Abstract
Restriction in nutrient acquisition is one of the primary causes for reduced growth and yield in water deficient soils. Sulfur (S) is an important secondary macronutrient that interacts with several stress metabolites to improve performance of food crops under various environmental stresses including drought. Increased S supply influences uptake and distribution of essential nutrients to confer nutritional homeostasis in plants exposed to limited water conditions. The regulation of S metabolism in plants, resulting in synthesis of numerous S-containing compounds, is crucial to the acclimation response to drought stress. Two different experiments were laid out in semi-controlled conditions to investigate the effects of different S sources on physiological and biochemical mechanisms of maize (Zea mays L. cv. P1574). Initially, the rate of S application in maize was optimized in terms of improved biomass and nutrient uptake. The maize seedlings were grown in sandy loam soil fertigated with various doses (0, 15, 30 and 45 kg ha−1) of different S fertilizers viz. K2SO4, FeSO4, CuSO4 and Na2SO4. The optimized S dose of each fertilizer was later tested in second experiment to determine its role in improving drought tolerance of maize plants. A marked effect of S fertilization was observed on biomass accumulation and nutrients uptake in maize. In addition, the optimized doses significantly increased the gas exchange characteristics and activity of antioxidant enzymes to improve yield of maize. Among various S sources, application of K2SO4 resulted in maximum photosynthetic rate (43%), stomatal conductance (98%), transpiration rate (61%) and sub-stomatal conductance (127%) compared to no S supply. Moreover, it also increased catalase, guaiacol peroxidase and superoxide dismutase activities by 55, 87 and 65%, respectively that ultimately improved maize yield by 33% with respect to control under water deficit conditions. These results highlight the importance of S fertilizers that would likely be helpful for farmers to get better yield in water deficient soils.
Subject terms: Plant physiology, Drought
Introduction
Maize (Zea mays L.) is one of the major cereal crops that provide food for humans and feed for livestock. The demand for maize seed has increased significantly during past few decades due to its consumption in poultry feed and wet milling industry. The importance of maize as a food crop is well recognized and is used as a staple food in many parts of the world1. Maize seed is an abundant source of energy as 100 g seed provides 365 kilocalories of energy2. However, it is an extensive nutrient crop and excessive use of fertilizers to obtain high yield has resulted in the depletion of nutrients particularly sulfur (S) in soils3. Moreover, water shortage due to climate change may induce further losses in maize production in future.
Adaptation of maize to limited water conditions has received great interest from farmers, researchers and policy makers considering its importance in nutritional food security. Since maize requires large quantities of water to complete its life cycle, water deficiency at critical growth stages significantly reduces maize yield4. Exposure to drought stress induces marked changes in water status, chlorophyll content and photosynthetic apparatus of plants5. It influences water use efficiency and biomass accumulation in plants6. Drought induced reduction in nutrients absorption, redistribution and transport adversely affects the plant production7,8. Due to utilization of large amount of nutrients, maize is considered sensitive to nutrient deficiency3, which may be further aggravated by limited water conditions. Increased deficiency of nutrients in agricultural soils is considered one of the major factors for reduced maize yield9.
Sulfur (S) is recognized as the fourth major nutrient after nitrogen (N), phosphorus (P) and potassium (K). It not only improves crop yield but also influences the quality due to its key role in protein synthesis. It is main constituent of proteins, thioredoxin (TRx), methionine (Met), cysteine (Cys), vitamins (Vit), sulfo-lipids (SL) and iron-sulfur (Fe-S) clusters system that play important role in regulation of physiological metabolism of plants10. Plants uptake S in metabolically inactive form known as sulfate (SO42−) from soil surface. It is reduced into sulfide (S−2) and assimilated into cysteine (Cys) by the activity of ATP-sulfuryase11. A variety of S compounds such as glutathione (Glu), Met, phytochelatins (PCs) are synthesized from Cys-residues which play an important role in alleviating the drastic effects of environmental stresses like drought12. Interestingly, S is the only macronutrient that accumulates in the xylem sap of water stressed maize plants13. Recent research suggests a coordinated action of several drought-responsive stress metabolites with S assimilation in plants exposed to drought stress14. For example, abscisic acid (ABA) induced closure of stomata is directly linked with S metabolism in maize13. The increased S demand in drought-stressed plants reflects the regulatory importance of S in ABA signalling and detoxification of reactive oxygen species (ROS)15. The highly reductive glutathione or GSH scavenges ROS such as OH•, O•−2 and H2O2 through activation of enzymatic antioxidants viz. catalase (CAT), guaiacol peroxidase (GPX) and superoxide dismutase (SOD). Both CAT and GPX eliminate excess H2O2 by generating H2O and O2, whereas SOD prevents OH• formation by removing O•−216,17. Additionally, S metabolism is linked to polyamine and ethylene through salvage pathway involved in maize response to drought stress3,11.
Studies involving the use of S fertilizers to improve crop produce and productivity are well documented18–22. However, the questions pertaining to comparative effects of S fertilizers on uptake and metabolism of other nutrients have largely remained unanswered. It is momentous to unravel the effects of S on mineral elements particularly nitrogen (N), potassium (K) and phosphorus (P). Evidence suggests that decrease in SO42− availability during drought may restrict nitrate (NO3−) uptake due to limited CO2 fixation and decreased flux of SO42− into cysteine in maize11. Therefore, a balanced N:S ratio is essential to obtain high yield and quality in cereal crops23. Remarkably, rhizospheric S availability regulates K content in shoot indicating that K+ acts as counter-ion to compensate for SO42− deficiency in soil24. Likewise, remobilization of vacuolar SO42− during S deprivation was compensated osmotically by accumulation of NO3− and phosphate (PO43−) in vacuole to sustain plant growth25.
A constant decline of water resources, due to climate change, is one of the major threats to the future food security of ever increasing world population. The challenge of water scarcity is more urgent than ever: acute water shortage pose serious threats to productivity of major food crops; decreasing water flows put us in seriously water-scarce countries. The frequent shortage of water and deterioration of eco-environment due to progressive global climate change have greatly influenced agricultural production in arid and semi-arid regions of the world. Adoption of drought mitigation approaches such as selection for physiologically efficient S fertilizers may have value in management programs aimed at improving drought stress tolerance to increase grain yield of food crops like maize. In this study, we hypothesized that S induced improvement in drought tolerance may be attributed to increased photosynthetic activity and activation of antioxidant machinery. To test this hypothesis, we firstly optimized doses for S fertilization in maize seedlings that were later used in second experiment to evaluate the effects of S on physiological and biochemical processes of maize under drought stress.
Materials and Methods
Experimental material and conditions
Two pot experiments were conducted in wire house of MNS-University of Agriculture, Multan (MNS-UAM), Pakistan using completely randomized design with factorial arrangement and three replications. Seeds of indigenous maize hybrid viz. P1574 characterized as drought sensitive by Majeed et al.26 were obtained from local seed dealer of Pioneer Pakistan Pvt. Ltd. It is a highly digestible spring maize hybrid also used for silage purposes by local farmers. The seeds were initially treated with recommended doses of Topsin-M-70-WP (fungicide) and Imidacloprid (insecticide) for disinfection. The pots were filled with sandy loam soil collected from research field area of MNS-UAM. Before filling the pots, soil samples were randomly taken from collected soil to determine the physicochemical characteristics following the procedure reported by Jackson and Barak27 (Table 1).
Table 1.
Physicochemical characteristics of soil used for the pot experiments.
| Physical Characteristics | Chemical Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Texture | Saturation percentage | pH | Organic matter (%) | Nitrogen (mg kg−1) | Phosphorus (mg kg−1) | Potassium (mg kg−1) | Sulfur (mg kg−1) |
| Sandy loam | 24 | 8.1 | 0.79 | 102 | 8.50 | 240 | 2.1 |
Pot experiment-I
First pot experiment was carried out to optimize sulfur (S) dose for selected maize hybrid using different S sources. Randomly selected healthy, uniform ten seeds were sown in plastic pots of 10 kg capacity of soil (25 cm diameter × 45 cm length). The seedlings were thinned later and only five seedlings were maintained after emergence in each pot. Nutrient solutions containing N, P and K were applied as fertilizers at the start of the experiment using urea (0.6 kg per pot), diammonium phosphate (0.3 kg per pot) and potassium oxide (0.2 kg per pot). Sulfur (S) fertilization was done one week after seedling emergence (V4, four leaf stage) through fertigation using various sources of S viz. K2SO4, CuSO4, FeSO4 and Na2SO4 applied at different rates of 15, 30 and 45 kg ha−1 (75, 150 and 225 mg per pot). All pots were weighed daily to estimate water lost through evapotranspiration and supplied with required amount of water. After five weeks of seedling emergence (V10), the seedlings were harvested for estimation of biomass attributes and later dried in an oven at 65 °C for at least 72 h to record dry weight and NPK analysis.
