Abstract
The bicyclo[3.3.1]nonane architecture is a privileged structural motif found in over 1000 natural products with relevance to neurodegenerative disease, bacterial and parasitic infection, and cancer among others. Despite disparate biosynthetic machinery, alkaloid, terpene, and polyketide-producing organisms have all evolved pathways to incorporate this carbocyclic ring system. Natural products of mixed polyketide/terpenoid origins (meroterpenes) are a particularly rich and important source of biologically active bicyclo[3.3.1]nonane-containing molecules. Herein we detail a fully synthetic strategy toward this broad family of targets based on an abiotic annulation/rearrangement strategy resulting in a 10-step total synthesis of garsubellin A, an enhancer of choline acetyltransferase and member of the large family of polycyclic polyprenylated acylphloroglucinols. This work solidifies a strategy for making multiple, diverse meroterpene chemotypes in a programmable assembly process involving a minimal number of chemical transformations.
Subject terms: Natural product synthesis, Synthetic chemistry methodology, Organic chemistry
Meroterpenes are a particularly important class of biologically active bicyclo[3.3.1]nonane-containing molecules. Here, the authors report a general strategy toward these valuable targets based on abiotic annulation/rearrangement steps resulting in a concise total synthesis of garsubellin A.
Introduction
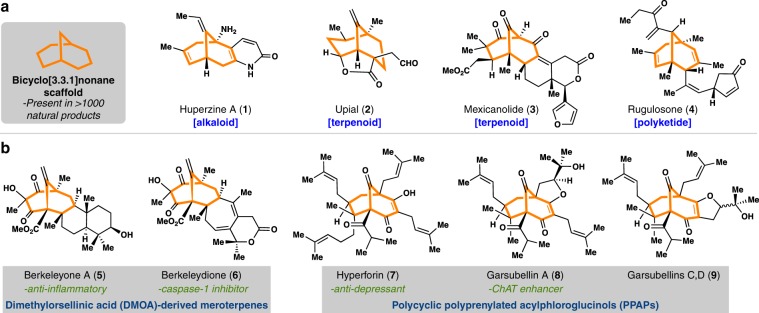
Nature remains the consummate master assembler of intricate polycyclic molecules, and natural products have—and continue to—provide the impetus for the design and discovery of many of the strategies and tactics pertinent to ring formation in organic synthesis1. Embedded in over 1000 natural products is the bicyclo[3.3.1]nonane ring system, which provides a unique spatial arrangement for up to 16 potential substituents to decorate its periphery (Fig. 1a). Despite the significant differences in synthesis capabilities between alkaloid, terpene, and polyketide biosynthetic machinery, all of these natural product classes claim bicyclo[3.3.1]nonane-containing members. Examples include the neuroprotective alkaloid huperzine (1), unusual sesquiterpene upial (2), cytotoxic limonoid mexicanolide (3), and the anti-malarial polyketide rugulosone (4).
Fig. 1. Complex bicyclo[3.3.1]nonane-containing natural products.
a Members are found across all areas of natural product space and therapeutic area. b The large family of terpene-polyketide hybrids (meroterpenes) as a particularly prolific sources of bicyclo[3.3.1] nonanes.
Owing to both their sheer number as well as biological importance, polyketide/terpenoid hybrids (meroterpenes) represent the flagship subset of bicyclo[3.3.1]nonane-containing natural products (Fig. 1b). Metabolites formed through the coupling of isoprenoid side chains with either 3,5-dimethylorsellinic acid (DMOA)2 or acylphloroglucinol derivatives3,4 constitute the majority of such members. The DMOA-derived members berkeleyone A (5) and berkeleydione (6), which possess anti-inflammatory and caspase-1 inhibitory properties5,6, and the neuroactive polycyclic polyprenylated acylphloroglucinols (PPAPs) hyperforin (7)7 and the garsubellins (8–9)8,9 are representative of the structural complexity these targets possess. Moreover, PPAPs in particular induce wide and diverse biological effects and thus represent lead structures for neuroscience, infectious disease, and oncology drug discovery programs4. Hundreds of bicyclo[3.3.1]nonane-containing PPAPs exists and it would appear that nature modulates PPAP function by varying the groups decorating the conserved bicyclo[3.3.1]nonane core. Yet how function is systematically encoded by these groups remains enigmatic. While natural products are unquestionably rich sources of medicinal leads for drug discovery10,11, in most cases they are still not easily constructed in a step-economical and components-based mix and match format as is typical in many drug discovery programs using small, planar compounds connected through C(sp2)–C(sp2) linkages.
Not surprisingly, DMOA-derived meroterpenes12–14, and especially the PPAPs15–50, have proven to be popular targets for total synthesis, and a wide range of creative synthetic approaches have been developed for this purpose to date. Herein, we solidify a broad strategy for bicyclo[3.3.1]nonane-containing meroterpene construction, which in the case of PPAPs, offers 10-step entry into synthetically challenging and biologically active type A members in a modular and predictable fashion. Using this strategy, a few simple fragments can be identified as the building blocks for a large proportion of family members. Finally, our work further underscores how following nature’s molecular design process can lead to efficient abiotic total synthesis tactics and the development of C–C bond-forming methodology.
Results
Synthetic design
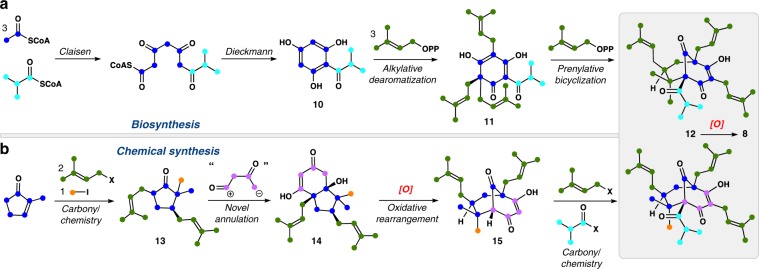
Nature’s assembly process of PPAPs served as initial inspiration for our meroterpene synthetic program as it has for others. Although detailed, enzyme-level blueprints are absent for complex PPAPs, labeling studies have provided a likely biosynthetic scenario, which is shown for the garsubellin A nucleus (12) (Fig. 2)51. In the initial polyketide assembly phase, three molecules of acetyl-CoA (shown in blue) and one molecule of isobutyryl-CoA (shown in turquoise) are joined and then cyclized to phloroglucinol 10 (Fig. 2a). Notably, the isobutyryl group can be swapped with an assortment of other acyl groups leading to early diversification of the PPAPs. Next, 10 is merged with three equivalents of dimethylallyl pyrophosphate (shown in green) in a dearomative alkylation event forming polyprenylated acylphloroglucinol 11. Finally, a fourth molecule of dimethylallyl pyrophosphate activates a single prenyl side chain, leading to a presumed cationic intermediate that is trapped by the pendant enol ether event, thus forging two C–C bonds and the hallmark bicyclo[3.3.1]nonane core (see 12) swiftly from 11. To produce garsubellin A (8) from 12, a chemo- and diastereoselective oxidation of a single prenyl side chain can be invoked followed by regioselective opening of the epoxide by the pendant vinylogous acid. Overall, nine C–C bonds are forged in only a few steps.
