Abstract
Background:
Increasing evidence points to the role of tumor immunologic environment on urothelial bladder cancer prognosis. This effect might be partly dependent on the host genetic context. We evaluated the association of SNPs in inflammation- related genes with non-muscle-invasive bladder cancer (NMIBC) risk-of-recurrence and risk-of-progression.
Methods:
We considered 822 NMIBC included in the SBC/EPICURO Study followed-up >10 years. We selected 1,679 SNPs belonging to 251 inflammatory genes. The association of SNPs with risk-of-recurrence and risk-of-progression was assessed using Cox regression single-marker (SMM) and multimarker methods (MMM) Bayes A and Bayesian LASSO. Discriminative abilities of the models were calculated using the c index and validated with bootstrap cross-validation procedures.
Results:
While no SNP was found to be associated with risk-of-recurrence using SMM, three SNPs in TNIP1, CD5, and JAK3 showed very strong association with posterior probabilities >90% using MMM. Regarding risk-of-progression, one SNP in CD3G was significantly associated using SMM (HR, 2.69; P = 1.55 × 10−5) and two SNPs in MASP1 and AIRE, showed a posterior probability ≥80% with MMM. Validated discriminative abilities of the models without and with the SNPs were 58.4% versus 60.5% and 72.1% versus 72.8% for risk-of-recurrence and risk-of-progression, respectively.
Conclusions:
Using innovative analytic approaches, we demonstrated that SNPs in inflammatory-related genes were associated with NMIBC prognosis and that they improve the discriminative ability of prognostic clinical models for NMIBC.
Impact:
This study provides proof of concept for the joint effect of genetic variants in improving the discriminative ability of clinical prognostic models. The approach may be extended to other diseases.
Introduction
Urothelial bladder carcinoma (UBC) is the fifth most common neoplasm in terms of incidence in industrialized countries. UBCis a multifactorial complex disease, tobacco and occupation exposure to aromatic amines being the two best established environmental risk factors (1, 2). In addition, UBC has a genetic component, and candidate gene and genome-wide association studies so far have identified 16 loci associated with UBC risk (3–13)
The majority of UBC are non-muscle-invasive (NMIBC). These tumors are heterogeneous regarding their clinical, pathologic, molecular, and genetic features. Management of NMIBC poses challenges because of their propensity to recur, requiring a long-term surveillance, and their risk to progress to muscle invasion, showing a poor 5-year survival rate (14). The current prognosticators do not completely discriminate between patients who will suffer from a tumor recurrence/progression and patients who will remain stable after the first transurethral resection of the bladder (TURB); thus justifying the need of prognostic biomarkers to guide the clinical management of patients with NMIBC (15).
Inflammation and cancer are deeply intricate. Not only local inflammation can promote tumor development but also systemic or tumor immune reaction has been shown to have either promoting or opposing cancer effects (16–18). These reactions are however dependent on the host genetic context (19). Previous studies have assessed SNPs involved in inflammatory pathways as prognostic markers for UBC (20–22). Those studies have had limited success, as they have applied simplistic models analyzing each SNP individually, therefore ignoring the complexity of the disease likely underlined by many genetic variants with relatively low effects (23). A recent study has shown the usefulness of multi-marker methods (MMM) able to handle large amount of SNPsç often exceeding the number of individuals, to assess associations between SNPs in inflammatory genes and UBC risk (24).
The objective of this study was to evaluate the association of SNPs in inflammation-related genes with the risk of NMIBC to recur and/or progress by extending the application of MMM to the prognostic field for the first time. We compared results with those coming from the classical single-marker method (SMM) accounting for the time-to-event nature of the data.
Materials and Methods
Ethics statement
Informed consent was obtained from study participants in accordance with the Institutional Review Board of the U.S. National Cancer Institute and the Ethics Committees of each participating hospital.
Study population and tissue samples
We primarily considered the 995 newly diagnosed patients with NMIBC included in the Spanish Bladder Cancer (SBC)/EPICURO Study, a multicenter hospital-based study conducted in 1997–2001 in 18 hospitals (3). Tumors were reviewed and confirmed by trained uropathologists who classified their stage and grade homogeneously using TNM 1997 AJCC and 1973 and 2004 WHO grade classifications. All tumors were transitional cell carcinomas (TCC). Clinical data and information on primary treatment were retrieved from the hospital charts by trained monitors using a structured questionnaire. Patients with NMIBC were classified at high (HiR, n = 284) or low (LR, n = 538) risk of progression according to the EAU guidelines (25). Low-risk patients consisted of PUNLMP, Ta G1, and G2/low grade and high-risk patients included all T1, G2, and G3/high grade and carcinoma in situ (CIS). The intermediate-risk group was not considered herein due to reduced sample size. Patients were followed up for >10 years using both the hospital charts and through direct telephone calls to patients/families. The follow-up rate for patients with NMIBC was 94%.