Pot experiment-II
The second pot experiment was conducted to evaluate the effects of optimized S doses on physiological and biochemical processes of maize under drought stress. Ten seeds of same maize hybrid were sown in earthern pots (diameter 45 cm × length 60 cm) filled with 24 kg soil. Only three seedlings per pot were maintained after emergence, which were later reduced to only one healthy seedling in each pot. Recommended doses of P and K (80 kg ha−1 each) and 1/8th N (120 kg ha−1) were fertigated at the time of sowing using diammonium phosphate, potassium oxide (0.96 g per pot) and urea (0.18 g per pot), whereas remaining N was applied at in three equal split doses of 0.42 g per pot as described by Naeem et al.28.
Drought stress and sulfur fertilization
Drought stress was imposed one week after seedling emergence by keeping one set of plants (normal plants) at 100% water holding capacity (WHC), whereas water stressed plants were kept at 30% WHC based on gravimetric method as described by Nachabe29. The soil moisture content was measured daily using soil moisture meter ML-3 Theta Probe (Delta-T Devices, United Kingdom) and maintained by adding the amount of water lost through evapotranspiration.
Sulfur fertilization was done through fertigation, before initiation of drought stress, using optimized doses of K2SO4 (30 kg ha−1), Na2SO4 (30 kg ha−1), CuSO4 (45 kg ha−1) and FeSO4 (45 kg ha−1). The youngest mature leaves from each experimental unit were selected for the estimation of water status, SPAD value and activity of antioxidative enzymes at tasseling (VT) stage. The plants were harvested at physiological maturity and data regarding yield attributes was recorded from harvested plant material following standard procedures.
Determination of NPK content
The above ground plant material including leaves (0.5 g) was dried in an oven and later grounded using Willey mill. The dried material was used for the determination of N, P and K content following the method described by Wolf30. Briefly, the powdered plant tissue was acid-digested with 5 ml of H2SO4 using BD50 digestion block (SEAL Analytical, Malaysia). Then 2 ml of H2O2 was added in tubes and heated at 350 °C for three and half hours until fumes were produced. Volume of extract was maintained by adding distilled water up to 50 ml in volumetric flask. The extract was filtered with Whatman-40 filter paper and N was determined using Kjeldhal method. The vanadium molybdate yellow colorimetric method was used for P determination, whereas K content was assayed using flame photometer (Sherwood M410, UK).
Estimation of leaf relative turgidity and SPAD value
The detached youngest, fully expanded leaf was weighed immediately to record fresh weight (FW) and then dipped in distilled water for 24 h at 4 °C. Later, the leaves were taken out from distilled water, wiped with tissue paper and weighed to determine turgid weight (TW), Then same leaves were placed in an oven for 72 h at 65 °C to record dry weight (DW). Leaf relative turgidity (RT) was measured using following formula reported by Barrs31:
The fully expanded young leaves were used to estimate leaf chlorophyll content expressed as SPAD value. The observations were made early in the morning between 9.00 and 11.00 a.m. using chlorophyll meter (SPAD-502, Minolta Corp.).
Gas exchange measurements
The gas exchange characteristics were measured with a CIRAS-3 portable open-flow gas exchange system (PP Systems, Amesbury, USA). The system was used to record net photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs) and sub-stomatal conductance (Ci) of uppermost fully expanded leaf of each seedling. The chamber was adjusted at 100 mL min−1 mL airflow rate, 1200 μmol∙m−2∙s−1 density of photosynthetic photon flux, 390 ± 5 μmol∙mol−1 CO2 concentration rate, 99.9 kPa atmospheric pressure.
Assay of antioxidative enzymes
Leaf samples were homogenized (1:5) in pestle and mortar using 50 mM Na2HPO4, pH 7.0 containing 1 M Sodium chloride,1 mM EDTA and 1% polyvinylpyrroldone. Enzyme activities were determined using supernatant of enzyme extract (EE) produced from sample solution after centrifugation (20,000 × g, 15 min) at 4 °C.
Catalase activity (CAT) activity was determined by monitoring the degradation of hydrogen peroxide (H2O2) according to Chance and Maehly32. Enzyme extract (200 µL) was added in reaction mixture (1.8 mL), which contained H2O2 (30 mM) and K-P-buffer (50 mM) of 7.0 pH. The decline in H2O2 was estimated as a reduction in optical density at 240 nm.
The procedure reported by Urbanek et al.33 was followed to estimate guaiacol peroxidase (GPX) activity. The reaction mixture (2 mL) was prepared by mixing 50 mM K-P buffer (pH 6.8) with H2O2 (20 mM) and guaiacol (20 Mm). The mixture was incubated at room temperature for 10 min. The reaction was stopped by adding 0.5 mM H2SO4 (5%) and absorbance was measured at 480 nm.
The enzymatic activity of superoxide dismutase (SOD) was recorded following the method of Van Rossun et al.34. The enzyme extract (50 µL) containing 50 mM K-P-buffer (pH 7.8). was added to 2 μM riboflavin, 75 μM nitroblue tetrazolium chloride (NBT), 100 μM EDTA and 13 mM L-methionine. The reaction was initiated in a chamber under illumination of a 30 W-fluorescent lamp for 10 min. The blue color formazane, produced as a result of photoreduction caused by nitroblue tetrazolium (NBT), was noted as increase in absorbance at 560 nm. No enzyme extract was added in reaction mixture used as control and kept in the dark. One SOD unit was defined as the quantity of enzyme required to inhibit 50% photoreduction of the NBT.
Economic analysis
A benefit-cost analysis was carried out to conclude the economic feasibility of various sulfate fertilizers to alleviate the drastic influence of drought stress in maize. The rate of each S fertilizer i.e. K2SO4, FeSO4, CuSO4 and Na2SO4 used in this experiment was 30, 45, 45 and 30 kg ha−1 respectively. The cost of K2SO4, FeSO4, CuSO4 and Na2SO4 was 176, 1200, 1200 and 65 kg−1 in PKR (Pakistani rupees) respectively. Land preparation, sowing seed, irrigation, fertilizing, plant protection measures (insecticide, herbicide), harvesting and threshing was included in fixed cost. The gross income was estimated by using prevailing average marketing price in Pakistan, PKR 900 per 40 kg.
Statistical analysis
All collected data were analyzed statistically using Fisher’s Analysis of Variance (ANOVA) technique on computer programme Statistix (version 9.1). The treatment means were compared using Tukey’s post-hoc test at 0.05 probability level.
Results
Biomass accumulation
Application of S fertilizers significantly (P < 0.01) influenced biomass attributes i.e. shoot length (SL), root length (RL), shoot fresh weight (SFW), root fresh weight (RFW), shoot dry weight (SDW) and root dry weight (RDW) of maize seedlings (Suppl. Table 1). Maize seedlings fertilized with K2SO4 at 30 kg ha−1 exhibited the highest increase in SL (119.24%) RL (71.08%), SFW (124.46%), RFW (68.59%), SDW (136.17%) and RDW (62.43%) compared to control (no S supply). Higher concentration of K2SO4 and Na2SO4 significantly (P < 0.01) reduced biomass accumulation, whereas application of CuSO4 and FeSO4 at 45 kg ha−1 gave maximum values for these attributes with respect to no S supply (Table 2).
Table 2.
Biomass attributes of maize seedlings applied with various sources of sulfur fertilizers viz. K2SO4, FeSO4, CuSO4 and Na2SO4 at 0, 15, 30 and 45 kg ha−1. Shoot length = SL, Root length = RL, Shoot fresh weight = SFW, Root fresh weight = RFW, Shoot dry weight = SDW and Root dry weight = RDW. Different alphabets represent significant difference between mean values ± standard error.
