Abstract
Cortical networks receive a highly variable stream of inputs from internal and external influences, and must flexibly adapt their operations on a short timescale. Recent work has highlighted this state-dependent functional flexibility of cortical circuits and provided initial insights into underlying circuit-level mechanisms. Transitions from quiescent to aroused or task-engaged behavioral states are associated with common motifs of network activity, including changes in correlations and enhanced sensory encoding. Evidence points to a key role for selective activation of specific GABAergic interneuron populations in mediating mode-switching in cortical networks. Finally, inhibitory interneurons may function as a critical target for convergent state-dependent neuromodulatory sculpting of cortical circuits.
Introduction
A mammalian brain made up of specialized circuits to accommodate each possible environment, sensory context, and behavioral state the organism might experience over its lifetime would be enormous and untenable. Instead, neural circuits in the cerebral cortex, which performs sensory and cognitive functions, are generalist computational units whose operations can adjust rapidly to adapt to changes in externally or internally generated inputs. External inputs from the outside world can change on a millisecond timescale, providing an ever-changing regime of input. Similarly, internally generated states, such as attention, affect, and arousal, vary rapidly. Even though the structural components of the physical circuit, the neurons and synaptic connections, do not change very rapidly, the functional circuit, those cells that are actively participating in computational operations, may change on a moment-to-moment basis to support functional flexibility. Indeed, this type of flexibility is not specific to mammalian circuits but rather a core property of neural circuits originally identified in invertebrates, where pattern generation is precise but different modes of circuit operation can be selected from the same circuit by external influences [1].
If functional flexibility is a core element of cortical circuit operation, it should be observed at several levels. To support rapid adaptation to changing cognitive and sensory processing demands, cortical circuits should exhibit rapid mode switching and changes in computational operations, such as sensory encoding, on a fast timescale. In addition, flexibility in the functional circuit may be detectable in the activity of cellular populations whose contributions to the circuit vary with behavioral state and cognitive demands. Finally, this flexibility may extend beyond cell assemblies to the level of large-scale cortical networks. Recent work has provided evidence for cortical functional flexibility at multiple scales and highlighted the contributions of GABAergic interneurons and neuromodulatory systems in the online functional sculpting of cortical circuits.
State-dependent circuit performance
Cortical network state fluctuates rapidly during wakefulness with changes in behavioral state, such as quiescence, motor activity, and focused attention. The statistics of cortical spiking are modulated in association with these fluctuations. In turn, behavioral state-dependent changes in cortical activity are correlated with predictable changes in sensory encoding [2–4] (Fig. 1). Recent studies in rodents have identified that arousal, as measured by pupil dilation and motor activity, causes changes in the activity of both excitatory and inhibitory cortical neurons at both the local [3,4] and global [5–7] scales.
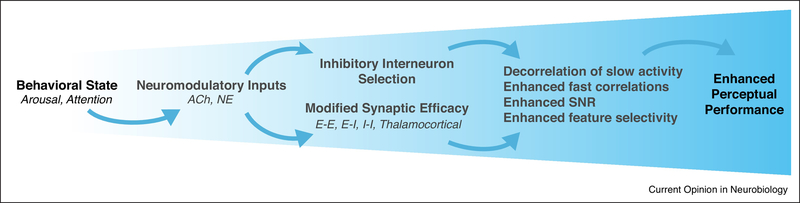
Figure 1.
Cortical function is flexibly modulated by behavioral state. Changes in behavioral state during wakefulness, such as increased arousal or attention, are associated with increased release of acetylcholine (ACh) and norepinephrine (NE). These neuromodulators have many effects in the cortex, including depolarization of inhibitory interneurons, such as the VIP and SST cells, and regulation of cortico-cortical synapses among excitatory (E) and inhibitory (I) cells as well as thalamocortical synapses. These cellular effects lead to altered circuit activity, including modulation of pairwise temporal correlations between neurons and enhancement of sensory encoding. Together, state-dependent modulation of cellular and circuit interactions leads to enhanced performance of perceptual and cognitive tasks.
