other dairy products (milk, cheese), eggs, cereals and grains (breakfast cereals, rice, white bread and wheat bread), meat intake (lamb/mutton, pork, beef, fish, poultry, other seafood) and chocolate. Coding was performed according to serving size (Supporting Table 1), based on daily or >1/week frequencies, depending on the food item. In the decompensated group, prior clinical events included variceal bleeding, HE, jaundice and ascites, as well as the presence of varices had been recorded13. Laboratory values (complete blood count, MELD score, serum albumin, serum sodium) at the time of sample collection were recorded. In addition, current medications [lactulose, rifaximin, fiber supplements, proton pump inhibitors (PPI), non-selective beta-blockers (NSBB), antiviral therapy for hepatitis B], as well as lifestyle issues (smoking and alcohol history) were obtained at the same time14. Co-morbid conditions such as diabetes and metabolic syndrome and history of sustained virological response (SVR) from hepatitis C were also assessed15,16.
Predicted functionality changes of the microbiota
All patients underwent stool collection for microbiota analysis. Microbial DNA were isolated from stool samples and analysed using 16srRNA analysis as previously described and reported17. We performed PiCRUST7 to determine predicted functionality differences between sites. We compared PiCRUST data using Linear discriminant analysis effect size (LEFSe) for the entire group for compensated, decompensated and control subjects and between cirrhotic patients within and between sites18.
Metabolomic analysis
Plasma collected in a fasted state from all individuals was analysed using NMR spectroscopy to determine metabolomics using lipidomic and small metabolite platforms. Signal intensities of metabolite regions were obtained using intellibucketing within BioRad KnowItAll® Informatics, Metabolomics Edition v17.0. Principal component analysis (PCA) between six groups (USA and Turkey: control, compensated and decompensated) was performed after the data were mean-centred and normalised. The binned NMR spectral data was uploaded into MetaboAnalyst v4.0 program19 for partial least squares-discriminant analysis (PLS-DA),where Q2 >0.40 indicates good separation, and orthogonal partial least squares discriminant analysis (OPLS-DA). The variable importance in projection (VIP) scores allowed an assessment of the contribution of each metabolite region to the PLS-DA model. We also performed these analyses between patients with and without specific medication when differences were found between groups. Signal levels of selected metabolites, relative to total signal intensity, were compared using post-hoc ANOVA and t-tests with Benjamini-Hochberg correction for multiple comparisons. Further details are in supporting data. We also compared the metabolites that emerged as different between patients with cirrhosis who got hospitalized in 90 days versus those who remained free of hospitalization using Mann-Whitney tests. In addition, individual metabolites for controls underwent stepwise linear regression using age, gender, USA/Turkey as independent variables. Individual metabolites in cirrhotic patients were analysed using stepwise linear regression with age, gender, compensated/decompensated and USA/Turkey as independent variables.
Correlation networks
Plasma metabolomics were linked with microbial taxa at the genus level using an R program and visualized in Cytoscape. Correlations between microbiota and metabolites that were different within USA and Turkey at p<0.001 significance level were analysed. Dietary components were not added to this given that most subjects within the country-specific cohorts consumed a similar diet. Therefore, dividing the population as USA vs Turkey was enough to represent the underlying dietary changes.
Multi-variable analyses:
MaAsLin (Multivariate Association with Linear Models), which studies associations between clinical metadata and microbial community abundance20, was performed for the controls and the cirrhosis group separately. For patients with cirrhosis, MaAsLin inputs included demographics, cirrhosis severity details (MELD score, prior complications, medications, alcoholic etiology) and changes between USA and Turkey, while for controls it only included demographics. A p-value <0.05 with a false discovery rate q-value<0.05 was considered significant.
As published before6, we performed multi-variable binary logistic regression analyses for all-cause hospitalization, the variables were divided into Clinical (Age, Male Gender, MELD, Smoking, Diabetes, Albumin, Na, Prior Ascites, Prior HE, Prior Variceal Bleed, Prior Jaundice, Alcohol etiology and Presence of Varices), medicines (Lactulose, Rifaximin, PPI, Beta Blockers), diet (Coffee/Tea, Yogurt, Milk, Fruits, Vegetables, Eggs, Cheese, Rice, Cereals, Bread-White, Bread-Whole Wheat, Bread-Other, Lamb, Pork, Beef, Fish, Other Seafood, Poultry, Caffeinated Carbonated Drinks, Decaffeinated Carbonated Drinks, Alcohol and Chocolate) and Shannon diversity. Due to this large number of variables, separately, for each domain, a backwards elimination procedure was performed with the logistic regression model where any variable significant at the p<0.20 significance level was retained. Ultimately 90-day hospitalization was used as the dependent variable.
Study approval
Written informed consent was obtained from all subjects and the protocols were approved by the local IRBs of each institution.
