Significance
Microorganisms can be rationally engineered to convert CO2 and H2O into chemicals, replacing those made from fossil fuels today. Sometimes such chemicals are poorly soluble in water or negatively affect the growth of the microorganism, resulting in cost-inefficient manufacturing. In nature, this problem is often solved by converting incompatible chemicals into those more compatible with the host and/or environment. Inspired by this, we propose a similar strategy for engineered biotechnology, whereby biochemical conversion inside the microorganism is followed by chemical reversal once outside. The principle was demonstrated with 1-octanol by implementing two different conversion methods in two different species, showing enhanced bioproduction in most cases. The approach may stimulate commercialization of sustainable and renewable production of chemicals.
Keywords: bioderivatization, 1-octanol, toxicity, solubility, bioproduction
Abstract
Bio-based production technologies may complement or replace petroleum-based production of chemicals, but they face a number of technical challenges, including product toxicity and/or water insolubility. Plants and microorganisms naturally biosynthesize chemicals that often are converted into derivatives with reduced toxicity or enhanced solubility. Inspired by this principle, we propose a bioderivatization strategy for biotechnological chemicals production, defined as purposeful biochemical derivatization of intended target molecules. As proof of principle, the effects of hydrophobic (e.g., esterification) and hydrophilic (e.g., glycosylation) bioderivatization strategies on the biosynthesis of a relatively toxic and poorly soluble chemical, 1-octanol, were evaluated in Escherichia coli and Synechocystis sp. PCC 6803. The 1-octanol pathway was first optimized to reach product titers at which the host displayed symptoms of toxicity. Solvent overlay used to capture volatile products partially masked product toxicity. Regardless of whether solvent overlay was used, most strains with bioderivatization had a higher molar product titer and product yield, as well as improved cellular growth and glucose consumption, compared with strains without bioderivatization. The positive effect on bioproduction was observed with both the hydrophobic and hydrophilic strategies. Interestingly, in several combinations of genotype/induction strength, bioderivatization had a positive effect on productivity without any apparent effect on growth. We attribute this to enhanced product solubility in the aqueous or solvent fraction of the bioreactor liquid phase (depending on the derivative and medium used), with consequent enhanced product removal. Overall, under most conditions, a benefit of bioproduction was observed, and the bioderivatization strategy could be considered for other similar chemicals as well.
Microbial biotechnology offers an attractive method for renewable production of chemicals that replace those currently sourced from fossil fuel feedstocks (e.g., monomers for plastic or textile polymer synthesis) (1) or nature (e.g., vanillin) (2, 3). A critical factor determining success with such a process is the compatibility between the engineered metabolism and its microbial host (4). If the target molecule or its metabolic intermediates are toxic to the host organism, then the maximum potential for cost-effective production is likely not achieved. For lower- value chemicals, economics really matter in the face of competition from fossil fuels (5). Nonetheless, implementation of strategies to enhance product tolerance has been found to improve productivity (6).
In nature, many organisms naturally synthesize very toxic molecules (7) but yet have survived throughout evolution and perhaps even prospered because of this. If we look closer, however, in many cases these chemicals accumulate as chemical derivatives—for example, glucosides that are synthesized by plants (8, 9). In some cases, these detoxification mechanisms are so effective that chemicals (e.g., cyanogenic glucosides) that without derivatization would certainly kill the plant itself can accumulate up to 30% of dry weight (10). Other examples are esters synthesized by yeasts using native alcohol acyltransferases (AATs). It has been argued that ester synthesis is also a detoxification mechanism to convert more toxic metabolites into those that are less harmful (11). If this process is so successful, why not also adopt it for biotechnology?
Herein we propose and define bioderivatization as the purposeful in vivo transformation of chemicals into chemical derivatives by modification of functional groups. Often these functional groups (e.g., hydroxyl groups, −OH) are central to rendering a chemical toxic toward cells (12). At the same time, bioderivatization may also radically change the chemical properties (e.g., water solubility) of the target chemical and/or protect the molecule from further conversion (e.g., oxidation). Bioderivatization could also open new opportunities for strategic product:process separation that is more cost-efficient. Once the derivative has been isolated outside of the biological host, it would need to be converted back to its original form, unless the particular derivative in question is also an attractive product. If the original form of the derivatized products are desired, the final end-products (i.e., aglycons or acids and alcohols) can be obtained through either hydrogenation or hydrolysis following isolation from the bioreactor. The general bioderivatization concept is illustrated in Fig. 1.
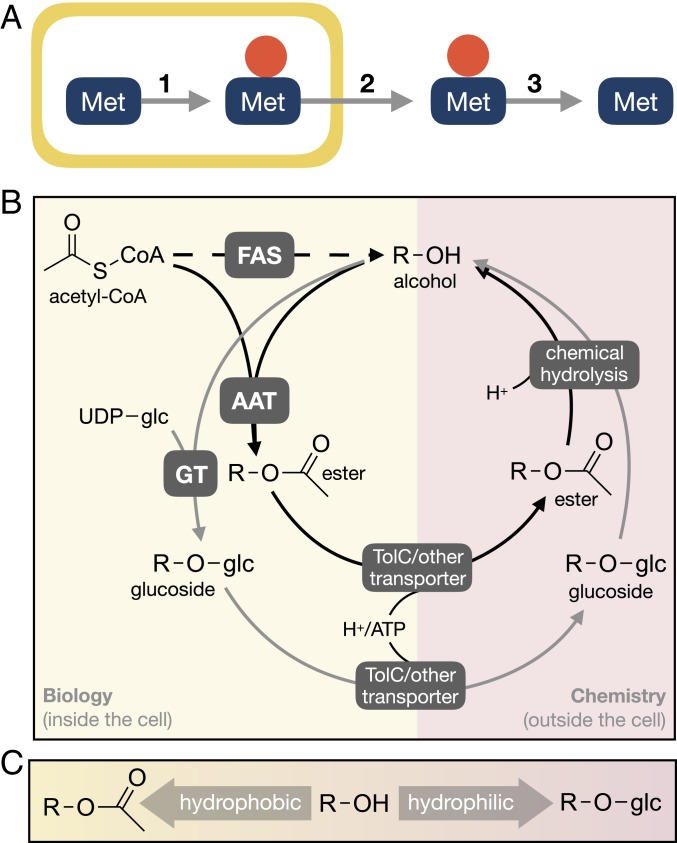
Fig. 1.
Overview of the bioderivatization process. (A) Concept scheme. Inside the cell, the target molecule is enzymatically converted into a derivative (e.g., ester) (1), with potential consequences for both toxicity and water solubility. After the product has been excreted from the cell (2), the original target molecule can be recovered by enzymatic or chemical processes that remove the conjugate group (3). The yellow box illustrates a cell or a production system. The red filled in circle represents the conjugate (e.g., acid). Met represents the target chemical in question. (B) Nonstoichiometric overview of the two derivatization processes considered in this study, esterification and O-glucosylation. FAS, fatty acid synthesis. (C) The varying effects of bioderivatization on the water solubility of the target molecule.
There are several instances where bioderivatization was implemented without a rationale (e.g., vanillin glucoside biosynthesis as opposed to synthesizing plain vanillin) (2) or occurred by chance through interactions with native metabolism in the biotechnological host (e.g., geraniol synthesis resulting in geranyl acetate formation through the action of native AATs) (13). To our knowledge, however, the impact of this process on biotechnological objectives has not yet been systematically studied.
We assumed that only toxic or labile products, or those that are expensive to separate (e.g., organic acids) (14), were likely to benefit from the strategy. For example, shorter-chain alcohols, such as l-butanol and ethanol, have relatively low toxicity and effective production systems already in place. Thus, in the present work, the hypothesis that bioderivatization offers benefits for a microbial biotechnological process was tested using 1-octanol (15, 16) as the model product using two different model biotechnological hosts, Escherichia coli and Synechocystis sp. PCC 6803. Because only efficient pathways are likely to result in the accumulation of sufficient concentration(s) of compounds to elicit a toxic response, the metabolic systems were first optimized.