Increased arousal is correlated at the level of individual neurons with depolarization and changes in membrane potential fluctuations, indicating that the statistics of network synaptic input to single neurons are altered in a state-dependent manner [3,8–10]. Pairwise correlations in the activity of cortical neurons decrease during periods of arousal and task engagement [4,11]. These changes in cortical circuit activity vary across cortical areas, with locomotion causing an increase in both excitatory and inhibitory synaptic activity in primary visual cortex [10] but a scaled decrease in primary auditory cortex [12]. Changes in neural activity with changes in waking behavioral states, such as attentional modulation, may also affect neural circuits differently across cortical layers [13*]. One caveat to interpreting results across species and experimental paradigms is that not all instances of behavioral state transition are equivalent. For instance, in mouse primary visual cortex (V1), arousal, as measured by pupil dilation, and motor activity, as measured by locomotion, cause distinct changes in the statistics of cortical activity at the single-neuron, local circuit, and population levels [4].
These state-dependent fluctuations in cortical network activity are associated with alterations in the mode of cortical operations, such as sensory stimulus encoding. Periods of high arousal, as measured by pupil dilation or locomotion, are correlated with enhanced cortical stimulus representation in both excitatory and inhibitory neurons [3,4]. Individual spikes generated by cortical neurons encode more information during running than during quiescent periods [14], and neural activity during locomotion provides better decoding of stimulus presence than that during quiescence [15]. In comparison, locomotion is associated with suppression, rather than enhancement, of auditory responses in A1 cortex [12,16].
Changes observed at the level of cortical circuits as animals toggle between wakeful states are associated with distinct functional outcomes at the level of behavior. Mice perform better at perceptual tasks during periods of arousal than during quiescence [10], although very high arousal levels may ultimately impair performance [8]. State-dependent fluctuations in rodent V1 cortex activity, as measured by changes in excitatory neuron firing rates and local field potential oscillations across cortical layers, are strongly associated with trial-by-trial fluctuations in visual detection performance [17]. In A1 cortex, behavioral engagement via self-initiation enhances both auditory representations and behavioral performance on auditory tasks, partially by changing spontaneous activity and partially by enhancing evoked responses [18]. State fluctuations observed in primate cortex are likewise associated with modulation of perceptual performance [2,19*]. Together, these findings suggest a common motif of state-dependent changes in cortical circuit activity patterns, with arousal potentially predisposing the cortex towards enhanced encoding and promoting perceptual performance.
State-dependent regulation of stimulus encoding may allow the cortex to switch between modes optimized for detection of prominent stimuli, as when an animal is resting but needs to detect predators, and discrimination between stimuli, as when an animal is foraging and needs to evaluate complex scenes for food, social, or environmental cues. Alternatively, arousal may signal the onset of a new context for decision making and facilitate the discarding of prior information that is no longer useful in guiding behavior [20].
State- and context-dependent cortical ensembles
In addition to overall regulation of cortical activity, recent work has suggested that selective ensembles of cortical neurons may be recruited at specific times in a context-dependent manner. In secondary motor cortex, the occurrence of two distinct modes of ensemble activity is correlated with behavioral shifts between two variants of an auditory-motor task [21]. In superficial retrosplenial cortex, ensembles of neurons that encode spatial information are activated sequentially, firing in sequences during movement [22]. Interestingly, flexible representation of information by cortical ensembles may be regulated by parvalbumin-expressing (PV) interneurons, suggesting that inhibition may play a key role in the engagement of ensemble activity by environmental stimuli [23]. Such state-dependent ensemble representations are not restricted to neurons whose firing rates are modulated by stimulus presentation, as neurons carrying information in the relative timing or pattern of spikes also contribute to ensemble encoding of relevant information during task performance [24].
The state-dependent sculpting of functional cortical interactions may occur across multiple spatial scales. Small numbers of spikes from single neurons can restructure the information encoded by surrounding neurons in the local circuit, highlighting the power of very local circuit interactions in shaping population encoding [25]*. State-dependent changes in functional connectivity also occur at the level of large-scale networks across cortical areas. During locomotion as compared to quiescence, large-scale cortico-cortical network interactions are substantially reweighted, increasing the strength of functional interactions between V1 neurons and those in higher-order sensory and motor areas [26]*.
