Abstract
Thermophilic Campylobacter species are clinically important aetiologies of gastroenteritis in humans throughout the world. The colonization of different animal reservoirs by Campylobacter poses an important risk for humans through shedding of the pathogen in livestock waste and contamination of water sources, environment, and food. A review of published articles was conducted to obtain information on the prevalence and antimicrobial resistance (AMR) profiles of thermophilic Campylobacter species in humans and animals in sub-Saharan Africa (SSA). Electronic databases, namely, PubMed, Google Scholar, Research4life-HINARI Health, and Researchgate.net, were searched using the following search terms “thermophilic Campylobacter,” “Campylobacter jejuni,” “Campylobacter coli,” “diarrhea/diarrhoea,” “antimicrobial resistance,” “antibiotic resistance,” “humans,” “animals,” “Sub-Saharan Africa,” and “a specific country name.” Initially, a total of 614 articles were identified, and the lists of references were screened in which 22 more articles were identified. After screening, 33 articles on humans and 34 on animals and animal products were included in this review. In humans, Nigeria reported the highest prevalence (62.7%), followed by Malawi (21%) and South Africa (20.3%). For Campylobacter infections in under-five children, Kenya reported 16.4%, followed by Rwanda (15.5%) and Ethiopia (14.5%). The country-level mean prevalence in all ages and under-five children was 18.6% and 9.4%, respectively. The prevalence ranged from 1.7%–62.7% in humans and 1.2%–80% in animals. The most reported species were C. jejuni and C. coli. The AMR to commonly used antimicrobials ranged from 0–100% in both humans and animals. Poultry consumption and drinking surface water were the main risk factors for campylobacteriosis. The present review provides evidence of thermophilic Campylobacter occurrence in humans and animals and high levels of AMR in SSA, emphasizing the need for strengthening both national and regional multisectoral antimicrobial resistance standard surveillance protocols to curb both the campylobacteriosis burden and increase of antimicrobial resistance in the region.
1. Introduction
Diarrhoea remains the main cause of morbidity and mortality in low- and middle-income countries (LMICs) [1–3]. Worldwide, under-five children experience approximately 1.4 billion episodes of diarrhoea each year, with several medical checks, hospitalizations, and around two million deaths. Over 78% of diarrhoea cases are found in the LMICs [4]. The burden of diarrhoeal diseases is complicated by the lack of appropriate case management [5], limited ability to detect the aetiologies [6], and antimicrobial resistance [7].
The most common aetiologies of diarrhoea include bacteria such as Escherichia coli, Vibrio cholerae, Campylobacter jejuni, Salmonella spp., Aeromonas spp., and Yersinia enterocolitica; viruses mainly rotavirus, norovirus, sapovirus, and adenovirus; and protozoa largely Entamoeba histolytica, Giardia spp., and Cryptosporidium spp. [8, 9]. Of the bacterial aetiologies, Campylobacter is a leading cause of gastroenteritis in both high-, middle-, and low- income countries, responsible for 400–500 million cases of diarrhoea each year [10]. The clinically important Campylobacter species are C. jejuni and C. coli, which are responsible for about 98% of all human Campylobacter gastroenteritis cases [11, 12].
In most cases, campylobacteriosis does not require any antimicrobial therapy except in severe cases, especially in immune-deficient or immune-suppressed individuals [13, 14]. The recommended drugs are macrolides (mostly erythromycin), fluoroquinolones (mainly ciprofloxacin), and tetracycline [10, 15, 16]. Nevertheless, there is an escalating number of Campylobacter isolates resistant to these drugs [17, 18] due to the immeasurable and misuse of antimicrobials [19], not only in animals but also in humans [20]. Several factors have been associated with occurrence of Campylobacter infections. They include consumption of different food items like undercooked poultry meat and pork, red meat at barbecue, grapes, and drinking unpasteurized milk, having a chronic illness [21–23], drinking contaminated water, type of water source, animal contact, young age, eating prepared salad, latrine usage, bottle feeding, and nutritional status [24–26]. There is a wide range of natural reservoirs for Campylobacter including chicken and other poultry, wild birds, pigs, dogs, cats, sheep, and cows [27, 28]. Consequently, colonization of different reservoirs by Campylobacter poses an important risk for humans through shedding of the pathogen in livestock waste and water sources contamination, environment, and food [29, 30].
In LMICs, studies on thermophilic Campylobacter species are few due to limited capacity in laboratory diagnosis [31] and lack of surveillance of enteric diseases [32]. The objective of this review was to gather information on the prevalence, risk factors, and antimicrobial resistance profiles of thermophilic Campylobacter species in humans and animals in SSA. The findings of this review are expected to provide evidence for policy formulation, prevention, and control of Campylobacter infections and increase awareness of the AMR issue.
2. Methods
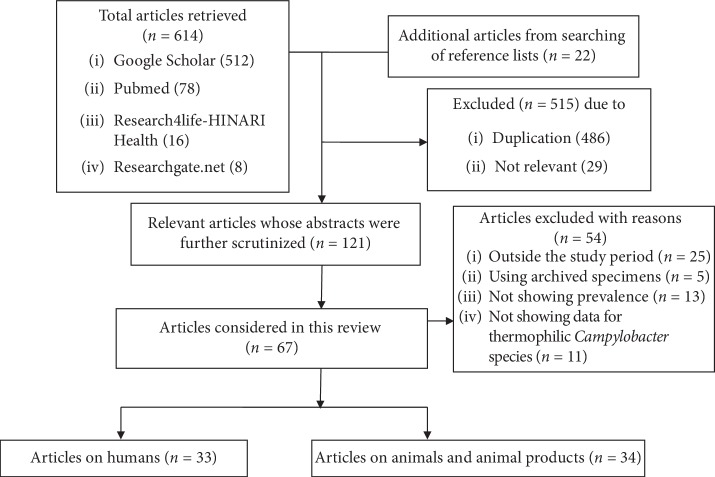
The data were collected by searching articles published in English from electronic databases, namely, PubMed, Google Scholar, Research4life-HINARI Health, and Researchgate.net. The search terms were “thermophilic Campylobacter,” “Campylobacter jejuni,” “Campylobacter coli,” “diarrhea/diarrhoea,” “antimicrobial resistance,” “antibiotic resistance,” “humans,” “animals,” “Sub-Saharan Africa,” and “a specific country name.” Initially, a total of 614 articles were identified, and the lists of references were screened in which 22 more articles were identified. After screening, 33 articles on humans and 34 on animals and animal products were included in this review (Figure 1). The reviewed articles were those published from 1997 to 2018. During the review process, the data extracted included title, country, sex and age distribution, sample size, isolation and identification methods, isolation rates, and antimicrobial resistance profiles. Articles for which the sample size was not shown or which used archived Campylobacter cultures were excluded from this review.
Figure 1.
Flowchart showing article selection process.
3. Results
3.1. Campylobacter Infections in Humans
Of the 47 SSA countries [33], data on human campylobacteriosis were available from 15 (31.9%) countries. The prevalence of thermophilic Campylobacter in humans was reported in 33 articles (Table 1). Nigeria reported the highest overall prevalence of thermophilic Campylobacter (62.7%); followed by Malawi (21%) and South Africa (20.3%). Kenya reported the highest prevalence (16.4%) of Campylobacter infections in under-five children; followed by Rwanda (15.5%) and Ethiopia (14.5%). The mean prevalence in all ages and under-five children was 18.6% and 9.4%, respectively. Burkina Faso and Mozambique had the lowest prevalence of campylobacteriosis for all ages (2.3%) and under-five (1.7%), respectively. Of the 33 articles reviewed, 16 (48.5%) presented data on distribution of Campylobacter infections by sex but the difference was not statistically significant. Of these 16 articles, campylobacteriosis was more prevalent among males (22.7%; n = 3966) than females (17.7%; n = 3705). Culture methods on selective media, biochemical tests, molecular, and biotyping techniques were used for identification of Campylobacter (Table 1). Of the 33 articles, 27 studies were carried out at clinical settings (hospitals and health centres) while 6 were community-based studies. Probability sampling methods were adopted in 5 articles while the remaining used convenience sampling. Although C. jejuni and C. coli were isolated in the mentioned articles, 15 articles reported other enteric pathogens as probable aetiologies of diarrhoea. Furthermore, more than 85% of the articles considered diarrhoeic cases while the remaining included even asymptomatic participants.
Table 1.
