Abstract
Background
Palmoplantar pustulosis is a chronic inflammatory disease in which sterile, relapsing pustules appear on the palms and soles, possibly in conjunction with other symptoms. The previous Cochrane Review on this topic was published in 2006, before biological treatments were extensively used.
Objectives
To assess the effects of interventions for chronic palmoplantar pustulosis to induce and maintain complete remission.
Search methods
We searched the following databases up to March 2019: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers and checked the reference lists of the included studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria
We considered RCTs including people with palmoplantar pustulosis or chronic palmoplantar pustular psoriasis assessing topical therapy, systemic therapy, combinations of topical or systemic therapies, or non‐pharmacological therapies compared with placebo, no intervention, or each other.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our outcomes included 'Proportion of participants cleared or almost cleared', 'Proportion of participants with adverse effects serious or severe enough to cause withdrawal', 'Proportion of participants with at least 50% improvement in disease severity', and 'Proportion of participants with adverse effects'.
Main results
We included 37 studies (1663 participants; mean age 50 years (range 34 to 63); 24% males). These studies reported condition severity differently. Around half of the included trials stated the setting (hospitals, community clinics, or both). More than half of the studies were at high risk of bias in at least one domain.
Our included studies assessed mainly systemic treatments (retinoids, ciclosporin, biologics, etretinate + PUVA (combination of psoralens and long‐wave ultraviolet radiation) therapy combined, and antibiotics), but also topical treatments (dermocorticoids, vitamin D) and phototherapy (PUVA, ultraviolet A1 (UVA1)). Other interventions were assessed by single studies. The most common comparator was placebo.
All results presented in this abstract were assessed in the short term (mean treatment duration was 11 weeks (range 8 to 24 weeks)) and are based on participants with chronic palmoplantar pustulosis. All outcome time point measurements were taken from baseline and assessed at the end of treatment. Short‐term and long‐term outcomes were defined as measurement up to 24 weeks after randomisation and between 24 and 104 weeks after randomisation, respectively.
One trial (188 participants) assessed the topical vitamin D derivative maxacalcitol versus placebo and found that maxacalcitol may be more effective than placebo in achieving clearance (risk ratio (RR) 7.83, 95% confidence interval (CI) 1.85 to 33.12; low‐quality evidence), and the risk of adverse effects (such as mild local irritation, pruritus, and haematological or urinary test abnormalities) is probably similar in both groups (RR 0.87, 95% CI 0.64 to 1.19; moderate‐quality evidence). Severity was not reported.
Two trials (49 participants) assessed PUVA therapy versus placebo or no treatment, providing very low‐quality evidence. Adverse effects were reported with oral PUVA (including nausea, ankle swelling, and non‐purulent conjunctivitis) and with local PUVA (including blistering, erythema, and pruritus).
With regard to the systemic retinoid alitretinoin, one trial (33 participants; moderate‐quality evidence) showed that alitretinoin probably makes little or no difference in reducing severity when compared to placebo (RR 0.69, 95% CI 0.36 to 1.30). A similar number of adverse events were reported in both treatment groups, including headache, cheilitis, nausea, arthralgia, and nasopharyngitis (RR 0.84, 95% CI 0.61 to 1.17). Clearance was not reported.
There may be little or no difference between etanercept and placebo in achieving clearance (RR 1.64, 95% CI 0.08 to 34.28; 1 study; 15 participants; low‐quality evidence); however, the 95% CI was very wide, showing there may be a difference between groups. Severity was not measured.
More patients treated with placebo may achieve reduced severity than those treated with ustekinumab, but the wide 95% CI indicates there might be little or no difference between groups and there might be greater effect with ustekinumab (RR 0.48, 95% CI 0.11 to 2.13; 1 study; 33 participants; low‐quality evidence). Clearance was not reported.
It is uncertain whether guselkumab increases clearance when compared to placebo (2 studies; 154 participants) because the quality of evidence is very low, but guselkumab probably better reduces disease severity (RR 2.88, 95% CI 1.24 to 6.69; 1 study; 49 participants; moderate‐quality evidence).
Secukinumab is probably superior to placebo in reducing severity (RR 1.55, 95% CI 1.02 to 2.35; 1 study; 157 participants; moderate‐quality evidence), but our clearance outcome was not reported. None of these trials reported on occurrence of adverse effects.
Only two of the studies discussed above reported adverse effects serious or severe enough to cause withdrawal. Guselkumab may cause more serious adverse events when compared to placebo, but there is uncertainty due to the very wide 95% CI showing there may be little or no difference and showing more events with placebo (RR 2.88, 95% CI 0.32 to 25.80; 1 study; 49 participants; low‐quality evidence). Secukinumab probably causes more serious adverse events than placebo (RR 3.29, 95% CI 1.40 to 7.75; 1 study; 157 participants; moderate‐quality evidence).
Authors' conclusions
Evidence is lacking for major chronic palmoplantar pustulosis treatments such as superpotent corticosteroids, phototherapy, acitretin, methotrexate, and ciclosporin. Risk of bias and imprecision limit our confidence.
Maxacalcitol may be more effective than placebo in achieving clearance in the short term (low‐quality evidence), and the risk of adverse effects is probably similar (moderate‐quality evidence). Oral alitretinoin is probably no more effective than placebo in reducing severity, with a similar risk of adverse effects (moderate‐quality evidence).
Regarding biological treatments, we are uncertain of the effect of etanercept on clearance and the effect of ustekinumab on severity (low‐quality evidence). Secukinumab and guselkumab are probably superior to placebo in reducing severity (moderate‐quality evidence). Adverse events not requiring withdrawal were not reported for these treatments.
Reporting of serious adverse effects was incomplete: compared to placebo, secukinumab probably caused more participant withdrawals (moderate‐quality evidence), but we are uncertain of the effect of guselkumab (low‐quality evidence).
Future trials should assess commonly used treatments using validated severity and quality of life scales.
Plain language summary
Treating long‐term palmoplantar pustulosis (pustules on the hands and feet)
Review question
We wanted to assess treatments for palmoplantar pustulosis (a persistent condition characterised by small, pus‐filled blisters on the hands and feet), when compared to an inactive substance (placebo), no intervention, or each other. We included 37 studies.
Background
Palmoplantar pustulosis negatively affects a person's life; there is no cure or standard treatment. Over time, the skin becomes thicker and redder, and may develop cracks or flake off as scales.
Symptoms are treated with topical medicines (usually corticosteroids), systemic medicines (medicines injected or taken by mouth that work throughout the entire body; usually medicines based on vitamin A or D), or phototherapy (ultraviolet light treatment).
Study characteristics
The studies involved 1663 adults (mostly women) 34 to 63 years of age (average age 50 years). In 19 studies, participants had had palmoplantar pustulosis from two to 16 years (average 6.4 years).
Participants had palmoplantar pustular psoriasis (6 studies), palmoplantar pustulosis (29 studies), or both (2 studies). Study authors reported condition severity differently.
The included studies assessed a variety of different treatments: mainly systemic treatments (including biologic medicines, vitamin A medicines, immunosuppressants, antibiotics, and light therapy combined with a vitamin A medicine), but also topical medicines (containing steroids or vitamin D) and light treatments. Single studies assessed other treatments.
Treatments were most commonly compared against placebo. Treatment length varied; for our key results, this ranged from 8 to 24 weeks (average 11 weeks). When reported, studies were conducted in hospitals, community clinics, or both.
Pharmaceutical companies funded 18 studies.
Key results
Low‐quality evidence suggests that maxacalcitol (a topical vitamin D derivative) may work better than placebo in achieving clearance; moderate‐quality evidence indicates that the number of side effects is probably similar in both groups (participants experienced itching, irritation, and blood or urine test abnormalities) (1 trial; 188 participants). Severity was not measured.
We found very low‐quality evidence for PUVA therapy (i.e. psoralen, a drug to sensitise the skin, and ultraviolet light A) versus placebo or no treatment (2 studies; 49 participants), so we are unable to draw conclusions. Side effects with PUVA included skin blisters, redness, itching, swelling, and feeling sick.
Oral alitretinoin probably makes little or no difference in reducing severity when compared to placebo (moderate‐quality evidence; 1 study; 33 participants). A similar result was found for side effects, with headache, sickness, joint pain, high cholesterol, and colds reported in both groups. Clearance was not reported.
Five studies assessed biological treatments (etanercept, ustekinumab, guselkumab, secukinumab), which use substances made from living organisms, or synthetic versions, to target the immune system.
Low‐quality evidence (1 study; 15 participants) suggests that etanercept may make little or no difference in clearance when compared to placebo, but we are very uncertain of this result. Side effects and severity were not measured.
We found low‐quality evidence suggesting that ustekinumab may be worse than placebo in reducing disease severity, but we are very uncertain of this result. Side effects and clearance were not reported (1 study; 33 participants).
Compared to placebo, guselkumab probably reduces severity (moderate‐quality evidence; 1 study; 49 participants), but its effects on clearance are uncertain (very low‐quality evidence; 2 studies; 154 participants). Side effects were not measured.
Moderate‐quality evidence shows that secukinumab was probably superior to placebo in reducing severity, but skin clearance and side effects were not reported (1 study; 157 participants).
Only two studies described above reported withdrawals from treatment due to serious side effects; these are probably more frequent with secukinumab than with placebo (157 participants), and may occur more often with guselkumab than with placebo (49 participants), but we are very uncertain of the guselkumab result.
For these key results, outcomes were assessed between 8 and 24 weeks, which we deemed short term.
This evidence is current to March 2019.
Quality of the evidence
The key comparisons reported clearance most often, but evidence quality was mainly very low. Only two key studies reported side effects causing withdrawal (low‐ and moderate‐quality evidence). The evidence underlying our severity and side effects outcomes was variable in quality (very low to moderate).
Small participant numbers, results with wide margins of error, and selective reporting have limited our confidence in the evidence.
Summary of findings
Summary of findings for the main comparison. Triamcinolone acetonide 0.1% cream with occlusive dressing compared to clobetasol cream 0.05% cream for chronic palmoplantar pustulosis.
| Triamcinolone acetonide 0.1% cream with occlusive dressing compared to clobetasol cream 0.05% cream for chronic palmoplantar pustulosis | ||||||
| Patient or population: for chronic palmoplantar pustulosis Setting: not reported Intervention: triamcinolone acetonide 0.1% cream with occlusive dressing Comparison: clobetasol cream 0.05% cream | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with clobetasol cream 0.05% cream | Risk with triamcinolone acetonide 0.1% cream with occlusive dressing | |||||
| Proportion of participants cleared or almost cleared ‐ assessed with overall assessment 5‐point scale at 4 weeks | In the triamcinolone side, 13/19 cleared or almost cleared compared to 3/19 in the clobetasol side (P = 0.26, when within‐participant unit of analysis is taken into account) | RR 1.20 (0.72 to 2.00) | 19 (1 RCT) |
⊕⊝⊝⊝ Very lowa | Within‐participant study, left or right side randomised | |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 8 weeks | Study author reported no adverse events and no skin atrophy in both groups | Not estimable | 19 (1 RCT) |
⊕⊝⊝⊝ Very lowa | Within‐participant study, left or right side randomised | |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by three levels to very low‐quality evidence. Two levels due to study limitations because of high risk of bias for blinding and unclear risk of bias for other items, and one level due to imprecision because the comparison was assessed in a single small study.
Summary of findings 2. Topical vitamin D derivative compared to placebo for chronic palmoplantar pustulosis.
| Topical vitamin D derivative compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: outpatients Intervention: topical vitamin D derivative Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with topical vitamin D derivative | |||||
| Proportion of participants cleared or almost cleared in the short term (8 weeks) | Study population | RR 7.83 (1.85 to 33.12) | 188 (1 RCT) | ⊕⊕⊝⊝ Lowa | Another study compared topical vitamin D derivative to placebo (within‐study design; side randomised) (Muro 2016). Co‐intervention (topical betamethasone butyrate propionate) was applied on both sides. Combined therapy was reported as significantly superior to monotherapy for each assessed symptom (erythema, pustules/vesicles, hyperkeratosis). | |
| 22 per 1000 | 168 per 1000 (40 to 712) | |||||
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 8 weeks | Study population | RR 0.87 (0.64 to 1.19) | 188 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Reported adverse events were mild local irritation, pruritus, and mild haematological or urinary test abnormalities In Muro 2016, none of the participants in both groups reported any side effects. |
|
| 495 per 1000 | 430 per 1000 (317 to 589) | |||||
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels to low‐quality evidence. One level due to study limitations because of incomplete reporting and other items were rated as unclear risk of bias. One further level due to imprecision as there is a large confidence interval for this result.
bDowngraded by one level to moderate‐quality evidence for study limitations because of incomplete reporting and other items rated as unclear risk of bias.
Summary of findings 3. Puvatherapy compared to placebo or no treatment for chronic palmoplantar pustulosis.
| Puvatherapy compared to placebo or no treatment for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: outpatient department Intervention: PUVA therapy Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with PUVA therapy | |||||
| Proportion of participants cleared or almost cleared at 8 weeks | In Murray 1980, clearance was obtained in 12/22 PUVA‐treated sides and in 0/22 non‐irradiated sides. In Layton 1991, clearance was not achieved in any palms and soles for local PUVA therapy sides nor for placebo sides (26 soles; 18 palms). | Not estimable | 22 (44 treated sides) ‐ Murray 1980 ‐ and 27 ‐ Layton 1991 (26 soles; 18 palms) (2 RCTs) | ⊕⊝⊝⊝ Very lowa | Two within‐participant trials (data to undertake analysis considering within‐participant variability were not available). | |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity at 8 weeks | In Murray 1980, 50% improvement was achieved by 10/22 treated sides and by 13/22 untreated sides. | Not estimable | 22 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 8 weeks | In Murray 1980, with oral psoralen, 1 participant was burned, 4 had nausea, 4 had ankle swelling, and 6 got non‐purulent conjunctivitis. With topical psoralen, 4 participants were burned. In Layton 1991, in the local PUVA group, 4 participants had blistering on the feet (3 on the hands), 3 had pruritus on the feet (2 on the hands), and 3 had erythema on the feet (2 on the hands). |
Not estimable | 22 ‐ Murray 1980 ‐ and 27 (26 soles; 18 palms) ‐ Layton 1991 (2 RCTs) | ⊕⊝⊝⊝ Very lowa | ‐ | |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PUVA: combination of psoralens and long‐wave ultraviolet radiation; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by three levels to very low‐quality evidence: one level due to study limitations because of unclear risk of bias for four out of five items, one level due to inconsistency (efficacy and type of adverse events were substantially different in these two trials), and one level due to imprecision because the comparison was assessed in two small studies.
Summary of findings 4. UVA1 compared to narrowband UVB for chronic palmoplantar pustulosis.
| UVA1 compared to narrowband UVB for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: Department of Dermatology Intervention: UVA1 Comparison: Narrowband UVB | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with narrowband UVB | Risk with UVA1 | |||||
| Proportion of participants cleared or almost cleared at 10 weeks | Data provided did not allow analysis taking account of intra‐participant variability. 22/33 were markedly improved (PPASI score) in UVA1‐treated sides and 11/33 in UVB‐treated sides. | Not estimable | 33 (1 RCT) |
⊕⊝⊝⊝ Very lowa | Within‐participant study (right/left side) | |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects measured over 10 weeks | Out of 33 UVA1‐treated sides, 6 had a burning sensation and 2 had hyperpigmentation. In UVB‐treated sides, 9/33 had xerosis. | Not estimable | 33 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; UCA1: ultraviolet A1; UVB: ultraviolet B. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by three levels to very low quality evidence: two levels due to study limitations because of high risk of bias for blinding and one level due to imprecision because the comparison was assessed in a single study involving 33 participants.
Summary of findings 5. Etretinate compared to placebo or no treatment for chronic palmoplantar pustulosis.
| Etretinate compared to placebo or no treatment for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: not reported Intervention: etretinate Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with etretinate | |||||
| Proportion of participants cleared or almost cleared in the short term (10 weeks or 4 months) | Study population | RR 3.48 (0.82 to 14.80) | 40 (2 RCTs) | ⊕⊝⊝⊝ Very lowa | Another study assessing this comparison ‐ White 1986 (20 participants) ‐ reported zero participants cleared in both groups. | |
| 100 per 1000 | 348 per 1000 (82 to 1000) | |||||
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term (6 months) | Study population | RR 2.39 (0.92 to 6.17) | 26 (1 RCT) | ⊕⊝⊝⊝ Very lowb | ‐ | |
| 267 per 1000 | 637 per 1000 (245 to 1000) | |||||
| Proportion of participants with adverse effects ‐ measured over 12 weeks | Study population | RR 3.50 (0.95 to 12.90) | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Four participants had cheilitis, 2 had facial dermatitis, and 1 developed some hair loss in the etretinate group compared with 2 participants with cheilitis in the placebo group (White 1986). | |
| 200 per 1000 | 700 per 1000 (190 to 1000) | |||||
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by three levels to very low‐quality evidence: two levels due to study limitations as the two trials are at risk of bias for blinding because of systematic visible adverse events due to etretinate, and one further level for imprecision because both trials included a small number of participants.
bDowngraded by three levels to very low‐quality evidence: two levels because of study limitations (high risk of bias for blinding and incomplete outcome data) and one level due to imprecision because only one trial including a small number of participants assessed this comparison.
Summary of findings 6. Etretinate with PUVA therapy as co‐intervention compared to placebo with PUVA therapy as co‐intervention for chronic palmoplantar pustulosis.
| Etretinate with PUVA therapy as co‐intervention compared to placebo with PUVA therapy as co‐intervention for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: Department of Dermatology Intervention: etretinate with PUVA therapy as co‐intervention Comparison: placebo with PUVA therapy as co‐intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo with PUVA therapy as co‐intervention | Risk with etretinate with PUVA therapy as co‐intervention | |||||
| Proportion of participants cleared or almost cleared in the short term (20 weeks) | Study population | RR 1.91 (1.04 to 3.50) | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 500 per 1000 | 955 per 1000 (520 to 1000) | |||||
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 20 weeks | There were zero events in the placebo group, so we were unable to calculate the assumed risk. Side effects in the etretinate group: 6 had cheilitis, 4 had hair loss, 2 had peeling of the palmoplantar skin, 1 had generalised peeling of the skin with pruritus, and 1 had dryness of the nasal mucosa. |
RR 17.00 (1.11 to 259.87) | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa | ‐ | |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PUVA: combination of psoralens and long‐wave ultraviolet radiation; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by four levels to very low‐quality evidence: two levels for study limitations because the trial was at high risk of bias for blinding and unclear risk for all other items, and a further two levels for imprecision because the result was based on a small trial with few participants and had a large 95% confidence interval.
Summary of findings 7. Etretinate compared to PUVA therapy for chronic palmoplantar pustulosis.
| Etretinate compared to PUVA therapy for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: not reported Intervention: etretinate Comparison: PUVA therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PUVA therapy | Risk with etretinate | |||||
| Proportion of participants cleared or almost cleared in the short term (12 weeks) | Study population | RR 11.20 (4.16 to 30.18) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 63 per 1000 | 700 per 1000 (260 to 1000) | |||||
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 12 weeks | Study population | RR 11.54 (5.17 to 25.74) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowa | In the etretinate group, 2 participants had severe hair loss and 1 had severe drying of the mucosa. One‐third of participants developed mild erythema and scaling of healthy skin, and all had mild drying of the lips and nasal mucosa. In the oral PUVA therapy group, 3 participants had nausea and 2 had pruritus. | |
| 78 per 1000 | 902 per 1000 (404 to 1000) | |||||
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PUVA: combination of psoralens and long‐wave ultraviolet radiation; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by three levels to very low‐quality evidence: two levels due to study limitations because of high risk of bias for blinding and incomplete outcome data, and one level due to imprecision because the comparison was assessed in a single study and the result had a very large confidence interval.
Summary of findings 8. Alitretinoin compared to placebo for chronic palmoplantar pustulosis.
| Alitretinoin compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: not reported Intervention: alitretinoin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with alitretinoin | |||||
| Proportion of participants cleared or almost cleared ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity in the long term (24 weeks) | Study population | RR 0.69 (0.36 to 1.30) | 33 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 667 per 1000 | 460 per 1000 (240 to 867) | |||||
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ measured over 24 weeks | Study population | RR 0.84 (0.61 to 1.17) | 33 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Adverse effects in the alitretinoin group included headache, nasopharyngitis, cheilitis, nausea, arthralgia, and hypercholesterolaemia. | |
| 889 per 1000 | 747 per 1000 (542 to 1000) | |||||
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level to moderate‐quality evidence because this comparison was assessed in only one trial involving 33 participants (imprecision).
Summary of findings 9. Etanercept compared to placebo for chronic palmoplantar pustulosis.
| Etanercept compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: not reported Intervention: etanercept Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with etanercept | |||||
| Proportion of participants cleared or almost cleared in the short term (3 months) | There were no events in the placebo group; hence, we could not calculate the assumed risk. | RR 1.64 (0.08 to 34.28) | 15 (1 RCT) | ⊕⊕⊝⊝ Lowa | ‐ | |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels to low‐quality evidence because only one study involving 15 participants assessed this comparison, and the result displayed a very large 95% confidence interval (imprecision).
Summary of findings 10. Ustekinumab compared to placebo for chronic palmoplantar pustulosis.
| Ustekinumab compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: not reported Intervention: ustekinumab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with ustekinumab | |||||
| Proportion of participants cleared or almost cleared ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity in the short term (16 weeks) | Study population | RR 0.48 (0.11 to 2.13) | 33 (1 RCT) | ⊕⊕⊝⊝ Lowa | ‐ | |
| 278 per 1000 | 133 per 1000 (31 to 592) | |||||
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels to low‐quality evidence because of study limitations (risk of reporting bias) and imprecision (only one trial; 33 participants).
Summary of findings 11. Guselkumab compared to placebo for chronic palmoplantar pustulosis.
| Guselkumab compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: outpatients/hospital Intervention: guselkumab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with guselkumab | |||||
| Proportion of participants cleared or almost cleared assessed with: PGA Follow‐up: 16 weeks | Study population | RR 1.17 (0.15 to 9.30) | 154 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ‐ | |
| 65 per 1000 | 76 per 1000 (10 to 604) | |||||
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal Follow‐up: over 24 weeks | Study population | RR 2.88 (0.32 to 25.80) | 49 (1 RCT) | ⊕⊕⊝⊝ Lowc | ‐ | |
| 42 per 1000 | 120 per 1000 (13 to 1000) | |||||
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants achieving a 50% reduction in disease severity assessed with PPPASI Follow‐up: 16 weeks | Study population | RR 2.88 (1.24 to 6.69) | 49 (1 RCT) | ⊕⊕⊕⊝ Moderated | ‐ | |
| 208 per 1000 | 600 per 1000 (258 to 1000) | |||||
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PGA: physicians' global assessment; PPPASI: Palmo‐Plantar Pustular Area and Severity Index; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels to low‐quality evidence because of imprecision (only two trials, 154 participants) and large CI.
bDowngraded by one level for inconsistency (different direction of treatment effect in the two studies; I² = 59%).
cDowngraded by two levels to low‐quality evidence because of imprecision (only one trial, 49 participants).
dDowngraded by one level to low‐quality evidence because of imprecision (only one trial, 49 participants).
Summary of findings 12. Secukinumab compared to placebo for chronic palmoplantar pustulosis.
| Secukinumab compared to placebo for chronic palmoplantar pustulosis | ||||||
| Patient or population: chronic palmoplantar pustulosis Setting: outpatients/hospital Intervention: secukinumab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with secukinumab | |||||
| Proportion of participants cleared or almost clear ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects serious or severe enough to have caused withdrawal Follow‐up: over 16 weeks | Study population | RR 3.29 (1.40 to 7.75) | 157 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 77 per 1 000 | 253 per 1 000 (108 to 596) | |||||
| Proportion of participants with at least 50% improvement in their quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with a 50% reduction in disease severity assessed with PPPASI Follow‐up: 16 weeks | Study population | RR 1.55 (1.02 to 2.35) | 157 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 295 per 1 000 | 457 per 1 000 (301 to 693) | |||||
| Proportion of participants without relapse in the long term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Proportion of participants with adverse effects in the short term ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Ease of compliance to an intervention or a treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level to moderate‐quality evidence because of imprecision (only one trial).
Background
Please refer to Table 13 for an explanation of the terms used in this review.
1. Glossary of terms.
| Term | Explanation |
| Acrosyringium | The most superficial portion of the eccrine gland (sweat gland) duct |
| Alitretinoin | A medicinal form of vitamin A that is taken orally (by mouth) to treat psoriasis and other skin conditions |
| Anti‐tumour necrosis factor | A class of drugs that target an inflammation‐causing substance called tumour necrosis factor (TNF) to reduce inflammation in many inflammatory conditions, such as rheumatoid arthritis, psoriatic arthritis, juvenile arthritis, Crohn's colitis, ankylosing spondylitis, and psoriasis |
| Cochran's Q | Q is the weighted sum of squares on a standardised scale. It is reported with a P value, with low P values indicating the presence of heterogeneity. This test, however, is known to have low power to detect heterogeneity, and it is suggested that a value of 0.10 is used as a cut‐off for significance. Conversely, Q has too much power as a test for heterogeneity if the number of studies is large |
| Concomitant | Something that accompanies something else |
| Corticosteroid cream | A cream formulation containing a steroid medicine that can be applied to the skin to treat inflammation in conditions such as psoriasis or eczema |
| Cytokines | Proteins involved in cell signalling |
| Dermis | The middle layer of the skin |
| Desquamation | The shedding of the outer layers of the skin |
| Dyslipidaemia | Abnormal blood lipid levels |
| Epidermis | The upper layer of the skin |
| Erythema | Redness of the skin or mucous membranes |
| Fissuring | Having a deep groove or tear in the skin |
| Heterogeneity | Presence of variation in true effect sizes underlying different studies |
| Hyperkeratosis | Thickening of the stratum corneum (the outermost layer of the skin) |
| Genetic susceptibility locus | Place on the gene coding for psoriasis vulgaris |
| Monoclonal antibody | An antibody produced by a single clone of cells and consisting of identical antibodies |
| Narrow‐band UVB | A type of light therapy that uses just a small part of the ultraviolet B wavelengths of light to treat skin conditions such as psoriasis |
| Occlusion | Using a topical treatment "under occlusion" means that the medication has been covered after being applied to the skin surface keeping it on the affected site |
| Placebo | A medicine prescribed for a patient for its psychological effect more than for its physiological benefit |
| Proteolysis | Breakdown of proteins into smaller parts |
| Spongiform | Having a porous structure; multi‐locular |
| Systemic therapy | Treatment that goes through the bloodstream to reach its target in the body |
| Topical vitamin D derivative | A compound similar in structure to vitamin D, which can be applied to the skin to treat skin conditions such as psoriasis |
| Unilocular | Characterised by 1 cavity: single‐chambered |
| White blood cells | Neutrophils, mast cells, T lymphocytes, eosinophils |
Description of the condition
Definition
Palmoplantar pustulosis is a chronic inflammatory disease in which a number of sterile pustules appear abruptly on the palms of the hands and the soles of the feet. These pustules relapse over time, possibly in conjunction with hyperkeratosis, erythema, scaling, and fissuring (Wolff 2008). Whether palmoplantar pustulosis is a variant of psoriasis or is a separate condition is still open to discussion.
Palmoplantar pustulosis most commonly presents in the fifth or sixth decade of life, and the median age of onset varies between 45 and 65 years, according to published reports (Brunasso 2013; Hellgren 1971), with between 58% and 94% of those affected being women (Brunasso 2013; Michaëlsson 2007). According to available data, palms are exclusively affected in 5% to 32% of cases, and the soles of the feet in 14% to 36% of cases. Palms and soles are concomitantly affected in 47% to 73% of cases (Brunasso 2013). Brunasso 2013 compared data from various publications and found that nails are involved in 30% to 76% of palmoplantar pustulosis cases, and that arthritis was noted in 13% to 65% of cases (Brunasso 2010; Brunasso 2013; Burden 1996; Miot 2009).
Involvement of the palms and soles negatively impacts the quality of life of people with this condition (Pettey 2003). Symptoms are usually limited to an itching or burning sensation that may precede eruption of new lesions. However, in severe cases, especially when cracking and fissuring occur, intense pain along with an inability to stand up, walk, or manipulate things interferes with everyday activities (Wolff 2008). Palmoplantar pustulosis is a chronic disease that persists for decades with periods of partial or complete remission interrupted by intermittent exacerbations (Wolff 2008). Because palmoplantar pustulosis is a chronic disease, it can affect not only a person's private life and relationships but also his or her professional life, especially when handling and manipulating materials is necessary.