Inhibitory control of cortical circuit mode
One current theory for how cortical circuits can switch efficiently between different functional modes on a short timescale is that the diversity of GABAergic inhibitory interneurons promotes flexible function. Because cortical GABAergic cell types comprise several groups with distinct intrinsic and biophysical properties, morphology, synaptic targeting, and even neuromodulatory receptor expression, different sources of synaptic inhibition could be recruited into ongoing cortical ensemble activity at different times to promote distinct computational operations. Inhibitory interneurons are reciprocally connected with each other and with pyramidal neurons, making them prominent regulators of nearby networks [27]. Indeed, recent work found that context-dependent activation of specific inhibitory interneuron populations promotes context-dependent behavioral switching in an auditory task [28]*. Although most computational models of circuit function do not include different inhibitory or excitatory cell types, studies incorporating contributions from realistically connected distinct populations of inhibitory interneurons support the idea that distinct sources of inhibition can differentially regulate network statistics, encoding, and feature selectivity [28**,29].
Recent work has pointed to GABAergic interneurons that express vasoactive intestinal peptide (VIP) as a powerful regulator of cortical mode, despite their low numbers. VIP interneurons in mouse V1 cortex are largely quiet when animals are quiescent, but highly active during locomotion periods (Fig. 2). In turn, loss of VIP interneurons causes loss of the increased sensory response gain in local pyramidal neurons during running [30]. Together, these data suggest that VIP interneurons may play a key regulatory role, at very specific time-points, in setting the mode of cortical computational operations.
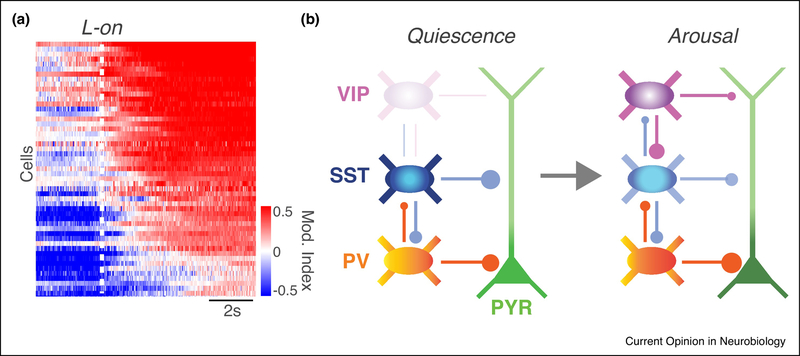
Figure 2.
Online sculpting of the functional cortical circuit. A. Activity of GCamp6-expressing VIP interneurons in primary visual cortex of an awake behaving mouse around the time of locomotion onset (L-on) as compared to a preceding quiescent period, quantified as a modulation index. The majority of VIP cells exhibit increased activity during locomotion, and some cells also show anticipatory activity preceding motor output. Adapted from [50]. B. Schematic of a local cortical circuit with PV (orange), SST (blue), and VIP (purple) -expressing interneurons and a pyramidal neuron (green). Under quiescent conditions, VIP interneurons may be minimally participatory in the local circuit. Upon arousal, VIP cells are robustly recruited into the functional local circuit, potentially altering the balance of dendrite- and soma-targeting inhibition and increasing pyramidal neuron output.
In addition to locomotion, VIP cells are also activated by stimuli of negative valence, such as punishment. They suppress both somatostatin-expressing (SST) and a subset of PV interneurons [31], leading to disinhibition of pyramidal neurons and enhancement of their sensory responses [32]. Activation of VIP cells improves perceptual performance for contrast detection, whereas PV and SST interneuron stimulation impairs performance [33]. Intriguingly, VIP cells may selectively innervate superficial, but not deep, layer SST cells, suggesting separate subnetworks in layers 2/3 and 5 that are differentially sculpted by behavioral state and local inhibition [34].
Together, these experimental data suggest that state-dependent recruitment of VIP interneurons selectively suppresses other inhibitory populations, potentially changing the distribution of dendrite- and soma-targeting synaptic inhibition received by pyramidal neurons (Fig. 2B). This shift in inhibitory interneuron activity patterns is associated with disinhibition of pyramidal neurons and a change in feature selectivity and sensory encoding, along with perceptual performance. Further work using computational models of cortical circuits suggests that VIP interneurons may play a key role in mode-switching in V1 cortex, promoting feature-specific enhancement of visual encoding by principal neurons [35,36]. One potential caveat is that VIP interneurons also directly inhibit pyramidal neuron dendrites [37], potentially making the impact of VIP activation on the pyramidal neurons a complex mix of disinhibition and direct inhibition. Indeed, the disinhibition model of VIP cell action does not hold for all stimulus conditions [38].