Prevalence of thermophilic Campylobacter spp. in humans in sub-Saharan Africa, 1997–2018.
| Country | Age group (sample size) | Number of articles | Prevalence (%) | Detection method | References |
|---|---|---|---|---|---|
| Uganda | Children <5 (226) | 1 | 9.3 (C. jejuni: 80.9%; C. coli: 4.8%) | Culture, biochemical | [34] |
| Tanzania | Children <5 (1,512) | 5 | 8.8 (2.6–19) (C. jejuni: 89.2%; C. coli: 9.8%) | Culture, biochemical, Gram staining, molecular | [8, 35–38] |
| Kenya | Children <5 (2,550) | 1 | 16.4 | Culture, biochemical, serotyping | [39] |
| Rwanda | Children <5 (706) | 1 | 15.5 (C. jejuni: 100%) | Molecular | [40] |
| Madagascar | Children <5 (5,620) | 2 | 9.4 (9.3–9.5) (C. jejuni: 73.6; C. coli: 24.3%) | Culture, serotyping, molecular | [41, 42] |
| Burkina Faso | Children <5 (283) | 1 | 2 (C. jejuni: 60%; C. coli: 40%) | Culture, molecular | [43] |
| Ethiopia | Children <5 (670) | 2 | 14.5 (12.7–16.7) (C. jejuni: 71.1%; C. coli: 21.1%) | Culture, biochemical, Gram staining | [25, 44, 45] |
| Nigeria | Children <5 (1,311) | 3 | 4.4 (0.5–8.2) (C. jejuni: 28%; C. coli: 72%) | Culture, biochemical, biotyping, Gram staining | [46–48] |
| Niger | Children <5 (260) | 1 | 11.4 (C. jejuni: 100%) | Culture, biochemical, Gram staining | [49] |
| Mozambique | Children <5 (529) | 1 | 1.7 | Culture, biochemical, Gram staining | [50] |
| Cameroon | Children <5 (260) | 1 | 9.6 (C. jejuni: 100%) | Culture, biochemical, Gram staining | [51] |
| Botswana | Under 15 years | 1 | 14 | Molecular | |
| Tanzania | All ages (2,487) | 4 | 11.1 (1.9–21.6) (C. jejuni: 93.3%; C. coli: 6.1%) | Culture, biochemical, Gram staining, molecular | [26, 52–54] |
| Kenya | All ages (4,274) | 2 | 9.2 (8.5–9.8) (C. jejuni: 76.2; C. coli: 12.7%) | Culture | [55, 56] |
| Burkina Faso | All ages (1,246) | 1 | 2.3 (C. jejuni: 51.8%; C. coli: 13.8%) | Culture, biochemical, Gram staining | [57] |
| Ethiopia | All ages (640) | 2 | 9.8 (8–11.6) (C. jejuni:94.1%; C. coli: 5.9%) | Culture, biochemical, Gram staining | [58, 59] |
| Nigeria | All ages (150) | 1 | 62.7 (C. jejuni: 24.5%; C. coli: 62.3%) | Culture, biochemical, Gram staining | [60] |
| Ghana | All ages (202) | 1 | 17.3 (C. jejuni: 42.8%; C. coli: 37%) | Culture, biochemical, Gram staining | [61] |
| Malawi | All ages (1,941) | 1 | 21 (C. jejuni: 85%; C. coli: 14%) | Molecular | [62] |
| South Africa | All ages (565) | 1 | 20.3 (C. jejuni: 85%; C. coli: 15%) | Culture, biochemical, molecular | [63] |
Of the 33 articles, only four reported on risk factors of campylobacteriosis in humans. In Tanzania, Campylobacter infections were associated with sex, young age, poultry meat consumption, and eating of salads [26, 38]. In Ethiopia, human campylobacteriosis was significantly associated with nonuse of latrines, water source, drinking unboiled water, bottle feeding, nutritional status, and exposure to domestic animals including cats, dogs, poultry, and pigeons [25]. In Burkina Faso, Campylobacter infections were most common among under-fives and those aged 21–40 years with more pet contacts [57].
3.2. Campylobacter spp. in Animals and Contamination of Animal Products
Of the 34 articles from which data on animals were extracted, 17 collected faeces from live animals, while 16 collected samples from meat or caeca at abattoirs. In 2 articles, samples were collected from both markets and abattoirs. Probability sampling methods were used in 6 articles while the remaining used convenience sampling.
Data on Campylobacter in cattle were obtained from ten articles published from studies conducted in six countries. The overall mean prevalence was 17.6% and C. jejuni had higher prevalence (70%) than C. coli (23.5%). The highest [64] and the lowest overall prevalence [52] were reported from Tanzania. Furthermore, Tanzania and Ghana showed higher prevalence for C. jejuni and C. coli, respectively (Table 2).
Table 2.
Prevalence of Campylobacter spp. in domestic animals and animal products.
| Animal type | Sample type | Country | Overall prevalence | C. jejuni (%) | C. coli (%) | References |
|---|---|---|---|---|---|---|
| Cattle | Faeces | South Africa | 19.3 | 72.4 | 27.6 | [18] |
| Nigeria | 18.5 | 80 | 20 | [65] | ||
| 12.9 | 65.1 | 23 | [66] | |||
| Tanzania | 2.3 | 100 | 0 | [52] | ||
| 5.6 | 83.3 | 16.7 | [67] | |||
| 32.5 | 65.5 | 27.3 | [64] | |||
| Ghana | 13.2 | 25 | 43.8 | [68] | ||
| Ethiopia | 12.7 | 53.8 | 38.5 | [69] | ||
| 48 | 75.3 | 17.6 | [70] | |||
| Mozambique | 11 | 80 | 20 | [71] | ||
| Average | 17.6 | 70 | 23.5 | |||
|
| ||||||
| Cattle | Meat | Tanzania | 2.8 | 100 | 0 | [67] |
| Ethiopia | 6.2 | 85.7 | 14.3 | [72] | ||
| Kenya | 2 | 100 | 0 | [73] | ||
| Average | 5.5 | 95.2 | 4.8 | |||
|
| ||||||
| Cattle | Carcasses | Tanzania | 3.7 | 75 | 25 | [67] |
| 9.5 | 62.5 | 29.2 | [74] | |||
| Ghana | 34.5 | 84.2 | 13.1 | [68] | ||
| Average | 15.9 | 73.9 | 22.4 | |||
|
| ||||||
| Sheep | Faeces | Tanzania | 31.6 | 55.6 | 44.4 | [75] |
| Ethiopia | 38 | 59.3 | 40.7 | [69] | ||
| 39 | 84.6 | 15.4 | [70] | |||
| Ghana | 18.6 | 27.2 | 40.9 | [68] | ||
| Average | 31.8 | 56.7 | 35.4 | |||
|
| ||||||
| Sheep | Carcasses | Ethiopia | 10.6 | 73.9 | 26.1 | [76] |
| Ghana | 35.9 | 92.8 | 0 | [68] | ||
| Average | 23.3 | 83.4 | 13.1 | |||
|
| ||||||
| Sheep | Meat | Ethiopia | 10.5 | 83.3 | 0 | [72] |
| Pig | Faeces | Nigeria | 92.7 | 14 | 78.7 | [60] |
| Ethiopia | 50 | 0 | 100 | [69] | ||
| Tanzania | 66.7 | 81.8 | 18.2 | [77] | ||
| 32.5 | 2.7 | 91.9 | [64] | |||
| Ghana | 28.7 | 48.2 | 48.2 | [68] | ||
| South Africa | 2.3 | 16.7 | 83.3 | [78] | ||
| Average | 45.5 | 27.2 | 70.1 | |||
|
| ||||||
| Pig | Carcasses | Ghana | 36.3 | 28.4 | 10.8 | [68] |
| Pig | Pork | Ethiopia | 8.5 | 25 | 50 | [72] |
| Chicken | Faeces | Burkina Faso | 68 | 70 | 30 | [79] |
| Tanzania | 69.8 | 91.2 | 8.8 | [53] | ||
| 42.5 | 87.1 | 12.9 | [38] | |||
| 77.8 | 91.1 | 7.3 | [54] | |||
| South Africa | 35.3 | 84.9 | 15.1 | [18] | ||
| 49.7 | 100 | 0 | [80] | |||
| 54.8 | 54.8 | 40.2 | [81] | |||
| Ethiopia | 72.7 | 92.5 | 7.5 | [59] | ||
| 68.1 | 80.8 | 16.2 | [69] | |||
| 86.6 | 86.9 | 11.9 | [70] | |||
| Ivory Coast | 63.8 | 51.3 | 48.7 | [82] | ||
| Average | 62.6 | 81 | 18.1 | |||
|
| ||||||
| Chicken | Colon | South Africa | 14.2 | 68.8 | 31.2 | [78] |
| Chicken | Carcasses | Burkina Faso | 50 | 100 | 0 | [79] |
| Chicken | Meat | Ethiopia | 21.7 | 84 | 8 | [72] |
| Kenya | 77 | 59 | 39 | [73] | ||
| Average | 49.4 | 71.5 | 23.5 | |||
|
| ||||||
| Goat | Faeces | DRC | 41.7 | 32.7 | 59.4 | [83] |
| Ghana | 18.5 | 36 | 56 | [68] | ||
| Ethiopia | 33.3 | 100 | 0 | [70] | ||
| Average | 31.2 | 56.2 | 38.5 | |||
|
| ||||||
| Goat | Carcasses | Ethiopia | 9.4 | 70.6 | 29.4 | [76] |
| Ghana | 23.9 | 81.3 | 0 | [68] | ||
| Average | 16.7 | 76 | 14.7 | |||
|
| ||||||
| Goat | Meat | DRC | 37.3 | 21.3 | 74.7 | [83] |
| Ethiopia | 7.6 | 71.4 | 28.6 | [72] | ||
| Average | 22.5 | 46.4 | 51.7 | |||
|
| ||||||
| Cattle | Milk | Tanzania | 13.4 | 55.3 | 31.6 | [74] |
Data on Campylobacter in goats were reported in three articles from three different countries. The overall mean prevalence was 31.2%, and C. jejuni presented with a higher prevalence (56.2%) than C. coli (38.5%). The highest and lowest prevalence were reported from the Democratic Republic of Congo (DRC) [83] and Ghana [83], respectively. Ethiopia [70] and DRC [83] had the highest frequencies for C. jejuni and C. coli, respectively (Table 2). For sheep, data were reported in four articles from three countries. The overall mean prevalence was 31.8%, with C. jejuni being reported at a higher frequency (56.7%) than C. coli (35.4%). The highest and lowest prevalence were reported from Ethiopia [70] and Ghana [70], respectively. Ethiopia [70] and Tanzania [75] had the highest prevalence for C. jejuni and C. coli, respectively (Table 2).
