Keywords: kidney, nucleotides, P2 receptors, renal hemodynamics, transport function
Abstract
The understanding of the nucleotide/P2 receptor system in the regulation of renal hemodynamics and transport function has grown exponentially over the last 20 yr. This review attempts to integrate the available data while also identifying areas of missing information. First, the determinants of nucleotide concentrations in the interstitial and tubular fluids of the kidney are described, including mechanisms of cellular release of nucleotides and their extracellular breakdown. Then the renal cell membrane expression of P2X and P2Y receptors is discussed in the context of their effects on renal vascular and tubular functions. Attention is paid to effects on the cortical vasculature and intraglomerular structures, autoregulation of renal blood flow, tubuloglomerular feedback, and the control of medullary blood flow. The role of the nucleotide/P2 receptor system in the autocrine/paracrine regulation of sodium and fluid transport in the tubular and collecting duct system is outlined together with its role in integrative sodium and fluid homeostasis and blood pressure control. The final section summarizes the rapidly growing evidence indicating a prominent role of the extracellular nucleotide/P2 receptor system in the pathophysiology of the kidney and aims to identify potential therapeutic opportunities, including hypertension, lithium-induced nephropathy, polycystic kidney disease, and kidney inflammation. We are only beginning to unravel the distinct physiological and pathophysiological influences of the extracellular nucleotide/P2 receptor system and the associated therapeutic perspectives.
This is a concise review of recent advances in renal functions of extracellular nucleotides and P2 receptors. This article also provides details on the cellular release and metabolism of extracellular nucleotides, physiological and pathophysiological functions of P2 receptors, and disease conditions associated with their deranged functions.
I. INTRODUCTION AND OVERVIEW
The kidneys adapt renal excretion to the body’s needs to maintain fluid and electrolyte homeostasis. In addition to systemic neurohumoral control, primary local mechanisms are important to regulate and fine tune renal function and maintain integrity. Various autacoids have been established to contribute to those local mechanisms, including the nucleoside adenosine (457, 525). The latter is released from cells, depending on their metabolic status, but extracellular adenosine is also formed from the cellular release and local breakdown of the nucleotide ATP. The role of ATP as a critical intracellular energy source for most cellular processes is well established. ATP and other nucleotides, however, are also released from cells to exert paracrine and autocrine effects (63). The critical role of ADP acting on the P2Y12 and P2Y1 receptors in platelet aggregation is one example that also led to successful therapeutic targeting (501). More and more evidence is accumulating that extracellular ATP and other nucleotides are also part of the local intrarenal mechanisms that help regulate kidney function and cellular integrity.
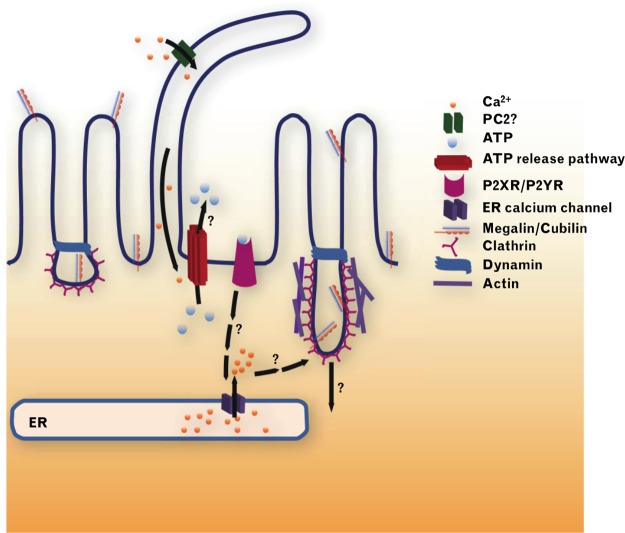
The concept of ATP as an extracellular autocrine and paracrine signaling molecule was proposed by Burnstock and co-workers in the early 1970s (64, 70). ATP’s breakdown product, the nucleoside adenosine, which lacks the phosphate groups of ATP, had been shown to have extracellular effects almost half a century earlier (127). Given that estimated concentrations of ATP inside cells vary from 1 to 10 mM, and are thus more than 1,000-fold higher than in the extracellular space, any form of tissue injury, with cell damage or lysis, including platelet aggregation and thrombosis, will result in significant local release of ATP. There is, however, also controlled and physiological release of nucleotides provoked by mechanical stimulation like cell swelling, shear stress, or agonist activation, mediated by conductive or vesicular nucleotide release (11, 148, 233, 415). Whereas extracellular adenosine acts on P1 receptors, released ATP and other nucleotides act on metabotropic G protein-coupled P2Y receptors or ionotropic P2X receptors to affect vascular, glomerular, tubular, and other kidney functions (86, 520) (FIGURE 1). P2 receptors are differentially expressed in cell membranes throughout the kidney. Moreover, ectonucleotidases can breakdown extracellular nucleotides, in part to adenosine, and thus affect the extracellular availability of P2 and P1 receptor agonists, and thereby the pattern of receptor activation in a local microenvironment (530) (FIGURE 1). Accordingly, it is reasonable to conclude that P2 receptors are part of a subtle autocrine or paracrine feedback system, integrating signals based on the local patterns of nucleotide secretion, release, and degradation. As well as in normal physiology, it is also becoming clear that deleterious signals mediated through P2 receptors may make an important contribution to renal pathophysiology. Overall, ATP seems to be more proinflammatory, whereas adenosine is often anti-inflammatory. In this review, we aim to summarize and integrate key knowledge and recent developments related to the extracellular nucleotide and P2 receptor system in the kidney.
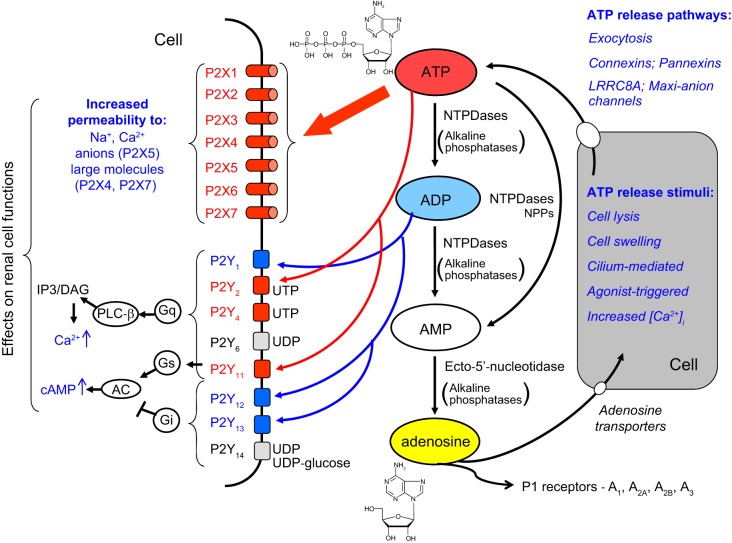
FIGURE 1.
The integrated extracellular nucleotides/P2 receptor system. A variety of stimuli including volume or flow-mediated stretch, damage, or agonist binding can trigger ATP release into the extracellular space via exocytosis or through specific channels. Ectonucleotidases present on the plasma membrane of the same or adjacent cells rapidly catalyze the sequential hydrolysis of ATP to ADP, AMP, and ultimately adenosine. Adenosine acts on P1 receptors, whereas ATP acts on P2X and some P2Y receptors, while ADP acts on P2Y receptors. Activation of P2X receptors leads to increased ion permeability and an increase in cytosolic calcium; P2X7 and P2X4 receptors are also potentially pore-forming and can increase permeability to larger molecules. P2Y receptor activation couples through G proteins to phospholipase C or adenylate cyclase activation, also leading to increases in intracellular calcium, as well as in cAMP for downstream signaling and effects on renal cell functions. The figure indicates the primary agonists in humans; see TABLE 1 for more details. IP3, inositol trisphosphate; DAG, diacylglycerol. [Modified from Shirley et al. (474), with permission from Elsevier.]
To this end, we first discuss determinants of extracellular nucleotide concentrations, which includes cellular release mechanisms and enzyme systems that metabolize extracellular nucleotides, thereby forming other nucleotides or nucleosides such as adenosine. We then describe the renal distribution and function of specific P2X and P2Y receptors in the vasculature, glomeruli, and the tubular system and discuss their role in integrated kidney and body functions, including salt and water homeostasis and blood pressure control. Finally, we outline the implications of extracellular nucleotides and their receptors in the pathophysiology of the kidney and briefly discuss therapeutic perspectives. The interested reader is also referred to recent reviews on this topic (25, 181, 272, 277, 334, 396, 415, 480, 512, 523, 527).
II. DETERMINANTS OF EXTRACELLULAR NUCLEOTIDE CONCENTRATIONS IN THE KIDNEY
P2 receptors are stimulated by ATP, ADP, UTP, UDP, or UDP-glucose, and the concentrations of these nucleotides in the extracellular space required to activate the receptors typically range from 0.1 to 10 μM (with the exception of P2X7 receptors, which require low millimolar concentrations) (4) (TABLE 1). The measurements of extracellular nucleotide concentrations in tissue and body fluids are a challenging task, and a comprehensive review of the methodologies has been published (413). Commercially available ATP biosensors have recently been used to assess extracellular ATP concentrations in isolated perfused kidneys and in vivo blood‐perfused kidneys (382, 384). Nevertheless, the lack of easy to use methods to quantify nucleotide concentrations at defined locations and in the different body fluids continues to impede our ability to understand this signaling system.
Table 1.
Characteristics, preferred agonists, and signaling of cloned P2 receptors
| Preferred Agonists (Potency EC50) |
Useful Agonist | Useful Antagonist | Transduction Mechanism | |
|---|---|---|---|---|
| P2Y1 | ADP (10 µM) > ATP (Ap4U ~1 µM) | 2-MeS-ADP; MRS2365 | MRS2500; BPTU | Gq/G11; PLC-β↑ |
| P2Y2 | ATP (0.1 µM) = UTP (0.01 µM) | PSB-1114 | AR-C1189251XX | Gq/G11 (Gi/Go?); PLC-β↑ |
| P2Y4 | UTP (human) (~1 µM); UTP = ATP (rodent) | MRS4062 | Gq/G11 (Gi?); PLC-β↑ | |
| P2Y6 | UDP (~0.3 µM) > UTP > ADP | MRS2957; PSB-0474 | MRS2578 | Gq/G11; PLC-β↑ |
| P2Y11 | ATP (~10 µM) and ADP (and UTP (547) (human) | γ-Thio-ATP; NF546 | NF340 | Gq/G11 and GS; PLC-β↑ |
| P2Y12 | ADP (0.1 µM) > ATP | 2-MeS-ADP | PSB-0739; ticagrelor | Gi/others |
| P2Y13 | ADP (0.01 µM) > ATP | 2-MeS-ADP | MRS2211 | Gi/Go/others |
| P2Y14 | UDP (0.1 µM) UDP-glucose (0.3 µM) and UDP-galactose | MRS2690 | PPTN | Gi/Go/others |
| P2X1 | ATP (0.1–0.7 µM) | ATP; 2-MeS-ATP | NF449 | ICC (Ca2+ and Na+) |
| P2X2 | ATP (2–8 µM) | ATP | PSB-1011; NF770 | IIC (particularly Ca2+) |
| P2X3 | ATP (~1 µM) | ATP; 2-MeS-ATP | A-317491; R0-4 | ICC |
| P2X4 | ATP (1–10 µM) | ATP; γ-Thio-ATP | AF353; R0-51; AF906 | IIC (especially Ca2+) |
| P2X5 | ATP (0.5 µM) | Benzoyl-ATP* | 5-BDBD; PSB-12054 | IIC |
| P2X6 | Functions poorly as homomultimer | BX-430 | IIC | |
| P2X7 | ATP (2–4 mM) | ATP; benzoyl-ATP | A-740003; A438079; AZ11657312 | ICN; large pore |
Due to their cellular release, nucleotide concentrations in the bulk phase are thought to underestimate the true values at the cell membrane receptor by at least 20-fold (4, 251). Most of the available information on extracellular nucleotides refers to ATP and its breakdown products. It is important, however, that other endogenous agonists of P2Y receptors, like UTP (and probably other nucleotides as well), are also released into the extracellular space by similar mechanisms.
A. Cellular Nucleotide Release Mechanisms in the Kidney
1. Basic stimuli and molecular mechanisms
The widespread and striking biological effects of nucleotides in all organs including the kidney explain the marked interest to unravel the physiology of cellular nucleotide release. In non-neuronal cells, physiological release of nucleotides is a general phenomenon. Several reviews have discussed this topic with the intention of sorting out the essential molecular, regulatory and physiological elements of this process (105, 148, 294, 413, 465, 466). Unfortunately, the molecular physiology of cellular nucleotide release mechanisms remains insufficiently understood. Nonetheless, general agreement exists on the main building blocks of nucleotide release from nonexcitable cells. 1) The source of extracellular nucleotides is the high cytosolic ATP concentration. 2) The regulated pathways that permit ATP release are either a conductive pore in the plasma membrane or intracellular storage vesicles that contain high nucleotide concentrations, which can be released into the extracellular space via exocytosis. Cellular/physiological ATP release is ubiquitous and can be triggered by 1) mechanical stress and cell swelling (293, 344, 382, 411), 2) hypoxia (484), 3) a large variety of classical transmitters and agonists (413), and 4) is intracellular Ca2+ dependent (344) (FIGURE 1). Intriguingly, after cellular stimulation/activation with local agonists or systemic hormones, the associated ATP release often mediates further cell activation and amplification of the signal.
a) a conductive pathway for nucleotide release.
Suggested conductive molecular pathways for nucleotide release relevant for the kidney will be reviewed and updated (293, 413) (TABLE 2). The general idea is that a gated anion channel or a nonselective pore permits the anionic ATP molecule to leak into the interstitial compartment down a steep electrochemical gradient.
Table 2.
Nucleotide release channels in the renal epithelium
| Channel | Tubule Segment | Localization | Comments | Reference Nos. |
|---|---|---|---|---|
| Cx30 | Rat: mTAL, cTAL, DCT, CD; mouse, rabbit: β-IC of CNT and CD | Apical | Good functional evidence, KO control | 331, 340, 478, 496 |
| Cx37 | Rat, mouse: TAL, DCT; less in PT, CD | Basolateral | No functional evidence, localized to basolateral infoldings and interdigitations | 488 |
| Panx1 | Mouse: PT, tDL, CD | Apical | Lower urinary ATP in Panx1 KO mice | 188, 237 |
| LRRC8A | Rat: Henle’s loop, IMCD | Only mRNA | Function only in Xenopus oocytes | 160, 298 |
| Maxi anion channel | Mouse, rabbit: MD | Basolateral | Suggested plasma membrane variant of mitochondrial VDAC | 32, 371 |
PT, proximal tubule; tDL, thin descending limb; mTAL, medullary thick ascending limb; cTAL, cortical thick ascending limb; MD, macula densa; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; IMCD, inner medullary collecting duct; β-IC, β-intercalated cell; KO, knockout; VDAC, voltage-dependent anion channel. [Note from other organs: the CALHM channels were clearly identified as ATP release channels necessary for transduction of taste perception (503).]
I) Connexin hemichannels. Connexin hemichannels have received significant attention as possible cellular ATP release channels. In addition to being cell-cell junction channels, connexins can also be found in the plasma membrane as hemichannels (43). They can permit cellular ATP release and reciprocally allow indicator dye uptake into the cytosol (13, 43).
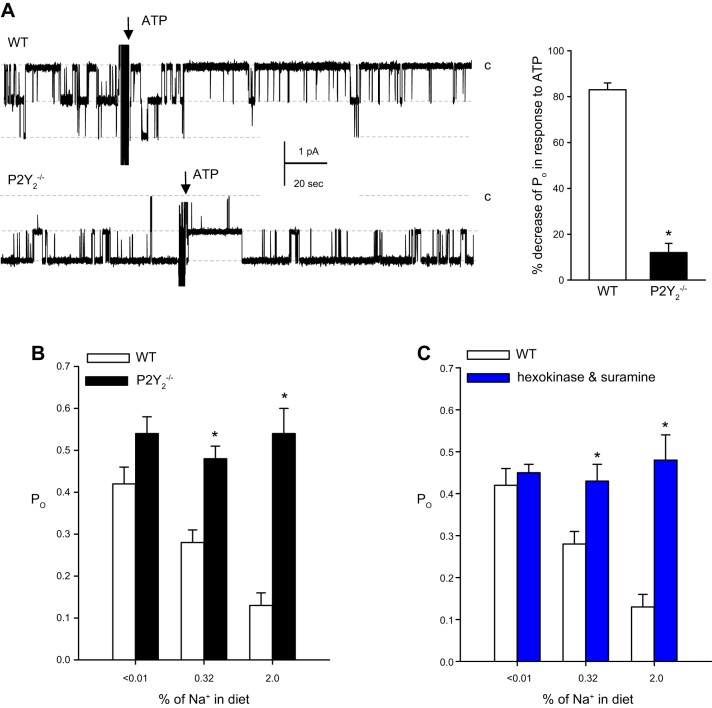
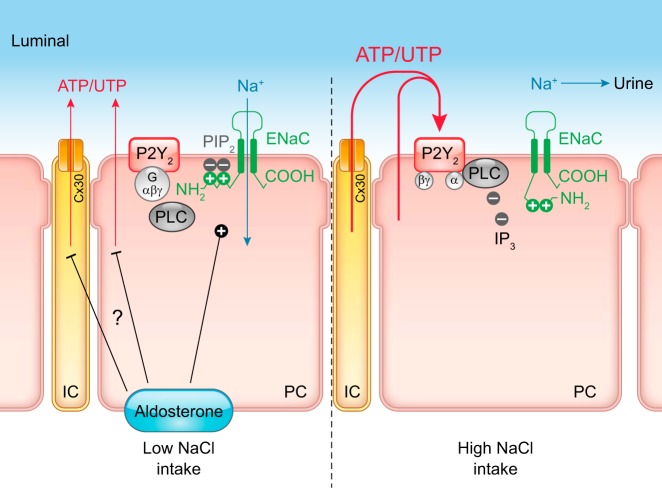
In the kidney, connexin 30 (Cx30) has been proposed to function as an ATP release channel that in rabbit and mouse kidney is expressed prominently in the apical membrane of pendrin-positive intercalated cells (IC) (331, 478, 487), whereas rats show a broader expression along the tubule and collecting duct (CD) system (331) (TABLE 2). In addition, in mouse inner medulla Cx30 staining was found near the apical membrane, and was markedly upregulated following a high-Na+ diet (331). Since Cx30 cannot function as a gap junction protein in the apical membrane of IC, it seems likely to have an alternative role. Indeed, using an ATP biosensor cell it was shown that a superfusion stimulus in split-opened CDs triggered ATP release in direct proximity to the apical surface of IC, which was absent in Cx30−/− mice (478). Moreover, a high dietary Na+ intake triggered an increase in urinary ATP concentration (from ~5 to ~50 nM), which was absent in Cx30−/− mice (340). Further details and the functional relevance of Cx30 in the CD is outlined in section VI.
A second example is the nonjunctional expression of Cx37 in the basolateral membrane of several tubular segments, particularly in the thick ascending limb (TAL) and the distal convoluted tubule (DCT) and weaker in proximal tubule (PT) and CD, which increased with a low-NaCl diet (488) (TABLE 2). Basolateral ATP release in renal epithelia is strongly suggested by several studies (165, 411, 415), and Cx37 is a candidate ATP conduit in this process.
II) Pannexin channels. In recent years, significant attention has focused on pannexin (Panx) membrane proteins. These connexin-like proteins function as plasma membrane channels and permit the release or uptake of larger molecules up to ~1 kDa, including nucleotides. Analogous to gap junctions, the terms pannexin channel or pannexons were coined: six pannexin subunits assemble to form a functional membrane conduit (93, 115). Remarkably, the hallmarks of cellular ATP release align with many key features attributed to pannexin channel function. Panx1 channels can be activated after mechanical stimulation, require an increase in cytosolic Ca2+ ([Ca2+]i), and are triggered to open after agonist stimulation or an increase in extracellular potassium (541).
Panx1 mRNA and protein have been identified in the kidney (27, 188, 298) (TABLE 2). RNAseq analysis identified Panx1 in rat renal tubules, mostly in the PT, the descending thin limb (dTL), and the CD system, however at a rather low expression (298). Of note, the same expression pattern was found in mouse tissue using immunohistochemistry, which also showed an apparent apical localization of Panx1 (188). Nanomolar ATP concentrations were found in fresh urine samples of wild-type (WT) mice and were slightly lower in Panx1−/− mice (188). In Panx1-expressing cultured or freshly isolated native cells agonists like norepinephrine, angiotensin II (ANG II), or UTP induce activation of carbenoxolone-sensitive channels (see below) and nonselective whole cell currents (93, 348, 575). A noticeable feature of these currents is their delayed activation, taking tens of seconds to minutes before their onset (93, 348, 575). In isolated perfused mouse medullary TAL (mTAL) and CD, arginine vasopressin (AVP)-stimulated ATP release into the tubular lumen occurs with a significant delay of 60–90 s (369). Since Panx1 channels appear to be activated by an array of agonists with a significant time delay, it is reasonable to speculate that AVP-stimulated nucleotide release may occur via Panx1 (see sects. V and VI), although more evidence is needed.
III) Cell swelling-induced nucleotide release. Cell swelling is a pronounced stimulus of nucleotide release. Swelling-induced anion currents have been studied for several decades (539) and received different names, the volume-sensitive organic osmolyte channel (VSOAC), the volume-sensitive outwardly rectifying channel (VSOR), or the volume-regulated anion channel (VRAC) (200). The idea that the cell swelling-induced anion currents could conduct nucleotides is not unreasonable because this conductance has a broad spectrum of anion permeabilities, including larger anions such as taurine and glutamate. Recently, the molecular basis for this ubiquitous current was identified, and it is composed of leucine-rich repeat-containing protein 8 (LRRC8) heteromers (418, 539). Cell swelling-induced anion currents require one obligatory LRRC8A subunit and at least one of the other LRRC8 subunits (B, C, D, or to E) (246). Studies in Xenopus oocytes expressing LRRC8A and E confirmed their involvement in swelling-induced cellular nucleotide release (160). Recent data also specified that LRRC8 anion channels allow specific permeation of neurotransmitters and other extracellular signaling modulators (316). Renal medullary epithelial cells are uniquely exposed to fluctuations in extracellular osmolarity due to extreme changes in interstitial osmolyte concentrations. Furthermore, high transcellular tubular solute transport rates may impose unbalanced intra- and extracellular osmolyte concentrations that require appropriate cell volume regulation. Thus it is not surprising to find ubiquitous expression of all LRRC8 subunits along the renal tubular system, with most pronounced subunit A and D mRNA expression along the entire loop of Henle and the CD, including the inner medullary CD (IMCD) (298). These results suggest that LRRC8 channels are suitable candidates for volume-regulated renal epithelial cell nucleotide release.
A hexameric structural anatomy with a central pore exists for connexins, pannexin, homomeric LRRC8 channels, and the calcium homeostasis modulator (CALHM) ion channels (258). It appears notable that evolution may have created hexameric plasma membrane release channels with a central pore allowing for small signaling molecule release and local intercellular communication (321).
Carbenoxolone (CBX) is an effective blocker of nucleotide release; however, CBX is not suitable for differentiating between connexins, pannexins, or LRRC8 channels as they are all readily blocked (160), although CALHM channels are not blocked by CBX (321).
In summary, significant progress has been made in defining the conductive cellular nucleotide release conduits, with connexins, Panx1, and LRRC8 channels being likely candidates in renal cells, but further work on this topic is still needed and may reveal yet more surprises.
B) a vesicular pathway for nucleotide release.
Vesicular release of nucleotides from neuronal or neuroendocrine cells is well recognized, and many studies have shown ATP as a cotransmitter in peripheral and central neurons as well as its release from various neuroendocrine cells (38, 366, 386). It is known that chromaffin cells and the closely related PC12 cells store large amounts of ATP (vesicular concentrations >100 mM) together with catecholamines (142, 360), and that insulin secretion from β-cells occurs together with ATP (368), and that α-dense granules in thrombocytes contain very high concentrations of ADP, a critical factor in thrombus formation following platelet activation (158). Vesicular nucleotide release is a feature of many cells (37, 46, 100, 185, 339, 389, 470, 510) except red blood cells (115). ATP is actively stored in intracellular vesicular organelles by a secondary active process exploiting the electrical driving force generated by the vacuolar H+-ATPase (590). The blocker of this pump, bafilomycin, is a useful tool to inhibit cellular nucleotide release (100, 326) in support of the vesicular nucleotide release hypothesis.
A major breakthrough was the discovery of VNUT (SLC17A9), a vesicular nucleotide transporter expressed in neuronal, neuroendocrine, and glial cells allowing nucleotide transport into secretory vesicles (9, 138, 290, 447). In secretory epithelial cells, SLC17A9’s function and transport of nucleotides into secretory vesicles are well documented (185, 470). Renal tubular epithelial cells do release ATP via a vesicular process (37), but VNUT mRNA expression is absent or in very low abundance (298).
In summary, strong and unequivocal evidence has identified vesicular nucleotide release as a ubiquitous phenomenon in cellular nucleotide release. A physiological role for vesicular nucleotide release is evolving and is better understood in pancreatic beta cell insulin secretion (68, 447), and in the role of astrocytes in providing purinergic dampening of neuronal network activity (389). In the kidney, however, a defined role of vesicular nucleotide release awaits further study.
2. Nucleotide release by renal vasculature and glomeruli
Activation of P2 receptors in the vasculature has dual effects, vasoconstriction or vasodilation (62) (see sect. IV). P2 receptor stimulation of vascular smooth muscle cells triggers increases of [Ca2+]i and vasoconstriction. One source of ATP is from sympathetic nerve endings that co-release ATP together with noradrenaline. Intriguingly, recent data suggest that adrenergic α1 receptor stimulation also triggers nucleotide release from vascular smooth muscle via Panx1 channels (34, 35). These results indicate an autocrine and paracrine amplification mechanism of vasocontraction and point to two sources of ATP for excitation/contraction coupling in vascular smooth muscle cells. In contrast, stimulation of P2 receptors in endothelial cells leads to formation of nitric oxide (NO) and consequent vasodilation. Nucleotide release from endothelial cells is triggered by laminar flow (542), a mechanosensory stimulus unique to blood vessels, ductal parts of exocrine glands, and the renal tubular system. The endothelial P2 receptor was suggested to be the P2X4 receptor (566), but recent results challenge this finding and provide good evidence for the P2Y2 receptor (432, 542), and both receptors may contribute (107). Endothelial nucleotide release purportedly involves Panx1 and Panx2, and the mechanosensory ion channel Piezo1 has been proposed as the endothelial molecular flow sensor (542). Recently, GPR68 was also identified as an essential endothelial flow sensor (564). In glomerular endothelial cells (GEC), P2 receptors have been identified, likely of the P2Y2 subtype. They stimulate an increase in [Ca2+]i and propagate [Ca2+]i waves (49, 511). Mechanically stimulated [Ca2+]i waves in GEC grown on glass coverslips depend on nucleotide release and traveling extracellular ATP waves. In GEC, Cx40 is a candidate for ATP secretion (511). GECs are positioned directly on the basal membrane of the glomerulus in close proximity to podocytes and mesangial cells. Thus glomerular endothelial to smooth muscle/mesangial and podocyte communication via ATP could exist thereby influencing the contractile state of the glomerulus and the filtration barrier (241, 242) (see sect. IVB for details).
Mechanically stimulated nucleotide release from podocytes has recently been demonstrated and found to trigger P2X4 receptor-dependent inward currents and cytoskeletal reorganization (150). A podocyte injury model targeting a single cell using two-photon microscopy triggered spreading of intercellular podocyte [Ca2+]i waves, which was markedly inhibited in podocytes that lack the P2Y2 receptor (55). In the nephrotoxic serum-induced glomerulonephritis model, podocytes show marked de novo expression of Cx43 and the nonspecific P2 receptor antagonist suramin ameliorates kidney damage (256). Cx43 was proposed as an ATP conduit in astrocytes, which, like podocytes, are a type of supporting cell (13). These results suggest a potential link between progressive Cx43 expression, cellular nucleotide release, and development of a P2 receptor-driven disease mechanism in podocytes (see sect. VII for details).
3. Nucleotide release by the macula densa
The macula densa (MD) cells sense the NaCl load at the end of the TAL. If the NaCl load is too high, the MD cells swell (174, 454) and release ATP into the basolateral compartment. ATP is subsequently broken down to adenosine, which then binds to adenosine A1 receptors on smooth muscle cells of the afferent arteriole to cause vasoconstriction and reduce single nephron glomerular filtration rate (GFR). The information flow of this paracrine signaling event is very well studied and paradigmatic for a purinergic extracellular signaling pathway (see sect. IVB). ATP release from the basolateral surface of MD cells has been demonstrated with the help of biosensor cells and could be stimulated by increased tubular NaCl at the MD (32). By similar maneuvers, a very large anion channel was identified in the basolateral membrane of MD cells that also permits ATP to pass through it (32). With the discovery of the LRRC8 volume-activated anion channels, a very suitable molecular candidate has been identified to address further the molecular basis of the “maxi anion channel” and define the MD cell-dependent ATP release mechanism.
4. Nucleotide release by tubules and collecting ducts
Release of nucleotides from renal epithelia is well documented and can occur either into the lumen or into the basolateral space (415, 466). P2Y and P2X receptors are readily found in both the apical and basolateral membranes of renal tubules and modulate tubular transport (303) (see sect. V). Ambient urinary ATP and UTP concentrations in mice are reported in the low nanomolar to low micromolar range, but show interesting variations, e.g., they are significantly elevated during increases in dietary Na+ intake (340, 405, 487) (for details, see sects. IIC and VI). Direct assessment of renal tubular fluid ATP concentrations collected by micropuncture have demonstrated values in the low micromolar range and thus are sufficiently high to activate apical P2 receptors (369, 531). Three hallmarks of cellular nucleotide release have also been identified in renal epithelia. 1) It occurs constitutively providing a basal P2 receptor-dependent influence (369). 2) It is further augmented by mechanical stimuli, which appears to be an important cellular input signal to trigger nucleotide release. Increases in interstitial ATP can also be triggered by the elevation of renal perfusion pressure (358, 382). An increase in tubular flow in native renal tubules triggers nucleotide release (245). Flow-dependent bending of the primary cilium in MDCK cells is a marked stimulus for nucleotide release, with stimulation of P2 receptors and a resulting increase in [Ca2+]i (414). Functional absence of ciliary sensory proteins like PC1, TRPP2, or TRPV4 removes the flow-induced increase in [Ca2+]i and also the release of ATP (280, 416, 562). 3) Cellular stimulation with various GPCR agonists triggers nucleotide release in most organs and cell systems, including renal tubules (413). In isolated perfused mouse TAL and cortical CD, antidiuretic hormone (ADH) in the bath triggered apical ATP secretion. Tubular ATP was sensed with “ATP sniffer cells” in the outflow of the tubule, and burstlike increases in ATP concentration, reaching up to 0.3 µM, could be triggered with ADH (369). The molecular mechanism of nucleotide release has been addressed in renal epithelia, and evidence for numerous candidates has been put forward, as discussed above, including Cx30 (478), Panx1 (188), vesicular nucleotide release (37), and possibly others. The functional relevance for tubular nucleotide release is discussed in sections V and VI.
5. Nucleotide release by renal nerves
Efferent renal sympathetic nerve activity (ERSNA) provides one control mechanism for volume and Na+ balance (125). Sympathetic nerve endings are found throughout renal tissues, including the vasculature, renin-producing juxtaglomerular cells, and the tubular system (125). Low-frequency ERSNA is well documented to mediate volume and Na+ conservation by activating renin secretion and tubular Na+ absorption (125). Sympathetic nerve endings commonly release noradrenaline together with other cotransmitters, and it is established that a major sympathetic cotransmitter is ATP (59, 61). In human renal cortex, adrenergic stimulation releases ATP from neuronal and non-neuronal sources (537). Activation of the tubular system by the sympathetic nervous system primarily induces antinatriuretic and antidiuretic effects (125). This contrasts with the natriuretic and diuretic effects of basolateral and apical P2 receptor stimulation in isolated renal tubule preparations (415, 527) (see sect. V). This raises the question whether sympathetic input could have dual effects, i.e., activation of tubular transport via adrenergic receptors and inhibition via P2 receptors. In rats subjected to renal denervation, the urinary sodium excretion response to high dietary Na+ intake was markedly decreased in the first 72 h, which may suggest an ATP/sympathetic element that serves rapid excretion of an oral Na+ load (177). Furthermore, there is considerable variation in the proportion of noradrenaline and ATP in sympathetic nerve terminals, and different nerve stimulation frequency patterns may lead to preferential release of one or the other neurotransmitters (61).
Another element for consideration is afferent renal nerve activity (ARNA). Afferent renal nerves mediate tonic inhibition of the contralateral ERSNA and promote excretion of Na+ and water on the contralateral side (250). The identified intrarenal sensors are either mechano- or chemoreceptors responding to an increase in pressure or environmental chemical cues. Extracellular ATP is an important activator of afferent neuronal pathways, suggesting its role in peripheral sensory physiology (7, 149, 364). It may, therefore, be speculated that intrarenal nucleotide release could play a role in activating ARNA to modulate reno-renal reflexes.
6. Nucleotide release in response to renal injury
Injury and death of any cell causes nonregulated release of copious amounts of ATP, which is expected to activate P2 receptors and, after extracellular conversion to adenosine (see sect. IIB), also P1 receptors on adjacent effector cells. Renal injury models have shown ample relevance for purinergic signaling in renal disease processes. In early ischemia-reperfusion studies, high concentrations of adenosine were found in renal tissue eliciting a reduction in renal blood flow (RBF) (376, 525). Also, chemical damage with, for example, maleic acid increased tissue and urinary adenosine levels (14). This likely reflects hypoxic/metabolic tubular cell injury, cell swelling, and possibly rupture with subsequent release of nucleotides and degradation to adenosine. Primary cellular adenosine release likely also contributed, since adenosine is also formed from ATP within cells and will accumulate intracellularly when energy supply is low, and facilitative adenosine transporters are well described in the renal tubular system (525). Importantly, adenosine appears to be tissue protective and anti-inflammatory (525) in contrast to the opposite effects attributed to high ATP concentrations. Extracellular ATP also contributes to renal fibrosis after ureter obstruction. Blocking of urinary outflow increases transtubular pressure gradients and stimulates epithelial nucleotide release (245, 411, 413). Quite dramatic results were obtained in P2X7−/− mice, in which ureteric obstruction failed to trigger fibrosis (173). In recent years, released extracellular nucleotides with their specific P2 receptor have been established as damage-associated molecular pattern (DAMP) molecules in relevant renal pathologies, as discussed in more detail in section VII.
B. Nucleotide Metabolizing Enzymes in the Kidney
1. Extracellular nucleotidases
To be effective as autocrine/paracrine regulators of cellular function, the concentrations of nucleotides in the extracellular milieu need to be controlled precisely within a narrow limit, usually in the range of the receptor’s affinity, i.e., in the low micromolar range (see TABLE 1). Furthermore, to quickly switch P2 receptor activation on and off, extracellular nucleotide concentrations must be able to change rapidly. This is accomplished by two mechanisms: one is the regulated cellular release of nucleotides (see sect. IIA), and the other is their rapid removal by regulated extracellular hydrolysis. The latter is achieved by extracellular nucleotidases, or ectonucleotidases. These enzymes are at the surface of plasma membranes with their catalytic domains facing the exterior to hydrolyze nucleotides in the extracellular milieu. The hydrolysis of extracellular nucleotides also contributes to the purine salvage pathway and recycling of nucleotides. Thus ectonucleotidases have both regulatory and metabolic functions (569). The ectonucleotidases belong to at least four distinct classes, namely, nucleoside triphosphate diphosphohydrolases (NTPDase 1, 2, 3, and 8), nucleotide pyrophosphatases (NPP1, 2, 3), ecto-5′-nucleotidase (CD73), and nonspecific alkaline phosphatases (AP) (FIGURE 1). As shown in TABLE 3, they vary in their substrate specificities.
Table 3.
Ectonucleotidases in the renal epithelium
| Collecting Duct |
|||||||
|---|---|---|---|---|---|---|---|
| Km, µM (593) | Proximal Tubule | Loop of Henle | CCD/OMCD | IMCD | Interstitial Cells | Peritubular Capillaries | |
| NTPDase1 (CD39) | ATP ~0–200 | tAL (269) | Low IC,PC (530); terminal part (269) | Cortical (269, 304, 530) | |||
| NTPDase2 (CD39L1) | ATP ~70 | TAL (A, B) (530) | Low IC,PC (530) | ||||
| NTPDase3 | ATP ~75 | TAL (A) (530) | IC (530) | Low PC (530) | |||
| NTPDase8 | ATP 81–226 | ||||||
| NPP1 (PC-1) | ATP ~50–500; ApnA ~1–20 | ||||||
| NPP2 | UDP-glucose ~100 | ||||||
| NPP3 | ~200 | PST (A) (530) | |||||
| AP | Low ATP ~40; high ATP ~1,000 | (A) (30) | |||||
| Ecto-5′ NT (CD73) | AMP 1–50 | Early PCT > late PCT > PST (A) (118, 161, 530) | IC (A) (118, 161, 530) | IC (A) (118, 161, 530) | css fibroblasts (161, 295) | ||
AP, alkaline phosphatase; (A), apical; (B), basolateral; CCD, cortical collecting duct; CNT, connecting tubule; css fibroblasts, cortical stellate-shaped fibroblasts; IC, intercalated cells; IMCD, inner medullary collecting duct; Km, the concentration of substrate that permits the enzyme to achieve half-maximal reaction velocity; low, low expression; OMCD, outer medullary collecting duct; PC, principal cell; PCT, proximal convoluted tubule; PST, proximal straight tubule; tAL, thin ascending limb; TAL; thick ascending limb. Studies were performed in rats (30, 118, 161, 269, 295, 530), mice (269), or pigs (304). All studies used immunohistochemistry except Ref. 30, which used alkaline phosphatase activity. Reference numbers are in parentheses. [Adapted from Vallon (523).]