Gene and SNP selection and genotyping
Germline DNA extracted from blood or saliva, in case blood was not available (4% of the patients), was used for genotyping (3). Genes (n = 251, Supplementary Table S1) were carefully selected according to current available evidence of their involvement in inflammatory processes, favoring those inflammatory genes showing association with cancer as described elsewhere (24). TagSNPs covering these genes were identified using SYSNP (26) and genotyped with the GoldenGate Illumina Genotyping Assay platform (27). On the basis of a literature review, we further included 3,628 SNPs in 52 inflammatory genes already genotyped in the same individuals with the Illumina Infinium HumanHap1M array (6). We excluded SNPs with a low genotyping rate (<95%) and minor allele frequency (MAF) <0.05. Missing genotypes were imputed with BEAGLE (28). To reduce both colinearity between variables and number of statistical tests, pairwise linkage disequilibrium (LD) between SNPs was estimated using the R-package GENETICS (http://cran.r-project.org/web/packages/genetics/index.html). We retained the SNPs with the highest MAF of each LD block when r2 > 0.5. At the end of the quality control process, 822 patients with 1,517 SNP genotypes and complete clinical and pathological information were available for the analysis. These patients were comparable with the whole series (n = 995) for age, gender, area, and tumor stage and grade.
Outcome definition
Time-to-first recurrence (TFR) was defined as time elapsed between first TURB and histologic diagnosis of a new NMIBC of any stage/grade. Time to progression (TP) was defined as time between first TURB and a subsequent histologic diagnosis of a muscle-invasive breast cancer (MIBC), occurrence of metastasis, or death due to bladder cancer.
Statistical analyses
Median follow-up times were obtained by using the reverse Kaplan-Meier method. We applied both an SMM based on a multivariate Cox regression and MMM based on Bayes A (BA) and Bayesian LASSO (BL; Supplementary Fig. S1). Cox proportional hazard regression was used to estimate the HR and 95% confidence intervals (CI) to assess the association between individual SNPs assuming both an additive and a dominant mode of inheritance and the outcomes of interest. Each SNP effect was adjusted for classical clinicopathologic prognosticators for TFR and TP (Supplementary Table S2). TFR analyses did not include patients who received radical cystectomy as first treatment (n = 12). TP was analyzed using all available patients with NMIBC and stratified according to HiR/LR. Stratification was not performed for TFR because survival curves of LRand HiR patients overlapped. Analyses were run in R-language (http://www.R-project.org). SNPs with P < 0.05, 2-sided test, after Bonferroni’s correction were kept for comparison with the results from the MMM.
Multimarker methods
Both Bayes A (29) and Bayesian LASSO (30) were applied coupled with a sequential threshold model to analyze each time-to-event data (See Supplementary Methods and ref. 31. This model (32) has been previously used in quantitative genetics settings (33, 34). This is the first time the method is applied in the prognosis field. The same clinicopathologic adjusting variables were used in the MMM (Supplementary Table S2). Because BA and BL do not provide P value, the association strength was estimated using a posterior probability that the SNP is associated with the outcome. An arbitrary threshold of 80% was deemed as significant. Analyses were done using an ad-hoc made Fortran program.
Discrimination ability of the model calculation and validation
The discrimination ability of the models including the SNPs showing association with the different outcomes was evaluated by estimating the c index. Briefly, a Cox model including the clinical variables only was compared through the c index with a model including also the previously associated SNPs for each outcome of interest. The c index is the frequency of concordant pairs (i.e., the risk of the event predicted by a model is lower for the patient who experiences the event at a later time point) among all pairs of subjects. Three c indexes were calculated: the apparent c index (calculated with the original data), the bootstrap cross-validation c index (calculated using the observations that are not in the bootstrap sample, obtained through a random sampling with replacement), and the bootstrap alone c index (i.e., a weighted average of the discrimination in the original dataset and the discrimination in the observations that were not included in the m-th bootstrapped sample; as in ref. 35) and R-package ‘pec’ http://cran.r-project.org/web/packages/pec/pec.pdf).
Results
The median age at diagnosis of the 822 patients with NMIBC was 68 years; 12% of cases were women and 65% of the patients presented LR tumors. Patient and treatment characteristics are displayed in Table 1 and SupplementaryTableS3, respectively. Up to July 2007, median follow-up period for the whole series and for patients “free of disease” were 80.4 and 77.5 months, respectively, with a total of 8 (1.0%) deaths due to UBC as first event. According to the abovementioned definitions, 324 (39.4%) patients suffered, at least, one event. Survival functions for each event are in Supplementary Figs. S2 and S3.
Table 1.