A second example of inhibitory sculpting comes from work on gamma oscillations in V1 cortex. Interactions between PV interneurons and pyramidal neurons generate the classic gamma rhythm (30–80Hz), but SST interneuron activation and the influence of SST interneurons on PV interneurons can shift the ongoing mode of temporal patterning to lower frequencies [27,39,40]*. The relative degree of recruitment of different inhibitory populations into ongoing network activity could thus allow frequency switching and potentially provide a mechanism for selecting channels of communication to downstream target structures.
Neuromodulatory circuit sculpting
Neuromodulatory influences may be a key mechanistic link between behavioral state and GABAergic interneurons in the cortex. The combined influence of several neuromodulatory systems may contribute to functional sculpting of the online cortical circuit. Indeed, changes in neuromodulatory tone are associated with behavioral state transitions during wakefulness. Recent work found that fluctuations in noradrenergic activity were correlated with phasic changes in pupil diameter, whereas cholinergic fluctuations were correlated with locomotion and tonic pupil dilation [41]. Cholinergic axon activity in primary somatosensory cortex in rodents is correlated with whisking, and cholinergic release shifts cortical network synaptic activity from a mode dominated by slow activity to a mode dominated by fast activity [42]. The relationship between neuromodulatory inputs and behavioral state is likely complex, as cholinergic neurons are activated during motor activity, such as running and licking, as well as in response to punishment [43,44]. In addition, neuromodulatory activity changes both the spatiotemporal structure and information content of cortical activity. Cholinergic signaling regulates neuronal correlation structure, reducing noise correlations [45,46]. Increased cholinergic release also enhances the signal:noise of evoked responses, leading to changes in sensory encoding [45,46].
Several lines of recent evidence have pointed to inhibitory interneurons as a critical intersection point between neuromodulatory inputs and local cortical circuits. One notable example is the VIP interneurons, which receive cholinergic input and are depolarized by activation of nicotinic acetylcholine receptors, leading to increased inhibition of SST interneurons [30,47]. However, desynchronization of cortical activity by cholinergic action requires SST interneuron activity and can be mimicked by SST stimulation [45], suggesting a complex relationship between neuromodulators and the reciprocally inhibitory local GABAergic populations in the cortex. There is some evidence for cholinergic activation of all three major IN types in sensory cortex, and these effects may drive inhibition-dependent changes in circuit activity during behavior [28]. Finally, neuromodulatory sculpting may occur even within a nominal population. Recent work highlighted two functional subpopulations of PV interneurons in sensory cortex, one group that increased activity with arousal in association with noradrenergic input and one group that was suppressed during locomotion in association with cholinergic input [48]. These findings suggest key links between behavioral state and neuromodulatory activity and highlight a potential role for interneurons as intermediaries, but the precise combination of neuromodulatory cellular mechanisms underlying these effects remain poorly understood.
Conclusions and open questions
Together, the recent studies highlighted above have examined state-dependent flexible function in cortical circuits in unprecedented detail. However, several important questions remain to be fully addressed. As shown by recent work using large-scale recording and imaging techniques, not all cortical areas are modulated the same way during state transitions [26,49], suggesting simultaneous enhancement or suppression of different functions in different circuits. These observations raise intriguing possibilities that primary sensory and high-order cortical circuits may be differentially flexible in response to ongoing behavioral and cognitive demands. One potential issue in comparing such data across findings is that the experimental paradigms and methods for identifying state transitions or different modes of cortical activity may vary across groups. Likewise, criteria for identification of cortical ensembles also vary, posing a challenge to interpretation.
Cortical networks incorporate extensive recursive connectivity, and reciprocal interactions between inhibitory populations make it unlikely that activation of any individual cell type will have only one action on the local network. It remains unclear how or whether different functional connections within the local network, such as the ‘disinhibition circuit’ mediated by VIP interneurons, are selectively activated without engaging competing mechanisms. These issues may be further resolved by future efforts to use locally targeted perturbations of small numbers of neurons in vivo.
