Abstract
Background and Aims
Chronic diarrhea affects about 5% of the population overall. Altered bile acid metabolism is a common but frequently undiagnosed cause.
Methods
We performed a systematic search of publication databases for studies of assessment and management of bile acid diarrhea (BAD). The certainty (quality) of evidence and strength of recommendations were rated according to the Grading of Recommendation Assessment, Development and Evaluation approach. Patient population, intervention, comparator and outcome questions were developed through an iterative process and were voted on by a group of specialists.
Results
The certainty of evidence was generally rated as very low. Therefore, 16 of 17 recommendations are conditional. In patients with chronic diarrhea, consideration of risk factors (terminal ileal resection, cholecystectomy or abdominal radiotherapy), but not additional symptoms, was recommended for identification of patients with possible BAD. The group suggested testing using 75selenium homocholic acid taurine (where available) or 7α-hydroxy-4-cholesten-3-one, including patients with irritable bowel syndrome with diarrhea, functional diarrhea and Crohn’s disease without inflammation. Testing was suggested over empiric bile acid sequestrant therapy (BAST). Once remediable causes are managed, the group suggested cholestyramine as initial therapy, with alternate BAST when tolerability is an issue. The group suggested against BAST for patients with extensive ileal Crohn’s disease or resection and suggested alternative antidiarrheal agents if BAST is not tolerated. Maintenance BAST should be given at the lowest effective dose, with a trial of intermittent, on-demand administration, concurrent medication review and reinvestigation for patients whose symptoms persist despite BAST.
Conclusions
Based on a systematic review, BAD should be considered for patients with chronic diarrhea. For patients with positive results from tests for BAD, a trial of BAST, initially with cholestyramine, is suggested.
Keywords: C4, Fibroblast growth factor 19, FGF19, IBS, SeHCAT
Diarrhea is a common symptom in the general population of developed countries. Among community-dwelling persons the 1-month rate of diarrhea was 7.6% in Canada and the United States, 6.4% in Australia and 3.4% in Ireland; approximately 20% of subjects sought medical care for this symptom (1). The prevalence of chronic diarrhea has been estimated to affect approximately 5% of this population overall (2), and may be higher among older individuals (3).
The most common causes of chronic diarrhea in clinical practice are functional disorders (e.g., irritable bowel syndrome [IBS]), and inflammatory diseases (e.g., Crohn’s disease, celiac disease) (3). However, a common but frequently underdiagnosed cause of chronic diarrhea is dysregulated bile acid recycling within the enterohepatic circulation: either excessive biosynthesis/secretion of bile acids, or malabsorption of bile acids by the ileum. Unabsorbed bile acids in the colon appear to cause diarrhea by stimulating fluid, mucus, or sodium secretion; increasing gastrointestinal motility; damaging the mucosa or stimulating defecation (3,4).
Three subtypes of bile acid diarrhea (BAD) have been described: type 1, patients with terminal ileal disease (e.g., Crohn’s disease, resection) or radiation injury resulting in impaired reabsorption of bile acids; type 2, idiopathic or primary; and type 3, other conditions (e.g., celiac disease, cholecystectomy) that alter intestinal motility or bile acid absorption (3,5). BAD has been reported in approximately 25% to 35% of patients with chronic diarrhea or diarrhea-predominant IBS (IBS-D) (6). Rates are even higher in patients with underlying terminal ileal disease, or other conditions such as cholecystectomy.
The diagnosis of BAD continues to be a challenge, although this may be improved in the future with the general availability of screening serologic tests and other diagnostic tests (discussed later). Although a treatment trial with bile acid sequestrant therapy (BAST) often is used, this approach has not been studied adequately, and likely os imprecise, and may lead to both under-treatment and overtreatment. Specific diagnostic tests are under investigation, particularly radiodiagnostic measurement of bile acid pool loss with 75selenium homocholic acid taurine (SeHCAT; GE Healthcare Canada, Inc, Ontario, Canada), or measurement of serum levels of biomarkers of bile acid synthesis including 7α-hydroxy-4-cholesten-3-one (C4) or the ileal regulatory hormone, fibroblast growth factor 19 (FGF19). SeHCAT testing is unavailable in some countries (including the United States).
BAD generally is not cured, and as is the case with many chronic gastrointestinal diseases or disorders, many patients will require lifelong treatment (7,8). Treatment is generally with BAST, but also is dependent on the underlying causes of BAD, severity of symptoms or the presence of other comorbid illnesses (e.g., Crohn’s disease, celiac disease).
BAD is an understudied, often underappreciated condition, and questions remain regarding its diagnosis and treatment. There have been guidelines on the management of chronic diarrhea from the American Gastroenterological Association (9), and the British Society of Gastroenterology (10), but diagnosis and management of BAD was not assessed extensively in these publications. The British Society of Gastroenterology updated guidelines on the investigation of chronic diarrhea in adults (11), published after the consensus meeting, addressed some issues related to BAD.
The purpose of this guideline is to critically review the literature relating to diagnostic testing, and the induction and maintenance treatment of BAD, with the aim of developing specific consensus recommendations for patients with BAD.
Methods
Scope and Purpose
These consensus statements focused on specific issues pertaining to the medical management of BAD, which the participants and GRADE experts (F.T., G.I.L.) identified a priori.
Sources, Literature Searches and Systematic Reviews
The Editorial Office of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group at McMaster University performed a systematic search of Medline, Embase, and the Cochrane Central Register of Controlled Trials for literature published between 1990 and September 2017. Key search terms were as follows: bile acid, cholecystectomy, cholestyramine, colestipol, colesevelam, diarrhea, loperamide, malabsorption, resection, SeHCAT and sequestrants. An additional search of the databases for SeHCAT trials published before 1990 (database inception as start date) also was performed. Only human studies published in English were considered. Further details of the search strategies are provided in Supplementary Appendix 1.
Assessment of the Certainty (Quality) of Evidence
Before the face-to-face meeting, the statements were converted to specific patient population, intervention, comparator and outcome (PICO) questions by the two nonvoting methodologists (F.T., G.I.L.). The overall certainty of evidence (CoE) was determined using the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach (12) to assess risk of bias (of individual studies and overall across studies), indirectness, inconsistency, imprecision, as well as other considerations (including publication bias). As described in GRADE (12,13) and used in previous consensus guidelines from the Canadian Association of Gastroenterology (CAG) (14–18), CoE was graded as very low, low, moderate or high. GRADE evaluations for each statement were provided before the consensus meeting and discussed during the consensus meeting.
The consensus group agreed that four statements (Statements 11, 13, 14 and 17) met GRADE criteria for good practice statements that these recommendations were clinically obvious, and that collection and GRADE assessment of evidence for these statements was not a good use of resources (19). Although formal GRADE evaluations were not performed, details of these statements are provided in the text.
Approved product labeling from government regulatory agencies varies from country to country, and although not ignored, recommendations are based on evidence from the literature and consensus discussion and may not fully reflect the product labeling for a given country.
Consensus Process
A face-to-face consensus meeting was held in Toronto, Canada, in February 2018. The international consensus group comprised five voting gastroenterologists (including the chair: D.C.S.), from Canada, the United States and the United Kingdom. Other participants included a nonvoting moderator (J.K.M.), the two GRADE experts (F.T., G.I.L.) and a nonvoting observer.
The consensus process was facilitated by the CAG via a web-based consensus platform (ECD Solutions, Atlanta, GA). The platform allowed consensus participants to review results of the initial literature searches and select and link the references to specific statements. Copies of the selected references were available to all members of the consensus group. The full consensus group voted anonymously on their level of agreement with the individual statements using a modified Delphi process (20,21). Participants suggested revisions and commented on the statements, after which, the specific statements were revised through two iterations.
At the 1-day consensus meeting, evidence for each of the PICO questions was presented, after which an Evidence-to-Decision framework was completed (22). Each PICO question was discussed and revised, and voting members anonymously indicated their level of agreement on a scale of 1 to 5. In favour of a specific strategy was defined as 75% or more of votes being 5 (strongly yes) or 4 (yes). A vote against the strategy was defined as 75% or more of votes being 1 (strongly no) or 2 (no). A vote of 3 indicated neutrality. Once reaching agreement on the PICO question, the strength of the recommendation (strong versus conditional) was determined based on the following four components: (i) CoE, (ii) benefit/harm balance, (iii) patients’ values/preferences and (iv) resource requirements (23). When the CoE was low or very low, unless at least one of the other three factors was overwhelmingly strong, the strength of the recommendation typically would default (without a vote) to conditional, using the phrasing ‘we suggest’. If the statement warranted a vote, and 75% or more of participants voted as strong, then the recommendation would be designated as strong and the phrasing contained ‘we recommend’.
During the meeting, consensus was not reached on four of the PICO questions; therefore, no statement was developed and no recommendations were made. Evidence and subsequent discussion pertaining to these four questions is summarized briefly in the text.
The manuscript was drafted initially by the meeting chair (D.C.S.), and then reviewed and revised by the remaining members of the consensus group. The manuscript then was made available to all CAG members for comment over a 2-week period before submission for publication.
In accordance with CAG policy, written disclosures of any potential conflicts of interest for the 24 months before the consensus meeting were provided by all participants, reviewed by the CAG ethics committee and made available to all group members.
Recommendation Statements
The individual recommendation statements are provided and include the strength of recommendation and certainty of supporting evidence (according to the GRADE approach), and the voting result. This is followed by a discussion of the evidence considered for the specific statement. A summary of the recommendation statements is provided in Table 1. See Supplementary Appendix 1 for detailed CoE assessments (including a description of the study limitations, inconsistency, indirectness, imprecision and publication bias) and the Evidence-to-Decision frameworks.
Table 1.