Patient and tumor characteristics and number of outcomes according to the NMIBC risk group
| All (N = 822) | Low-risk (n = 538) | High-risk (n = 284) | P | |
|---|---|---|---|---|
| Age, y | 7.6 × 10−5 | |||
| Median [IQR] | 68 (60–73) | 67 (58–73) | 69 (63–75) | |
| Mean (SD) | 65.5 (10.2) | 64.5 (10.8) | 67.5 (8.7) | |
| Gender | 0.49 | |||
| Male | 722 (88%) | 469 (87%) | 253 (89%) | |
| Female | 100 (12%) | 69 (13%) | 31 (11%) | |
| TG | - | |||
| PUNLMP | 41 (5%) | 41 (7.6%) | - | |
| Ta G1 | 311 (37.8%) | 311 (57.8%) | - | |
| Ta G2 | 253 (30.8%) | 186 (34.6%) | 67 (23.6%) | |
| Ta G3 | 85 (10.3%) | - | 85 (29.9%) | |
| T1 G2 | 20 (2.4%) | - | 20 (7.1%) | |
| T1 G3 | 106 (13%) | - | 106 (37.3%) | |
| Tis G2 | 1 (0.1%) | - | 1 (0.34%) | |
| Tis G3 | 5 (0.6%) | - | 5 (1.76%) | |
| Multiplicity | 4.2 × 10−5 | |||
| ≤3 tumors | 543 (66%) | 383 (71.2%) | 160 (56.3%) | |
| >3 tumors | 236 (29%) | 130 (24.2%) | 106 (37.3%) | |
| Missing values | 43 (5%) | 25 (4.6%) | 18 (6.4%) | |
| Size | 9.9 × 10−5 | |||
| ≤3 cm | 480 (58.4%) | 341 (63.4%) | 139 (48.9%) | |
| >3 cm | 112 (13.6%) | 58 (10.8%) | 54 (19%) | |
| Missing values | 230 (28%) | 139 (25.8%) | 91 (32.1%) | |
| Number of patients with tumor recurrence | 268 (32.6) | 190 (35.3) | 78 (27.5) | |
| Number of patients with progression | 76 (9.2) | 24 (4.5) | 52 (18.3) | |
Abbreviation: IQR, interquartile range.
Time to first recurrence
No SNP was found to be associated with TFR by SMM additive model after Bonferroni’s correction (P > 3 × 10−5, Supplementary Table S4). Using a dominant mode of inheritance, we found that an SNP in CARD4/NOD1 (rs10267377) was significantly associated with TRF (HR, 0.58; 95% CI, 0.45–0.75; P = 0.000026, Padjusted = 0.039). Using MMM, 43 SNPs had PP > 80% of being associated with TFR (Supplementary Table S5). Among them, three had probabilities >90% using both BA and BL, pointing to a very strong association (TNIP1-rs2277940, CD5-rs7104333, JAK3-rs6523, Table 2). The same SNP in CARD4 had PP of 88% and 90% of being associated with risk of recurrence using BA and BL, respectively.
Table 2.
SNPs with a strong posterior probability (PP > 90%, BA and BL analyses) of being associated with risk of recurrence in NMIBC
| Gene | SNP | MAF | BA |
BL |
Cox regression |
|||
|---|---|---|---|---|---|---|---|---|
| HRaa_AA | PP > 90% | HRaa_AA | PP > 90% | HR | P | |||
| JAK3 | rs6523a | 0.40 | 1.69 | 98 | 1.17 | 92 | 1.20 | 0.0488 |
| TNIP1 | rs2277940 | 0.07 | 1.50 | 91 | 1.24 | 94 | 1.74 | 0.0001 |
| CDS | rs7104333 | 0.49 | 0.72 | 90 | 0.86 | 92 | 0.81 | 0.0126 |
NOTE: The last column displays the adjusted Cox regression results from models including individual SNPs and covariates.
Previously as rs2286662. Analyses were adjusted for geographical area, gender, multiplicity, tumor stage and grade, tumor size, and treatment (see Supplementary Table S2).
Time to progression
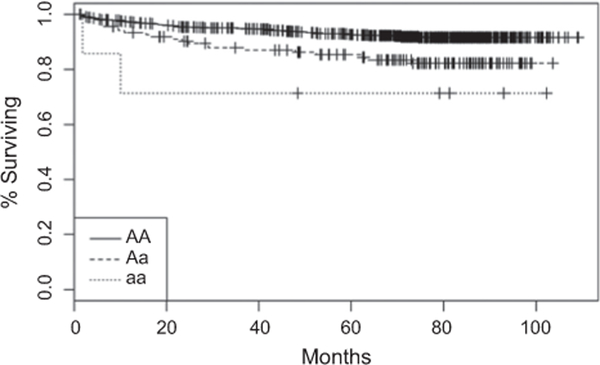
Only one SNP identified by SMM additive model showed a significant association after Bonferroni’s correction: CD3G-rs3212262 (HR, 2.69; 95% CI, 1.72–4.23; P = 1.55 × 10−5, Padjusted = 0.023; Table 3). Five-year progression-free survival rate was 92% for the AA genotype versus 84% for the Aa and 71% for the aa (log-rank P = 0.001, Fig. 1). No SNP was associated with TP using a dominant model. Using MMM, 2 additional SNPs had PP ≥ 80% with both BA and BL: MASP1-rs698079 and AIRE-rs941405 (Table 4). When assessing only HiR tumors, MMM identified 3 SNPs with PP between 77–80%: CARD4-rs2256023, MAP2K3-rs9901404, and TMEM189-rs2269217 (Supplementary Table S6). Neither SMM nor MMM identified any SNP associated with TP among LR tumors, CD68-rs12942088 (Supplementary Table S7) presenting the highest PP (75% with BL). This SNP was one of the top 2 SNPs associated with TP using an additive SMM.