2. Expression and localization of ectonucleotidases in the kidney
As shown in TABLE 3, all classes of ectonucleotidases are expressed along the nephron and CD system, but to different degrees. The NTPDases have eight subfamilies, of which only NTPDase1, 2, 3, and 8 are ecto-NTPDases (E-NTPDases) (286, 593). All four NTPDases are efficient in hydrolyzing ATP and UTP, thus terminating the activation of P2 receptors. NTPDase1 or CD39 is unique as it sequentially hydrolyzes ATP to ADP and then to AMP, and thus prevents any accumulation of ADP, the ligand for the P2Y1 and P2Y12 receptors. CD39 is the most widely distributed and studied E-NTPDase. In the rat renal cortex, CD39 is expressed in vascular smooth muscle cells and endothelium of interlobular arteries, afferent arterioles, and peritubular capillaries. In the inner medulla, CD39 is expressed in the thin ascending limb of Henle’s loop, ducts of Bellini, and the pelvic wall. NTPDase2 [or CD39-L1 (CD39 like protein-1)], is also expressed in several renal structures (TABLE 3) (269).
Ecto-5′-nucleotidase or CD73 is expressed in all cells and is attached to the cell membrane through a glycosylphosphatidylinositol (GPI) anchor and hydrolyzes extracellular AMP to adenosine and phosphate (490, 593). CD73 is widely distributed in the kidney (296). While CD39 sequentially hydrolyzes ATP to ADP, and then to AMP, thereby effectively terminating P2 receptor signaling, the conversion of AMP to adenosine by the action of CD73 marks the beginning of P1 (adenosine) receptor signaling (287) (FIGURE 1). In many tissues, the physiological processes initiated by P2 receptor signaling are opposed by the activation of P1 receptors by adenosine. Hence, in conjunction with CD39, CD73 plays a decisive role in regulating extracellular nucleotide signaling (102).
Nucleotide pyrophosphatases/phosphodiesterases (NPPs) hydrolyze ATP to AMP and pyrophosphate (PPi). They can be either membrane-bound or soluble enzymes. NPPs also convert cAMP to AMP and release AMP from diadenosine polyphosphates. NPP1 to NPP3 are the better characterized isoenzymes (39, 593); however, compared with CD73 or CD39, relatively little is known about the renal physiology and pathophysiology of NPPs, apart from their localization along the nephron (530). The Km values for NTPDases and NPPs are in the micromolar range (20–50 µM). Potent and selective inhibitors of NPPs have been identified (299, 425).
Alkaline phosphatases are a group of cell surface isoenzymes that catalyze the hydrolysis of organic phosphate esters in the extracellular spaces. They are widely distributed in the body and are classified as tissue-specific and -nonspecific types (593). Those found in the intestines, placenta, and germinal tissue are tissue specific. The tissue-nonspecific alkaline phosphatases, which are found circulating in plasma, make up the bulk of alkaline phosphatases and have clinical relevance. Irrespective of this classification, all alkaline phosphates catalyze the same reaction (472), converting ATP all the way to adenosine (FIGURE 1). Unlike for NTPDases and NPPs, Km values for alkaline phosphatases have been reported in the millimolar range, and therefore their physiological role in purinergic signaling may be limited, although high-affinity sites have also been described (593).
As further discussed in section IIC, significant amounts of ATP (and probably other nucleotides) are present in the tubular fluid along the nephron, and the concentrations may decrease over the length of the nephron and CD. However, it is still difficult to understand or even speculate as to how exactly they regulate purinergic signaling at the tubular level. While much is known about the expression and function of ectonucleotidases in the normal kidney, we know very little about the regulation of their expression and how they are deranged in pathophysiological conditions.
3. The CD39-adenosinergic axis
The CD39-adenosinergic axis mediated by the coordinated actions of CD39 and CD73 has emerged as an important local regulatory system of purinergic signaling with potentially significant impact on renal physiology, pathophysiology, and novel therapies. Thanks to the availability of CD39 or CD73 gene knockout mice or transgenic mice overexpressing human CD39 (hCD39), and reagents such as potato apyrase, soluble engineered ectonucleotidases, and small molecules that selectively interact with CD39 or P1 or P2 receptors, investigators were able to gain significant insights into how the CD39-adenosinergic axis operates under physiological conditions and how it affects renal pathophysiology (reviewed in Ref. 272). Evidence is accumulating that an optimum balance between extracellular nucleotides and adenosine is needed for maintaining health and that a tilt in favor of extracellular nucleotide signaling may lead to pathophysiological conditions. In this regard, it has been shown that the CD39-adenosinergic axis plays a critical role in the regulation of water and sodium transport (433), tubuloglomerular feedback (see sect. IVB), renin secretion, ischemia-reperfusion injury, renal fibrosis, hypertension, diabetic nephropathy, transplantation, inflammation, and macrophage transformation (reviewed in Ref. 272). New therapeutic modalities for these diseases could be based on modulation of the CD39-adenosinergic axis.
C. Nucleotide Concentrations in Tubular Fluid and Renal Interstitium
Renal sources of extracellular ATP include perivascular and peritubular nerve terminals, erythrocytes, aggregating platelets, and renal endothelial and epithelial cells. Renal micropuncture has been performed in anesthetized rats to collect tubular fluid from renal proximal and distal tubules and measure intraluminal ATP concentrations (531). The studies indicated that ATP is present in the glomerular filtrate at concentrations of ~200 nM. Moreover, PTs release and degrade ATP in the lumen with ambient tubular fluid ATP concentrations along the proximal convoluted tubule ranging from 100 to 300 nM. In comparison, ATP concentrations in early distal tubular fluid were significantly lower at ~30 nM. Another study used microdialysis to measure ATP in the interstitial fluid of rat renal cortex and reported concentrations of ~7 nM (357). These numbers would be consistent with a proposed greater ATP release from the apical versus the basolateral membrane in renal epithelia (465). Moreover, nucleotide concentrations in the bulk phase underestimate the true values at the cell membrane receptor by at least 20-fold, which places nucleotide concentrations near membrane P2 receptors well within the affinity range for nucleotides (~0.1–10 µM) (4, 251). New technologies may help refine measurements of extracellular nucleotide concentrations, including in the pericellular space (143).
As discussed in section IIB, ectonucleotidases degrade nucleotides, thereby modulating the ligand availability at nucleotide and nucleoside receptors. The expression pattern of these enzymes along the nephron and CD system may provide some clues as to their function and their effect on luminal and interstitial nucleotide concentrations (269, 523, 530). Based on current knowledge, ATP released into the lumen of the proximal convoluted tubule could be relatively stable, whereas the apical membranes of the proximal straight tubule, the thin ascending limb (tAL), and the TAL have significant capacities to break down ATP and lower its luminal availability (523). This would be consistent with the above-described ATP profile from micropuncture studies showing lower tubular fluid levels in early distal versus proximal tubules (531). In other words, the luminal nucleotide milieu of the aldosterone-sensitive distal nephron (ASDN) may be isolated from the PT. Together with a low apical expression of ectonucleotidases in cortical and outer medullary CDs (CCD, OMCD) (523), this may facilitate a nucleotide-mediated autocrine or paracrine control of transport mechanisms in the ASDN that is physiologically regulated by nucleotide release. Urine ATP concentrations in mice on a normal sodium diet were reported in the range of ~15 nM (340, 487) to ~1 µM (405). Moreover, and as discussed in section VIA, urinary ATP and UTP concentrations vary positively with dietary sodium intake, consistent with ATP and UTP release-dependent regulation of sodium transport in the ASDN.
D. Section Concluding Remarks
We are only just beginning to understand the determinants and the regulation of nucleotide concentrations at their membrane receptors in the kidney, let alone intracellular nucleotide transport (99) or nucleotide concentrations in different subcellular compartments (215). Progress in this area is constrained because currently there are no noninvasive techniques available to detect extracellular nucleotides in live animals or organs down to the cellular level in real time. There is also a need to develop new technologies to study the regulation of the release and/or activities of ectonucleotidases in cell or tissue culture models in real time, and to develop activators or inhibitors that specifically target only one or one type of ectonucleotidase.
III. NUCLEOTIDE P2 RECEPTOR EXPRESSION IN THE KIDNEY
Burnstock proposed a broad division between those receptors sensitive to ATP and other nucleotides (ADP, UTP, UDP and UDP-glucose), named P2, and those sensitive to the nucleoside adenosine, named P1 (57, 164). P2 receptors were initially thought to be postjunctional and P1 prejunctional, both controlling neurotransmitter release. Their tissue distribution was soon found to be widespread, and they were eventually subdivided on the basis of their pharmacology. It wasn’t until much later that the actual subtypes for each class were cloned and identified (4).
So far, only four P1 or adenosine receptors have been cloned and identified as G protein-coupled adenosine A1, A2A, A2B, and A3 receptors that either stimulate or inhibit adenylyl cyclase and thus alter intracellular cAMP levels (234); the ATP breakdown product AMP is also an A1 agonist (436). In comparison, extracellular nucleotides activate two families of P2 receptors, namely, P2Y receptors or P2X receptors (4, 60, 135). In contrast to the small number of adenosine receptors, there are eight metabotropic P2Y receptors (P2Y1,2,4,6 and 11–14), which are also G protein coupled, and seven inotropic P2X ion channel receptors (P2X1–7) that have structural similarities to the epithelial sodium channel (ENaC) (235). Each of these P2 receptor subtypes represents a specific gene (for phylogenetic trees for both P2X and P2Y receptors, see Refs. 204, 457). TABLE 1 lists the corresponding natural ligands and half-maximal effective concentrations (EC50) for these receptors; note that P2X receptors are exclusively ATP gated.
The molecular properties of P2 receptors and their ligands are described in detail in the IUPHAR/BPS Guide to Pharmacology accessible online at https://www.guidetopharmacology.org. P2 receptors have now been identified on almost every cell type in the body, particularly nerve, muscle, and immune cells, but also on most kidney cell types (67, 520). P2Y and P2X receptors that have been detected and identified along the nephron are shown in FIGURE 2, the relevant receptor pharmacology is listed in TABLE 1, and some of the reported functional effects of P2 receptor stimulation are illustrated in FIGURES 3–10. For P2Y and P2X receptors, most of the renal expression data have been generated in male mice and rats, and some information is available in humans. While age and sex have been reported to influence the expression of P2 receptors in many tissues [including mouse microglia (108)] and ovarian hormones can affect renal P2 receptor signaling (170), no systematic analysis of P2 receptor expression in the kidney has been performed with regard to age and sex.
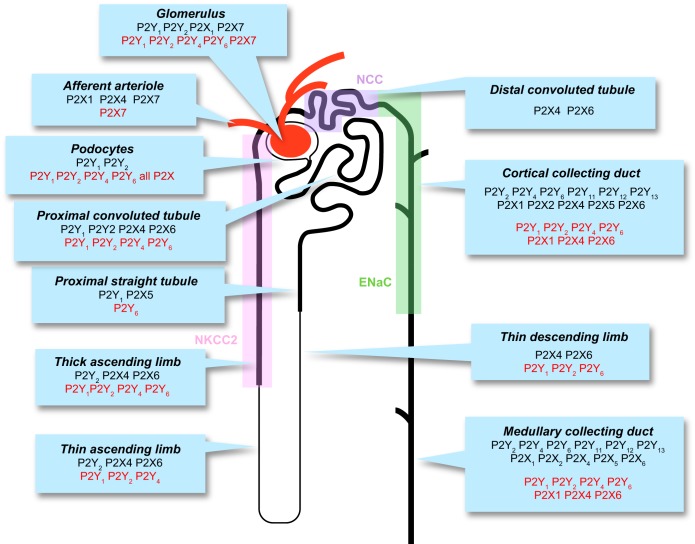
FIGURE 2.
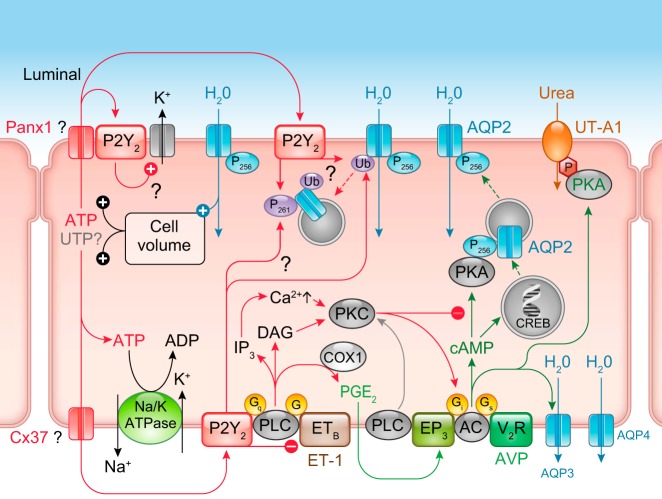
P2 receptor expression in the renal epithelium. Most P2X and P2Y receptor subtypes can be found throughout the nephron, although their relative expression has been difficult to define, as well as their exact function in each nephron segment. There is clearly some overlap in function and even between P2X and P2Y receptors. Message (mRNA in red) detection has been easier than protein (in black) by immunohistochemistry. Also shown are the corresponding renal tubular segment expression patterns for Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and epithelial sodium channel (ENaC), which have been proposed to be P2 receptor regulated. [Modified from Shirley et al. (474), with permission from Elsevier.]
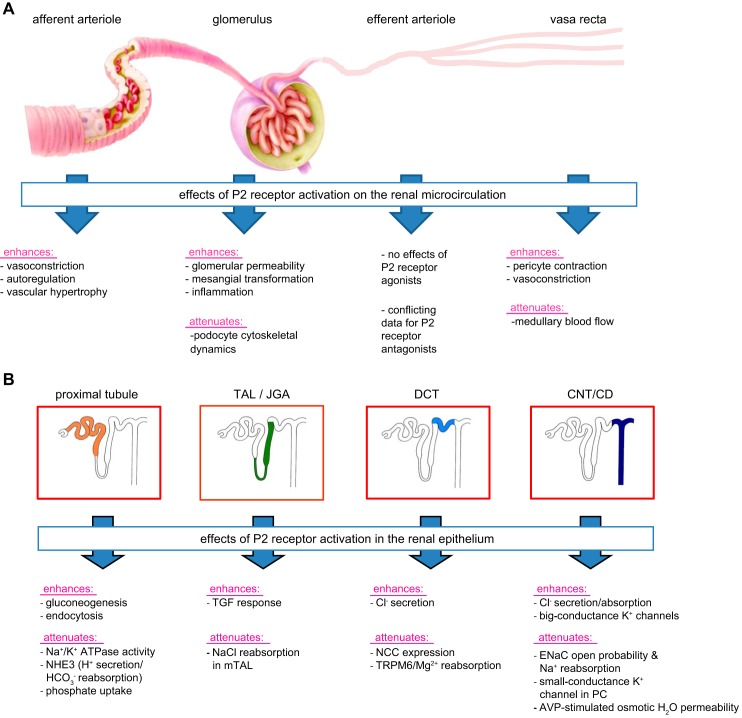
FIGURE 3.
Some examples of P2 receptor-mediated effects of ATP on the vascular segments (A) and the nephron and collecting duct system (B). A: P2 receptor activation induces distinct effects on the renal vasculature, including constriction of the afferent arteriole and vasa recta, with the former contributing to renal autoregulation of glomerular filtration rate and renal blood flow. B: P2 receptor activation affects multiple transport processes along the nephron and collecting duct system, including the inhibition of Na+ reabsorption in multiple segments and of water reabsorption in the connectin tubule (CNT)/cortical collecting duct (CCD). TAL, thick ascending limb; JGA, juxtaglomerular apparatus; DCT, distal convoluted tubule; TGF, tubuloglomerular feedback; ENaC, epithelial sodium channel; PC, principal cell; AVP, arginine vasopressin. See text for details.
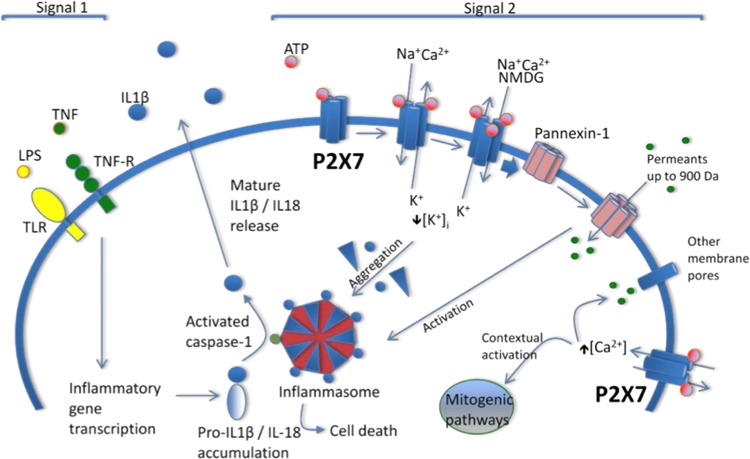
FIGURE 10.
A model of P2X7 receptor participation in cell proliferation, inflammation, and cell death in an archetypal cell, typically, though not exclusively, an immune cell, e.g., a macrophage. Release of mature interleukin (IL)-1β from macrophages requires a cellular priming signal delivered via Toll-like receptors in the case of microbial infection or inflammatory cytokine receptors (e.g., tumor necrosis factor receptor) in sterile inflammation, which leads to inflammatory gene transcription and accumulation of pro-IL-1β (‟Signal 1ˮ). P2X7 receptor activation requires relatively high local concentrations of ATP (in the high micromolar to millimolar range) usually released following cell injury, although localized cell surface concentrations may be sufficiently high in other settings. P2X7 receptor stimulation leads to an initial inorganic cation permeability when two ATP binding sites are occupied, progressing to pore formation when all three binding sites are occupied. An associated decrease in intracellular potassium is required for assembly of the NLRP3 inflammasome, the central caspase-1 activating platform. The P2X7 receptor then triggers opening of large-diameter membrane pores, including the pannexin-1 hemichannel. This event leads through still undefined mechanisms to activation of caspase-1, rapid maturation and release of IL-1β, and the related cytokine IL-18, and cell death (“Signal 2”). Elevations of cytosolic calcium in the absence of large membrane pore opening are thought to underlie the seemingly paradoxical mitogenic effect to P2X7 receptor activation seen in some settings (e.g., tumor growth). This P2X7 receptor-activated processes are believed to be important in a number of inflammatory diseases, including nephropathies in which inflammation, proliferation, and cell death may play a part, for example, glomerulonephritis, diabetic nephropathy, and polycystic kidney disease. See text for details. LPS, lipopolysaccharide; TNF, tumor necrosis factor; TLR, Toll-like receptor; TNF-R, tumor necrosis factor receptor; NMDG, N-methyl-d-glucamine. [Modified from Booth et al. (42), with permission from Dustri-Verlag.]
A. P2Y Receptors
Two distinct P2Y receptor subgroups have been identified that are characterized by a high level of sequence divergence. The first subgroup includes P2Y1,2,4,6,11 subtypes and the second subgroup the P2Y12,13,14 subtypes. These two P2Y receptor subgroups also diverge in their primary coupling to G proteins. P2Y1,2,4,6,11 receptors all principally use Gq/G11 to activate the phospholipase C (PLC) β isotype/inositol 1,4,5-trisphosphate (IP3) pathway and increase [Ca2+]i, whereas P2Y12,13,14 receptors almost exclusively couple to members of the Gi/o family of G proteins and inhibit adenylyl cyclase and thus lower cAMP levels (4, 60, 135). In some epithelia, the Gq-coupled receptors can also indirectly elicit cAMP-regulated cellular functions via activation of phospholipase A2, and generation and release of prostaglandin E, which then activates EP receptors (408) (see sect. VIB).
Insights on P2Y receptors were gained from experiments studying their functional expression. This experimental strategy uses the nearly ubiquitous coupling of P2Y receptors to PLC and the subsequent increase in [Ca2+]i (4). The participation of specific P2Y receptor subtypes has been implicated depending on the responsiveness to adenine or uracil nucleotides or sugar nucleotides (UDP-glucose, UDP-galactose; see TABLE 1) (4, 61). For example, the P2Y2 receptor responds to similar concentrations of ATP and UTP: EC50 values of the P2Y2 receptor for ATP have been reported in the range of 0.085–0.23 µM in human and 0.7–18 µM in mouse with similar values observed for UTP (for review, see Ref. 52). The P2Y11 receptor differs from all the other P2Y receptors, including both sequence and pharmacological differences between species (e.g., canine vs. human) and its absence in the murine and rat genomes (4). More recent studies made use of the availability of P2Y receptor knockout mouse models. The evidence for the renal expression of P2Y receptors is discussed together with their function in sections IV–VII.
B. P2X Receptors
P2X receptors share ~50% homology in amino acid sequence and are membrane ion channels that open in response to the binding of extracellular ATP as their principal ligand (for review, see Refs. 61, 363). All P2X receptors are permeable to small monovalent cations; some have significant Na+, K+, Ca2+, or anion permeability and upon activation cause cell depolarization (see TABLE 1). Under prolonged agonist exposure, P2X4 and P2X7 receptors also can become permeable to large organic cations such as N-methyl-d-glucamine (363).
EC50 values for ATP range from 0.7 to 15 µM for cloned P2X1 through P2X6 receptors (54), whereas P2X7 receptors require concentrations greater than 100 µM (322). Although several selective agonists and antagonists have been identified (see TABLE 1), the pharmacology of P2 receptors is still not completely elucidated, especially for P2X receptors, and there are currently no truly, or only limited, selective agonists or antagonists available (51, 291), which is not the case for adenosine receptors (235). This has made progress in understanding the function of P2X receptors particularly difficult; moreover, even the availability of P2X receptor knockout mouse models has not provided all the answers (253). The evidence for the renal expression of P2X receptors is discussed together with their function in sections IV–VII.
C. Heteromeric Receptor Complexes
G protein-coupled receptors can function as homomeric or heteromeric dimers or even higher-structure oligomers (10, 417). Therefore, it does not come as a surprise that also P2Y receptor subtypes are able to form heteromeric complexes (4). The breakdown of extracellular ATP or ADP is not just forming adenosine, but adenosine A1 receptors can form a heteromeric complex with P2Y1 receptors. Such an adenosine A1 receptor has P2Y1 receptor-like agonistic pharmacology: ATP or ADP binds to an adenosine A1 receptor binding pocket, activates the Gi/o protein-linked effector system, and inhibits adenylyl cyclase, and the effect is inhibited by adenosine A1 receptor blockade (570, 571). Furthermore, combined agonist stimulation promoted the hetero-oligomerization of these two purinergic receptors (572). Also, the P2Y2 receptor can form functional heterodimers with the adenosine A1 receptor (349, 571). Likewise, P2X receptors have a trimeric structure (cf. ENaC) and usually exist as homomers, but have the potential to exist as heteromers, e.g., P2X2/3, P2X2/5, and P2X1/5 all with different properties (36, 363, 535). The formation of receptor heteromultimers enhances the biological complexity and may serve to fine-tune the regulation of physiological processes, but complicates the pharmacology of these receptors and could contribute to unexpected pharmacological effects, especially in vivo. It remains to be determined to which extent the quality of effects of nucleotides on kidney function depend on, and are mediated by, G protein-coupled receptors other than P2, e.g., the adenosine A1 receptor (525).
IV. EXTRACELLULAR NUCLEOTIDES/P2 RECEPTORS AND RENAL VASCULAR FUNCTION
In 1926, Drury and Szent-Györgyi (127) described the influence of nucleotides/nucleosides on renal hemodynamics. They infused the nucleoside adenosine or the nucleotide adenylic acid (AMP) directly into the femoral vein and found that the purified extracts reduced RBF and “secretion of urine” and stated: “At the moment we have no evidence of an action upon the kidney cells as the results obtained can be explained by an action on the blood vessels leading to a fall in glomerular capillary pressure” (127). This paper marked the beginning of investigations into the impact of adenosine-based substances on renal hemodynamics (525).
In 1964, Harvey (192) showed that ATP infusion increased RBF, but slightly reduced GFR with little effect on urine composition, suggesting a primary hemodynamic effect. GFR remained relatively stable in the face of the increase in RBF leading to their conclusion that ATP may relax efferent arterioles and influence renal autoregulation. In 1970, Tagawa and Vander (497) examined the effect of ATP, AMP, and adenosine on renal hemodynamics and renal function in the canine kidney. They found that adenosine reduced GFR, filtration fraction, sodium excretion, and renin secretion, while RBF was only modestly affected. Responses to AMP and ATP were overall similar to adenosine, except that ATP caused a larger increase in RBF. While these early studies on purinergic regulation of renal function did not consider adenosine as a vasoactive breakdown product, they did reveal the possibility for ATP-dependent regulation of renal function.
Subsequent studies established that the renal hemodynamic response to ATP is affected by experimental conditions (like basal vascular tone), and species differences have also been described. For example, in contrast to the increase in RBF described in canines, intrarenal infusion of ATP into rat kidneys produced a pronounced renal vasoconstriction (446). Therefore, attention must be given to the experimental conditions and species being studied. Most importantly, and as discussed below, effects of ATP on renal hemodynamics can be mediated by its breakdown product adenosine, which acts on P1 receptors, rather than through P2 receptors, consistent with complex vascular interactions between these two systems. This aspect may also contribute to the observed species differences. Finally, Schwartz and Malic (464) demonstrated that sympathetic nerve stimulation produced a pronounced, frequency-dependent vasoconstriction in the rat kidney. They noted that this vasoconstriction was largely P2 receptor-mediated at lower levels of stimulation (~0.5 Hz) and shifted to a more adrenergic mechanism at higher levels of stimulation (6–10 Hz). Thus ATP may represent an important neurotransmitter in the kidney for regulating renal vascular resistance in response to renal nerve stimulation. The following sections will detail the influence extracellular nucleotides and P2 receptors have in regulating renal vascular function.
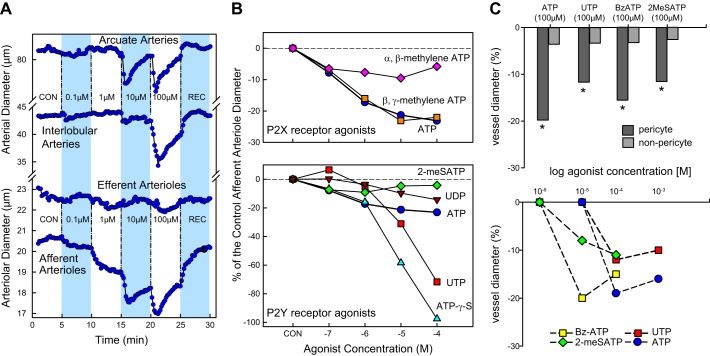
A. General Considerations for Studying Renal Responses to ATP
The renal hemodynamic response to infused ATP can vary depending on the species and conditions being studied and the method by which baseline vascular resistance is established (e.g., preconstriction of the renal vasculature). The majority of the available data were collected using rat kidneys, but kidneys from rabbits, dogs, cats and mice have also been examined (132, 323, 324, 355, 432, 443, 453, 529). In isolated rat kidneys perfused with a Tyrode solution, ATP infusion evoked a renal vasoconstriction consistent with the vasodilated status of the kidney (132, 529). However, when the kidney was “preconstricted” with norepinephrine or phenylephrine, ATP infusion yielded both vasoconstriction at low infusion doses and vasorelaxation at higher infusion doses (132, 529). Similarly, P2X7 receptor blockade improved renal perfusion and renal tissue oxygenation in ANG II-infused rats, but not in the absence of ANG II infusion (338).
ATP infusion into isolated perfused rabbit kidneys perfused with a Krebs-Henseleit solution produced a slight renal vasoconstriction coincident with rapid release of “prostaglandin-like substances” (355). In contrast, in vivo, blood-perfused canine kidneys responded to intrarenal infusion of ATP with a pronounced vasodilation that was NO mediated and could be completely blocked by the NO synthase inhibitor nitro-l-arginine (Nω-nitro-l-arginine) (323, 324). Moreover, during NO synthase inhibition, the renal vasodilation produced by infused ATP reversed to vasoconstriction. Thus NO is an important modulator of renal vascular responses to intraluminal ATP in the canine kidney, and blocking NO unmasked its vasoconstrictor influence.
Exposure of isolated perfused preconstricted (norepinephrine) rabbit or human renal artery segments yielded vasodilation in response to ATP, and this dilation could be blocked by nonspecific adenosine P1 receptor blockade (443). In these arterial segments, the vasodilation was largely eliminated by removal of the endothelium, but only slightly attenuated by inhibitors of NO synthesis, indicating that the P1 receptor-mediated, endothelium-dependent vasodilation did not rely on NO. These studies made the important point that effects of ATP can reflect activation of P1 receptors, secondarily to the conversion of ATP to adenosine, as discussed in more detail below (FIGURE 1).
The intrarenal source of released ATP is important when considering the mechanisms by which extracellular nucleotides influence renal function. Intrarenal perivascular nerves could release ATP along with other neurotransmitters. The released nucleotides could be delivered directly to the adjacent microvascular smooth muscle cells and modulate renal vascular resistance (464, 469). ATP released to the intrarenal interstitial fluid space from endothelial cells, vascular smooth muscle cells, or renal tubular epithelial cells could have access to vascular P2 receptors and influence renal microvascular function (357, 358, 465, 466, 469, 481) (see sect. IIA). Infused nucleotides encounter the renal microvascular endothelium first, followed possibly by access to the underlying vascular smooth muscle (370). Renal vascular responses to intravascular nucleotides might differ from responses derived from ATP delivered to the adventitial side of the blood vessel, as might occur with neurotransmission (57, 58, 66, 69) or autocrine and paracrine actions of ATP released from nearby tubular cells (31, 32, 47, 104, 175, 245, 278, 369, 394, 478) (see sect. IIA). Accordingly, investigation of renal microvascular reactivity to P2 receptor activation should consider extravascular delivery of P2 agonists in addition to intraluminal/intravascular delivery of ATP and P2 ligands. P2 receptor antagonists can be used to gain insights into the contribution of endogenous P2 receptor tone.
The regional distribution of P2 receptors along the renal vascular tree is not yet definitively worked out. Most of the data are derived from functional responses as will be evident from subsequent sections in this review. Nevertheless, there are some data that catalog P2 receptor expression by the renal vasculature (see below). An important deficiency for much of those data is limited information on receptor expression by the renal vascular/microvascular smooth muscle cells versus endothelial cells.
B. Extracellular Nucleotides/P2 Receptors and Glomerular Function
Glomeruli express several subtypes of P2X and P2Y receptors, and our understanding of the distribution of these glomerular receptors is continuously evolving. P2 receptors are expressed by different cell types found in healthy glomeruli and the expression profile changes under pathological conditions (20, 21, 24, 65, 208, 392, 393, 519–521, 538) (see TABLE 4). While considerable work has already been done, more specific investigations are needed to clarify the respective roles of P2 receptors on glomerular cell types in health and disease (see also sect. IIA2).
Table 4.
Renal expression of P2 receptors
| Vasculature/Glomeruli | Mesangial Cells | Podocytes | Proximal Tubules | Loop of Henle | Distal Convoluted Tubule | Collecting Duct | |
|---|---|---|---|---|---|---|---|
| P2Y1 | IM: glomeruli (24, 153); mRNA: (24) | IM (24, 520); WB: mouse culture (437); mRNA: human culture (536) | IM (24, 214); mRNA (214); mouse (150); mouse immortalized (147) | IM: PST-A (520); mRNA: PCT (21) | mRNA: tDL, tAL, mTAL (21) | mRNA: mouse immortalized DCT (159) | mRNA: OMCD (21); mouse IMCD cell line (330) |
| P2Y2 | IM, mRNA: glomeruli (24) | IM (24); cultured cells (440); WB: mouse culture (437); mRNA (191); cultured cells (440); human culture (536); mouse culture (437) | IM (24); mRNA: mouse immortalized (147) | mRNA: PCT (21, 267); PST (267) | IM: tAL, mTAL (intra), cTAL (intra) (520); mRNA: tDL, tAL (21, 267), mTAL (21, 267), cTAL (267); mouse mTAL (327) | mRNA: mouse immortalized DCT (116, 159) | IM: CCD (PC and IC, PC: intra) (553); OMCD (PC and IC, PC: intra-A/B) (553); MCD IC (520), IMCD (A > B) (267); IMCD (PC and IC, PC: B > A) (553); WB: IMCD (267); mRNA: CD (553); CCD (267), OMCD (21, 267), IMCD (267), mouse ISOM (327) |
| P2Y4 | mRNA: glomeruli (24); mRNA: vasa recta (110) | WB: mouse culture (437); mRNA (191); human culture (536); mouse culture (437) | IM: PCT (B) (520); mRNA: PCT (21) | mRNA: tAL, mTAL (21) | IM: CCD, OMCD, IMCD (PC and IC, PC: all A) (554); mRNA: OMCD (21); CD (NaCl restriction) (554) | ||
| P2Y6 | mRNA: glomeruli (24); mRNA: vasa recta (110) | IM: cultured cells (440); mRNA: cultured cells (440); human culture (536) | mRNA: mouse immortalized (147) | mRNA: PCT, PST (22) | mRNA: tDL (22), mTAL (22), cTAL (22); mouse mTAL (327) | mRNA: mouse immortalized DCT (159) | IM: CCD, OMCD, IMCD (PC and IC, PC: all intra-A) (554); mRNA: CD (554); CCD, OMCD (22); mouse ISOM (327) |
| P2Y11 | mRNA: human culture (536) | ||||||
| P2Y12 | mRNA: human culture (536) | mRNA: mouse ISOM (327) | |||||
| P2Y13 | mRNA: mouse ISOM (327) | ||||||
| P2Y14 | mRNA: mouse ISOM (327) | ||||||
| P2X1 | IM, autoradiography: VSMC arcuate and interlobular arteries (86, 336); VSMC afferent arterioles (86, 336, 589); IM: renal arteries (305); IM: glomeruli (153); mRNA: vasa recta (110) | mRNA: human culture (536); mouse culture (437) | mRNA: mouse (150) | mRNA: mouse mTAL (327) | mRNA: mouse immortalized DCT (116) | NaCl restriction: IM: CCD, OMCD, IMCD (all IC); mRNA: CD (554); mouse ISOM (327) | |
| P2X2 | WB: mouse culture (437); mRNA: human culture (536); mouse culture (437) | mRNA: mouse (150) | mRNA: mouse immortalized DCT (116) | mRNA: mouse ISOM (327) | |||
| P2X3 | mRNA: vasa recta (110) | mRNA: mouse culture (437) | mRNA: mouse (150) | mRNA: mouse immortalized DCT (116) | mRNA: mouse IMCD cell line (330); mouse ISOM (327) | ||
| P2X4 | IM: EC renal artery (305, 336) | WB: mouse culture (437); mRNA: human culture (536); mouse culture (437) | mRNA: mouse (150) | IM: low level (520) | IM: tDL (520); mRNA: mouse mTAL (327) | IM: B (520); mRNA (520); mouse (119); mouse immortalized DCT (116, 159) | IM: low level (520); CCD, OMCD, IMCD (all PC and IC, PC: A/B) (554); mRNA: CD (554) |
| P2X5 | mRNA: human culture (536) | mRNA: mouse (150) | IM: PST-A (520) | mRNA: mouse mTAL (327) | mRNA: mouse immortalized DCT (116, 159) | IM: CCD << OMCD < IMCD principal cells (520); | |
| P2X6 | mRNA: human culture (536) | mRNA: mouse (150) | IM: low level (520) | IM: B (520); mRNA (520); mouse (119); | IM: low level (520); CCD, OMCD, IMCD (all PC and IC, PC: A/B) (554); mRNA: CD (NaCl restriction) (554) | ||
| P2X7 | IM: EC renal artery (305, 336); IM, mRNA: glomeruli (538); mRNA: vasa recta (110); IM: EC vasa recta (335) | IM (191); mRNA (191); human culture (536); mouse culture (437) | mRNA: mouse (150); mouse immortalized (147) | IM: mouse (198); mouse ISOM (327) | |||
Data are from rat kidney unless otherwise stated. Reference numbers are in parentheses. (A), apical; (B), basolateral; CCD, cortical collecting duct; CD, collecting duct; cTAL, cortical thick ascending limb; EC, endothelial cells; IC, intercalated cells; IM, immunostaining; IMCD, inner medullary CD; (intra), intracellular; ISOM, inner stripe of outer medulla; OMCD, outer medullary CD; PC, principal cell; PCT, proximal convoluted tubule; PST, proximal straight tubule; tAL, thin ascending limb; mTAL, medullary thick ascending limb; tDL, thin descending limb; WB, Western blotting. [Updated and modified from Vallon (523).]
1. Extracellular nucleotides/P2 receptors and mesangial cells
The glomerular surface area is a determinant of GFR and believed to be controlled by the intraglomerular mesangium. Mesangial cells express multiple P2 receptor subtypes that are responsive to ATP, UTP, and even diadenosine polyphosphates (24, 169, 184, 208, 211, 212, 274, 397, 398, 400, 437, 440, 452, 458, 460, 462, 463, 499, 591). RT-PCR products for P2Y1,2,4,6,11,12 receptors and P2X1,2,4,5,6,7 receptors were detected in cultured human mesangial cells processed from fresh human kidneys (536).
Exposure of isolated glomeruli to ATP, 2-methylthio-ATP (P2Y agonist), or β,γ-methylene ATP (P2X agonist) reduced glomerular volume similarly to that observed with ANG II (243). The contractile response to P2Y receptor activation involved activation of the Rho-kinase pathway (240). Preconstriction of isolated glomeruli with ANG II reversed the contractile effects of ATP and 2-methylthio-ATP, and produced a concentration-dependent relaxation that involved induction of NO synthase activity and cGMP production (238, 242, 243). Therefore, in vitro evidence suggests that P2 receptor activation can influence glomerular volume, probably by inducing mesangial cell contraction or relaxation, depending on the context.
Western blot analysis of cultured mouse mesangial cells indicated expression of P2X2, P2X4, P2X7, P2Y2, and P2Y4, but did not detect evidence of P2X1 or P2X3 protein expression (437). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis revealed mRNA expression for P2X1, P2X2, P2X3, P2X4, P2X7, P2Y2, and P2Y4 receptors (549). Activation of these receptors with reasonably selective P2 receptor agonists yielded strong increases in [Ca2+]i with the exception of 2-methylthio-ATP, which had no detectable effect. These data are generally consistent with earlier work using cultured rat mesangial cells, where it was shown that ATP and UTP evoked transient or sustained membrane depolarization, respectively, that was accompanied by an increase in [Ca2+]i (208, 398).
