Summary
Nutritional psychiatry is a growing area of research, with several nutritional factors implicated in the aetiology of psychiatric ill health. However, nutritional research is highly complex, with multiple potential factors involved, highly confounded exposures and small individual effect sizes. This paper considers whether Mendelian randomization provides a solution to these difficulties, by investigating causality in a low risk and low-cost way. Current studies using MR in nutritional psychiatry are reviewed, along with the potential opportunities and challenges of using this approach for investigating the causal effects of nutritional exposures. Several studies have identified potentially causal nutritional exposures using Mendelian randomisation in psychiatry, offering opportunities for further mechanistic research, intervention development, and replication. Using Mendelian randomisation as a foundation for intervention development facilitates the best use of resources in an emerging discipline in which opportunities are rich, but resources are often poor.
Keywords: Mendelian randomization, nutritional psychiatry, causality
Introduction
The founding of the International Society for Nutritional Psychiatry Research1 reflects an increasing recognition of nutrition as a modifiable risk factor for mental ill-health, and the need for good quality research in this area. Whilst the adverse psychological effects of severe nutritional deficiency are well established,2 the extent to which subtle nutritional factors might have on cognitive and affective processes, or on the increasing burden of psychological ill health at the population level remains unclear. As wholefood diets have been replaced by processed foods - high in sugar and low in essential fats, vitamins and minerals - many argue that subtle malnutrition may exist even in the presence of calorie-abundance,3, 4 with unclear repercussions for population mental health. 5, 6 Several meta-analyses of prospective studies suggest that a high-quality diet can reduce the risk of mental illness,5, 6 warranting further investigation of specific nutritional factors and mechanisms. Conventional epidemiological associations between nutritional intake or status and psychiatric outcomes are highly prone to confounding by lifestyle and correlated dietary factors.7 Furthermore, as many aspects of nutrition are affected by mental ill-health,8 it is likely that reverse causality, or at least a bi-directional relationship, explains some of these associations. Finally, as individual nutrients have small effect sizes, large sample sizes are required to explore such associations with adequate statistical power, in which accurate dietary measurement is difficult. Despite the best efforts of researchers to control for these limitations, nutritional epidemiology is limited by issues of residual confounding, biological complexities and limited power.
Interventional research in nutritional psychiatry is a potential solution to these limitations, as good quality randomized controlled trials (RCTs) eliminate issues of confounding and reverse causality. There are a growing number of RCTs in nutritional psychiatry. Although many studies have focused on individual nutritional supplements - probably reflecting the parallels with a pharmacological research model,9–11 there are few supplements that have been robustly identified as beneficial in psychiatry.11 Results are often inconsistent, and it is unclear which interventions are worth further investment. Given the complexities and inter-relatedness of dietary composition, a more comprehensive nutritional approach may be preferable. Combination micronutrient supplement interventions12–14 and interventions focused on making broader changes to dietary patterns might be advantageous.15, 16 Dietary pattern interventions offer a potential solution to this complexity, with supporting meta-analytical evidence in both observational5 and interventional research17. However, selecting the right intervention and participants, and accounting for behaviour change and attrition, make the planning and evaluation of such trials complex and costly. With a multitude of potential nutritional interventions, it can be difficult to prioritise the most likely to be effective. False negatives from underpowered designs or minor aberrations in a complex intervention, might hinder the development of potentially beneficial interventions. Conversely, false positives due to biased designs, compounded by publication bias, lead to wasted expenditure and potential harm in repeated trials. Further evidence to establish likely causality for specific nutritional factors to underpin nutritional interventions and identify the most likely beneficial components would prevent wasted time and expenditure.
This paper considers whether ‘Mendelian randomization’ is a viable method to inform intervention development in nutritional psychiatry, in a low-cost and low-risk way. We review existing Mendelian randomization studies in nutritional psychiatry, the challenges faced, and opportunities for further research.
Mendelian Randomization
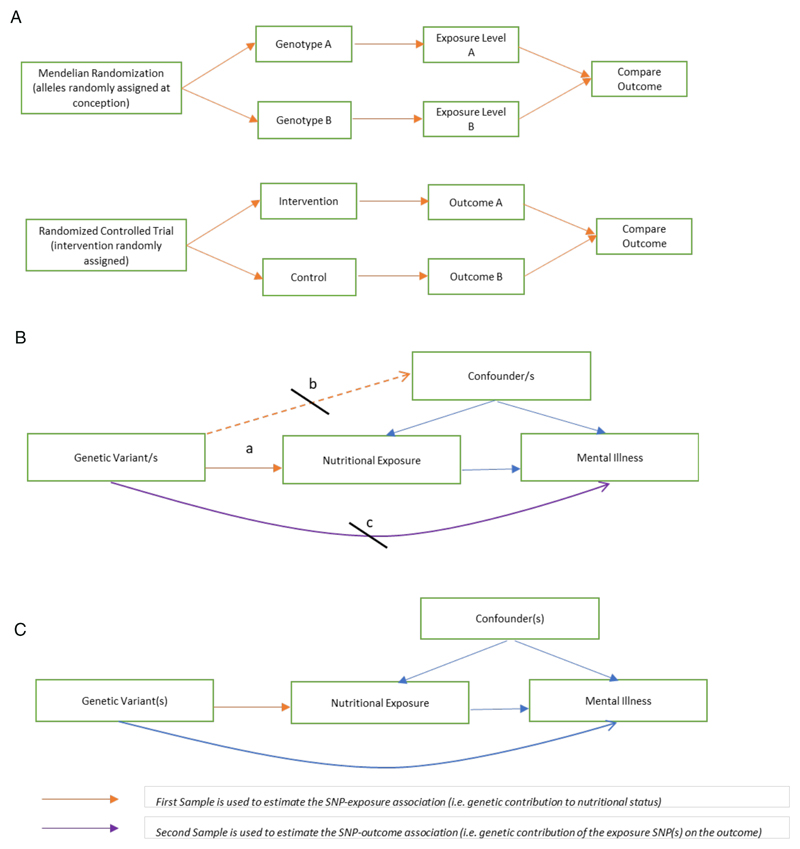
Mendelian randomization (MR) is a method that is increasingly used to infer causality in epidemiological research. MR uses genetic markers that are robustly associated with a particular potentially modifiable exposure as ‘instrumental variables’ in assessing the relationship between an exposure and an outcome.18, 19 As genetic markers (or ‘alleles’) are randomly allocated at conception, many have compared MR to a natural RCT, in which variant alleles rather than interventions, are randomized (figure 1a). The individual variations in genetic alleles are referred to as single nucleotide polymorphisms (SNPs). MR exploits this natural genetic variation to circumvent the problem of confounding and reverse causality (figure 1a).
Figure 1. Mendelian Randomization: comparisons and assumption.
1a Mendelian Randomization as a ‘natural’ Randomized Controlled Trial
MR has been compared to a randomized controlled trial, with random allocation of genetic alleles at conception could be considered analogous to random allocation of interventions in a trial.
1b Assumptions in Mendelian Randomization
MR assumes that the genetic variants are: a. associated with the exposure of interest; b. not associated with confounders; and c. only associated with the outcome through the exposure
1c Two-sample Mendelian Randomization
Two-sample MR takes estimates of the SNP-exposure association from one population (e.g. a nutritional exposure GWAS) and the SNP-outcomes association from a separate sample (e.g. a psychiatric outcome GWAS).
