Abstract
Adult neurogenesis, the production of newborn neurons from neural stem cells (NSCs) has been suggested to be decreased in patients with schizophrenia. A similar finding was observed in an animal model of schizophrenia, as indicated by decreased bromodeoxyuridine (BrdU) labelling cells in response to a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist. The antipsychotic drug clozapine was shown to counteract the observed decrease in BrdU-labelled cells in hippocampal dentate gyrus (DG). However, phenotypic determination by immunohistochemistry analysis could not reveal whether BrdU-positive cells were indeed NSCs. Using a previously established cell model for analysing NSC protection in vitro, we investigated a protective effect of clozapine on NSCs.
Primary NSCs were isolated from the mouse subventricular zone (SVZ), we show that clozapine had a NSC protective activity alone, as evident by employing an ATP cell viability assay. In contrast, haloperidol did not show any NSC protective properties. Subsequently, cells were exposed to the non-competitive NMDA-receptor antagonist ketamine. Clozapine, but not haloperidol, had a NSC protective/anti-apoptotic activity against ketamine-induced cytotoxicity. The observed NSC protective activity of clozapine was associated with increased expression of the anti-apoptotic marker Bcl-2, decreased expression of the pro-apoptotic cleaved form of caspase-3 and associated with decreased expression of the autophagosome marker 1A/1B-light chain 3 (LC3-II).
Collectively, our findings suggest that clozapine may have a protective/anti-apoptotic effect on NSCs, supporting previous in vivo observations, indicating a neurogenesis-promoting activity for clozapine. If the data are further confirmed in vivo, the results may encourage an expanded use of clozapine to restore impaired neurogenesis in schizophrenia.
Keywords: autophagy, clozapine, fluoxetine, haloperidol, ketamine, neuroprotection
Introduction
Schizophrenia is a devastating mental disorder characterised by severe cognitive impairments, involving severe deterioration of executive function, attention and memory [1]. Moreover, a body of evidence indicates that patients with schizophrenia have disturbed neural plasticity, generally defined as a process responsible for the ability of the brain to adapt to new environmental changes and/or recover from injuries by restructuring itself [2,3]. Neural plasticity has recently been shown to be dependent on adult neurogenesis [4].
Neurogenesis, i.e. the production of new neurons from neural stem cells (NSCs), has been shown to be a continuing process throughout life in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and in the subventricular zone (SVZ) of the lateral ventricle. The vast majority of cells from the SVZ provide cellular turnover in the olfactory bulb (OB) in the rodent brain [5], and in the striatum in the human brain [6]. The SGZ provides cells to the hippocampus in both humans and rodents [7,8]. Impaired neurogenesis and/or insufficient regenerative activity, has been observed in various CNS diseases/disorders, including Alzheimer’s disease and schizophrenia [9–11], as well as in a number of experimental animal models [12,13]. Whether this impairment is causative and/or the result of the CNS disorders themselves is unknown. However, studies indicate that impaired functional neurogenesis may be restored by the use of neuroprotective agents [14,15]. This may be in accordance with current schizophrenia literature, where neuroprotection has been suggested to be a novel drug target for the treatment of this disease [16].
Due to the complexity to study the role of neurogenesis in human schizophrenia, several animal models have been designed. Administration of a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist, ketamine and phencyclidine (PCP) [17,18] are commonly used to mimic symptoms in rodents similar to those associated with schizophrenia in humans [17]. Moreover, animals exposed to ketamine acutely and repeatedly, have shown to develop behavior changes and brain morphological changes similar to those observed in schizophrenic patients [17,19]. We and others have shown that ketamine has dose-dependent cytotoxic activity on numerous cell types, including adult NSCs. The associated toxicity correlated with endoplasmic reticulum stress, mTOR activity, mitochondrial dysfunction and apoptosis [20–26]. Moreover, in the ketamine animal model of schizophrenia, neurogenesis has been shown to be activated, but without the capacity to overcome the simultaneous ketamine-mediated loss of parvalbumin expressing neurons, indicating a deficiency and/or impairment in neurogenesis [12].
Antipsychotics and antidepressants (as adjunctive agents), are commonly used in the treatment of schizophrenia [27–29]. Antidepressants have been shown to promote neurogenesis in hippocampus and SVZ [30,31] by promoting NSC proliferation. Antidepressants have also been shown to promote cell protection on hippocampus-derived NSCs [32]. Antipsychotics, the mainstay of treatment for schizophrenia, are commonly divided into two classes: the first-generation (FGA), including haloperidol and the second-generation (SGA) including risperidone, olanzapine and clozapine [29]. It is well-established that SGA are more potent than FGA in treating symptoms of schizophrenia. The reason for the difference has not been clarified. However, recent studies indicate that they may differ in their capacity to promote neurogenesis [28,33].
Clozapine, the most effective drug for treatment-resistant schizophrenia [34], has been shown to stimulate cell proliferation in the hippocampus, as indicated by increased labelling of bromodeoxyuridine (BrdU), suggesting a neurogenic-promoting activity for clozapine [35,36]. However, phenotypic analysis by immunohistochemistry staining of BrdU-positive cells could not clarify that the proliferating cells were indeed NSCs [35]. Moreover, in an animal model of schizophrenia induced by the NMDA-receptor antagonist PCP, clozapine was shown to normalise/counteract a decrease in neurogenesis in DG [37], indicating that clozapine has a NSC-protective activity [34]. However, phenotypic analysis by immunohistochemistry could not reveal that the protective activity of clozapine on BrdU-positive cells were indeed NSCs. In the same study, clozapine alone could not be shown to promote an increased number of BrdU-positive cells, as previously observed.
Phenotypic analysis by immunohistochemistry to detect NSCs in vivo has been shown to be difficult due to the deficiency of reliable NSCs markers, thus making cellular in vitro cell models essential.