RESULTS
Cohort characteristics
Subjects:
The etiology of cirrhosis was predominantly hepatitis C and alcohol in USA, while it was more related to hepatitis B (HBV), hepatitis C (HCV) and non-alcoholic steatohepatitis (NASH) in Turkey (Table 1). The US and Turkish cirrhotic population were statistically similar in age, MELD score, PPI use, metabolic syndrome, and proportion with decompensated cirrhosis (Table 1). The Turkish controls were significantly younger than all other groups. The American cirrhotic cohort had a higher proportion of men, those with recent alcohol use, lower serum sodium and platelet counts, NSBB use and prior variceal bleeding, and those with prior HE and those on lactulose and rifaximin. There was a higher proportion with prior jaundice in the Turkish cohort. SVR rates, prior ascites and other blood indices were similar between groups. Over 90 days, 12 patients in the Turkish and 29 in the USA cohort (p=0.016) were hospitalized as previously reported, but given the overall low numbers, we divided the groups into hospitalized vs not-hospitalized for the entire cohort6. The mean time between enrolment and hospitalization was 46.8±31.9 days. The major causes of hospitalization were acute kidney injury/ascites (n=13), infections (n=7), hepatic encephalopathy (n=6), HCC-related (n=4), variceal bleeding (n=3) and liver-unrelated in the rest. Patients who were ultimately hospitalized demonstrated a lower Shannon diversity index (6.6±1.8 vs 7.3±1.3, p=0.02). None of these patients received a liver transplant or died prior to the hospitalization, which is why we only used hospitalization as the end-point.
Table 1:
Characteristics of the cohort
| USA (n=157) | Turkey (n=139) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Control (n=48) | Comp (n=59) | Decomp (n=50) | All | Control (n=46) | Comp (n=50) | Decomp (n=43) | |
| Age† | 62.1±13.6 | 61.0±7.9 | 61.0±6.8 | 42.4±8.0 | 59.9±10.2 | 60.4±12.2* | ||
| Males† | 121 | 27 | 48 | 46* | 79 | 23 | 29 | 27 |
| BMI | 25.6±5.8 | 26.3±7.4 | 27.9±11.9 | 26.2±6.0 | 27.0±9.2 | 29.0±6.1 | ||
| Diabetes | 29 | - | 14 | 15 | 28 | - | 17 | 11 |
| Metabolic syndrome | 35 | - | 16 | 19 | 20 | - | 12 | 8 |
| MELD score | - | 8.7±2.9 | 12.6±5.3* | - | 8.4±2.4 | 11.2±3.5* | ||
| Serum albumin (g/dL) | - | 3.8±0.5 | 3.2±0.6* | - | 4.0±0.42 | 3.4±0.53* | ||
| Etiology (HCV/Alc/HCV+Alc/NASH/HBV/Others)† | 45/24/15/16/3/6 | - | 27/7/7/12/2/4 | 18/17/8/4/1/2 | 20/10/2/30/24/7 | - | 11/3/0/17/15/4 | 9/7/2/13/9/3 |
| PPI Use | 55 | 10 | 19 | 26* | 56 | 8 | 17 | 31 |
| Lactulose† | 38 | 0 | 0 | 38 | 19 | 0 | 0 | 19 |
| Rifaximin† | 31 | 0 | 0 | 31 | 8 | 0 | 0 | 8 |
| Alcohol use† | 17 | 10 | 5 | 2* | 6 | 6 | 0 | 0* |
| NSBB† | 44 | - | 15 | 29* | 17 | - | 9 | 8 |
| Prior Ascites | 34 | - | 0 | 34* | 24 | - | 0 | 24* |
| Prior HE† | 40 | - | 0 | 40* | 8 | - | 0 | 8* |
| Prior Variceal bleeding† | 20 | - | 0 | 20* | 8 | - | 0 | 8* |
| Prior jaundice† | 2 | - | 0 | 2 | 14 | - | 0 | 14 |
| Presence of Varices | 41 | - | 9 | 32* | 47 | - | 14 | 23* |
| Smoking | 27 | 2 | 16 | 9* | 24 | 13 | 9 | 12 |
| Fiber supplement | 7 | 0 | 4 | 3 | 3 | 0 | 0 | 5 |
| Total daily caloric intake | 2361±801 | 2461±1201 | 2260±740 | 2373±995 | 2399±890 | 2292±1015 | ||
| % calorie protein | 16.5±4.6 | 15.9±5.3 | 15.7±6.2 | 16.1±5.2 | 17.2±9.4 | 14.9±8.3 | ||
| % calories fats | 30.3±7.6 | 33.1±5.9 | 32.1±8.2 | 29.6±8.9 | 30.1±6.7 | 31.2±7.4 | ||
| % calories carbohydrates | 48.1±9.8 | 49.4±6.0 | 46.8±12.1 | 49.2±11.6 | 47.9±9.6 | 45.7±6.1 | ||
| Shannon diversity index† | 7.31±1.05 | 6.86±1.23 | 6.02±1.52* | 7.83±0.91 | 8.04±0.30 | 7.86±0.97 | ||
Comp: compensated, decomp: decompensated, MELD: model for end-stage liver disease, HCV: hepatitis C virus, Alc: alcoholic liver disease, NASH: non-alcoholic steatohepatitis, PPI: proton pump inhibitor, NSBB: non-selective beta-blockers, HE: hepatic encephalopathy.
