Abstract
This study reports the development and application of a liquid chromatography method coupled to electrospray tandem mass spectrometry (LC-MS/MS) for the identification and quantification of the five most common juvenile hormone (JH) homologs and methyl farnesoate (MF). The protocol allows the simultaneous analysis in a single LC run of JH I, JH II, JH III, JH III bisepoxide (JHB3) and JH III skipped bisepoxide (JHSB3). The identification of JHs is based on multiple reaction monitoring (MRM), using two of the most abundant fragmentation transitions for each hormone. Addition of deuterated JH III as an internal standard permits the absolute quantification of the different JHs. The JH homologs common structural features led to similar chromatographic behavior, as well as related fragmentation patterns, which facilitated the simultaneous detection of all the homologs in a single LC-MS/MS run. The protocol detects JHs in the low femtomole range, allowing often the analysis of JH in individual insects. Fragmentation of each of the JH homologs generates unique diagnostic ions that permitted the identification and quantification of JHs from samples of different species of Diptera, Lepidoptera, Heteroptera and Hymenoptera. Having a simple protocol, which can undisputedly determine the identity of the homologs present in a particular species, provides us with the opportunity to identify and quantify JHs existing in insects that are pests, vector of diseases or important research models.
Keywords: Juvenile hormone, homologs, liquid chromatography, MRM, quantification
Graphical Abstract
INTRODUCTION
Juvenile hormones (JHs) are synthesized by the corpora allata glands (CA). They play key roles in many processes in insect development and reproduction, including inhibition of metamorphosis, caste determination and differentiation, stimulation of flight and migration, regulation of reproduction, control of diapause, stress resistance, and aging (Goodman and Cusson, 2012, Zhu and Noriega, 2016). Consequently, JHs have been considered as targets for the development of novel insecticides (Cusson et al., 2013). Several JH homologs have been identified in insects. The first two JHs, JH I and II, were isolated from the moth Hyalophora cecropia (Röller et al., 1967 and Meyer et al., 1968). JH III, the homolog found in most insects, was described from the moth Manduca sexta (Judy et al., 1973). In addition, two double-epoxidated compounds were later reported, JH III bisepoxide (JHB3) in Drosophila melanogaster (Richard et al., 1989), and JH III skipped bisepoxide (JHSB3) in the heteropteran Plautia stali (Kotaki et al., 2009, 2011). JH titers in insects are often in the femtomole to picomole range, which makes it challenging to measure them by most typical analytical techniques, such as radioimmunoassay and mass spectrometry (MS) coupled with gas or liquid chromatography and capillary electrophoresis (reviewed in Rivera-Perez et al., 2014). Previously, we described the detection and quantification of JH III using a liquid chromatography method coupled to electrospray tandem mass spectrometry analysis (LC-ESI-MS/MS) that increased sensitivity and reproducibility, while reducing the analysis time (Ramirez et al., 2016). In the present study, we optimized a LC-MS/MS method to identify and quantify simultaneously several different JH homologs. The protocol allows the concurrent analysis in a single LC run of the five most common JH homologs: JH I, JH II, JH III, JH III bisepoxide (JHB3) and JH III skipped bisepoxide (JHSB3), as well as methyl farneosate (MF). We utilized multiple reaction monitoring (MRM), selecting two of the most abundant fragmentation transitions for each hormone. Including a deuterated JH III as an internal standard permitted the absolute quantification of the different JHs. The protocol detects JHs in the low femtomole range (pg/ml), allowing often the analysis of JH in individual insects. Fragmentation of each of the JH homologs produced unique diagnostic ions that allowed the identification and quantification of JHs from samples of species of Diptera, Lepidoptera, Heteroptera and Hymenoptera. This simple protocol can unquestionably determine the identity of the JH homolog present in a particular species, and provides the opportunity to identify and quantify JHs existing in insects that are pests, vector of diseases or important research models.
MATERIALS AND METHODS
Materials and reagents:
Certified standard solutions for JH I, JH II, JH III skipped bisepoxide (JHSB3), JH III and its deuterated analog (JH III-D3) were obtained from Toronto Research Chemicals (Toronto, Canada). JH III bisepoxide (JHB3) and methyl farnesoate (MF) were from Echelon (Salt Lake City, Utah). Sodium chloride, potassium chloride, hydrochloric acid, sodium hydroxide, ammonium acetate, ammonium formate and ammonium hydroxide salts were analytical grade or better (Fisher Scientific, Pittsburgh, PA). Water, methanol, hexane and acetonitrile were all Optima grade or better (Fisher Scientific). Chromatographic mobile phases (0.1% formic acid in water, and 0.1% formic acid in acetonitrile) of Optima LC-MS grade were obtained from Fisher Scientific. Tissue culture media Gibco M-199, silanized LC vials and silanized LC vials with fused 250 μL inserts were also from Fisher Scientific.