The debate about whether psoriasis and palmoplantar pustulosis should be considered as variants of the same disease or separate conditions is ongoing. Palmoplantar pustulosis was originally described as a local variant of psoriasis (Barber 1930). The proportion of people with palmoplantar pustulosis who also have psoriatic lesions elsewhere on the body is highly variable, ranging from 8% in Burden 1996 to 73% in Brunasso 2010. In 2013, a case series study compared clinical and epidemiological data of those affected by palmoplantar plaque psoriasis and palmoplantar pustulosis. This study showed that 90% of people with a diagnosis of palmoplantar pustulosis had evidence of palmoplantar plaque psoriasis at baseline or during follow‐up (Brunasso 2013). No statistical difference was found between palmoplantar pustulosis and palmoplantar plaque psoriasis in terms of age at onset of disease, disease duration, family history of psoriasis, concomitant arthritis, or smoking habits, which was consistent with previously published data (Brunasso 2013).
In 2007, the International Psoriasis Council stated that palmoplantar pustulosis should still be considered as a separate disease, especially because genetic studies have failed to demonstrate an association between palmoplantar pustulosis and the psoriasis susceptibility gene 1 (PSORS 1) (Griffiths 2007), which is acknowledged to be the most important genetic susceptibility locus for psoriasis vulgaris (Asumalahti 2003). Those supporting the hypothesis that the two conditions are different diseases believe that palmoplantar pustulosis is an innate immune disorder mainly affecting women with a high prevalence of autoimmune disease (Michaëlsson 2007).
Histologically, the presence of unilocular (single cavity) pustules containing neutrophils (a type of white blood cell) characterises palmoplantar pustulosis. Small spongiform (or multi‐locular) pustules may be present in the epidermal wall of the pustule and within the surrounding epidermis, along with slight epidermal thickening (Elder 2008). Another hallmark of palmoplantar pustulosis is lack of visibility of the epidermal part of the eccrine duct, denoting involvement of the acrosyringium (the most superficial portion of the eccrine gland duct) (Eriksson 1998).
Physiopathology
The physiopathology of palmoplantar pustulosis is still not fully understood, but the condition is characterised by infiltration of white blood cells (mast cells, eosinophils, and T lymphocytes) into the dermis, along with accumulation of neutrophils and eosinophils in the pustules (Eriksson 1998; Uehara 1974). Over‐expression of kallikrein‐related peptidases (enzymes that break down proteins) has been shown to be responsible for shedding of layers of skin, which frequently accompanies this condition (Kaneko 2012).
In addition, findings suggest that the most superficial portion of the sweat gland duct is the major site of vesicle or pustule formation in palmoplantar pustulosis (Murakami 2010). Those with palmoplantar lesions have increased levels of the cytokine interleukin (IL)‐17 in both tissue and serum (Murakami 2011).
Trigger factors
Palmoplantar pustulosis has been reported as a condition triggered in some cases by focal infection, such as dental infection or infection of the palatine tonsils (Kikushi 2013).
Tobacco smoke has also been suspected to be involved in the pathogenesis of palmoplantar pustulosis (Miot 2009). The relative risk of developing palmoplantar pustulosis is 74 times higher among active smokers compared with non‐smokers (Hagforsen 2002).
Palmoplantar pustulosis may also occur as an adverse reaction to anti‐tumour necrosis factor‐alpha (anti‐TNF‐α) biological agents (Moustou 2009; Puig 2012).
Description of the intervention
Palmoplantar pustulosis is a challenging disease for dermatologists to manage, and even though many treatments have been used over the years, no gold standard therapy has yet been identified, and no treatments are curative (Chalmers 2006). Most of the treatments used are those indicated in psoriasis.
Topical agents
The most commonly used treatments remain topical agents, mainly topical corticosteroids, such as triamcinolone acetonide cream, clobetasol propionate, and betamethasone dipropionate, which are considered even more effective if applied under occlusion (Kragballe 1991); vitamin D derivatives (e.g. maxacalcitol); and topical retinoids (e.g. tazarotene, tretinoin) (Adisen 2010).
Topical corticosteroids cause several side effects including skin atrophy, tachyphylaxis, and rebound effects (Saurat 2009).
Topical vitamin D derivatives (e.g. maxacalcitol) are mainly indicated in localised psoriasis. However, they are contraindicated in cases of pregnancy, lactation, hypercalcaemia, and renal and hepatic insufficiency. Topical vitamin D may cause local irritation of the skin as it needs to be applied twice per day (Saurat 2009).
Several topical retinoids (tretinoin, isotretinoin, alitretinoin, retinol, retinaldehyde, adapalene, and tazarotene) are available for various indications. The most frequent side effect is irritation that is experienced during the first weeks of treatment. Topical retinoids are contraindicated in cases of pregnancy (Saurat 2009).
Phototherapy
Phototherapy is also used and includes ultraviolet A photochemotherapy (UVA) associated with topical or oral psoralen (PUVA (combination of psoralens and long‐wave ultraviolet radiation) therapy) and narrowband ultraviolet B (NB‐UVB) phototherapy. Phototherapy can induce side effects similar to intense sun exposure: erythema, burns, pigmentation, skin cancer (mainly melanoma), etc. UVB phototherapy is usually administered three to four times per week. Photochemotherapy UVA is usually administered three times per week. History of skin cancer is an absolute contraindication for both (Saurat 2009).
Systemic agents
Many systemic agents are used as well, including systemic retinoids (etretinate (which is the same as Tigason and oral RO 10‐9359) has been removed from the market because of its long half‐life (120 days)); acitretin (RO 10‐1670), which is the acid form of the ethyl ester etretinate, formed by hydrolysis in the body of etretinate and considered as the main active principle of the latter but with a shorter elimination half‐life (50 to 60 hours); alitretinoin, an oral retinoid authorised for use in severe chronic hand eczema; and liarozole, an all‐trans retinoic acid) (Adisen 2010). It is important to note that acitretin is converted to etretinate in the liver during concomitant alcohol intake, and an efficient contraceptive method is required for a period of two years after discontinuation of treatment as systemic retinoids are teratogenic and thus are contraindicated in women of childbearing age. Other side effects include mucocutaneous xerosis and dyslipidaemia. Oral retinoids may interact with cyclines and treatments that compete for cytochrome 3A4 (Saurat 2009).
Tetracycline antibiotics are also used for this indication. Cyclines are contraindicated in pregnancy and in children younger than eight years. Side effects are mainly nausea, abdominal pain, and genital candidosis (Saurat 2009).
In severe forms of palmoplantar pustulosis, or forms resistant to previously mentioned treatments, immunosuppressive treatments such as methotrexate or ciclosporin can be used (Adisen 2010). Biologics used in psoriasis treatment are also used in refractory and severe cases. The main side effects of ciclosporin are nephrotoxicity and hypertension; for methotrexate, they are haematological and hepatological toxicities; and the main side effect of biologics is immunosuppression that can lead to an increased incidence of infection and cancer (Saurat 2009).
All treatments used in palmoplantar pustulosis are symptomatic treatments and thus do not affect the course of the disease.
How the intervention might work
Topical corticosteroids have anti‐inflammatory, antiproliferative, immunosuppressive, and vasoconstrictive actions affecting cutaneous T cells, macrophages, and dendritic cells, thus reducing the inflammatory reaction in the skin and the symptoms of patients with palmoplantar pustulosis (Saurat 2009). Topical retinoids can be used in combination with topical corticosteroids or as topical corticosteroid‐sparing agents and act by directly suppressing the inflammatory reaction and normalising epidermal differentiation (Kurian 2011). Topical vitamin D derivatives reduce hyperkeratosis and induce normal epidermal differentiation by modulating the transcription of various genes (Saurat 2009).
Topical or oral PUVA therapy induces inhibition of DNA synthesis and immunosuppression (Saurat 2009).
Narrowband ultraviolet B (NB‐UVB) phototherapy might also act by decreasing CCR4+ CD8+ T cells, a subtype of white blood cells found in excess in patients with palmoplantar pustulosis, thereby reducing inflammation (Otsuka 2010).
Systemic retinoids exert their effects by binding specific nuclear receptors belonging to the superfamily of glucocorticosteroid, thyroid hormone, and vitamin D receptors (Saurat 2009). These receptors are expressed in the skin and act on cell differentiation and apoptosis. Systemic retinoids are efficient in all types of psoriasis but especially in pustular and palmoplantar forms (Saurat 2009).
Ciclosporin is an immunosuppressive agent that inhibits the initial phase of activation of CD4 T cells, leading to the absence of synthesis of IL‐2 and thus preventing activation and proliferation of T cells, the main component of the inflammatory infiltrate in palmoplantar pustulosis (Ho 1996).
Of the newer biological therapies, ustekinumab is a fully human immunoglobulin (Ig)G1/κ monoclonal antibody targeting the p40 subunit shared by IL‐12 and IL‐23, thus blocking the immunological sequence of events leading to psoriasis plaques and recruitment of neutrophils in pustular forms of psoriasis and in palmoplantar pustulosis (Di Cesare 2009; Martin 2013; Morales‐Múnera 2013; Watanabe 2009; Yilmaz 2012). Tocilizumab (TCZ), a humanised monoclonal antibody against the IL‐6 receptor, is used mainly for treatment of anti‐TNF‐α‐induced palmoplantar pustulosis (Fujishima 2010). Etanercept is a synthetic antibody that competitively inhibits binding of TNF‐α to its receptor, thereby preventing its inflammatory effects. Etanercept has been reported as a potential treatment for palmoplantar pustulosis (Floristan 2011), even though palmoplantar pustulosis may paradoxically appear or worsen as a result of anti‐TNF‐α therapy for other inflammatory diseases (Rueda‐Gotor 2012). Secukinumab is a human monoclonal antibody that targets IL‐17A selectively and is highly efficacious in moderate to severe cases of plaque psoriasis (Langley 2014). Guselkumab is a fully human IgG1/λ monoclonal antibody that binds the p19 subunit of IL‐23, thereby inhibiting binding of IL‐23 to the receptor and subsequently inhibiting the terminal differentiation of IL‐17‐producing cells (McGeachy 2009). Guselkumab has showed efficacy in moderate to severe cases of plaque psoriasis (McGeachy 2009).
Smoking cessation was associated with a significant reduction in pustule number (Michaëlsson 2006).
Why it is important to do this review
Palmoplantar pustulosis is a chronic condition that has a negative impact on a person's quality of life. Although many treatments are used for this condition, a Cochrane Review on 'Interventions for chronic palmoplantar pustulosis' concluded that there was an absence of either a gold standard treatment or a standardised method for assessing response to treatment in any of the conducted clinical trials (Chalmers 2006). The previous review is now out of date, and furthermore, it assessed the evidence before biological treatments were extensively used.
This review has been updated by way of a new protocol (Obeid 2015), but with different primary and secondary outcomes.
Objectives
To assess the effects of interventions for chronic palmoplantar pustulosis to induce and maintain complete remission.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and within‐patient RCTs (e.g. right foot compared with left foot in the same person).
Cross‐over trials were also eligible as we considered only the first period data.
Types of participants
People with palmoplantar pustulosis or chronic palmoplantar pustular psoriasis (including cases associated with plaque‐psoriasis lesions) who were recruited either before the induction phase or whilst in the maintenance phase (see below). We excluded studies that included patients with non‐pustular palmoplantar psoriasis. In cases where studies included only a subset of relevant participants, we included those studies if the characteristics of participants and results were provided separately or were obtained through contact with study authors.
We excluded participants with palmoplantar pustulosis triggered by anti‐TNF‐α therapy, acute pustular bacterid (a condition triggered by a streptococcal infection), acropustulosis (an idiopathic self‐limited vesiculopustular eruption on the palms and soles, occurring mainly in infants), and acrodermatitis continua of Hallopeau (an inflammatory disease wherein pustular eruptions begin in the tips of the fingers and toes).
Types of interventions
We considered trials that assessed the following.
Any topical therapy versus placebo or no treatment.
Any systemic therapy versus placebo or no treatment.
Comparison of two or more topical therapies.
Comparison of two or more systemic therapies.
Comparison of systemic therapies versus topical therapies.
Non‐pharmacological therapies (such as quitting smoking).
Types of outcome measures
Timings
We evaluated all outcomes at two different timings.
Induction phase: evaluation up to 24 weeks after randomisation (short term).
Maintenance phase: evaluation between 24 and 104 weeks after randomisation (long term).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: short term and long term
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
We define 'serious' adverse effects as events that pose a threat to a patient's life or functioning, whereas we define 'severe' adverse effects by their intensity.
Secondary outcomes
Proportion of participants with at least 50% improvement in quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or the Pain Disability Index (PDI), evaluated in the short term and in the long term
Proportion of participants achieving a 50% reduction in disease severity in the short term
Proportion of participants without relapse in the long term
Proportion of participants with adverse effects in the short term and in the long term
Ease of compliance with an intervention or a treatment
We expressed all outcomes as a percentage of participants randomised (intention‐to‐treat analysis).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 12 March 2019 using strategies based on the draft strategy for MEDLINE in our published protocol (Obeid 2015).
Cochrane Skin Group Specialised Register via the search strategy in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 3), in the Cochrane Library, via the strategy in Appendix 2.
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3.
Embase via Ovid (from 1974) using the strategy in Appendix 4.
Latin American and Caribbean Health Science Information database (LILACS; from 1982) using the strategy in Appendix 5.
Trials registers
We (GO and LLC) searched the following trials registers (on 30 March 2019) using the search terms (palmoplantar pustulosis and palmoplantar pustular psoriasis).
International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com).
ClinicalTrials.gov (www.clinicaltrials.gov).
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
GO also searched the trials databases of relevant pharmaceutical companies (Novartis (https://www.novctrd.com/CtrdWeb/trialresults.nov) and Pfizer (https://www.pfizer.com/science/research_clinical_trials/trial_results)) on 23 March 2019, using the search terms 'palmoplantar pustulosis' and 'palmoplantar pustular psoriasis' to identify ongoing and unpublished trials. We planned to search relevant trials submitted to the US Food and Drug Administration (FDA) for drug registration (www.accessdata.fda.gov/scripts/cder/drugsatfda/), but we did not search this source because all drugs assessed were old or had received no approval for this indication.
Searching other resources
References from included studies
We checked the bibliographies of the included studies for further references to relevant trials.
Unpublished literature
We contacted research leaders in the field to identify additional published or unpublished data.
We contacted by email the authors of papers published in or after 2007 to request information regarding the primary outcomes of interest in our review.
Conference proceedings
We searched the proceedings of the following conferences from 2004 to 2016, except those years that the Cochrane Skin Group had already handsearched.
American Academy of Dermatology (AAD) (except the years 2006, 2007, 2010, and 2011).
Society for Investigative Dermatology (SID) (except the years 2004, 2005, 2006, 2010, and 2011).
European Academy of Dermatology and Venereology (EADV) (from 2008 to 2016, except the years 2004, 2005, 2006, and 2007), searched via CD‐ROM on 29 February 2016.
Adverse effects
We did not perform a separate search for rare or delayed adverse effects of interventions used for treatment of patients with chronic palmoplantar pustulosis. We considered only adverse effects and side effects described in the included studies.
Data collection and analysis
Some parts of the methods section of this review use text that was originally published in another Cochrane protocol (Le Cleach 2011).
We included 12 'Summary of findings' tables in our review. In these tables, we summarised our primary outcomes and secondary outcomes for the most clinically important comparisons.
Triamcinolone acetonide 0.1% cream with occlusive dressing compared to clobetasol cream 0.05% cream.
Topical vitamin D derivative compared to placebo.
Puvatherapy compared to placebo or no treatment.
Ultraviolet A1 (UVA1) compared to narrowband ultraviolet B (UVB).
Etretinate compared to placebo or no treatment.
Etretinate with PUVA therapy as co‐intervention compared to placebo with PUVA therapy as co‐intervention.
Etretinate compared to PUVA therapy.
Alitretinoin compared to placebo.
Etanercept compared to placebo.
Ustekinumab compared to placebo.
Guselkumab compared to placebo.
Secukinumab compared to placebo.
We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence (Schünemann 2013). We used this assessment, which two review authors conducted, to inform the main text of the Discussion section.
Selection of studies
Two review authors (GO and GD) independently examined each title and abstract and excluded obviously irrelevant reports. These two review authors independently examined full‐text articles to determine eligibility. They aimed to reach consensus by discussion but consulted a third review author (LLC) when they could not reach agreement. We contacted study authors for clarification when necessary.
We listed excluded studies and documented the primary reasons for exclusion.
Data extraction and management
Two review authors (GO and GD or LK) independently extracted data from published and unpublished reports using a standardised form. LLC piloted this data extraction form on a set of included trials and resolved any disagreements between the two review authors who extracted the data. We extracted the following data for each study.
Data publication characteristics.
Study design.
Inclusion and exclusion criteria.
Characteristics of the included population.
Details of interventions.
Number of randomised participants per group.
Number and reasons for losses to follow‐up.
For each outcome, results per group (intention‐to‐treat (ITT) and per protocol).
Risk of bias across six specific domains, based on the Cochrane ’Risk of bias’ assessment tool (Higgins 2011).
One review author (GO) checked and entered data into the Cochrane RevMan software to populate the Characteristics of included studies tables (RevMan 2014). We contacted the authors of these trials to request missing data when required.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to assess the risk of bias. Two review authors (GO and GD or LK) independently assessed the risk of bias for each study. We resolved disagreements between two review authors through discussion with a third review author (LLC). We graded each of the following domains as 'low', 'high', or 'unclear' and according to the following general principles (Section 8.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Selection bias
Was the allocation sequence adequately generated? We considered randomisation as adequate if the allocation sequence was generated from a table of random numbers or by computer. We considered randomisation as inadequate if sequences could be related to prognosis, and we considered it unclear if the paper stated that the trial was randomised but did not describe the method.
Was allocation adequately concealed? We deemed allocation concealment as adequate if the report stated that it was undertaken by means of sequentially pre‐numbered, sealed opaque envelopes or by a centralised system. We considered a double‐blind double‐dummy process as at low risk of bias even if the method of allocation concealment was not described.
Performance and detection bias
Was knowledge of the allocated intervention adequately prevented during the study? We evaluated the risk of bias separately for personnel and participants (performance bias) and for outcome assessors (detection bias).
Attrition bias
Were incomplete outcome data adequately addressed? We examined if there was imbalance across intervention groups in numbers or reasons for missing data, type of measure undertaken to handle missing data, and whether the analysis was carried out on an intention‐to‐treat basis. We assessed the use of strategies to handle missing data.
Reporting bias
Are reports of the study free of the suggestion of selective outcome reporting? We evaluated if each outcome was measured, analysed, and reported. We compared outcomes specified in study protocols (if available on the FDA website (www.fda.gov) or ClinicalTrials.gov)) and in the materials and methods section of the publication with outcomes presented in the results section (Ioannidis 2007).
We did not anticipate any other specific risk of bias; hence, we did not assess the domain of 'other sources of bias'.
Measures of treatment effect
We extracted numbers of events and non‐events in each study. We defined an event as a severity assessment (e.g. predefined disease severity score). For each pair‐wise comparison and each dichotomous outcome, we used risk ratios (RRs) with 95% confidence intervals (CIs) as a measure of treatment effect.
Unit of analysis issues
In cases of cluster‐randomised studies or individually randomised trials with clustering, if the data are available, we plan to extract risk ratios (RRs) with their 95% confidence intervals (CIs) accounting for the cluster design (i.e. we plan to use information based on a 'multi‐level model' or a 'variance components analysis', or we may use 'generalised estimating equations') (Section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). If the data are not available, we plan to conduct the analysis at the same level as the allocation, using a summary measurement from each cluster and reporting data separately (Section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
In the case of trials with multiple intervention groups, we plan to divide the trial into pair‐wise comparisons (that is A vs control, B vs control, A vs B) and to conduct a meta‐analysis for each comparison so we will not include a group of participants twice in the same meta‐analysis.
In case of trials with a within‐participant design, we aimed to take into account the within‐participant variability. When the P value had been computed, we reconstructed the paired data table to calculate the risk ratio and the confidence interval (Hirji 2011). When the P value had not been computed, we described the results without a P value or 95% CI.
When results are estimated for individual studies with low numbers of outcome events (< 10 in total), or where the total sample size is fewer than 30 participants and a risk ratio is used, we will report the proportion of events in each treatment group together with a P value from Fisher's exact test.
For cross‐over trials, we included only data from the first period for analysis.
Dealing with missing data
We performed an evaluation of the number of randomised and analysed participants. When required, we requested missing data (numbers of events and numbers of participants for important dichotomous clinical outcomes) from trial authors or sponsors by email. For the main analysis, we assumed that any participant with missing outcome data has experienced treatment failure, whatever the group. We planned to also synthesise data as analysed in each trial (complete cases); however, considering the few meta‐analysis possible we did not perform this.
Assessment of heterogeneity
We assessed statistical heterogeneity by visually inspecting the forest plots and by calculating the Q and I² statistics. We will interpret the I² statistic value according to the following thresholds (Higgins 2008; Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
0% to 40% might not be important.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100% represents considerable heterogeneity.
We undertook meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (Section 9.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Assessment of reporting biases
To address publication bias, we planned to draw contour‐enhanced funnel plots for each meta‐analysis if 10 or more studies had contributed data. However, due to the low number of studies in each meta‐analyses, we were not able to do this.
Data synthesis
For each pair‐wise comparison and each dichotomous outcome, we presented results as risk ratios (RRs) with 95% confidence intervals (CIs) as a measure of treatment effect. We performed pair‐wise meta‐analyses for all outcomes and comparisons, provided at least two studies were available, using a random‐effects model. If meta‐analysis was not appropriate, we used a narrative synthesis. We assessed heterogeneity using Cochran's Q test (Cochran 1950).
Subgroup analysis and investigation of heterogeneity
As we identified insufficient trials, we could not investigate the influence of doses, the therapeutic schemes, the duration of the condition, the weight of participants, and the presence or absence of psoriasis in sites other than palms and soles via meta‐regression or subgroup analyses.
Sensitivity analysis
Because of insufficient data, we did not perform sensitivity analyses to assess adequate and inadequate randomisation.
Results
Description of studies
Results of the search
The Electronic searches yielded 640 records, and we identified an additional 17 records through trial registry searches and screening of conference proceedings. After removing duplicates, we had a total of 401 unique records.
We excluded 331 records based on titles and/or abstracts. We examined the full texts of the remaining 70 records: 24 did not meet the inclusion criteria and we excluded them (see Characteristics of excluded studies). Four trials were classified as awaiting classification (see Characteristics of studies awaiting classification), and we identified two records related to ongoing trials (see Characteristics of ongoing studies). The remaining 40 references reported the 37 included studies (see Characteristics of included studies).
Please see Figure 1 for our study flow diagram.
1.
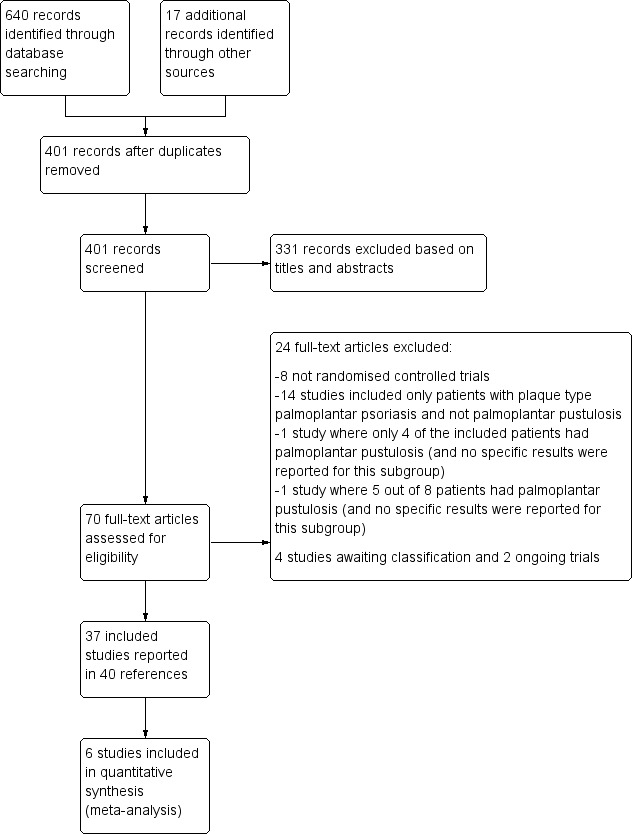
Study flow diagram.
Included studies
We included a total of 37 studies. Twenty‐seven were published in 2006 or earlier.
Design
A total of 16 trials, in two parallel groups, compared an active treatment to placebo (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Erkko 1998; Fairris 1984; Foged 1983; Jansen 1979; Lassus 1983; Reich 2016; Reitamo 1993; Rodriguez 2000; Schroder 1989; Terui 2018; Umezawa 2016; White 1985; White 1986). Two trials had a parallel‐group design, with both arms comparing active treatment (Fredriksson 1978; Lassus 1988). One trial used a four‐arm parallel‐group design (Lassus 1985), and two used a three‐arm parallel‐group design (Mrowietz 2019; NCT02641730 Guselkumab). A total of six trials used a cross‐over design (Hattel 1974; Nielsen 1995; Thestrup‐Pedersen 1984; Thomsen 1973; Thune 1982; Ward 1976), and eight were within‐patient trials (Cazzaniga 2014; Kragballe 1991; Layton 1991; Lindelof 1990; Muro 2016; Murray 1980; Rosen 1987; Su 2017).
Fourteen trials were multi‐centre trials (2 to 61 centres per trial, mainly in Europe) (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Cazzaniga 2014; Erkko 1998; Foged 1983; Mrowietz 2019; NCT02641730 Guselkumab; Reich 2016; Schroder 1989; Su 2017; Terui 2018; Umezawa 2016; Ward 1976), seven were single‐centre trials (Hattel 1974; Lawrence 1984; Lindelof 1990; Muro 2016; Murray 1980; Rodriguez 2000; White 1986), and the number of centres was unspecified in 16 trials.
Trial settings
Thirteen studies were conducted in one or multiple hospitals in a variety of countries: United Kingdom, Italy, Finland, Sweden, Denmark, Japan, Spain, and China (Bhushan 2001; Cazzaniga 2014; Erkko 1998; Hattel 1974; Lawrence 1984; Lindelof 1990; Muro 2016; Murray 1980; Rodriguez 2000; Su 2017; Terui 2018; Ward 1976; White 1986); in the community (clinics) in four trials, conducted in Canada, France, Germany, the Netherlands, and the United Kingdom (Bissonnette 2008; Bissonnette 2014; Schroder 1989; Reich 2016); and in both hospitals and clinics in one study, conducted in Japan (Umezawa 2016). No details were provided for the remaining studies.
Funding
Eighteen studies declared pharmaceutical company funding (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Cazzaniga 2014; Erkko 1998; Hattel 1974; Lawrence 1984; Mrowietz 2019; NCT02641730 Guselkumab; Reich 2016; Reitamo 1993; Rosen 1987; Su 2017; Terui 2018; Thune 1982; Umezawa 2016; White 1985; White 1986).
Participants
We included 37 studies with 1663 participants.
Mean sample size per study was 45 (ranging from 6 to 237 participants). The mean age of participants across studies was 50 years (ranging from 34 to 63 years) (this information was available for only 24 trials) (Bissonnette 2014; Erkko 1998; Fredriksson 1978; Jansen 1979; Kragballe 1991; Lassus 1985; Lassus 1988; Lawrence 1984; Matsunami 1990; Mrowietz 2019; Muro 2016; Murray 1980; NCT02641730 Guselkumab; Nielsen 1995; Reich 2016; Reitamo 1993; Rodriguez 2000; Rosen 1987; Su 2017; Thomsen 1973; Umezawa 2016; Ward 1976; White 1985; White 1986). All studies included participants of both sexes, with males representing 24% of the participants (n = 413) across studies (this information is available for only 30 trials).