Data on presence of thermophilic Campylobacter in pigs were available from six articles from five countries. The overall mean prevalence was 45.5% and contrary to other animals, C. coli occurred at a higher prevalence (70.1%) than C. jejuni (27.2%). The highest and lowest prevalence were reported from Nigeria [60] and South Africa [78], respectively. Ethiopia [69] had both higher and lower values for C. jejuni and C. coli (Table 2).
Data on thermophilic Campylobacter in chickens were obtained from 11 articles from five different countries. In this review, the number of articles on chickens was the highest compared to other reservoirs. The overall mean prevalence was 62.6% which was the highest in all animal reservoirs documented in this review. Campylobacter jejuni was reported in higher prevalence (81.0%) than C. coli (18.1%). The highest and lowest prevalence rates were reported in Ethiopia [70] and South Africa [18], respectively (Table 2).
As regards to animal products, data on cattle meat were reported in three articles from three countries. The overall mean prevalence was 5.5%, and C. jejuni had higher prevalence (95.2%) than C. coli (4.8%). The highest and lowest prevalence rates were reported in Ethiopia [72] and Kenya [73], respectively. For cattle carcasses, data were reported by two articles from two countries with a mean prevalence of 15.9%. Ghana [68] reported a higher prevalence of C. jejuni while Tanzania [74] observed a higher prevalence of C. coli (Table 2).
Data on sheep meat were reported by a single article from Ethiopia [72] with the prevalence of 10.5%. In sheep carcasses, the mean prevalence was 23.3% computed using two articles from two countries. Ghana [68] showed a higher prevalence of C. jejuni while Ethiopia [76] reported a higher prevalence of C. coli. In pork, the prevalence was 8.5% in one article from Ethiopia [72] with C. coli being more prevalent than C. jejuni. In pig carcasses, the prevalence was 36.3% from one article reporting a study carried out in Ghana [68]. In chicken meat, the mean prevalence was 49.4% reported by two articles from two countries. Dadi and Asrat in a study conducted in Ethiopia [72] indicated a higher prevalence for C. jejuni while a study in Kenya [73] found a higher prevalence for C. coli. For chicken carcasses, the prevalence was 50% from one article in Burkina Faso [79] and all isolates were C. jejuni. In goat meat, the mean prevalence was 22.5% reported by only one article from Ethiopia [72]. In goat carcasses, the mean prevalence was 16.7% reported by two articles from two countries. A study conducted in Ghana [76] reported a higher prevalence for C. jejuni while that in Ethiopia [76] found a higher prevalence for C. coli (Table 2).
The overall prevalence of thermophilic Campylobacter in cats [84] and dogs [84, 85] were 18.3% and 20%, respectively. Of the reviewed articles, some presented data on companion, wild, and other animals (Table 3).
Table 3.
Prevalence of Campylobacter spp. in companion, wild, and other animals.
| Animal type | Specimen | Country | Overall prevalence | C. jejuni (%) | C. coli (%) | References |
|---|---|---|---|---|---|---|
| Companion animals | ||||||
| Cat | Faeces | Nigeria | 18.3 | 21.1 | [66] | |
| Dog | Faeces | Nigeria | 27.7 | 23.1 | 0 | [66] |
| 12.3 | 53.8 | 30.8 | [85] | |||
| Average | 20 | 38.5 | 15.4 | |||
|
| ||||||
| Other animals | ||||||
| Crow | Faeces | Tanzania | 72.8 | 93.8 | 6.2 | [53] |
| Duck | Faeces | Tanzania | 80 | 81.5 | [86] | |
| Greater crested tern | Faeces | South Africa | 16 | 15 | 1 | [87] |
| Kelp gull | Faeces | South Africa | 12.4 | 11.6 | 0.8 | [87] |
| Quail | Caeca | Nigeria | 31.1 | 81 | 19 | [88] |
| Horse | Faeces | Tanzania | 60 | 66.7 | 33.3 | [75] |
| Guinea pig | Faeces | Tanzania | 26.7 | 50 | 50 | [75] |
| Rat | Faeces | Tanzania | 1.2 | 66.7 | 33.3 | [75] |
3.3. Antimicrobial Resistance Profiles of C. jejuni and C. coli in Humans and Animals
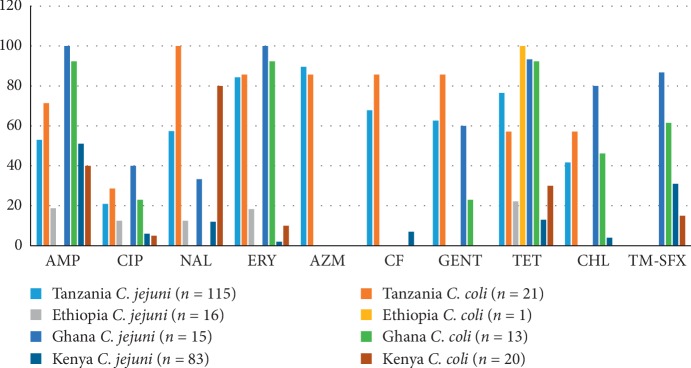
In humans, the AMR profiles, determined using disk diffusion, were available in 4 articles from four different countries (Figure 2), while the remaining did not specify the species. The antimicrobials considered in this review for the ease of comparison were ampicillin (AMP), erythromycin (ERY), tetracycline (TET), cefalotin (CF), nalidixic acid (NAL), azithromycin (AZM), gentamicin (GEN), ciprofloxacin (CIP), chloramphenicol (CHL), and trimethoprim-sulfamethoxazole (TM-SFX).
Figure 2.
Antimicrobial resistance data in humans by the disk diffusion method.
The percentage of antimicrobial resistant isolates ranged from 2–100% for C. jejuni and 0–100% for C. coli. The AMR data for CIP and ERY, which are drugs of choice for treating Campylobacter infections, showed that Ghana [61] and Tanzania [26] reported higher values for both C. jejuni and C. coli. Resistance of Campylobacter jejuni to GEN was similar for both Tanzania and Ghana while for C. coli, it was higher in Tanzania compared eith that of Ghana [26, 61]. Higher frequencies of resistance were also reported for TET and AMP which have been in use for many years. In general, higher levels of AMR were reported in C. jejuni than C. coli.