Data are presented as subject numbers or as mean±standard deviation.
p<0.05 between USA and Turkey,
p<0.05 within the same population.
Dietary details:
All subjects were non-vegetarian and had similar caloric intake and macronutrient proportions (Table 1). Western diet was the norm in the US population with relatively low consumption of fermented foods and high coffee, pork and fish consumption (Supporting Table 2). In patients with cirrhosis, US patients consumed more carbonated drinks compared to Turkish patients. The Turkish cirrhotic cohort consumed a diet approximating to a modified Mediterranean diet, which was rich in fermented foods such as yogurt and vegetables. In addition, they demonstrated a higher tea consumption, as well as avoidance of pork and alcohol. In controls recruited from Turkey, there was a greater coffee and carbonated drink consumption and lower tea and fermented milk product consumption than either of the Turkish cirrhotic patient groups.
Predicted functionality changes of the microbiota
Comparison of subject groupings between countries (Figure 1):
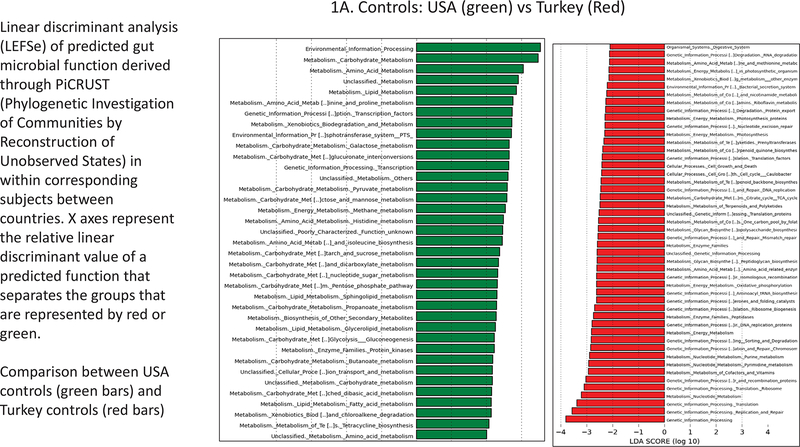
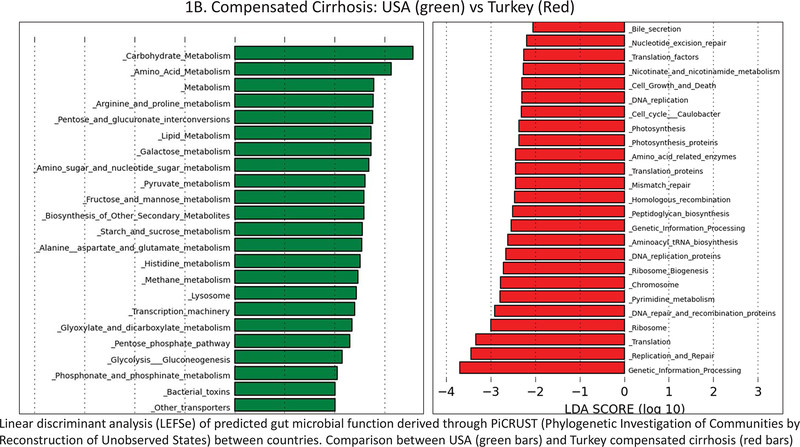
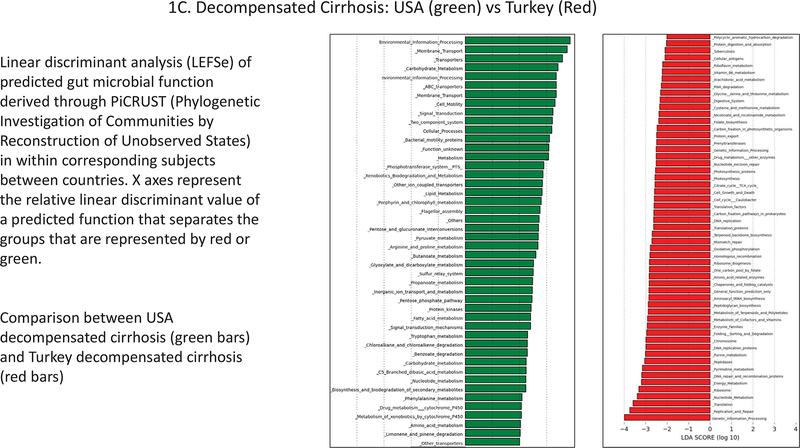
Figure 1.
Linear discriminant analysis (LEFSe) of predicted gut microbial function derived through PiCRUST (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) within corresponding subjects between countries. X axes represent the relative linear discriminant value of a predicted function that separates the groups that are represented by red or green. A. USA controls (green bars) and Turkey controls (red bars); B. Compensated Cirrhosis: USA (green) vs Turkey (Red); C. Decompensated Cirrhosis: USA (green) vs Turkey (Red).