Summary of consensus recommendations for the management of BAD
| Diagnosis of BAD |
|---|
| Statement 1. In patients with chronic nonbloody diarrhea, we recommend using risk factors (history of terminal ileal resection, cholecystectomy, or radiotherapy) as the initial assessment to identify patients with possible BAD. |
| GRADE. Strong recommendation, very low-certainty evidence. Vote on PICO question: strongly yes, 60%; yes, 40%. |
| Statement 2. In patients with chronic nonbloody diarrhea, we suggest against using symptom presentation as the initial assessment to identify patients with possible BAD. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: no, 100%. |
| Statement 3. In patients with chronic diarrhea including IBS-D and functional diarrhea, we suggest SeHCAT testing to identify patients with BAD. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 20%; yes, 80%. |
| Statement 4. In patients with small intestinal Crohn’s disease without objective evidence of inflammation who have persistent diarrhea, we suggest SeHCAT testing. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 20%; yes, 80%. |
| Statement 5. In patients with chronic diarrhea including IBS-D and functional diarrhea, we suggest using a C4 assay to identify possible BAD. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 20%; yes, 60%; neutral, 20%. |
| Statement 6. In patients with suspected BAD, we suggest against initiating empiric BAST over performing SeHCAT to establish a diagnosis of BAD. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: yes, 20%; no, 40%; strongly no, 40%. |
| Induction therapy for BAD (BAST) |
| Statement 7. In patients with type 1 or type 3 BAD, we suggest the use of treatments for remediable causes (e.g., Crohn’s disease, microscopic colitis, SIBO) in addition to treatment for BAD for induction of clinical response. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 80%; yes, 20%. |
| Statement 8. In patients with BAD, we suggest using cholestyramine over no treatment for induction of clinical response. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 60%; yes, 40%. |
| Statement 9. In patients with BAD, we suggest using cholestyramine over other BASTs as initial therapy for induction of clinical response. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: yes, 80%; neutral, 20%. |
| Statement 10. In patients with BAD who are unable to tolerate cholestyramine, we suggest using an alternate BAST for induction of clinical response. |
| GRADE. Conditional recommendation, low-certainty evidence. Vote on PICO question: strongly yes, 40%; yes, 60%. |
| Statement 11. In patients with BAD receiving empiric BAST, gradual daily dose titration should be used to minimize side effects. Designated a good practice statement |
| Statement 12. In patients with Crohn’s disease with extensive ileal involvement or resection, we suggest against using BAST. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question (should we use BAST?): yes, 20%; no, 80%. |
| Maintenance therapy for BAD (BAST) |
| Statement 13. In patients with BAD who respond to BAST, we suggest that intermittent, on-demand dosing be tried. |
| GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: yes, 80%; neutral, 20%. |
| Statement 14. In patients with BAD who are unable to tolerate BAST, we suggest using alternative antidiarrheal agents versus no treatment for long-term symptomatic therapy.GRADE. Conditional recommendation, very-low-certainty evidence. Vote on PICO question: yes, 100%. |
| Statement 15. In patients with BAD receiving empiric BAST, maintenance therapy should be used at the lowest dose needed to minimize symptoms. Designated a good practice statement |
| Maintenance therapy for BAD (BAST) |
| Statement 16. In patients with BAD and recurrent or worsening symptoms despite stable BAST, diagnostic re-evaluation should be conducted. Designated a good practice statement |
| Statement 17. In patients being considered for BAST, a review of concurrent medications should be conducted to minimize the potential for drug interactions. Designated a good practice statement |
| Statements with no recommendations |
| No recommendation A. In patients with chronic diarrhea including IBS-D and functional diarrhea, the consensus group could not make a recommendation for or against the use of FGF19 assay to identify possible BAD. |
| GRADE. NO recommendation, very-low-certainty evidence. Vote on PICO question: strongly yes, 20%; neutral, 80%. |
| No recommendation B. In patients receiving long-term maintenance therapy with BAST, the consensus group could not make a recommendation for or against measuring fat-soluble vitamin levels at baseline and annually thereafter. |
| GRADE. NO recommendation; very-low-certainty evidence. Vote on PICO question: yes, 20%; neutral, 80%. |
The strength of each recommendation was assigned by the consensus group, per the GRADE system, as strong (“we recommend...”) or conditional (“we suggest...”). A recommendation could be classified as strong despite low-certainty evidence to support it, or conditional despite the existence of high-certainty evidence due to the 4 components considered in each recommendation (risk:benefit balance, patients’ values and preferences, cost and resource allocation and certainty of evidence).
BAD, bile acid diarrhea; BAST, bile acid sequestrant therapy; C4, 7α-hydroxy-4-cholesten-3-one; FGF19, fibroblast growth factor 19; GRADE, Grading of Recommendation Assessment, Development and Evaluation; IBS-D, diarrhea-predominant irritable bowel syndrome; PICO, patient population, intervention, comparator, and outcome; SeHCAT, 75selenium homocholic acid taurine; SIBO, small intestinal bacterial overgrowth.
Diagnosis of BAD
Statement 1. In patients with chronic nonbloody diarrhea, we recommend using risk factors (history of terminal ileal resection, cholecystectomy, or abdominal radiotherapy) as the initial assessment to identify patients with possible BAD.
GRADE: Strong recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 60%; yes, 40%.
Statement 2. In patients with chronic nonbloody diarrhea, we suggest against using symptom presentation as the initial assessment to identify patients with possible BAD.
GRADE: conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: no, 100%.
Key Evidence
No published randomized controlled trials (RCTs) were available comparing the clinical impact of using versus not using risk factors or symptom presentation for the diagnosis of BAD, therefore evidence from observational, diagnostic test accuracy (DTA) studies was evaluated. Overall, studies have shown that history of terminal ileal resection, cholecystectomy or radiotherapy are the risk factors associated most commonly with having a positive SeHCAT test suggestive of a BAD diagnosis (Table 2) (24–30).
Table 2.
Risk factors in patients with chronic nonbloody diarrhea most commonly associated with having a positive SeHCAT test suggestive of a BAD diagnosis
| Risk factor | SeHCAT, <10% (at least moderate) | SeHCAT, <15% (at least mild) |
|---|---|---|
| Cholecystectomy | 78% (26) 68%; OR, 5.70; 95% CI, 2.42–13.46 (25) 21% (24) | 86%; 95% CI, 71%–95% (26) 68%; OR, 2.51; 99% CI, 1.10–5.77 (24) 57%; OR, 2.54; 95% CI, 1.36–4.74 (29) |
| TI resection or right hemicolectomy for Crohn’s disease | 100% (44)97% (28) 91%; OR, 15.83; 95% CI, 2.62–95.69 (25) 87% (24) | 92%; OR, 12.4; 99% CI, 2.42–63.8 (24)91%; 95% CI, 78%–87% (26) 87%; OR, 5.0; 95% CI, 2.20–11.4 (29) |
| TI resection or right hemicolectomy for reasons other than Crohn’s disease | 76% (24) 71% (29) | 82%; OR, 7.94; 99% CI, 1.02– 61.6 (24) |
| Radiotherapy without resection | 18% (30) | 62% (27) 36% (30) |
| Radiotherapy with resection | 71% (30) | 88% (30) |
BAD, bile acid diarrhea; OR, odds ratio; SeHCAT, 75selenium homocholic acid taurine; TI, terminal ileum.
No symptoms have consistently been predictive of a greater likelihood of having SeHCAT-diagnosed BAD among patients with chronic diarrhea. Rates of abdominal pain or discomfort, distension, bloating, flatulence and urgency, were similar or less frequent among patients with BAD and those with diarrhea resulting from other causes (24,31–33). Some studies have reported an association between stool weight, consistency or frequency, and a higher risk of BAD among patients with chronic diarrhea, but no diagnostic accuracy data, or definitions, are available (7,31–35).
All studies had either a high or unclear risk of bias, inconsistency (with respect to the specific symptoms and clinical characteristics as risk factors for BAD) and imprecision.
Discussion
In patients presenting with nonbloody chronic diarrhea or IBS-D, rates of SeHCAT retention suggestive of BAD are much higher in those with risk factors compared with those in whom other possible causes have been excluded. Rates of BAD were lower in patients without compared with those with risk factors, specifically rates of severe BAD (SeHCAT retention, <5%) were approximately 10% (6,36) compared with 24% to 48% (24,26,29), rates of at least moderate BAD (SeHCAT retention, <10%) were 19% to 39% (6,25,34,36) compared with 38% to 58% (24,26,29), and rates of at least mild BAD (SeHCAT retention, <15%) were 24% to 27% (6,24,36) compared with 46% to 68% (24,26,29). The risk factors most commonly identified are shown in Table 2. In patients with ileal resection, BAD appeared to be independent of resection length; resections of less than 10 cm were sufficient to cause BAD (26).
The potential harms of using clinical risk factors as a triage test for BAD could include overdiagnoses leading to unnecessary diagnostic tests and/or treatments, or underdiagnoses leading to ongoing patient suffering. In patients with ileal resection, there is an extremely high risk of BAD, and diagnostic testing may not be necessary before treatment, whereas patients with chronic diarrhea after a cholecystectomy or after radiotherapy may warrant diagnostic tests. No consistent correlation has been found between the length of resection and SeHCAT retention, therefore, all patients should be considered at high risk after resection (25,26,37).
Other conditions such as diabetes, pancreatitis, small intestinal bacterial overgrowth (SIBO), microscopic colitis, vagotomy and celiac disease have been associated occasionally, but not consistently, with an increased risk of BAD (26,38).
No symptoms have been identified that reliably will predict a diagnosis of BAD. In fact, data suggest that reliance on symptoms can lead to underdiagnosis in clinical practice; one survey found that 44% of patients reported they had experienced symptoms for more than 5 years before diagnosis (39). Although symptom presentation is inaccurate for BAD, it continues to play a role in the differential diagnosis to rule out other conditions.
Based on the available data, the consensus group recommends that in patients with chronic nonbloody diarrhea, a history of terminal ileal resection, cholecystectomy or radiotherapy, but not symptom presentation, be used during the initial assessment to help identify patients with BAD.
Statement 3. In patients with chronic diarrhea including IBS-D and functional diarrhea, we suggest SeHCAT testing to identify patients with BAD.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 20%; yes, 80%.
Key Evidence
Data on the diagnostic accuracy of the SeHCAT retention test (as an initial test for diagnosis) were derived from two prospective DTA studies, both conducted by Sciarretta et al. (37,40) in Italy. These were designed as case–control studies to assess the ability of SeHCAT retention to discriminate between cases and controls. However, using other secondary results, a 2013 Health Technology Assessment calculated the diagnostic accuracy of SeHCAT retention for predicting the response to BAST (41). In the first study, the sensitivity and specificity of SeHCAT retention (cut-off value, <5%) were 85.7% (95% confidence interval [CI], 42.1 to 99.6) and 100% (95% CI, 54.1 to 100), respectively, in a subgroup of patients (n = 13) with diarrhea without evidence of intestinal or extraintestinal pathology (37,41). The second study, which included 46 patients with IBS-D or cholecystectomy, found the sensitivity and specificity of SeHCAT retention (cut-off value, <8%) were 95.0% (95% CI, 75.1 to 99.9) and 96.2% (95% CI, 80.4 to 99.9), respectively (40,41). In both studies, a response to BAST was defined as the disappearance of diarrhea. No studies were found that measured the diagnostic accuracy of SeHCAT in patients with chronic diarrhea, which avoided a case–control design and used a proven reference standard (because there is currently no such reference standard, apart from the surrogate response to BAST).
