Abstract
Studies in rodents with reduced nephron mass have suggested a strong positive correlation between dietary phosphate consumption and CKD progression. Prior work by our group demonstrated that dietary phosphate restriction can prevent tubular injury and microcyst formation in rodents with glomerulonephritis. Tubular injury and cystic dilation of tubules are key contributors to kidney function decline in polycystic kidney disease (PKD). Here, we determined whether dietary phosphate restriction slows renal cyst growth and fibrosis in a mouse model of PKD. Pcy/pcy mice received a normal phosphate (0.54%) or a phosphate-restricted (0.02%) diet (n = 10/group) from 7 to 20 wk of age. All of the other major dietary constituents, including protein source and content, were comparable between the two diets. At 20 wk, body weight, kidney weight-to-body weight ratio (KW/BW), cystic area, cyst number, and kidney fibrosis were quantified. Pcy/pcy mice fed a phosphate-restricted diet had lower serum phosphate, fibroblast growth factor 23, and parathyroid hormone levels, along with elevated serum calcium levels and increased kidney Klotho gene expression compared with mice that consumed the control diet. Dietary phosphate restriction resulted in a 25% lower KW/BW ratio and reduced the cyst number, cystic index, and gene expression for the tubular injury markers neutrophil gelatinase-associated lipocalin and interleukin-18. Mice fed the phosphate-restricted diet exhibited lower kidney expression for pathways involved in collagen deposition and myofibroblast activation (collagen type I-α1, phosphorylated SMAD3, and α-smooth muscle actin); however, histological differences in kidney fibrosis were not appreciated. Dietary phosphate restriction slows cystogenesis and inhibits the activation of key pathways in the generation of kidney fibrosis in PKD mice.
Keywords: chronic kidney disease, fibroblast growth factor 23, fibrosis, phosphate, polycystic kidney disease
INTRODUCTION
In polycystic kidney disease (PKD) and other forms of chronic kidney disease (CKD), there is a progressive rise in phosphaturic hormones, including fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH), beginning in the very early stages of kidney dysfunction (10, 15, 36). The resulting increase in urinary phosphate excretion is an important mechanism for maintaining systemic phosphate homeostasis in the setting of reduced functional nephron numbers. As a result, overt hyperphosphatemia is not observed until late-stage disease, when the renal capacity for phosphate excretion is insufficient to match the phosphate load generated from dietary intake and skeletal resorption.
Studies in CKD rodent models have suggested a strong positive correlation between dietary phosphate consumption and CKD progression (2, 3, 14, 18, 19, 24, 25, 48). Moreover, serum concentrations of FGF23 in patients predict a higher risk for loss of kidney function in both native and transplanted kidneys (11, 30, 43). Previously, we investigated the impact of dietary phosphate restriction on FGF23 production in a mouse model of Alport syndrome (48). An unexpected observation in this investigation was a dramatic preservation of renal tubule architecture in mice that consumed a phosphate-restricted diet, while the severity of glomerular injury appeared to be unaffected. We speculated that the potential renoprotective effect of dietary phosphate restriction was related to mechanisms directly preventing tubular injury.
Tubular injury plays an important role in the pathophysiology of kidney function decline in PKD. In PKD kidneys, the progressive expansion of fluid-filled cysts encroaches on surrounding parenchyma leading to further tubular injury, interstitial inflammation, and fibrosis. Moreover, induction of tubular injury by either experimental hypoxia or the administration of exogenous toxins accelerates cystogenesis and nephron loss in PKD mice (17, 22, 37). To determine the role of dietary phosphate content on tubular injury and kidney disease progression in PKD, we tested the impact of dietary phosphate restriction on cyst growth and fibrosis in pcy/pcy mice, a slowly progressive model of PKD.
METHODS
Animal preparation and study protocol.
All mice were maintained in accordance with recommendations in the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (1996), and all animal protocols were reviewed and approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee before the commencement of this research.