The concept of MR relies on key assumptions for validity (figure 1b). Whilst a comprehensive review of MR is beyond the scope of this review, some key terms used to describe aspects of MR studies relevant to this review are explained in Table 1. For more detail, see Zheng et al 201720 and the MR Dictionary.21
Table 1. Glossary of MR Terms and potential uses in nutritional psychiatry 20 For more information about other terms and the.
| Term | Explanation |
|---|---|
| F-Statistic | The F-statistic measures the strength of genetic instruments. F<10 is suggestive of weak instrument bias. |
| Multivariable MR | Multivariable MR is a technique to account for pleiotropy due to multiple correlated exposures. |
| MR-PheWAS (MR Phenome Wide Association Study) | MR PheWAS is a method using a hypothesis-free approach to scan many outcomes for a given exposure using MR methodology. Such approaches could be used to test for and identify any potential adverse off-target effects of dietary supplementation, providing genetic instruments exist. |
| Pleiotropy | Horizontal Pleiotropy is where the SNP or SNPs related to the exposure are associated with the outcome through a pathway independent of the exposure (i.e. a violation of assumption c in figure 1b). Pleiotropy can be demonstrated by several methods, including Cochran’s Q statistic testing heterogeneity in causal estimates from each SNP, MREgger intercept, and leave-one-out analysis to identify influential outliers |
| Population Stratification | Spurious associations may arise in MR where the genetic variant and the outcome are associated with ancestral background in a mixed or stratified sample. Using genetic associations from within homogenous populations, or checking that the GWAS has controlled for population substructure in the analysis is important. |
| One-sample MR | Conventional one-sample MR uses a single sample in which exposure, outcome and genetic instrument are measured within the same population. One-sample MR may have power issues due to inadequate sample sizes of studies that are required to have genotype, exposure and outcome data. |
| Two-Sample MR | The estimates of the SNP-exposure and SNP-outcome associations used in MR analyses are identified in independent studies (usually genome-wide association studies) |
| Two-Step/Mediation MR | Two-step MR can be used to identify mediating mechanisms between an exposure and outcome using two steps- the first to assess the causal effect of the exposure on the potential mediator, and the second to assess the causal effect of the mediator on the outcome |
There are potential benefits to applying MR methodology to nutritional psychiatry, as a cheap and powerful method for attributing causality to putative exposures, and it enables the exploration of multiple avenues for intervention development in a low-cost and low-risk way. This is particularly true with the development of two-sample MR, in which exposures and outcomes need not be measured in the same sample (figure 1c). Two-sample MR takes estimates of the SNP-exposure association from a one population (for example a genome-wide association study (GWAS) of a nutritional exposure), and the SNP-outcome association from another (for example, a GWAS of a given psychiatric outcome). This allows for the possibility of utilising the increasing sample sizes provided by large psychiatric genetic consortia, without the need to access individual-level data on specific nutritional measures. Given the relatively small effect sizes, and modest genetic contribution to nutritional exposures, a two-sample MR methodology using large outcome samples should provide adequate power to investigate them.
One advantage of MR is that, providing appropriate genetic instruments are available, it is theoretically possible to model the results of certain randomised trials, thereby reducing unnecessary potential harms and expenditure. One example in the context of nutritional epidemiology was given by a recent MR study to model the Selenium and Vitamin E Cancer Prevention Trial (SELECT) for prostate cancer, which was based on extensive epidemiological evidence at that time. The SELECT trial, randomised 35,533 men to use selenium supplementation, to investigate whether increasing selenium levels might prevent prostate cancer.22 The $114 million trial ended prematurely as results showed that selenium supplementation not only failed to reduce prostate cancer risk, it was likely to increase the risk of advanced prostate cancer and type 2 diabetes mellitus. These results were replicated by MR, using genetic instruments for circulating selenium in the PRACTICAL consortium.23 Although retrospective, the MR study took a fraction of the financial and time burden of a trial, and more importantly avoided any potential harm to participants.23
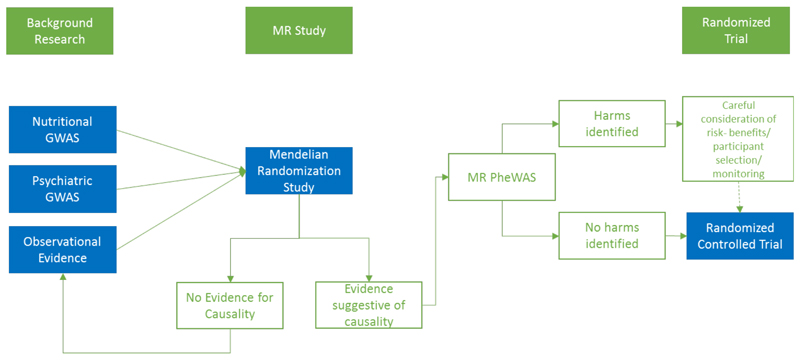
A comparison between MR and a naturalised RCT, has its limitations. Firstly, as genetic variants reflect lifetime exposures rather than short durations of therapeutic intervention, MR may produce a stronger effect than in the best approximation of a time-limited intervention. Conversely, individual adaptation to genotype may reduce the effect of the SNP on the exposure and so may underestimate the effect (also known as canalization (see table 1)). Rather than a replacement for RCTs, MR might be viewed as a foundation from which interventions for further development can be identified, in combination with epidemiology and basic science, also referred to as triangulation24 (figure 2).
Figure 2. A theoretical pipeline for the use of MR studies in intervention development.
Whilst many have compared MR to ‘nature’s RCT’, it may be more realistic to see MR studies as an interim step in intervention development.
MR may be particularly useful for a field such as nutritional psychiatry, in which many of the interventional trials have small to modest sample sizes. A well-powered MR study can be used to verify results in a potentially underpowered study, as well as to inform future studies. MR studies showing no evidence for a causal effect need careful consideration about whether it is possible to rule out a clinically significant effect based on the available parameters, and whether replication using updated background literature would be beneficial at a future date. This includes whether the methods and instruments are valid, power is adequate, and whether biological complexity might complicate results. This is particularly relevant in psychiatry, where diagnostic categorisation is yet to account for the diversity of symptoms and presentations categorized by a single ‘disorder’. Studies showing strong evidence for an effect need equal consideration before intervention development is considered, - such as how to increase the nutritional exposure in the desired way, whether participants are selected based on deficiency states, and whether supplementation might have potential adverse effects.
Mendelian Randomization Studies in Nutritional Psychiatry
We identified 26 studies using MR to investigate causality in nutritional psychiatry (Table 2). Many have investigated a single exposure or outcome, but some have investigated multiple exposures and outcomes within the same paper. The studies are broadly grouped into three main psychiatric outcomes - cognitive impairment and dementia, schizophrenia, and mood disorders.
Table 2. Studies using Mendelian randomization in nutritional psychiatry.
Table summarizes current MR studies in nutritional psychiatry. Discrepancies exist between disorders, and the applicability of existing instruments to other outcomes, or to a combined ‘cross disorder’ cohort may be fruitful. Results are given as odds ratios per standard deviation change in the exposure unless otherwise specified. Abbreviations: IGAP (International Genomics of Alzheimer’s), Psychiatric Genomics Consortium (PGC). For further details of instrument rsids, genes and beta coefficients please refer to the original publication.