In vitro studies have shown that clozapine may also have cell protective properties in various cells types including microglia cells and rat pheochromocytoma cells (PC12) [38–40]. However, it is not known whether clozapine also has NSC protective properties, involving up-regulation of an anti-apoptotic response in vitro. Haloperidol, the most well-representated FGA drug used worldwide has shown opposing activity to clozapine. In contrast with clozapine, haloperidol has shown neurotoxic activity both in vitro and in vivo and no obvious effect on neurogenesis [35–37].
In the present study, we have tested the hypothesis that clozapine has NSC protective activity, involving up-regulation of an anti-apoptotic response on adult NSCs. We show that clozapine had NSC-protective activity alone and against ketamine-induced cytotoxicity in vitro. Moreover, this activity of clozapine was associated with attenuated expression of apoptosis markers and decreased expression of the autophagosome marker 1A/1B-light chain 3 (LC3-II). In summary, our findings indicate that clozapine may play a protective/anti-apoptotic role on NSCs, supporting previous in vivo observations of neurogenic-promoting activity for clozapine. If these data are confirmed in vivo, the results may encourage an expanded use of clozapine to restore impaired neurogenesis in schizophrenia.
Materials and methods
NSCs isolation and cell cultures
The SVZ of the lateral brain ventricles of adult male mice 6 weeks of age (five C57 BL6/SCA mice in each experiment) was micro-dissected by using a micro-dissector scissor and enzymatically dissociated in 0.5 mg/ml trypsin, 0.8 mg/ml hyaluronidase and 80 U/ml deoxyribonuclease I (Sigma–Aldrich, St. Louis, MO) in DMEM/F12 containing B27 supplement, 4.5 mg/ml glucose, 100 U/ml penicillin, 100 μg/ml streptomycin sulphate and 12.5 mM HEPES buffer solution (Invitrogen, Stockholm, Sweden). The enzymatic digestion was carried out at 37°C for 20 min. After a gentle trituration with a pipette and mixing, cells were passed through a 70-μm strainer (BD Biosciences, Stockholm, Sweden) and pelleted at 1000 rpm for 12 min. The centrifugation step was repeated once more after removing the supernatant by adding fresh cold DMEM/F12. The supernatant was then removed, and cells were re-suspended in DMEM/F12 supplemented with B27 and 18 ng/ml human epidermal growth factor (EGF) (R&D Systems, Oxon, U.K.). Cells were plated in a 10-cm Petri dish and incubated at 37°C for 7 days in order for neurospheres (NSs) to be developed. After 7 days, the NSs were collected and centrifuged at 1000 rpm for 10 min. NSs were re-suspended in 0.5% trypsin/EDTA (Invitrogen, Stockholm, Sweden), by incubating at 37°C for 2 min and triturated gently to aid dissociation. After a further 2-min incubation at 37°C, the cell preparation was diluted 1/20 in DMEM/F12 at 37°C. Cells were then pelleted at 1000 rpm for 10 min and re-suspended in fresh DMEM/F12 containing 18 ng/ml EGF and 16 ng/ml human basic fibroblast growth factor (bFGF) (R&D Systems, Oxon, U.K.) before plating. NSCs were split every 5 days for 4 weeks and all experiments were performed between passages 2 and 8. The NSC culture has been characterised and validated in a previous work by Mercer et al. [41].
All experiments were conducted according to the regional ethics committee for animal experimentation conforming to the ‘Guide for the Care and Use of Laboratory Animals’ published by U.S. National Institutes of Health (NIH publication # 80-23, revised 1996). The C57 BL6/J mice were imported from Nova-SCB, Stockholm, Sweden. The regional ethical committee (Stockholm Södra djurförsöksetiska nämnd, ethical permit number N422/12 and 423/12) approved all animal studies presented in the manuscript. Animals were killed in a CO2 chamber by cervical dislocation at the Animal Department, Karolinska Institutet, Stockholm, Sweden.
Ketamine medium
Previous work by our group have demonstrated a cytotoxic effect of ketamine on NSCs [24]. NSCs were treated with s-ketamine, NMDA receptor antagonist, which in mice have shown to induce hippocampal atrophy and pathology in parvalbumin-expressing interneurons, and cognitive deficits in a similar way to those observed in schizophrenic patients [17,19]. The indicated ketamine concentration (see ‘Results’ section) was added to DMEM/F12 and supplemented with B27 and 0.01 ng/ml of EGF.
ATP assay
Previous studies have demonstrated that intracellular ATP levels correlate to cell numbers [42]. In order to measure NSC viability, NSCs were plated as single cells (see above) into 96-well plates (Corning B.V. Life Sciences, Amsterdam, Netherlands) at a final concentration of 50000 cells/well with DMEM supplemented with B27 and low EGF (0.01 ng/ml). To note, the supplementation of low EGF concentration has been adjusted to a minimum level, to produce an experimental condition, where no proliferation is occurring (including the control cells, clozapine, fluoxetine and haloperidol alone and treated cells). The rational for this has been to produce a cellular protection assay in absence of proliferation. Clozapine (1, 10, 100, 500 nM, 2 μM), fluoxetine (1, 10, 100 nM) and haloperidol (250, 500 nM, 1 μM) were co-treated with or without ketamine. After 24 h of incubation at 37°C (5% CO2, 98% humidity), intracellular ATP levels were measured using the Cellular ATP Kit HTS according to the manufacturer’s instructions (BioThema, Stockholm, Sweden). In these experiments, the effect of each treatment at a certain concentration was determined in quadruplicates in six to seven different sets of experiments.