Pcy/pcy mice possess a deletion of nephrocystin-3 (Nphp3) and develop kidney cysts that primarily originate in the collecting duct. Beginning at 7 wk of age, both male and female pcy/pcy littermates (CD1 background) were randomized in a 1:1 distribution to receive a control diet (normal phosphate content) or a phosphate-restricted diet for a total of 13 wk. Mice were euthanized by exsanguination, and tissue was collected at 20 wk of age. Mouse diets were custom made by Envigo (Madison, WI) and consisted of highly refined, phytate-free ingredients containing equal amounts of calcium (0.6%), vitamin D (2200 IU), fats (5.0%), carbohydrates (70%), and protein (16.3%) per kilogram of diet. The phosphate content of the diets was 0.54% for the control diet (TD.06009) and 0.02% for the phosphate-restricted diet (TD.80235). Both diets utilized an egg white solid protein source.
Tissue processing and histology.
Kidneys were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and cut into 4-µm sections. For quantification of cyst burden, tissue sections were stained with hematoxylin and eosin, and images were collected using a dissecting microscope connected to a digital camera (Leica Microsystems, Buffalo Grove, IL). Total number of cysts and cystic cross-sectional surface area (SA) per kidney section were determined by a blinded observer using a morphometric analysis system (AnalySis, Soft Imaging System, Lakewood, CO). Interstitial fibrosis was assessed by picrosirius red staining with subsequent imaging using ImageJ 64 (http://rsb.info.nih.gov). Total area and area of interstitial fibrosis were quantified by a renal pathologist (T. A. Fields) blinded to specimen identity. The method of fibrosis assessment was adapted from prior recommendations for renal biopsy evaluation of PKD kidneys (42). Briefly, images were imported into ImageJ (v1.43, NIH), midsagittal kidney sections stained with picrosirius red were visually inspected, and areas of pathological fibrosis were outlined. Following identification of fibrotic areas, ImageJ was used to calculate the area of fibrosis as a percentage of the total cross-sectional area of the kidney.
Serum biochemistries.
Blood urea nitrogen (BUN), creatinine, calcium, and phosphate were measured using an Integra 400 Plus Bioanalyzer (Roche Diagnostics, Indianapolis, IN). Intact FGF23 was measured by ELISA (Kainos Laboratory, Tokyo), and PTH was measured by the Mouse Intact PTH ELISA Kit (Alpco Diagnostics, Salem, NH).
Quantitative real-time PCR.
Kidney specimens for gene expression analysis were snap frozen in liquid nitrogen and stored at −80°C until further processing. Total RNA was extracted following homogenization using TRI-Reagent (Molecular Research Center, Cincinnati, OH) and treated with RNase-free DNase (Qiagen, Valencia, CA). First-strand cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), with 1 μg of RNA used for each reverse transcriptase reaction. PCRs contained 100 ng cDNA, 300 nM of each primer, and 1× iQ SYBR Green Supermix (Bio-Rad) in 50 μl. The threshold cycle (Ct) of each gene product was normalized to the Ct for hypoxanthine guanine phosphoribosyl transferase (HPRT) for all quantitative RT-PCR experiments. Gene primer sequences were as follows: HPRT, forward 5′-TGATAGATCCATTCCTATGACTGTAGA-3′ and reverse 5′-AAGACATTCTTTCCAGTTAAAGTTGAG-3′; collagen type I-α1 (Col1α1), forward 5′-CATGTTCAGCTTTGTGGACCT-3′ and reverse 5′-GCAGCTGACTTCAGGGATGT-3′; transforming growth factor-β (TGFβ), forward 5′-TGGAGCAACATGTGGAACTC-3′ and reverse 5′-GTCAGCAGCCGGTTACCA-3′; α-smooth muscle actin (α-SMA; Acta2), forward 5′-CTCTCTTCCAGCCATCTTTCAT-3′ and reverse 5′-TATAGGTGGTTTCGTGGATGC-3′; Klotho, forward 5′-TCTCAAGAAGTTCATAATGGAAACC-3′ and reverse 5′-CAGAAAGTCAACGTAGAAGAGTCCT-3′; neutrophil gelatinase-associated lipocalin (NGAL; Lcn2), forward 5′-CCATCTATGAGCTACAAGAGAACAAT-3′ and reverse 5′-TCTGATCCAGTAGCGACAGC-3′; IL-18, forward 5′-CAAACCTTCCAAATCACTTCCT-3′ and reverse 5′-TCCTTG AATTGACGCAAGA-3′; and periostin (Postn), forward 5′-AAGCTGCGGCAAGACAAG-3′ and reverse 5′-TCAAATCTGCAGCTTCAAGG-3′.