Insect samples:
Aedes aegypti mosquitoes (Culicidae, Diptera) of the Rockefeller strain were reared at 28 °C and 80% humidity as previously described (Nouzova et al., 2018). Grey flesh fly, Sarcophaga bullata (Sarcophagidae, Diptera), milkweed bug, Oncopeltus fasciatus (Lygaeidae, Hemiptera), tobacco hornworm, Manduca sexta (Sphingidae, Lepidoptera) and silkworms, Bombyx mori (Bombycidae, Lepidoptera) were purchased from Carolina Biological (Burlington, NC), and raised following the supplier instructions. Hemolymph samples from Drosophila melanogaster (cantonized w ▪1118) (Drosophilidae, Diptera) were provided by Lacy Barton. Hemolymph samples from sweat bee, Megalopta genalis (Halictidae, Hymenoptera) were provided by Callum Kingwell. Hemolymph samples from Anopheles albimanus (Culicidae, Diptera) were provided by Salvador Hernandez-Martinez. Hemolymph samples from Dipetalogaster maxima (Heteroptera, Reduvidae) were provided by Fabian Orlando Ramos.
Hemolymph collection:
Hemolymph samples were collected directly on a glass silanized tube (Thermo Scientific) placed on ice, containing 100 μl of phosphate-buffered saline (PBS, pH 7.2) and 10 μL of 6.25 ppb JH III-D3 in acetonitrile (Nouzova et al., 2018). Hexane (600 μL) was added; samples were vortexed for 1 minute, and spun for 5 minutes at 4 °C and 2000 g. The organic phase was transferred to a new silanized vial. Acetonitrile (100 μl) was added, samples were vortexed 10 sec, spun for 1 min at 4 °C and 2000 g and the hexane phase was dried under nitrogen flow. The remained acetonitrile fraction was reduced under nitrogen flow to a final volume of 50 μL and transferred to a new silanized vial with a fused 250 μL insert. Samples were stored at −20°C until analysis.
Juvenile hormone biosynthesis assay:
JH biosynthesis assays by corpora allata-corpora cardiaca (CA-CC) complexes connected to the brain and head capsule (BR-CA-CC) were performed as described in Li et al (2003). The aorta-CA-CC was left connected to the intact head capsule to facilitate the visualization and transfer of the glands. BR-CA-CC were dissected in a drop of mosquito saline-buffer containing 138 mM NaCl, 8.4 mM KCl, 4 mM CaCl2, 12 mM NaH2PO4 and 42.5 mM sucrose. After dissection, the BR-CA-CC complexes were incubated in 150 μL of tissue culture media M-199, containing 2% Ficoll 400 and 50 μM methionine. Incubations of BR-CA-CC complexes were carried out in a humid chamber in silanized 2 mL vials for 4 h in the dark at 32 °C, and under continuous gentle agitation. After incubation, 10 μL of 6.25 ppb JH III-D3 in acetonitrile were added to each sample, followed by 600 μL of hexane. Samples were vortexed for 1 minute, and spun for 5 minutes at 4 °C and 2000 g. The organic phase was transferred to a new silanized vial, dried under nitrogen flow and stored at −20 °C until analysis.
Mass Spectrometry Analysis:
Fourier-transform ion cyclotron resonance (FT-ICR) was used to confirm the identity of the JH homologs and collision-induced dissociation (CID) fragments. We infused 10 mg/L solutions of single JH homologs in 0.1% formic acid in acetonitrile into a Solarix 7T FT-ICR mass spectrometer (Bruker Daltonics Inc, Billerica, MA, USA) equipped with an electrospray ionization (ESI) source (summarized in supplemental Fig. 1).
Liquid chromatography (LC) separations were performed by an Advance UHPLC system (Bruker Daltonics Inc, Billerica, MA, USA), equipped with an Xbridge BEH Phenyl Column (4.6 mm X 150 mm, 3.5 μm) protected by a VanGuard cartridge 3.9 mm X 5 mm, 3.5 μm) (Waters, MA, USA). Column temperature was kept at 30 °C. Gradient separation was performed between 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B). We employed the following program: hold 10% B for 0.25 min, increase to 25% B in 3.75 min, increase to 99% B in 4.00 min and hold for another 4.00 min, return to 10% B in 0.50 min and hold for 2.5 min for a total run time of 15 min. Flow rate was changed according to the following program: hold 0.8 mL/min for 10.50 min, increase to 1.25 mL/min in 0.20 min and hold for another 1.8 min, decrease to 1 mL/min in 0.10 min and decrease to 0.80 mL/min in 2.4 min.