Fig. 2. Approaches to the synthesis of bicyclo[3.3.1]nonane-containing meroterpenes.
a Nature’s presumed synthetic route employing polyketide assembly of an aromatic precursor followed by various C–C bond-forming prenylation events. b An abiotic synthetic strategy allows for similarly rapid and modular assembly of complex meroterpene architectures.
Our approach toward PPAPs, including 8, did not seek to emulate nature’s reaction pathway, but rather took inspiration from the basic building blocks employed (Fig. 2b). In particular, the use of fully intact prenyl groups in the construction of PPAPs had great synthetic appeal both for elegance and simplicity reasons. We envisioned that 2-methylcyclopentenone (shown in blue) could be converted into ketone 13 via straightforward conjugate addition and enolate alkylation chemistry, involving two prenyl equivalents (shown in green) and a one-carbon electrophile (shown in orange). From this simple, but highly substituted, cyclopentanone building block, a reaction was desired that could append on a 4-carbon 1,3-diketone equivalent generating 5,6-fused bicycle 14 in analogy to the Robinson-annulation process, but employing an electrophile of a higher oxidation state. From 14, a formal oxidative isomerization of the 5,6-fused skeleton could construct the bicyclo[3.3.1]nonane skeleton directly (see 14 to 15). Finally, late-stage scaffold-decorating steps, in this case attachment of the final prenyl group and isobutyryl unit, would generate 12. In this approach, seven C–C bonds could be forged in a minimal number of steps.
Enolate annulation chemistry
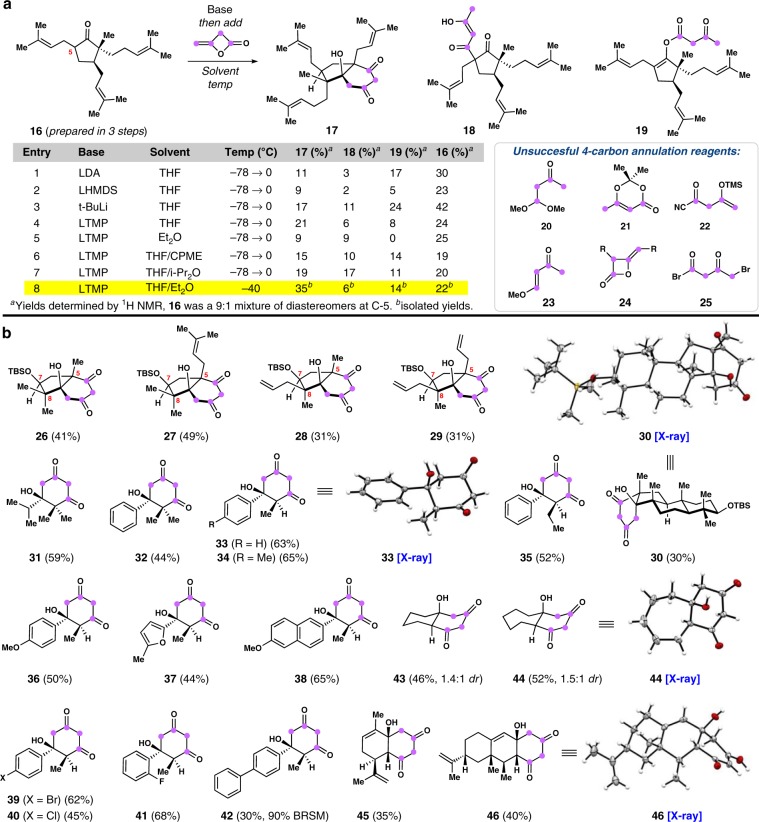
A major hurdle in the implementation of the proposed reaction plan was the four-carbon annulation reaction. Our efforts began with the identification and optimization of a suitable coupling reagent capable of reacting with the enolate of ketone 16 itself, prepared in three steps (Fig. 3a)43. Cyclopentanone 16 represents one of the two building blocks that comprise the majority of the PPAPs amenable to our abiotic strategy (vide infra). After significant experimentation, we identified the strained, feedstock chemical diketene (see Supplementary Note 1 for preparation) as a unique and highly reactive four-carbon unit capable of forming bicycle 17 in 11% yield (see Fig. 3a inset, entry 1). Lithium enolates were found to be critical in this process, which also formed C-acylated, but uncyclized, product 18 (3%) as well as O-acylated product 19 (17%). Similar results were also obtained using lithium hexamethyldisilazide (LHMDS) as base (see Fig. 3a inset, entry 2). Given the ability of diketene to react with diisopropylamine, we also evaluated tert-Butyllithum (t-BuLi) and lithium tetramethylpiperidine (LTMP) as bases finding both boosted the amount of annulated product formed (see Fig. 3a inset, entries 3 and 4). In an effort to reduce the O-acylation product, we evaluated ethereal solvents of varying polarity. Although pure diethyl ether showed promise in this regard (see Fig. 3a inset, entry 5), the reaction efficiency was low and almost equal amounts of 17 and 18 were formed highlighting the importance of solvent in the ring-closing step. A variety of mixed ethereal solvents were examined (see Fig. 3a inset, entries 6–8) ultimately arriving at an optimal THF/Et2O mixture for this specific substrate. After temperature optimization, we were able to produce 17 in 35% isolated yield and as a single diastereomer. It should be stressed that diketene is a highly unique reagent in this annulation process. A variety of other four-carbon conjunctive reagents (see 20–25), including the unstrained diketene acetone adduct (21) as well as substituted diketenes (24), were all examined and did not produce the desired cyclic product.