Both DTA studies were found to be at serious risk of bias with respect to the index tests and reference standards used, serious indirectness of the study populations and index tests, and very serious imprecision as a result of the very small sample sizes, and the lower limit of the CI crossing the threshold for a clinically useful diagnostic test. This suggests that the data are insufficient to support or refute the clinical utility of SeHCAT in patients with IBS-D. Therefore, other factors and indirect supportive evidence were considered.
Discussion
Overall, the CoE for the diagnostic accuracy of SeHCAT was determined to be very low. As discussed in Statement 1, the prevalence data suggest that up to 40% of patients with functional diarrhea or IBS-D may have at least moderate BAD as assessed by a SeHCAT cut-off value less than 10% (6,25,34,36).
In addition, a systematic review (SR) including 15 observational studies showed a correlation between the severity of SeHCAT loss and response to treatment with BAST: response to cholestyramine was 96% in patients with less than 5% retention, 80% at less than 10% retention and 70% at less than 15% retention (6). This was not confirmed by a newer SR of 21 studies that found response rates with BAST of 67% at less than 5% retention, 73% at less than 8% to 11.7% retention and 59% at less than 15% retention (42). However, one study (26) published after the earlier SR, which included a large number of patients with secondary BAD, made a disproportionately large contribution to the group with less than 5% retention in this second analysis. Response rates were much lower in patients with negative SeHCAT tests; only 15% of patients had a good or partial response compared with 65.6% of patients with a SeHCAT retention less than 15% (29). A study has been proposed to evaluate the diagnostic accuracy of SeHCAT retention in which the test result will be concealed from clinicians and patients, and all patients will receive BAST (43).
Cost effectiveness and feasibility also were considered. The Health Technology Assessment assessed the cost effectiveness of SeHCAT testing compared with response to BAST based on data from three small trials and rather limited assumptions (41). They concluded that for the short term (first 6 months), the optimal choice between SeHCAT testing and no SeHCAT testing depended on willingness to pay, but that a trial of BAST would be more cost effective. From the long-term perspective, the optimal choice was a trial of BAST, no SeHCAT testing, or SeHCAT testing with a cut-off retention value of less than 15% depending on the scenario. Feasibility can be an issue in some areas because nuclear medicine facilities or the isotope may not be available.
BAST has poor tolerance and a high dropout rate; a positive SeHCAT test may have the additional benefit of providing the clinician with a stronger argument to encourage patients to stay on therapy when a definite diagnosis of BAD has been made (44). Other factors to consider are the potential harms of SeHCAT use, such as radiation risk, patient inconvenience and anxiety and loss of opportunity to use BAST in cases of false-negative results. Cut-off values to initiate treatment are sometimes inconsistent (45), and the role of borderline SeHCAT retention in therapeutic decisions is ill defined.
Taking all of these issues together, the consensus group concluded that SeHCAT retention is a relatively safe test, BAST is a relatively safe treatment (although poorly tolerated), and the anticipated benefit of SeHCAT retention testing likely outweighs the uncertainty of the evidence. Although other tests show promise for the future, SeHCAT retention has been the most widely tested, with consistent results.
Statement 4. In patients with small intestinal Crohn’s disease without objective evidence of inflammation who have persistent diarrhea, we suggest SeHCAT testing.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 20%; yes, 80%.
Key Evidence
An observational cohort study included a subgroup of 44 patients with unoperated Crohn’s disease in clinical remission (other than diarrhea) who had normal hematology and C-reactive protein levels (28). SeHCAT retention was abnormal (<10%) in 54% of patients. Of the 24 patients with abnormal SeHCAT retention, 20 received initial conventional treatment (prednisolone ± mesalamine), followed by BAST when conventional treatment failed. Response rates were 55% with conventional treatment, and 40% with BAST, with 5% failing both treatments. The treatment duration and outcome assessments, as well as the use of BAST in patients with normal SeHCAT retention, were not clearly described. The diagnostic accuracy and the effects of using test results to inform management choices could not be calculated because of the lack of a control group.
The CoE was downgraded to very low because of a very serious risk of bias (with regard to the reference standard, patient flow and timing) and very serious imprecision (very small sample size).
Discussion
Although there is very-low-certainty evidence supporting the use of SeHCAT testing to guide management decisions in patients with Crohn’s disease, testing may play a role in patients with ileal Crohn’s disease in complete remission who have ongoing chronic diarrhea.
Observational studies have suggested that almost half of the patients with ileal Crohn’s disease who have not undergone resection will have a positive SeHCAT, suggestive of a diagnosis of at least moderate BAD (Table 3) (25,26,28,29). These patients may have a two to four times greater likelihood of having a positive SeHCAT compared with having a negative test (25,29).
Table 3.
Prevalence of positive SeHCAT tests in patients with ileal Crohn’s disease who have not undergone resection
| SeHCAT | Prevalence |
|---|---|
| <10% (at least moderate) | 80%; OR, 3.76; 95% CI, 1.10–12.60 (25) 54% (28) 52% (26) 43% (29) 35% (24) |
| <15% (at least mild) | 76%; 95% CI, 57%–90% (26) 52%; OR, 1.88; 95% CI, 1.04‒3.41 (29) 35% (24) |
OR, odds ratio; SeHCAT, 75selenium homocholic acid taurine.
Given the association between positive SeHCAT testing and response to BAST in patients with Crohn’s disease who continue to have persistent diarrhea despite conventional treatments, the consensus group made a conditional recommendation in favour of SeHCAT testing in patients with Crohn’s disease who have no objective evidence of active inflammation.
Statement 5. In patients with chronic diarrhea including IBS-D and functional diarrhea, we suggest using a C4 assay to identify possible BAD.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 20%; yes, 60%; neutral, 20%.
No recommendation A: In patients with chronic diarrhea including IBS-D and functional diarrhea, the consensus group could not make a recommendation for or against the use of the FGF19 assay to identify possible BAD.
GRADE: No recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 20%; neutral, 80%.
Key Evidence
The majority of published DTAs compared C4(46–48) and FGF19(32,49,50) assays with SeHCAT testing. These showed good inverse correlation between C4 and SeHCAT testing, and between FGF19 and SeHCAT testing, however, the overall CoE for the diagnostic accuracy of SeHCAT was assessed for Statement 3 and determined to be very low. Therefore, the true diagnostic accuracy of these tests cannot be estimated from these studies.
One study assessing C4 and FGF19 assays used direct measurement of 48-hour fecal bile acid as a reference standard (51). This prospective DTA study included 30 patients with IBS-D who had replicate C4 and FGF19 samples 5 years apart that could be compared with fecal bile acid levels. When patients with a prior cholecystectomy were excluded, the sensitivity and specificity of serum C4 were 40% and 85%, respectively, with a 40% positive predictive value and an 85% negative predictive value for the diagnosis of BAD. For FGF19, the sensitivity and specificity were 20% and 75%, respectively, with a 17% positive predictive value and a 79% negative predictive value for the diagnosis of BAD.
The CoE was downgraded to very low because of the moderately serious risk of bias and very serious imprecision (CI lower limits crossed the threshold for clinically useful diagnostic tests, small sample sizes) in the DTA that used fecal bile acid levels as the reference standard (51). Similarly, there was very serious risk of bias and serious indirectness in the studies that used SeHCAT retention as the reference standard.
Discussion
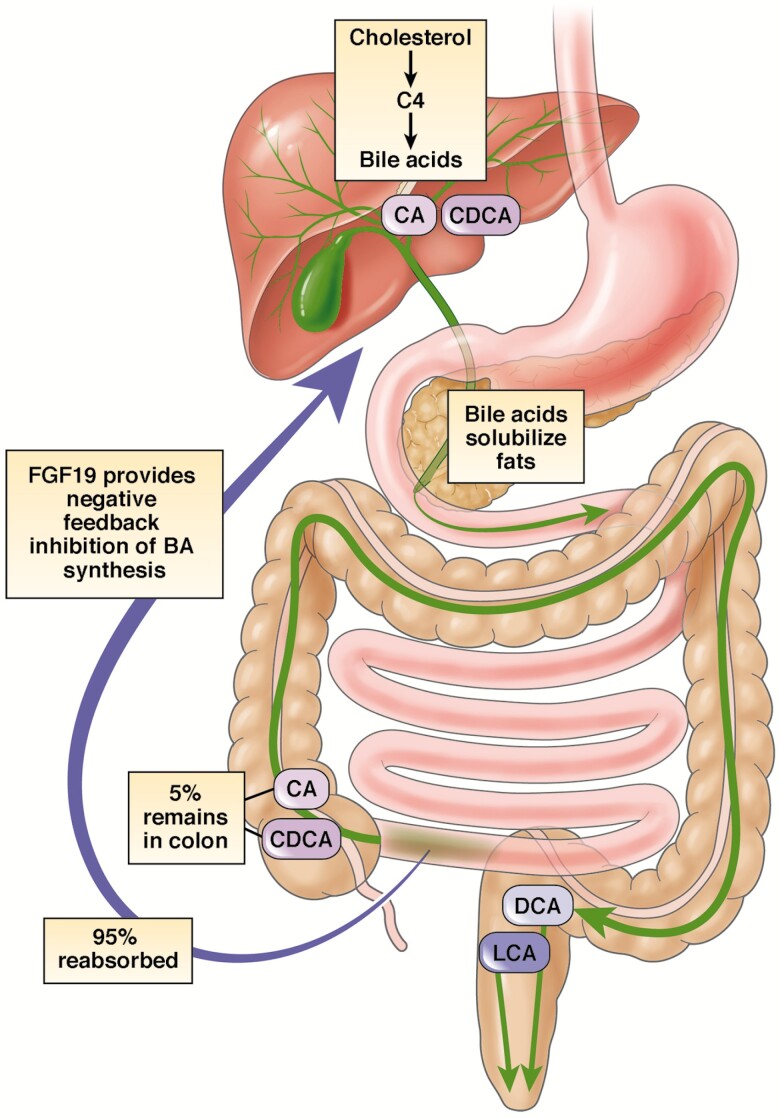
Although there appears to be a good correlation (inverse) between C4 and SeHCAT results, and between FGF19 and SeHCAT results, SeHCAT retention has not been validated adequately as a reference standard. Theoretically, C4 and FGF19 should be good markers of bile acid loss. C4 is a metabolic intermediate in the rate-limiting step for the synthesis of bile acids from hepatic cholesterol. FGF19 is a hormone released by ileal enterocytes after stimulation of nuclear farnesoid X receptors, typically by absorbed bile acids. Both markers have been correlated with fecal loss of bile acids (Figure 1) (51–53). In addition, FGF19 levels have been shown to correlate with C4 levels (54).
