Abstract
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder with a multifactorial pathophysiology. The gut microbiota differs between patients with IBS and healthy individuals. After a bout of acute gastroenteritis, postinfection IBS may result in up to approximately 10% of those affected. Small intestinal bacterial overgrowth (SIBO) is more common in patients with IBS than in healthy individuals, and eradication of SIBO with systemic antibiotics has decreased symptoms of IBS in some patients with IBS and SIBO. The nonsystemic (i.e. low oral bioavailability) antibiotic rifaximin is indicated in the United States and Canada for the treatment of adults with IBS with diarrhea (IBS-D). The efficacy and safety of 2-week single and repeat courses of rifaximin have been demonstrated in randomized, placebo-controlled studies of adults with IBS. Rifaximin is widely thought to exert its beneficial clinical effects in IBS-D through manipulation of the gut microbiota. However, current studies indicate that rifaximin induces only modest effects on the gut microbiota of patients with IBS-D, suggesting that the efficacy of rifaximin may involve other mechanisms. Indeed, preclinical data reveal a potential role for rifaximin in the modulation of inflammatory cytokines and intestinal permeability, but these two findings have not yet been examined in the context of clinical studies. The mechanism of action of rifaximin in IBS is likely multifactorial, and further study is needed.
Keywords: antibiotic, irritable bowel syndrome, microbiota, mechanism, pathophysiology, rifaximin
Introduction
Irritable bowel syndrome (IBS) is a disorder of gut–brain interactions characterized by recurrent bouts of abdominal pain and altered bowel habits; patients also often experience bloating.1 IBS is further characterized by the predominant stool form observed: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), and IBS with a mixture of constipation and diarrhea (IBS-M).1 IBS is one of the most common gastrointestinal (GI) conditions, with a pooled worldwide prevalence ranging from 8.8% to 11.2%.2,3 A three-country survey using ROME IV IBS diagnostic criteria reported estimated prevalence rates overall, and specifically for females, at 5.5% and 7.5%, respectively (United Kingdom), 5.7% and 7.8% (Canada), and 6.1% and 7.1% (United States).4 The pathophysiology of IBS is multifactorial in nature, and it is thought to include contributions from factors such as gut microbiota dysbiosis, altered intestinal and colonic permeability, GI immune cell activation, visceral hypersensitivity, and abnormal gut–brain interactions.1,5 The goal of this narrative review is to provide an overview of the factors proposed to be involved in IBS pathophysiology, and to discuss the role rifaximin may play in modulating these pathophysiologic factors and improving symptoms in patients with IBS.
Methods
A PubMed search of all available English-language articles to date was conducted on 6 May 2019, using the following search terms: ‘irritable bowel syndrome,’ ‘pathophysiology,’ ‘pathogenesis,’ ‘rifaximin,’ ‘mechanism of action,’ ‘pharmacology,’ ‘pharmacokinetics,’ ‘microbiota,’ ‘bacteria,’ ‘inflammation,’ ‘immunology,’ ‘cytokines,’ ‘hypersensitivity,’ ‘permeability,’ ‘small intestinal bacterial overgrowth,’ and ‘motility.’ Reference lists from review articles were used to identify additional publications for inclusion.
Pathophysiology of IBS
Role of gut microbiota in IBS
Alterations in the gut microbiota are thought to be involved in the pathophysiology of IBS. Indeed, the gut microbiota is altered in patients with IBS compared with healthy individuals, as was demonstrated by a 2019 systematic review of 24 studies.6 In that publication, patients with IBS had increased levels of potentially harmful bacteria from the Enterobacteriaceae family (n = 4 studies) and Bacteroides species (n = 4 studies), compared with healthy individuals.6 Levels of the bacteria from the Clostridiales order and Faecalibacterium genus were decreased in patients with IBS versus healthy individuals (3 studies each).6
Emerging data suggest that specific subgroups of patients with IBS might be characterized by a distinct gut microbiota profile.7–9 In one study, the intensity of IBS symptoms was associated with a specific fecal-microbiota profile in patients with IBS (n = 110); the abundance of bacteria of the Prevotella genus decreased with increasing symptom intensity (p < 0.05).8 In a prospective study of women with IBS (n = 76), both fecal microbiota composition and lower diversity were associated with an increase in extraintestinal pain symptoms (composite assessment) and reduced quality of life (p < 0.05 for both); however, the composition and diversity of fecal microbiota were not associated with daily abdominal pain, bloating, flatulence, or psychologic distress.9 An increased ratio of Firmicutes:Bacteroidetes was associated with looser stool forms, with the mean ratio higher in patients with IBS-D and IBS-M compared with IBS-C (21.0 and 19.0 versus 9.9; p = 0.02).9 A meta-analysis of 13 studies comparing GI bacterial gene expression profiles in tissue and fecal samples found significantly lower concentrations of some bacterial strains in patients with IBS (n = 360) compared with healthy individuals (n = 268): Lactobacillus genus [standardized mean difference (SMD), –0.8; p < 0.001], Bifidobacterium genus (SMD, –1.2; p < 0.001), and Faecalibacterium prausnitzii (SMD, –1.0; p < 0.001).7 In subgroup analyses (n = 4 studies), patients with IBS-D had significantly lower concentrations of Lactobacillus and Bifidobacterium compared with healthy individuals (Lactobacillus: SMD, –1.8; p < 0.001; Bifidobacterium: SMD, –1.4; p < 0.001), while patients with IBS-C did not.7
A study comparing the fecal- and mucosa-associated microbiota between patients with IBS-D (n = 23 for both samples) and healthy individuals (n = 19 and n = 24, respectively) reported a decrease in fecal microbial richness in patients with IBS-D compared with healthy individuals (p < 0.05).10 Further, while bacteria of the Faecalibacterium genus were present in all fecal samples, the proportion of Faecalibacterium was lower in patients with IBS-D (0.04) compared with healthy individuals (0.05; p = 0.02), and the proportion of Enterobacteriaceae was greater in patients with IBS-D (0.003) versus healthy individuals (0.0006; p = 0.03). In that study, mucosal bacterial richness did not differ between the two groups.
Antibacterial gene expression was altered in patients with IBS (n = 31) compared with healthy individuals (n = 16), with 15 antibacterial genes downregulated (most associated with the interferon regulatory factor 7 pathway) and 1 upregulated (suppressor of G2 allele of SKP1 homolog gene).11 In addition, antibacterial gene expression differed between patients with IBS considered to have an overall immune activity profile similar to healthy individuals and those with IBS with an increased overall immune activity profile.11 In healthy women (n = 53), stool consistency was associated with fecal microbial richness; fecal microbial richness decreased in individuals reporting more solid stool form or slower GI transit (r = −0.4; p = 0.0007).12 Further, solid stool forms were associated with the presence of specific microbial populations (i.e. genera Methanobrevibacter, Akkermansia), whereas loose stool forms were associated with the presence of Bacteroides.12 Of note, data regarding the role of the viruses (microvirome) and fungi (mycobiome) in the pathogenesis of IBS are currently lacking.