| Exposure | Study | Measure | Sample | N | MR Method | SNPs | Results Reported OR / beta/ Hazard ratio/ Risk difference (95% confidence intervals) p-value | |
|---|---|---|---|---|---|---|---|---|
| VITAMINS | Vitamin D | Maddock 2017 29 | Global Cognitive tests | Cross cohort | 172,349 | Two-sample | 2 | β 0.00 points per 25(OH)D decreasing allele (0.01, 0.01) p>0.99 |
| Memory tests | β 0.00 points per 25(OH)D decreasing allele (-0.01, 0.01) p=0.6 | |||||||
| Jorde 2015 28 | Cognitive Tests | Tromso Study | 5,980 | One-sample | 4 | No overall association | ||
| Mokry 2016 25 | Alzheimer’s Diagnosis | IGAP1 | 54,162 | Two-sample | 4 | OR 0.8 per SD (0.97, 0.66) p=0.021 | ||
| Olsson 2017 27 | Dementia Diagnosis | Uppsala Longitudinal Study | 1,087 | One-sample | 2 | HR 1.04 points per effect allele (0.91, 1.19) | ||
| Cognitive Impairment (MMSE) | 408 | One-sample | 2 | OR 1.03 per effect allele (0.80, 1.34) | ||||
| Larsson 2018 26 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 7 | OR 0.86 per SD (0.78, 0.94) p = 0.002 | ||
| Taylor 2016 48 | Schizophrenia Diagnosis | PGC2 | 79,845 | Two-sample | 4 | OR 0.99 per 10% increase (0.97, 1.0) | ||
| Michaelsson 2018 52 | Major Depression Diagnosis | PGC | 173,005 | Two-sample | 6 | OR 1.02 per SD (0.97, 1.08) p = 0·44 | ||
| Vitamin E | Liu 2018 41 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 3 | OR 0·96 per SD (0·47,1·94) p =0·936 | |
| Vitamin B6 | Tomioka 2018 43 | Schizophrenia Diagnosis | Tokushima University Hospital | 10,689 | One-sample | 1 | OR 0·99 per SD log(B6) (0·65, 1·51) p= 0·96 | |
| Folate | Larsson 2017 32 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 2 | OR 0·98 per SD (0·72, 1·33) p=0·89 | |
| Mollehave 2017 50 | Depression (SLCR90_r3) | Health 2006 & Inter 99 | 4,126 | One-sample | 2 | OR 1·18 per effect allele (0·18, 7·66), P=0·86 | ||
| Homocysteine | Hu 2016 33 | Alzheimer’s Diagnosis | 34 studies | 9,397 | Two-Sample | 1 | OR 3·37 per SD (1·90, 5·95) p = 2·9×10-5 | |
| Larsson 2017 32 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 18 | OR 0·99 per SD (0·88, 1·11) 0·86 | ||
| Roostaei 2018 67 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 13 | OR 1·01 per SD (0·89, 1·15), p=0·84 | ||
| Numata 2015 44 | Schizophrenia Diagnosis | 36 Studies | 25,599 | Two-sample | 1 | OR 2·15 per SD (1·39, 3·32) p=5·3x10-4 | ||
| Kinoshita 201545 | Schizophrenia Diagnosis | Meta-analysis | 10,378 | One-sample | 1 | OR 1.14 per SD (1.03-1.27), p=1.6x10-2 | ||
| Wu 201735 | Vascular Dementia Diagnosis | Meta-analysis | 1,880 | Two-sample | 1 | OR 4·29 per SD log (hcy) (1·11,16·57) P = 0·03 | ||
| B12 | Mollehave 2017 50 | Depression (SLCR90_r4) | Health 2006 & Inter 99 | 4,126 | One-sample | 12 | 0·96 (0·52,1·79), P=0·91 | |
| Larsson 2018 32 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 7 | OR 1·11 per SD (0·95, 1·30) p=0·18 | ||
| MINERALS | Calcium | Cheng 201942 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 6 | OR 0·74 per SD (0·45, 1·22) p=0·23 |
| Major Depression Diagnosis | PGC | 10,640 | Two-sample | 6 | OR 0·92 per SD (0·67, 1·28) p=0·63 | |||
| Bipolar Disorder Diagnosis | PGC | 41,653 | Two-sample | 7 | OR 1·85 per SD (0·74, 4·65) p=0·19 | |||
| Schizophrenia Diagnosis | PGC | 65,967 | Two-sample | 7 | OR 1·85 per SD (0·74, 4·65) p=0·19 | |||
| Copper | Cheng 201942 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 2 | OR 0·87 per SD (0·75, 1·00) p=0·05 | |
| Bipolar Disorder Diagnosis | PGC | 41,653 | Two-sample | 2 | OR 0·87 per SD (0·79, 0·97) p=0·01 | |||
| Schizophrenia Diagnosis | PGC | 65,967 | Two-sample | 2 | OR 0·96 per SD (0·85, 1·08) p=0·47 | |||
| Magnesium | Cheng 201942 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 4 | OR 0·43 per SD (0·08-2·44) p=0·34 | |
| Major Depression Diagnosis | PGC | 10,640 | Two-sample | 3 | OR 1·19 per SD (0·22, 6·61) p=0·84 | |||
| Bipolar Disorder Diagnosis | PGC | 41,653 | Two-sample | 4 | OR 8·78 per SD (1·16, 66·26) p=0·04 | |||
| Schizophrenia Diagnosis | PGC | 65,967 | Two-sample | 4 | OR 0·87 per SD (0·24, 3·19) p=0·83 | |||
| Iron | Cheng 201942 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 11 | OR 1·02 per SD (0·94, 1·14) p=0·48 | |
| Major Depression Diagnosis | PGC | 10,640 | Two-sample | 9 | OR 0·98 per SD (0·91, 1·05) p=0·60 | |||
| Bipolar Disorder Diagnosis | PGC | 41,653 | Two-sample | 11 | OR 1·17 per SD (0·89, 1·29) p=0·45 | |||
| Schizophrenia Diagnosis | PGC | 65,967 | Two-sample | 10 | OR 1·04 per SD (0·92, 1·18) p=0·55 | |||
| Zinc | Cheng 201942 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 2 | OR 0·99 per SD (0·85, 1·14) p=0·85 | |
| Major Depression Diagnosis | PGC | 10,640 | Two-sample | 2 | OR 0·99 per SD (0·95, 1·03) p=0·66 | |||
| Bipolar Disorder Diagnosis | PGC | 41,653 | Two-sample | 2 | OR 1·02 per SD (0·91, 1·14) p=0·70 | |||
| Schizophrenia Diagnosis | PGC | 65,967 | Two-sample | 2 | OR 0·94 per SD (0·86, 1·02) p=0·11 | |||
| LIPID FAT AND GLUCOSE HOMEOSTASIS | Isoleucine | Larsson 2017 36 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 4 | OR 1·35 per SD (1·08,1·69) p=0·007 |
| Leucine | 1 | OR 1·16 per SD (95% CI, 0·78–1·72) p=0·46 | ||||||
| Valine | 1 | OR 1·13 per SD (95% CI, 0·82–1·57 p=0·46 | ||||||
| Fasting Glucose | Weslowska 2017 53 | Depression (BDI) | Young Finns Study | 1,217 | One-Sample | 35 | -0·43 (-0·79, -0·07) p=0·02 | |
| Li 2018 46 | Schizophrenia Diagnosis | PGC | 77,096 | Two-sample | 30 | OR 0·84 per SD, (0·71,0·99) p=0·038 | ||
| BIO-X | 26,026 | 14 | OR 1·04 per SD (0·84,1·27) p=0·737 | |||||
| Ostegaard 201540 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 36 | OR 1·12 per SD (0·97, 1·30) p=0·112 | ||
| Fasting insulin and insulin sensitivity | Ostegaard 201540 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 10 | OR 1·32 per SD (0·88, 1·98) p=0.18 | |
| Walter 2016 68 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 9 | OR 1·17 per unit (1·02,1·34) p=0.02 | ||
| Li 2018 46 | Schizophrenia Diagnosis | PGC | 77,096 | Two-sample | 13 | OR 2·33 per SD (1·40, 3·90) p=0.001 | ||
| DHA (Omega 3) | Sallis 2014 51 | Perinatal Depression (EPDS) | ALSPAC mothers | 2,378 | One-sample | 4 | RD 0·08 (-0·05, 0·22) p=0·21 | |
| Plasma APOE | Rasmussen37 | Alzheimer’s Diagnosis | Copenhagen General Population Study and Copenhagen City Heart Study | 106,562 | One-sample | 5 | OR 1.41 per mg/dL (1.27, 1.57) | |
| All Dementia | OR 1.33 per mg/dL (1.25, 1.43) | |||||||
| Cholesterol & Triglycerides | Proitsi 2014 39 | Alzheimer’s Diagnosis | Cross Cohort | 10,578 | Two-Sample | 70 | OR 0·95 per unit (0·76,1·21) p=0·69 Total Cholesterol | |