There were major differences in PiCRUST between controls from USA versus controls from Turkey (Figure 1A). Microbiota in the controls from the USA had a greater metabolism related to amino acid, bioenergetics and lipid pathways while Turkish controls had microbiota that were more likely to express functions related to vitamin B, glycan, xenobiotic metabolism, and DNA/RNA synthesis. The USA compensated patients had higher predicted microbial functionality, related to bioenergetics, bacterial toxin production and amino acid metabolism and lower bile, peptidoglycan, and RNA/DNA synthesis, compared to Turkish compensated patients (Figure 1B). Differences were even greater when decompensated patients were compared across countries. Again, the USA decompensated patients had greater predicted microbial functionality related to bioenergetics, amino acids, bacterial motility and assembly with xenobiotic handling and lower RNA/DNA and peptidoglycan metabolism, compared to their Turkish counterparts (Figure 1C).
Controls vs cirrhosis (Supporting Figure 1):
There were significant differences in the predicted functionality of microbiota for the control group compared to each of the compensated and decompensated cirrhosis groups, with the populations combined (Supporting Figure 1A and 1B). In controls compared to compensated cirrhosis, there was higher amino acid, glycan and lipid metabolic pathway expression, while environmental processing systems were overexpressed in compensated cirrhosis. Similarly, amino acid, glycan and lipid pathways were more expressed and environmental pathways and purine metabolism were less expressed in controls compared to decompensated cirrhotic patients.
Compensated vs decompensated cirrhosis (Figure 2):
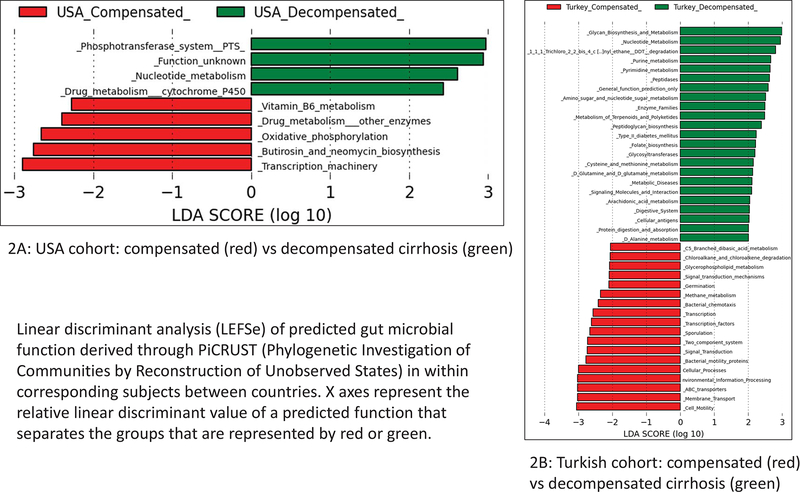
Figure 2.
Linear discriminant analysis (LEFSe) of predicted gut microbial function derived through PiCRUST (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) within corresponding subjects between countries. X axes represent the relative linear discriminant value of a predicted function that separates the groups that are represented by red or green. 2A. USA cohort: compensated (red) vs decompensated cirrhosis (green); 2B. Turkish cohort: compensated (red) vs decompensated cirrhosis (green).
In the USA, decompensated cirrhotic patients had greater vitamin B6, neomycin and drug metabolism and lower cytochrome p450 and nucleotide metabolism compared to compensated patients (Figure 2A). In the Turkish cohort, there was again a higher nucleotide metabolism along with amino acid metabolism with lower predicted microbial functionality related to bacterial motility/chemotaxis and intracellular processes in decompensated patients (Figure 2B).
Metabolomics
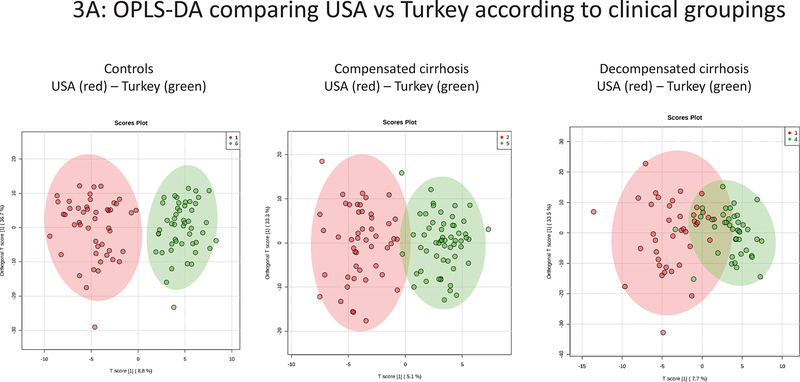
Comparison of subject groupings between countries (Figure 3A):
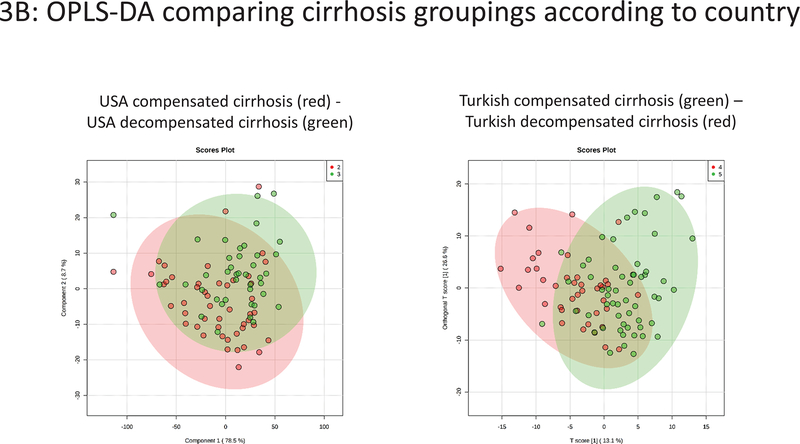
Figure 3.