Detection was performed by a Bruker EvoQ LC-TQ Elite triple quadrupole mass spectrometer (Bruker Daltonics Inc., Billerica, MA., USA.) equipped with a heated electrospray ionization (HESI) interface. The instrument was operated under positive mode ionization with multiple reaction monitoring (MRM) of two transitions per compound. Optimization of MRM collision-induced dissociation (CID) energies were performed by infusing 5 mg/L solutions of single JH homologs in 0.1% formic acid in acetonitrile. Heated electrospray ionization (HESI) source parameters were optimized with multiple LC-MS/MS runs using final gradient and flow rate conditions. Source parameters were: Spray voltage 4500V; Cone temperature: 350 °C; cone gas flow: 20 (arbitrary); Heated probe temperature: 350 °C; probe gas flow: 30 (arbitrary); nebulizer flow: 30 (arbitrary).
RESULTS AND DISCUSSION
The common structural features of the JH homologs facilitated their simultaneous analysis.
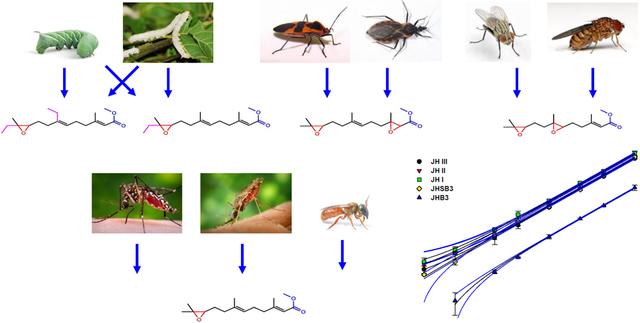
The five JH homologs analyzed are sesquiterpenes (16C) that have a methyl ester (α, β-unsaturated) at the C1 position and an epoxide ring at the C10-C11 position; with the different JH homologs displaying changes in the numbers and positions of carbons and epoxide groups (Fig. 1). These structural similarities, which include the 2E,6E geometry of the JH skeleton, dictated by two stereogenic double bonds, are critical for any JH to exert its agonist activity, through binding to the JH receptor (Bittova et al., 2019). A single intracellular receptor of JH has been described in insects, the methoprene-tolerant (Met) protein, which in D. melanogaster has a paralog (Gce) (Jindra et al., 2015). All known JH homologs are interacting with this unique Met protein. Significant changes in the JH “basic” structural features (2E,6E geometry, C10-C11 epoxide ring and C1 methyl ester), might reduce interactions with the receptor; either by preventing the hormone entry to the hormone-binding cavity, or establishing proper interactions with critical residues in the pocket (Charles et al., 2011; Bittova et al., 2019). Additional constrains on the evolution of JH homologs structural features might have been imposed by their transport in the hemolymph by JH-binding proteins; which show preference toward epoxidated and enantiospecific JH forms (Suzuki et al, 2011; Kim et al., 2017); as well as by the well-conserved architecture of the JH biosynthetic pathway (Noriega, 2014).
Fig. 1: Chemical structures of JH homologs:
JHB3: juvenile hormone III bisepoxide. JHSB3: juvenile hormone III skipped bisepoxide. JH III: juvenile hormone III. JH II: juvenile hormone II. JH I: juvenile hormone I. MF: methyl farnesoate. Epoxide groups are in red. Methyl esters groups are in blue. Ethyl groups are in magenta.
These similar structural features facilitated the goal of these studies, which was to develop a protocol that would allow for the simultaneous identification and quantification of multiple JHs from insect samples. Having similar structures and masses resulted in similar behaviors for the six compounds in the HPLC system, as well as generated fragments with similar masses, which facilitates the MS/MS analysis. Nevertheless, the unique structural features of each JH homolog and MF resulted in slight differences in chromatographic elution times, as well as unique diagnostic fragmentation patterns that permitted the identification and quantification of each compound simultaneously. Based on the present results, we could predict that the protocol could also resolve and detect additional unknown JH homologs, which should still maintain the “basic” structural features described above.