| 40 | OR 1·10 per unit (0·89,1·37) p=0·36 Triglycerides | |||||||
| 69 | 1·01 per unit (0·82,1·24) p=0·96 HDL-c | |||||||
| 55 | 0·90 per unit (0·65,1·25) p=0·53 LDL-c | |||||||
| Ostergaard 201540 | Alzheimer’s Diagnosis | IGAP | 54,162 | Two-sample | 73 | OR 1·94 per SD (1·79-2·10) p=3·1x10-56 Total Cholesterol | ||
| 39 | OR 0·96 per SD (0·87,1·07) p=0·48 Triglycerides | |||||||
| 71 | OR 0·75 per SD (0·69, 0·82) p=1x10-11 HDL-c | |||||||
| 57 | OR 2·31 per SD (2·12, 2·50) p=3x10-87 LDL-c | |||||||
| Benn 201738 | Alzheimer’s Diagnosis | Copenhagen General Population Study and Copenhagen City Heart Study | 111,194 | One-sample | 380 | OR 0·57 per mmolL-1 (0·27, 1·17) LDL-c | ||
| Vascular Dementia | OR 0·81 per mmolL-1 (0·34, 1·89) LDL-c | |||||||
| All Dementia | OR 0·66 per mmolL-1 (0·34, 1·26) LDL-c | |||||||
| Khandaker 201954 | Major Depression | UK Biobank | 367,703 | Two-sample | 76 | OR 1.02 per SD (0.91–1.14) LDL-c | ||
| 86 | OR 0.97 per SD (0.91–1.03) HDL-c | |||||||
| 51 | OR 1.18 per SD (1.09–1.27) Triglycerides | |||||||
IGAP International Genomics of Alzheimer’s Project
PGC Psychiatric Genomics Consortium
SLCR90_r diagnosis depression…
SLCR90_r diagnosis depression…
Dementia and Cognition
We identified 17 studies using MR to investigate the causality of nutritional factors on dementia and cognitive outcomes. Evidence suggesting a protective effect of 25-hydroxyvitamin D (25(OH)D) in Alzheimer’s disease has been shown in two studies in the International Genomics of Alzheimer’s Project (IGAP) Cohort (OR 0·86 per SD increase in vitamin D, 95% CI 0·78 to 0·94),25, 26 but not replicated in the Uppsala Longitudinal Study (Hazard ratio per allele 1.04, 95% CI 0.91 to 1.19).27 Studies investigating 25(OH)D as a causal factor in cognitive function have found no evidence for an association.27–29 It may be that vitamin D is particularly relevant to Alzheimer’s pathology, or that larger sample size or stronger genetic instruments are required to identify the effects in non-clinical population samples. Furthermore, a possible non-linear observational association between vitamin D and cognition, with both deficiency and excess associated with poor cognition, was noted by Maddock et al 2017.29 This raises important considerations about the ability of traditional MR techniques to detect causality for cognitive outcomes,29 as well as other associations in which a similar relationship has been noted.30 Novel methods are being developed to manage non-linearity in MR,31 but are not commonly employed.
Studies investigating the causal role for B vitamin pathways in dementia have had mixed results. A study looking at multiple exposures using the IGAP cohort did not provide evidence for folate (OR 0·98 per SD, 95% CI 0·72 to 1·33), homocysteine (OR 0·99 per SD, 95% CI 0·88 to 1·11) or vitamin B12 (OR 1·11 per SD, 95% CI 0·95 to 1·30) in Alzheimer’s disease.32 However, previous studies looking at homocysteine using a single SNP in the methylenetetrahydrofolate reductase (MTHFR) gene have suggested strong evidence of causality.33 The MTHFR gene produces an enzyme which activates folate to metabolise homocysteine, and SNPs in this gene have been identified in GWAS of both homocysteine and circulating folate levels. However, some have suggested caution in the use of the MTHFR gene for MR due to a complex interaction with folate intake, in which the same polymorphism leading to reduced enzymatic activity in low-intake states (and therefore low blood folate and high homocysteine), may not have any effect on blood folate or high homocysteine in high-intake states.34 Several MR studies of homocysteine using a single SNP relating to MTHFR have failed to replicate using instruments containing more SNPs and explaining a greater variation in homocysteine levels, suggesting that this SNP may be acting via a different mechanistic pathway. A meta-analysis of the results for homocysteine in Alzheimer’s disease using the different instruments suggests some causal evidence for homocysteine (pooled effect 1.34 per SD, 95% CI 1.03 to 1.66), but in light of the complex biology, this may be misleading. Another study investigating vascular dementia using the same single SNP in the MTHFR gene also showed strong causal evidence for homocysteine (OR 4·29 per SD log(homocysteine), 95% CI 1·11 to 16·57).35 However, the same caveats apply.
A single identified study has investigated amino acids in psychiatric disease, suggesting a potential causal role for isoleucine in Alzheimer’s disease (OR 1·35 per SD, 95% CI 1.08 to 1.69), though not for other branched chain amino acids such as valine and leucine.36
The established link between APOE genotype and Alzheimer’s has been corroborated using MR studies (OR 1.41 per mg/dL of APOE, 95% CI 1.27 to 1.57).37 Further exploration of the role of lipids in dementia have not shown evidence for a causal role for any specific lipid faction when the APOE SNPs are excluded from analysis.38, 39 MR studies investigating fasting glucose (OR 1·12 per SD, 95% CI 0·97 to 1·30),40 and vitamin E levels (OR 0·96 per SD, 95% CI 0·47 to 1·94),41 have not found any evidence for a causal association. A single study investigating minerals using several psychiatric outcomes including Alzheimer’s disease found no causal evidence for magnesium (0.43 per SD, 95% CI 0.08 to 2.44), calcium (Ca 0·74 per SD, 95% CI 0·45 to 1·22), Iron (1·02 per SD, 95% CI 0·94 to 1·14) or zinc (0·99 per SD, 95% CI 0·85 to 1·14), with weak evidence for low copper (0·87 per SD, 95% CI 0·75 to 1·00).42
Schizophrenia
We identified six studies that have investigated nutritional exposures in schizophrenia using MR. There was weak causal evidence for vitamin B6 (OR 0·99 per SD log(B6), 95% CI 0·65 to 1·51),43 and for serum minerals (Calcium, Serum Magnesium, Copper, Iron and Zinc, see Table 2) in schizophrenia.42 Two studies have identified an association between homocysteine and schizophrenia, in European (2·15 per SD, 95% CI 1·39 to 3·32)44 and Japanese populations (1.14 per SD, 95% CI 1.03 to 1.27);45 however, both used a single SNP related to the MTHFR gene, with the aforementioned limitations. A study looking at the causal role of glucose and insulin related traits found some evidence for fasting glucose (OR 0·84 per SD, 95% CI 0·71 to 0·99), but strong evidence for fasting insulin levels (OR 2·33 per SD, 95% CI 1·40 to 3·90).46 Given the discrepancy with the strength of effect of fasting glucose in the same study, it is likely that insulin partially acts through an independent pathway to glucose, possibly related to a direct action as a ‘neuropeptide’, involved in neuroplasticity and modulation.