Fig. 3. Discovery of a lithium enolate 4-carbon annulation reaction.
a Key optimization efforts and findings. b Successfully prepared cyclohexane-1,3-diones.
The annulation reaction of lithium enolates proved amenable to the preparation of a variety of diverse, cyclic 1,3-diketones (Fig. 3b). The starting enolates can be generated by either reacting the free ketone with strong base (LTMP) or by treating the corresponding silyl enol ether derivative with methyllithium (see Supplementary Notes 3 and 4). Cyclopentanones with varying C-5, C-7, and C-8 substitution patterns (see products 26–29) were tolerated, highlighting how different inputs can be easily inserted into our PPAP synthetic blueprint. Sterically congested polycycle 30 was also formed in 30% and forms the basis for a DMOA-derived meroterpene synthetic program13,14. Diketones 31 and 32, which contain a gem-dimethyl group in close proximity to either an isopropyl or phenyl substituent also highlight the ability of this method to forge highly hindered C–C linkages. A variety of propio- or butyrophenone derivatives with diverse substituents were also annulated, leading to cyclic products 33–42 in good yields (30–65%) and with high diastereoselectivity. We attribute the lower isolated yield of product 42 to poor lithium enolate solubility in the ethereal solvent mixture employed. Cyclic aliphatic ketones could also be annulated leading to products 43 and 44; a small preference for the trans diastereomer was noted in these cases. Cyclic enone-containing natural products, such as carvone and nootkatone, could be annulated to produce adducts 45 and 46, respectively. All of the compounds prepared using this chemistry were previously inaccessible chemical entities.
The total synthesis of garsubellin A
Although we had previously employed building block 16 in a short synthesis of the antidepressant PPAP hyperforin (7)43, we turned our attention toward garsubellin A (8). In a search for naturally occurring small molecules to treat neurodegenerative disease, Fukuyama and co-workers isolated 8 from the wood of Garcinia subelliptica, finding that it increased choline acetyltransferase (ChAT) levels 154% relative to control in P10 rat septal neurons8. Notably, ChAT is attenuated in neurological pathologies such as Alzheimer’s disease52,53.
Not surprisingly, garsubellin A has attracted substantial worldwide synthetic interest20,54–59, yet to date, only the groups of Shibasaki15, Danishefsky16, and Nakada36 have documented full total syntheses of this target. Unlike hyperforin, in 8 a single prenyl side chain has been chemo- and stereoselectively oxidized and then cyclized resulting in a complex cyclic ether motif. Mimicking similar oxidative processes in the laboratory in a diastereo-controlled manner has proven challenging in a number of contexts15,20,36,37, and given the presence of garsubellins C and D in Garcinia subelliptica (see 9, Fig. 1)9. Nature may also struggle with this problem in regio- and stereoselective chemical synthesis. Notably, isomers 9 are not reported as ChAT enhancers.
Synthesis of the key THF substructure
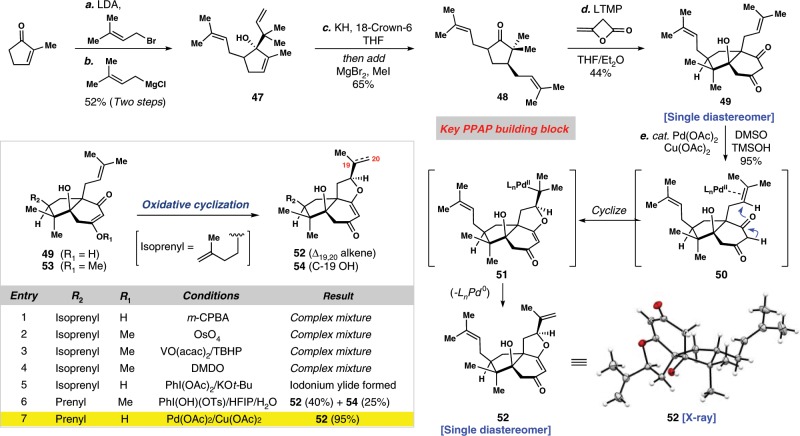
We began our synthetic efforts by preparing key diprenylated cyclopentanone building block 48 (Fig. 4). In doing so, we developed a straightforward route to this motif, diverging from our previous conjugate addition strategy used to produce 16. Thus, commercially available 2-methyl-cyclopenten-1-one was first α-alkylated with prenyl bromide and then treated with prenylmagnesium chloride inducing a highly diastereoselective reverse ketone prenylation. The sterically congested tertiary alcohol formed (see 47) then underwent a facile anionic oxy-Cope rearrangement when exposed to potassium hydride and 18-crown-6. After treatment with MgBr2, the resulting magnesium enolate was alkylated at carbon with methyl iodide affording 48. Small amounts (~10%) of the regioisomeric alkylation product as well doubly alkylated materials were also formed in this transformation; whereas these materials could not be cleanly separated by silica gel chromatography, they were effectively removed following the subsequent transformation. To that effect, employing the diketene annulation on the lithium enolate derived from ketone 48 smoothly generated 5,6-fused bicycle 49 in 44% isolated yield and as a single diastereomer; interestingly, this process was noticeably more efficient than with the related ketone 16.
Fig. 4. Diastereoselective synthesis of a key tricyclic intermediate.
A diketene annulation and unusual vinylogous acid Wacker-type cyclization forge 52 in five chemical steps.
Although a number of synthetic intermediates could be imagined as suitable starting points for construction of the key tetrahydrofuran (THF) motif embedded in garsubellin A, our final successful route incorporated this structure from bicycle 49 (Fig. 4). After extensive experimentation, we found that catalytic quantities of palladium acetate in the presence of a stoichiometric copper-based oxidant (Cu(OAc)2) led to the formation of 52 as a single diastereomer (confirmed by X-ray crystallographic analysis) in very high yield (95%), presumably via an unusual Wacker-type cyclization of the vinylogous acid (see 50→51→52, only an anti-oxypalladation pathway is depicted for simplicity)60. Typically Pd(II)-activated alkenes are attacked by the carbon atom of 1,3-dicarbonyl-containing compounds61–64, but geometric constraints presumably disfavor this process in this system.