In 29 trials, inclusion criteria stated that participants had palmoplantar pustulosis; in six trials, they had palmoplantar pustular psoriasis (Bhushan 2001; Lawrence 1984; Mrowietz 2019; Nielsen 1995; White 1985; White 1986); and in two trials, they had palmoplantar pustular psoriasis or palmoplantar pustulosis (Bissonnette 2014; Kragballe 1991). There was no clear clinical definition to distinguish palmoplantar pustular psoriasis from palmoplantar pustulosis, except in Bissonnette 2014. The proportion of participants having psoriatic lesions elsewhere was specified in 10 trials (n = 108/466) (Bhushan 2001; Bissonnette 2008; Hattel 1974; Lawrence 1984; Mrowietz 2019; Rosen 1987; Thestrup‐Pedersen 1984; Thomsen 1973; Thune 1982; White 1986), varying from 0% in Hattel 1974 to 53% in Bissonnette 2008.
Duration of the condition in participants at baseline was defined as mean duration of 6.4 years (ranging from 2 to 16 years) (this information was available for only 19 trials (Erkko 1998; Fredriksson 1978; Jansen 1979; Kragballe 1991; Lassus 1985; Lawrence 1984; Matsunami 1990; Mrowietz 2019; Muro 2016; Murray 1980; Reitamo 1993; Rodriguez 2000; Rosen 1987; Su 2017; Terui 2018; Thomsen 1973; Thune 1982; White 1985; White 1986)), or as median duration of the condition of three years (ranging from 3 to 10 years) (this information was available for only four trials (Bhushan 2001; Cazzaniga 2014; Foged 1983; Lindelof 1990)).
Baseline severity was reported by different scores that were detailed in each of the trials. No standardised score was used across studies.
Both palms and soles were affected in 253 participants (57%) (Fairris 1984; Foged 1983; Hattel 1974; Kragballe 1991; Lawrence 1984; Lindelof 1990; Matsunami 1990; Murray 1980; Nielsen 1995; Reitamo 1993; Rodriguez 2000; Thomsen 1973; Thune 1982; Ward 1976), whereas palms were exclusively affected in 24 participants (5%) (Fairris 1984; Foged 1983; Kragballe 1991; Muro 2016; Murray 1980; Rosen 1987; Thune 1982; Ward 1976), and soles were exclusively affected in 166 participants (38%) (Cazzaniga 2014; Fairris 1984; Foged 1983; Kragballe 1991; Lawrence 1984; Lindelof 1990; Muro 2016; Murray 1980; Nielsen 1995; Reitamo 1993; Rodriguez 2000; Rosen 1987; Thune 1982; Ward 1976). The number of participants having palmoplantar pustulosis on the palms, the soles, or both, was not specified (or was not clearly specified) in 20 trials (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Erkko 1998; Fredriksson 1978; Jansen 1979; Lassus 1983; Lassus 1985; Lassus 1988; Layton 1991; Mrowietz 2019; NCT02641730 Guselkumab; Reich 2016; Schroder 1989; Su 2017; Terui 2018; Thestrup‐Pedersen 1984; Umezawa 2016; White 1985; White 1986).
Interventions
Most interventions were compared against placebo; other comparators were phototherapy, no treatment, etretinate, and cotton fabric socks. Included trials evaluated interventions given over a period of 8 to 24 weeks from baseline to end of treatment (mean 11 weeks), except for one study which assessed the effects of long‐term treatment on one outcome.
Topical treatment
Dermocorticoids
Triamcinolone acetonide versus clobetasol cream
One study compared occlusive dressing plus triamcinolone acetonide 0.1% cream every third day versus clobetasol 0.05% cream twice per day for four weeks (Kragballe 1991).
Topical vitamin D
Vitamin D versus placebo
Two studies compared topical vitamin D (Oxarol ointment 25 microg/g) once daily versus no treatment (Muro 2016) or twice daily or placebo for eight weeks (Umezawa 2016).
Phototherapy
Oral PUVA therapy versus placebo
One study compared oral PUVA therapy (oral 8‐methoxypsoralen (8‐MOP) two hours before UVA irradiation (four times per week) for 30 treatments) versus no treatment (Murray 1980).
Local PUVA therapy versus placebo
One study compared local PUVA therapy (0.75% 8‐methoxypsoralen in hydrophilic water/oil emulsion and UVA phototherapy three times per week) versus placebo for eight weeks (Layton 1991).
Local PUVA therapy versus bath PUVA therapy versus oral PUVA therapy versus etretinate
Lassus 1985 compared four treatments: local PUVA therapy (local methoxsalen 1% one hour before UVA irradiation), bath PUVA therapy (trioxsalen bath (0.33 mg per one litre of water) 15 minutes before UVA irradiation), oral PUVA therapy (oral methoxsalen (0.6 mg/kg) two hours before irradiation with UVA), and etretinate (0.9 to 1 mg/kg/d for two weeks, then 0.6 to 0.7 mg/kg/d for 12 weeks).
UVA1 versus narrowband UVB
One study compared UVA1 (80 J/cm², three times weekly for up to 30 sessions) versus narrowband UVB (the initial dose was 0.3 J/cm², and doses were increased by 0.1 J/cm² every two weeks to a maximum dose of 0.7 J/cm², three times weekly for up to 30 sessions) (Su 2017).
PUVA versus no treatment
One study compared short‐term PUVA therapy (8‐methoxypsoralen (Puvamet) 0.4 to 0.6 mg per kg body weight, followed by an irradiation dose of on average 2.25 J/cm², twice a week for three weeks) versus no treatment with topical clobetasol propionate as a co‐intervention (Nielsen 1995).
Systemic treatment
Classical treatment for psoriasis (retinoids, ciclosporin, biologics, antibiotics, other treatments)
Retinoids (etretinate, acitretin, alitretinoin, and liarozole)
Four different retinoids are examined in the included studies.
Etretinate (also known as Tigason and oral RO 10‐9359).
Acitretin (RO 10‐1670).
Alitretinoin.
Liarozol.
Etretinate versus placebo
Eight studies compared oral etretinate versus placebo (1 mg/kg/d for 10 weeks (White 1985), 30 mg/d for 12 weeks (White 1986), 1 mg/kg once daily for 8 weeks (Foged 1983), 1 mg/kg/d for 20 weeks (Lawrence 1984), 25 to 100 mg/d (depending on tolerance) for four months (Jansen 1979), 25 mg thrice daily and reduced according to efficacy for 12 weeks (Thune 1982), 0.14 to 0.38 mg/kg/d for six months (Lassus 1983)) or no treatment (1 mg/kg for four weeks, then 0.5 mg/kg for eight weeks (Matsunami 1990)).
Etretinate 25 mg versus etretinate 200 mg
One study compared oral RO 10‐9359 25 mg thrice per day versus oral RO 10‐9359 200 mg twice per week for eight weeks (Fredriksson 1978).
Acitretin versus placebo
One study compared acitretin (50 mg once per day) versus placebo for four weeks (Schroder 1989).
Alitretinoin versus placebo
One study compared alitretinoin (30 mg once daily) versus placebo for 24 weeks (Reich 2016).
Acitretin versus etretinate
One study compared acitretin (3 capsules of 10 mg each, once per day) versus etretinate (3 capsules of 10 mg each, once per day) for 12 weeks (Lassus 1988).
Liarozole versus placebo
One study compared liarozole (75 mg twice daily) versus placebo for 12 weeks (Bhushan 2001).
Etretinate versus PUVA therapy versus etretinate + PUVA therapy versus placebo
One study compared the four treatments: oral etretinate twice per day (0.6 mg/kg/d), PUVA therapy three times per week (methoxsalen one and a half hours before UVA at a dose of 20 kJ/m² increased at each treatment session by 10 kJ/m², except between 40 and 60 kJ/m², where the light dose was increased by 5 kJ/m²), etretinate plus PUVA therapy, and placebo for 14 weeks (Rosen 1987).
Ciclosporin
Ciclosporin versus placebo
Two studies compared ciclosporin (2.5 mg/kg per day for four weeks (Reitamo 1993), and 1 mg/kg/d twice daily for one month (Erkko 1998)) versus placebo.
Biologics
Etanercept versus placebo
One study compared etanercept (50 mg subcutaneously twice per week) versus placebo for three months (Bissonnette 2008).
Ustekinumab versus placebo
One study compared ustekinumab (45 mg or 90 mg (based on weight)) versus placebo for 16 weeks (Bissonnette 2014).
Secukinumab versus placebo
One study compared secukinumab 300 mg versus secukinumab 150 mg (at weeks 1, 2, 3, and 4, then at four‐week intervals) versus placebo for 16 weeks (Mrowietz 2019).
Guselkumab versus placebo
One study compared guselkumab 200 mg subcutaneously (given on week 0 and week 4) versus placebo for 24 weeks (Terui 2018), and another study evaluated guselkumab 200 mg subcutaneously (given on weeks 0, 4, and 12) versus guselkumab 100 mg subcutaneously (given on weeks 0, 4, and 12) versus placebo for 16 weeks (NCT02641730 Guselkumab).
Antibiotics
Tetracycline versus placebo
One study compared tetracycline (250 mg twice daily) versus placebo for 12 weeks (Thomsen 1973).
Clomocycline (a tetracycline) versus placebo
One study compared clomocycline (170 mg thrice daily for two weeks, then twice a day for 10 weeks) versus placebo (Ward 1976).
Other treatments
Hydroxyurea versus placebo
One study compared hydroxyurea (0.5 g thrice daily) versus placebo for three weeks (Hattel 1974).
Colchicine versus placebo
One study compared colchicine (0.5 mg (three to four times per day according to weight)) versus placebo for eight weeks (Thestrup‐Pedersen 1984).
Grenz ray therapy versus placebo
One study compared grenz ray therapy (4 Gy once per week) versus placebo for six weeks (Lindelof 1990).
Fluorine‐synthetic fibre socks versus cotton fabric socks
One study compared socks made of fluorine‐synthetic fibre versus socks made of cotton fabric for four weeks (Cazzaniga 2014).
Aluminium chloride versus placebo
One study compared 20% aqueous solution of aluminium chloride hexahydrate versus placebo for five months (Rodriguez 2000).
Superficial X‐ray therapy versus placebo
One study compared superficial X‐ray therapy versus placebo for 18 weeks (Fairris 1984).
Outcome measures
Fifteen out of the 37 included studies reported our first primary outcome 'Proportion of participants cleared or almost cleared' (Bissonnette 2008; Cazzaniga 2014; Jansen 1979; Kragballe 1991; Lassus 1985; Lawrence 1984; Layton 1991; Murray 1980; NCT02641730 Guselkumab; Su 2017; Terui 2018; Thestrup‐Pedersen 1984; Umezawa 2016; White 1985; White 1986), and only three reported our second primary outcome 'Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study' (Mrowietz 2019; NCT02641730 Guselkumab; Terui 2018).
One study addressed our secondary outcome 'Proportion of participants with at least 50% improvement in their quality of life' (Terui 2018), whereas five reported the outcome 'Proportion of participants achieving a 50% reduction in disease severity' (Bissonnette 2014; Mrowietz 2019; Murray 1980; Reich 2016; Terui 2018). One study addressed the outcome 'Proportion of participants without relapse in the long term' (Lassus 1983); 15 studies reported the outcome 'Proportion of participants with adverse effects' (Kragballe 1991; Lassus 1985; Lawrence 1984; Layton 1991; Muro 2016; Murray 1980; Reich 2016; Reitamo 1993; Su 2017; Thestrup‐Pedersen 1984; Thomsen 1973; Umezawa 2016; Ward 1976; White 1985; White 1986); and the outcome 'Ease of compliance to an intervention or a treatment' was never reported.
Six trials include the percentage of change in the Palmo‐Plantar Pustular Area and Severity Index (PPPASI) scale (modified Psoriasis Area and Severity Index (PASI) calculated by scoring signs of PPP (erythema, pustules, desquamation) on a four‐point scale and the area involvement on a six‐point scale separately for each palm and sole, with scores ranging from 0 to 72) as an outcome (Bissonnette 2008; Bissonnette 2014; NCT02641730 Guselkumab; Reich 2016; Su 2017; Terui 2018); 10 studies include the reduction in the number of fresh pustules (Bhushan 2001; Erkko 1998; Fredriksson 1978; Lassus 1985; Lassus 1988; Layton 1991; Reich 2016; Reitamo 1993; Schroder 1989; White 1986), and 23 studies report the change in a pre‐fixed severity index (Bhushan 2001; Cazzaniga 2014; Erkko 1998; Fairris 1984; Foged 1983; Jansen 1979; Kragballe 1991; Lassus 1985; Lassus 1988; Layton 1991; Lindelof 1990; Matsunami 1990; Murray 1980; Nielsen 1995; Reitamo 1993; Rosen 1987; Schroder 1989; Thestrup‐Pedersen 1984; Thune 1982; Umezawa 2016; Ward 1976; White 1985; White 1986). The pre‐fixed severity index was however different among trials and was not based on a validated score.
All outcomes were clinician‐assessed except quality of life, which was assessed by study participants. Outcomes were always assessed at the end of the treatment period (for our key results, this ranged from 8 to 24 weeks). We could not assess long‐term results as we deemed all treatment durations to be short term, except for one study which assessed one outcome in the long‐term.
We emailed the authors of trial reports published in or after 2007 to request information regarding the primary outcomes of interest in our review. We have summarised the responses of study authors in Table 14.
2. Responses of contacted authors.
| Author | Requested information | Contacted | Reply |
| Dr. Luigi Naldi | “Randomized, within‐patient, clinical trial comparing fluorine‐synthetic fiber socks with standard cotton socks in improving plantar pustulosis”, published in Dermatology in 2014, vol 228, N°2 Outcome: proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity Outcome: proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study |
1 October 2017 | None of the treated sides cleared in the included patients |
| Pr. Diamant Thaci | “A phase IIIb, multicentre, randomised, double‐blind, vehicle‐controlled study of the efficacy and safety of adalimumab with and without calcipotriol ⁄betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study”, published in British Journal of Dermatology in 2010, vol 62 Info requested: Did the included patients have palmoplantar plaque psoriasis, palmoplantar pustular psoriasis, or a combination of both? |
1 October 2017 | All included patients had plaque‐type psoriasis; thus the study was excluded |
| Dr. Bissonnette | “Etanercept in the treatment of palmoplantar pustulosis”, published in Journal of Drugs in Dermatology in 2008, vol 7, N°10 Outcome: proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity Outcome: proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study “Increased expression of IL‐17A and limited involvement of IL‐23 in patients with palmo‐plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial”, published in Journal of the European Academy of Dermatology and Venereology in 2013, vol 28, N°10 Outcome: proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity Outcome: proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study |
1 October 2017 11 October 2017 |
One patient (treated with etanercept) achieved PPPASI > 75% No serious or severe adverse effects that caused withdrawal from the study |
| Dr. Reich | “Oral alitretinoin treatment in patients with palmoplantar pustulosis inadequately responding to standard topical treatment: a randomised phase II study”, published in British Journal of Dermatology in 2016, vol 174 Outcome: proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity Outcome: proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study |
1 October 2017 11 October 2017 25 October 2017 |
No reply |
| Dr Wilson | “APRICOT ‐ Anakinra for pustular psoriasis”, Trial Nb: ISRCTN13127147 Info requested: status of the study and the results, mainly the proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity as well as the proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study |
1 October 2017 11 October 2017 25 October 2017 |
The trial is still in progress at the moment, so study authors had no results to share |
| Dr. Petzelbauer | "Comparison of fumaric acid ester‐PUVA versus PUVA‐etretinate in palmoplantar pustolosis," unpublished study EudraCT 2006‐004519‐23, registered in 2009 (EudraCT Number: 2006‐004519‐23. Sponsor Protocol Number: 08/08) Info requested: any unpublished results |
12 June 2018 | No reply |
Excluded studies
We excluded 24 studies from the review: eight studies for not being RCTs (Aso K 1983; Carr 2008; Dupre 1973; Fritsch 1978; Gjertsen 1980; Gupta 2011; Yaniv 2012; Zhang Jun 2007); 14 studies as they addressed only patients with plaque‐type palmoplantar psoriasis ‐ not palmoplantar pustulosis or palmoplantar pustular psoriasis (Cassano 2010; Duweb 2001; Grundmann‐Kollmann 1999; Janagond 2013; Khandpur 2011; Kumar 1997; Mehta 2011; Neumann 2006; Orfanos 1978; Papp 2012; Rosen 1988; Schiener 2005; Sezer 2007; Thaci 2010); and one study as only four of the included patients had palmoplantar pustulosis (and no specific results were reported for this subgroup (Engin 2005)); we excluded another study as only five out of eight participants had palmoplantar pustulosis (and no specific results were reported for the subgroup of palmoplantar pustulosis) (Hofer 2006).
Studies awaiting classification
Four studies are awaiting classification: two studies were registered as completed but still were not published (EudraCT 2006‐004519‐23; NCT03135548 BI 655130), and it is not clear whether two other studies are RCTs as study details are reported only in an abstract, which provides limited information (Fenton 1983; Mann 1982).
Ongoing studies
Two trials are ongoing (ISRCTN13127147 APRICOT; NCT03633396).
Risk of bias in included studies
We report these assessments in the ’Risk of bias’ table associated with each study, as well as in the 'Risk of bias' summary (Figure 2; Figure 3). We assessed only two studies as being at low risk of bias in all domains (Cazzaniga 2014; Reich 2016).
2.
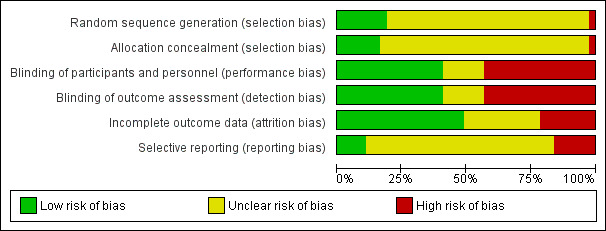
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
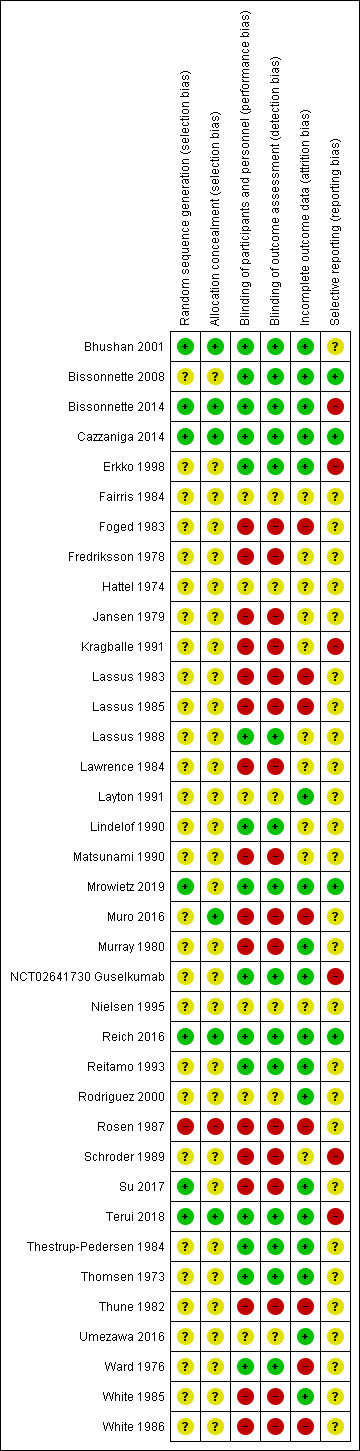
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged seven studies to be at low risk for this domain (Bhushan 2001; Bissonnette 2014; Cazzaniga 2014; Mrowietz 2019; Reich 2016; Su 2017; Terui 2018). All clearly specified the method of sequence generation. For example, "Centralised telephone randomisation procedures were adopted" (Cazzaniga 2014). We assessed one study as high risk (Rosen 1987), and we judged the remaining 29 studies as having unclear risk.
Allocation concealment
We assessed six studies as being at low risk with regard to allocation concealment, as they provided a clear description of their allocation concealment method (Bhushan 2001; Bissonnette 2014; Cazzaniga 2014; Muro 2016; Reich 2016; Terui 2018). We assessed one study as high risk as "The patients were allocated to treatment groups according to year of birth (even or odd)" (Rosen 1987). We assessed the risk to be unclear for 30 studies as the method to guarantee allocation concealment was not described.
Blinding
Performance bias
We assessed 15 studies as being at low risk of bias for this domain (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Cazzaniga 2014; Erkko 1998; Lassus 1988; Lindelof 1990; Mrowietz 2019; NCT02641730 Guselkumab; Reich 2016; Reitamo 1993; Terui 2018; Thestrup‐Pedersen 1984; Thomsen 1973; Ward 1976), as they are double‐blind placebo‐controlled trials and we consider blinding at low risk for study drug versus placebo with no obvious systematic clinical adverse events or known specific taste of experimental drug. We assessed 16 studies as being at high risk because blinding was impossible due to the medication's side effects (Foged 1983; Jansen 1979; Lassus 1983; Lawrence 1984; Matsunami 1990; Muro 2016; Rosen 1987; Schroder 1989; Thune 1982; White 1985; White 1986), or because there was no mention of blinding (Fredriksson 1978; Lassus 1985; Su 2017), or because blinding was impossible due to the study's design (Kragballe 1991; Murray 1980). We assessed six studies as being at unclear risk because the method of blinding was not described (Hattel 1974; Rodriguez 2000; Umezawa 2016; Layton 1991; Fairris 1984), or because the study was single‐blinded (Nielsen 1995).
Detection bias
We assessed 15 studies as being at low risk of bias for this domain as the outcome assessors were blinded (Bhushan 2001; Bissonnette 2008; Bissonnette 2014; Cazzaniga 2014; Erkko 1998; Lassus 1988; Lindelof 1990; Mrowietz 2019; NCT02641730 Guselkumab; Reich 2016; Reitamo 1993; Terui 2018; Thestrup‐Pedersen 1984; Thomsen 1973; Ward 1976). We assessed 16 studies as being at high risk because blinding was impossible due to the medication's side effects (Foged 1983; Jansen 1979; Lassus 1983; Lawrence 1984; Matsunami 1990; Rosen 1987; Schroder 1989; Thune 1982; White 1985; White 1986), or because there was no mention of blinding (Fredriksson 1978; Kragballe 1991; Lassus 1985; Muro 2016; Murray 1980; Su 2017). We assessed six studies as being at unclear risk (Fairris 1984; Hattel 1974; Layton 1991; Nielsen 1995; Rodriguez 2000; Umezawa 2016).
Incomplete outcome data
We assessed 18 studies as being at low risk of attrition bias because they accounted for all participants in the analysis (Bissonnette 2014; Cazzaniga 2014; Mrowietz 2019; Reich 2016; Terui 2018; Thomsen 1973; NCT02641730 Guselkumab), there were no missing data (no dropouts) (Bhushan 2001; Bissonnette 2008; Erkko 1998; Layton 1991; Murray 1980; Rodriguez 2000), or the number of participants unaccounted for was very low (Reitamo 1993; Su 2017; Thestrup‐Pedersen 1984; Umezawa 2016; White 1985).
We considered eight studies at high risk as more than 10% of participants dropped out and they showed no precision on how they did deal with the missing data (Foged 1983; Lassus 1983; Lassus 1985; Muro 2016; Rosen 1987; Thune 1982; Ward 1976; White 1986).
The risk was unclear for the remaining 11 studies as there was no clear mention of the missing data.
Selective reporting
We assessed four studies as being at low risk of selective reporting (Bissonnette 2008; Cazzaniga 2014; Mrowietz 2019; Reich 2016), as the protocols are available and all of the pre‐specified outcomes of interest in the review were reported in the pre‐specified way.
We considered six studies to be at high risk either because not all of the study’s pre‐specified outcomes in the protocol were reported in the pre‐specified way (Bissonnette 2014; NCT02641730 Guselkumab; Terui 2018), or because not all pre‐specified outcomes are reported and we found no protocol to guarantee that all planned outcomes are presented in the results (Erkko 1998; Kragballe 1991; Schroder 1989). We considered this risk as unclear in 27 studies as we found no protocol to guarantee that all planned outcomes were presented in the results.
Other potential sources of bias
We did not anticipate any other specific risk of bias; hence, we did not assess this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12
Fourteen studies provided no useful data and did not contribute further to the results of this review (Table 15). The main reasons for considering these studies as non‐usable were the lack of numerical results for outcomes addressed, limited available data, and non‐pertinent outcomes (e.g. decrease in the number of fresh pustules).
3. No contributive studies.
| Study ID | Interventions and comparisons | Number randomised | Comments |
| Fairris 1984 | Superficial X‐ray therapy vs placebo | 6 | Lack of numerical results |
| Hattel 1974 | Hydroxyurea vs placebo | 13 | Lack of numerical results |
| Fredriksson 1978 | Oral RO 10‐9359 (25 mg thrice per day) vs oral RO 10‐9359 (200 mg twice per week) | 30 | Non‐pertinent outcome (decreased number of pustules) |
| Thune 1982 | Tigason vs placebo | 42 | Lack of numerical results for addressed outcomes (for remission, we have results for Tigason group but not for placebo group) |
| Foged 1983 | Etretinate vs placebo | 50 | Lack of numerical results |
| Schroder 1989 | Etretin vs placebo | 30 | Lack of numerical results and non‐pertinent outcome (decreased number of pustules) |
| Lassus 1988 | Acitretin vs etretinate | 60 | Lack of numerical results |
| Lindelof 1990 | Grenz rays vs placebo | 17 | Lack of numerical results |
| Matsunami 1990 | Etretinate vs no treatment | 20 | Lack of numerical results |
| Erkko 1998 | Ciclosporin vs placebo | 58 | Non‐pertinent primary outcome (reduction of 50% or more in the number of fresh pustules) and lack of numerical results for other outcomes |
| Bhushan 2001 | Liarozole vs placebo | 15 | Numerical results available only for patients achieving PPASI 75 (not 100% or 50%) |
| Nielsen 1995 | PUVA therapy vs placebo (with clobetasol as co‐intervention) | 22 | Cross‐over study results from first phase not reported separately |
| Rodriguez 2000 | Aluminium chloride hexahydrate vs placebo | 12 | Lack of numerical results |
| Rosen 1987 | Etretinate + PUVA therapy vs etretinate vs PUVA therapy vs placebo | 20 | Randomisation of participants, then of sides; number of randomised participants in UVA or no treatment was not available |
PPASI: Palmo‐Plantar Area and Severity Index.
PUVA: combination of psoralens and long‐wave ultraviolet radiation.
UVA: ultraviolet A.
Among the remaining 22 studies, we were able to pool the following.
Two studies comparing etretinate (aromatic retinoid ethyl ester (Ro 10‐9359) 25 to 100 mg depending on tolerance and etretinate (1 mg/kg/d)) versus placebo over a period of four months and 10 weeks, respectively, for the primary outcome 'proportion of participants cleared or almost cleared in the short term' (Jansen 1979; White 1985).
Two studies comparing tetracyclines (tetracycline 250 mg twice daily and clomocycline 170 mg thrice daily for two weeks, then twice a day for 10 weeks) versus placebo over a period of 12 weeks and three months, respectively, for the secondary outcome 'proportion of participants with adverse effects in the short term' (Thomsen 1973; Ward 1976).
Two studies comparing guselkumab at S0, S4, S12 versus placebo over a period of 16 weeks for the primary outcome 'proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at the short term' (NCT02641730 Guselkumab; Terui 2018).
We assessed outcomes at the end of the treatment period. We intended to evaluate all outcomes at two different timings:
induction phase: evaluation up to 24 weeks after randomisation (short term); and
maintenance phase: evaluation between 24 and 104 weeks after randomisation (long term).
Topical treatment
1. Triamcinolone acetonide cream with occlusive dressing versus clobetasol cream
One study compared triamcinolone acetonide 0.1% cream with occlusive dressing changed every third day versus clobetasol 0.05% cream twice per day in 19 patients over four weeks (left/right comparison, within‐patient study) (Table 1) (Kragballe 1991).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. pre‐defined disease severity score) at two timings: the short term and the long term
Thirteen out of 19 patients cleared in the triamcinolone acetonide 0.1% cream with occlusive dressing side compared with three out of 19 in the clobetasol side at week 4 (risk ratio (RR) 1.20, 95% confidence interval (CI) 0.72 to 2.00; P = 0.26) ‐ calculated using the methods described in Hirji 2011.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
No adverse events were reported in both groups.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
Study authors reported no adverse events and no skin atrophy in both groups. They reported loosening of the dressing (n = 2), hydrocolloid outside the dressing (n = 2), and sweating (n = 1) in the triamcinolone acetonide 0.1% cream with occlusive dressing side.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
2. Topical vitamin D versus placebo
Two studies compared topical vitamin D (maxacalcitol ointment) versus placebo in Umezawa 2016, or no treatment in Muro 2016, over eight weeks. One randomised participants (Umezawa 2016), and the other randomised the treated side (within‐patient study) (Muro 2016). In Muro 2016, on both sides, participants received betamethasone butyrate propionate ointment as a co‐intervention (Table 2).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was assessed in Umezawa 2016, where 16 out of 95 patients in the maxacalcitol group were markedly improved compared to two out of 93 in the placebo group at eight weeks (RR 7.83, 95% CI 1.85 to 33.12; Analysis 1.1). Information (i.e. the P value) needed to calculate the confidence interval was not available for Muro 2016. Combined therapy was reported as significantly superior to monotherapy for each of the assessed symptoms (erythema, pustules/vesicles, hyperkeratosis).