Stimulation of mesangial cells with ATP or UTP also activated other intracellular second messenger systems such as the p38-stress-activated protein kinase cascade (211–213) and induced mesangial cell migration through sphingosine kinase-1 expression (273) (FIGURE 3A). P2 receptor activation of mesangial cells also induced additional signaling pathways, including the mitogen-activated protein kinase cascade (211, 461); protein kinase C (400); phospholipases A2, C, and D (400, 456, 461); polyphosphoinositide hydrolysis (398); suppression of cAMP accumulation and inducible NO synthase activity via P2Y2 receptor activation (345, 460, 461); and stimulation of immediate early genes c-fos, c-jun, and Egr-1 (461). Activation of these signaling pathways can lead to formation of extracellular signaling molecules with autocrine or paracrine effects on local and more distant cellular and tissue function. Indeed, mesangial cell P2 receptor activation with ATP stimulated the synthesis and release of prostaglandin E2 (398, 462). Released prostaglandins could directly modulate local cell and tissue signaling in glomeruli or stimulate proinflammatory processes that contribute to tissue injury (191, 461). P2Y2 and possibly P2Y4 and P2Y6 receptors promote mesangial cell proliferation (191, 440, 462), whereas P2X7 receptors appear to promote mesangial cell apoptosis and necrosis (191, 458) (see sect. VII). Thus P2 receptor-dependent effects on mesangial cells provide mechanisms by which extracellular nucleotides could influence the intraglomerular mesangium, glomerular volume, glomerular filtration barrier integrity, and/or regulate glomerular hemodynamics (176, 212, 213, 238, 273, 391, 399, 437, 479) (FIGURE 3A).
2. Extracellular nucleotides/P2 receptors and podocytes
a) p2 receptor expression in podocytes.
RT-qPCR studies detected the expression of mRNAs of P2Y1,2,4,6,11 and all P2X receptors in podocytes (24, 67, 147, 150, 181, 520). P2Y1 and P2Y2 receptor protein expression has been detected in podocytes (and mesangial cells, see above) using pharmacological tools, knockout mice, intact rat glomeruli, or immunohistochemistry (24, 55, 214) (TABLE 4). As to intraglomerular expression of P2X receptors, the main findings have been for P2X7, and more recently P2X4 receptors, and possibly P2X2 receptors (150, 538); however, the data have come largely from models of glomerular injury (538). P2X7 receptor expression is very low in normal kidneys, but is consistently upregulated in podocytes, mesangial cells, and tubular epithelium of diabetic kidneys (337, 538); but also in endothelial and vascular smooth cells in a model of severe hypertension (337, 538). The data indicate a dynamic remodeling of purinergic signaling in glomerular cells as well as expression of specific P2X receptors under pathological conditions (see sect. VII).
b) p2 receptor function in podocytes.
Podocytes maintain the permselectivity and provide physical support of the glomerular filtration barrier. Nonmetabolized ATP analogs 2-meS-ATP and ATPγS increased convectional albumin permeability in isolated rat glomeruli (254) (FIGURE 3A). As discussed above, [Ca2+]i waves can be propagated to podocytes during tubuloglomerular feedback (TGF) activation by an ATP- and P2 receptor-dependent mechanism. While the source of ATP reaching the podocytes remains unclear, adjacent glomerular endothelial cells, which can secrete ATP through connexin and/or pannexin hemichannels (511), may contribute. Injuring one single podocyte using two-photon microscopy triggered spreading of intercellular podocyte [Ca2+]i waves, which was absent in P2Y2−/− mice (55). Using patch-clamp and fura 2 fluorescence techniques, it was observed that nucleotides depolarized immortalized mouse podocytes in culture and increased [Ca2+]i. The rank order of potency of various nucleotides on these two parameters indicated that extracellular nucleotides modulate podocyte function mainly by activation of P2Y2 and P2Y6 receptors (147). Another study indicated functional expression of P2Y1 and P2Y2/P2Y4, but not P2Y6, receptors in intact rat glomeruli by measuring the relative stimulation of the inositol phosphate pathway in response to selective agonists of a particular receptor subtype. The latter study also used available polyclonal antibodies and suggested the expression of P2Y1 and P2Y2 receptors in mesangial cells and podocytes (24). Subsequent studies in podocytes of freshly isolated rat glomeruli indicated that the P2Y1 receptor is the dominant receptor to mediate Ca2+ influx based on comprehensive pharmacological profiling and immunolocalization (214).
ATP induced TRPC6 currents in immortalized mouse podocytes and in primary rat podocytes still attached to the glomerular basement membrane (439) (FIGURE 3A). These effects were blocked by the P2 antagonist suramin and mimicked by P2Y agonists such as ADP and UTP, implicating a role for P2Y receptors, potentially P2Y1 and P2Y2 receptors. Furthermore, the studies implicated phospholipase, podocin, and reactive oxygen species (ROS) in the signal transduction cascade leading from P2Y receptors to TRPC6 (439). Podocin is required for colocalization of TRPC6 channels and NOX2 in podocytes (262) and thus may allow local generation of ROS as a physiological modulator of TRPC6 gating. Extracellular ATP increases superoxide production in cultured human podocytes (178). On the other hand, activation of P2 receptors by ATP stimulated AMP-activated protein kinase and suppressed superoxide generation in cultured mouse podocytes (401), indicating a potential negative feedback regulation of superoxide generation or species-related differences.
A role for P2X1, P2X4, and P2X7 receptors has been proposed in controlling glomerular hemodynamics under conditions of ANG II-driven hypertension (153), with P2X1 and P2X7 receptors causing vasoconstriction, and P2X4 receptors appearing to have an opposing action (154). In podocytes, the P2X4 receptor, and perhaps also P2X2, reportedly behave as mechanotransducers, responding to autocrine ATP release after mechanical stimulation with an increase in [Ca2+]i coupled to potentially damaging cytoskeletal rearrangements (150). This effect appears to be independent of TRP channel activation and may contribute to glomerular hypertensive damage. However, P2X4−/− mice have no obvious gross glomerular phenotype and are not known to be proteinuric under basal conditions (150). The role of P2 receptors in glomerular pathophysiology is discussed in section VII.
3. Extracellular nucleotides/P2 receptors and renal microvascular effects
Renal perfusion is influenced by humoral factors, paracrine and autocrine factors, neural factors, and intrinsic factors (73, 124, 351, 354, 550) that impinge on the renal microvasculature to influence RBF regulation, glomerular capillary pressure, and effective filtration pressure. Initial interest in the renal vascular effects of ATP arose from the observations that infused ATP affected RBF, GFR, and renal microvascular resistance and led to the idea that nucleotides might exert distinct effects on renal hemodynamics that are different from responses to nucleosides, and involve disparate receptor families and/or signaling mechanisms.
a) p2 receptor distribution in the renal vasculature.
mRNA coexpression for P2X1, P2X2, and P2X4 receptors was detected in rat renal arteries but no distinction was made for vascular smooth muscle and endothelium (361). With the study of the expression of the seven P2X receptors in the rat vascular system, it was observed that immunoreactivity for P2X1 receptors was greatest in small and medium intrarenal arteries along with some detection of P2X4 receptors. Barely detectable levels of immunoreactivity were reported for P2X3 and P2X5 receptors in small intrarenal arteries, but no distinction was made for vascular smooth muscle versus endothelium (305). Other studies in rats confirmed prominent P2X1 receptor immunostaining in renal arteries, including arcuate arteries, interlobular arteries, and afferent arterioles, but no staining was detected on efferent arterioles (86, 589). Moreover, it was noted that P2X1 receptor staining was limited to the vascular smooth muscle layer of rat intrarenal arteries and afferent arterioles (336). In comparison, P2X4 and P2X7 receptors were immunlocalized to the endothelium of large- to medium-caliber intrarenal arteries in the rat (305) and throughout the rat preglomerular vasculature (336), respectively. P2X7 receptor protein expression has also been reported on the endothelial lining of the vasa recta in the rat inner medulla (335). Clearly more work is needed to better define the cell type-specific distribution of P2 receptors in the renal vasculature, and their role in endothelial and smooth muscle cell function. The same issue applies to P1 receptors in the renal vasculature where no distinction has been made between vascular smooth muscle expression and endothelial expression.
Defining the segmental influences of nucleotides along the renal vascular tree came from the use of isolated renal vascular or microvascular segments. For example, introduction of ATP or 2-methylthio-ATP (P2Y agonist) to the lumen of isolated perfused rabbit renal arteries treated with indomethacin and preconstricted with norepinephrine yielded consistent vasorelaxation that was attenuated by the nonselective P2Y receptor antagonist Reactive Blue 2 (442). In the same study, similarly prepared human renal arteries (preconstricted with prostaglandin F2α) also vasodilated in response to luminally applied ATP (442). Rump et al. (443) followed up on that study to demonstrate that ATP-mediated vasodilation of isolated perfused human and rabbit renal arteries involved a P1 receptor-dependent mechanism that appeared to be independent of NO; however, the vasorelaxation to 2-methylthio-ATP was largely eliminated by the NO synthase inhibitor NG-nitro-l-arginine. Thus, in rabbit and human renal arteries, the vasodilatory effect of exogenous ATP mainly results from P1 receptor activation and that P2Y receptor-induced renal dilation is mediated by NO.
As mentioned above, ambient vascular tone affects the response to intravascular ATP. For example, renal artery infusion of ATP or the P2X agonist α,β-methylene ATP in an isolated perfused rat kidney with ambient vascular tone results in vasoconstriction (97, 132, 144, 529). However, when the kidney is preconstricted with norepinephrine (132) or phenylephrine (528, 529), the renal vascular response to infused ATP becomes biphasic with both vasoconstriction and vasodilation observed, although some variability exists in the responses observed by different investigators. This could have important implications for the renal vascular responses to P2 receptor stimulation under conditions of high sympathetic tone or circulating levels of vasoactive hormones like ANG II or endothelin that can elevate renal vascular resistance and alter renal responses to P2 receptor activation.
b) p2 receptors on afferent arterioles.
Control of renal and glomerular hemodynamics arises primarily from regulation of afferent arteriole resistance and to a lesser degree efferent arteriole resistance. The majority of the intrarenal data on P2 receptor-mediated effects arise from studies using rat and mouse afferent arterioles with more limited information available for other renal microvascular segments.
Aside from whole kidney studies described above, one of the earliest reports visually demonstrating P2 receptor actions on the renal vasculature contrasted P1 and P2 receptor-dependent responses (219). It demonstrated in rat juxtamedullary afferent arterioles that poorly hydrolyzable P1 and P2 receptor agonists yielded different afferent arteriole responses: P2 receptor agonists caused stable, concentration-dependent reductions in afferent arteriole diameter (FIGURE 3A), while the adenosine analog 2-chloroadenosine produced vasoconstriction at lower concentrations (via high-affinity A1 receptors) that reversed to significant relaxation at the highest concentration (via lower affinity A2 receptors). One of the P2 receptor agonists employed was the P2X1 receptor agonist α,β-methylene ATP (also has some affinity for P2X3 receptors with normal usage but in high concentrations it may activate most P2X receptors), which evoked a dramatic and rapid initial vasoconstriction that gradually declined to a smaller, sustained vasoconstriction. Similar P1 and P2X receptor-dependent responses were also reported using isolated perfused rabbit afferent arterioles (545). Interestingly, ATP-mediated vasoconstriction of blood-perfused, rat juxtamedullary afferent arterioles was enhanced during nonselective P1 receptor blockade with 1,3-dipropyl-8-p-sulfophenylxanthine (226, 353). Notably, the adenosine A1 receptor blocker inhibited ATP-dependent vasoconstriction of the proximal aspect of isolated-perfused rabbit arterioles while vasoconstriction of the distal aspect of the arteriole was retained (226, 545). Collectively, these observations suggested that afferent arterioles express P2X1 receptors. This was later substantiated autoradiographically showing P2X1 receptor expression in the preglomerular vasculature, including afferent arterioles (86). The same group also employed immunohistochemistry showing strong positive staining along the entire preglomerular vascular tree, but no detectable staining of efferent arterioles. These data showed that P2X1 receptors were present and functional in the preglomerular vasculature, but the existence of other P2 receptors remained unknown. Accordingly, while involvement of adenosine-sensitive P1 receptors in regulating preglomerular resistance had been established (190, 455, 525), these new observations suggest that both P1 and P2 receptor systems are present in the renal microcirculation and could influence renal microvascular function.
To begin to address how P2 receptors may participate in regulating renal hemodynamics, Inscho et al. (226) directly determined the segmental, renal microvascular response to exogenous ATP administration in the rat. In this experimental setting, the vascular elements were perfused with blood and exhibited endogenous tone, so both vasodilation and vasoconstriction could be detected. As shown in FIGURE 4A, and consistent with the noted receptor expression profiles (86), all preglomerular microvascular segments responded to ATP superfusion with biphasic vasoconstriction manifested as a rapid initial vasoconstriction that gradually waned. Depending on the vascular segment being studied, and the ATP concentration being administered, the initial vasoconstriction either waned completely or decreased to a smaller, but sustained, reduction in vessel diameter. In contrast, efferent arterioles were unresponsive to exogenously applied ATP up to 100 μM. Arcuate arteries exhibited only transient initial vasoconstrictions, whereas the next downstream element, interlobular arteries, did exhibit a sustained vasoconstriction at the highest ATP concentration tested. In contrast to the larger arterial segments, afferent arterioles responded to ATP with both transient initial vasoconstriction and a sustained vasoconstriction, even at the lowest ATP concentration tested. These data defined the segmental intrarenal microvascular reactivity to P2 receptor activation in the rat, and indicated that ATP, or another endogenous P2 receptor agonist (see TABLE 1), could regulate preglomerular resistance and participate in regulating renal and glomerular hemodynamics.
FIGURE 4.
Renal microvascular responses to ATP or P2 receptor activation. A: changes in intrarenal microvessel diameter in response to exogenous ATP in blood-perfused rat juxtamedullary nephrons. Represented are arcuate arteries, interlobular arteries, and efferent and afferent arterioles. Exogenous ATP was applied from the adventitial surface via superfusion. Following a 5-min control period (CON), increasing concentrations of ATP from 0.1 to 100 μM were applied at 5-min intervals as indicated by the dashed lines and shaded bars. Each protocol ended with a 5-min recovery period (REC). [Modified from Inscho et al. (226).] B: sustained afferent arteriole responses to ATP and selected P2X and P2Y receptor agonists. Data are expressed as percentage of control diameter before agonist exposure. Top panel illustrates responses to P2X agonists, α,β-methylene ATP, and β,γ-methylene ATP compared with ATP. Bottom panel illustrates responses to P2Y agonists 2-methylthio-ATP (2-MeSATP), UDP, UTP, and ATP-γ-S compared with ATP. [Modified from Inscho et al. (223).] C: in situ effects of P2 receptor agonists on vasa recta diameter. P2 receptor agonists ATP, UTP, BzATP, and 2-MeSATP evoked a greater constriction of vasa recta at pericyte sites (dark gray bars) compared with non‐pericyte sites (light gray bars). *P < 0.01 vs. non-pericyte. Line graph shows concentration‐dependent effect of agonist‐evoked change in vasa recta diameter at pericyte sites for all P2 receptor agonists shown above. [Modified from Crawford et al. (110), with permission from John Wiley and Sons.]
To clarify the P2 receptors involved in ATP-mediated renal vasoconstriction, renal microvascular reactivity was assessed by using rank-order potency profiles (223). As shown in FIGURE 4B, P2X receptor agonists α,β-methylene ATP and β,γ-methylene ATP and ATP produced concentration-dependent vasoconstrictions of afferent arterioles: the effect began with a rapid initial response followed by a smaller, sustained vasoconstriction (shown for ATP in FIGURE 4A); ADP and AMP were without effect (220, 223). The responses to α,β-methylene ATP and β,γ-methylene ATP resemble the sustained vasoconstrictor response previously reported for α,β-methylene ATP in isolated Tyrode solution-perfused kidneys with raised tone, but no evidence of a vasorelaxation was observed for ATP in the blood-perfused kidney setting (223). The response to β,γ-methylene ATP closely mimicked the response to ATP, consistent with P2X1 receptor activation (223, 545).
In the blood-perfused juxtamedullary nephron setting, the P2Y agonists 2-methylthio-ATP, ATP, ADP, UDP, UTP, and ATPγS produced disparate agonist-specific results (FIGURE 4B) (219, 223). Afferent arteriole diameter was reduced slightly by 2-methylthio-ATP, while the response to UDP was ~50% of that evoked by ATP, but was significant only at the 100 µM concentration (223). UTP and adenosine 5′-O-(3-thiotriphoshate) (ATPγS) were not as potent in stimulating vasoconstriction of afferent arterioles at lower concentrations, but with concentrations of 10 and 100 µM, very large monophasic vasoconstriction was observed (223). There is general agreement between these results and those obtained with the Tyrode solution-perfused kidney preparation (132), with the exception of not seeing vasorelaxation to these P2Y agonists. It is important to note that the vasorelaxation observed in the isolated perfused rat kidney with raised vascular tone (norepinephrine) occurred at concentrations much lower (estimated ED50 in the 0.1–1 nM range) than have been tested in the blood-perfused setting (132). In addition, the isolated perfused kidney provides integrated information of the vascular resistance response of the entire renal vasculature and not individual vascular segments.
c) p2 receptors on efferent arterioles.
Harvey (192) reported that intrarenal infusion of ATP increased RBF and reduced GFR and ureteral stop flow pressure and suggested that this effect could be explained by vasodilation of the efferent arteriole. Indeed, efferent arterioles express vasodilatory adenosine A2 receptors (232, 356, 483, 525). Studies in blood-perfused rat juxtamedullary nephrons found that selective A2 receptor blockade yielded an adenosine-induced vasoconstriction of afferent and efferent arterioles. These studies provided compelling functional evidence that efferent arterioles express vasodilatory P1 receptors that are activated when the applied ATP is broken down to adenosine. Rat afferent and efferent arterioles (but not arcuate arteries) are also invested with membrane-bound CD73 (161, 296) that catabolizes extracellular ATP to its breakdown products, including adenosine, that can influence efferent arteriole resistance (75). Efferent arterioles receive innervation from type II axons and could participate in purinergic neurotransmission (333). Despite this, there is little clear evidence that efferent arterioles exhibit pronounced reactivity to P2 receptor stimulation (FIGURE 3A). Efforts to define P2 receptor distribution along the normal renal microcirculation using a variety of techniques have failed to identify P2 receptor expression by efferent arterioles. Chan et al. (86) showed positive P2X1 immunostaining of the intrarenal vasculature from the main renal branches to the afferent arteriole, but glomeruli and efferent arterioles were negative. Radioligand binding studies with [3H]α,β-methylene ATP revealed P2 receptors along the preglomerular microvasculature but not efferent arterioles or glomeruli (86). Turner et al. (520) confirmed lack of P2X1 or P2X2 receptor expression along efferent arterioles, but they did note expression of P2Y1 receptors. These studies were performed using normal animals and various staining techniques, which may impact the results if expression levels are low or if conditions change under stressed or pathological conditions (538). However, consistent with the expression data, Inscho et al. (226) demonstrated that direct application of ATP to blood-perfused efferent arterioles had no effect on efferent arteriole diameter, even though there was a pronounced vasoconstriction of preglomerular arteries and arterioles.
Renal micropuncture was employed to assess the impact of nonselective P2 receptor blockade with pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) (PPADS) on renal and glomerular hemodynamics in sham and ANG II-infused hypertensive rats (153). In sham rats, PPADS significantly increased afferent and efferent arteriolar resistances, leading to reduced glomerular plasma flow, reduced ultrafiltration coefficient, and a decline in single-nephron GFR, without any significant changes in blood pressure (153). In contrast, when PPADS was given to ANG II hypertensive rats, the renal microvascular responses were quite different. Basal afferent and efferent arteriolar resistances were higher in the ANG II-hypertensive rats (153). PPADS actually reduced these resistances to values similar to those calculated for sham controls. Importantly, in the ANG II hypertensive setting, nonselective P2 receptor blockade promoted renal microvascular relaxation that involved an l-NAME-sensitive mechanism (153). More recently, it was reported that P2X1 or P2X7 blockade with NF449 and A438079, respectively, reduced afferent and efferent resistance and increased glomerular plasma flow (154). The explanation for these apparently discrepant data with regard to the effect of P2 receptor activation on efferent arteriole resistance remains unclear (FIGURE 3A), but could reflect greater resolution with more selective receptor antagonists. Finally, the efferent arteriole response to the diadenosine polyphosphates Ap3A and Ap5A was studied using the rat hydronephrotic kidney model (157). With this model (485), renal microvessels can be visualized and studied using transillumination microscopy. Efferent arterioles were found to be essentially unresponsive to Ap3A and Ap5A, consistent with what others have observed for ATP.
Collectively, these data indicate that P2X and P2Y receptors are expressed by rat afferent arterioles. One subtype is the P2X1 receptor, but the presence of other P2X isoforms such as P2X7 cannot be ruled out. P2X1 receptors respond strongly to ATP and also α,β-methylene ATP and β,γ-methylene ATP (1–4, 139, 140, 363, 365). In addition, it is probable that the P2Y2 receptor is also expressed, based on the powerful response elicited by UTP and ATPγS. Combining results from isolated perfused kidneys and in vitro blood-perfused juxtamedullary nephrons, it is also possible that there are regional differences in receptor expression, which could impact renal hemodynamic responses to P2 receptor activation. Such differences in receptor expression may represent a novel mechanism for regulating regional renal tissue perfusion, for example, unique regulation of renal cortical and medullary perfusion, as also proposed for P1 receptors (525).
4. Extracellular nucleotides/P2 receptors and tubuloglomerular feedback
The MD cells sense the tubular NaCl load at the end of the TAL and generate a response that primarily affects afferent arteriolar tone, such that an inverse relationship is established between this tubular NaCl load and the GFR of the same nephron. The tubuloglomerular feedback (TGF) thus coordinates GFR with the tubular transport activity or capacity upstream of the MD and thereby contributes to the autoregulation of GFR and RBF. Changes in the luminal NaCl concentration trigger the basolateral release of ATP from MD cells (32, 278) (see sect. IIA and FIGURE 5). The released ATP is converted to ADP and adenosine by local CD39 (373) and CD73 (82, 207, 429, 509). Adenosine activates the adenosine A1 receptor on extraglomerular mesangial cells to raise [Ca2+]i, which induces the propagation of a Ca2+ wave to the vascular smooth muscle cells in the afferent arteriole, causing vasoconstriction and a reduction in GFR (525). Alternatively or in addition, extracellular adenosine directly activates the adenosine A1 receptor on the VSMC of the afferent arteriole (525). Local changes in the formation of adenosine and in adenosine A1 receptor activation are required for a normal TGF response (50, 491, 509, 526). Gene knockout of CD73 only partially inhibited the TGF response with the remaining activity being also sensitive to adenosine A1 receptor blockade (207). Based on these data it has been hypothesized that part of the adenosine that mediates the TGF derives from direct MD release. Overall, a model is envisioned in which both ATP and adenosine would be considered mediators of TGF, since both are released or generated, respectively, in accordance with the NaCl concentration sensed at the MD (522, 525).
FIGURE 5.
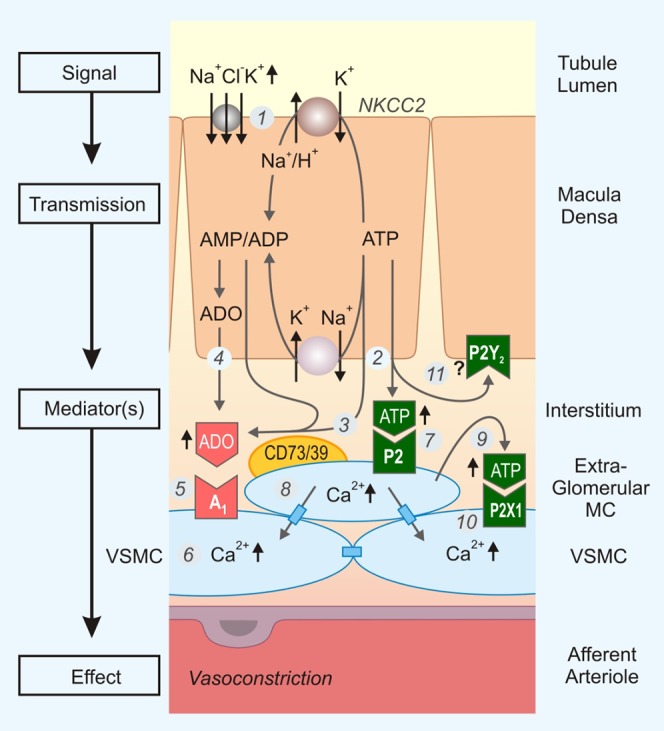
Nucleotide/P2 receptor system and tubuloglomerular feedback. Numbers in circles refer to the following sequence of events: 1) increase in concentration-dependent uptake of Na+, K+, and Cl− via the furosemide-sensitive Na+-K+-2Cl− cotransporter (NKCC2) in macula densa cells; 2) basolateral release of ATP; 3 and 4) transport-dependent, intra- and/or extracellular generation of adenosine (ADO); 5 and 6) the extracellular generation involves local ecto-NTPDase1 (CD39) and ecto-5′-nucleotidase (CD73); 7) extracellular ADO activates adenosine A1 receptors triggering an increase in cytosolic Ca2+ on extraglomerular mesangium cells (MC; not shown) and smooth muscle cells of the afferent arteriole (VSMC) resulting in afferent arteriolar vasoconstriction; 8) extracellular ATP activates P2 receptors on extraglomerular MC and increases cytosolic Ca2+; the intensive coupling between extraglomerular MC, intraglomerular MC (not shown), and VSMC of the afferent arteriole by gap junctions contributes to the propagation of the Ca2+ signal; 9 and 10) ATP release and P2 receptor activation also contribute to the cell to cell propagation of the Ca2+ signal, including to VSMC where activation of P2X1 receptors causes afferent arteriolar vasoconstriction; 11) extracellular ATP may also activate P2Y2 receptors on the basolateral membrane of macula densa cells with unknown function. See text for further explanations. [Modified from Vallon et al. (525).]
Functional studies suggested the expression of P2Y2-like receptors at the basolateral membrane of the MD (311), consistent with the general concept that released ATP acts in an autocrine or paracrine fashion on P2 receptors. In this regard, a more direct role of P2 receptors in the mediation of TGF has been proposed (221, 359, 395). Mesangial cells play a key role in mediating the TGF response, and gap junctions among mesangial cells and between mesangial cells and vascular smooth muscle cells of the afferent arteriole communicate the TGF signal to the afferent arteriole (426). This communication involves propagation of [Ca2+]i waves between these cells as well as intraglomerular elements and podocytes, a process that is blocked by ATP scavenging or by pharmacological P2 receptor inhibition (395, 568) (FIGURE 5). Renal autoregulation, which in part depends on TGF, was found to be impaired in P2X1−/− mice (220) (FIGURE 3A). Furthermore, two maneuvers that can inhibit the TGF response, namely, papillectomy and furosemide treatment, both attenuated the pressure-induced reduction of afferent arteriolar diameter in WT control mice but had no detectable effect on the pressure or diameter response in P2X1−/− mice (220), suggesting that the TGF component of renal autoregulation was attenuated in the absence of the P2X1 receptor. On the other hand, systemic infusion of the P2 receptor inhibitors PPADS or suramin, at doses that significantly reduced the blood pressure response to the P2X agonist α,β-methylene ATP, did not affect the TGF response. The latter was assessed using renal micropuncture, i.e., by measuring the proximal tubule stop flow pressure change (as a surrogate for glomerular capillary pressure) caused by a saturating increase in loop of Henle fluid flow, which maximizes NaCl delivery to the MD. Also the intratubular application of PPADS did not affect the TGF response (453). It seems possible that the micropuncture studies, which used TGF-saturating increases in loop of Henle flow, generated sufficient extracellular adenosine in the juxtaglomerular apparatus to directly activate adenosine A1 receptors on the vascular smooth muscle cells of the afferent arteriole, thereby bypassing the P2X1 receptor-mediated propagation of the Ca2+ wave, which may serve to amplify the TGF signal. Further studies are needed to test this hypothesis and resolve the discrepant or dual mechanisms.
5. Extracellular nucleotides/P2 receptors and renal autoregulation
Autoregulation of blood flow is defined as the inherent ability of a tissue or organ to maintain a stable/constant blood flow, despite changes in perfusion pressure. The kidney exhibits an exquisitely sensitive autoregulatory capability that is achieved through the combined influences of the myogenic response and the TGF mechanism (33, 56, 73, 76, 350, 351, 354, 427, 428, 455) and to a less well-known degree by the connecting tubule glomerular feedback mechanism (427, 428). The myogenic response involves a signaling system, intrinsic to vascular smooth muscle, which transduces mechanical stretch of the vascular wall into active [Ca2+]i-dependent tension “calibrated” to offset the pressure-induced stretch and increase vascular resistance such that blood flow through that vascular element remains essentially constant. For this to occur, there must be a sensing system (mechanosensor) and a signal transduction step coupled to second messenger systems such as changes in [Ca2+]i, to initiate this pressure-mediated vasoconstriction and increase in vascular resistance (15, 73, 351, 354). As described above, TGF is mediated by P1/adenosine A1 receptors and perhaps indirectly involves P2 receptors. As discussed below, ATP and P2 receptor activation have been proposed to play critical roles in renal autoregulation, including the myogenic component. In comparison, the influence of P1/adenosine A1 receptors on renal autoregulation appears to be primarily due to their role in TGF (194, 252). For a comprehensive presentation of renal autoregulation and the underlying mechanisms, the reader is directed to many excellent articles (15, 33, 56, 73, 76, 350, 351, 354, 427, 428, 455).
Earlier observations that intrarenal infusion of ATP could alter renal function by influencing renal hemodynamics, specifically RBF, led to the general idea that extravascular ATP could manipulate renal vascular resistance (192, 446, 464, 497). As more specific information became available, including what segments of the intrarenal vascular architecture were responsive to P2 receptor stimulation, it became apparent that P2 receptor-mediated regulation of renal vascular resistance was predominantly (if not exclusively) a preglomerular effect (223). As discussed above, the afferent arteriole is the vascular segment displaying the greatest reactivity to P2 receptor stimulation (FIGURE 4A) (223, 226, 545). Moreover, the intracellular signaling mechanisms employed include the same mechanisms essential for autoregulatory resistance adjustments (73, 77, 80, 195, 196, 222, 225, 341, 352, 354, 448). Based on this information, the idea arose that P2 receptors may be involved in autoregulation of RBF and GFR (224) (FIGURE 3A).
The blood-perfused juxtamedullary nephron preparation (81) had previously been used for assessing autoregulatory control and the impact of P2 receptor activation on renal microvascular diameter, resistance, blood flow, and/or GFR, so it provided an excellent platform to determine the relationship between P2 receptor functionality and autoregulatory capability (44, 45, 74, 76, 78, 79, 218, 346, 347, 352, 388). At the time, P2 receptor “inactivation” was the best method to link loss of P2 signaling with loss of autoregulatory function. Collectively, the data support the idea that autoregulatory renal vascular resistance adjustments involve local release of ATP with subsequent activation of P2, probably P2X1, receptors. This conclusion is based on the inhibitory effects of P2 receptor desensitization or saturation (224), P2 receptor blockade (216, 224), and P2 receptor deletion (220, 221) on afferent arteriole autoregulatory behavior and calcium signaling (217, 220, 548). In the in vivo setting, the P2X1 receptor antagonist IP5I potently inhibited whole kidney autoregulation of RBF in the rat, and P2 receptor “saturation” using an ATP clamp approach inhibited autoregulation of whole kidney RBF in the canine kidney, consistent with the predictions made in vitro on afferent arteriole function (323, 375). While these data strongly support an important role for P2X1 receptors in autoregulation, it is important to remember that the involvement of other P2 receptors in renal autoregulatory resistance adjustments has not been exhaustively studied, so other receptors could participate, such as P2Y6 receptors, which reportedly influence mesenteric myogenic reactivity (255).
C. Extracellular Nucleotides/P2 Receptors and Medullary Blood Flow
Much of what is known about the roles of P2 receptors in influencing renal hemodynamics pertains to whole kidney renal hemodynamics or in vitro assessment of microvascular function in the renal cortex. There is comparatively little known about P2 receptor-dependent regulation of blood flow in the renal medulla, but P2 receptors could certainly have an influence on renal medullary perfusion and the concentrating mechanism for water and electrolyte homeostasis. Indeed, most of the data examining the impact of P2 receptor activation on renal microvascular function were obtained from afferent and efferent arterioles associated with juxtamedullary nephrons in the inner cortex. The efferent arterioles that exit these juxtamedullary glomeruli provide the blood supply to the vasa recta of the renal medulla. Consequently, P2 receptor-dependent effects in the upstream vasculature could have profound effects on renal medullary hemodynamics and tubular function. Intramedullary infusion of UTP can increase sodium excretion, possibly by activating P2Y2/4 receptors (171, 172). This natriuretic response was prevented by P2 receptor blockade with suramin, or by combined endothelin A/endothelin B receptor blockade, suggesting a novel endothelin and P2 receptor interaction (see sects. V and VI for details on P2 receptors and renal transport). In addition, this interaction may be influenced by ovarian hormones (170). Endothelin is a potent vasoactive peptide, so there may be important interactions between the endothelin and P2 receptor systems in the regulation of medullary perfusion.
Vasa recta are unique microvascular elements that do not possess a complete smooth muscle layer but, rather, more closely resemble capillaries with periodic distribution of smooth muscle-like pericytes along their length (261, 378–380). Pericytes can contract/relax in response to vasoactive agents to produce constriction/dilation of vasa recta diameter. Using an “intact kidney slice” preparation, Peppiatt-Wildman and co-workers (109, 111) showed that pericyte-mediated constrictions on the order of 10–30% could be recorded in response to vasoactive agents like endothelin, ANG II, or norepinephrine. In addition, tyramine-induced stimulation of norepinephrine and ATP release from adjacent sympathetic nerve terminals evoked pericyte-mediated constriction of the vasa recta that was reduced by ~50% in the presence of suramin, with the remainder likely reflecting adrenergic contributions (109, 111) (FIGURE 3A). The magnitude of the tyramine-induced vasoconstriction was considerably smaller than responses evoked by high concentrations of ATP (100 μM) (111). Further characterization of vasa recta reactivity to P2 agonists revealed that significant vasoconstriction could be detected at sites where pericytes were located compared with non-pericyte locations (FIGURE 4C). The rank order potency reported was benzylbenzyl-ATP = 2-methylthio-ATP > ATP = UTP. Pericyte contractions to ATP were blocked by suramin, PPADS, or Reactive Blue 2, whereas the response to UTP was blocked by suramin or Reactive Blue 2, but not by PPADS, potentially indicating a role for P2Y receptors in modulating medullary vascular resistance. Applying suramin or PPADS in the absence of P2 receptor agonists yielded a small but significant vasoconstriction, suggesting some tonic P2 receptor-dependent vasorelaxation due to endogenous nucleotides. P2 receptors expressed by vasa recta include P2X1, 3, and 7 and P2Y4 and 6 (110) (TABLE 4).
Extrapolating these in vitro findings to the in vivo setting, it was reported that acute renal nerve stimulation reduced total RBF, as well as cortical and medullary blood flow (laser Doppler flowmetry) in the rabbit kidney (133). Infusion of α,β-methylene ATP to desensitize P2X1 receptors did not alter the blood flow responses to renal nerve stimulation. Interestingly, when small boluses of ATP, β,γ-methylene ATP, or adenosine were infused into the renal artery, biphasic reductions in total and medullary blood flow were observed, followed by a hyperemic response (134). Under similar conditions, α,β-methylene ATP only produced a reduction in blood flow. These data indicated that in the rabbit kidney in vivo, α,β-methylene ATP-sensitive receptors mediate vasoconstriction presumably by activating P2X1 receptors, whereas β,γ-methylene ATP and ATP tend to produce a more sustained vasodilation mediated at least partly through adenosine receptors.
Studies in the anesthetized rat showed that suprarenal aortic infusion of ATP increased inner medullary blood flow in a NO-dependent manner in rats fed a low-salt diet (126). In contrast, ATP delivery to kidneys of rats fed a high-salt diet reduced outer and inner medullary blood flow (FIGURE 3A) and did so through a mechanism involving cytochrome P-450 metabolites. PPADS prevented the decline in medullary blood flow in response to ATP in high-salt rats, indicating the likely involvement of P2X receptors. More recently, the same group showed that intramedullary infusion of the P2X1 receptor antagonist MRS2159 increased inner medullary blood flow, suggesting that tonic activation of P2X1 receptors contributes to the regulation of medullary perfusion (285). From a similar perspective, but focusing on P2X7 receptors, Menzies et al. (338) demonstrated that infusion of AZ11657312, a P2X7 receptor antagonist, increased medullary blood flow and tissue oxygenation in ANG II-infused rats, but not control rats. This led to the conclusion that in the high ANG II setting, activation of P2X7 receptors reduced renal medullary perfusion and contributed to the renal injury induced by chronic ANG II (338) (see sect. VII). Further studies are needed to test the relevance of this interaction in response to endogenous ANG II, e.g., in response to a low salt intake.
D. Section Concluding Remarks
Recognition of the functional role of P2 receptors in the renal vasculature has expanded considerably in the last 20 plus years. What began with much skepticism at the prospect that ATP could serve as an extracellular signaling molecule has now grown into a large area of investigation into P2 receptor-dependent mechanisms in regulating microvascular and tubular (see below) function in physiological and pathophysiological conditions. FIGURE 3A highlights some of the effects of P2 receptor activation on the renal microcirculation. Separate control mechanisms are at work for regulating renal vascular function, and these systems can be compromised in disease states. More work is needed to resolve how P2 receptors influence whole kidney hemodynamics.
V. EXTRACELLULAR NUCLEOTIDES/P2 RECEPTORS AND TUBULAR FUNCTION
The lack of P2 receptor subtype-specific antagonists has made it difficult to define the functional contribution to tubular function regulation of extracellular nucleotides and their specific P2 receptor subtypes. Furthermore, nucleotides are degraded and interconverted to other nucleotides or nucleosides by ectonucleotidases, and thereby agonists are generated for other subtypes of P2 receptors or for adenosine P1 receptors. Nevertheless, insights on the involvement of specific P2 receptor subtypes can be gained due to their differences in agonist preference. For example, in rodents, only the P2Y2 receptors and P2Y4 receptors are activated to a similar extent by ATP and UTP, and in early studies target structures sensitive to both ATP and UTP were proposed to have functional evidence for “P2Y2-like” receptors (4, 60). This approach was applied to identify and characterize P2Y receptor subtypes in several renal cell lines and primary cell cultures of almost all nephron segments. Studies in MDCK cells especially, which have a distal tubular phenotype, have implicated roles for ATP and nucleotide receptors in regulating epithelial cell signaling and transport mechanisms (146, 227, 377, 408, 409, 513, 574). These insights triggered studies in intact nephrons. Studies on the effects of nucleotides in renal cell lines and cell cultures have been reviewed (228, 466, 521). More recently, the generation of gene knockout animals has provided invaluable tools to better define the function and relevance of P2 receptors. The role of P2 receptors in epithelial transport (53, 303, 468) and in renal epithelia (23, 415, 433, 465, 466, 521, 523) has been reviewed.
