Abstract
Background
Adolescent vaccination has received increased attention since the Global Vaccine Action Plan's call to extend the benefits of immunisation more equitably beyond childhood. In recent years, many programmes have been launched to increase the uptake of different vaccines in adolescent populations; however, vaccination coverage among adolescents remains suboptimal. Therefore, understanding and evaluating the various interventions that can be used to improve adolescent vaccination is crucial.
Objectives
To evaluate the effects of interventions to improve vaccine uptake among adolescents.
Search methods
In October 2018, we searched the following databases: CENTRAL, MEDLINE Ovid, Embase Ovid, and eight other databases. In addition, we searched two clinical trials platforms, electronic databases of grey literature, and reference lists of relevant articles. For related systematic reviews, we searched four databases. Furthermore, in May 2019, we performed a citation search of five other websites.
Selection criteria
Randomised trials, non‐randomised trials, controlled before‐after studies, and interrupted time series studies of adolescents (girls or boys aged 10 to 19 years) eligible for World Health Organization‐recommended vaccines and their parents or healthcare providers.
Data collection and analysis
Two review authors independently screened records, reviewed full‐text articles to identify potentially eligible studies, extracted data, and assessed risk of bias, resolving discrepancies by consensus. For each included study, we calculated risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CI) where appropriate. We pooled study results using random‐effects meta‐analyses and assessed the certainty of the evidence using GRADE.
Main results
We included 16 studies (eight individually randomised trials, four cluster randomised trials, three non‐randomised trials, and one controlled before‐after study). Twelve studies were conducted in the USA, while there was one study each from: Australia, Sweden, Tanzania, and the UK. Ten studies had unclear or high risk of bias. We categorised interventions as recipient‐oriented, provider‐oriented, or health systems‐oriented.
The interventions targeted adolescent boys or girls or both (seven studies), parents (four studies), and providers (two studies). Five studies had mixed participants that included adolescents and parents, adolescents and healthcare providers, and parents and healthcare providers. The outcomes included uptake of human papillomavirus (HPV) (11 studies); hepatitis B (three studies); and tetanus–diphtheria–acellular–pertussis (Tdap), meningococcal, HPV, and influenza (three studies) vaccines among adolescents.
Health education improves HPV vaccine uptake compared to usual practice (RR 1.43, 95% CI 1.16 to 1.76; I² = 0%; 3 studies, 1054 participants; high‐certainty evidence). In addition, one large study provided evidence that a complex multi‐component health education intervention probably results in little to no difference in hepatitis B vaccine uptake compared to simplified information leaflets on the vaccine (RR 0.98, 95% CI 0.97 to 0.99; 17,411 participants; moderate‐certainty evidence).
Financial incentives may improve HPV vaccine uptake compared to usual practice (RR 1.45, 95% CI 1.05 to 1.99; 1 study, 500 participants; low‐certainty evidence). However, we are uncertain whether combining health education and financial incentives has an effect on hepatitis B vaccine uptake, compared to usual practice (RR 1.38, 95% CI 0.96 to 2.00; 1 study, 104 participants; very low certainty evidence).
Mandatory vaccination probably leads to a large increase in hepatitis B vaccine uptake compared to usual practice (RR 3.92, 95% CI 3.65 to 4.20; 1 study, 6462 participants; moderate‐certainty evidence).
Provider prompts probably make little or no difference compared to usual practice, on completion of Tdap (OR 1.28, 95% CI 0.59 to 2.80; 2 studies, 3296 participants), meningococcal (OR 1.09, 95% CI 0.67 to 1.79; 2 studies, 3219 participants), HPV (OR 0.99, 95% CI 0.55 to 1.81; 2 studies, 859 participants), and influenza (OR 0.91, 95% CI 0.61 to 1.34; 2 studies, 1439 participants) vaccination schedules (moderate‐certainty evidence).
Provider education with performance feedback may increase the proportion of adolescents who are offered and accept HPV vaccination by clinicians, compared to usual practice. Compared to adolescents visiting non‐participating clinicians (in the usual practice group), the adolescents visiting clinicians in the intervention group were more likely to receive the first dose of HPV during preventive visits (5.7 percentage points increase) and during acute visits (0.7 percentage points for the first and 5.6 percentage points for the second doses of HPV) (227 clinicians and more than 200,000 children; low‐certainty evidence).
A class‐based school vaccination strategy probably leads to slightly higher HPV vaccine uptake than an age‐based school vaccination strategy (RR 1.09, 95% CI 1.06 to 1.13; 1 study, 5537 participants; moderate‐certainty evidence).
A multi‐component provider intervention (including an education session, repeated contacts, individualised feedback, and incentives) probably improves uptake of HPV vaccine compared to usual practice (moderate‐certainty evidence).
A multi‐component intervention targeting providers and parents involving social marketing and health education may improve HPV vaccine uptake compared to usual practice (RR 1.41, 95% CI 1.25 to 1.59; 1 study, 25,869 participants; low‐certainty evidence).
Authors' conclusions
Various strategies have been evaluated to improve adolescent vaccination including health education, financial incentives, mandatory vaccination, and class‐based school vaccine delivery. However, most of the evidence is of low to moderate certainty. This implies that while this research provides some indication of the likely effect of these interventions, the likelihood that the effects will be substantially different is high. Therefore, additional research is needed to further enhance adolescent immunisation strategies, especially in low‐ and middle‐income countries where there are limited adolescent vaccination programmes. In addition, it is critical to understand the factors that influence hesitancy, acceptance, and demand for adolescent vaccination in different settings. This is the topic of an ongoing Cochrane qualitative evidence synthesis, which may help to explain why and how some interventions were more effective than others in increasing adolescent HPV vaccination coverage.
Keywords: Adolescent, Child, Humans, Controlled Before‐After Studies, Health Education, Health Education/methods, Health Personnel, Health Personnel/education, Parents, Parents/education, Randomized Controlled Trials as Topic, Vaccination, Vaccination/statistics & numerical data, Vaccination/trends
Plain language summary
Improving vaccination uptake among adolescents
This Cochrane Review aimed to assess the effects of approaches to increase the number of adolescents who get vaccinated. Cochrane researchers collected and analysed all relevant studies to answer this question and found 16 studies.
Key messages
This review showed that several different approaches may increase the number of adolescents who get their recommended vaccines. These include giving health education, offering gifts, and passing laws. However, more research is needed to understand what approaches work best, especially in low‐ and middle‐income countries.
What was studied in the review?
The World Health Organization recommends several vaccines for children aged between 10 and 19 years (adolescents). Some of these vaccines are mainly offered to this age group, such as the human papillomavirus (HPV; a viral infection that is passed between people through skin‐to‐skin contact and can cause genital warts and cancer) vaccine. Others are booster vaccines and are also given to younger children, such as hepatitis B vaccines, diphtheria, tetanus, and pertussis (whooping cough) vaccines.
Many adolescents do not get their recommended vaccines. Governments and organisations have tried different approaches to change this. One approach is to target adolescents and their parents and communities. This can be done, for instance, by giving them information about vaccines; reminding them when the vaccines are due; or giving them gifts. Another approach is to target healthcare providers, for instance through information, reminders, or feedback about their practice. A third approach is to make vaccines more accessible to people. This can be done, for instance, by making vaccines free or cheap, or by offering vaccines closer to home, including at schools. A fourth approach is to pass laws about vaccination. For instance, in some countries, students have to prove that they have been vaccinated before they can attend school.
What were the main results of the review?
The review authors found 16 relevant studies. Twelve of the studies were from the USA. The other studies were one each from Australia, Sweden, Tanzania, and the UK. These studies showed the following.
When adolescents (girl or boys, or both) and their parents were given vaccination information and education, more adolescents got HPV vaccines (high‐certainty evidence).
When adolescents were given gift vouchers, more adolescents may have got HPV vaccines (low‐quality evidence). However, we were uncertain whether giving adolescents and their parents health education, cash, and gift packages led to more adolescents getting hepatitis B vaccines (very low certainty evidence).
When laws were passed stating that adolescents must be vaccinated to go to school, substantially more adolescents probably got hepatitis B vaccines (moderate‐certainty evidence).
When healthcare providers were reminded to vaccinate adolescents when they opened their electronic medical charts, this probably had little or no effect on the number of adolescents who got tetanus–diphtheria–pertussis, meningococcal, HPV, or influenza vaccines (moderate‐certainty evidence).
When healthcare providers were given education with performance feedback, more adolescents may have got HPV vaccines (low‐certainty evidence).
When healthcare providers were given education, individualised feedback, frequent visits, and incentives, more adolescents probably got HPV vaccines (moderate‐certainty evidence).
When healthcare providers and parents were targeted in several ways, including through education, telephone calls, and radio messages, more adolescents may have got HPV vaccines (low‐certainty evidence).
These studies compared the use of these approaches (health education, gifts and rewards, laws, or reminders) to using no approaches.
In addition, one study from Tanzania gave vaccination information to all girls that were in school class six but were not necessarily of the same age. They were compared to girls who were given vaccination information because they were all born in the same year, but were not necessarily in the same class. This study showed that the class‐based approach probably led to slightly more girls getting HPV vaccines (moderate‐certainty evidence).
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 31 October 2018.
Summary of findings
Summary of findings for the main comparison. Health education compared to usual practice.
| Comparison 1: health education compared to usual practice | ||||||
| Population: adolescents and parents Setting: Sweden and USA intervention: health education Comparison: usual practice | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With usual practice | With health education | |||||
| Uptake of HPV vaccinea | 209 per 1000 | 298 per 1000 (242 to 367) | RR 1.43 (1.16 to 1.76) | Health education improves uptake of HPV vaccine compared to usual practice. | 1054 (3)b | ⊕⊕⊕⊕ Highc,d,e |
|
CI: confidence interval; HPV: human papillomavirus; RR: risk ratio. *The anticipated absolute effect in the intervention group (and its 95% confidence interval) is based on the assumed likelihood of being vaccinated in the usual care group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence.: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
a The lag‐time between delivery of the intervention and assessment of outcomes ranged from three months (Grandahl 2016) to 11 months (Winer 2016 )
bDiclemente 2015 (randomised trial); Grandahl 2016 (cluster‐randomised trial); Winer 2016 (cluster‐randomised trial). c Well conducted randomised trials with consistent findings (I2 = 0%).
d The findings from the one non‐randomised trial that assessed this comparison were similar to the findings of the randomised trials.
eOne study reported that health education did not have any adverse events in relation to usual practice (Rickert 2015).
Summary of findings 2. Complex compared to simplified health education.
| Comparison 2: complex compared to simplified health education | ||||||
| Population: adolescents Setting: Australia Intervention: multi‐component health educationa Comparison: simplified health education | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With simplified education | With complex health education | |||||
| Uptake of hepatitis B vaccineb | 756 per 1000 | 741 per 1000 (726 to 748) | RR 0.98 (0.96 to 0.99) | A complex multi‐component health education programme probably results in little or no difference in uptake of 3 doses of hepatitis B vaccine compared to simplified health education. | 17,411 (1)c | ⊕⊕⊕⊝ Moderated |
|
CI: confidence interval; RR: risk ratio. *The anticipated absolute effect in the intervention group (and its 95% confidence interval) is based on the assumed likelihood of being vaccinated in the simplified health education group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
a Health education kit with 4‐lesson structured multi‐component intervention that included: a resource fact sheet and assessment, an information video and questions designed to engage an adolescent audience, small group discussion, and an activity to locate resource information on the Internet.
bThe lag‐time between delivery of the intervention and assessment of outcomes was not provided.
cSkinner 2000 (randomised trial). d Downgraded one level due to study limitations, as the included study has an unclear risk of bias.
Summary of findings 3. Financial incentives compared to usual practice.
| Comparison 3: financial incentives compared to usual practice | ||||||
| Patient or population: adolescents Setting: UK intervention: financial incentivea Comparison: usual practice | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With usual practice | With financial incentives | |||||
| Uptake of HPV vaccineb | 196 per 1000 | 284 per 1000 (206 to 390) | RR 1.45 (1.05 to 1.99) | Financial incentives may improve uptake of HPV vaccine compared to usual practice. | 500 (1)c | ⊕⊕⊝⊝ Lowd,e |
|
CI: confidence interval; HPV: human papillomavirus; RR: risk ratio. *The anticipated absolute effect in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the usual practice group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence.: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
aThe financial incentive involved an offer of shopping vouchers worth GBP 45 upon completion of 3 HPV vaccination doses.
bThe lag‐time between delivery of the intervention and assessment of outcomes was one to seven months. Invitation letters promising incentives were sent in February‐March of 2010 and vaccination sessions were conducted between March and September 2010
cMantzari 2015 (randomised trial). d Downgraded one level for study limitations (unclear risk of bias in the included study). e Downgraded one level for imprecision of findings.
Summary of findings 4. Health education plus financial incentives compared to usual practice.
| Comparison 4: health education plus financial incentives compared to usual practice | ||||||
| Population: adolescents and parents Setting: USA Intervention: health education plus financial incentivesa Comparison: usual practice | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With usual practice | With health education and incentives | |||||
| Uptake of hepatitis B vaccineb | 451 per 1000 | 622 per 1000 (433 to 902) | RR 1.38 (0.96 to 2.00) | We are uncertain about the effects of health education plus financial incentives on the uptake of 3 doses of hepatitis B vaccine compared to usual practice. | 104 (1)c | ⊕⊝⊝⊝ Very lowd,e,f |
|
CI: confidence interval; HPV: human papillomavirus; RR: risk ratio. *The anticipated absolute effects in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the usual practice group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
aThe intervention involved (1) an educational video and PowerPoint presentation for caregivers and adolescents about hepatitis B infection and the importance of hepatitis B vaccination, (2) free vaccination, and (3) financial incentives. When the adolescents received each vaccine dose, their caregivers were given cash incentives of USD 10 for the first dose, USD 10 for the second dose, and USD 30 for the third dose. In addition, at each visit, adolescents and caregivers were given gift packages containing cosmetics for adults and sweets and toothbrushes for the children.
bThe lag‐time between delivery of the intervention and assessment of outcomes was three months.
cSchwarz 2008 (randomised trial). d Downgraded one level for serious study limitations (unclear risk of bias in the included study). e Downgraded two levels for imprecision of findings with a wide confidence interval that includes both benefit and harm as well as a very small number of participants.
f Downgraded two levels for serious indirectness, given that this finding is based on one small study from one setting.
Summary of findings 5. Mandatory vaccination versus usual practice.
| Comparison 5: mandatory vaccination vs usual practice | ||||||
| Population: adolescents Setting: USA Intervention: school entry law mandating vaccination Comparison: usual practice in other classes in the same schools | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With usual practice | With mandatory vaccination | |||||
| Uptake of hepatitis B vaccinea | 248 per 1000 | 728 per 1000 (677 to 784) | RR 2.94 (2.66 to 3.25) | Mandatory vaccination probably leads to a large increase in uptake of 3 doses of the hepatitis B vaccine compared to usual practice in other classes in the same schools. | 2642 (1)b | ⊕⊕⊕⊝ Moderated |
| Population: adolescents Setting: USA Intervention: school entry law mandating vaccination Comparison: usual practice in areas not affected by the mandatory vaccination law | ||||||
| Uptake of hepatitis B vaccinea | 186 per 1000 | 728 per 1000 (678 to 780) |
RR 3.92 (3.65 to 4.20) |
Mandatory vaccination probably leads to a large increase in uptake of 3 doses of the hepatitis B vaccine compared to usual practice in areas not affected by the mandatory vaccination law. | 6462 (1)c | ⊕⊕⊕⊝ Moderated |
|
CI: confidence interval; RR: risk ratio. *The anticipated absolute effects in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the no‐intervention group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
a The lag‐time between delivery of the intervention and assessment of outcomes was 6‐8 years.
bWilson 2005 (non‐randomised trial) compared students in the ninth grade (affected by the hepatitis B law) and 12th grade (not affected by the law) in the state of Missouri . cWilson 2005 (non‐randomised trial) compared the ninth grade in the state of Missouri (affected by the hepatitis B vaccination law) to the ninth grade in the state of Kansas (not affected by the law).
d As a non‐randomised trial, these outcomes were initially graded as low certainty evidence and then upgraded by one level for very large effect sizes.
Summary of findings 6. Provider prompts compared to usual practice.
| Comparison 6: provider prompts compared to usual practice | ||||
| Population: healthcare workers Setting: USA Intervention: provider promptsa Comparison: usual practice | ||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |
| Relative effect (95% CI) | Narrative results | |||
| Uptake of HPV vaccineb | aOR 0.99 (0.55 to 1.81)e |
Provider prompts probably make little or no difference to uptake of 3 doses of HPV vaccine among adolescents compared to usual practice. | 859 (2)c | ⊕⊕⊕⊝ Moderated |
| Uptake of Tdap vaccineb | aOR 1.28 (0.59 to 2.80) |
Provider prompts probably make little or no difference to uptake of Tdap vaccine among adolescents compared to usual practice. | 3296 (2)c | ⊕⊕⊕⊝ Moderated |
| Uptake of meningococcal conjugate vaccineb | aOR 1.09 ,(0.67 to 1.79) | Provider prompts probably make little or no difference to uptake of the meningococcal conjugate vaccine among adolescents compared to usual practice. | 3219 (2)c | ⊕⊕⊕⊝ Moderated |
| Uptake of seasonal influenza vaccineb |
aOR 0.91 (0.61 to 1.34) |
Provider prompts probably make little or no difference to uptake of the seasonal influenza vaccine among adolescents compared to usual practice. | 1439 (2)c | ⊕⊕⊕⊝ Moderated |
|
CI: confidence interval; HPV: human papillomavirus; aOR: adjusted odds ratio; Tdap: tetanus–diphtheria–acellular–pertussis. *The anticipated absolute effects in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the usual practice group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence.: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||
a When a healthcare provider opened a patient's electronic medical record, there was a screen display of the list of vaccines that were due at that visit. At the beginning of the study, a 1‐2 hour educational session was given to the providers to inform them about the electronic health record based prompts.
bThe lag‐time between delivery of the intervention and assessment of outcomes was 12 months.
cSzilagyi 2015 conducted two separate randomised trials, one in a local and one in a national network, and then reported these in one paper. d Downgraded one level for imprecision of findings.
e All odds ratios were adjusted based on a multilevel mixed‐effect logistic regression model with covariates for pair assignment, study time period, group assignment, and an interaction between time and group assignment.
Summary of findings 7. Provider education with performance feedback compared to usual practice.
| Comparison 7: provider education with performance feedback compared to usual practice | |||
| Population: paediatricians and nurse practitioners Setting: USA Intervention: education with performance feedback Comparison: usual practice | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)* |
| Uptake of HPV vaccinationa | Provider education with performance feedback may increase the proportion of adolescents who are offered and accept HPV vaccination by clinicians, compared to usual practice. Compared to adolescents visiting non‐participating clinicians (in the usual practice group), the adolescents visiting clinicians in the intervention group were more likely to receive the first dose of HPV during preventive visits (5.7 percentage points increase) and during acute visits (0.7 percentage points for the first and 5.6 percentage points for the second doses of HPV). | > 200,000 children (1 CBA)b | ⊕⊕⊝⊝ Lowc |
|
HPV: human papillomavirus; CBA: controlled before‐after study *GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | |||
a There was no lag‐time between delivery of the intervention and assessment of outcomes. The intervention period ran from 01 January to 30 November 2013. Outcomes were assessed throughout this period, starting from day 1.
bFiks 2016 (controlled before‐after study). c This is a non‐randomised study.
Summary of findings 8. Class‐based compared to age‐based HPV vaccination in schools.
| Comparison 8: class‐based compared to age‐based HPV vaccination in schools | ||||||
| Population: adolescents Setting: Tanzania Intervention: class‐based vaccination Comparison: age‐based vaccination | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With age‐based delivery | With class‐based delivery | |||||
| HPV vaccine uptakea | 721 per 1000 | 786 per 1000 (764 to 815) | RR 1.09 (1.06 to 1.13) | Class‐based vaccination probably leads to slightly higher HPV vaccine uptake than age‐based vaccination. | 5537 (1)b | ⊕⊕⊕⊝ Moderatec |
|
CI: confidence interval; HPV: human papillomavirus; RR: risk ratio. *The anticipated absolute effects in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the comparison group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision | ||||||
a The lag‐time between delivery of the intervention and assessment of outcomes was 12 months.
bWatson‐Jones 2012 (cluster‐randomised trial). c Downgraded one level for indirectness, given that the outcome is based on one study from one setting.
Summary of findings 9. Multi‐component provider intervention compared to usual practice.
| Comparison 9: multi‐component provider intervention compared to usual practice | |||
| Population: healthcare providers and their adolescent patients (boys and girls aged 11–21 years) Setting: USA Intervention: multi‐component performance improvement continuing medical education interventiona Comparison: usual practice | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)* |
| HPV vaccine uptakeb | A multi‐component provider intervention (including an education session, repeated contacts, individualised feedback, and incentives) probably improves uptake of HPV vaccine compared to usual practice. Girls in the intervention group are probably more likely to receive their next HPV vaccine dose than those in the comparison group (odds ratio 1.6, 95% CI 1.1 to 2.2). The effects are probably larger for boys (odds ratio 25.00, 95% CI 15.00 to 40.00), and this may be because publicly funded HPV vaccination for boys became available during the study. | 15,849 adolescents (1)c | ⊕⊕⊕⊝ Moderated |
|
HPV: human papillomavirus. *GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | |||
aThe intervention involved: (1) 6–8 education visits over 12 months by an HPV physician‐educator; (2) focused education sessions on HPV‐related topics designed to change the way providers viewed the importance of HPV vaccination and responded to parents' hesitation toward HPV vaccines; (3) individualised feedback where providers and practices received individual reports that showed their performance compared to other providers in their practice on HPV vaccination coverage; and (4) quality improvement incentives whereby physicians were eligible to receive maintenance‐of‐registration credits, which fulfilled requirements for maintaining board certification.
b The lag‐time between delivery of the intervention and assessment of outcomes was six months.
cPerkins 2015 (cluster‐randomised trial). d Downgraded one level because of serious indirectness, given that this finding is based on one study from one setting.
Summary of findings 10. Multi‐component provider and parent intervention compared to usual practice.
| Comparison 10: multi‐component provider and parent intervention compared to usual practice | ||||||
| Population: healthcare workers and parents Setting: USA Intervention: multi‐component provider and parent interventiona Comparison: usual practice | ||||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE)** | |||
| Absolute effects* (95% CI) | Relative effect (95% CI) | Narrative results | ||||
| With usual practice | With multi‐faced intervention | |||||
| HPV vaccine uptake at 3 months | 25 per 1,000 | 57 per 1000 (18 to 180) |
RR 2.34 (0.75 to 7.32) |
A multi‐component intervention involving healthcare providers and parents may improve uptake of the HPV vaccine compared to usual practice. | 337
(1)b Randomised trial |
⊕⊕⊝⊝ Lowd |
| HPV vaccine uptake at 6 months | 52 per 1,000 | 73 per 1000 (65 to 83) |
RR 1.41 (1.25 to 1.59) |
25,869 (1)cNon‐randomised trial | ⊕⊕⊝⊝ Lowe | |
|
CI: confidence interval; HPV: human papillomavirus; RR: risk ratio. *The risk in the intervention group (and its 95% CI) is based on the likelihood of being vaccinated in the usual practice group and the relative effect of the intervention (and its 95% CI). **GRADE Working Group grades of evidence: High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. †Substantially different = a large enough difference that it might affect a decision. | ||||||
a In the randomised trial (Paskett 2016), healthcare providers received a one‐hour PowerPoint presentation and handouts on the HPV vaccine, focusing on current evidence‐based HPV vaccine information and strategies designed to assist physicians in discussing HPV vaccination with parents. In addition, parents were mailed a packet that included an educational brochure and DVD video about HPV infection and HPV vaccination as well as a CDC HPV vaccine information statement. Furthermore, health educators conducted an education session with parents about the HPV vaccine via telephone to reinforce the message in the educational materials regarding the need for the vaccine and addressed any vaccination barriers or questions.