| Observations | K2SO4 (kg ha−1) | FeSO4 (kg ha−1) | CuSO4 (kg ha−1) | Na2SO4 (kg ha−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 0 | 15 | 30 | 45 | 0 | 15 | 30 | 45 | 0 | 15 | 30 | 45 | |
| SL | 26.0 ± 1.76 g | 39.7 ± 4.17c–f | 57.0 ± 2.12a | 54.3 ± 3.9ab | 28.3 ± 1.8fg | 38.3 ± 2.65c-f | 40 ± 1.8c-f | 46.7 ± 2.37a-c | 31.0 ± 1.17e-g | 31.0 ± 1.17e-g | 39.0 ± 1.17c-f | 43.0 ± 2.7b-e | 30.0 ± 1.15fg | 38.6 ± 1.5c-f | 44.7 ± 4.5b-d | 32.7 ± 2.06d-g |
| RL | 27.7 ± 2.44c | 36.0 ± 3.57a-c | 47.3 ± 6.12a | 45.0 ± 4.07ab | 25.66 ± 1.48c | 36.3 ± 1.8a-c | 34.3 ± 2.96a-c | 35.3 ± 2.37a-c | 29.0 ± 0.58c | 28.0 ± 1.17c | 34.0 ± 1.76a-c | 34.0 ± 2.56a-c | 28.7 ± 0.89c | 33.7 ± 1.48a-c | 39.0 ± 4.11a-c | 31.3 ± 1.79bc |
| SFW | 2.60 ± 0.38bc | 3.40 ± 0.35bc | 5.82 ± 0.38a | 5.82 ± 0.45a | 2.64 ± 0.32bc | 3.40 ± 0.53bc | 3.66 ± 0.17bc | 4.30 ± 0.54ab | 2.34 ± 0.06c | 3.61 ± 0.33bc | 3.61 ± 0.26bc | 4.30 ± 0.36bc | 2.69 ± 0.23bc | 3.61 ± 0.27bc | 3.91 ± 0.32bc | 3.51 ± 0.27bc |
| RFW | 2.07 ± 0.08fg | 2.29 ± 0.08e-g | 3.49 ± 0.07ab | 3.27 ± 0.06bc | 2.11 ± 0.09e-g | 2.40 ± 0.12e-g | 2.88 ± 0.10 cd | 3.75 ± 0.12a | 2.15 ± 0.09e-g | 2.28 ± 0.07e-g | 2.57 ± 0.08de | 3.21 ± 0.11bc | 2.05 ± 0.10 g | 2.53 ± 0.08d-f | 3.10 ± 0.10bc | 2.96 ± 0.09 cd |
| SDW | 0.47 ± 0.04c | 0.54 ± 0.02c | 1.11 ± 0.22a | 0.97 ± 0.10ab | 0.50 ± 0.06c | 0.55 ± 0.03bc | 0.58 ± 0.05bc | 0.67 ± 0.02bc | 0.54 ± 0.04c | 0.58 ± 0.05bc | 0.59 ± 0.06bc | 0.71 ± 0.03a-c | 0.44 ± 0.05c | 0.71 ± 0.06a-c | 0.83 ± 0.03a-c | 0.60 ± 0.05bc |
| RDW | 1.04 ± 0.03de | 1.19 ± 0.04c-e | 1.77 ± 0.09a | 1.72 ± 0.09a | 0.96 ± 0.11e | 1.08 ± 0.06de | 1.35 ± 0.07b-d | 1.75 ± 0.08a | 1.04 ± 0.04de | 1.14 ± 0.07de | 1.32 ± 0.05b-e | 1.56 ± 0.06ab | 1.02 ± 0.08de | 1.15 ± 0.08de | 1.55 ± 0.06a-c | 1.57 ± 0.06ab |
NPK content
Fertigation with various S sources markedly (P < 0.01) affected NPK accumulation in shoots of maize seedlings (Suppl. Table 1). Excess S supply significantly (P < 0.001) improved N content and resulted in mean maximum N accumulation in seedlings treated with K2SO4 (53.67 mg kg−1), FeSO4 (53.0 mg kg−1) and CuSO4 (51.50 mg kg−1) at 45 kg ha−1. However, high dose of Na2SO4 i.e. 45 kg ha−1 reduced N content by 23% compared to 30 kg ha−1 that exhibited maximum N accumulation (52.0 mg kg−1) in shoot (Fig. 1a). Similar trend was noted for shoot P and K content as application of K2SO4, FeSO4 and CuSO4 at high S dose of 45 kg ha−1 significantly (P < 0.05) improved P accumulation by 53, 51 and 48% in comparison with no S supply, whereas mean maximum P content (15.25 mg kg−1) using Na2SO4 was recorded at 30 kg ha−1 and it declined significantly by 22% at higher dose of 45 kg ha−1 (Fig. 1b). Similarly, relative to no S treatment, maize seedlings fertigated with K2SO4, FeSO4 and CuSO4 at 45 kg ha−1 exhibited 56, 54 and 49% higher K content in shoot. In maize seedlings treated with Na2SO4, the highest K content (102.50 mg kg−1) was recorded at 30 kg ha−1 in comparison with 45 kg ha−1 that considerably reduced shoot K content by 17% (Fig. 1c).
Figure 1.
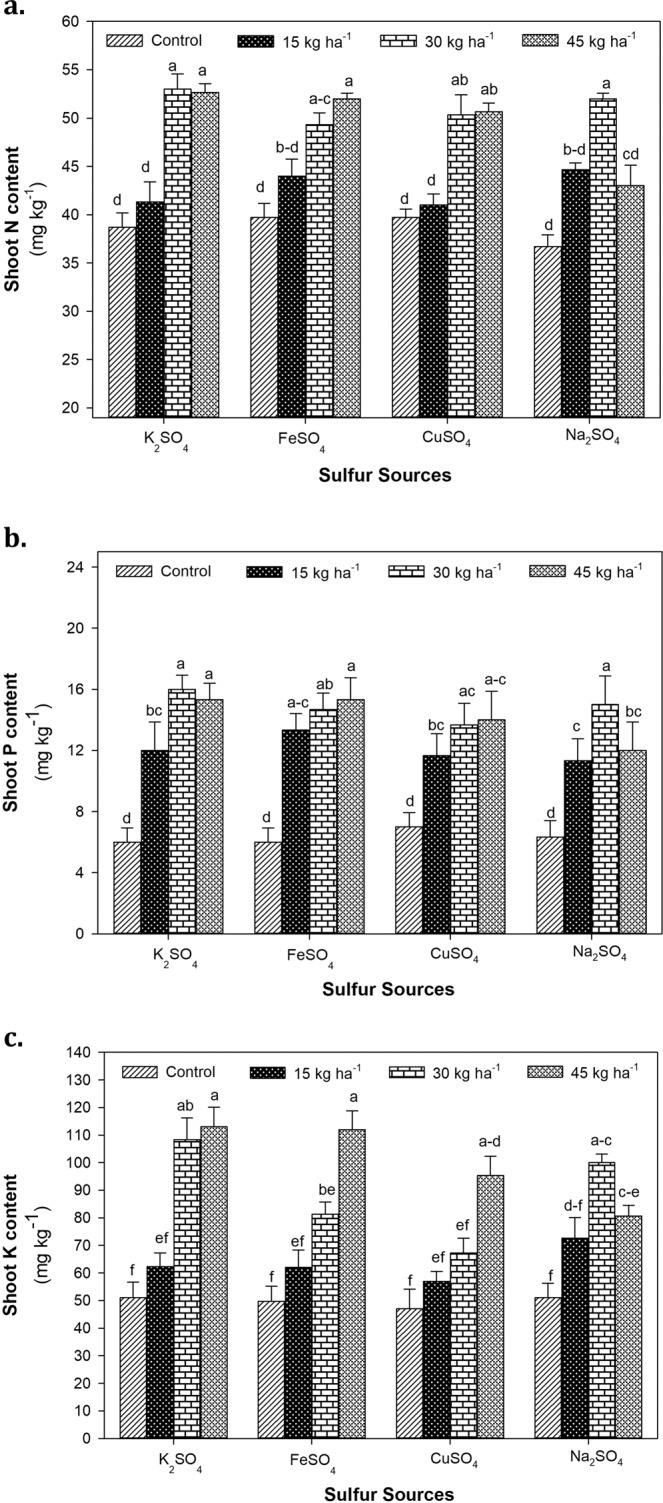
(a) The nitrogen (N), (b) phosphorous (P) and (c) potassium (K) content of maize seedlings affected by the application of various doses (0, 15, 30 and 45 kg ha−1) of sulfate fertilizers (K2SO4, FeSO4, CuSO4 and Na2SO4). The mean values with different letters indicate significant difference (P ≤ 0.05), according to post hoc Tukey’s test.
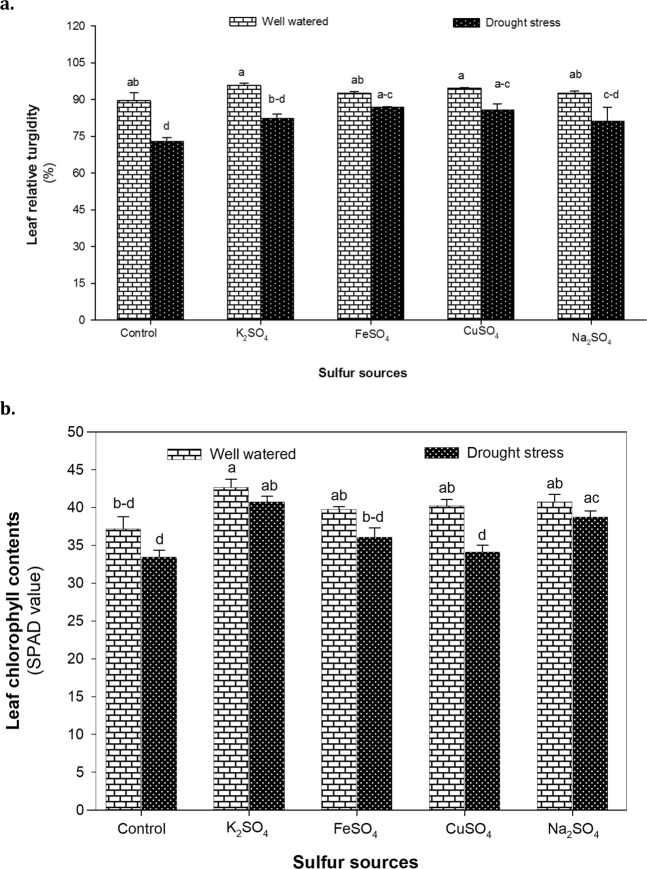
Leaf relative turgidity and SPAD value
The main effects of drought stress (D) and sulfur sources (S) were found significant (P < 0.01) for leaf relative turgidity (RT) and chlorophyll content (Chl) (Suppl. Table 2). Drought stress caused a substantial decline (P < 0.01) in RT and Chl of maize seedlings by 13 and 10%, respectively. Application of S fertilizers significantly (P < 0.01) ameliorated the drastic effects of drought stress. Maize plants treated with FeSO4 and CuSO4 maintained the highest RT i.e. 86.78 and 85.68%, respectively under water deficit conditions. Similarly, K2SO4 and Na2SO4 application increased RT by 12 and 10% compared to no S supply in maize under drought stress (Fig. 2a). The highest Chl was recorded in maize plants fertilized with K2SO4 under normal (42.67 SPAD value) as well as drought stress (40.73 SPAD value) conditions. Interestingly, CuSO4 application negatively influenced Chl and resulted in the lowest values along with control in both normal (37.17 SPAD value) and water stressed (31.78 SPAD value) maize plants (Fig. 2b).