An additional point of interest in this study was the role of solvent overlay. For hydrophobic products, solvent overlay offers an opportunity to reduce toxicity, most likely simply by facilitating product removal. However, situations may arise when solvent overlay is undesirable—for example, when attempting to separate a volatile product in the off-gas or when the energetic cost of solvent-product separation is excessive. Thus, the presence or absence of solvent overlay was also evaluated.
Materials and Methods
Strains and Plasmids.
E. coli DH5α (Thermo Fisher Scientific) was used to propagate all the plasmids used in this work. Two strains of E. coli (E. coli C43 [DE3; Lucigen] and BW25113 [Keio collection]) and one species of cyanobacteria (Synechocystis sp. PCC 6803) were used as hosts for 1-octanol and octyl acetate production. All E. coli and cyanobacterial strains used in this study are listed in SI Appendix, Tables S1 and S2, respectively. The genes encoding thioesterase Tes3, phosphopantetheinyl transferase Sfp, and carboxylic acid reductase (CAR) were obtained from plasmid pET-TPC3 (15), whereas the chloramphenicol acyltransferase (CAT) gene (cat) was amplified from plasmid pACYC-petF-fpr (17). A strawberry AAT (SAAT) gene (saat) from Fragaria x ananassa (18) (UniProtKB accession no. Q9FVF1), an alcohol O-acetyltransferase (ATF1) gene (atf1) from Saccharomyces cerevisiae (UniProtKB accession no. P40353), and C8-preferring thioesterases (CpFatB1 from Cuphea palustris and CaFatB3 from Cuphea avigera pulcherrima), including their variants (‘CpFatB1-4 and ‘CpFatB3-5), were chemically synthesized from Integrated DNA Technologies (IDT) and codon-optimized for E. coli. Five genes encoding glycosyl transferases and one gene encoding sucrose synthase (SUS) (SI Appendix, Table S3) were also synthesized from IDT for octyl glucoside production.
Plasmids used for gene expression were constructed using Biopart Assembly Standard for Idempotent Cloning (BASIC) (19) or traditional restriction enzyme ligase-based cloning. The strains and plasmid construction methods are described in detail in SI Appendix. The UniProtKB accession numbers for all of the genes used in this study are listed in SI Appendix, Table S3, and the primers used for PCR analysis are listed in SI Appendix, Table S4. All linkers used for BASIC are listed in SI Appendix, Table S5. The amino acid sequence alignments of the C8-preferring thioesterases are shown in SI Appendix, Fig. S1.
Evaluating the Effect of 1-Octanol, Octyl Acetate, and Octyl Glucoside on Growth and Liquid-Phase Partitioning.
E. coli strain C43 (DE3) was cultivated in 10 mL of lysogeny broth (LB broth; Sigma-Aldrich) overnight at 37 °C and 180 rpm. The overnight culture was washed twice with fresh M9 minimal medium (47.8 mM Na2HPO4, 22 mM KH2PO4, 8.55 mM NaCl, 18.69 mM NH4Cl, 2 mM MgSO4, 0.1 mM CaCl2 and 2% [wt/vol] glucose) and resuspended in the same medium to an initial OD600 of ∼0.1. The liquid cultures were spiked with different concentrations of 1-octanol (0 to 50 mM), octyl acetate (0 to 200 mM), and octyl glucoside (0 to 50 mM) and transferred (200 μL) into a well in 96-well microtiter plate. The plate was incubated in a Tecan Infinite M200 Pro Spectrophotometer at 37 °C with continuous shaking at 432 rpm, and OD600 was measured every hour for 15 h. The specific growth rates were calculated for each treatment.
Production of 1-Octanol, Octyl Acetate, and Octyl Glucoside in Engineered E. coli.
Overnight cultures were grown in LB media (10 mL) containing appropriate antibiotics with final concentrations as follows: kanamycin 50 μg/mL, spectinomycin 50 μg/mL, and carbenicillin 100 μg/mL The overnight cultures were washed twice with fresh M9 minimal medium and resuspended in 2 mL of M9 minimal medium before inoculation in 25 mL of M9 minimal medium with a starting OD600 of ∼0.1 in 100-mL Erlenmeyer flasks supplied with appropriate antibiotics. For 1-octanol and octyl acetate production experiments, the liquid cultures were cultivated at 37 °C and 180 rpm for 4 h and then induced with various concentrations of isopropyl β-d-1-thiogalactopyranoside (IPTG: 0.02, 0.05, 0.2, and 0.5 mM). After induction, 10% (vol/vol) hexadecane solvent overlay (Sigma-Aldrich) was added, and the liquid cultures were cultivated at 30 °C and 150 rpm. OD600 was measured every 24 h after inoculation for 48 h unless stated otherwise. Samples from the liquid cultures and hexadecane solvent overlay were collected every 24 h after inoculation for HPLC and GC-MS analysis, respectively. For octyl glucoside production, the cultures were cultivated at 37 °C and 180 rpm for 4 h and induced with 0.5 mM IPTG before continuing the incubation at 30 °C and 150 rpm for 48 h. Samples were centrifuged, and the supernatants were analyzed by HPLC. Sucrose (15 or 100 mM) was supplemented to the culture medium at the time of induction when the function of AtSUS1 was assessed. Solvent overlay was used when the production of 1-octanol and octyl glucoside was compared.
Production of 1-Octanol and Octyl Acetate in Engineered Cyanobacteria.
All cyanobacterial strains were cultivated in BG11 medium without cobalt (hereinafter BG11-Co; SI Appendix, Text), as cobalt was used as an inducer. The preculture was grown in 5 mL of BG11-Co containing appropriate antibiotic(s) (final concentrations: kanamycin, 10 μg/mL; spectinomycin, 10 μg/mL; erythromycin, 20 μg/mL) in a six-well plate at 30 °C and 180 rpm, with continuous illumination (warm-white LED) at 60 μmol photons/m2⋅s and 1% (vol/vol) CO2 in an Algaetron AG 230 (Photon Systems Instruments). When the OD730 reached 3 to 4, the liquid preculture was transferred into an autoclaved 100-mL Erlenmeyer flask covered with aluminum foil. The OD730 was adjusted to 0.2 by adding BG11-Co to a final volume of 25 mL, and antibiotics were added accordingly. The liquid culture was induced on day 2 with 15 μM nickel and 625 nM cobalt, and the OD730 was monitored for 20 d in the presence or absence of a 30% (vol/vol) hexadecane solvent overlay. On day 20, the liquid culture was transferred into fresh BG11-Co medium containing antibiotics with initial OD730 ∼0.2. When the OD730 reached 1 to 1.5, the liquid culture was induced with 15 μM nickel and 625 nM cobalt, and hexadecane solvent overlay 30% (vol/vol) was added. The hexadecane solvent overlay was sampled at 16 d after induction for GC-MS analysis. In a separate experiment, production cultures were inoculated directly from the initial preculture and cultured for 10 d in the presence of 30% (vol/vol) hexadecane solvent overlay, with induction of protein expression on day 2, as described above. The toxicity of the different products to Synechocystis sp. PCC 6803 were evaluated as described in SI Appendix, Fig. S2.
Quantification and Analysis of 1-Octanol, Octyl Acetate, Octyl Glucoside, and Other Metabolites.
GC-MS.