Table 3.
Top 10 autosomal SNPs associated with risk of progression in the whole NMIBC series using multivariable Cox regression additive model
| Gene | SNP | HR | P | MAF |
|---|---|---|---|---|
| CD3G | rs3212262 | 2.70 | 1.5 × 10−5 | 0.09 |
| HLA-B | rs9266462 | 2.67 | 0.0026 | 0.06 |
| CCL-2 | rs929259 | 0.52 | 0.0081 | 0.37 |
| FAS | rs1571014 | 1.73 | 0.0089 | 0.36 |
| PPARG | rs7626560 | 1.85 | 0.0015 | 0.19 |
| CXCR4 | rs778192 | 0.55 | 0.0017 | 0.38 |
| SOCS5 | rs973491 | 2.11 | 0.0025 | 0.07 |
| CXCR4 | rs16834018 | 2.10 | 0.0035 | 0.08 |
| CD8B1 | rs13024609 | 1.70 | 0.0044 | 0.20 |
| IL6 | rs2069827 | 2.00 | 0.0053 | 0.06 |
NOTE: Analyses were adjusted for geographical area, age, multiplicity, tumor stage and grade, number of recurrences, and treatment (see Supplementary Table S2).
Figure 1.
Progression-free survival of the 822 NMIBC according to CD3G-rs3212262 genotypes. Five-year progression-free survival was 92% for AA, 85% for Aa, and 71% for aa genotypes (log-rank P = 8.4 × 10−4, adjusted Cox P = 0.023).
Table 4.
SNPs with strong posterior probability (PP > 80%, BA and BL analyses) of being associated with risk of progression in patients with NMIBC
| Gene | SNP | MAF | BA |
BL |
Cox regression |
|||
|---|---|---|---|---|---|---|---|---|
| HRaa_AA | PP > 80% | HRaa_AA | PP > 80% | HR | P | |||
| MASP1 | rs698079 | 0.18 | 1.31 | 83 | 1.10 | 80 | 1.58 | 0.0178 |
| AIRE | rs941405 | 0.37 | 1.31 | 86 | 1.11 | 83 | 1.56 | 0.0116 |
NOTE: Analyses were adjusted for geographical area, age, multiplicity, tumor stage and grade, number of recurrences, and treatment (see Supplementary Table S2). The last column displays the adjusted Cox regression results from models including individual SNPs and covariates.
Models’ discriminative ability
The clinical parameter model for TFR showed a moderate discriminatory ability (validated c index = 0.58; Table 5). By adding the 3 SNPs showing a PP > 90% (TNIP1-rs2277940, CD5-rs7104333, and JAK3-rs6523), the c index raised to 0.61. Adding each SNP to the clinical variables increased the predictive ability compared with the model including clinical variables only, showing that the predictive ability of the SNPs does not overlap (see Supplementary Table S8).
Table 5.
Apparent and validated c index using Bootstrap alone or 1,000 bootstrap cross-validation indicating the discriminatory ability of the models for each outcome of interest
| Apparent c index |
Bootstrap alone c index |
Bootstrap cross- validation c index |
|
|---|---|---|---|
| Recurrence ALL (N = 268 events) | |||
| CV | 62.6 | 60.1 | 58.4 |
| CV + SNPs (JAK3, TNIP1, CD5) | 65 | 62.5 | 60.5 |
| Progression ALL (n = 76 events) | |||
| CV | 77.5 | 74.7 | 72.1 |
| CV + SNPs (MASP1, AIRE) | 78.7 | 75.4 | 72.8 |
| Progression HiR (n = 52 events) | |||
| CV | 69.4 | 63.6 | 60.1 |
| CV + SNPs (TMEM189, MAP2K3, CARD4) | 76 | 70.1 | 66.4 |
| Progression LR (n = 24 events) | |||
| CV | 74.2 | 66.6 | 62.6 |
| CV + SNPs (CD68) | 80.8 | 73.5 | 69 |
Abbreviations: CV, clinical variable; n, number of events.
The clinical parameter model for TP showed good discrimination ability (validated c index = 0.72, Table 5). By adding the 2 SNPs showing PP > 80% (MASP1-rs698079 and AIRE-rs941405), the c index raised to 0.73. The TP predictive ability was also calculated for the HiR and LR subgroups. Adding the SNPs to the clinical variables improved their discrimination ability by 10.5 % for HiR and by 10.2 % for LR in the validation set. As forTFR, the predictive ability of the SNPs did not overlap for TP considering all and HiR patients (see Supplementary Table S8).