A. Extracellular Nucleotides/P2 Receptors in PT
1. P2Y receptor expression in PT
Autoradiographical studies in 1998 showed binding of the stable ATP analog ATPγS to the basolateral membrane of rat PT, which was competitively inhibited by UTP, suggesting the presence of P2Y2-like receptors (87), i.e., P2Y2 and/or P2Y4 receptor. In accordance, ATP or UTP added to the bath of isolated proximal convoluted tubules (including S1 segments) increased [Ca2+]i (21, 84). Subsequent studies demonstrated the presence of mRNA for P2Y2 receptors in rat proximal convoluted and straight tubules (21, 267) (see TABLE 4, FIGURE 2).
2. Activation of P2Y2-like receptors stimulates gluconeogenesis
Studies in freshly prepared rat renal cortical tubule suspensions showed that ATP increases [Ca2+]i and stimulates gluconeogenesis via P2Y receptor activation (83) (FIGURE 3B). Studies in isolated rat PTs revealed that UTP and the stable analog UTPγS stimulated gluconeogenesis, indicating a P2Y2-like receptor. Moreover, these responses were dependent on PLC activation and an increase in [Ca2+]i. Based on additional pharmacological maneuvers, the authors concluded that P2Y2 receptors rather than P2Y4 receptors contributed to stimulation of gluconeogenesis (343), but more direct evidence is needed.
3. Activation of P2Y receptors inhibits proximal tubular reabsorption
In primary rabbit renal PT cell cultures, ATP inhibited Pi uptake through a protein kinase C (PKC) and p38 mitogen-activated protein kinase (MAPK)-dependent mechanism, potentially mediated by P2Y receptor activation, since suramin (nonspecific P2 receptor antagonist) and RB-2 (P2Y receptor antagonist) blocked the effect (300). Studies in immortalized early PT primary cell cultures from the Wistar-Kyoto rat strain indicated that activation of a P2Y receptor (potency order was ATP > ADP >> β,γ-methylene ATP = UTP) acutely inhibited Na+-K+-ATPase, and this effect did not require an elevation of [Ca2+]i (249). Studies in polarized A6 cells (an amphibian cell line that forms a polarized and high-resistance epithelium) with stable transfection and expression of rat Na+/H+-exchanger 3 (NHE3) indicated that basolateral activation of P2Y1 receptors, which have a greater sensitivity to ADP than to ATP (4, 61), inhibited NHE3 activity by a cAMP/protein kinase A (PKA)-dependent mechanism (18). Subsequent studies using stationary in vivo microperfusion techniques in the rat PT indicated that activation of apical P2Y1 receptors inhibits bicarbonate reabsorption and potentially NHE3 activity (19) (FIGURE 3B).
4. Fluid shear stress induces ATP release to activate P2Y receptors and facilitate endocytosis in PT
The PT is the primary site for reabsorption of low-molecular-weight proteins by endocytosis. PT cells are known to rapidly modulate transport capacity in response to the fluid shear stress that accompanies changes in GFR. Studies in opossum kidney (OK) cells supported a model in which exposure to fluid shear stress increases apical endocytic capacity in proximal tubular cells (420). The underlying mechanism includes flow-induced ciliary bending and entry of extracellular Ca2+ via a ciliary-localized cation channel [possibly polycystin-2 (PC2)], which increases [Ca2+]i and triggers actin dynamics to facilitate endocytosis. Moreover, bending of the primary cilium also releases ATP to the apical surface, which by activation of P2Y receptors further increases [Ca2+]i (420) (FIGURE 6).
FIGURE 6.
Regulation of renal transport processes by flow-induced and cilium-mediated nucleotide release. Model of ciliary-activated release of ATP, which in turn activates P2X and P2Y receptors to increase intracellular calcium from endoplasmatic reticulum (ER) stores and stimulation of inositol trisphosphate (IP3) receptor-mediated enhancement of, in this example, apical endocytosis in the proximal tubule. 1) Exposure to flow-induced shear stress (FSS) bends the apical cilium, which causes entry of extracellular Ca2+ via a ciliary-localized cation channel [possibly polycystin-2 (PC2)] thereby increasing intracellular Ca2+ ([Ca2+]i). 2) Bending of the primary cilium causes release of ATP into the tubular lumen (via nucleotide transporters or other mechanisms) which in turn activates P2Y or P2X receptors in the apical membrane and increases [Ca2+]i. 3) The latter enhances the endocytic capacity at the base of microvilli, possibly by modulating actin dynamics to increase the size and volume of individual apical clathrin-coated pits. Addition of ATP bypasses the need for FSS in enhancing endocytic capacity. [Modified from Raghavan et al. (420) and Raghavan and Weisz (421), with permission from Wolters Kluwer Health, Inc.]
5. P2X receptor expression and function in PT
Immunohistochemical studies identified P2X6 receptors in the basolateral membrane and P2X5 receptors in the apical membrane of the rat proximal convoluted tubule (PCT), the latter being limited to the S3 segment (520). P2X4 protein expression has also been detected in the PCT, although the expression level is low and appears to be intracellular (520).
The physiological role of P2X receptors in the PT has not been unequivocally defined. Nevertheless, in isolated PTs, adding synthetic, nonselective P2X agonists to the bath reduced Na+-K+-ATPase activity by ~20%, which was inhibited by the nonselective P2 antagonist PPADS (239). These compounds infused in vivo increased sodium and lithium clearance, consistent with reduced sodium reabsorption in the PT (239). Moreover, this effect of P2X stimulation was enhanced by renal denervation (281). Similar natriuretic effects reportedly occur with intravenous infusion of P1,P4-diadenosine tetraphosphate (Ap4A) (486). Ap4A is also released from PT cells (244) and can activate both P2X4 and P2X5 receptors with high affinity (260).
In summary, available rodent studies indicate an overall inhibitory effect of both P2Y and P2X receptor activation on proximal tubular reabsorption. This may involve P2Y and P2X receptors in both the apical and basolateral membrane.
B. Extracellular Nucleotides/P2 Receptors in Thin Limbs of Henle’s Loop
1. P2Y receptor expression and function in thin limbs
Both thin descending limbs (tDL) and tAL of Henle’s loop of the rat express mRNA for the P2Y2 receptor (21) (TABLE 4). Accordingly, application of ATP, UTP, and ATPγS to these freshly isolated segments was equipotent in increasing [Ca2+]i, indicating the basolateral presence of P2Y2-like receptors in tDL and tAL (21). However, the functional relevance of P2Y receptors in the thin limbs of Henle is unknown.
2. P2X receptor expression and function in thin limbs
P2X4 and P2X6 receptors have been immunolocalized to the thin descending limb in the rat, but expression was not resolved to a specific membrane compartment (520), and the role of these receptors in this segment is also unknown.
C. Extracellular Nucleotides/P2 Receptors in TAL
1. P2Y receptor expression in TAL
Autoradiographical studies in rat TAL detected ATPγS binding sites in the basolateral membrane that were competitively inhibited by UTP, suggesting the presence of P2Y2-like receptors (20, 87). Accordingly, adding ATP or UTP to the superfusate was equally effective to transiently increase [Ca2+]i in mouse cortical TAL, while ADP had little or no effect (390), consistent with a basolateral P2Y2-like receptor. More recently, measurement of ATP- and UTP-induced changes in [Ca2+]i in perfused mTAL of P2Y2−/− and WT mice established a functional role of basolateral P2Y2 receptors (245). The same study showed that apical application of UTP or ATP increased [Ca2+]i in WT mice but never in P2Y2−/− mice. Other nucleotides such as UDP or ADP were without effect, suggesting that the P2Y2 receptor is the dominant P2 receptor expressed on the apical membrane of mouse medullary TAL (245). This study also showed that apical P2Y2 receptors promoted larger [Ca2+]i elevations compared with basolateral receptors, and provided evidence that P2Y2 receptor activation increases [Ca2+]i via release of Ca2+ from internal stores, and also activation of store-operated Ca2+ entry (245). The presence of P2Y2 receptor mRNA (21, 267) and protein (predominantly intracellular) (520) was demonstrated in medullary and cortical TAL in the rat, and P2Y6 receptor mRNA was detected in mouse TAL (327) (TABLE 4).
2. P2X receptor expression in TAL
In TAL, the P2X4 receptor was detected in the basolateral membrane only (245, 327). mRNA expression of P2X1 and P2X5 receptors was also found in TAL, but the function of these receptors is undefined (327).
3. P2Y and P2X receptor function in TAL
The first record of nucleotide-induced cellular effects in the TAL was reported in 1995 and described basolateral ATP- and UTP-triggered elevations of intracellular [Ca2+]i in nonperfused mouse cortical TAL (390). Subsequent molecular studies and functional work in isolated perfused medullary TAL (mTAL) from mice identified the above-described P2 receptors and characterized their localization to either the apical or basolateral side. The P2Y2 receptor functioned in the apical and basolateral membrane, while the P2X4 receptor was expressed only in the basolateral membrane (245, 327). When the mouse mTAL was used to study flow-stimulated increases in epithelial [Ca2+]i, it could be shown that flow-triggered [Ca2+]i responses were markedly reduced in mTAL from P2Y2−/− mice. These results fostered the concept of flow-stimulated nucleotide release followed by autocrine/paracrine stimulation of epithelial purinergic receptors (245). Intriguingly, P2Y2 receptor stimulation, either by increasing tubular fluid flow (72) or direct application of ATP/UTP, also stimulates tubular production of NO (475), and NO reportedly inhibits NaCl absorption in the mTAL (374). However, the main mTAL function, i.e., the absorption of NaCl, remained unaffected by stimulation of P2Y2 receptors or addition of NO donors (327, 328, 495). It may be that the mTAL is the source of P2Y2 receptor-stimulated NO production, but is not its target. Possibly, NO generated from the mTAL epithelium elicits its effect on the neighboring vasculature and is therefore involved in the regulation of medullary blood flow. Two studies found increased renal cortical and medullary expression of Na+-K+-2Cl− cotransporter (NKCC2) in P2Y2−/− mice (431, 579), associated in one study with a greater natriuretic response to the NKCC2 inhibitor furosemide compared with WT controls (431). A third study, however, could not confirm enhanced medullary NKCC2 expression in P2Y2−/− mice and, as outlined above, basal and ADH-induced salt transport in isolated perfused mTALs were unaffected by the absence of P2Y2 receptors (328). Thus the ATP/UTP/P2Y2 receptor system in mTAL may inhibit and limit NaCl reabsorption, but the inhibitory influence may be variable or small, not primarily due to effects on P2Y2 receptors in the TAL itself, or not captured in an isolated perfused TAL in vitro.
The situation contrasts sharply with distinct inhibitory NaCl transport effects in TAL after stimulation of P2X receptors (477), more specifically basolateral P2X4 receptors (327) (FIGURE 3B). The underlying P2X receptor-related intracellular signaling events remain unresolved, but are NO independent (495). Serendipitous results found that transport inhibition after P2X receptor stimulation is paralleled by a sizeable intracellular alkalization caused by activation of the apical NHE3 transporter (121). This points to a possible role of basolateral ATP in HCO3− reabsorption in the mTAL and potentially renal acid/base excretion. Surprisingly, P2X receptor-dependent NaCl transport inhibition is confined to the medullary part of the TAL, because almost no basolateral ATP effects on NaCl absorption could be observed in the cTAL (J. Leipziger and S. L. Svendsen, unpublished findings). Taken together, the medullary part of the TAL is subject to basolateral P2X4 receptor-mediated inhibitory transport regulation. Stimuli for ATP release include increased flow (72), as well as hypotonicity, the latter involving activation of the transient receptor potential cation channel TRPV4 osmosensor (476). Inhibitory effects of extracellular nucleotides may serve to limit transport work and oxygen consumption in the vulnerable mTAL, which may support and interact with the adenosine system in the metabolic control of TAL function (525), although the role of the apical and/or basolateral P2Y2 receptors in the TAL is not fully understood.
D. Extracellular Nucleotides/P2 Receptors in DCT
1. P2Y receptor expression and function in the DCT
By RT-qPCR, the presence of several subtypes of P2Y receptors was detected in an immortalized mouse DCT cell line, among which P2Y1, P2Y2, and P2Y6 were most prominent (159) (TABLE 4). Basolateral application of ATP to isolated rat DCT promotes a weak calcium transient (21). Stimulation of immortalized mouse DCT cells with ATP or UTP promoted Ca2+ transients and decreased the expression of NCC at both mRNA and protein levels (29, 159), indicating a role for a P2Y2-like receptor. Mouse DCT cells coexpressed P2Y2 receptors and NCC in the apical membrane, and specific siRNA-mediated silencing of P2Y2 receptors almost completely abolished ATP/UTP-induced Ca2+ transients, and significantly reduced ATP/UTP-induced decreases in NCC expression. The decrease in NCC mRNA on nucleotide stimulation was abolished in cells overexpressing the Ca2+-binding protein parvalbumin in the cytosol, and high [Ca2+]i induced instability of NCC mRNA. The authors concluded that P2Y2 receptor activation in immortalized mouse DCT by ATP or UTP increases [Ca2+]i, which downregulates NCC expression, at least in part, via destabilization of its mRNA (159) (FIGURE 3B). Downregulation by extracellular nucleotide-induced Ca2+ transients adds another level of complexity to the regulation of NCC expression in the distal nephron. Studies in P2Y2−/− mice reported that the acute natriuretic response to an NCC inhibitor was similar compared with WT mice (431). In contrast to mice, the highest expression of the nucleotide release channel Cx30 in the distal nephron and CD of the rat kidney was found in the apical membrane of the DCT (331). Further studies are needed to determine the quantitative relevance of extracellular nucleotides to the in vivo NCC function in rats and human, and under conditions of enhanced NaCl load and reabsorption by the DCT.
2. P2X receptor expression and function in the DCT
Based on RT-qPCR analyses, P2X4 and P2X5 receptors were the most highly expressed P2X receptors in an immortalized mouse DCT cell line (159). Mouse DCT expressed mRNA for P2X4 and P2X6 (119). The rat DCT was found to express mRNA for P2X4 and P2X6 receptors, which were immunolocalized to the basolateral membrane (520). Transient receptor potential melastatin type 6 (TRPM6) channels are the rate-limiting step for magnesium transport in the DCT. In an immortalized mouse DCT cell line, synthetic P2X receptor agonists inhibited magnesium uptake (116) (FIGURE 3B). Coexpression of the P2X4 receptor with TRPM6 caused a constitutive inhibition of magnesium currents in HEK293 cells (119). The P2X6 receptor did not interact with the TRPM6 current (119) in this bioassay, and P2X6 knockout mice show no abnormalities in the excretion of magnesium (120). The in vivo relevance of a potential inhibition of magnesium reabsorption by P2X4 receptor activation in the DCT remains to be determined.
E. Extracellular Nucleotides/P2 Receptors in CNT and CD
1. P2Y receptor expression in the CNT and CD
Autoradiographical studies suggested the presence of P2Y2-like receptors in the basolateral membrane of rat CD segments (87). Subsequent studies demonstrated the presence of P2Y2 receptor mRNA (267, 553) and protein (553) in rat CD (TABLE 4). The proposed localization of P2Y2 receptors to both apical and basolateral CD membranes is consistent with the expression pattern found in other epithelia (202, 445). P2Y14 receptors appear specifically and highly expressed in the apical membrane of ICs in the CD, where they can be activated by UDP-glucose, a DAMP molecule released by injured cells (17) (see sect. VII).
2. ATP via activation of P2Y2 receptors acts in an autocrine/paracrine manner to tonically inhibit ENaC open probability in mammalian CD
Regulation of the amiloride-sensitive ENaC in principal cells (PC) of the ASDN is essential for adapting renal sodium reabsorption to the body’s needs. In 1996, studies in cultured rabbit CNT and cortical CD (CCD) cells presented the first evidence that ATP inhibits ENaC-mediated sodium transport (279). Short-circuit current measurements in M-1 mouse CCD cells indicated that activation of P2Y2-like receptors in the apical and basolateral membrane by extracellular ATP inhibited amiloride-sensitive sodium absorption (112). The P2Y receptor agonist ATPγS reduced the open probability (Po) of ENaC in the apical membrane of A6 cells (318). Studies in isolated perfused CCD of mice, receiving a low-salt diet to enhance ENaC activity, also found that apical and basolateral application of ATP or UTP (100 µM) reduced amiloride-sensitive short-circuit currents by 30–50% and 20%, respectively (302). While ATP and UTP can increase [Ca2+]i in CCD cells (122, 441), their effect on ENaC was independent of changes in [Ca2+]i (279, 302, 508).
In vivo microperfusion experiments in rats kept on a low-sodium diet confirmed an inhibitory effect of apical ATP on sodium reabsorption in the CD (473). Moreover, plasma aldosterone levels and renal expression of the α-subunit of ENaC were reduced in mice lacking P2Y2 receptors, but whole kidney amiloride-sensitive sodium reabsorption was not different compared with WT mice (431). These in vivo findings supported the hypothesis that lack of P2Y2 receptor activation resulted in greater ENaC Po, while the observed compensatory decreases in circulating aldosterone concentrations and ENaC expression served to normalize net ENaC activity. The studies further revealed that P2Y2−/− mice required smaller increases in plasma aldosterone to adapt renal sodium excretion to a reduced dietary sodium intake, indicating a greater aldosterone sensitivity and facilitated renal sodium retention in the absence of P2Y2 receptors (431).
Patch-clamp studies in freshly isolated split-open CNTs and CCDs demonstrated that ATP decreases ENaC Po and activity in both mouse and rat PC. Moreover, UTP mimicked the effects of ATP and studies in P2Y2−/− mice indicated that the decrease in ENaC activity was primarily mediated by apical P2Y2 receptors (404, 405) (FIGURE 7). Concentration-response curves for the inhibitory effect of ATP and UTP on ENaC activity revealed IC50 values of 33 ± 2 and 49 ± 2 nM, respectively (487). The studies also provided the first evidence for a tonic regulation of ENaC in the CD via locally released ATP, inasmuch as ENaC resting Po and activity were greater in CNT/CCD from P2Y2−/− mice, and scavenging endogenous ATP and inhibiting P2Y receptors rapidly increased ENaC Po and activity in WT mice (404). Thus locally released ATP via activation of P2Y2 receptors acts in an autocrine/paracrine manner to tonically inhibit ENaC Po in mammalian CD. The molecular mechanism of the ATP release that inhibits ENaC in this segment includes Cx30, as discussed in section VIA3.
FIGURE 7.
P2Y2 receptor activation determines the inhibitory effect of ATP as well as of dietary salt on epithelial sodium channel (ENaC) open probability (Po) in connecting tubule (CNT)/cortical collecting duct (CCD). A: representative continuous current traces from cell-attached patches on principal cells (containing ≥2 ENaCs) before and after application of ATP (100 µM) in CNTs/CCDs harvested from wild-type (WT) and P2Y2−/− mice fed a regular 0.32% Na+ diet. Patches clamped to voltage potential of −60 mV and inward Li+ currents through ENaC are downward. Dashed lines indicate the respective current levels with c denoting the closed state. The summary graph on the right shows that ATP reduced ENaC Po, which was blunted in the absence of P2Y2 receptors. *P < 0.05 vs. wild type (WT). B: an increase in dietary NaCl intake for 1 wk reduced ENaC Po in WT. This effect was absent in P2Y2−/− mice. *P < 0.05 vs. WT. C: inhibition of local ATP signaling [hexokinase (+glucose) to degrade local ATP plus suramin to prevent P2 receptor activation] prevented the regulation of ENaC Po by dietary NaCl intake in cell-attached patches from WT mice. *P < 0.05 vs. WT control. [Modified from Pochynyuk et al. (405), with permission from FASEB; permission conveyed through Copyright Clearance Center, Inc.]
3. Inhibition of ENaC Po by P2Y2 receptors involves activation of PLC and hydrolysis of anionic phospholipids
Studies in the A6 cell line showed the reduction in Po of apical ENaC in response to the P2Y receptor agonist ATPγS involved the activation of PLC (318). Furthermore, inhibition of ENaC by PLC-coupled receptors in A6 cells involved hydrolysis of the anionic phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2). These effects were independent of ENaC trafficking (319). Application of total internal reflection fluorescence (TIRF) microscopy and a PIP2 reporter in mpkCCDc14 PCs revealed that ATP rapidly decreased plasma membrane PIP2 levels and ENaC Po with similar time courses (404). Furthermore, direct stimulation of PLC mimicked the ATP effect on ENaC in isolated CNT/CCD of WT mice, and decreased ENaC activity in the absence of P2Y2 receptors, indicating that signaling pathways downstream of the P2Y2 receptor were intact in CNT/CCD of P2Y2−/− mice (404). Studies using pharmacological tools and whole cell patch clamp of PCs of split-open CDs from sodium-restricted rats also indicated that PLC-dependent activation of P2 receptors (most likely the P2Y2 and/or P2Y4 subtype) inhibited ENaC activity (553).
In 2005, a mechanism was proposed for the regulation of ENaC by P2Y2-like receptors (288, 317) based on experiments in Xenopus oocytes and M-1 mouse CCD cells (FIGURE 8): under resting conditions the inner leaflet of the lipid bilayer contains a high concentration of PIP2, the latter binding the NH2 terminus of the β-subunit of ENaC, holding the ENaC channel open. Stimulation of P2Y2-like receptors activates PLC, which hydrolyzes and lowers the concentration of PIP2 with resultant decreases in PIP2 binding to the NH2 terminus of β-ENaC. The latter lowers ENaC activity by decreasing Po. A role for the cytosolic NH2 terminus of β-ENaC for PIP2 regulation is consistent with previous studies (573).
FIGURE 8.
The inhibition of epithelial sodium channel (ENaC) open probability (Po) by dietary salt is mediated by the ATP/UTP/Cx30/P2Y2 receptor system in the aldosterone-sensitive distal nephron. Low salt intake enhances aldosterone concentrations and suppresses apical release of ATP and UTP. This reduces activation of apical P2Y2 receptors on principal cells (PC), and the inner leaflet of the lipid bilayer maintains a high concentration of negatively charged phosphatidylinositol 4,5-bisphosphate (PIP2), which binds to positively charged domains of the NH2 terminus of the β-subunit of ENaC, thereby keeping the ENaC channel open to reabsorb Na+. High salt intake enhances the apical release of ATP and UTP, at least in part via connexin30 (Cx30) in intercalated cells (IC). The nucleotides activate P2Y2 receptors and, thereby, phospholipase C (PLC), which hydrolyzes and lowers the concentration of negatively charged PIP2. This releases the NH2 terminus of β-ENaC and induces a conformation change that lowers ENaC Po and activity, thereby increasing Na+ excretion. See text for details. IP3, inositol trisphosphate. [Modified from Vallon and Rieg (527).]
4. A proposed role for P2Y receptors in stimulating chloride secretion in CD
Studies in an Ussing chamber system and a mouse inner medullary CD cell line (mIMCD-K2) expressing P2X and P2Y receptors on the apical plasma membrane provided evidence that nucleotide agonists inhibited sodium reabsorption, but stimulated chloride secretion (330) (FIGURE 3B). Similar studies performed during inhibition of ENaC indicated that activation of basolateral P2Y1 and P2Y2 receptors, as well as apical P2Y2 receptors, can induce chloride secretion through both PLC and intracellular Ca2+ to activate Ca2+-activated chloride channels (CACC) (422). The effect on chloride secretion may complement the inhibitory effect on ENaC to enhance urinary NaCl excretion. Using the same setup, extracellular ATP also stimulated chloride secretion in a mouse CCD cell line [mpkCCD(c14)] (423).
5. A proposed role for P2Y2 receptors in the regulation of potassium secretion in the ASDN
In vitro studies in clonal kidney cells from African green monkey suggested that the activation of P2Y2 receptors can increase the activity of big-conductance Ca2+-activated potassium (BK) channels (186) (FIGURE 3B). The BK channel is of particular interest because it contributes to both basal and flow-induced renal potassium secretion (434). Accordingly, an increase in tubular fluid flow may increase apical ATP release, which activates P2Y2 receptors and increases [Ca2+]i (245) to stimulate potassium secretion via the BK channel in the ASDN, known to be activated by a rise in [Ca2+]i (403, 434, 560). Moreover, flow-induced ATP release in CD PCs involves the primary cilium (205), and studies in MDCK cells showed that the increased [Ca2+]i associated with bending of the primary cilium activates BK channels (412). P2Y2−/− mice reportedly exhibit reduced renal outer medullary potassium channel (ROMK) expression in kidney cortex (412).
In comparison, patch-clamp studies of the apical membrane in split-open mouse CCD implicated a P2Y2-like receptor as a negative regulator of renal potassium secretion, inasmuch as both ATP and UTP inhibited the small-conductance potassium channel in PCs (314) (FIGURE 3B). The underlying signaling cascade may include an increase in protein kinase G-sensitive phosphatase activity that dephosphorylates the channel (314). P2Y2−/− mice have normal total renal excretion of potassium, despite lower plasma concentrations of both potassium and aldosterone, which are primary stimulators of renal potassium excretion (431). This provided indirect evidence for facilitated renal potassium excretion in P2Y2−/− mice on a standard potassium diet. Since net ENaC activity seems normal in P2Y2−/− mice, greater ENaC activity is unlikely to explain this effect. However, it would be consistent with the proposed inhibitory influence of P2Y2-like receptors on the small-conductance potassium channel in PCs discussed above. Facilitation of urinary potassium excretion in P2Y2−/− versus WT mice disappeared during high potassium intake (431). This may reflect increasing contributions of P2Y2 receptor-stimulated BK channels under these conditions, but further studies are needed to provide more direct evidence for the relevance of extracellular nucleotides and P2 receptors in the regulation of renal potassium handling.
6. P2X receptor expression in the CD
Immunohistochemical studies identified P2X4 and P2X6 receptors on the basolateral membrane of rodent CDs (520). They may exist as heteromers, together with a wide range of P2X receptors identified in the CD (25). Immunohistochemistry established expression of P2X1 (only in ICs of sodium-restricted rats), P2X2, P2X4, P2X5 and P2X6 receptors in CCD and medullary CD (520, 553). In IC, using an enrichment technique based on enhanced green fluorescent protein (EGFP) expression driven by the promoter of the H+-ATPase B1 subunit in α-IC, higher P2X4 receptor mRNA expression was found in cells of the medulla compared with the cortex, whereas P2X5 receptor expression was similar in cortex and medulla (17). P2X4 and P2X6 receptors were found in both apical and basolateral membranes of PC, whereas staining for P2X2 and P2X5 receptors appeared to be ‟intracellularˮ (553). In the mouse, immunohistochemistry localized P2X1 and P2X4 receptors to the apical membrane of medullary CD cells (306). Analysis of the human kidney transcriptome found that of tags for 258 genes conferring transport properties, the only P2X receptor detected in significant amounts in the CD was the P2X4 receptor (85). Studies in vitro suggest that P2X4 receptor activation also inhibits ENaC (473, 553), and studies in a P2X4 receptor null mouse suggest that this receptor may be important in the modulation of sodium transport by aldosterone (107). On the other hand, activation of basolateral P2X4 receptors has been found to stimulate ENaC activity in Xenopus CCD A6 cells (507).
F. Section Concluding Remarks
Both P2X and P2Y receptors are expressed along the nephron and collecting duct system of the kidney. The functional relevance of these receptors is starting to emerge (FIGURE 3B). Much has been learned about the role of P2 receptors in the regulation of renal transport in the TAL and in the ASDN. In both segments, P2 receptor activation has an inhibitory influence on sodium reabsorption. In particular, the contribution of the ATP/UTP/P2Y2 receptor system in the regulation of the open probability of the epithelial sodium channel ENaC in the ASDN has been well established. Implications for body homeostasis are discussed in the following section.
VI. EXTRACELLULAR NUCLEOTIDES/P2 RECEPTORS AND INTEGRATED KIDNEY FUNCTIONS
A. Sodium Homeostasis, Pressure Natriuresis, and Blood Pressure
As outlined in section IV, extracellular nucleotides and P2 receptors have well-established effects on renal hemodynamics, including autoregulation of GFR and RBF, tubuloglomerular feedback, and the regulation of medullary blood flow. By regulating these processes and mechanisms, extracellular nucleotides and P2 receptors have the potential to affect sodium homeostasis, pressure natriuresis, and blood pressure. Where links between specific P2 receptors in the vasculature and these parameters have been established, this has been outlined in section IV. The following discussion is focusing primarily on the role of extracellular nucleotides and P2 receptors in the tubular and collecting duct system.
1. Dietary salt inhibits the open probability of ENaC in CNT/CCD by enhancing apical P2Y2 receptor activation
ENaC is regulated by protein trafficking (71), which determines the number of ENaCs in the apical membrane of the ASDN, as well as by controlling ENaC Po, which is mediated by proteolytic cleavage (275). Patch-clamp studies in freshly isolated mouse CNT/CCD showed that ENaC Po is negatively regulated by dietary salt (405, 524), i.e., an increase in dietary salt intake lowers ENaC Po consistent with a contribution to sodium homeostasis. Furthermore, absence of P2Y2 receptors in mice did not affect the inhibitory effect of dietary salt on the number of ENaCs per patch (N), but prevented the reduction in ENaC Po in response to increasing salt intake (FIGURE 7) (405). Switching WT mice from control to high-salt diet lowered ENaC activity (product of N and Po) by 76%, whereas ENaC activity was only reduced by 44% in P2Y2−/− mice. As a consequence, ENaC activity was 150% higher in P2Y2−/− versus WT mice on a high-salt diet (405). Similar effects on ENaC Po were observed with apical ATP scavenging or pharmacological inhibition of apical P2Y-receptors in WT mice (FIGURE 7). These studies demonstrated that an increase in dietary NaCl inhibits renal sodium reabsorption by activating the apical ATP/UTP/P2Y2 receptor system in the ASDN, which reduces ENaC Po (405) (FIGURE 8).
In accordance, the same study showed that increasing dietary salt intake was associated with increased urinary excretion of UTP as well as the ATP hydrolytic product ADP, which may reflect greater ATP release in the ASDN followed by hydrolysis to ADP (see sects. II and III and FIGURE 1) (405). Urinary ATP showed the highest urinary levels in response to a high-salt diet, but the values were more variable and lower than levels of UTP and ADP (405). Subsequent studies could detect a significantly enhanced urinary ATP excretion in response to a high-salt diet (487). Thus increasing dietary salt increased the urinary concentrations of both primary P2Y2 receptor agonists (FIGURE 8). Furthermore, the urinary concentrations of both ATP and UTP were within the range of concentrations shown to alter ENaC activity in freshly isolated split-open CNT/CCD (487).
2. The relevance of the ATP/UTP/P2Y2 receptor system for salt homeostasis, blood pressure regulation, and aldosterone escape
P2Y2−/− mice presented with salt-resistant hypertension (431). However, and in contrast to WT mice, heart rate was reduced and inversely related to salt intake in P2Y2−/− mice, indicating activation of baroreceptors. Moreover, plasma aldosterone levels were consistently lower in P2Y2−/− mice (431). Thus the hypertension in P2Y2−/− mice appears “salt resistant” because the baroreceptor response to changes in salt intake is intact, thus allowing maintenance of blood pressure by lowering heart rate and increasing salt excretion by lowering sympathetic nerve activity and aldosterone (431).
P2Y2−/− mice have increased blood pressure but amiloride-sensitive sodium excretion is not enhanced compared with WT mice on a regular-NaCl diet (431). Lower plasma aldosterone concentrations can explain the lower renal αENaC expression observed in P2Y2−/− mice, which normalized net ENaC activity despite greater ENaC Po (405). Accordingly, preventing the suppression of mineralocorticoid tone in P2Y2−/− versus WT mice by application of DOCA unmasked a greater net ENaC activity and NaCl sensitivity of blood pressure compared with WT mice (405). DOCA also sensitized expression of active ENaC in the apical membrane to a P2Y2 receptor-mediated inhibitory influence, indicating that the ATP/UTP/P2Y2 receptor system can affect ENaC beyond regulation of its Po (405).
The above and subsequent studies (405, 487) expose a prominent role of P2Y2 receptors in suppressing ENaC activity when sodium transport is activated by high levels of mineralocorticoids in combination with high NaCl intake as observed in aldosterone escape. Many other factors can regulate ENaC and affect salt balance (e.g., NO, endothelin), and further studies are needed to establish the relationship between these systems and P2Y receptor activation.
Confirming the natriuretic potential of extracellular ATP, mice overexpressing human CD39, an ectonucleotidase that hydrolyzes extracellular ATP/ADP to AMP (see sect. IIB and FIGURE 1), showed evidence for an impaired natriuretic response to a high-salt diet and concomitant aldosterone infusion, including potential dysregulation in the expression of NKCC2 and NCC (586). Proposed dysregulation of NKCC2 is reminiscent of enhanced renal NKCC2 expression observed in P2Y2−/− mice (431, 578).
Mineralocorticoid tone also induces NaCl appetite or intake, i.e., when treated with DOCA and given the choice of water or NaCl solution, WT mice prefer to drink the NaCl solution, whereas P2Y2−/− mice preferred tap water. The underlying mechanism remains unclear, but the lower NaCl intake likely prevented an even greater increase in blood pressure in P2Y2−/− mice (405). The molecular mechanisms that contribute to mineralocorticoid effects in the ASDN are similar to those involved in salt appetite in the brain (156). The roles of extracellular nucleotides and P2Y2 receptors in the brain for salt balance and blood pressure regulation remain to be determined.
3. Cx30 contributes to the dietary salt-induced increase of luminal ATP that inhibits ENaC in CNT/CCD and prevents blood pressure increase
The molecular mechanisms involved in regulation of ATP/UTP concentrations at the apical membrane of the ASDN may include changes in secretion or breakdown. The mechano-sensitive gap junction protein Cx30 purportedly functions as a hemichannel permitting apical release of ATP in murine CD (478). Accordingly, flow-induced ATP release and [Ca2+]i oscillations in CDs were attenuated, and urinary ATP excretion was reduced under control diet in Cx30−/− versus WT mice (487, 496). Importantly, the increase in urinary ATP observed in WT mice in response to high salt intake was largely prevented in Cx30−/− mice, supporting the concept that Cx30 expression, directly or indirectly, contributed to regulation of renal epithelial ATP release by dietary salt (487).
Patch-clamp studies in isolated split-open CNT/CCD in Cx30−/− mice revealed that ENaC activity and ENaC Po remained sensitive to changes in dietary salt intake, but ENaC Po remained greater on normal and high salt intake, and total ENaC activity was greater on high-salt diet in Cx30−/− versus WT mice (340). Moreover, Cx30−/− mice showed a salt-sensitive increase in blood pressure that was prevented by the ENaC inhibitor benzamil (478). Overall, these findings implicated a critical role for Cx30 in the local ATP release and inhibition of ENaC in the ASDN by dietary salt (FIGURE 8). Cx30 is also required for normal DOCA escape of ENaC (340). These data mirror those observed in P2Y2 knockout mice, the P2 receptor mediating ENaC inhibition (302, 404, 523). In both, the Cx30−/− and the P2Y2−/− mouse, the local apical ATP-mediated regulation of ENaC appears to malfunction, and this is associated with increased ENaC activity. These observations are of great importance because they point to a first molecular ATP release mechanism in renal epithelial cells.
In contrast to Cx30−/− mice, mice lacking P2Y2 receptors have completely lost the downregulation of ENaC Po by dietary salt or the upregulation of ENaC Po by aldosterone. This indicates the existence of additional, Cx30-independent pathways of ATP or UTP release, potentially including Cx30.3 and Cx37, which both appear to be expressed in the distal nephron. It remains to be determined whether they contribute to nucleotide release and/or formation of heteromers with Cx30 (189), and whether Cx30 is an actual conduit for ATP secretion or a regulator of nucleotide release.
4. Role of nucleotide release and P2 receptors in pressure natriuresis
Pressure natriuresis plays an important role in regulating salt homeostasis (231). In addition to hemodynamic factors such as medullary blood flow and renal interstitial hydrostatic pressure, the underlying mechanism includes inhibitory effects on proximal and distal tubular transport, in part mediated by paracrine factors, including extracellular nucleotides (586). Microdialysis experiments indicated a positive relationship between renal artery perfusion pressure and the concentration of ATP in the interstitial fluid of the kidney cortex (357, 382). As mentioned in section V and FIGURE 3B, extracellular ATP can inhibit some of the key sodium transporters in the PT (18, 19, 249, 300). This natriuretic effect of ATP is supported by its inhibition of sodium transport in the distal nephron, where physical stimulation promotes ATP and UTP secretion to inhibit ENaC (527), and thus extracellular nucleotides may contribute to pressure natriuresis. Furthermore, pressure-induced natriuresis was strongly inhibited in Cx30−/− mice (478), implicating Cx30-mediated ATP release in pressure natriuresis. The mechanical stimulation and opening of Cx30 hemichannels may be induced by increases in interstitial pressure or tubular fluid flow and could involve the sensing of shear or hydrodynamic impulses by primary cilia (e.g., in PT or PC) and microvilli (e.g., in IC) (312).
Recent studies suggested another pathway for inhibition of renal sodium reabsorption by nucleotide receptor signaling during pressure natriuresis: in inner medullary CD cells (IMCD3 cells), an increase in flow, via polycystin-2 and P2X7 and P2Y2 receptors, engages Ca2+-dependent signaling pathways that stimulate endothelin-1 synthesis (385), an autocrine inhibitor of sodium and water reabsorption. Consistent with this postulate is the finding that acute antagonism of P2X7 receptors improved the pressure natriuresis relationship and medullary perfusion in ANG II-treated rats (338).
5. Functional implications of nucleotide release from intercalated cells
Notably, the renal expression of rabbit and murine Cx30 appears largely restricted to ICs of the CNT/CCD (331), indicating a potential role of these cells in the regulation of apical ATP release (FIGURE 8). As noted in section IIB, the apical expression of ecto-ATPases and 5′-ectonucleotidase (converts AMP to adenosine) appears primarily localized to intercalated cells, though expression levels are rather low. The relevance is unclear but could indicate that the luminal availability of ATP/UTP in these segments is regulated by secretion and local breakdown of these nucleotides by ICs. In comparison, high apical membrane abundance of Cx30 was found in rat kidney continuously from the medullary TAL to the medullary CD system, including PC, with the highest level in the DCT (331) indicating significant species differences and the need to define the expression pattern of these systems in the human kidney. The prominent expression of Cx30 in rat DCT may support a role of apical P2Y2 receptor activation in the suppression of NCC expression (159) (see sect. VD).