Mass spectrometry analysis:
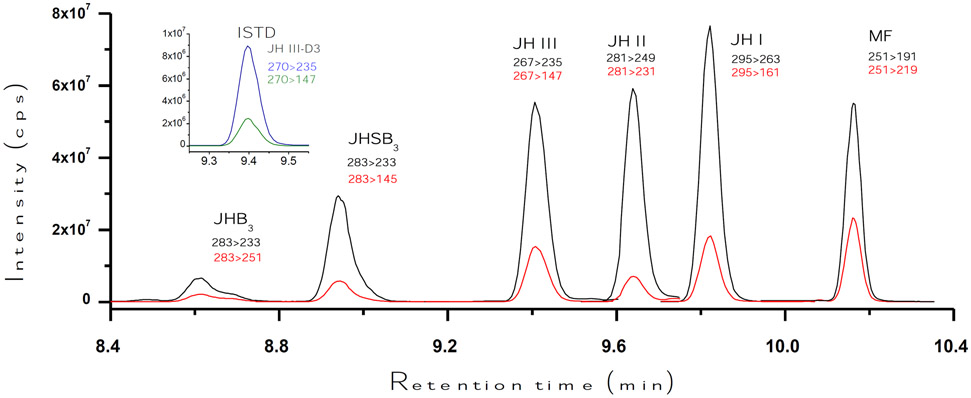
The HPLC-MS/MS workflow was developed using standards for the five JH analytes, as well as MF (Fig. 1). The HPLC program consisted of a short 15 minutes run, with the different five JH homologs and MF eluting between 8.4 and 10.4 minutes (Fig. 2). JH III and the internal standard JH III-D3 co-eluted.
Fig. 2: LC separation of JH homologues:
Typical LC-MS/MS peaks of JH homologues and MF. It shows the relationships between the retention times in minutes (X axis) and the signal intensity (cps; counts per second) (Y axis). Injected mass was 125 pg (325 pg for MF and 32 pg for the internal standard –ISTD-). Retention times are in minutes. Black lines represent the signal intensity of the primary transition, and red lines represent the intensity of the secondary transition. The inset shows the corresponding chromatograms for JH III-D3 (blue is the primary and green is the secondary transitions).
All JH homologs and MF were detected in the protonated form ([M+H]+. The [M+H]+ molecular ions underwent fragmentation via collision induced activation, allowing the construction of the different JHs [M+H]+ fragmentation pathways (Supplemental. Fig. 1). The fragmentation of the protonated JH forms provided a variety of signature fragmentation ions that were used to selectively identify each of the different JH homologs. From those fragmentation patterns, we selected two transitions (ions or fragments) to be used for the quantification (primary ion) and confirmation (secondary ion) of JHs in biological samples (Table 1). The selection of the two transitions to perform the quantification of each JH homolog was primarily based on their relative abundance and diagnostic usefulness. Primary MS/MS transitions were an average 3-6 fold more abundant than the secondary ions (Table 1 and Supplemental Fig. 1). The most common fragmentation was the loss of a CH3OH, with a reduction in a mass of 32; therefore the primary ions of JH I, JH II and JH III had masses of 263, 249 and 235 respectively (Supplemental Fig. 1).
Table 1.
Different critical parameters for multiple reaction monitoring (MRM) detection of the JHs and MF.
| Analyte | Ret. time | Parent ion | Primary fragment | Secondary fragment | |||
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | (m/z) | CID (V) | (m/z) | CID (V) | % | |
| JHB3 | 8.60 | 283 | 233 | 10.0 | 251 | 7.0 | 34 |
| JHSB3 | 8.94 | 283 | 233 | 9.0 | 145 | 18.0 | 19 |
| JH III* | 9.40 | 267 | 235 | 5.0 | 147 | 10.0 | 29 |
| JH II | 9.64 | 281 | 249 | 5.0 | 231 | 6.0 | 14 |
| JH I | 9.82 | 295 | 263 | 5.0 | 161 | 12.0 | 25 |
| MF | 10.16 | 251 | 191 | 9.0 | 219 | 6.0 | 40 |
Retention times are in minutes. Collision-induced dissociation (CID) energies are in volts (V). %: represents the relative abundance of secondary fragments as % of the signal intensity of the primary fragment. Qualitative analysis tolerances were ± 0.2 min from retention times and ± 5% of relative abundances of secondary fragments. LC peak width at base was 0.15 min or lower for all compounds.
Detection of JH III-D3 internal standard was done on parent ion m/z 270 with same retention time, fragment ions and collision energies than JH III.
The heavy isotopomer JH III-D3 was utilized as an internal standard to normalize all sample preparation, extraction and analysis steps, in order to accurately quantify the amount of analyte present in biological samples (Ramirez et al., 2016). The concentration of JH III-D3 was constant in biological samples and calibration solutions (625 pg/mL).