Plasma NMR metabolomics analysis showing orthogonal partial least squares discriminant analysis (OPLS-DA) results. 3A: USA vs Turkey according to clinical groupings. 3B: Compensated versus decompensated cirrhosis groupings according to country.
There were differences between controls and decompensated patients from USA and Turkey (PLS-DA Q2 0.60, 0.45 respectively), although less pronounced differences between compensated patient groups (PLS-DA Q2 0.29), mainly due to a higher lactate levels in each Turkey sub-groups compared to the USA groupings. OPLS-DA plots between corresponding cohorts between countries are shown in Figure 3A and representative NMR spectra in Supporting Figure 2.
We also compared those taking, or not taking, rifaximin or lactulose for patients with decompensated cirrhosis between countries (Supporting Figure 3). The results demonstrated that, regardless of rifaximin and lactulose use, the major comparison between patients with decompensated cirrhosis not taking rifaximin was similar to the overall comparison between decompensated cirrhosis patients between USA and Turkey (Figure 3A). Therefore, this suggests taking rifaximin or lactulose was not the main factor contributing to the metabolomic differences seen between patients with decompensated cirrhosis from USA vs Turkey (Supporting Figure 3)
Comparison of controls and cirrhosis within countries (Supporting Figure 4):
Within the USA group, the difference in plasma metabolites was most pronounced between controls and decompensated patients (PLS-DA Q2 0.59), rather than controls and compensated patients (PLS-DA Q2 0.17). There were differences in plasma metabolites within the Turkish cohort, despite similar microbial diversity, which was marked between controls and compensated cirrhosis but greatest between controls vs decompensated cirrhosis (PLS-DA Q2 control-compensated 0.40, PLS-DA Q2 control-decompensated 0.69).
Cirrhosis comparison within countries (Figure 3B):
There was some overlap between compensated and decompensated cirrhotic patients within both the USA and Turkish cohorts (PLS-DA Q2=0.28 and 0.28 respectively).
Individual metabolites driving the differences between subject groupings (Table 2 and Supporting Table 3):
Table 2:
Specific Plasma Metabolites Driving the Differences Between and Within Cohorts from VIP scores in PLS-DA (Q2>0.40).
| Turkey vs USA | Within Turkey | Within USA | |||
|---|---|---|---|---|---|
| Metabolite | Control | Decomp | Control vs comp | Ctrl vs decomp | Ctrl vs decomp |
| Lactate (1.34–1.32ppm) | ↑ | ↑ | ↑ | ↑ | - |
| Lipid CH2 (1.32–1.22ppm) | ↓ | ↑ | ↑ | ↑ | ↑ |
| Lipid CH3 LDL (0.86–0.78ppm) | ↑ | ↑ | - | ↑ | ↑ |
| Lipid CH3 VLDL (0.92–0.86ppm) | ↓ | - | ↑ | ↑ | ↑ |
| Glucose (5.25–5.22ppm) | ↓ | ↓ | ↓ | ↓ | ↓ |
| Phosphocholine (3.22ppm) | ↑ | - | ↑ | ↑ | ↑ |
Lower in the group/cohort mentioned first compared to the one mentioned second,
Higher in the group/cohort mentioned first compared to the one mentioned second,
similar between groups.
The Turkish control group had higher relative lactate levels but lower glucose levels compared to the USA controls, with a trend for lower lipid-CH2 and lipid-CH3 (VLDL) levels. Lactate levels were also higher in decompensated patients from Turkey compared to the USA. Within the USA group, relative lipid-CH2, lipid-CH3 and phosphocholines were higher in controls compared to decompensated cirrhosis; and relative glucose levels were lowest in controls. Within the Turkish cohort, relative levels of lipid-CH2 and phosphocholines were higher in controls compared to cirrhotics and in compensated compared to decompensated cirrhotics; relative lactate levels were higher in controls compared to cirrhotic patients; relative glucose levels were higher in both cirrhosis groups compared to controls. Representative NMR spectra are shown in Supporting Figure 3.