Figure 2.
(a) The leaf relative turgidity (RT) and (b) the chlorophyll content (Chl) of maize plants affected by the application of optimized doses of sulfate fertilizers (K2SO4, FeSO4, CuSO4 and Na2SO4) under normal (100% WHC) and drought stress (30% WHC) conditions. The mean values with different letters indicate significant difference (P ≤ 0.05), according to post hoc Tukey’s test.
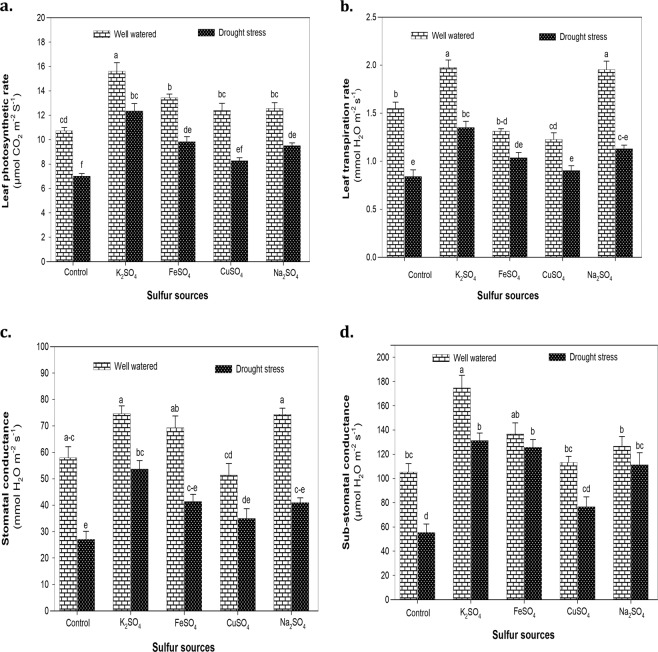
Gas exchange characteristics
Exposure to drought stress considerably (P < 0.001) reduced A (37.75%), E (52.47%), gs (31.24%) and Ci (65.49%) of maize plants compared to normal conditions, irrespective of S application (Suppl. Table 2). Exogenous S fertilization with K2SO4 resulted in the highest increase in A (43%) and E (61%) of maize plants subjected to drought stress (Fig. 3a,b). A marked increase of 43 and 23% in A and E, respectively was also observed by FeSO4 application under water deficit conditions (Fig. 3a,b).
Figure 3.
(a) The leaf photosynthetic rate (A), (b) transpiration rate (E), (c) stomatal conductance (gs) and (d) sub-stomatal conductance (Ci) of maize plants affected by the application of optimized doses of sulfate fertilizers (K2SO4, FeSO4, CuSO4 and Na2SO4) under normal (100% WHC) and drought stress (30% WHC) conditions. The mean values with different letters indicate significant difference (P ≤ 0.05), according to post hoc Tukey’s test.
The regulation of stomatal apparatus was also significantly (P < 0.001) influenced by the application of various S fertilizers (Suppl. Table 2). Application of K2SO4 effectively improved gs and Ci of maize plants by 98 and 127%, respectively under drought stress. Fertilization with FeSO4 and Na2SO4 also increased gs by 53% (Fig. 3c), whereas application of Na2SO4 enhanced Ci by 105% under water deficit conditions (Fig. 3d).
Antioxidant enzyme activities
Drought stress markedly (P < 0.001) markedly increased the activities CAT (131%), GPX (79%) and SOD (137%) compared to well-watered conditions (Suppl. Table 2). The highest CAT activity (253.33 μmol H2O2 min−1 mg−1 protein) was noted in leaves of water stressed maize plants supplemented with K2SO4 and did not differ significantly from FeSO4 (237.0 μmol H2O2 min−1 mg−1 protein) and Na2SO4 (215.0 μmol H2O2 min−1 mg−1 protein) (Fig. 4a). Similarly, the maize plants treated with K2SO4, FeSO4 and Na2SO4 exhibited 87, 34 and 30% higher GPX activity, respectively in comparison to plants with no S application (control) (Fig. 4b). Application of S fertilizers effectively enhanced SOD activity (P < 0.001) and gave the maximum increase (65%) in plants supplemented with K2SO4 under water deficit conditions. Likewise, Na2SO4, FeSO4 and CuSO4 upregulated SOD activity by 43, 31 and 29% compared to control under drought stress (Fig. 4c).
Figure 4.
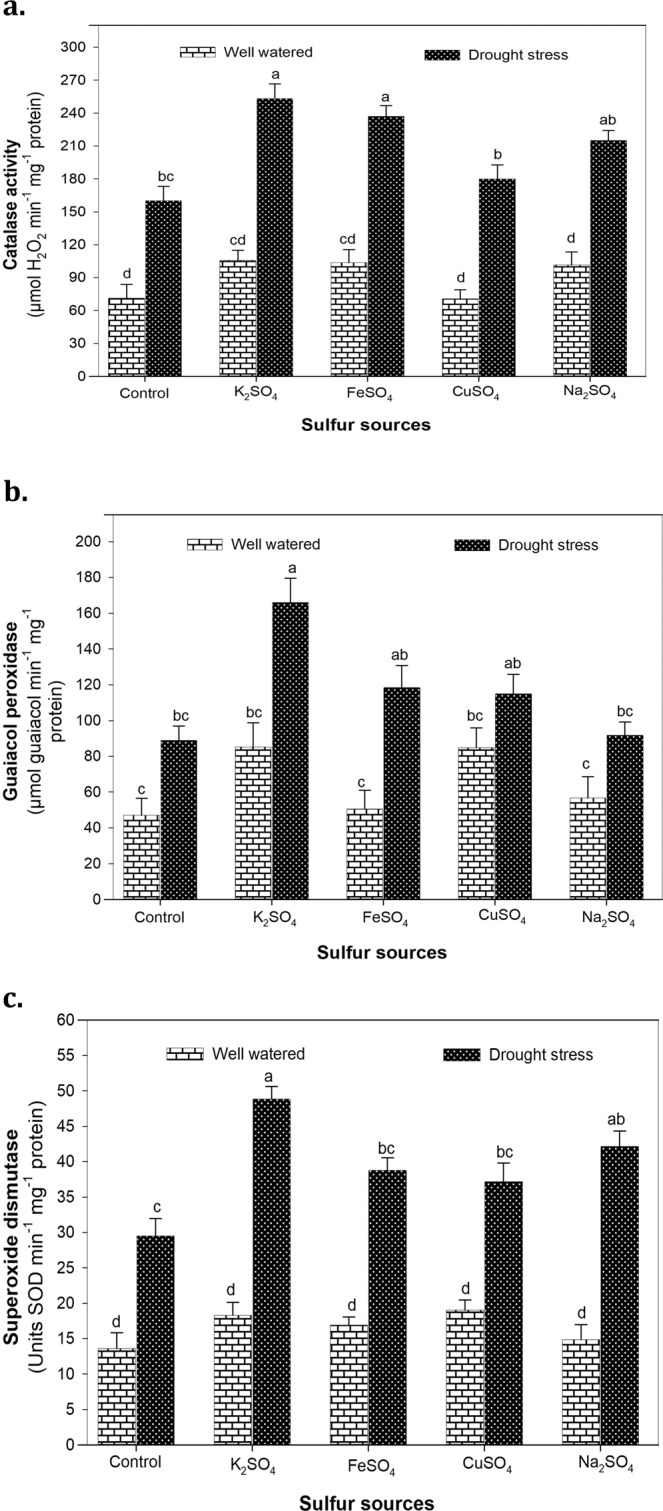
(a) The catalase (CAT), (b) guaiacol peroxidase (GPX) and (c) superoxide dismutase (SOD) activity of maize plants affected by the application of optimized doses of sulfate fertilizers (K2SO4, FeSO4, CuSO4 and Na2SO4) under normal (100% WHC) and drought stress (30% WHC) conditions. The mean values with different letters indicate significant difference (P ≤ 0.05), according to post hoc Tukey’s test.