Western blot analysis.
Soluble proteins were resolved by SDS-PAGE using the Bio-Rad Mini-Protean III cell system and then transferred from the gels to nitrocellulose membranes. Membranes were blocked at room temperature for 1 h, incubated with primary antibody for the target of interest overnight at 4°C, rinsed, and incubated with horseradish peroxidase-labeled anti-rabbit IgG secondary antibody (Cell Signaling Technology, Danvers, MA; no. 7074). Primary antibodies used for Western blot analyses were anti-phosphorylated (p)SMAD3 (Abcam, Cambridge, MA; ab52903), anti-Col1α1 (Abcam; ab34710), and α-SMA (Abcam; ab5694). Proteins were visualized using the ECL system, and images were captured for analysis. Protein content for individual bands of interest was normalized to the quantified total protein expression for the corresponding sample lane using AzureSpot 2.0 software (Azure Biosystems, Dublin, CA).
Statistical analysis.
Differences between multiple groups at different time points were evaluated by two-way ANOVA, while differences between two groups at a single time point were evaluated by a two-sided Student’s t test (for data with a Gaussian distribution) or Mann-Whitney U test (for data with a non-Gaussian distribution). Computations were performed using Prism 5 software (GraphPad Software, San Diego, CA) and presented as means ± SD unless otherwise specified.
RESULTS
Phosphate-restricted mice exhibit lower body weight and kidney-to-body weight ratio.
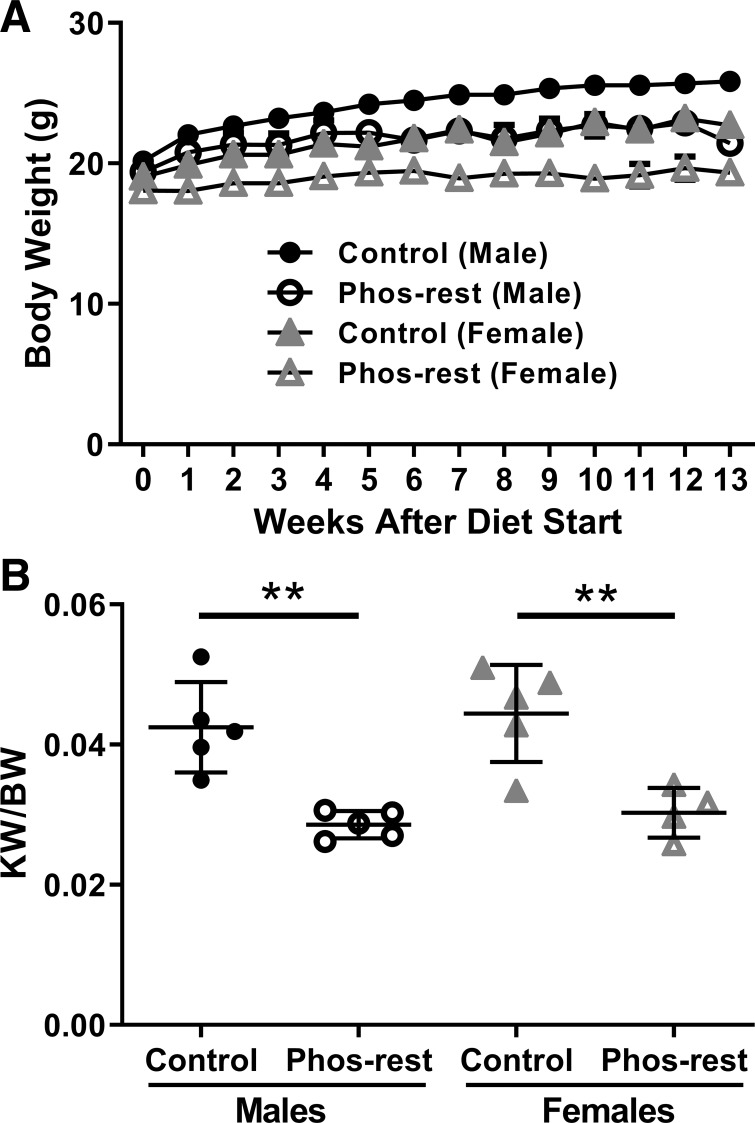