The construction of this cyclic ether motif has previously proven challenging to access by epoxidation of a prenyl side chain and acid-promoted cyclization–poor diastereoselectivity in the oxidation has historically led to low overall efficiency15,20,36. Moreover, facile oxidative degradation of PPAPs further complicates such transformations. We also encountered similar problems in our exploratory studies toward this key ring (see Fig. 4 inset). A variety of oxidation conditions were evaluated on 1,3-diketone-containing substrate 49 or its corresponding vinylogous methylester (53). Epoxidation (m-CPBA, DMDO, or VO(acac)2/TBHP) and dihydroxylation (OsO4) conditions all afforded complex reaction mixtures containing cyclized and non-cyclized materials; a poorly diastereoselective and chemoselective oxidation was noted here as well (see Fig. 4 inset, entries 1–4). Seeking to avoid these reaction shortcomings, we reasoned that activation of the prenyl group and direct cyclization by the pendant vinylogous acid in an overall oxidative process would be a more prudent strategy. Interestingly, we initially discovered that treatment of 53 with Koser’s reagent (PhI(OH)OTs) in mixtures of hexafluoroisopropanol/water could promote the direct formation of tertiary alcohol 54 (25%) along with alkene 52 (40%) (see Fig. 4 inset, entry 6). Notably, the other electronically similar prenyl group was not oxidized, suggesting this process involved π-bond activation and cyclization; unfortunately, we were unable to further bias the reaction profile toward 54. Of note, the free 1,3-diketone (i.e., 49) reacted to form an iodonium ylide under related conditions (see Fig. 4 inset, entry 5). Ultimately, these findings assisted in the discovery of the high-yielding Pd(II)-catalyzed oxidative cyclization of 49–52 (see Fig. 4 inset, entry 7).
Completion of a 10-step synthesis of garsubellin A
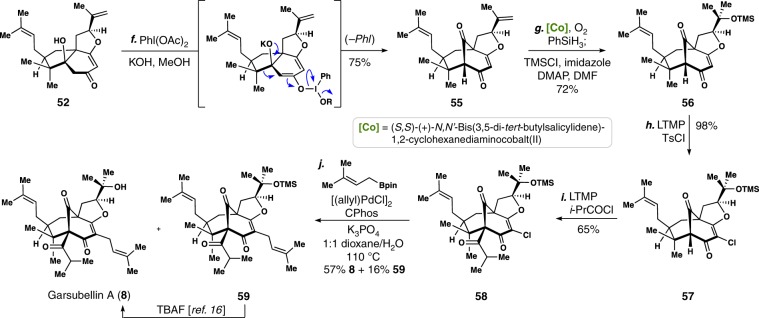
With a robust, five-step route to tricycle 52, we were poised to evaluate the second key transformation in our programmable route to meroterpenes, namely oxidative isomerization of the 5,6-fused bicycle to the bicyclo[3.3.1]nonane (Fig. 5)13,43. Despite the presence of the additional rigidifying THF ring, treatment of this substrate with (diacetoxyiodo)benzene under basic conditions cleanly forged bicyclo[3.3.1]nonane 55 in good yield (75%). Cobalt(II)-salen-catalyzed Mukaiyama hydration of 55 chemoselectively furnished a tertiary alcohol, which could be silylated in the same pot (TMSCl, imidazole, DMAP) to afford 5665. Chlorination of the C-2 position (LTMP, TsCl) allowed for the challenging bridgehead (i.e., C-6) functionalization process (LTMP then i-PrCOCl) to proceed smoothly (see 57→58)66. We note that in prior work toward hyperforin, only one of two possible vinylogous ester regioisomers took part in the bridgehead deprotonation reaction, an unfortunate finding since they were formed unselectively43,67. By incorporating the THF ring prior to the C-6 deprotonation, this problem is obviated in this context16,18.
Fig. 5. Total synthesis of garsubellin A.
An oxidative ring expansion constructs the key bicyclo[3.3.1] nonane core framework and enables a 10-step route to the target.
With 58 in hand, all that remained was C-2 prenylation and cleavage of the silyl ether. Transition metal-catalyzed cross-coupling methods offer perhaps the most-attractive solution to this problem owing to their potential for easy and rapid compound diversification late-stage. Yet palladium-catalyzed cross-coupling methods, especially direct prenylation, have historically proven challenging on similar bicyclo[3.3.1] nonane-containing PPAP scaffolds and we assumed deactivated vinyl chloride 58 would be no different15. Although recent advances in ligand-controlled, linear-selective prenylation methodology boded well for such endeavors68–70, the sensitivity of vinylogous ester 58 to hydrolysis and base-induced decomposition proved problematic. After significant experimentation, we successfully applied Buchwald’s Pd/CPhos-system71 to the Suzuki coupling of 58 and prenylBpin, affording garsubellin A (8) directly in 57% yield. It should be noted that the successful reactivity window for this transformation was minute and deviating even slightly from the optimized conditions proved quite deleterious. For example, heating the reaction mixture for 8 h instead of four led to an ~10% yield. Similarly, changing the solvent mixture from 1:1 dioxane:H2O to 4:1 dioxane:H2O lead primarily to reduction of the vinyl chloride. A small amount of material (17%) still containing the TMS group (see 59) was also formed under our optimized conditions, but can be converted into 8 via the procedure of Danishefsky16. Overall, the synthesis of 8 required 10 steps (3% overall yield) and is nearly half the steps of the previously reported shortest total synthesis of this complex PPAP.