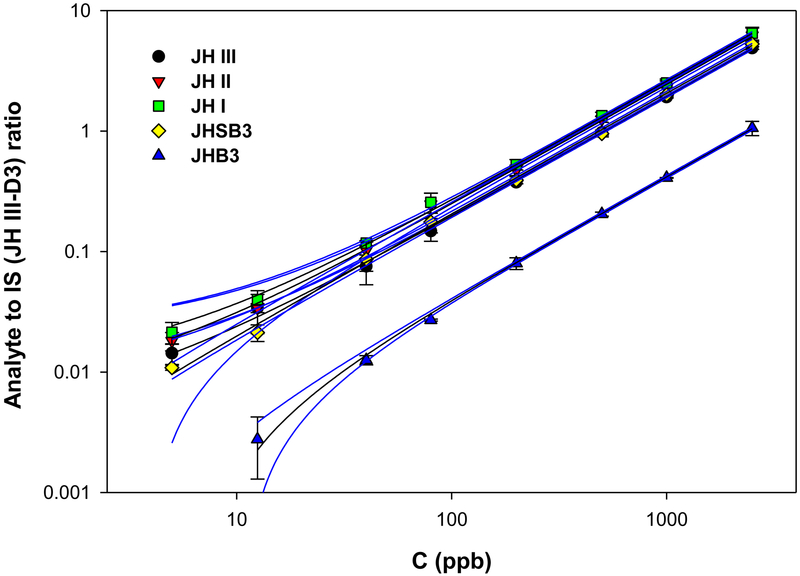
The HPLC-MS/MS method limit of detection (LOD), defined as three times the background analytical response, was determined experimentally for each JH form and MF (Fig. 3 and Supplemental Fig. 2). Calibration curves were obtained by plotting the peak area ratio of the individual JH homologs (expressed as the ratio between the signal intensities of the analyte and the deuteriated internal standard) as a function of the analyte concentration expressed in parts per billion (ppb). Average calibration curves for each of the JH homologs were constructed using between 21 and 54 different standard curve replicates (Fig. 3). Linearity was observed over a wide concentration range for all the compounds (R2 > 0.999). LODs for the different JHs were in a range of 3.5 to 8.7 pg/mL (0.48 to 1.23 fmols on the column) (Table 2). In addition, in the same LC run we also detected MF, a JH precursor in insects, which is present in the hemolymph of immature stages (Wen et al 2015), and it is considered the JH of crustacean (Cusson et al., 1991; Qu et al., 2015). The LOD for MF was 7.0 pg/mL (1.12 fmol on the column) (Supplemental Fig. 2).
Fig. 3: STD curves:
relationships between the concentration of each of the five JH standards (C), in parts per billions (ppb) (X-axis), and the signal intensities expressed as the ratio between the JH standard and the internal standard (IS, JH III-D3) (Y-axis). JH III (black circle). JH II (red inverted triangle). JH I (green square), JHSB3 (yellow diamond) and JHB3 (green triangle). Calibration curves for each of the JH homologs were constructed using between 21 and 54 different standard curves replicates. Linearity were observed over a wide concentration range. The blue lines represent the 95% confidence bands, depicting the upper and lower confidence bounds for all points on a fitted line within the range of data.
Table 2:
Limits of detection (LOD) for the different JH homologs and MF.
| JH Homolog | LOD (pg/ml) | LOD test range (pg/ml) | LOD (fmol on column) | n |
|---|---|---|---|---|
| JH I | 3.5 | 5-500 | 0.48 | 43 |
| JH II | 4.0 | 5-500 | 0.56 | 43 |
| JH III | 3.6 | 5-200 | 0.55 | 54 |
| JHSB3 | 7.8 | 5-500 | 1.10 | 51 |
| JHB3 | 8.7 | 5-1000 | 1.23 | 21 |
| MF | 7.0 | 10-600 | 1.12 | 46 |
Limit of detections (LOD), defined as three times the background analytical response, were determined experimentally for each JH form and MF (pg/ml). Compounds were tested in the LOD test range (pg/ml). The LOD is also expresses as fmol detected on the column. LOD values were based in 21-54 replicates (n)
Analysis of JH homologs from biological samples.