Western blotting
NSCs were plated as single cells and expanded in a 10-cm Petri dish with EGF/bFGF (see under cell cultures). After 3–4 days when NSs were formed, the different treatments were added for 24 h with low EGF concentration (0.01 ng/ml). After exposure, cells were washed with PBS and lysed in a buffer containing 150 mM NaCl, 20 mM Tris, 0.1% SDS, 1% Triton X-100, 0.25% Na-deoxycholate, 1 mM Na3VO4, 50 mM NaF, 2 mM EDTA, and Protease inhibitory cocktail (Sigma–Aldrich) on ice for 30 min. Samples were clarified by centrifugation. The supernatants were transferred to new tubes and the total protein concentration was determined by Lowry protein assay (Bio-Rad Laboratories, Stockholm, Sweden). Samples were then mixed with reducing SDS/PAGE sample buffer and boiled for 5 min before performing SDS/PAGE. After electrophoresis, proteins were transferred on to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories). Immunoblot analyses were performed with antibodies against the cleaved form of caspase-3 (1:1000, polyclonal), LC3-II (1:1000, polyclonal) (Cell Signaling Technology, Danvers, MA, U.S.A.), and Bcl-2 (1:1000, monoclonal rabbit) (Abcam, Cambridge, MA, U.S.A.). Immuno-reactive bands were developed using ECL (GE Healthcare, Stockholm, Sweden), imaged with a GelDoc system and quantified with Quantity One software (Bio-Rad Laboratories). After imaging, in order to verify equal protein loading, the PDVF membranes were stained with β-actin (1:800, polyclonal) (Santa Cruz Biotechnology. Inc, Germany) or Coomassie Blue (Fermentas, St. Leon-Rot, Germany) as demonstrated in previous studies [24,43–45]. In these experiments, the effect of each treatment at a certain concentration was determined in single/double samples in three to five different sets of experiments.
Mitochondrial DNA analysis
NSCs were plated as single cells and expanded in a 10-cm Petri dish with EGF/bFGF (see under cell cultures) for 3–4 days. When NSs were formed, the different treatments were added for 24 h with low EGF concentration (0.01 ng/ml). After exposure, the number of mitochondrial DNA (mtDNA) copies per diploid nucleus in cells were purified and determined by real-time PCR absolute quantification using the ABI 7500 Fast system (Applied Biosystems). Total genomic DNA was purified from mouse stem cells using the DNeasy blood and tissue kit (Qiagen). A total of 10 ng of genomic DNA was used in each reaction. Primers and probe for mouse mt-ND1 gene (mitochondrial encoded NADH dehydrogenase 1; primers, mt-ND1-F: 5′-TCG ACC TGA CAG AAG GAG AAT CA-3′ and mt-ND1-R: 5′-GGG CCG GCT GCG TAT T-3′; probe, mt-ND1: FAM-AATTAGTATCAGGGTTTAACG-TAMRA) and for single-copy mouse RPPH1 gene (nuclear-encoded ribonuclease P RNA component H1; primers, RPPH1-F: 5′-GGA GAG TAG TCT GAA TTG GGT TAT GAG-3′ and RPPH1-R: 5′-CAG CAG TGC GAG TTC AAT GG-3′; probe, RPPH1: FAM-CCGGGAGGTGCCTC-TAMRA) were used. For each DNA sample, the mitochondrial gene mt-ND1 and the nuclear gene RPPH1 were quantified separately. Standard curves were generated using known numbers of a plasmid containing one copy of each of the two mouse genes. According to the standard curve, the number of copies from each gene was calculated for each sample, and the number of mtDNA copies per diploid nucleus was calculated according to the formula: mtDNA copies per diploid nucleus = 2 × (mt-ND1 gene copies/RPPH1 gene copies). In these experiments, the effect of each treatment at a certain concentration was determined in single samples in three to five different sets of experiments.
Statistical analysis
The differences between groups were tested with one-way ANOVA followed by post hoc Fisher LSD test or Kruskal–Wallis followed by Dunn’s test if data were not normally distributed. All statistical analyses were performed using Sigma Plot software v. 11. Data are presented as mean ± SEM. P<0.05 was considered statistically significant.
Results
Clozapine and fluoxetine, but not haloperidol increases NSC viability
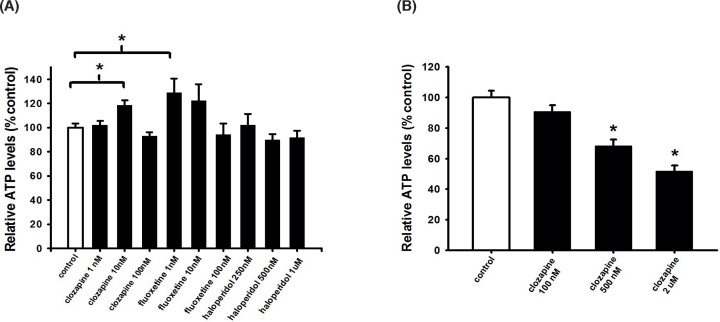
To determine the potential cell protective activity of clozapine on NSCs, NSCs were isolated from the SVZ and exposed to the indicated concentrations of clozapine. For comparison, we also tested the FGA drug haloperidol and the antidepressant drug fluoxetine, drugs commonly used in the treatment of schizophrenia. NSC viability was assessed after 24 h by measuring intracellular ATP levels. The results in Figure 1A show that both clozapine and fluoxetine significantly increased NSC viability at doses of 10 nM (clozapine) and 1 nM (fluoxetine), respectively, while none of the haloperidol concentrations increased NSC viability. At higher concentrations of clozapine, 500 nM and 2 μM, respectively, NSCs viability was significantly decreased (Figure 1B).
Figure 1. Low clozapine and fluoxetine concentrations increase NSC viability, while haloperidol did not.
NSCs were plated as single cells treated with (A) 1, 10, 100 nM of clozapine or 1, 10, 100 nM of fluoxetine or 250, 500 nM, 1 μM of haloperidol or (B) 100, 500 nM, 2 μM clozapine. To measure cell viability, intracellular ATP levels were measured after 24 h. Data are shown as mean ± SEM ((A), n=22–35; (B), n=15–29). Fisher LSD test and Kruskal–Wallis followed by Dunn’s test was used. Differences were considered significant at P<0.05. * denotes P<0.05 compared with control.