In the non‐randomised trial (Cates 2014), the intervention included: (1) distribution of HPV vaccination posters and brochures with the risk‐related message to health departments and healthcare providers; (2) two radio public service announcements designed to raise awareness about HPV vaccine for boys among parents of preteen boys; (3) an online continuing medical education training with video demonstrating communication among providers, parents, and preteen boys available to enrolled health providers; (4) one‐page tip sheet for providers to discuss HPV vaccination with parents and boys; and (5) a website with links to credible information sources useful for both parents and providers.
bPaskett 2016 (randomised trial).
cCates 2014 (non‐randomised trial).
d Downgraded by two levels for serious imprecision and serious study limitations (unclear risk of selection bias in the included study) (Paskett 2016).
e Downgraded by two levels for non‐randomised study design (Cates 2014).
Background
Description of the condition
The World Health Organization (WHO) defines adolescents as people aged between 10 and 19 years (WHO 2019a). Targeting adolescents with relevant vaccines offers three benefits: catch‐up on missed vaccinations, boosting of waning immunity, and primary immunisation with new vaccines (Brabin 2008; Mackroth 2010). Vaccines given during adolescence include, but are not limited to, those against human papillomavirus (HPV), diphtheria, tetanus, pertussis, measles, mumps, rubella, varicella, hepatitis B, poliomyelitis, and meningococcal disease (Gilkey 2014; Harris 2009; Lee 2005; Mavundza 2019; Piot 2019; SAHM 2013; WHO 2019b). Future vaccines against HIV and Mycobacterium tuberculosis are likely to target adolescents as the primary population (Gowda 2012; Zipursky 2010).
In many settings, adolescents usually turn to physicians only when they are ill and so there are limited opportunities to inform them that vaccines are important and should be administered (Cawley 2010; Principi 2013). In such instances, adolescents may be more interested in their current health condition than possible benefits of preventing future vaccine‐preventable diseases (VPDs) (Principi 2013). Schools have been used extensively as a delivery platform for vaccinating large numbers of school‐aged children (Barry 2013; Cawley 2010; Harris 2009; Robbins 2011; Tsu 2009). However, school‐based vaccination programmes may not be entirely successful in countries with suboptimal school attendance rates (Mackroth 2010; Warren 2004). For instance, school attendance rates in many low‐ and middle‐income countries (LMICs) are variable due to factors such as geographical location, socioeconomic status, and gender (Mackroth 2010; Warren 2004; Zipursky 2010). Strategies such as mass immunisation campaigns can be used to complement school‐based vaccination programmes in settings with poor school attendance rates (Clements 2004; Piot 2019).
Data on vaccination coverage among adolescents are limited, but coverage is generally low in this group (Brotherton 2015; Loke 2017; Newman 2018). For example, it is estimated that only 6.1% of adolescent girls worldwide completed the full series of HPV vaccination in 2014; with wide variation between LMICs and high‐income countries (Bruni 2016). HPV vaccination coverage was only 1.1% in Asia and 1.2% in Africa, compared to 35.6% in North America and 35.9% in Oceania. Overall, HPV vaccination coverage in 2014 was 33.6% in high‐income countries, compared with only 2.7% in LMICs (Bruni 2016). The most commonly reported barriers to adolescent vaccination include lack of knowledge about vaccines and VPDs; negative attitudes towards vaccination from adolescents, parents, teachers, and healthcare providers; poor vaccine infrastructure; and financial constraints (Adamu 2019; Gowda 2012; Ngcobo 2018; SAHM 2013).
Description of the intervention
Interventions to enhance the uptake of vaccines by adolescents may have multiple components, targeting adolescents and their communities, healthcare providers, the health system, or a combination of these (Wiysonge 2012).
Recipient‐oriented interventions
Interventions targeting adolescents and their communities (including their parents and teachers) may include education, reminders, incentives, and mandatory vaccination.
Educational interventions enable adolescents and their communities to understand the meaning and relevance of vaccination to their health (Willis 2013; Kaufman 2017). Such interventions may be delivered face‐to‐face or via written mail, telephone conversation, audiovisual presentation or drama, printed materials, websites, multi‐media campaigns, or community events (Willis 2013; Kaufman 2017). These types of interventions may be directed at individuals or groups, and may include information about VPDs; the risks and benefits of vaccines; where, how, and when to access vaccine services; who should be vaccinated; or a combination of these (Oyo‐Ita 2016; Williams 2011; Willis 2013; Kaufman 2017). Adolescents and communities may receive education about vaccines through prominently displayed posters in waiting rooms, brochures, e‐mails, and website resources (Stinchfield 2008).
Client reminder interventions involve reminding members of a target population that vaccinations are due or have been missed. Reminders are delivered using various methods, such as telephone calls, letters, or postcards (Jacobson Vann 2018). The contents of the reminders may include personalised information related to a specific upcoming or missed appointment (Stinchfield 2008; Willis 2013; Kaufman 2017).
Adolescent or community incentives involve providing financial or other incentives to motivate people to accept vaccinations (Briss 2000; Oyo‐Ita 2016; TFCPS 2000). Incentives can be rewards or gifts (TFCPS 2000).
Mandatory vaccination refers to a law or policy that requires students to show proof of immunisation records prior to school admission with failure to do this resulting in school admission being denied (Briss 2000; Oyo‐Ita 2016; TFCPS 2000).
Provider‐oriented interventions
Provider‐oriented interventions may include reminders, audit and feedback, and education.
Provider reminder interventions inform vaccinators that individual clients are due for vaccinations. Reminders may be delivered through client charts, computer, e‐mail, or postal mail, among many others (Briss 2000; TFCPS 2000; Ward 2012).
Audit and feedback for vaccinators involves retrospectively evaluating the performance of the vaccinators in administering vaccines and providing feedback to them (Oyo‐Ita 2016; Stinchfield 2008; Williams 2011). This information is given to providers to motivate them to improve immunisation services.
Provider education involves giving information regarding vaccinations to providers to increase their knowledge and to encourage them to adopt positive attitudes towards vaccination. Techniques by which information is delivered can include written materials, videos, lectures, continuing medical education programmes, and computerised software (TFCPS 2000; Ward 2012; Williams 2011).
Health system interventions
Outreach programmes include school‐based immunisation and mass campaigns. School‐based immunisation outreach is intended to improve delivery of vaccinations to school‐going children (TFCPS 2000). School‐based interventions usually include vaccination‐related education of students about either provision of vaccinations or referral for vaccinations (Briss 2000; Oyo‐Ita 2016; TFCPS 2000). Mass campaign programmes target adolescents both in school and out of school (Clements 2004).
Expanding access in healthcare settings is used to increase the availability of vaccines in the medical or public health settings in which vaccinations are offered. This can be achieved using several methods such as: increasing or changing the hours during which vaccination services are provided; delivering vaccinations in clinical settings in which they were previously not provided (e.g. emergency departments, inpatient units, or subspeciality clinics); or reducing administrative barriers to obtaining vaccination services within clinics (e.g. developing a 'drop‐in' clinic or an 'express lane' vaccination service) (Briss 2000; Stinchfield 2008; TFCPS 2000).
Reducing out‐of‐pocket costs can be implemented by subsiding the costs of vaccines, paying for vaccinations, providing insurance coverage, or reducing copayments for vaccinations at the point of service (Briss 2000; Oyo‐Ita 2016; TFCPS 2000).
Multi‐component interventions
Multi‐component interventions are approaches that include more than one tactic, with the aim of addressing a variety of barriers to adolescent vaccine uptake. Such interventions could enable communities to be aware of the immunisation services available to them, demonstrate the utility and relevance of these services, provide community members with the knowledge and information base to effectively take advantage of the services, or incorporate a variety of associated provider or health system strategies to improve immunisation uptake (Briss 2000; Oyo‐Ita 2016; TFCPS 2000).
How the intervention might work
We have proposed a logic model which suggests how the strategies described in the Description of the intervention section may, alone or in combination, influence adolescent vaccination uptake and other outcomes (Figure 1).
1.
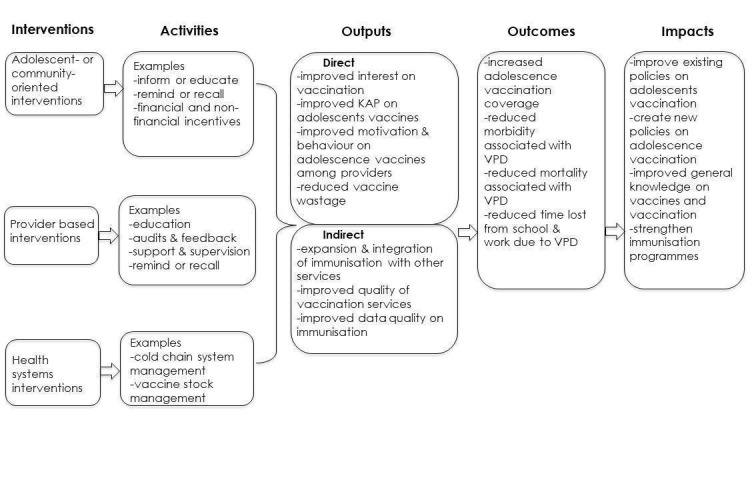
Logic framework on interventions for improving uptake of adolescent vaccines. KAP: knowledge, attitudes and practices; VPD: vaccine‐preventable disease.
Parents, including legal guardians or other people assuming the parental role, are routinely involved in the decision‐making process about vaccine administration to their children (Kaufman 2018). Teachers can also play a crucial role in adolescent vaccination uptake, especially where school‐based vaccination programmes are a popular platform for vaccination of adolescents (Barry 2013; Tsu 2009). In some situations, the final decision on whether an adolescent will be vaccinated or not may be entirely dependent on the parents, as adolescents may not have an independent final decision on whether to get vaccinated (Barry 2013; WHO 2019a). Hence, adequate knowledge and positive attitudes towards vaccination among parents, teachers, and adolescents may improve the uptake of vaccines among adolescents (Abdullahi 2016; Gowda 2012; Mahomed 2008). It is likely that more vaccine‐informed adolescents may be more able to positively guide and influence their parents and peers on vaccinations compared to peers who are less well informed. In addition, adolescents are future parents and investing resources in educating adolescents about vaccination may lead to improved uptake of vaccines by their children (Barry 2013). Therefore, educating adolescents about vaccination may have long‐term positive benefits on vaccine uptake in general (Principi 2013).
Healthcare providers give advice to parents and adolescents on vaccination. The ability of healthcare providers to keep up‐to‐date with knowledge on vaccines is essential, particularly when new vaccines are recommended (Gowda 2012; Principi 2013). Careful and factual advice on vaccination to adolescents and their parents by healthcare providers can result in more willingness to get vaccinated by adolescents. Health system interventions ensure that vaccines are available when adolescent girls and boys, and their communities, demand them (Kaddar 2013).
Why it is important to do this review
Adolescents represent 25% of the global population, but vaccination coverage among them is very low (Brotherton 2015; Bruni 2016; Loke 2017; Newman 2018). There is a knowledge gap around interventions to improve vaccine uptake among adolescents, especially in LMICs. Our review evaluated the evidence on strategies that can be adopted to improve vaccine uptake among adolescents. Such strategies will improve the uptake of current vaccines among adolescents, and may also increase the uptake of future vaccines. In addition, this review could be used to advocate for strengthening existing adolescent vaccination policies and to formulate new policies on the vaccination of adolescents where none currently exist. We are not aware of any previous systematic review that has assessed interventions to improve adolescent immunisation coverage across all country income categories. However, a number of reviews have assessed various strategies to improve immunisation coverage in children or the whole population (Jacobson Vann 2018; Kaufman 2018; Oyo‐Ita 2016; Saeterdal 2012; Williams 2011). These reviews considered general barriers to immunisation and assessed the effects of a variety of interventions. In our review, we used a similar approach among the adolescent population.
Objectives
To evaluate the effects of interventions to improve vaccine uptake among adolescents.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials, non‐randomised trials, interrupted time series studies, and controlled before‐after studies that met the quality criteria used by Cochrane Effective Practice and Organisation of Care (EPOC) (EPOC 2019a). We included both individually randomised and cluster‐randomised trials. For cluster‐randomised trials, we only included those with at least two intervention and two control clusters. Following the EPOC criteria, we included interrupted time series studies only if outcomes were measured during at least three points before and three points after the intervention. For a controlled before‐after study to be included in the review, it must have included at least two intervention groups and at least two comparable control groups, with simultaneous data collection.
We excluded simple pre–post designs; cluster‐randomised and non‐randomised trials with only one intervention or control site; and controlled before‐after studies without concurrent data collection in intervention and comparison groups in accordance with the EPOC criteria for inclusion of studies in systematic reviews of effects (EPOC 2019a).
Types of participants
Girls or boys (or both) aged 10 to 19 years eligible for WHO‐recommended vaccines and their parents or healthcare providers. In the case of studies with interventions directed at mixed populations of children and adolescents or adolescents and adults, we excluded a study if specific data for adolescents were not reported.
Types of interventions
Intervention
-
Recipient‐oriented interventions (i.e. interventions targeting adolescents or their communities, or both), for example:
interventions to communicate with adolescents or their parents (or both) about adolescent immunisation;
financial and non‐financial incentives for adolescents or their parents (or both); and
mandatory vaccination: vaccination requirement for high school and university attendance.
-
Provider‐oriented interventions, for example:
any intervention to reduce missed opportunities for vaccination (e.g. audit and feedback); and
health education, training, and supportive supervision.
-
Health system interventions, for example:
interventions to improve the quality of services, such as provision of reliable cold chain systems, provision of transport for vaccination, vaccine stock management;
outreach programmes, for example, school‐based immunisation and mass vaccination campaign for out‐of‐school adolescents;
expanded services, for example, extended hours for immunisation services;
increased immunisation budget; and
integration of immunisation services with other services.
Multi‐component interventions.
Exclusions
We excluded interventions to remind recipients or providers of immunisation services, as there is already a Cochrane Review on this topic (Jacobson Vann 2018).
Comparisons
Standard immunisation practices in the study setting.
Alternative interventions.
Similar interventions implemented with different degrees of intensity.
Types of outcome measures
Primary outcomes
Adolescent vaccination coverage, that is, the proportion of adolescents who have received the recommended dose(s) of the vaccine(s) studied.
Secondary outcomes
Proportion of adolescents completing the schedule.
Equitable uptake of immunisation (as defined by the study authors).
Knowledge, attitudes, and beliefs.
Adverse effects of the intervention.
Cost of the intervention.
Incidence of VPDs.
Search methods for identification of studies
With the assistance of the Cochrane EPOC Information Specialist, we developed search strategies, with no restrictions on language or publication date. The search strategies for the electronic databases incorporated the Cochrane EPOC search strategy for randomised trials, non‐randomised trials, interrupted time series studies, and controlled before‐after studies (EPOC 2019a), and combined selected MeSH and free‐text terms relating to adolescent vaccination uptake literature globally.
Electronic searches
We searched the following databases for primary studies:
Cochrane Central Register of Controlled Trials (CENTRAL) 2017, Issue 1; part of the Cochrane Library (www.cochranelibrary.com; searched 31 October 2018);
MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and MEDLINE (1946 to 31 October 2018);
Embase Ovid (1974 to 31 October 2018);
CINAHL EBSCOhost (1981 to 31 October 2018);
Africa‐Wide Information EBSCOhost (19th century to 31 October 2018);
Global Health Ovid (1973 to 31 October 2018);
Scopus, Elsevier (searched 31 October 2018); and
Science Citation Index Expanded; Social Sciences Citation Index (1987 to October 2018), and Emerging Sources Citation Index (2015 to October 2018), Web of Science Core Collection, Thompson Reuters (searched 24 April 2017) (for papers citing any of the included studies in the review 31 April 2019).
We searched the following databases for related reviews:
Cochrane Database of Systematic Reviews (CDSR), 2017, Issue 3, part of the Cochrane Library (www.cochranelibrary.com; searched 31 October 2018);
Database of Abstracts of Reviews of Effects (DARE), 2015, Issue 2, part of the Cochrane Library (www.cochranelibrary.com; searched 31 October 2018);
Health Technology Assessment Database (HTA), 2016, Issue 4 (searched 31 October 2018);
PDQ‐Evidence (searched 31 October 2018).
In addition, in May 2019, we did a citation search using: Science Citation Index Expanded; Social Sciences Citation Index (from 1987), and Emerging Sources Citation Index (from 2015), Web of Science Core Collection, and Clarivate Analytics.
See Appendix 1 for search strategies used.
Searching other resources
Grey literature
We searched the following grey literature (31 October 2018):
WHO (www.who.int/);
Gavi, the Vaccine Alliance (www.gavi.org);
United Nations Children's Funds (UNICEF; www.unicef.org/);
PATH Vaccine Resources Library (www.path.org/);
US Centers for Disease Control and Prevention (CDC; www.cdc.gov/);
The Communication Initiative Network (www.comminit.com/);
Grey Literature Report (www.greylit.org);
OpenGrey (www.opengrey.eu/);
Eldis (www.eldis.org/);
Immunization Basics (www.immunizationbasics.jsi.com).
Trial registries
We searched the following trial registries (31 October 2018):
WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/);
ClinicalTrials.gov, US National Institutes of Health (NIH; clinicaltrials.gov/).
Reference lists
We searched the reference lists of potentially eligible studies and relevant previous reviews.
Data collection and analysis
Selection of studies
Two review authors (LA and BK) screened titles and abstracts to select potentially eligible studies. One review author (LA) then obtained the full text of potentially eligible studies and two review authors (LA and VN) independently conducted the final study selection for inclusion in the review. We resolved any disagreements regarding the inclusion of studies by discussion or by consulting a third review author (BK and CW). We used a PRISMA flow chart (Moher 2009) to summarise the search and selection of studies for the review (Figure 2).
2.
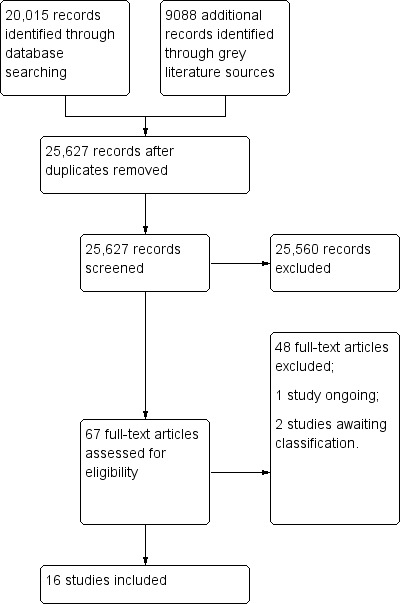
Study flow diagram.
Data extraction and management
Two review authors (LA and VN) independently extracted data from selected studies using an adapted version of the Cochrane data extraction form. Disagreements on study selection and data extraction were resolved by consensus between the two review authors, failing which a third review author (BK) arbitrated. Prior to use, we piloted the data extraction form on four studies identified randomly from the list of included studies.
The data extraction form included the following items.
Setting of the study (city and country).
Type of study: randomised trials, non‐randomised trials, interrupted time series studies, and controlled before‐after studies.
Type of participants: adolescents, parents, healthcare providers.
Type of interventions: name of intervention, frequency, timing, delivery method, venue of delivery.
Type of outcomes measured: vaccine coverage, knowledge, attitudes and beliefs, cost of intervention, adverse effects of the intervention, equity.
When dichotomous outcome data were presented as percentages, we multiplied the percentages by the number of participants in the study arm to obtain the approximate number of events.
Assessment of risk of bias in included studies
We applied the Cochrane EPOC 'Risk of bias' criteria for randomised trials, non‐randomised trials, interrupted time series studies, and controlled before‐after studies, as appropriate (EPOC 2019b). For each included study, we reported our assessment of risk of bias (low, high, or unclear risk) for each domain, together with a descriptive summary of the information that influenced our judgement. Any study that was assigned a high risk of bias for allocation concealment, blinding of outcome assessment, completeness of outcome data, or a combination of these was considered to have a high risk of bias. Studies with low risk of bias for all three domains were considered to have a low risk of bias, and all other studies were considered to have an unclear risk of bias. Two review authors (LA and VN) applied the criteria independently and a third review author (CW) arbitrated any disagreements.
Measures of treatment effect
We used raw dichotomous data reported in each study to express the study's result as a risk ratio (RR) with its corresponding 95% confidence interval (CI). However, one study reported adjusted odds ratios (ORs) and we calculated the natural logarithm of the OR and its standard error for each outcome in this study. We then expressed the intervention effect for each outcome in this study as an OR with its 95% CI using inverse variance. We grouped studies with broadly similar types of participants, interventions, study designs, and outcomes to get feasible results for an overall estimate of effect. See Appendix 2 for measures of effect specified in the protocol (Abdullahi 2015), but not used in the review.
Unit of analysis issues
We did not encounter unit‐of‐analysis issues in this review. Two included studies were cluster‐randomised trials based on matched pairs of clusters (Perkins 2015; Watson‐Jones 2012). We did not reanalyse these data as matching cannot be taken into account in reanalyses in such studies unless the raw data are available. However, the studies conducted appropriate analyses of the data, and we provided the results as reported in the studies. See Appendix 2 for methods specified in the protocol (Abdullahi 2015), but not used in the review.
Dealing with missing data
For the current version of the review, we did not experience any missing data thus we did not contact the primary study authors for missing data. In Appendix 2, we indicated methods specified in the protocol (Abdullahi 2015), but not used in the review.
Assessment of heterogeneity
We reviewed heterogeneity in the type of intervention, type of setting, study design, and risk of bias of included studies in order to make an assessment of the extent to which the included studies were similar to each other. We examined the levels of heterogeneity between study results using the Chi² test of homogeneity (with significance defined at the alpha level of 10%). We quantified any statistical heterogeneity between study results using the I² statistic. We regarded heterogeneity as substantial if the I² was greater than 50% (Higgins 2019).
Assessment of reporting biases
Test for asymmetry with a funnel plot was not feasible because the number of included studies for each meta‐analysis was less that the recommended 10 studies. We have archived methods for assessing reporting biases in Appendix 2 , for use in updates of this review.
Data synthesis
We pooled data from studies of similar study designs, similar interventions, similar participants, and similar outcomes in a meta‐analysis using the random‐effects model if there was no significant statistical heterogeneity, methodological difference, or high risk of bias. For outcomes with substantial variation between studies in the reported interventions, participants, study designs, and outcome measures, we did not pool the results but summarised the findings in a narrative format. Overall, we interpreted the study findings by taking into account the methodological quality of the studies and the strength of the evidence. For each observed effect, we explicitly stated the strength of evidence and drew conclusions. See Appendix 2 for data synthesis methods specified in the protocol (Abdullahi 2015), but not used in the review.
'Summary of findings' tables
We created 'Summary of findings' tables for the main intervention comparisons and included the primary outcome: vaccination coverage. We used the GRADE approach to assess the certainty of evidence at outcome level (Guyatt 2008). Two review authors (LA and CW) independently assessed the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), and the EPOC worksheets (EPOC 2019c), and used GRADEpro software. We resolved disagreements on certainty ratings by discussion and provided justification for decisions to downgrade the ratings using footnotes in the table and made comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2019d; Santesso 2019).
Subgroup analysis and investigation of heterogeneity
We did not have sufficient data to conduct planned subgroup analyses (Appendix 2). However, we conducted a posthoc subgroup analysis exploring the effect of variations in the intervention (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4) or comparison (Analysis 5.1) groups on vaccination coverage. We used the Chi² test for subgroup differences to test for subgroup interactions.
6.1. Analysis.
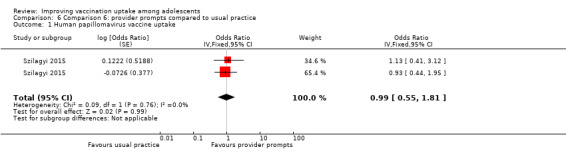
Comparison 6 Comparison 6: provider prompts compared to usual practice, Outcome 1 Human papillomavirus vaccine uptake.
6.2. Analysis.
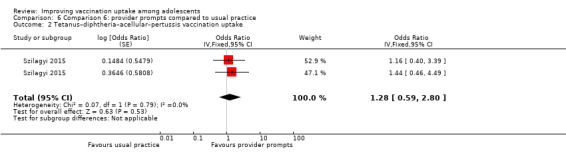
Comparison 6 Comparison 6: provider prompts compared to usual practice, Outcome 2 Tetanus–diphtheria–acellular–pertussis vaccination uptake.