To validate the protocol we analysed biological samples from nine different insect species. First, we extracted and examined hemolymph from eight insect species, belonging to four orders: four species of Diptera, D. melanogaster (3rd instar larvae), Sarcophaga bullata, (3rd instar larvae), Ae. aegypti (sugar-fed adult females) and Anopheles albimanus (sugar-fed adult females), two species of Lepidoptera, Manduca sexta (4th instar larvae) and Bombyx mori (4th instar larvae), as well as one species of Hymenoptera, Megalopta genalis (reproductive queens), and one species of Heteroptera, Dipetalogaster maxima (adult vitellogenic females). In addition, we evaluated in vitro JH biosynthesis by BR-CA-CC complexes of two species, the Diptera Ae. aegypti, (CA from sugar-fed adult females), and the Heteroptera, Oncopeltus fasciatus (CA from adult males and females). Because these experiments represented a proof of concept, we studied one developmental stage for each species, and therefore sometimes we did not detect all the expected JH homologs present in that particular species (for example, JH III was not detected in Sarcophaga). These results are presented in Table 3. The combined analysis of samples from the nine species allowed the detection of the five major JH homologs and MF, validating the capability of the protocol to detect all of them from biological samples (Fig. 4). In addition, for each of the species we detected only those homologs that were previously described (Rivera Perez et al., 2014). JH III was present in dipteran (Shapiro et al., 1986; Hernandez-Martinez et al., 2019; Richard et al. 1989) and hymenopteran samples (Westerlund and Hoffman, 2004). JH I and JH II only in the Lepidoptera (Judy et al., 1973, Furuta et al., 2013). Among the Diptera, JHB3 was found in the Brachycera (Sarcophaga and Drosophila) (Yin et al., 1995; Richards et al., 1989), but not in the Nematocera (Aedes and Anopheles) (Shapiro et al., 1985; Hernandez-Martinez et al., 2019). JHSB3 was exclusively present in Heteroptera (Kotaki et al., 2009). In each individual analysis, the program runs standard curves for each of the six hormones and quantifies all the transitions (ions) described in Table 1; in other words, our protocol not only provides accurate quantitative information on the JHs present in the biological sample, but also rules out the presence of the additional homologs examined.
Table 3:
JH homologs detected in hemolymph samples or synthesized by CA from different insect species.
| JH III | JHB3 | JH I | JH II | JHSB3 | |
|---|---|---|---|---|---|
| Drosophila1 | 0.11 ± 0.01 | 0.79 ± 0.07 | - | - | - |
| Sarcophaga2 | - | 14.1 ± 1.5 | - | - | - |
| Aedes3 | 18.0 ± 2.4 | - | - | - | - |
| Anopheles4 | 2.8 ± 0.2 | - | - | - | - |
| Manduca5 | - | - | 30.9 ± 26.9 | 8.0 ± 5.3 | - |
| Bombyx6 | - | - | 46.3 ± 11.5 | 24.0 ± 15.0 | - |
| Megalopta7 | 36.5 ± 6.2 | - | - | - | - |
| Dipetalogaster8 | - | - | - | - | 43.2 ± 3.3 |
| A: Hemolymph JH titers. | |||||
| B: JH synthesis. | |||||
| JH III | JHB3 | JH I | JH II | JHSB3 | |
| Aedes9 | 33.5 ± 4.0 | - | - | - | - |
| Oncopeltus10 | - | - | - | - | 16.9 ± 3.9 |
Hemolymph values are fmol/insect (mean ± SEM). JH biosynthesis values are fmol/CA/h (mean ± SEM). “-”: not detected
Drosophila melanogaster, 3rd instar larvae.
Sarcophaga bullata, 3rd instar larvae.
Aedes aegypti, sugar-fed adult females.
Anopheles albimanus, sugar-fed adult females.
Manduca sexta, 4th instar larvae.
Bombyx mori, 4th instar larvae.
Megalopta genalis, reproductive queens.
Dipetalogaster maxima, reproductive females
Aedes aegypti, CA from sugar-fed adult females.
Oncopeltus fasciatus, CA from adult males and females.
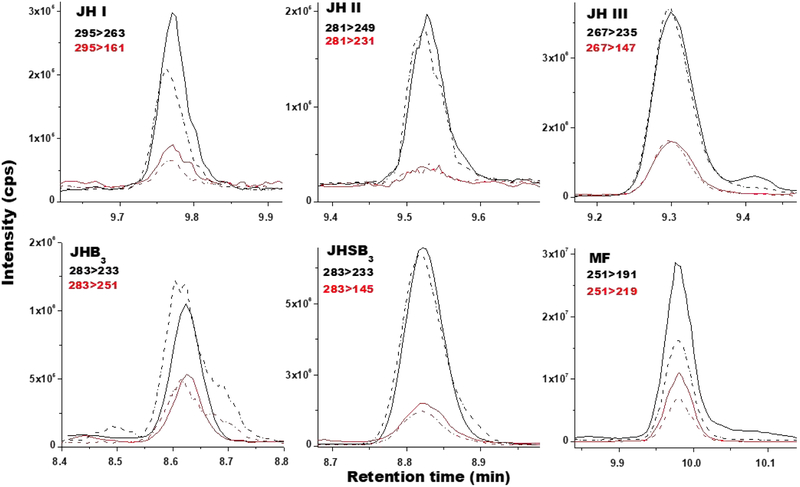
Fig. 4: Analysis of JH homologs from biological samples:
Typical ion extracted chromatograms comparing the relationships between retention times in minutes (X-axis) and signal intensities (cps; counts per second) (Y-axis) for JHs from biological samples (solid line) and from standard solutions (dashed lines). MRM transitions: primary (black) and secondary (red). JH I and II are from B. mori larval hemolymph. JH III is from Ae. aegypti adult female hemolymph. JHB3 is from D. melanogaster larval hemolymph. JHSB3 is from Dipetalogaster maxima adult female hemolymph. MF was synthesized in vitro by the CA of Ae. aegypti 4th instar larvae. Primary transitions masses evaluated are in black and secondary transitions are in red.