Figure 1.
Enterohepatic circulation of bile acids. C4 is a metabolic intermediate in the rate-limiting step for the synthesis of bile acids from hepatic cholesterol. FGF19 is a hormone released by ileal enterocytes after stimulation of nuclear farnesoid X receptors by absorbed bile acids. BA, bile acid; C4, 7 α-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; FGF19, fibroblast growth factor 19; LCA, lithocholic acid. Reprinted with permission from Vijayvargiya and Camilleri (53).
Currently, there are no well-defined cut-off values for the diagnosis of BAD. In one prospective study, a C4 level of 52.5 ng/mL or greater and a FGF19 level of 61.7 pg/mL or less were diagnostic for BAD (51). Other observational studies have used cut-off values of 30 to 48 ng/mL for C4 (46,55). One study found a wide range of normal values for C4 (corrected for cholesterol) from 0.76 to 8.0 mg/mol and for FGF19 from 48 to 343 pg/mL (33).
Insufficient evidence is available with C4, and even less with FGF19. In addition, the FGF19 assay was not available as a commercial clinical test at the time of the meeting, which impacts the feasibility of implementing that test. Therefore, the consensus group made a conditional recommendation in favour of C4, but was unable to make a recommendation for or against the use of the FGF19 assay to identify BAD.
Statement 6. In patients with suspected BAD, we suggest against initiating empiric BAST over performing SeHCAT to establish a diagnosis of BAD.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question (in patients with suspected BAD, should we initiate empiric BAST over performing SeHCAT to establish a diagnosis of BAD?): yes, 20%; no, 40%; strongly no, 40%.
Key Evidence
No direct comparative or DTA studies were found to inform this statement. As described in Statement 3, the two studies on the diagnostic accuracy of SeHCAT testing for predicting response to BAST yielded very low-certainty evidence in favour of using SeHCAT testing (37,40). The cost-effectiveness analysis included in the Health Technology Assessment conducted by Riemsma et al. (41) found that in the short term, a trial of BAST may be the optimal choice. However, over the long term, the optimal choice (trial of BAST, no SeHCAT testing, or SeHCAT at a cut-off retention value 15%) varied depending on the scenario. The analysis provided very low CoE regarding the optimal strategy.
Discussion
There is very little evidence to determine the relative role of testing with SeHCAT testing versus using an empiric trial of BAST to make a diagnosis of BAD. Other factors were considered when making a conditional recommendation against empiric treatment.
A poor response to a therapeutic trial of BAST could be related to noncompliance and early discontinuation, which could result in a falsely negative diagnosis with patients being denied other effective alternative BAST that may be better tolerated (38,56). As discussed in Statement 3, a definitive diagnosis of BAD may help educate and motivate patients to adhere to treatment (38,44).
Conversely, in patients in whom there is a very high index of suspicion (in whom a positive SeHCAT test is found in >90%), such as terminal ileum resection or right hemicolectomy, early initiation of therapy may be preferred. In addition, although a test-and-treat strategy was preferred for most patients, it was recognized that SeHCAT testing or other diagnostic tests are not available in some areas. In these cases, a trial of BAST may be the only option.
Induction Therapy for BAD
Statement 7. In patients with type 1 or type 3 BAD, we suggest the use of treatments for remediable causes (e.g., Crohn’s disease, microscopic colitis, SIBO) in addition to treatment for BAD for induction of clinical response.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 80%; yes, 20%.
Key Evidence
No RCTs or directly applicable cohort studies were identified in which treatment for remediable causes was compared with BAST in patients with type 1 or type 3 BAD. A cohort study (described in Statement 4), included subgroups of patients with IBS-D (n = 65, n = 40 treated) and unoperated Crohn’s disease in clinical remission (other than diarrhea, n = 24, n = 20 treated) who were diagnosed with BAD (SeHCAT retention, <10%) (28). The rates of response to initial conventional treatment (prednisone ± mesalamine for Crohn’s disease patients, or antidiarrheal agents for non-Crohn’s disease patients) were 55% among treated Crohn’s disease patients, and 15% among treated IBS-D patients. Conventional therapy followed by BAST was successful in 40% of treated Crohn’s disease patients and 70% of treated IBS-D patients.
This study lacked a control group and blinding, and had a subjective outcome measure. No evidence was found for other conditions (e.g., microscopic colitis, SIBO). The CoE was downgraded to very low because of serious risk of bias, indirectness and imprecision.
Discussion
Little data were available to define the role of other non-BAST treatments in patients with BAST and comorbid conditions. Specific treatments for comorbid conditions that may cause diarrhea (e.g., Crohn’s disease, microscopic colitis, SIBO) may achieve control of diarrhea and other symptoms, but, conversely, this may delay BAST for BAD. In addition, depending on the condition, the treatment (e.g., corticosteroids, immunosuppressive agents, biologics or antibiotics) may be associated with more risks or side effects than BAST treatment, and the investigations may be more invasive and costly (e.g., colonoscopy).
As mentioned in Statement 4, patients with Crohn’s disease with continuing diarrhea have a high rate of BAD. These patients were still more likely to benefit from conventional treatment, although some did benefit from BAST (28).
Some studies have suggested that BAD and collagenous colitis are associated, but likely are independent diseases (57–59). In case series of collagenous colitis, BAST improved symptoms, but had no effect on histopathology (57). In another case series, 86% of patients with microscopic colitis who had BAD benefited from BAST, whereas no patients with collagenous colitis without BAD improved (59). The etiology of microscopic colitis is not well defined, and may include infectious agents, medications, or other causes in some patients, which may require other specific treatments. Other treatments that may be beneficial include corticosteroids, antibiotics, antidiarrheal agents or immunosuppressive therapies (59,60).
In a large case series, 36% of patients with SIBO who were tested had SeHCAT retention less than 10% (26). These patients may benefit from BAST, but antibiotic therapy is the current standard for SIBO (61). The etiology of SIBO is very complex and may involve disorders of protective antibacterial mechanisms, anatomic abnormalities, or motility disorders. Patients with SIBO require treatment of the underlying disease, as well as nutritional support (62).
Although there is little evidence to guide therapeutic decisions, in patients with comorbid conditions, BAD may not be the sole cause of symptoms. Although some patients will respond to BAST for BAD, others might not, or may have other symptoms in addition to diarrhea that will not benefit from BAST. Therefore, the consensus group agreed that it was prudent to individualize therapy and address other remedial causes of gastrointestinal symptoms, with the order of therapy guided by severity of each condition.
Statement 8. In patients with BAD, we suggest using cholestyramine over no treatment for induction of clinical response.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: strongly yes, 60%; yes, 40%.
Statement 9. In patients with BAD, we suggest using cholestyramine over other BASTs as initial therapy for induction of clinical response.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: yes, 80%; neutral, 20%.
Key Evidence
One RCT compared cholestyramine with hydroxypropyl cellulose (HPC) (63). Although HPC was chosen as a placebo, it may be pharmacologically active, and a small case series suggested it may be effective in BAD (63,64). The RCT was an 8-week study in 26 patients with chronic functional watery diarrhea or IBS-D, of which 77% of the cholestyramine-treated and 54% of HPC-treated patients had a SeHCAT retention rate of 10% or less (63). There was no significant difference in clinical remission rates (defined as <3 bowel movements/d over 1 week, with <1 watery stool/d) between treatments (53.8% versus 38.4%; P = 0.43). However, there was a significant improvement in the decrease in watery stools per day (−92.4% ± 3.5% versus −75.8% ± 7.1%; P = 0.048). Because HPC binds bile acids and may have a bulking effect, it may have some efficacy for BAD (63–66); this makes it difficult to interpret the lack of significant differences in clinical remission rates with HPC compared with cholestyramine.
A SR of 23 cohort studies including 801 patients with BAD found that first-line cholestyramine was successful in 69.8% of patients overall, 67% of those with SeHCAT retention less than 5%, 73% of those with SeHCAT retention less than 8% to 11.7%, and 59% of those with SeHCAT retention less than 15% (42). Study designs, patient populations, inclusion and exclusion criteria, diagnostic tests and cut-off values for BAD, cholestyramine dosing and timing of administration, and definitions of clinical response varied widely among the studies. An additional cohort study published after the SR reported a response rate of 56% with first-line cholestyramine in 87 patients with BAD (defined as SeHCAT <15%) (67).
Although the RCT found that the rate of drug-related adverse events did not differ between cholestyramine and hydroxypropyl cellulose (63), the SR of cohort studies reported that 11% of patients found cholestyramine intolerable because of unpalatability or side effects (range, 0% to 46%) (42). The most common side effects included abdominal bloating and pain, dyspepsia, nausea/vomiting, flatulence, borborygmi, abdominal distension, constipation and increased severity of diarrhea. In the additional cohort study, almost half (45%) of treatment failures were related to medication intolerance (67). However, both studies had no control group for comparisons, and relationships to the study drug were not assessed.
RCTs assessing the efficacy of cholestyramine compared with other BAST in patients with BAD were not found. Evidence for using cholestyramine over other BASTs as initial therapy considered other factors such as adverse events, clinical experience and cost. There is no direct evidence that cholestyramine is associated with more side effects than other BAST. However, an RCT of BAST for cardiovascular disease prevention reported higher rates of gastrointestinal side effects (55% versus 16%), and lower rates of compliance (53% versus 77%) with adjunctive cholestyramine compared with monotherapy with a statin (68). In contrast, a SR of 6 RCTs in patients with diabetes found that adverse rates with adjunctive colesevelam were similar to placebo (relative risk, 1.06; 95% CI, 0.97 to 1.15), with the most common events with colesevelam being gastrointestinal-related (e.g., constipation, dyspepsia and nausea) and minor in nature (69). The majority of clinical experiences with BASTs in BAD has been with cholestyramine, with few data on the other agents; in addition, colesevelam and colestipol tend to be more costly compared with cholestyramine.
The overall CoE was very low. Very serious indirectness and serious imprecision were found in the RCT (63), with a serious risk of bias, indirectness and imprecision in the cohort studies (42,67).
Discussion
Clear RCT evidence showing the benefits of BAST was not available, however, case series and SRs of observational studies support a dramatic and rapid response for many patients. Although no patient preference data were found, the high dropout rates in all of these studies suggest that some patients may place a greater value on being free of the side effects or unpalatability of cholestyramine compared with reduction in their diarrhea frequency or severity. However, because BAST targets the problem, the potential higher response rates in patients with more severe BAD (as measured by SeHCAT retention) (6) and the lack of response in patients who test negative for BAD (29) (see Statement 3), the consensus group suggested that patients with BAD receive treatment with BAST over no treatment. This was a conditional recommendation because of the very low CoE and poor tolerability profile, making it important to discuss the benefits and side effects with patients.