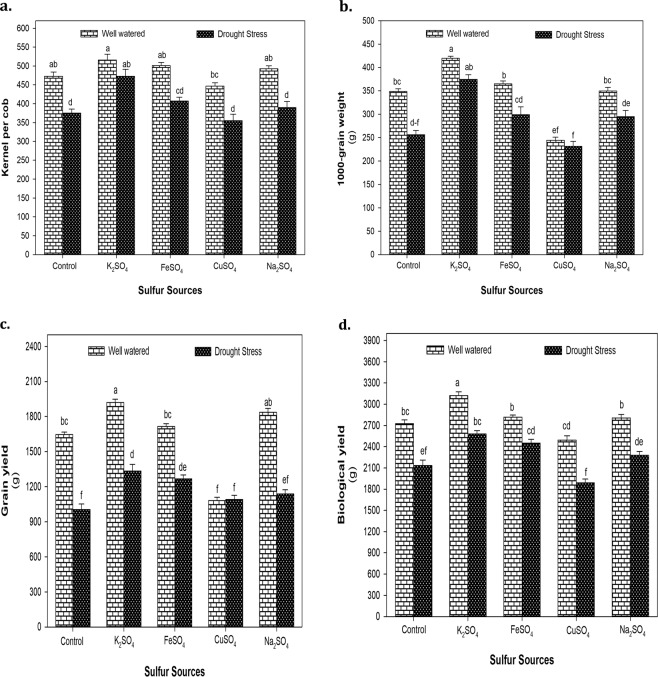
Yield and yield components
The main effects of D and S were significant (P < 0.01) for all maize yield attributes viz. kernels per cob (KC), 1000-grain weight (GW), grain yield (GY) and biological yield (BY), however, significant (P < 0.05) two way interaction (D × S) was only observed for KC, GW and GY (Suppl. Table 3). Exposure to drought stress considerably reduced KC, GW, GY and BY by 24, 19, 23 and 41%, respectively with respect to normal conditions. The highest increase in KC (41%) and GW (27%) was recorded by K2SO4 application compared to control (no S supply) under drought stress conditions (Fig. 5a,b). Interestingly, CuSO4 application reduced GW by 11% in water stressed maize plants compared to normal ones (Fig. 5b). Similar trend was observed for GY and BY as K2SO4 supply significantly increased GY by 17 and 33% compared to control under normal and water deficit conditions, respectively (Fig. 5c). Likewise, it improved BY by 15 and 21% in normal and water stressed maize plants (Fig. 5d). A marked increase in GY and BY was also observed by FeSO4 (26 and 15%) and Na2SO4 (13 and 7%) application under drought stress conditions (Fig. 5c,d).
Figure 5.
(a) The number of kernels per cob (KC), (b) 1000-grain weight (GW), (c) grain yield (GY) and (d) biological yield (BY) of maize plants affected by the application of optimized doses of sulfate fertilizers (K2SO4, FeSO4, CuSO4 and Na2SO4) under normal (100% WHC) and drought stress (30% WHC) conditions. The mean values with different letters indicate significant difference (P ≤ 0.05), according to post hoc Tukey’s test.
Application of various sulfate fertilizers improved the net benefit cost ratio, however, high market cost of FeSO4 resulted in negative net income. Similarly, CuSO4 and Na2SO4 induced toxicity also negatively influenced the net benefit ratio, whereas K2SO4 application was found to be most economical for improving maize yield under water deficit conditions (Table 3).
Table 3.
Effect of various sulfate fertilizers application on net income and benefit-to cast ratio of maize under normal and drought conditions.
| Treatment | Total expenditure (*PKR ha−1) | Grass income (PKR ha−1) | Net Income (PKR ha-1) | Benefit: cost ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Normal | Drought | Normal | Drought | Normal | Drought | Normal | Drought | |
| Control | 134394 | 132754 | 174020 | 97900 | 39626 | −34854 | 1.29 | 0.74 |
| K2SO4 | 139794 | 138154 | 229526 | 166100 | 89732 | 27946 | 1.64 | 1.20 |
| FeSO4 | 188394 | 186754 | 208560 | 143726 | 20166 | −43028 | 1.11 | 0.77 |
| CuSO4 | 188394 | 186754 | 155540 | 95700 | 32854 | −91054 | 0.83 | 0.51 |
| Na2SO4 | 137394 | 135754 | 185174 | 121000 | 47780 | −14754 | 1.35 | 0.89 |
*1.00 USD = 159.7 PKR.
Discussion
This study presents data to interpret firstly the effects of various S fertilizers and their doses on biomass accumulation and NPK uptake in maize seedlings. Secondly, the physiological and biochemical significance of S fertilizers in drought stress tolerance, with a particular focus on regulation of gas exchange characteristics and enzymatic antioxidants to improve maize yield will be discussed. Exogenous S application significantly (P < 0.01) enhanced SL, RL, SFW, RFW, SDW and RDW of maize seedlings (Suppl. Table 1). As biomass attributes were increased by S fertilization, it may be concluded that S availability enhances photosynthetic rate and stimulates translocation of photosynthates towards sink22. However, this stimulating effect of S fertilizers was found to be dose dependent and considerable variation was observed among various doses (Table 2). High doses of K2SO4 and Na2SO4 (45 kg ha−1) showed adverse effects on biomass accumulation as reported earlier in studies involving maize35 and Brassica rapa36. The possible general explanation for this reduction may be the altered toxicity of sulfate anion (SO42−) by the presence of Na+37.
Addition of sufficient amount of nutrients is first agricultural measure to increase crop performance38, however, the uptake of mineral nutrients is considerably influenced by the interactions between metal ions at different physiological levels24. This is particularly true for S as drought stress results in low SO42− absorption and its subsequent translocation to leaves that also limits NO3− translocation, consequently reducing nitrogen use efficiency39. Similarly, S-deprivation influences K accumulation in shoots providing evidence that K+ acts as counter-cation in the absence of SO42− in leaf tissue24,40. The results in present study showed that low water availability considerably (P < 0.01) restricts NPK accumulation in shoots of maize seedlings (Fig. 1a–c). Drought induced restriction in nutrient accumulation might be associated to reduced E, resulting in limited root absorbing power to uptake N, P and K41. Drought stress drastically influences maize at early growth stages by changing root maintenance system and architecture42. Recent studies by Studer et al.43 confirmed that the seedling growth of maize is most sensitive to nutrient deficiency, particularly under drought stress conditions. In agreement with the findings of Raza et al.22 in sesame, our results showed that S fertilization significantly (P < 0.05) increased NPK content in shoots of maize seedlings (Suppl. Table 1), this may be associated with improved nutrient acquisition and utilization of these macronutrients with S application. Shoot N content were considerably (P < 0.001) increased by S application (Fig. 1a) providing further evidence that these nutrients are highly inter-related32 and significantly influence protein metabolism, thereby quality of crop plants44,45. Lošák et al.46 found positive correlation between N and S to increase camelina seed yield in S deficient soils. They suggested that combined S and N application could be utilized as an effective approach to improve oil and protein yield. High concentration of these nutrients obtained by K2SO4 might also be attributed to presence of K+ considering the importance of this cation to improve drought tolerance in crop plants47,48. Previous studies on Eruca sativa49 sesame50 and maize51 also reported an increased P and K content by S application. It may be inferred that S fertilization after biochemical oxidation produces H2SO4, which improves nutrient availability to plants52,53. Recent report of Reich et al.31 showed that S deficiency reduced K+ accumulation in shoots of Chinese cabbage highlighting the role of S in xylem loading and translocation of K+ to the shoot. Positive effect of FeSO4 on nutrient accumulation in maize seedlings (Fig. 1a-c) suggests the cooperative role of S and Fe in plant metabolism, for example Fe-S clusters in the electron transport chain54. Cross talk between S and Fe uptake and metabolism in plants is particularly important because S deficiency not only limits the synthesis of Fe-S cluster proteins but also reduces the translation in general55.
Maintenance of leaf RT and Chl indicate plant ability to tolerate water stress conditions28,56. In the present study, drought induced reduction (P < 0.001) in leaf RT (Suppl. Table 2) might be the result of loss in turgor or impaired photosynthetic rate or combination of these factors57,58. Leaf RT is often used as a criterion to determine the degree of drought stress as loss in RT leads to protoplasm dehydration59 and decrease in cell enlargement and expansion60. Application of S fertilizers significantly (P < 0.01) enhanced leaf RT (Fig. 2a) indicating that S availability restricts the movement of ions and solutes in the cells associated with reduced osmotic potential under water deficit conditions49. High RT by S application might be associated with increased water uptake by roots as drought stress reduces translocation of newly absorbed SO42− to shoot also reported in B. napus61.