Expression of P2Y2 receptors was detected in mouse ICs (17), which agrees with pharmacological studies showing functional P2Y2 receptors in the apical membrane of ICs in rabbit CCDs (561) (TABLE 4). Functional evidence for ATP-mediated signaling is reported for mouse β-ICs, presumably via apical and basolateral P2Y2 receptors, leading to paracrine release of PGE2 that inhibits sodium and water transport in adjacent PC. This seems to depend on normal function of the H+-ATPase and is a proposed mechanism to explain the sodium and potassium losses often seen in patients with distal renal tubular acidosis (182). The roles of P2Y14 receptors in ICs and inflammation are discussed in section VII.
6. What regulates the release of ATP/UTP in DCT/CNT/CD in response to salt intake or during aldosterone escape?
As outlined above, Cx30 in ICs potentially contributes to ATP/UTP release triggered by dietary salt. Nonetheless, PCs in the ASDN (205) as well as upstream tubular segments, including DCT (165, 245, 331, 531), are additional potential sources of nucleotide release following an increase in salt intake. Cell swelling can induce tubular and CD ATP release and the apical monocilia in the CD may sense tubular fluid flow to trigger ATP release (165, 245, 444, 478) (similar to FIGURE 6). ENaC-mediated sodium uptake in PCs is expected to be reduced in response to a high-salt diet, which should reduce rather than increase cell volume. If an increase in dietary salt intake is associated with an increase in GFR and thus the filtered salt load to the tubule, then due to glomerulotubular balance the absolute salt reabsorption in most tubular segments (including the PT) is expected to be increased, which may enhance cell volume. Whether a high-salt diet induces a sustained cell volume increase in ICs to trigger ATP/UTP release via Cx30 remains to be determined. Furthermore, Cx30 can mediate flow-induced ATP release from the CD (478), and increased salt intake associates with a higher flow through the tubular segments and CD, although not all changes in flow through the CD are associated with enhanced urinary ATP excretion (433).
Alternatively or in addition, hormones that regulate salt homeostasis may directly or indirectly control the release and possibly the breakdown of ATP or UTP in the ASDN. In the isolated perfused rat kidney, ANG II induced rapid release of ATP via the AT1 receptor (384). Studies in A6 cells have shown that mineralocorticoids enhance ENaC Po (128, 259, 307). As discussed above, results obtained in P2Y2−/− mice are consistent with the notion that mineralocorticoids increase ENaC Po by suppressing the inhibitory influence of P2Y2 receptor activation (405). It remains to be tested whether mineralocorticoids also reduce ATP/UTP release or enhance their breakdown (FIGURE 8). In mineralocorticoid escape (e.g., DOCA plus high-salt diet) ATP and/or UTP release could also reflect increases in cell volume (431, 433, 504, 543) as a consequence of DOCA-induced overactive sodium transport and/or increases in tubular fluid flow.
B. Water Homeostasis
1. Regulating water reabsorption in the kidney
Water accounts for ~60% of the total body weight. Humans filter ~180 L of water per day in the kidneys, with more than 99% being reabsorbed along the tubular and CD system, such that ~1.5 L are excreted daily as urine. However, the kidneys can vary the amount of water excreted based on the needs of the body, making this route the only one with regulated water excretion, as compared with water loss through feces, lungs, or skin. The neurohypophysial hormone arginine vasopressin (AVP), acting through the V2 receptor-cAMP pathway, is central to fine tuning of water excretion by the kidneys and thus for maintaining water homeostasis (40, 449, 557) (FIGURE 9). The AVP-V2 receptor-cAMP pathway represents the primary hormonal system that increases urinary concentrating ability, acting on the TAL, CNT, and CD. However, a number of intrarenal autocrine or paracrine agents also regulate renal handling of water, especially at the levels of the CNT and CD. These include, but are not limited to, endothelin, prostaglandin E2 (PGE2), and extracellular nucleotides. These agents, acting through their respective G protein-coupled receptors, counter the action of AVP by decreasing cellular cAMP levels (271, 276, 372, 433) (FIGURE 9). It appears that the balance between cAMP and phosphoinositides in the CNT and CD is critical in determining water absorption. In response to changes in plasma osmolality, rapid adjustments in renal reabsorption of free water are necessary, but AVP has a relatively long half-life of 10–35 min (113). Therefore, local mechanisms fine tune CD water transport to bodily needs until changes in AVP levels can rebalance water status. In addition, local factors may attenuate AVP actions to protect CD cells from excessive perturbations in cell volume as a consequence of rapid changes in extracellular osmolality in the renal inner medulla. Increases in CD cell volume may release local factors and serve as a sensor of changes in plasma osmolality to accelerate AVP responses as well to maintain cell volume and integrity (see below). There are two well-studied nucleotide receptors that affect water transport in the CNT and CD, namely, the P2Y2 and P2Y12 receptors, as discussed in the following.
FIGURE 9.
A proposed model for cell volume-dependent ATP release and water transport inhibition by P2Y2 receptor activation in inner medullary collecting duct (IMCD) cells. Arginine vasopressin (AVP) activates vasopressin V2 receptor (V2R) to stimulate aquaporin-2-mediated water entry which increases cell volume. The latter increases basolateral and apical release of ATP (and potentially UTP?) by unknown mechanisms. P2Y2 receptor activation inhibits water reabsorption via multiple signaling pathways (see text for details) and stimulates volume regulatory potassium channels as shown in other cell types. In response to water loading, the ATP/UTP/P2Y2 receptor system maintains cell volume, which tends to increase by the relative hypotonic extracellular fluid, and accelerates the excretion of free water before circulating AVP levels drop. AC, adenylyl cyclase; AQP2, -3, -4, aquaporin-2, -3, -4; COX1, cyclooxygenase 1; CREB, cAMP responsive element binding protein; Cx37, connexin 37; DAG, diacylglycerol; EP3, PGE2 E3 receptor; ET-1, endothelin-1; ETB, endothelin B receptor; Gi, inhibitory G protein; Gq, Gs, stimulatory G protein; IP3, inositol trisphosphate; P256, P261, phosphorylation of AQP2; Panx1, pannexin 1; PGE2, prostaglandin E2; PLC, phospholipase C; PKA, protein kinase A; PKC, protein kinase C; Ub, ubiquitin; UT-A1, urea transporter A1. [Modified from Vallon and Rieg (527).]
2. P2Y2 receptor activation inhibits water reabsorption in the CD
The first evidence for a potential role of extracellular nucleotides and P2Y receptors in regulating CNT/CD water reabsorption came from isolated microperfusion experiments, where ATP added to the basolateral side of medullary CDs decreased the AVP-stimulated water absorption in a PKC-dependent fashion (265, 441). ATP also decreased AVP-stimulated cAMP production in freshly isolated medullary CDs of rats (265). Subsequently, with the use of a peptide-derived antibody specific for P2Y2 receptors, the receptor protein has been localized to the apical and basolateral domains of the CD, along with other structures of the rat kidney (267). Later it was shown that basolateral but not apical ATP inhibits AVP-stimulated water transport in the CD (129). Studies in a highly differentiated mouse clonal CCD principal cell line (mpkCCD) indicate that ATP counteracts AVP-induced water permeability by increasing aquaporin-2 (AQP2) degradation in lysosomes, preceded by ubiquitin-dependent internalization, and by decreasing AQP2 gene transcription by reducing AVP-induced cAMP levels. Moreover, ATP also increased AQP2 phosphorylation at S261, which followed ubiquitination, although this was not essential for hormone-induced AQP2 degradation (41). Studies in the same cell line indicated a role for P2Y2 or P2Y4 receptors in the ATP effects on AQP2 since UTP yielded the same results as ATP, and these studies proposed a role for an apical receptor (551) (FIGURE 9).
In line with a role of the P2Y2 receptor, P2Y2−/− mice exhibited a distinct renal water handling phenotype. Under basal conditions with free access to water and food, P2Y2−/− mice do not show abnormalities in urine concentration, but, when they are given gelled diet as the sole ration to limit water intake in relation to calorie intake, P2Y2−/− mice significantly concentrated the urine more with increased AQP2 water channel protein abundance, despite no differences in circulating AVP levels compared with WT mice (587). Studies in freshly isolated IMCD showed that ATPγS enhanced the EC50 for the stimulation of cAMP formation by the V2 receptor agonist 1-desamino-8-d-arginine vasopressin (dDAVP) in WT mice, but not in P2Y2−/− mice (431). To determine the ambient contribution of V2 receptor activation on water transport in WT and P2Y2−/− mice, acute responses to the V2 receptor antagonist SR121463 were assessed. In WT animals, SR121463 increased urine flow and electrolyte free water clearance (Cle-H2O). These changes in WT mice were associated with reduced urinary ATP, implying a tonic stimulation of ATP release by V2 receptor activation. In P2Y2−/− mice, SR121463 elicited a significantly greater diuresis and Cle-H2O compared with WT, indicating greater basal reabsorption of fluid in the CD of P2Y2−/− mice, associated with greater renal AQP2 expression in the latter group (431). Together these data support the concept that P2Y2 receptor activation inhibits the cAMP-mediated effects of AVP on AQP2 and water transport in the CD (FIGURE 9).
How is this local ATP-P2Y2 receptor system activated? ATP release in the CD is triggered by changes in tubular fluid flow, cell volume, and AVP stimulation (see sect. II). IMCDs respond to AVP stimulation with an increase in cell volume (162). Cells of various organs respond to changes in cell volume with a release of ATP (148, 543). Measuring urinary ATP excretion in P2Y2−/− and WT mice under two different conditions provided indirect evidence for volume-dependent ATP release in CD in vivo (431, 527). V2 receptor blockade and acute water loading both increased urine flow but had opposite effects on ATP excretion. The authors proposed that 1) urine flow alone is not a good predictor of urinary ATP excretion; 2) acute water loading increases ATP release, which may reflect increases in CD cell volume due to a reduction in extracellular tonicity; 3) acute pharmacological blockade of V2 receptors reduces cell volume by blocking the apical water entry, thus off-setting the basal, AVP-, and cell volume-induced ATP release; and 4) urothelial cells of the lower urinary tract, which were exposed to similar increases in fluid flow and therefore similar distension and hypotonicity, may not play a dominant role in urinary ATP excretion. Moreover, consistent with cell volume regulation of the local ATP-P2Y2 receptor system, urinary ATP excretion was similar in P2Y2−/− and WT mice during inhibition of water transport by V2 receptor blockade but modestly greater under basal conditions and much greater in response to acute water loading in P2Y2−/− compared with WT. This has been discussed and illustrated in Reference 527.
Another important aspect of P2Y2 receptors in the kidney is the nature of their interactions with AVP and the prostanoid system. Briefly, addition of ATPγS or UTP to rat IMCDs enhanced the production of PGE2, and the response was greater in hydrated rats (sucrose water drinking) and suppressed in dehydrated rats (water deprivation) (492). This was associated with higher expression of P2Y2 receptor mRNA and protein expression in IMCDs of hydrated versus dehydrated rats (270). Chronic infusion of dDAVP in rats decreased P2Y2 receptor protein abundance in IMCD, associated with lesser ATPγS-stimulated production of PGE2 (493). Notably, the ATPγS-induced production of PGE2 in IMCD was significantly enhanced following induction of lithium-induced nephrogenic diabetes insipidus (NDI) or postobstructive uropathy (577, 580). Increased renal PGE2 production is implicated in AVP resistance in NDI and could reflect increased P2Y2 receptor activity. Furthermore, ATP can inhibit endothelin-1 production by rat IMCD independent of cyclooxygenase or NO (209). These interactions among different systems in the IMCD microenvironment may impact CD water reabsorbing capability in health and disease (264, 271).
ATP has a short extracellular half-life of <5 min and can be broken down to form adenosine, another well-established local regulator of renal water transport (for review, see Ref. 525). Integrative aspects of water transport regulation in the CD by ATP and adenosine have been reviewed (433).
3. Activation of P2Y12 receptors increases AVP expression and effects on the CD
Also known as the platelet ADP receptors, P2Y12 receptors are phylogenetically different from the P2Y2 receptor (see sect. III). ADP preferentially activates the P2Y12 receptor to inhibit adenylyl cyclase and reduces cellular cAMP levels. Recently, P2Y12 receptor protein expression was detected in rodent CDs (582, 583). The availability of the United States Food and Drug Administration (FDA)-approved thienopyridine class of antithrombotic drugs that selectively and irreversibly block the P2Y12 receptor, such as clopidogrel or prasugrel, has immensely advanced understanding of the physiological and pathophysiological roles of P2Y12 receptors. In normal rats, clopidogrel bisulfate significantly increased urine osmolality and AQP2 protein abundance in the kidney in an AVP-dependent fashion. Administration of clopidogrel or prasugrel also elevated urinary excretion of AVP (582). The AVP dependency of the inhibitory effect of P2Y12 receptor blockade on urine concentration was further confirmed in Brattleboro rats, which genetically lack AVP and were unresponsive to clopidogrel bisulfate (582). In vitro experiments in primary cultures of rat IMCD cells revealed that PSB-0739, a potent and reversible antagonist of P2Y12 receptors, had no effect on AQP2 gene expression in the absence of dDAVP, a synthetic analog of AVP. But PSB-0739 significantly enhanced the effects of dDAVP on the medullary CD in terms of AQP2 gene expression and cAMP production (582). Moreover, treating primary cultures of rat hypothalamic cells with PSB-0739 significantly increased AVP gene expression (582). Thus these studies suggested that P2Y12 receptor blockade has dual effects with regard to AVP and renal water handling: it increases the production of AVP in the hypothalamus and enhances AVP’s effects in the CD. These two actions potentiate each other. Accordingly, preliminary data showed that P2Y12−/− mice have higher urinary cAMP levels, associated with greater dDAVP-induced AQP2 mRNA expression and cAMP production in primary cultures of IMCD cells in P2Y12−/− mice versus WT (268).
4. Pathophysiological implications of the regulation of CNT/CD water transport by nucleotides
Studies on the pathophysiology of P2Y2 receptors have been severely hampered by the lack of FDA-approved selective receptor antagonists. Recently, AR-C118925, a selective and potent P2Y2 receptor antagonist, has been made commercially available as an experimental compound (419), although its bioavailability and pharmacokinetics need to be further established. In view of this limitation, the most unequivocal evidence regarding the role of P2Y2 receptors in physiology and pathophysiology comes from studies in knockout mice. As discussed in section VIIC, P2Y2−/− mice are resistant to lithium-induced polyuria (584). In comparison, the availability of FDA-approved P2Y12 receptor antagonists critically helped to define the role of this receptor in lithium-induced nephrogenic diabetes insipidus (264, 581–583).
D. Section Concluding Remarks
The physiological role of cellular nucleotide release and the subsequent activation of P2 receptors is increasingly appreciated. This includes the dietary salt-dependent regulation of ENaC through the apical ATP/UTP/P2Y2 receptor system in the CNT/CCD, which affects blood pressure. The identification of Cx30-mediated nucleotide release in response to dietary salt advanced the molecular understanding of renal epithelial nucleotide release pathways and provides opportunities to better define signals and signaling cascades implicated in the regulation of nucleotide release in the kidney. Likewise, the identification of key roles for P2Y2 and P2Y12 receptors in the AVP-regulated renal water transport critically expanded the understanding of renal transport physiology.
VII. EXTRACELLULAR NUCLEOTIDES/P2 RECEPTORS: ROLE IN RENAL PATHOPHYSIOLOGY AND THERAPEUTIC POTENTIAL
We are only starting to define the role of extracellular nucleotides and their P2 receptors in disease states (26, 136, 506), including the kidney.
A. A Role for P2Y2, P2X4, and P2X7 Receptors in Arterial Hypertension
Renal function and blood pressure control are almost inseparable; hypertension is a major modifiable risk factor for cardiovascular and renal disease and is highly prevalent (257). In addition to their role in facilitating renal salt and fluid handling, P2Y2 receptors are also implicated in ATP-evoked relaxation of the murine aorta (183). P2Y2 receptors are required for the acute NO-independent blood pressure lowering effect of the P2Y2/P2Y4 receptor agonist P1-(inosine 5′-)P4-(uridine 5′-)tetraphosphate (Ip4U), implicating P2Y2 receptors in the vascular response to endothelium-derived hyperpolarizing factor (432). In addition to vasodilation, the latter studies also implicated P2Y2 receptors in Ip4U-induced inhibition of renal sodium reabsorption, suggesting the potential utility of P2Y2 agonists in the treatment of hypertension (432). This is consistent with an emerging role of P2 receptors in the physiology and pathophysiology of the cardiovascular system (137). Studies are needed to determine the therapeutic potential and safety of P2Y2 receptor agonists and define the relevance of first genetic studies indicating an association between polymorphisms in the P2Y2 receptor gene with blood pressure in African Americans (152) and with essential hypertension in Japanese male individuals (544).
Human genetic studies have also found an association between single nucleotide polymorphisms in P2X receptors encoding genes and blood pressure or cardiovascular disease. The loss of function variant rs28360472 in the P2X4 receptor associates with increased pulse pressure (489), itself an important cardiovascular risk factor. P2X4 receptor blockade with PSB12054 in ANG II-infused rats increased afferent and efferent arteriole resistance by 78 and 100%, respectively, leading to 51% reduction in single-nephron GFR (154). The elevated blood pressure may reflect endothelial dysfunction (impaired NO release), as has also been found in P2X4 receptor knockout mice (566), although this model also shows salt sensitivity (107), which is probably NO independent (495).
An intronic single nucleotide polymorphism rs591874 in the gene encoding for the P2X7 receptor is also associated with an increase in blood pressure (381). rs3751143 is a common loss of function variant in P2X7 (heterozygosity of 25% and up to 3% homozygosity) that is protective against ischemic stroke (167). rs3751143 reduces the sensitivity to P2X7 receptor antagonism (332), but does not associate with an impairment in endothelial dysfunction or vascular stiffness in essential hypertension (166), indicating that the physiology of genetic variation of the P2X7 receptor is both subtle and complex.
Although P2X7 receptor activation contributes to the physiological control of blood pressure by the kidney, sustained activation of the receptor, which does not desensitize with repeated exposure to ATP, can promote hypertensive renal injury. Thus prophylactic P2X7 receptor antagonism (247) or ‟knockoutˮ of murine P2X7k transcript (248), which leaves several other functional P2X7 receptor transcripts intact (329), protects against the injury associated with salt-sensitive hypertension. In these studies, P2X7 receptor antagonism or deletion reduced albuminuria and interstitial fibrosis, lowered blood pressure, and reduced the infiltration of T and B cells, macrophages, and leukocytes. In the rat, P2X7 receptor blockade with A438079 had little effect on afferent or efferent arteriole resistance in normotensive sham rats, but markedly reduced those resistances in ANG II hypertensive rats (154). The mechanisms underpinning these effects are unknown. However, available data suggest that P2X7 receptor expression in the renal vasculature and microvasculature may impair blood pressure regulation by the kidney (338).
Elevated renal expression of P2X7 (and P2X4) receptor as a candidate gene for hypertensive renal vascular injury has also been identified in rats (336). The P2X7 receptor localized to the vascular and microvascular endothelium down to the afferent arterioles. The selective P2X7 receptor antagonist AZ11657312 increased renal medullary perfusion and improved tissue oxygenation in ANG II-treated rats (338); these beneficial effects were in part NO mediated. A more direct contribution to medullary perfusion via vasa recta control has been proposed for ATP (111), and pharmacological data indicated possible P2X1 and P2X7 receptor expression on descending vasa recta pericytes (110) (see sect. IVC). Overall, activation of the P2X7 receptor seems to induce renal microvascular dysfunction and regional hypoxia, particularly under high ANG II tone. These effects are also pro-inflammatory and may contribute to renal injury being consistently linked to P2X7 receptor activation (see below).
B. P2 Receptors and Renal Disease
The release of ATP and other nucleotides from injured and dying cells at sites of tissue injury and inflammation as danger molecules, and from red blood cells and platelets following platelet aggregation and in thrombosis, means that the extracellular nucleotide/P2 receptor system could serve a local pathophysiological role, including in the kidney (87). In addition to the many effects of P2 receptors on renal hemodynamics and tubular transport, stimulation of P2 receptors can also affect renal cell growth acutely (191, 229, 230, 459), although possibly not chronically (210). In particular, the P2X7 receptor can also promote cell death and inflammation (197, 458). Signaling through different P2 receptors can lead to contrasting and even antagonistic effects (481). In broad terms, many P2X receptors are proinflammatory and some P2Y receptors are anti-inflammatory, and thus they can have opposite effects (480).
Infusion of ATP into pregnant rats increased 24-h protein excretion, while having no effect in nonpregnant controls (141). The proteinuria was accompanied by increased neutrophil counts, monocyte infiltration into glomeruli, reduced staining density for glomerular sialoglycoproteins, and increased expression of glomerular intracellular adhesion protein-1 (141). These observations suggest that ATP infusion promoted a proinflammatory response that included a compromised glomerular filtration barrier leading to proteinuria, particularly in the pregnant rats.
The published literature in support of a pathophysiological role for P2 receptors in the kidney, although steadily increasing, is still largely circumstantial and speculative. However, two forms of renal disease have continued to be a focus of interest for P2 receptors: renal cystic disease (424) and glomerular injury with inflammation (480). The first began with studies of P2 receptors in secretory epithelia (292), in particular the airway epithelium in cystic fibrosis (367), and was later extrapolated to a potential role for P2 receptors in renal cyst growth and expansion in polycystic kidney disease (467). The second stemmed from the broadly pro-proliferative effect of stimulating various P2Y receptor subtypes (440), and the pro-apoptotic effect of activating P2X7 receptors, particularly when expressed by glomerular cells (538), inflammatory macrophages (517), and interstitial fibroblasts (407). Moreover, P2X7 and P2X4, which have some properties in common and may even have a coupled and inverse relationship (106), are the P2 receptors that seem more likely to have a pathophysiological role in the kidney.
1. Glomerular disease and renal inflammation and fibrosis
Studies in rats suggested that activation of vascular and glomerular purinergic P2 receptors may contribute to the mesangial cell transformation, renal inflammation, and vascular hypertrophy in response to ANG II-induced hypertension (176). Injuring one single podocyte using two-photon microscopy triggered spreading of podocyte [Ca2+]i waves that depended on the presence of P2Y2 receptors, although the functional relevance remains unclear (55). An early study in the rat anti-Thy1 model of glomerulonephritis (GN; with acute mesangiolysis) showed a transient increase in glomerular P2Y2 and P2Y6 expression that might have been endothelial in origin (168), together with P2 receptor-dependent (using the nonselective P2 inhibitor PPADS) mesangial cell proliferation (440). Activation of these P2Y receptors may oppose or blunt the contractile actions of P2X receptor stimulation, in part via NO release (238), and might even help to protect from hyperfiltration injury (410). On the other hand, induction of an experimental antibody-mediated GN model in mice increased urinary ATP levels and upregulated P2Y2 receptor expression in both resident kidney cells and infiltrating leukocytes, while P2Y2−/− mice were protected. Additional experiments indicated that P2Y2 receptors on hematopoietic cells drove the ATP/P2Y2 receptor-mediated disease progression in this GN model (430). In another inflammatory immune-related model of GN, P2Y1−/− mice were protected, indicating that activation of this receptor may promote capillary loss, fibrosis, and death by renal failure, although expression on which cell type (resident or infiltrating inflammatory cells) was unclear (201). Thus no integrated picture emerges for P2Y receptors in GN, and the likely interplay among their subtypes has yet to be fully explored and defined.
a) ics as sensors, mediators, and effectors of inflammation via p2y14 receptors.
The P2Y2 receptor has been implicated in bacterial toxin-induced ATP release from renal epithelia in general and subsequent proinflammatory responses in a urosepsis model in mice (96). ICs have specifically been implicated in the defense against ascending pathogens, which involves bacterial Toll-like receptor TLR4 activation, followed by stimulation of the NF-κB pathway and release of the bacteriostatic LCN2 (also known as NGAL) and protons into the lumen (387). The P2Y14 receptor is specifically and highly expressed in the apical membrane of CD ICs (17, 90), and recent studies implicated its role in mediating a parallel non-TLR-mediated sterile inflammation in the kidney (17, 48). The P2Y14 receptor is activated by UDP-glucose, a DAMP molecule. UDP-glucose, extracellular ATP, and adenosine are emerging as immune-regulatory factors known as DAMP molecules that are released by injured cells and can initiate sterile inflammatory reactions (91, 131). While most extracellular nucleotides are rapidly degraded by ectonucleotidases, UDP-glucose resists hydrolysis by these enzymes (592). UDP-glucose increased proinflammatory chemokine expression in ICs, as well as MDCK-C11 cells, and UDP-glucose activated the MEK1/2-ERK1/2 pathway in MDCK-C11 cells (17). These effects were prevented following inhibition of P2Y14 receptors with PPTN (17), a small molecule previously shown to be a selective high-affinity antagonist of P2Y14 receptors that inhibits UDP-glucose-stimulated chemotaxis of human neutrophils (28). Furthermore, tail vein injection of mice with UDP-glucose induced the recruitment of neutrophils to the renal medulla, and the authors proposed that ICs act as immune defense cells by creating a chemotaxic gradient favorable to neutrophil recruitment. The renal medulla is the primary site of exposure to urinary ascending pathogens. It has been proposed that the higher P2Y14 receptor mRNA expression in ICs in renal medulla versus cortex could be explained by the necessity to synthesize a functional protein immediately following infection to facilitate a rapid response to an ascending infection (17). Thus ICs can be viewed as “purinergic” immune-relay cells picking up local or systemic cues of cellular stress and damage, in part via activation of apical P2Y14 receptors. The studies identified P2Y14 receptors as a potential therapeutic target for the prevention or treatment of sterile inflammation, and potentially pathogen-associated inflammation in the kidney (17). Further studies are needed to test whether ascending bacteria themselves can trigger an apical UDP-glucose release that initiates an inflammatory response in ICs and determine the role of the UDP-glucose/P2Y14 receptor signaling pathway in the physiology and pathophysiology of the human kidney (17).
b) p2x4 and p2x7 receptors and inflammation and cell death.
P2X7 receptor expression is evident during kidney development but is normally barely detectable in healthy adult kidney (197, 494). However, its expression is increased in rodent models of glomerular injury, diabetes mellitus, and renin-dependent hypertension (538). As well as the ability to form a nonselective cation channel or a larger membrane pore leading to cell death by necrosis or apoptosis, the P2X7 receptor can mediate an inflammatory response by causing release of interleukin (IL)-1β (145) (FIGURE 10). Factors that determine whether P2X7 receptor stimulation will cause cell necrosis or apoptosis, and/or inflammatory cytokine release, include the cell type, the concentration of ATP, and duration of nucleotide exposure, as well as the level of P2X7 receptor surface expression. In cultured human embryonic kidney cells expressing P2X7 receptors, membrane blebbing and microvesiculation occur within seconds to minutes of receptor stimulation and are associated with cell death by apoptosis (556). More prolonged receptor stimulation leads to formation of a large membrane pore that permits leakage of vital intracellular components (including ATP) and loss of membrane potential, ultimately leading to cell death and necrosis (363). The ERK pathway purportedly mediates P2X7 receptor expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells (406). Somewhat paradoxically, in some cell types, lower ATP concentrations (and perhaps tonic P2X7 receptor stimulation) promote cell proliferation, rather than inducing cell death (6).
Both interferon-γ and tumor necrosis factor-α (TNF-α) can enhance P2X7 receptor expression and P2X7 receptor activation increases the release of mature IL-1β, IL-6, and IL-18 from activated macrophages (191, 289, 308, 534), suggesting that this receptor is regulated by, and can regulate, inflammatory cytokine processing and release. A marked increase in glomerular expression of P2X7 receptors has been described in a rodent model of proliferative GN, reaching a peak that coincides with the onset of proteinuria (519). Macrophage infiltration and release of inflammatory cytokines are characteristic features of glomerular damage in many forms of GN (500). P2X7 receptor gene knockout or treatment with a P2X7 receptor antagonist significantly reduced the severity of proliferative GN in mouse and rat models of nephrotoxic nephritis (NTN), respectively, as shown by reduced renal expression of CC chemokine ligand 2 (CCL2; monocyte chemoattractant-1 MCP-1), reductions in glomerular macrophage infiltration, glomerular capillary thrombosis and proteinuria, and a smaller rise in serum creatinine concentration (505). In the same model in WKY rats, which are known to be more sensitive to this form of renal injury when compared with Lewis (LEW) rats, infiltrating macrophages show NLRP3 inflammasome activation. Moreover, these strains have identical MHC genes, but a genetic difference in their expression of P2X7 receptors that is linked to more caspase-1 activation (123). However, ATP binding to P2X7 receptors usually activates the inflammasome in endotoxin-primed macrophages (145), but endotoxin as a trigger is not a feature of antibody-mediated NTN, so exactly how the protective effect of P2X7 receptor antagonism is mediated is unclear. Moreover, a recent paper has confirmed the earlier finding that P2X7 receptor blockade can ameliorate inflammatory GN using a novel approach with a highly specific nanobody that can selectively block P2X7 receptor activation (117). Using this nanobody, the authors report beneficial effects in an immune-mediated form of murine GN targeting podocytes, and in a mouse model of contact dermatitis. When using a P2X receptor agonist nanobody, they were able to show a worsening of inflammatory injury. From these studies, they concluded that the effect was IL-1β dependent and involved P2X7 receptor expressing macrophages and regulatory T cells. The P2X7 receptor is also linked to lupus nephritis, and its blockade was shown to be protective in a mouse model (130, 588). Thus ATP-induced activation of P2X7 receptors could interact with, and control, the inflammatory response, and eventually causes cell death. The latter potentially serves to delete damaged cells without spreading toxic cell contents. A wider and potentially more fundamental role for the P2X7 receptor in tissue inflammation is suggested by two auto-inflammatory rheumatic diseases, SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) and Schnitzler’s syndrome, which involve dysregulation and enhanced interaction of the P2X7 receptor with the inflammasome, causing massive IL-1β production and release (103, 402).
In the rat streptozotocin (STZ) diabetic model, both glomerular epithelial (podocytes) and mesangial cells express P2X receptors (538): damaged podocytes can release inflammatory cytokines found in diabetic glomeruli (193). Broader P2X7 receptor expression has also been detected in human diabetic tissue and may be linked to both mesangial expansion and macrophage infiltration (337). In a high dietary fat mouse model of metabolic disease with proteinuria and inflammasome activation, P2X7−/− mice were protected (482). The vascular ectonucleotidase CD39 appears to be a renoprotective factor in diabetic nephropathy that modulates glomerular inflammation and thromboregulation (155). Protective effects were also observed in diabetic mice lacking CD73 (498), consistent with potentially opposing effects of P2 and P1 receptor activation in kidney disease. The latter includes protective effects of adenosine A2A and A2B receptor activation (163, 498, 515).
P2X4 receptor signaling can also mediate inflammasome activation (89). Proximal tubular cells from patients with type 2 diabetic kidney disease show increased expression of P2X4 receptors, which is positively correlated with urinary IL-1β and IL-18 levels and tubular NLRP3 staining. A similar pattern was observed in proximal tubular cells in culture exposed to high glucose (89). Both P2X4 and P2X7 receptor expression are increased in endothelial cells exposed to high glucose, and their inhibition reduces proinflammatory IL-6 levels (450). Recently, in an accelerated rat model of STZ-induced diabetes with uninephrectomy, an inverse relationship was reported between the renal expression of klotho, an anti-aging factor with pleiotropic effects, and the P2X7 receptor: reduced klotho expression associated with increased P2X7 receptor expression (438). Klotho’s function is best characterized in relation to phosphate homeostasis, where it acts as a cofactor for fibroblast growth factor (FGF)-23 and can increase phosphate excretion (206). In chronic kidney disease, circulating FGF-23 levels increase, whereas renal klotho levels decrease (206). A speculation is that by controlling phosphate availability, and thereby ATP synthesis, klotho can limit renal P2X7 receptor expression and function (438). Please see References 68 and 151 for a more detailed discussion of P2 and P1 receptor signaling in diabetes.
More recently, the P2X7 receptor was reported to have a role in the response to sepsis, but confusingly the potential effect of P2X7 receptor inhibition may be beneficial (16) or harmful (179), once again illustrating how complex and challenging this receptor is as a potential therapeutic target. The canine epithelial cell line MDCK expresses functional P2X7 receptors, as well as TLR4 and molecules associated with the NALP3 inflammasome, which may also help elucidate the complex role of these molecules in kidney epithelial cells and in renal disorders in dogs and humans (236).
Renal cell cancer is another area in which P2X receptor modulation may play a role. P2X7 receptor expression can predict postoperative cancer-specific survival, which is thought to be related to its local proinflammatory effect (313); this makes the P2X7 receptor another possible target in this form of cancer, perhaps as an adjunctive therapy.
c) panx1-mediated nucleotide release and p2x7 receptors in renal ischemia.
Pharmacological and genetic approaches were used to determine the role of nucleotide release via Panx1 in kidney ischemia-reperfusion injury (IRI), a mouse model of acute kidney injury (AKI). Pharmacological inhibition of gap junctions, including Panx1, by carbenoxolone protected mice from IRI (237). Furthermore, kidney function and morphology were preserved and the expression of proinflammatory molecules diminished after IRI in mice with tissue-specific knockout of Panx1 in the PT or vascular endothelium. The studies indicated that Panx1 is involved in regulating ATP release from hypoxic cells, and reducing this ATP release may protect kidneys from AKI, potentially in part by retaining more ATP under hypoxic stress (237). Studies in mice indicated that the application of adult renal progenitor cells (MRPC) together with the nonselective P2 receptor blocker suramin enhanced kidney recovery after IRI (187).
Inhibition of P2X7 receptors in a mouse model of IRI and AKI has been shown to be protective, although there was a narrow time window in which to intervene, and treatment had to be given within 6 h of injury for any protection; injury was associated with increased tubular expression of P2X7 receptors (567).
d) deleterious effects of p2x7 receptor activation in transplantation.
In organ transplantation, P2X7 receptors could also have a role in both the alloimmune response and in any associated IRI. An increase in P2X7 receptor expression was reported in allograft rejection (310), and also in graft-versus-host disease (555). A prominent role has been proposed for the extracellular ATP/P2X7 receptor axis, including studies showing that inhibition of this pathway is associated with long-term graft function and reduced graft-versus-host disease severity in murine models (114, 310, 532, 533). In contrast to P2X and P2Y receptors, P1 receptors can exert an inhibitory effect on the immune response (471), which also implicates the functional relevance or therapeutic perspectives of the ectoenzymes involved in the conversion of nucleotides to adenosine (see also sect. IIB).
e) p2x7 and p2x4 receptors and fibrosis.
Fibrosis often follows inflammation, and it is still a major goal to elucidate which factors during inflammation determine its resolution and repair and which ones lead to chronicity and progressive fibrosis. The potential role of the P2X7 receptor in renal fibrosis has been investigated in a mouse model of unilateral ureteric obstruction (UUO), a popular experimental model causing fibrosis. Transient expression of P2X7 receptors was detected in tubular epithelial cells following ureteric obstruction, and the severity of tubulo-interstitial macrophage infiltration and expression of TGF-β and fibrosis were reduced in P2X7−/− mice (173). In the kidney, interstitial fibroblasts are important in normal repair, and their P2X7-mediated loss might contribute to postinjury fibrosis and scarring in this model (407). A similar protective effect of P2X7 gene deletion has been reported recently in a model of lung fibrosis (435). In contrast, the P2X4 receptor may be protective in the UUO model (263). T cell cytotoxic function is thought to constrain fibrosis by inducing apoptosis in fibroblasts (540). The P2X4 receptor has been shown to control T cell migration (297), which might explain the exaggerated fibrotic injury observed in its absence (263). This is another example of the complex physiological and pathophysiological interplay between these closely related P2X receptors.
f) a proposed proinflammatory effect of atp and p2y2 receptor activation against bacterial infections in uroepithelial cells.
Studies using exposure to uropathogenic Escherichia coli of human uroepithelial A498 cells indicated that the enhanced ATP release and P2Y receptor activation during urinary tract infection may represent a non-TLR4-mediated mechanism for production of proinflammatory IL-8 in human urinary tract epithelial cells (451). ATP-induced P2Y2 receptor activation induced proinflammatory IL-8 release in uroepithelial cells (284). Moreover, renal epithelial responses elicited by bacteria could be mediated by bacteria- or host-derived ATP (283).
2. Polycystic kidney disease
Mutations in the membrane receptor polycystin 1 or the putative calcium channel polycystin 2 are responsible for polycystic kidney disease (PKD). This condition is associated with uncontrolled proliferation of renal epithelial cells and disordered fluid transport, leading to tubular dilatation and formation and expansion of fluid-filled cysts that eventually compress and destroy adjacent normal tissue, thereby compromising renal function (559). Cyst-lining cells are thought to exhibit disordered regulation of proliferation and apoptosis, abnormal secretion of fluid and electrolytes, and disturbed polarity of membrane transport proteins and receptors, which is believed to be due to a defect in mechanosensation (including detection of tubular fluid flow) as a result of mislocalization and dysfunction of the epithelial cell primary cilium (563).
Human PKD cells in culture release more ATP (predominantly from their apical surface) than normal proximal tubular cells (558). In the cysts, ecto-ATPase activity (which degrades ATP) seems to be reduced (467) and ATP is accumulating (0.5–1 mM). By activation of P2Y receptors, and possibly P2X receptors, on cyst-lining cells, extracellular ATP may promote fluid secretion and cell growth; moreover, by acting on P2X7 receptors, ATP may also induce apoptosis and thus cell loss, which may be part of the remodeling required for progressive cyst expansion; in this regard, PKD progression was slowed down by inhibition of caspase-mediated apoptosis (502). However, less indirect and circumstantial evidence is needed to support this hypothesis. An in vitro analysis of P2 receptor signaling in cyst-derived epithelial cells confirmed ATP release and P2 receptor-mediated stimulation of short-circuit current (chloride secretion), and also documented mRNA expression for several P2Y and P2X receptor subtypes (467). At the same time, the expression of the P2X7 receptor (at both mRNA and protein levels) was demonstrated in the cystic epithelium of the cpk/cpk mouse model of autosomal recessive PKD (198). With the use of cells isolated from the cpk/cpk mouse, a three-dimensional suspension model of cyst development was generated to study the effects of P2X7 receptor activation on cyst development. The widely used P2X7 receptor agonist BzATP (but which is not selective for this receptor, and can also act as an antagonist at P2Y1 and P2Y4 receptors) reduced cyst number, but not cyst size, in this model (199). However, in another three-dimensional model of cyst formation using MDCK cells, cyst expansion was inhibited by BzATP and appeared to depend on P2Y, rather than P2X, stimulation (518), which subsequent work identified as probably P2Y2 dependent (282). Ex vivo analyses of cystic epithelia in a rat model of autosomal recessive PKD indicated an age-dependent increase of P2X4 and/or P2X7 receptor signaling (383). In a rat model (Han-SPRD) of autosomal dominant PKD, although P2X7 receptor was detected in cyst-lining cells, and its mRNA level increased, other P2X and P2Y receptors were also detected, with particular increases in P2Y2, P2Y4, and P2Y6 receptors (516). However, all this suggests a complex interplay of P2 receptor subtypes in PKD (probably favoring more P2Y receptors) with potentially dissimilar effects on cyst size and number.