Mechanisms of postinfection IBS
Postinfection IBS (PI-IBS) is a diagnosis that may be established in patients who meet Rome criteria for IBS soon after they experience an episode of acute gastroenteritis (positive stool culture test result in symptomatic patient, or presence of at least two of the three symptoms of fever, vomiting, or diarrhea); notably, IBS symptoms were not present before the occurrence of gastroenteritis.13 A systematic review of 45 studies (n = 21,421 patients) estimated a 10.1% prevalence rate of PI-IBS for individuals within 12 months after a case of gastroenteritis.14 The analysis also indicated that the 1-year relative risk (RR) for PI-IBS was significantly higher for patients who had gastroenteritis versus those who did not in studies conducted in Asia or Europe, versus those conducted in North America. Based on data from 23 of the studies, individuals with gastroenteritis were at a 4.2-fold greater risk of developing IBS compared with unaffected individuals.14 Another systematic review of six studies reported that patients with travelers’ diarrhea were at a 3.4-fold greater risk of developing PI-IBS compared with healthy individuals.15 Further, a prospective cohort study identified an increased risk of developing PI-IBS in patients who had Salmonella exposure in childhood compared with individuals without known Salmonella exposure [odds ratio (OR) 1.9; 95% confidence interval (CI), 1.2–3.0].16 Survey data suggested that individuals who experienced acute gastroenteritis attributed mostly to Campylobacter, Salmonella, or Shigella, with diarrhea lasting >2 weeks, were at an increased risk of developing PI-IBS compared with patients with diarrhea lasting ⩽1 week [RR 6.5 (95% CI, 1.3–34) for diarrhea duration 15–21 days; RR 11.4 (95% CI, 2.2–58) for duration ⩾22 days].17 A prospective study reported that 16.5% of 345 patients with acute gastroenteritis and 2.6% of 345 age- and sex-matched controls developed PI-IBS after 12 months [p < 0.001; RR, 6.4 (95% CI, 3.2–12.7)].18 In addition to the role of bacteria in development of PI-IBS, norovirus was significantly associated with development of PI-IBS in individuals affected during a waterborne gastroenteritis outbreak in Italy in 2009 compared with unaffected individuals (OR, 11.4; 95% CI, 3.4–37.8; p < 0.0001).19 As well, a prospective study found that a food-borne norovirus illness was associated with a higher risk of PI-IBS at 3 months after the illness compared with individuals who did not contract norovirus during a Canadian outbreak in 2002 (OR, 6.9; 95% CI, 1.0–48.7).20
Parasitic infection has been associated with development of PI-IBS, although this may be limited to specific parasites and is not fully understood.21–23 In the literature, 13.9% of 72 patients with a confirmed infection of Trichinella britovi during an outbreak in Turkey developed PI-IBS after 6 months.21 While results of a population-based, case-control study conducted in Denmark showed that a significant percentage of fecal samples collected over 3 months from 124 patients with IBS had evidence of the parasites Dientamoeba fragilis, Blastocystis, or both, compared with samples from 204 individuals without GI symptoms (D. fragilis: 23.4% versus 34.8%, respectively; p = 0.03; Blastocystis: 14.5% versus 22.1%, p = 0.09; both: 4.8% versus 11.8%, p = 0.04),22 a subsequent study of the same cohort indicated that the greatest percentage of parasitic colonization was in asymptomatic individuals (52.2% of 186) compared with patients with IBS (38.7% of 119).23 Similarly, more asymptomatic individuals than patients with IBS were positive for D. fragilis (38.2% versus 24.4%, respectively) and Blastocystis (27.4% versus 17.6%). Thus, results of these studies indicate that colonization of the GI tract by specific parasites appears to be associated with a healthy gut microbiome, whereas there is less parasitic colonization in patients with IBS.23 Additional studies are warranted to fully understand the association between parasites and bacteria in the human GI tract and development of PI-IBS.
Susceptibility to GI infection resulting in the development of PI-IBS may, in part, depend on an individual’s gut microbiota composition. Indeed, susceptibility to Campylobacter infection was associated with increased GI microbial levels of Bacteroides and Escherichia species.24 Further, individuals who developed Campylobacter or Salmonella infection while traveling had lower baseline fecal microbiota diversity than those who remained infection-free.25 Patients with PI-IBS have GI microbial profiles that can be distinguished from those of healthy individuals, with a 12-fold increase in Bacteroidetes and a 35-fold decrease in Clostridiales in PI-IBS.26 Interestingly, in one study, travel from the United States to Central America or India was associated with gut microbiota dysbiosis in both individuals who developed travelers’ diarrhea (n = 99) and travelers who remained diarrhea-free (n = 12); however, healthy travelers had a significantly lower percentage of Bacteroidetes (0.5%) compared with individuals developing pathogen-associated travelers’ diarrhea (15%; p = 0.0002) or travelers’ diarrhea with no identified pathogen (23%; p = 0.01).27 Gut microbiota dysbiosis observed in healthy travelers may be related to the travel itself disrupting GI homeostasis or related to consumption of food and water at the travel destination. Due to a lack of follow-up data in the study, it is also unknown whether healthy travelers eventually developed diarrhea.27
Interestingly, there are data to suggest that the risk of IBS may not be limited to enteric infections alone. A population-based study reported that a greater percentage of patients with IBS had a history of non-enteric infections compared with individuals without IBS (76.3% versus 66.4%, respectively; OR, 1.7; 95% CI, 1.1–2.7; p = 0.01).28 In another study, a greater percentage of individuals with non-enteric infections developed IBS within 3 months compared with individuals without enteric infections (14.8% versus 1.9%, respectively; OR, 6.1; 95% CI, 1.3–29.1).29
Development of small intestinal bacterial overgrowth in patients with IBS
Cytolethal distending toxin B (CdtB) is a bacterial toxin that is detected after the onset of acute gastroenteritis.30,31 Based on an animal model of Campylobacter jejuni gastroenteritis, effects of Cdt after clearance of infection included development of altered stool form and inflammation, but not long-term histologic changes in the GI epithelium.31,32 Further, this animal model of C. jejuni showed that acute gastroenteritis can lead to the development of bacterial overgrowth.33,34 Circulating levels of anti-CdtB antibodies were elevated in rats infected with C. jejuni, a finding that correlated with development of small intestinal bacterial overgrowth (SIBO).35 Further, anti-CdtB antibodies bind to the host cell adhesion protein vinculin, which plays a role in smooth muscle contraction. Reduced vinculin levels, which could reduce GI motility, were associated with development of SIBO in this model.35