Discussion
Classical studies looking for associations between individual SNPs in inflammatory genes and UBC prognosis have had limited success (20–22). While some variants have been previously associated with UBC prognosis (19, 36), significance was in most cases limited to univariate analysis and none of the variant was replicated in independent studies. Moreover, Cox regression is limited by the number of variables the model permits (37). In general, the lack of associations found by SMM outlines its inefficiency to pinpoint variants with small effects in complex traits. To explore the joint effect of multiple SNPs, we have applied MMM strategies mimicking the polygenic scenario that features UBC prognosis. MMM identified, with strong evidence, inflammatory genes with variants individually conferring a small risk of NMIBC recurrence or progression.
A larger number of inflammatory variants showed association with TFR than with TP: 44 SNPs with PP > 80%, 3 of them with PP > 90%, have been associated with TFR. Among them, JAK3-rs6523 and CD5-rs7104333 were already identified as associated with UBC risk (24). Only 2 SNPs were associated with TP using MMM. This could be explained by the lower rate of progression events (n = 76) compared with the number of recurrences (n = 268) that may affect the power of tests in detecting associations. Most of the SNPs/genes associated with TFR were not associated with TP and vice versa, which may indicate that different inflammatory genes trigger distinct NMIBC outcomes. The small correlation between SNP effects obtained with MMM for TFR and TP (data not shown) also support this hypothesis. However, it is noteworthy that genetic variation in CARD4/NOD1 was both associated with TR of the whole cohort and TP of high-risk patients. Most of the SNPs identified by MMM also ranked in the first positions when Cox regression was applied. Only two SNPs in CD3G and CARD4 identified by SMM, as associated with TP and TR, passed Bonferroni’s correction and their PPs were high: PPBA = 0.76 and PPBL = 0.79 for CD3G and PPBA = 0.88 and PPBL = 0.90 for CARD4. Potential explanations for the different SNP ranking for CD3G between tests is the small MAF (0.09) of this variant with very few events in the aa genotype group (Fig. 1; ref. 38) and the adjustment by other SNPs included in the MMMs.
Inflammatory SNPs were not strongly associated with outcome in both HiR and LR subcohorts, probably because of the limited sample size, too. Polymorphisms in inflammatory genes were differently associated with TP in patients at HiR versus LR. Correlation between SMM and MMM estimates of TP in both subcohorts disagreed (Pearson correlation between SNPs effect estimates = −0.01 for BA and −0.03 for Cox regression), this suggesting that the difference in prognosis of both groups may, at least, partially be mediated by inflammatory genes. Risk of TP is highly influenced by Bacillus Calmette-Guérin (BCG) administration (39). Focusing on the HiR subcohort, no BCG*SNP interaction using MMMs was found (results not shown). Larger cohorts might be needed to pinpoint these potential interactions.
SNPs were included as they tagged the selected inflammatory genes, what does not imply a potential function. Since it is difficult to know whether those variants identified are causative or are in high LD with the real ones, they should merely be considered as biomarkers of prognosis. While most of the selected SNPs tagged genes of particular interest in cancer biology, the position of some of the genes changed according to the new version of the human genome release, a fact that misplaced SNPs from the initial selection window (i.e., rs2269217). Noteworthy, most of the significant variants are placed in genes involved in immune tolerance processes: Janus kinase 3 (JAK3) participates in intra-cellular signal transduction after activation of immune cells. Lower levels of JAK3 might be responsible for the defective reactivity of T lymphocytes in patients with cancer (40). CD5 is known as a negative regulator of T- and B-cell receptor signaling. Its expression has been shown to be implicated in T lymphocytes tolerance toward tumor cells (41). AIRE encodes a transcription factor that regulates the expression of tissue antigens in the thymus and plays an important role in the development of organ-specific T regulatory lymphocytes (42). Those T regulators are thought to be major barrier impeding antitumor immune response (42). In melanoma, polymorphisms in AIRE may variably affect the selection of melanoma-associated antigen-specific thymocytes, generating T-cell repertoires protecting or predisposing individuals to cancer (43). CARD4/NOD1 is a member of the NOD receptor family that plays a major role in innate and adaptive immunities. Polymorphisms in those genes have been shown to be associated with multiple cancer risk including UBC (22, 44). NOD receptors were demonstrated to be involved in antitumor cytotoxicity through the potentiation of human natural killer cells and macrophage activities (45). MAP2K3 pathways play a critical role in carcinogenesis. MAP2K3 has been shown to suppress the growth of breast cancer cells (46) and alterations in its pathway are frequent in UBC (47). Finally, the protein encoded by CD3G is part of the T-cell receptor-CD3 complex. Prognostic impact of T lymphocytes’ infiltration is now being investigated in multiple cancers, and CD3 expression has been shown to be associated with UBC risk of recurrence and mortality (48).