Hexadecane solvent overlay was used to capture 1-octanol and octyl acetate from the liquid culture. Alternatively, 0.1 volume of hexadecane was used to extract samples of the aqueous phase from mock or production cultures. One hundred microliters of hexadecane overlay or extract was transferred into an insert in a 2-mL screw top GC vial (Agilent). Samples were analyzed using an Agilent 7890B gas chromatograph with an HP-5 ms column, a 7693 autosampler, and a 5977B MSD system. Then 1 µL of sample was injected using a pulsed split ratio of 10:1 and split flow at 10 min/mL. The GC was programmed with an initial temperature of 70 °C for 30 s, followed by a first ramp at 30 °C/min to 250 °C before ramping up to 300 °C with a final hold for 2 min at 40 °C/min. Target products were identified by comparing mass spectra and retention times with external standards. Serial dilutions of 1-octanol (≥99%; Sigma-Aldrich) and octyl acetate (≥99%; ACROS Organics) standards were used to quantify the concentrations of 1-octanol and octyl acetate in the sample.
HPLC.
An Agilent 1200 series HPLC instrument equipped with different columns and a reflective index detector was used to determine the concentrations of octyl glucoside, glucose, fructose, sucrose and fermentation products in the E. coli sample every 24 h. One milliliter of liquid cultures was sampled at 24 h and 48 h and centrifuged at 17,000 × g for 15 min to separate the aqueous and hexadecane layers. The supernatant was transferred into a 2-mL HPLC vial. For octyl glucoside detection, samples (20 μL) were analyzed with a Zorbax XDB-C18 column (Agilent). The flow rate was set at 1 mL/min with a column temperature of 30 °C (20). For the analysis of sugars (glucose, sucrose, and fructose) from samples supplemented with sucrose, an Aminex HPX-87P column (Bio-Rad) was used to analyze the samples (20 μL) with the flow rate of 0.6 mL/min, and the column temperature was set at 85 °C. For glucose and other fermentation products when sucrose was not supplemented, samples (100 μL) were analyzed with an Aminex HPX-87H column (Bio-Rad), and the flow rate and column temperature were set at 0.6 mL/min and 60 °C, respectively. Serial dilutions of glucose (Sigma-Aldrich), sucrose (Sigma-Aldrich), fructose (Sigma-Aldrich), sodium acetate (Sigma-Aldrich), sodium lactate (Sigma-Aldrich), and absolute ethanol (VWR) were used to determine the amounts of these compounds in the sample.
Statistical Treatment and Data.
Three biological replicates were used for each treatment and/or condition. Unless stated otherwise, symbols or bar graphs represent the mean ± SD from three biological replicates. Individual data from line graphs are presented in SI Appendix, Fig. S3. Because all samples were collected from cultures that most likely were reasonably homogenous, normality was assumed in all cases, as discussed by Fay and Gerow (21). With selected data, indicated in the text or figure legends with P values obtained, a two-sided Student’s t test was used, with asterisks indicating significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005).
The data supporting the findings of this study are available within the paper and its Supplemental Information files. All other data that support the findings of this study are available from the corresponding author upon reasonable request.
Results and Discussion
Comparing the Toxicity of 1-Octanol, Octyl Acetate, and Octyl Glucoside.
In previous studies (15, 16), we observed that 1-octanol was toxic, resulting in reduced growth and also genetic instability in cyanobacteria (16). Alcohols are known to compromise the integrity of cell membranes (22), thereby causing cellular toxicity that is most commonly observed as a growth defect. With 1-octanol having only a single hydroxyl-group as a functional group, we initially considered two different types of derivatization: O-glucosylation and esterification. For ester synthesis, the most valuable organic acid moiety that could be recovered following hydrolysis of the bioderivative would make the most sense economically; however, although acetate is not a particularly valuable end product, the biosynthesis of acetate esters is easiest to implement and thus served as the starting point.
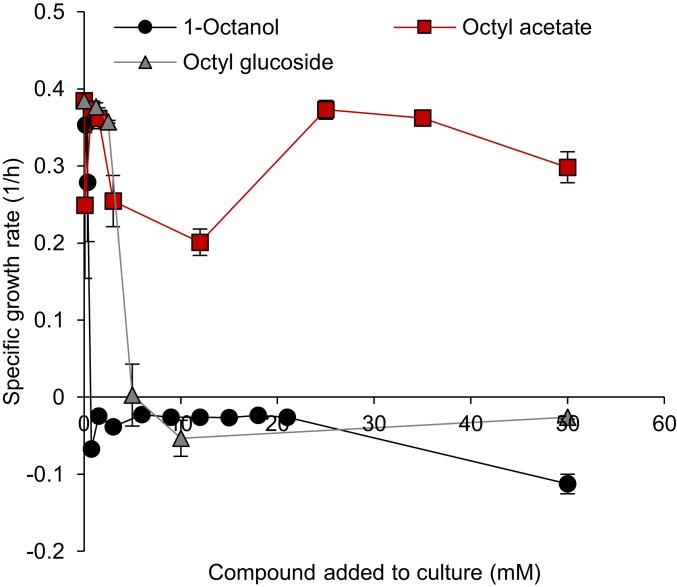
Before commencing with metabolic engineering, we evaluated the tolerance of the E. coli host strain to 1-octanol and its two proposed derivatives (octyl glucoside and octyl acetate) in 96-well microtiter plates. The cells were unable to grow when the concentration of 1-octanol in the liquid culture was >0.75 mM (Fig. 2 and SI Appendix, Fig. S4A). In contrast, growth was observed at all tested concentrations of octyl acetate (0 to 50 mM) (Fig. 2 and SI Appendix, Fig. S4B) and above (SI Appendix, Fig. S5), and up to 2.5 mM for octyl glucoside (Fig. 2 and SI Appendix, Fig. S4C). In previous studies, alcohols displayed varying (sometimes more, sometimes less) toxicity relative to its corresponding esters, depending on the specific product and derivative in question (12); for example, butyl acetate was found to be more toxic than l-butanol. To further complicate matters, the apparent toxicity of externally applied substances is likely influenced by environmental factors, including varying types of solvent overlay, and the toxicity of internally accumulated 1-octanol, octanal, and octanoic acid it remains unknown. Nevertheless, at least in the case of externally applied chemicals, both the corresponding acetyl ester and glucoside were less toxic than 1-octanol under the tested conditions. Based on this experiment, both derivatives were pursued in vivo as a model system, although clearly a “one size fits all” generalization is not possible.
Fig. 2.
Toxicity of 1-octanol, octyl acetate, and octyl glucoside. Different concentrations of each compound (0 to 50 mM) were added at the beginning of cultivation. The specific growth rate was determined by calculating the slopes of average growth curves (SI Appendix, Fig. S4) within 1 to 4 h.
Selection of AAT for Octyl Acetate Biosynthesis.
Octyl acetate is naturally found in wild strawberry (Fragaria vesca) (23). For use as a food flavor additive, it is also synthesized by a direct esterification reaction between acetic acid and octyl alcohol, catalyzed by acids, ion-exchange resins, or ionic liquids (24). Until now, we have found no reports of microbial in vivo production of octyl acetate using a renewable substrate.
The TPC3 pathway was extended with an AAT under the assumption that native acetyl-CoA was not limiting (Fig. 3A). Three AAT enzymes—CAT (25), SAAT (18), and ATF1 (26)—were selected based on the literature. CAT has previously been used in E. coli for ester biosynthesis (27), while both SAAT and ATF1 have been reported to use 1-octanol as a substrate (18, 27, 28). Before the in vivo production of 1-octanol and octyl acetate, a mock experiment with spiked 1-octanol (1 or 3.84 mM) and octyl acetate (1 or 2.9 mM) was carried out to investigate whether 1-octanol or octyl acetate remained in the aqueous or solvent phases after a 24-h incubation with solvent overlay. None of the compounds was detected in the aqueous phase at the lower concentration (SI Appendix, Fig. S6 A and B), while ∼10% (vol/vol) of 1-octanol was found in the aqueous phase at the higher concentration (SI Appendix, Fig. S6 C and D).