To gain mechanistic insights, a COOH-terminal polycystin 1 construct that was fused to a membrane expression cassette was expressed in mouse M1 cells (CD cell line) and found to increase the ATP-stimulated rise in [Ca2+]i (due to increased calcium entry) and enhance chloride secretion (203, 552). On the other hand, recent studies in cultured human PKD cells reported a loss of flow-induced ATP release and rise in [Ca2+]i, but with impaired recovery of [Ca2+]i that was attributed to reduced P2X7 expression and function (562, 563). In this same model, expression of the ectonucleotidase CD39 was also reduced, which would be expected to lead to an increase in local ATP levels. These human PKD cells have also been shown to have defective cilia, which are thought to be sensors for flow-activated cell signaling. In ciliated airway epithelia, cilial motion is increased by ATP, and these cilia express a P2X receptor that may be a heteromer of P2X4/7 receptors (320) or their colocalization (12), since both P2X subtypes are also found in PKD cells. Moreover, in a zebrafish model of PKD2, the P2X7 receptor antagonist oxidized ATP or the putative blocker A-438079, as well as the knockdown of the receptor, reduced cyst formation that depended on ERK activation (88). Although highly suggestive, these findings pose more questions than provide answers, and the exact role of P2 receptors in PKD pathogenesis is still unclear (424). The heterogeneity of the animal models, which form renal cysts in different locations and with likely different pathogenesis, remains a challenge and may affect the role of P2 receptors.
C. Lithium and Impaired Renal Function
1. Lithium as therapeutic agent and its effects on the kidney
Introduced in 1949 into the modern psychiatric practice, lithium has proven to be a very effective drug for the treatment of manic-depressive illness and thus bipolar disorder (342). Despite the introduction of new drugs, lithium retained its position because it effectively counters suicidal tendencies among bipolar patients, which no other drug does (98). In recent years, lithium found potential new applications for the treatment of acute brain injury (ischemic stroke) or chronic neurodegenerative disease (92) or even traumatic brain injury (301). This is due to the unique property of lithium to prevent apoptosis of neurons. In fact, lithium is the only substance known to prevent apoptosis of neurons.
Despite its therapeutic potentials, chronic use of lithium is associated with nephrogenic diabetes insipidus (NDI), a debilitating condition with considerable morbidity, and maybe even mortality in elderly patients (180). Patients with NDI, especially the elderly ones, often exhibit dehydration, hypernatremia, alterations in consciousness, and hemodynamic instability from hypovolemia (315). In animal models, lithium-induced NDI is characterized by AVP-resistant polyuria, natriuresis, kaliuresis, and decreased protein abundance of AQP2 water channel in the kidney as well as sodium channels or urea transporters (266). Lithium-induced NDI is mainly due to resistance of the kidney to circulating AVP (180, 266). Lithium treatment also causes renal tubular acidosis (RTA), which may be related to CD remodeling. Structurally, lithium treatment results in proliferation of CD cells, as well as CD remodeling, whereby the percentage of AQP2-positive PCs is reduced with a proportionate increase of H+-ATPase-positive ICs (94, 95, 546). Finally, chronic lithium treatment in humans and experimental animals leads to interstitial nephritis and rarely kidney failure (325). At the cellular and molecular level, lithium is known to induce several abnormalities. Notable among them is increased production of PGE2, an antagonist of vasopressin V2 receptor signaling, due to increased expression of cyclooxygenase-1 and -2 (COX1 and COX2) in the kidney. Thus PGE2 has become a target for the treatment of lithium-induced NDI in bipolar patients on lithium therapy (8). In addition to causing an AVP-resistant state accounting for polyuria, lithium appears to induce aldosterone resistance in the CD (514).
2. Purinergic signaling and lithium-induced NDI
Two P2 receptors, namely, the UTP/ATP-activated P2Y2 receptor and the ADP-activated P2Y12 receptor, have been shown to play significant roles in lithium-induced NDI (264). Both receptors have been localized in the CD (267, 582, 583). Although they do not belong to the same branch on the phylogenetic tree, they have one common feature, i.e., opposing the cAMP signaling that enhances AQP2 activity (see sect. III). While P2Y2 receptors counter cAMP signaling through enhanced phosphoinositide signaling (FIGURE 9), P2Y12 receptor activation decreases the activity of adenylyl cyclase. On the basis of the studies conducted in rodent models, it appears that both P2Y2 and P2Y12 receptors exert tonic inhibition on the action of AVP in the CD. Thus suppression of their activity appears to sensitize the CD to the action of AVP. Accordingly, genetic deletion of P2Y2 or P2Y12 receptors or pharmacological inhibition of P2Y12 receptors in rats or mice using antithrombotic agents (clopidogrel or prasugrel) have been shown to significantly reduce lithium-induced polyuria and preserve AQP2 protein abundance (581–584). Interestingly, although rat models of lithium-induced NDI revealed enhanced release of PGE2 from the medullary CDs (580), the amelioration of polyuria in lithium-treated P2Y2−/− mice was not due to suppression of PGE2 production. Instead, it appears to be altered prostanoid receptor signaling in the medullary CD duct resulting in enhanced production of cAMP when challenged with PGE2 (584).
Studies performed on P2Y2−/− mice showed that they are also resistant to development of lithium-induced natriuresis and kaliuresis, apparently due to blunted alterations in the protein abundances of sodium or potassium channels/transporters (578). The protection offered against polyuria, natriuresis, and kaliuresis by genetic deletion of the P2Y2 receptor is long lasting (5 mo) in mice (585). Interestingly, P2Y2−/− mice were also protected against the development of lithium-induced CD remodeling and prevented proliferation of CD cells (585). On the contrary, genetic deletion of P2Y12 receptors, which significantly ameliorated lithium-induced polyuria, did not affect lithium-induced natriuresis (576, 583). The protection offered in the genetic or pharmacological models was not associated with altered lithium disposition in the body, and the animals have comparable serum and/or inner medullary tissue lithium levels versus their respective controls (576, 582, 584). This is an important finding with translational significance, because to be useful as a therapeutic approach for the prevention of lithium-induced NDI requires that lithium disposition in the body is not altered. Thus purinergic antagonism may be a viable and alternative approach for the treatment of lithium-induced NDI as compared with the currently available therapies, such as the administration of cyclooxygenase inhibitors or amiloride or thiazides, which are not suitable in all types of patients (264, 576). The current therapeutic options also pose the risk of lithium intoxication. Furthermore, the therapeutic modality of purinergic antagonism shifts the current focus of countering anti-AVP effects of lithium to enhancing the sensitivity of the kidney to AVP action, i.e., relieving the AVP-resistant state in NDI.
D. Section Concluding Remarks
Although the availability of P2 receptor subtype selective agonists or antagonists that can be used in vivo is still limited, significant progress has been made to unravel the role of these receptors in renal pathophysiological conditions. While genetically modified mouse models can provide important insights, experience has shown that they can differ at times from the pharmacological models. Hence, the availability of specific pharmacological tools is imperative for further progress. Nevertheless, modulation or blockade of P2 receptors may hold promise to develop novel therapeutic drugs for the treatment of glomerular diseases, renal inflammation and fibrosis, PKD, and lithium-induced NDI.
VIII. SUMMARY AND PERSPECTIVES
The cellular processes and functions in the kidney that are regulated by physiological nucleotide release and the subsequent activation of plasma membrane P2 receptors are increasingly appreciated.
Physiological nucleotide release provides a key initiation event for the P2 receptor-mediated intrarenal regulation of kidney function, including the regulation of the amounts of fluid and salt that are first being filtered by the glomeruli and subsequently being reabsorbed by the tubular and collecting duct system. Several conductive membrane nucleotide release channels have been identified in this regard. Mechanical but also hormonal cues are transduced into nucleotide release with P2 receptor-mediated signaling often servo-controlling or amplifying a distinct functional response. We are only beginning to understand the specific conditions and triggers that call upon intrarenal nucleotide/P2 receptor-mediated signaling. Does the nucleotide/P2 receptor system provide a tonic regulatory input that is “holding” certain functions “in balance” or is it only activated in response to specific conditions? The answer to this question likely depends on the specific P2 receptor subtype involved.
P2X1 and P2Y2 receptors have been implicated in the regulation of renal vascular resistance and autoregulatory capability, potentially including medullary vasa recta, but considerable more work is needed, also to define the role of other P2X and P2Y receptors. ATP and adenosine are considered mediators of the GFR regulation by tubuloglomerular feedback. Because impaired regulation of renal microvascular function contributes to the renal pathology of diseases like hypertension, diabetes, sepsis, or ischemia-reperfusion injury, a better understanding holds the potential to identify P2-receptor-dependent interventions that provide therapeutic benefit and renal protection.
Considerable advances have been made with regard to the identification of the apical ATP/UTP/P2Y2 receptor system in the ASDN as a key regulator of ENaC: depending on dietary salt intake, the system sets channel open probability and thus the ENaC-mediated sodium reabsorption thereby facilitating salt balance. Since ATP/UTP release in the ASDN is related to salt intake and thus to the homeostasis of the whole organism, this example indicates that local nucleotide/P2 receptor systems have functions beyond preserving cell volume and integrity. Moreover, the identification of Cx30-mediated nucleotide release in response to dietary salt advanced the molecular understanding of renal epithelial nucleotide release pathways and provides opportunities to better define signals and signaling cascades implicated in their regulation. Dual effects of P2Y2 receptor activation on both the vasculature and renal salt reabsorption implicate these receptors as potential therapeutic targets in hypertension. First insights have been gained on the renal localization of P2Y2 receptor expression and their function under basal condition, but little is known about how the expression is regulated and affected by physiological conditions other than changes in salt intake, or by pathophysiological conditions.
The availability of FDA-approved selective P2Y12 receptor inhibitor drugs and their use in experimental studies has facilitated our functional understanding of this receptor in the kidney, including its role in AVP-regulated water reabsorption in the collecting duct. A pathophysiological role of the P2Y12 receptor in the kidney has been established in the development of lithium-induced NDI, but little is known about its role in the kidney beyond that. As outlined in this review, many other P2 receptors have been implicated in the regulation of renal transport process, but much more granularity is needed.
When and how do renal nucleotide/P2 receptor systems go wrong and become drivers of relevant disease processes? Strong evidence implicates changes in renal P2X7 receptor expression and function in pathophysiological conditions, including cell proliferation, inflammation, and cell death; however, the impact of P2X7 receptors on the regulation of renal microvascular function and renal hemodynamics and tubular function in pathological conditions is still poorly understood. A major problem confronting P2X7 receptor biology and this receptor as a therapeutic target is the existence of several splice variants that can determine its sensitivity to ATP and its proinflammatory and other biological effects. These are only just beginning to be better defined and explored in rodents and humans. Moreover, these variants may be expressed in a cell-specific fashion, which has been a particular problem with the mouse knockout models available and used to date. Because they are not true knockouts, their use to determine the role of the P2X7 receptor in a given physiological and pathophysiological setting is still open to question (101, 565).
Another important factor is ATP as a ligand, its metabolism, and the necessarily contemporaneous actions of the adenosine P1 receptor system that is always operating in tandem (480, 525). Little is known about the regulation along the tubular and CD system of the metabolizing enzymes involved in nucleotide degradation and interconversion or the interactions between P2 receptor- and P1 receptor-mediated signaling including the formation of receptor heteromultimers [e.g., for P2Y2 and adenosine A1 receptors (4, 349)]. We need to be open-minded with regard to other renal functions that could be under nucleotide/P2 receptor-mediated control. One example is the recent identification of P2Y14 receptors in ICs of the CD as sensors, mediators, and effectors of inflammation and as a potential therapeutic target for the prevention or treatment of sterile inflammation, and potentially pathogen-associated inflammation in the kidney (17). Further progress in understanding the role of P2X and P2Y receptors in normal renal physiology and in renal disease will depend crucially on increasing knowledge of their variants and structure, and the development and availability of more stable and highly selective antagonists and agonists (362).
GRANTS
V. Vallon is supported by National Institutes of Health (NIH) Grants R01DK112042, R01DK106102, RF1AG061296, and R01HL142814; the UAB/UCSD O’Brien Center of Acute Kidney Injury Grant NIH-P30DK079337, and the Department of Veterans Affairs. R. Unwin is supported by Medical Research Council; Kidney Research UK; St Peter’s Trust for Bladder, Prostate and Kidney Research; and Wellcome Trust. E. W. Inscho is supported by NIH Grants RO1DK044628, 1PO1HL095499, and RO1DK106500 and an American Heart Association Strategically Focused Hypertension Research Center. J. Leipziger is supported by the Danish Medical Research Council Grant 6110-00131B. B. K. Kishore is supported by Department of Veterans Affairs Merit Review program.
DISCLOSURES
Over the past 36 mo, V. Vallon has served as a consultant and received honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Janssen Pharmaceutical, Eli Lilly, and Merck and has received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen. R. Unwin is an employee of AstraZeneca IMED ECD CVRM (Gothenburg, Sweden). B. K. Kishore received research grant support and is collaborating with AstraZeneca and has United States patents issued for the use of P2Y2 and P2Y12 antagonists for the treatment of nephrogenic diabetes insipidus. E. W. Inscho and J. Leipziger have no conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: V. Vallon, Div. of Nephrology & Hypertension, Dept. of Medicine, Univ. of California San Diego & VA San Diego Healthcare System, 3350 La Jolla Village Dr. (9151), San Diego, CA 92161 (e-mail: vvallon@ucsd.edu).
REFERENCES
- 1.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24: 52–55, 2003. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64: 445–475, 1994. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol 78: 113–145, 1998. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelrahman A, Namasivayam V, Hinz S, Schiedel AC, Köse M, Burton M, El-Tayeb A, Gillard M, Bajorath J, de Ryck M, Müller CE. Characterization of P2X4 receptor agonists and antagonists by calcium influx and radioligand binding studies. Biochem Pharmacol 125: 41–54, 2017. doi: 10.1016/j.bcp.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, Rizzuto R, Di Virgilio F. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J Biol Chem 284: 10120–10128, 2009. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adriaensen D, Brouns I, Timmermans JP. Sensory input to the central nervous system from the lungs and airways: a prominent role for purinergic signalling via P2X2/3 receptors. Auton Neurosci 191: 39–47, 2015. doi: 10.1016/j.autneu.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Allen HM, Jackson RL, Winchester MD, Deck LV, Allon M. Indomethacin in the treatment of lithium-induced nephrogenic diabetes insipidus. Arch Intern Med 149: 1123–1126, 1989. doi: 10.1001/archinte.1989.00390050095019. [DOI] [PubMed] [Google Scholar]
- 9.Angelova PR, Iversen KZ, Teschemacher AG, Kasparov S, Gourine AV, Abramov AY. Signal transduction in astrocytes: localization and release of inorganic polyphosphate. Glia 66: 2126–2136, 2018. doi: 10.1002/glia.23466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435, 2002. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 11.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105: 18770–18775, 2008. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonio LS, Stewart AP, Xu XJ, Varanda WA, Murrell-Lagnado RD, Edwardson JM. P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 163: 1069–1077, 2011. doi: 10.1111/j.1476-5381.2011.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arend LJ, Thompson CI, Brandt MA, Spielman WS. Elevation of intrarenal adenosine by maleic acid decreases GFR and renin release. Kidney Int 30: 656–661, 1986. doi: 10.1038/ki.1986.236. [DOI] [PubMed] [Google Scholar]
- 15.Arendshorst WJ, Navar LG. Renal circulation and glomerular hemodynamics. In: Diseases of the Kidney, edited by Schrier RW, Gottschalk C. Boston, MA: Little, Brown, 1993, p. 65–117. [Google Scholar]
- 16.Arulkumaran N, Sixma ML, Pollen S, Ceravola E, Jentho E, Prendecki M, Bass PS, Tam FWK, Unwin RJ, Singer M. P2X7 receptor antagonism ameliorates renal dysfunction in a rat model of sepsis. Physiol Rep 6: e13622, 2018. doi: 10.14814/phy2.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azroyan A, Cortez-Retamozo V, Bouley R, Liberman R, Ruan YC, Kiselev E, Jacobson KA, Pittet MJ, Brown D, Breton S. Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor. PLoS One 10: e0121419, 2015. doi: 10.1371/journal.pone.0121419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagorda A, Guerra L, Di Sole F, Helmle-Kolb C, Favia M, Jacobson KA, Casavola V, Reshkin SJ. Extracellular adenine nucleotides regulate Na+/H+ exchanger NHE3 activity in A6-NHE3 transfectants by a cAMP/PKA-dependent mechanism. J Membr Biol 188: 249–259, 2002. doi: 10.1007/s00232-001-0189-8. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287: F789–F796, 2004. doi: 10.1152/ajprenal.00033.2004. [DOI] [PubMed] [Google Scholar]
- 20.Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst 81: 264–270, 2000. doi: 10.1016/S0165-1838(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 21.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000. doi: 10.1111/j.1523-1755.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 22.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ. Evidence for basolateral P2Y(6) receptors along the rat proximal tubule: functional and molecular characterization. J Am Soc Nephrol 12: 1640–1647, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signal 5: 473–480, 2009. doi: 10.1007/s11302-009-9149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ. P2Y receptors present in the native and isolated rat glomerulus. Nephron, Physiol 96: 79–90, 2004. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- 25.Bailey MA, Unwin RJ, Shirley DG. P2X receptors and kidney function. WIREs Membr Transp Signal 1: 503–511, 2012. doi: 10.1002/wmts.40. [DOI] [Google Scholar]
- 26.Baldini C, Rossi C, Ferro F, Santini E, Seccia V, Donati V, Solini A. The P2X7 receptor-inflammasome complex has a role in modulating the inflammatory response in primary Sjögren’s syndrome. J Intern Med 274: 480–489, 2013. doi: 10.1111/joim.12115. [DOI] [PubMed] [Google Scholar]
- 27.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83: 706–716, 2004. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK. A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol 84: 41–49, 2013. doi: 10.1124/mol.113.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belge H, Gailly P, Schwaller B, Loffing J, Debaix H, Riveira-Munoz E, Beauwens R, Devogelaer JP, Hoenderop JG, Bindels RJ, Devuyst O. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci USA 104: 14849–14854, 2007. doi: 10.1073/pnas.0702810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beliveau R, Brunette MG, Strevey J. Characterization of phosphate binding by alkaline phosphatase in rat kidney brush border membrane. Pflugers Arch 398: 227–232, 1983. doi: 10.1007/BF00657156. [DOI] [PubMed] [Google Scholar]
- 31.Bell PD, Komlosi P, Zhang ZR. ATP as a mediator of macula densa cell signalling. Purinergic Signal 5: 461–471, 2009. doi: 10.1007/s11302-009-9148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci Signal 8: ra17, 2015. doi: 10.1126/scisignal.2005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ Res 109: 80–85, 2011. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birch RE, Schwiebert EM, Peppiatt-Wildman CM, Wildman SS. Emerging key roles for P2X receptors in the kidney. Front Physiol 4: 262, 2013. doi: 10.3389/fphys.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjaelde RG, Arnadottir SS, Overgaard MT, Leipziger J, Praetorius HA. Renal epithelial cells can release ATP by vesicular fusion. Front Physiol 4: 238, 2013. doi: 10.3389/fphys.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 26: 959–969, 2001. doi: 10.1023/A:1012388618693. [DOI] [PubMed] [Google Scholar]
- 39.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol 35: 393–432, 2000. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 40.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch 456: 1005–1024, 2008. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boone M, Kortenoeven ML, Robben JH, Tamma G, Deen PM. Counteracting vasopressin-mediated water reabsorption by ATP, dopamine, and phorbol esters: mechanisms of action. Am J Physiol Renal Physiol 300: F761–F771, 2011. doi: 10.1152/ajprenal.00247.2010. [DOI] [PubMed] [Google Scholar]
- 42.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol 78: 154–163, 2012. doi: 10.5414/CN107325. [DOI] [PubMed] [Google Scholar]
- 43.Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev 91: 1393–1445, 2011. doi: 10.1152/physrev.00027.2010. [DOI] [PubMed] [Google Scholar]
- 44.Bouriquet N, Casellas D. Chronic l-NAME hypertension in rats and autoregulation of juxtamedullary preglomerular vessels. Am J Physiol Renal Physiol 269: F190–F197, 1995. doi: 10.1152/ajprenal.1995.269.2.F190. [DOI] [PubMed] [Google Scholar]
- 45.Bouriquet N, Casellas D. Interaction between cGMP-dependent dilators and autoregulation in rat preglomerular vasculature. Am J Physiol Renal Physiol 268: F338–F346, 1995. doi: 10.1152/ajprenal.1995.268.2.F338. [DOI] [PubMed] [Google Scholar]
- 46.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 129: 485–491, 2007. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyce AKJ, Kim MS, Wicki-Stordeur LE, Swayne LA. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J 470: 319–330, 2015. doi: 10.1042/BJ20141551. [DOI] [PubMed] [Google Scholar]
- 48.Breton S, Brown D. Novel Proinflammatory Function of Renal Intercalated Cells. Ann Nutr Metab 72, Suppl 2: 11–16, 2018. doi: 10.1159/000488303. [DOI] [PubMed] [Google Scholar]
- 49.Briner VA, Kern F. ATP stimulates Ca2+ mobilization by a nucleotide receptor in glomerular endothelial cells. Am J Physiol Renal Physiol 266: F210–F217, 1994. doi: 10.1152/ajprenal.1994.266.2.F210. [DOI] [PubMed] [Google Scholar]
- 50.Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 51.Browne LE, Jiang L-H, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31: 229–237, 2010. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunschweiger A, Müller CE. P2 receptors activated by uracil nucleotides–an update. Curr Med Chem 13: 289–312, 2006. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- 53.Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol 555: 311–321, 2004. doi: 10.1113/jphysiol.2003.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. Eur J Neurosci 8: 2221–2228, 1996. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 55.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, Shankland SJ, Peti-Peterdi J. Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014. doi: 10.1172/JCI71702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burnstock G. Purinergic nerves. Pharmacol Rev 24: 509–581, 1972. [PubMed] [Google Scholar]
- 58.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach, edited by Bolis L, Straub RCO. New York: Raven, 1978, p. 107–118. [Google Scholar]
- 59.Burnstock G. Noradrenaline and ATP: cotransmitters and neuromodulators. J Physiol Pharmacol 46: 365–384, 1995. [PubMed] [Google Scholar]
- 60.Burnstock G. Purinergic signalling. Br J Pharmacol 147, Suppl 1: S172–S181, 2006. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 62.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep 60: 12–20, 2008. [PubMed] [Google Scholar]
- 63.Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. BioEssays 34: 218–225, 2012. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 64.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol 40: 668–688, 1970. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnstock G, Evans LC, Bailey MA. Purinergic signalling in the kidney in health and disease. Purinergic Signal 10: 71–101, 2014. doi: 10.1007/s11302-013-9400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burnstock G, Kennedy C. A dual function for adenosine 5′-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res 58: 319–330, 1986. doi: 10.1161/01.RES.58.3.319. [DOI] [PubMed] [Google Scholar]
- 67.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240: 31–304, 2004. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 68.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal 9: 307–324, 2013. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg 47: 527–543, 1994. doi: 10.1016/0007-1226(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 70.Burnstock G, Nistri A, Khakh BS, Giniatullin R. ATP-gated P2X receptors in health and disease. Front Cell Neurosci 8: 204, 2014. doi: 10.3389/fncel.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol 303: F194–F200, 2012. doi: 10.1152/ajprenal.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carmines PK, Inscho EW. Perfusate composition modulates in vitro renal microvascular pressure responsiveness in a segment-specific manner. Microvasc Res 43: 347–351, 1992. doi: 10.1016/0026-2862(92)90031-J. [DOI] [PubMed] [Google Scholar]
- 75.Carmines PK, Inscho EW. Renal arteriolar angiotensin responses during varied adenosine receptor activation. Hypertension 23, Suppl: I114–I119, 1994. doi: 10.1161/01.HYP.23.1_Suppl.I114. [DOI] [PubMed] [Google Scholar]
- 76.Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol Renal Physiol 258: F94–F102, 1990. doi: 10.1152/ajprenal.1990.258.1.F94. [DOI] [PubMed] [Google Scholar]
- 77.Carmines PK, Mitchell KD, Navar LG. Effects of calcium antagonists on renal hemodynamics and glomerular function. Kidney Int Suppl 36, Suppl 36: S43–S48, 1992. [PubMed] [Google Scholar]
- 78.Casellas D, Carmines PK. Control of the renal microcirculation: cellular and integrative perspectives. Curr Opin Nephrol Hypertens 5: 57–63, 1996. doi: 10.1097/00041552-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Casellas D, Moore LC. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol Renal Physiol 258: F660–F669, 1990. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- 80.Casellas D, Morsing P, Stenberg A, and Persson AEG. The juxtamedullary nephron preparation: a tool in tubuloglomerular feedback studies. In: The Juxtaglomerular Apparatus, edited by Persson AEG, Boberg U. Amsterdam: Elsevier Science, 1988, p. 189–199. [Google Scholar]
- 81.Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol Renal Physiol 246: F349–F358, 1984. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- 82.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cha SH, Jung KY, Endou H. Effect of P2Y-purinoceptor stimulation on renal gluconeogenesis in rats. Biochem Biophys Res Commun 211: 454–461, 1995. doi: 10.1006/bbrc.1995.1835. [DOI] [PubMed] [Google Scholar]
- 84.Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998. doi: 10.1152/ajprenal.1998.274.6.F1006. [DOI] [PubMed] [Google Scholar]
- 85.Chabardès-Garonne D, Mejéan A, Aude J-C, Cheval L, Di Stefano A, Gaillard M-C, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, Ségurens B, Wincker P, Weissenbach J, Doucet A, Elalouf J-M. A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci USA 100: 13710–13715, 2003. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol Renal Physiol 274: F799–F804, 1998. doi: 10.1152/ajprenal.1998.274.4.F799. [DOI] [PubMed] [Google Scholar]
- 87.Chan CM, Unwin RJ, Burnstock G. Potential functional roles of extracellular ATP in kidney and urinary tract. Exp Nephrol 6: 200–207, 1998. doi: 10.1159/000020524. [DOI] [PubMed] [Google Scholar]
- 88.Chang M-Y, Lu J-K, Tian Y-C, Chen Y-C, Hung C-C, Huang Y-H, Chen Y-H, Wu M-S, Yang C-W, Cheng Y-C. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol 22: 1696–1706, 2011. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45: 932–943, 2013. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 92.Chiu CT, Wang Z, Hunsberger JG, Chuang DM. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65: 105–142, 2013. doi: 10.1124/pr.111.005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiu YH, Jin X, Medina CB, Leonhardt SA, Kiessling V, Bennett BC, Shu S, Tamm LK, Yeager M, Ravichandran KS, Bayliss DA. A quantized mechanism for activation of pannexin channels. Nat Commun 8: 14324, 2017. doi: 10.1038/ncomms14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006. doi: 10.1152/ajprenal.00383.2005. [DOI] [PubMed] [Google Scholar]
- 95.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004. doi: 10.1152/ajpcell.00266.2003. [DOI] [PubMed] [Google Scholar]
- 96.Christensen MG, Fagerberg SK, de Bruijn PI, Bjaelde RG, Jakobsen H, Leipziger J, Skals M, Praetorius HA. [Ca2+]i Oscillations and IL-6 Release Induced by α-Hemolysin from Escherichia coli Require P2 Receptor Activation in Renal Epithelia. J Biol Chem 290: 14776–14784, 2015. doi: 10.1074/jbc.M115.639526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Churchill PC, Ellis VR. Pharmacological characterization of the renovascular P2 purinergic receptors. J Pharmacol Exp Ther 265: 334–338, 1993. [PubMed] [Google Scholar]
- 98.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 346: f3646, 2013. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 99.Clémençon B, Babot M, Trézéguet V. The mitochondrial ADP/ATP carrier (SLC25 family): pathological implications of its dysfunction. Mol Aspects Med 34: 485–493, 2013. doi: 10.1016/j.mam.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 101.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63: 641–683, 2011. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colina M, Pizzirani C, Khodeir M, Falzoni S, Bruschi M, Trotta F, Di Virgilio F. Dysregulation of P2X7 receptor-inflammasome axis in SAPHO syndrome: successful treatment with anakinra. Rheumatology (Oxford) 49: 1416–1418, 2010. doi: 10.1093/rheumatology/keq074. [DOI] [PubMed] [Google Scholar]
- 104.Contreras-Sanz A, Scott-Ward TS, Gill HS, Jacoby JC, Birch RE, Malone-Lee J, Taylor KMG, Peppiatt-Wildman CM, Wildman SSP. Simultaneous quantification of 12 different nucleotides and nucleosides released from renal epithelium and in human urine samples using ion-pair reversed-phase HPLC. Purinergic Signal 8: 741–751, 2012. doi: 10.1007/s11302-012-9321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craigie E, Birch RE, Unwin RJ, Wildman SS. The relationship between P2X4 and P2X7: a physiologically important interaction? Front Physiol 4: 216, 2013. doi: 10.3389/fphys.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Craigie E, Menzies RI, Larsen CK, Jacquillet G, Carrel M, Wildman SS, Loffing J, Leipziger J, Shirley DG, Bailey MA, Unwin RJ. The renal and blood pressure response to low sodium diet in P2X4 receptor knockout mice. Physiol Rep 6: e13899, 2018. doi: 10.14814/phy2.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crain JM, Watters JJ. Microglial P2 Purinergic Receptor and Immunomodulatory Gene Transcripts Vary By Region, Sex, and Age in the Healthy Mouse CNS. Transcr Open Access 3: 124, 2015. doi: 10.4172/2329-8936.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron, Physiol 120: 17–31, 2012. doi: 10.1159/000339110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crawford C, Kennedy-Lydon TM, Callaghan H, Sprott C, Simmons RL, Sawbridge L, Syme HM, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. Extracellular nucleotides affect pericyte-mediated regulation of rat in situ vasa recta diameter. Acta Physiol (Oxf) 202: 241–251, 2011. doi: 10.1111/j.1748-1716.2011.02310.x. [DOI] [PubMed] [Google Scholar]
- 111.Crawford C, Wildman SS, Kelly MC, Kennedy-Lydon TM, Peppiatt-Wildman CM. Sympathetic nerve-derived ATP regulates renal medullary vasa recta diameter via pericyte cells: a role for regulating medullary blood flow? Front Physiol 4: 307, 2013. doi: 10.3389/fphys.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czaczkes JW, Kleeman CR, Koenig M, Boston R. Physiological studies of antidiuretic hormone by its direct measurement in human plasma. J Clin Invest 43: 1625–1640, 1964. doi: 10.1172/JCI105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Addio F, Vergani A, Potena L, Maestroni A, Usuelli V, Ben Nasr M, Bassi R, Tezza S, Dellepiane S, El Essawy B, Iascone M, Iacovoni A, Borgese L, Liu K, Visner G, Dhe-Paganon S, Corradi D, Abdi R, Starling RC, Folli F, Zuccotti GV, Sayegh MH, Heeger PS, Chandraker A, Grigioni F, Fiorina P. P2X7R mutation disrupts the NLRP3-mediated Th program and predicts poor cardiac allograft outcomes. J Clin Invest 128: 3490–3503, 2018. doi: 10.1172/JCI94524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370: 20140191, 2015. doi: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dai LJ, Kang HS, Kerstan D, Ritchie G, Quamme GA. ATP inhibits Mg2+ uptake in MDCT cells via P2X purinoceptors. Am J Physiol Renal Physiol 281: F833–F840, 2001. doi: 10.1152/ajprenal.0349.2000. [DOI] [PubMed] [Google Scholar]
- 117.Danquah W, Meyer-Schwesinger C, Rissiek B, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop J-H, Hammel A, Bergmann P, Schwarz N, Assunção J, Rotthier W, Haag F, Tolosa E, Bannas P, Boué-Grabot E, Magnus T, Laeremans T, Stortelers C, Koch-Nolte F. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med 8: 366ra162, 2016. doi: 10.1126/scitranslmed.aaf8463. [DOI] [PubMed] [Google Scholar]
- 118.Dawson TP, Gandhi R, Le Hir M, Kaissling B. Ecto-5′-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem 37: 39–47, 1989. doi: 10.1177/37.1.2535703. [DOI] [PubMed] [Google Scholar]
- 119.De Baaij JHF, Blanchard MG, Lavrijsen M, Leipziger J, Bindels RJM, Hoenderop JGJ. P2X4 receptor regulation of transient receptor potential melastatin type 6 (TRPM6) Mg2+ channels. Pflugers Arch 466: 1941–1952, 2014. doi: 10.1007/s00424-014-1440-3. [DOI] [PubMed] [Google Scholar]
- 120.De Baaij JHF, Kompatscher A, Viering DHHM, Bos C, Bindels RJM, Hoenderop JGJ. P2X6 Knockout Mice Exhibit Normal Electrolyte Homeostasis. PLoS One 11: e0156803–e0156816, 2016. doi: 10.1371/journal.pone.0156803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Bruijn PI, Bleich M, Praetorius HA, Leipziger J. P2X receptors trigger intracellular alkalization in isolated perfused mouse medullary thick ascending limb. Acta Physiol (Oxf) 213: 277–284, 2015. doi: 10.1111/apha.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deetjen P, Thomas J, Lehrmann H, Kim SJ, Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11: 1798–1806, 2000. [DOI] [PubMed] [Google Scholar]
- 123.Deplano S, Cook HT, Russell R, Franchi L, Schneiter S, Bhangal G, Unwin RJ, Pusey CD, Tam FWK, Behmoaras J. P2X7 receptor-mediated Nlrp3-inflammasome activation is a genetic determinant of macrophage-dependent crescentic glomerulonephritis. J Leukoc Biol 93: 127–134, 2013. doi: 10.1189/jlb.0612284. [DOI] [PubMed] [Google Scholar]
- 124.DiBona GF. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 289: R633–R641, 2005. doi: 10.1152/ajpregu.00258.2005. [DOI] [PubMed] [Google Scholar]
- 125.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 126.Dobrowolski L, Walkowska A, Kompanowska-Jezierska E, Kuczeriszka M, Sadowski J. Effects of ATP on rat renal haemodynamics and excretion: role of sodium intake, nitric oxide and cytochrome P450. Acta Physiol (Oxf) 189: 77–85, 2007. doi: 10.1111/j.1748-1716.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 127.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol 68: 213–237, 1929. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duchatelle P, Ohara A, Ling BN, Kemendy AE, Kokko KE, Matsumoto PS, Eaton DC. Regulation of renal epithelial sodium channels. Mol Cell Biochem 114: 27–34, 1992. doi: 10.1007/BF00240294. [DOI] [PubMed] [Google Scholar]
- 129.Edwards RM. Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438: 179–181, 2002. doi: 10.1016/S0014-2999(02)01293-1. [DOI] [PubMed] [Google Scholar]
- 130.Elliott JI, McVey JH, Higgins CF. The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: a hypothesis and comparison of murine allelic products. Arthritis Res Ther 7: R468–R475, 2005. doi: 10.1186/ar1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eltze M, Ullrich B. Characterization of vascular P2 purinoceptors in the rat isolated perfused kidney. Eur J Pharmacol 306: 139–152, 1996. doi: 10.1016/0014-2999(96)00244-0. [DOI] [PubMed] [Google Scholar]
- 133.Eppel GA, Ventura S, Denton KM, Evans RG. Lack of contribution of P2X receptors to neurally mediated vasoconstriction in the rabbit kidney in vivo. Acta Physiol (Oxf) 186: 197–207, 2006. doi: 10.1111/j.1748-1716.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 134.Eppel GA, Ventura S, Evans RG. Regional vascular responses to ATP and ATP analogues in the rabbit kidney in vivo: roles for adenosine receptors and prostanoids. Br J Pharmacol 149: 523–531, 2006. doi: 10.1038/sj.bjp.0706901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch 452: 552–562, 2006. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 136.Erlinge D. P2Y receptors in health and disease. Adv Pharmacol 61: 417–439, 2011. doi: 10.1016/B978-0-12-385526-8.00013-8. [DOI] [PubMed] [Google Scholar]
- 137.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4: 1–20, 2008. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Estévez-Herrera J, Domínguez N, Pardo MR, González-Santana A, Westhead EW, Borges R, Machado JD. ATP: The crucial component of secretory vesicles. Proc Natl Acad Sci USA 113: E4098–E4106, 2016. doi: 10.1073/pnas.1600690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol 48: 178–183, 1995. [PubMed] [Google Scholar]
- 140.Evans RJ, Surprenant A, North RA. P2X receptors: cloned and expressed. In: The P2 Nucleotide Receptors, edited by Turner JT, Weisman GA, Fedan JS. Totowa, NJ: Humana, 1998, p. 43–61. [Google Scholar]
- 141.Faas MM, van der Schaaf G, Borghuis T, Jongman RM, van Pampus MG, de Vos P, van Goor H, Bakker WW. Extracellular ATP induces albuminuria in pregnant rats. Nephrol Dial Transplant 25: 2468–2478, 2010. doi: 10.1093/ndt/gfq095. [DOI] [PubMed] [Google Scholar]
- 142.Fabbro A, Skorinkin A, Grandolfo M, Nistri A, Giniatullin R. Quantal release of ATP from clusters of PC12 cells. J Physiol 560: 505–517, 2004. doi: 10.1113/jphysiol.2004.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Falzoni S, Donvito G, Di Virgilio F. Detecting adenosine triphosphate in the pericellular space. Interface Focus 3: 20120101, 2013. doi: 10.1098/rsfs.2012.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernández O, Wangensteen R, Osuna A, Vargas F. Renal vascular reactivity to P(2)-purinoceptor activation in spontaneously hypertensive rats. Pharmacology 60: 47–50, 2000. doi: 10.1159/000028346. [DOI] [PubMed] [Google Scholar]