6.3. Analysis.
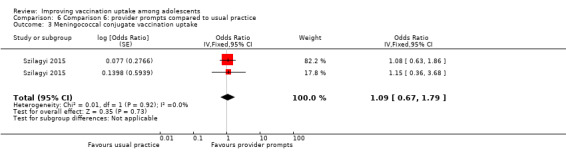
Comparison 6 Comparison 6: provider prompts compared to usual practice, Outcome 3 Meningococcal conjugate vaccination uptake.
6.4. Analysis.
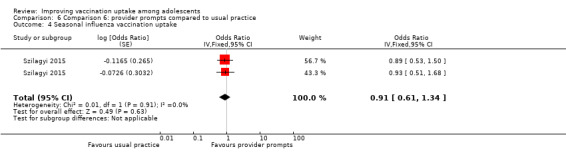
Comparison 6 Comparison 6: provider prompts compared to usual practice, Outcome 4 Seasonal influenza vaccination uptake.
5.1. Analysis.
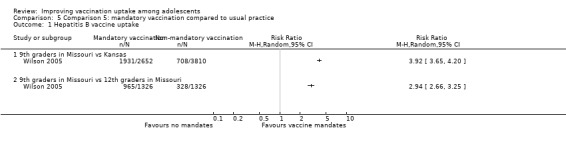
Comparison 5 Comparison 5: mandatory vaccination compared to usual practice, Outcome 1 Hepatitis B vaccine uptake.
Sensitivity analysis
We planned to perform sensitivity analyses based on unit of analysis errors, risk of bias, and missing data (Appendix 2). However, available data were insufficient to perform these analyses.
Results
Description of studies
Results of the search
We identified 29,103 records from the electronic databases and other sources. After excluding 3476 duplicates, we screened 25,627 records, and found that 25,560 records were not relevant to our review question. We reviewed the remaining 67 potentially eligible full‐text articles for inclusion and excluded 48 of them for the reasons given in the Characteristics of excluded studies table.
Sixteen studies met the inclusion criteria and were included in the review (Table 11). Two studies are awaiting classification (Dempsey 2018; Esposito 2018; Characteristics of studies awaiting classification table), and one study is ongoing (Skinner 2015; Characteristics of ongoing studies table). The search process and selection of studies is presented in Figure 2.
1. Summary of included studies.
| Study ID | Study design | Country | Participants | Intervention | Comparison | Duration of intervention | Vaccine target |
| Cates 2014 | Non‐randomised trial | USA | Parents and health providers | Multi‐component providers and parents | Usual practice | 3 months | HPV |
| Diclemente 2015 | Randomised trial | USA | Adolescents | Health education | Usual practice | 30 minutes | HPV |
| Fiks 2016 | Controlled before‐after study | USA | Health provider | Provider education with performance feedback | Usual practice | 1 month | HPV |
| Gargano 2015 | Randomised trial | USA | Parents | Health education | Usual practice | 2 months | Tdap, MCV, HPV, Influenza |
| Grandahl 2016 | Cluster‐randomised trial | Sweden | Adolescents | Health education | Usual practice | 30 minutes | HPV |
| Mantzari 2015 | Randomised trial | UK | Adolescents | Financial incentives | Usual practice | 6 months | HPV |
| Paskett 2016 | Randomised trial | USA | Parents and health providers | Multi‐component providers and parents | Usual practice | — | HPV |
| Perkins 2015 | Cluster‐randomised trial | USA | Adolescent and health providers | Multi‐component provider intervention | Usual practice | 1 months | HPV |
| Rickert 2015 | Randomised trial | USA | Parents | Health education | Usual practice | 1 hour | HPV |
| Schwarz 2008 | Randomised trial | USA | Adolescents and caregivers | Health education plus financial incentives | Usual practice | 1 hour | HepB |
| Skinner 2000 | Randomised trial | Australia | Adolescents | Complex health education | Simplified health education | 1 hour | HepB |
| Staras 2015 | Non‐randomised trial | USA | Adolescents | Health education | Usual practice | 3 months | HPV |
| Szilagyi 2015 | Randomised trial | USA | Health providers | Provider prompts | Usual practice | 2 months | Tdap, MCV, HPV, Influenza |
| Wilson 2005 | Non‐randomised trial | USA | Adolescent | Mandatory school entry vaccination | Usual practice | — | HepB, Td, and MMR |
| Winer 2016 | Cluster‐randomised trial | USA | Parents | Health education | Usual practice | 30–40 minutes | HPV |
| Watson‐Jones 2012 | Cluster‐randomised trial | Tanzania | Adolescents | Class‐based vaccination | Age‐based vaccination | 12 months | HPV |
HepB: hepatitis B virus; HPV: human papillomavirus; MCV: meningococcal conjugate vaccine; MMR: measles–mumps–rubella; Td: tetanus–diphtheria; Tdap: tetanus–diphtheria–acellular–pertussis.
Included studies
Study design and setting
Sixteen studies met the inclusion criteria. Eight studies were randomised trials with individuals as the unit of randomisation (Diclemente 2015; Gargano 2015; Mantzari 2015; Paskett 2016; Rickert 2015; Schwarz 2008; Skinner 2000; Szilagyi 2015); four studies were cluster‐randomised trials that used health facilities or schools as the unit of randomisation (Grandahl 2016; Perkins 2015; Watson‐Jones 2012; Winer 2016); three studies were non‐randomised trials with at least two intervention and two control arms (Cates 2014; Staras 2015; Wilson 2005); and one study was a controlled before‐after study with two intervention and two control arms (Fiks 2016).
Twelve studies were conducted in the USA (Cates 2014; Diclemente 2015; Fiks 2016; Gargano 2015; Paskett 2016; Perkins 2015; Rickert 2015; Schwarz 2008; Staras 2015; Szilagyi 2015; Wilson 2005; Winer 2016); one study was conducted in Australia (Skinner 2000); one study was conducted in Sweden (Grandahl 2016); one study was conducted in the UK (Mantzari 2015); and one study was conducted in Tanzania (Watson‐Jones 2012).
Participants
Two studies enrolled girls only (Diclemente 2015; Watson‐Jones 2012), five studies enrolled boys and girls (Grandahl 2016; Mantzari 2015; Skinner 2000; Staras 2015; Wilson 2005), three studies enrolled parents (Gargano 2015; Rickert 2015; Winer 2016), and two studies enrolled healthcare providers (Fiks 2016; Szilagyi 2015).
Four studies enrolled mixed participants, comprising of adolescents and parents (Schwarz 2008), adolescents and healthcare providers (Perkins 2015), and parents and healthcare providers (Cates 2014; Paskett 2016). The healthcare providers included physicians, nurses, and physician assistants.
Interventions and comparisons
We present a summary of the interventions and comparisons used in the included studies in Table 11 and a detailed description in the Characteristics of included studies table.
Recipient‐oriented interventions
The recipient‐oriented intervention studies compared the following to usual care: health education (Diclemente 2015; Gargano 2015; Grandahl 2016; Rickert 2015; Staras 2015; Winer 2016), financial incentives (Mantzari 2015), health education and financial incentives (Schwarz 2008), and a school entry law mandating vaccination (Wilson 2005). The seventh health education study compared a multi‐component intervention to simplified information leaflets (Skinner 2000).
In six health education studies, participants in the intervention arm received structured 30 to 40 minute (Diclemente 2015; Grandahl 2016; Staras 2015; Winer 2016), one hour (Rickert 2015), or two to three day (Gargano 2015) interactive education on the target disease, vaccine recommendations, vaccine schedule, vaccine efficacy, and vaccine safety. Participants in the comparison 'usual care' arm received group general health education or education on the prevention of a specific non‐vaccine‐related condition. In the seventh study, participants in the education arm received a complex multi‐component intervention that included a resource fact sheet and assessment; an information video and questions designed to engage the adolescent audience; small group discussions; and an activity to locate resource information on the Internet. However, both the intervention and comparison arms received information brochures consisting of one‐page folded coloured leaflets, outlining in simple terms the risks of the target disease and the benefits and adverse effects of vaccination (Skinner 2000).
Provider‐oriented interventions
The provider‐oriented intervention studies assessed provider prompts (Szilagyi 2015), provider education with performance feedback (Fiks 2016), and a multi‐faceted intervention (Perkins 2015), compared to usual care.
Health system intervention
One study compared a class‐based vaccination strategy to an age‐based strategy (Watson‐Jones 2012).
Multi‐component interventions
Two studies assessed multi‐faceted interventions aimed at both recipients and providers of vaccination services compared to usual care (Cates 2014; Paskett 2016).
Outcomes
Fifteen studies reported data on our primary outcome, vaccination coverage. Eleven studies evaluated completion of the HPV vaccination schedule (Cates 2014; Diclemente 2015; Fiks 2016; Grandahl 2016; Mantzari 2015; Paskett 2016; Perkins 2015; Rickert 2015; Staras 2015; Watson‐Jones 2012; Winer 2016). Three studies assessed uptake of vaccines against hepatitis B virus (Schwarz 2008; Skinner 2000; Wilson 2005). One study examined uptake of tetanus–diphtheria (Td) and measles–mumps–rubella (MMR) vaccines (Wilson 2005). Finally, two studies reported data on uptake of tetanus–diphtheria–acellular–pertussis (Tdap), meningococcal conjugate, HPV, and influenza vaccines (Gargano 2015; Szilagyi 2015).
Other predefined outcome measures reported by the included studies were:
knowledge, attitudes, and beliefs (Gargano 2015; Paskett 2016; Schwarz 2008; Skinner 2000);
cost of the intervention (Fiks 2016; Watson‐Jones 2012); and
adverse effects of the intervention (Rickert 2015).
Predefined outcomes not reported by the included studies were:
incidence of VPDs; and
equitable uptake of immunisation.
The following predefined outcome was considered in a posthoc assessment as not relevant to the review: adverse events following immunisation (AEFI). An AEFI is any undesirable medical incident which follows administration of a vaccine, but is not necessarily caused by the vaccination (WHO 2019c). It is thus not a relevant outcome in this review, given that we are assessing interventions to improve vaccination uptake rather than the effects of the vaccine itself.
The studies did not report the lag‐time between delivery of interventions and assessment of outcomes.
Excluded studies
We excluded 48 studies for reasons given in the Characteristics of excluded studies table. The most common reasons for exclusion were ineligible study designs and ineligible interventions.
Risk of bias in included studies
Allocation
The risk of selection bias (random sequence generation) was low for nine studies (Diclemente 2015; Grandahl 2016; Mantzari 2015; Paskett 2016; Perkins 2015; Rickert 2015; Skinner 2000; Szilagyi 2015; Winer 2016), unclear for three studies (Gargano 2015; Schwarz 2008; Watson‐Jones 2012), and high for four studies (Cates 2014; Fiks 2016; Staras 2015; Wilson 2005).
The risk of selection bias (allocation concealment) was low for seven studies (Diclemente 2015; Grandahl 2016; Mantzari 2015; Perkins 2015; Rickert 2015; Szilagyi 2015; Watson‐Jones 2012), unclear for six studies (Cates 2014; Gargano 2015; Paskett 2016; Schwarz 2008; Skinner 2000; Winer 2016), and high for three studies (Fiks 2016; Staras 2015; Wilson 2005).
Blinding
For the types of intervention assessed in this review, blinding of participants and personnel was not possible. However, since vaccination coverage is an objective measure, we considered all studies to be at low risk of performance and detection biases.
Incomplete outcome data
The risk of attrition bias (incomplete outcome data) was low for 12 studies (Diclemente 2015; Fiks 2016; Mantzari 2015; Paskett 2016; Perkins 2015; Rickert 2015; Schwarz 2008; Skinner 2000; Szilagyi 2015; Watson‐Jones 2012; Wilson 2005; Winer 2016), unclear for one study (Cates 2014), and high for two studies (Gargano 2015; Staras 2015).
Selective reporting
Selective reporting was categorised as low risk in 12 studies (Cates 2014; Diclemente 2015; Fiks 2016; Grandahl 2016; Mantzari 2015; Paskett 2016; Perkins 2015; Schwarz 2008; Staras 2015; Szilagyi 2015; Watson‐Jones 2012; Wilson 2005), and unclear in four studies (Gargano 2015; Rickert 2015; Skinner 2000; Winer 2016).
Other potential sources of bias
None of the studies had evidence of other biases.
Summary of risk of bias assessments
We have summarised the risk of bias assessment in each of the included studies in Figure 3 and Figure 4. Overall, three studies had low risk of bias (Diclemente 2015; Grandahl 2016; Szilagyi 2015), nine studies had unclear risk of bias (Gargano 2015; Mantzari 2015; Paskett 2016; Perkins 2015; Rickert 2015; Schwarz 2008; Skinner 2000; Watson‐Jones 2012; Winer 2016), and four studies had high of bias (Cates 2014; Fiks 2016; Staras 2015; Wilson 2005).
3.
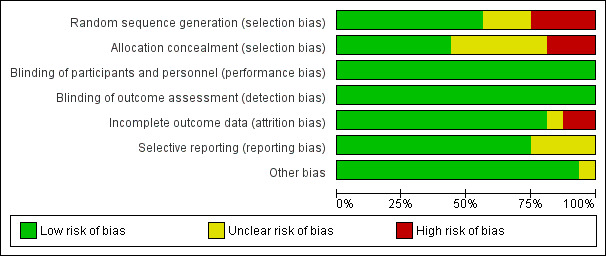
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
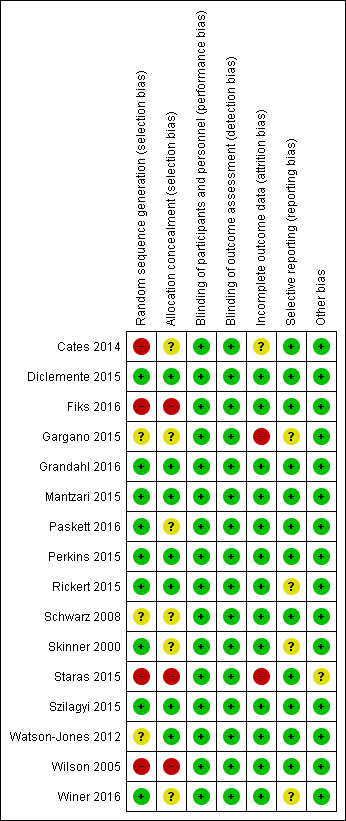
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
We present a summary of the effects of the interventions in Table 12. In this table we report the direction of the results and the certainty of the evidence for both primary and secondary outcomes.
2. Intervention‐outcome matrix.
| Intervention | Vaccination coverage | Equity | Knowledge | Attitudes | Beliefs | Adverse effects | Cost | Vaccine‐preventable diseases |
| Recipient‐oriented interventions | ||||||||
| 1 Health education vs usual practice | ✔㊉㊉㊉㊉1 | NR | ✔㊉㊉㊀㊀2 | NR | NR | NG | NR | NR |
| 2. Complex vs simplified health education | Ø㊉㊉㊉㊀3 | NR | ✔㊉㊉㊀㊀3 | NR | NR | NR | NR | NR |
| 3. Financial incentives vs usual practice | ✔㊉㊉㊀㊀4 | NR | NR | NR | NR | NR | NR | NR |
| 4. Health education plus financial incentives vs usual practice | ?㊉㊀㊀㊀5 | NR | ?㊉㊀㊀㊀5 | NR | NR | NR | NR | NR |
| 5. Mandatory vaccination vs usual practice | ✔㊉㊉㊉㊀6 | NR | NR | NR | NR | NR | NR | NR |
| Provider‐oriented interventions | ||||||||
| 6. Provider prompts vs usual practice | ✔㊉㊉㊉㊀7 | NR | NR | NR | NR | NR | NR | NR |
| 7. Provider education plus performance feedback vs usual practice | ✔㊉㊉㊀㊀8 | NR | NR | NR | NR | NR | NG | NR |
| Health system interventions | ||||||||
| 8. Class‐based vs age‐based HPV vaccination in schools | ✔㊉㊉㊉㊀9 | NR | NR | NR | NR | NR | NG | NR |
| Multi‐component interventions | ||||||||
| 9. Multi‐component provider intervention vs usual practice | ✔㊉㊉㊀㊀10 | NR | NR | NR | NR | NR | NR | NR |
| 10. Multi‐component provider and parent intervention vs usual practice | ✔㊉㊉㊀㊀11 | NR | ✔㊉㊉㊀㊀12 | NR | NR | NR | NR | NR |
✔ = a desirable effect
Ø = little or no effect
? = uncertain effect
x = undesirable effect
vs = Compared to
NR = not reported
NG = outcome not graded
1Diclemente 2015 (randomised trial), Grandahl 2016 (cluster‐randomised trial), and Winer 2016 (cluster‐randomised trial).
2Gargano 2015 (randomised trial).
3Skinner 2000 (randomised trial).
4Mantzari 2015 (randomised trial).
5Schwarz 2008 (randomised trial).
6Wilson 2005 (non‐randomised trial).
7Szilagyi 2015 (randomised trial).
8Fiks 2016 (controlled before‐after study).
9Watson‐Jones 2012 (cluster‐randomised trial).
10Perkins 2015 (cluster‐randomised trial).
11Paskett 2016 (randomised trial) and Cates 2014 (non‐randomised trial).
12Paskett 2016 (randomised trial)
⊕⊕⊕⊕ = High‐certainty evidence
Definition: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low.
Implications: this research provides a very good basis for making a decision about whether to implement the intervention. Impact evaluation and monitoring of the impact are unlikely to be needed if it is implemented.
⊕⊕⊕⊖ = Moderate‐certainty evidence
Definition: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate.
Implications: this evidence provides a good basis for making a decision about whether to implement the intervention. Monitoring of the impact is likely to be needed and impact evaluation may be warranted if it is implemented.
⊕⊕⊖⊖ = Low‐certainty evidence
Definition: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high.
Implications: this evidence provides some basis for making a decision about whether to implement the intervention. Impact evaluation is likely to be warranted if it is implemented.
⊕⊖⊖⊖ = Very low certainty evidence
Definition: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high.
Implications: this evidence does not provide a good basis for making a decision about whether to implement the intervention. Impact evaluation is very likely to be warranted if it is implemented.
Recipient‐oriented interventions
Comparison 1: health education compared to usual practice
1.1. Vaccination coverage
Three randomised trials (Diclemente 2015; Grandahl 2016; Winer 2016) and one non‐randomised trial (Staras 2015) reported vaccination coverage. A meta‐analysis of the randomised trials showed that health education improves HPV vaccination uptake compared to usual practice (RR 1.43, 95% CI 1.16 to 1.76; I² = 0%; high‐certainty evidence; 1054 participants). The non‐randomised study had similar findings, suggesting that health education may improve HPV vaccination uptake compared to usual practice (RR 1.84, 95% CI 1.34 to 2.54; low‐certainty evidence; 2822 participants)(Analysis 1.1; Table 1).
1.1. Analysis.
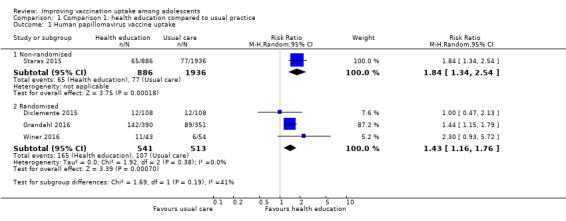
Comparison 1 Comparison 1: health education compared to usual practice, Outcome 1 Human papillomavirus vaccine uptake.
1.2. Secondary outcomes
Knowledge, attitude, and beliefs
One randomised trial suggested that health education may improve knowledge of vaccines (HPV, meningococcal conjugate, Tdap, and Influenza) and corresponding VPDs compared to usual practice (Gargano 2015). We downgraded the certainty of the evidence to low because of serious study limitations (high risk of bias) and serious indirectness (given that this finding is based on one study from one setting).
Adverse effects of the intervention
One study reported that health education did not have any adverse events in relation to usual practice (Rickert 2015). The remaining studies did not provide any information on adverse effects.
Comparison 2: complex health education programme compared to simplified health education
2.1. Vaccination coverage
One large randomised trial (Skinner 2000) suggested that a multi‐component health education intervention probably results in little or no difference in the uptake of three doses of the hepatitis B vaccine compared to simplified information leaflets (RR 0.98, 95% CI 0.96 to 0.99; 17,411 participants; Analysis 2.1). We judged the certainty of the evidence as moderate because of serious study limitations, as the included study had an unclear risk of bias (Table 2).
2.1. Analysis.
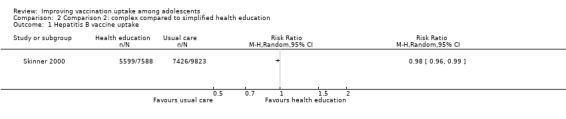
Comparison 2 Comparison 2: complex compared to simplified health education, Outcome 1 Hepatitis B vaccine uptake.
2.2. Secondary outcomes
Knowledge, attitude, and beliefs
The randomised trial showed that multi‐component health education may improve knowledge of the targeted vaccine and disease compared to simplified information leaflets (Skinner 2000). We downgraded the certainty of evidence to moderate because of serious study limitations (unclear risk of bias) and serious indirectness (given that this finding is based on one study from one setting).
Comparison 3: financial incentives compared to usual practice
3.1. Vaccination coverage
One randomised trial (Mantzari 2015) found that financial incentives may improve uptake of the first dose of the HPV vaccine compared to usual practice (RR 1.45, 95% CI 1.05 to 1.99; 500 participants; Analysis 3.1). We judged the certainty of the evidence as low, because of concerns regarding study limitations (unclear risk of bias) and imprecision of the effect (Table 3).
3.1. Analysis.
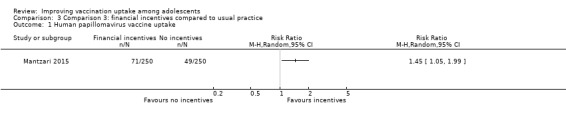
Comparison 3 Comparison 3: financial incentives compared to usual practice, Outcome 1 Human papillomavirus vaccine uptake.
3.2. Secondary outcomes
None of the included studies reported relevant secondary outcomes for this comparison.
Comparison 4: health education plus financial incentives compared to usual practice
4.1. Vaccination coverage
From the findings of one small randomised trial (Schwarz 2008), we are uncertain about the effects of combining health education and financial incentives on the completion of three doses of the hepatitis B vaccine, compared to usual practice (RR 1.38, 95% CI 0.96 to 2.00; 104 participants; Analysis 4.1). We judged the certainty of the evidence as very low because of concerns regarding risk of bias in the included study, very serious imprecision of the findings, and very serious indirectness (given that this finding is based on one small study from one setting) (Table 4).
4.1. Analysis.
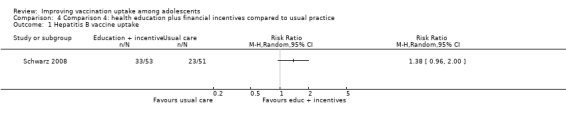
Comparison 4 Comparison 4: health education plus financial incentives compared to usual practice, Outcome 1 Hepatitis B vaccine uptake.
4.2. Secondary outcomes
Knowledge, attitude, and beliefs
One study (Schwarz 2008) assessed this outcome. We are uncertain about the effects of health education plus financial incentives on vaccine knowledge compared to usual practice because the certainty of the evidence was very low. We judged the certainty of the evidence as very low because of serious study limitations and very serious indirectness.
Comparison 5: mandatory vaccination compared to usual practice
5.1. Vaccination coverage
Wilson 2005 assessed the effects of mandating hepatitis B vaccination by law for elementary school entry in the state of Missouri in the USA. This non‐randomised trial compared students in the ninth grade (affected by the hepatitis B law) and 12th grade (not affected by the law) in the state and showed that making vaccinations mandatory probably leads to substantial improvements in vaccination uptake (RR 2.94, 95% CI 2.66 to 3.25; 2642 participants; Analysis 5.1).