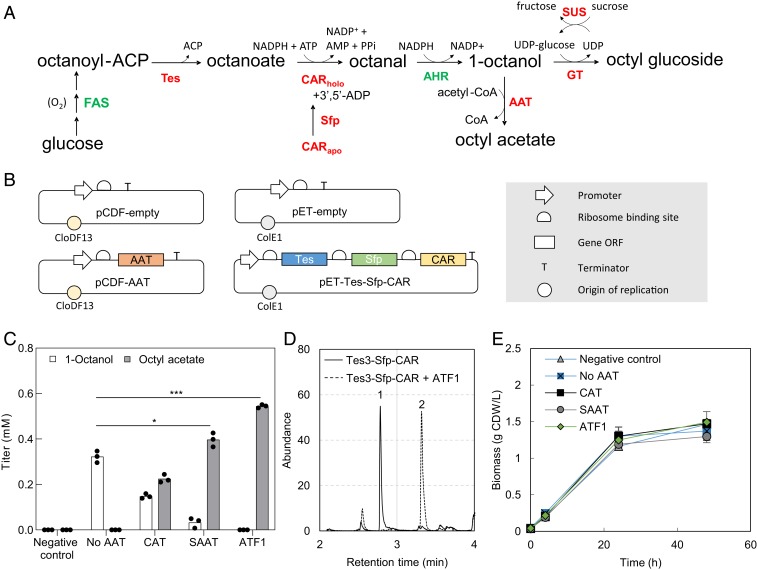
Fig. 3.
Pathway engineering for the synthesis of octyl glucoside and octyl acetate. (A) Metabolic scheme for in vivo production of bioderivatives. AHR, aldehyde reductase. (B) Plasmids used to generate strains 1 to 5 as listed in SI Appendix, Table S1. (C) Production of octyl acetate by introducing AAT enzymes in E. coli C43 (DE3). (D) Chromatogram of overlay sampled from Tes3-Sfp-CAR and Tes3-Sfp-CAR + ATF1 at 48 h. Peak identification: (1) 1-octanol; (2) octyl acetate. (E) Biomass accumulation of strains in C.
The majority of both compounds migrated to the solvent phase under all tested conditions while 10 to 20% was lost, presumably by volatilization. Therefore, the analytical detection of products was carried out only in the solvent overlay phase. Strains with all genetic combinations and controls were thereafter prepared and cultivated in M9 minimal medium with 2% (wt/vol) glucose and 10% (vol/vol) hexadecane overlay. Analysis of the solvent overlay indicated that all the tested AAT enzymes were able to convert 1-octanol and acetyl-CoA into octyl acetate; however, SAAT and ATF1 were more effective than CAT (P = 0.003 and 0.0002, respectively) (Fig. 3B). The highest octyl acetate titer (0.54 ± 0.01 mM [93.82 mg/L]) at 48 h after inoculation) and yield (12.54 mmol/mol glucose) were found in cultures of the strain expressing ATF1 (strain no. 5; SI Appendix, Table S1). The introduction of AAT did not result in marked changes in growth (Fig. 3C), and esterification of 1-octanol had only a small positive impact on the final product titer. There may be at least two possible explanations for this, including (i) the hexadecane solvent overlay reduced the toxicity of the products by in situ product removal (27) or (ii) the 1-octanol–producing strain reached a final titer of only 0.32 ± 0.03 mM, which is lower than the concentration that affected the growth of E. coli, as shown in Fig. 2.
To more comprehensively evaluate the impact of bioderivatization, we hypothesized that it was important to exceed the titer at which the underivatized product affected the growth of the host, at least in the absence of solvent overlay. Thus, the next task was to improve flux through the 1-octanol pathway.
Identification of Limiting Factor(s) for 1-Octanol Pathway Flux.
The titers of 1-octanol and octyl acetate in the previous experiment were low, indicating that one or more reactions in the introduced pathway prevented efficient pathway flux. In cyanobacteria, the availability of octanoic acid was found to limit the biosynthesis of 1-octanol (16). To evaluate whether the supply of octanoic acid was also limiting in E. coli, octanoic acid was added externally to the strains expressing the first-generation 1-octanol pathway with (strain no. 4) or without (strain no. 2) coexpression of SAAT (SI Appendix, Table S1). Substantially more of each product was observed in cultures to which octanoic acid was added, confirming that the supply of acid was indeed limiting the 1-octanol pathway (Fig. 4A). As all the 1-octanol was converted into the corresponding ester in SAAT-expressing strains, this experiment also confirmed that both acetyl-CoA and the AAT activity were not limiting (Fig. 4B).
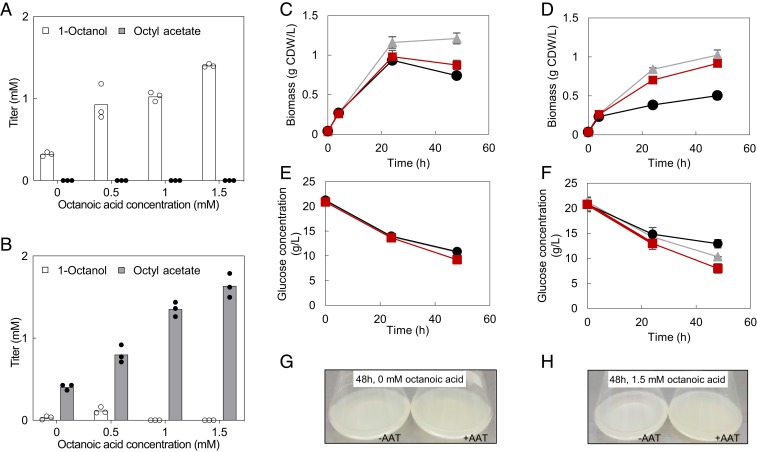
Fig. 4.
(A and B) Identification of limiting substrates in 1-octanol and octyl acetate production by substrate feeding and effect of bioderivatization on E. coli growth and glucose consumption without overlay. E. coli C43 (DE3) Tes3-Sfp-CAR (A) and Tes3-Sfp-CAR + SAAT (B) cultivated with 10% (vol/vol) hexadecane overlay. Different concentrations of octanoic acid were added to the cultures. (C–F) Growth and metabolism of E. coli C43 (DE3) fed with 0 mM (C and E) and 1.5 mM (D and F) octanoic acid in the absence of solvent overlay. Three strains were tested: negative control (gray triangles), Tes3-Sfp-CAR (black circles), and Tes3-Sfp-CAR + SAAT (red squares). (G and H) Photographs of liquid cultures taken at 48 h when supplemented with 0 mM octanoic acid (G) and 1.5 mM octanoic acid (H) in the absence of solvent overlay.
A similar substrate feeding experiment was also carried out in the absence of the hexadecane solvent overlay. However, this precluded quantification of the products, since both 1-octanol and octyl acetate are volatile. Thus, growth and glucose consumption were instead evaluated as indicators of cellular and metabolic activity. The experiment indicated that octanoic acid feeding reduced the growth of E. coli even without conversion to 1-octanol and octyl acetate (compare the negative control in Fig. 4 C and D at 48 h; P = 0.027). This is in line with a previous report by Wilbanks and Trinh (12). The 1-octanol–producing strain (Tes3-Sfp-CAR) showed noticeably lower cell density (Fig. 4 C, D, G, and H) and glucose consumption (Fig. 4 E and F), an effect that was largely alleviated by esterification (Tes3-Sfp-CAR + SAAT), thereby providing early insight into the main hypothesis of the work. The differences between the alcohol- and ester-forming strains were reduced in the absence of externally added octanoic acid (Fig. 4 C, D, G, and H), supporting the idea that further pathway optimization was essential to evaluate the effect of bioderivatization under conditions that were more likely to be relevant for application.