Biosynthesis and maintenance of photosynthetic pigments is considered to be a potential indicator of drought tolerance in crop plants4. Measurement of total Chl using chlorophyll meters such as SPAD-502 is an effective, non-destructive and inexpensive method that provides absolute values of Chl per unit leaf area62. A marked decline in SPAD value (P < 0.001) was noted in S deficient maize plants (control) under drought stress (Fig. 2b), which may be due to decrease in S compounds such as Cys and Met that serve as integral component of chloroplast targeted proteins. Our findings are in line with reports of Houhou et al.63 in E. sativa and Kassem et al.64 in Lycopersicum esculentum providing further evidence that S starvation influences the coordination between light reaction and Calvin cycle in chloroplast ultimately reducing Chl in leaves. In rice, Lunde et al.65 found a significant decrease in Chl of S deficient plants followed by a noteable reduction in photochemical performance and decreased photosynthetic activity. Maize seedlings supplemented with CuSO4 exhibited a significant (P < 0.001) decrease in Chl (Fig. 2b) that might be attributed to Cu ion toxicity leading to ultra-structural alterations and photochemical oxidation in chloroplast66. Excess Cu inhibits carboxylase activity and causes swelling of thylakoids as reported by Ibrahim et al.67 in Gynura procumbens.
The reduction in gas exchange attributes viz. A, E, gs and Ci is generally considered to be the first effects of drought stress due to chlorophyll degradation and restriction in available CO268,69. Reduced gas exchange under S deficient conditions has been previously reported in several plant species such as barley70, mustard71 and rape72. In this study, application of S fertilizers considerably (P < 0.001) increased A (Fig. 3a) suggesting that S availability improves CO2 assimilation and protein abundance to alleviate drought induced inhibition of photosynthetic capacity in maize plants. Adequate S-supplementation favors formation of S containing amino acids (Cys and Met) and reduces oxidative stress (high CAT, GPX and SOD activity) to stabilize Rubisco and thylakoid membrane proteins under drought stress conditions14. Compared to no S supply, application of K2SO4 and FeSO4 markedly (P < 0.001) enhanced gs and Ci (Fig. 3c,d) accompanied by high E (Fig. 3b) suggesting that these fertilizers influence stomatal regulation and further stabilize Fe-S clusters to improve the functioning of vital cellular processes such as photosynthesis and respiration under water deficit conditions73,74. In a study involving contrasting B. napus genotypes (Mosa and Saturnin), Lee et al.39 reported higher photosynthetic activity in genotype (Saturnin) with high sulfur use efficiency that ultimately contributed to better drought tolerance.
Drought stress as well as S deprivation causes metabolic imbalance that leads to oxidative burst in plant cell75. S deficiency stimulates peroxidation of biomolecules due to excessive accumulation of reactive oxygen species (ROS) and reduced synthesis of S-containing compounds76. In contrast to no S supply, application of S fertilizers considerably (P < 0.001) increased the activities of CAT, GPX and SOD (Suppl. Table 2), which was consistent with the maintenance of photosynthetic capacity following S supplementation under drought stress as reported by Ma et al.77 in wheat. Adequate S supply helps to counteract the drastic effects of ROS on nucleic acids and proteins through upregulation of antioxidant enzymes such as CAT, GPX and SOD78. These antioxidative enzymes serve as scavengers of O2 and H2O2 and help to prevent the production of toxic HO̅̅̅̅̅79. It is evident from the results that S mediated high antioxidant activity (Fig. 4a–c) corresponds to drought tolerance in maize. The combined action of CAT and SOD converts highly toxic O2− and H2O2 into molecular oxygen and water, respectively to prevent ROS induced cellular damage in plants41,80. The availability of S promotes photosynthetic assimilation of SO42− to produce Cys that may be used to synthesize Met or converted into glutathione to regulate protein and cell function81 under environmental stresses like drought11. Compared to other S sources, higher antioxidant activity by K2SO4 application is in agreement with the recent reports of Zareei et al.82 in black grapes and Marques et al.83 in eggplant suggesting the coordinated action of K+ and SO42 to regulate photosynthesis and translocation of photosynthates from roots to shoots, thereby alleviating drought induced oxidative damage in maize plants.
The overall impact of drought stress on yield and yield attributes of maize plants was highly significant (P < 0.001) with no S supply (Suppl. Table 3) that might be associated with reduced translocation of sugars to developing kernels under water deficit conditions84. This poor supply of sugars starves embryo and induces abortion of ovary ultimately affecting grain formation and yield of maize85. Positive effects of S fertilization on yield (Fig. 5a-d) suggest that SO42 availability stimulates movement of assimilates from phloem into the apoplast due to higher photosynthetic activity and enhanced stomatal regulation (also observed in present study) under drought stress conditions. In line with our findings, early reports of Dewal and Pareek86 and Shahzad et al.47 showed ameliorative effects of K2SO4 application to improve GY in water stressed wheat and maize, respectively. Our results also showed a significant effect of FeSO4 application on maize yield attributes (Fig. 5a-d) as reported by Farokhi et al.87 in sunflower and Heidari et al.50 in sesame. The possible explanation for this increased yield by S fertilization could be the involvement of S-containing compounds in vital physiological and biochemical processes to modulate stress response under water deficit conditions88. The reduction in GY by CuSO4 application might be due to toxic effects of Cu2+ on photosynthetic electron transport chain resulting in protein denaturation and deactivation of antioxidant enzymes in plant cell89.
Conclusion
The present study concludes that S starvation has a diverse impact on physiological and biochemical processes with important implications for maize yield under drought stress conditions. In contrast, S availability positively influenced the leaf water status, gas exchange characteristics and antioxidative machinery in water stressed maize plants. Among various S sources, K2SO4 application resulted in the maximum increase in yield providing further evidence that K+ and SO42− are strongly correlated to improve yield in crop plants. A marked increase in growth and yield was also noted by FeSO4 fertigation indicating that application of this fertilizer influences vital cellular processes including synthesis of Fe-S cluster proteins to improve drought tolerance in maize. However, high market cost of this fertilizer resulted in negative cost:benefit ratio for this fertilizer. Similary, Na2SO4 supply improved maize yield but high dose of this fertilizer induces toxicity, which may be due to accumulation of Na+ in rhizosphere. Similarly, CuSO4 application causes toxicity and significantly reduced yield compared to other S fertilziers. Moreover, these fertilzers were not found to be economical for improving yield under drought stress conditions.
Supplementary information
Author contributions
F.N. designed and supervised the experiments and wrote the manuscript. M.M.U. performed the experiments and analytical work. S.M. and K.S.A. provided the reagents for analytical work and assisted in preparing the final draft of manuscript. R.N.S and M.A. provided inputs for experiments and guided in preparing figures. M.A.S. and G.A. co-supervised the experiments carried out as a part of MSc. (Hons.) thesis work of M. M.U.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58169-2.
References
- 1.Boomsma CR, Vyn TJ. Maize drought tolerance: potential improvements through arbuscular mycorrhizal symbiosis. Field Crops Res. 2008;108:14–31. doi: 10.1016/j.fcr.2008.03.002. [DOI] [Google Scholar]
- 2.Nuss ET, Tanumihardjo SA. Maize a paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Safety. 2010;9:417–436. doi: 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- 3.Sutar RK, Pujar AM, Kumar BA, Hebsur. NS. Sulphur Nutrition in Maize-A Critical Review. Int. J. Pure Applied Biosci. 2017;5:1582–1596. doi: 10.18782/2320-7051.6092. [DOI] [Google Scholar]
- 4.Naeem M, Naeem MS, Ahmad R, Ahmad R. Foliar-applied calcium induces drought stress tolerance in maize by manipulating osmolyte accumulation and antioxidative responses. Pak. J. Bot. 2017;49:427–434. [Google Scholar]
- 5.Yang X, et al. Drought induced root aerenchyma foration restricts water uptake in rice seedling supplied with nitrate. Plant Cell Physiol. 2012;53:495–504. doi: 10.1093/pcp/pcs003. [DOI] [PubMed] [Google Scholar]
- 6.Farooq WA, et al. Elemental analysis of fertilizer using laser induced breakdown spectroscopy. Optics Spec. 2012;112:874–880. doi: 10.1134/S0030400X12060082. [DOI] [Google Scholar]
- 7.Rouphael, Y., Cardarelli, M., Schwarz, D., Franken P. & Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In: Plant responses to drought stress (Ed. Aroca, R). 171–195 (Springer Nature, 2012).