The healthy human gut is inhabited by an estimated 3.8 × 1013 bacteria.36 Bacterial concentrations increase from the duodenum and jejunum (103 to 104/ml) to the ileum (108/ml) to the colon (1011/ml).36 Patients with SIBO have an overabundance of bacteria in the small intestine, potentially due to GI dysmotility, immune activation, or increased GI permeability.37 The odds of SIBO developing in patients with IBS is significantly greater than those for healthy individuals (OR, 4.7; 95% CI, 3.1–7.2).38 The prevalence of SIBO in patients with IBS has varied widely in the literature, ranging from 4% to 84%,37,38 with an estimated pooled prevalence rate of 38% (n = 50 studies).38 The wide range in prevalence rates is thought to be the result of variation in study populations, criteria for establishing a diagnosis of IBS, and methods used to diagnose SIBO.37,38 Demographic and disease characteristics associated with SIBO in patients with IBS include being female (OR, 1.5; 95% CI, 1.0–2.1), older in age (SMD, 3.1 years; 95% CI, 0.9–5.4 years), and having IBS-D (OR, 1.7; 95% CI, 1.3–2.3).38
The recommended method for diagnosing SIBO is breath testing.39 Individuals undergo breath testing in the fasted state, consuming a carbohydrate substrate (e.g. lactulose 10 g, glucose 75 g) that can be metabolized to hydrogen and methane by intestinal microbes; these gases are detectable during exhalation.39 It remains unclear what effects, if any, demographic characteristics (e.g. age, ethnicity, sex) have on breath testing; further, the effects of prebiotics, probiotics, and antibiotics are also unknown.39 Small bowel culture is another diagnostic tool for SIBO, provided a threshold of >103 colony forming units/mL is achieved from a duodenal aspirate.39 However, small bowel culture is limited by its invasive nature, a lack of practical techniques allowing for acquisition of aspirates under sterile conditions, and difficulties in accessing the mid and distal small intestinal segments.39
Antibiotic use and its paradoxical association with IBS
Antibiotic use within the previous year has been associated with an increased risk of development of IBS (RR, 1.9; 95% CI, 1.1–3.1).40 For development of PI-IBS, the odds increased if there was antibiotic use at the time of gastroenteritis, based on a meta-analysis of seven studies (OR, 1.7; 95% CI, 1.2–2.4).14 In addition, findings of a population-based study reported that treatment of nonenteric infections with antibiotics was significantly associated with development of IBS at a later date (OR, 2.3; 95% CI, 1.2–4.3; p = 0.01).28
Paradoxically, treatment of IBS may include antibiotic therapy.41,42 Indeed, eradication of SIBO by antibiotics (e.g. ciprofloxacin, doxycycline, metronidazole, neomycin, rifaximin) has been shown to improve IBS symptoms in a subset of patients.41–43 In one study, eradication of SIBO (i.e. based on lactulose hydrogen breath test results no longer showing two peaks, no hydrogen production <90 min after lactulose consumption, and an absolute change in hydrogen concentration ⩽20 ppm) in patients with IBS was associated with a significant decrease from baseline in abdominal pain (p < 0.001) and diarrhea (p < 0.05).41 Moreover, almost half of the patients who achieved eradication of SIBO in that study no longer met Rome criteria for IBS. In a randomized, double-blind, placebo-controlled study, a greater percentage of patients with IBS and comorbid SIBO (duodenal aspirate of ⩾105 cfu/ml; n = 8) receiving norfloxacin 400 mg twice daily for 10 days achieved global symptom relief compared with patients with IBS without SIBO at 1 month post-treatment (<103 cfu/ml; n = 21; 87.5% versus 14.3%, respectively; p = 0.001).42 Further, norfloxacin was more efficacious than placebo for providing global symptom relief each month for up to 6 months.42 In addition, a meta-analysis of 26 randomized, controlled studies of adults with a diagnosis of SIBO (n = 1141) reported a high rate of SIBO eradication (based on glucose or lactulose breath testing) with rifaximin (pooled rate, 70.8%; 95% CI, 61.4–78.2).43 In the six SIBO studies that included patients with IBS (n = 311), the pooled SIBO eradication rate with rifaximin was similar, at 71.6% (95% CI, 56.7–84.4). Thus, while antibiotics as a broad class are associated with development of IBS, potentially through the enabling of gut microbiota dysbiosis,14,28,40 antibiotics may also improve GI symptoms in a subset of patients with IBS. This symptom improvement certainly could be a consequence of eradicating comorbid SIBO, but it might also result from other mechanisms (e.g. modifying the intestinal or colonic gut microbiota composition in favor of beneficial bacteria, altering expression of virulence factors of pathogenic bacteria).41–45
Evidence of GI mucosal inflammation in IBS
A meta-analysis of 16 studies reported that low-grade mucosal inflammation may play a role in the pathogenesis of IBS, and is associated with IBS symptoms.46 Gastrointestinal immune activation is one factor that may be involved in the pathophysiology of IBS,1,5 and may be linked to visceral hypersensitivity, given that activated immune cells are localized near enteric nerves.47 Visceral hypersensitivity (i.e. altered pain perception to normal physiologic stimulation) is increased in patients with IBS compared with healthy individuals, and approximately half of patients with IBS exhibit hypersensitivity to rectal distension.48–50 Further, 18% of patients with IBS (n = 50) included in a prospective study had visceral hypersensitivity.51 Preclinical data indicated that rats subjected to chronic and repeated stress had increased visceral hypersensitivity compared with control animals, which was significantly decreased by treatment with the antibiotic rifaximin (p < 0.05)52; however, clinical studies are warranted to confirm these effects in patients with IBS.
Mast cell counts were significantly increased in mucosal biopsies from the proximal descending colon of patients with IBS (n = 44) compared with healthy individuals (n = 22; p < 0.001); the number of degranulating mast cells, indicative of mast cell activation, was increased 150% in patients with IBS compared with healthy individuals (p = 0.03).47 Patients with IBS had 223% more mast cells located <5 µm from colonic nerves compared with healthy individuals, a finding that correlated positively with the rate of mast cell degranulation (r = 0.7; p = 0.002).47 The proximity of mast cells to nerves positively correlated with both the intensity and frequency of abdominal pain in patients with IBS (r = 0.8, p = 0.001 and r = 0.7, p = 0.003, respectively).47 Mast cell counts from cecal biopsies were significantly greater in patients with IBS (n = 34) than in healthy individuals (n = 15; p = 0.001); patients with IBS-D and IBS-M had the greatest difference versus healthy individuals (p = 0.001, for both comparisons).53 In patients with IBS, the mast cell count correlated significantly with paracellular permeability (r = 0.4; p = 0.03) and disease intensity (r = 0.6; p = 0.0001).53
Toll-like receptors (TLR) are membrane-bound receptors that recognize and bind microbial components in the GI mucosa, leading to an inflammatory response.54 Women with IBS had a significantly greater expression of TLR-4 and TLR-5 genes in the mucosa of the sigmoid colon and rectum compared with healthy women (p < 0.0001 and p = 0.001, respectively); further, TLR-4 gene expression was significantly greater in women with IBS versus healthy women (p < 0.01).55 Expression levels of the TLR-4 and TLR-5 genes in biopsy samples from the sigmoid colon of patients with IBS (n = 47) were 1.2- and 6-fold greater, respectively, compared with those of healthy individuals (n = 25; p = 0.01 and p < 0.001, respectively); further, for patients with IBS-D (n = 20), TLR-4, and TLR-5 gene expression levels were increased 1.3- and 8-fold, respectively, compared with healthy individuals (p < 0.02 for both).56 As well, TLR-4 and TLR-5 protein levels in colonic crypts and the luminal surface were 4.2- and 6.6-fold greater, respectively, in patients with IBS-D compared with healthy individuals, and TLR-4 gene expression levels correlated with stool frequency in patients with IBS.56