The first-generation 1-octanol pathway was also evaluated in two different E. coli strain backgrounds: E. coli B strain C43 (DE3) and K-12 strain BW25113. As the latter strain (0.74 mM, 96.47 mg/L, 27 mmol/mol glucose) displayed greater alcohol yield (P = 0.042) than the former (0.35 mM, 45.44 mg/L, 10.15 mmol/mol glucose) (SI Appendix, Fig. S7), the E. coli BW25113 strain background was used in the subsequent experiments.
Thioesterase Selection for 1-Octanol Production and Growth and Metabolism in Response to Bioderivatization.
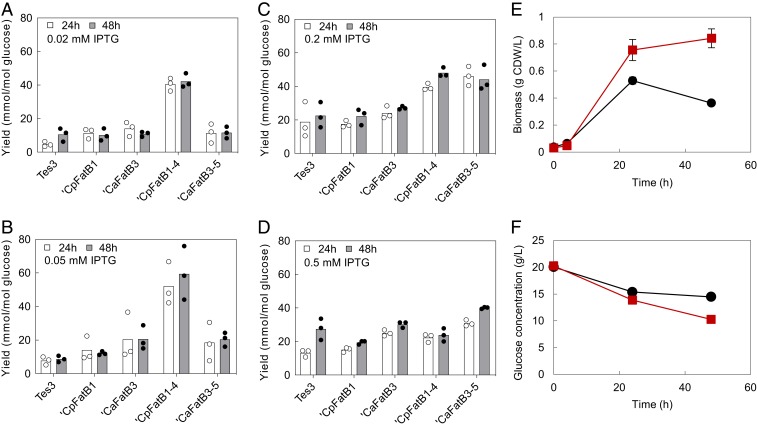
The finding that exogenous addition of octanoic acid greatly enhanced octyl acetate productivity (Fig. 4) suggested thioesterase as the primary limitation for the pathway. Four different C8-preferring thioesterases were therefore evaluated at varying levels of protein expression. The new thioesterases were based on Cuphea palustris (CpFatB1) (29) and Cuphea avigera pulcherrima (CaFatB3) (30) with varying mutations and truncations (SI Appendix, Fig. S1), two of which were designed according to recent work of Lozada et al. (31). Surprisingly, all new thioesterases showed an improvement in 1-octanol production compared with Tes3 (15), but the performance varied widely in response to changes in the inducer concentration (Fig. 5). ‘CpFatB1-4 was the best performer with the highest concentration of 1-octanol (4.29 mM, 558.2 mg/L, 59.48 mmol/mol glucose) after 48 h of inoculation with 0.05 mM IPTG, a concentration of 1-octanol ∼8 times higher than that obtained with Tes3 under the same conditions (P = 0.03 comparing ‘CpFatB1-4 with Tes3 and P = 0.044 comparing ‘CpFatB1-4 with ‘CaFatB3-5, all at 48 h) (Fig. 5B). ‘CaFatB3-5 was not as effective but reached the maximum titer of 1-octanol (2.90 mM, 377.37 mg/L, 44.20 mmol/mol glucose) at the higher IPTG concentration of 0.2 mM (Fig. 5C). With these improvements, the internally produced 1-octanol now exceeded the titer (0.75 mM) at which the underivatized product affected the growth of the strain in the absence of solvent overlay.
Fig. 5.
Optimization of 1-octanol production and effect of bioderivatization on E. coli growth and metabolism. (A–D) 1-octanol yield from E. coli BW25113 strains 7 to 11 (SI Appendix, Table S1) at 24 h and 48 h when cultivated with 10% (vol/vol) hexadecane overlay with different IPTG concentrations: 0.02 mM (A), 0.05 mM (B), 0.2 mM (C), and 0.5 mM (D). (E) Average growth curves of E. coli BW25113 strains ‘CpFatB1-4-Sfp-CAR (black circles) and ‘CpFatB1-4-Sfp-CAR + ATF1 (red squares). (F) Glucose consumption when cultivated in the absence of solvent overlay.
Lozada et al. (31) reported that ‘CpFatB1-4 was most effective when the expression level was low, as the strain expressing it showed a growth defect at higher expression levels. This was also observed in our study (SI Appendix, Fig. S8A). Since the same promoter was also used in the second AAT-encoding plasmid, we speculated that an imbalance in pathway enzyme activities may compromise the final outcome. Thus, the second-best thioesterase (‘CaFatB3-5) was also used in further production experiments, given that growth of the ‘CaFatB3-5 strain was not greatly influenced by the IPTG concentration (SI Appendix, Fig. S8B).
Following optimization of the 1-octanol pathway, the effect of esterification on growth and glucose consumption of the biocatalytic host was evaluated. Strains harboring ‘CpFatB1-4, with or without ATF1, were induced at the IPTG concentration found to be optimal for ‘CpFatB1-4 (0.05 mM) and grown in the absence of a hexadecane overlay. The presence of ATF1 enhanced both growth and glucose consumption (Fig. 5 E and F), as was also observed in the previous octanoic acid feeding experiments (Fig. 4) with P = 0.006 and 0.003 when comparing growth and glucose consumption at 48 h, respectively.
Effect of Bioderivatization on Production with Enhanced Pathway Flux.
Would bioderivatization also influence C8 productivity now that pathway flux was up to 8 times greater? To answer this question, the effect of ATF1 in the improved (high-flux) ‘CpFatB1-4 and ‘CaFatB3-5 strains was evaluated in the presence of a hexadecane solvent overlay at three different IPTG levels. The results were complex depending on the conditions, but a number of interesting observations were made. Cellular growth and glucose consumption were positively influenced by ATF1 in some of the induction/thioesterase combinations (e.g., ‘CafatB3-5 at 0.2 and 0.5 mM IPTG) but not in others (e.g., none of the strains at 0.05 mM IPTG) (SI Appendix, Figs. S9 and S10). Microtiter plate well growth at 0.2 mM IPTG also indicated enhanced growth in the first 24 h (SI Appendix, Fig. S11). In contrast, no difference in growth was observed in response to esterification at low IPTG induction, as was also found with the first-generation low-flux strains (Fig. 3), despite the fact that a substantial impact on growth was observed in the absence of solvent overlay (Fig. 5 E and F). Thus, the solvent overlay partially mitigated the “growth and metabolism” defect caused by the pathway and/or its product, at least when compared with the same strain cultured in the absence of solvent overlay, that is, 0.05 mM IPTG with the ‘CpFatB1-4 strain.
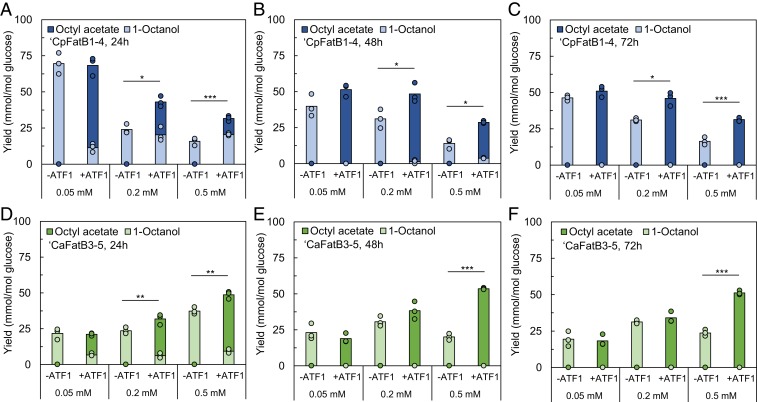
At the lowest protein expression inducer level (0.05 mM IPTG), esterification had no impact on yield (Fig. 6) or titer (SI Appendix, Fig. S12) for both strains. In contrast, at the higher IPTG levels, almost all IPTG/strain/time combinations showed both improved titer and yield when ATF1 was coexpressed. Interestingly, this means that all combinations of IPTG dosage and strain sampled at 24 and 48 h except one (‘CpFatB3-5, 0.2 mM, 48 h), had increased C8 productivity, even with the variable impacts on growth or glucose consumption. In other words, the effect of esterification under these conditions was partially independent of any effect on cellular activity, and thus the effect of esterification on product toxicity was not the sole reason for the improved productivity under these conditions.