Clozapine counteracts ketamine-induced decrease in NSC viability
Ketamine administration is a well-established approach to experimentally mimic some aspects of schizophrenia [17]. Previous work by us has shown that ketamine has a dose-dependent cytotoxic activity on mouse adult NSCs after 24-h treatment. Our previous work indicated that 400 μM ketamine induced a significant and submaximal negative effect on NSC cell viability [24]. This cytotoxic concentration is in accordance with several animal and human clinical studies [19,46–49]. In the present study we showed, with this specific concentration, that the copy-number of mtDNA increased significantly after 24-h treatment (data not shown), a sensitive index indicating cellular oxidative stress, inflammation and mitochondrial dysfunction [50,51]. Our result is in accordance with previous work showing an association between an altered mtDNA copy-number and cellular stress/schizophrenia pathology [52–54]. We found that treatment for 24 h significantly increased the copy number of mtDNA.
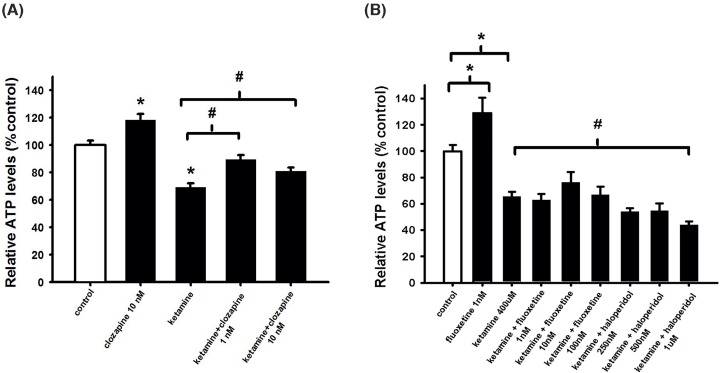
In subsequent experiments, 400 μM of ketamine was selected. As shown in (Figure 2B), both haloperidol and fluoxetine were unable to counteract the ketamine-induced toxic effect on NSCs, while low concentrations of clozapine (1, 10 nM) were able to revert the cytotoxic effect induced by ketamine (Figure 2A).
Figure 2. Ketamine-induced cell death counteracted by clozapine, while fluoxetine and haloperidol cannot.
(A,B) NSCs were plated as single cells and treated with 400 μM ketamine and/or treated with 1, 10 nM clozapine or 1, 10, 100 nM of fluoxetine or 250, 500 nM, 1 μM of haloperidol. After 24-h incubation, intracellular ATP levels were measured. Data are shown as mean ± SEM ((A), n=23–39; (B), n=4–7). Kruskal–Wallis followed by Dunn’s test was used. Differences were considered significant at P<0.05. * denotes P<0.05 compared with control, # denotes P<0.05 compared with ketamine.
Clozapine counteracts ketamine-induced decrease in NSC viability in correlation to decreased apoptosis and autophagy
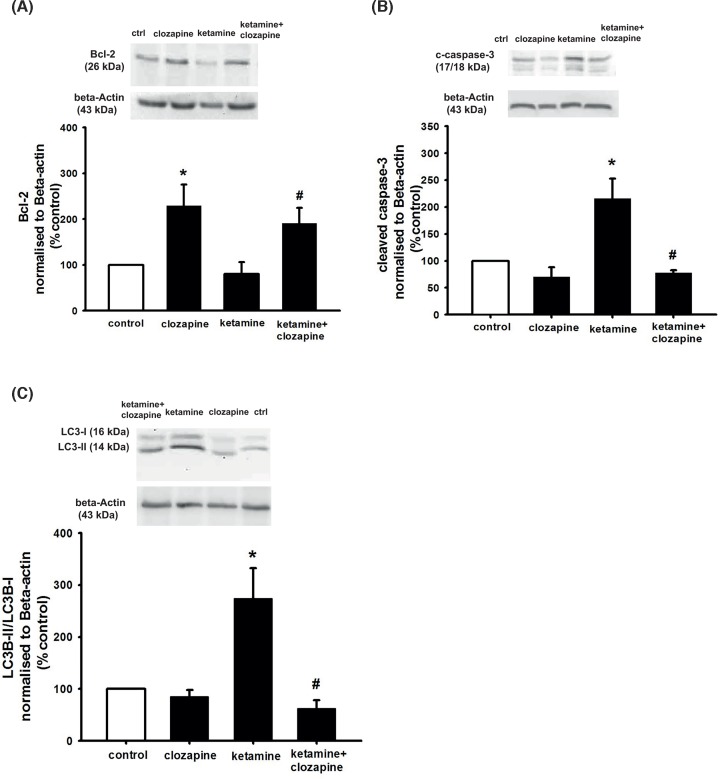
To study the potential involvement of apoptosis in the counteracting effect of clozapine on NSC viability, we assessed the protein levels of the anti-apoptotic marker Bcl-2 and the pro-apoptotic marker cleaved form of caspase-3 after 24 h of incubation by Western blotting analysis. As shown in Figure 3A clozapine alone increased Bcl-2 expression, while the levels of cleaved form of caspase-3 was unchanged (Figure 3B). Moreover, ketamine-induced a significant decrease in Bcl-2 protein levels and an increase in levels of cleaved form of caspase-3. Finally, co-treatment with clozapine was able to revert these effects (Figure 3A,B). Similar results were obtained with Coomassie normalisation (Supplementary Figure S1A,B).
Figure 3. Clozapine counteracts the impaired NSC viability induced by ketamine, in correlation with decreased apoptosis and autophagy.