Statistically significant results and potential biologic relevance are not enough criteria to reach clinical utility. Marker(s) have to be clinically actionable and cost efficient. While we identified SNPs strongly associated with NMIBC outcomes, they contributed little (<3.6%) to the higher event prediction ability provided by clinicopathologic variables. Furthermore, these estimates have to be taken cautiously, as their added value might be overestimated mainly because of the relatively low number of progression events in the whole cohort and mainly in the HiR/LR subcohorts. We cannot discard that the performances of the models are still magnified even though their predictive ability was tested by applying bootstrapped cross-validation samples (49). Therefore, an external validation would be advisable to confirm the added predictive value of the identified SNPs. However, heterogeneity across studies regarding patient recruitment, treatment, patient management, or availability of the genotypes data for the same set of SNPs limit the potential success of the replication stage. Furthermore, the definition of inflammatory genes was itself challenging. The list of potential genes is tremendously large, and the edges of the definition are difficult to delineate due to the crosstalk between inflammatory pathways and other cellular functions. It is possible that potential susceptibility markers identified in other studies were not included here, although we estimate these are a minority. Moreover, incomplete mapping of the genes might have occurred as a result of using a previous HapMap genome reference release or filtering by LD, what might have led to missing SNPs of interest. Finally, we did not explore all genetic mechanisms sustaining UBC prognosis, as the genetic architecture and correlations between genes involving complex interactions and epigenetic regulation are still unknown (50).
Despite that, this study reports valuable findings and has noteworthy strengths. The cohort used was built upon strong methodology. All the patients had complete and homogeneously collected clinical, pathologic, and genetic information, with long enough follow-up to investigate NMIBC prognosis. Using innovative MMM that identified many SNPs in inflammatory genes, we provide further evidence of the complex and heterogeneous nature of UBC prognosis and enable to find associations that were not found by applying restrictive SMM.
Conclusion
Considering multiple genetic information jointly is key to understand its influence on complex traits such as UBC outcome. Innovative analytic approaches were essential to demonstrate that several SNPs in inflammatory genes were differently associated with risk of TFR and TP in NMIBC. Although external validation is warranted, this study provides proof of concept for the joint effect of few genetic variants in improving the discriminative ability of clinical prognostic models.
Supplementary Material
Acknowledgments
The authors thank the coordinators, field and administrative workers, technicians, secretaries, and study participants of the Spanish Bladder Cancer/EPICURO study.
Spanish Bladder Cancer (SBC)/EPICURO Investigators: Institut Municipal d’Investigació Mèdica, Universitat Pompeu Fabra, Barcelona—Coordinating Center (M. Kogevinas, N. Malats, F.X. Real, M. Sala, G. Castaño, M. Torà, D. Puente, C. Villanueva, C. Murta-Nascimento, J. Fortuny, E. López, S. Hernández, R. Jaramillo, G. Vellalta, L. Palencia, F. Fermández, A. Amorós, A. Alfaro, G. Carretero); Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona (J. Lloreta, S. Serrano, L. Ferrer, A. Gelabert, J. Carles, O. Bielsa, K. Villadiego); Hospital Germans Trias i Pujol, Badalona, Barcelona (L. Cecchini, J.M. Saladié, L. Ibarz); Hospital de Sant Boi, Sant Boi de Llobregat, Barcelona (M. Céspedes); Consorci Hospitalari ParcTaulí, Sabadell (C. Serra, D. García, J. Pujadas, R. Hernando, A. Cabezuelo, C. Abad, A. Prera, J. Prat); Centre Hospitalari i Cardiològic, Manresa, Barcelona (M. Domènech, J. Badal, J. Malet); Hospital Universitario de Canarias, La Laguna, Tenerife (R. García-Closas, J. Rodríguez deVera, A.I. Martín); Hospital Universitario Nuestra Señora de la Candelaria, Tenerife (J. Taño, F. Cáceres); Hospital General Universitario de Elche, Universidad Miguel Hernández, Elche, Alicante (A. Carrato, F. García-López, M. Ull, A. Teruel, E. Andrada, A. Bustos, A. Castillejo, J.L. Soto); Universidad de Oviedo, Oviedo, Asturias (A. Tardón); Hospital San Agustín, Avileós, Asturias (J.L. Guate, J.M. Lanzas, J. Velasco); Hospital Central Cova-donga, Oviedo, Asturias (J.M. Fernández, J.J. Rodríguez, A. Herrero); Hospital Central General, Oviedo, Asturias (R. Abascal, C. Manzano, T. Miralles); Hospital de Cabueñes, Gijón, Asturias (M. Rivas, M. Arguelles); Hospital de Jove, Gijón, Asturias (M. Díaz, J. Sánchez, O. González); Hospital de Cruz Roja, Gijón, Asturias (A. Mateos, V. Frade); Hospital Alvarez-Buylla, Mieres, Asturias (P. Muntañola, C. Pravia); Hospital Jarrio, Coaña, Asturias (A.M. Huescar, F. Huergo); Hospital Carmen y Severo Ochoa, Cangas, Asturias (J. Mosquera).