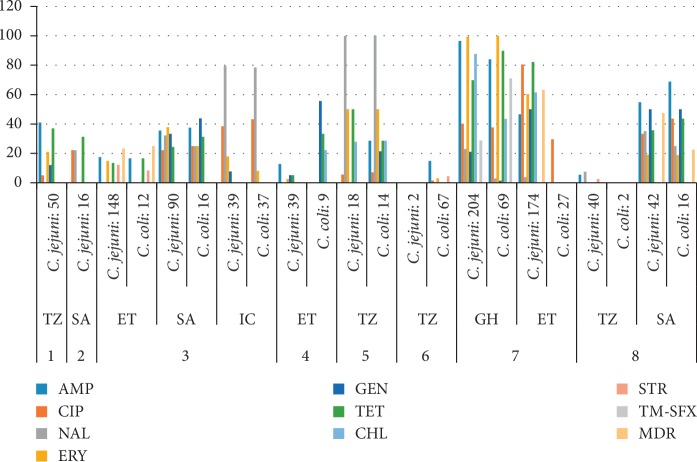
In animal and animal products, the following antimicrobials were used in the reviewed articles: chloramphenicol (CHL), ampicillin (AMP), erythromycin (ERY), ciprofloxacin (CIP), nalidixic acid (NAL), streptomycin (STR), tetracycline (TET), gentamicin (GEN), and trimethoprim-sulfamethoxazole (TM-SFX) (Figure 3).
Figure 3.
Antimicrobial resistance data in animals by the disk diffusion method. 1: duck, 2: sea birds, 3: chicken, 4: raw meat, 5: laboratory and farm animals, 6: pig, 7: food animals, 8: cattle; TZ: Tanzania, SA: South Africa, ET: Ethiopia, IC: Ivory Coast, GH: Ghana.
In animals, the percentage of resistant isolates varied from 0–100%. Resistance to CIP was in the range of 0–80.5% and 0–68.8% for C. jejuni and C. coli, respectively. Resistance to ERY varied from 0–99.5% and 0–100% for C. jejuni and C. coli, respectively. Resistance to GEN was <55.6% for both C. jejuni and C. coli. The highest resistance to most of the drugs was seen in Ghana [68] while the lowest resistance was observed in Tanzania [74, 89]. Resistance to nalidixic acid was high for both C. jejuni and C. coli in a study conducted in Tanzania [75]. Data on multidrug resistance were available from three studies in which values ranged from 23.3% to 63.3% for C. jejuni [18, 59, 74] and from 0–25% for C. coli [59, 70]. There were variations in resistance levels to commonly used antimicrobials in animal species depending on the species tested.
4. Discussion
The overall mean prevalence of thermophilic Campylobacter in humans ranged from 9.6–18.5% and is within the ranges reported elsewhere in LMICs [31] and in Poland [90]. However, the prevalence was higher than that reported from Korea [91], and was lower than that reported from the USA [92]. This variation may be attributed to the fact that campylobacteriosis is hyperendemic in LMICs probably due to poor sanitation and close proximity of humans and domestic animals [31]. The risk factors for human infections highlighted in this review partly explain this. They include consumption of poultry meat, drinking surface water, and animal contact, which is in agreement with other studies with consumption of poultry being the major risk factor [24, 93].
The prevalence of thermophilic Campylobacter in animals varied between 1.2% and 80%. The mean prevalence recorded in chickens (60.3%) concurs with findings from other LMICs such as Thailand [94], Sri Lanka [95], and Vietnam [96]. The mean prevalence of thermophilic Campylobacter in pigs was comparable to what was reported in Spain and Vietnam [30, 96] but lower than those reported in Norway and the Netherlands [97, 98]. The prevalence of Campylobacter in goats and sheep was slightly higher than the prevalence reported in Germany and Trinidad [99, 100] but lower than the prevalence reported in Spain [30]. The prevalence in cattle (17.6%) was lower than those reported in the USA and Iran [101, 102] but higher than the prevalence reported in another paper in the USA [103].
Although thermophilic Campylobacter species are frequently isolated from animal faeces, this review showed that they are also present in considerable amounts in a number of animal products. The reported prevalence of Campylobacter in cattle and goat carcasses in sub-Saharan Africa was higher compared to the prevalence in Poland for cattle [104] and in Canada for goat [105]. The contamination of carcasses may result from contact with gut contents during manual skin removal, cleaning, and processing in the slaughter house [106]. The prevalence rates in beef, pork, and mutton were slightly higher compared to those observed in other countries [107–109]. The variation could be influenced by the differences in husbandry practices which determine exposure of the animals to the bacteria. Partly, this could also be attributable to slaughter and animal product handling practices which enhance the contamination of the products.
Campylobacter jejuni and C. coli were the most frequently encountered species from both human and animals. Similar observations have been reported by other authors [30, 110]. The predominance of C. jejuni in various animals, other than pigs, in sub-Saharan Africa has been previously reported [31, 111]. The possible explanation is that most of the studies rely on culture and biochemical tests which may not correctly identify some species. Another reason is the use of selective media containing antibiotics to which some other Campylobacter species are sensitive to. Furthermore, higher incubation temperatures may limit the growth of some thermophilic Campylobacter species like C. lari and C. upsaliensis [112, 113].
In pigs, C. coli showed higher prevalence (67.4%) than C. jejuni (27.2%) which is in agreement with reports in Canada and the USA that C. coli is a normal flora of pigs' intestines [114, 115]. Furthermore, some studies show that C. jejuni and C. coli may cohabit in pigs but usually C. jejuni is always present in lower frequencies than C. coli [116, 117].
The results on AMR in both humans and animals highlight that resistance to mostly used antimicrobials is frequent. The resistance ranged from 0 to 100%, and higher resistance rates were reported in C. jejuni than in C. coli. The antimicrobials to which resistance was high included AMP, TET, ERY, and TET. The findings concur with the reports from other studies in both LMICs and high-income countries showing an increment in the number of Campylobacter strains resistant to most of the antimicrobials used in treating human campylobacteriosis [118–120]. The increase in resistance to most antimicrobial agents and emergence of MDR isolates could be associated with extensive use of antimicrobials not only as therapeutic agents for human infections [20] but also for prophylaxis and growth promotion in animal husbandry [68]. However, there are challenges in surveillance, differences in design and predominance of the disk diffusion method and not using globally accepted methods. These may cause differences within and between countries and certainly limit comparability with data reported in other parts of the world. The resistance to TET was comparable with the findings reported from Poland [121] and the USA [122] and the pooled estimate prevalence worldwide (94.3%) [120]. This resistance may be due to wide use of tetracycline in both human and veterinary medicine [20]. The proportion of isolates resistant to macrolides (ERY) ranged from 0 to 100% in both humans and animals for C. jejuni while the range was from 0 to 92.3% for C. coli. The frequency of isolates resistant to fluoroquinolone was relatively lower in humans which is comparable to rates described in Western Europe [118, 121]. The resistance to both erythromycin and ciprofloxacin is of public health concern as there are currently limited options in the choice of treatment of Campylobacter infections. The proportion of multidrug resistance (MDR) isolates varied between 23.3 and 63.3% (Figure 3) which falls within the range of 37–90% from studies in China, Korea, and France [123–125].
There are no internationally agreed criteria of susceptibility testing and breakpoint assessment for Campylobacter spp. [126]. Therefore, it is difficult to interpret the available data and draw conclusion. Several laboratory standards have been applied for the susceptibility testing of Campylobacter species. Although disk diffusion was used in some studies, it should be used only as a screening method for resistance to erythromycin and ciprofloxacin [127].