1.1. Analysis.
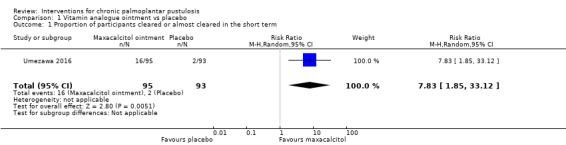
Comparison 1 Vitamin analogue ointment vs placebo, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
The incidence of adverse events was not different between two groups in Umezawa 2016 (RR 0.87, 95% CI 0.64 to 1.19; Analysis 1.2). Reported adverse events were mild local irritation, pruritus, and mild haematological or urinary test abnormalities. In Muro 2016, none of the participants in both groups reported any side effects.
1.2. Analysis.
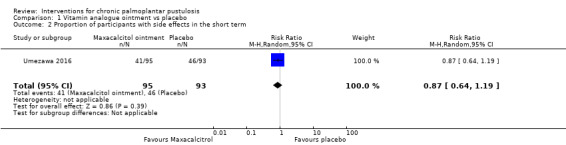
Comparison 1 Vitamin analogue ointment vs placebo, Outcome 2 Proportion of participants with side effects in the short term.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Phototherapy
3. Oral PUVA therapy or local PUVA therapy versus placebo or no treatment
One study compared UVA four times per week for 30 treatments on one side versus no treatment on the other side (within‐patient comparison) + oral psoralen in all participants (Murray 1980).
One study compared local PUVA therapy on one side versus placebo (excipient of psoralen and sham irradiation) three times per week on the other side, over a period of eight weeks (within‐patient comparison) (Table 3) (Layton 1991).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
Clearance was seen in 12 of 22 treated sides (psoralen + UVA) compared to no clearance in the no irradiated side (psoralen alone) (Murray 1980).
Clearance, according to a grade calculated for palmoplantar pustulosis, was not achieved in any palms or soles for the local PUVA therapy side or the placebo side (Layton 1991).
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants achieving a 50% reduction in disease severity in the short term
A 50% improvement in the visual analogue score used was achieved by 10 of 22 patients in the oral PUVA therapy group compared to 13 of 22 in the placebo group (Murray 1980). This outcome was not reported in Layton 1991.
Proportion of participants with adverse effects in the short term and in the long term
In Murray 1980, in the oral PUVA therapy group: one participant got burned, four got nausea, four had ankle swelling, and six got non‐purulent conjunctivitis, whereas in Layton 1991, in the local PUVA therapy group: four participants had blistering on the feet (three on the hands), three had pruritus on the feet (two on the hands), and three had erythema on the feet (two on the hands).
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
4. UVA1 versus narrowband UVB
One within‐participant study compared UVA1 versus narrowband UVB done three times per week for 30 treatments (total of 10 weeks of treatment) (Su 2017). Information (i.e. the P value) needed to calculate the confidence interval was not available (Table 4).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
Twenty‐two out of 33 sides were markedly improved in PPPASI score in the UVA1 group versus 11 of 33 narrowband UVB‐treated sides.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
In the UVA1 side: six participants had a burning sensation and two experienced hyperpigmentation. In the narrowband UVB‐treated side: nine participants had xerosis.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Oral retinoids
5. Etretinate versus placebo
Data were available for four studies comparing etretinate to placebo with no co‐intervention in both groups over a period of 10 weeks (1 mg/kg/d), 12 weeks (30 mg/d), four months (25 to 100 mg/d according to the individual participant's tolerance), and six months (0.14 to 0.38 mg/kg/d), respectively (Table 5) (Jansen 1979; Lassus 1983; White 1985; White 1986).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
Two studies ‐ Jansen 1979 and White 1985 ‐ were pooled: 7 of 20 participants in etretinate group had clearance (or almost) compared to 2 of 20 in the placebo group (RR 3.48, 95% CI 0.82 to 14.80; I² = 0; Analysis 3.1). White 1986 (20 participants) was not pooled with the previous studies as zero participants cleared in both groups. Lassus 1983 did not report this outcome.
3.1. Analysis.
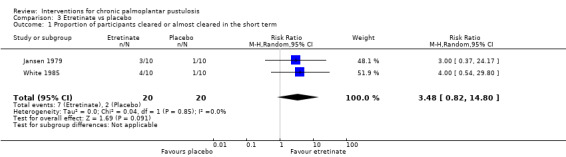
Comparison 3 Etretinate vs placebo, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants without relapse in the long term
In Lassus 1983, 40 participants received etretinate for 16 weeks, then responders (26 participants) were allocated to either etretinate or placebo. At six months, 7 of 11 participants in the etretinate group were in remission versus 4 of 15 in the placebo group (RR 2.39, 95% CI 0.92 to 6.17; Analysis 3.2).
3.2. Analysis.
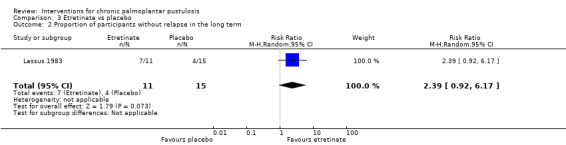
Comparison 3 Etretinate vs placebo, Outcome 2 Proportion of participants without relapse in the long term.
Proportion of participants with adverse effects in the short term and in the long term
In White 1985, side effects were reported (cheilitis, hair loss, and others), along with the number of participants having each side effect, but we lack the total number of participants who developed side effects in both groups.
In White 1986, 7 of 10 participants had side effects (four patients had cheilitis, two had facial dermatitis, and one developed some hair loss) versus 2 of 10 in the placebo group (cheilitis) (RR 3.50, 95% CI 0.95 to 12.90; Analysis 3.3). Fisher's exact test: P = 0.0698.
3.3. Analysis.
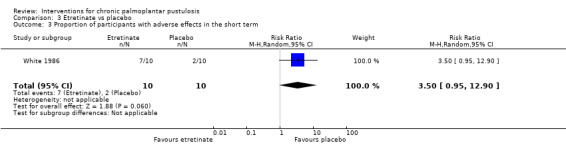
Comparison 3 Etretinate vs placebo, Outcome 3 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Ease of compliance to an intervention or a treatment.
6. Etretinate versus placebo with PUVA therapy
One study compared etretinate (1 mg/kg/d) versus placebo with PUVA therapy as co‐intervention in both groups over a period of 20 weeks (Table 6) (Lawrence 1984).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
A total of 10 of 10 participants cleared in the etretinate group and 5 of 10 in the placebo group (RR 1.91, 95% CI 1.04 to 3.50; Analysis 4.1). This study was not pooled with the previous studies because PUVA was a co‐intervention in both groups.
4.1. Analysis.
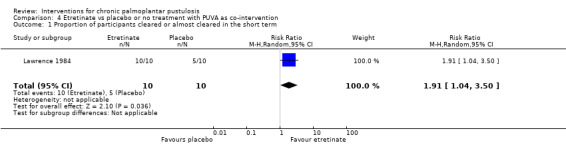
Comparison 4 Etretinate vs placebo or no treatment with PUVA as co‐intervention, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects
In Lawrence 1984, eight out of 10 participants in the etretinate group experienced side effects (six had cheilitis, four had hair loss, two had peeling of the palmoplantar skin, one had generalised peeling of the skin with pruritus, and one had dryness of the nasal mucosa) compared to zero out of 10 in the placebo group (RR 17.00, 95% CI 1.11 to 259.87; Analysis 4.2). Fisher's exact test: P = 0.0007.
4.2. Analysis.
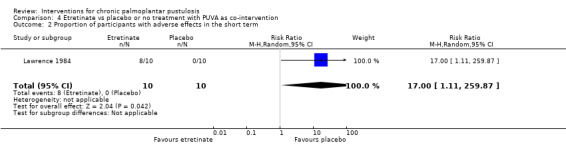
Comparison 4 Etretinate vs placebo or no treatment with PUVA as co‐intervention, Outcome 2 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term, Ease of compliance to an intervention or a treatment.
7. Alitretinoin versus placebo
One study compared alitretinoin 30 mg once daily versus placebo over a period of 24 weeks (Table 8) (Reich 2016).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was not reported.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants achieving a 50% reduction in disease severity in the short term
In the alitretinoin group, 11 of 24 patients achieved 50% reduction in disease severity compared to 6 of 9 in the placebo group (RR 0.69, 95% CI 0.36 to 1.30; Analysis 5.1).
5.1. Analysis.
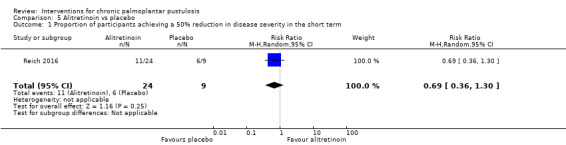
Comparison 5 Alitretinoin vs placebo, Outcome 1 Proportion of participants achieving a 50% reduction in disease severity in the short term.
Proportion of participants with adverse effects in the short term and in the long term
Eighteen out of 24 participants in the alitretinoin group reported side effects (headache, nasopharyngitis, cheilitis, nausea, arthralgia, hypercholesterolaemia) compared to eight out of nine participants in the placebo group (RR 0.84, 95% CI 0.61 to 1.17; Analysis 5.2).
5.2. Analysis.
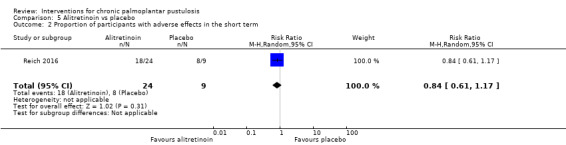
Comparison 5 Alitretinoin vs placebo, Outcome 2 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
8. Etretinate versus local PUVA therapy versus bath PUVA therapy versus oral PUVA therapy
One study compared etretinate (0.9 to 1 mg/kg two weeks, then 0.6 to 0.7 mg/kg) versus local, oral, and bath PUVA therapy over 12 weeks (Table 7) (Lassus 1985).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
Clearance was obtained in 14 out of 20 participants in the etretinate group compared to four out of 64 in the PUVA therapy group (local, bath, or oral psoralen) (RR 11.20, 95% CI 4.16 to 30.18; Analysis 6.1).
6.1. Analysis.
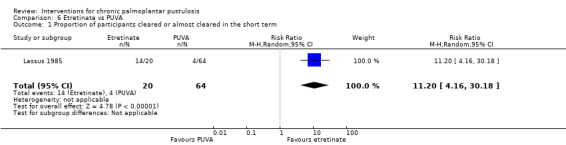
Comparison 6 Etretinate vs PUVA, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
In the etretinate group, two participants had severe hair loss and one had severe drying of the mucosa. One‐third of participants developed mild erythema and scaling of the healthy skin, and all participants had mild drying of the lips and nasal mucosa. In the oral PUVA therapy group, three participants had nausea and two had pruritus (RR 11.54, 95% CI 5.17 to 25.74; Analysis 6.2).
6.2. Analysis.
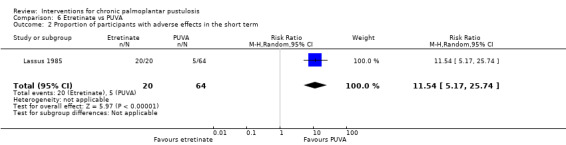
Comparison 6 Etretinate vs PUVA, Outcome 2 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Ciclosporine
9. Ciclosporin versus placebo
Data were available for one study that compared ciclosporin (2.5 mg/kg/d) versus placebo over a period of four weeks (Reitamo 1993).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was not reported.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
In Reitamo 1993, seven out of 20 participants in the ciclosporin group reported side effects compared to six out of 20 in the placebo group (RR 1.17, 95% CI 0.48 to 2.86; Analysis 7.1).
7.1. Analysis.
Comparison 7 Oral ciclosporin vs placebo, Outcome 1 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Biologic treatments
10. Etanercept versus placebo
One study compared etanercept 50 mg subcutaneously twice per week versus placebo over a period of three months (Table 9) (Bissonnette 2008).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
Clearance was obtained in one out of 10 participants in the etanercept group but in zero out of five in the placebo group (RR 1.64, 95% CI 0.08 to 34.28; Analysis 8.1). Fisher's exact test: P = 1.00. This information was provided by the authors of the paper upon our request.
8.1. Analysis.
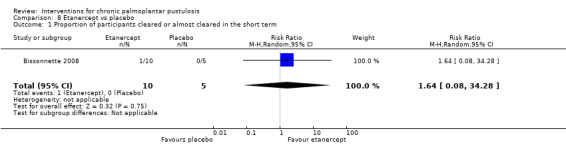
Comparison 8 Etanercept vs placebo, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
None of our secondary outcomes were reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Proportion of participants with adverse effects in the short term and in the long term, Ease of compliance to an intervention or a treatment.
11. Ustekinumab versus placebo
One study compared ustekinumab 45 mg or 90 mg (based on the weight) versus placebo over a period of 16 weeks (Table 10) (Bissonnette 2014).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was not reported.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants achieving a 50% reduction in disease severity in the short term
In the ustekinumab group, 2 of 15 participants had 50% reduction in disease severity (Palmo‐Plantar Pustular Area and Severity Index (PPPASI) 50) in the short term (16 weeks) compared to 5 of 18 in the placebo group (RR 0.48, 95% CI 0.11 to 2.13; Analysis 9.1). Fisher's exact test: P = 0.4134.
9.1. Analysis.
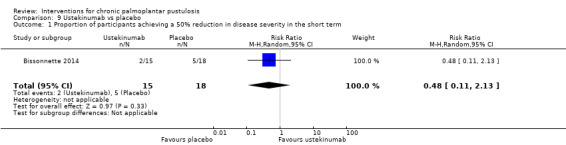
Comparison 9 Ustekinumab vs placebo, Outcome 1 Proportion of participants achieving a 50% reduction in disease severity in the short term.
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants without relapse in the long term, Proportion of participants with adverse effects in the short term and in the long term, Ease of compliance to an intervention or a treatment.
12. Guselkumab versus placebo
Two studies compared guselkumab 200 mg versus placebo over a period of 16 weeks (Terui 2018: one injection at 0 and 4 weeks; NCT02641730 Guselkumab: one injection at 0, 4, and 12 weeks) (Table 11).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
In the guselkumab 200 mg group, 7 of 77 participants achieved clear or almost clear status in the short term (physicians' global assessment (PGA) ≤ 1) (16 weeks) compared to 5 of 77 in the placebo group (2 studies; 154 participants; pooled RR 1.17, 95% CI 0.15 to 9.30; I² = 59%; Analysis 10.1).
10.1. Analysis.
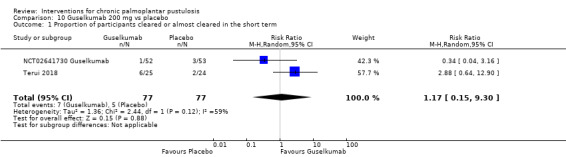
Comparison 10 Guselkumab 200 mg vs placebo, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
NCT02641730 Guselkumab also compared guselkumab 100 mg against placebo. In the guselkumab 100‐mg group, 4 of 54 participants achieved clear or almost clear status in the short term (PGA ≤ 1) (16 weeks) compared to 3 of 53 in the placebo group (RR 1.31, 95% CI 0.31 to 5.57; Analysis 11.1)
11.1. Analysis.
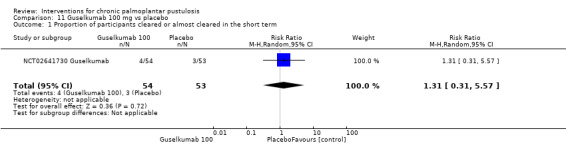
Comparison 11 Guselkumab 100 mg vs placebo, Outcome 1 Proportion of participants cleared or almost cleared in the short term.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was reported for one of the two studies (Terui 2018). In the guselkumab group, 3 of 25 participants had emergent serious adverse events (pyelonephritis and gastric cancer), and 1 withdrew because of urticaria compared to 1 of 24 (pustular psoriasis) in the placebo group (RR 2.88, 95% CI 0.32 to 25.80; Analysis 10.2).
10.2. Analysis.
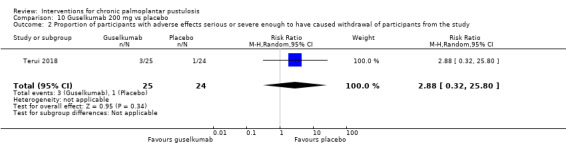
Comparison 10 Guselkumab 200 mg vs placebo, Outcome 2 Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study.
NCT02641730 Guselkumab reported adverse events occurring only in the period of 0 to 52 weeks, which included a non‐randomised period.
Secondary outcomes
Proportion of participants achieving a 50% reduction in disease severity in the short term
This outcome was reported for one of the two studies (Terui 2018). In the guselkumab 200‐mg group, 15 of 25 participants had a 50% reduction in disease severity (PPPASI 50) in the short term (16 weeks) compared to 5 of 24 in the placebo group (RR 2.88, 95% CI 1.24 to 6.69; Analysis 10.3).
10.3. Analysis.
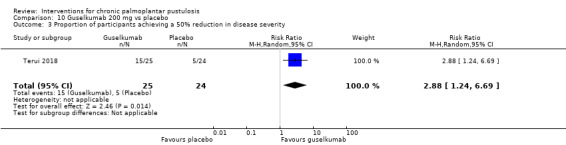
Comparison 10 Guselkumab 200 mg vs placebo, Outcome 3 Proportion of participants achieving a 50% reduction in disease severity.
Proportion of participants with at least 50% improvement in their quality of life (Dermatology Life Quality Index (DLQI))
Mean decrease from baseline in DLQI score (0 to 30; higher score indicates more severe disease) was ‐1.8 in the placebo group compared to 3.1 in the guselkumab group.
Other secondary outcomes were not reported:Proportion of participants without relapse in the long term, Proportion of participants with adverse effects in the short term and in the long term, Ease of compliance to an intervention or a treatment.
13. Secukinumab versus placebo
One study compared secukinumab 150 mg or 300 mg versus placebo over a period of 16 weeks (Table 12) (Mrowietz 2019).
(Results below involved 300 mg ‐ the approved dose for psoriasis.)
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was not reported.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
In the secukinumab group, 20 of 79 participants had serious adverse events (seven cardiac disorders, one multiple organ dysfunction syndrome, four infections and infestations, four pustular psoriasis, four others), and 6 of 78 in the placebo group (one cardiac disorder, one drug‐induced liver injury, one infections and infestations, one cerebrovascular accident, two others) (RR 3.29, 95% CI 1.40 to 7.75; Analysis 12.1).
12.1. Analysis.
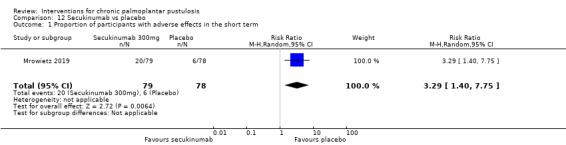
Comparison 12 Secukinumab vs placebo, Outcome 1 Proportion of participants with adverse effects in the short term.
(Data from clinicaltrial.gov posted results.)
Secondary outcomes
Proportion of participants achieving a 50% reduction in disease severity in the short term
In the secukinumab group, 36 of 79 participants had a 50% reduction in disease severity (PPPASI 50) in the short term (16 weeks) compared to 23 of 78 in the placebo group (RR 1.55, 95% CI 1.02, 2.35; Analysis 12.2).
12.2. Analysis.
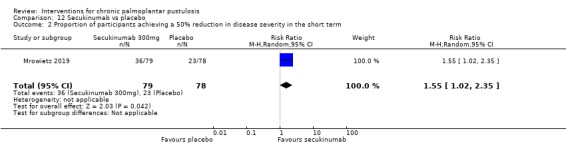
Comparison 12 Secukinumab vs placebo, Outcome 2 Proportion of participants achieving a 50% reduction in disease severity in the short term.
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants without relapse in the long term, Proportion of participants with adverse effects in the short term and in the long term, Ease of compliance to an intervention or a treatment.
Antibiotics
14. Tetracyclines versus placebo
One study compared tetracycline 250 mg twice daily to placebo over a period of 12 weeks with no co‐intervention in both groups (Thomsen 1973). Another study compared clomocycline 170 mg thrice daily for two weeks, then twice a day for 10 weeks versus placebo with a co‐intervention in both groups (emulsifying ointment or dilute Betnovate 1/4 in petrolatum) (Ward 1976).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
This outcome was not reported.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
Side effects were reported in 21 of 100 participants in the tetracycline group versus 4 of 100 in the placebo group (RR 4.91, 95% CI 1.00 to 24.07; I2 = 48%; Analysis 13.1).
13.1. Analysis.
Comparison 13 Tetracyclines vs placebo, Outcome 1 Proportion of participants with adverse effects in the short term.
Other outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Other treatments
15. Colchicine versus placebo
One study compared colchicine 0.5 mg (three to four times per day according to weight) versus placebo over a period of eight weeks (Thestrup‐Pedersen 1984).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
None of the patients in both groups had clearance of their disease.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
In the colchicine group, 10 of 27 participants had side effects versus 3 of 27 in the placebo group (RR 3.33, 95% CI 1.03 to 10.79; Analysis 14.1).
14.1. Analysis.
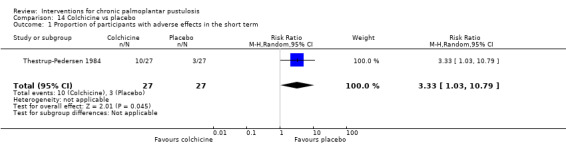
Comparison 14 Colchicine vs placebo, Outcome 1 Proportion of participants with adverse effects in the short term.
Other secondary outcomes were not reported:Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Ease of compliance to an intervention or a treatment.
16. Fluorine‐synthetic fibre socks versus cotton fabric socks
One study compared socks made of fluorine‐synthetic fibre versus socks made of cotton fabric over a period of four weeks (Cazzaniga 2014).
Primary outcomes
Proportion of participants cleared or almost cleared, preferably measured as an objective measure of disease severity (e.g. predefined disease severity score) at two timings: the short term and the long term
None of the participants in both groups had clearance of their disease.
Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study
This outcome was not reported.
Secondary outcomes
Proportion of participants with adverse effects in the short term and in the long term
None of the participants reported any side effects.
Other secondary outcomes were not reported: Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term; Proportion of participants achieving a 50% reduction in disease severity in the short term; Proportion of participants without relapse in the long term; Ease of compliance to an intervention or a treatment.
Discussion
Summary of main results
We included 37 studies assessing the following treatments for palmoplantar pustulosis.
Topical treatments (superpotent corticosteroids: triamcinolone acetonide and clobetasol, and vitamin D derivative: maxacalcitol).
Phototherapy (oral and local PUVA (combination of psoralens and long‐wave ultraviolet radiation), ultraviolet A1 (UVA1), and PUVA).
Systemic treatments (oral retinoids: etretinate (alone or in combination with PUVA), alitretinoin, and liarozole; ciclosporin; oral antibiotics: tetracyclines; biologics: ustekinumab, etanercept, guselkumab, secukinumab).
Other treatments (hydroxyurea, Grenz ray therapy, colchicine, X‐ray therapy, fluorine‐synthetic fibre socks, aluminium chloride).
A large majority of studies were conducted before 2006 (n = 27), and the mean number of participants per study was 45. Few meta‐analyses were performed because only 18 trials reported one of our pre‐specified outcomes, and among them few assessed the same comparison and a common outcome. Please see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; and Table 12 for details of our key comparisons.
Our primary efficacy outcome 'Proportion of participants cleared or almost clear in the short term' was available for seven of our key comparisons and was the most‐often measured outcome.
This outcome may be more favourable with the topical vitamin D derivative maxacalcitol than with placebo, but there may be little or no difference between etanercept and placebo (however, there is uncertainty in this result due to the 95% confidence interval showing there may be a difference) (low‐quality evidence).
We are uncertain of the results from the following comparisons for this outcome: (1) PUVA therapy compared to placebo or no treatment, and (2) guselkumab compared to placebo (very low‐quality evidence).
This outcome was not reported by studies comparing oral alitretinoin to placebo, ustekinumab to placebo, or secukinumab to placebo.
The secondary outcome ‘Proportion of participants achieving a 50% reduction in disease severity in the short term’ was available for five of our key comparisons.
This outcome is probably more favourable with secukinumab or guselkumab than with placebo (moderate‐quality evidence).
There is probably little or no difference in achieving the outcome when oral alitretinoin is compared to placebo (moderate‐quality evidence).
This outcome may be more favourable with placebo than with ustekinumab (however, there is uncertainty in this result due to the 95% confidence interval showing there may be little or no difference, as well as a greater effect with ustekinumab) (low‐quality evidence).
We are uncertain of the results of PUVA therapy compared to placebo or no treatment for this outcome (very low‐quality evidence).
This outcome was not reported by studies comparing (1) etanercept against placebo, or (2) the topical vitamin D derivative maxacalcitol against placebo.
Our secondary outcome ‘Proportion of participants without relapse in the long term’ was reported for only one comparison in the review.
None of the included studies measured or reported the following secondary outcome: ‘Ease of compliance to an intervention or a treatment’, and only one study reported ‘Proportion of participants with at least 50% improvement in their quality of life measured by a specific validated scale, such as the Dermatology Life Quality Index (DLQI), Skindex, or Pain Disability Index (PDI), evaluated in the short term and in the long term’.
Adverse events were poorly reported. Only three studies reported our primary safety outcome 'Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study’. We found low‐quality evidence suggesting that guselkumab may cause more serious adverse events leading to withdrawal than placebo, but the results are very imprecise, showing some uncertainty. We found moderate‐quality evidence showing that the proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study was probably superior with secukinumab compared to placebo.
Regarding the proportion of participants with adverse effects not requiring withdrawal, we found very low‐quality evidence for the comparison of PUVA therapy versus placebo or no treatment, showing we are uncertain of their effects on the occurrence of adverse events. We found moderate‐quality evidence to show that the proportion of participants with adverse effects is probably similar when maxacalcitol (a vitamin D derivative) is compared to placebo, with mild local irritation, pruritus, and haematological or urinary test abnormalities reported in study participants. We also found moderate‐quality evidence showing that there is probably little or no difference in the number of adverse events caused by oral alitretinoin compared to placebo. Adverse events reported were headache, cheilitis, nausea, arthralgia, hypercholesterolaemia, and nasopharyngitis. This outcome was not reported for etanercept compared to placebo, ustekinumab compared to placebo, guselkumab compared to placebo, or secukinumab compared to placebo.
Overall completeness and applicability of evidence
The evidence that we found and included in this review is not sufficient to address all of its objectives. Absent interventions, infrequent assessment of our pre‐specified outcomes, and short‐term appraisal of effects meant that we cannot draw conclusions on the efficacy of the included interventions to induce and maintain complete remission.
Evidence is lacking for major (i.e. common) treatments used in chronic palmoplantar pustulosis such as topical corticosteroids (assessed by one study), phototherapy (assessed by three studies, but light therapy was also used as a comparator), acitretin (assessed by two studies, but neither contributed data), methotrexate (not assessed), and ciclosporin (assessed by two studies, but one did not contribute data). The quality of evidence for topical vitamin D derivative, etretinate, and biologics was very low to moderate.
Many of our pre‐specified outcomes were not reported or were reported infrequently. Our primary efficacy outcome 'Proportion of participants cleared or almost cleared' was reported in 15 of 37 of the included studies.
Only 3 of the 37 included studies reported our primary safety outcome 'Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study'. More studies (15/37) reported short‐term or long‐term adverse effects not causing withdrawal. With 22 studies not reporting such adverse events, there is incomplete coverage of safety effects.