Discussion
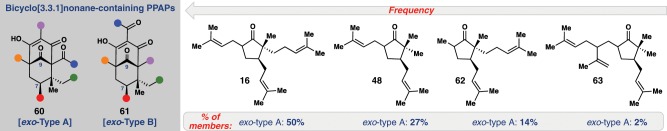
The described total synthesis of garsubellin A combined with our prior work on hyperforin and the DMOA-derived meroterpene berkeleyone A solidifies a general strategy for bicyclo[3.3.1]nonane-containing meroterpene construction and reduces the complexity of many of these targets to that of a substituted cyclopentanone. Recent scholarly analyses of all PPAPs by Grossman and Xu highlighted ~350 natural products with intact bicyclo[3.3.1]nonane ring systems, which can be classified as either type A or type B, depending on the placement of the acyl group (Fig. 6)3,4. More than half of these members are further designated “exo-type” PPAPs (see 60 and 61, Fig. 6) signifying that the C-7 substituent resides on the same face as the C-9 carbonyl group as is found in garsubellin and hyperforin. From the exo-type A grouping, of relevance to the work demonstrated herein, we estimate that only four building blocks, namely 16, 48, 62, and 63 are required to access over 90% of members using the strategy documented here in combination with side chain manipulations. Moreover, building blocks 16 and 48 alone, which we have already successfully employed, cover over 75% of exo-Type A PPAP space. In broader terms, our work in combination with Porco’s efficient biomimetic assembly of type B PPAPs40, and Plietker’s modular route to endo-type PPAPs30,37,38, means that a large swath of all of PPAP chemical space can potentially be covered synthetically in ten or fewer steps.
Fig. 6. Building block analysis of bicyclo[3.3.1]nonane-containing exo-type A PPAPs.
Four simple building blocks are embedded in over 90% of members using this strategy.
We note in closing that calls to increase three-dimensional complexity in drug discovery programs are intensifying72, further highlighting the need for modular routes to spatially multifaceted small molecules in rapid fashion. Nature routinely provides such complexity, and in many cases, also clues to its synthetic design and diversification process. Although directly mimicking such chemistry in the laboratory can prove fruitful, abiotic synthesis strategies also offer opportunities for new reaction discovery and the prospect of accessing diverse naturally occurring secondary metabolites and their derivatives with efficiencies that rival nature.
Methods
General
All air and moisture sensitive reactions were performed in flame-dried glassware under an atmosphere of dry nitrogen or argon. Dry THF, dichloromethane, diethyl ether, toluene, dimethylformamide, and hexane were obtained by passing these previously degassed solvents through activated alumina columns. When mentioned, further solvent degassing was performed by bubbling a stream of argon through the solvent in an ultrasound bath for a period of 10 min. Anhydrous dimethyl sulfoxide was purchased from Aldrich, stored over molecular sieves, and used without further purification. All other reagents were used as received, unless stated otherwise. Reactions were monitored by thin-layer chromatography (TLC) on Silicycle SiliaplateTM glass backed TLC plates (250 μm thickness, 60 Å porosity, F-254 indicator) and visualized by UV irradiation or development with an anisaldehyde or phosphomolybdic/cerium sulfate stain. Volatile solvents were removed under reduced pressure with a rotary evaporator. All flash column chromatography was performed using Silicycle SiliaFlash F60, 230–400 mesh silica gel (40–63 μm). 1H NMR and 13C NMR spectra were recorded with Bruker AV, AVQ, and DRX spectrometers operating at 400, 500, 600, or 700 MHz for 1H (100, 125, 150, 175 MHz for 13C) in CDCl3, C6D6, CD2Cl2, CD3OD, or (CD3)2CO. Chemical shifts are reported relative to the residual solvent signal (1H NMR: δ = 7.26 (CDCl3); 13C NMR: δ = 77.16 (CDCl3)). NMR data are reported as follows: chemical shift (multiplicity, coupling constants where applicable, number of hydrogens). Splitting is reported with the following symbols: s = singlet, bs = broad singlet, d = doublet, t = triplet, app t = apparent triplet, dd = doublet of doublets, ddd = doublet of doublet of doublets, dt = doublet of triplets, hept = heptet, m = multiplet. IR spectra were recorded on a Nicolet 380 spectrometer as thin films and are reported in frequency of absorption (cm−1). High-resolution mass spectra were obtained by the mass spectrometry facility at the University of California, Berkeley using a Finnigan LTQ FT mass spectrometer.
Experimental data
For experimental procedures and spectroscopic data of the compounds, see Supplementary Information. For general procedures for preparing diketene and its employment in enolate annulations, see Supplementary Notes 1–4 and Supplementary Fig. 1. For general procedures to prepare garsubellin A and intermediates, see Supplementary Fig. 2 and Supplementary Note 5. For NMR spectra of synthetic intermediates, see Supplementary Figs. 3–70. For the comparison of synthetic and natural garsubellin A NMR spectra see Supplementary Tables 1–2, Supplementary Fig. 71, and the Supplementary References.
Supplementary information
Acknowledgements
We thank the National Science Foundation (NSF) for funding this work (CAREER award #155454 to T.J.M.). C.P.T. also acknowledges the NSF for providing a Graduate Research Fellowship (DGE-1106400). G.X. thanks the Swiss National Science Foundation (SNSF) for a postdoctoral mobility fellowship (P2BEP2_162076). T.J.M. is an Arthur C. Cope Scholar and acknowledges unrestricted grant support from Bristol-Myers Squibb, Amgen, Novartis, and Eli Lilly. We thank Dr. Hasan Celik for NMR spectroscopic assistance and NIH grant S10OD024998. Dr. Nicholas Settineri is acknowledged for X-ray crystallographic analysis wherein support from NIH Shared Instrument Grant S10RR027172 is also acknowledged.
Author contributions
T.J.M. and C.P.T. conceived of the project. X.S., C.P.T. and G.X. performed all of the synthetic experiments. All authors analyzed the data. T.J.M. directed the project and wrote the manuscript with assistance from X.S. and C.P.T.
Data availability
The X-ray crystallographic coordinates for structures 30, 33, 46, 44, and 52 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession codes CCDC #1521625, 1521624, 1956231, 1521627, and 1879639, respectively. (www.ccdc.cam.ac.uk/data_request/cif). All other relevant data supporting the findings of this study are available within the article and its Supplementary Information files.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Xiaoguang Lei, Hong-Xi Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xingyu Shen, Chi P. Ting.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14354-5.