Grant Support
The project was funded partiallyby Fondo de Investigaciones Sanitarias (FIS, #PI00–0745, #PI05–1436, and #PI06–1614) and RedTemóticade Investigación Cooperativa en Cáncer (RTICC, #RD12/0036/0050 and #RD12/0036/0034), Instituto de Salud Carlos III; and Asociación Española Contra el Cancer (AECC), Spain; and EU-FP7-HEALTH-F2–2008-201663-UROMOL and EU-7FP- HEALTH-TransBíoBC #601933. D. Silverman, N. Rothman, and S.J. Chanock received funding from the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (contract NCI NO2-CP-11015). A. Masson-Lecomte was awarded with a fellowship of the European Urological Scholarship Program for Research (EUSP Scholarship S-01–2013) andE.Lòpez de Maturana with a Sara Borrell fellowship, Instituto de Salud Carlos III, Spain.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
References
- 1.Samanic C, Kogevinas M, Dosemeci M, Malats N, Real FX, Garcia-Closas M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev 2006;15:1348–54. [DOI] [PubMed] [Google Scholar]
- 2.Silverman D, Devesa S, Morore L, Rothman N. Bladder cancer. Cancer Epidemiol Prev 2006;1101–27. [Google Scholar]
- 3.García-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 2006;366:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Closas M,Ye Y, Rothman N, Figueroa JD, Malats N, Dinney CP, et al. A genome-wide association study of bladder cancer identifies a new susceptibility locus within SLC14A1, a urea transporter gene on chromosome 18q12.3. Hum Mol Genet 2011;20:4282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KKH, Stacey SN, et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet 2008;40:1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet 2010;42: 978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Ye Y, Kiemeney LA, Sulem P, Rafnar T, Matullo G, et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat Genet 2009;41:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiemeney LA, Sulem P, Besenbacher S, Vermeulen SH, Sigurdsson A, Thorleifsson G, et al. A sequence variant at 4p16.3 confers susceptibility to urinary bladder cancer. Nat Genet 2010;42:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafnar T, Vermeulen SH, Sulem P, Thorleifsson G, Aben KK, Witjes JA, et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Hum Mol Genet 2011;20: 4268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang W, Fu Y-P, Figueroa JD, Malats N, Garcia-Closas M, Chatterjee N, et al. Mapping of the UGT1A locus identifies an uncommon coding variant that affects mRNA expression and protects from bladder cancer. Hum Mol Genet 2012;21:1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, et al. Sequence variants attheTERT-CLPTM1L locus associate with many cancer types. Nat Genet 2009;41:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafnar T, Sulem P, Thorleifsson G, Vermeulen SH, Helgason H, Saemundsdottir J, et al. Genome-wide association study yields variants at 20p12.2 that associate with urinary bladder cancer. Hum Mol Genet 2014;23:5545–57. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa JD, Ye Y, Siddiq A, Garcia-closas M, Chatterjee N, Prokunina-olsson L, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum Mol Genet 2014;23:1387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrier BP, Hollander MP, Van Rhijn BWG, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol 2004; 45:292–6. [DOI] [PubMed] [Google Scholar]
- 15.Di Martino E, Tomlinson DC, Knowles MA. A decade of FGF receptor research in bladder cancer: Past, present, and future challenges. Adv Urol 2012;2012:429213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009;27:2217–24. [DOI] [PubMed] [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 18.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol 2014;66:1157–64. [DOI] [PubMed] [Google Scholar]
- 19.Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, et al. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J Clin Oncol 2005; 23:5746–56. [DOI] [PubMed] [Google Scholar]
- 20.Ahirwar DK, Agrahari A, Mandhani A, Mittal RD. Cytokine gene polymorphisms are associated with risk of urinary bladder cancer and recurrence after BCG immunotherapy. Biomarkers 2009;14:213–8. [DOI] [PubMed] [Google Scholar]
- 21.Andrew AS, Gui J, Sanderson AC, Mason RA, Morlock EV, Schned AR, et al. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet 2009;125:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guirado M, Gil H, Saenz-Lopez P, Reinboth J, Garrido F, Cozar JM, et al. Association between C13ORF31, NOD2, RIPK2 and TLR10 polymorphisms and urothelial bladder cancer. Hum Immunol 2012;73:668–72. [DOI] [PubMed] [Google Scholar]
- 23.Malats N Genetic epidemiology of bladder cancer: scaling up in the identification of low-penetrance genetic markers of bladder cancer risk and progression. Scand J Urol Nephrol Suppl 2008;42:131–40. [DOI] [PubMed] [Google Scholar]