When regressions were performed for individual metabolites using age, gender and USA/Turkey in the control population, lipid-CH2,, lipid -CH=CH-CH2-CH=CH- (polyunsaturated lipid, L6) and lactate were significantly differentially predicted by USA vs Turkey (p<0.001) in addition to the age variables. In the cirrhotic group, lipid- CH2 L6, lactate, acetate, and phosphocholine were significantly predicted by USA vs Turkey (p<0.001) while compensated/decompensated status was additionally associated with formate, lactate, L6, GPC, choline, alanine and phosphocholine (all p<0.01).
Comparison of metabolites in patients with and without hospitalizations:
We found significantly higher phosphocholine in the patients who were not hospitalized [median (IQR): hospitalized 0.014(0.05) vs not hospitalized 0.011(0.06), p=0.03]. Neither lactate, lipid-CH2 and lipid-CH3 were significantly different between those who experienced hospitalization at 90 days compared to the rest.
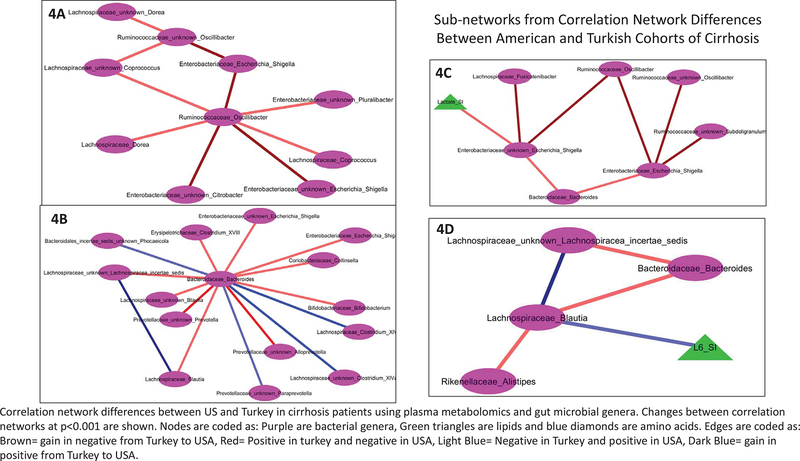
Correlation Network differences (Figure 4/Supporting Figure 5):
Figure 4.
Correlation network differences between US and Turkey in cirrhosis patients using plasma metabolomics and gut microbial genera. Changes between correlation networks at p<0.001 are shown. Nodes are coded as: purple (bacterial genera), green triangles (lipids), blue diamonds (amino acids). Edges are coded as: brown (gain in negative from Turkey to USA), red (positive in turkey and negative in USA), light blue (negative in Turkey and positive in USA), dark blue (gain in positive from Turkey to USA). 4A-D. Sub-networks from Correlation Network Differences Between American and Turkish Cohorts of Cirrhosis.
We found several changes between the relationship of plasma metabolites and gut microbiota in Turkish compared to US compensated and decompensated cirrhotic patients (SupportingFigure 5). Specifically, we created sub-networks based on specific microbial genera (Figure 4A–D). Oscillibacter, and SCFA-producing genus, had relationships with constituents of Enterobacteriaceae that were more negative in the American cohorts (Figure 4A). The linkage between Oscillibacter and genera with Lachnospiraceae were positive in the Turkish, but negative in the US patients (Figure 4C). Blautia, another SCFA-producing genus, was negatively associated with relative plasma lipid -CH=CH-CH2-CH=CH- levels (polyunsaturated lipid, L6) in Turkey, but not in US-based cohorts (Figure 4D). Interactions between Lachnospiraceae components were positive in both cohorts (Figure 4D). Linkages between Lachnospiraceae taxa and Bacteroides and Alistipes were positive in Turkey and turned negative in USA. Using Bacteroides as the sub-network (Figure 4B), Clostridium taxa were more positive in USA but genera belonging to Lachnospiraceae, Escherichia/Shigella and Colinsella were negative in USA and positive in Turkey. When Escherichia/Shigella were used the focus, Ruminococaceae components were even more negative in USA compared to Turkey, while lactate was negatively correlated with Escherichia and Shigella in USA without any relationship in the Turkish cohort. Relative plasma formate was negatively linked with Parasutterella in Turkey, which became positive in USA cohort (Supporting Figure 5).
Multi-variable analyses:
Logistic regression:
The variables that were retained for each domain (Clinical: MELD, Prior Ascites; Treatment: lactulose and Non-selective Beta Blockers; Diet: Vegetables, Cereals and Coffee/tea) were then placed into a model simultaneously and another backwards elimination logistic regression model was performed, this time retaining variables significant at the p < 0.05 significance level. The variables significant at this stage were MELD, cereals, vegetables, coffee/tea and prior ascites. When Shannon index was introduced, the significant model consisted of diversity (OR 0.78, CI 0.59–0.96, p=0.04), cereals (0.19, CI 0.05–0.77,p=0.02), vegetables (0.17, CI 0.05–0.65,p=0.008), coffee/tea (0.38, CI:0.16–0.91, p=0.03) and prior ascites (4.72, CI 2.08–10.73, p<0.001).