Although the data are inconsistent, increased serum levels of proinflammatory cytokines have been reported in patients with IBS compared with healthy individuals.57,58 In one study, patients with IBS (n = 74) had significantly greater serum levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α compared with healthy individuals (n = 75; p < 0.001 for IL-6 and IL-8; p = 0.04 for TNF-α).57 This finding was also shown in a subgroup of patients with IBS-D, as serum levels of IL-6, IL-8, and TNF-α were significantly greater in patients with IBS-D compared with healthy individuals (p < 0.001 for all comparisons).57 In another study, patients with IBS-D (n = 60) had significantly higher median serum cytokine concentrations compared with healthy individuals (n = 32) for IL-1β (p = 0.004), IL-6 (p < 0.001), IL-8 (p < 0.001), macrophage inflammatory protein-1α (MIP-1α; p < 0.001), and TNF-α (p < 0.001).58 A meta-analysis of four studies reported that serum IL-6 concentrations were increased in patients with IBS compared with healthy controls (SMD, 2.4; 95% CI, 0.5–4.3; p = 0.01).59 A meta-analysis of six studies showed that serum levels of the anti-inflammatory cytokine IL-10 did not differ between patients with IBS (n = 317) and healthy individuals (n = 319; SMD, –0.2; 95% CI, –0.4 to 0.1; p = 0.3); however, a meta-analysis of three studies of patients with IBS (not including PI-IBS) showed that colonic IL-10 mRNA expression was increased in healthy individuals (n = 80) compared with patients with IBS (n = 82; SMD, –0.3; 95% CI, –0.6 to 0.01; p = 0.05).60 Conversely, results of a meta-analysis (n = 6 studies) indicated there was no difference in serum concentrations of the proinflammatory cytokine TNF-α between patients with IBS and healthy individuals (SMD, 0.5; 95% CI, –0.1 to 1.1; p = 0.09).60 Apparent differences in the data regarding cytokine profiles in patients with IBS could be attributed not only to the multifactorial nature of IBS pathophysiology, but also variation in patient populations and research methods across studies.59,60
Evidence of GI permeability in IBS
Intestinal permeability is increased in patients with IBS compared with healthy individuals.61 In one study, patients with IBS-D (n = 40) had significantly increased small intestinal permeability, but not colonic permeability, compared with healthy volunteers (n = 10; p = 0.01).62 Patients with IBS have increased proteolytic activity in the colon, which is associated with increased membrane permeability and visceral hypersensitivity.63 Trypsin-3 has been associated with increased epithelial permeability, increased signaling to submucosal neurons in colonic biopsies, and induced visceral hypersensitivity in vivo in patients with IBS.63 Trypsin-3 protein expression in the colonic epithelium was greater in patients with IBS than in healthy individuals.63
Increased intestinal membrane permeability in patients with IBS was associated with greater translocation of bacteria across the GI epithelium compared with healthy individuals.64 Movement of commensal (e.g. Escherichia coli) and pathogenic (e.g. Salmonella typhimurium) bacteria across the colonic mucosa and epithelium of 37 women with IBS was significantly greater compared with 20 healthy, age-matched women [2-fold (E. coli) and 2.8-fold (S. typhimurium); p < 0.0005, for both comparisons]. These findings suggest a role for bacteria or bacterial metabolites in the modulation of transepithelial and submucosal targets in patients with IBS.64 However, it is unclear how epithelial permeability might impact clinical symptom development in patients with IBS.64
Rifaximin
Multiple randomized, placebo-controlled trials have found rifaximin to improve overall symptoms in a subset of patients with IBS-D.65–67 On the strength of the available data, rifaximin is currently approved for the treatment of adults with IBS-D in the United States and Canada. Though the efficacy of rifaximin has been clearly demonstrated, the mechanisms responsible for the clinical benefits of rifaximin in patients with IBS-D have not been firmly established. With this in mind, we have reviewed current knowledge regarding the pharmacology, clinical impact, impact on the microbiota, inflammatory activity in the GI tract, and intestinal permeability of rifaximin in patients with IBS-D.
Pharmacology
Rifaximin is a nonsystemic antibiotic with low oral bioavailability; generally <0.01% of a single orally administered 400 mg dose is detected in the plasma and urine of healthy volunteers 48 h after administration.68 The presence of bile acids increases the solubility of rifaximin 70- to 120-fold, which may increase the availability of rifaximin to exert antimicrobial and other effects in the small intestine.69 The detection of unchanged drug in stool samples following oral administration indicates that rifaximin has high availability in the GI tract, considered to be a factor in the minimum inhibitory concentrations observed against human GI bacteria (Table 1).68,70–73
Table 1.
In vitro activity of rifaximin against anaerobic bacteria found in the human GI tract.73
| Anaerobe (number of strains tested) | MIC50 (µg/ml) | MIC90 (µg/ml) | Range (µg/ml) |
|---|---|---|---|
| Bacteroides fragilis (20) | 0.25 | >1024 | 0.25 to >1024 |
| Bacteroides ovatus (11) | 1 | 1 | 0.25 to >1024 |
| Bacteroides thetaiotaomicron (10) | 1 | >1024 | 0.25 to >1024 |
| Bacteroides vulgatus (11) | 0.25 | 0.5 | 0.25 to 4 |
| Parabacteroides distasonis/merdae/goldsteinii (17) | 0.25 | 1 | 0.25 to 1 |
| Other Bacteroides species (18) | 0.25 | 0.5 | 0.25 to 0.5 |
| All Bacteroides species (87) | 0.25 | 1 | 0.25 to >1024 |
| Bilophila wadsworthia (13) | 32 | 64 | 32 to 64 |
| Desulfovibrio species (17) | 16 | 32 | 0.25 to 64 |
| Fusobacterium nucleatum (10) | 2 | 8 | 0.5 to 8 |
| Other Fusobacterium species (24) | 16 | >1024 | 0.25 to >1024 |
| All Fusobacterium species (34) | 8 | >1024 | 0.25 to >1024 |
| Porphyromonas species (16) | 0.25 | 0.5 | 0.25 to 1 |
| Prevotella species (31) | 0.25 | 0.5 | 0.25 to 1 |
| All gram-negative species (198) | 0.5 | 64 | 0.25 to >1024 |
| Clostridium clostridioforme (11) | 0.25 | 0.25 | 0.25 to 0.25 |
| Clostridium difficile (10) | 0.25 | 0.25 | 0.25 to 0.25 |
| Clostridium hathewayi (10) | 0.25 | 0.25 | 0.25 to 0.25 |
| Clostridium innocuum (10) | >1024 | >1024 | >1024 to >1024 |
| Clostridium orbiscindens (10) | >1024 | >1024 | 1024 to >1024 |
| Clostridium perfringens (12) | 0.25 | 0.25 | 0.25 to 0.25 |
| Other Clostridium species (106) | 0.25 | >1024 | 0.25 to >1024 |
| All Clostridium species (169) | 0.25 | >1024 | 0.25 to >1024 |
| Gram-positive nonspore-forming rods (107) | 0.5 | >1024 | 0.25 to >1024 |
| Anaerobic gram-positive cocci (62) | 0.25 | 4 | 0.25 to 16 |
| All gram-positive strains (338) | 0.25 | >1024 | 0.25 to >1024 |
| All strains (536) | 0.25 | 256 | 0.25 to >1024 |
GI, gastrointestinal; MIC, minimum inhibitory concentration.