Although the consensus group suggested that cholestyramine be used initially over the other BAST agents (colesevelam or colestipol), there are few comparative data. Compared with cholestyramine, colesevelam has a four to six times stronger binding affinity to bile acids. It may be better tolerated and have fewer clinical interactions (67). The majority of clinical experiences to date are with cholestyramine, with a limited number of cases using other BAST agents (29,33,38,70). Response rates with first-line use of other BASTs have been reported at 67% with colesevelam (70) and 55% with colestipol (33). Although cholestyramine appears to be less costly than colesevelam or colestipol, the lack of comparative data casts doubt on whether cholestyramine should be preferred; therefore, this was a conditional recommendation.
Statement 10. In patients with BAD who are unable to tolerate cholestyramine, we suggest using an alternate BAST for induction of clinical response.
GRADE: Conditional recommendation, low-certainty evidence.
Vote: on PICO question: strongly yes, 40%; yes, 60%.
Key Evidence
No RCT data were available comparing alternate BASTs with either placebo or other treatments as second-line therapy in patients with BAD who are unable to tolerate cholestyramine. One RCT compared first-line colesevelam and placebo for BAD-associated diarrhea in 26 patients with Crohn’s disease in remission (70). There was a statistically nonsignificant improvement in the primary end point (proportion of patients with >30% reduction of liquid stools/day) with colesevelam (66.7%) versus placebo (27.3%) based on intention-to-treat analysis (risk difference, 0.394; 95% CI, −0.012 to 0.706; P = 0.0566). Colesevelam significantly improved the secondary end points of the reduction in the number of liquid stools per day and improvement in stool consistency compared with placebo. This trial did not assess colesevelam as second-line therapy, and had a very small sample size; therefore, the CoE was downgraded to low for serious indirectness and imprecision.
Additional evidence comes from a SR of four observational cohort studies (n = 63) that assessed the efficacy of second-line colesevelam after failure of cholestyramine and reported a success rate of 57% (range, 42% to 100%) (42). One other cohort study published after the SR included 15 patients who had not responded to cholestyramine and received second-line treatment with colesevelam (67). Of these patients, 47% had a successful response. The CoE from the observational trials was downgraded to very low for serious risks of bias and imprecision.
There is no direct evidence that colesevelam is associated with a higher or lower frequency of adverse effects than cholestyramine or other BASTs. In the RCT, colesevelam generally was well tolerated; adverse events were mild (constipation, bloating and nausea) and occurred in similar proportions to colesevelam and placebo groups (40.0% versus 36.4%) (70). For safety, the SR included one RCT and four observational cohort studies, and found that 9% were unable to tolerate colesevelam because of unpalatability or side effects (42). In the additional observational study, no patients reported treatment intolerance with colesevelam (67). As discussed in Statement 8, tolerability data for BASTs in nongastrointestinal conditions suggested high rates of gastrointestinal side effects with cholestyramine, while colesevelam had side effects rates similar to placebo (68,69).
There have been limited reports describing the use of colestipol as second-line therapy after failure of cholestyramine (42,71).
Discussion
Case series data have suggested that patients who fail or are unable to tolerate cholestyramine may benefit from second-line BAST (29). In a large series of patients given one or more BAST, there were no significant differences in good/partial response rates between the cholestyramine (74%) and colesevelam (73%). However, whether alternate BAST was used as first- or second-line therapy was not described (29). Although, not regulatory approved for BAD, use of second-line colesevelam in clinical practice appears to be quite common. In a survey of patients followed up for up to 13 years, 38% of respondents continued with cholestyramine, while 32% had switched to colesevelam (56). The consensus group agreed that compared with cholestyramine, colesevelam has a favourable benefit:risk profile and greater ease of administration (tablet versus granules/powder). However, because of the limited clinical experience and higher cost, it is suggested that it be reserved for second-line use.
Statement 11. In patients with BAD receiving empiric BAST, gradual daily dose titration should be used to minimize side effects.
Designated a good practice statement
Key Evidence
Good practice statement, CoE not assessed.
Discussion
In general, most cohort studies reported gradual dose titration for cholestyramine to clinical response (42,67). However, there was no mention of dose titration of colesevelam or colestipol.
In BAD studies, cholestyramine generally was started at a low dose of 2 to 4 g/day and titrated based on response (maximum, 4 to 24 g/day) (42,67). In an open-label study, the colestipol dose was initiated at 1 g twice daily, with an increase of 1 g/d every other day (33). In BAD studies, colesevelam has been prescribed in a dose of two tablets (625 mg) three times per day (70,72).
Product labeling for BAST agents recommends that cholestyramine be started at one 4-g dose daily and titrated to effect with a maximum of 24 g/day for all patients (73). Initiation of colestipol granules (tablets) is recommended at 5 g (2 g) either once or twice daily, increasing by 5 g/day (2 g once or twice per day), but no more frequently than 1 per month, with a maximum of 30 g/day (16 g/day). No dose titration is recommended for colesevelam. Colesevelam is dosed at 3.75 g/day as three 625-mg tablets twice daily, 6 tablets once daily, or one 3.75-g powder packet once daily. These colestipol and colesevelam doses are regulatory approved for cholesterol-lowering indications.
Generally, it is intuitive to gradually titrate medication to maximize symptom relief and minimize side effects. This is particularly relevant with BAST because of the high frequency of side effects and intolerance (42). Gradual dose titration of BAST may reduce the risks of side effects, increase compliance and potentially reduce costs.
Statement 12. In patients with Crohn’s disease with extensive ileal involvement or resection, we suggest against using BAST.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question (In patients with Crohn’s disease with extensive ileal involvement or resection, should we use BAST vs no BAST?): yes, 20%; no, 80%.
Key Evidence
There are no long-term studies assessing the safety of cholestyramine in patients with extensive ileal resection. It has been suggested that use of BAST in these patients can lead to an increased rate of steatorrhea (74,75). A small series of nine patients, in whom three had ileal resection greater than 100 cm and steatorrhea greater than 20 g/day, found that the use of cholestyramine led to a small decrease in diarrhea, but an increase in steatorrhea with substantial caloric loss (74,75).
Discussion
It is unclear how extensive a resection is required to produce negative consequences with BAST. In the case reports, the risk of steatorrhea was increased in patients with resections of greater than 100 cm (74,75).
Other data have shown no correlation between the length of resection, SeHCAT retention and response to BAST. In case series of patients with ileal resection of up to 200 cm, the majority had severe BAD and responded to BAST (26,76,77). In one case series, the mean length of resection was not significantly different in patients who did or did not respond to BAST (35 versus 46 cm) (78).
SeHCAT testing in patients with large ileal resection almost universally will indicate severe bile acid wasting and is unlikely to be of discriminatory clinical value. Although there are very few reports of adverse consequences of BAST use in patients with extensive resection, the consensus group concluded that the risk of steatorrhea makes it prudent to err on the side of caution and avoid BAST in this patient group. Furthermore, there is concern that these patients may have extensive inflammatory disease that should be identified and treated with anti-inflammatory approaches rather than BAST. However, in some cases, the benefits may outweigh the risks, and patients should be evaluated on a case-by-case basis.
Maintenance Therapy for BAD
Statement 13. In patients with BAD who respond to BAST, we suggest that intermittent, on-demand dosing be tried.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: yes, 80%; neutral, 20%.
Key Evidence
No studies were found that directly compared different dosing strategies in patients with BAD who had responded to BAST. Two small cohort studies suggested that for some patients, BAD symptoms could remain controlled with on-demand therapy or no therapy at all (7,8). In a prospective cohort study of patients with postcholecystectomy BAD, cholestyramine (2 to 12 g/day for 1‒6 months) was effective in 23 of 26 patients, and 9 of 23 (39%) patients experienced recurrent diarrhea when treatment was withdrawn. Bowel habit remained regular in 14 patients (61%) who took the drug occasionally (on demand) in the event of sporadic episodes of slight diarrhea (8). In the other cohort study in patients with BAD and IBS-D, recurrent diarrhea occurred in 33 of 35 (94%) of patients when cholestyramine (2‒8 mg/day for 1 month) was withdrawn, and the drug was prescribed again at the dose that controlled the patient’s symptoms (7). Only 6% of patients were able to discontinue therapy without suffering recurrent diarrhea.
Discussion
Evidence suggests that some patients with BAD will require regular daily dosing, whereas others may be able to discontinue completely or use on-demand therapy for symptom control. The dose or frequency of BAST required to control symptoms may be dependent on the severity of symptoms, underlying causes of BAD, or the presence of other comorbid illnesses (e.g., gastroenteritis, Clostridium difficile infection). The need for BAST also may be affected by use of medications that cause constipation, which may reduce the need for BAST, or by medications that cause diarrhea, which may increase the need for BAST.
Long-term use of BAST should balance the potentially high rate of relapse of diarrhea against the high rate of adverse events, poor palatability and uncertainty around long-term harms (e.g., malabsorption of fat and vitamins). Therefore, the consensus group suggested that during ongoing long-term therapy, intermittent, on-demand therapy should be attempted to minimize exposure to BAST, encourage compliance and minimize costs.
Statement 14. In patients with BAD who are unable to tolerate BAST, we suggest using alternative anti-diarrheal agents vs. no treatment for long-term symptomatic therapy.
GRADE: Conditional recommendation, very-low-certainty evidence.
Vote: on PICO question: yes, 100%
Key Evidence
No studies were found that systematically assessed the effectiveness of other antidiarrheal agents in patients with BAD who are unable to tolerate BAST. As described in Statement 8, 1 RCT that compared cholestyramine with HPC found no difference in clinical remission (53.8% versus 38.4%) or adverse events (63).
Three cohort studies assessed first-line loperamide in patients with BAD; however, the effectiveness was difficult to estimate because of differences in patient populations, study designs and outcome measurements (mainly subjective improvement of symptoms) (28,79,80). A randomized, double-blind, cross-over RCT in 18 patients with chronic diarrhea resulting from chronic radiation enteritis compared loperamide (3 mg twice daily) and placebo for 14 days (79). The study did not include dichotomized response rates, but did report significant improvements in stool frequency, stool weight and SeHCAT retention with loperamide as compared with placebo. In a prospective cohort study of 19 patients with chronic diarrhea resulting from ileal irradiation and/or resection, 13 patients with resections of 20 to 50 cm (n = 7) or no resection (n = 6) showed normalized or improved SeHCAT retention, with symptomatic improvement while on loperamide (80). In six patients with resection greater than 80 cm, SeHCAT retention remained abnormal, and only three patients had slight improvement of diarrhea with loperamide. In another cohort study, 27 of 96 (28%) patients reported improvement with conventional antidiarrheal agents; however, this included codeine, loperamide or prednisolone (not considered an antidiarrheal agent), and did not specify response to individual medications (28).