In addition, the following secondary outcome 'Ease of compliance to an intervention or a treatment' was not assessed by any of the included studies, and only one study reported ‘Quality of life’, which is a major outcome to assess in chronic palmoplantar pustulosis. Our secondary efficacy outcome 'Proportion of participants achieving a 50% reduction in disease severity in the short term' was reported in only five studies.
Long‐term assessment, which is mandatory in such a chronic disease, was performed in only one study via reporting of 'Proportion of participants without relapse in the long term'. This means that we could not evaluate maintenance of remission.
Globally, when described, study participants were representative of people with chronic palmoplantar pustulosis in terms of sex ratio (with about two‐thirds women), age (mean age 50 years), and long‐lasting chronic disease (mean duration of evolution: six years), and both palms and soles were affected for more than half of study participants.
Reflecting the ongoing debate about the relationship between psoriasis and palmoplantar pustulosis, the inclusion criteria of included studies were declared as palmoplantar pustulosis, palmoplantar pustular psoriasis, or both. Information on the presence of concomitant psoriasis lesions elsewhere was available for only nine trials, with highly variable results from 0% to 53% of participants.
One main issue in the included studies was the lack of validated scales to assess clinical severity; we found many means of evaluation such as various non‐validated scales and scores and global assessment.
Quality of the evidence
For the comparison topical vitamin D derivative as maxacalcitol versus placebo, we downgraded to low‐quality evidence for the outcome 'Proportion of participants cleared or almost cleared': by one level because of incomplete reporting, and we rated all other items as having unclear risk of bias, and we downgraded one further level due to imprecision as there is a large confidence interval for this result. For the outcome 'Proportion of participants with adverse events', we downgraded by one level because of incomplete reporting and other items were rated as having unclear risk of bias. For the comparison of superpotent corticosteroid cream with occlusive dressing (clobetasol propionate) versus another superpotent corticosteroid cream without occlusion (triamcinolone acetonide), we downgraded by three levels to very low‐quality evidence for the outcomes 'Proportion of participants cleared or almost cleared' and 'Proportion of participants with adverse effects': two levels due to study limitations because of high risk of bias for blinding and unclear risk of bias for other items; and one level due to imprecision because the comparison was assessed in a single small study.
For the comparison PUVA versus placebo or no treatment, we downgraded by three levels to very low‐quality evidence for three outcomes 'Proportion of participants cleared or almost cleared', 'Proportion of participants achieving a 50% reduction in disease severity' and 'Proportion of participants with adverse effects': one level due to study limitations because of unclear risk of bias for four out of five items, one level due to inconsistency (efficacy and types of adverse events were substantially different in these two trials), and one level due to imprecision because the comparison was assessed in two small studies. For the comparison UVA1 versus ultraviolet B (UVB), we downgraded by three levels for two outcomes 'Proportion of participants cleared or almost cleared' and 'Proportion of participants with adverse effects' to very low‐quality evidence: two levels due to study limitations because of high risk of bias for blinding, and one level due to imprecision because the comparison was assessed in a single study involving 33 participants.
For the comparison etretinate versus placebo, we downgraded to very low‐quality evidence for the three available outcomes ('Proportion of participants cleared or almost cleared', 'Proportion of participants with adverse events', and 'Proportion of participants without relapse in the long term'): two levels because of high risk of bias for blinding because of systematic visible adverse events due to etretinate in the context of subjective outcome, and one level because of imprecision.
For the comparison etretinate versus placebo with PUVA therapy as co‐intervention, we downgraded to very low‐quality evidence for the two available outcomes ('Proportion of participants cleared or almost cleared', 'Proportion of participants with adverse events'): two levels because of high risk of bias for blinding because of systematic visible adverse events due to etretinate in the context of subjective outcomes, and two levels because of imprecision.
For the comparison etretinate versus PUVA therapy, we downgraded to very low‐quality evidence for the two available outcomes ('Proportion of participants cleared or almost cleared', 'Proportion of participants with adverse events'): two levels because of high risk of bias for blinding because of systematic visible adverse events due to etretinate in the context of subjective outcome, and one level because of imprecision.
For the comparison alitretinoin versus placebo, we downgraded to moderate‐quality evidence for the two available outcomes ('Proportion of participants achieving a 50% reduction in disease severity in the long term', 'Proportion of participants with adverse events'): one level because of imprecision.
For the comparison etanercept versus placebo, we downgraded to low‐quality evidence for the available outcome ('Proportion of participants cleared or almost cleared'): two levels because of imprecision.
For the comparison ustekinumab versus placebo, we downgraded to low‐quality evidence for the available outcome ('Proportion of participants achieving a 50% reduction in disease severity in the short term'): one level because of high risk of reporting bias, and one level because of imprecision.
For the comparison guselkumab versus placebo, we downgraded to very low‐quality evidence for one outcome ('Proportion of participants cleared or almost cleared') because of imprecision and inconsistency; low‐quality evidence for one outcome ('Proportion of participants with adverse effects serious or severe enough to have caused withdrawal') because of imprecision; and moderate‐quality evidence for one outcome ('Proportion of participants achieving a 50% reduction in disease severity') because of high risk of imprecision.
For the comparison secukinumab versus placebo, we downgraded to moderate‐quality evidence for the available outcomes ('Proportion of participants with a 50% improvement in disease severity', 'Proportion of participants with adverse effects serious or severe enough to have caused withdrawal') because of imprecision.
Globally, levels of evidence were downgraded for all comparisons because of imprecision as they were underpowered and were unlikely to detect a difference.
Potential biases in the review process
We performed a search in a large range of databases and other sources to limit the risk of publication bias. We found one unpublished study registered in 2009 comparing acitretin to fumaric acid ester with PUVA therapy as co‐intervention in both groups, for which we found no report (EudraCT 2006‐004519‐23). We did not receive a response after contacting the study authors. Due to the small number of studies assessing the same comparison, we were unable to test for publication bias by funnel plot.
We obtained complementary information after contacting study authors (Table 14).
We decided to not consider as a relevant means of assessment the count of fresh pustules for the outcome 'Proportion of participants achieving a 50% reduction in disease severity in the short term' (Table 15), which meant that we did not include data from some studies.
Results of the two ongoing studies, when available, could alter the conclusions of this review (ISRCTN13127147 APRICOT; NCT03633396).
Agreements and disagreements with other studies or reviews
The previous version of this review concluded that "there is no standardised method for assessing response to treatment, and reductions in pustule counts or other empirical semi‐quantitative scoring systems may be of little relevance to the patient. This review has shown that the ideal treatment for PPP remains elusive and that the standards of study design and reporting need to be improved to inform patients and those treating them of the relative merits of the many treatments available to them" (Chalmers 2006). We included 14 additional trials, including eight that assessed new treatments (topical vitamin D derivative, ustekinumab, guselkumab, secukinumab, etanercept, alitretinoin, fluorine‐synthetic fibre socks). We used a more stringent and precise efficacy outcome (clear or almost clear and 50% reduction in disease severity) and did not consider the outcome 'Count of new fresh pustules' to be relevant, which review authors included in analysis in the previous version of this review. New trials included in our review led to a different conclusion; however, we still agree with the conclusion of Chalmers 2006 that "the ideal treatment for PPP remains elusive."
A Cochrane Review ‐ Chen 2013 ‐ assessing the effects of narrowband ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen ultraviolet A photochemotherapy for psoriasis included one study including participants with palmoplantar psoriasis (Sezer 2007). We excluded this study because we found no mention of pustular lesions in the inclusion criteria or in the description of included participants.
Authors' conclusions
Implications for practice.
Evidence is lacking for major treatments used in chronic palmoplantar pustulosis including superpotent dermocorticoids such as clobetasol propionate and betamethasone dipropionate, phototherapy, acitretin, methotrexate, and ciclosporin.
We found low‐quality evidence suggesting that the topical vitamin D derivative maxacalcitol may be more effective in achieving clear or almost clear status than placebo, and the likelihood of adverse events (e.g. mild local irritation, pruritus) is probably similar between the two groups (moderate‐quality evidence). For this comparison, the outcome ‘Proportion of participants achieving a 50% reduction in disease severity’ was not reported.
We found moderate‐quality evidence indicating that oral alitretinoin probably is no more effective than placebo in achieving 50% reduction in disease severity, and there is probably no difference in adverse effects (e.g. headache, cheilitis, nausea) between groups. The outcome ‘Proportion of participants cleared or almost cleared’ was not reported.
Regarding biological treatments (etanercept (anti‐tumour necrosis factor (TNF), ustekinumab (anti‐interleukin (IL)‐17/IL‐23), guselkumab (anti‐IL‐23), and secukinumab), low‐quality evidence suggests that etanercept may be no more effective than placebo in achieving clear or almost clear status, but this suggestion is based on a study of only 15 participants. The outcome ‘Proportion of participants achieving a 50% reduction in disease severity’ was not reported. We are unsure of the effect of ustekinumab in achieving a 50% reduction in disease severity when compared to placebo due to the small number of participants in the evidence base (low‐quality evidence). The outcome ‘Proportion of participants cleared or almost cleared' was not reported.
We are not certain of the effect that guselkumab has on clearance as the evidence was of very low quality, but guselkumab probably increases the chance of achieving 50% reduction in disease severity when compared to placebo (moderate‐quality evidence). Moderate‐quality evidence indicates that secukinumab (anti‐IL‐17) is probably more effective than placebo in achieving 50% reduction in disease severity. The outcome ‘Proportion of participants cleared or almost cleared’ was not reported. None of these biologics resulted in adverse events not requiring withdrawal.
Only two studies (5.4%) reported adverse effects serious or severe enough to have caused withdrawal. Guselkumab may cause more serious adverse events compared to placebo, but there is uncertainty due to the small number of participants included (low‐quality evidence). Secukinumab probably causes more serious adverse events than placebo (moderate‐quality evidence).
Limited evaluation of "classical" topical or systemic treatments and assessment of safety and quality of life and lack of long‐term efficacy precluded conclusions about efficacy and tolerance of treatments used in palmoplantar pustulosis.
The two ongoing studies may alter the conclusions of this review.
Implications for research.
Further studies are needed to assess efficacy and tolerance of interventions for chronic palmoplantar pustulosis.
Future randomised controlled trials should ensure that they follow CONSORT guidelines (Moher 2001). This includes ensuring that the trial protocol is made publicly available before the trial commences, and that the published report includes all pre‐planned outcomes. Trials should include a number of participants that allows sufficient power to detect a difference for the outcomes assessed. To reduce bias, trialists should ensure that participants, study personnel, and outcome assessors are blinded to treatment allocation.
Participants
Inclusion criteria should describe precise clinical diagnostic criteria and should state whether or not specific clinical manifestations of psoriasis are required.
Intervention
Interventions preferred for future research include superpotent topical corticosteroids under occlusion; vitamin D derivatives; the association of topical corticosteroids and vitamin D derivatives; acitretin, methotrexate, ciclosporin, phototherapy, and biologics; and combined treatment such as acitretin + phototherapy should be explored. The first comparisons to be assessed should include superpotent topical corticosteroids under occlusion, acitretin, and methotrexate.
Comparator
These treatments are currently used for this indication; therefore, use of placebo could be considered as unethical despite absence of a clear demonstration of efficacy.
Outcomes
A core outcome set is needed in chronic palmoplantar pustulosis, including a validated scale to assess severity, quality of life, work impairment, pain, and pruritus and ease of compliance to the intervention or treatment. We advise that this should be done in consultation with the Cochrane Skin Outcomes Set Initiative (CS‐COUSIN). Safety should be monitored and fully reported in all future trials.
Timing
Assessment should be conducted at short term for remission (within three months) and at long term (at least one year) to assess maintenance of remission.
Acknowledgements
The Cochrane Skin Editorial Base wishes to thank Michael Bigby, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the Statistical Editor; the clinical referees, Ignacio Garcia‐Doval, Jerry Brewer, and one who prefers to remain anonymous; and Dolores P Matthews, who copy‐edited this review.
Appendices
Appendix 1. Cochrane Skin (CRSW) search strategy
#1 (((palm* or sole* or pustul*) and psoria*) or (pustul* and palmoplant*) or (Pustulosis of palm* and sole*) or (Pustulosis palmaris et plantaris)) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (the Cochrane Library) search strategy
#1 psoria*:ti,ab,kw #2 MeSH descriptor: [Psoriasis] explode all trees #3 #1 or #2 #4 (palm* or sole* or pustul*):ti,ab,kw #5 #3 and #4 #6 Pustulosis palmaris et plantaris:ti,ab,kw #7 (pustul* and palmoplant*):ti,ab,kw #8 (Pustulosis of palm* and sole*):ti,ab,kw #9 {or #5‐#8}
Appendix 3. MEDLINE (Ovid) search strategy
1. Pustulosis palmaris et plantaris.ti,ab. 2. Psoriasis/ 3. psoriasis.ti,ab. 4. 2 or 3 5. (palm$ or sole$1 or pustul$).ti,ab. 6. 4 and 5 7. (pustul$ and palmoplant$).ti,ab. 8. (Pustulosis of palm$ and sole$).ti,ab. 9. 1 or 6 or 7 or 8 10. randomised controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. clinical trials as topic.sh. 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp animals/ not humans.sh. 19. 17 not 18 20. 9 and 19
[Lines 10‐19: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. Pustulosis palmaris et plantaris.ti,ab. 2. Psoriasis/ 3. psoriasis.ti,ab. 4. 2 or 3 5. (palm$ or sole$1 or pustul$).ti,ab. 6. 4 and 5 7. (pustul$ and palmoplant$).ti,ab. 8. (Pustulosis of palm$ and sole$).ti,ab. 9. pustulosis palmoplantaris/ 10. 1 or 6 or 7 or 8 or 9 11. crossover procedure.sh. 12. double‐blind procedure.sh. 13. single‐blind procedure.sh. 14. (crossover$ or cross over$).tw. 15. placebo$.tw. 16. (doubl$ adj blind$).tw. 17. allocat$.tw. 18. trial.ti. 19. randomized controlled trial.sh. 20. random$.tw. 21. or/11‐20 22. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 23. human/ or normal human/ 24. 22 and 23 25. 22 not 24 26. 21 not 25 27. 10 and 26
Appendix 5. LILACS search strategy
((palm$ or sole$1 or pustul$) and psoria$) or (pustul$ and palmoplant$) or (Pustulosis of palm$ and sole$)
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1. Vitamin analogue ointment vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 7.83 [1.85, 33.12] |
| 2 Proportion of participants with side effects in the short term | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.19] |
Comparison 2. UVA vs narrowband UVB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Within‐participant study | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.17, 3.43] |
2.1. Analysis.
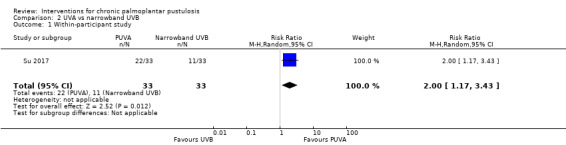
Comparison 2 UVA vs narrowband UVB, Outcome 1 Within‐participant study.
Comparison 3. Etretinate vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.48 [0.82, 14.80] |
| 2 Proportion of participants without relapse in the long term | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.92, 6.17] |
| 3 Proportion of participants with adverse effects in the short term | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [0.95, 12.90] |
Comparison 4. Etretinate vs placebo or no treatment with PUVA as co‐intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.04, 3.50] |
| 2 Proportion of participants with adverse effects in the short term | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 17.0 [1.11, 259.87] |
Comparison 5. Alitretinoin vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants achieving a 50% reduction in disease severity in the short term | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.30] |
| 2 Proportion of participants with adverse effects in the short term | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.17] |
Comparison 6. Etretinate vs PUVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 11.2 [4.16, 30.18] |
| 2 Proportion of participants with adverse effects in the short term | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 11.54 [5.17, 25.74] |
Comparison 7. Oral ciclosporin vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with adverse effects in the short term | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.48, 2.86] |
Comparison 8. Etanercept vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.08, 34.28] |
Comparison 9. Ustekinumab vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants achieving a 50% reduction in disease severity in the short term | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.11, 2.13] |
Comparison 10. Guselkumab 200 mg vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.15, 9.30] |
| 2 Proportion of participants with adverse effects serious or severe enough to have caused withdrawal of participants from the study | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.32, 25.80] |
| 3 Proportion of participants achieving a 50% reduction in disease severity | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.24, 6.69] |
Comparison 11. Guselkumab 100 mg vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants cleared or almost cleared in the short term | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.31, 5.57] |
Comparison 12. Secukinumab vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with adverse effects in the short term | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [1.40, 7.75] |
| 2 Proportion of participants achieving a 50% reduction in disease severity in the short term | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.02, 2.35] |
Comparison 13. Tetracyclines vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with adverse effects in the short term | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 4.91 [1.00, 24.07] |
Comparison 14. Colchicine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with adverse effects in the short term | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [1.03, 10.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhushan 2001.
| Methods | Randomised, parallel‐arm, placebo‐controlled trial Two centres in the UK Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomly assigned to either liarozole 75 mg × 2 (n = 7) or placebo treatment (n = 8)
Withdrawal: no dropouts in the intervention group nor in the control group during the 12‐week period |
|
| Interventions |
Intervention A: oral liarozole 75 mg twice daily (7 participants) Control intervention B: placebo (8 participants) Co‐interventions: none Duration of treatment: 12 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Drug and placebo were supplied by the Janssen Research Foundation (Beerse, Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to either liarozole or placebo treatment on the basis of a computer‐generated randomisation code" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned to either liarozole or placebo treatment on the basis of a computer‐generated randomisation code. Patients, study co‐ordinator and study physician were unaware as to which group each patient had been assigned" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The capsules, whether placebo or liarozole, were identical in appearance and taste" Comment: double‐blind placebo‐controlled. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The capsules, whether placebo or liarozole, were identical in appearance and taste" Comment: double‐blind placebo‐controlled. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ''All subjects who entered the study completed 12 weeks of treatment" Comment: no missing data (no dropouts neither in the intervention group nor in the control group) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes were presented in the results |
Bissonnette 2008.
| Methods | Placebo‐controlled, parallel‐arm trial Three centres in Canada (community centre clinics) Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: participants were randomised 2:1 to subcutaneous injections of either etanercept 50 mg twice per week (n = 10) or placebo (n = 5)
Withdrawal: no dropouts |
|
| Interventions |
Intervention A: etanercept 50 mg subcutaneously twice per week (10 participants) Control intervention B: placebo (5 participants) Co‐interventions: none Duration of treatment: 3 months |
|
| Outcomes |
Primary outcome
Other outcome
|
|
| Notes | The study was funded by Amgen Canada Inc and Wyeth Pharmaceuticals Clinical trial: NCT00353119 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised 2:1 to receive subcutaneous injections of either etanercept 50 mg or a placebo" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomised 2:1 to receive subcutaneous injections of either etanercept 50 mg or a placebo" Comment: the method use to guarantee concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" Comment: double‐blind placebo‐controlled. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind study" Comment: double‐blind placebo‐controlled. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All randomised subjects completed the study" Comment: no missing data (no dropouts neither in the intervention group nor in the control group) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes of interest in the review have been reported in the pre‐specified way |
Bissonnette 2014.
| Methods | Randomised, parallel‐arm trial Five centres in Canada (community centre clinics) Period of inclusion: March 2010 to August 2011 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: palmoplantar pustular psoriasis and palmoplantar pustulosis randomised 1:1 to ustekinumab 45 mg or 90 mg (based on weight) (15 participants) or placebo (18 participants). Total number of participants = 33
Withdrawal: 1 patient in the ustekinumab group discontinued treatment because of side effects, as did 3 in the placebo group (1 because of side effects and 2 for withdrawal of consent) |
|
| Interventions |
Intervention A: ustekinumab 45 mg or 90 mg (based on weight) (15 participants) Control intervention B: placebo (18 participants) Co‐interventions: none Duration of treatment: 16 weeks |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Research funded and medication provided by Janssen Inc. Canada. Funders were involved in study design but not in data collection, manuscript preparation, or publication decisions Clinical trial: NCT01091051 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Palmoplantar pustular psoriasis and palmoplantar pustulosis patients were randomised (1:1) to receive either ustekinumab or placebo. The sponsor generated the non‐blocked random allocation sequences for each cohort using Excel (Microsoft, Richmond, WA, USA) and provided them to sites in sequentially numbered envelopes" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Palmoplantar pustular psoriasis and palmoplantar pustulosis patients were randomised (1:1) to receive either ustekinumab or placebo. The sponsor generated the non‐blocked random allocation sequences for each cohort using Excel (Microsoft, Richmond, WA, USA) and provided them to sites in sequentially numbered envelopes" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomised to receive ustekinumab or placebo. Patients randomised to placebo received an equal volume injection of bacteriostatic sodium chloride at day 0 and week 4 followed by ustekinumab at weeks 16 and 20. The un‐blinded pharmacist who had access to the randomisation codes was the only person knowing the nature of the treatment dispensed to patients" Comment: participants were blind but the pharmacist was not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were randomised to receive ustekinumab or placebo. Patients randomised to placebo received an equal volume injection of bacteriostatic sodium chloride at day 0 and week 4 followed by ustekinumab at weeks 16 and 20. The un‐blinded pharmacist who had access to the randomisation codes was the only person knowing the nature of the treatment dispensed to patients" Comment: the pharmacist was the only person knowing the nature of the treatment delivered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one participant in the ustekinumab group discontinued treatment because of side effects as did 3 in the placebo group (1 because of side effects and 2 for withdrawal of consent). All participants were included in the 'intention‐to‐treat' population and in the safety population |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available but not all of the study’s pre‐specified secondary outcomes (PPPASI‐75 at week 16) have been reported in the pre‐specified way |
Cazzaniga 2014.
| Methods | Randomised, within‐patient clinical trial Three centres in Italy Period of inclusion: March 2010 to June 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: dressing sides were uniformly randomised on a 1:1 basis to sock made of fluorine‐synthetic fibre (17 soles) or sock made of cotton fabric (17 soles)
Withdrawal: 3 participants were lost to follow‐up because of bad compliance; 1 withdrew from the study after the second week for worsening of pathological conditions |
|
| Interventions |
Intervention A: sock made of fluorine‐synthetic fibre (17 soles) Control Intervention B: sock made of cotton fabric (17 soles) Co‐interventions: topical corticosteroids or vitamin D derivatives Duration of treatment: 4 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This trial was supported by a grant from Lenzi Egisto S.p.A. Clinical trial: NCT01197989 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Dressing sides were uniformly randomised on a 1: 1 basis. Centralised telephone randomisation procedures were adopted" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Centralized telephone randomisation procedures were adopted, and both investigators and outcomes assessor were blinded to the randomisation rule" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both socks were tailor‐made by the study sponsor in order to be as similar as possible regarding colour, model and texture of fabric" Comment: patients and personnel were unaware of the socks used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both socks were tailor‐made by the study sponsor in order to be as similar as possible regarding colour, model and texture of fabric. Both investigators and outcomes assessor were blinded to the randomisation rule" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients dropped out of the study for low compliance rate, one after baseline, one after the first week and one after the second week, while one patient withdrew from the study after the second week for worsening of pathological conditions (loss of 23.5% of enrolled patients). An intention‐to‐treat approach was adopted in the primary analysis. In this analysis, patients lost to follow‐up were recovered by the last observation carried forward technique. Intention‐to‐treat analysis was then complemented by per‐protocol analyses, which considered only those patients who completed the study period" Comment: intention‐to‐treat approach was adopted. So, we know how they dealt with missing data |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes of interest in the review have been reported in the pre‐specified way |
Erkko 1998.
| Methods | Randomised, double‐blind, parallel‐arm trial Three centres in Helsinki, Gothenburg, and Stockholm Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised to ciclosporin at a dose of 1.0 mg/kg per day (n = 27) or placebo (n = 31)
Withdrawal: no dropouts |
|
| Interventions |
Intervention A: ciclosporin 1 mg/kg/d twice daily for 1 month (27 participants) Control intervention B: placebo (31 participants) Co‐interventions: none Duration of treatment: 1 month |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This work was supported by Novartis Pharma Ltd, Basel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were given numbers in consecutive order at the various centres; each number had been pre‐assigned to start the study with treatment of either the traditional formulation of cyclosporin (Sandimmun) 1·0 mg/kg per day or placebo (vehicle without cyclosporin)" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were given numbers in consecutive order at the various centres; each number had been pre‐assigned to start the study with treatment of either the traditional formulation of cyclosporin (Sandimmun) 1·0 mg/kg per day or placebo (vehicle without cyclosporin)" Comment: the method to guarantee allocation concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded study" Comment: double‐blind placebo‐controlled trial. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blinded study" Comment: double‐blind placebo‐controlled trial. We consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 58 patients completed the double‐blind placebo‐controlled part 1 of the study" Comment: no dropouts |
| Selective reporting (reporting bias) | High risk | Comment: not all pre‐specified secondary outcomes are reported (scores), and no protocol is available to guarantee that all planned outcomes are presented in the results |
Fairris 1984.
| Methods | Placebo‐controlled (double‐blind), parallel‐group trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated Baseline data Not stated (n = 6 participants) Withdrawal: no dropouts |
|
| Interventions |
Intervention A: superficial X‐ray therapy (N = 9 sites) Control intervention B: placebo (N = 9 sites) Co‐interventions: none Duration of treatment: 18 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified Grade of severity at each visit (no precision) Participants were reviewed 6, 9, and 18 weeks after the start of X‐ray therapy. During each visit, patient and observer separately graded severity of the disease at both sites |
|
| Notes | Available only as an abstract; hence, many details are missing and some are not clear Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no indication on randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no indication on the process to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the intervention is radiotherapy on the treated side and placebo on the other side No indication of the measures used to guarantee blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the intervention is radiotherapy on the treated side and placebo on the other side. No indication of the measures used to guarantee blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no indication on dropout nor on eventual ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol; no primary outcome specified |
Foged 1983.
| Methods | Double‐blind, randomised, parallel‐arm trial Multi‐centre Period of inclusion: winter 1980 to 1981 |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria
Baseline data: randomised to etretinate 1 mg/kg once daily (n = 24) or placebo (n = 26)
Withdrawal: etretinate (n = 4); placebo (n = 5)
|
|
| Interventions |
Intervention A: etretinate 1 mg/kg once daily (24 participants) Control intervention B: placebo (26 participants) Co‐interventions: none Duration of treatment: 8 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent, 24 patients were allocated to 8 weeks of oral etretinate treatment and 26 patients to placebo" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear because the method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind" Comment: likely that blinding could have been broken because of etretinate's side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind" Comment: likely that blinding could have been broken because of etretinate's side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "9 patients withdrawn during trial were used in analyses of side effects only: etretinate (4); placebo (5)
Comment: 20% of missing data with unbalanced reason by group; not specified how study authors dealt with it |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Fredriksson 1978.
| Methods | Parallel‐arm randomised controlled trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated Baseline data: randomised to RO 10‐9359 (25 mg thrice per day) (n = 15) or RO 10‐9359 (200 mg twice per week) (n = 15)
Withdrawal: no mention of missing data |
|
| Interventions |
Intervention 1 A: oral RO 10‐9359 (25 mg thrice per day) (15 participants) Intervention 2 B: oral RO 10‐9359 (200 mg twice per week) (15 participants) Co‐interventions: none Duration of treatment: 8 weeks |
|
| Outcomes | No primary or secondary outcomes pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 patients randomly allocated to two treatment groups" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "30 patients randomly allocated to two treatment groups (one group treated with etretinate 75 mg daily and the other with 200 mg twice weekly)" Comment: the method to guarantee allocation concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Hattel 1974.
| Methods | Randomised controlled trial with cross‐over One centre in Copenhagen Period of inclusion: year 1972 |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data Not stated but typical psoriatic lesions were absent Withdrawal: no mention of missing data |
|
| Interventions |
Intervention A: hydroxyurea 0.5 g thrice daily (13 participants, cross‐over design) Control Intervention B: placebo (13 participants, cross‐over design) Co‐interventions: none Duration of treatment: 3 weeks |
|
| Outcomes | No primary or secondary outcomes pre‐specified
Photographs were taken before and after each 3‐week period |
|
| Notes | Hydrea was supplied by Squibb and Sons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method to guarantee allocation concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind, cross‐over" Comment: use of placebo but insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind, cross‐over" Comment: use of placebo but insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Jansen 1979.