References
- 1.Armaly AM, DePorre YC, Groso EJ, Riehl PS, Schindler CS. Disca. Chem. Rev. 2015;115:9232–9276. doi: 10.1021/acs.chemrev.5b00034. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda Y, Abe I. Biosynthesis of overy of novel synthetic methodologies and reagents during natural product synthesis in the post-palytoxin erungal meroterpenoids. Nat. Prod. Rep. 2016;33:26–53. doi: 10.1039/C5NP00090D. [DOI] [PubMed] [Google Scholar]
- 3.Ciochina R, Grossman RB. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2006;106:3963–3986. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 4.Yang X-W, Grossman RB, Xu G. Research progress of polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2018;118:3508–3558. doi: 10.1021/acs.chemrev.7b00551. [DOI] [PubMed] [Google Scholar]
- 5.Stierle DB, Stierle AA, Hobbs JD, Stokken J, Clardy J. Berkeleydione and berkeleytrione, new bioactive metabolites from an acid mine organism. Org. Lett. 2004;6:1049–1052. doi: 10.1021/ol049852k. [DOI] [PubMed] [Google Scholar]
- 6.Stierle DB, et al. Berkeleyones and related meroterpenes from a deep water acid mine waste fungus that inhibit the production of interleukin 1-β from induced inflammasomes. J. Nat. Prod. 2011;74:2273–2277. doi: 10.1021/np2003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richard Jean-Alexandre. Chemistry and Biology of the Polycyclic Polyprenylated Acylphloroglucinol Hyperforin. European Journal of Organic Chemistry. 2013;2014(2):273–299. doi: 10.1002/ejoc.201300815. [DOI] [Google Scholar]
- 8.Fukuyama Y, Kuwayama A, Minami H. Garsubellin A, a novel polyprenylated phloroglucin derivative, increasing choline acetyltransferase (ChAT) activity in postnatal rat septal neuron cultures. Chem. Pharm. Bull. 1997;45:947–949. doi: 10.1248/cpb.45.947. [DOI] [PubMed] [Google Scholar]
- 9.Fukuyama Y, Minami H, Kuwayama A. Garsubellins, polyisoprenylated phloroglucinol derivatives from Garcinia Subelliptica. Phytochemistry. 1998;49:853–857. doi: 10.1016/S0031-9422(98)00126-5. [DOI] [Google Scholar]
- 10.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 11.Kearney SE, et al. Canvass: a crowd-sourced, natural product screening library for exploring biological space. ACS Cent. Sci. 2018;4:1727–1741. doi: 10.1021/acscentsci.8b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkin M, Szewczyk SM, Scruse AC, Newhouse TR. Total synthesis of (±)-berkeleyone A. J. Am. Chem. Soc. 2017;139:1790–1793. doi: 10.1021/jacs.6b12914. [DOI] [PubMed] [Google Scholar]
- 13.Ting CP, Xu G, Zeng X, Maimone TJ. Annulative methods enable a total synthesis of the complex meroterpene berkeleyone A. J. Am. Chem. Soc. 2016;138:14868–14871. doi: 10.1021/jacs.6b10397. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Elkin M, Tantillo DJ, Newhouse TR, Maimone TJ. Traversing biosynthetic carbocation landscapes in the total synthesis of andrastin and terretonin meroterpenes. Angew. Chem. Int. Ed. 2017;56:12498–12502. doi: 10.1002/anie.201705654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuramochi A, Usuda H, Yamatsuga K, Kanai M, Shibasaki M. Total synthesis of (±)-garsubellin A. J. Am. Chem. Soc. 2005;127:14200–14201. doi: 10.1021/ja055301t. [DOI] [PubMed] [Google Scholar]
- 16.Siegel DR, Danishefsky SJ. Total synthesis of garsubellin A. J. Am. Chem. Soc. 2016;128:1048–1049. doi: 10.1021/ja057418n. [DOI] [PubMed] [Google Scholar]
- 17.Rodeschini V, Ahmad NM, Simpkins NS. Synthesis of (±)-clusianone: high-yielding bridgehead and diketone substitutions by regioselective lithiation of enol ether derivatives of bicyclo[3.3.1]nonane-2,4,9-triones. Org. Lett. 2006;8:5283–5285. doi: 10.1021/ol0620592. [DOI] [PubMed] [Google Scholar]
- 18.Tsukano C, Siegel D, Danishefsky SJ. Differentiation of nonconventional “carbanions”-the total synthesis of nemorosone and clusianone. Angew. Chem. Int. Ed. 2007;46:8840–8844. doi: 10.1002/anie.200703886. [DOI] [PubMed] [Google Scholar]
- 19.Qi J, Porco JA., Jr. Rapid access to polyprenylated phloroglucinols via alkylative dearomatization-annulation: total synthesis of (±)-clusianone. J. Am. Chem. Soc. 2007;129:12682–12683. doi: 10.1021/ja0762339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Blake AJ. Synthesis of polyprenylated acylphloroglucinols using bridgehead lithiation: the total synthesis of racemic clusianone and a formal synthesis of racemic garsubellin A. J. Org. Chem. 2007;72:4803–4815. doi: 10.1021/jo070388h. [DOI] [PubMed] [Google Scholar]
- 21.Nuhant P, David M, Pouplin T, Delpech B, Marazano C. α,α‘-annulation of 2,6-prenyl-substituted cyclohexanone derivatives with malonyl chloride: application to a short synthesis of (±)-clusianone. formation and rearrangement of a biogenetic-like intermediate. Org. Lett. 2007;9:287–289. doi: 10.1021/ol062736s. [DOI] [PubMed] [Google Scholar]
- 22.Simpkins, N. S., Taylor, J. D., Weller, M. D. & Hayes, C. J. Synthesis of nemorosone via a difficult bridgehead substitution reaction. Synlett 639–643 (2010).