In contrast to findings in multiple sclerosis47 and Alzheimer’s Disease,26, 27 no strong causal evidence has been found for vitamin D in schizophrenia (OR 0·99 per 10% increase in 25(OH)D, 95% CI 0·97 to 1·01).48 This may suggest that the observational estimate is the result of confounding or reverse causality, but it is also possible that standard MR techniques have been unable to detect a true causal association due to limited power, population stratification, or biological complexities (table 4). Although the power of the study appears more than adequate (example sample sizes based on MR power calculations are shown in table 3), diagnosis of schizophrenia is comparatively vague, and more subject to symptom interpretation that for an outcome such as multiple sclerosis or Alzheimer’s Disease. This heterogeneity may require larger sample sizes to identify causal effects of a similar magnitude. A second limitation is MR results represent the causal impact of a lifetime exposure on an outcome, it is unable to account for exposures that are time-limited or during a sensitive period.
Table 4. Limitations of MR 20.
| Limitation | Description | Relevance to Nutritional Psychiatry | Potential Solution |
|---|---|---|---|
| Lack of Available Instruments | Genetic instruments are unavailable for certain exposures | Lack of GWAS for certain nutritional exposures· Also due to poor measurement of particular nutritional exposures (e·g· serum versus intracellular magnesium). | Choose a proxy exposure for which data is available. Continue to review instruments as nutritional GWAS are published. |
| Weak instrument Bias | Genetic variants that are weakly associated with an exposure (e.g. F-statistic <10) will bias estimates towards the observational estimate in one-sample MR, and to the null in Two-sample MR | Weak instruments for nutritional exposures often result from limited sample sizes of pre-existing GWAS, as well as having a small proportion of variance explained by genetic variation. | Increase sample sizes (e·g· through publicly available GWAS datasets and consortia). Explain more variation in the exposure using allele scores. |
| Low Power | May be caused by small sample size, low variance explained in the exposure by the SNP, confounding and type 1 error rate. | Inadequate power may result in null results and hinder important further research. | Increase sample size or instrument strength where possible Power for one-sample MR can be calculated using free web application at http://cnsgenomics.com/shiny/mRnd/69 |
| Horizontal Pleiotropy | The association between the genetic variant and the outcome of interest goes through an alternative pathway to the exposure. | Violates a core assumption of MR (figure 1c). | Understand underlying biological function of genetic variants. Use variants directly coding for exposure of interest where possible. Use MR-Egger estimation. |
| Linkage Disequilibrium | Non-random allocation of alleles in close proximity during meiosis. | Confounding can be introduced by using an allele close to another allele, which affects the outcome of interest through another pathway. | Omit alleles in close genetic proximity to others. Utilise genetic alleles on separate chromosomes Use homogeneous populations where LD structures will be similar |
| Developmental Compensation (Canalization) | Individual adaptation to a genetic change, which reduces the phenotypic effect of the genetic change· | MR may produce causal estimates that are below the effect achieved by modifying the exposure. | The extent of the impact of canalization on MR is currently unclear. |
| Population Stratification | Spurious results may result from using mixed populations in which the genetic variant and outcome are associated with a particular genetic background. | Possible limitation of vitamin D in schizophrenia. | Use genetic associations derived from within homogenous populations only· Use summary results statistics that have adequately controlled for population substructure through e.g. principal components analysis or linear mixed models. |
| Biological Complexity | MR may give misleading results due to overly simplistic interpretation of complex biological pathways. | Several studies have suggested a non-linear association between vitamin D and various outcomes, but standard MR techniques are not able to detect this. Likewise, MR is unable to account for timelimited exposures or sensitive periods, such as intrauterine exposures and psychiatric outcomes. | Improved understanding of biological pathways. Use of novel methods to account for non-linear associations. |
Table 3. A rough guide to sample size requirements for MR studies.
An illustration of minimum sample sizes required for MR studies, taken from the online calculator available at http://cnsgenomics.com/shiny/mRnd/ 69 Results shown are for a binary outcome, assuming 25% cases in study, 0.8 power and alpha 0.05.
| Variance explained | Estimated Effect Size (OR) | Minimum Sample size |
|---|---|---|
| 1% | 1.01 | 42,069,473 |
| 1.1 | 439,015 | |
| 1.5 | 20,408 | |
| 2 | 5,756 | |
| 5% | 1.01 | 8,413,895 |
| 1.1 | 87,803 | |
| 1.5 | 4,082 | |
| 2 | 1,152 | |
For example, if the sensitive period for vitamin D deficiency is intrauterine, as suggested by the higher prevalence among winter-born individuals,49 an MR analysis would not reflect this. Finally, standard MR techniques assume a linear relationship between exposure and outcome, which in the case of vitamin D might be a fallacy, as both deficiency and excess states may be harmful.30 Standard MR techniques assume a linear association between the exposure and outcome, and whilst novel methods are being developed to overcome this limitation, they are not yet standard practice.31
Mood Disorders
Several nutritional factors have been investigated using MR in major depression samples, with no strong evidence of effect. Nutritional factors include vitamins B12 and folate,50 omega 3 fatty acids,51 and 25-hydroxyvitamin D.52 The five minerals investigated in Cheng 2019 did not show evidence of causality, though the sample used as the outcome is small (N=10,640) in comparison to the latest PGC Major Depression sample (N=807,553).42 An MR study using the Young Finns study 53 showed an inverse association between fasting glucose and depressive symptoms measured using the Beck Depression Inventory, (−0·43 BDI points per weighted effect allele, 95% CI −0·79 to 0·07), which the authors hypothesise to relate to the cognitive effects of hypoglycaemia. A study in UK Biobank suggested a potentially causal role for elevated triglycerides (but not LDL- or HDL-cholesterol) in the development of lifetime major depression (OR 1.18 per SD (1·09–1·27)).54
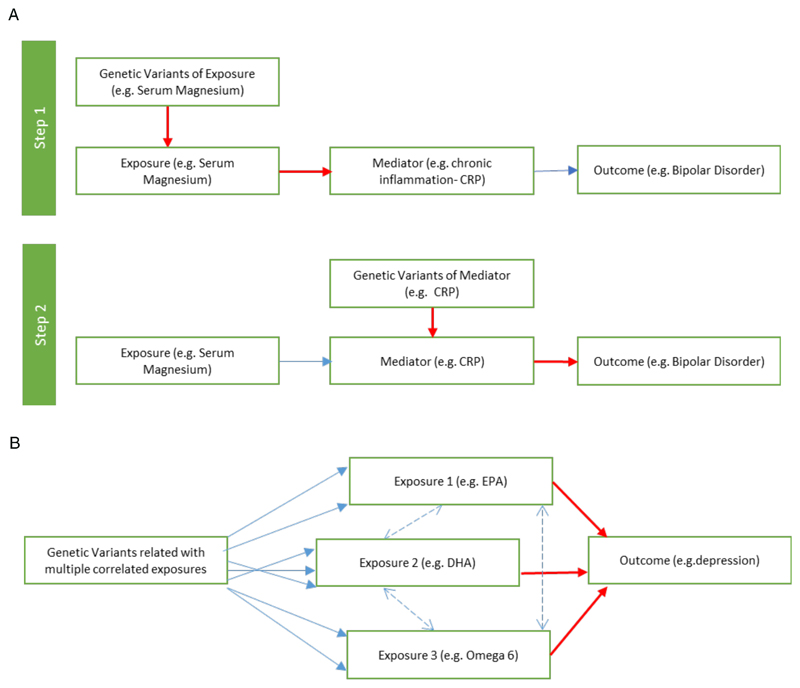
An MR study looking at multiple minerals identified a potentially causal role for low copper (OR 0·87 per SD, 95% CI 0·79 to 0·97) and for high serum magnesium (OR 8·78 per SD, 95% CI 1·16 to 66·26) in bipolar disorder using the Bipolar Disorder Working Group sample of the Psychiatric Genomics Consortium. Both findings warrant replication and further investigation. Some observational literature has suggested higher serum magnesium (though lower intracellular magnesium) levels in bipolar disorder, and the pathophysiological mechanisms behind this could be further explored using two-step MR (figure 3a).