| Methods | Parallel‐arm RCT Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: by random allocation, 25‐mg capsules of Ro 10‐9359 or placebo in identical capsules were each supplied to 10 participants
Withdrawal: 1 dropout case in the Ro 10‐9359 group was a woman aged 72 who had a mild transient ischaemic attack |
|
| Interventions |
Intervention 1 A: aromatic retinoid ethyl ester (Ro 10‐9359) (10 participants) Dose of Ro 10‐9359 varied between 25 and 100 mg per day Intervention 2 B: placebo (10 participants) Co‐intervention: none Duration of treatment: 4 months |
|
| Outcomes | No primary or secondary outcomes pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By random allocation, either 25mg capsules of Ro 10‐9359 or placebo, in identical capsules, was supplied" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind comparison" Comment: likely that blinding could have been broken because of Ro 10‐9359 side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind comparison" Comment: likely that blinding could have been broken because of Ro 10‐9359 side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "one drop‐out case in the Ro 10‐9359 was a woman aged 72 who had mild transient ischemic attack" Comment: probably not included in the analysis, but this was not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Kragballe 1991.
| Methods | Randomised, open, prospective, right‐left comparative trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised comparison of Actiderm plus triamcinolone acetonide 0.1% cream applied every third day (19) vs clobetasol 0.1% cream applied twice daily (19)
Withdrawal: no dropouts |
|
| Interventions |
Intervention 1 A: Actiderm plus triamcinolone acetonide (TAA) 0.1% cream every third day (19 participants) Intervention 2 B: clobetasol 0.05% cream twice per day (19 participants) Co‐interventions: none Duration of treatment: 4 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
Outcomes were evaluated at each visit (‐2, 0, 2, 4, and 8 weeks) |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a randomised comparison" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Actiderm plus TAA 0.1% cream was replaced every third day. Clobetasol 0.05% cream was applied twice daily" Comment: no evidence of blinding, which is very difficult in this case |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no evidence of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of missing data |
| Selective reporting (reporting bias) | High risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results and not all outcomes cited in the methods were reported in the results (skin atrophy) |
Lassus 1983.
| Methods | Randomised, parallel‐arm trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised to Tigason 0.14 to 0.38 mg/kg/d (n = 11) or placebo (n = 14)
Withdrawal: 4 participants (in the placebo group) were lost because of adverse events (marked hair loss) but not specified if they were included in the analysis |
|
| Interventions |
Intervention A: oral Tigason 0.14 to 0.38 mg/kg/d (11 participants) Control intervention B: placebo (14 participants) Co‐interventions: none Duration of treatment: 6 months |
|
| Outcomes | No primary or secondary outcomes pre‐specified | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to either Tigason or placebo for 6 months" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled" Comment: likely that blinding could have been broken because of Tigason's side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled" Comment: likely that blinding could have been broken because of etretinate's side effects, mainly symptoms from skin and/or nasobuccal mucous membranes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In the placebo group, there were initially 14 patients but 4 of them were dropouts (side effects = marked hair loss)" Comment: all dropouts were in the placebo group, and not clearly mentioned if they were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Lassus 1985.
| Methods | Randomised, parallel‐arm trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated Baseline data: randomly assigned to 1 of 4 regimens: local methoxsalen 1% 1 hour before UVA irradiation (N = 33); trioxsalen bath (0.33 mg per 1 litre of water) 15 minutes before UVA irradiation (N = 18); oral methoxsalen (0.6 mg/kg) 2 hours before irradiation with UVA (N = 13); or etretinate 0.9 to 1 mg/kg/d for 2 weeks, then 0.6 to 0.7 mg/kg/d (N = 20)
Withdrawal: 5 (lack of efficacy); 9 (lack of efficacy); 4 (lack of efficacy); 3 (adverse events: 2 had severe hair loss, and 1 had severe drying of the lips and oral mucosa, cheilitis, and conjunctivitis) |
|
| Interventions |
Intervention 1 A: local methoxsalen 1% 1 hour before UVA irradiation (33 participants) Intervention 2 B: trioxsalen bath (0.33 mg per 1 litre of water) 15 minutes before UVA irradiation (18 participants) Intervention 3 C: oral methoxsalen (0.6 mg/kg) 2 hours before irradiation with UVA (13 participants) Intervention 4 D: etretinate 0.9 to 1 mg/kg/d for 2 weeks, then 0.6 to 0.7 mg/kg/d (20 participants) Co‐interventions: none Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treated patients were randomly allocated to one of four different treatment regimens" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The treated patients were randomly allocated to one of four different treatment regimens" Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of blinding and impossibility of blinding because of the different treatment regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Severe adverse effects were observed only in the group treated with etretinate. Two patients developed severe hair loss and one severe drying of the lips, oral mucosa and cheilitis and conjunctivitis. All three patients discontinued the treatment. Altogether eighteen of the patients treated with PUVA therapy discontinued the treatment after 8 weeks because of lack of effect. Four of these were on systemic PUVA therapy, five on local methoxsalen and nine on local trioxsalen treatment" Comment: 21 participants dropped out (3 from the etretinate group and 18 from the other groups), but no clear mention if they were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Lassus 1988.
| Methods | Parallel‐arm trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria
Participants were examined before treatment and at weeks 2, 4, 8, and 12 Baseline data: randomised to acitretin (n = 30) or etretinate (n = 30)
Withdrawal: 1 in the acitretin group (complete remission after week 4) and 1 in the etretinate group (failed to return for follow‐up visits) |
|
| Interventions |
Intervention 1 A: acitretin (3 capsules of 10 mg each, once per day) (30 participants) Intervention 2 B: etretinate (3 capsules of 10 mg each, once per day) (30 participants) Co‐interventions: topical corticosteroids, oral antibiotics Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified ‐ Decrease in the number of fresh pustules ‐ Severity score depending on intensity of erythema, scaling, and infiltration (3 = severe, 2 = moderate, 1 = mild, 0 = none) ‐ Area of involvement ‐ Adverse events |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to one of the two retinoid treatment groups" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated to one of the two retinoid treatment groups" Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind comparative trial" Comment: unlikely that blinding could have been broken because both treatments are retinoids and have identical side effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind comparative trial" Comment: unlikely that blinding could have been broken because both treatments are retinoids and have identical side effects |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two patients left the study before 12 weeks of treatment; one from the etretinate group failed to return for follow‐up visits after the week 2 check while the other patient, who belonged to the acitretin group, discontinued treatment because of complete remission after 4 weeks" Comment: 2 participants dropped out but unclear how study authors dealt with it, especially that the dropout in the acitretin group was cleared, whereas we have no precision regarding the dropout in the etretinate group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Lawrence 1984.
| Methods | Randomised, parallel‐arm trial One centre in the UK Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised to PUVA therapy‐etretinate (1 mg/kg) (n = 10) or PUVA therapy‐placebo (n = 10)
Withdrawal: 1 dropout in the PUVA therapy‐placebo group (diverticulitis) |
|
| Interventions |
Intervention 1 A: etretinate (1 mg/kg/d) (10 participants) Intervention 2 B: placebo (10 participants) Co‐interventions: oral PUVA therapy Duration of treatment: 20 weeks (2 weeks of etretinate or placebo alone and 18 weeks maximum of PUVA therapy‐etretinate or PUVA therapy‐placebo) |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Roche Products Ltd supported the study and provided the etretinate. Dr Parker was supported by the Newcastle Health Authority Research Committee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to either PUVA plus placebo (PUVA‐placebo) or PUVA plus etretinate (PUVA‐etretinate)" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial was conducted as if double‐blind, although in most patients differences in side‐effects and patient response made a double‐blind assessment impossible" Comment: blinding probably broken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The trial was conducted as if double‐blind, although in most patients differences in side‐effects and patient response made a double‐blind assessment impossible" Comment: blinding probably broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Nineteen patients completed the study. One patient, who was receiving placebo, was withdrawn during the sixth week of the study because she developed acute diverticulitis, presumably unrelated to treatment" Comment: 1 participant dropped out because of diverticulitis, but not mentioned whether she was included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Layton 1991.
| Methods | Placebo‐controlled trial, within participants Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: topical PUVA therapy (26 soles; 18 palms) vs placebo (26 soles; 18 palms)
Withdrawal: no dropouts |
|
| Interventions |
Intervention 1 A: 0.75% 8‐methoxypsoralen in hydrophilic water/oil emulsion and UVA phototherapy 3 times per week (N = 26 soles; 18 palms) Intervention 2 B: placebo (hydrophilic water/oil emulsion before UVA phototherapy, with a Perspex plate inserted between palm/sole and UVA light source) 3 times per week (N = 26 soles; 18 palms) Co‐interventions: none Duration of treatment: 8 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the initial visit each side was randomly allocated active or placebo therapy" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "To the placebo‐treated side, the emulsion base only was applied for 10 min prior to exposure to the light source. A Perspex plate was inserted between the palm/sole and the UVA light source at the placebo‐treated side. These procedures were carried out so that the patient was unaware which side received active treatment" Comment: patients were probably blinded; however no details regarding blinding of personnel, so hence the risk is unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To the placebo‐treated side, the emulsion base only was applied for 10 min prior to exposure to the light source. A Perspex plate was inserted between the palm/sole and the UVA light source at the placebo‐treated side" Comment: no details on how the assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the patients had treatment three times a week over the course of 8 weeks and all completed the trial" Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Lindelof 1990.
| Methods | Within‐patient, randomised trial One centre in Stockholm Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria
Baseline data: active treatment of lesions on 1 side of the body vs control on the other side (17 participants)
Withdrawal: 2 dropouts (1 because of flare‐up at other parts of the body, and 1 elderly participant because of illness) |
|
| Interventions |
Intervention 1 A: Grenz ray therapy (4 Gy) once per week Intervention 2 B: placebo Co‐interventions: none Duration of treatment: 6 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The nurse treating the patient gave the active radiation or placebo treatment according to a randomised predetermined code" Comment: no precision on how the code was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled. Neither the patient nor the evaluating doctor knew which side had received active Grenz ray therapy" Comment: probably done, but nurse treating the patient was not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled. Neither the patient nor the evaluating doctor knew which side had received active Grenz ray therapy" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the 17 patients who started the trial, 2 patients failed to participate throughout the study" Comment: no clear mention of how study authors deals with dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Matsunami 1990.
| Methods | Randomised, parallel‐arm study Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: participants were randomly divided into 2 groups: Re (+) (n = 10) and Re (‐) (n = 10)
Withdrawal: unclear |
|
| Interventions |
Intervention 1 A: etretinate 1 mg/kg for 4 weeks, then 0.5 mg/kg (10 participants) Intervention 2 B: no treatment (10 participants) Co‐interventions: PUVA therapy once per week on the right side (for 12 weeks) Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified ‐ Degree of severity based on various factors with the conclusion of no change, beginning of resolution, or complete clearance |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into two groups" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no mention of blinding and etretinate side effects making blinding impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no mention of blinding and etretinate side effects making blinding impossible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Mrowietz 2019.
| Methods | Randomised, controlled, parallel‐arm study 61 centres in Europe Period of inclusion: December 26, 2013 to January 20, 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: secukinumab 300 mg (n = 79)/secukinumab 150 mg (n = 80)/placebo (n = 78)
Withdrawal: 15 (withdrawal by participant 7, AE 8)/15 (withdrawal by participant 7, AE 6, pregnancy 1, physician decision 1)/12 (withdrawal by participant 5, AE 6, physician decision 1) |
|
| Interventions |
Intervention 1 Secukinumab, 300 mg subcutaneously at baseline; weeks 1, 2, 3, and 4 Intervention 2 Secukinumab, 150 mg, subcutaneously at baseline; weeks 1, 2, 3, and 4 Intervention 3 Placebo subcutaneously at baseline; weeks 1, 2, 3, and 4 Duration of treatment: 4 weeks for period 1 |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Novartis sponsored the 2PRECISE study and provided funding for conduct of the study, data analysis, and medical writing assistance for the study’s publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by using interactive response technology" Comment: randomisation method was described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no indication of measure undertaken to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: pre‐filled syringe, placebo‐controlled probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: pre‐filled syringe, placebo‐controlled probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy analyses were based on the full analysis set, comprising all subjects to whom study treatment was assigned. Subjects with missing PPPASI assessments at week 16 were considered responders if they met the response criteria by the time of dropout; otherwise, they were considered nonresponder (last observation carried forward)" Comment: all included participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes pre‐specified in registration file were reported |
Muro 2016.
| Methods | Randomised, within‐patient study (left, right) One centre in Tokyo Period of inclusion: August 2010 to November 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: treatment sites were randomised to maxacalcitol + betamethasone butyrate propionate ointments or betamethasone butyrate propionate ointment alone
Withdrawal: 2 participants did not return to our hospital after the start of the study; 4 did not visit at week 8; 2 changed therapeutic strategy |
|
| Interventions |
Intervention 1 A: maxacalcitol ointment (Oxarol ointment 25 mg/g; MXA) (27 participants, within‐participant design) Intervention 2 B: no treatment (27 participants, within‐participant design) Co‐interventions: betamethasone butyrate propionate ointment (Antebate ointment 0.05%; BBP). In addition, any phototherapy or systemic therapy begun before treatment would be continued without any change to the dosage regimen Duration of treatment: 8 weeks Seven out of 27 participants received a concomitant therapy during the study: ‐ Methotrexate (n = 2) ‐ Etretinate (n = 2) ‐ Local bath‐PUVA (n = 1) ‐ Etretinate, local bath‐PUVA (n = 1) ‐ Etretinate, methotrexate (n = 1) |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The type of therapy was randomly assigned" Comment: no precision |
| Allocation concealment (selection bias) | Low risk | Quote: "The type of therapy was randomly assigned via the sealed envelope method" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "On one side two topical treatments were applied whereas on the other just one was applied making the blinding impossible " Comment: blinding impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "No double blind" Comment: no blinding was done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty one patients were completed" Comment: 21 participants completed the study instead of 29, and study authors did not include 27 in the analysis ‐ only 21 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Murray 1980.
| Methods | Within‐patient, controlled trial One centre in London Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Participants were seen weekly by a single observer Baseline data
Withrawal: no dropouts |
|
| Interventions | Oral 8‐methoxypsoralen (8‐MOP) 2 hours before UVA irradiation (4 times per week) for 30 treatments administered to all 22 participants Intervention 1 UVA irradiation (22 palms or soles, or both) Intervention 2 Other side was covered (22 palms or soles, or both) Duration of treatment: 30 treatments (sessions) (7.5 weeks) |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were irradiated four times per week on one randomly selected side" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The opposite side was covered and served as a control" Comment: as 1 side was covered, blinding was impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The treated side cleared completely in twelve patients, almost cleared in five patients and improved in four. One patient improved on both sides" Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
NCT02641730 Guselkumab.
| Methods | Randomized, double‐blind, placebo‐controlled study Thirty‐three centres in Japan Period of inclusion: January 2016 to January 2018 |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Baseline data: participants were randomised to placebo (n = 53) or guselkumab 100 mg (n = 54) or guselkumab 200 mg (n = 52) for up to 16 weeks
Withdrawal: placebo n = 2 (adverse event n = 2); guselkumab 100 n = 1 (adverse event n = 1); guselkumab 200 n = 2 (adverse event n = 2) |
|
| Interventions |
Intervention 1 A: guselkumab 200 mg at weeks 0, 4, 12 Intervention 2 B: guselkumab 100 mg at weeks 0, 4, 12 Intervention 3 C: placebo at weeks 0, 4, and 12 At week 16, placebo participants will be randomised in a 1:1 ratio to guselkumab 200 mg arm or 100 mg arm for 44‐week open‐label extension |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Funding: Janssen Pharmaceutical K.K. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" Comment: results from clinicaltrials.gov, which give no methodological details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised" Comment: results from clinicaltrials.gov, which give no methodological details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: results from clinicaltrials.gov; no methodological details but placebo‐controlled, so assumed blinding of participants and personnel was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: results from clinicaltrials.gov; no methodological details but placebo‐controlled, so assumed blinding of outcome assessment was probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were analysed. Missing data were imputed or 'last observation carried forward' was considered |
| Selective reporting (reporting bias) | High risk | Percentage of participants who achieved a PPPASI‐50 response at week 16 was one of the outcomes described in study details, and this outcome was reported for 12 and 20 weeks but not for 16 weeks |
Nielsen 1995.
| Methods | Cross‐over study Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: random allocation to the sequence of treatment: clobetasol propionate ointment occluded with hydrocolloid dressing v/s clobetasol propionate ointment occluded with hydrocolloid dressing followed by PUVA therapy (n = 22)
Withdrawal: 1 participant did not tolerate 8‐methoxypsoralen and was withdrawn |
|
| Interventions |
Intervention 1 A: oral PUVA therapy (22 participants, cross‐over design) Intervention 2 B: no treatment (22 participants, cross‐over design) Co‐interventions: clobetasol propionate under occlusion Duration of treatment: 3 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "with random allocation to the sequence of treatments" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "with random allocation to the sequence of treatments" Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The trial was designed as a single‐blind crossover study" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The trial was designed as a single‐blind crossover study" Comment: unclear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One patient did not tolerate 8‐methoxypsoralen and was withdrawn" Comment: unclear how study authors dealt with the dropout |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Reich 2016.
| Methods | Phase II, randomised, placebo‐controlled, parallel‐arm study Seven centres in France, Germany, the Netherlands, and the UK (community centre clinics) Period of inclusion: 26 April 2011 to 16 April 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: participants were randomised to alitretinoin 30 mg once daily (n = 24) or placebo (n = 9) for up to 24 weeks
Withdrawal: 10 (lack of efficacy: n = 4; adverse event: n = 3; reason not specified: n = 3); 3 (lack of efficacy: n = 2; adverse event: n = 1) |
|
| Interventions |
Intervention 1 A: alitretinoin 30 mg once daily PO (24 participants) Intervention 2 B: placebo (9 participants) Co‐interventions: none Duration of treatment: 24 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This study was funded by Stiefel, a GSK company, and Basilea Pharmaceutica Deutschland GmbH NCT01245140 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to treatment by use of a computer‐generated randomisation code, and were assigned a patient number sequentially in order of enrolment" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were assigned to treatment by use of a computer‐generated randomisation code, and were assigned a patient number sequentially in order of enrolment. Alitretinoin and placebo had indistinguishable physical characteristics and were provided in packaging that did not reveal the study product identity" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled with no major side effects" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The full analysis set (used for analysis of all efficacy end points) included all patients in the safety population with at least one efficacy assessment" Comment: even though there were 13 dropouts, the full analysis set (used for analysis of all efficacy endpoints) included all participants in the safety population with at least 1 efficacy assessment. In the alitretinoin group, 10 dropouts (lack of efficacy: n = 4; adverse event: n = 3; reason not specified: n = 3); in the placebo group, 3 dropouts (lack of efficacy: n = 2; adverse event: n = 1) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes of interest in the review have been reported in the a pre‐specified way |
Reitamo 1993.
| Methods | Randomised, placebo‐controlled, parallel‐arm trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised to ciclosporin 2.5 mg/kg/d (n = 20) or placebo (n = 20)
Withdrawal: 2 dropouts (unknown reason) |
|
| Interventions |
Intervention 1 A: oral ciclosporin twice daily (2.5 mg/kg/d) (20 participants) Intervention 2 B: placebo (20 participants) Co‐interventions: none Duration of treatment: 4 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This investigation was supported by Sandoz Pharma, Basel, Switzerland; and Finska Làkaresallskapet and the Paulo Foundation, Helsinki, Finland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were given numbers 1 through 40 in consecutive order; each number had been pre assigned to treatment with either cyclosporine or placebo" Comment: unclear of how the list was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Cyclosporine or placebo (i.e., vehicle without cyclosporine) was administered twice daily as capsules" Comment: unclear whether or not allocation concealment was present |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thirty‐eight of the 40 patients were considered valid for the statistical analysis at week 4" Comment: the analysis was an ITT |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Rodriguez 2000.
| Methods | Placebo‐controlled, parallel‐arm study One centre in Spain Period of inclusion: June to October 1999 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: participants received 20% aqueous solution of aluminium chloride hexahydrate (n = 6) or placebo (n = 6)
Withdrawal: no dropouts |
|
| Interventions |
Intervention 1 A: 20% aqueous solution of aluminium chloride hexahydrate (6 participants) Intervention 2 B: placebo (6 participants) Co‐interventions: none Duration of treatment: 5 months |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study authors mentioned that the study is blinded, randomised, and controlled with placebo |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: study authors mention in the methods that the study is blinded, even though in the abstract they say it is an open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: study authors mention in the methods that the study is blinded, even though in the abstract they say it is an open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Rosen 1987.
| Methods | Placebo‐controlled, randomised, within‐participants study Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Clinical situation was assessed by the same investigator (KR) before treatment (2 weeks), after 2 weeks (0 weeks), and then every 3 weeks by judging the severity of lesions on either side Baseline data: randomly allocated to etretinate 0.6 mg/kg/d (23 palms/soles) or placebo (14 palms/soles) or etretinate 0.6 mg/kg/d and PUVA therapy 3 times per week (23 palms/soles) or PUVA therapy 3 times per week (14 palms/soles)
Withdrawal: 7 participants were lost because of adverse events (5 in the etretinate/etretinate + PUVA therapy group: reasons for ending therapy were suddenly increased light sensitivity, with development of bullae on the feet, in combination with dry and scaly dermatitis, itching, and cheilitis (2 participants); extreme dryness of the skin and mucous membranes (1 participant); pain under all fingernails after 3 weeks of etretinate treatment (no UVA to the hands), without signs of paronychia (1 participant); and thrombophlebitis on the lower leg, considered unrelated to therapy (2 participants and 2 in the placebo/PUVA therapy group). One placebo‐treated participant withdrew from the trial because he did not tolerate the psoralen tablets, and another because he could not return regularly |
|
| Interventions |
Randomisation of participants to etretinate vs placebo, then for each group randomisation of 1 side (UVA irradiation or not) Intervention 1 A: oral etretinate twice per day (0.6 mg/kg/d) (23 palms/soles) Intervention 2 B: placebo (14 palms/soles) Intervention 3 C: oral etretinate (0.6 mg/kg/d) + UVA (3 times per week) (23 palms/soles) Intervention 4 D: UVA irradiation (3 times per week) (14 palms/soles) Co‐interventions: none Duration of treatment: 14 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | AB Draco, Lund, Sweden, provided the methoxsalen (Puvamet) tablets; and AB Hoffmann‐La Roche, Skärholmen, Sweden, the etretinate (Tigason) tablets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were allocated to treatment groups according to year of birth (even or odd)" Comment: non‐random component in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Quote: "The patients were allocated to treatment groups according to year of birth (even or odd)" Comment: participants and investigators could foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "placebo‐controlled" Comment: placebo‐controlled but obvious side effects of etretinate, especially mucocutaneous side effects (dry lips and skin) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "placebo‐controlled" Comment: placebo‐controlled but obvious side effects of etretinate, especially mucocutaneous side effects (dry lips and skin) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "7 patients were lost because of adverse events (5 in the etretinate/etretinate +PUVA group: the reasons for ending therapy were suddenly increased light sensitivity, with development of bullae on the feet in combination with dry and scaly dermatitis, itching, and cheilitis (two patients); extreme dryness of the skin and mucous membranes (one patient); pain under all fingernails after three weeks of etretinate treatment (no UVA to the hands), without signs of paronychia (one patient); and thrombophlebitis on the lower leg, considered unrelated to therapy (one patient) and 2 in the placebo/PUVA group: One placebo treated patient withdrew from the trial because he did not tolerate the psoralen tablets, and another because he could not return regularly). The number of treatment‐related dropouts in the two groups was not statistically different" Comment: more than 10% of participants dropped out and there was no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Schroder 1989.
| Methods | Randomised, parallel‐arm trial Three centres in Germany (community centre clinics) Period of inclusion: March 1986 to February 1988 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: participants were randomised to receive etretinate (n = 15) or placebo (n = 15)
Withdrawal: not stated |
|
| Interventions |
Intervention 1 A: etretinate 50 mg once per day (15 participants) Intervention 2 B: placebo (15 participants) Co‐interventions: 2% salicylic ointment Duration of treatment: 4 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were admitted using a list of randomisation" Comment: no precision on how it was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "either etretin or placebo" Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind study" Comment: however, 65% of participants treated with Tigason had side effects (mainly mucocutaneous side effects), so likely that blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind study" Comment: however, 65% of participants treated with Tigason had side effects (mainly mucocutaneous side effects), so likely that blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of missing data |
| Selective reporting (reporting bias) | High risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results. Not all outcomes cited in the methods were found in the results (clinical aspect: 4‐point‐score) |
Su 2017.
| Methods | Pilot randomised controlled parallel‐arm trial; within‐participants study Two centres in China (Shanghai and Huashan) Period of inclusion: May 2015 to April 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: sides were randomised to receive UVA1 (n = 33) or NB‐UVB treatment (n = 33) according to a left‐right randomisation table
Withdrawal: 2 dropouts (1 in the UVA1 group discontinued for non‐compliance, and 1 in the NB‐UVB group dropped out for personal reasons) |
|
| Interventions |
Intervention 1 A: UVA1 phototherapy (32 participants) Intervention 2 B: Narrowband UVB phototherapy (32 participants) Co‐interventions: none Duration of treatment: 30 sessions (3 sessions per week) |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | This study was supported by a grant from the Natural Science Foundation of Shanghai Municipal Commission of Health and Family Planning (201640188) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomly assigned to either UVA1 or NB‐UVB treatment according to a left‐right randomisation table" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient of the UVA1 group discontinued for non‐compliance, and one patient of the NB‐UVB group dropped out for personal reason. Therefore, 64 completed the study" Comment: only 2 dropouts; we know the reasons |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol and no registration found to guarantee that all planned outcomes are presented in the results |
Terui 2018.
| Methods | Double‐blind, randomised, placebo‐controlled, parallel‐group trial 11 centres in Japan Period of inclusion: 14 May 2013 to 27 September 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: guselkumab/placebo
Withdrawal: in guselkumab group: 2 participants discontinued (1 adverse event, 1 physician’s decision); in the placebo group: 6 discontinued (4 withdrew consent, 2 Initiated protocol‐prohibited medication) |
|
| Interventions |
Intervention 1 200 mg of guselkumab subcutaneously week 0 and week 4 (52 participants) Intervention 2 100 mg guselkumab (54 participants) Control intervention Placebo subcutaneously week 0 and week 4 (53 participants) Duration of treatment: 4 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This study was funded by Janssen Pharmaceutical K.K., Tokyo, Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects will be randomly assigned to 1 of 2 treatment groups based on a randomization schedule prepared before the study by or under the supervision of the sponsor. The randomization will be balanced by using randomly permuted blocks and will be stratified by study site (supplementary appendix)" Comment: randomisation method described |
| Allocation concealment (selection bias) | Low risk | Quote: "The unblinded pharmacy staff (Pharmacists or medically licensed individuals) responsible for the preparation of study drugs at each site will be unblinded to treatment assignment throughout the study and will prepare, dispense, and account for all study drugs. These individuals should have no other contact with the subject during the study other than study drug administration, should not communicate their knowledge of treatment assignment to any other study personnel. An independent, unblinded drug monitor will monitor any study drug preparation and accountability data.(supplementary appendix)" Comment: allocation was likely concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blinded, controlled study with no expected specific adverse effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double‐blinded, controlled study with no expected specific adverse effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The primary and other efficacy endpoints were analyzed on the full analysis set (FAS) that consisted of all randomised patients. The last observation carried forward (LOCF) approach was used to impute missing data" Comment: withdrawals were 2 in active treatment and 6 in placebo |
| Selective reporting (reporting bias) | High risk | Proportions of participants with ≥ 75% improvement from baseline in PPPASI score (PPPASI‐75), change from baseline in physician’s assessment, patient’s visual analogue scale assessment for PPP and pustulotic arthro‐osteitis activity and pain, change in Dermatology Life Quality Index, and responses to the Short Form Health Survey were also investigated. However, these assessments are not included in the present report |
Thestrup‐Pedersen 1984.
| Methods | Cross‐over trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Clinical evaluation was performed before and after 4 and after 8 weeks Baseline data: colchicine 0.5 mg 3 to 4 times per day according to weight vs placebo (n = 27)
Withdrawal: 1 dropout (reason not mentioned) |
|
| Interventions |
Intervention 1 A: colchicine 0.5 mg (3 to 4 times per day according to weight) (27 participants, cross‐over design) Intervention 2 B: placebo (3 to 4 times per day) (27 participants, cross‐over design) Co‐interventions: none Duration of treatment: 8 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients received Tablet A for four weeks and Tablet B for another four weeks" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled. Drugs were added to placebo to give a bitter taste equal to tablets with colchicine" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient received colchicine, but not placebo" Comment: only 1 dropout in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Thomsen 1973.