- 23.George JH, Hesse MD, Baldwin JE, Adlington RM. Biomimetic synthesis of polycyclic polyprenylated acylphloroglucinol natural products isolated from Hypericum papuanum. Org. Lett. 2010;12:3532–3535. doi: 10.1021/ol101380a. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu Y, Shi S-L, Usuda H, Kanai M, Shibasaki M. Catalytic asymmetric total synthesis of ent-hyperforin. Angew. Chem. Int. Ed. 2010;49:1103–1106. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu Y, Shi S-L, Usuda H, Kanai M, Shibasaki M. The first catalytic asymmetric total synthesis of ent-hyperforin. Tetrahedron. 2010;66:6569–6584. doi: 10.1016/j.tet.2010.05.086. [DOI] [PubMed] [Google Scholar]
- 26.Garnsey MR, Matous JA, Kwiek JJ, Coltart DM. Asymmetric total synthesis of (+)- and (−)-clusianone and (+)- and (−)-clusianone methyl enol ether via ACC alkylation and evaluation of their anti-HIV activity. Bioorg. Med. Chem. Lett. 2011;21:2406–2409. doi: 10.1016/j.bmcl.2011.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnsey MR, Lim D, Yost JM, Coltart DM. Development of a strategy for the asymmetric synthesis of polycyclic polyprenylated acylphloroglucinols via n-amino cyclic carbamate hydrazones: application to the total synthesis of (+)-clusianone. Org. Lett. 2010;12:5234–5237. doi: 10.1021/ol1022728. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Mitasev B, Qi J, Porco JA., Jr. Total synthesis of plukenetione A. J. Am. Chem. Soc. 2010;132:14212–14215. doi: 10.1021/ja105784s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi J, Beeler AB, Zhang Q, Porco JA., Jr. Catalytic enantioselective alkylative dearomatization–annulation: total synthesis of absolute configuration assignment of hyperibone K. J. Am. Chem. Soc. 2010;132:13642–13644. doi: 10.1021/ja1057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biber N, Moews K, Plietker B. The total synthesis of hyperpapuanone, hyperibone L, epi-clusianone, and oblogifolin A. Nat. Chem. 2011;3:938–942. doi: 10.1038/nchem.1170. [DOI] [PubMed] [Google Scholar]
- 31.Pepper HP, Lam HC, Bloch WM, George JH. Biomimetic total synthesis of (±)-garcibracteatone. Org. Lett. 2012;14:5162–5164. doi: 10.1021/ol302524q. [DOI] [PubMed] [Google Scholar]
- 32.Uwamori M, Saito A, Nakada M. Stereoselective total synthesis of nemorosone. J. Org. Chem. 2012;77:5098–5107. doi: 10.1021/jo300646j. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Porco JA., Jr. Total synthesis of (±)-7-epi-nemorosone. Org. Lett. 2012;14:1796–1799. doi: 10.1021/ol300386t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparling BA, Moebius DC, Shair MD. Enantioselective total synthesis of hyperforin. J. Am. Chem. Soc. 2013;135:644–647. doi: 10.1021/ja312150d. [DOI] [PubMed] [Google Scholar]
- 35.Uwamori M, Nakada M. Stereoselective total synthesis of (±)-hyperforin via intramolecular cyclopropanation. Tetrahedron Lett. 2013;54:2022–2025. doi: 10.1016/j.tetlet.2013.02.021. [DOI] [Google Scholar]
- 36.Uwamori M, Nakada M. Stereoselective total synthesis of garsubellin A. J. Antibiot. 2013;66:141–145. doi: 10.1038/ja.2012.125. [DOI] [PubMed] [Google Scholar]
- 37.Lindermayr K, Plietker B. The bidirectional total synthesis of sampsonione P and hyperibone I. Angew. Chem. Int. Ed. 2013;52:12183–12186. doi: 10.1002/anie.201306256. [DOI] [PubMed] [Google Scholar]
- 38.Horeischi F, Biber N, Plietker B. The total syntheses of guttiferone A and 6-epi-guttiferone A. J. Am. Chem. Soc. 2014;136:4026–4030. doi: 10.1021/ja500063a. [DOI] [PubMed] [Google Scholar]
- 39.Pepper HP, Tulip SJ, Nakano Y, George JH. Biomimetic total synthesis of (±)-doitunggarcinone A and (+)-garcibracteatone. J. Org. Chem. 2014;79:2564–2573. doi: 10.1021/jo500027k. [DOI] [PubMed] [Google Scholar]
- 40.Boyce JH, Porco JA., Jr. Asymmetric, stereodivergent synthesis of (−)‐clusianone utilizing a biomimetic cationic cyclization. Angew. Chem. Int. Ed. 2014;53:7832–7837. doi: 10.1002/anie.201404437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellavance G, Barriault L. Total syntheses of hyperforin and papuaforins |A–C, and formal synthesis of nemorosone through a gold(I)‐catalyzed carbocyclization. Angew. Chem. Int. Ed. 2014;53:6701–6704. doi: 10.1002/anie.201403939. [DOI] [PubMed] [Google Scholar]
- 42.Uetake Y, Uwamori M, Nakada M. Enantioselective approach to polycyclic polyprenylated acylphloroglucinols via catalytic asymmetric intramolecular cyclopropanation. J. Org. Chem. 2016;80:1735–1745. doi: 10.1021/jo5026699. [DOI] [PubMed] [Google Scholar]
- 43.Ting CP, Maimone TJ. Total synthesis of hyperforin. J. Am. Chem. Soc. 2015;137:10516–10519. doi: 10.1021/jacs.5b06939. [DOI] [PubMed] [Google Scholar]
- 44.Sparling BA, Tucker JK, Moebius DC, Shair MD. Total synthesis of (−)-nemorosone and (+)-secohyperforin. Org. Lett. 2015;17:3398–3401. doi: 10.1021/acs.orglett.5b01121. [DOI] [PubMed] [Google Scholar]
- 45.Horeischi F, Guttroff C, Plietker B. The enantioselective total synthesis of (+)-clusianone. Chem. Commun. 2015;51:2259–2261. doi: 10.1039/C4CC09701G. [DOI] [PubMed] [Google Scholar]
- 46.Socolsky C, Plietker B. Total synthesis and absolute configuration assignment of MRSA active garcinol and isogarcinol. Chem. Eur. J. 2015;21:3053–3061. doi: 10.1002/chem.201406077. [DOI] [PubMed] [Google Scholar]
- 47.Boyce JH, Eschenbrenner-Lux V, Porco JA., Jr. Syntheses of (+)-30-epi-, (−)-6-epi-, (±)-6,30-epi-13,14-didehydroxyisogarcinol and (±)-6,30-epi-garcimultiflorone A utilizing highly diastereoselective, lewis acid-controlled cyclizations. J. Am. Chem. Soc. 2016;138:14789–14797. doi: 10.1021/jacs.6b09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellavance G, Barriault L. Modular total syntheses of hyperforin, papuaforins A, B, and C via gold(I)-catalyzed carbocyclization. J. Org. Chem. 2018;83:7215–7230. doi: 10.1021/acs.joc.8b00426. [DOI] [PubMed] [Google Scholar]