MaAsLin outputs for the patients with cirrhosis demonstrated changes in species level based on (a) MELD score (b) age and (c) USA vs Turkey. Species associated with age belonged to Lachnspiraceae (Clostridium symbiosium and Dorea formicigenerans) with Shigella Boydii (Supporting table 4). The MELD score was associated with Enterobacteriaceae_Klebseilla (p value 5.12E-05 and q value 0.02). For USA vs Turkey comparisons, the two species that were important were Veillonellaceae_Dialister (p value 9.55E-06, q value 0.003), and Prevotella_copri (p value 5.48E-07, q value 0.0009). None of the other variables, including gender, alcohol etiology, individual medication use, history of cirrhosis complications or diabetes showed multi-variable associations with the microbiota.
MaAsLin results between controls did not show any microbiota that were different between groups that were specifically associated with age, gender or ethnicity.
DISCUSSION
In this study we found differences in predicted gut microbiota functionality, plasma metabolomic profiles and correlation networks between and within Turkish and USA cirrhotic cohorts. Differences in gut microbiota functionality and plasma metabolites were also observed between Turkish and USA control groups, which could factor into the overall benefit of the Mediterranean diet in cirrhosis in the Turkish cohort.
Alterations in gut microbial composition and function can be affected by multiple factors, including diseases, ethnicity, and diet21 . Performing a comparison between countries, the Turkish controls showed lower predicted microbial functionality related to amino acid, bioenergetics and lipid metabolism but were more likely to express functions related to vitamin B and glycan metabolism. Relative plasma lactate levels were greater in the Turkish control group, compared to the USA cohort, reflecting increases in consumption of lactate present in fermented dairy products in the diet8,22. On the other hand, in the USA control group, relative lipid-CH2 and lipid-CH3 (VLDL) levels tended to be higher when compared to the Turkish control group, even though both diets had a similar total calorie proportions from fat. As the USA control group had a richer abundance of bacterial functions relating to lipid metabolism, this suggests a different mechanism for lipid nutrient metabolism compared to the Turkish counterparts, which is likely related to the Western diet23,24.
These changes were further illustrated by the higher energy harvesting (bioenergetics, amino acid production) and lower bile acid metabolism, peptidoglycan and RNA/DNA synthesis in USA cirrhotic compared to Turkish compensated and decompensated patients. In addition, USA groups showed higher potentially harmful bacterial functionality related to toxin production, chemotaxis/motility and xenobiotic handling25. These findings in the Turkish cohort suggest greater bacterial metabolism of bile acids likely related to more gram positive rather than gram-negative taxa due to the peptidoglycan cell wall and greater bacterial replication, which are associated with an optimal intestinal milieu26. When patients with decompensated cirrhosis were compared, we found significant plasma metabolite differences with higher lactate and lipid-CH2 levels in decompensation in Turkey. Since the Turkish cirrhotic population had a lower 90-day hospitalization, these microbial functional changes and plasma metabolomic features could likely contribute towards this benefit27.
This study on predicted microbiota functionality and plasma metabolomics demonstrated systematic differences related to dietary changes in two distinct populations that were variably modulated by the cirrhotic state. Changes between microbial-metabolite linkages were observed in cirrhosis with genera associated with benefit (Oscillibacter, Blautia) and those associated with negative consequences such as Escherichia/Shigella and Bacteroides that differed between USA and Turkey. Blautia are significant acetate producers, which are more common in the presence of a fermented milk-containing and fiber-rich diets28, and acetate is the principal gut microbial-based stimulus for beta-oxidation of fatty acids. Thus, in the Turkish diet, the acetate produced by Blautia may be the stimulus for burning fat, which would be consistent with the negative correlation noted between L6 and Blautia. On the other hand, a positive correlation was noted between L6 and Blautia.in the USA cohort. Other species belonging to Prevotellaceae and Veillonellaceae were also different between USA and Turkey on multi-variable modeling in the cirrhosis group. These are associated with greater “oralization”, worsening intestinal inflammation and poor outcomes29,30. The effect of diet and microbiota was also confirmed on multi-variable logistic regression, where a higher microbial diversity, along with cereals, vegetables and coffee/tea intake was associated with lower hospitalization independent of cirrhosis severity, demographics and concomitant medication use. These altered diet-microbial interactions could contribute towards the lower 90-day hospitalizations previously observed in those with higher microbial diversity27.
Despite the between country changes above, there were also patterns by which cirrhotic patients differed from controls regardless of USA or Turkey. In both the American and Turkish cohorts, reduced lipid-CH2 and phosphocholine were observed in decompensated patients compared to controls. Phosphocholines, associated with cell and hepatic regeneration, were more abundant in controls and compensated patients compared to cirrhotic and decompensated patients respectively31. Higher phosphocholines were also protective against 90-day hospitalizations in the entire cohort extending a study of survival in decompensated cirrhotic patients into an outpatient compensated/decompensated group32. While the mechanisms are unclear, these could be linked with in vivo hepatic MR spectroscopy findings that reflect hepatic accumulation of both phosphoethanolamines and phosphocholines and be an index of hepatocyte regeneration33.