- 8.Osakabe Y, Osakabe K, Shinozaki K, Tran LSP. Response of plants to water stress. Front. Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MJ, Khan MH, Khattak RA, Jan MT. Response of maize to different levels of sulfur. Commun. Soil Sci. Plant Anal. 2006;37:41–51. doi: 10.1080/00103620500403804. [DOI] [Google Scholar]
- 10.Khan NA, et al. Salinity tolerance in plants: revisiting the role of sulfur metabolites. J. Plant Biochem. Physiol. 2014;2:120. doi: 10.4172/2329-9029.1000e124. [DOI] [Google Scholar]
- 11.Ahmad N, Malagoli M, Wirtz M, Hell R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016;16:247. doi: 10.1186/s12870-016-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anjum NA, et al. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015;6:210. doi: 10.3389/fpls.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst L, et al. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010;61:3395–3405. doi: 10.1093/jxb/erq160. [DOI] [PubMed] [Google Scholar]
- 14.Chan KX, Wirtz M, Phua SY, Estavillo GM, Pogson BJ. Balancing metabolites in drought: the sulfur assimilation conundrum. Trends Plant Sci. 2013;18:18–29. doi: 10.1016/j.tplants.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Nawaz, F., et al. Reactive Sulfur Species-Key Regulators of Abiotic Stress Tolerance in Plants. In: Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms (Ed. Hasanuzzaman et al.) pp. 685-713 (2019).
- 16.Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 17.Sarker U, Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018;8:16496. doi: 10.1038/s41598-018-34944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazin F. Effect of zinc sulfate on quantitative and qualitative characteristics of corn (Zea mays) in drought stress. Cer. Agron. Mold. 2012;45:15–24. [Google Scholar]
- 19.Bahadur L, Tiwari DD. Nutrient management in mung bean (Vigna radiata L.) through sulphur and biofertilizers. Legume Res. 2014;37:180–187. doi: 10.5958/j.0976-0571.37.2.027. [DOI] [Google Scholar]
- 20.Kausar A, et al. Alleviation of salt stress by K2SO4 in two wheat (Triticum aestivum L.) cultivars. Appl. Ecol. Environ. Res. 2016;14:137–147. doi: 10.15666/aeer/1405_137147. [DOI] [Google Scholar]
- 21.Khan R, et al. Effect of foliar application of zinc and manganese on growth and some biochemical constituents of Brassica junceae grown under water stress. Americ-Eura. J. Agric. Environ. Sci. 2016;16:984–997. [Google Scholar]
- 22.Raza M, et al. Effect of sulphur application on photosynthesis and biomass accumulation of sesame varieties under rainfed conditions. Agron. 2018;8:149. doi: 10.3390/agronomy8080149. [DOI] [Google Scholar]
- 23.Klikocka H, Marx M. Sulphur and nitrogen fertilization as a potential means of agronomic biofortification to improve the content and uptake of microelements in spring wheat grain DM. J. Chem. 2018;2018:1–12. doi: 10.1155/2018/9326820. [DOI] [Google Scholar]
- 24.Reich M, et al. Interactions of sulfate with other nutrients as revealed by H2S fumigation of Chinese cabbage. Front. Plant Sci. 2016;7:541. doi: 10.3389/fpls.2016.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorin E, et al. Effect of sulphur deprivation on osmotic potential components and nitrogen metabolism in oilseed rape leaves: identification of a new early indicator. J. Exp. Bot. 2015;66:6175–6189. doi: 10.1093/jxb/erv321. [DOI] [PubMed] [Google Scholar]
- 26.Majeed S, Nawaz F, Naeem M, Ashraf MY. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol. Plantar. 2018;40:206. doi: 10.1007/s11738-018-2780-y. [DOI] [Google Scholar]
- 27.Jackson, M. L. & Barak, P. Soil chemical analysis: advanced course. Madison (WI): UW-Madison Libraries Parallel Press (2005).
- 28.Naeem M, et al. Improving drought tolerance in maize by foliar application of boron: water status, antioxidative defense and photosynthetic capacity. Arch. Agron. Soil Sci. 2018;64:626–639. doi: 10.1080/03650340.2017.1370541. [DOI] [Google Scholar]
- 29.Nachabe MH. Refining the definition of field capacity in the literature. J. Irrig. Drain. Engin. 1998;124:230–232. doi: 10.1061/(ASCE)0733-9437(1998)124:4(230). [DOI] [Google Scholar]
- 30.Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982;13:1035–1059. doi: 10.1080/00103628209367332. [DOI] [Google Scholar]
- 31.Barrs, H. D. Determination of water deficits in plant tissues. In: Water Deficits and Plant Growth (Ed. T.T. Kozlowski), Vol. I, 235 (Academic Press London, 1968).
- 32.Chance B, Maehly C. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- 33.Urbanek H, Kuzniak-Gebarowska E, Herka K. Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plantar. 1991;13:43–50. [Google Scholar]
- 34.Van Rossum MWPC, Alberda M, van der Plas LHW. Role of oxidative damage in tulip bulb scale micro propagation. Plant Sci. 1997;130:207–216. doi: 10.1016/S0168-9452(97)00215-X. [DOI] [Google Scholar]
- 35.Tariq MU, Saeed A, Nisar MU, Mian IA, Afzal M. Effect of potassium rates and sources on the growth performance and on chloride accumulation of maize in two different textured soils of Haripur, Hazara division. Sarhad J. Agric. 2011;27:415–22. [Google Scholar]
- 36.Reich M, et al. Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil. 2017;411:319–332. doi: 10.1007/s11104-016-3026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamal A, Moon YS, Abdin MZ. Enzyme activity assessment of peanut (Arachis hypogea) under slow-release sulphur fertilization. Aust. J. Crop Sci. 2010;4:169–174. [Google Scholar]
- 38.Bloem E, Haneklaus S, Schnug E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015;5:779. doi: 10.3389/fpls.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee BR, Zaman R, Avice JC, Ourry A, Kim TH. Sulfur use efficiency is a significant determinant of drought stress tolerance in relation to photosynthetic activity in Brassica napus cultivars. Front. Plant Sci. 2015;7:459. doi: 10.3389/fpls.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasanuzzaman M, et al. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal. Behav. 2018;13:1477905. doi: 10.1080/15592324.2018.1477905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shabbir RN, et al. Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.) Environ. Sci. Pollut. Res. 2016;23:2651–2662. doi: 10.1007/s11356-015-5452-0. [DOI] [PubMed] [Google Scholar]
- 42.Hu YC, Burucs Z, von Tucher S, Schmidhalter U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007;60:268–275. doi: 10.1016/j.envexpbot.2006.11.003. [DOI] [Google Scholar]
- 43.Studer C, Hu Y, Schmidhalter U. Interactive effects of N-, P-and K-nutrition and drought stress on the development of maize seedlings. Agriculture. 2017;7:90. doi: 10.3390/agriculture7110090. [DOI] [Google Scholar]
- 44.Fazili IS, et al. Interactive effect of sulfur and nitrogen on growthand yield attributes of oilseed crops (Brassica campestris L.and Eruca sativa Mill.) Differing in yield potential. J. Plant Nutri. 2010;33:1216–1228. doi: 10.1080/01904161003765745. [DOI] [Google Scholar]
- 45.Fazili IS, et al. Oil Biosynthesis and its Related Variables in Developing Seeds of Mustard (Brassica juncea L.) as Influenced by Sulphur Fertilization. J. Crop Sci. Biotech. 2010;13:39–46. doi: 10.1007/s12892-009-0117-5. [DOI] [Google Scholar]
- 46.Lošák T, et al. Effect of combined nitrogen and sulphur fertilization on yield and qualitative parameters of Camelina sativa [L.] Crtz.(false flax) Acta Agric. Scand. Section B-Soil Plant Sci. 2011;61:313–321. [Google Scholar]
- 47.Shahzad AN, et al. Foliar application of potassium sulfate partially alleviates pre-anthesis drought-induced kernel abortion in maize. Int. J. Agric. Biol. 2017;19:495. doi: 10.17957/IJAB/15.0317. [DOI] [Google Scholar]
- 48.Adhikari, B., Dhungana, S. K., Kim, I. D. & Shin, D. H. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J. Saudi Soc. Agric. Sci. In press (2019).
- 49.Singh S, Pareek BL. Effect of different levels of nitrogen and sulphur on content and uptake of nutreint and quality of taramira. Annu. Agric. Res. New Series. 2003;24:200–202. [Google Scholar]
- 50.Heidari M, Galavi M, Hassani M. Effect of sulfur and iron fertilizers on yield, yield components and nutrient uptake in sesame (Sesamum indicum L.) under water stress. African J. Biotech. 2011;10:8816–8822. doi: 10.5897/AJB11.854. [DOI] [Google Scholar]
- 51.Rahman MM, Soaug AA, Darwish FHA, Golam F, Sofian-Azirun M. Growth and nutrient uptake of maize plants as affected by elemental sulfur and nitrogen fertilizer in sandy calcareous soil. African J. Biotech. 2011;10:12882–12889. doi: 10.5897/AJB11.2075. [DOI] [Google Scholar]
- 52.El-Tarabily KA, Abdou AS, Maher ES, Satoshi M. Isolation and characterization of sulfur-oxidizing bacteria, including strains of Rhizobium from calcareous sandy soils and their effects on nutrient uptake and growth of maize. Aust. J. Agril. Res. 2006;57:10–111. [Google Scholar]
- 53.Abdou, A. S. Effect of applied elemental sulfur and sulfur-oxidizing bacteria (Parococcus versutus) into calcareous sandy soils on the availability of native and applied phosphorus and some micronutrients. In: 18th World Congress of Soil Science, Philadelphia,Pennsylvania, USA. July 9-15. Aebi, H., 1984. Catalase in vitro. Meth. Enzymol. 105, 121-126 (2006).