Table adapted with permission from Finegold and colleagues.73
Improvement in IBS symptoms
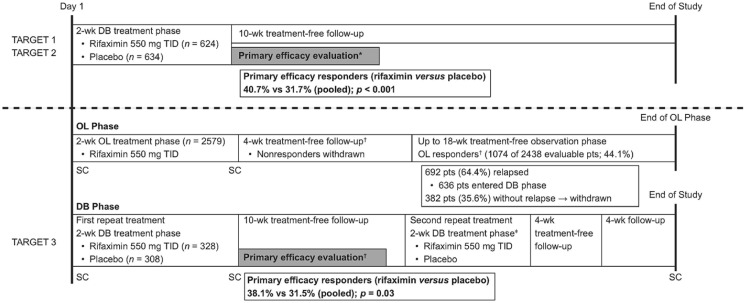
In two identically designed, phase III, double-blind, placebo-controlled studies [Targeted, Nonsystemic Antibiotic Rifaximin Gut-Selective Evaluation of Treatment for IBS-D (TARGET) 1 and TARGET 2; Figure 1],65,66 a significantly greater percentage of patients with IBS-D randomly assigned to receive a 2-week course of rifaximin 550 mg three times daily achieved adequate relief of global IBS symptoms for ⩾2 of the first 4 weeks post-treatment (primary efficacy endpoint) compared with placebo (pooled data; 40.7% versus 31.7%, respectively; p < 0.001).65 The safety profile of rifaximin was generally comparable with that of placebo; the most common adverse events (AEs) reported in patients receiving rifaximin versus placebo included headache (6.1% versus 6.6%, respectively), upper respiratory tract infection (5.6% versus 6.2%), and abdominal pain (4.6% versus 5.5%)65; serious AEs occurred in 1.6% and 2.4% of patients receiving rifaximin and placebo, respectively; no patients in either study developed Clostridium difficile-associated colitis or ischemic colitis.65
Figure 1.
Study design and summary of findings from phase III studies of rifaximin (TARGET 1–3).65,66
DB, double-blind; OL, open-label; SC, stool collection; TARGET, Targeted, Nonsystemic Antibiotic Rifaximin Gut-Selective Evaluation of Treatment for IBS-D; TID, three times daily.
*Primary efficacy end point defined as adequate relief of global IBS symptoms for ⩾2 of the first 4 weeks post-treatment.
†Response defined as simultaneously achieving both a ⩾30% decrease from baseline in the mean weekly abdominal pain score and ⩾50% decrease from baseline in the number of days/week with Bristol Stool Scale type 6 or 7 stool for ⩾2 of the first 4 weeks posttreatment. The primary efficacy evaluation period occurred after the first double-blind repeat treatment phase.
‡The second repeat treatment course was included in the study to evaluate the safety of repeat rifaximin treatment.
In a randomized, double-blind, placebo-controlled phase III repeat treatment study (TARGET 3), patients received open-label rifaximin 550 mg three times daily for 2 weeks; patients who responded to treatment and relapsed during a subsequent observation phase (⩽18 weeks) were randomly assigned to receive up to two 2-week double-blind repeat courses of rifaximin or placebo.66 The percentage of patients with response (i.e. simultaneously achieving a ⩾30% decrease from baseline in the mean weekly abdominal pain score and ⩾50% decrease from baseline in the number of days/week with Bristol Stool Scale type 6 or 7 stool for ⩾2 of the first 4 weeks posttreatment) to repeat rifaximin treatment was significantly greater compared with placebo (first repeat treatment: 38.1% versus 31.5%, respectively; p = 0.03; Figure 1).66 AEs were reported by similar percentages of patients receiving rifaximin or placebo during the double-blind phase (following open-label rifaximin; up to two treatment courses), with nausea (3.7% versus 2.3%, respectively), upper respiratory tract infection (3.7% versus 2.6%), urinary tract infection (3.4% versus 4.9%), and nasopharyngitis (3.0% versus 2.9%) reported in ⩾3.0% of patients in the double-blind rifaximin group.66 Serious AEs occurred in 1.2% (n = 4) and 1.3% (n = 4) of patients receiving rifaximin or placebo, respectively, in the double-blind phase; no serious AEs were considered related to treatment.66 C. difficile colitis infection developed in one patient after 37 days of rifaximin repeat treatment; the patient had a medical history of C. difficile infection and had completed a 10-day course of cefdinir just prior to C. difficile infection development.66
In addition to these three phase III studies in IBS-D, there have also been published randomized, double-blind, placebo-controlled studies of rifaximin at unapproved dosing regimens, in combination with systemic antibiotics, and/or in patients with IBS-C.67,74 Patients with IBS receiving rifaximin 400 mg three times daily for 10 days experienced significant global improvement in symptoms compared with those receiving placebo 10 weeks after treatment (mean improvement: 36.4% versus 21.0%, respectively; p = 0.02).67 Further, improvement in bloating during the 10 weeks of post-treatment follow up achieved significance with rifaximin versus placebo (p = 0.01).67 In that study, the most common AEs with rifaximin were abdominal pain and a bad taste in the mouth, although these AEs were rare and there were no differences in incidence of these, or any, AEs between rifaximin and placebo.67 In a study of patients with IBS-C receiving rifaximin 550 mg three times daily plus neomycin 500 mg twice daily for 14 days, combination therapy resulted in significantly greater improvement of constipation severity compared with neomycin alone 1 week post-treatment (visual analog scale score: 28.6 versus 61.2, respectively; p = 0.002).74 During the 2-week treatment period, nausea, bloating/distension, and abdominal pain were the most common AEs occurring with either rifaximin plus neomycin, or neomycin alone, although there were no significant differences in incidence of AEs between groups.74
In summary, a meta-analysis of five studies demonstrated that rifaximin was significantly associated with improvement of global IBS symptoms compared with placebo [42.2% versus 32.4%, respectively; OR, 1.6 (95% CI, 1.2–2.0); p < 0.001]; data from four studies showed rifaximin was significantly associated with improvement in bloating versus placebo 10–14 days after treatment [41.6% versus 31.7%; OR, 1.6 (95% CI, 1.2–2.0); p < 0.001].75 The number needed to treat for rifaximin has been reported as 8 (n = 7 studies) to 11 (n = 4–6 studies).76,77 Overall, the number needed to stop for rifaximin (based on discontinuation due to an AE) was 8971, with an estimated 846 patients benefiting from rifaximin before an AE resulting in treatment discontinuation would be observed.76
Modulation of the gut microbiota in IBS
Recent studies have provided insight into the impact of rifaximin on the colonic luminal microbiota. In one study, Bacteroidetes (64.6%), Firmicutes (26.1%), Fusobacteria (5.2%), and Proteobacteria (3.7%) were the most common bacterial phyla detected in fecal samples from patients with IBS-D (n = 27); levels of Firmicutes and Bacteroidetes were significantly decreased and increased, respectively, in patients with IBS-D compared with healthy individuals (n = 13; p = 0.046 and p = 0.02, respectively).78 Analysis of fecal samples randomly selected from patients with IBS-D receiving a 2-week course of rifaximin 400 mg twice daily (n = 15) showed a significant increase from baseline in the phyla Chloroflexi (p = 0.008), Deinococcus-Thermus (p = 0.04), and Acidobacteria (p = 0.04).78 In another study, more than half of patients with IBS-C (n = 11), IBS-D (n = 31), or IBS-M (n = 30) receiving rifaximin 1200 mg/day for approximately 10 days achieved adequate relief of symptoms and improvement in symptom intensity for 10–12 weeks post-treatment (64%, 68%, and 53% for each IBS subtype, respectively).79 In that study, rifaximin did not affect the Bacteroidetes/Firmicutes ratio.79 Species richness (i.e. total number of species/sample) was significantly greater in fecal samples from patients with IBS compared with healthy individuals (p = 0.01), and was significantly decreased from baseline after rifaximin treatment (p = 0.0003).79 It is important to note that these studies are observational and do not prove cause and effect. Thus, it is not possible to know whether differences in the gut microbiota observed after treatment with rifaximin are indeed linked to improvement in clinical symptoms in patients with IBS.