CONCLUSIONS
We developed an analytical workflow for the fast, ultra-trace quantitation of the five most common JHs described in insect samples. The protocol was optimized for accurate quantitative analysis, with higher sensitivity and a reduced number of sample preparation steps. The protocol detects the hormones in the low femtomole range, allowing the analysis of JH in individual insects. The method is highly reproducible, with little variation among different individual experiments. The common structural features facilitated the simultaneous identification and quantification of the five most common juvenile hormones, as well as MF, and most likely will resolve and detect additional unknown JH homologs, which should still maintain the “basic” structural features described.
Supplementary Material
Figure S1. Optimization of multiple reaction monitoring (MRM) detection on the Bruker EvoQ LC-TQ Elite Triple Quadrupole Mass Spectrometer and confirmation experiments using the Bruker Solarix 7T Fourier-transform ion cyclotron resonance (FT-ICR) ultra high-resolution mass spectrometer (UHRMS).
Figure S2. Relationship between the concentration of standard and signal intensity for MF.
Highlights.
This protocol allows the simultaneous analysis in a single LC-MS/MS run of JH I, JH II, JH III, JHB3 and JHSB3.
The JH homologs common structural features led to similar chromatographic behavior, as well as related fragmentation patterns
Addition of deuterated JH III as an internal standard permits the absolute quantification of the different JHs.
The protocol detects JHs in the low femtomole range, allowing often the analysis of JH in individual insects.
We identified and quantified JHs from samples of different species of Diptera, Lepidoptera, Heteroptera and Hymenoptera.
ACKNOWLEDGEMENTS
We thank Lacy Barton, Callum Kingwell, Salvador Hernandez-Martinez and Fabian Ramos for providing hemolymph from different insects. This work was supported by the National Institute of Health (Grant No. 2R01AI045545-19 to FGN), and the Advanced Mass Spectrometry Facility of Florida International University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bittova L, Jedlicka P, Dracinsky M, Kirubakaran P, Vondrasek J, Hanus R, Jindra M, 2019. Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem 294, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J-P, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M, 2011. Ligand-binding properties of a juvenile hormone receptor, methoprene-tolerant. Proc. Natl. Acad. Sci. USA 108, 21128–21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusson M, Yagi KJ, Ding Q, Duve H, Thorpe A, McNeil JN, and Tobe SS, 1991. Biosynthesis and release of juvenile hormone and its precursors in insects and crustaceans: the search for a unifying arthropod endocrinology. Insect Biochem. 21, 1–6. [Google Scholar]
- Cusson M, Sen SE, Shinoda T, 2013. Juvenile hormone biosynthetic enzymes as targets for insecticide discovery In: Ishayya I, Palli SR, Horowitz AR (Eds.), Advanced Technologies for Managing Insect Pests. Springer, Netherlands, pp. 31–55. [Google Scholar]
- Furuta K, Ichikawa A, Murata M, Kuwano E, Shinoda T, Shiotsuki T, 2013. Determination by LC-MS of juvenile hormone titers in hemolymph of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem 77, 988–991. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Cusson M, 2012. The Juvenile Hormones In: Gilbert LI, editor. Insect Endocrinology. San Diego: Academic Press; p. 310–65. [Google Scholar]
- Hernández-Martinez S, Cardozo-Jaime V, Nouzova M, Michalkova V, Ramirez CE, Fernandez-Lima F, Noriega FG, 2019. Juvenile hormone controls ovarian development in female Anopheles albimanus mosquitoes. Nature Scientific Reports. 9:2127 ∣ 10.1038/s41598-019-38631-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy KJ, Schooley DA, Dunham LL, Hall MS, Bergot BJ, Siddall JB, 1973. Isolation, structure, and absolute configuration of a new natural insect juvenile hormone from Manduca sexta. Proc Natl Acad Sci USA. 70, 1509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindra M, Palli SR, Riddiford LM, 2013The juvenile hormone signaling pathway in insect development. Ann Review Entomol. 58, 181–204. [DOI] [PubMed] [Google Scholar]