NSCs were plated as single cells. Cells were treated with 400 μM ketamine and 10 nM clozapine for 24 h. After 24-h incubation, cells were harvested for Western blot experiments. To obtain quantitative measurements (A) Bcl-2 protein levels and (B) cleaved caspase 3 (C) LC3-II/LC3-I ratio and were normalised against β-actin. Data are shown as mean ± SEM (A), n=3–4; (B), n=3–4; (C), n=3–5. Kruskal–Wallis followed by Dunn’s test was used. Differences were considered significant at P<0.05. * denotes P<0.05 compared with control, # denotes P<0.05 compared with ketamine.
Autophagy is crucial in maintaining cellular homoeostasis. It constitutes a major protective mechanism that allows cells to survive in response to multiple stressors and helps to defend organisms against degenerative, inflammatory, infectious and neoplastic diseases [55–57]. Importantly, several studies have shown its crucial role in the development and pathology of schizophrenia [58,59]. To study whether ketamine induces autophagy, NSCs were incubated with 400 μM ketamine for 24 h and/or co-treated with 10 nM clozapine whereupon the level of the autophagosome marker LC3-II was assessed. Results in Figure 3C show LC3-II levels to be significantly higher in cells treated with ketamine compared with control, while ketamine combined with clozapine treatment inhibited the ketamine-induced autophagy activity. Clozapine alone did not affect LC3-II activity.
Discussion
Several studies have reported neurogenesis impairment in schizophrenic patients and in established animal models of schizophrenia induced by non-competitive NMDA-receptor antagonist models of schizophrenia [9,11,12]. Interestingly, in an animal model of schizophrenia, induced by non-competitive NMDA-receptor antagonist PCP, clozapine was shown to counteract a decrease in BrdU-labelling cells of the DG [37] suggesting that clozapine have neurogenesis protective activity. However, phenotypic determination by immunohistochemistry analysis of BrdU-positive cells could not clarify whether BrdU positive cells were indeed NSCs and consequently could not clarify neuroprotective activity of NSCs [37]. Therefore, the aim of the present study was to elucidate whether clozapine has NSC-protective activity and whether this involves an up-regulation of an anti-apoptotic response. To avoid the complexity of an in vivo condition, we tested the NSC activity of clozapine in an in vitro model. Here we present data indicating that clozapine exerts NSC protective effect, that was associated with an up-regulation of an anti-apoptotic response. We also observed that clozapine may decrease cellular stress, as evident by an attenuated autophagy. Our data are in accordance with previous studies where clozapine has shown cell protective properties in various other cell types in vitro, mediated by an anti-apoptotic activity and associated with up-regulation of Bcl-2 activity [38,39,60,61]. However, we cannot conclusively prove at this stage which specific cells are responsible for the reported effects due to the heterogeneity population of NSCs [62]. Therefore, the results of the present study are preliminary and further single cell analysis with appropriate markers will need to be performed to investigate whether the neuroprotective effect of clozapine is linked to a specific subcell population. Overall, our observation of a neuroprotective NSC activity, may thus give further support to the previous in vivo study, by Maeda et al. [37], suggesting that clozapine may have a neurogenic-protective activity.
In a previous in vivo study by Halim et al. [35] they demonstrated that clozapine may also promote neurogenesis stimulating cell proliferation. However, they were not able to distinguish whether this involved NSC proliferation. In the present study, we used a previously established ATP cell viability assay, specially developed to study cell protection of adult NSCs in vitro [24,43,63–65], involving an assay condition omitting cell proliferation. Thus, further studies need to be conducted to clarify whether clozapine also stimulates NSC proliferation, using assay conditions optimised for the analysis of NSC proliferation.
We and others have previously shown that ketamine causes apoptosis [24,66] and up-regulates autophagy [67]. To explore the underlying mechanism of observed NSC protection and anti-apoptotic activity, induced by clozapine, we investigated the effect of clozapine on autophagy. This was conducted by analysing expression levels of LC3-II, a phosphatidylethanolamine modified isoform of the microtubule-associated protein LC3-I, which is generated and translocated to nascent autophagosomes upon macroautophagy induction. Thus, LC3-II is considered a biochemical marker evidence for autophagy in many studies [68–70]. Our results showed that the anti-apoptotic activity of clozapine was associated with attenuated autophagy evident by decreased protein levels of LC3-II. The result was in accordance with previous studies where agents that attenuated ketamine neurotoxicity were associated with decreased apoptosis and autophagy [66,67]. These data suggest that clozapine may inhibit the accumulation of toxic protein aggregates and defective organelles that ketamine introduces to the cells, causing an accumulation of dysfunctional autophagosomes. However, several reports have described that blockage of autophagy in neurons leads to cell death and neurodegeneration in rodents and significant reduction in autophagy in post-mortem hippocampus of schizophrenia patients [58,71,72]. This suggests that autophagy may have a dual role [73], and both be important for the removal of damaged proteins/organelles and promoting cellular injury. Thus, increased or decreased autophagy may be dependent on different injury models and injured cells, although the reasons for this are still unknown [70].
We were also able to detect a cytotoxic activity when NSCs were exposed to ≥500 nM of clozapine. A cytotoxic activity for clozapine is well-established in the literature and has been observed in numerous in vitro [68,74,75] and in vivo studies [76]. Importantly, a study by Park et al. [68], showed a neurotoxic activity when primary neurons were exposed to ≥10 μM of clozapine in vitro, the observed neurotoxic activity was also associated with increased apoptosis and autophagy. Hematopoietic toxicity is the major limiting factor for a general use of clozapine for treating schizophrenia. Patients with schizophrenia treated with clozapine in therapeutic concentrations, have serum levels in the concentration range of 1–2 μM [77,78]. In a study by Nordin et al. [77], the levels of clozapine in cerebrospinal fluid (CSF) of nine treated patients were found to be much lower, in the range of 16–120 nM. Although speculative, if linking these therapeutic concentrations of clozapine to the concentrations of clozapine used in the current study, it may indicate that therapeutic concentrations have the possibility to elicit either cell protective or cytotoxic activity on adult NSCs. This assumption needs to be validated in vivo by investigating whether disturbed neurogenesis occurs in the OB and striatum in animal models of schizophrenia. If neurogenesis is disturbed in these areas, it would be of interest to administer clozapine and see whether it can normalise the impaired neurogenesis and neural plasticity in schizophrenia.