5. Conclusion
This review indicates that C. jejuni and C. coli are frequently isolated from humans, food animals, and animal products in sub-Saharan Africa. Isolates from the different sources display varying degrees of resistance to commonly used antimicrobial agents. The findings of this review suggest that the disease burden due to thermophilic Campylobacter species in SSA is of public and economic importance. Therefore, routine diagnosis of C. jejuni and C. coli, appropriate use of antimicrobials, educating communities on hygienic practices, establishment of both national and regional multisectoral antimicrobial resistance standard surveillance protocols are necessary to curb both the campylobacteriosis burden, and increase of antimicrobial resistance in the region.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Bern C., Martines J., de Zoysa I., Glass R. I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bulletin of the World Health Organization. 1992;70(6):705–714. [PMC free article] [PubMed] [Google Scholar]
- 2.Walker C. L. F., Rudan I., Liu L., et al. Global burden of childhood pneumonia and diarrhoea. The Lancet. 2013;381(9875):1405–1416. doi: 10.1016/s0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L., Oza S., Hogan D., et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet. 2015;385(9966):430–440. doi: 10.1016/s0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Ahs J. W., Tao W., Löfgren J., Forsberg B. C. Diarrheal diseases in low- and middle-income countries: incidence, prevention and management. The Open Infectious Diseases Journal. 2010;4(1):113–124. doi: 10.2174/1874279301004020113. [DOI] [Google Scholar]
- 5.Carvajal-Vélez L., Amouzou A., Perin J., et al. Diarrhea management in children under five in sub-Saharan Africa: does the source of care matter? A countdown analysis. BMC Public Health. 2016;16(1):p. 830. doi: 10.1186/s12889-016-3475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petti C. A., Polage C. R., Quinn T. C., Ronald A. R., Sande M. A. Laboratory medicine in Africa: a barrier to effective health care. Clinical Infectious Diseases. 2006;42(3):377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T. V., Le P. V., Le C. H., Weintraub A. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrobial Agents and Chemotherapy. 2005;49(2):816–819. doi: 10.1128/aac.49.2.816-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargas M., Vila J., Casals C., et al. Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. The American Journal of Tropical Medicine and Hygiene. 2004;70(5):536–539. doi: 10.4269/ajtmh.2004.70.536. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Duarte O. G., Bai J., Newell E. Detection of Escherichia coli, Salmonella spp., Shigella spp., Yersinia enterocolitica, Vibrio cholerae, and Campylobacter spp. enteropathogens by 3-reaction multiplex polymerase chain reaction. Diagnostic Microbiology and Infectious Disease. 2009;63(1):1–9. doi: 10.1016/j.diagmicrobio.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Palacios G. M. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clinical Infectious Diseases. 2007;44(5):701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 11.Allos B. M., Blaser M. J. Campylobacter jejuni and the expanding spectrum of related infections. Clinical Infectious Diseases. 1995;20(5):1092–1101. doi: 10.1093/clinids/20.5.1092. [DOI] [PubMed] [Google Scholar]
- 12.Gilliss D., Cronquist A. B., Cartter M., et al. Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. Sites, 1996–2012. Morbidity and Mortality Weekly Report. 2013;62(15):283–287. [PMC free article] [PubMed] [Google Scholar]
- 13.Guévremont E., Nadeau É., Sirois M., Quessy S. Antimicrobial susceptibilities of thermophilic Campylobacter from humans, swine, and chicken broilers. Canadian Journal of Veterinary Research. 2006;70(2):81–86. [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur S., Zhao S., McDermott P. F., et al. Antimicrobial resistance, virulence, and genotypic profile comparison of Campylobacter jejuni and Campylobacter coli isolated from humans and retail meats. Foodborne Pathogens and Disease. 2010;7(7):835–844. doi: 10.1089/fpd.2009.0487. [DOI] [PubMed] [Google Scholar]
- 15.Guerrant R. L., Van Gilder T., Steiner T. S., et al. Practice guidelines for the management of infectious diarrhea. Clinical Infectious Diseases. 2001;32(3):321–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 16.Aarestrup F. M., McDermott P. F., Wegener H. C. Transmission of Antibiotic Resistance from Food Animals to Humans. 3rd. Washington, DC, USA: American Society for Microbiology; 2008. [Google Scholar]
- 17.Aarestrup F. M., Engberg J. R. Antimicrobial resistance of thermophilic Campylobacter. Veterinary Research. 2001;32(3/4):311–321. doi: 10.1051/vetres:2001127. [DOI] [PubMed] [Google Scholar]
- 18.Uaboi-Egbenni. Potentially pathogenic Campylobacter species among farm animals in rural areas of Limpopo province, South Africa: a case study of chickens and cattles. African Journal of Microbiology Research. 2012;6(12) doi: 10.5897/ajmr11.891. [DOI] [Google Scholar]
- 19.Padungton P., Kaneene J. B. Campylobacter spp. in human, chickens, pigs and their antimicrobial resistance. Journal of Veterinary Medical Science. 2003;65(2):161–170. doi: 10.1292/jvms.65.161. [DOI] [PubMed] [Google Scholar]
- 20.Iovine N. M. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013;4(3):230–240. doi: 10.4161/viru.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neimann J., Engberg J., Mølbak K., Wegener H. C. A case–control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiology and Infection. 2003;130(3):353–366. doi: 10.1017/S0950268803008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doorduyn Y., Van Den Brandhof W. E., Van Duynhoven Y. T. H. P., Breukink B. J., Wagenaar J. A., Van Pelt W. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in the Netherlands: a case-control study. Epidemiology and Infection. 2010;138(10):1391–1404. doi: 10.1017/s095026881000052x. [DOI] [PubMed] [Google Scholar]
- 23.Mossong J., Mughini-Gras L., Penny C., et al. Human campylobacteriosis in Luxembourg, 2010-2013: a case-control study combined with multilocus sequence typing for source attribution and risk factor analysis. Scientific Reports. 2016;6(1) doi: 10.1038/srep20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapperud G. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. American Journal of Epidemiology. 2003;158(3):234–242. doi: 10.1093/aje/kwg139. [DOI] [PubMed] [Google Scholar]
- 25.Lengerh A., Moges F., Unakal C., Anagaw B. Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatrics. 2013;13(1):p. 82. doi: 10.1186/1471-2431-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komba E. V. G., Mdegela R. H., Msoffe P. L. M., Nielsen L. N., Ingmer H. Prevalence, antimicrobial resistance and risk factors for thermophilic Campylobacter infections in symptomatic and asymptomatic humans in Tanzania. Zoonoses and Public Health. 2015;62(7):557–568. doi: 10.1111/zph.12185. [DOI] [PubMed] [Google Scholar]
- 27.Skirrow M. B. Epidemiology of Campylobacter enteritis. International Journal of Food Microbiology. 1991;12(1):9–16. doi: 10.1016/0168-1605(91)90044-p. [DOI] [PubMed] [Google Scholar]
- 28.Workman S. N., Mathison G. E., Lavoie M. C. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. Journal of Clinical Microbiology. 2005;43(6):2642–2650. doi: 10.1128/JCM.43.6.2642-2650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luechtefeld N. W., Reller L. B., Blaser M. J., Wang W. L. Comparison of atmospheres of incubation for primary isolation of Campylobacter fetus subsp. jejuni from animal specimens: 5% oxygen versus candle jar. Journal of Clinical Microbiology. 1982;15(1):53–57. doi: 10.1128/jcm.15.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oporto B., Esteban J. I., Aduriz G., Juste R. A., Hurtado A. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms. Journal of Applied Microbiology. 2007;103(4):977–984. doi: 10.1111/j.1365-2672.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 31.Coker A. O., Isokpehi R. D., Thomas B. N., Amisu K. O., Obi C. L. Human campylobacteriosis in developing countries. Emerging Infectious Diseases. 2002;8(3):237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO/FAO/OIE. The Global View of Campylobacteriosis: Report of an Expert Consultation. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 33.Mullan F., Frehywot S. Non-physician clinicians in 47 sub-Saharan African countries. The Lancet. 2007;370(9605):2158–2163. doi: 10.1016/s0140-6736(07)60785-5. [DOI] [PubMed] [Google Scholar]