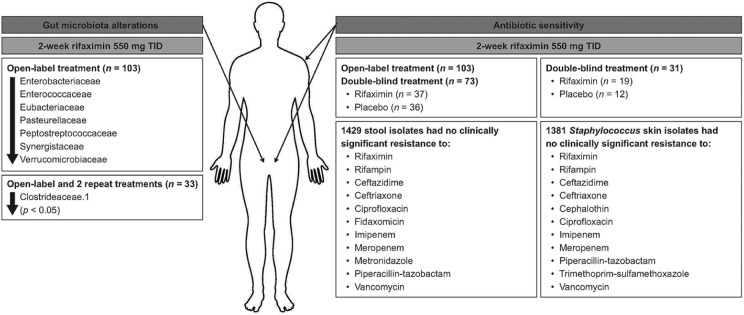
In the TARGET 3 study, the gut microbiota of patients with IBS-D who received up to three 2-week courses of rifaximin 550 mg three times daily (n = 103 patients; n = 675 fecal samples) was stable.80 However, a small, transient decrease in the relative abundance of specific taxa was observed at the end of the first course (2-week open-label rifaximin) compared with baseline (Figure 2).80–82 For 33 patients who received three rifaximin courses, Clostridiaceae.1 was the only taxon that was significantly decreased from baseline following the first and second courses of treatment (p < 0.05, for both courses; Figure 2).80 One putative mechanism of action for rifaximin may be modulation of the observed taxa that transiently decreased in this study, although additional research is needed to confirm this hypothesis.80 Data analyzing the effects of rifaximin on alterations in gut microbiota in patients with IBS and psychologic comorbidities are not currently available. Overall, although it is apparent that rifaximin has some modest effect on the gut microbiota in patients with IBS, the efficacy of rifaximin in IBS likely involves several factors.
Figure 2.
Summary of gut microbiota alterations and antibiotic sensitivity in adults with IBS-D (TARGET 3).80–82
IBS-D, irritable bowel syndrome with diarrhea; TARGET, Targeted, Nonsystemic Antibiotic Rifaximin Gut-Selective Evaluation of Treatment for IBS-D; TID, 3 times daily.
Impact on GI mucosal inflammation and visceral hypersensitivity
Preclinical findings suggest that rifaximin has anti-inflammatory effects in the GI tract. In chronically stressed rats, rifaximin improved GI mucosal inflammation (i.e. decreased levels of IL-6, IL-17, and TNF-α genes) and normalized visceral hypersensitivity following a decrease in concentrations of ileal bacteria.52 Rifaximin is a GI-specific human pregnane-X-receptor (PXR) ligand83; activation of PXR by rifaximin regulates the innate immune response.84 Normal human colonic epithelial cells with decreased PXR expression (using anti-PXR small interfering RNA) had a 50% reduction in concentrations of transforming growth factor-β and an 18% reduction in interferon gamma-induced protein 10 kDa concentrations compared with control cells (p < 0.05 for both); expression levels of TNF-α, IL-8, MIP-3α, and IL-6 genes were increased compared with control cells (p < 0.05 for all).84 In human colonic epithelial cells, the increased production of chemokines and cytokines following activation of TLR-4 by lipopolysaccharide (LPS) stimulation was abrogated by rifaximin treatment.84 Further, ex vivo exposure of human colonic tissue to rifaximin decreased the expression of IL-8, MIP-3α, RANTES, and TNF-α genes following LPS-stimulated induction.84 A preclinical study of intestinal epithelial cells showed that rifaximin decreased production of TLR-4 in a dose-dependent manner, downregulating the nuclear factor-κB pathway through a PXR-related mechanism, which is associated with inflammation.85 However, it is currently unclear whether these preclinical findings can be translated to patients with IBS.
Prevention of intestinal permeability
Data are limited on the effects of rifaximin on intestinal permeability. Chronically stressed rats have increased GI permeability compared with control rats, indicative of impaired barrier function, and treatment with rifaximin prevented development of increased GI permeability in the chronically stressed model.52 However, a 2-week course of rifaximin 550 mg three times daily in a randomized, double-blind, placebo-controlled study of patients with nonconstipation IBS (n = 24) did not significantly impact colonic mucosal permeability compared with placebo.86
Conclusion
The pathophysiology of IBS is multifactorial in nature, with interplay among several factors. For example, the gut microbiota plays a role in visceral hypersensitivity and immune activation. The gut microbiota of patients with IBS is altered compared with that of healthy individuals. Acute gastroenteritis is one etiologic factor involved in the development of IBS (i.e. PI-IBS), and CdtB levels may be increased following acute gastroenteritis; this rise in CdtB levels has been associated with the development of SIBO in an animal model. SIBO is more prevalent in patients with IBS versus healthy individuals, and was associated with specific demographic and disease characteristics (e.g. female sex, older age, IBS-D).
Systemic antibiotic use is associated with development of IBS. However, treatment for IBS (e.g. IBS-D) includes nonsystemic antibiotic therapy. Short (2-week) courses of the nonsystemic (poorly absorbed) antibiotic rifaximin are efficacious and well tolerated for improving symptoms of IBS in adults with IBS-D. While the mechanisms of action of rifaximin have not been fully elucidated, indirect evidence suggests the drug has beneficial effects on SIBO, mucosal inflammation, and microbiota stabilization. Based on available data, it is apparent that the mechanism of action of rifaximin extends beyond its role as a GI-targeted antibiotic. Additional research is needed to address the outstanding knowledge gaps related to the role rifaximin plays in IBS (Table 2). Preclinical and clinical studies suggest that rifaximin may also function to normalize visceral hypersensitivity, reduce mucosal inflammation, alter expression of immune modulators, and inhibit GI permeability. Clinical studies that include surrogate markers are warranted to fully elucidate the role of rifaximin in modulating these etiologic factors thought to be involved in the pathophysiology of IBS.
Table 2.