Conclusions
We report that clozapine may have a protective/anti-apoptotic activity on NSCs, alone and against ketamine-induced cytotoxicity, as demonstrated by increased protein expression of the anti-apoptotic marker Bcl-2 and decreased protein expression of the pro-apoptotic marker cleaved form of caspase-3, respectively. Moreover, clozapine’s cell protective activity was associated with decreased protein expression of the autophagasome marker LC3-II. The demonstrated NSC-protective activity, involving up-regulation of an anti-apoptotic response by clozapine, give further support to previous in vivo observations indicating a neurogenesis-promoting activity for clozapine. If these data are confirmed in vivo, they may encourage an expanded use of clozapine to restore impaired neurogenesis in schizophrenia.
Supplementary Material
Acknowledgements
We thank Dr. Fuad Bahram (Södersjukhuset AB) for laboratory technical assistance.
Abbreviations
- Bcl-2
B-cell lymphoma 2
- bFGF
basic fibroblast growth factor
- BrdU
bromodeoxyuridine
- CNS
central nervous system
- DG
dentate gyrus
- EGF
epidermal growth factor
- FGA
first-generation
- LC3-II
1A/1B-light chain 3
- mtDNA
mitochondrial DNA
- mt-ND1
mitochondrial encoded NADH dehydrogenase 1
- NMDA
N-methyl-d-aspartate
- NS
neurosphere
- NSC
neural stem cell
- OB
olfactory bulb
- PCP
phencyclidine
- RPPH1
nuclear-encoded ribonuclease P RNA component H1
- SGA
second-generation
- SGZ
subgranular zone
- SVZ
subventricular zone
Contributor Information
Mathias Lundberg, Email: mathias.lundberg@ki.se.
Shiva Mansouri, Email: shiva.mansouri@ki.se.
Funding
This work was supported by the Anders Paulssons Gåvor Fond [grant number D0042]; the Swedish Research Council FORMAS, Thurings Stiftelse Grant and the Karolinska Institutet Funds.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
Mathias Lundberg assisted with the concept of design research, interpretation of data, writing of manuscript and main applicant for Anders Paulssons Gåvor Fond and Thurings Stiftelse grant. Sophie Curbo assisted with acquisition of data, interpretation of data and writing of manuscript. Hannes Bohman assisted with the writing of manuscript. Ingrid Agartz assisted with the writing of manuscript and was the main applicant for Swedish Research Council FORMAS grant. Sven-Ove Ögren assisted with concept of design research and interpretation of data. Cesare Patrone assisted with interpretation of data and writing of manuscript. Shiva Mansouri assisted with concept of design research, acquisition of data, interpretation of data, writing of manuscript and was the main applicant for Karolinska Institutet research grant.
References
- 1.Ettinger U. et al. (2015) Cognition and brain function in schizotypy: a selective review. Schizophr. Bull. 41, S417–S426 10.1093/schbul/sbu190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voineskos D. et al. (2013) A review of evidence linking disrupted neural plasticity to schizophrenia. Can. J. Psychiatry 58, 86–92 10.1177/070674371305800205 [DOI] [PubMed] [Google Scholar]
- 3.Zhou D. et al. (2017) Altered motor-striatal plasticity and cortical functioning in patients with schizophrenia. Neurosci. Bull. 33, 307–311 10.1007/s12264-016-0079-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adlaf E.W. et al. (2017) Adult-born neurons modify excitatory synaptic transmission to existing neurons. Elife 6, 1–25 10.7554/eLife.19886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun S.M. and Jessberger S. (2014) Adult neurogenesis: mechanisms and functional significance. Development 141, 1983–1986 10.1242/dev.104596 [DOI] [PubMed] [Google Scholar]
- 6.Ernst A. et al. (2014) Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- 7.Eriksson P.S. et al. (1998) Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- 8.Cameron H.A. and McKay R.D. (1999) Restoring production of hippocampal neurons in old age. Nat. Neurosci. 2, 894–897 10.1038/13197 [DOI] [PubMed] [Google Scholar]
- 9.Reif A. et al. (2006) Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 11, 514–522 10.1038/sj.mp.4001791 [DOI] [PubMed] [Google Scholar]
- 10.Curtis M.A., Eriksson P.S. and Faull R.L. (2007) Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin. Exp. Pharmacol. Physiol. 34, 528–532 10.1111/j.1440-1681.2007.04609.x [DOI] [PubMed] [Google Scholar]
- 11.Reif A. et al. (2007) Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur. Arch. Psychiatry Clin. Neurosci. 257, 290–299 10.1007/s00406-007-0733-3 [DOI] [PubMed] [Google Scholar]
- 12.Keilhoff G. et al. (2004) Increased neurogenesis in a rat ketamine model of schizophrenia. Biol. Psychiatry 56, 317–322 10.1016/j.biopsych.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Winner B., Kohl Z. and Gage F.H. (2011) Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 33, 1139–1151 10.1111/j.1460-9568.2011.07613.x [DOI] [PubMed] [Google Scholar]
- 14.Sun Y. et al. (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 111, 1843–1851 10.1172/JCI200317977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin K. et al. (2005) FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. U.S.A. 102, 18189–18194 10.1073/pnas.0506375102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y.K. and Na K.S. (2017) Neuroprotection in schizophrenia and its therapeutic implications. Psychiatry Investig. 14, 383–391 10.4306/pi.2017.14.4.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker A. et al. (2003) Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 687–700 10.1016/S0278-5846(03)00080-0 [DOI] [PubMed] [Google Scholar]