- 34.Mshana S., Joloba M., Kakooza A., Kaddu-Mulindwa D. Campylobacter spp among children with acute diarrhea attending Mulago hospital in Kampala-Uganda. African Health Sciences. 2009;9(3):201–205. [PMC free article] [PubMed] [Google Scholar]
- 35.Kingamkono R., Sjögren E., Svanberg U. Enteropathogenic bacteria in faecal swabs of young children fed on lactic acid-fermented cereal gruels. Epidemiology and Infection. 1999;122(1):23–32. doi: 10.1017/s0950268898001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oketcho R., Nyaruhucha C., Taybali S., Karimuribo E. Influence of enteric bacteria and parasite infection and nutritional status on diarrhoea occurrence in six to 60 month old children admitted at Morogoro regional hospital of Tanzania. Tanzania Journal of Health Research. 2012;14(2) doi: 10.4314/thrb.v14i2.3. [DOI] [PubMed] [Google Scholar]
- 37.Deogratias A. P., et al. Prevalence and determinants of Campylobacter infection among under five children with acute watery diarrhea in Mwanza, North Tanzania. Archives of Public Health. 2014;72(1) doi: 10.1186/2049-3258-72-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuma I. S., Nonga H. E., Mdegela R. H., Kazwala R. R. Epidemiology and RAPD-PCR typing of thermophilic campylobacters from children under five years and chickens in Morogoro Municipality, Tanzania. BMC Infectious Diseases. 2016;16(1):1–11. doi: 10.1186/s12879-016-2031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty M., et al. Sporadic paediatric diarrhoeal illness in urban and rural sites in Nyanza province, Kenya. East African Medical Journal. 2010;86(8) doi: 10.4314/eamj.v86i8.54159. [DOI] [PubMed] [Google Scholar]
- 40.Kabayiza J.-C., Andersson M. E., Nilsson S., Bergström T., Muhirwa G., Lindh M. Real-time PCR identification of agents causing diarrhea in Rwandan children less than 5 years of age. The Pediatric Infectious Disease Journal. 2014;33(10):1037–1042. doi: 10.1097/inf.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 41.Randremanana R., Randrianirina F., Gousseff M., et al. Case-control study of the etiology of infant diarrheal disease in 14 districts in Madagascar. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044533.e44533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randremanana R. V., Randrianirina F., Sabatier P., et al. Campylobacter infection in a cohort of rural children in Moramanga, Madagascar. BMC Infectious Diseases. 2014;14(1) doi: 10.1186/1471-2334-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonkoungou I. J. O., Haukka K., Österblad M., et al. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatrics. 2013;13(1):p. 36. doi: 10.1186/1471-2431-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tafa B., Sewunet T., Tassew H., Asrat D. Isolation and antimicrobial susceptibility patterns of Campylobacter species among diarrheic children at Jimma, Ethiopia. International Journal of Bacteriology. 2014;2014:7. doi: 10.1155/2014/560617.560617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Getamesay M., Getenet B., Ahmed Z. Prevalence of Shigella, Salmonella and Campylobacter species and their susceptibility patters among under five children with diarrhea in Hawassa town, South Ethiopia. Ethiopian Journal of Health Sciences. 2014;24(2):p. 101. doi: 10.4314/ejhs.v24i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adekunle O., Coker A., Kolawole D. Incidence, isolation and characterization of Campylobacter species in Osogbo. Biology and Medicine. 2009;1(1):24–27. [Google Scholar]
- 47.Samuel S. O., Aboderin A. O., Akanbi A. A., Adegboro B., Smith S. I., Coker A. O. Campylobacter enteritis in Ilorin, Nigeria. East African Medical Journal. 2006;83(9):478–484. doi: 10.4314/eamj.v83i09.46770. [DOI] [PubMed] [Google Scholar]
- 48.Aboderin A. O., Smith S. I., Oyelese A. O., Onipede A. O., Zailini S. B., Coker A. O. Role of Campylobacter jejuni/coli in Ile-Ife, Nigeria. East African Medical Journal. 2002;79(8):423–426. doi: 10.4314/eamj.v79i8.8829. [DOI] [PubMed] [Google Scholar]
- 49.Langendorf C., Le Hello S., Moumouni A., et al. Enteric bacterial pathogens in children with diarrhea in Niger: diversity and antimicrobial resistance. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120275.e0120275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandomando I. M., Alonso P. L., Macete E. V., et al. Etiology of diarrhea in children younger than 5 years of age admitted in a rural hospital of southern Mozambique. The American Journal of Tropical Medicine and Hygiene. 2007;76(3):522–527. doi: 10.4269/ajtmh.2007.76.522. [DOI] [PubMed] [Google Scholar]
- 51.Yongsi H. B. N. Pathogenic microorganisms associated with childhood diarrhea in low-and-middle income countries: case study of Yaoundé-Cameroon. International Journal of Environmental Research and Public Health. 2008;5(4):213–229. doi: 10.3390/ijerph5040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusiluka L. J. M., Karimuribo E. D., Mdegela R. H., et al. Prevalence and impact of water-borne zoonotic pathogens in water, cattle and humans in selected villages in Dodoma rural and Bagamoyo districts, Tanzania. Physics and Chemistry of the Earth, Parts A/B/C. 2005;30(11–16):818–825. doi: 10.1016/j.pce.2005.08.025. [DOI] [Google Scholar]
- 53.Mdegela R. H., Nonga H. E., Ngowi H. A., Kazwala R. R. Prevalence of thermophilic Campylobacter infections in humans, chickens and crows in Morogoro, Tanzania. Journal of Veterinary Medicine Series B. 2006;53(3):116–121. doi: 10.1111/j.1439-0450.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 54.Jacob P., Mdegela R. H., Nonga H. E. Comparison of Cape Town and Skirrow’s Campylobacter isolation protocols in humans and broilers in Morogoro, Tanzania. Tropical Animal Health and Production. 2011;43(5):1007–1013. doi: 10.1007/s11250-011-9799-z. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro R. L., Kumar L., Phillips-Howard P., et al. Antimicrobial-resistant bacterial diarrhea in rural western Kenya. The Journal of Infectious Diseases. 2001;183(11):1701–1704. doi: 10.1086/320710. [DOI] [PubMed] [Google Scholar]
- 56.Brooks J. T., Ochieng J. B., Kumar L., et al. Surveillance for bacterial diarrhea and antimicrobial resistance in rural western Kenya, 1997–2003. Clinical Infectious Diseases. 2006;43(4):393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 57.Sangaré L., Nikiéma A. K., Zimmermann S., et al. Campylobacter spp. epidemiology and antimicrobial susceptibility in a developing country, Burkina Faso (West Africa) African Journal of Clinical and Experimental Microbiology. 2012;13(2):110–117. doi: 10.4314/ajcem.v13i2.9. [DOI] [Google Scholar]
- 58.Beyene G., Haile-Amlak A. Antimicrobial sensitivity pattern of Campylobacter species among children in Jimma university specialized hospital, Southwest Ethiopia. Ethiopian Journal of Health Development. 2004;18(3):185–189. doi: 10.4314/ejhd.v18i3.9958. [DOI] [Google Scholar]
- 59.Ewnetu D., Mihret A. Prevalence and antimicrobial resistance of Campylobacter isolates from humans and chickens in bahir dar, Ethiopia. Foodborne Pathogens and Disease. 2010;7(6):667–670. doi: 10.1089/fpd.2009.0433. [DOI] [PubMed] [Google Scholar]
- 60.Gwimi P. B., Faleke O. O., Salihu M. D., et al. Prevalence of Campylobacter species in fecal samples of pigs and humans from Zuru Kebbi State, Nigeria. International Journal of One Health. 2015;1:1–5. doi: 10.14202/ijoh.2015.1-5. [DOI] [Google Scholar]
- 61.Karikari A. B., Obiri-Danso K., Frimpong E. H., Krogfelt K. A. Antibiotic resistance in Campylobacter isolated from patients with gastroenteritis in a teaching hospital in Ghana. Open Journal of Medical Microbiology. 2017;7(1):1–11. doi: 10.4236/ojmm.2017.71001. [DOI] [Google Scholar]
- 62.Mason J., Iturriza-Gomara M., O’Brien S. J., et al. Campylobacter infection in children in Malawi is common and is frequently associated with enteric virus co-infections. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059663.e59663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samie A., Ramalivhana J., Igumbor E. O., Obi C. L. Prevalence, haemolytic and haemagglutination activities and antibiotic susceptibility profiles of Campylobacter spp. isolated from human diarrhoeal stools in Vhembe district, South Africa. Journal of Health, Population and Nutrition. 2007;25(4):406–413. [PMC free article] [PubMed] [Google Scholar]