Higher levels of lipid-CH3 (LDL) were also observed in relatively healthier groups within and between countries. Synthesis of LDL is a key hepatic function affected by the diet34. However, further studies are required to define a causal relationship between dyslipidemia and apoptosis/necrosis at the hepatic level25. This pattern also demonstrates that there do remain some underlying metabolic abnormalities related to cirrhosis that are stable across countries, remained significant on multi-variable analysis and therefore are unlikely to be related to diet or microbiota differences.
The study may be limited overall by differences in disease etiology and medication use between cirrhosis cohorts and possibly by the younger age of Turkish controls. However, comparison between subgroups of decompensated cirrhosis on or not on rifaximin or lactulose and the multi-variable analyses did not change the overall differences. However, between USA and Turkish cohorts, measures such as the MELD score, metabolic syndrome, diabetes and BMI were statistically similar between groups. We performed multi-variable regression to further confirm these changes. We only focused on hospitalizations given the relatively small number of patients and further studies to evaluate liver transplant and risk of death are required. Given the uniform dietary pattern within a population, we used a categorization of yes/no in the dietary evaluation, rather than a quantitative measure, and therefore this was not specifically added to the correlation network. There could be factors other than diet, including environmental that could impact these correlations and the results need to be interpreted in that light.
We conclude that Mediterranean and Western diets contribute to alterations in microbial function and metabolomics in patients with cirrhosis that were linked with beneficial microbiota in subjects on the Mediterranean diets. These differential effects could contribute to the relatively better prognosis of patients on a Mediterranean diet and should increase insight into designing dietary interventions across international cohorts of patients with cirrhosis.
Supplementary Material
Statement of financial support:
This work was partly supported by grants from VA Merit Review I0CX001076, R21TR002024 from NCATS to JSB and a grant from the Turkish Association for the Study of the Liver to RI. SDT-R is grateful to the United Kingdom National Institute for health Research Biomedical Facility at Imperial College London for infrastructure support. The NMR Facility of the Centre for Biomolecular Spectroscopy was funded by British Heart Foundation, Wellcome Trust and King’s College London.
List of Abbreviations:
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HE
hepatic encephalopathy
- LEFSe
linear discriminant function effect size
- MaAsLin
Multivariate Association with Linear Models
- MELD
Model for End-Stage Liver Disease
- NASH
non-alcoholic steatohepatitis
- NMR
nuclear magnetic resonance
- NSBB
nonselective beta-blockers
- OPLS-DA
orthogonal partial least squares discriminant analysis
- PiCRUST
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- PLS-DA
partial least squares-discriminant analysis
- SVR
sustained virological response
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists
Trial registration: Not applicable; this is an observational cohort study
References
- 1.Acharya C, Bajaj JS. The Microbiome in Cirrhosis and its Complications. Clin Gastroenterol Hepatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–572. [DOI] [PubMed] [Google Scholar]
- 3.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. [DOI] [PubMed] [Google Scholar]
- 4.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013;31(9):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen SM, Nielsen NC, Andersen HJ, et al. The serum metabolite response to diet intervention with probiotic acidified milk in irritable bowel syndrome patients is indistinguishable from that of non-probiotic acidified milk by 1H NMR-based metabonomic analysis. Nutrients. 2010;2(11):1141–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi R, Gibson GR, Vulevic J, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. 2018;6(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Wang B, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105(6):2117–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wishart DS, Jewison T, Guo AC, et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41(Database issue):D801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albilllos A, Garcia-Tsao G. Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers. 2011;31(3):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merli M, Lucidi C, Di Gregorio V, et al. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 2015;35(2):362–369. [DOI] [PubMed] [Google Scholar]
- 15.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidrich B, Vital M, Plumeier I, et al. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7(5):1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome biology. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome biology. 2012;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allaband C, McDonald D, Vazquez-Baeza Y, et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin Gastroenterol Hepatol. 2019;17(2):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vrese M, Barth CA. Postprandial plasma D-lactate concentrations after yogurt ingestion. Z Ernahrungswiss. 1991;30(2):131–137. [DOI] [PubMed] [Google Scholar]
- 23.Park Y, Kim SB, Wang B, et al. Individual variation in macronutrient regulation measured by proton magnetic resonance spectroscopy of human plasma. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kindt A, Liebisch G, Clavel T, et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat Commun. 2018;9(1):3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shawcross DL. Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy? Expert Rev Gastroenterol Hepatol. 2015;9(5):539–542. [DOI] [PubMed] [Google Scholar]
- 26.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology. 2018;68(1):234–247. [DOI] [PubMed] [Google Scholar]
- 28.Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajaj JS, Acharya C, Fagan A, et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Agellon LB, Allen TM, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3(5):321–331. [DOI] [PubMed] [Google Scholar]
- 32.McPhail MJ, Shawcross DL, Lewis MR, et al. Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J Hepatol. 2016;64(5):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon DK, Sargentoni J, Taylor-Robinson SD, et al. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21(2):417–427. [PubMed] [Google Scholar]
- 34.McIntyre N Plasma lipids and lipoproteins in liver disease. Gut. 1978;19(6):526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.