- Jindra M, Uhlirova M, Charles J-P, Smykal V, Hill RJ, 2015. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Pham V, Jablonka W, Goodman WG, , Ribeiro JMC, Andersen JF, 2017. A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J. Biol. Chem 292, 15329–15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaki T, Shinada T, Kaihara K, Ohfune Y, Numata H, 2009. Structure determination of a new juvenile hormone from a Heteropteran insect. Organic Letters. 11, 5234–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaki T, Shinada T, Kaihara K, Ohfune Y, Numata H, 2011. Biological activities of juvenile hormone III skipped bisepoxide in last instar nymphs and adults of a stink bug, Plautia stali. J. Insect Physiol 57,147–152. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. 2003. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Bioch Mol Biol. 33, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Schneiderman HA, Hanzmann E, Ko JH, 1968. The two juvenile hormones from the Cecropia silk moth. Proc Natl Acad Sci USA. 60, 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, 2014. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? International Scholarly Research Notices, 967361. doi: 10.1155/2014/967361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Michalkova V, Hernandez-Martinez S, Rivera-Perez C, Ramirez CE, Fernandez-Lima F, Noriega FG, 2018. JH biosynthesis and hemolymph titers in adult male Aedes aegypti mosquitoes. Insect Biochem Molec Biol. 95, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Kenny NJ, Lam HM, Chan TF, Chu KH, Bendena WG, Tobe SS, Hui JHL, 2015. How did arthropod sesquiterpenoids and ecdysteroids arise? Comparison of hormonal pathway genes in noninsect arthropod genomes. Genome Biol. Evol 7, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez CE, Nouzova M, Benigni P, Quirke JM, Noriega FG, Fernandez-Lima F, 2016. Fast, ultra-trace detection of juvenile hormone III from mosquitoes using mass spectrometry. Talanta. 159, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DS, Applebaum SW, Sliter TJ, Baker FC, Schooley DA, Reuter CC, Henrich VC, Gilbert LI, 1989. Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila melanogaster: a putative juvenile hormone in the higher Diptera. Proc Natl Acad Sci USA. 86, 1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Noriega FG, 2014. New approaches to study juvenile hormone biosynthesis in insects. In: Short Views on Insect Biochemistry and Molecular Biology. Chapter 7. 185–216. [Google Scholar]
- Röller H, Dahms KH, Sweely CC, Trost BM, 1967. The structure of the juvenile hormone. Angewandte Chemie International Edition. 6, 1966–1967. [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA, 1986. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol 32, 867–877. [Google Scholar]
- Suzuki R, Fujimoto Z, Shiotsuki T, Tsuchiya W, Momma M, Tase A, Miyazawa M, Yamazaki T, 2011. Structural mechanism of JH delivery in hemolymph by JHBP of silkworm, Bombyx mori. Sci. Rep 1, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CM, Zou BX, Jiang M, Li MF, Qin W, Potter TL, Stoffolano JG, 1995. Identification of juvenile hormone III bisepoxide (JHB3), juvenile hormone III and methyl farnesoate secreted by the corpus allatum of Phormia regina (Meigen) in vitro and function of JHB3 either applied alone or as part of juvenoid blend. J. Insect Physiol 41, 473–479. [Google Scholar]
- Wen D, Rivera-Perez C, Abdou M, Jia Q, He Q, Zyaan O, Bendena WB, Tobe SS, Noriega FG, Palli SR, Wang J, Li S, 2015. Methyl Farnesoate Plays a Dual Role in Regulating Drosophila Metamorphosis. PLoS Genet 11(3): e1005038. doi: 10.1371/journal.pgen.1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund SA, Hoffman KH, 2004. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography-mass spectrometry (LC-MS). Anal. Bioanal. Chem 379, 540–543. [DOI] [PubMed] [Google Scholar]
- Zhu J, Noriega FG, 2016. The role of juvenile hormone in mosquito development and reproduction Adv. Insect Physiol. Progress in Mosquito Research Editor. Alex Raikhel. 51, 93–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Optimization of multiple reaction monitoring (MRM) detection on the Bruker EvoQ LC-TQ Elite Triple Quadrupole Mass Spectrometer and confirmation experiments using the Bruker Solarix 7T Fourier-transform ion cyclotron resonance (FT-ICR) ultra high-resolution mass spectrometer (UHRMS).
Figure S2. Relationship between the concentration of standard and signal intensity for MF.