In addition, the study compared the ninth grade in Missouri (affected by the mandatory hepatitis B vaccination law) to the ninth grade in the state of Kansas (not affected by the law) and confirmed that mandating vaccination probably leads to a large increase in the uptake of hepatitis B vaccine (RR 3.92, 95% CI 3.65 to 4.20; 6462 participants; Analysis 5.1). This was a well conducted non‐randomised study and, for both outcome assessments, we upgraded the certainty of the evidence to moderate because of very large intervention effects (Table 5).
5.2. Secondary outcomes
The study reported no relevant secondary outcomes.
Provider‐oriented interventions
Comparison 6: provider prompts compared to usual practice
6.1. Vaccination coverage
Szilagyi 2015 assessed the impact of provider prompts compared to usual practice on the uptake of various recommended adolescent vaccines through two parallel randomised trials: one in a national network of paediatric clinics, covering 36 states in the USA, and one in a local network of primary care practices, based in one county in New York state in the USA.
In the national network of paediatric clinics, provider prompts probably made little or no difference to the uptake of three doses of HPV (adjusted OR 1.13 95% CI 0.68 to 1.88; 437 participants), Tdap (adjusted OR 1.16, 95% CI 0.68 to 1.99; 1746 participants), meningococcal conjugate (adjusted OR 1.08, 95% CI 0.82 to 1.41; 1752 participants), and seasonal influenza (adjusted OR 0.89, 95% CI 0.69 to 1.16; 878 participants) vaccines. These adjusted odds ratios were based on a multilevel mixed‐effect logistic regression model with covariates for pair assignment, study time period, group assignment, and an interaction between time and group assignment.
In the local network of primary care practices, provider prompts probably made little or no difference to the uptake of three doses of HPV (adjusted OR 0.93, 95% CI 0.64 to 1.34; 422 participants), Tdap (adjusted OR 1.44, 95% CI 0.82 to 2.56; 1550 participants), meningococcal conjugate (adjusted OR 1.15, 95% CI 0.64 to 2.05; 1467 participants), and seasonal influenza (adjusted OR 0.93, 95% CI 0.69 to 1.25; 561 participants) vaccines. The odds ratios were adjusted as for the national network results above.
Pooling the data from the two networks, this randomised trial shows that provider prompts probably make little or no difference to the uptake of three doses of HPV (OR 0.99, 95% CI 0.55 to 1.81; 859 participants; Analysis 6.1), Tdap (OR 1.28, 95% CI 0.59 to 2.80; 3296 participants; Analysis 6.2), meningococcal conjugate (OR 1.09, 95% CI 0.67 to 1.79; 3219 participants; Analysis 6.3), and seasonal influenza (OR 0.91, 95% CI 0.61 to 1.34; 1439 participants; Analysis 6.4) vaccines.
We judged the certainty of the evidence as moderate because of concerns regarding imprecision of findings (Table 6).
6.2. Secondary outcomes
The study reported no relevant secondary outcomes.
Comparison 7: provider education with performance feedback compared to usual practice
7.1. Vaccination coverage
One controlled before‐after study looked at the effects of education and performance feedback on the uptake of HPV vaccination (Fiks 2016). Provider education with performance feedback may increase the proportion of adolescents who are offered and accept HPV vaccination by clinicians, compared to usual practice. Compared to adolescents visiting non‐participating clinicians (in the usual practice group), the adolescents visiting clinicians in the intervention group were more likely to receive the first dose of HPV during preventive visits (5.7 percentage points increase) and during acute visits (0.7 percentage points for the first and 5.6 percentage points for the second doses of HPV)(227 clinicians and more than 200,000 children). We judged the certainty of the evidence as low because this is a non‐randomised study (Table 7).
7.2. Secondary outcomes
Cost
Fiks 2016 evaluated the costs required to implement the education and performance feedback programme. The authors calculated the total cost of each of the following components: creation of the performance feedback reports; time spent on creating and delivering the educational content; and time spent by participating providers on group calls, reviewing data, and planning/implementing practice change. The estimated total cost of the intervention was USD 17,887 (USD 662 per participant), of which USD 17,064 was for participant time spent on the programmes (Fiks 2016).
Health system interventions
Comparison 8: class‐based compared to age‐based HPV vaccination in schools
8.1. Vaccination coverage
Watson‐Jones 2012 assessed the effect of two HPV vaccine delivery strategies in a cluster randomised trial and showed that a class‐based delivery tactic probably leads to slightly higher HPV vaccine uptake than an age‐based delivery strategy (RR 1.09, 95% CI 1.06 to 1.13; 1 study, 5537 participants; Analysis 7.1). We judged the certainty of the evidence as moderate because of serious indirectness, given that this finding is based on one study from one setting (Table 8).
7.1. Analysis.
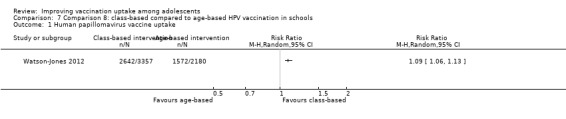
Comparison 7 Comparison 8: class‐based compared to age‐based HPV vaccination in schools, Outcome 1 Human papillomavirus vaccine uptake.
8.2. Secondary outcomes
Cost
Watson‐Jones 2012 collected data on the costs of class‐based versus age‐based delivery of the HPV vaccine in Tanzania and found the class‐based vaccination strategy to be less expensive. In urban schools, the cost was USD 52 per girl vaccinated in a class‐based strategy compared to USD 87 for the age‐based delivery system. In rural schools, the cost was USD 67 per girl vaccinated in a class‐based strategy compared to USD 98 for the age‐based delivery system.
Multi‐component interventions
Comparison 9: multi‐component provider intervention compared to usual practice
9.1. Vaccination coverage
Perkins 2015 (cluster randomised trial) assessed the effects of a four‐component provider intervention package (education session, repeated contacts, individualised feedback, and incentives) and found that the intervention probably improves HPV vaccination coverage compared to usual practice. Girls in the intervention group are probably more likely to receive their next HPV vaccine dose than those in the control group (odds ratio 1.6, 95% CI 1.1 to 2.2; 5786 participants). The effects are probably larger for boys (odds ratio 25.00, 95% CI 15.00 to 40.00; 7332 participants), and this may be because publicly funded HPV vaccination for boys became available during the study. We judged the certainty of the evidence as moderate because of serious indirectness, given that this finding is based on one study from one setting (Table 9).
9.2. Secondary outcomes
The study reported no relevant secondary outcomes.
Comparison 10: multi‐component provider and parent intervention compared to usual practice
10.1. Vaccination coverage
One non‐randomised trial showed that a social marketing intervention directed to parents and providers may improve HPV vaccination uptake compared to usual practice (RR 1.41, 95% CI 1.25 to 1.59; 25,869 participants; Cates 2014). The intervention included: distribution of HPV vaccination pamphlets to healthcare providers; radio messages directed at adolescents and their parents to raise awareness about HPV vaccination; an online continuing medical education training on HPV vaccination for health providers; simplified information sheet for adolescents and parents; and a website with links to credible information sources for parents and providers.
A very small randomised trial (Paskett 2016) also suggested that a multi‐component educational intervention directed to both providers and parents may improve HPV vaccination uptake (RR 2.34, 95% CI 0.75 to 7.32; 337 participants). Healthcare providers received a one‐hour presentation and handouts on HPV vaccination, and parents were mailed simplified educational material on HPV vaccination followed by a phone call to emphasise the importance of HPV vaccination.
Overall, the two studies show that using a multi‐faceted provider and parent intervention may improve HPV vaccination uptake compared to usual practice (Analysis 8.1). We judged the certainty of the evidence as low because of concerns regarding serious imprecision and serious study limitations (unclear risk of selection bias in the included study) (Table 10).
8.1. Analysis.
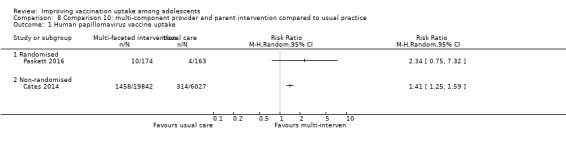
Comparison 8 Comparison 10: multi‐component provider and parent intervention compared to usual practice, Outcome 1 Human papillomavirus vaccine uptake.
10.2. Secondary outcomes
Knowledge, attitude, and beliefs
Paskett 2016 reported that a multi‐component educational intervention directed to both providers and parents may increase knowledge about HPV infection and HPV vaccine among providers and parents compared to usual practice. The average number of correct answers about HPV infection and HPV vaccination (out of 10) post‐intervention among parents in the intervention group was 9.4 (standard deviation 1.0), compared to 7.3 (standard deviation 1.9) among parents in the comparison group (P = 0.001). We judged the certainty of the evidence as low because of serious indirectness (given that this finding is based on one study from one setting) and serious study limitations.
Discussion
Summary of main results
We found that educating adolescents and their parents about the importance of vaccinations; passing laws stating that adolescents must be vaccinated to go to school; using a multi‐faceted package of interventions for providers of vaccination services, including education, repeated contacts, individualised feedback, and incentives; or using class‐based rather than age‐based approaches for delivering vaccines probably improve adolescent vaccination coverage. Adolescent vaccination coverage may also be improved through targeting parents and healthcare providers with a combination of vaccination education, telephone calls, and radio messages. In addition, providing adolescents and their parents with financial incentives may improve adolescent vaccination coverage. However, reminding healthcare providers to vaccinate adolescents when they open their electronic medical charts, probably makes little or no difference to adolescent vaccination coverage. From the data provided in the studies included in this review, we were uncertain about the costs of the interventions tested and their effects on knowledge and attitudes regarding adolescent vaccination. Table 12 shows a summary of the effects of the interventions, indicating the certainty of the evidence and gaps in the evidence base.
Overall completeness and applicability of evidence
Our systematic review was comprehensive as we included all known types of interventions for improving vaccination coverage (except recipient‐oriented reminders (Jacobson Vann 2018)), all vaccines recommended by WHO for boys and girls aged 10 to 19 years (WHO 2019b), and all country settings. However, we identified only 16 eligible studies, mostly conducted in high‐income countries.
There may be multiple barriers and facilitators of vaccine uptake among adolescents, from the logistics of ensuring access and the affordability of vaccination services, to the psychosocial factors that influence vaccination‐seeking behaviours and individual acceptance of vaccination. Therefore, multiple interventions may be required to achieve optimal vaccination coverage among adolescents. This review showed promising findings for a range of interventions including recipient‐oriented education, vaccine mandates, and financial incentives; provider education and a combination of interventions targeting providers alone or together with parents; and tailored school outreach programmes.
The most promising intervention was mandatory vaccination, which increased hepatitis B vaccination coverage more than four‐fold (Wilson 2005). This study was conducted in the US states of Missouri (which had a law mandating hepatitis B vaccination for school entry) and Kansas (which did not have mandatory hepatitis B vaccination at the time of the study). The study authors noted that the vaccination coverage achieved in this study (i.e. 72.8%) did not reach the high coverage levels obtained following a similar vaccination mandate in California. This was probably because, unlike in Missouri, mandatory vaccination in California was stringently enforced through measures such as expulsion of unvaccinated students and inspections to verify that vaccination coverage levels reported by schools were correct. Various countries have mandates for several vaccines (Omer 2019). However, there is considerable variation between and within countries in the implementation of mandatory vaccination; including (but not limited to) variation in what is required of people, the penalties imposed if requirements are not met, and the age groups and populations covered by vaccine mandates; (Attwell 2019; Omer 2019). Therefore, countries considering mandatory vaccination, as part of a multi‐intervention approach to reach optimal immunisation coverage among adolescents, may want to ensure that the process is consultative, involving relevant national stakeholders in its planning, implementation, and monitoring. National immunisation decision makers should bear in mind that mandatory vaccination is unlikely to be effective in settings where healthcare facility obstacles and other access issues are major drivers of sub‐optimal vaccination coverage (Adamu 2019; Nnaji 2020). In addition, the Wilson 2005 study was conducted more than 15 years ago, and it is possible that changes in health systems arrangements and other contextual changes since the time of the study may impact on the applicability of this evidence.
We also found health education to be very effective, leading to a relative increase of 43% in the completion of a three‐dose schedule of HPV vaccination (Diclemente 2015; Grandahl 2016; Staras 2015; Winer 2016). In addition, we found little or no difference in effects between a complex multi‐component health education intervention and simplified information leaflets; suggesting that interventions need to be tailored to local vaccination barriers.
This review has several limitations in relation to the applicability of this evidence. First, 15/16 included studies were conducted in high‐income countries, mainly the USA, in which vaccination services are readily available to adolescent girls and boys. The findings from these studies need to be interpreted with caution when applied to settings with different health system arrangements and access to vaccination services. Second, there was limited information from the studies on the cost‐effectiveness of the interventions tested. Only two studies reported the costs of interventions, including provider education with performance feedback (Fiks 2016) and a health system intervention (Watson‐Jones 2012). Therefore, when applying the findings of this review to any setting, local costing should be undertaken, particularly in settings differing from those of the original investigations. Third, the studies included in this review did not report information on equity. It is possible that the implementation of interventions may increase inequity if they are not adapted to populations in remote and under‐served areas in countries or if there is substantial variability in socioeconomic characteristics among populations receiving the interventions. Given these contextual issues, any adolescent vaccination programmes implemented based on our review findings should include a monitoring component to assess the performance of the intervention within the given context.
One study in our review was conducted in a country defined by the World Bank as low‐income or middle‐income (Watson‐Jones 2012). The study compared class‐based and age‐based strategies for delivering HPV vaccines among 5532 girls in 134 primary schools in northwest Tanzania (a low‐income country). There was a 9% relative increase in vaccination coverage among eligible girls in schools assigned to a class‐based approach compared to girls in schools using an age‐based strategy. This finding may be relevant to (low‐ or middle‐income) countries that do not have established healthcare programmes for adolescent boys and girls, but have introduced or are contemplating to introduce HPV (Oberlin 2018) and other vaccines for adolescents. School health programmes can have an advantage of integrating various existing health services at the same or a minimal increase in cost (Robbins 2011). In line with our findings, one previous review of school‐based programmes in 17 countries found that such programmes led to substantial increases in HPV vaccination coverage rates (Paul 2014).
Although the effect sizes reported in this review were small to moderate, even relatively small effects for interventions aimed at increasing uptake of adolescent vaccines may be important from a health service perspective, when applied across large populations. Therefore, we believe that this review is an important resource for countries and international organisations in the context of the "Immunization Agenda 2030", a global strategy which envisions a "world where everyone, everywhere, at every age, fully benefits from vaccines for good health and well‐being" (WHO 2019).
Three quarters of the studies in this review assessed the effects of various interventions on HPV vaccination coverage. Despite a global expansion of HPV vaccination programmes in recent years, HPV vaccination coverage remains sub‐optimal worldwide (Brotherton 2015; Bruni 2016; Loke 2017; Newman 2018). The current review will be supplemented by a Cochrane qualitative evidence synthesis which will explore the factors that influence acceptance of adolescent HPV vaccination (Cooper 2019). The findings may help to explain why some interventions in the current review were more effective than others, and may contribute to the development of more effective and contextualised interventions for improving HPV vaccination uptake among adolescents.
Certainty of the evidence
The certainty of the evidence on the effects of included interventions on our primary outcome (adolescent vaccination coverage) varied widely, from high to very low. Among the interventions targeting adolescents and their communities, we judged the certainty of the evidence as high for education (Table 1), moderate for multi‐component health education (Table 2) and legislation mandating vaccination (Table 5), low for financial incentives (Table 3), and very low for a combination of health education and financial incentives (Table 4). Regarding provider‐oriented interventions, we assessed the certainty as moderate for provider prompts (Table 6) and multi‐component performance improvement continuing medical education intervention (Table 9) and low for provider education with performance feedback (Table 7). For the combination of recipient and provider interventions, we assessed the certainty of evidence as moderate (Table 10). On health system interventions, we judged the certainty of the evidence as moderate for class‐based compared to age‐based delivery of vaccines to adolescents (Table 8). Our main concerns with the evidence related to study limitations (risk of bias; Figure 3; Figure 4), indirectness (for findings based on single studies from one setting), and imprecision.
Potential biases in the review process
We minimised potential biases in the review process by adhering to Cochrane guidelines (Higgins 2019). We conducted comprehensive searches without limiting the searches to a specific language. Two review authors independently assessed study eligibility, extracted data, and assessed the risk of bias in each included study. The eligible cluster‐randomised trials reported that they adjusted for cluster effects. However, there was some level of subjectivity in the determination of concerns that were serious enough to require rating down the evidence; and it is possible that other authors would have arrived at slightly different levels of certainty of evidence.
The searches for the main databases were done in October 2018. It is possible that some relevant studies published after the last search date have not been included in the review and the review authors acknowledge this limitation, However, we do not think that this limitation has an impact on the reliability of the main findings and conclusions of the review.
Agreements and disagreements with other studies or reviews
Few recent systematic reviews have assessed the effectiveness of interventions for improving adolescent immunisation coverage (Das 2016; Jacobson Vann 2018; Smulian 2016). Das and colleagues searched three databases for studies published up to December 2014 and included 23 studies on the effectiveness of interventions to improve vaccination coverage among adolescents. The authors reported that evidence of moderate certainty from 13 studies suggested that mandatory vaccination in schools, reminders, and national permissive recommendation increased vaccination coverage in adolescents (Das 2016). Smulian and colleagues searched five databases and included 34 intervention studies published from June 2006 to May 2015. The authors reported that many types of intervention strategies (targeting recipients, providers, and the health system) led to increases in HPV vaccination coverage in different settings (Smulian 2016). The Das 2016 and Smulian 2016 reviews had some overlap with our review in terms of included studies, but many studies included in the two reviews do not meet the EPOC criteria for inclusion of studies in systematic reviews of effects (EPOC 2019a).
Jacobson Vann and colleagues searched four databases to January 2017 for trials, controlled before‐after studies, and interrupted time series evaluating vaccination‐focused recipient reminders in children, adolescents, and adults in any setting. Based on 10 studies, the authors reported high‐certainty evidence that reminders improved adolescent vaccination coverage (Jacobson Vann 2018). There was no overlap between our review and the Jacobson Vann 2018 review since we excluded reminders of this kind.
Overall, our systematic review complements earlier relevant reviews, and is the most comprehensive systematic review to date on the effects of interventions for improving uptake of vaccines among adolescent boys and girls.
Authors' conclusions
Implications for practice.
We found that educating adolescents and their parents about the importance of vaccinations; passing laws requiring adolescents to be vaccinated as a condition for school enrolment; using a multi‐faceted package of interventions for providers of vaccination services, including education, repeated contacts, individualised feedback, and incentives; or using a class‐based approach for delivering vaccines probably increase the uptake of vaccines among adolescent girls and boys. The certainty of the evidence for these interventions was moderate, implying that monitoring and evaluation of the impact is likely to be needed.
In addition, we found low‐certainty evidence that adolescent vaccination coverage may be improved through providing adolescents and their parents with financial incentives; and giving education and feedback to providers of vaccination services. The low certainty of the evidence for these interventions implies that an impact evaluation is warranted if any of these interventions is implemented to improve adolescent vaccination coverage.
However, these are complex interventions which may be implemented in many different ways. For example, mandatory vaccination is context‐specific and can be operationalised through (but not limited to) schools, childcare, social welfare, and criminal justice (Attwell 2019; Omer 2019). The study that assessed mandatory vaccination in this review reported two key implementation challenges (Wilson 2005). Firstly, it was complicated to track the three dose series of hepatitis B vaccinations throughout the year; especially when students moved from one school to another. Secondly, school funding was tied to school attendance, a situation which may have deterred school personnel from enforcing mandatory vaccination through exclusion of unvaccinated students.
It is critical to understand the factors that influence hesitancy, acceptance, and demand for adolescent vaccination in different settings. An ongoing Cochrane qualitative evidence synthesis of factors that influence acceptance of adolescent HPV vaccination (Cooper 2019) may help to explain why and how some of the interventions in the current review were more effective than others in improving uptake of HPV vaccines.
Implications for research.
Most of the currently available evidence on interventions for improving adolescent vaccination coverage are from high‐income countries. In order to understand the effects of these interventions across a range of settings, there is a need for rigorous evaluations of adolescent vaccination interventions in low‐ and middle‐income countries. Given that there is little or no evidence from existing studies on cost and (gender, socioeconomic, and geographical) equity (Table 12), the challenge for the future is to design rigorous evaluations and report results in ways that can assess costs and equity impacts clearly, in addition to vaccination knowledge, intentions, and coverage.
In addition, there is a need for appropriately designed, implemented, and reported evaluations of interventions for which this review found low‐certainty evidence of benefits (e.g. recipient incentives, provider education and performance feedback, optimal combination of effective interventions, etc.), moderate‐certainty evidence of little or no benefits (provider prompts), and interventions for which we found no eligible studies (e.g. expansion of access to adolescent vaccination services, integration of adolescent vaccination with other services).
Acknowledgements
We are grateful to the Cochrane EPOC editorial base for assistance in the preparation of this review. We thank Marit Johansen who advised on the search strategy and helped with computerised searches, as well as the following editors and peer referees, who provided comments to improve the review: Simon Lewin, Kent Ranson, Chris Rose, Ntombenhle Ngcobo, Sabrina Bakeera‐Kitaka, Xavier Bosch‐Capblanch, Newton Opiyo, Claire Glenton, and Heather Ames.
The South African Medical Research Council and the National Research Foundation of South Africa supported Leila H Abdullahi and Charles S Wiysonge (grant numbers: 106035 and 119467).
The Norwegian Satellite of the EPOC Group receives funding from the Norwegian Agency for Development Cooperation (Norad), via the Norwegian Institute of Public Health, to support review authors in the production of their reviews.