- 49.Wen S, et al. Regiodivergent photocyclization of dearomatized acylphloroglucinols: asymmetric syntheses of (–)-nemorosone and (–)-6-epi-garcimultiflorone A. J. Am. Chem. Soc. 2019;141:11315–11321. doi: 10.1021/jacs.9b05600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, et al. Me2AlSEt-promoted domino dieckmann cyclization enables the total synthesis of polycyclic polyprenylated acylphloroglucinols. Org. Lett. 2019;21:8075–8079. doi: 10.1021/acs.orglett.9b03078. [DOI] [PubMed] [Google Scholar]
- 51.Adam P, Arigoni D, Bacher A, Eisenreich W. Biosynthesis of hyperforin in Hypericum perforatum. J. Med. Chem. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- 52.Roberson MR, Harrell LE. Cholinergic activity and amyloid precursor protein metabolism. Brain. Res. Rev. 1997;30:50–69. doi: 10.1016/S0165-0173(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 53.Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert. Rev. Neurother. 2008;8:1703–1708. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolaou KC, Pfefferkorn JA, Kim S, Wei HX. Synthesis of the fully functionalized bicyclic core of garsubellin A. J. Am. Chem. Soc. 1999;121:4724–4725. doi: 10.1021/ja9905445. [DOI] [Google Scholar]
- 55.Spessard SJ, Stoltz BM. Progress toward the synthesis of garsubellin A and related phloroglucins: the direct diastereoselective synthesis of the bicyclo[3.3.1]nonane core. Org. Lett. 2002;4:1943–1946. doi: 10.1021/ol025968+. [DOI] [PubMed] [Google Scholar]
- 56.Mehta G, Bera MK. Towards an enantiospecific total synthesis of garsubellin A and related phloroglucin natural products: the α-pinene approach. Tetrahedron Lett. 2003;45:1113–1116. doi: 10.1016/j.tetlet.2003.12.023. [DOI] [Google Scholar]
- 57.Mehta G, Bera MK. A rapid acquisition of the bicyclo[3.3.1]nonan-9-one core present in garsubellin A and related phloroglucins. Tetrahedron Lett. 2006;47:689–692. doi: 10.1016/j.tetlet.2005.11.120. [DOI] [Google Scholar]
- 58.Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Wilson C. Synthetic studies towards garsubellin A: synthesis of model systems and potential mimics by regioselective lithiation of bicyclo[3.3.1]nonane-2,4,9-trione derivatives from catechinic acid. Org. Biomol. Chem. 2007;5:1924–1934. doi: 10.1039/b704311b. [DOI] [PubMed] [Google Scholar]
- 59.Mehta G, Bera MK. An approach toward the synthesis of PPAP natural product garsubellin A: construction of the tricyclic core. Tetrahedron. 2013;69:1815–1821. doi: 10.1016/j.tet.2012.12.066. [DOI] [Google Scholar]
- 60.Kočovsky P, Bäckvall J-E. The syn/anti-dichotomy in the palladium-catalyzed addition of nucleophiles to alkenes. Chem. Eur. J. 2015. 2015;21:36–56. doi: 10.1002/chem.201404070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi T, Hegedus LS. Palladium(II) assisted alkylation of olefins with stabilized carbanions. J. Am. Chem. Soc. 1977;99:7093–7094. doi: 10.1021/ja00463a069. [DOI] [Google Scholar]
- 62.Hegedus LS, Williams RE, McGuire MA, Hayashi T. Palladium-assisted alkylation of olefins. J. Am. Chem. Soc. 1980;102:4973–4979. doi: 10.1021/ja00535a026. [DOI] [Google Scholar]
- 63.Pei T, Widenhoefer RA. Palladium-catalyzed intramolecular addition of 1,3-diones to unactivated olefins. J. Am. Chem. Soc. 2001;123:11290–11291. doi: 10.1021/ja011764x. [DOI] [PubMed] [Google Scholar]
- 64.Pei T, Wang X, Widenhoefer RA. Palladium-catalyzed intramolecular oxidative alkylation of unactivated olefins. J. Am. Chem. Soc. 2003;125:648–649. doi: 10.1021/ja026317b. [DOI] [PubMed] [Google Scholar]
- 65.Crossley SWM, Obradors C, Martinez RM, Shenvi RA. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 2016;116:8912–9000. doi: 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simpkins NS. Adventures in bridgehead substitution chemistry: synthesis of polycyclic polyprenylated acylphloroglucinols (PPAPs) Chem. Commun. 2013;49:1042–1051. doi: 10.1039/C2CC37914G. [DOI] [PubMed] [Google Scholar]
- 67.Ting CP, Maimone TJ. The total synthesis of hyperforin. Synlett. 2016;27:1443–1449. doi: 10.1055/s-0035-1561619. [DOI] [Google Scholar]
- 68.Farmer JL, Hunter H, Organ M. Regioselective cross-coupling of allylboronic acid pinacol ester derivatives with aryl halides via Pd-PEPPSI-IPent. J. Am. Chem. Soc. 2012;134:17470–17473. doi: 10.1021/ja308613b. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Buchwald SL. Ligand-controlled palladium-catalyzed regiodivergent suzuki–miyaura cross-coupling of allylboronates and aryl halides. J. Am. Chem. Soc. 2013;135:10642–10645. doi: 10.1021/ja405950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y, Mustard TJL, Cheong PH-Y, Buchwald SL. Palladium-catalyzed completely linear-selective negishi cross-coupling of allylzinc halides with aryl and vinyl electrophiles. Angew. Chem. Int. Ed. 2013;52:14099–14102. doi: 10.1002/anie.201308585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han C, Buchwald SL. Negishi couping of secondary alkyzinc halides with aryl bromides and chlorides. J. Am. Chem. Soc. 2009;131:7532–7533. doi: 10.1021/ja902046m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lovering F. Escape from flatland 2: complexity and promiscuity. Med. Chem. Comm. 2013;4:515–519. doi: 10.1039/c2md20347b. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures 30, 33, 46, 44, and 52 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession codes CCDC #1521625, 1521624, 1956231, 1521627, and 1879639, respectively. (www.ccdc.cam.ac.uk/data_request/cif). All other relevant data supporting the findings of this study are available within the article and its Supplementary Information files.