| Methods | Cross‐over trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: tetracycline 250 mg twice daily vs placebo (n = 40)
Withdrawal: 2 participants stopped taking treatment after 2 courses because of side effects |
|
| Interventions |
Intervention 1 A: tetracycline 250 mg twice daily (40 participants, cross‐over design) Intervention 2 B: placebo twice daily (40 participants, cross‐over design) Co‐interventions: none Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treatment was randomised in three periods of 4 weeks each. Thus, each patient had one course of tetracycline and two courses of placebo or vice versa. The treatment periods followed immediately upon each other, but the sequence was accidental" Comment: unclear how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind technique was applied. Tetracycline, 250 mg, and identical‐looking lactose capsules were used, the dosage being one capsule twice daily" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind technique was applied" Comment: we consider blinding at low risk for trial vs placebo with no obvious clinical adverse events or known specific taste of experimental drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the forty patients treated thirty‐eight completed the study. Two patients stopped taking the drug after two courses because of side effects. Nevertheless, these two patients are included in the analysis" Comment: the 2 dropouts were included in the statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Thune 1982.
| Methods | Cross‐over trial Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated Baseline data: Tigason 75 mg per day vs placebo (n = 42)
Withdrawal: 3 female participants stopped Tigason because of side effects (2 because of alopecia and 1 because of development of pustules and abscesses in the perineal area) |
|
| Interventions |
Intervention 1 A: Tigason 25 mg thrice daily and reduced according to efficacy (42 participants, cross‐over design) Intervention 2 B: placebo (42 participants, cross‐over design) Co‐interventions: none Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Tigason tablets were supplied by F.Hoffmann La Roche and Co Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For each treatment period all patients were given one box containing 100 capsules. At the end of each treatment period the patient was supplied with a new box of capsules" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled" Comment: likely that blinding could have been broken because of Tigason's side effects |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled" Comment: likely that blinding could have been broken because of Tigason's side effects |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "30 (19 men and 11 women) of the 42 patients were regularly assessed after 4, 8 and 12 weeks of treatment and their data were analysed" Comment: data analysis was done on only 30 participants and no ITT analysis was done |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Umezawa 2016.
| Methods | Randomised, placebo‐controlled trial 26 centres, Tokyo (both hospital and community centre clinics) Period of Inclusion: September 2005 to August 2006 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised on a 1:1 basis to OCT (a white translucent ointment containing 25 lg/g of maxacalcitol) (n = 95) group or placebo group (n = 93)
Withdrawal: 1 participant in the OCT group was not included in the FAS due to lack of evaluable data after investigational treatment |
|
| Interventions |
Intervention 1 A: OCT (25 microg/g of maxacalcitol ointment) (94 participants) Intervention 2 B: placebo (93 participants) Co‐interventions: none Duration of treatment: 8 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | This study was financially supported by Maruho. The topical study drug was provided by Chugai Pharmaceutical. YU has served as a paid speaker for Maruho. HN has served as a paid speaker, advisory board member, and consultant for Maruho | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "white translucent ointment" Comment: double‐blind, placebo‐controlled trial and both OCT and placebo were presented as white translucent ointment. However, no information on allocation concealment or packaging |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: double‐blind, placebo‐controlled trial and both OCT and placebo were presented as white translucent ointment. No information on measure applied to guarantee blind assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One subject in the OCT group was not included in the FAS due to a lack of evaluable data after the investigational treatment" Comment: only 1 participant left and was not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
Ward 1976.
| Methods | Cross‐over trial Three centres in London Period of inclusion: February 1973 to May 1974 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Participants were seen by an observer at intervals of 6 weeks Baseline data: clomocycline 170 mg thrice daily vs placebo (n = 60)
Withdrawal: 6 participants did not attend (4 after the first visit), 14 discontinued because of side effects (1 was on placebo and complained of heartburn and 13 were on clomocycline and complained of nausea and vomiting (6), vaginal thrush (1), constipation (1), heartburn (1), and miscellaneous symptoms (4)) |
|
| Interventions |
Intervention 1 A: clomocycline 170 mg thrice daily for 2 weeks, then twice a day for 10 weeks (60 participants; cross‐over design) Intervention 2 B: placebo (60 participants, cross‐over design) Co‐interventions: ointment or dilute Betnovate in petrolatum Duration of treatment: 3 months |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient received 3 months each of clomocycline and placebo in random order" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The paired packs were randomly labelled A and B using a restricted randomisation in tens, so that when treatment was started with pack A neither investigator nor patient knew whether it contained clomocycline or placebo" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The paired packs were randomly labelled A and B using a restricted randomisation in tens, so that when treatment was started with pack A neither investigator nor patient knew whether it contained clomocycline or placebo" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of sixty patients entering the trial twenty failed to complete the treatment. Six patients did not attend, four after the first visit. The remaining fourteen discontinued with side effects: one of these was on placebo and complained of heartburn; while the thirteen on clomocycline complained of nausea and vomiting (6), vaginal thrush (1), constipation (1), heartburn (1), miscellaneous symptoms (4)" Comment: 20 dropouts and no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
White 1985.
| Methods | Controlled, parallel‐arm study Number of centres not stated Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria Not stated Baseline data: randomised to oral etretinate 1 mg/kg/d (n = 10) or placebo (n = 10)
Withdrawal: 1 participant in the etretinate group failed to attend after the first week because he moved to another part of the country |
|
| Interventions |
Intervention 1 A: etretinate (1 mg/kg/d) (10 participants) Intervention 2 B: placebo (10 participants) Co‐interventions: none Duration of treatment: 10 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Quote from the paper: "Roche Products Ltd supplied the etretinate and Dr AJ Miller of Roche Products Ltd supported the trial" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated, according to a random number system, to oral etretinate (1 mg/kg/day) or to placebo" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were allocated, according to a random number system, to oral etretinate (1 mg/kg/day) or to placebo. Neither the doctor nor the patient knew which treatment the patient was receiving until after completion of the trial" Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled. Neither the doctor nor the patient knew which treatment the patient was receiving until after completion of the trial" Comment: blinding likely broken because of frequent side effects in the etretinate group, mainly mucocutaneous side effects |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "double‐blind placebo‐controlled. Neither the doctor nor the patient knew which treatment the patient was receiving until after completion of the trial" Comment: blinding likely broken because of frequent side effects in the etretinate group, mainly mucocutaneous side effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in the etretinate group failed to attend after the first week, because he moved to another part of the country, and was not included in subsequent analyses. Four other patients withdrew before the end of the trial because they were dissatisfied; one in the test group and three in the placebo group" Comment: only 1 participant was not included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
White 1986.
| Methods | Randomised, parallel‐arm trial One centre in Newcastle, UK Period of inclusion not stated |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline data: randomised to etretinate 30 mg/d (n = 10) or placebo (n = 10)
Withdrawal: 2 in each group were lost (1 for an episode of chest pain and the other who felt treatment was not controlling the disease in the etretinate group; 1 for cellulitis of the leg and the other for poor compliance in the placebo group) |
|
| Interventions |
Intervention 1 A: etretinate 30 mg/d (10 participants) Intervention 2 B: placebo (10 participants) Co‐interventions: none Duration of treatment: 12 weeks |
|
| Outcomes | No primary or secondary outcome pre‐specified
|
|
| Notes | Quote: "Thanks to Dr Alan Miller and Miss Sandy Jones and to Roche Products Limited for support" Comment: it appears that the study had industry support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After an initial 4‐week period of taking 70 mg etretinate per day, patients were allocated at random to one of two treatment regimens, receiving either 30 mg etretinate per day or identical placebo capsules" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on method to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither patient nor doctor knew which patients were allocated to which group" Comment: however, blinding was likely broken because of obvious side effects in the etretinate group, mainly mucocutaneous side effects |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Neither patient nor doctor knew which patients were allocated to which group" Comment: however, blinding was likely broken because of obvious side effects in the etretinate group, mainly mucocutaneous side effects |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eight patients in the etretinate group completed the trial. Of the others, one had an episode of chest pain 2 weeks after starting the maintenance dose and was advised by her general practitioner to stop the tablets. Data from this patient were not included in subsequent analyses. Another patient withdrew after 4 weeks on the low‐dose regimen because she felt the treatment was not controlling her condition. Eight patients in the placebo group completed the trial. The two who did not were one with poor compliance and one who was withdrawn when she developed cellulitis of her leg after 4 weeks. This settled with antibiotics. Her data were not included in the analyses from that point" Comment: 2 of the 4 dropouts were not included in subsequent analyses, but unsure if the other 2 were included |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found to guarantee that all planned outcomes are presented in the results |
AE: adverse event.
BBP: betamethasone butyrate propionate.
DLQI: Dermatology Life Quality Index.
FAS: full analysis set.
IL: interleukin.
ITT: intention‐to‐treat.
LOCF: last observation carried forward.
mPASI: modified Psoriasis Area and Severity Index.
NB‐UVB: narrowband ultraviolet B.
PAO: pustulotic arthro‐osteitis.
PGA: physicians' global assessment.
PPP: palmoplantar pustulosis.
PPPASI: Palmo‐Plantar Pustular Area and Severity Index.
PPPGA: Palmo‐Plantar Physician Global Assessment.
PPQoLI: Palmoplantar Quality of Life Index.
PPSI: Palmo‐Plantar Severity Index.
PUVA: combination of psoralens and long‐wave ultraviolet radiation.
RCT: randomised controlled trial.
SD: standard deviation.
TAA: triamcinolone acetonide.
UVA: ultraviolet A.
UVA1: ultraviolet A1.
WPAI:PSO: Work Productivity and Activity Impairment questionnaire:Psoriasis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aso K 1983 | Not an RCT |
| Carr 2008 | Not an RCT |
| Cassano 2010 | All included patients had palmoplantar psoriasis ‐ not palmoplantar pustulosis |
| Dupre 1973 | Not an RCT |
| Duweb 2001 | All cases concerned patients with palmoplantar psoriasis ‐ not palmoplantar pustulosis |
| Engin 2005 | Only 4 of the included patients had palmoplantar pustulosis (and results are not specified clearly for them); the others had other types of palmoplantar dermatosis |
| Fritsch 1978 | Not an RCT |
| Gjertsen 1980 | Not an RCT |
| Grundmann‐Kollmann 1999 | None of the selected patients had palmoplantar pustulosis |
| Gupta 2011 | Not an RCT |
| Hofer 2006 | Only 5 out of 8 participants had palmoplantar pustulosis and 3 had hyperkeratotic plaque psoriasis with no differentiation in results between the 2 subgroups |
| Janagond 2013 | None of the selected patients had palmoplantar pustulosis |
| Khandpur 2011 | All included patients had palmoplantar psoriasis, with no mention of the presence of pustulosis |
| Kumar 1997 | All included patients had plaque‐type ‐ not pustular ‐ palmar and/or plantar psoriasis |
| Mehta 2011 | All included patients had plaque‐type palmar and/or plantar psoriasis, and patients with palmoplantar pustulosis were excluded |
| Neumann 2006 | None of the selected patients had palmoplantar pustulosis |
| Orfanos 1978 | Trial includes different types of psoriasis |
| Papp 2012 | All included patients had plaque psoriasis of palms, soles, and scalp, but not palmoplantar pustulosis |
| Rosen 1988 | Study of epidermal Langerhans cells on skin biopsies |
| Schiener 2005 | None of the selected patients had palmoplantar pustulosis |
| Sezer 2007 | None of the selected patients had palmoplantar pustulosis (palmoplantar psoriasis ‐ no pustulosis described in inclusion criteria or patient description at baseline) |
| Thaci 2010 | All patients had plaque‐type psoriasis, which may have involved palms or soles |
| Yaniv 2012 | Not a randomised controlled trial |
| Zhang Jun 2007 | Not an RCT |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
EudraCT 2006‐004519‐23.
| Methods | Randomised, controlled trial One centre in Austria Period of inclusion: not stated |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions |
Intervention 1 A: acitretin (Neotigason) and PUVA therapy Intervention 2 B: fumaric acid ester and PUVA therapy |
| Outcomes |
Primary outcome of the trial
Secondary outcome of the trial Not stated |
| Notes | Study registered in 2009 and never published An email was sent to study authors with no reply (12 June 2018) |
Fenton 1983.
| Methods | "Not clear if an RCT, and unable to obtain further information as only the abstract is available" Cross‐over, placebo‐controlled trial Number of centres: not stated Period of inclusion: not stated |
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated |
| Interventions |
Intervention 1 A: benoxaprofen (600 mg) Intervention 2 B: placebo |
| Outcomes | No primary or secondary outcome pre‐specified |
| Notes | ‐ |
Mann 1982.
| Methods | "Not clear if an RCT, and unable to obtain further information as only the abstract is available" Cross‐over trial Number of centres: not stated Period of inclusion: not stated |
| Participants |
Inclusion criteria Not stated Exclusion criteria Not stated |
| Interventions |
Intervention 1 A: oral colchicine (5 mg twice daily) Intervention 2 B: placebo |
| Outcomes | No primary or secondary outcome pre‐specified
|
| Notes | ‐ |
NCT03135548 BI 655130.
| Methods | Double‐blind, randomised, placebo‐controlled, phase IIa study Multi‐centre Period of inclusion: starting 30 May 2017 |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions |
Intervention 1 A: BI 655130 (low‐dose) 12 weeks of treatment Intervention 2 B: Placebo 12 weeks of treatment Intervention 3 C: BI 655130 (high‐dose) 12 weeks of treatment |
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
| Notes | Sponsor: Boehringer Ingelheim |
AE: adverse event.
PPP: palmoplantar pustulosis.
ppPASI: Palmoplantar Pustular Psoriasis Area and Severity Index.
PPPGA: Palmoplantar Pustulosis Physicians Global Assessment.
PUVA: combination of psoralens and long‐wave ultraviolet radiation.
RCT: randomised controlled trial.
TNF: tumour necrosis factor.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN13127147 APRICOT.
| Trial name or title | APRICOT ‐ Anakinra for Pustular Psoriasis |
| Methods | Randomised controlled trial, interventional Four centres in the UK Period of inclusion: November 2011 to May 2019 |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions |
Intervention 1 A: anakinra (Kineret) 100 mg/0.67 mL Intervention 2 B: placebo (matched 0.67‐mL vehicle solution) Duration of treatment: 8 weeks |
| Outcomes |
Primary outcome of the trial
Secondary outcome of the trial
|
| Starting date | November 2011 (estimated completion date May 2019) |
| Contact information | Miss Rosemary Wilson rosemary.wilson@gstt.nhs.uk |
| Notes | National Institute for Health Research (UK) is funding this trial Trial No.: ISRCTN13127147 |
NCT03633396.
| Trial name or title | A Study to Evaluate the Efficacy and Safety of ANB019 in Subjects With Palmoplantar Pustulosis (PPP) |
| Methods | Phase II, randomised, placebo‐controlled, double‐blind study Period of inclusion: starting 20 November 2018 |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions |
Intervention 1 A: ANB019 (humanised monoclonal antibody) as subcutaneous (SC) injection every 4 weeks Intervention 2 B: placebo solution as subcutaneous (SC) injection every 4 weeks |
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
| Starting date | 20 November 2018 (estimated study completion date: December 2019) |
| Contact information | Contact: Cherie Robbins, BScN (clinicaltrialsinfo@anaptysbio.com) Contact: Irina Khanskaya, MD (clinicaltrialsinfo@anaptysbio.com) |
| Notes | Sponsor: AnaptysBio, Inc. |
AE: adverse event.
ALT: alanine aminotransferase.
AST: aspartate aminotransferase.
CrCl: creatinine clearance.
DLQI: Dermatology Life Quality Index.
EQ‐5D‐5L: EuroQoL Group Quality of Life Questionnaire based on five‐level scale.
HIV: human immunodeficiency virus.
IL: interleukin.
PASI: Psoriasis Area and Severity Index.
PPP: palmoplantar pustulosis.
PPPASI: Palmoplantar Pustular Psoriasis Area and Severity Index.
PPP‐IGA: Palmoplantar Pustulosis Investigators' Global Assessment.
PPSI: Palmo‐Plantar Severity Index.
PUVA: combination of psoralens and long‐wave ultraviolet radiation.
TB: tuberculosis.
TNF: tumour necrosis factor.
ULN: upper limit of normal.
Differences between protocol and review
Methods > Criteria for considering studies for this review > Types of outcomes: We changed the outcomes compared with the previous Cochrane Review. This was done in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which advised that our primary outcomes needed to include one efficacy outcome and one safety outcome. Also, for assessing clinical improvement, we assessed clearance of the disease (as in the previous version), but we were more precise for the other clinical improvement outcomes (e.g. adding 'Proportion of participants achieving a 50% reduction in disease severity in the short term'), so we were able to synthesise results that show a clinically meaningful treatment effect.
Primary outcomes were as follows.
Improvement in disease severity as assessed by objective severity assessment, preferably as measured by reduction in objective measure of disease severity (e.g. pre‐defined semi‐quantitative disease severity score).
Clearance of disease as assessed by objective severity assessment, preferably as measured as an objective measure of disease severity (e.g. pre‐defined semi‐quantitative disease severity score).
Patient satisfaction and quality of life scores: improvement in patient satisfaction measures and quality of life assessment measures over the time course of the intervention.
Secondary outcomes were as follows.
Maintenance of reduction of disease severity from baseline as assessed by objective measure of disease severity (e.g. pre‐defined semi‐quantitative disease severity score).
Maintenance of patients' satisfaction and quality of life scores.
Relapse rates as measured by proportion of patients relapsing to baseline scores during continued treatment or following discontinuation of treatment.
Tertiary outcome measures were as follows.
Adverse events and side effects.
Methods > Criteria for considering studies for this review > participants: we added the following two sentences: "We excluded studies that included patients with non‐pustular palmoplantar psoriasis" because we had not anticipated that trials could have this specific criterion of inclusion, and our review was focused on palmoplantar pustulosis ‐ not on the non‐pustular form of palmoplantar psoriasis; and "In cases where studies included only a subset of relevant participants, we included the study only if the characteristics of patients and results were provided separately or were obtained through contact with study authors."
Methods > Search methods for identification of studies > Electronic searches > Trials registers: We planned to search relevant trials submitted to the US Food and Drug Administration (FDA) for drug registration (www.accessdata.fda.gov/scripts/cder/drugsatfda/), but we did not search this source because all drugs assessed were old or had no approval for this indication.
Methods > Data collection and analysis: We also included our secondary outcomes in the 'Summary of findings' tables.
Methods > Unit of analysis: We did not anticipate within‐participant trial design. We added the following sentence: "In case of trials with a within‐participant design, we aimed to take into account the within‐participant variability. When the P value had been computed, we reconstructed the paired data table to calculate the risk ratio and the confidence interval (Hirji 2011). When the P value had not been computed, we described the results without a P value or 95% CI."
Methods > Subgroup analysis and investigation of heterogeneity: We could conduct only two meta‐analyses (each containing two studies); hence, we could not investigate heterogeneity as planned in our protocol.
Methods > Data synthesis: We deleted the following as these methods were not used; instead, we used the random‐effects mode: "In case of homogeneity, we will pool treatment effect estimates using the Mantel‐Haenszel method described as follows (Mantel 1959): ѰMH =(Σai di/ni )/(Σbici/ni ). Please see Table 2. The Mantel‐Haenszel method is more robust than the Woolf method for small numbers of participants in control groups and can be used without modification in case of no events, unlike the Peto method (Deeks 2001)."
Contributions of authors
GO was the contact person with the Editorial Base. LLC and GO co‐ordinated contributions from the co‐authors and wrote the final draft of the review. GO, GD, KL, and LLC screened papers against eligibility criteria. GO obtained data on ongoing and unpublished studies. GO, GD, KL, and LLC appraised the quality of papers. GO, GD, KL, and LLC extracted data for the review and sought additional information about papers. GO and LLC entered data into RevMan. GO, LLC, and ES analysed and interpreted data. LLC and GD worked on the methods sections. GO, GD, and LLC drafted the clinical sections of the background and responded to the clinical comments of the referees. GO and LLC responded to the methodology and statistics comments of the referees. CH was the consumer co‐author and checked the review for readability and clarity, as well as ensuring that outcomes are relevant to consumers. LLC is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of Cochrane Skin
Declarations of interest
Grace Obeid: none known. Giao Do: none known. Lisa Kirby: none known. Carolyn Hughes: none known. Emilie Sbidian: none known. Laurence Le Cleach: none known.
New
References
References to studies included in this review
Bhushan 2001 {published data only}
- Bhushan M, Burden AD, McElhone K, James R, Vanhoutte FP, Griffiths CE. Oral liarozole in the treatment of palmoplantar pustular psoriasis: a randomized, double‐blind, placebo‐controlled study. British Journal of Dermatology 2001;145(4):546‐53. [CENTRAL: CN‐00375323; PUBMED: 11703279] [DOI] [PubMed] [Google Scholar]
- Bhushan M, Burden AD, McElhone K, Vanhoutte FP, Griffiths CEM. A double‐blinded placebo controlled study of oral liarozole in the treatment of palmoplantar pustular psoriasis. Abstract 34, International Congress 'Psoriasis: from gene to clinic'. British Journal of Dermatology 1999;141(5):978. [CENTRAL: CN‐00428744] [DOI] [PubMed] [Google Scholar]
Bissonnette 2008 {published data only}
- Bissonnette R, Poulin Y, Bolduc C, Maari C, Provost N, Syrotuik J, et al. Etanercept in the treatment of palmoplantar pustulosis. Journal of Drugs in Dermatology 2008;7(10):940‐6. [CENTRAL: CN‐00664757; PUBMED: 19112757] [PubMed] [Google Scholar]
Bissonnette 2014 {published data only}
- Bissonnette R, Lynde CW, Krueger JG, Tan J, Nigen S, Langley R.B. Interleukins‐23 and ‐17 in patients with palmoplantar pustular psoriasis and palmoplantar pustulosis. Journal of the European Academy of Dermatology and Venereology 2014;28(10):1298‐305. [PUBMED: 24112799] [DOI] [PubMed] [Google Scholar]
Cazzaniga 2014 {published data only}
- Cazzaniga S, Lo Scocco G, Schincaglia E, Mercuri SR, Chimenti S, Saraceno R, et al. Randomized, within‐patient, clinical trial comparing fluorine‐synthetic fiber socks with standard cotton socks in improving plantar pustulosis. Dermatology (Basel, Switzerland) 2014;228(2):166‐71. [CENTRAL: CN‐00991953; PUBMED: 24434748] [DOI] [PubMed] [Google Scholar]
Erkko 1998 {published data only}
- Erkko P, Granlund H, Remitz A, Rosen K, Mobacken H, Lindelof B, et al. Double‐blind placebo‐controlled study of long‐term low‐dose cyclosporin in the treatment of palmoplantar pustulosis. British Journal of Dermatology 1998;139(6):997‐1004. [CENTRAL: CN‐00159869; PUBMED: 9990362] [DOI] [PubMed] [Google Scholar]
Fairris 1984 {published data only}
- Fairris GM, Jones DH, Mack DP, Rowell NR. Superficial X‐ray therapy in the treatment of palmoplantar pustulosis. British Journal of Dermatology 1984;111(4):499‐500. [CENTRAL: CN‐00568893; PUBMED: 6386034] [DOI] [PubMed] [Google Scholar]
Foged 1983 {published data only}
- Foged E, Holm P, Larsen PO, Laurberg G, Reymann F, Roesdahle K, et al. A randomized trial of etretinate (Tigason) in palmoplantar pustulosis. Dermatologica 1983;166(4):220‐3. [CENTRAL: CN‐00031137; PUBMED: 6343149] [DOI] [PubMed] [Google Scholar]
Fredriksson 1978 {published data only}
- Fredriksson T. Oral treatment of psoriasis and pustulosis palmo‐plantaris with Ro 10‐9359. Dermatologica 1978;157(Suppl 1):13‐8. [CENTRAL: CN‐00499373; PUBMED: 150982] [DOI] [PubMed] [Google Scholar]
- Fredriksson T, Pettersson U. Oral treatment of pustulosis palmo‐plantaris with a new retinoid, Ro 10‐9359. Dermatologica 1979;158(1):60‐4. [CENTRAL: CN‐00019698; Pubmed: 153863] [DOI] [PubMed] [Google Scholar]
Hattel 1974 {published data only}
- Hattel T, Sondergaard J. Pustulosis palmaris et plantaris treated with hydroxyurea. Acta Dermato‐venereologica 1974;54(2):152‐4. [CENTRAL: CN‐00010203; PUBMED: 4133022] [PubMed] [Google Scholar]
Jansen 1979 {published data only}
- Jansen C, Hollmén A, Pajarre R. Peroral aromatic retinoid treatment of palmoplantar pustulosis: double‐blind comparison of Ro 10‐9359 and placebo. Acta Dermato‐venereologica 1979;59(3):271‐3. [CENTRAL: CN‐00020396; Pubmed: 87093] [PubMed] [Google Scholar]
Kragballe 1991 {published data only}
- Kragballe K, Larsen FG. A hydrocolloid occlusive dressing plus triamcinolone acetonide cream is superior to clobetasol cream in palmo‐plantar pustulosis. Acta Dermato‐venereologica 1991;71(6):540‐2. [CENTRAL: CN‐00081448; PUBMED: 1685841] [PubMed] [Google Scholar]
Lassus 1983 {published data only}
- Lassus A, Lauharanta J, Juvakoski T, Kanerva L. Efficacy of etretinate (Tigason) in clearing and prevention of relapse of palmoplantar pustulosis. Dermatologica 1983;166(4):215‐9. [CENTRAL: CN‐00031136; PUBMED: 6343148] [DOI] [PubMed] [Google Scholar]
Lassus 1985 {published data only}
- Lassus A, Lauharanta J, Eskelinen A. The effect of etretinate compared with different regimens of PUVA in the treatment of persistent palmoplantar pustulosis. British Journal of Dermatology 1985;112(4):455‐9. [CENTRAL: CN‐00568680; PUBMED: 3888246] [DOI] [PubMed] [Google Scholar]
Lassus 1988 {published data only}
- Lassus A, Geiger JM. Acitretin and etretinate in the treatment of palmoplantar pustulosis: a double‐blind comparative trial. British Journal of Dermatology 1988;119(6):755‐9. [CENTRAL: CN‐00057015; PUBMED: 2974306] [DOI] [PubMed] [Google Scholar]
Lawrence 1984 {published data only}
- Lawrence CM, Marks J, Parker S, Shuster S. A comparison of PUVA‐etretinate and PUVA‐placebo for palmoplantar pustular psoriasis. British Journal of Dermatology 1984;110(2):221‐6. [CENTRAL: CN‐00033676; PUBMED: 6696838] [DOI] [PubMed] [Google Scholar]
Layton 1991 {published data only}
- Layton AM, Sheehan‐Dare R, Cunliffe WJ. A double‐blind, placebo‐controlled trial of topical PUVA in persistent palmoplantar pustulosis. British Journal of Dermatology 1991;124(6):581‐4. [CENTRAL: CN‐00076516; PUBMED: 2064943] [DOI] [PubMed] [Google Scholar]
Lindelof 1990 {published data only}
- Lindelof B, Beitner H. The effect of grenz ray therapy on pustulosis palmoplantaris. A double‐blind bilateral trial. Acta Dermato‐venereologica 1990;70(6):529‐31. [CENTRAL: CN‐00073682; PUBMED: 1981434] [PubMed] [Google Scholar]
Matsunami 1990 {published data only}
- Matsunami E, Takashima A, Mizuno N, Jinno T, Ito H. Topical PUVA, etretinate, and combined PUVA and etretinate for palmoplantar pustulosis: comparison of therapeutic efficacy and the influences of tonsillar and dental focal infections. Journal of Dermatology 1990;17(2):92‐6. [CENTRAL: CN‐00067169; PUBMED: 2184179] [DOI] [PubMed] [Google Scholar]
Mrowietz 2019 {published data only}
- Mrowietz U, Bachelez H, Burden AD, Rissler M, Sieder C, Orsenigo R, et al. Secukinumab for moderate‐to‐severe palmoplantar pustular psoriasis: results of the 2PRECISE study. Journal of the American Academy of Dermatology 2019;80(5):1344‐52. [PUBMED: 30716404] [DOI] [PubMed] [Google Scholar]
Muro 2016 {published data only}
- Muro M, Kawakami H, Matsumoto Y, Abe N, Tsuboi R, Okubo Y. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomised,left‐right comparison study. Journal of Dermatological Treatment 2016;27(1):51‐3. [CENTRAL: CN‐01257303; PUBMED: 26108445] [DOI] [PubMed] [Google Scholar]
Murray 1980 {published data only}
- Murray D, Corbett MF, Warin AP. A controlled trial of photochemotherapy for persistent palmoplantar pustulosis. British Journal of Dermatology 1980;102(6):659‐63. [CENTRAL: CN‐00023617; PUBMED: 7000136] [DOI] [PubMed] [Google Scholar]
- Murray D, Warin AP. Photochemotherapy for persistent palmoplantar pustulosis (PPP). British Journal of Dermatology 1979;101(Suppl 17):13‐4. [CENTRAL: CN‐00568675; Pubmed: 380623] [PubMed] [Google Scholar]
NCT02641730 Guselkumab {unpublished data only}
- NCT02641730. An efficacy and safety of guselkumab in participants with palmoplantar pustulosis. clinicaltrials.gov/ct2/show/NCT02641730 (first received 29 December 2015).