Fig. 6.
Comparison of in vivo 1-octanol and octyl acetate production from E. coli BW25113 in the presence of solvent overlay. Shown is 1-octanol and octyl acetate production by E. coli strains ‘CpFatB1-4-Sfp-CAR and ‘CpFatB1-4-Sfp-CAR + ATF1 at 24 h (A), 48 h (B), and 72 h (C), and by strains ‘CaFatB3-5-Sfp-CAR and ‘CaFatB3-5-Sfp-CAR + ATF1 at 24 h (D), 48 h (E), and 72 h (F).
Most likely, the additional positive effect of bioderivatization on productivity can be explained by esterification enhancing product removal, either by enhancing the compatibility with native efflux transporter(s) or, more likely, by enhancing product solubility in the solvent overlay and thereby facilitating the transfer away from the cell. In turn, this would reduce the local product concentration, with consequences for both toxicity and pathway thermodynamics (due to a reduction in the actual free energy change of the entire pathway including efflux). The lack of effect of bioderivatization on C8 productivity at the low (0.05 mM) IPTG induction level might be explained by an imbalance between pathway catalysts complicated by the contrasting impact of IPTG on the two different thioesterases. This difference is illustrated by the change in the product titer ratio between strains with and without ATF1 in response to the concentration of IPTG used for induction (SI Appendix, Figs. S13 and S14).
To ensure that the measurements from the solvent phase were representative of the total bioproduction system, the distribution of products between the liquid and solvent phases was quantified with a 1-octanol– and octyl acetate-producing strain. Similar to the mock experiments (SI Appendix, Fig. S6), the majority of 1-octanol (84%; mole 1-octanol in solvent phase/mole 1-octanol in aqueous and solvent phases) and all the octyl acetate were found in the solvent phase (SI Appendix, Fig. S15).
Transfer of the Bioderivatization Concept to a Different Organism.
We recently reported the introduction of 1-octanol biosynthesis into cyanobacteria (16). The use of solvent overlay was found to be important, as the cyanobacterium (Synechocystis sp. PCC 6803) was even more sensitive than E. coli to 1-octanol. Given the positive impact of esterification on C8 biosynthesis in E. coli, we wondered whether similar benefits could also be observed in Synechocystis sp. PCC 6803. External addition of 1-octanol and octyl acetate indicated reduced sensitivity to the ester compared with 1-octanol (SI Appendix, Fig. S2), although Synechocystis sp. PCC 6803 was clearly more sensitive than E. coli to octyl acetate.
Heterologous expression of SAAT in combination with the ‘CpFatB1-4 thioesterase, Sfp, and CAR enabled complete conversion of 1-octanol into octyl acetate in this species as well. Strains expressing ATF1 could not be obtained despite repeated transformation attempts. Cultivation of the two strains in the absence of a solvent overlay resulted in marked differences in growth and appearance by day 10 (Fig. 7 A and B), but by day 20 the 1-octanol strain had caught up, and both cultures were vibrant green. The day 20 culture was then used as a preculture for a new culture with fresh medium that contained hexadecane solvent overlay.
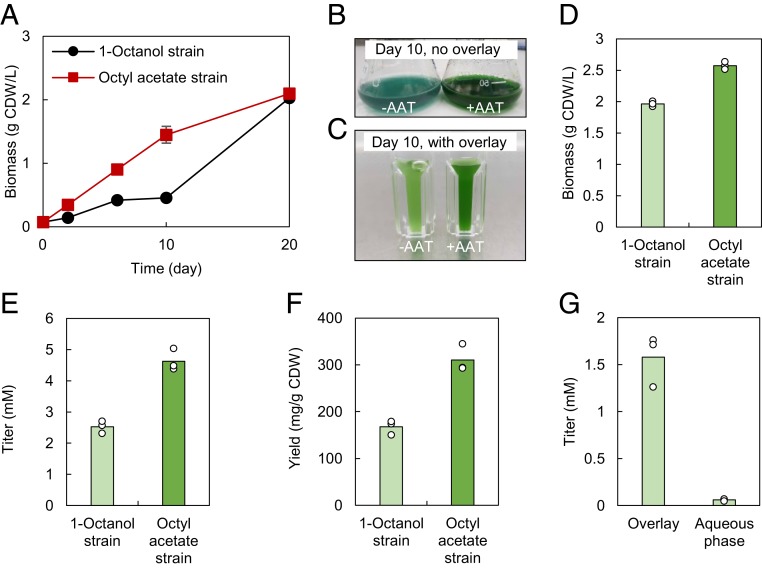
Fig. 7.
Effect of bioderivatization on product titer, yield, and cellular growth of engineered Synechocystis sp. PCC 6803. (A) Biomass accumulation of Δaas-PnrsB-Sfp-CAR-Pcoa-‘CpFatB1-4 and Δaas-PnrsB-Sfp-CAR-Pcoa-‘CpFatB1-4-SAAT cultivated in the absence of overlay for 20 d. All strains were induced to express recombinant proteins on day 2 with 625 nM cobalt and 15 µM nickel. After 20 d of cultivation in the absence of overlay, each culture was used to inoculate fresh cultures with overlay and induced to express recombinant proteins as above. (B and C) Photographs of the 1-octanol strain (−AAT) and octyl acetate strain (+AAT) on day 10 when cultivated in the absence (B) or presence (C) of 30% (vol/vol) hexadecane solvent overlay. (D–F) Biomass accumulation (D), product titer (E), and product yield (F) in solvent overlay cultures sampled on day 16. (G) Localization of 1-octanol from a 1-octanol–producing strain with solvent overlay sampled on day 10. Student’s t test analyses on all data shown in D–F were statistically significant (P ≤ 0.01).
In the presence of solvent overlay, the addition of SAAT resulted in enhanced growth (Fig. 7 C and D), C8 product titer (Fig. 7E), and yield (Fig. 7F) after 16 d of cultivation and induction of protein expression on day 2. Surprisingly, none of the 1-octanol–producing strains lost the ability to accumulate its product in this study, in contrast to what we observed earlier (16). The initial no-induction precultures of the same strains were also used to inoculate cultures that were immediately provided a solvent overlay and then induced for protein expression and cultured for 10 d. Such cultures accumulated 1.6 ± 0.4 mM 1-octanol and 2.4 ± 0.5 mM octyl acetate of each respective product. Similar to what was found with E. coli (SI Appendix, Fig. S15), the majority of the 1-octanol (96%; mole 1-octanol in solvent phase/mole 1-octanol in aqueous and solvent phases) produced by the strain lacking AAT accumulated in the solvent phase (Fig. 7G).
Effect of Conjugate Type: Hydrophilic Instead of Hydrophobic Bioderivatization.
Other conjugation types are also possible—for example, glycosylation. This requires changes in metabolic engineering and has implications for both cellular efflux and product separation. The simplest glycosylation to implement is O-glucosylation, thereby resulting in the formation of octyl glucoside, a nonionic alkyl glucoside used as a surfactant (32). Octyl glucoside was only slightly less toxic to E. coli than 1-octanol (Fig. 2). The glucoside is much more soluble in water than the alcohol—5.5 g/L (33) vs. 0.5 g/L (34), respectively—and will require a different choice of downstream processing for separation compared with esters.