- 145.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 146.Firestein BL, Xing M, Hughes RJ, Corvera CU, Insel PA. Heterogeneity of P2u- and P2y-purinergic receptor regulation of phospholipases in MDCK cells. Am J Physiol Renal Physiol 271: F610–F618, 1996. doi: 10.1152/ajprenal.1996.271.3.F610. [DOI] [PubMed] [Google Scholar]
- 147.Fischer KG, Saueressig U, Jacobshagen C, Wichelmann A, Pavenstädt H. Extracellular nucleotides regulate cellular functions of podocytes in culture. Am J Physiol Renal Physiol 281: F1075–F1081, 2001. doi: 10.1152/ajprenal.2001.281.6.F1075. [DOI] [PubMed] [Google Scholar]
- 148.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118: 199–208, 2007. [PMC free article] [PubMed] [Google Scholar]
- 149.Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci 7: 267, 2013. doi: 10.3389/fncel.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, Reiser J, Gudermann T, Mederos y Schnitzler M, Storch U. Podocyte Purinergic P2X4 Channels Are Mechanotransducers That Mediate Cytoskeletal Disorganization. J Am Soc Nephrol 27: 848–862, 2016. doi: 10.1681/ASN.2014111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fotino C, Dal Ben D, Adinolfi E. Emerging Roles of Purinergic Signaling in Diabetes. Med Chem 14: 428–438, 2018. doi: 10.2174/1573406414666180226165204. [DOI] [PubMed] [Google Scholar]
- 152.Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, Liu K, Morrison AC, Ganesh S, Kutlar A, Ramachandran VS, Polak JF, Fabsitz RR, Dries DL, Farlow DN, Redline S, Adeyemo A, Hirschorn JN, Sun YV, Wyatt SB, Penman AD, Palmas W, Rotter JI, Townsend RR, Doumatey AP, Tayo BO, Mosley TH Jr, Lyon HN, Kang SJ, Rotimi CN, Cooper RS, Franceschini N, Curb JD, Martin LW, Eaton CB, Kardia SL, Taylor HA, Caulfield MJ, Ehret GB, Johnson T, Chakravarti A, Zhu X, Levy D; International Consortium for Blood Pressure Genome-wide Association Studies (ICBP-GWAS) . Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet 20: 2273–2284, 2011. doi: 10.1093/hmg/ddr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franco M, Bautista R, Tapia E, Soto V, Santamaría J, Osorio H, Pacheco U, Sánchez-Lozada LG, Kobori H, Navar LG. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 300: F1301–F1309, 2011. doi: 10.1152/ajprenal.00367.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Franco M, Bautista-Pérez R, Cano-Martínez A, Pacheco U, Santamaría J, Del Valle Mondragón L, Pérez-Méndez O, Navar LG. Physiopathological implications of P2X1 and P2X7 receptors in regulation of glomerular hemodynamics in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 313: F9–F19, 2017. doi: 10.1152/ajprenal.00663.2016. [DOI] [PubMed] [Google Scholar]
- 155.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56: 2371–2379, 2007. doi: 10.2337/db06-1593. [DOI] [PubMed] [Google Scholar]
- 156.Fu Y, Vallon V. Mineralocorticoid-induced sodium appetite and renal salt retention: evidence for common signaling and effector mechanisms. Nephron, Physiol 128: 8–16, 2014. doi: 10.1159/000368264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gabriëls G, Endlich K, Rahn KH, Schlatter E, Steinhausen M. In vivo effects of diadenosine polyphosphates on rat renal microcirculation. Kidney Int 57: 2476–2484, 2000. doi: 10.1046/j.1523-1755.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 158.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol 46: 277–300, 2006. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 159.Gailly P, Szutkowska M, Olinger E, Debaix H, Seghers F, Janas S, Vallon V, Devuyst O. P2Y2 receptor activation inhibits the expression of the sodium-chloride cotransporter NCC in distal convoluted tubule cells. Pflugers Arch 466: 2035–2047, 2014. doi: 10.1007/s00424-013-1438-2. [DOI] [PubMed] [Google Scholar]
- 160.Gaitán-Peñas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernández-Dueñas V, Barrallo-Gimeno A, Ciruela F, Lakadamyali M, Pusch M, Estévez R. Investigation of LRRC8-Mediated Volume-Regulated Anion Currents in Xenopus Oocytes. Biophys J 111: 1429–1443, 2016. doi: 10.1016/j.bpj.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gandhi R, Le Hir M, Kaissling B. Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry 95: 165–174, 1990. doi: 10.1007/BF00266589. [DOI] [PubMed] [Google Scholar]
- 162.Ganote CE, Grantham JJ, Moses HL, Burg MB, Orloff J. Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol 36: 355–367, 1968. doi: 10.1083/jcb.36.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garcia GE, Truong LD, Chen JF, Johnson RJ, Feng L. Adenosine A(2A) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int 80: 378–388, 2011. doi: 10.1038/ki.2011.101. [DOI] [PubMed] [Google Scholar]
- 164.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch 452: 513–537, 2006. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 165.Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA. Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch 455: 1105–1117, 2008. doi: 10.1007/s00424-007-0366-4. [DOI] [PubMed] [Google Scholar]
- 166.Ghiadoni L, Rossi C, Duranti E, Santini E, Bruno RM, Salvati A, Taddei S, Solini A. P2X7 receptor polymorphisms do not influence endothelial function and vascular tone in neo-diagnosed, treatment-naive essential hypertensive patients. J Hypertens 31: 2362–2369, 2013. doi: 10.1097/HJH.0b013e3283653ff5. [DOI] [PubMed] [Google Scholar]
- 167.Gidlöf O, Smith JG, Melander O, Lövkvist H, Hedblad B, Engström G, Nilsson P, Carlson J, Berglund G, Olsson S, Jood K, Jern C, Norrving B, Lindgren A, Erlinge D. A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One 7: e37491, 2012. doi: 10.1371/journal.pone.0037491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Glass R, Loesch A, Bodin P, Burnstock G. P2X4 and P2X6 receptors associate with VE-cadherin in human endothelial cells. Cell Mol Life Sci 59: 870–881, 2002. doi: 10.1007/s00018-002-8474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gloy J, Greger R, Schollmeyer P, Huber M, Pavenstädt H. Influence of cell culture conditions and passage number on the response of membrane voltage to ATP and angiotensin II in rat mesangial cells. Ren Physiol Biochem 17: 62–72, 1994. doi: 10.1159/000173789. [DOI] [PubMed] [Google Scholar]
- 170.Gohar EY, Kasztan M, Becker BK, Speed JS, Pollock DM. Ovariectomy uncovers purinergic receptor activation of endothelin-dependent natriuresis. Am J Physiol Renal Physiol 313: F361–F369, 2017. doi: 10.1152/ajprenal.00098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gohar EY, Kasztan M, Pollock DM. Interplay between renal endothelin and purinergic signaling systems. Am J Physiol Renal Physiol 313: F666–F668, 2017. doi: 10.1152/ajprenal.00639.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gohar EY, Speed JS, Kasztan M, Jin C, Pollock DM. Activation of purinergic receptors (P2) in the renal medulla promotes endothelin-dependent natriuresis in male rats. Am J Physiol Renal Physiol 311: F260–F267, 2016. doi: 10.1152/ajprenal.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gonçalves RG, Gabrich L, Rosário A Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M Jr. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70: 1599–1606, 2006. doi: 10.1038/sj.ki.5001804. [DOI] [PubMed] [Google Scholar]
- 174.González E, Salomonsson M, Müller-Suur C, Persson AE. Measurements of macula densa cell volume changes in isolated and perfused rabbit cortical thick ascending limb. II. Apical and basolateral cell osmotic water permeabilities. Acta Physiol Scand 133: 159–166, 1988. doi: 10.1111/j.1748-1716.1988.tb08395.x. [DOI] [PubMed] [Google Scholar]
- 175.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J 233: 309–319, 1986. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008. doi: 10.1152/ajprenal.00281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Greenberg SG, Tershner S, Osborn JL. Neurogenic regulation of rate of achieving sodium balance after increasing sodium intake. Am J Physiol Renal Physiol 261: F300–F307, 1991. doi: 10.1152/ajprenal.1991.261.2.F300. [DOI] [PubMed] [Google Scholar]
- 178.Greiber S, Münzel T, Kästner S, Müller B, Schollmeyer P, Pavenstädt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int 53: 654–663, 1998. doi: 10.1046/j.1523-1755.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- 179.Greve A-S, Skals M, Fagerberg SK, Tonnus W, Ellermann-Eriksen S, Evans RJ, Linkermann A, Praetorius HA. P2X1, P2X4, and P2X7 Receptor Knock Out Mice Expose Differential Outcome of Sepsis Induced by α-Haemolysin Producing Escherichia coli. Front Cell Infect Microbiol 7: 113, 2017. doi: 10.3389/fcimb.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol 5: 270–276, 2009. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 181.Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med (Maywood) 232: 715–726, 2007. [PubMed] [Google Scholar]
- 182.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol 147: 569–574, 2006. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gutierrez AM, Lou X, Erik A, Persson G, Ring A. Ca2+ response of rat mesangial cells to ATP analogues. Eur J Pharmacol 369: 107–112, 1999. doi: 10.1016/S0014-2999(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 185.Haanes KA, Kowal JM, Arpino G, Lange SC, Moriyama Y, Pedersen PA, Novak I. Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal 10: 431–440, 2014. doi: 10.1007/s11302-014-9406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hafting T, Sand O. Purinergic activation of BK channels in clonal kidney cells (Vero cells). Acta Physiol Scand 170: 99–109, 2000. doi: 10.1046/j.1365-201x.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 187.Han X, Zhao L, Lu G, Ge J, Zhao Y, Zu S, Yuan M, Liu Y, Kong F, Xiao Z, Zhao S. Improving outcomes of acute kidney injury using mouse renal progenitor cells alone or in combination with erythropoietin or suramin. Stem Cell Res Ther 4: 74, 2013. doi: 10.1186/scrt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303: F1454–F1459, 2012. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol 285: F590–F599, 2003. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 191.Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57: 949–958, 2000. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 192.Harvey RB. Effects of adenosinetriphosphate on autoregulation of renal blood flow and glomerular filtration rate. Circ Res 15, Suppl 1: 178–182, 1964. [PubMed] [Google Scholar]
- 193.Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40: 1007–1012, 1991. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 194.Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol 290: F888–F891, 2006. doi: 10.1152/ajprenal.00381.2005. [DOI] [PubMed] [Google Scholar]
- 195.Hayashi K, Epstein M, Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Circ Res 65: 1475–1484, 1989. doi: 10.1161/01.RES.65.6.1475. [DOI] [PubMed] [Google Scholar]
- 196.Hayashi K, Epstein M, Saruta T. Altered myogenic responsiveness of the renal microvasculature in experimental hypertension. J Hypertens 14: 1387–1401, 1996. doi: 10.1097/00004872-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 197.Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron, Exp Nephrol 101: e24–e30, 2005. doi: 10.1159/000086036. [DOI] [PubMed] [Google Scholar]
- 198.Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS. P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol 10: 34–42, 2002. doi: 10.1159/000049896. [DOI] [PubMed] [Google Scholar]
- 199.Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun 322: 434–439, 2004. doi: 10.1016/j.bbrc.2004.07.148. [DOI] [PubMed] [Google Scholar]
- 200.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 201.Hohenstein B, Renk S, Lang K, Daniel C, Freund M, Léon C, Amann KU, Gachet C, Hugo CP. P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol 18: 494–505, 2007. doi: 10.1681/ASN.2006050439. [DOI] [PubMed] [Google Scholar]
- 202.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150: 1349–1360, 2000. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hooper KM, Unwin RJ, Sutters M. The isolated C-terminus of polycystin-1 promotes increased ATP-stimulated chloride secretion in a collecting duct cell line. Clin Sci (Lond) 104: 217–221, 2003. doi: 10.1042/CS20020239. [DOI] [PubMed] [Google Scholar]
- 204.Hou Z, Cao J. Comparative study of the P2X gene family in animals and plants. Purinergic Signal 12: 269–281, 2016. doi: 10.1007/s11302-016-9501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol 180: 47–63, 2013. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 291: F282–F288, 2006. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- 208.Huber-Lang M, Fischer KG, Gloy J, Schollmeyer P, Krämer-Guth A, Greger R, Pavenstädt H. UTP and ATP induce different membrane voltage responses in rat mesangial cells. Am J Physiol Renal Physiol 272: F704–F711, 1997. doi: 10.1152/ajprenal.1997.272.6.F704. [DOI] [PubMed] [Google Scholar]
- 209.Hughes AK, Stricklett PK, Kishore BK, Kohan DE. Adenosine triphosphate inhibits endothelin-1 production by rat inner medullary collecting duct cells. Exp Biol Med (Maywood) 231: 1006–1009, 2006. [PubMed] [Google Scholar]
- 210.Humes HD, Cieslinski DA. Adenosine triphosphate stimulates thymidine incorporation but does not promote cell growth in primary cultures of renal proximal tubule cells. Ren Physiol Biochem 14: 253–258, 1991. doi: 10.1159/000173413. [DOI] [PubMed] [Google Scholar]
- 211.Huwiler A, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol 113: 1455–1463, 1994. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huwiler A, van Rossum G, Wartmann M, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br J Pharmacol 120: 807–812, 1997. doi: 10.1038/sj.bjp.0700979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huwiler A, Wartmann M, van den Bosch H, Pfeilschifter J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br J Pharmacol 129: 612–618, 2000. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol 305: C1050–C1059, 2013. doi: 10.1152/ajpcell.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Imamura H, Huynh Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA 106: 15651–15656, 2009. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280: F927–F944, 2001. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- 217.Inscho EW. Mysteries of renal autoregulation. Hypertension 53: 299–306, 2009. doi: 10.1161/HYPERTENSIONAHA.108.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Inscho EW, Carmines PK, Cook AK, Navar LG. Afferent arteriolar responsiveness to altered perfusion pressure in renal hypertension. Hypertension 15: 748–752, 1990. doi: 10.1161/01.HYP.15.6.748. [DOI] [PubMed] [Google Scholar]
- 219.Inscho EW, Carmines PK, Navar LG. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension 17: 1033–1037, 1991. doi: 10.1161/01.HYP.17.6.1033. [DOI] [PubMed] [Google Scholar]
- 220.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004. doi: 10.1111/j.1365-201X.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 222.Inscho EW, Cook AK, Mui V, Imig JD. Calcium mobilization contributes to pressure-mediated afferent arteriolar vasoconstriction. Hypertension 31: 421–428, 1998. doi: 10.1161/01.HYP.31.1.421. [DOI] [PubMed] [Google Scholar]
- 223.Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol Renal Physiol 274: F718–F727, 1998. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- 224.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Physiol 271: F1077–F1085, 1996. doi: 10.1152/ajprenal.1996.271.5.F1077. [DOI] [PubMed] [Google Scholar]
- 225.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol 296: F590–F597, 2009. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am J Physiol Renal Physiol 263: F886–F893, 1992. doi: 10.1152/ajprenal.1992.263.5.F886. [DOI] [PubMed] [Google Scholar]
- 227.Insel PA, Firestein BL, Xing M, Post SR, Jacobson JP, Balboa MA, Hughes RJ. P2-purinoceptors utilize multiple signalling pathways in MDCK-D1 cells. J Auton Pharmacol 16: 311–314, 1996. doi: 10.1111/j.1474-8673.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 228.Insel PA, Ostrom RS, Zambon AC, Hughes RJ, Balboa MA, Shehnaz D, Gregorian C, Torres B, Firestein BL, Xing M, Post SR. P2Y receptors of MDCK cells: epithelial cell regulation by extracellular nucleotides. Clin Exp Pharmacol Physiol 28: 351–354, 2001. doi: 10.1046/j.1440-1681.2001.03452.x. [DOI] [PubMed] [Google Scholar]
- 229.Ishikawa S, Kawasumi M, Kusaka I, Komatsu N, Iwao N, Saito T. Extracellular ATP promotes cellular growth of glomerular mesangial cells mediated via phospholipase C. Biochem Biophys Res Commun 202: 234–240, 1994. doi: 10.1006/bbrc.1994.1917. [DOI] [PubMed] [Google Scholar]
- 230.Ishikawa S, Higashiyama M, Kusaka I, Saito T, Nagasaka S, Fukuda S, Saito T. Extracellular ATP promotes cellular growth of renal inner medullary collecting duct cells mediated via P2u receptors. Nephron 76: 208–214, 1997. doi: 10.1159/000190170. [DOI] [PubMed] [Google Scholar]
- 231.Ivy JR, Bailey MA. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 592: 3955–3967, 2014. doi: 10.1113/jphysiol.2014.271676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- 233.Jackson MF. Interdependence of ATP signalling and pannexin channels; the servant was really the master all along? Biochem J 472: e27–e30, 2015. doi: 10.1042/BJ20151016. [DOI] [PubMed] [Google Scholar]
- 234.Jacobson KA, Gao Z-G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: 247–264, 2006. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jacobson KA, Müller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104: 31–49, 2016. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jalilian I, Spildrejorde M, Seavers A, Curtis BL, McArthur JD, Sluyter R. Functional expression of the damage-associated molecular pattern receptor P2X7 on canine kidney epithelial cells. Vet Immunol Immunopathol 150: 228–233, 2012. doi: 10.1016/j.vetimm.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 237.Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD. Epithelial and Endothelial Pannexin1 Channels Mediate AKI. J Am Soc Nephrol 29: 1887–1899, 2018. doi: 10.1681/ASN.2017121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jankowski M. Purinergic regulation of glomerular microvasculature and tubular function. J Physiol Pharmacol 59, Suppl 9: 121–135, 2008. [PubMed] [Google Scholar]
- 239.Jankowski M, Szamocka E, Kowalski R, Angielski S, Szczepańska-Konkel M. The effects of P2X receptor agonists on renal sodium and water excretion in anaesthetized rats. Acta Physiol (Oxf) 202: 193–201, 2011. doi: 10.1111/j.1748-1716.2011.02276.x. [DOI] [PubMed] [Google Scholar]
- 240.Jankowski M, Szczepańska-Konkel K, Kalinowski L, Angielski S. Involvement of Rho-kinase in P2Y-receptor-mediated contraction of renal glomeruli. Biochem Biophys Res Commun 302: 855–859, 2003. doi: 10.1016/S0006-291X(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 241.Jankowski M, Szczepańska-Konkel M, Kalinowski L, Angielski S. Bidirectional action of extracellular ATP on intracapillary volume of isolated rat renal glomeruli. J Physiol Pharmacol 51: 491–496, 2000. [PubMed] [Google Scholar]
- 242.Jankowski M, Szczepanska-Konkel M, Kalinowski L, Angielski S. Cyclic GMP-dependent relaxation of isolated rat renal glomeruli induced by extracellular ATP. J Physiol 530: 123–130, 2001. doi: 10.1111/j.1469-7793.2001.0123m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jankowski M, Szczepańska-Konkel M, Kalinowski L, Angielski S. The role of P2Y-receptors in the regulation of glomerular volume. Med Sci Monit 7: 635–340, 2001. [PubMed] [Google Scholar]
- 244.Jankowski V, Karadogan S, Vanholder R, Nofer JR, Herget-Rosenthal S, van der Giet M, Tölle M, Tran TN, Zidek W, Jankowski J. Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells. Kidney Int 71: 994–1000, 2007. doi: 10.1038/sj.ki.5002186. [DOI] [PubMed] [Google Scholar]
- 245.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 246.Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17: 293–307, 2016. doi: 10.1038/nrm.2016.29. [DOI] [PubMed] [Google Scholar]
- 247.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res 35: 173–179, 2012. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- 248.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012. doi: 10.1152/ajprenal.00051.2012. [DOI] [PubMed] [Google Scholar]
- 249.Jin W, Hopfer U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: elevated cytosolic Ca2+ is not required. Am J Physiol Cell Physiol 272: C1169–C1177, 1997. doi: 10.1152/ajpcell.1997.272.4.C1169. [DOI] [PubMed] [Google Scholar]
- 250.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 251.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342, 2003. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 252.Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489–F1500, 2007. doi: 10.1152/ajprenal.00256.2007. [DOI] [PubMed] [Google Scholar]
- 253.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors–recent progress and persisting challenges. Purinergic Signal 8: 375–417, 2012. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kasztan M, Piwkowska A, Kreft E, Rogacka D, Audzeyenka I, Szczepanska-Konkel M, Jankowski M. Extracellular purines’ action on glomerular albumin permeability in isolated rat glomeruli: insights into the pathogenesis of albuminuria. Am J Physiol Renal Physiol 311: F103–F111, 2016. doi: 10.1152/ajprenal.00567.2015. [DOI] [PubMed] [Google Scholar]
- 255.Kauffenstein G, Tamareille S, Prunier F, Roy C, Ayer A, Toutain B, Billaud M, Isakson BE, Grimaud L, Loufrani L, Rousseau P, Abraham P, Procaccio V, Monyer H, de Wit C, Boeynaems JM, Robaye B, Kwak BR, Henrion D. Central Role of P2Y6 UDP Receptor in Arteriolar Myogenic Tone. Arterioscler Thromb Vasc Biol 36: 1598–1606, 2016. doi: 10.1161/ATVBAHA.116.307739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kavvadas P, Abed A, Poulain C, Authier F, Labéjof LP, Calmont A, Afieri C, Prakoura N, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN. J Am Soc Nephrol 28: 2915–2930, 2017. doi: 10.1681/ASN.2016111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 258.Kefauver JM, Saotome K, Dubin AE, Pallesen J, Cottrell CA, Cahalan SM, Qiu Z, Hong G, Crowley CS, Whitwam T, Lee WH, Ward AB, Patapoutian A. Structure of the human volume regulated anion channel. eLife 7: e38461, 2018. doi: 10.7554/eLife.38461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kemendy AE, Kleyman TR, Eaton DC. Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol Cell Physiol 263: C825–C837, 1992. doi: 10.1152/ajpcell.1992.263.4.C825. [DOI] [PubMed] [Google Scholar]
- 260.Kennedy C, Chootip K, Mitchell C, Syed NI, Tengah A. P2X and P2Y nucleotide receptors as targets in cardiovascular disease. Future Med Chem 5: 431–449, 2013. doi: 10.4155/fmc.13.6. [DOI] [PubMed] [Google Scholar]
- 261.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM. Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 207: 212–225, 2013. doi: 10.1111/apha.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kim EY, Anderson M, Wilson C, Hagmann H, Benzing T, Dryer SE. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol 305: C960–C971, 2013. doi: 10.1152/ajpcell.00191.2013. [DOI] [PubMed] [Google Scholar]
- 263.Kim MJ, Turner CM, Hewitt R, Smith J, Bhangal G, Pusey CD, Unwin RJ, Tam FWK. Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol Dial Transplant 29: 1350–1361, 2014. doi: 10.1093/ndt/gfu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kishore BK, Carlson NG, Ecelbarger CM, Kohan DE, Müller CE, Nelson RD, Peti-Peterdi J, Zhang Y. Targeting renal purinergic signalling for the treatment of lithium-induced nephrogenic diabetes insipidus. Acta Physiol (Oxf) 214: 176–188, 2015. doi: 10.1111/apha.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Physiol 269: F863–F869, 1995. doi: 10.1152/ajprenal.1995.269.6.F863. [DOI] [PubMed] [Google Scholar]
- 266.Kishore BK, Ecelbarger CM. Lithium: a versatile tool for understanding renal physiology. Am J Physiol Renal Physiol 304: F1139–F1149, 2013. doi: 10.1152/ajprenal.00718.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y(2) purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000. doi: 10.1152/ajprenal.2000.278.1.F43. [DOI] [PubMed] [Google Scholar]
- 268.Kishore BK, Hansson KM, Tao L, Magnell K, Carlson NG, Zhang Y. Renal phenotype of P2Y12 receptor knockout mice. J Am Soc Nephrol 28: 71, 2017. [Google Scholar]
- 269.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sévigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 270.Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 288: F1164–F1172, 2005. doi: 10.1152/ajprenal.00199.2004. [DOI] [PubMed] [Google Scholar]
- 271.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y(2) receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kishore BK, Robson SC, Dwyer KM. CD39-adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signal 14: 109–120, 2018. doi: 10.1007/s11302-017-9596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Klawitter S, Hofmann LP, Pfeilschifter J, Huwiler A. Extracellular nucleotides induce migration of renal mesangial cells by upregulating sphingosine kinase-1 expression and activity. Br J Pharmacol 150: 271–280, 2007. doi: 10.1038/sj.bjp.0706983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kleta R, Hirsch J, Heidenreich S, Schlüter H, Zidek W, Schlatter E. Effects of diadenosine polyphosphates, ATP and angiotensin II on membrane voltage and membrane conductances of rat mesangial cells. Pflugers Arch 430: 713–720, 1995. doi: 10.1007/BF00386166. [DOI] [PubMed] [Google Scholar]
- 275.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kohan DE. Endothelin and collecting duct sodium and water transport. Contrib Nephrol 172: 94–106, 2011. doi: 10.1159/000328687. [DOI] [PubMed] [Google Scholar]
- 277.Komlosi P, Fintha A, Bell PD. Renal cell-to-cell communication via extracellular ATP. Physiology (Bethesda) 20: 86–90, 2005. doi: 10.1152/physiol.00002.2005. [DOI] [PubMed] [Google Scholar]
- 278.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054–F1058, 2004. doi: 10.1152/ajprenal.00336.2003. [DOI] [PubMed] [Google Scholar]
- 279.Koster HP, Hartog A, van Os CH, Bindels RJ. Inhibition of Na+ and Ca2+ reabsorption by P2u purinoceptors requires PKC but not Ca2+ signaling. Am J Physiol Renal Physiol 270: F53–F60, 1996. doi: 10.1152/ajprenal.1996.270.1.F53. [DOI] [PubMed] [Google Scholar]
- 280.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kowalski R, Kreft E, Kasztan M, Jankowski M, Szczepanska-Konkel M. Chronic renal denervation increases renal tubular response to P2X receptor agonists in rats: implication for renal sympathetic nerve ablation. Nephrol Dial Transplant 27: 3443–3448, 2012. doi: 10.1093/ndt/gfs087. [DOI] [PubMed] [Google Scholar]
- 282.Kraus A, Grampp S, Goppelt-Struebe M, Schreiber R, Kunzelmann K, Peters DJ, Leipziger J, Schley G, Schödel J, Eckardt KU, Buchholz B. P2Y2R is a direct target of HIF-1α and mediates secretion-dependent cyst growth of renal cyst-forming epithelial cells. Purinergic Signal 12: 687–695, 2016. doi: 10.1007/s11302-016-9532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kruse R, Demirel I, Säve S, Persson K. IL-8 and global gene expression analysis define a key role of ATP in renal epithelial cell responses induced by uropathogenic bacteria. Purinergic Signal 10: 499–508, 2014. doi: 10.1007/s11302-014-9414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kruse R, Säve S, Persson K. Adenosine triphosphate induced P2Y2 receptor activation induces proinflammatory cytokine release in uroepithelial cells. J Urol 188: 2419–2425, 2012. doi: 10.1016/j.juro.2012.07.095. [DOI] [PubMed] [Google Scholar]
- 285.Kuczeriszka M, Dobrowolski L, Walkowska A, Sadowski J. Influence of P2X receptors on renal medullary circulation is not altered by angiotensin II pretreatment. Pharmacol Rep 68: 1230–1236, 2016. doi: 10.1016/j.pharep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 286.Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal 1: 193–204, 2005. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kukulski F, Lévesque SA, Sévigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol 61: 263–299, 2011. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 288.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143, 2005. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 289.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168: 6436–6445, 2002. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 290.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. [Correction at https://doi.org/10.1371/journal.pbio.1001857.] PLoS Biol 12: e1001747, 2014. doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lambrecht G. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn Schmiedebergs Arch Pharmacol 362: 340–350, 2000. doi: 10.1007/s002100000312. [DOI] [PubMed] [Google Scholar]
- 292.Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci 16: 1–5, 2001. [DOI] [PubMed] [Google Scholar]
- 293.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8: 359–373, 2012. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 295.Le Hir M, Kaissling B. Distribution of 5′-nucleotidase in the renal interstitium of the rat. Cell Tissue Res 258: 177–182, 1989. doi: 10.1007/BF00223156. [DOI] [PubMed] [Google Scholar]
- 296.Le Hir M, Kaissling B. Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. Am J Physiol Renal Physiol 264: F377–F387, 1993. doi: 10.1152/ajprenal.1993.264.3.F377. [DOI] [PubMed] [Google Scholar]
- 297.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicoló S, Arbab M, Hubner J, Konrad K, Fakhari M, Lederer JA, Robson SC, Visner GA, Junger WG. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 128: 3583–3594, 2018. doi: 10.1172/JCI120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lee SY, Sarkar S, Bhattarai S, Namasivayam V, De Jonghe S, Stephan H, Herdewijn P, El-Tayeb A, Müller CE. Substrate-Dependence of Competitive Nucleotide Pyrophosphatase/Phosphodiesterase1 (NPP1) Inhibitors. Front Pharmacol 8: 54, 2017. doi: 10.3389/fphar.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lee YJ, Park SH, Jeung TO, Kim KW, Lee JH, Han HJ. Effect of adenosine triphosphate on phosphate uptake in renal proximal tubule cells: involvement of PKC and p38 MAPK. J Cell Physiol 205: 68–76, 2005. doi: 10.1002/jcp.20367. [DOI] [PubMed] [Google Scholar]
- 301.Leeds PR, Yu F, Wang Z, Chiu CT, Zhang Y, Leng Y, Linares GR, Chuang DM. A new avenue for lithium: intervention in traumatic brain injury. ACS Chem Neurosci 5: 422–433, 2014. doi: 10.1021/cn500040g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002. [DOI] [PubMed] [Google Scholar]
- 303.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 304.Lemmens R, Kupers L, Sévigny J, Beaudoin AR, Grondin G, Kittel A, Waelkens E, Vanduffel L. Purification, characterization, and localization of an ATP diphosphohydrolase in porcine kidney. Am J Physiol Renal Physiol 278: F978–F988, 2000. doi: 10.1152/ajprenal.2000.278.6.F978. [DOI] [PubMed] [Google Scholar]
- 305.Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res 38: 332–340, 2001. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- 306.Li L, Lynch IJ, Zheng W, Cash MN, Teng X, Wingo CS, Verlander JW, Xia S-L. Apical P2XR contribute to [Ca2+]i signaling and Isc in mouse renal MCD. Biochem Biophys Res Commun 359: 438–444, 2007. doi: 10.1016/j.bbrc.2007.05.143. [DOI] [PubMed] [Google Scholar]
- 307.Ling BN, Kemendy AE, Kokko KE, Hinton CF, Marunaka Y, Eaton DC. Regulation of the amiloride-blockable sodium channel from epithelial tissue. Mol Cell Biochem 99: 141–150, 1990. doi: 10.1007/BF00230344. [DOI] [PubMed] [Google Scholar]
- 308.Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 4: 5–14, 2007. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu K, Vergani A, Zhao P, Ben Nasr M, Wu X, Iken K, Jiang D, Su X, Fotino C, Fiorina P, Visner GA. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol 51: 300–310, 2014. doi: 10.1165/rcmb.2013-0362OC. [DOI] [PubMed] [Google Scholar]
- 311.Liu R, Bell PD, Peti-Peterdi J, Kovacs G, Johansson A, Persson AE. Purinergic receptor signaling at the basolateral membrane of macula densa cells. J Am Soc Nephrol 13: 1145–1151, 2002. doi: 10.1097/01.ASN.0000014827.71910.39. [DOI] [PubMed] [Google Scholar]
- 312.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 313.Liu Z, Liu Y, Xu L, An H, Chang Y, Yang Y, Zhang W, Xu J. P2X7 receptor predicts postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma. Cancer Sci 106: 1224–1231, 2015. doi: 10.1111/cas.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lu M, MacGregor GG, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116: 299–310, 2000. doi: 10.1085/jgp.116.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Luckey AE, Parsa CJ. Fluid and electrolytes in the aged. Arch Surg 138: 1055–1060, 2003. doi: 10.1001/archsurg.138.10.1055. [DOI] [PubMed] [Google Scholar]
- 316.Lutter D, Ullrich F, Lueck JC, Kempa S, Jentsch TJ. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J Cell Sci 130: 1122–1133, 2017. doi: 10.1242/jcs.196253. [DOI] [PubMed] [Google Scholar]
- 317.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 318.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002. doi: 10.1152/ajprenal.00147.2001. [DOI] [PubMed] [Google Scholar]
- 319.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 320.Ma W, Korngreen A, Weil S, Cohen EB-T, Priel A, Kuzin L, Silberberg SD. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 571: 503–517, 2006. doi: 10.1113/jphysiol.2005.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ma Z, Tanis JE, Taruno A, Foskett JK. Calcium homeostasis modulator (CALHM) ion channels. Pflugers Arch 468: 395–403, 2016. doi: 10.1007/s00424-015-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.MacKenzie AB, Surprenant A, North RA. Functional and molecular diversity of purinergic ion channel receptors. Ann N Y Acad Sci 868, 1 MOLECULAR AND: 716–729, 1999. doi: 10.1111/j.1749-6632.1999.tb11351.x. [DOI] [PubMed] [Google Scholar]
- 323.Majid DSA, Inscho EW, Navar LG. P2 purinoceptor saturation by adenosine triphosphate impairs renal autoregulation in dogs. J Am Soc Nephrol 10: 492–498, 1999. [DOI] [PubMed] [Google Scholar]
- 324.Majid DSA, Navar LG. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol Renal Physiol 262: F40–F46, 1992. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- 325.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000. [DOI] [PubMed] [Google Scholar]
- 326.Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem 276: 23867–23872, 2001. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- 327.Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J. Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL). Am J Physiol Renal Physiol 302: F487–F494, 2012. doi: 10.1152/ajprenal.00570.2011. [DOI] [PubMed] [Google Scholar]
- 328.Marques RD, Praetorius HA, Leipziger J. P2Y2 receptor knock-out mice display normal NaCl absorption in medullary thick ascending limb. Front Physiol 4: 280, 2013. doi: 10.3389/fphys.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Masin M, Young C, Lim K, Barnes SJ, Xu XJ, Marschall V, Brutkowski W, Mooney ER, Górecki DC, Murrell-Lagnado R. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: re-evaluation of P2X7 knockouts. Br J Pharmacol 165: 978–993, 2012. doi: 10.1111/j.1476-5381.2011.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol Renal Physiol 277: F552–F559, 1999. doi: 10.1152/ajprenal.1999.277.4.F552. [DOI] [PubMed] [Google Scholar]
- 331.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 332.McHugh SM, Roman S, Davis B, Koch A, Pickett AM, Richardson JC, Miller SR, Wetten S, Cox CJ, Karpe F, Todd JA, Bullmore ET. Effects of genetic variation in the P2RX7 gene on pharmacodynamics of a P2X(7) receptor antagonist: a prospective genotyping approach. Br J Clin Pharmacol 74: 376–380, 2012. doi: 10.1111/j.1365-2125.2012.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McLachlan EM, Luff SE. Sympathetic innervation of renal and extra-renal arterial vessels. Kidney Int Suppl 37, Suppl 37: S56–S60, 1992. [PubMed] [Google Scholar]
- 334.Menzies RI, Tam FW, Unwin RJ, Bailey MA. Purinergic signaling in kidney disease. Kidney Int 91: 315–323, 2017. doi: 10.1016/j.kint.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 335.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiol (Oxf) 213: 232–241, 2015. doi: 10.1111/apha.12412. [DOI] [PubMed] [Google Scholar]
- 336.Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW Jr, Carlson BE, Mullins JJ, Bailey MA. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front Physiol 4: 305, 2013. doi: 10.3389/fphys.2013.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Menzies RI, Booth JWR, Mullins JJ, Bailey MA, Tam FWK, Norman JT, Unwin RJ. Hyperglycemia-induced Renal P2X7 Receptor Activation Enhances Diabetes-related Injury. EBioMedicine 19: 73–83, 2017. doi: 10.1016/j.ebiom.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Menzies RI, Howarth AR, Unwin RJ, Tam FWK, Mullins JJ, Bailey MA. Inhibition of the purinergic P2X7 receptor improves renal perfusion in angiotensin-II-infused rats. Kidney Int 88: 1079–1087, 2015. doi: 10.1038/ki.2015.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol 589: 3471–3482, 2011. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mitchell KD, Navar LG. Tubuloglomerular feedback responses during peritubular infusions of calcium channel blockers. Am J Physiol Renal Physiol 258: F537–F544, 1990. doi: 10.1152/ajprenal.1990.258.3.F537. [DOI] [PubMed] [Google Scholar]
- 342.Mitchell PB. On the 50th anniversary of John Cade’s discovery of the anti-manic effect of lithium. Aust N Z J Psychiatry 33: 623–628, 1999. [DOI] [PubMed] [Google Scholar]
- 343.Mo J, Fisher MJ. Uridine nucleotide-induced stimulation of gluconeogenesis in isolated rat proximal tubules. Naunyn Schmiedebergs Arch Pharmacol 366: 151–157, 2002. doi: 10.1007/s00210-002-0571-9. [DOI] [PubMed] [Google Scholar]
- 344.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mohaupt MG, Fischer T, Schwöbel J, Sterzel RB, Schulze-Lohoff E. Activation of purinergic P2Y2 receptors inhibits inducible NO synthase in cultured rat mesangial cells. Am J Physiol Renal Physiol 275: F103–F110, 1998. doi: 10.1152/ajprenal.1998.275.1.F103. [DOI] [PubMed] [Google Scholar]
- 346.Moore LC, Casellas D. Tubuloglomerular feedback dependence of autoregulation in rat juxtamedullary afferent arterioles. Kidney Int 37: 1402–1408, 1990. doi: 10.1038/ki.1990.129. [DOI] [PubMed] [Google Scholar]
- 347.Moore LC, Rich A, Casellas D. Ascending myogenic autoregulation: interactions between tubuloglomerular feedback and myogenic mechanisms. Bull Math Biol 56: 391–410, 1994. doi: 10.1007/BF02460464. [DOI] [PubMed] [Google Scholar]
- 348.Murali S, Zhang M, Nurse CA. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J Physiol 592: 4747–4762, 2014. doi: 10.1113/jphysiol.2014.279299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Namba K, Suzuki T, Nakata H. Immunogold electron microscopic evidence of in situ formation of homo- and heteromeric purinergic adenosine A1 and P2Y2 receptors in rat brain. BMC Res Notes 3: 323, 2010. doi: 10.1186/1756-0500-3-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol Renal Physiol 234: F357–F370, 1978. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 351.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The Renal Microcirculation. In: Handbook of Physiology: Microcirculation, edited by Tuma RF, Duran WN, Ley K. San Diego, CA: Elsevier, 2008, p. 550–683. [Google Scholar]
- 352.Navar LG, Champion WJ, Thomas CE. Effects of calcium channel blockade on renal vascular resistance responses to changes in perfusion pressure and angiotensin-converting enzyme inhibition in dogs. Circ Res 58: 874–881, 1986. doi: 10.1161/01.RES.58.6.874. [DOI] [PubMed] [Google Scholar]
- 353.Navar LG, Inscho EW, Ibarrola M, Carmines PK. Communication between the macula densa cells and the afferent arteriole. Kidney Int Suppl 32, Suppl 32: S78–S82, 1991. [PubMed] [Google Scholar]
- 354.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 355.Needleman P, Minkes MS, Douglas JR Jr. Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res 34: 455–460, 1974. doi: 10.1161/01.RES.34.4.455. [DOI] [PubMed] [Google Scholar]
- 356.Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol 280: F406–F414, 2001. doi: 10.1152/ajprenal.2001.280.3.F406. [DOI] [PubMed] [Google Scholar]
- 357.Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000. doi: 10.1161/01.RES.86.6.656. [DOI] [PubMed] [Google Scholar]
- 358.Nishiyama A, Majid DS, Walker M III, Miyatake A, Navar LG. Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37: 753–759, 2001. doi: 10.1161/01.HYP.37.2.753. [DOI] [PubMed] [Google Scholar]