- 64.Kashoma I. P., Kassem I. I., Kumar A., et al. Antimicrobial resistance and genotypic diversity of Campylobacter isolated from pigs, dairy, and beef cattle in Tanzania. Frontiers in Microbiology. 2015;6:1–11. doi: 10.3389/fmicb.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ngulukun S. S., Oboegbulem S. I., Fagbamila I. O., Bertu W., Odugbo M. O. Prevalence and molecular characterization of thermophilic Campylobacter species isolated from cattle in Plateau State, Nigeria. Nigerian Veterinary Journal. 2011;32(4):349–356. [Google Scholar]
- 66.Salihu M. D., Abdulkadir J. U., Oboegbulem S. I., et al. Isolation and prevalence of Campylobacter species in cattle from Sokoto State, Nigeria. Veterinaria Italiana. 2009;45(4):501–505. [PubMed] [Google Scholar]
- 67.E Nonga H., Sells P., Karimuribo E. D. Occurrences of thermophilic Campylobacter in cattle slaughtered at Morogoro municipal abattoir, Tanzania. Tropical Animal Health and Production. 2010;42(1):73–78. doi: 10.1007/s11250-009-9387-7. [DOI] [PubMed] [Google Scholar]
- 68.Karikari A. B., Obiri-Danso K., Frimpong E. H., Krogfelt K. A. Antibiotic resistance of Campylobacter recovered from faeces and carcasses of healthy livestock. BioMed Research International. 2017;2017:9. doi: 10.1155/2017/4091856.4091856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kassa T., Gebre-selassie S., Asrat D. The prevalence of thermotolerant Campylobacter species in food animals in Jimma Zone, Southwest Ethiopia. Ethiopian Journal of Health Development. 2005;19(3):225–229. doi: 10.4314/ejhd.v19i3.10002. [DOI] [Google Scholar]
- 70.Abamecha A., Assebe G., Tafa B., Wondafrash B. Prevalence of thermophilic Campylobacter and their antimicrobial resistance profile in food animals in Lare District, Nuer Zone, Gambella, Ethiopia. Journal of Drug Research and Development. 2016;1(2) doi: 10.16966/2470-1009.108. [DOI] [Google Scholar]
- 71.Achá S., Kühn I., Jonsson P., Mbazima G., Katouli M., Möllby R. Studies on calf diarrhoea in Mozambique: prevalence of bacterial pathogens. Acta Veterinaria Scandinavica. 2004;45(1):27–36. doi: 10.1186/1751-0147-45-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dadi L., Asrat D. Prevalence and antimicrobial susceptibility profiles of thermotolerant Campylobacter strains in retail raw meat products in Ethiopia. Ethiopian Journal of Health Development. 2008;22(2):195–200. doi: 10.4314/ejhd.v22i2.10072. [DOI] [Google Scholar]
- 73.Osano O., Arimi S. M. Retail poultry and beef as sources of Campylobacter jejuni. East African Medical Journal. 1999;76(76):141–143. [PubMed] [Google Scholar]
- 74.Kashoma I. P., Kassem I. I., John J., et al. Prevalence and antimicrobial resistance of Campylobacter isolated from dressed beef carcasses and raw milk in Tanzania. Microbial Drug Resistance. 2016;22(1):40–52. doi: 10.1089/mdr.2015.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komba E. V. G., Mdegela R. H., Msoffe P. L. M., Matowo D. E., Maro M. J. Occurrence, species distribution and antimicrobial resistance of thermophilic Campylobacter isolates from farm and laboratory animals in Morogoro, Tanzania. Veterinary World. 2014;7(8):559–565. doi: 10.14202/vetworld.2014.559-565. [DOI] [Google Scholar]
- 76.Woldemariam T., Asrat D., Zewde G. Prevalence of thermophilic Campylobacter species in carcasses from sheep and goats in an abattoir in Debre Zeit area, Ethiopia. Ethiopian Journal of Health Development. 2009;23(3):229–233. doi: 10.4314/ejhd.v23i3.53245. [DOI] [Google Scholar]
- 77.Mdegela R. H., Laurence K., Jacob P., Nonga H. E. Occurrences of thermophilic Campylobacter in pigs slaughtered at Morogoro slaughter slabs, Tanzania. Tropical Animal Health and Production. 2011;43(1):83–87. doi: 10.1007/s11250-010-9657-4. [DOI] [PubMed] [Google Scholar]
- 78.Jonker A., Picard J. A. Antimicrobial susceptibility in thermophilic Campylobacter species isolated from pigs and chickens in South Africa. Journal of the South African Veterinary Association. 2010;81(4):228–236. doi: 10.4102/jsava.v81i4.153. [DOI] [PubMed] [Google Scholar]
- 79.Kagambèga A., Thibodeau A., Trinetta V., et al. Salmonella spp. and Campylobacter spp. in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Science & Nutrition. Jul. 2018;6(6):1601–1606. doi: 10.1002/fsn3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bester L. A., Essack S. Y. Prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry suppliers in KwaZulu-Natal, South Africa. Journal of Antimicrobial Chemotherapy. 2008;62(6):1298–1300. doi: 10.1093/jac/dkn408. [DOI] [PubMed] [Google Scholar]
- 81.Bester L. A., Essack S. Y. Observational study of the prevalence and antibiotic resistance of Campylobacter spp. from different poultry production systems in KwaZulu-Natal, South Africa. Journal of Food Protection. 2012;75(1):154–159. doi: 10.4315/0362-028x.jfp-11-237. [DOI] [PubMed] [Google Scholar]
- 82.Gblossi Bernadette G., Eric Essoh A., Elise Solange K.-N. G., et al. Prevalence and antimicrobial resistance of thermophilic Campylobacter isolated from chicken in côte d’Ivoire. International Journal of Microbiology. 2012;2012:5. doi: 10.1155/2012/150612.150612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.A Mpalang R. K., Boreux R., Melin P., Akir Ni Bitiang K. M., Daube G., De Mol P. Prevalence of Campylobacter among goats and retail goat meat in Congo. The Journal of Infection in Developing Countries. 2014;8(2):168–175. doi: 10.3855/jidc.3199. [DOI] [PubMed] [Google Scholar]
- 84.Salihu M. D., Magaji A. A., Abdulkadir J. U., Kolawale A. Survey of thermophilic Campylobacter species in cats and dogs in North‐Western Nigeria. Veterinaria Italiana. 2010;46:p. 6. [PubMed] [Google Scholar]
- 85.Karshima N. S., Benshak J. A., Bata S. I., Barde I. J., Aaron S. Molecular characterization of thermophilic Campylobacter species isolated from companion dogs presented to a veterinary facility in Jos, Nigeria. Tropical Veterinarian. 2014;32(1-2):17–27. [Google Scholar]
- 86.Nonga H. E., Muhairwa A. P. Prevalence and antibiotic susceptibility of thermophilic Campylobacter isolates from free range domestic duck (Cairina moschata) in Morogoro municipality, Tanzania. Tropical Animal Health and Production. 2010;42(2):165–172. doi: 10.1007/s11250-009-9401-0. [DOI] [PubMed] [Google Scholar]
- 87.Moré E., Ayats T., Ryan P. G., et al. Seabirds (Laridae) as a source of Campylobacter spp., Salmonella spp. and antimicrobial resistance in South Africa. Environmental Microbiology. 2017;19(10):4164–4176. doi: 10.1111/1462-2920.13874. [DOI] [PubMed] [Google Scholar]
- 88.Ngulukun S. S., Oboegbulem S. I., Fagbamila I. O., et al. Isolation of thermophilic Campylobacter species from Japanese quails (Coturnix coturnix) in Vom, Nigeria. Veterinary Record. 2010;166(5):147–148. doi: 10.1136/vr.b4787. [DOI] [PubMed] [Google Scholar]
- 89.Kashoma I. P., Kumar A., Sanad Y. M., et al. Phenotypic and genotypic diversity of thermophilic Campylobacter spp. in commercial Turkey flocks: a longitudinal study. Foodborne Pathogens and Disease. 2014;11(11):850–860. doi: 10.1089/fpd.2014.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szczepanska B., Andrzejewska M., Spica D., Klawe J. J. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiology. 2017;17(1) doi: 10.1186/s12866-017-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang Y.-S., Cho Y.-S., Yoon S.-K., et al. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw chicken meat and human stools in Korea. Journal of Food Protection. 2006;69(12):2915–2923. doi: 10.4315/0362-028X-69.12.2915. [DOI] [PubMed] [Google Scholar]
- 92.Kendall M. E., Crim S., Fullerton K., et al. Travel-associated enteric infections diagnosed after return to the United States, foodborne diseases active surveillance network (FoodNet), 2004–2009. Clinical Infectious Diseases. 2012;54(5):S480–S487. doi: 10.1093/cid/cis052. [DOI] [PubMed] [Google Scholar]
- 93.Evans M. R., Ribeiro C. D., Salmon R. L. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerging Infectious Diseases. 2003;9(10):1219–1225. doi: 10.3201/eid0910.020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Padungtod P., Kaneene J. B. Campylobacter in food animals and humans in Northern Thailand. Journal of Food Protection. 2005;68(12):2519–2526. doi: 10.4315/0362-028x-68.12.2519. [DOI] [PubMed] [Google Scholar]