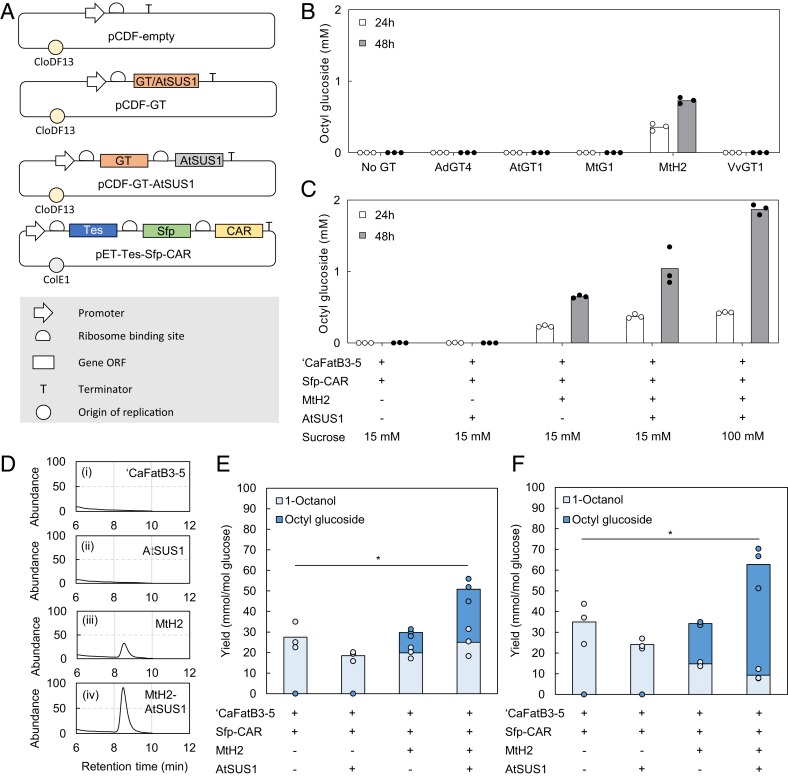
The ‘CaFatB3-5 thioesterase 1-octanol pathway was extended by overexpression of a glycosyltransferase (GT) (Fig. 3A). Five candidates were selected based on their reported activity toward longer-chain alcohols (35, 36). By combining a glycosyltransferase from Medicago truncatula (MtH2) (37) with the 1-octanol pathway, 0.73 mM (214 mg/L) octyl glucoside was produced after 48 h (Fig. 8B). Therefore, MtH2 was selected for further investigations. The octyl glucoside titers were low in comparison with the octyl acetate pathway if the amount of available 1-octanol was taken into consideration (Figs. 3A and 8). A possible explanation for this may be inadequate GT activity, as reported previously (38).
Fig. 8.
Engineering a synthetic pathway for octyl glucoside biosynthesis in E. coli. (A) Schematic diagram of plasmids used to generate strains 13 and 16 to 22 (SI Appendix, Table S1). (B) Selection of GT for octyl glucoside production with ‘CaFatB3-5-Sfp-CAR, ‘CaFatB3-5-Sfp-CAR + AdGT4, ‘CaFatB3-5-Sfp-CAR + AtGT1, ‘CaFatB3-5-Sfp-CAR + MtG1, ‘CaFatB3-5-Sfp-CAR + MtH2, and CaFatB3-5-Sfp-CAR + VvGT1 in the absence of solvent overlay. (C) Octyl glucoside production with strains ‘CaFatB3-5-Sfp-CAR, ‘CaFatB3-5-Sfp-CAR + MtH2, ‘CaFatB3-5-Sfp-CAR + AtSUS1, and ‘CaFatB3-5-Sfp-CAR + MtH2 + AtSUS1, supplemented with 15 mM and 100 mM sucrose. (D) Chromatograms from cultures shown in C at 48 h when supplemented with 15 mM sucrose. (E and F) 1-Octanol and octyl glucoside production in the presence of solvent overlay comparing ‘CaFatB3-5-Sfp-CAR), ‘CaFatB3-5-Sfp-CAR + AtSUS1, ‘CaFatB3-5-Sfp-CAR + MtH2, and ‘CaFatB3-5-Sfp-CAR + MtH2 + AtSUS1, cultured with 15 mM sucrose at 24 h (E) and 48 h (F).
Insufficient glucosylation may be due to a limitation in the supply of substrates, (i.e., UDP-glucose) or inhibition by UDP (38), one of the products of the glucosylation reaction. Another possible limiting factor is UDP-glucose availability. Naturally, UDP-glucose is involved in bacterial cell wall synthesis and produced at a basal level in E. coli (39). Given the relatively low UDP-glucose pool, enhanced regeneration may be important to achieve high glucoside productivity. SUS catalyzes the reversible conversion of sucrose and UDP into UDP-glucose and has been shown to enhance glucoside production (40). Thus, the SUS from Arabidopsis thaliana (AtSUS1) was coexpressed with the GT and 1-octanol pathway (Fig. 3A), resulting in improved octyl glucoside production (Fig. 8C) dependent on the external supply of sucrose. Notably, SUS not only helps maintain the supply of UDP-glucose, but also lowers the intracellular concentration of UDP, thereby diminishing any inhibitory effect it may have on MtH2. Without further investigation, it is difficult to fully elucidate the cause of inefficiency in the octyl glucoside pathway; however, sufficient production was achieved, answering the question of whether glucosylation enhances net 1-octanol production.
Even though octyl glucoside is secreted and soluble in the aqueous culture medium (SI Appendix, Fig. S16), hexadecane was used as an overlay of the cultures to quantify any 1-octanol that was excreted too quickly and thereby evaded glucosylation. Surprisingly, more octyl glucoside was produced in the presence of solvent overlay than in its absence (P < 0.001 at 48 h); compare Fig. 8C and SI Appendix, Fig. S17. As the solvent overlay had no impact on the growth of these strains, this could not be explained by any differential toxicity. An alternative possibility is that the solvent overlay captures 1-octanol and enables its reuse by the cells and conversion into glucosides in the stationary phase. The yield of 1-octanol was improved, with P = 0.049 and 0.040 at 24 and 48 h, respectively (Fig. 8 E and F), in strains combining the 1-octanol pathway with both MtH2 and AtSUS1 compared with the strains carrying only the 1-octanol pathway. Similarly, the product titers were also improved by bioderivatization (P = 0.0009 and 0.008 at 24 and 48 h, respectively) (SI Appendix, Fig. S17).
The combined analysis suggests that bioderivatization has the potential to enhance at least some biotechnological production systems in which product toxicity and/or insufficient product solubility places excessive limits on bio-based production of valuable chemicals. In most circumstances, the growth defect caused by the target product (or pathway/parts) was overcome either by adding solvent overlay or by bioderivatization. This is most clearly shown in SI Appendix, Fig. S2 or by comparing Fig. 5E with SI Appendix, Fig. S9A. In several instances, bioderivatization enhanced productivity (Figs. 6 and 8) without also enhancing growth (e.g., SI Appendix, Figs. S9, S11, and S18).
The increased water solubility of octyl-β-glucoside relative to 1-octanol, along with the small difference in toxicity between the two compounds, support the notion that enhanced product solubility also plays an important role—that is, bioderivatization does not improve productivity solely by mitigating product toxicity. Regardless, and most importantly, the addition of solvent overlay alone did not result in the same level of productivity enhancement—the ultimate objective of the biotechnological application—as bioderivatization (e.g., Fig. 7). Thus, even if solvent overlay can help overcome the growth defect, bioderivatization achieves the same effect and also improves productivity. In addition, as mentioned in the introductory paragraph, solvent overlay is not always going to be a suitable universal solution.
In future studies, it would be interesting to evaluate also how this approach may influence product:process separation, as illustrated in Fig. 1. For example, esters are attractive in this respect, as they have lower water solubility than their corresponding alcohols, which is likely to reduce the energetic cost of the product separation process from the aqueous media (41). The postbiology separation strategy and capability for cellular excretion will also influence whether to opt for a more hydrophobic or a more hydrophilic derivative. At least in the case of 1-octanol, the host cells evaluated in this work were able to excrete both conjugate types, but this might not apply for all target chemicals.