Figure 3. Advanced MR methodologies.
3a Two-step Mendelian Randomization
Two-step MR can be used to identify mediating mechanisms between an exposure and outcome using separate MR analyses which are combined in a traditional mediation analysis.
3b Multivariable Mendelian Randomization
Multivariable MR can be used where genetic variants are related to multiple correlated exposures. For example, a SNP for one lipid will often be correlated with others. This technique could be used to untangle potentially opposing associations between omega 3(EPA/ DHA) and omega 6 fatty acids.
Opportunities for MR in Nutritional Psychiatry
Although one of the biggest challenges for MR in nutritional psychiatry to date has been the lack of appropriate genetic instruments, nutritional genetics is evolving. Instruments for many nutritional exposures are being utilised in MR studies outside psychiatry or applied to only one of a multitude of psychiatric outcomes. In addition to biological nutritional markers, GWAS of dietary intake,55 dietary patterns,56 and even gut microbial diversity,57 may provide useful potential instruments for future MR studies aiming to assess the impact of nutritional characteristics on psychiatric ill-health. For example, evidence suggests that gut microbial diversity and abundance is influenced by human genetics,57 making MR studies of this exposure possible, with examples of causal relationships being identified using MR in other areas of medicine.58 MR studies of the gut microbiome characterised in different ways may help to explain the association between reduced gut microbiome diversity and the presence of specific bacterial taxa in psychiatric disease,59 and the apparent benefits of probiotics in psychiatry.60, 61
MR methods are continually evolving (see table 1), with several techniques relevant to research in nutritional psychiatry. An example is multivariable MR (see figure 3b), which can be employed in situations where genetic variants are related to several correlated exposures. Multivariable MR has been used successfully in untangling the association between high density lipoprotein cholesterol, low density lipoprotein cholesterol, and triglycerides with cardiovascular disease62 and depression.54 Multivariable MR could similarly be used to unpick potentially complex associations, such as between omega 3 and 6 fatty acids, or B vitamin pathways, in psychiatry. For positive findings in nutritional psychiatry, potential off-target adverse effects of nutritional supplementation could be identified using MR phenome-wide association study (MR-PheWAS).63 An MR-PheWAS uses a hypothesis-free approach to scan many outcomes for a given exposure, and could have potentially pre-empted the increased risk of diabetes with selenium supplementation seen in the SELECT trial.23 As well as informing intervention development, MR can also be used to investigate biological mechanisms in psychiatry including metabolomic, microbiomic, proteomic and epigenomic intermediates, using two-step MR.64 Two-step MR is a relatively new method for identifying and quantifying mediating mechanisms between an exposure and outcome using an MR framework (figure 3a). Novel MR methods to analyse gene-environment interactions are also under development, and may be particularly useful in the context of nutritional psychiatry. Finally, using MR of the human proteome in relation to psychiatric outcomes may identify novel drug targets.
Standard MR methods rely on a single exposure-outcome framework, which many consider to be oversimplified when in the context of complex nutritional biology. Many nutritional epidemiologists have moved beyond a single nutrient approach to consider whole dietary patterns, adiposity, and the inherently complex interaction between diet, hormones and physical activity.65 It is possible that future MR methods could consider interactions between other nutritional exposures, as well as with gene-environment interactions considering nutritional intake or other lifestyle factors. Techniques such as machine learning and data mining using nutritional exposures, genetic data, dietary intake and psychiatric diagnoses and symptoms might be necessary for unpicking complex associations and gene-environment interactions further. Machine learning has already been suggested for augmenting MR, by predicting the most appropriate model to optimise power and detect pleiotropy, and could potentially enhance MR in the complex arena of nutritional psychiatry.66
Challenges for MR in Nutritional Psychiatry
With increasing availability of genetic instruments, genetic samples, and platforms for MR analysis, false results can be obtained quickly. Results need careful consideration as to validity of the methods, samples, and instruments used, irrespective of their strength or direction. Subsequent replication in independent cohorts remains crucial.19
Several limitations of traditional MR methods may hinder the application to nutritional psychiatry (see table 4). The lack of valid, robust genetic instruments for many nutritional exposures is arguably one of the most fundamental limitations. GWAS studies identifying SNPs robustly associated with nutritional exposures depend on adequately sized genotyped samples of nutritional factors. Difficulties identifying robust and reliable nutritional biomarkers reflecting nutritional status may underlie this, along with the availability of such nutritional measures in adequately sized genotyped cohorts. Instruments that are only weakly associated with the exposure of interest (e.g. F-statistic <10, see table 2) will bias estimates in different directions depending on whether a one-sample or two-sample methodology is used (table 4).
Nutritional genetic epidemiology is a developing field and the expectation is that good quality, validated instruments for nutritional exposures should emerge and evolve. However, even where genetic instruments appear to exist, some consideration needs to be given to whether they are valid for the specific association being tested with MR analyses, checking as far as possible that the assumptions of MR hold, and by understanding their underlying biological function.
With the increasing development of large psychiatric genomics consortia samples, outcome sample sizes are rapidly increasing. At first glance these appear to provide ample power to detect nutritional exposures, even those with a very small effect (see table 3.)19 However, as sample sizes increase, it is important to consider the extent to which the genetic heterogeneity of the population has increased, and the validity of the genetic instrument within this new population structure. Furthermore, the risk of overlapping exposure and outcome samples may invalidate some of the assumptions of two-sample MR. The relative benefits of using small samples with precisely measured nutritional exposures and psychiatric symptomatology, compared to large samples with imprecise measures and heterogenous samples are not always clearly defined.
Future Directions
Genetic epidemiology is evolving. Sample sizes, genetic markers and MR techniques are continuing to increase in both number and complexity. Negative early findings need careful consideration, and positive findings warrant replication in independent cohorts. As sample sizes and genetic instruments develop, formal repetition of earlier studies and independent replication remains essential. Given the relative ease with which analyses can be conducted once an instrument is identified, a more systematic and thorough approach to evaluating nutritional factors in psychiatry would be beneficial, perhaps considering individual psychiatric presentations along with a ‘cross-disorder’ approach. Opportunities for undertaking GWAS of nutritional biomarkers should be sought and validated, to make future MR studies possible. Future MR studies should consider novel MR techniques such as multivariable MR where appropriate, techniques for accounting for non-linear associations, as well as two-step MR to identify causal mechanisms. Further understanding of gene-environment interactions using large biobanks with data on genetics as well as nutritional and lifestyle measures might be useful for triangulating with nutritional MR studies. Finally, as the research landscape evolves, replication of earlier studies using larger samples and improved genetic instruments, continues to be of value.
Beyond genetics, ongoing research from a broad range of disciplines including epidemiology, basic sciences, and clinical trials is needed to identify novel biomarkers of nutritional intake and status, to develop new technologies for accurate dietary assessment, and to apply the results of MR studies to inform and conduct large-scale pragmatic trials.
Conclusion
Nutritional psychiatry, nutritional genetic epidemiology and psychiatric genetics are all at relatively early stages in their understanding. MR in nutritional psychiatry sits at the centre of these emerging disciplines, providing a unique way to investigate causality in nutritional psychiatry and understand its mechanisms. Despite some challenges in this area, emerging MR evidence for nutritional factors including vitamin D, folate, serum magnesium, copper, triglycerides, and glucose metabolic pathways on psychiatric outcomes highlight the potential utility of this technique for identifying causal factors in nutritional psychiatry and developing a firm evidence base for the causality of nutritional exposures from which successful interventions can develop.