- 24.de Maturana EL, Ye Y, Calle ML, Rothman N, Urrea V, Kogevinas M, et al. Application of multi-SNP approaches Bayesian LASSO and AUC-RF to detect main effects of inflammatory-gene variants associated with bladder cancer risk. PLoS One 2013;8:e83745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BWG, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639–53. [DOI] [PubMed] [Google Scholar]
- 26.Galdos BL, Medina I, Suarez CM, Heredia T, Torres Ác, Sangrós R, et al. Select your SNPs (SYSNPs): a web tool for automatic and massive selection of SNPs. Int J Data Min Bioinform 2012;6:324–34. [DOI] [PubMed] [Google Scholar]
- 27.García-Closas M, Malats N, Real FX, Yeager M, Welch R, Silverman D, et al. Large-scale evaluation of candidate genes identifies associations between VEGF polymorphisms and bladder cancer risk. PLoS Genet 2007;3:0287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 2007;81: 1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meuwissen THE, Hayes BJ, Goddard ME. Prediction oftotal genetic value using genome-wide dense marker maps. Genetics 2001;157:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park T, Casella G. The Bayesian Lasso. J Am Stat Assoc 2008;103:681–6. [Google Scholar]
- 31.López de Maturana E, Ibáñez-Escriche N, González-Recio O, Marenne G, Mehrban H, Chanock SJ, et al. Next generation modeling in GWAS: comparing different genetic architectures. Hum Genet 2014;133: 1235–53. [DOI] [PubMed] [Google Scholar]
- 32.Albert JH, Chib S. Sequential ordinal modeling with applications to survival data. Biometrics 2001;57:829–36. [DOI] [PubMed] [Google Scholar]
- 33.González-Recio O, Gianola D, Long N, Weigel KA, Rosa GJM, Avendaño S. Nonparametric methods for incorporating genomic information into genetic evaluations: an application to mortality in broilers. Genetics 2008; 178:2305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goddard ME. Optimal effective population size for the global population of black and white dairy cattle. J Dairy Sci 1992;75:2902–11. [DOI] [PubMed] [Google Scholar]
- 35.Gerds TA, Kattan MW, Schumacher M, Yu C. Estimating a time-dependent concordance index for survival prediction models with covariate dependent censoring. Stat Med 2013;32:2173–84. [DOI] [PubMed] [Google Scholar]
- 36.Ahirwar D, Kesarwani P, Manchanda PK, Mandhani A, Mittal RD. Anti- and proinflammatory cytokine gene polymorphism and genetic predisposition: association with smoking, tumor stage and grade, and bacillus Calmette-Guérin immunotherapy in bladder cancer. Cancer Genet Cyto-genet 2008;184:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
- 38.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–10. [DOI] [PubMed] [Google Scholar]
- 39.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesmann JE, Lowe BA, et al. Maintenance bacillus calmette-guerin immunotherapy for recurrent Ta, T1 and carcinoma in situ of the bladder: A Randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9. [PubMed] [Google Scholar]
- 40.Klink M, Kielbik M, Nowak M, Bednarska K, Sulowska Z. JAK3, STAT3 and CD3-zeta signaling proteins status in regard to the lymphocytes function in patients with ovarian cancer. Immunol Invest 2012;41:382–98. [DOI] [PubMed] [Google Scholar]
- 41.Dalloul A CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev 2009;8:349–53. [DOI] [PubMed] [Google Scholar]
- 42.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire- dependent thymic development of tumor-associated regulatory T cells. J Urol 2013;190:1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conteduca G, Ferrera F, Pastorino L, Fenoglio D, Negrini S, Sormani MP, et al. The role of AIRE polymorphisms in melanoma. Clin Immunol 2010;136:96–104. [DOI] [PubMed] [Google Scholar]
- 44.Kutikhin AG. Role of NOD1/CARD4 and NOD2/CARD15 gene polymorphisms in cancer etiology. Hum Immunol 2011;72:955–68. [DOI] [PubMed] [Google Scholar]
- 45.Qiu F, Maniar A, Diaz MQ, Chapoval AI, Medvedev AE. Activation of cytokine-producing and antitumor activities of natural killer cells and macrophages by engagement of Toll-like and NOD-like receptors. Innate Immun 2011;17:375–87. [DOI] [PubMed] [Google Scholar]
- 46.MacNeil AJ, Jiao SC, McEachern LA, Yang YJ, Dennis A, Yu H, et al. MAPK kinase 3 Is a tumor suppressor with reduced copy number in breast cancer. Cancer Res 2014;74:162–72. [DOI] [PubMed] [Google Scholar]
- 47.Otto KB, Acharya SS, Robinson VL. Stress-activated kinase pathway alteration is a frequent event in bladder cancer. Urol Oncol Semin Orig Investig 2012;30:415–20. [DOI] [PubMed] [Google Scholar]
- 48.Otto W, Denzinger S, Wieland WF, Hartmann A. First analysis of immune cell infiltration in stage pT1 urothelial bladder carcinoma: CD3 positivity as a prognostic marker for cancer-specific survival. World J Urol 2012; 30:875–7. [DOI] [PubMed] [Google Scholar]
- 49.Bleeker SE, Moll HA, Steyerberg EW, Donders AR, Derksen-Lubsen G, Grobbee DE, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol 2003;56:826–32. [DOI] [PubMed] [Google Scholar]
- 50.Besaratinia A, Cockburn M, Tommasi S. Alterations of DNA methylome in human bladder cancer. Epigenetics 2013;8:1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.