- 18.Beraki S. et al. (2008) Repeated low dose of phencyclidine administration impairs spatial learning in mice: blockade by clozapine but not by haloperidol. Eur. Neuropsychopharmacol. 18, 486–497 10.1016/j.euroneuro.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Schobel S.A. et al. (2013) Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93 10.1016/j.neuron.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mak Y.T. et al. (2010) The toxic effect of ketamine on SH-SY5Y neuroblastoma cell line and human neuron. Microsc. Res. Tech. 73, 195–201 [DOI] [PubMed] [Google Scholar]
- 21.Bosnjak Z.J. et al. (2012) Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr. Drug Saf. 7, 106–119 10.2174/157488612802715663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong C., Rovnaghi C.R. and Anand K.J. (2012) Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells. Crit. Care Med. 40, 2407–2416 10.1097/CCM.0b013e318253563c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H., Uchida T. and Makita K. (2015) Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLoS ONE 10, e0128445 10.1371/journal.pone.0128445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri S. et al. (2017) PACAP protects adult neural stem cells from the neurotoxic effect of ketamine associated with decreased apoptosis, ER stress and mTOR pathway activation. PLoS ONE 12, e0170496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faizi M. et al. (2014) Schizophrenia induces oxidative stress and cytochrome C release in isolated rat brain mitochondria: a possible pathway for induction of apoptosis and neurodegeneration. Iran. J. Pharm. Res. 13, 93–100 [PMC free article] [PubMed] [Google Scholar]
- 26.de Oliveira L. et al. (2011) Behavioral changes and mitochondrial dysfunction in a rat model of schizophrenia induced by ketamine. Metab. Brain Dis. 26, 69–77 10.1007/s11011-011-9234-1 [DOI] [PubMed] [Google Scholar]
- 27.Helfer B. et al. (2016) Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am. J. Psychiatry 173, 876–886 10.1176/appi.ajp.2016.15081035 [DOI] [PubMed] [Google Scholar]
- 28.Newton S.S. and Duman R.S. (2007) Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs 21, 715–725 10.2165/00023210-200721090-00002 [DOI] [PubMed] [Google Scholar]
- 29.Lally J. and MacCabe J.H. (2015) Antipsychotic medication in schizophrenia: a review. Br. Med. Bull. 114, 169–179 10.1093/bmb/ldv017 [DOI] [PubMed] [Google Scholar]
- 30.Malberg J.E. et al. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 10.1523/JNEUROSCI.20-24-09104.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohira K. and Miyakawa T. (2011) Chronic treatment with fluoxetine for more than 6 weeks decreases neurogenesis in the subventricular zone of adult mice. Mol. Brain 4, 10 10.1186/1756-6606-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiou S.H. et al. (2006) Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem. Biophys. Res. Commun. 343, 391–400 10.1016/j.bbrc.2006.02.180 [DOI] [PubMed] [Google Scholar]
- 33.Chen A.T. and Nasrallah H.A. (2019) Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 208, 1–7 10.1016/j.schres.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 34.Lewis S.W. et al. (2006) Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr. Bull. 32, 715–723 10.1093/schbul/sbj067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halim N.D. et al. (2004) Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 29, 1063–1069 10.1038/sj.npp.1300422 [DOI] [PubMed] [Google Scholar]
- 36.Chikama K. et al. (2017) Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 1676, 77–82 10.1016/j.brainres.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 37.Maeda K. et al. (2007) Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J. Pharmacol. Sci. 103, 299–308 10.1254/jphs.FP0061424 [DOI] [PubMed] [Google Scholar]
- 38.Bai O. et al. (2002) Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J. Neurosci. Res. 69, 278–283 10.1002/jnr.10290 [DOI] [PubMed] [Google Scholar]
- 39.Qing H. et al. (2003) The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur. J. Neurosci. 17, 1563–1570 10.1046/j.1460-9568.2003.02590.x [DOI] [PubMed] [Google Scholar]
- 40.Hu X. et al. (2012) Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J. Neuroimmune Pharmacol. 7, 187–201 10.1007/s11481-011-9309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercer A. et al. (2004) PACAP promotes neural stem cell proliferation in adult mouse brain. J. Neurosci. Res. 76, 205–215 10.1002/jnr.20038 [DOI] [PubMed] [Google Scholar]
- 42.Crouch S.P. et al. (1993) The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88 10.1016/0022-1759(93)90011-U [DOI] [PubMed] [Google Scholar]
- 43.Mansouri S. et al. (2013) GalR3 activation promotes adult neural stem cell survival in response to a diabetic milieu. J. Neurochem. 127, 209–220 10.1111/jnc.12396 [DOI] [PubMed] [Google Scholar]
- 44.Mansouri S., Darsalia V., Eweida M., Lundberg M., Nathanson D. and Patrone C. (2014) Exendin-4 protects neural progenitor cells from glucolipoapoptosis. J. Diabetes Metab. 5, 10.4172/2155-6156.1000409 [DOI] [Google Scholar]
- 45.Gilda J.E. and Gomes A.V. (2013) Stain-free total protein staining is a superior loading control to beta-actin for Western blots. Anal. Biochem. 440, 186–188 10.1016/j.ab.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vranken J.H. et al. (2005) Neuropathological findings after continuous intrathecal administration of S(+)-ketamine for the management of neuropathic cancer pain. Pain 117, 231–235 10.1016/j.pain.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 47.Keilhoff G. et al. (2004) Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience 126, 591–598 10.1016/j.neuroscience.2004.03.039 [DOI] [PubMed] [Google Scholar]
- 48.Yaksh T.L. et al. (2008) Toxicology profile of N-methyl-D-aspartate antagonists delivered by intrathecal infusion in the canine model. Anesthesiology 108, 938–949 10.1097/ALN.0b013e31816c902a [DOI] [PubMed] [Google Scholar]