This Cochrane Review is associated with the Research, Evidence and Development Initiative (READ‐It) project. READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search strategies
PDQ Evidence
Title/Abstract: ("vaccination uptake" OR "vaccination up take" OR "vaccination coverage" OR "vaccine uptake" OR "vaccine up take" OR "vaccine coverage")
CDSR, the Cochrane Library
| ID | Search | Hits |
| #1 | (vaccin* and (uptake or coverage)):ti,ab | 507 |
| #2 | (vaccin* next uptake or vaccin* next coverage):ti,ab | 265 |
| #3 | #1 or #2 | 507 |
| #4 | MeSH descriptor: [Immunization] this term only | 661 |
| #5 | MeSH descriptor: [Immunization Schedule] this term only | 984 |
| #6 | MeSH descriptor: [Immunization, Secondary] this term only | 794 |
| #7 | MeSH descriptor: [Immunization Programs] this term only | 391 |
| #8 | MeSH descriptor: [Immunotherapy, Active] this term only | 111 |
| #9 | MeSH descriptor: [Vaccination] this term only | 2456 |
| #10 | MeSH descriptor: [Mass Vaccination] this term only | 78 |
| #11 | #4 or #5 or #6 or #7 or #8 or #9 or #10 | 4580 |
| #12 | MeSH descriptor: [Diphtheria] this term only | 90 |
| #13 | MeSH descriptor: [Tetanus] this term only | 166 |
| #14 | MeSH descriptor: [Bordetella Infections] this term only | 5 |
| #15 | MeSH descriptor: [Bordetella pertussis] this term only | 118 |
| #16 | MeSH descriptor: [Whooping Cough] this term only | 228 |
| #17 | MeSH descriptor: [Measles] this term only | 219 |
| #18 | MeSH descriptor: [Mumps] this term only | 68 |
| #19 | MeSH descriptor: [Rubella] this term only | 107 |
| #20 | MeSH descriptor: [Poliomyelitis] this term only | 120 |
| #21 | MeSH descriptor: [Poliomyelitis, Bulbar] this term only | 0 |
| #22 | MeSH descriptor: [Tuberculosis] this term only | 743 |
| #23 | MeSH descriptor: [Tuberculosis, Pulmonary] this term only | 937 |
| #24 | MeSH descriptor: [Mycobacterium tuberculosis] this term only | 375 |
| #25 | MeSH descriptor: [Hepatitis A] this term only | 238 |
| #26 | MeSH descriptor: [Hepatitis A virus] this term only | 9 |
| #27 | MeSH descriptor: [Hepatitis A Virus, Human] this term only | 32 |
| #28 | MeSH descriptor: [Hepatitis B] this term only | 1238 |
| #29 | MeSH descriptor: [Hepatitis B, Chronic] this term only | 933 |
| #30 | MeSH descriptor: [Hepatitis B virus] this term only | 764 |
| #31 | MeSH descriptor: [Chickenpox] this term only | 141 |
| #32 | MeSH descriptor: [Papillomavirus Infections] this term only | 724 |
| #33 | MeSH descriptor: [Herpesviridae Infections] this term only | 62 |
| #34 | MeSH descriptor: [Herpes Simplex] this term only | 230 |
| #35 | MeSH descriptor: [Herpes Genitalis] this term only | 366 |
| #36 | MeSH descriptor: [Herpes Labialis] this term only | 134 |
| #37 | MeSH descriptor: [Herpes Zoster] this term only | 361 |
| #38 | MeSH descriptor: [Meningococcal Infections] this term only | 156 |
| #39 | MeSH descriptor: [Meningitis, Meningococcal] this term only | 127 |
| #40 | MeSH descriptor: [Neisseria meningitidis] this term only | 166 |
| #41 | MeSH descriptor: [HIV Infections] explode all trees | 9351 |
| #42 | MeSH descriptor: [HIV] this term only | 402 |
| #43 | MeSH descriptor: [HIV‐1] this term only | 2542 |
| #44 | MeSH descriptor: [HIV‐2] this term only | 25 |
| #45 | MeSH descriptor: [Neoplasms] this term only | 5682 |
| #46 | #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 | 21,667 |
| #47 | #11 and #46 | 1462 |
| #48 | MeSH descriptor: [Diphtheria‐Tetanus‐acellular Pertussis Vaccines] this term only | 182 |
| #49 | MeSH descriptor: [Diphtheria‐Tetanus‐Pertussis Vaccine] this term only | 486 |
| #50 | MeSH descriptor: [Diphtheria‐Tetanus Vaccine] this term only | 63 |
| #51 | MeSH descriptor: [Pertussis Vaccine] this term only | 196 |
| #52 | MeSH descriptor: [Vaccines, Combined] this term only | 427 |
| #53 | MeSH descriptor: [Diphtheria Toxoid] this term only | 177 |
| #54 | MeSH descriptor: [Tetanus Toxoid] this term only | 385 |
| #55 | MeSH descriptor: [Measles‐Mumps‐Rubella Vaccine] this term only | 152 |
| #56 | MeSH descriptor: [Measles Vaccine] this term only | 231 |
| #57 | MeSH descriptor: [Mumps Vaccine] this term only | 59 |
| #58 | MeSH descriptor: [Rubella Vaccine] this term only | 114 |
| #59 | MeSH descriptor: [Poliovirus Vaccines] this term only | 32 |
| #60 | MeSH descriptor: [Poliovirus Vaccine, Oral] this term only | 149 |
| #61 | MeSH descriptor: [Poliovirus Vaccine, Inactivated] this term only | 258 |
| #62 | MeSH descriptor: [Tuberculosis Vaccines] this term only | 48 |
| #63 | MeSH descriptor: [BCG Vaccine] this term only | 745 |
| #64 | MeSH descriptor: [Viral Hepatitis Vaccines] this term only | 275 |
| #65 | MeSH descriptor: [Hepatitis A Vaccines] this term only | 263 |
| #66 | MeSH descriptor: [Hepatitis B Vaccines] this term only | 883 |
| #67 | MeSH descriptor: [Chickenpox Vaccine] this term only | 139 |
| #68 | MeSH descriptor: [Papillomavirus Vaccines] this term only | 384 |
| #69 | MeSH descriptor: [Human Papillomavirus Recombinant Vaccine Quadrivalent, Types 6, 11, 16, 18] this term only | 55 |
| #70 | MeSH descriptor: [Meningococcal Vaccines] this term only | 331 |
| #71 | MeSH descriptor: [AIDS Vaccines] this term only | 391 |
| #72 | #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 | 4335 |
| #73 | ((diphtheria* or tetanus or bordetella or pertussis or "whooping cough" or measles or mumps or rubella* or rubeola or mmr or polio* or "infantile paralysis" or tuberculosis or tuberculoses or bcg or calmette* or hepatitis or chickenpox or varicella or papilloma* or hpv or herpes or meningococcal or meningitidis or meningitis or "acquired immunodeficiency syndrome" or aids or "human immunodeficiency virus" or hiv or cancer* or neoplasm*) near/3 (vaccin* or revaccinat* or immunization or immunisation or immunotherapy)):ti,ab | 6557 |
| #74 | ((tripe or combin*) next vaccin*):ti,ab | 339 |
| #75 | #73 or #74 | 6622 |
| #76 | #3 or #47 or #72 or #75 | 7842 |
| #77 | MeSH descriptor: [Adolescent] this term only | 89,560 |
| #78 | MeSH descriptor: [Adolescent Health Services] this term only | 177 |
| #79 | (adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles):ti,ab | 20,966 |
| #80 | #77 or #78 or #79 | 101,978 |
| #81 | #76 and #80 in Cochrane Reviews (Reviews and Protocols) | 7 |
CENTRAL, DARE and HTA, Cochrane Library
| ID | Search | Hits |
| #1 | vaccin* and (uptake or coverage) | 942 |
| #2 | (vaccin* next uptake or vaccin* next coverage) | 459 |
| #3 | #1 or #2 | 942 |
| #4 | MeSH descriptor: [Immunization] this term only | 659 |
| #5 | MeSH descriptor: [Immunization Schedule] this term only | 981 |
| #6 | MeSH descriptor: [Immunization, Secondary] this term only | 792 |
| #7 | MeSH descriptor: [Immunization Programs] this term only | 390 |
| #8 | MeSH descriptor: [Immunotherapy, Active] this term only | 111 |
| #9 | MeSH descriptor: [Vaccination] this term only | 2450 |
| #10 | MeSH descriptor: [Mass Vaccination] this term only | 78 |
| #11 | #4 or #5 or #6 or #7 or #8 or #9 or #10 | 4569 |
| #12 | MeSH descriptor: [Diphtheria] this term only | 90 |
| #13 | MeSH descriptor: [Tetanus] this term only | 166 |
| #14 | MeSH descriptor: [Bordetella Infections] this term only | 5 |
| #15 | MeSH descriptor: [Bordetella pertussis] this term only | 118 |
| #16 | MeSH descriptor: [Whooping Cough] this term only | 227 |
| #17 | MeSH descriptor: [Measles] this term only | 219 |
| #18 | MeSH descriptor: [Mumps] this term only | 68 |
| #19 | MeSH descriptor: [Rubella] this term only | 107 |
| #20 | MeSH descriptor: [Poliomyelitis] this term only | 120 |
| #21 | MeSH descriptor: [Poliomyelitis, Bulbar] this term only | 0 |
| #22 | MeSH descriptor: [Tuberculosis] this term only | 740 |
| #23 | MeSH descriptor: [Tuberculosis, Pulmonary] this term only | 937 |
| #24 | MeSH descriptor: [Mycobacterium tuberculosis] this term only | 375 |
| #25 | MeSH descriptor: [Hepatitis A] this term only | 237 |
| #26 | MeSH descriptor: [Hepatitis A virus] this term only | 8 |
| #27 | MeSH descriptor: [Hepatitis A Virus, Human] this term only | 32 |
| #28 | MeSH descriptor: [Hepatitis B] this term only | 1237 |
| #29 | MeSH descriptor: [Hepatitis B, Chronic] this term only | 931 |
| #30 | MeSH descriptor: [Hepatitis B virus] this term only | 760 |
| #31 | MeSH descriptor: [Chickenpox] this term only | 141 |
| #32 | MeSH descriptor: [Papillomavirus Infections] this term only | 722 |
| #33 | MeSH descriptor: [Herpesviridae Infections] this term only | 62 |
| #34 | MeSH descriptor: [Herpes Simplex] this term only | 230 |
| #35 | MeSH descriptor: [Herpes Genitalis] this term only | 365 |
| #36 | MeSH descriptor: [Herpes Labialis] this term only | 134 |
| #37 | MeSH descriptor: [Herpes Zoster] this term only | 361 |
| #38 | MeSH descriptor: [Meningococcal Infections] this term only | 156 |
| #39 | MeSH descriptor: [Meningitis, Meningococcal] this term only | 127 |
| #40 | MeSH descriptor: [Neisseria meningitidis] this term only | 166 |
| #41 | MeSH descriptor: [HIV Infections] explode all trees | 9328 |
| #42 | MeSH descriptor: [HIV] this term only | 402 |
| #43 | MeSH descriptor: [HIV‐1] this term only | 2535 |
| #44 | MeSH descriptor: [HIV‐2] this term only | 25 |
| #45 | MeSH descriptor: [Neoplasms] this term only | 5662 |
| #46 | #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 | 21,613 |
| #47 | #11 and #46 | 1459 |
| #48 | MeSH descriptor: [Diphtheria‐Tetanus‐acellular Pertussis Vaccines] this term only | 182 |
| #49 | MeSH descriptor: [Diphtheria‐Tetanus‐Pertussis Vaccine] this term only | 486 |
| #50 | MeSH descriptor: [Diphtheria‐Tetanus Vaccine] this term only | 63 |
| #51 | MeSH descriptor: [Pertussis Vaccine] this term only | 196 |
| #52 | MeSH descriptor: [Vaccines, Combined] this term only | 427 |
| #53 | MeSH descriptor: [Diphtheria Toxoid] this term only | 177 |
| #54 | MeSH descriptor: [Tetanus Toxoid] this term only | 385 |
| #55 | MeSH descriptor: [Measles‐Mumps‐Rubella Vaccine] this term only | 152 |
| #56 | MeSH descriptor: [Measles Vaccine] this term only | 231 |
| #57 | MeSH descriptor: [Mumps Vaccine] this term only | 59 |
| #58 | MeSH descriptor: [Rubella Vaccine] this term only | 114 |
| #59 | MeSH descriptor: [Poliovirus Vaccines] this term only | 32 |
| #60 | MeSH descriptor: [Poliovirus Vaccine, Oral] this term only | 149 |
| #61 | MeSH descriptor: [Poliovirus Vaccine, Inactivated] this term only | 258 |
| #62 | MeSH descriptor: [Tuberculosis Vaccines] this term only | 48 |
| #63 | MeSH descriptor: [BCG Vaccine] this term only | 743 |
| #64 | MeSH descriptor: [Viral Hepatitis Vaccines] this term only | 275 |
| #65 | MeSH descriptor: [Hepatitis A Vaccines] this term only | 262 |
| #66 | MeSH descriptor: [Hepatitis B Vaccines] this term only | 883 |
| #67 | MeSH descriptor: [Chickenpox Vaccine] this term only | 139 |
| #68 | MeSH descriptor: [Papillomavirus Vaccines] this term only | 381 |
| #69 | MeSH descriptor: [Human Papillomavirus Recombinant Vaccine Quadrivalent, Types 6, 11, 16, 18] this term only | 55 |
| #70 | MeSH descriptor: [Meningococcal Vaccines] this term only | 331 |
| #71 | MeSH descriptor: [AIDS Vaccines] this term only | 391 |
| #72 | #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 | 4329 |
| #73 | (diphtheria* or tetanus or bordetella or pertussis or "whooping cough" or measles or mumps or rubella* or rubeola or mmr or polio* or "infantile paralysis" or tuberculosis or tuberculoses or bcg or calmette* or hepatitis or chickenpox or varicella or papilloma* or hpv or herpes or meningococcal or meningitidis or meningitis or "acquired immunodeficiency syndrome" or aids or "human immunodeficiency virus" or hiv or cancer* or neoplasm*) near/3 (vaccin* or revaccinat* or immunization or immunisation or immunotherapy) | 8741 |
| #74 | (tripe or combin*) next vaccin* | 362 |
| #75 | #73 or #74 | 8791 |
| #76 | #3 or #47 or #72 or #75 | 9346 |
| #77 | MeSH descriptor: [Adolescent] this term only | 89,013 |
| #78 | MeSH descriptor: [Adolescent Health Services] this term only | 177 |
| #79 | (adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles) | 141,801 |
| #80 | #77 or #78 or #79 | 141,801 |
| #81 | #76 and #80 in Technology Assessments | 4 |
| #82 | #76 and #80 in Other Reviews | 33 |
| #83 | #76 and #80 in Trials | 1807 |
MEDLINE, Ovid
| # | Searches | Results |
| 1 | (vaccin* and (uptake or coverage)).ti. | 2490 |
| 2 | (vaccin* adj (uptake or coverage)).ab. | 6767 |
| 3 | or/1‐2 | 7780 |
| 4 | Immunization/ | 47,410 |
| 5 | Immunization Schedule/ | 9559 |
| 6 | Immunization, Secondary/ | 7528 |
| 7 | Immunization Programs/ | 8736 |
| 8 | Immunotherapy, Active/ | 2415 |
| 9 | Vaccination/ | 70,957 |
| 10 | Mass Vaccination/ | 2622 |
| 11 | or/4‐10 | 133,949 |
| 12 | Diphtheria/ | 6480 |
| 13 | Tetanus/ | 9227 |
| 14 | Bordetella Infections/ | 924 |
| 15 | Bordetella Pertussis/ | 4880 |
| 16 | Whooping Cough/ | 7684 |
| 17 | Measles/ | 12,546 |
| 18 | Mumps/ | 4237 |
| 19 | Rubella/ | 7497 |
| 20 | Poliomyelitis/ | 18,660 |
| 21 | Poliomyelitis, Bulbar/ | 908 |
| 22 | Tuberculosis/ | 98,038 |
| 23 | Tuberculosis, Pulmonary/ | 71,501 |
| 24 | Mycobacterium Tuberculosis/ | 43,342 |
| 25 | Hepatitis A/ | 20,034 |
| 26 | Hepatitis A virus/ | 1000 |
| 27 | Hepatitis A Virus, Human/ | 519 |
| 28 | Hepatitis B/ | 39,639 |
| 29 | Hepatitis B, Chronic/ | 12,448 |
| 30 | Hepatitis B virus/ | 22,921 |
| 31 | Chickenpox/ | 6995 |
| 32 | Papillomavirus Infections/ | 20,045 |
| 33 | Herpesviridae Infections/ | 13,580 |
| 34 | Herpes Simplex/ | 13,421 |
| 35 | Herpes Genitalis/ | 4426 |
| 36 | Herpes Labialis/ | 1139 |
| 37 | Herpes Zoster/ | 9345 |
| 38 | Meningococcal Infections/ | 5655 |
| 39 | Meningitis, Meningococcal/ | 4875 |
| 40 | Neisseria meningitidis/ | 7575 |
| 41 | exp HIV Infections/ | 253,907 |
| 42 | HIV/ | 17,423 |
| 43 | HIV‐1/ | 71,579 |
| 44 | HIV‐2/ | 3963 |
| 45 | Neoplasms/ | 370,682 |
| 46 | or/12‐45 | 1,012,945 |
| 47 | 11 and 46 | 33,251 |
| 48 | Diphtheria‐Tetanus‐Acellular Pertussis Vaccines/ | 979 |
| 49 | Diphtheria‐Tetanus‐Pertussis Vaccine/ | 2633 |
| 50 | Diphtheria‐Tetanus Vaccine/ | 376 |
| 51 | Pertussis Vaccine/ | 4842 |
| 52 | Vaccines, Combined/ | 2155 |
| 53 | Diphtheria Toxoid/ | 2985 |
| 54 | Tetanus Toxoid/ | 9063 |
| 55 | Measles‐Mumps‐Rubella Vaccine/ | 2382 |
| 56 | Measles Vaccine/ | 6252 |
| 57 | Mumps Vaccine/ | 1604 |
| 58 | Rubella Vaccine/ | 2895 |
| 59 | Poliovirus Vaccines/ | 1471 |
| 60 | Poliovirus Vaccine, Oral/ | 3743 |
| 61 | Poliovirus Vaccine, Inactivated/ | 2696 |
| 62 | Tuberculosis Vaccines/ | 1509 |
| 63 | BCG Vaccine/ | 18,209 |
| 64 | Viral Hepatitis Vaccines/ | 3366 |
| 65 | Hepatitis A Vaccines/ | 1553 |
| 66 | Hepatitis B Vaccines/ | 8401 |
| 67 | Chickenpox Vaccine/ | 1791 |
| 68 | Papillomavirus Vaccines/ | 5705 |
| 69 | Human Papillomavirus Recombinant Vaccine Quadrivalent, Types 6, 11, 16, 18/ | 630 |
| 70 | Meningococcal Vaccines/ | 2900 |
| 71 | AIDS Vaccines/ | 7326 |
| 72 | or/48‐71 | 78,770 |
| 73 | ((diphtheria? or tetanus or bordetella or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio* or infantile paralysis or tuberculosis or tuberculoses or bcg or calmette* or hepatitis or chickenpox or varicella or papilloma* or hpv or herpes or meningococcal or meningitidis or meningitis or acquired immunodeficiency syndrome or aids or human immunodeficiency virus or hiv? or cancer? or neoplasm?) adj3 (vaccin* or revaccinat* or immunization or immunisation or immunotherapy)).ti,ab,kf. | 83,990 |
| 74 | ((tripe or combin*) adj vaccin*).ti,ab,kf. | 1865 |
| 75 | or/73‐74 | 84,971 |
| 76 | 3 or 47 or 72 or 75 | 129,195 |
| 77 | Adolescent/ | 1,791,256 |
| 78 | Adolescent Health Services/ | 4970 |
| 79 | (adolescent? or youth? or young adult? or teenager? or teen? or juvenile?).ti,ab,kf. | 370,765 |
| 80 | or/77‐79 | 1,944,236 |
| 81 | 76 and 80 | 21,377 |
| 82 | randomized controlled trial.pt. | 450,371 |
| 83 | controlled clinical trial.pt. | 92,108 |
| 84 | multicenter study.pt. | 220,188 |
| 85 | pragmatic clinical trial.pt. | 527 |
| 86 | non‐randomized controlled trials as topic/ | 124 |
| 87 | interrupted time series analysis/ | 241 |
| 88 | controlled before‐after studies/ | 216 |
| 89 | (randomis* or randomiz* or randomly).ti,ab. | 727,552 |
| 90 | groups.ab. | 1,678,666 |
| 91 | (trial or intervention? or effect? or impact? or multicenter or multi center or multicentre or multi centre).ti. | 1,999,737 |
| 92 | (controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 3,813,640 |
| 93 | or/82‐92 | 6,451,399 |
| 94 | exp Animals/ | 20,778,909 |
| 95 | Humans/ | 16,453,168 |
| 96 | 94 not (94 and 95) | 4,325,741 |
| 97 | review.pt. | 2,231,738 |
| 98 | meta analysis.pt. | 74,571 |
| 99 | news.pt. | 180,934 |
| 100 | comment.pt. | 680,643 |
| 101 | editorial.pt. | 426,869 |
| 102 | cochrane database of systematic reviews.jn. | 12,989 |
| 103 | comment on.cm. | 680,642 |
| 104 | (systematic review or literature review).ti. | 90,912 |
| 105 | or/96‐104 | 7,545,359 |
| 106 | 93 not 105 | 4,704,292 |
| 107 | 81 and 106 | 7694 |
Embase, Ovid
| # | Searches | Results |
| 1 | (vaccin* and (uptake or coverage)).ti. | 2874 |
| 2 | (vaccin* adj (uptake or coverage)).ab. | 7778 |
| 3 | or/1‐2 | 8995 |
| 4 | vaccination/ | 141,285 |
| 5 | immunization/ | 101,664 |
| 6 | mass immunization/ | 3442 |
| 7 | or/4‐6 | 222,281 |
| 8 | 3 or 7 | 224,178 |
| 9 | adolescent/ | 1,421,541 |
| 10 | juvenile/ | 62,746 |
| 11 | (adolescent? or youth? or young adult? or teenager? or teen? or juvenile?).ti,ab. | 450,617 |
| 12 | or/9‐11 | 1,599,735 |
| 13 | 8 and 12 | 19,041 |
| 14 | Randomized Controlled Trial/ | 479,705 |
| 15 | Controlled Clinical Trial/ | 475,294 |
| 16 | Quasi Experimental Study/ | 4422 |
| 17 | Pretest Posttest Control Group Design/ | 353 |
| 18 | Time Series Analysis/ | 24,354 |
| 19 | Experimental Design/ | 25,555 |
| 20 | Multicenter Study/ | 164,862 |
| 21 | (randomis* or randomiz* or randomly).ti,ab. | 964,283 |
| 22 | groups.ab. | 2,227,946 |
| 23 | (trial or intervention? or effect? or impact? or multicenter or multi center or multicentre or multi centre).ti. | 2,390,553 |
| 24 | (controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 5,069,452 |
| 25 | or/14‐24 | 8,222,057 |
| 26 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 24,477,256 |
| 27 | human/ or normal human/ or human cell/ | 18,584,467 |
| 28 | 26 and 27 | 18,537,629 |
| 29 | 26 not 28 | 5,939,627 |
| 30 | (systematic review or literature review).ti. | 108,093 |
| 31 | "cochrane database of systematic reviews".jn. | 5634 |
| 32 | or/29‐31 | 6,052,569 |
| 33 | 25 not 32 | 6,461,120 |
| 34 | 13 and 33 | 7340 |
| 35 | limit 34 to embase | 1961 |
| 36 | limit 34 to embase status | 651 |
| 37 | 35 or 36 | 2134 |
CINAHL, EBSCOhost
| # | Query | Results |
| S31 | S29 AND S30 | 17 |
| S30 | EM 201510‐ | 173,379 |
| S29 | S11 AND S27 [Limiters: Exclude MEDLINE records] | 194 |
| S28 | S11 AND S27 | 1430 |
| S27 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 | 1,355,101 |
| S26 | TI ( controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur* ) OR AB ( controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur* ) | 378,261 |
| S25 | TI ( trial or intervention* or effect* or impact* or multicenter or "multi center" or multicentre or "multi centre" ) OR AB ( trial or intervention* or effect* or impact* or multicenter or "multi center" or multicentre or "multi centre" ) | 668,977 |
| S24 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 120,324 |
| S23 | (MH "Health Services Research") | 7568 |
| S22 | (MH "Multicenter Studies") | 21,717 |
| S21 | (MH "Quasi‐Experimental Studies+") | 8885 |
| S20 | (MH "Pretest‐Posttest Design+") | 28,056 |
| S19 | (MH "Experimental Studies") | 15,256 |
| S18 | (MH "Nonrandomized Trials") | 183 |
| S17 | (MH "Intervention Trials") | 6179 |
| S16 | (MH "Clinical Trials") | 87,623 |
| S15 | (MH "Randomized Controlled Trials") | 30,257 |
| S14 | PT research | 995,947 |
| S13 | PT clinical trial | 52,904 |
| S12 | PT randomized controlled trial | 30,868 |
| S11 | S6 AND S10 | 2949 |
| S10 | S7 OR S8 OR S9 | 257,799 |
| S9 | TI ( adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles ) OR AB ( adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles ) | 80,203 |
| S8 | (MH "Adolescent Health Services") | 1661 |
| S7 | (MH "Adolescence") | 236,921 |
| S6 | S1 OR S2 OR S3 OR S4 | 16,710 |
| S5 | TI ( vaccin* and (uptake or coverage) ) OR AB ( vaccin* and (uptake or coverage) ) | 2167 |
| S4 | (MH "Immunization Programs") | 3166 |
| S3 | (MH "Immunization, Secondary") | 93 |
| S2 | (MH "Immunization Schedule") | 2003 |
| S1 | (MH "Immunization") | 13,171 |
Global Health, Ovid
| # | Searches | Results |
| 1 | (vaccin* and (uptake or coverage)).ti. | 1705 |
| 2 | (vaccin* adj (uptake or coverage)).ab. | 5122 |
| 3 | or/1‐2 | 5604 |
| 4 | vaccination.cw,hw,id. | 53,695 |
| 5 | (immunisation or immunization).cw,hw,id. | 59,234 |
| 6 | or/4‐5 | 68,004 |
| 7 | 3 or 6 | 68,533 |
| 8 | adolescents/ | 45,303 |
| 9 | young adults/ | 9363 |
| 10 | youth/ | 9910 |
| 11 | (adolescent? or youth? or young adult? or teenager? or teen? or juvenile?).ti,ab,cw,hw,id. | 81,376 |
| 12 | or/8‐11 | 81,376 |
| 13 | 7 and 12 | 3563 |
| 14 | (trial or intervention? or effect? or impact? or multicenter or multi center or multicentre or multi centre).ti. | 307,550 |
| 15 | (randomiz* or randomis* or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or evaluat* or time series or time point? or repeated measur*).ti,ab,cw,hw,id. | 637,049 |
| 16 | or/14‐15 | 819,614 |
| 17 | 13 and 16 | 1294 |
Africa‐Wide Information, EBSCOhost
| # | Query | Results |
| S20 | S8 AND S13 AND S19 | 72 |
| S19 | S14 OR S15 OR S16 OR S17 OR S18 | 599,804 |
| S18 | TI ( (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) ) OR AB ( (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) ) | 557,235 |
| S17 | TP (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 948 |
| S16 | SU (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 73,881 |
| S15 | SM (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 9392 |
| S14 | KW (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or controlled or control W0 group* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 74,045 |
| S13 | S9 OR S10 OR S11 OR S12 | 76,308 |
| S12 | TI ( (adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles) ) OR AB ( (adolescent* or youth* or young adult* or teenager* or teen or teens or juvenile or juveniles) ) OR AB ( (adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles) ) OR AB ( (adolescent* or youth* or young adult* or teenager* or teen or teens or juvenile or juveniles) ) | 43,801 |
| S11 | SU adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles | 64,994 |
| S10 | SM adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles | 62,957 |
| S9 | KW adolescent* or youth* or "young adult" or "young adults" or teenager* or teen or teens or juvenile or juveniles | 64,992 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 6952 |
| S7 | TI ( vaccin* and (uptake or coverage) ) OR AB ( vaccin* and (uptake or coverage) ) | 2129 |
| S6 | SU immunization | 994 |
| S5 | SM immunization | 0 |
| S4 | KW immunization | 2012 |
| S3 | SU vaccination | 3036 |
| S2 | SM vaccination | 0 |
| S1 | KW vaccination | 3365 |
Scopus, Elsevier
( TITLE‐ABS‐KEY ( ( vaccination W/3 adolescent* ) OR ( vaccination W/3 youth* ) OR ( vaccination W/3 {young adult} ) OR ( vaccination W/3 {young adults} ) OR ( vaccination W/3 teenager* ) OR ( immunization W/3 adolescent* ) OR ( immunization W/3 youth* ) OR ( immunization W/3 {young adult} ) OR ( immunization W/3 {young adults} ) OR ( immunization W/3 teenager* ) OR ( immunisation W/3 adolescent* ) OR ( immunisation W/3 youth* ) OR ( immunisation W/3 {young adult} ) OR ( immunisation W/3 {young adults} ) OR ( immunisation W/3 teenager* ) ) ) AND ( ( TITLE‐ABS‐KEY ( randomis* OR randomiz* OR randomly OR intervention* OR control* OR evaluat* OR {before and after} OR ( ( {pre test} OR {pretest} ) AND ( {post test} OR posttest ) ) OR quasiexperiment* OR ( quasi W/0 experiment* ) OR {time series} OR ( time W/0 point* ) OR ( repeated W/0 measur* ) ) ) OR ( TITLE ( trial OR effect* OR impact* ) ) )
Grey literature
(Interventions) AND (adolescent) AND (immunisation)
Trial registries:
(With all the words (( interventions AND adolescent AND immunisation)))
Appendix 2. Table of unused methods
| Method | Approach |
| Measures of treatment effects | We will express the result of each study as a mean difference with its 95% confidence intervals for continuous data. We will analyse interrupted time series (ITS) studies using a regression analysis with time trends before and after the interventions. We will present the results for the outcomes as change in level and slope (Ramsay 2003). |