Discussion
Given the poor tolerability and high discontinuation rates with BAST, alternative treatments often are needed. HPC may improve diarrhea in patients with BAD through its bulking effects and its ability to bind bile acids (63–66). In addition, some patients may benefit from loperamide; given its low cost and relatively good safety profile (although no cost-effectiveness data are available), a treatment trial may be warranted.
Statement 15. In patients with BAD receiving empiric BAST, maintenance therapy should be used at the lowest dose needed to minimize symptoms.
Designated a good practice statement
Key Evidence
Good practice statement, CoE not assessed.
Discussion
The importance of minimizing exposure to BAST was discussed in Statement 11 (dose titration during induction) and Statement 13 (use of intermittent or discontinuing dosing during maintenance therapy).
Cohort studies have reported the use of cholestyramine for 6 to 44 months, which was titrated to response (42). In one study, patients were allowed to titrate their own dose of cholestyramine (between 2 and 16 g/day) and sustained responses for over 1 year (81). Colesevelam has been used for up to 44 months with some patients titrating the dose down (72).
Statement 16. In patients with BAD and recurrent or worsening symptoms despite stable BAST, diagnostic re-evaluation should be conducted.
Designated a good practice statement
Key Evidence
Good practice statement, CoE not assessed.
Discussion
Other diagnoses are common in patients with BAD, and a diagnosis of BAD is seen frequently in patients with other conditions (see Statement 1). As discussed in Statement 7, some patients may need specific treatments for other causes of chronic diarrhea.
BAD can have a variable course, and fat intake can cause fluctuations in SeHCAT retention and severity of BAD. Low-fat dietary interventions can improve gastrointestinal symptoms for some patients (82). However, sudden worsening of symptoms not related to dietary changes should prompt re-evaluation. The differential diagnosis should consider conditions such as microscopic colitis, Crohn’s disease, celiac disease, SIBO and functional bowel disease. Strategies in patients with worsening symptoms might include repeating SeHCAT testing with an escalation of therapy if needed, as well as other tests, such as stool tests for infectious etiologies, blood tests, colonoscopy, hydrogen breath tests as determined by the underlying cause of BAD, and the patient’s history, risk factors and symptoms.
Statement 17. In patients being considered for BAST, a review of concurrent medications should be conducted to minimize the potential for drug interactions.
Designated a good practice statement
Key Evidence
Good practice statement, CoE not assessed.
Discussion
BAST agents may bind other drugs given concurrently, which necessitates separating administration to minimize the risk of reduced absorption of the concomitant medication. Health Canada recommends that when a drug interaction cannot be excluded, patients should take other drugs at least 1 hour before or 4 to 6 hours after the BAST (73,83,84). Gastric emptying studies have suggested that a window of 3 hours between administration of BAST and other medications is adequate to avoid potential interactions such as binding (85).
Examples of some medications that may interact when coadministered with cholestyramine or colestipol include thyroid preparations, warfarin, hydrochlorothiazide, furosemide, phenylbutazone, phenobarbital, tetracycline, penicillin G, digoxin, mycophenolic acid and estrogen-containing drugs (3,73,84). Colesevelam has a different structure that maximizes interactions with bile salt and reduces the potential for interactions with other drugs (86,87). Colesevelam does not appear to interact with some medications (e.g., digoxin, fenofibrate, lovastatin, metoprolol, pioglitazone, quinidine, repaglinide, valproic acid, verapamil), but has been found to reduce the absorption of others (e.g., glyburide, levothyroxine and oral contraceptives), and may interact with warfarin and phenytoin (83).
No recommendation B. In patients receiving long-term maintenance therapy with BAST, the consensus group could not make a recommendation for or against measuring fat-soluble vitamin levels at baseline and annually thereafter.
GRADE: NO recommendation; very-low-certainty evidence.
Vote: on PICO question: yes, 20%; neutral, 80%
Key Evidence
The literature search failed to identify any relevant article assessing fat-soluble vitamin levels before and after initiation of long-term maintenance therapy with BAST. Because of the action of BAST agents in sequestering bile acids, these agents theoretically may interfere with normal fat absorption, thus reducing absorption of folic acid and fat-soluble vitamins A, D and K (73,83,84). Whether this interference can result in clinical consequences is based on rare case reports. Since 1970, there have been only a few reports of hypoprothrombinemia or hemorrhage in adults (88,89), and of hypoprothrombinemia, hemorrhage or folate deficiency in paediatric patients (90–92) taking cholestyramine.
Discussion
Cholestyramine has been associated with reduced vitamin and folate levels during long-term use (73). However, colestipol use for 1 to 2 years had no effect on vitamin A or folic acid levels, and only a small effect on vitamin D levels (84). Colesevelam was not associated with significant reductions in the absorption of vitamins A, D, E or K during clinical studies of up to 1 year (83). In general, the approved product labels recommend supplementation of vitamins A, D and K only if a deficiency occurs (73,83,84).
The rare cases of vitamin K deficiency resulting in increased risk of coagulopathy have occurred within a few weeks to months or years after the start of therapy (89), and generally can be corrected with oral vitamin K. Although during long-term use periodic monitoring of vitamin levels and prothrombin time sometimes are advised (3,93), the group did not reach consensus on the value of annual routine monitoring. Most of the consensus participants were neutral on this issue, although it was suggested that performing an international normalized ratio at intervals during long-term treatment may be prudent.
Conclusions
The group recognized that specific, high-certainty evidence was lacking in many areas and recommended further studies that would improve the data available in future methodological evaluations.
In DTA studies, the diagnostic accuracy of an index test (a test under evaluation) is determined by comparing its results with that of a reference standard (best available method to determine the presence or absence of a target condition), by applying both in individuals who are suspected of having the target condition of interest. However, if the reference standard does not correspond perfectly to a true target condition, estimates of the accuracy of the index test can be biased. The main challenge in conducting DTA studies for BAD is the lack of a widely accepted or universally agreed-upon reference standard because the condition is defined and classified based on pathophysiologic mechanisms and its response to treatment (BAST). In addition, the index tests (SeHCAT, C4, FGF19, fecal bile acid assay) provide a continuous measure of metabolic function. Hence, DTA studies are not the most appropriate study design (41). In studies in which all patients are tested with the index tests and all patients are treated with BAST, response to treatment can provide an imperfect, but the best available, reference standard. This is because patients responding to BAST may be true-positive patients with a true response, but also may be false-positive patients with a placebo response. To date, only two small DTA studies have reported information on the probability of response to treatment with BAST for people with a negative SeHCAT test, and no DTA studies have incorporated a blinded placebo arm (37,40). Consequently, the lack of evidence of the accuracy of the SeHACT test based on a reference standard and the variation in cut-off values of test results led to important uncertainties in the cost-effectiveness analyses in determining the optimal strategy in investigating BAD (41). Therefore, one of the research priorities in BAD is for the scientific and clinical communities to agree on a reference standard that best represents BAD (e.g., response to BAST), with full understanding that the reference standard is and likely will be imperfect.
Given the paucity of high-certainty evidence on diagnostic tests, there is also a need for well-designed DTA studies comparing SeHCAT, C4 assay, FGF19, and total and primary bile acid measurement in stool, with a reference standard for BAD (e.g., response to BAST) by applying both the index tests and reference standard to all patients (94,95), as well as RCTs comparing SeHCAT testing versus an empiric trial of BAST in patients with suspected BAD including an assessment of objective clinical efficacy and safety outcome measures. A placebo-controlled RCT of BAST (colesevelam) in patients with evidence of BAD, based on fecal bile acid measurements, is ongoing (NCT03270085) and the results will help to inform the role of fecal bile acids as a diagnostic test for BAD (96).
It is important to note that the diagnostic accuracy of total and primary bile acid excretion has not been assessed formally by GRADE for this guideline because it was not a topic initially proposed for inclusion a priori. Nevertheless, there have been recent publications on assessing 48-hour total and primary bile acid fecal excretion (a test available in North America) as a diagnostic test for BAD (95). Recent advances also have assessed whether this test could be optimized by including assays of primary bile acids (95). Most (if not all) have been observational studies that have found significant correlation or association between increased fecal bile acids and certain conditions that can cause diarrhea (i.e., IBS-D, chronic functional diarrhea) (95,97–99). Although observational studies can provide evidence of significant association or correlation between predictor and outcome variables, they cannot prove causality because there are always residual confounding variables (unmeasured or imprecisely measured) that may have affected the results. Spurious associations also can arise with reverse causality. Future prospective studies are required to validate the diagnostic accuracy for BAD of primary bile acids at various cut-off concentrations in a single stool sample against a reference standard (i.e., the ability of this test to accurately predict response to BAST).
RCTs are needed to compare cholestyramine with other BASTs for the treatment of BAD. In addition, evidence is needed to guide dosing schedules. This includes assessment of whether there is any advantage to morning versus evening dosing and once-daily versus divided doses of BAST to maximize benefits and minimize interactions with other medications. Theoretically, there may be some efficacy benefits to targeting dosing to times of maximum gallbladder emptying, such as postprandially or in the morning, but more research is needed. In hypercholesterolemia there were no significant variations in the hypocholesterolemic effects when cholestyramine was timed with meals to optimize exposure to bile in the duodenum that followed gallbladder emptying (100). However, the relevant mechanisms in BAD may be different, particularly because the therapeutic aim is to reduce the effects of free secretory bile acid in the colon.
In conclusion, current evidence suggests that the accuracy of diagnostic tests (e.g., SeHCAT, C4) in predicting BAD or response to treatment are highly uncertain. Economic evaluation suggests that strategies of either an empiric trial of BAST or performing SeHCAT testing may be cost effective depending on the scenarios and society’s willingness to pay. Therefore, either strategy may be used to identify patients with possible BAD depending on cost, available resources, local expertise and patient preferences.