Finally, most methods used in vivo capture only spiking activity and infer functional connections through correlations. However, much of the online sculpting of the functional cortical circuit from the physical circuit probably occurs at the level of short-term synaptic dynamics, such as synaptic depression following repeated spiking. This level of circuit interaction largely remains to be fully investigated in active circuits in vivo.
Highlights.
Cortical circuits exhibit flexible activity patterns and encoding of sensory stimuli.
Inhibitory interneurons may mediate cortical mode transitions.
Neuromodulatory inputs sculpt the functional cortical circuit.
Acknowledgements
This work was supported by NIH R01 MH102365, NIH R01 EY022951, NIH R01 MH113852, a SFARI Research Grant from the Simons Foundation, a McKnight Fellowship, and a grant from the Ludwig Family Foundation to J.A.C. The author thanks Dr. M.J. Higley for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Marder E: Modifiability of pattern generation. Curr Opin Neurobiol 1991, 1:571–576. [DOI] [PubMed] [Google Scholar]
- 2.Engel TA, Steinmetz NA, Gieselmann MA, Thiele A, Moore T, Boahen K: Selective modulation of cortical state during spatial attention. Science 2016, 354:1140–1144. [DOI] [PubMed] [Google Scholar]
- 3.Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS: Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 2014, 84:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinck M, Batista-Brito R, Knoblich U, Cardin JA: Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 2015, 86:740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK: Single-trial neural dynamics are dominated by richly varied movements. bioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD: Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019, 364:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barson D, Hamodi AS, Shen XY, Lur G, Constable RT, Cardin JA, Crair MC, Higley MJ: Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. bioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGinley MJ, David SV, McCormick DA: Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron 2015, 87:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack PO, Friedman J, Golshani P: Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 2013, 16:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett C, Arroyo S, Hestrin S: Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron 2013, 80:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downer JD, Niwa M, Sutter ML: Task engagement selectively modulates neural correlations in primary auditory cortex. J Neurosci 2015, 35:7565–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI: Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci 2014, 17:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Nandy AS, Nassi JJ, Reynolds JH: Laminar Organization of Attentional Modulation in Macaque Visual Area V4. Neuron 2017, 93:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study using laminar probe recordings in macaque cortex to examine the laminar distribution of attentional state modulation.
- 14.Dadarlat MC, Stryker MP: Locomotion Enhances Neural Encoding of Visual Stimuli in Mouse V1. J Neurosci 2017, 37:3764–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mineault PJ, Tring E, Trachtenberg JT, Ringach DL: Enhanced Spatial Resolution During Locomotion and Heightened Attention in Mouse Primary Visual Cortex. J Neurosci 2016, 36:6382–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider DM, Nelson A, Mooney R: A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 2014, 513:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speed A, Del Rosario J, Burgess CP, Haider B: Cortical State Fluctuations across Layers of V1 during Visual Spatial Perception. Cell Rep 2019, 26:2868–2874 e2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carcea I, Insanally MN, Froemke RC: Dynamics of auditory cortical activity during behavioural engagement and auditory perception. Nat Commun 2017, 8:14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Gutnisky DA, Beaman C, Lew SE, Dragoi V: Cortical response states for enhanced sensory discrimination. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Electrophysiological recordings in monkey V1 revealed that the preceding state of the cortex predisposed the ability of the cortical network to accurately encode sensory stimuli.
- 20.Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI: Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci 2012, 15:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siniscalchi MJ, Phoumthipphavong V, Ali F, Lozano M, Kwan AC: Fast and slow transitions in frontal ensemble activity during flexible sensorimotor behavior. Nat Neurosci 2016, 19:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao D, Kandler S, McNaughton BL, Bonin V: Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat Commun 2017, 8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agetsuma M, Hamm JP, Tao K, Fujisawa S, Yuste R: Parvalbumin-Positive Interneurons Regulate Neuronal Ensembles in Visual Cortex. Cereb Cortex 2018, 28:1831–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insanally MN, Carcea I, Field RE, Rodgers CC, DePasquale B, Rajan K, DeWeese MR, Albanna BF, Froemke RC: Spike-timing-dependent ensemble encoding by non-classically responsive cortical neurons. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Chettih SN, Harvey CD: Single-neuron perturbations reveal feature-specific competition in V1. Nature 2019, 567:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]; Used targeted optical stimulation of single neurons to reveal profound influences on activity in the surrounding circuit.