Search Strategy and Selection Criteria
References for this review were identified through systematic searches of OVID Medline (1946 to January 2019,) PsycINFO (1808 to January 2019) and EMBASE (1974-2019) database for articles published from by use of the terms “Mendelian randomization”, “Psychiatry OR Psychology”, and other diagnostic terms (see Appendix 1). All abstracts identified were screened to include any exposure related to nutrition. Exposures were included if they measured any factor that was directly related to nutritional components or nutritional status, including micronutrients (including vitamins and minerals), macronutrients (including glucose homeostatic markers, amino acids and peptides and lipids), and biological markers of nutritional status. These factors were not selected a priori, but identified post-hoc based on the MR exposures available. Studies using psychiatric diagnosis as an exposure rather than outcome, addressing broader lifestyle exposures such as body mass index, physical activity or alcohol, and considered inter-generational exposures (such as offspring outcomes of pregnancy exposures) were excluded. A full search strategy is given in Appendix 1, with a flowchart of included studies in Appendix 2. No exclusions were made on the basis of language.
Supplementary Material
Research in Context.
Evidence before this study
Nutritional psychiatry is an emerging area of research, but its complexities are numerous. Several nutritional factors have been implicated in psychiatric aetiology, but causal evidence remains scarce. Mendelian randomization (MR) is an epidemiological method that can help investigate causality. Outside of psychiatry, MR has identified likely causal associations between low vitamin D and multiple sclerosis, low serum iron and Parkinson’s disease, and low serum magnesium and cardiovascular disease. We searched the OVID Medline database for studies using “Mendelian randomization” with any outcome related to “Psychiatry OR Psychology”. We excluded studies in which psychiatric conditions were used as an exposure rather than outcome, which used broader lifestyle exposures such as body mass index, physical activity or alcohol, and for which the exposure and outcome was inter-generational (such as offspring outcomes of pregnancy exposures).
Added value of this study
Several studies have investigated potential causal nutritional factors in psychiatry using MR. This study summarizes the current evidence and explores the opportunities and challenges in using this method to underpin intervention development. This paper also summarises some of the novel methods in MR, and how they might overcome issues with correlated nutritional exposures, non-linear effects, and to identify potential harms of supplementation.
Implications of all the available evidence
Several MR studies have shown evidence for causal nutritional factors in psychiatry. A comprehensive approach to investigating nutritional exposures psychiatry would be beneficial for the current evidence base and would help to inform intervention development in a resource-constrained field. It is important to consider the validity of findings irrespective of the direction or strength of evidence, and to replicate results as new samples, methods and biological insights become available.
Footnotes
Conflict of Interest
RC was funded by a Wellcome Trust GW4 CAT Fellowship (grant number WT 203918/Z/16/Z), during the conduct of the study. The other authors declare no conflicts of interest.
Contributors
RC was primary author and undertook the literature search. JZ, KW, HS and HJ provided help and training in MR methodology. All other authors contributed through advice on content and editing drafts. All authors had approval of final draft for submission.
References
- 1.Sarris J, Logan AC, Akbaraly TN, Paul Amminger G, Balanza-Martinez V, Freeman MP, et al. International Society for Nutritional Psychiatry Research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. 2015;14(3):370–1. doi: 10.1002/wps.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobey JA. The Biology of Human Starvation. Am J Public Health N. 1951;41(2):236–7. [Google Scholar]
- 3.Monteiro CA, Levy RB, Claro RM, de Castro IRR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 2011;14(1):5–13. doi: 10.1017/S1368980010003241. [DOI] [PubMed] [Google Scholar]
- 4.Sarris J. Nutritional Psychiatry: From Concept to the Clinic. Drugs. 2019;79(9):929–34. doi: 10.1007/s40265-019-01134-9. [DOI] [PubMed] [Google Scholar]
- 5.Lassale C, Batty GD, Baghdadli A, Jacka F, Sanchez-Villegas A, Kivimaki M, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. 2019;24(7):965–86. doi: 10.1038/s41380-018-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean Diet, Stroke, Cognitive Impairment, and Depression: A Meta-Analysis. Ann Neurol. 2013;74(4):580–91. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis JPA. The Challenge of Reforming Nutritional Epidemiologic Research. Jama-J Am Med Assoc. 2018;320(10):969–70. doi: 10.1001/jama.2018.11025. [DOI] [PubMed] [Google Scholar]
- 8.Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: A systematic review. J Psychiatr Res. 2013;47(2):197–207. doi: 10.1016/j.jpsychires.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D Supplementation for Depressive Symptoms: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychosomatic Medicine. 2014;76(3):190–6. doi: 10.1097/PSY.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatr. 17(12):1272–82. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, et al. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016;173(6):575–87. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- 12.Rucklidge JJ, Eggleston MJF, Johnstone JM, Darling K, Frampton CM. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: a fully blinded, randomized, placebo-controlled trial. J Child Psychol Psychiatry. 2018;59(3):232–46. doi: 10.1111/jcpp.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rucklidge JJ, Kaplan BJ. Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: a systematic review. Expert Rev Neurother. 2013;13(1):49–73. doi: 10.1586/ern.12.143. [DOI] [PubMed] [Google Scholar]
- 14.Kimball SM, Mirhosseini N, Rucklidge J. Database Analysis of Depression and Anxiety in a Community Sample-Response to a Micronutrient Intervention. Nutrients. 2018;10(2) doi: 10.3390/nu10020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial) Bmc Med. 2017;15 doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca M, Kohls E, Gili M, Watkins E, Owens M, Hegerl U, et al. Prevention of depression through nutritional strategies in high-risk persons: rationale and design of the MooDFOOD prevention trial. Bmc Psychiatry. 2016;16 doi: 10.1186/s12888-016-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosomatic Medicine. 2019;81(3):265–80. doi: 10.1097/PSY.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human Molecular Genetics. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haycock PC, Burgess S, Wade KH, Bowden J, Relton C, Smith GD. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am J Clin Nutr. 2016;103(4):965–78. doi: 10.3945/ajcn.115.118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4(4):330–45. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A Lawlor D, Wade K, Borges M, Palmer T, Hartwig F, Hemani G, et al. A Mendelian Randomization dictionary: Useful definitions and descriptions for undertaking, understanding and interpreting Mendelian Randomization studies. 2019 [Google Scholar]
- 22.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers The Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama-J Am Med Assoc. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarmolinsky J, Bonilla C, Haycock PC, Langdon RJQ, Lotta LA, Langenberg C, et al. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. J Natl Cancer Inst. 2018;110(9):1035–8. doi: 10.1093/jnci/djy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–86. doi: 10.1093/ije/dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, Richards JB. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology. 87(24):2567–74. doi: 10.1212/WNL.0000000000003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Traylor M, Markus HS, Michaelsson K. Serum Parathyroid Hormone, 25-Hydroxyvitamin D, and Risk of Alzheimer's Disease: A Mendelian Randomization Study. Nutrients. 2018;10(9) doi: 10.3390/nu10091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson E, Byberg L, Karlstrom B, Cederholm T, Melhus H, Sjogren P, et al. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. American Journal of Clinical Nutrition. 2017;105(4):936–43. doi: 10.3945/ajcn.116.141531. [DOI] [PubMed] [Google Scholar]