| Unit of analysis issues | If investigators report cluster‐randomised trial data as if the randomisation was performed on the individuals rather than the clusters, we will request the intracluster correlation coefficient (ICC) from the study authors; failing this, we will obtain external estimates of the ICC from similar studies or available resources (Campbell 2000). Once established, we will use the ICC to reanalyse the trial data to obtain approximate correct analyses. We will adjust the data by inflating the standard errors, i.e. multiplying them by the square root of the design effect (Higgins 2019). We plan to report the effect estimates and the corrected standard errors from cluster‐randomised trials with those from parallel‐group design trials, noting that the analysis of data from that specific study had a unit of analysis error (Higgins 2019). If insufficient information is available to control for clustering in this way, we will enter data into Review Manager 5 using individuals as the unit of analysis (Review Manager 2014). We will then perform sensitivity analyses to assess the potential bias that may have occurred as a result of the inadequately controlled clustered trials. We will also perform sensitivity analyses if we obtained the ICCs from external sources, to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2001). |
| Dealing with missing data | Where necessary, we will contact the corresponding authors of included studies to supply any unreported data. We will describe missing data and dropouts for each included study in a 'Risk of bias' table, and discuss the extent to which the missing data could alter our results. For controlled before‐after studies where relative measures are not available, we will estimate the difference between outcome measures at 2 time points for both baseline and after the intervention and then compare the difference between the groups. In contrast, if interrupted ITS are incorrectly analysed by the authors and provide the data points, we will reanalyse them using a regression analysis with time trends before and after the intervention, which adjust for autocorrelation and any periodic change (Ramsay 2003). |
| Assessment of reporting bias | We will use a funnel plot to investigate the risk of publication bias by intervention type, provided ≥ 10 studies are included in the analysis for each intervention type. We will critically examine the funnel plot for asymmetry both visually and with the use of formal tests. For continuous outcomes, we will use the test proposed by Egger (Egger 1997), and for dichotomous outcomes, we will use the test proposed by Harbord (Higgins 2019). In situations where asymmetry is detected by either test or by visual assessment, we will perform further exploratory analyses to investigate it. This will include reviewing the included studies for small sample size studies and their intervention effect. |
| Data synthesis | We will report unit of analysis error studies as changes in level and slope. If ITS studies are incorrectly analysed by the authors and provide the data points, we will reanalyse them using a regression analysis with time trends before and after the intervention, which adjust for autocorrelation and any periodic change. |
| Subgroup analysis and investigation of heterogeneity | Where sufficient data are available, we will conduct subgroup analyses, which will explore the effects of: vaccine given including frequency of the vaccine; availability of a policy on adolescent vaccination including vaccination schedule; equity (school‐based interventions or mass campaign programmes); and country income status (World Bank classification as either high‐income countries or low‐ to middle‐income countries). |
| Sensitivity analysis | Where sufficient data are available, we will conduct, if applicable, a sensitivity analysis to establish whether the meta‐analysis results for the treatment effect are influenced by study designs and overall risk of bias. We will perform sensitivity analyses by excluding studies with a particular study design and studies with high risk of bias. |
Data and analyses
Comparison 1. Comparison 1: health education compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Human papillomavirus vaccine uptake | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐randomised | 1 | 2822 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.34, 2.54] |
| 1.2 Randomised | 3 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.16, 1.76] |
Comparison 2. Comparison 2: complex compared to simplified health education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatitis B vaccine uptake | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Comparison 3: financial incentives compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Human papillomavirus vaccine uptake | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Comparison 4: health education plus financial incentives compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatitis B vaccine uptake | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Comparison 5: mandatory vaccination compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatitis B vaccine uptake | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 9th graders in Missouri vs Kansas | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 9th graders in Missouri vs 12th graders in Missouri | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Comparison 6: provider prompts compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Human papillomavirus vaccine uptake | 1 | Odds Ratio (Fixed, 95% CI) | 0.99 [0.55, 1.81] | |
| 2 Tetanus–diphtheria–acellular–pertussis vaccination uptake | 1 | Odds Ratio (Fixed, 95% CI) | 1.28 [0.59, 2.80] | |
| 3 Meningococcal conjugate vaccination uptake | 1 | Odds Ratio (Fixed, 95% CI) | 1.09 [0.67, 1.79] | |
| 4 Seasonal influenza vaccination uptake | 1 | Odds Ratio (Fixed, 95% CI) | 0.91 [0.61, 1.34] |
Comparison 7. Comparison 8: class‐based compared to age‐based HPV vaccination in schools.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Human papillomavirus vaccine uptake | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Comparison 10: multi‐component provider and parent intervention compared to usual practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Human papillomavirus vaccine uptake | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Randomised | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐randomised | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cates 2014.
| Methods | Non‐randomised trial conducted in the USA | |
| Participants |
Participants: parents and health providers Number per group: 19,842 boys in intervention group and 6027 boys in control group Total number enrolled: 28,869 Study population: healthcare providers and parents of boys aged 9–13 year in a 13‐county region in North Carolina |
|
| Interventions |
Intervention: social marketing intervention Description: intervention included: (1) distribution of HPV vaccination posters and brochures with the risk‐related message to health departments plus health providers; (2) 2 radio public service announcements in both English and Spanish designed to raise awareness about HPV vaccine for boys among parents of preteen boys; (3) an online continuing medical education training with video demonstrating communication among providers, parents, and preteen boys available to enrolled health providers; (4) 1‐page tip sheet for providers to discuss HPV vaccination with parents and boys; and (5) a website (protecthim.org) with links to credible information sources useful for both parents and providers. Duration: 3 months Comparison: usual practice Description of comparison: none Vaccine target: HPV vaccines Disease targeted: cervical cancer Number of doses: 3 |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | No evidence of other biases. |
Diclemente 2015.
| Methods | Randomised trial conducted in the USA | |
| Participants |
Participants: adolescent girls Number per group: 108 in "Girls OnGuard" intervention group and 108 in health promotion comparison group Total number enrolled: 216 participants Study population: African American adolescent girls attending 5 health clinics in metropolitan Atlanta between 2010 and 2012. |
|
| Interventions |
Intervention: "Girls OnGuard" Description: participants viewed a 12‐minute interactive computer‐delivered media presentation on HPV vaccination designed to enhance initial uptake and completion of the full series of 4 doses of HPV. This presentation was culture‐ and gender‐sensitive. Study procedures were initiated and completed while participants waited in the clinic waiting area to receive health services. Duration: 30 minutes Comparison: usual practice Description of comparison: participants viewed a time‐equivalent health promotion media presentation on physical activity and nutrition. The videos were designed to be gender and culturally appropriate, beneficial, and engaging. Vaccine target: HPV vaccine Disease targeted: cervical cancer Number of doses: 4 doses |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using prepackaged unmarked envelopes containing solid blue (intervention group) or purple (comparison group) slips of paper. |
| Allocation concealment (selection bias) | Low risk | Unmarked envelopes with colour coding that was based on a randomisation scheme that was created by computer algorithm, designed to eliminate bias in assigning participants to study conditions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinic providers were blind to study intervention conditions and provided standard of care counselling to all participants in accordance with clinic protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete. |
| Selective reporting (reporting bias) | Low risk | No follow‐up assessments incorporated into the study procedure, as this was a purposive method to minimise reactivity and reporting bias, such as social desirability bias. |
| Other bias | Low risk | No evidence of other biases. |
Fiks 2016.
| Methods | Controlled before‐after study conducted in the USA | |
| Participants |
Participants: providers Number per group: 27 MOC paediatricians and 200 "usual care" paediatricians Total number enrolled: 227 paediatricians Study population: Children's Hospital of Philadelphia primary care network comprising 227 primary care clinicians practicing at 27 practices at 31 sites, caring for > 200,000 children in Pennsylvania and New Jersey. All practices shared a common EHR (EpicCare, Verona, WI). |
|
| Interventions |
Intervention: MOC programmes using education and performance feedback Description: paediatricians received education and EHR‐generated performance feedback reports with their rates of captured HPV immunisation opportunities (dose given at eligible visit). The educational component consisted of a 1‐hour webinar that described current vaccination rates in the network, data on vaccine safety and efficacy, and strategies for overcoming barriers to vaccine receipt. Providers enrolled in the MOC project received quarterly performance feedback reports, extracted from EHRs, summarising their own, their practices', and the network's rates of missed HPV vaccination opportunities. Participating clinicians, drawn from practices across the network, met quarterly in a lunch‐hour teleconference to review the results of performance feedback and decide on an area of improvement for the next quarter. Duration: 1 year Comparison: usual practice Description of comparison: paediatricians did not receive the MOC programme. Vaccine target: HPV vaccine Disease targeted: cervical cancer Number of doses: 3 doses |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | No evidence of other biases. |
Gargano 2015.
| Methods | Randomised trial in the USA between November 2011 and July 2013 | |
| Participants |
Participants: parents of middle‐ and high‐school students Number enrolled: 6 middle‐ and 5 high‐schools Study population: parents of middle‐ and high‐school students enrolled in schools that were participating in a study focused on evaluating approaches to promoting adolescent vaccination. |
|
| Interventions |
Intervention: 1. parent‐only adolescent vaccination education and 2. parent and adolescent education Description: 3‐ to 5‐month health education for parents only or parents and adolescents. The intervention consisted of a brochure for parents and a 2‐ to 3‐day curriculum for adolescents. The parent education package included an invitation letter and a gift card valued at USD 20. Duration: 2 years Comparison: usual practice Description of the comparison: none. Vaccine target: Tdap, meningococcal, HPV, and influenza vaccines Disease targeted: not specified Number of doses: not stated |
|
| Outcomes | Parent willingness to have their adolescent vaccinated in a school‐located vaccination clinic Attitudes and beliefs towards vaccination |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low response rate. |
| Selective reporting (reporting bias) | Unclear risk | No description. |
| Other bias | Low risk | No evidence of other biases. |
Grandahl 2016.
| Methods | Cluster randomised trial conducted in Sweden | |
| Participants |
Participants: 16‐year old girls and boys Number per group: 390 students from 60 classes in intervention group and 351 students from 53 classes in a control group. Total number enrolled: 741 adolescents Study population: Swedish upper secondary school adolescents aged 16–19 years |
|
| Interventions |
Intervention: face‐to‐face health education Description: face‐to‐face structured information about HPV, including cancer risks and HPV prevention, by propagating condom use and HPV vaccination. Duration: 30 minutes Comparison: usual practice Description of comparison: general information, including sexual health Vaccine target: HPV vaccine Disease targeted: cervical cancer Number of doses: not specified |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in 2 steps. First, in order to avoid contamination, the schools were randomised into either the intervention group or the control group. Second, 113 school classes within these schools were randomly selected to be included in the study. |
| Allocation concealment (selection bias) | Low risk | Schools were randomly drawn by administrative personnel not involved in the project. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant who recorded the data from the participants did not possess this knowledge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Due to the self‐reported questionnaires, there was a risk of participants' over‐reporting or under‐reporting or having recall bias; however, we consider this risk to be small in this study. |
| Other bias | Low risk | No evidence of other biases. |
Mantzari 2015.
| Methods | Parallel‐group randomised controlled trial conducted in the UK | |
| Participants |
Participants: girls aged 16–18 years Number per group: 500 girls had not yet received an invitation to attend the vaccination programmes (first‐time invitees), and 500 girls had previously received an invitation to get vaccinated, but had failed to attend the first vaccination appointment (previous non‐attenders). Total number enrolled: 1000 girls Study population: all girls lived in Birmingham, UK, and were registered with general practitioners; were eligible to be vaccinated; and had not been vaccinated against HPV before. |
|
| Interventions |
Intervention: financial incentive with standard practice in combination with reminder text messages Description: participants received invitation letters addressed to them and inviting them to attend first HPV vaccination session. The letters included the date, time, and venue of their allocated vaccination appointment. In addition to the invitation letters, all participants were sent a standard leaflet containing information about HPV and the HPV vaccine. Participants in the intervention groups received an invitation letter with an enclosed offer of Love2Shop vouchers worth GBP 45 upon completion of 3 HPV vaccination doses. Duration: 6 months Comparison: standard practice with no incentives and no reminder system Description of comparison: letters, addressed to participants, inviting them to attend their first HPV vaccination session. In addition, the participants were sent a leaflet containing information about HPV and the HPV vaccine. Vaccine target: HPV vaccines Disease targeted: cervical cancer, genital wart Number of doses: 1‐3 doses |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation done using the RAND function in Excel. |
| Allocation concealment (selection bias) | Low risk | Participants allocated to the intervention and control group using the RAND function in Excel. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vaccinations administered by nurses working with Heart of Birmingham Primary Care Trust. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other biases. |
Paskett 2016.
| Methods | Randomized trial conducted in the USA | |
| Participants |
Participants: parents and providers Number per group: of the 337 parents, 174 in intervention group and 163 in control group. Of the 119 providers, 57 in intervention group and 62 in control group Total number enrolled: 337 parents and 119 providers Study population: a group‐randomised trial among 12 counties in Appalachian Ohio were conducted. Parents who had a daughter aged 9–17 years who had not received the HPV vaccine were recruited. Providers from these 12 county clinics were also recruited. |
|
| Interventions |
Intervention: educational HPV vaccine intervention Description: the intervention included:
Duration: no description Comparison: educational intervention on influenza vaccine Description of comparison:
Vaccine target: cervical cancer Disease targeted: HPV vaccine Number of doses: 3 doses |
|
| Outcomes | HPV vaccine uptake Knowledge on HPV vaccination |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Counties were pair‐matched based on cervical cancer incidence rates and location. 1 county from each pair was randomly assigned to receive the intervention whereas the other county was assigned to the comparison condition. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias. |
| Other bias | Low risk | No evidence of other biases. |
Perkins 2015.
| Methods | Cluster randomised trial in the USA | |
| Participants |
Participants: healthcare providers and their patients including boys and girls aged 11–21 years Number per group: 2 intervention health centres (4093 participants) and 6 control health centres (9025 participants) Total number enrolled: 13,118 participants Study population: healthcare workers (physicians, nurse practitioners, nurses, physician assistants, and medical assistants). Only physicians, nurse practitioners, and physician assistants with their own patient panels were eligible to receive personalised feedback on vaccination rates. As an incentive, physicians were eligible to receive maintenance of certification (MOC) Part IV credits, which fulfilled the requirements for maintaining board certification. Boys and girls aged 11–21 years from low‐income populations who received primary care in the Pediatric/Adolescent Departments at an intervention or control practice. |
|
| Interventions |
Intervention: multi‐component performance improvement continuing medical education intervention Description: intervention included:
Comparison: usual practice Description of comparison: none Duration: 2 years Vaccine target: HPV vaccines Disease targeted: HPV infection Number of doses: 3 doses |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Selection of the practice for the intervention or control condition was random. |
| Allocation concealment (selection bias) | Low risk | Included centres allocated randomly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available through a common electronic medical records' system. |
| Selective reporting (reporting bias) | Low risk | All practices used the same electronic medical records' system. |
| Other bias | Low risk | No evidence of other biases. |
Rickert 2015.
| Methods | Randomised trial conducted in the USA | |
| Participants |
Participants: parents Number per group: parents were randomised into 4 groups: 106 parents in rhetorical question (RQ) intervention with 2‐sided message intervention; 114 parents in 2‐sided message‐only intervention; and controls 109 parents in RQ with 1‐sided message; 116 parents in 1‐sided message‐only. Total number enrolled: 445 parents Study population: parents of boys and girls aged 11–15 years who had not previously received the HPV vaccine. |
|
| Interventions |
Intervention: 2 intervention groups:
Description: The RQ approach involved first asking a general question that the participant was likely to endorse, followed by a more targeted question or request in a 2‐sided message. A 2‐sided message listed supporting arguments, but also acknowledged (and usually rebutted) ≥ 1 potential arguments against the advocated behaviour. Duration: 1 hour Comparison: 2 groups that received 1‐sided messages only:
Description of comparison: The RQ involved asking a general question that the participant was likely to endorse, followed by a 1‐sdied message. A 1‐sided message presented only the arguments supporting the advocated behaviour. Vaccine target: HPV vaccine Disease targeted: cervical cancer Number of doses: not specified |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done into 4 groups, RQ intervention with 2‐sided message intervention, 2‐sided message‐only intervention, RQ with 1‐sided message, and 1‐sided message. |
| Allocation concealment (selection bias) | Low risk | Randomisation was built into the programmes using a random numbers table. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No description. |
| Other bias | Low risk | No evidence of other biases. |
Schwarz 2008.
| Methods | Randomised trial conducted in the USA | |
| Participants |
Participants: adolescents (girls and boys) and caregivers Number per group: 37 caregivers and 41 adolescents aged 10–18 years in intervention group and 39 caregivers and 39 adolescents aged 10–18 years in control group Total number enrolled: 328 children and 170 caregivers Study population: homeless shelter children and adolescents and their caregivers |
|
| Interventions |
Intervention: hepatitis B education intervention Description: the videos were "Respect Yourself/Protect Yourself" by the Hepatitis Foundation International (to instruct the person about hepatitis B infection and the importance of hepatitis B vaccine). The interventions included: health education, cash incentives, and free vaccination. 1 of the interventions was health education on hepatitis B vaccine for caregivers and adolescents. The caregivers and adolescents were exposed to an educational video called "Respect Yourself/Protect Yourself" which educated the participants about hepatitis B infection and the importance of hepatitis B vaccination. After the video presentation, the research nurse reviewed the contents of the video with a 5‐minute PowerPoint summary and encouraged the participants to ask questions to increase their understanding of the information presented. The families were given the vaccine information sheet for hepatitis B. Thereafter, free vaccination was offered as an incentive. The investigators offered free hepatitis B vaccine, provided caregivers with USD 10, and gave adolescents and caregivers gift packages containing cosmetics for the adults and sweets and toothbrushes for the children. All families were instructed to return after 1 month for the second visit (second dose), and after 2 months for a third dose. During the second visit, the caregivers were paid USD 10 and during the third visit they were paid USD 30 and adolescents and caregivers were given gift packages for both visits. Duration: 21 months Comparison 1: usual practice Description of comparison 1: education about the deleterious health consequences of cigarette smoking. Vaccine target: hepatitis B Disease targeted: hepatitis B Number of doses: 3 doses |
|
| Outcomes | Hepatitis B vaccine uptake Hepatitis B knowledge score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study was free from selective outcome reporting. |
| Other bias | Low risk | No evidence of other bias. |
Skinner 2000.
| Methods | Randomised trial conducted in Australia | |
| Participants |
Participants: school‐going girls and boys (class 7) Number per group: 458 students in intervention group and 467 students in control group Total number enrolled: 66 intervention schools (7588 students) AND 69 control schools (9823 students) aged 11–13 years Study population: Melbourne metropolitan secondary schools school‐going children in class 7 |
|
| Interventions |
Intervention: complex hepatitis B education Description: health education kit with 4‐lesson structured multi‐component intervention that included:
The intervention group received the health educational in addition to the usual government student and parent information brochures. Duration: 1 year Comparison: simplified hepatitis B education Description of comparison: brochures were 1‐page folded coloured leaflets, outlining in simple terms, the risks of hepatitis B and benefits and adverse effects of vaccination. Vaccine target: hepatitis B vaccine Disease targeted: hepatitis B Number of doses: 3 doses |
|
| Outcomes | Hepatitis B vaccine uptake Hepatitis B vaccine knowledge and attitude |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation to intervention and control was done. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All schools recruited were included in the analysis on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Unclear risk | No description. |
| Other bias | Low risk | No evidence of other bias. |
Staras 2015.