- 49.Cohen M.L. et al. (1973) Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology 39, 370–376 10.1097/00000542-197310000-00003 [DOI] [PubMed] [Google Scholar]
- 50.Liu C.S. et al. (2003) Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic. Res. 37, 1307–1317 10.1080/10715760310001621342 [DOI] [PubMed] [Google Scholar]
- 51.Malik A.N. and Czajka A. (2013) Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13, 481–492 10.1016/j.mito.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 52.Chestkov I.V. et al. (2018) ROS-induced DNA damage associates with abundance of mitochondrial DNA in white blood cells of the untreated schizophrenic patients. Oxid. Med. Cell Longev. 2018, 8587475 10.1155/2018/8587475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kakiuchi C. et al. (2005) Quantitative analysis of mitochondrial DNA deletions in the brains of patients with bipolar disorder and schizophrenia. Int. J. Neuropsychopharmacol. 8, 515–522 10.1017/S1461145705005213 [DOI] [PubMed] [Google Scholar]
- 54.Rajasekaran A. et al. (2015) Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 48, 10–21 10.1016/j.neubiorev.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 55.Menzies F.M., Fleming A. and Rubinsztein D.C. (2015) Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 16, 345–357 10.1038/nrn3961 [DOI] [PubMed] [Google Scholar]
- 56.Kroemer G., Marino G. and Levine B. (2010) Autophagy and the integrated stress response. Mol. Cell 40, 280–293 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizushima N. et al. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merenlender-Wagner A. et al. (2015) Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 20, 126–132 10.1038/mp.2013.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider J.L., Miller A.M. and Woesner M.E. (2016) Autophagy and schizophrenia a closer look at how dysregulation of neuronal cell homeostasis influences the pathogenesis of schizophrenia. Einstein J. Biol. Med. 31, 34–39 10.23861/EJBM201631752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vargas F. et al. (2005) Clozapine prevents apoptosis and enhances receptor-dependent respiratory burst in human neutrophils. Pharmazie 60, 364–368 [PubMed] [Google Scholar]
- 61.Zeng Z. et al. (2017) The atypical antipsychotic agent, Clozapine, protects against corticosterone-induced death of PC12 cells by regulating the Akt/FoxO3a signaling pathway. Mol. Neurobiol. 54, 3395–3406 10.1007/s12035-016-9904-4 [DOI] [PubMed] [Google Scholar]
- 62.Gil-Perotin S. et al. (2013) Adult neural stem cells from the subventricular zone: a review of the neurosphere assay. Anat. Rec. (Hoboken) 296, 1435–1452 10.1002/ar.22746 [DOI] [PubMed] [Google Scholar]
- 63.Mansouri S. et al. (2012) Pituitary adenylate cyclase-activating polypeptide counteracts the impaired adult neural stem cell viability induced by palmitate. J. Neurosci. Res. 90, 759–768 10.1002/jnr.22803 [DOI] [PubMed] [Google Scholar]
- 64.Mansouri S. et al. (2016) Pituitary adenlylate cyclase activating peptide protects adult neural stem cells from a hypoglycaemic milieu. PLoS ONE 11, e0156867 10.1371/journal.pone.0156867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertilsson G. et al. (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 86, 326–338 10.1002/jnr.21483 [DOI] [PubMed] [Google Scholar]
- 66.Li X. et al. (2018) Administration of ketamine causes autophagy and apoptosis in the rat fetal hippocampus and in PC12 cells. Front. Cell Neurosci. 12, 21 10.3389/fncel.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y. et al. (2018) Midazolam attenuates autophagy and apoptosis caused by ketamine by decreasing reactive oxygen species in the hippocampus of fetal rats. Neuroscience 388, 460–471 [DOI] [PubMed] [Google Scholar]
- 68.Park J. et al. (2012) Haloperidol and clozapine block formation of autophagolysosomes in rat primary neurons. Neuroscience 209, 64–73 10.1016/j.neuroscience.2012.02.035 [DOI] [PubMed] [Google Scholar]
- 69.Wang C.Q. et al. (2017) Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 343, 30–38 10.1016/j.neuroscience.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 70.Lipinski M.M. et al. (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid. Redox Signal. 23, 565–577 10.1089/ars.2015.6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hara T. et al. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 72.Mortensen M. et al. (2010) Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 832–837 10.1073/pnas.0913170107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shintani T. and Klionsky D.J. (2004) Autophagy in health and disease: a double-edged sword. Science 306, 990–995 10.1126/science.1099993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahdelma L. et al. (2010) Clozapine is cytotoxic to primary cultures of human bone marrow mesenchymal stromal cells. J. Clin. Psychopharmacol. 30, 461–463 10.1097/JCP.0b013e3181e6a082 [DOI] [PubMed] [Google Scholar]
- 75.Kumar P. et al. (2018) Mitochondrial DNA copy number is associated with psychosis severity and anti-psychotic treatment. Sci. Rep. 8, 12743 10.1038/s41598-018-31122-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baldessarini R.J. and Frankenburg F.R. (1991) Clozapine. A novel antipsychotic agent. N. Engl. J. Med. 324, 746–754 10.1056/NEJM199103143241107 [DOI] [PubMed] [Google Scholar]
- 77.Nordin C., Alme B. and Bondesson U. (1995) CSF and serum concentrations of clozapine and its demethyl metabolite: a pilot study. Psychopharmacology (Berl.) 122, 104–107 10.1007/BF02246083 [DOI] [PubMed] [Google Scholar]
- 78.Greenwood-Smith C., Lubman D.I. and Castle D.J. (2003) Serum clozapine levels: a review of their clinical utility. J. Psychopharmacol. 17, 234–238 10.1177/0269881103017002014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.