- 54.Liu J, Zhang H, Yin Y, Chen H. Effects of exogenous hydrogen sulfide on antioxidant metabolism of rice seed germinated under drought stress. J. South Agric. 2017;48:31–37. [Google Scholar]
- 55.Forieri I, Wirtz M, Hell R. Toward new perspectives on the interaction of iron and sulfur metabolism in plants. Front. Plant Sci. 2013;4:357. doi: 10.3389/fpls.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nawaz F, et al. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015;175:350–357. doi: 10.1016/j.foodchem.2014.11.147. [DOI] [PubMed] [Google Scholar]
- 57.Hajiboland R, Farhanghi F. Effect of low boron supply in turnip plants under drought stress. Biol Plant. 2011;55:775. doi: 10.1007/s10535-011-0186-4. [DOI] [Google Scholar]
- 58.Hussain RA, Ahmad R, Nawaz F, Ashraf MY, Warraich EA. Foliar NK application mitigates drought effects in sunflower (Helianthus annuus L.) Acta Physiol. Plantar. 2016;38:83. doi: 10.1007/s11738-016-2104-z. [DOI] [Google Scholar]
- 59.Yagmur M, Kaydan D. Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr. J. Biotechnol. 2008;7:2156–2162. [Google Scholar]
- 60.Živčák M, Repková J, Olšovská K, Brestič M. Osmotic adjustment in winter wheat varieties and its importance as a mechanism of drought tolerance. Cereal Res. Commun. 2009;37:569–572. [Google Scholar]
- 61.Lee BR, et al. Genotypic variation in N uptake and assimilation estimated by 15N tracing water deficit-stressed Brassica napus. Envrion. Exp. Bot. 2015;109:73–79. doi: 10.1016/j.envexpbot.2014.08.004. [DOI] [Google Scholar]
- 62.Shah S, Houborg R, McCabe M. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.) Agron. 2017;7:61. doi: 10.3390/agronomy7030061. [DOI] [Google Scholar]
- 63.Houhou M, Joutei KA, Louhalia S. Biomass production, chlorophyll content and morphorogical parameters are affected by sulfur deficiency in Eruca sativa L. Int. J. Ecol. Environ. Sci. 2018;44:67–75. [Google Scholar]
- 64.Kassem AS, Mohammed HFA, EL-Sayed SAA. Influence of sulfur deprivation on biomass allocation, mineral composition and fruit quality of tomato plants. Middle East J. Agric. Res. 2015;4:42–48. [Google Scholar]
- 65.Lunde C, et al. Sulfur starvation in rice: the effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiol. Plantar. 2008;134:508–521. doi: 10.1111/j.1399-3054.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 66.Jain P, Kachhwaha S, Kothari SL. Chloroplast ultra structure, photosynthesis and enzyme activities in regenerated plants of Stevia rebaudiana (Bert.) Bertoni as influenced by copper sulphate in the medium. Ind. J. Exp. Bio. 2014;52:898–904. [PubMed] [Google Scholar]
- 67.Ibrahim M, Chee Kong Y, Mohd Zain N. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung Nyawa (Gynura procumbens (Lour.) Merr. Molecules. 2017;22:1623. doi: 10.3390/molecules22101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pietrini F, Iannelli MA, Pasqualini S, Massacci A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmite saustralis (Cav.) Trin. exsteudel. Plant Physiol. 2003;133:829–837. doi: 10.1104/pp.103.026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee BR, et al. Increased proline loading to phloem and its effect on nitrogen uptake and assimilation in water stressed white clover (Trifolium repens) New Phytol. 2009;182:654–663. doi: 10.1111/j.1469-8137.2009.02795.x. [DOI] [PubMed] [Google Scholar]
- 70.Astolfi S, et al. Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J. Exp. Bot. 2012;63:1241–1250. doi: 10.1093/jxb/err344. [DOI] [PubMed] [Google Scholar]
- 71.Fatma M, Asgher M, Masood A, Khan NA. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014;107:55–63. doi: 10.1016/j.envexpbot.2014.05.008. [DOI] [Google Scholar]
- 72.Muneer S, et al. Involvement of sulphur nutrition in modulating iron deficiency responses in photosynthetic organelles of oilseed rape (Brassica napus L.) Photosynth. Res. 2014;119:319–329. doi: 10.1007/s11120-013-9953-8. [DOI] [PubMed] [Google Scholar]
- 73.Balk J, Pilon M. Ancient and essential, the assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011;16:218–226. doi: 10.1016/j.tplants.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Duan J, et al. Response of gas-exchange characteristics and chlorophyll fluorescence to acute sulfur dioxide exposure in landscape plants. Ecotox. Environ. Saf. 2019;171:122–129. doi: 10.1016/j.ecoenv.2018.12.064. [DOI] [PubMed] [Google Scholar]
- 75.Chandra, N. & Pandey, N. Influence of Sulfur Induced Stress on Oxidative Status and Antioxidative Machinery in Leaves of Allium cepa L. Inter. Schol. Res. Noti. Article ID 568081 (2014). [DOI] [PMC free article] [PubMed]
- 76.Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013;94:19–32. doi: 10.1016/j.envexpbot.2012.05.004. [DOI] [Google Scholar]
- 77.Ma D, et al. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PloS one. 2016;11:0163082. doi: 10.1371/journal.pone.0163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasan MK, et al. Melatonin alleviates low-sulfur stress by promoting sulfur homeostasis in tomato plants. Sci. Rep. 2018;8:10182. doi: 10.1038/s41598-018-28561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Tahi H, Wahbi S, El Modafar C, Aganchich A, Serraj R. Changes in antioxidant activities and phenol content in tomato plants subjected to partial root drying and regulated deficit irrigation. Plant Biosys. 2008;142:550–562. doi: 10.1080/11263500802410900. [DOI] [Google Scholar]
- 81.Gotor C, et al. Signaling in the plant cytosol: cysteine or sulfide? Amino Acids. 2015;47:2155–2164. doi: 10.1007/s00726-014-1786-z. [DOI] [PubMed] [Google Scholar]
- 82.Zareei E, Javadi T, Aryal R. Biochemical composition and antioxidant activity affected by spraying potassium sulfate in black grape (Vitis vinifera L. cv. Rasha) J. Sci. Food Agric. 2018;98:5632–5638. doi: 10.1002/jsfa.9107. [DOI] [PubMed] [Google Scholar]
- 83.Marques DJ, et al. Effect of potassium sources on the antioxidant activity of eggplant. Rev. Brasil. Ciên. Solo. 2014;38:1836–1842. doi: 10.1590/S0100-06832014000600018. [DOI] [Google Scholar]
- 84.Hütsch BW, Jung S, Schubert S. Comparison of Salt and Drought‐Stress Effects on Maize Growth and Yield Formation with Regard to Acid Invertase Activity in the Kernels. J. Agron. Crop Sci. 2015;201:353–367. doi: 10.1111/jac.12111. [DOI] [Google Scholar]
- 85.Setter TL, Flannigan BA, Melkonian J. Loss of kernel set due to water deficit and shade in maize. Crop Sci. 2001;41:1530–1540. doi: 10.2135/cropsci2001.4151530x. [DOI] [Google Scholar]
- 86.Dewal GS, Pareek RG. Effect of phosphorus, sulphur and zinc on growth, yield and nutrient uptake of wheat (Triticum aestivum) Indian J. Agron. 2004;49:160–162. [Google Scholar]
- 87.Farokhi H, Shirzadi MH, Afsharmanesh G, Ahmadizadeh M. Response of azargol sunflower cultivar to different micronutrients in Jiroft region, southeast of Iran. South West. J. Hortic. Biol. Environ. 2015;6:53–64. [Google Scholar]
- 88.Hasanuzzaman Mirza, Hossain Mohammad Anwar, da Silva Jaime A. Teixeira, Fujita Masayuki. Crop Stress and its Management: Perspectives and Strategies. Dordrecht: Springer Netherlands; 2011. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor; pp. 261–315. [Google Scholar]
- 89.Barbosa RH, et al. Foliar copper uptake by maize plants: effects on growth and yield. Ciência Rural. 2013;43:1561–1568. doi: 10.1590/S0103-84782013000900005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.