| Methods | Non‐randomised trial in the USA | |
| Participants |
Participants: girls and boys aged 11–17 years Number per group: 1387 girls and 1764 boys, 400 parents Total number enrolled: 2773 girls and 3350 boys assigned to 4 groups: postcard campaign, in‐clinic HIT system, postcard campaign and in‐clinic HIT system, and usual care. Study population: adolescents who were enrolled in Medicaid or Children’s Health Insurance Program (CHIP) in June 2013; who had a residential zip code in North Central Florida defined as within Gainesville, Florida, or a surrounding Primary Care Service Area (Chiefland, Citra, Crescent City, Cross City, Interlachen, Keystone Heights, Lake Butler, Lake City, Live Oak, Mayo, Ocala, Palatka, Starke, Steinhatchee, and Williston); and had ≥ 1 regular clinic visit between 1 July 2011 and 1 August 2013. |
|
| Interventions |
Intervention: multi‐level intervention called Protect Me from HPV, with 2 components: a system‐level postcard campaign and an in‐clinic health information technology (HIT) reminder system Description: the interventions were offered in 3 groups:
The postcard campaign contained healthcare information about vaccine benefits, costs, adverse effects, and safety and was designed to prompt parents and adolescents to discuss the vaccine with their doctor. The HIT system contained health risk questions for adolescents to verify vaccination history and indicate interest in learning about the vaccine. The HIT system summarised adolescent responses for providers in real time via colour‐coded system. Duration: 3 months Comparison: usual practice Description: Providers were asked to follow usual care for adolescents in this group Vaccine target: HPV vaccines Disease targeted: HPV infection Number of doses: 1 dose |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation of HIT. Thus it was possible that some of the effects of the HIT system could have been be attributed to differences between the HIT and non‐HIT provider practices or participants rather than the HIT system itself. |
| Allocation concealment (selection bias) | High risk | The HIT system was offered only to adolescents attending specific providers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Like all single‐system records of vaccination, the Medicaid and CHIP records likely contain incomplete information as suggested by patient interactions with the HIT system. For example, adolescents who received the HPV vaccine at the Health Department may not have claimed through Medicaid and CHIP. |
| Selective reporting (reporting bias) | Low risk | Potential for selection bias was evaluated by comparing vaccine initiation between HIT and non‐HIT providers. |
| Other bias | Unclear risk | No description. |
Szilagyi 2015.
| Methods | Randomised trial in the USA | |
| Participants |
Participants: providers Number per group: PBRN consisted of 10 practices; 5 intervention and 5 controls while from the national paediatric continuity clinic PBRN (CORNET) consisted of 12 practices; 6 intervention, 6 controls. Number enrolled: 22 practices Study population: 22 practices were allocated in 1 of 2 PBRNs to provider prompts or standard‐of‐care control. 10 primary care practices participated, 5 intervention and 5 controls, each matched in pairs on urban, suburban, or rural location and practice type (paediatric or family medicine), from a PBRN in Greater Rochester, NY (GR‐PBRN); and 12 practices, 6 intervention, 6 controls, similarly matched, from a national paediatric continuity clinic PBRN (CORNET). |
|
| Interventions |
Intervention: EHR prompt Description: the EHR display a prompt on the screen when a healthcare provider opens each of the patient's EMRs. In the study, all prompts used the same algorithm and displayed a list of vaccines due at that visit. Prompts did not generally show prior vaccinations and did not include standing orders. For each intervention practice, between 1‐ and 2‐hour educational sessions was given to the providers to inform them about EHR‐based prompts. Duration: 12‐month Comparison: usual practice Description: providers in the control practices received standard of care, which did not include prompts Vaccine target: Tdap, MCV4, HPV, and influenza vaccines Disease targeted: meningitis, HPV, influenza, tetanus, diphtheria, pertussis Number of doses: 3 doses for HPV. Others not specified |
|
| Outcomes | Uptake of Tdap; MCV4; HPV1, 2, and 3; and influenza vaccines | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SAS software program used to randomly allocate to the intervention or control. |
| Allocation concealment (selection bias) | Low risk | Using Stata 12.1, 1 author (AB) randomly assigned practices within each PBRN and practice pair to be an intervention or a standard of care control practice. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Healthcare providers were unaware of group assignment and the intervention was delivered by trained patient immunisation navigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 1 practice pair from the GR‐PBRN and refusal rates were similar for intervention and control practices. |
| Selective reporting (reporting bias) | Low risk | Probably no selective reporting. |
| Other bias | Low risk | No other bias. |
Watson‐Jones 2012.
| Methods | Cluster‐randomised trial in Tanzania | |
| Participants |
Participants: girls enrolled in primary school grade 6 or girls born in 1998. Number enrolled: 134 schools (60 urban government, 60 rural government, and 14 private) and 5532 eligible girls Study population: in the city of Mwanza and the neighbouring district of Misungwi in northwest Tanzania, 134 primary schools were randomly assigned to class‐based (girls enrolled in primary school grade (class) 6) or age‐based (girls born in 1998; 67 schools per arm) vaccine delivery. |
|
| Interventions |
Intervention: provision of HPV vaccine through a class‐based strategy (targeting girls in school class 6). Comparison: provision of HPV vaccine through an age‐based strategy (targeting girls born in 1998). Description: teachers, parents, and girls in the target vaccination group were provided with verbal and written information about HPV vaccination through school, parent, and community meetings; leaflets and posters; radio messages; and through community drama troupes. Duration: 12 months Vaccine target: HPV Disease targeted: cervical cancer Number of doses: 3 doses |
|
| Outcomes | HPV vaccine uptake Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Low risk | Allocation done by an independent statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was cluster randomised and the outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HPV uptake is an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | No other bias observed. |
Wilson 2005.
| Methods | Non‐randomised trial in the USA | |
| Participants |
Participants: ninth grade and 12th grade girls and boys Number per group: 2652 students from intervention group (Missouri state) and 3810 students from the control group (Kansas state) Total number enrolled: 6462 student from 12 accepted (4 urban public, 4 suburban public, 2 rural public, and 2 private) were evaluated. Study population: ninth and 12th grade students attending schools in the Kansas City, Missouri metropolitan area both affected and unaffected by the hepatitis B vaccination school entry law. |
|
| Interventions |
Intervention: school entry law mandating hepatitis B vaccination Description: hepatitis B vaccination required, by law, for seventh grade entry. The study compared vaccination coverage among adolescent students in ninth grade (affected by a new hepatitis B law) and 12th grade (not affected by the law) from 11 schools in 2 states of the USA. The intervention state mandated hepatitis B vaccination for elementary school entry in 1997 and for middle school in 1999 while in the control state, the elementary school did not mandate school entry vaccination. Duration: Missouri‐mandated hepatitis B vaccination for elementary school entry in 1997 and for middle school in 1999 and data collection occurred in 2003. Comparison: usual practice Description: no hepatitis B school entry law. Kansas had not mandated hepatitis B vaccination for elementary school entry at the time this study was conducted. Vaccine target: hepatitis B vaccine Disease targeted: hepatitis B Number of doses: 3 |
|
| Outcomes | Hepatitis B vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a non‐randomised study. However, it was a well conducted study that used "retrospective design with purposive school sampling, using location of residence to determine study group. In each school, immunization records from a random sample of up to 75 students" were reviewed. |
| Allocation concealment (selection bias) | High risk | This is a non‐randomised study. However, it was a well conducted study that used "retrospective design with purposive school sampling, using location of residence to determine study group. In each school, immunization records from a random sample of up to 75 students" were reviewed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99.5% of participants had outcome data available. |
| Selective reporting (reporting bias) | Low risk | Outcomes/hypotheses were all reported. |
| Other bias | Low risk | No evidence of other biases. |
Winer 2016.
| Methods | Cluster‐randomised trial in the USA | |
| Participants |
Participants: parents Number per group: 43 parents in intervention group and 54 parents in control group Total number enrolled: 97 parents Study population: mother or female legal guardian of a girl aged 9–12 years enrolled in the Hopi Tribe |
|
| Interventions |
Intervention: educational presentations on HPV Description: PowerPoint presentation with information on HPV prevalence and transmission, HPV vaccine recommendations, dosage schedule, and vaccine efficacy and safety. An educational brochure with similar content was also created to accompany the presentation. Parents attended mother–daughter dinners featuring educational presentations for mothers on HPV. Educational PowerPoint presentations provided information on HPV prevalence and transmission, HPV vaccine recommendations, dosage schedule, vaccine efficacy, and vaccine safety. An educational brochure with similar content was created to accompany the presentation. Duration: 30–40 minutes Comparison: no education on HPV Description of comparison: parents attended mother–daughter dinners featuring educational presentations for mothers on juvenile diabetes. Educational PowerPoint presentations focused on risk factors for type 2 juvenile diabetes, healthy nutrition, physical activity, and what parents could do to prevent or manage diabetes for their children. Vaccine target: HPV vaccine Disease targeted: cervical cancer Number of doses: not specified |
|
| Outcomes | HPV vaccine uptake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study randomly assigned 2 clusters to the intervention group and 2 to the control group. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is an objective measure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No description. |
| Other bias | Low risk | No evidence of other biases. |
CDC: Centers for Disease Control and Prevention; CHIP: Children's Health Insurance Program; EHR: electronic health record; HIT: health information technology; HPV: human papillomavirus; MCV4: meningococcal conjugate vaccine; MOC: maintenance of certification; PBRN: practice‐based research network; RQ: rhetorical questions; Tdap: tetanus–diphtheria–acellular–pertussis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anjum 2012 | Simple pre‐ and post cross‐sectional survey with no controls. |
| Bar‐Shain 2015 | Intervention was a reminder. |
| Bennett 2015 | Age of participants was 18–26 years, not separated to cater for 18–19 years. |
| Broutet 2013 | Review. |
| Catledge 2014 | Pre‐ and postsurvey. |
| Chan 2015 | Pre‐ and poststudy. |
| Chapman 2010 | Pre‐ and postcross‐sectional survey. |
| Chaves 2000 | The study is in Spanish |
| Chou 2014 | Pre‐ and postconsultation surveys. |
| Chung 2015 | Intervention was a reminder. |
| Dawson 2015 | Pre‐ and postintervention study. |
| Dempsey 2015a | Pre‐ and postintervention study. |
| Dempsey 2015b | Pre‐ and poststudy. |
| Donahue 2016 | Intervention was a reminder. |
| Dorji 2015 | Descriptive study, with no intervention and control arms. |
| Farmar 2016 | Pre‐ and postintervention study. |
| Fujiwara 2013 | Questionnaire survey, with no intervention and control. |
| Furlan 2010 | Descriptive study with no intervention. |
| Gargano 2014 | Descriptive study with no intervention. |
| Gillespie 2011 | Questionnaire survey in an ineligible age group. |
| Gordon 2013 | Descriptive study, with no intervention. |
| Gottvall 2010 | Quasi‐experimental intervention study. |
| Hadley 2014 | Descriptive study. |
| Hofman 2013 | Pre‐ and post‐test evaluation. |
| Hull 2016 | Pilot cross‐over study. |
| Iqbal 2016 | Age group is 11–25 years and data not stratified by age group. |
| Kim 2015 | Pre‐ and post‐test study. |
| Kwan 2011 | Pre‐ and postevaluation study. |
| Kwang 2016 | Pre‐ and poststudy in an ineligible age group. |
| Lai 2013 | Quasi‐experimental time series. |
| LaMontagne 2011 | Cross‐sectional study. |
| Marek 2012 | Simple pre–post survey. |
| Meneses Echavez 2015 | Pre‐ and postintervention with no control. |
| Moss 2012 | Pre‐ and postintervention survey. |
| Ortiz 2016 | Pilot cross‐sectional study. |
| Perkins 2016 | Cross‐sectional survey. |
| Pierre‐Victor 2017 | Pre‐ and poststudy. |
| Reiter 2011 | Pre‐ and postevaluation study. |
| Ruffin 2015 | Intervention was a reminder. |
| Sales 2011 | Pre‐ and poststudy. |
| Soldan 2006 | Review. |
| Spleen 2012 | Pretest/post‐test assessment. |
| Stokley 2015 | Review. |
| Szilagyi 2011 | Intervention was a reminder. |
| Tiro 2016 | Pre‐ and postsurvey. |
| Unti 1997 | Pre‐ and postsurvey. |
| Won 2015 | Outcomes were trust and participation in school‐located immunisation programmes, and none of our outcomes of interest was reported. |
| Zhou 2003 | Pre‐ and postintervention survey. |
Characteristics of studies awaiting assessment [ordered by study ID]
Dempsey 2018.
| Methods | Cluster‐randomised trial in the USA |
| Participants |
Participants: providers Number per group: 8 providers in intervention group and 8 providers in control group Total number enrolled: 188 providers Study population: 16 practices (4 family medicine and 12 paediatrics) that included 188 medical professionals with ≥ 400 active adolescents (aged 11–17 years). |
| Interventions |
Intervention: 5‐component communication intervention on HPV Description: the intervention included the following:
Duration: 6 months Comparison: no communication on HPV Description of comparison: practices in the control arm continued usual care with regard to communication about HPV vaccines. Vaccine target: HPV vaccine, meningococcal conjugate vaccine and ≥ 1 dose of the HPV vaccine series Disease targeted: cervical cancer and meningitis and tetanus, pertussis and diphtheria Number of doses: ≥ 1 dose the HPV vaccine series |
| Outcomes | HPV vaccine uptake Uptake of 2 other adolescent vaccines, i.e. meningococcal conjugate vaccine (MenACWY) and the tetanus–diphtheria–acellular pertussis vaccine (Tdap) |
| Notes |
Esposito 2018.
| Methods | Randomised trial in Italy |
| Participants |
Participants: adolescents aged 11–13 years Number per group: 334 participants in no intervention group, 281 participants in website educational programme only, and 302 participants in website educational programme plus the face‐to‐face lesson Total number enrolled: 917 adolescents Study population: over 1 school year, involving 4 secondary schools for adolescents aged 11–13 years and 8 schools for adolescents 14–18 years old in Milan, Italy. |
| Interventions |
Intervention: education intervention on adolescent vaccines Description:
Duration: 1 school year Comparison: no education intervention on adolescent vaccines Description of comparison: registration of vaccination coverage and attitudes toward vaccination at the beginning and at the end of the school year, but no intervention Vaccine target: diphtheria, tetanus, pertussis and HPV vaccines Disease targeted: cervical cancer and tetanus, pertussis and diphtheria Number of doses: not specified |
| Outcomes | Adolescent vaccines uptake Knowledge and attitude on adolescent vaccines |
| Notes |
HPV: human papillomavirus.
Characteristics of ongoing studies [ordered by study ID]
Skinner 2015.
| Trial name or title | HPV.edu |
| Methods | Cluster‐randomised trial |
| Participants |
Participants: adolescents in their first year of high school (year 8 in participating states) Number enrolled: 40 schools with year‐8 enrolments above 100 students Study population: adolescents attending high school in each of 2 states, western Australia and south Australia |
| Interventions |
Intervention: 3 main components: adolescent intervention; HPV vaccine parent/adolescent decision support tool; and logistical strategies; methods for increasing consent form return such as direct mail‐out of forms to parents. Description:
Duration: conducted over 2 school years: 2013 and 2014. Comparison: usual practice Vaccine target: HPV vaccination Disease targeted: cervical cancer Number of doses: no description |
| Outcomes | HPV vaccine uptake HPV vaccination knowledge |
| Starting date | 1 February 2013 |
| Contact information | Prof Rachel Skinner Discipline of Paediatrics and Child Health, University of Sydney, Children's Hospital at Westmead, Locked Bag 4001, Westmead, NSW 2054, Australia Email: Rachel.Skinner@health.nsw.gov.au |
| Notes | Trial registered on ANZCTR with registration number ACTRN12614000404628 |
HPV: human papillomavirus.
Differences between protocol and review
New author added during the review process (i.e. Valantine N Ndze).
In the review, we did not conduct subgroup and sensitivity analyses, as specified in the protocol, due to lack of data. See Table 1 for methods specified in the protocol (Abdullahi 2015), but not used in the review.
We removed adverse events following immunisation as an outcome as we realised posthoc that it was not a relevant outcome for this review.
Contributions of authors
LA, BK, GH, and CW conceived the review, participated in the development of the protocol (Abdullahi 2015), and approved the final version for publication.
LA, BK, VN, GH, and CW participated in the review and approved the final version for publication.
Sources of support
Internal sources
Vaccines for Africa Initiative, University of Cape Town, South Africa.
External sources
-
Department for International Development, UK.
Project number 300342‐104
Declarations of interest
LA: none.
BK: I am employed by the University to conduct research and aid in supervision of postgraduate students. I am a co‐supervisor of Leila Abdullahi, the first author of the review. I do not have any conflicts of interest for the review.
VN: none.
GH: none.
CW: none.
New
References
References to studies included in this review
Cates 2014 {published data only}
- Cates JR, Diehl SJ, Crandell JL, Coyne‐Beasley T. Intervention effects from a social marketing campaign to promote HPV vaccination in preteen boys. Vaccine 2014;32(33):4171‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diclemente 2015 {published data only}
- Diclemente RJ, Murray CC, Graham T, Still J. Overcoming barriers to HPV vaccination: a randomized clinical trial of a culturally‐tailored, media intervention among African American girls. Human Vaccines and Immunotherapeutics 2015;11(12):2883‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiks 2016 {published data only}
- Fiks AG, Luan X, Mayne SL. Improving HPV vaccination rates using maintenance‐of‐certification requirements. Pediatrics 2016;137(3):e20150675. [DOI] [PubMed] [Google Scholar]
Gargano 2015 {published data only}
- Gargano L, Weiss P, Underwood N, Seib K, Sales J, Vogt T, et al. School‐located vaccination clinics for adolescents: correlates of acceptance among parents. Journal of Community Health 2015;40(4):660‐9. [DOI] [PubMed] [Google Scholar]
Grandahl 2016 {published data only}
- Grandahl M, Rosenblad A, Stenhammar C, Tyden T, Westerling R, Larsson M, et al. School‐based intervention for the prevention of HPV among adolescents: a cluster randomised controlled study. BMJ Open 2016;6(1):e009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantzari 2015 {published data only}
- Mantzari E, Vogt F, Marteau TM. Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial. Health Psychology 2015;34(2):160‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paskett 2016 {published data only}
- Paskett ED, Krok‐Schoen JL, Pennell ML, Tatum CM, Reiter PL, Peng J, et al. Results of a multilevel intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiology Biomarkers and Prevention 2016;25(4):593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins 2015 {published data only}
- Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS. Effectiveness of a provider‐focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015;33(9):1223‐9. [DOI] [PubMed] [Google Scholar]
Rickert 2015 {published data only}
- Rickert VI, Auslander BA, Cox DS, Rosenthal SL, Rupp RE, Zimet GD. School‐based HPV immunization of young adolescents: effects of two brief health interventions. Human Vaccines and Immunotherapeutics 2015;11(2):315‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2008 {published data only}
- Schwarz K, Garrett B, Lee J, Thompson D, Thiel T, Alter MJ, et al. Positive impact of a shelter‐based hepatitis B vaccine program in homeless Baltimore children and adolescents. Journal of Urban Health 2008;85(2):228‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2000 {published data only}
- Skinner SR, Imberger A, Nolan T, Lester R, Glover S, Bowes G. Randomised controlled trial of an educational strategy to increase school‐based adolescent hepatitis B vaccination. Australian and New Zealand Journal of Public Health 2000;24(3):298‐304. [DOI] [PubMed] [Google Scholar]
Staras 2015 {published data only}
- Staras SA, Vadaparampil S, Livingston IM, Thompson L, Sanders A, Shenkman E. A health information technology intervention increases HPV vaccine series initiation among Florida Medicaid and CHIP adolescents. Sexually Transmitted Diseases 2014;41:S9‐10. [Google Scholar]
- Staras SA, Vadaparampil ST, Livingston MD, Thompson LA, Sanders AH, Shenkman EA. Increasing human papillomavirus vaccine initiation among publicly insured Florida adolescents. Journal of Adolescent Health 2015;56(5):S40‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szilagyi 2015 {published data only}
- Szilagyi PG, Serwint JR, Humiston SG, Rand CM, Schaffer S, Vincelli P, et al. Effect of provider prompts on adolescent immunization rates: a randomized trial. Academic Pediatrics 2015;15(2):149‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson‐Jones 2012 {published data only}
- Watson‐Jones D, Baisley K, Ponsiano R, Lemme F, Remes P, Ross D, et al. Human papillomavirus vaccination in Tanzanian schoolgirls: cluster‐randomised trial comparing 2 vaccine‐delivery strategies. Journal of Infectious Diseases 2012;206(5):678‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2005 {published data only}
- Wilson TR, Fishbein DB, Ellis PA, Edlavitch SA. The impact of a school entry law on adolescent immunization rates. Journal of Adolescent Health 2005;37(6):511‐6. [DOI] [PubMed] [Google Scholar]
Winer 2016 {published data only}
- Winer RL, Gonzales AA, Noonan CJ, Buchwald DS. A cluster‐randomized trial to evaluate a mother‐daughter dyadic educational intervention for increasing HPV vaccination coverage in American Indian girls. Journal of Community Health 2016;41(2):274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anjum 2012 {published data only}
- Anjum S, Pirzada AG, Memon AA. Knowledge and educational intervention pertaining to viral hepatitis in adolescent male students of urban and rural Sindh, Pakistan. Journal of the Dow University of Health Sciences 2012;6(2):66‐9. [Google Scholar]
Bar‐Shain 2015 {published data only}
- Bar‐Shain DS, Stager MM, Runkle AP, Leon JB, Kaelber DC. Direct messaging to parents/guardians to improve adolescent immunizations. Adolescent Health 2015;56:S21‐6. [DOI] [PubMed] [Google Scholar]
Bennett 2015 {published data only}
- Bennett A, Patel D, Carlos R, Zochowski M, Pennewell S, Chi A, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. Journal of Women's Health 2015;24(11):950‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broutet 2013 {published data only}
- Broutet N, Lehnertz N, Mehl G, Camacho AV, Bloem P, Chandra‐Mouli V, et al. Effective health interventions for adolescents that could be integrated with human papillomavirus vaccination programs. Journal of Adolescent Health 2013;53(1):6‐13. [DOI] [PubMed] [Google Scholar]
Catledge 2014 {published data only}
- Catledge SW. Increasing HPV Vaccination Rates Using Social Marketing Strategies [Thesis]. 72. Hattiesburg (MS): University of Southern Mississippi, 2014. [Google Scholar]
Chan 2015 {published data only}
- Chan A, Brown B, Sepulveda E, Teran‐Clayton L. Evaluation of fotonovela to increase human papillomavirus vaccine knowledge, attitudes, and intentions in a low‐income Hispanic community. BMC Research Notes 2015;8(1):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2010 {published data only}
- Chapman E, Venkat P, Ko E, Orezzoli JP, Carmen M, Garner EI. Use of multimedia as an educational tool to improve human papillomavirus vaccine acceptability – a pilot study. Gynecologic Oncology 2010;118(2):103‐7. [DOI] [PubMed] [Google Scholar]
Chaves 2000 {published data only}
- Chaves Pérez JB, Perea‐Milla E, Cobos López JE. Educative intervention in systematic anti‐hepatitis B vaccination in a school community [Spanish]. Enfermeria Clinica 2000;10(4):142‐9. [Google Scholar]