Outstanding research questions regarding the role of rifaximin in patients with IBS.
| Number | Research question |
|---|---|
| Clinical benefit | |
| 1 Where is the optimal location of drug delivery as it pertains to clinical benefits for IBS? | |
| 2 What is the comparative effectiveness of rifaximin versus other treatments for IBS-D? | |
| 3 Does rifaximin offer clinical benefits to other IBS subgroups (i.e. IBS-C or IBS-M)? | |
| 4 What strategies can be employed to increase the durability of clinical benefit of rifaximin in patients with IBS? | |
| 5 What is the optimal approach to management of IBS with rifaximin, including dose, duration, and recurrent treatment to control symptoms long-term? | |
| 6 What, if any, is the role of breath testing or other biomarker measurements (e.g. fecal, serum) in identifying patients with IBS who would maximally benefit from rifaximin treatment? | |
| Mechanism of action | |
| 7 What changes and to what degree do the gut microbiota play in the clinical benefits observed with rifaximin? | |
| 8 In addition to its GI-specific effects, does rifaximin affect the gut-microbiota-brain axis? | |
| 9 Do characteristics of the gut microbiota or metabolome help identify patients who are more or less likely to experience symptom improvement with rifaximin? | |
| 10 What are the long-term consequences to the gut microbiota when taking repeated courses of rifaximin for IBS? | |
| 11 What are the important changes in the gut microbiota or metabolome in rifaximin responders versus nonresponders? | |
| 12 Does rifaximin exert effects on mucosal permeability or immune activation in patients with IBS, and, if so, do these changes predict clinical response? | |
GI, gastrointestinal; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, mixed form irritable bowel syndrome.
Acknowledgments
Technical editorial assistance and medical writing support was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, PA, USA.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: Funding for technical editorial assistance and medical writing support was provided by Salix Pharmaceuticals, Bridgewater, NJ. The authors did not receive any compensation for development of this manuscript.
Conflict of interest statement: WD Chey reports serving as a consultant for Allergan, Alnylam Pharmaceuticals, Biomerica, Inc., IM Health, Ironwood Pharmaceuticals, Outpost Medicine, Ritter Pharmaceuticals, Inc., Salix Pharmaceuticals, and Urovant Sciences, Inc. He also reports receiving funding from Biomerica, Inc., IM Health, Ironwood Pharmaceuticals, Nestlé, Salix Pharmaceuticals, and Urovant Sciences, Inc.
ED Shah reports having no relevant disclosures.
HL DuPont reports serving as a consultant for Aries and Salix Pharmaceuticals.
ORCID iD: William D. Chey  https://orcid.org/0000-0002-4584-4026.
https://orcid.org/0000-0002-4584-4026.
Contributor Information
William D. Chey, Department of Nutrition Sciences, Division of Gastroenterology, Michigan Medicine, 3912 Taubman Center, SPC 5362, Ann Arbor, MI 48109-5362, USA.
Eric D. Shah, Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
Herbert L. DuPont, Division of Epidemiology, Human Genetics and Environmental Sciences and Center for Infectious Diseases, University of Texas School of Public Health, Houston, TX, USA Mary W. Kelsey Chair in Medical Sciences, Division of Internal Medicine, University of Texas McGovern Medical School Houston, TX, USA; Kelsey Research Foundation, Houston, TX, USA.
References
- 1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 2. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721. [DOI] [PubMed] [Google Scholar]
- 3. Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome foundation working team literature review. Gut 2017; 66: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 4. Palsson OS, van Tilburg MA, Simren M, et al. Population prevalence of Rome IV and Rome III irritable bowel syndrome (IBS) in the United States (US), Canada and the United Kingdom (UK). Gastroenterology 2016; 150: S739–S740. [Google Scholar]
- 5. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 6. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology 2019; 157: 97–108. [DOI] [PubMed] [Google Scholar]
- 7. Liu HN, Wu H, Chen YZ, et al. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis 2017; 49: 331–337. [DOI] [PubMed] [Google Scholar]
- 8. Tap J, Derrien M, Törnblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017; 152: 111–123. [DOI] [PubMed] [Google Scholar]
- 9. Hollister EB, Cain KC, Shulman RJ, et al. Relationships of microbiome markers with extraintestinal, psychological distress and gastrointestinal symptoms, and quality of life in women with irritable bowel syndrome. J Clin Gastroenterol. Epub ahead of print 24 August 2018. DOI: 10.1097/MCG.0000000000001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maharshak N, Ringel Y, Katibian D, et al. Fecal and mucosa-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Dig Dis Sci 2018; 63: 1890–1899. [DOI] [PubMed] [Google Scholar]
- 11. Bennet SMP, Sundin J, Magnusson MK, et al. Altered intestinal antibacterial gene expression response profile in irritable bowel syndrome is linked to bacterial composition and immune activation. Neurogastroenterol Motil 2018; 30: e13468. [DOI] [PubMed] [Google Scholar]
- 12. Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016; 65: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 2019; 156: 46–58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klem F, Wadhwa A, Prokop L, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017; 152: 1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers’ diarrhoea. Aliment Pharmacol Ther 2015; 41: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 16. Cremon C, Stanghellini V, Pallotti F, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology 2014; 147: 69–77. [DOI] [PubMed] [Google Scholar]
- 17. Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 1997; 314: 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahman MM, Ghoshal UC, Sultana S, et al. Long-term gastrointestinal consequences are frequent following sporadic acute infectious diarrhea in a tropical country: a prospective cohort study. Am J Gastroenterol 2018; 113: 1363–1375. [DOI] [PubMed] [Google Scholar]
- 19. Zanini B, Ricci C, Bandera F, et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol 2012; 107: 891–899. [DOI] [PubMed] [Google Scholar]
- 20. Marshall JK, Thabane M, Borgaonkar MR, et al. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol 2007; 5: 457–460. [DOI] [PubMed] [Google Scholar]
- 21. Soyturk M, Akpinar H, Gurler O, et al. Irritable bowel syndrome in persons who acquired trichinellosis. Am J Gastroenterol 2007; 102: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 22. Krogsgaard LR, Engsbro AL, Stensvold CR, et al. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin Gastroenterol Hepatol 2015; 13: 507–513.e2. [DOI] [PubMed] [Google Scholar]
- 23. Krogsgaard LR, Andersen LO, Johannesen TB, et al. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin Transl Gastroenterol 2018; 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dicksved J, Ellstrom P, Engstrand L, et al. Susceptibility to Campylobacter infection is associated with the species composition of the human fecal microbiota. MBio 2014; 5: e01212–e01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kampmann C, Dicksved J, Engstrand L, et al. Composition of human faecal microbiota in resistance to Campylobacter infection. Clin Microbiol Infect 2016; 22: 61.e1–61.e8. [DOI] [PubMed] [Google Scholar]
- 26. Jalanka J, Salonen A, Fuentes S, et al. Microbial signatures in post-infectious irritable bowel syndrome - toward patient stratification for improved diagnostics and treatment. Gut Microbes 2015; 6: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Youmans BP, Ajami NJ, Jiang ZD, et al. Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes 2015; 6: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paula H, Grover M, Halder SL, et al. Non-enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neurogastroenterol Motil 2015; 27: 1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKeown ES, Parry SD, Stansfield R, et al. Postinfectious irritable bowel syndrome may occur after non-gastrointestinal and intestinal infection. Neurogastroenterol Motil 2006; 18: 839–843. [DOI] [PubMed] [Google Scholar]
- 30. Guerra L, Cortes-Bratti X, Guidi R, et al. The biology of the cytolethal distending toxins. Toxins (Basel) 2011; 3: 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pokkunuri V, Pimentel M, Morales W, et al. Role of cytolethal distending toxin in altered stool form and bowel phenotypes in a rat model of post-infectious irritable bowel syndrome. J Neurogastroenterol Motil 2012; 18: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morales W, Pimentel M, Hwang L, et al. Acute and chronic histological changes of the small bowel secondary to C. jejuni infection in a rat model for post-infectious IBS. Dig Dis Sci 2011; 56: 2575–2584. [DOI] [PubMed] [Google Scholar]