- *26.Clancy KB, Orsolic I, Mrsic-Flogel TD: Locomotion-dependent remapping of distributed cortical networks. Nat Neurosci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a combination of electrophysiology and wide-field calcium imaging, this study found a remapping of cortex-wide activity patterns during locomotion in mice, along with altered functional connectivity of individual cortical neurons.
- 27.Cardin JA: Inhibitory Interneurons Regulate Temporal Precision and Correlations in Cortical Circuits. Trends Neurosci 2018, 41:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Kuchibhotla KV, Gill JV, Lindsay GW, Papadoyannis ES, Field RE, Sten TA, Miller KD, Froemke RC: Parallel processing by cortical inhibition enables context-dependent behavior. Nat Neurosci 2017, 20:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provided links between fluctuations in cholinergic signaling in cortex and the recruitment of local inhibitory interneurons into the functional circuit during perceptual task performance. This study also includes a computational model incorporating three inhibitory interneuron populations.
- 29.Litwin-Kumar A, Rosenbaum R, Doiron B: Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. J Neurophysiol 2016, 115:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP: A cortical circuit for gain control by behavioral state. Cell 2014, 156:1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A: Cortical interneurons that specialize in disinhibitory control. Nature 2013, 503:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karnani MM, Jackson J, Ayzenshtat I, Hamzehei Sichani A, Manoocheri K, Kim S, Yuste R: Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J Neurosci 2016, 36:3471–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cone JJ, Scantlen MD, Histed MH, Maunsell JHR: Different Inhibitory Interneuron Cell Classes Make Distinct Contributions to Visual Contrast Perception. eNeuro 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz W, Tremblay R, Levenstein D, Rudy B: Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 2017, 355:954–959. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Koch C, Mihalas S: A Computational Analysis of the Function of Three Inhibitory Cell Types in Contextual Visual Processing. Front Comput Neurosci 2017, 11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Mihalas S: Visual processing mode switching regulated by VIP cells. Sci Rep 2017, 7:1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ, Higley MJ: Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 2018, 97:368–377 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pakan JM, Lowe SC, Dylda E, Keemink SW, Currie SP, Coutts CA, Rochefort NL: Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, Zhang Y, Li X, Zhao X, Ye Q, Lin Y, Tao HW, Rasch MJ, Zhang X: Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 2017, 96:1403–1418 e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veit J, Hakim R, Jadi MP, Sejnowski TJ, Adesnik H: Cortical gamma band synchronization through somatostatin interneurons. Nat Neurosci 2017, 20:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS: Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 2016, 7:13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggermann E, Kremer Y, Crochet S, Petersen CCH: Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep 2014, 9:1654–1660. [DOI] [PubMed] [Google Scholar]
- 43.Harrison TC, Pinto L, Brock JR, Dan Y: Calcium Imaging of Basal Forebrain Activity during Innate and Learned Behaviors. Front Neural Circuits 2016, 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monosov IE, Leopold DA, Hikosaka O: Neurons in the Primate Medial Basal Forebrain Signal Combined Information about Reward Uncertainty, Value, and Punishment Anticipation. J Neurosci 2015, 35:7443–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen N, Sugihara H, Sur M: An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci 2015, 18:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minces V, Pinto L, Dan Y, Chiba AA: Cholinergic shaping of neural correlations. Proc Natl Acad Sci U S A 2017, 114:5725–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E: Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci 1999, 19:5228–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Junco-Clemente P, Tring E, Ringach DL, Trachtenberg JT: State-Dependent Subnetworks of Parvalbumin-Expressing Interneurons in Neocortex. Cell Rep 2019, 26:2282–2288 e2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimaoka D, Harris KD, Carandini M: Effects of Arousal on Mouse Sensory Cortex Depend on Modality. Cell Rep 2018, 22:3160–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batista-Brito R, Vinck M, Ferguson KA, Chang JT, Laubender D, Lur G, Mossner JM, Hernandez VG, Ramakrishnan C, Deisseroth K, et al. : Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits. Neuron 2017, 95:884–895 e889. [DOI] [PMC free article] [PubMed] [Google Scholar]