Conclusions
In this work, two different strategies for bioderivatization were implemented, and the effect on bioproduction of the toxic chemical 1-octanol was systematically evaluated in two different species. In E. coli, the conversion of 1-octanol into octyl acetate resulted in enhanced product titer and yield in most cases and also enhanced growth and glucose consumption in one-half of the cases. Clear and more consistent positive effects on both growth and productivity were observed when the hydrophobic bioderivatization strategy was transferred to cyanobacteria or when a hydrophilic bioderivatization strategy was implemented. The impact of esterification on bioproduction was influenced by the presence or absence of solvent overlay, as well as by the species and induction level, while the positive effect of glucosylation could be observed under both environmental conditions. We assumed that any improvements in productivity that were accompanied by improvements in cellular activity were caused by reduced product toxicity.
Interestingly, closer inspection of the data suggested independent effects of bioderivatization on cellular activity (i.e., growth and glucose consumption) with or without an effect on productivity, or on productivity without any effect on cellular activity. The latter effect is likely related to enhanced product removal caused by the enhanced product solubility of derivatives, with esters more soluble in solvent overlay and glucosides more soluble in the aqueous phase. The results presented in this paper support the idea that engineered bioderivatization that mimics the evolved metabolism of many specialized metabolite accumulating species may offer benefits for biotechnology, even with entirely synthetic metabolism.
Supplementary Material
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme Project PHOTOFUEL under Grant 640720. P.S. and I.S.Y. have received doctoral scholarships from the Thai Development and Promotion of Science and Technology Talents Project, and Indonesia Endowment Fund for Education, respectively.
Footnotes
Competing interest statement: The authors are inventors on the UK patent application entitled “'Bio-based production of toxic chemicals.” The subject is a method of producing a derivative of 1-octanol, as well as microorganisms and expression vectors for use in said method.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914069117/-/DCSupplemental.
References
- 1.Bozell J. J., Petersen G. R., Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 12, 539–554 (2010). [Google Scholar]
- 2.Gallage N. J., et al. , Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 5, 4037 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyne M. E., Narcross L., Martin V. J. J., Engineering plant secondary metabolism in microbial systems. Plant Physiol. 179, 844–861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop M. J., et al. , Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 7, 487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer M., Porro D., Mattanovich D., Branduardi P., Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 26, 100–108 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A., Tolerance engineering in bacteria for the production of advanced biofuels and chemicals. Trends Microbiol. 23, 498–508 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Sirikantaramas S., Yamazaki M., Saito K., Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem. Rev. 7, 467–477 (2008). [Google Scholar]
- 8.de Roode B. M., Franssen M. C. R., van der Padt A., Boom R. M., Perspectives for the industrial enzymatic production of glycosides. Biotechnol. Prog. 19, 1391–1402 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Jones P., Vogt T., Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 213, 164–174 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Halkier B. A., Møller B. L., Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol. 90, 1552–1559 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saerens S. M. G., Delvaux F. R., Verstrepen K. J., Thevelein J. M., Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 3, 165–177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilbanks B., Trinh C. T., Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol. Biofuels 10, 262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W., et al. , Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 9, 131 (2016). Erratum in: Biotechnol. Biofuels9, 58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Garzón C. S., Straathof A. J. J., Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 32, 873–904 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Akhtar M. K., Dandapani H., Thiel K., Jones P. R., Microbial production of 1-octanol: A naturally excreted biofuel with diesel-like properties. Metab. Eng. Commun. 2, 1–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yunus I. S., Jones P. R., Photosynthesis-dependent biosynthesis of medium chain-length fatty acids and alcohols. Metab. Eng. 49, 59–68 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kallio P., Pásztor A., Thiel K., Akhtar M. K., Jones P. R., An engineered pathway for the biosynthesis of renewable propane. Nat. Commun. 5, 4731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aharoni A., et al. , Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12, 647–662 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storch M., et al. , BASIC: A new biopart assembly standard for idempotent cloning provides accurate, single-tier DNA assembly for synthetic biology. ACS Synth. Biol. 4, 781–787 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Wang Q., et al. , Significantly improved equilibrium yield of long-chain alkyl glucosides via reverse hydrolysis in a water-poor system using cross-linked almond meal as a cheap and robust biocatalyst. Chin. J. Catal. 33, 275–280 (2012). [Google Scholar]
- 21.Fay D. S., Gerow K., A biologist’s guide to statistical thinking and analysis. Wormbook 1–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S., Qureshi N., How microbes tolerate ethanol and butanol. N. Biotechnol. 26, 117–121 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Beekwilder J., et al. , Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 135, 1865–1878 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomke P. D., Rathod V. K., Enzyme as biocatalyst for synthesis of octyl ethanoate using acoustic cavitation: Optimization and kinetic study. Biocatal. Agric. Biotechnol. 7, 145–153 (2016). [Google Scholar]
- 25.Röttig A., Steinbüchel A., Acyltransferases in bacteria. Microbiol. Mol. Biol. Rev. 77, 277–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstrepen K. J., et al. , Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 69, 5228–5237 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez G. M., Tashiro Y., Atsumi S., Expanding ester biosynthesis in Escherichia coli. Nat. Chem. Biol. 10, 259–265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cumplido-Laso G., et al. , The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J. Exp. Bot. 63, 4275–4290 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Dehesh K., Edwards P., Hayes T., Cranmer A. M., Fillatti J., Two novel thioesterases are key determinants of the bimodal distribution of acyl chain length of Cuphea palustris seed oil. Plant Physiol. 110, 203–210 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjellström H., Strawsine M., Silva J., Cahoon E. B., Ohlrogge J. B., Disruption of plastid acyl:acyl carrier protein synthetases increases medium chain fatty acid accumulation in seeds of transgenic Arabidopsis. FEBS Lett. 587, 936–942 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Hernández Lozada N. J., et al. , Highly active C8-acyl-ACP thioesterase variant isolated by a synthetic selection strategy. ACS Synth. Biol. 7, 2205–2215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konidala P., He L., Niemeyer B., Molecular dynamics characterization of n-octyl-β-D-glucopyranoside micelle structure in aqueous solution. J. Mol. Graph. Model. 25, 77–86 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Focher B., et al. , Micelles of 1-alkyl glucoside and maltoside: Anomeric effects on structure and induced chirality. Chem. Phys. Lett. 158, 491–494 (1989). [Google Scholar]
- 34.Rumble J. R., CRC Handbook of Chemistry and Physics (CRC Press, 2019). [Google Scholar]
- 35.Bönisch F., et al. , Activity-based profiling of a physiologic aglycone library reveals sugar acceptor promiscuity of family 1 UDP-glucosyltransferases from grape. Plant Physiol. 166, 23–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X. Z., Wang X., Dixon R. A., Mutational analysis of the Medicago glycosyltransferase UGT71G1 reveals residues that control regioselectivity for (iso)flavonoid glycosylation. J. Biol. Chem. 281, 34441–34447 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Li L., et al. , Crystal structure of Medicago truncatula UGT85H2—Insights into the structural basis of a multifunctional (iso)flavonoid glycosyltransferase. J. Mol. Biol. 370, 951–963 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Masada S., et al. , An efficient chemoenzymatic production of small molecule glucosides with in situ UDP-glucose recycling. FEBS Lett. 581, 2562–2566 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Ruffing A., Chen R. R., Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microb. Cell Fact. 5, 25 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y., Anderson S., Zhang Y., Garavito R. M., The structure of sucrose synthase-1 from Arabidopsis thaliana and its functional implications. J. Biol. Chem. 286, 36108–36118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Berg C., Heeres A. S., van der Wielen L. A. M., Straathof A. J. J., Simultaneous Clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate. Biotechnol. Bioeng. 110, 137–142 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.