Nielsen 1995 {published data only}
- Nielsen PG, Madsen SM. Occlusive treatment of palmoplantar pustular psoriasis with clobetasol propionate ointment succeeded by short‐term PUVA. Journal of Dermatological Treatment 1995;6(2):77‐9. [CENTRAL: CN‐00199646] [Google Scholar]
Reich 2016 {published data only}
- Reich K, Graff O, Mehta N. Oral alitretinoin treatment in patients with palmoplantar pustulosis inadequately responding to standard topical treatment: a randomised phase II study. British Journal of Dermatology 2016;174(6):1277‐81. [CENTRAL: CN‐01166541] [DOI] [PubMed] [Google Scholar]
Reitamo 1993 {published data only}
- Reitamo S, Erkko P, Remitz A, Lauerma AI, Montonen O, Harjula K. Cyclosporine in the treatment of palmoplantar pustulosis. A randomized, double‐blind, placebo‐controlled study. Archives of Dermatology 1993;129(10):1273‐9. [CENTRAL: CN‐00096600; PUBMED: 8215491] [PubMed] [Google Scholar]
Rodriguez 2000 {published data only}
- Rodriguez MS, Martin‐Neda FG, Bustinduy MG, Alom SD, Garcia ME, Gonzalez RS, et al. Aluminium chloride hexahydrate in the treatment of palmoplantar pustulosis. A preliminary study [Cloruro de aluminio hexahidratado en el tratamiento de las pustulosis palmoplantares. Estudio preliminar]. Actas Dermo‐sifiliograficas 2000;91(9):416‐9. [CENTRAL: CN‐00424502] [Google Scholar]
Rosen 1987 {published data only}
- Rosen K, Mobacken H, Swanbeck G. PUVA, etretinate, and PUVA‐etretinate therapy for pustulosis palmoplantaris. A placebo‐controlled comparative trial. Archives of Dermatology 1987;123(7):885‐9. [CENTRAL: CN‐00048987; PUBMED: 3300565] [DOI] [PubMed] [Google Scholar]
Schroder 1989 {published data only}
- Schroder K, Zaun H, Holzmann H, Altmeyer P, el‐Gammal S. Pustulosis palmo‐plantaris. Clinical and histological changes during etretin (acitretin) therapy. Acta Dermato‐venereologica. Supplementum 1989;146:111‐6. [CENTRAL: CN‐00064908; PUBMED: 2532841] [PubMed] [Google Scholar]
Su 2017 {published data only}
- Su LN, Ren J, Cheng SM, Liu JL, Ding YF, Zhu NW. UVA1 vs. narrowband UVB phototherapy in the treatment of palmoplantar pustulosis: a pilot randomised controlled study. Lasers in Medical Science 2017;32(8):1819‐23. [CENTRAL: CN‐01446891; PUBMED: 28699044] [DOI] [PubMed] [Google Scholar]
Terui 2018 {published data only}
- Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Efficacy and safety of guselkumab, an anti‐interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatology 2018;154(3):309‐16. [PUBMED: 29417135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thestrup‐Pedersen 1984 {published data only}
- Thestrup‐Pedersen K, Reymann F. Treatment of pustulosis palmaris et plantaris with colchicine. A double‐blind cross‐over trial. Acta Dermato‐venereologica 1984;64(1):76‐8. [CENTRAL: CN‐00034679; PUBMED: 6203289] [PubMed] [Google Scholar]
Thomsen 1973 {published data only}
- Thomsen K, Osterbye P. Pustulosis palmaris et plantaris. British Journal of Dermatology 1973;89(3):293‐6. [CENTRAL: CN‐00009126; PUBMED: 4582718] [DOI] [PubMed] [Google Scholar]
Thune 1982 {published data only}
- Thune P. Treatment of palmoplantar pustulosis with Tigason. Dermatologica 1982;164(1):67‐72. [CENTRAL: CN‐00384407; PUBMED: 7040131] [DOI] [PubMed] [Google Scholar]
Umezawa 2016 {published data only}
- Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomised, double‐blind, placebo‐controlled trial. Journal of Dermatology 2016;43(3):288‐93. [CENTRAL: CN‐01141237; PUBMED: 26282062] [DOI] [PubMed] [Google Scholar]
Ward 1976 {published data only}
- Ward JM, Corbett MF, Hanna MJ. A double‐blind trial of clomocycline in the treatment of persistent palmoplantar pustulosis. British Journal of Dermatology 1976;95(3):317‐22. [CENTRAL: CN‐00014806; PUBMED: 788767] [DOI] [PubMed] [Google Scholar]
White 1985 {published data only}
- White SI, Marks JM, Shuster S. Etretinate in pustular psoriasis of palms and soles. British Journal of Dermatology 1985;113(5):581‐5. [CENTRAL: CN‐00040409; PUBMED: 3904810] [DOI] [PubMed] [Google Scholar]
White 1986 {published data only}
- White SI, Puttick L, Marks JM. Low‐dose etretinate in the maintenance of remission of palmoplantar pustular psoriasis. British Journal of Dermatology 1986;115(5):577‐82. [CENTRAL: CN‐00045794; PUBMED: 3539171] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aso K 1983 {published data only}
- Aso K, Amano N. Inhibitory effect of retinoid (RO 10‐9359) on chemotaxis and random migration of polymorphonuclear leucocytes in patients with pustulosis palmaris et plantaris. Nihon Hifuka Gakkai Zasshi 1983;93(3):333‐7. [PubMed] [Google Scholar]
Carr 2008 {published data only}
- Carr D, Tusa MG, Carroll CL, Pearce DJ, Camacho F, Kaur M, et al. Open label trial of alefacept in palmoplantar pustular psoriasis. Journal of Dermatological Treatment 2008;19(2):97‐100. [PUBMED: 18512272] [DOI] [PubMed] [Google Scholar]
Cassano 2010 {published data only}
- Cassano N, Mantegazza R, Battaglini S, Apruzzi D, Loconsole F, Vena GA. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open‐label study. Giornale Italiano di Dermatologia e Venereologia 2010;145(6):789‐92. [CENTRAL: CN‐00785934; Pubmed : 21139557] [PubMed] [Google Scholar]
Dupre 1973 {published data only}
- Dupre A, Christol B, Cassagnaveres E, Bazex J. Atypical palmoplantar psoriasis and related syndromes [Psoriasis palmo‐plantaires atypiques et syndromes apparentes]. Cahiers de Medecine 1973;14(8):681‐8. [PUBMED: 4602471] [PubMed] [Google Scholar]
Duweb 2001 {published data only}
- Duweb GA, Abuzariba O, Rahim M, al‐Taweel M, al‐Alem S, Abdulla SA. Occlusive versus nonocclusive calcipotriol ointment treatment for palmoplantar psoriasis. International Journal of Tissue Reactions 2001;23(2):59‐62. [CENTRAL: CN‐00373257; Pubmed : 11447774] [PubMed] [Google Scholar]
Engin 2005 {published data only}
- Engin B, Oguz O. Evaluation of time‐dependent response to psoralen plus UVA (PUVA) treatment with topical 8‐methoxypsoralen (8‐MOP) gel in palmoplantar dermatoses. International Journal of Dermatology 2005;44(4):337‐9. [CENTRAL: CN‐00575420; PUBMED: 15811091] [DOI] [PubMed] [Google Scholar]
Fritsch 1978 {published data only}
- Fritsch P, Honigsmann H, Jashcke E, Wolff K. Photochemotherapy of psoriasis: increasing its effectiveness with an oral aromatic retinoid (clinical results in 134 patients). Deutsche Medizinische Wochenschrift (1946) 1978;103(44):1731‐6. [CENTRAL: CN‐00019171] [DOI] [PubMed] [Google Scholar]
Gjertsen 1980 {published data only}
- Gjertsen BT, Nilsen A. Local treatment of psoriasis and pustulosis palmoplantaris with desoxymethasone ointment 0.25%. Tidsskr Nor Laegeforen 1980;100(11):632‐3. [PUBMED: 6992336] [PubMed] [Google Scholar]
Grundmann‐Kollmann 1999 {published data only}
- Grundmann‐Kollmann M, Behrens S, Peter RU, Kerscher M. Treatment of severe recalcitrant dermatoses of the palms and soles with PUVA‐bath versus PUVA‐cream therapy. Photodermatology, Photoimmunology & Photomedicine 1999;15(2):87‐9. [CENTRAL: CN‐00162994; PUBMED: 10321522] [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta SK, Singh KK, Lalit M. Comparative therapeutic evaluation of different topicals and narrow band ultraviolet B therapy combined with systemic methotrexate in the treatment of palmoplantar psoriasis. Indian Journal of Dermatology 2011;56(2):165‐70. [CENTRAL: CN‐00843779; PUBMED: 21716541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofer 2006 {published data only}
- Hofer A, Fink‐Puches R, Kerl H, Quehenberger F, Wolf P. Paired comparison of bathwater versus oral delivery of 8‐methoxypsoralen in psoralen plus ultraviolet: a therapy for chronic palmoplantar psoriasis. Photodermatology, Photoimmunology & Photomedicine 2006;22(1):1‐5. [CENTRAL: CN‐00562268; PUBMED: 16436174] [DOI] [PubMed] [Google Scholar]
Janagond 2013 {published data only}
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. Journal of the European Academy of Dermatology and Venereology 2013;27(3):e384‐9. [CENTRAL: CN‐01124551; PUBMED: 23066720] [DOI] [PubMed] [Google Scholar]
Khandpur 2011 {published data only}
- Khandpur S, Sharma VK. Comparison of clobetasol propionate cream plus coal tar vs. topical psoralen and solar ultraviolet A therapy in palmoplantar psoriasis. Clinical and Experimental Dermatology 2011;36(6):613‐6. [CENTRAL: CN‐00800550; PUBMED: 21507036] [DOI] [PubMed] [Google Scholar]
Kumar 1997 {published data only}
- Kumar B, Kumar R, Kaur I. Coal tar therapy in palmoplantar psoriasis: old wine in an old bottle?. International Journal of Dermatology 1997;36(4):309‐12. [CENTRAL: CN‐00140118; PUBMED: 9169338] [DOI] [PubMed] [Google Scholar]
Mehta 2011 {published data only}
- Mehta BH, Amladi ST. Evaluation of topical 0.1% tazarotene cream in the treatment of palmoplantar psoriasis: an observer‐blinded randomized controlled study. Indian Journal of Dermatology 2011;56(1):40‐3. [CENTRAL: CN‐00861405; PUBMED: 21572790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2006 {published data only}
- Neumann NJ, Mahnke N, Korpusik D, Stege H, Ruzicka T. Treatment of palmoplantar psoriasis with monochromatic excimer light (308‐nm) versus cream PUVA. Acta Dermato‐venereologica 2006;86(1):22‐4. [CENTRAL: CN‐00563926; PUBMED: 16585984] [DOI] [PubMed] [Google Scholar]
Orfanos 1978 {published data only}
- Orfanos CE, Goerz G. Oral psoriasis treatment with a new aromatic retinoid (Ro 10‐9359): a multi‐centre controlled study of 291 patients (preliminary results) (author's transl) [Orale psoriasis‐Therapie mit einem neuen aromatischen Retinoid (Ro 10‐9359). Eine multizentrische kontrollierte Studie an 291 Patienten in der Bundesrepublik. Vorlaufige Mitteilung]. Deutsche Medizinische Wochenschrift (1946) 1978;103(5):195‐9. [CENTRAL: CN‐00017768; PUBMED: 631039] [DOI] [PubMed] [Google Scholar]
Papp 2012 {published data only}
- Papp K, Hu A, Wasel N, Day R. Apremilast is effective in the treatment of scalp and palmoplantar psoriasis: results from a phase II2b randomized, controlled study. Journal of the American Academy of Dermatology. 2012; Vol. 66:AB185.
Rosen 1988 {published data only}
- Rosen K, Jontell M, Mobacken H, Rosdahl I. Epidermal Langerhans' cells in patients with pustulosis palmoplantaris treated with etretinate or etretinate + methoxsalen photochemotherapy. Acta Dermato‐venereologica 1988;68(3):218‐23. [CENTRAL: CN‐00054561; PUBMED: 2455414] [PubMed] [Google Scholar]
Schiener 2005 {published data only}
- Schiener R, Gottlober P, Muller B, Williams S, Pillekamp H, Peter RU, et al. PUVA‐gel vs. PUVA‐bath therapy for severe recalcitrant palmoplantar dermatoses. A randomized, single‐blinded prospective study. Photodermatology, Photoimmunology & Photomedicine 2005;21(2):62‐7. [CENTRAL: CN‐00513404; PUBMED: 15752122] [DOI] [PubMed] [Google Scholar]
Sezer 2007 {published data only}
- Sezer E, Erbil AH, Kurumlu Z, Tastan HB, Etikan I. Comparison of the efficacy of local narrowband ultraviolet B (NB‐UVB) phototherapy versus psoralen plus ultraviolet A (PUVA) paint for palmoplantar psoriasis. Journal of Dermatology 2007;34(7):435‐40. [CENTRAL: CN‐00610281; PUBMED: 17584319] [DOI] [PubMed] [Google Scholar]
Thaci 2010 {published data only}
- Thaci D, Xie J, Mulani P, Gupta S. Effects of adalimumab monotherapy versus adalimumab plus betamethasone/calcipotriol on patient‐reported outcomes in patients with palmoplantar, nail, or scalp psoriasis. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB 135. [CENTRAL: CN‐00843899] [Google Scholar]
Yaniv 2012 {published data only}
- Yaniv S, Gottlieb A, Wang A, Michelon M, Dumont N, Au SC, et al. An investigator‐initiated, open‐label study evaluating the efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis. Journal of the American Academy of Dermatology. 2012:AB185.
Zhang Jun 2007 {published data only}
- Zhang J, Hu F‐H, Li L‐M, Zhu W‐Y. Observation on topical tacalcitol ointment in the treatment of twenty‐seven patients with palmoplantar pustulosis. Journal of Clinical Dermatology 2007;36(5):328‐9. [CENTRAL: CN‐00640929] [Google Scholar]
References to studies awaiting assessment
EudraCT 2006‐004519‐23 {unpublished data only}
- EudraCT 2006‐004519‐23. Comparison of fumaric acid ester‐PUVA versus PUVA‐etretinate in palmoplantar pustulosis. www.clinicaltrialsregister.eu/ctr‐search/search?query=2006‐004519‐23 (first received 10 February 2009).
Fenton 1983 {published data only}
- Fenton DA, English JS, Wilkinson JD. A controlled trial using a lipoxygenase inhibitor (benoxaprofen) in the treatment of persistent palmoplantar pustulosis. British Journal of Dermatology 1983;109(Suppl 24):25‐6. [CENTRAL: CN‐00400885] [Google Scholar]
Mann 1982 {published data only}
- Mann RJ. Failure of colchicine for palmo‐plantar pustulosis. British Journal of Dermatology 1982;106(3):373. [CENTRAL: CN‐00568966; PUBMED: 7039659] [DOI] [PubMed] [Google Scholar]
NCT03135548 BI 655130 {unpublished data only}
- NCT03135548. Initial dosing of BI 655130 in palmoplantar pustulosis patients. clinicaltrials.gov/NCT03135548 (first received 1 May 2017).
References to ongoing studies
ISRCTN13127147 APRICOT {unpublished data only}
- ISRCTN13127147. Treatment of pustular psoriasis with the IL‐1 receptor antagonist anakinra: a randomised, placebo controlled trial and associated mechanistic studies. www.isrctn.com/ISRCTN13127147 (first received 1 August 2016).
NCT03633396 {published data only}
- NCT03633396. A study to evaluate the efficacy and safety of ANB019 in subjects with palmoplantar pustulosis (PPP) [A phase II, randomized, placebo‐controlled, double‐blind, multiple dose study to evaluate the efficacy and safety of ANB019 in subjects with palmoplantar pustulosis]. clinicaltrials.gov/ct2/show/NCT03633396 (first received 16 August 2018).
Additional references
Adisen 2010
- Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clinical & Experimental Dermatology 2010;35(3):219‐22. [PUBMED: 19874337] [DOI] [PubMed] [Google Scholar]
Asumalahti 2003
- Asumalahti K, Ameen M, Suomela S, Hagforsen E, Michaëlsson G, Evans J, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. Journal of Investigative Dermatology 2003;120(4):627‐32. [PUBMED: 12648227] [DOI] [PubMed] [Google Scholar]
Barber 1930
- Barber HW. Acrodermatitis continua vel perstans (dermatitis repens) and psoriasis pustule. British Journal of Dermatology 1930;42:500‐18. [DOI: 10.1111/j.1365-2133.1930.tb09380.x] [DOI] [Google Scholar]
Brunasso 2010
- Brunasso AM, Massone C. Can we really separate palmoplantar pustulosis from psoriasis?. Journal of the European Academy of Dermatology and Venereology 2010;24(5):619‐21. [PUBMED: 20337817] [DOI] [PubMed] [Google Scholar]
Brunasso 2013
- Brunasso AM, Puntoni M, Aberer W, Delfino C, Fancelli L, Massone C. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. British Journal of Dermatology 2013;168(6):1243‐51. [PUBMED: 23301847] [DOI] [PubMed] [Google Scholar]
Burden 1996
- Burden AD, Kemmett D. The spectrum of nail involvement in palmoplantar pustulosis. British Journal of Dermatology 1996;134(6):1079‐82. [PUBMED: 8763428] [PubMed] [Google Scholar]
Chen 2013
- Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen‐ultraviolet A photochemotherapy for psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD009481.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochran 1950
- Cochran WG. The comparison of percentages in matched samples. Biometrika 1950;37(3‐4):256–66. [PUBMED: 14801052] [PubMed] [Google Scholar]
CS‐COUSIN
- Cochrane Skin. Core Outcome Set Initiative. cs‐cousin.org (accessed 8 July 2019).
Di Cesare 2009
- Cesare A, Meglio P, Nestle FO. The IL‐23/Th17 axis in the immunopathogenesis of psoriasis. Journal of Investigative Dermatology 2009;129(6):1339‐50. [PUBMED: 19322214] [DOI] [PubMed] [Google Scholar]
Elder 2008
- Elder D, Elenitsas R, Johnson B, Murphy G, Xu G (editors). Lever's Histopathology of the Skin. 10th Edition. Philadelphia: Lippincott Williams & Wilkins, 2008. [Google Scholar]
Eriksson 1998
- Eriksson MO, Hagforsen E, Lundin IP, Michaëlsson G. Palmoplantar pustulosis: a clinical and immunohistological study. British Journal of Dermatology 1998;138(3):390‐8. [PUBMED: 9580788] [DOI] [PubMed] [Google Scholar]
Floristan 2011
- Floristan U, Feltes R, Ramírez P, Alonso ML, Lucas R. Recalcitrant palmoplantar pustular psoriasis treated with etanercept. Pediatric Dermatology 2011;28(3):349‐50. [PUBMED: 21371112] [DOI] [PubMed] [Google Scholar]
Fujishima 2010
- Fujishima S, Watanabe H, Kawaguchi M, Suzuki T, Matsukura S, Homma T, et al. Involvement of IL‐17F via the induction of IL‐6 in psoriasis. Archives of Dermatological Research 2010;302(7):499‐505. [PUBMED: 20148256] [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths CE, Christophers E, Barker JN, Chalmers RJ, Chimenti S, Krueger GG, et al. A classification of psoriasis vulgaris according to phenotype. British Journal of Dermatology 2007;156(2):258‐62. [PUBMED: 17223864] [DOI] [PubMed] [Google Scholar]
Hagforsen 2002
- Hagforsen E, Awder M, Lefvert AK, Nordlind K, Michaëlsson G. Palmoplantar pustulosis: an autoimmune disease precipitated by smoking?. Acta Dermato‐venereologica 2002;82(5):341‐6. [PUBMED: 12430732] [DOI] [PubMed] [Google Scholar]
Hellgren 1971
- Hellgren L, Mobacken H. Pustulosis palmaris et plantaris. Prevalence, clinical observations and prognosis. Acta Dermato‐venereologica 1971;51(4):284‐8. [PUBMED: 4105777] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5(3):225‐39. [PUBMED: 18559412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Hirji 2011
- Hirji KF, Fagerland MW. Calculating unreported confidence intervals for paired data. BMC Medical Research Methodology 2011;11:66. [PUBMED: 21569392] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 1996
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clinical Immunology & Immunopathology 1996;80(3 Pt 2):S40‐5. [PUBMED: 8811062] [DOI] [PubMed] [Google Scholar]
Ioannidis 2007
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ 2007;176(8):1091‐6. [PUBMED: 17420491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaneko 2012
- Kaneko T, Murakami M, Kishibe M, Brattsand M, Morhenn VB, Ishida‐Yamamoto A, et al. Over‐expression of kallikrein related peptidases in palmoplantar pustulosis. Journal of Dermatological Science 2012;67(1):73‐6. [PUBMED: 22520926] [DOI] [PubMed] [Google Scholar]
Kikushi 2013
- Kikuchi N, Yamamoto T. Dental infection as a triggering factor in palmoplantar pustulosis. Acta Dermato‐venereologica 2013;93(6):721‐2. [PUBMED: 23462950] [DOI] [PubMed] [Google Scholar]
Kurian 2011
- Kurian A, Barankin B. Current effective topical therapies in the management of psoriasis. Skin Therapy Letter 2011;16(1):4‐7. [PUBMED: 21293834] [PubMed] [Google Scholar]
Langley 2014
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis ‐ results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326‐38. [PUBMED: 25007392 ] [DOI] [PubMed] [Google Scholar]
Le Cleach 2011
- Cleach L, Trinquart L, Lebrun‐Vignes B, Giraudeau B, Chosidow O. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2013
- Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL‐17 in the pathogenesis of psoriasis: preclinical and clinical findings. Journal of Investigative Dermatology 2013;133(1):17‐26. [PUBMED: 22673731] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGeachy 2009
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce‐Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17‐producing effector T helper cells in vivo. Nature Immunology 2009;10(3):314‐24. [PUBMED: 19182808 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michaëlsson 2006
- Michaëlsson G, Gustafsson K, Hagforsen E. The psoriasis variant palmoplantar pustulosis can be improved after cessation of smoking. Journal of the American Academy of Dermatology 2006;54(4):737‐8. [PUBMED: 16546609] [DOI] [PubMed] [Google Scholar]
Michaëlsson 2007
- Michaëlsson G, Kristjánsson G, Pihl Lundin I, Hagforsen E. Palmoplantar pustulosis and gluten sensitivity: a study of serum antibodies against gliadin and tissue transglutaminase, the duodenal mucosa and effects of gluten‐free diet. British Journal of Dermatology 2007;156(4):659‐66. [PUBMED: 17263812] [DOI] [PubMed] [Google Scholar]
Miot 2009
- Miot HA, Miot LD, Lopes PS, Haddad GR, Marques SA. Association between palmoplantar pustulosis and cigarette smoking in Brazil: a case‐control study. Journal of the American Academy of Dermatology & Venereology 2009;23(10):1173‐7. [PUBMED: 19453779] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Morales‐Múnera 2013
- Morales‐Múnera C, Vilarrasa E, Puig L. Efficacy of ustekinumab in refractory palmoplantar pustular psoriasis. British Journal of Dermatology 2013;168(4):820‐4. [PUBMED: 23210683] [DOI] [PubMed] [Google Scholar]
Moustou 2009
- Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti‐tumor necrosis factor biologic therapy: a clinical review. Journal of the American Academy of Dermatology 2009;61(3):486‐504. [PUBMED: 19628303] [DOI] [PubMed] [Google Scholar]
Murakami 2010
- Murakami M, Ohtake T, Horibe Y, Ishida‐Yamamoto A, Morhenn VB, Gallo RL, et al. Acrosyringium is the main site of the vesicle/pustule formation in palmoplantar pustulosis. Journal of Investigative Dermatology 2010;130(8):2010‐6. [PUBMED: 20393482] [DOI] [PubMed] [Google Scholar]
Murakami 2011
- Murakami M, Hagforsen E, Morhenn V, Ishida‐Yamamoto A, Iizuka H. Patients with palmoplantar pustulosis have increased IL‐17 and IL‐22 levels both in the lesion and serum. Experimental Dermatology 2011;20(10):845‐7. [PUBMED: 21732985] [DOI] [PubMed] [Google Scholar]
Otsuka 2010
- Otsuka A, Miyachi Y, Kabashima K. Narrowband ultraviolet B phototherapy decreased CCR4+ CD8+ T cells in a patient with palmoplantar pustulosis. Journal of the European Academy of Dermatology & Venereology 2011;25(4):495‐6. [PUBMED: 20553356] [DOI] [PubMed] [Google Scholar]
Pettey 2003
- Pettey AA, Balkrishnan R, Rapp SR, Fleischer AB, Feldman SR. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. Journal of the American Academy of Dermatology 2003;49(2):271‐5. [PUBMED: 12894076] [DOI] [PubMed] [Google Scholar]
Puig 2012
- Puig L, Morales‐Múnera CE, López‐Ferrer A, Geli C. Ustekinumab treatment of TNF antagonist‐induced paradoxical psoriasis flare in a patient with psoriatic arthritis: case report and review. Dermatology 2012;225(1):14‐7. [PUBMED: 22890275] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rueda‐Gotor 2012
- Rueda‐Gotor J, González‐Gay MA, Blanco Alonso R, Gonzalez‐Vela C, Lopez‐Obregon C, González‐López MA. Successful effect of tocilizumab in anti‐TNF‐α‐induced palmoplantar pustulosis in rheumatoid arthritis. Joint, Bone, Spine: Revue du Rhumatisme 2012;79(5):510‐3. [PUBMED: 22857979] [DOI] [PubMed] [Google Scholar]
Saurat 2009
- Saurat JH, Lachapelle JM, Lipsker D, Thomas L. Dermatologie et Infections Sexuellement Transmissibles. 5th Edition. Paris: Elsevier Masson, 2009. [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Uehara 1974
- Uehara M, Ofuji S. The morphogenesis of pustulosis palmaris et plantaris. Archives of Dermatology 1974;109(4):518‐20. [PUBMED: 4819106] [PubMed] [Google Scholar]
Watanabe 2009
- Watanabe H, Kawaguchi M, Fujishima S, Ogura M, Matsukura S, Takeuchi H, et al. Functional characterization of IL‐17F as a selective neutrophil attractant in psoriasis. Journal of Investigative Dermatology 2009;129(3):650‐6. [PUBMED: 18830271] [DOI] [PubMed] [Google Scholar]
Wolff 2008
- Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D. Fitzpatrick's Dermatology in General Medicine. 7th Edition. New York: McGraw‐Hill Professional, 2008. [Google Scholar]
Yilmaz 2012
- Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL‐17 in different clinical subtypes of psoriasis. Archives of Dermatological Research 2012;304(6):465‐9. [PUBMED: 22426986] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chalmers 2006
- Chalmers R, Hollis S, Leonardi‐Bee J, Griffiths CEM, Marsland Bsc MRCP A. Interventions for chronic palmoplantar pustulosis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001433.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obeid 2015
- Obeid G, Do G, Katsahian S, Kirby L, Hughes C, Cleach L. Interventions for chronic palmoplantar pustulosis. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011628] [DOI] [PMC free article] [PubMed] [Google Scholar]