- 28.Jorde R, Mathiesen EB, Rogne S, Wilsgaard T, Kjaergaard M, Grimnes G, et al. Vitamin D and cognitive function: The Tromso Study. Journal of the Neurological Sciences. 2015;355(1–2):155–61. doi: 10.1016/j.jns.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Maddock J, Zhou A, Cavadino A, Kuzma E, Bao Y, Smart MC, et al. Vitamin D and cognitive function: A Mendelian randomisation study. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13189-3. 13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, et al. Neonatal Vitamin D Status and Risk of Schizophrenia: A Population-Based Case-Control Study. Schizophrenia Research. 2010;117(2–3):312. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- 31.Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol. 2017;41(4):341–52. doi: 10.1002/gepi.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS, et al. Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ. 2017;359 doi: 10.1136/bmj.j5375. j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Q, Teng W, Li J, Hao F, Wang N. Homocysteine and Alzheimer's Disease: Evidence for a Causal Link from Mendelian Randomization. Journal of Alzheimer's Disease. 2016;52(2):747–56. doi: 10.3233/JAD-150977. [DOI] [PubMed] [Google Scholar]
- 34.Schatzkin A, Abnet CC, Cross AJ, Gunter M, Pfeiffer R, Gail M, et al. Mendelian randomization: how it can--and cannot--help confirm causal relations between nutrition and cancer. Cancer Prev Res (Phila) 2009;2(2):104–13. doi: 10.1158/1940-6207.CAPR-08-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu SP, Ma JJ, Qi YW, Zhang JW. Plasma homocysteine levels and risk of vascular dementia: A Mendelian randomization study. International Journal of Clinical and Experimental Medicine. 2017;10(6):9142–51. [Google Scholar]
- 36.Larsson SC, Markus HS. Branched-chain amino acids and Alzheimer's disease: a Mendelian randomization analysis. Sci Rep-Uk. 2017;7 doi: 10.1038/s41598-017-12931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma apolipoprotein E levels and risk of dementia: A Mendelian randomization study of 106,562 individuals. Alzheimers Dement. 2018;14(1):71–80. doi: 10.1016/j.jalz.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjaerg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. BMJ. 2017;357 doi: 10.1136/bmj.j1648. j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proitsi P, Lupton MK, Velayudhan L, Newhouse S, Fogh I, Tsolaki M, et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. PLoS Medicine / Public Library of Science. 11(9):e1001713. doi: 10.1371/journal.pmed.1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostergaard SD, Mukherjee S, Sharp SJ, Proitsi P, Lotta LA, Day F, et al. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLoS Medicine / Public Library of Science. 12(6):e1001841. doi: 10.1371/journal.pmed.1001841. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G, Zhao Y, Jin S, Hu Y, Wang T, Tian R, et al. Circulating vitamin E levels and Alzheimer's disease: A Mendelian randomization study. Neurobiology of Aging. 2018;72:189. doi: 10.1016/j.neurobiolaging.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Cheng WW, Zhu Q, Zhang HY. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients. 2019;11(2):12. doi: 10.3390/nu11020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomioka Y, Numata S, Kinoshita M, Umehara H, Watanabe S, Nakataki M, et al. Decreased serum pyridoxal levels in schizophrenia: meta-analysis and Mendelian randomization analysis. J Psychiatr Neurosci. 2018;43(3):194–200. doi: 10.1503/jpn.170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Numata S, Kinoshita M, Tajima A, Nishi A, Imoto I, Ohmori T. Evaluation of an association between plasma total homocysteine and schizophrenia by a Mendelian randomization analysis. BMC Medical Genetics. 2015;16:54. doi: 10.1186/s12881-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinoshita M. One-carbon Metabolism and Schizophrenia. [Japanese] Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica. 2015;117(5):362–8. [PubMed] [Google Scholar]
- 46.Li Z, Chen P, Chen J, Xu Y, Wang Q, Li X, et al. Glucose and Insulin-Related Traits, Type 2 Diabetes and Risk of Schizophrenia: A Mendelian Randomization Study. Ebiomedicine. 2018;34:182–8. doi: 10.1016/j.ebiom.2018.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Leong A, et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. Plos Med. 2015;12(8) doi: 10.1371/journal.pmed.1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Davey Smith G, et al. Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep. 2016;6 doi: 10.1038/srep26496. 26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalen P. Month of Birth and Schizophrenia. Acta Psychiat Scand. 1968;S:55–+. doi: 10.1111/j.1600-0447.1968.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 50.Mollehave LT, Skaaby T, Simonsen KS, Thuesen BH, Mortensen EL, Sandholt CH, et al. Association studies of genetic scores of serum vitamin B12 and folate levels with symptoms of depression and anxiety in two danish population studies. Eur J Clin Nutr. 2017;71(9):1054–60. doi: 10.1038/ejcn.2017.97. [DOI] [PubMed] [Google Scholar]
- 51.Sallis H, Steer C, Paternoster L, Smith GD, Evans J. Perinatal depression and omega-3 fatty acids: A Mendelian randomisation study. J Affect Disorders. 2014;166:124–31. doi: 10.1016/j.jad.2014.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaelsson K, Melhus H, Larsson SC. Serum 25-Hydroxyvitamin D Concentrations and Major Depression: A Mendelian Randomization Study. Nutrients. 2018;10(12) doi: 10.3390/nu10121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesolowska K, Elovainio M, Hintsa T, Jokela M, Pulkki-Raback L, Pitkanen N, et al. Fasting Glucose and the Risk of Depressive Symptoms: Instrumental-Variable Regression in the Cardiovascular Risk in Young Finns Study. International Journal of Behavioral Medicine. 2017;24(6):901–7. doi: 10.1007/s12529-017-9639-2. [DOI] [PubMed] [Google Scholar]
- 54.Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatr. 2019;19:19. doi: 10.1038/s41380-019-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meddens SFW, Vlaming Rd, Bowers P, Burik CA, Linnér RK, Lee C, et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. bioRxiv. 2018 doi: 10.1038/s41380-020-0697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guenard F, Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Genome-Wide Association Study of Dietary Pattern Scores. Nutrients. 2017;9(7) doi: 10.3390/nu9070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human Genetics Shape the Gut Microbiome. Cell. 2014;159(4):789–99. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vila AV, Vosa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nature Genetics. 2019;51(4):600–+. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making Sense of … the Microbiome in Psychiatry. Int J Neuropsychoph. 2019;22(1):37–52. doi: 10.1093/ijnp/pyy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kali A. Psychobiotics: An emerging probiotic in psychiatric practice. Biomed J. 2016;39(3):223–4. doi: 10.1016/j.bj.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Annals of General Psychiatry. 2017;16 doi: 10.1186/s12991-017-0138-2. (vol 16, 14, 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG. Using Multivariable Mendelian Randomization to Disentangle the Causal Effects of Lipid Fractions. Plos One. 2014;9(10) doi: 10.1371/journal.pone.0108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Millard LA, Davies NM, Timpson NJ, Tilling K, Flach PA, Davey Smith G. MR-PheWAS: hypothesis prioritization among potential causal effects of body mass index on many outcomes, using Mendelian randomization. Sci Rep-Uk. 5 doi: 10.1038/srep16645. 16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41(1):161–76. doi: 10.1093/ije/dyr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giovannucci E. Nutritional epidemiology: forest, trees and leaves. Eur J Epidemiol. 2019;34(4):319–25. doi: 10.1007/s10654-019-00488-4. [DOI] [PubMed] [Google Scholar]
- 66.Hemani G, Bowden J, Haycock P, Zheng J, Davis O, Flach P, et al. Automating Mendelian randomization through machine learning to construct a putative causal map of the human phenome. bioRxiv. 2017 [Google Scholar]
- 67.Roostaei T, Felsky D, Nazeri A, De Jager PL, Schneider JA, Bennett DA, et al. Genetic influence of plasma homocysteine on Alzheimer's disease. Neurobiology of Aging. 62:243.e7–e14. doi: 10.1016/j.neurobiolaging.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walter S, Marden JR, Kubzansky LD, Mayeda ER, Crane PK, Chang S-C, et al. Diabetic phenotypes and late-life dementia risk: A mechanism-specific Mendelian Randomization study. Alzheimer Disease and Associated Disorders. 2016;30(1):15–20. doi: 10.1097/WAD.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brion MJA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.