- 33. Pimentel M, Chatterjee S, Chang C, et al. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci 2008; 53: 982–989. [DOI] [PubMed] [Google Scholar]
- 34. Sung J, Morales W, Kim G, et al. Effect of repeated Campylobacter jejuni infection on gut flora and mucosal defense in a rat model of post infectious functional and microbial bowel changes. Neurogastroenterol Motil 2013; 25: 529–537. [DOI] [PubMed] [Google Scholar]
- 35. Pimentel M, Morales W, Pokkunuri V, et al. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following Campylobacter jejuni infection in a rat model. Dig Dis Sci 2015; 60: 1195–1205. [DOI] [PubMed] [Google Scholar]
- 36. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016; 14: e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver 2017; 11: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen B, Kim JJ, Zhang Y, et al. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol 2018; 53: 807–818. [DOI] [PubMed] [Google Scholar]
- 39. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol 2017; 112: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krogsgaard LR, Engsbro AL, Bytzer P. Antibiotics: a risk factor for irritable bowel syndrome in a population-based cohort. Scand J Gastroenterol 2018; 53: 1027–1030. [DOI] [PubMed] [Google Scholar]
- 41. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000; 95: 3503–3506. [DOI] [PubMed] [Google Scholar]
- 42. Ghoshal UC, Srivastava D, Misra A, et al. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol 2016; 28: 281–289. [DOI] [PubMed] [Google Scholar]
- 43. Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther 2017; 45: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soldi S, Vasileiadis S, Uggeri F, et al. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol 2015; 8: 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang ZD, Ke S, DuPont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents 2010; 35: 278–281. [DOI] [PubMed] [Google Scholar]
- 46. Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol 2011; 46: 421–431. [DOI] [PubMed] [Google Scholar]
- 47. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004; 126: 693–702. [DOI] [PubMed] [Google Scholar]
- 48. Balemans D, Boeckxstaens GE, Talavera K, et al. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2017; 312: G635–G648. [DOI] [PubMed] [Google Scholar]
- 49. Kuiken SD, Lindeboom R, Tytgat GN, et al. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2005; 22: 157–164. [DOI] [PubMed] [Google Scholar]
- 50. van Wanrooij SJ, Wouters MM, Van Oudenhove L, et al. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am J Gastroenterol 2014; 109: 99–109. [DOI] [PubMed] [Google Scholar]
- 51. Melchior C, Bril L, Leroi AM, et al. Are characteristics of abdominal pain helpful to identify patients with visceral hypersensitivity in irritable bowel syndrome? Results of a prospective study. Neurogastroenterol Motil 2017; 30: e13290. [DOI] [PubMed] [Google Scholar]
- 52. Xu D, Gao J, Gillilland M, III, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014; 146: 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vivinus-Nébot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol 2012; 107: 75–81. [DOI] [PubMed] [Google Scholar]
- 54. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22: 240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brint EK, MacSharry J, Fanning A, et al. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol 2011; 106: 329–336. [DOI] [PubMed] [Google Scholar]
- 56. Shukla R, Ghoshal U, Ranjan P, et al. Expression of toll-like receptors, pro-, and anti-inflammatory cytokines in relation to gut microbiota in irritable bowel syndrome: the evidence for its micro-organic basis. J Neurogastroenterol Motil 2018; 24: 628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seyedmirzaee S, Hayatbakhsh MM, Ahmadi B, et al. Serum immune biomarkers in irritable bowel syndrome. Clin Res Hepatol Gastroenterol 2016; 40: 631–637. [DOI] [PubMed] [Google Scholar]
- 58. Katsumata R, Ishii M, Lee S, et al. Cytokine profile and immunoglobulin e-mediated serological food hypersensitivity in patients with irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil 2018; 24: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: a systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine 2017; 99: 132–138. [DOI] [PubMed] [Google Scholar]
- 60. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 2014; 26: 1036–1048. [DOI] [PubMed] [Google Scholar]
- 61. Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009; 58: 196–201. [DOI] [PubMed] [Google Scholar]
- 62. Li L, Xiong L, Yao J, et al. Increased small intestinal permeability and RNA expression profiles of mucosa from terminal ileum in patients with diarrhoea-predominant irritable bowel syndrome. Dig Liver Dis 2016; 48: 880–887. [DOI] [PubMed] [Google Scholar]
- 63. Rolland-Fourcade C, Denadai-Souza A, Cirillo C, et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 2017; 66: 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bednarska O, Walter SA, Casado-Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 2017; 153: 948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 66. Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 67. Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med 2006; 145: 557–563. [DOI] [PubMed] [Google Scholar]
- 68. Descombe JJ, Dubourg D, Picard M, et al. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res 1994; 14: 51–56. [PubMed] [Google Scholar]
- 69. Darkoh C, Lichtenberger LM, Ajami N, et al. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother 2010; 54: 3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kane JS, Ford AC. Rifaximin for the treatment of diarrhea-predominant irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 2016; 10: 431–442. [DOI] [PubMed] [Google Scholar]
- 71. Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion 2006; 73(Suppl. 1): 13–27. [DOI] [PubMed] [Google Scholar]
- 72. Jiang ZD, Ke S, Palazzini E, et al. In vitro activity and fecal concentration of rifaximin after oral administration. Antimicrob Agents Chemother 2000; 44: 2205–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Finegold SM, Molitoris D, Väisänen ML. Study of the in vitro activities of rifaximin and comparator agents against 536 anaerobic intestinal bacteria from the perspective of potential utility in pathology involving bowel flora. Antimicrob Agents Chemother 2009; 53: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pimentel M, Chang C, Chua KS, et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig Dis Sci 2014; 59: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 75. Menees SB, Maneerattannaporn M, Kim HM, et al. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 28–35. [DOI] [PubMed] [Google Scholar]
- 76. Shah E, Kim S, Chong K, et al. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012; 125: 381–393. [DOI] [PubMed] [Google Scholar]
- 77. Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018; 113: 1–18. [DOI] [PubMed] [Google Scholar]
- 78. Zhuang X, Tian Z, Li L, et al. Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front Microbiol 2018; 9: 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, et al. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes 2016; 7: 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fodor AA, Pimentel M, Chey WD, et al. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019; 10: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. DuPont HL, Wolf RA, Israel RJ, et al. Antimicrobial susceptibility of Staphylococcus isolates from the skin of patients with diarrhea-predominant irritable bowel syndrome treated with repeat courses of rifaximin. Antimicrob Agents Chemother 2017; 61: e02165–e02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pimentel M, Cash BD, Lembo A, et al. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci 2017; 62: 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ma X, Shah YM, Guo GL, et al. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 2007; 322: 391–398. [DOI] [PubMed] [Google Scholar]
- 84. Mencarelli A, Renga B, Palladino G, et al. Inhibition of NF-kB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol 2011; 668: 317–324. [DOI] [PubMed] [Google Scholar]
- 85. Esposito G, Nobile N, Gigli S, et al. Rifaximin improves clostridium difficile toxin A-induced toxicity in caco-2 cells by the PXR-dependent TLR4/MyD88/NF-kB pathway. Front Pharmacol 2016; 7: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Acosta A, Camilleri M, Shin A, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016; 7: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]