- 95.Kottawatta K., Van Bergen M., Abeynayake P., Wagenaar J., Veldman K., Kalupahana R. Campylobacter in broiler chicken and broiler meat in Sri Lanka: influence of semi-automated vs. wet market processing on Campylobacter contamination of broiler neck skin samples. Foods. 2017;6(12):p. 105. doi: 10.3390/foods6120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carrique-Mas J. J., Bryant J. E., Cuong N. V., et al. An epidemiological investigation of Campylobacter in pig and poultry farms in the Mekong Delta of Vietnam. Epidemiology and Infection. 2014;142(7):1425–1436. doi: 10.1017/s0950268813002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosef O., Gondrosen B., Kapperud G., Underdal B. Isolation and characterization of Campylobacter jejuni and Campylobacter coli from domestic and wild mammals in Norway. Applied and Environmental Microbiology. 1983;46(4):855–859. doi: 10.1128/aem.46.4.855-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oosterom J., Dekker R., de Wilde G. J. A., van Kempende Troye F., Engels G. B. Prevalence of Campylobacter jejuni and Salmonella during pig slaughtering. Veterinary Quarterly. 1985;7(1):31–34. doi: 10.1080/01652176.1985.9693950. [DOI] [PubMed] [Google Scholar]
- 99.Adesiyun A. A., Kaminjolo J. S., Loregnard R., Kitson-Piggott W. Campylobacter infections in calves, piglets, lambs and kids in Trinidad. British Veterinary Journal. 1992;148(6):547–556. doi: 10.1016/0007-1935(92)90011-o. [DOI] [PubMed] [Google Scholar]
- 100.Schilling A.-K., Hotzel H., Methner U., et al. Zoonotic agents in small ruminants kept on city farms in Southern Germany. Applied and Environmental Microbiology. 2012;78(11):3785–3793. doi: 10.1128/aem.07802-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanad Y. M., Kassem I. I., Abley M., Gebreyes W., LeJeune J. T., Rajashekara G. Genotypic and phenotypic properties of cattle-associated Campylobacter and their implications to public health in the USA. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025778.e25778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khoshbakht R., Tabatabaei M., Hoseinzadeh S., Raeisi M., Aski H. S., Berizi E. Prevalence and antibiotic resistance profile of thermophilic Campylobacter spp. of slaughtered cattle and sheep in Shiraz, Iran. Veterinary Research Forum. 2016;7(3):241–246. [PMC free article] [PubMed] [Google Scholar]
- 103.Hoar B. R., Atwill E. R., Elmi C., Farver T. B. An examination of risk factors associated with beef cattle shedding pathogens of potential zoonotic concern. Epidemiology and Infection. 2001;127(1):147–155. doi: 10.1017/s0950268801005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wieczorek K., Denis E., Lynch O., Osek J. Molecular characterization and antibiotic resistance profiling of Campylobacter isolated from cattle in polish slaughterhouses. Food Microbiology. 2013;34(1):130–136. doi: 10.1016/j.fm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 105.Prescott J. F., Bruin-Mosch C. W. Carriage of Campylobacter jejuni in healthy and diarrheic animals. American Journal of Veterinary Research. 1981;42(1):164–165. [PubMed] [Google Scholar]
- 106.Whyte P., McGill K., Collins J. D. An assessment of steam pasteurization and hot water immersion treatments for the microbiological decontamination of broiler carcasses. Food Microbiology. 2003;20(1):111–117. doi: 10.1016/s0740-0020(02)00084-9. [DOI] [Google Scholar]
- 107.Pezzotti G., Serafin A., Luzzi I., Mioni R., Milan M., Perin R. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. International Journal of Food Microbiology. 2003;82(3):281–287. doi: 10.1016/s0168-1605(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 108.Mayrhofer S., Paulsen P., Smulders F. J. M., Hilbert F. Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultry. International Journal of Food Microbiology. 2004;97(1):23–29. doi: 10.1016/j.ijfoodmicro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Hussain I., Shahid Mahmood M., Akhtar M., Khan A. Prevalence of Campylobacter species in meat, milk and other food commodities in Pakistan. Food Microbiology. 2007;24(3):219–222. doi: 10.1016/j.fm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Yang J.-R., Wu H.-S., Chiang C.-S., Mu J.-J. Pediatric campylobacteriosis in Northern Taiwan from 2003 to 2005. BMC Infectious Diseases. 2008;8:p. 151. doi: 10.1186/1471-2334-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Altekruse S. F., Stern N. J., Fields P. I., Swerdlow D. L. Campylobacter jejuni-an emerging foodborne pathogen. Emerging Infectious Diseases. 1999;5(1):28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lastovica A. J. Emerging Campylobacter spp.: the tip of the iceberg. Clinical Microbiology Newsletter. 2006;28(7):49–56. doi: 10.1016/j.clinmicnews.2006.03.004. [DOI] [Google Scholar]
- 113.Bessede E., Delcamp A., Sifre E., Buissonniere A., Megraud F. New methods for detection of Campylobacters in stool samples in comparison to culture. Journal of Clinical Microbiology. 2011;49(3):941–944. doi: 10.1128/JCM.01489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varela N. P., Friendship R. M., Dewey C. E. Prevalence of Campylobacter spp isolated from grower-finisher pigs in Ontario. The Canadian Veterinary Journal = La Revue Veterinaire Canadienne. 2007;48(48):515–517. [PMC free article] [PubMed] [Google Scholar]
- 115.Tadesse D. A., Bahnson P. B., Funk J. A., et al. Prevalence and antimicrobial resistance profile of Campylobacter spp. isolated from conventional and antimicrobial-free swine production systems from different U.S. regions. Foodborne Pathogens and Disease. 2011;8(3):367–374. doi: 10.1089/fpd.2010.0665. [DOI] [PubMed] [Google Scholar]
- 116.Jensen A. N., Andersen M. T., Dalsgaard A., Baggesen D. L., Nielsen E. M. Development of real-time PCR and hybridization methods for detection and identification of thermophilic Campylobacter spp. in pig faecal samples. Journal of Applied Microbiology. 2005;99(2):292–300. doi: 10.1111/j.1365-2672.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 117.Madden R. H., Moran L., Scates P. Optimising recovery of Campylobacter spp. from the lower porcine gastrointestinal tract. Journal of Microbiological Methods. 2000;42:115–119. doi: 10.1016/s0167-7012(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 118.Luber P., Wagner J., Hahn H., Bartelt E. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli strains isolated in 1991 and 2001-2002 from poultry and humans in Berlin, Germany. Antimicrobial Agents and Chemotherapy. 2003;47(12):3825–3830. doi: 10.1128/aac.47.12.3825-3830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lévesque S., Frost E., Michaud S. Comparison of antimicrobial resistance of Campylobacter jejuni isolated from humans, chickens, raw milk, and environmental water in Québec. Journal of Food Protection. 2007;70(3):729–735. doi: 10.4315/0362-028x-70.3.729. [DOI] [PubMed] [Google Scholar]
- 120.Signorini M. L., Rossler E., Díaz David D. C., et al. Antimicrobial resistance of thermotolerant Campylobacter species isolated from humans, food-producing animals, and products of animal origin: a worldwide meta-analysis. Microbial Drug Resistance. 2018;24(8):1174–1190. doi: 10.1089/mdr.2017.0310. [DOI] [PubMed] [Google Scholar]
- 121.Wieczorek K., Szewczyk R., Osek J. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter jejuni and C. coli isolated from retail raw meat in Poland. Veterinární Medicína. 2012;57(6):293–299. doi: 10.17221/6016-vetmed. [DOI] [Google Scholar]
- 122.Noormohamed A., Fakhr M. A higher prevalence rate of Campylobacter in retail beef livers compared to other beef and pork meat cuts. International Journal of Environmental Research and Public Health. 2013;10(5):2058–2068. doi: 10.3390/ijerph10052058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Payot S., Bolla J. M., Corcoran D., Fanning S., Mégraud F., Zhang Q. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes and Infection. 2006;8(7):1967–1971. doi: 10.1016/j.micinf.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 124.Chen X., Naren G.-W., Wu C.-M., et al. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Veterinary Microbiology. 2010;144(1-2):133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 125.Shin E., Lee Y. Characterization of erythromycin-resistant porcine isolates of Campylobacter coli. Microbial Drug Resistance. 2010;16(3):231–239. doi: 10.1089/mdr.2010.0039. [DOI] [PubMed] [Google Scholar]
- 126.Caprioli A. Monitoring of antibiotic resistance in bacteria of animal origin: epidemiological and microbiological methodologies. International Journal of Antimicrobial Agents. 2000;14(4):295–301. doi: 10.1016/S0924-8579(00)00140-0. [DOI] [PubMed] [Google Scholar]
- 127.Lehtopolku M., Kotilainen P., Puukka P., et al. Inaccuracy of the disk diffusion method compared with the agar dilution method for susceptibility testing of Campylobacter spp. Journal of Clinical Microbiology. 2012;50(1):52–56. doi: 10.1128/JCM.01090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]