Canadian Association of Gastroenterology Statement
This clinical practice guideline on the management of BAD was developed under the direction of Dr Daniel Sadowski, in accordance with the policies and procedures of the Canadian Association of Gastroenterology and under the direction of the Canadian Association of Gastroenterology Clinical Affairs. It has been reviewed by the Canadian Association of Gastroenterology Practice Affairs and Clinical Affairs Committees and the Canadian Association of Gastroenterology Board of Directors. The clinical practice guideline was developed after a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian, U.S. and UK panel comprising experts on this topic. The clinical practice guideline aims to provide a reasonable and practical approach to care for specialists, and allied health professionals are charged with the duty of providing optimal care to patients and families, and can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The clinical practice guideline is not intended to be a substitute for physicians using their individual judgment in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic and treatment options available and available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
Acknowledgments
The consensus group would like to thank Pauline Lavigne and Steven Portelance (unaffiliated) who provided medical writing services and editorial assistance on their behalf, supported by funds from the Canadian Association of Gastroenterology; and Paul Sinclair, Cindy Roll and Adria Cehovin (Canadian Association of Gastroenterology representatives: administrative and technical support, and logistical assistance).
As per Canadian Association of Gastroenterology policy for all clinical practice guidelines, the manuscript was made available to all Canadian Association of Gastroenterology members for commenting before submission for publication. Members were notified that the manuscript was available on the members-only section of the Canadian Association of Gastroenterology website and open for comment for a 2-week period.
Funding
This guideline was supported through unrestricted grants to the Canadian Association of Gastroenterology by Pendopharm and GE Healthcare Canada, neither of which had any involvement in the development of this guideline. The Canadian Association of Gastroenterology administered all aspects of the meeting, and the funding sources had no involvement in the process at any point, and were not made aware of any part of the process from the development of search strings and the statements, to drafting and approval of these guidelines.
Conflicts of interest
These authors disclose the following: John K. Marshall has served on the advisory boards of AbbVie, Allergan, AstraZeneca, Boehringer-Ingelheim, Celgene, Celltrion, Ferring, Hospira, Janssen, Merck, Pfizer, Pharmascience, Shire, and Takeda, has consulted for Lupin, Merck, and Pharmascience, and has served on the speaker’s bureau for AbbVie, Allergan, Ferring, Janssen, Shire, and Takeda; Michael Camilleri has served on the advisory boards of Allergan and BioKier, has consulted for AstraZeneca, Dignify Therapeutics, Elobix AB, Enterin, Ironwood, Shire, Takeda, and Theravance, and has received educational and/or research grants from Allergan, AstraZeneca, Elira, NGM Biopharma, Novartis Pharma, Novo Nordisk, and Rhythm; William D. Chey has served on the advisory boards of the American College of Gastroenterology, the American Neurogastroenterology and Motility Society, and the Rome Foundation, and has consulted for Allergan, Biomerican, IM Health, Ironwood, Nestle, Prometheus Diagnostics, QOL Medical, Ritter, Salix, and Shire; Julian R. F. Walters has consulted for ENYO Pharma, GE Healthcare, Intercept Pharma, Metacrine, Inc, Novartis Pharma, Pharmascience, Prometheus Diagnostics, and Zealand Pharma, has received educational and/or research grants from ENYO Pharma, GE Healthcare, Intercept Pharma, Novartis Pharma, and Prometheus Diagnostics, and has served on the speaker’s bureau of GE Healthcare; and Eldon A. Shaffer has served on the speaker’s bureau of Pendopharm. The remaining authors disclose no conflicts.
References
- 1. Scallan E, Majowicz SE, Hall G, et al. Prevalence of diarrhoea in the community in Australia, Canada, Ireland, and the United States. Int J Epidemiol 2005;34(2):454–60. [DOI] [PubMed] [Google Scholar]
- 2. Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999;116(6):1464–86. [DOI] [PubMed] [Google Scholar]
- 3. Barkun AN, Love J, Gould M, et al. Bile acid malabsorption in chronic diarrhea: Pathophysiology and treatment. Can J Gastroenterol 2013;27(11):653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: Diagnosis and management. Clin Gastroenterol Hepatol 2017;15(2):182–93.e3. [DOI] [PubMed] [Google Scholar]
- 5. Mottacki N, Simrén M, Bajor A. Review article: Bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2016;43(8):884–98. [DOI] [PubMed] [Google Scholar]
- 6. Wedlake L, A’Hern R, Russell D, et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30(7):707–17. [DOI] [PubMed] [Google Scholar]
- 7. Galatola G, Ferraris R, Pellerito R, et al. The prevalence of bile acid malabsorption in irritable bowel syndrome and the effect of cholestyramine: An uncontrolled open multicentre study. Eur J Gastroenterol Hepatol 1992;4:533–7. [Google Scholar]
- 8. Sciarretta G, Furno A, Mazzoni M, et al. Post-cholecystectomy diarrhea: Evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol 1992;87(12):1852–4. [PubMed] [Google Scholar]
- 9. American Gastroenterological Association medical position statement: Guidelines for the evaluation and management of chronic diarrhea. Gastroenterology 1999;116:1461–3. [DOI] [PubMed] [Google Scholar]
- 10. Thomas PD, Forbes A, Green J, et al. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut 2003;52(Suppl 5):v1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arasaradnam RP, Brown S, Forbes A, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018;67(8):1380–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultan S, Falck-Ytter Y, Inadomi JM. The AGA institute process for developing clinical practice guidelines part one: Grading the evidence. Clin Gastroenterol Hepatol 2013;11(4):329–32. [DOI] [PubMed] [Google Scholar]
- 14. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 2017;152(3):497–514. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology . The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016;150(3):734–57.e1. [DOI] [PubMed] [Google Scholar]
- 16. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of helicobacter pylori infection in adults. Gastroenterology 2016;151(1):51–69.e14. [DOI] [PubMed] [Google Scholar]
- 17. Bressler B, Marshall JK, Bernstein CN, et al. ; Toronto Ulcerative Colitis Consensus Group . Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: The Toronto consensus. Gastroenterology 2015;148(5):1035–58.e3. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014;146(3):835–48.e6. [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Schünemann HJ, Djulbegovic B, et al. Guideline panels should not GRADE good practice statements. J Clin Epidemiol 2015;68(5):597–600. [DOI] [PubMed] [Google Scholar]
- 20. Dalkey N. An experimental study of group opinion: The Delphi method. Futures 1969;1:408–26. [Google Scholar]
- 21. Cook DJ, Greengold NL, Ellrodt AG, et al. The relation between systematic reviews and practice guidelines. Ann Intern Med 1997;127(3):210–6. [DOI] [PubMed] [Google Scholar]
- 22. Alonso-Coello P, Schünemann HJ, Moberg J, et al. ; GRADE Working Group . GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . Going from evidence to recommendations. BMJ 2008;336(7652):1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gracie DJ, Kane JS, Mumtaz S, et al. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil 2012;24(11):983–e538. [DOI] [PubMed] [Google Scholar]
- 25. Kurien M, Evans KE, Leeds JS, et al. Bile acid malabsorption: An under-investigated differential diagnosis in patients presenting with diarrhea predominant irritable bowel syndrome type symptoms. Scand J Gastroenterol 2011;46(7–8):818–22. [DOI] [PubMed] [Google Scholar]
- 26. Borghede MK, Schlütter JM, Agnholt JS, et al. Bile acid malabsorption investigated by selenium-75-homocholic acid taurine ((75)SeHCAT) scans: Causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med 2011;22(6):e137–40. [DOI] [PubMed] [Google Scholar]
- 27. Ludgate SM, Merrick MV. The pathogenesis of post-irradiation chronic diarrhoea: Measurement of SeHCAT and B12 absorption for differential diagnosis determines treatment. Clin Radiol 1985;36(3):275–8. [DOI] [PubMed] [Google Scholar]
- 28. Smith MJ, Cherian P, Raju GS, et al. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond 2000;34(5):448–51. [PMC free article] [PubMed] [Google Scholar]
- 29. Murray IA, Murray LK, Woolson KL, et al. Incidence and predictive factors for positive 75SeHCAT test: Improving the diagnosis of bile acid diarrhoea. Scand J Gastroenterol 2017;52(6–7):698–703. [DOI] [PubMed] [Google Scholar]
- 30. Valdes Olmos R, den Hartog Jager F, Hoefnagel C, et al. Imaging and retention measurements of selenium 75 homocholic acid conjugated with taurine, and the carbon 14 glycochol breath test to document ileal dysfunction due to late radiation damage. Eur J Nucl Med 1991;18:124–8. [DOI] [PubMed] [Google Scholar]
- 31. Ung KA, Kilander AF, Lindgren A, et al. Impact of bile acid malabsorption on steatorrhoea and symptoms in patients with chronic diarrhoea. Eur J Gastroenterol Hepatol 2000;12(5):541–7. [DOI] [PubMed] [Google Scholar]
- 32. Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: A prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther 2013;38(8):967–76. [DOI] [PubMed] [Google Scholar]
- 33. Bajor A, Törnblom H, Rudling M, et al. Increased colonic bile acid exposure: A relevant factor for symptoms and treatment in IBS. Gut 2015;64(1):84–92. [DOI] [PubMed] [Google Scholar]
- 34. Stotzer PO, Abrahamsson H, Bajor A, et al. Are the definitions for chronic diarrhoea adequate? Evaluation of two different definitions in patients with chronic diarrhoea. United European Gastroenterol J 2015;3(4):381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption–a review of clinical presentation, diagnosis, and response to treatment. Gut 1991;32(9):1004–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aziz I, Mumtaz S, Bholah H, et al. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on Rome III Criteria. Clin Gastroenterol Hepatol 2015;13(9):1650–5.e2. [DOI] [PubMed] [Google Scholar]
- 37. Sciarretta G, Vicini G, Fagioli G, et al. Use of 23-selena-25-homocholyltaurine to detect bile acid malabsorption in patients with illeal dysfunction or diarrhea. Gastroenterology 1986;91(1):1–9. [DOI] [PubMed] [Google Scholar]
- 38. Lin S, Sanders DS, Gleeson JT, et al. Long-term outcomes in patients diagnosed with bile-acid diarrhoea. Eur J Gastroenterol Hepatol 2016;28(2):240–5. [DOI] [PubMed] [Google Scholar]