- 359.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R273–R275, 2002. doi: 10.1152/ajpregu.00071.2002. [DOI] [PubMed] [Google Scholar]
- 360.Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim Biophys Acta 853: 237–265, 1986. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 361.Nori S, Fumagalli L, Bo X, Bogdanov Y, Burnstock G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res 35: 179–185, 1998. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- 362.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol 83: 759–769, 2013. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 364.North RA. P2X receptors. Philos Trans R Soc Lond B Biol Sci 371: 20150427, 2016. doi: 10.1098/rstb.2015.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563–580, 2000. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 366.North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch 452: 479–485, 2006. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 367.Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci 18: 12–17, 2003. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 368.Obermüller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci 118: 4271–4282, 2005. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- 369.Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. Am J Physiol Renal Physiol 297: F341–F349, 2009. doi: 10.1152/ajprenal.00190.2009. [DOI] [PubMed] [Google Scholar]
- 370.Öhman J, Erlinge D. The touching story of purinergic signaling in epithelial and endothelial cells. Purinergic Signal 8: 599–608, 2012. doi: 10.1007/s11302-012-9316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124: 513–526, 2004. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Olesen ET, Fenton RA. Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013. doi: 10.1681/ASN.2012020217. [DOI] [PubMed] [Google Scholar]
- 373.Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann J. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol 294: F965–F970, 2008. doi: 10.1152/ajprenal.00603.2007. [DOI] [PubMed] [Google Scholar]
- 374.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. doi: 10.1152/ajprenal.0075.2001. [DOI] [PubMed] [Google Scholar]
- 375.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Osswald H, Schmitz HJ, Kemper R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflugers Arch 371: 45–49, 1977. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- 377.Ostrom RS, Gregorian C, Drenan RM, Gabot K, Rana BK, Insel PA. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol 281: C524–C531, 2001. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- 378.Pallone TL, Robertson CR, Jamison RL. Renal medullary microcirculation. Physiol Rev 70: 885–920, 1990. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- 379.Pallone TL, Silldorff EP, Turner MR. Intrarenal blood flow: microvascular anatomy and the regulation of medullary perfusion. Clin Exp Pharmacol Physiol 25: 383–392, 1998. doi: 10.1111/j.1440-1681.1998.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 380.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 381.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, Watkins H, Edwards CRW, Keavney B. Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension 52: 980–985, 2008. doi: 10.1161/HYPERTENSIONAHA.108.113282. [DOI] [PubMed] [Google Scholar]
- 382.Palygin O, Evans LC, Cowley AW Jr, Staruschenko A. Acute In Vivo Analysis of ATP Release in Rat Kidneys in Response to Changes of Renal Perfusion Pressure. J Am Heart Assoc 6: e006658, 2017. doi: 10.1161/JAHA.117.006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, Pavlov TS, Staruschenko A. Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal 14: 485–497, 2018. doi: 10.1007/s11302-018-9632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW Jr, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol Renal Physiol 305: F134–F141, 2013. doi: 10.1152/ajprenal.00129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F541–F552, 2015. doi: 10.1152/ajprenal.00456.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch 452: 589–597, 2006. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 387.Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D’Agati V, Lin CS, Qiu A, Al-Awqati Q, Ratner AJ, Barasch J. α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest 124: 2963–2976, 2014. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Park S, Bivona BJ, Feng Y, Lazartigues E, Harrison-Bernard LM. Intact renal afferent arteriolar autoregulatory responsiveness in db/db mice. Am J Physiol Renal Physiol 295: F1504–F1511, 2008. doi: 10.1152/ajprenal.90417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 390.Paulais M, Baudouin-Legros M, Teulon J. Extracellular ATP and UTP trigger calcium entry in mouse cortical thick ascending limbs. Am J Physiol Renal Physiol 268: F496–F502, 1995. doi: 10.1152/ajprenal.1995.268.3.F496. [DOI] [PubMed] [Google Scholar]
- 391.Pavenstädt H, Gloy J, Leipziger J, Klär B, Pfeilschifter J, Schollmeyer P, Greger R. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. Br J Pharmacol 109: 953–959, 1993. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Pavenstädt H, Henger A, Briner V, Greger R, Schollmeyer P. Extracellular ATP regulates glomerular endothelial cell function. J Auton Pharmacol 16: 389–392, 1996. doi: 10.1111/j.1474-8673.1996.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 393.Pavenstädt H, Späth M, Schlunck G, Nauck M, Fischer R, Wanner C, Schollmeyer P. Effect of nucleotides on the cytosolic free calcium activity and inositol phosphate formation in human glomerular epithelial cells. Br J Pharmacol 107: 189–195, 1992. doi: 10.1111/j.1476-5381.1992.tb14485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature 281: 384–386, 1979. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 395.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 396.Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of Vascular and Renal Function by Metabolite Receptors. Annu Rev Physiol 78: 391–414, 2016. doi: 10.1146/annurev-physiol-021115-105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. Biochem J 272: 469–472, 1990. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pfeilschifter J. Extracellular ATP stimulates polyphosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Involvement of a pertussis toxin-sensitive guanine nucleotide binding protein and feedback inhibition by protein kinase C. Cell Signal 2: 129–138, 1990. doi: 10.1016/0898-6568(90)90016-4. [DOI] [PubMed] [Google Scholar]
- 399.Pfeilschifter J, Huwiler A. Regulatory functions of protein kinase C isoenzymes in purinoceptor signalling in mesangial cells. J Auton Pharmacol 16: 315–318, 1996. doi: 10.1111/j.1474-8673.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 400.Pfeilschifter J, Merriweather C. Extracellular ATP and UTP activation of phospholipase D is mediated by protein kinase C-epsilon in rat renal mesangial cells. Br J Pharmacol 110: 847–853, 1993. doi: 10.1111/j.1476-5381.1993.tb13890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Piwkowska A, Rogacka D, Jankowski M, Angielski S. Extracellular ATP through P2 receptors activates AMP-activated protein kinase and suppresses superoxide generation in cultured mouse podocytes. Exp Cell Res 317: 1904–1913, 2011. doi: 10.1016/j.yexcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 402.Pizzirani C, Falzoni S, Govoni M, La Corte R, Donadei S, Di Virgilio F, Trotta F, Lo Monaco A. Dysfunctional inflammasome in Schnitzler’s syndrome. Rheumatology (Oxford) 48: 1304–1308, 2009. doi: 10.1093/rheumatology/kep222. [DOI] [PubMed] [Google Scholar]
- 403.Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- 404.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol 301: F650–F659, 2011. doi: 10.1152/ajprenal.00215.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011. doi: 10.1152/ajprenal.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Post SR, Jacobson JP, Insel PA. P2 purinergic receptor agonists enhance cAMP production in Madin-Darby canine kidney epithelial cells via an autocrine/paracrine mechanism. J Biol Chem 271: 2029–2032, 1996. doi: 10.1074/jbc.271.4.2029. [DOI] [PubMed] [Google Scholar]
- 409.Post SR, Rump LC, Zambon A, Hughes RJ, Buda MD, Jacobson JP, Kao CC, Insel PA. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J Biol Chem 273: 23093–23097, 1998. doi: 10.1074/jbc.273.36.23093. [DOI] [PubMed] [Google Scholar]
- 410.Potthoff SA, Stegbauer J, Becker J, Wagenhaeuser PJ, Duvnjak B, Rump LC, Vonend O. P2Y2 receptor deficiency aggravates chronic kidney disease progression. Front Physiol 4: 234, 2013. doi: 10.3389/fphys.2013.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Praetorius HA, Frøkiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- 412.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- 413.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 197: 241–251, 2009. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 415.Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010. doi: 10.1146/annurev-physiol-021909-135825. [DOI] [PubMed] [Google Scholar]
- 416.Praetorius HA, Leipziger J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 24: 3–10, 2013. doi: 10.1016/j.semcdb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 417.Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev 57: 289–298, 2005. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 418.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rafehi M, Burbiel JC, Attah IY, Abdelrahman A, Müller CE. Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal 13: 89–103, 2017. doi: 10.1007/s11302-016-9542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. [Correction in Proc Natl Acad Sci USA 113: E1587, 2016.] Proc Natl Acad Sci USA 111: 8506–8511, 2014. doi: 10.1073/pnas.1402195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Raghavan V, Weisz OA. Flow stimulated endocytosis in the proximal tubule. Curr Opin Nephrol Hypertens 24: 359–365, 2015. doi: 10.1097/MNH.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Rajagopal M, Kathpalia PP, Thomas SV, Pao AC. Activation of P2Y1 and P2Y2 receptors induces chloride secretion via calcium-activated chloride channels in kidney inner medullary collecting duct cells. Am J Physiol Renal Physiol 301: F544–F553, 2011. doi: 10.1152/ajprenal.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Rajagopal M, Kathpalia PP, Widdicombe JH, Pao AC. Differential effects of extracellular ATP on chloride transport in cortical collecting duct cells. Am J Physiol Renal Physiol 303: F483–F491, 2012. doi: 10.1152/ajprenal.00062.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Rangan G. Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease. Front Physiol 4: 218, 2013. doi: 10.3389/fphys.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Raza R, Akhtar T, Hameed S, Lecka J, Iqbal J, Sevigny J. Identification of potent and selective human ecto-nucleotide pyrophosphate/phosphodiesterases-3 inhibitors. Open Enzyme Inhib J 4: 17–22, 2011. doi: 10.2174/1874940201104010017. [DOI] [Google Scholar]
- 426.Ren Y, Carretero OA, Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney Int 62: 525–531, 2002. doi: 10.1046/j.1523-1755.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 427.Ren Y, D’Ambrosio MA, Garvin JL, Wang H, Carretero OA. Prostaglandin E2 mediates connecting tubule glomerular feedback. Hypertension 62: 1123–1128, 2013. doi: 10.1161/HYPERTENSIONAHA.113.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 429.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004. doi: 10.1111/j.1523-1755.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 430.Rennert L, Zschiedrich S, Sandner L, Hartleben B, Cicko S, Ayata CK, Meyer C, Zech A, Zeiser R, Huber TB, Idzko M, Grahammer F. P2Y2R Signaling Is Involved in the Onset of Glomerulonephritis. Front Immunol 9: 1589, 2018. doi: 10.3389/fimmu.2018.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 432.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am J Physiol Regul Integr Comp Physiol 301: R510–R518, 2011. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009. doi: 10.1152/ajpregu.90784.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 435.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VFJ, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 436.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J Biol Chem 287: 5301–5309, 2012. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Rivera I, Zhang S, Fuller BS, Edwards B, Seki T, Wang MH, Marrero MB, Inscho EW. P2 receptor regulation of [Ca2+]i in cultured mouse mesangial cells. Am J Physiol Renal Physiol 292: F1380–F1389, 2007. doi: 10.1152/ajprenal.00349.2006. [DOI] [PubMed] [Google Scholar]
- 438.Rodrigues AM, Serralha RS, Farias C, Punaro GR, Fernandes MJS, Higa EMS. P2X7 receptor and klotho expressions in diabetic nephropathy progression. Purinergic Signal 14: 167–176, 2018. doi: 10.1007/s11302-018-9602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014. doi: 10.1152/ajprenal.00661.2013. [DOI] [PubMed] [Google Scholar]
- 440.Rost S, Daniel C, Schulze-Lohoff E, Bäumert HG, Lambrecht G, Hugo C. P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kidney Int 62: 1659–1671, 2002. doi: 10.1046/j.1523-1755.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- 441.Rouse D, Leite M, Suki WN. ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol Renal Physiol 267: F289–F295, 1994. doi: 10.1152/ajprenal.1994.267.2.F289. [DOI] [PubMed] [Google Scholar]
- 442.Rump LC, Bohmann C, Schwertfeger E, Krumme B, von Kügelgen I, Schollmeyer P. Extracellular ATP in the human kidney: mode of release and vascular effects. J Auton Pharmacol 16: 371–376, 1996. doi: 10.1111/j.1474-8673.1996.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 443.Rump LC, Oberhauser V, von Kügelgen I. Purinoceptors mediate renal vasodilation by nitric oxide dependent and independent mechanisms. Kidney Int 54: 473–481, 1998. doi: 10.1046/j.1523-1755.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- 444.Sabirov RZ, Okada Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol 54: 7–14, 2004. doi: 10.2170/jjphysiol.54.7. [DOI] [PubMed] [Google Scholar]
- 445.Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol 185: 103–115, 2002. doi: 10.1007/s00232-001-0116-z. [DOI] [PubMed] [Google Scholar]
- 446.Sakai K, Akima M, Nabata H. A possible purinergic mechanism for reactive ischemia in isolated, cross-circulated rat kidney. Jpn J Pharmacol 29: 235–242, 1979. doi: 10.1254/jjp.29.235. [DOI] [PubMed] [Google Scholar]
- 447.Sakamoto S, Miyaji T, Hiasa M, Ichikawa R, Uematsu A, Iwatsuki K, Shibata A, Uneyama H, Takayanagi R, Yamamoto A, Omote H, Nomura M, Moriyama Y. Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Sci Rep 4: 6689, 2014. doi: 10.1038/srep06689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Sanchez-Ferrer CF, Roman RJ, Harder DR. Pressure-dependent contraction of rat juxtamedullary afferent arterioles. Circ Res 64: 790–798, 1989. doi: 10.1161/01.RES.64.4.790. [DOI] [PubMed] [Google Scholar]
- 449.Sands JM, Layton HE. Advances in understanding the urine-concentrating mechanism. Annu Rev Physiol 76: 387–409, 2014. doi: 10.1146/annurev-physiol-021113-170350. [DOI] [PubMed] [Google Scholar]
- 450.Sathanoori R, Swärd K, Olde B, Erlinge D. Correction: The ATP Receptors P2X7 and P2X4 Modulate High Glucose and Palmitate-Induced Inflammatory Responses in Endothelial Cells. PLoS One 10: e0133346, 2015. doi: 10.1371/journal.pone.0133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Säve S, Persson K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun 78: 3609–3615, 2010. doi: 10.1128/IAI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Schlatter E, Ankorina I, Haxelmans S, Kleta R. Effects of diadenosine polyphosphates, ATP and angiotensin II on cytosolic Ca2+ activity and contraction of rat mesangial cells. Pflugers Arch 430: 721–728, 1995. doi: 10.1007/BF00386167. [DOI] [PubMed] [Google Scholar]
- 453.Schnermann J. Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice. Am J Physiol Renal Physiol 300: F339–F344, 2011. doi: 10.1152/ajprenal.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int 74: 418–426, 2008. doi: 10.1038/ki.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65: 501–529, 2003. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 456.Scholz-Pedretti K, Pfeilschifter J, Kaszkin M. Potentiation of cytokine induction of group IIA phospholipase A2 in rat mesangial cells by ATP and adenosine via the A2A adenosine receptor. Br J Pharmacol 132: 37–46, 2001. doi: 10.1038/sj.bjp.0703774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Schöneberg T, Hermsdorf T, Engemaier E, Engel K, Liebscher I, Thor D, Zierau K, Römpler H, Schulz A. Structural and functional evolution of the P2Y12-like receptor group. Purinergic Signal 3: 255–268, 2007. doi: 10.1007/s11302-007-9064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol Renal Physiol 275: F962–F971, 1998. [DOI] [PubMed] [Google Scholar]
- 459.Schulze-Lohoff E, Schagerl S, Ogilvie A, Sterzel RB. Extracellular ATP augments mesangial cell growth induced by multiple growth factors. Nephrol Dial Transplant 10: 2027–2034, 1995. [PubMed] [Google Scholar]
- 460.Schulze-Lohoff E, Bitzer M, Ogilvie A, Sterzel RB. P2U-purinergic receptor activation mediates inhibition of cAMP accumulation in cultured renal mesangial cells. Ren Physiol Biochem 18: 219–230, 1995. [DOI] [PubMed] [Google Scholar]
- 461.Schulze-Lohoff E, Ogilvie A, Sterzel RB. Extracellular nucleotides as signalling molecules for renal mesangial cells. J Auton Pharmacol 16: 381–384, 1996. doi: 10.1111/j.1474-8673.1996.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 462.Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Extracellular ATP stimulates proliferation of cultured mesangial cells via P2-purinergic receptors. Am J Physiol Renal Physiol 263: F374–F383, 1992. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- 463.Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Vasoactive diadenosine polyphosphates promote growth of cultured renal mesangial cells. Hypertension 26: 899–904, 1995. doi: 10.1161/01.HYP.26.6.899. [DOI] [PubMed] [Google Scholar]
- 464.Schwartz DD, Malik KU. Renal periarterial nerve stimulation-induced vasoconstriction at low frequencies is primarily due to release of a purinergic transmitter in the rat. J Pharmacol Exp Ther 250: 764–771, 1989. [PubMed] [Google Scholar]
- 465.Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol 28: 340–350, 2001. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 466.Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001. doi: 10.1152/ajprenal.2001.280.6.F945. [DOI] [PubMed] [Google Scholar]
- 467.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 468.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615: 7–32, 2003. doi: 10.1016/S0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 469.Sehic E, Ruan Y, Malik KU. Attenuation by alpha,beta-methylenadenosine-5′-triphosphate of periarterial nerve stimulation-induced renal vasoconstriction is not due to desensitization of purinergic receptors. J Pharmacol Exp Ther 271: 983–992, 1994. [PubMed] [Google Scholar]
- 470.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol 304: C976–C984, 2013. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol 178: 4240–4249, 2007. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 472.Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 29: 269–278, 2014. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243–F1248, 2005. doi: 10.1152/ajprenal.00152.2004. [DOI] [PubMed] [Google Scholar]
- 474.Shirley DG, Bailey MA, Wildman SSP, Tam FWK, Unwin RJ. Extracellular nucleotides and renal function. In: Seldin & Giebisch’s The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW. New York: Academic, 2013, p. 511–537. [Google Scholar]
- 475.Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47: 563–567, 2006. doi: 10.1161/01.HYP.0000197954.93874.ef. [DOI] [PubMed] [Google Scholar]
- 476.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008. doi: 10.1152/ajprenal.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Silva GB, Garvin JL. Extracellular ATP inhibits transport in medullary thick ascending limbs: role of P2X receptors. Am J Physiol Renal Physiol 297: F1168–F1173, 2009. doi: 10.1152/ajprenal.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Solini A, Santini E, Chimenti D, Chiozzi P, Pratesi F, Cuccato S, Falzoni S, Lupi R, Ferrannini E, Pugliese G, Di Virgilio F. Multiple P2X receptors are involved in the modulation of apoptosis in human mesangial cells: evidence for a role of P2X4. Am J Physiol Renal Physiol 292: F1537–F1547, 2007. doi: 10.1152/ajprenal.00440.2006. [DOI] [PubMed] [Google Scholar]
- 480.Solini A, Usuelli V, Fiorina P. The dark side of extracellular ATP in kidney diseases. J Am Soc Nephrol 26: 1007–1016, 2015. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Solini A, Iacobini C, Ricci C, Chiozzi P, Amadio L, Pricci F, Di Mario U, Di Virgilio F, Pugliese G. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int 67: 875–885, 2005. doi: 10.1111/j.1523-1755.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 482.Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol 231: 342–353, 2013. doi: 10.1002/path.4237. [DOI] [PubMed] [Google Scholar]
- 483.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension 17: 117–130, 1991. doi: 10.1161/01.HYP.17.2.117. [DOI] [PubMed] [Google Scholar]
- 484.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Steinhausen M, Snoei H, Parekh N, Baker R, Johnson PC. Hydronephrosis: a new method to visualize vas afferens, efferens, and glomerular network. Kidney Int 23: 794–806, 1983. doi: 10.1038/ki.1983.98. [DOI] [PubMed] [Google Scholar]
- 486.Stiepanow-Trzeciak A, Jankowski M, Angielski S, Szczepanska-Konkel M. P1,P4-diadenosine tetraphosphate (Ap4A) inhibits proximal tubular reabsorption of sodium in rats. Nephron, Physiol 106: 13–18, 2007. doi: 10.1159/000101488. [DOI] [PubMed] [Google Scholar]
- 487.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010. doi: 10.1681/ASN.2010040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol 298: F216–F223, 2010. doi: 10.1152/ajprenal.00295.2009. [DOI] [PubMed] [Google Scholar]
- 489.Stokes L, Scurrah K, Ellis JA, Cromer BA, Skarratt KK, Gu BJ, Harrap SB, Wiley JS. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 58: 1086–1092, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176180. [DOI] [PubMed] [Google Scholar]
- 490.Sträter N. Ecto-5′-nucleotidase: Structure function relationships. Purinergic Signal 2: 343–350, 2006. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 289: F585–F592, 2005. doi: 10.1152/ajprenal.00050.2005. [DOI] [PubMed] [Google Scholar]
- 493.Sun R, Miller RL, Hemmert AC, Zhang P, Shi H, Nelson RD, Kishore BK. Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 release by IMCD. Am J Physiol Renal Physiol 289: F768–F776, 2005. doi: 10.1152/ajprenal.00177.2005. [DOI] [PubMed] [Google Scholar]
- 494.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 71: 333–359, 2009. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 495.Svendsen SL, Isidor S, Praetorius HA, Leipziger J. P2X Receptors Inhibit NaCl Absorption in mTAL Independently of Nitric Oxide. Front Physiol 8: 18, 2017. doi: 10.3389/fphys.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Svenningsen P, Burford JL, Peti-Peterdi J. ATP releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Front Physiol 4: 292, 2013. doi: 10.3389/fphys.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Tagawa H, Vander AJ. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ Res 26: 327–338, 1970. doi: 10.1161/01.RES.26.3.327. [DOI] [PubMed] [Google Scholar]
- 498.Tak E, Ridyard D, Kim JH, Zimmerman M, Werner T, Wang XX, Shabeka U, Seo SW, Christians U, Klawitter J, Moldovan R, Garcia G, Levi M, Haase V, Ravid K, Eltzschig HK, Grenz A. CD73-dependent generation of adenosine and endothelial Adora2b signaling attenuate diabetic nephropathy. J Am Soc Nephrol 25: 547–563, 2014. doi: 10.1681/ASN.2012101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Takeda M, Kawamura T, Kobayashi M, Endou H. ATP-induced calcium mobilization in glomerular mesangial cells is mediated by P2U purinoceptor. Biochem Mol Biol Int 39: 1193–1200, 1996. doi: 10.1080/15216549600201382. [DOI] [PubMed] [Google Scholar]
- 500.Tam FWK. Current pharmacotherapy for the treatment of crescentic glomerulonephritis. Expert Opin Investig Drugs 15: 1353–1369, 2006. doi: 10.1517/13543784.15.11.1353. [DOI] [PubMed] [Google Scholar]
- 501.Tang XF, Fan JY, Meng J, Jin C, Yuan JQ, Yang YJ. Impact of new oral or intravenous P2Y12 inhibitors and clopidogrel on major ischemic and bleeding events in patients with coronary artery disease: a meta-analysis of randomized trials. Atherosclerosis 233: 568–578, 2014. doi: 10.1016/j.atherosclerosis.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 502.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci USA 102: 6954–6959, 2005. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol Cell Physiol 275: C1391–C1406, 1998. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- 505.Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Tezza S, Ben Nasr M, D’Addio F, Vergani A, Usuelli V, Falzoni S, Bassi R, Dellepiane S, Fotino C, Rossi C, Maestroni A, Solini A, Corradi D, Giani E, Mameli C, Bertuzzi F, Pezzolesi MG, Wasserfall CH, Atkinson MA, Füchtbauer EM, Ricordi C, Folli F, Di Virgilio F, Pileggi A, Dhe-Paganon S, Zuccotti GV, Fiorina P. Islet-Derived eATP Fuels Autoreactive CD8+ T Cells and Facilitates the Onset of Type 1 Diabetes. Diabetes 67: 2038–2053, 2018. doi: 10.2337/db17-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Thai TL, Yu L, Eaton DC, Duke BJ, Al-Khalili O, Lam HYC, Ma H, Bao H-F. Basolateral P2X4channels stimulate ENaC activity in Xenopus cortical collecting duct A6 cells. Am J Physiol Renal Physiol 307: F806–F813, 2014. doi: 10.1152/ajprenal.00350.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Thomas J, Deetjen P, Ko WH, Jacobi C, Leipziger J. P2Y(2) receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol 183: 115–124, 2001. doi: 10.1007/s00232-001-0059-4. [DOI] [PubMed] [Google Scholar]
- 509.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem 285: 17406–17416, 2010. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294: R1769–R1776, 2008. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na(+) channel. Curr Opin Nephrol Hypertens 21: 52–60, 2012. doi: 10.1097/MNH.0b013e32834db4a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Torres B, Zambon AC, Insel PA. P2Y11 receptors activate adenylyl cyclase and contribute to nucleotide-promoted cAMP formation in MDCK-D(1) cells. A mechanism for nucleotide-mediated autocrine-paracrine regulation. J Biol Chem 277: 7761–7765, 2002. doi: 10.1074/jbc.M110352200. [DOI] [PubMed] [Google Scholar]
- 514.Transbøl I, Christiansen C, Baastrup PC, Nielsen MD, Giese J. Endocrine effects of lithium. III. Hypermagnesaemia and activation of the renin-aldosterone system. Acta Endocrinol (Copenh) 88: 619–624, 1978. [PubMed] [Google Scholar]
- 515.Truong LD, Trostel J, McMahan R, Chen JF, Garcia GE. Macrophage A2A Adenosine Receptors Are Essential to Protect from Progressive Kidney Injury. Am J Pathol 186: 2601–2613, 2016. doi: 10.1016/j.ajpath.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Turner CM, Ramesh B, Srai SKS, Burnstock G, Unwin RJ. Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs 178: 168–179, 2004. doi: 10.1159/000082247. [DOI] [PubMed] [Google Scholar]
- 517.Turner CM, Elliott JI, Tam FW. P2 receptors in renal pathophysiology. Purinergic Signal 5: 513–520, 2009. doi: 10.1007/s11302-009-9153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Turner CM, King BF, Srai KS, Unwin RJ. Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol 292: F15–F25, 2007. doi: 10.1152/ajprenal.00103.2006. [DOI] [PubMed] [Google Scholar]
- 519.Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22: 386–395, 2007. doi: 10.1093/ndt/gfl589. [DOI] [PubMed] [Google Scholar]
- 520.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 521.Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci 18: 237–241, 2003. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- 522.Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 18: 169–174, 2003. doi: 10.1152/nips.01442.2003. [DOI] [PubMed] [Google Scholar]
- 523.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 524.Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol 20: 721–729, 2009. doi: 10.1681/ASN.2008040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Vallon V, Mühlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 526.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflugers Arch 448: 214–221, 2004. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- 527.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Vargas F, Moreno MRR, Osuna A. Renal vascular reactivity to ATP in ageing rats. Med Sci Res 24: 263–265, 1996. [Google Scholar]
- 529.Vargas F, Osuna A, Fernández-Rivas A. Renal vascular reactivity to ATP in hyper- and hypothyroid rats. Experientia 52: 225–229, 1996. doi: 10.1007/BF01920711. [DOI] [PubMed] [Google Scholar]
- 530.Vekaria RM, Shirley DG, Sévigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290: F550–F560, 2006. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- 531.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006. doi: 10.1681/ASN.2005111171. [DOI] [PubMed] [Google Scholar]
- 532.Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 127: 463–475, 2013. doi: 10.1161/CIRCULATIONAHA.112.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Vergani A, Tezza S, Fotino C, Visner G, Pileggi A, Chandraker A, Fiorina P. The purinergic system in allotransplantation. Am J Transplant 14: 507–514, 2014. doi: 10.1111/ajt.12567. [DOI] [PubMed] [Google Scholar]
- 534.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol 170: 5728–5738, 2003. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 535.Volonté C, Amadio S, D’Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther 112: 264–280, 2006. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 536.Vonend O, Grote T, Oberhauser V, Von Kügelgen I, Rump LC. P2Y-receptors stimulating the proliferation of human mesangial cells through the MAPK42/44 pathway. Br J Pharmacol 139: 1119–1126, 2003. doi: 10.1038/sj.bjp.0705358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Vonend O, Oberhauser V, von Kügelgen I, Apel TW, Amann K, Ritz E, Rump LC. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int 61: 1617–1626, 2002. doi: 10.1046/j.1523-1755.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 538.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 539.Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344: 634–638, 2014. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 540.Wang H, Wang J, Bai Y, Li J, Li L, Dong Y. CD11c+ CD8+ T Cells Reduce Renal Fibrosis Following Ureteric Obstruction by Inducing Fibroblast Apoptosis. Int J Mol Sci 18: 1–12, 2016. doi: 10.3390/ijms18010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal 7: ra69, 2014. doi: 10.1126/scisignal.2005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest 125: 3077–3086, 2015. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020–12025, 1996. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Wang Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Ohta M, Soma M, Aoi N, Ozawa Y, Ma Y. The purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) gene associated with essential hypertension in Japanese men. J Hum Hypertens 24: 327–335, 2010. doi: 10.1038/jhh.2009.67. [DOI] [PubMed] [Google Scholar]
- 545.Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Vasomotor effects of purinergic agonists in isolated rabbit afferent arterioles. Am J Physiol Renal Physiol 263: F1026–F1033, 1992. doi: 10.1152/ajprenal.1992.263.6.F1026. [DOI] [PubMed] [Google Scholar]
- 546.Welsh-Bacic D, Nowik M, Kaissling B, Wagner CA. Proliferation of acid-secretory cells in the kidney during adaptive remodelling of the collecting duct. PLoS One 6: e25240, 2011. doi: 10.1371/journal.pone.0025240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.White PJ, Webb TE, Boarder MR. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol Pharmacol 63: 1356–1363, 2003. doi: 10.1124/mol.63.6.1356. [DOI] [PubMed] [Google Scholar]
- 548.White SM, Imig JD, Kim T-T, Hauschild BC, Inscho EW. Calcium signaling pathways utilized by P2X receptors in freshly isolated preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 280: F1054–F1061, 2001. doi: 10.1152/ajprenal.2001.280.6.F1054. [DOI] [PubMed] [Google Scholar]
- 549.Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24: 1810–1815, 2004. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- 550.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Wildman SS, Boone M, Peppiatt-Wildman CM, Contreras-Sanz A, King BF, Shirley DG, Deen PM, Unwin RJ. Nucleotides downregulate aquaporin 2 via activation of apical P2 receptors. J Am Soc Nephrol 20: 1480–1490, 2009. doi: 10.1681/ASN.2008070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wildman SS, Hooper KM, Turner CM, Sham JS, Lakatta EG, King BF, Unwin RJ, Sutters M. The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl- conductance through increased Ca2+ entry. Am J Physiol Renal Physiol 285: F1168–F1178, 2003. doi: 10.1152/ajprenal.00171.2003. [DOI] [PubMed] [Google Scholar]
- 553.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Wildman SSP, Turner CM, Marks J, Shirley DG, King BF, Unwin RJ. Differential regulation of renal ENaC by apical P2X and P2Y receptors. FASEB J 21: 937.3, 2007. [Google Scholar]
- 555.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 16: 1434–1438, 2010. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 556.Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem 277: 34017–34023, 2002. doi: 10.1074/jbc.M205120200. [DOI] [PubMed] [Google Scholar]
- 557.Wilson JL, Miranda CA, Knepper MA. Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013. doi: 10.1007/s10157-013-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999. [DOI] [PubMed] [Google Scholar]
- 559.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 560.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 561.Woda CB, Leite M Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002. doi: 10.1152/ajprenal.00316.2001. [DOI] [PubMed] [Google Scholar]
- 562.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Ca2+i signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 296: F1464–F1476, 2009. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol 292: F930–F945, 2007. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang FL, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, Patapoutian A. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173: 762–775.e16, 2018. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Xu XJ, Boumechache M, Robinson LE, Marschall V, Górecki DC, Masin M, Murrell-Lagnado RD. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J Cell Sci 125: 3776–3789, 2012. doi: 10.1242/jcs.099374. [DOI] [PubMed] [Google Scholar]
- 566.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12: 133–137, 2006. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 567.Yan Y, Bai J, Zhou X, Tang J, Jiang C, Tolbert E, Bayliss G, Gong R, Zhao TC, Zhuang S. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol 308: C463–C472, 2015. doi: 10.1152/ajpcell.00245.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res 93: 338–345, 2003. doi: 10.1161/01.RES.0000086802.21850.5D. [DOI] [PubMed] [Google Scholar]
- 569.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49: 473–497, 2014. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 570.Yoshioka K, Nakata H. ATP- and adenosine-mediated signaling in the central nervous system: purinergic receptor complex: generating adenine nucleotide-sensitive adenosine receptors. J Pharmacol Sci 94: 88–94, 2004. doi: 10.1254/jphs.94.88. [DOI] [PubMed] [Google Scholar]
- 571.Yoshioka K, Saitoh O, Nakata H. Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci USA 98: 7617–7622, 2001. doi: 10.1073/pnas.121587098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yoshioka K, Saitoh O, Nakata H. Agonist-promoted heteromeric oligomerization between adenosine A(1) and P2Y(1) receptors in living cells. FEBS Lett 523: 147–151, 2002. doi: 10.1016/S0014-5793(02)02965-4. [DOI] [PubMed] [Google Scholar]
- 573.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 574.Zambon AC, Hughes RJ, Meszaros JG, Wu JJ, Torres B, Brunton LL, Insel PA. P2Y(2) receptor of MDCK cells: cloning, expression, and cell-specific signaling. Am J Physiol Renal Physiol 279: F1045–F1052, 2000. doi: 10.1152/ajprenal.2000.279.6.F1045. [DOI] [PubMed] [Google Scholar]
- 575.Zhang M, Piskuric NA, Vollmer C, Nurse CA. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. J Physiol 590: 4335–4350, 2012. doi: 10.1113/jphysiol.2012.236265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Zhang Y, Hansson KM, Liu T, Magnell K, Huang Y, Carlson NG, Kishore BK. Genetic deletion of ADP-activated P2Y12 receptor ameliorates lithium-induced nephrogenic diabetes insipidus in mice. Acta Physiol (Oxf) 225: e13191, 2019. doi: 10.1111/apha.13191. [DOI] [PubMed] [Google Scholar]
- 577.Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK. Potential involvement of P2Y2 receptor in diuresis of postobstructive uropathy in rats. Am J Physiol Renal Physiol 298: F634–F642, 2010. doi: 10.1152/ajprenal.00382.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK. Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol 305: F407–F416, 2013. doi: 10.1152/ajprenal.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F657–F668, 2011. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194–F1201, 2009. doi: 10.1152/ajprenal.90774.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Zhang Y, Peti-Peterdi J, Brandes AU, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK. Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice. Purinergic Signal 13: 239–248, 2017. doi: 10.1007/s11302-017-9555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhang Y, Peti-Peterdi J, Heiney KM, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK. Clopidogrel attenuates lithium-induced alterations in renal water and sodium channels/transporters in mice. Purinergic Signal 11: 507–518, 2015. doi: 10.1007/s11302-015-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Zhang Y, Peti-Peterdi J, Müller CE, Carlson NG, Baqi Y, Strasburg DL, Heiney KM, Villanueva K, Kohan DE, Kishore BK. P2Y12 Receptor Localizes in the Renal Collecting Duct and Its Blockade Augments Arginine Vasopressin Action and Alleviates Nephrogenic Diabetes Insipidus. J Am Soc Nephrol 26: 2978–2987, 2015. doi: 10.1681/ASN.2014010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Zhang Y, Pop IL, Carlson NG, Kishore BK. Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302: F70–F77, 2012. doi: 10.1152/ajprenal.00444.2011. [DOI] [PubMed] [Google Scholar]
- 585.Zhang Y, Riquier-Brison A, Liu T, Huang Y, Carlson NG, Peti-Peterdi J, Kishore BK. Genetic Deletion of P2Y2 Receptor Offers Long-Term (5 Months) Protection Against Lithium-Induced Polyuria, Natriuresis, Kaliuresis, and Collecting Duct Remodeling and Cell Proliferation. Front Physiol 9: 1765, 2018. doi: 10.3389/fphys.2018.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Zhang Y, Robson SC, Morris KL, Heiney KM, Dwyer KM, Kishore BK, Ecelbarger CM. Impaired natriuretic response to high-NaCl diet plus aldosterone infusion in mice overexpressing human CD39, an ectonucleotidase (NTPDase1). Am J Physiol Renal Physiol 308: F1398–F1408, 2015. doi: 10.1152/ajprenal.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008. doi: 10.1152/ajprenal.90311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum 65: 3176–3185, 2013. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension 46: 562–568, 2005. doi: 10.1161/01.HYP.0000179584.39937.41. [DOI] [PubMed] [Google Scholar]
- 590.Zhong XZ, Cao Q, Sun X, Dong XP. Activation of lysosomal P2X4 by ATP transported into lysosomes via VNUT/SLC17A9 using V-ATPase generated voltage gradient as the driving force. J Physiol 594: 4253–4266, 2016. doi: 10.1113/JP271893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Zhu D, Bousamra M II, Zeldin DC, Falck JR, Townsley M, Harder DR, Roman RJ, Jacobs ER. Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 278: L335–L343, 2000. doi: 10.1152/ajplung.2000.278.2.L335. [DOI] [PubMed] [Google Scholar]
- 592.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362: 299–309, 2000. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 593.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]