Chou 2014 {published data only}
- Chou TI, Lash DB, Malcolm B, Yousify L, Quach JY, Dong S, et al. Effects of a student pharmacist consultation on patient knowledge and attitudes about vaccines. Journal of the American Pharmacists Association 2014;54(2):130‐7. [DOI] [PubMed] [Google Scholar]
Chung 2015 {published data only}
- Chung RJ, Walter EB, Kemper AR, Dayton A. Keen on teen vaccines: improvement of adolescent vaccine coverage in rural North Carolina. Adolescent Health 2015;56:S14‐6. [DOI] [PubMed] [Google Scholar]
Dawson 2015 {published data only}
- Dawson RS, Hansen S. Promoting adolescent vaccination and wellness throughout military treatment facilities. Journal of Adolescent Health 2015;1:S116‐7. [Google Scholar]
Dempsey 2015a {published data only}
- Dempsey AF, Maertens J, Beaty BL, O'Leary ST. Understanding how different recruitment strategies impact parent engagement with an iPad‐based intervention to provide personalized information about adolescent vaccines. Journal of Adolescent Health 2015;56(5):S7‐13. [DOI] [PubMed] [Google Scholar]
Dempsey 2015b {published data only}
- Dempsey AF, Maertens J, Beaty B, O'Leary ST. Characteristics of users of a tailored, interactive website for parents and its impact on adolescent vaccination attitudes and uptake. BMC Research Notes 2015;8:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donahue 2016 {published data only}
- Donahue KL, Hendrix KS, Sturm LA, Zimet GD. The effect of provider communication about vaccination on mothers' willingness to vaccinate their children against HPV and influenza: a randomized trial of illustrated health messaging vignettes. Journal of Adolescent Health 2016;58(2):S117. [Google Scholar]
Dorji 2015 {published data only}
- Dorji T, Tshomo U, Phuntsho S, Tamang TD, Tshokey T, Baussano I, et al. Introduction of a national HPV vaccination program into Bhutan. Vaccine 2015;33(31):3726‐30. [DOI] [PubMed] [Google Scholar]
Farmar 2016 {published data only}
- Farmar AL, Love‐Osborne K, Chichester K, Breslin K, Bronkan K, Hambidge SJ. Achieving high adolescent HPV vaccination coverage. Pediatrics 2016;138(5):e20152653. [DOI] [PubMed] [Google Scholar]
Fujiwara 2013 {published data only}
- Fujiwara H, Takei Y, Ishikawa Y, Saga Y, Machida S, Taneichi A, et al. Community‐based interventions to improve HPV vaccination coverage among 13‐ to 15‐year‐old females: measures implemented by local governments in Japan. PloS One 2013;8(12):e84126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furlan 2010 {published data only}
- Furlan MC, Rissardo LK, Oliveira RG, Ferrer AL, Marcon SS. Impact of compulsory school vaccination against hepatitis B in adolescents: exploratory‐descriptive study [Portuguese]. Online Brazilian Journal of Nursing 2010;9(2):1‐1. [Google Scholar]
Gargano 2014 {published data only}
- Gargano LM, Herbert NL, Painter JE, Sales JM, Vogt TM, Morfaw C, et al. Development, theoretical framework, and evaluation of a parent and teacher‐delivered intervention on adolescent vaccination. Health Promotion Practice 2014;15(4):556‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillespie 2011 {published data only}
- Gillespie L, Hicks CW, Santana M, Worley SE, Banas DA, Holmes S, et al. The acceptability of human papillomavirus vaccine among parents and guardians of newborn to 10‐year‐old children. Journal of Pediatric and Adolescent Gynecology 2011;24(2):66‐70. [DOI] [PubMed] [Google Scholar]
Gordon 2013 {published data only}
- Gordon J, Lansley M, Mitchell D. Combining the delivery of the human papillomavirus vaccine and the Td/IPV teenage booster. British Journal of School Nursing 2013;8(1):20‐4. [Google Scholar]
Gottvall 2010 {published data only}
- Gottvall RM, Tyden T, Hoglund AT, Larsson M. Knowledge of human papillomavirus among high school students can be increased by an educational intervention. International Journal of STD & AIDS 2010;21(8):558‐62. [DOI] [PubMed] [Google Scholar]
Hadley 2014 {published data only}
- Hadley L. Increasing HPV Vaccination Rates among Adolescent Males: a Toolkit for Parents [Thesis]. Minneapolis (MN): Walden University, 2014. [Google Scholar]
Hofman 2013 {published data only}
- Hofman R, Schiffers PA, Richardus JH, Raat H, Kok IM, Ballegooijen M, et al. Increasing girls' knowledge about human papillomavirus vaccination with a pre‐test and a national leaflet: a quasi‐experimental study. BMC Public Health 2013;13:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hull 2016 {published data only}
- Hull PC, Williams EA, Khabele D, McAfee CR, Sanderson M. Bug Your Doc: Get 3 shots! – a culturally appropriate social marketing intervention to increase HPV vaccination. Cancer Epidemiology, Biomarkers & Prevention 2016;25(3 Suppl):A57. [Google Scholar]
Iqbal 2016 {published data only}
- Iqbal MS, Iqbal MZ, Bahari MB. Effectiveness of pharmacists‐led provider‐focused interventions to improve HPV vaccination rates in adults in Malaysia. Value in Health 2016;19(7):A424. [Google Scholar]
Kim 2015 {published data only}
- Kim HW. Awareness of human papillomavirus and factors associated with intention to obtain HPV vaccination among Korean youth: quasi experimental study. BMC International Health and Human Rights 2015;15(4):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwan 2011 {published data only}
- Kwan TT, Tam KF, Lee PW, Chan KK, Ngan HY. The effect of school‐based cervical cancer education on perceptions towards human papillomavirus vaccination among Hong Kong Chinese adolescent girls. Patient Education and Counseling 2011;84(1):118‐22. [DOI] [PubMed] [Google Scholar]
Kwang 2016 {published data only}
- Kwang NB, Mahayudin T, Yien HL, Abdul Karim AK, Teik CK, Shan LP. Effect of an educational intervention on knowledge of human papillomavirus vaccination among pre‐university students in Malaysia. Asian Pacific Journal of Cancer Prevention 2016;17(1):267‐74. [DOI] [PubMed] [Google Scholar]
Lai 2013 {published data only}
- Lai CY, Wu WW, Cheng SF. The effectiveness of innovative integrated teaching method on female adolescents' knowledge of cervical cancer, health belief, and the intention of HPV vaccination. Supportive Care in Cancer 2013;21:S48. [Google Scholar]
LaMontagne 2011 {published data only}
- LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low‐ and middle‐income countries. World Health Organization Bulletin 2011;89(11):821‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marek 2012 {published data only}
- Marek E, Dergez T, Rebek‐Nagy G, Szilard I, Kiss I, Ember I, et al. Effect of an educational intervention on Hungarian adolescents' awareness, beliefs and attitudes on the prevention of cervical cancer. Vaccine 2012;30(48):6824‐32. [DOI] [PubMed] [Google Scholar]
Meneses Echavez 2015 {published data only}
- Meneses Echavez J, Ramirez‐Velez R. Promoting knowledge and beliefs about human papillomavirus‐related cancers and vaccination strategies in adolescents from Colombia through a health‐education intervention. Supportive Care in Cancer 2015;1(1):S69. [Google Scholar]
Moss 2012 {published data only}
- Moss JL, Reiter PL, Dayton A, Brewer NT. Increasing adolescent immunization by webinar: a brief provider intervention at federally qualified health centers. Vaccine 2012;30(33):4960‐3. [DOI] [PubMed] [Google Scholar]
Ortiz 2016 {published data only}
- Ortiz R, Downs S, Shafer A, Cates J, Coyne‐Beasley T. Engaging adolescents through social media to improve HPV vaccination: findings from a pilot Facebook intervention. Journal of Adolescent Health 2016;58(2):S92‐3. [Google Scholar]
Perkins 2016 {published data only}
- Perkins RB, Lin M, Wallington SF, Hanchate AD. Impact of school‐entry and education mandates by states on HPV vaccination coverage: analysis of the 2009–2013 National Immunization Survey‐Teen. Human Vaccines and Immunotherapeutics 2016;12(6):1615‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierre‐Victor 2017 {published data only}
- Pierre‐Victor D, Page TF, Trepka MJ, Stephens DP, Li T, Madhivanan P. Impact of Virginia's school‐entry vaccine mandate on human papillomavirus vaccination among 13‐17‐year‐old females. Journal of Women's Health 2017;26(3):266‐75. [DOI] [PubMed] [Google Scholar]
Reiter 2011 {published data only}
- Reiter PL, Stubbs B, Panozzo CA, Whitesell D, Brewer NT. HPV and HPV vaccine education intervention: effects on parents, healthcare staff, and school staff. Cancer Epidemiology Biomarkers and Prevention 2011;20(11):2354‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruffin 2015 {published data only}
- Ruffin MT, Plegue MA, Rockwell PG, Young AP, Patel DA, Yeazel MW. Impact of an electronic health record (EHR) reminder on human papillomavirus (HPV) vaccine initiation and timely completion. Journal of the American Board of Family Medicine 2015;28(3):324‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sales 2011 {published data only}
- Sales JM, Painter JE, Pazol K, Gargano LM, Orenstein WA, Hughes JM, et al. Rural parents' vaccination‐related attitudes and intention to vaccinate middle and high school children against influenza following educational influenza vaccination intervention. Human Vaccines 2011;7(11):1146‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soldan 2006 {published data only}
- Soldan K, Dillner J. Comparable strategies needed to evaluate human papillomavirus vaccine efficiency across Europe. Euro Surveillance 2006;11(11):E061123.061123. [DOI] [PubMed] [Google Scholar]
Spleen 2012 {published data only}
- Spleen AM, Kluhsman BC, Clark AD, Dignan MB, Lengerich EJ, Force AH. An increase in HPV‐related knowledge and vaccination intent among parental and non‐parental caregivers of adolescent girls, age 9‐17 years, in Appalachian Pennsylvania. Journal of Cancer Education 2012;27(2):312‐9. [DOI] [PubMed] [Google Scholar]
Stokley 2015 {published data only}
- Stokley S. Interventions to improve adolescent vaccination coverage. Journal of Adolescent Health 2015;56(5):S3‐4. [DOI] [PubMed] [Google Scholar]
Szilagyi 2011 {published data only}
- Szilagyi PG, Humiston SG, Gallivan S, Albertin C, Sandler M, Blumkin A. Effectiveness of a citywide patient immunization navigator program on improving adolescent immunizations and preventive care visit rates. Archives of Pediatrics and Adolescent Medicine 2011;165(6):547‐53. [DOI] [PubMed] [Google Scholar]
Tiro 2016 {published data only}
- Tiro JA, Lee SC, Farrell D, Marks EG, Baldwin AS, Denman DC. Development of a tablet‐based application to elicit self‐persuasion about HPV vaccination among undecided parents. Cancer Epidemiology, Biomarkers & Prevention 2016;25(3 Suppl):A87. [Google Scholar]
Unti 1997 {published data only}
- Unti LM, Coyle KK, Woodruff BA, Boyer‐Chuanroong L. Incentives and motivators in school‐based hepatitis B vaccination programs. Journal of School Health 1997;67(7):265‐8. [DOI] [PubMed] [Google Scholar]
Won 2015 {published data only}
- Won TL, Middleman AB, Auslander BA, Short MB. Trust and a school‐located immunization program. Journal of Adolescent Health 2015;56(5 Suppl):S33‐9. [DOI] [PubMed] [Google Scholar]
Zhou 2003 {published data only}
- Zhou F, Euler GL, McPhee SJ, Nguyen T, Lam T, Wong C, et al. Economic analysis of promotion of hepatitis B vaccinations among Vietnamese‐American children and adolescents in Houston and Dallas. Pediatrics 2003;111(6 Pt 1):1289‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dempsey 2018 {published data only}
- Dempsey AF, Pyrznawoski J, Lockhart S, Barnard J, Campagna EJ, Garrett K, et al. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: a cluster randomized clinical trial. JAMA Pediatrics 2018;172(5):e180016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2018 {published data only}
- Esposito S, Bianchini S, Tagliabue C, Umbrello G, Madini B, Pietro G, et al. Impact of a website based educational program for increasing vaccination coverage among adolescents. Human Vaccines & Immunotherapeutics 2018;14(4):961‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Skinner 2015 {published data only}
- Skinner SR, Davies C, Cooper S, Stoney T, Marshall H, Jones J, et al. HPV.edu study protocol: a cluster randomised controlled evaluation of education, decisional support and logistical strategies in school‐based human papillomavirus (HPV) vaccination of adolescents. BMC Public Health 2015;15:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdullahi 2016
- Abdullahi LH, Kagina BM, Cassidy T, Adebayo EF, Wiysonge CS, Hussey GD. Knowledge, attitudes and practices on adolescent vaccination among parents, teachers and adolescents in Africa: a systematic review. Vaccine 2016;34(34):3950‐60. [DOI] [PubMed] [Google Scholar]
Adamu 2019
- Adamu AA, Sarki AM, Uthman OA, Wiyeh AB, Gadanya MA, Wiysonge CS. Prevalence and dynamics of missed opportunities for vaccination among children in Africa: applying systems thinking in a systematic review and meta‐analysis of observational studies. Expert Review of Vaccines 2019;18:547‐558. [DOI] [PubMed] [Google Scholar]
Attwell 2019
- Attwell KC, Navin M. Childhood Vaccination Mandates: Scope, Sanctions, Severity, Selectivity, and Salience. Milbank Quarterly 2019;16 September:[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 2013
- Barry D. Increasing knowledge about HPV and the HPV vaccine amongst adolescents and adults through a school‐based setting: a Capstone project, Doctor of Nursing Practice (DNP) Capstone Projects. Paper 31, 2013. scholarworks.umass.edu/nursing_dnp_capstone/31 (accessed prior to 21 August 2019).
Brabin 2008
- Brabin L, Greenberg DP, Hessel L, Hyer R, Ivanoff B, Damme P. Current issues in adolescent immunization. Vaccine 2008;26:4120‐34. [DOI] [PubMed] [Google Scholar]
Briss 2000
- Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. American Journal of Preventive Medicine 2000;18:97‐140. [DOI] [PubMed] [Google Scholar]
Brotherton 2015
- Brotherton JM, Ogilvie GS. Current status of human papillomavirus vaccination. Current Opinion in Oncology 2015;27(5):399‐404. [DOI] [PubMed] [Google Scholar]
Bruni 2016
- Bruni L, Diaz M, Barrionuevo‐Rosas L, Herrero R, Bray F, Bosch FX, Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016;4:e453‐63. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Services Research and Policy 2000;5:12‐6. [DOI] [PubMed] [Google Scholar]
Cawley 2010
- Cawley J, Hull HF, Rousculp MD. Strategies for implementing school‐located influenza vaccination of children: a systematic literature review. Journal of School Health 2010;80:167‐75. [DOI] [PubMed] [Google Scholar]
Clements 2004
- Clements C, Abdool‐Karim Q, Chang M, Nkowane B, Esparza J. Breaking new ground – are changes in immunization services needed for the introduction of future HIV/AIDS vaccines and other new vaccines targeted at adolescents?. Vaccine 2004;22:2822‐6. [DOI] [PubMed] [Google Scholar]
Cooper 2019
- Cooper S, Schmidt BM, Ryan J, Leon N, Mavundza E, Burnett R, Tanywe AC, Wiysonge CS. Factors that influence acceptance of human papillomavirus (HPV) vaccination for adolescents: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2019, Issue 9. [DOI: 10.1002/14651858.CD013430] [DOI] [Google Scholar]
Das 2016
- Das JK, Salam RA, Arshad A, Lassi ZS, Bhutta ZA. Systematic review and meta‐analysis of interventions to improve access and coverage of adolescent immunizations. Journal of Adolescent Health 2016;59(4S):S40‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donner 2001
- Donner A, Piaggio G, Villar J. Statistical methods for the meta‐analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10:325‐38. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PMC2127453; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2019a
- Cochrane Effective Practice, Organisation of Care (EPOC). What study designs can be considered for inclusion in an EPOC review and what should they be called? EPOC resources for review authors. epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 9 August 2019).
EPOC 2019b
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 9 August 2019).
EPOC 2019c
- Cochrane Effective Practice, Organisation of Care (EPOC). Worksheets for preparing summary of findings (SoF) tables using GRADE. EPOC resources for review authors. Available at epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 9 August 2019).
EPOC 2019d
- Cochrane Effective Practice, Organisation of Care (EPOC). How to report the effects of an intervention. EPOC resources for review authors. Available at epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 9 August 2019).
Gilkey 2014
- Gilkey MB, Dayton AM, Moss JL, Sparks AC, Grimshaw AH, Bowling JM, et al. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics 2014;134:e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gowda 2012
- Gowda C, Schaffer S, Dombkowski K, Dempsey A. Understanding attitudes toward adolescent vaccination and the decision‐making dynamic among adolescents, parents and providers. BMC Public Health 2012;12:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2009
- Harris KM, Laurie TM, Nicole L. Strategies and models for promoting adolescent vaccination for low‐income populations (published in 2009 by the RAND Corporation). www.rand.org/content/dam/rand/pubs/documented_briefings/2009/RAND_DB577.pdf (accessed 30 August 2018).
Higgins 2019
- Higgins JP, Thomas J (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Jacobson Vann 2018
- Jacobson Vann JC, Jacobson RM, Coyne‐Beasley T, Asafu‐Adjei JK, Szilagyi PG, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD003941.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaddar 2013
- Kaddar M, Schmitt S, Makinen M, Milstien J. Global support for new vaccine implementation in middle‐income countries. Vaccine 2013;31S:B81‐96. [DOI] [PubMed] [Google Scholar]
Kaufman 2017
- Kaufman, J, Ames, H, Bosch‐Capblanch, X, et al. The comprehensive ‘Communicate to Vaccinate’ taxonomy of communication interventions for childhood vaccination in routine and campaign contexts.. BMC Public Health 2017;17:423. [DOI: 10.1186/s12889-017-4320-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 2018
- Kaufman J, Ryan R, Walsh L, Horey D, Leask J, Robinson P, et al. Face‐to‐face interventions for informing or educating parents about early childhood vaccination. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD010038.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2005
- Lee G, LeBaron C, Murphy T, Lett S, Schauer S, Lieu T. Pertussis in adolescents and adults: should we vaccinate?. Pediatrics 2005;115:1675. [DOI] [PubMed] [Google Scholar]
Loke 2017
- Loke AY, Kwan ML, Wong YT, Wong AKY. The uptake of Human Papillomavirus Vaccination and its associated factors among adolescents: a systematic review. Journal of Primary Care & Community Health 2017;8(4):349‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackroth 2010
- Mackroth M, Irwin K, Vandelaer J, Hombach J, Eckert L. Immunizing school‐age children and adolescents: experience from low‐ and middle‐income countries. Vaccine 2010;28:1138‐47. [DOI] [PubMed] [Google Scholar]
Mahomed 2008
- Mahomed H, Shea J, Kafaar F, Hawkridge T, Hanekom WA, Hussey GD. Are adolescents ready for tuberculosis vaccine trials?. Vaccine 2008;26:4725‐30. [DOI] [PubMed] [Google Scholar]
Mavundza 2019
- Mavundza EJ, Wiyeh AB, Mahasha PW, Halle‐Ekane G, Wiysonge CS. A systematic review of immunogenicity, clinical efficacy and safety of human papillomavirus vaccines in people living with the human immunodeficiency virus. Human Vaccines and Immunotherapeutics 2019;20 September:1‐10. doi: 10.1080/21645515.2019.1656481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2018
- Newman PA, Logie CH, Lacombe‐Duncan A, Baiden P, Tepjan S, Rubincam C, et al. Parents' uptake of human papillomavirus vaccines for their children: a systematic review and meta‐analysis of observational studies. BMJ Open 2018;8(4):e019206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngcobo 2018
- Ngcobo NJ, Burnett RJ, Cooper S, Wiysonge CS. Human papillomavirus vaccination acceptance and hesitancy in South Africa: research and policy agenda. South African Medical Journal 2018;109:13‐5. [DOI] [PubMed] [Google Scholar]
Nnaji 2020
- Nnaji CA, Owoyemi AJ, Amaechi UA, Wiyeh AB, Ndwandwe DE, Wiysonge CS. Taking stock of global immunisation coverage progress: the gains, the losses and the journey ahead. International Health 2020;January:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oberlin 2018
- Oberlin AM, Rahangdale L, Chinula L, Fuseini NM, Chibwesha CJ. Making HPV vaccination available to girls everywhere. International Journal of Gynaecology and Obstetrics 2018;143(3):267‐76. [DOI: 10.1002/ijgo.12656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omer 2019
- Omer SB, Betsch C, Leask J. Mandate vaccination with care. Nature 2019;571:469‐72. [DOI] [PubMed] [Google Scholar]
Oyo‐Ita 2016
- Oyo‐Ita A, Wiysonge CS, Oringanje C, Nwachukwu CE, Oduwole O, Meremikwu MM. Interventions for improving coverage of childhood immunisation in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD008145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2014
- Paul P, Fabio A. Literature review of HPV vaccine delivery strategies: considerations for school‐ and non‐school based immunization program. Vaccine 2014;32(3):320‐6. [DOI] [PubMed] [Google Scholar]
Piot 2019
- Piot P, Larson HJ, O'Brien KL, N'kengasong J, Ng E, Sow S, Kampmann B. Immunization: vital progress, unfinished agenda. Nature 2019;575:119‐129. [DOI] [PubMed] [Google Scholar]
Principi 2013
- Principi N, Esposito S. Adolescents and vaccines in the western world. Vaccine 2013;31:5366‐74. [DOI] [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behaviour change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):613‐23. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robbins 2011
- Robbins Cooper SC, Ward K, Skinner R. School‐based vaccination: a systematic review of process evaluation. Vaccine 2011;29:9588‐99. [DOI] [PubMed] [Google Scholar]
Saeterdal 2012
- Saeterdal I, Glenton C, Austvoll‐Dahlgren A, Munabi‐Babigumira S, Lewin S. Community‐directed interventions for informing and/or educating about early childhood vaccination. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010232] [DOI] [PMC free article] [PubMed] [Google Scholar]
SAHM 2013
- Society for Adolescent Health and Medicine. Adolescent consent for vaccination: a position paper of the Society for Adolescent Health and Medicine. Journal of Adolescent Health 2013;53:550‐3. [DOI] [PubMed] [Google Scholar]
Santesso 2019
- Santesso N, Glenton C, Dahm P, Garner P, Akl E, Alper B, Brignardello‐Petersen R, Carrasco‐Labra A, Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ, GRADE Working Group. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2019;pii:S0895‐4356(19)30416‐0. [DOI] [PubMed] [Google Scholar]
Smulian 2016
- Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Human Vaccines & Immunotherapeutics 2016;12(6):1566‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stinchfield 2008
- Stinchfield PK. Practice‐proven interventions to increase vaccination rates and broaden the immunization season. American Journal of Medicine 2008;121:S11‐21. [DOI] [PubMed] [Google Scholar]
TFCPS 2000
- Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. American Journal of Preventive Medicine 2000;18:92‐6. [PubMed] [Google Scholar]
Tsu 2009
- Tsu VD. Overcoming barriers and ensuring access to HPV vaccines in low‐income countries. American Journal of Law & Medicine 2009;35:401‐13. [DOI] [PubMed] [Google Scholar]
Ward 2012
- Ward K, Chow MY, King C, Leask J. Strategies to improve vaccination uptake in Australia, a systematic review of types and effectiveness. Australian and New Zealand Journal of Public Health 2012;36:369‐77. [Google Scholar]
Warren 2004
- Warren S. Adolescent vaccination in the developing world: time for serious consideration?. Vaccine 2004;22:781‐5. [DOI] [PubMed] [Google Scholar]
WHO 2019
- WHO. Immunization Agenda 2030: A Global Strategy To Leave No One Behind. World Health Organization, 2019; Geneva, Switzerland https://www.who.int/immunization/immunization_agenda_2030/en/ (accessed 17 November 2019).
WHO 2019a
- World Health Organization. Adolescent health. www.who.int/maternal_child_adolescent/adolescence/en/ (accessed 7 August 2019).
WHO 2019b
- World Health Organization. WHO recommendations for routine immunization. www.who.int/immunization/policy/Immunization_routine_table1.pdf (accessed 7 August 2019).
WHO 2019c
- World Health Organization. Adverse events following immunisation (AEFI). https://www.who.int/vaccine_safety/initiative/detection/AEFI/en/ (accessed 4 December 2019).
Williams 2011
- Williams N, Woodward H, Majeed A, Saxena S. Primary care strategies to improve childhood immunisation uptake in developed countries: systematic review. Journal of the Royal Society of Medicine 2011;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willis 2013
- Willis N, Hill S, Kaufman J, Lewin S, Kis‐Rigo J, Castro Freire SB, et al. "Communicate to vaccinate": the development of a taxonomy of communication interventions to improve routine childhood vaccination. BMC International Health and Human Rights 2013;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiysonge 2012
- Wiysonge CS, Ngcobo NJ, Jeena PM, Madhi SA, Schoub BD, Hawkridge A, et al. Advances in childhood immunisation in South Africa: where to now? Programme managers' views and evidence from systematic reviews. BMC Public Health 2012;12:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zipursky 2010
- Zipursky S, Wiysonge CS, Hussey G. Knowledge and attitudes towards vaccines and immunization among adolescents in South Africa. Human Vaccines 2010;6:455‐61. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdullahi 2015
- Abdullahi LH, Kagina BMN, Wiysonge CS, Hussey GD. Improving vaccination uptake among adolescents. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011895] [DOI] [PMC free article] [PubMed] [Google Scholar]