- 39. Bannaga A, Kelman L, O’Connor M, et al. How bad is bile acid diarrhoea: An online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol 2017;4(1):e000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sciarretta G, Fagioli G, Furno A, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut 1987;28(8):970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riemsma R, Al M, Corro Ramos I, et al. SeHCAT [tauroselcholic (selenium-75) acid] for the investigation of bile acid malabsorption and measurement of bile acid pool loss: A systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17(61):1–236.m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilcox C, Turner J, Green J. Systematic review: The management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther 2014;39(9):923–39. [DOI] [PubMed] [Google Scholar]
- 43. Reid F, Peacock J, Coker B, et al. A multicenter prospective study to investigate the diagnostic accuracy of the SeHCAT test in measuring bile acid malabsorption: Research protocol. JMIR Res Protoc 2016;5(1):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn’s disease and indications for its assessment using SeHCAT. Gut 1994;35(1):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Summers JA, Peacock J, Coker B, et al. Multicentre prospective survey of SeHCAT provision and practice in the UK. BMJ Open Gastroenterol 2016;3(1):e000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sauter GH, Münzing W, von Ritter C, et al. Bile acid malabsorption as a cause of chronic diarrhea: Diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci 1999;44(1):14–9. [DOI] [PubMed] [Google Scholar]
- 47. Färkkilä MA, Kairemo KJ, Taavitsainen MJ, et al. Plasma lathosterol as a screening test for bile acid malabsorption due to ileal resection: Correlation with 75SeHCAT test and faecal bile acid excretion. Clin Sci (Lond) 1996;90(4):315–9. [DOI] [PubMed] [Google Scholar]
- 48. Brydon WG, Nyhlin H, Eastwood MA, et al. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol 1996;8(2):117–23. [DOI] [PubMed] [Google Scholar]
- 49. Johnston IM, Nolan JD, Pattni SS, et al. Characterizing factors associated with differences in fgf19 blood levels and synthesis in patients with primary bile acid diarrhea. Am J Gastroenterol 2016;111(3):423–32. [DOI] [PubMed] [Google Scholar]
- 50. Borup C, Syversen C, Bouchelouche P, et al. Diagnosis of bile acid diarrhoea by fasting and postprandial measurements of fibroblast growth factor 19. Eur J Gastroenterol Hepatol 2015;27(12):1399–402. [DOI] [PubMed] [Google Scholar]
- 51. Vijayvargiya P, Camilleri M, Carlson P, et al. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Aliment Pharmacol Ther 2017;46(6):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Camilleri M. Physiological underpinnings of irritable bowel syndrome: Neurohormonal mechanisms. J Physiol 2014;592(14):2967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156(5):1233–8. [DOI] [PubMed] [Google Scholar]
- 54. Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 and 7α-hydroxy-4-cholesten-3-one in the diagnosis of patients with possible bile acid diarrhea. Clin Transl Gastroenterol 2012;3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brydon WG, Culbert P, Kingstone K, et al. An evaluation of the use of serum 7-alpha-hydroxycholestenone as a diagnostic test of bile acid malabsorption causing watery diarrhea. Can J Gastroenterol 2011;25(6):319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Damsgaard B, Dalby HR, Krogh K, et al. Long-term effect of medical treatment of diarrhoea in 377 patients with SeHCAT scan diagnosed bile acid malabsorption from 2003 to 2016; a retrospective study. Aliment Pharmacol Ther 2018;47(7):951–7. [DOI] [PubMed] [Google Scholar]
- 57. Ung KA, Kilander A, Nilsson O, et al. Long-term course in collagenous colitis and the impact of bile acid malabsorption and bile acid sequestrants on histopathology and clinical features. Scand J Gastroenterol 2001;36(6):601–9. [DOI] [PubMed] [Google Scholar]
- 58. Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol 2017;112(1):78–85. [DOI] [PubMed] [Google Scholar]
- 59. Fernandez-Bañares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46(10):2231–8. [DOI] [PubMed] [Google Scholar]
- 60. Baert D, Coppens M, Burvenich P, et al. Chronic diarrhoea in non collagenous microscopic colitis: Therapeutic effect of cholestyramine. Acta Clin Belg 2004;59(5):258–62. [DOI] [PubMed] [Google Scholar]
- 61. Fan X, Sellin JH. Review article: Small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Aliment Pharmacol Ther 2009;29(10):1069–77. [DOI] [PubMed] [Google Scholar]
- 62. Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol 2010;16(24):2978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fernández-Bañares F, Rosinach M, Piqueras M, et al. Randomised clinical trial: Colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther 2015;41(11):1132–40. [DOI] [PubMed] [Google Scholar]
- 64. Brydon WG, Walters JR, Ghosh S, et al. Letter: Hydroxypropyl cellulose as therapy for chronic diarrhoea in patients with bile acid malabsorption - possible mechanisms. Aliment Pharmacol Ther 2016;44(3):306–7. [DOI] [PubMed] [Google Scholar]
- 65. Torcello-Gómez A, Fernández Fraguas C, Ridout MJ, et al. Effect of substituent pattern and molecular weight of cellulose ethers on interactions with different bile salts. Food Funct 2015;6(3):730–9. [DOI] [PubMed] [Google Scholar]
- 66. Tsuchida E, Yamamoto K, Miyatake K, et al. Molecular assembly of cholesterol-bearing poly (allylamine) for binding bile salts in water. Macromolecules 1997;30:4235–7. [Google Scholar]
- 67. Orekoya O, McLaughlin J, Leitao E, et al. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med (Lond) 2015;15(3):252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eriksson M, Hådell K, Holme I, et al. Compliance with and efficacy of treatment with pravastatin and cholestyramine: A randomized study on lipid-lowering in primary care. J Intern Med 1998;243(5):373–80. [DOI] [PubMed] [Google Scholar]
- 69. Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012;12:CD009361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beigel F, Teich N, Howaldt S, et al. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled study. J Crohns Colitis 2014;8(11):1471–9. [DOI] [PubMed] [Google Scholar]
- 71. Ung KA, Gillberg R, Kilander A, et al. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut 2000;46(2):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wedlake L, Thomas K, Lalji A, et al. Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: A retrospective chart review and patient questionnaire. Clin Ther 2009;31(11):2549–58. [DOI] [PubMed] [Google Scholar]
- 73. Olestyr (cholestyramine) Product Monograph. Montreal, Quebec, Canada: Pharmascience, Inc, 2017. [Google Scholar]
- 74. Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology 1972;62(5):918–34. [PubMed] [Google Scholar]
- 75. Poley JR, Hofmann AF. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology 1976;71(1):38–44. [PubMed] [Google Scholar]
- 76. Williams CN, Dickson RC. Cholestyramine and medium-chain triglyceride in prolonged management of patients subjected to ileal resection or bypass. Can Med Assoc J 1972;107(7):626–31. [PMC free article] [PubMed] [Google Scholar]
- 77. Ford GA, Preece JD, Davies IH, et al. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J 1992;68(798):272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Worobetz L, Wilkinson A, Chmielowiec C, et al. Evaluation of SeHCAT test in determining ileal involvement and dysfunction in Crohn’s disease. Can J Gastroenterol 1993;7:597–601. [Google Scholar]
- 79. Yeoh EK, Horowitz M, Russo A, et al. Gastrointestinal function in chronic radiation enteritis–effects of loperamide-N-oxide. Gut 1993;34(4):476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Valdés Olmos RA, Taal BG, Hoefnagel CA, et al. 75SeHCAT in the assessment of improved bile acid absorption in patients with ileal damage by delaying bowel motility with loperamide. Eur J Gastroenterol Hepatol 1993;5:941–6. [Google Scholar]
- 81. Menon S, Jones BJ. Postinfective bile acid malabsorption: Is this a long-term condition? Eur J Gastroenterol Hepatol 2011;23(4):308–10. [DOI] [PubMed] [Google Scholar]
- 82. Watson L, Lalji A, Bodla S, et al. Management of bile acid malabsorption using low-fat dietary interventions: A useful strategy applicable to some patients with diarrhoea-predominant irritable bowel syndrome? Clin Med (Lond) 2015;15(6):536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lodalis (colesevelam) Product Monograph. Laval, Quebec, Canada: Valeant Canada LP, October 16, 2014. [Google Scholar]
- 84. Colestid (colestipol) Product Monograph. Kirkland, Quebec, Canada: Pfizer Canada, Inc, October 7, 2013. [Google Scholar]
- 85. Camilleri M. Drug-resin drug interactions in patients with delayed gastric emptying: What is optimal time window for drug administration? Neurogastroenterol Motil 2016;28(8):1268–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scaldaferri F, Pizzoferrato M, Ponziani FR, et al. Use and indications of cholestyramine and bile acid sequestrants. Intern Emerg Med 2013;8(3):205–10. [DOI] [PubMed] [Google Scholar]
- 87. Steinmetz KL, Schonder KS. Colesevelam: Potential uses for the newest bile resin. Cardiovasc Drug Rev 2005;23(1):15–30. [DOI] [PubMed] [Google Scholar]
- 88. Gross L, Brotman M. Hypoprothrombinemia and hemorrhage associated with cholestyramine therapy. Ann Intern Med 1970;72(1):95–6. [DOI] [PubMed] [Google Scholar]
- 89. Vroonhof K, van Rijn HJ, van Hattum J. Vitamin K deficiency and bleeding after long-term use of cholestyramine. Neth J Med 2003;61(1):19–21. [PubMed] [Google Scholar]
- 90. Shojania AM, Grewar D. Hypoprothrombinemic hemorrhage due to cholestyramine therapy. CMAJ 1986;134(6):609–10. [PMC free article] [PubMed] [Google Scholar]
- 91. Sadler LC, Lane M, North R. Severe fetal intracranial haemorrhage during treatment with cholestyramine for intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol 1995;102(2):169–70. [DOI] [PubMed] [Google Scholar]
- 92. West RJ, Lloyd JK. The effect of cholestyramine on intestinal absorption. Gut 1975;16(2):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Westergaard H. Bile Acid malabsorption. Curr Treat Options Gastroenterol 2007;10(1):28–33.m [DOI] [PubMed] [Google Scholar]
- 94. Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol 2014;109(10):1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vijayvargiya P, Camilleri M, Chedid V, et al. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin Gastroenterol Hepatol 2019;17(5):922–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Camilleri M. Trial to understand efficacy of colesevelam in diarrhea predominant IBS patients with bile acid malabsorption. Last update 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03270085. Accessed December 6, 2018.
- 97. Dior M, Delagrèverie H, Duboc H, et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil 2016;28(9):1330–40. [DOI] [PubMed] [Google Scholar]
- 98. Hou RG, Fan L, Liu JJ, et al. Bile acid malabsorption is associated with diarrhea in acute phase of colitis. Can J Physiol Pharmacol 2018;96(12):1328–36. [DOI] [PubMed] [Google Scholar]
- 99. Arya LA. Rationale for investigating stool metabolites and microbiota in women with fecal incontinence. Dis Colon Rectum 2017;60(2):249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sirtori M, Pazzucconi F, Gianfranceschi G, et al. Efficacy of cholestyramine does not vary when taken before or during meals. Atherosclerosis 1991;88(2–3):249–52. [DOI] [PubMed] [Google Scholar]