Pcy/pcy mice that consumed the phosphate-restricted diet had a modestly lower final body weight compared with age- and sex-matched littermates (male mice: 25.8 ± 1.7 g for control diet vs. 21.4 ± 1.4 g for phosphate-restricted diet; female mice: 22.7 ± 0.7 g for control diet vs. 19.3 ± 1.0 g for phosphate-restricted diet) (Fig. 1A). Pcy/pcy mice that consumed the phosphate-restricted diet had a 25% lower kidney weight corrected for body weight (KW/BW) compared with mice that consumed the control diet (male mice: 4.2 ± 0.6% for control diet vs. 2.9 ± 0.2% for phosphate-restricted diet; female mice: 4.4 ± 0.7% for control diet vs. 3.0 ± 0.4% for phosphate-restricted diet) (Fig. 1B). No sex difference was observed for other outcomes of interest, so the remaining analyses are presented as pooled data containing both sexes.
Fig. 1.
Impact of phosphate restriction on kidney and body weight in pcy/pcy mice. A: mice that consumed the phosphate-restricted (Phos-rest) diet demonstrated reduced body weight compared with age- and sex-matched mice fed the control diet (n ≥ 4 per group; at study end, P < 0.001 for male mice versus control and P < 0.001 for female mice versus control by 2-way ANOVA; data are plotted without error bars to preserve figure clarity). B: mice that consumed the phosphate-restricted diet demonstrated a reduced kidney weight-to-body weight ratio (KW/BW) compared with age- and sex-matched mice fed the control diet (**P < 0.01 by Student’s t-test; n ≥ 4 per group).
Phosphate restriction reduces renal cyst burden and markers of tubular injury.
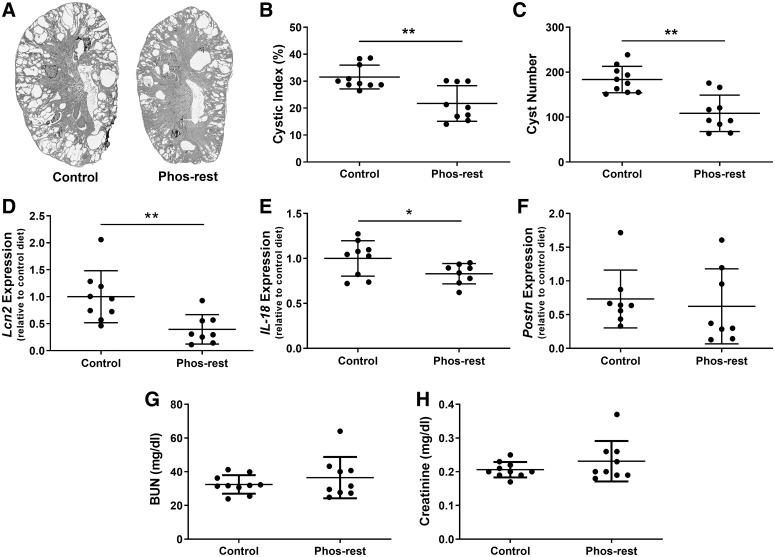
Histological analysis of kidneys demonstrated reduced cyst burden in pcy/pcy mice that consumed the phosphate-restricted diet (Fig. 2A). Cyst burden was quantified by calculating the cystic index (ratio of cyst area to total kidney area multiplied by 100) and counting cyst numbers in midsagittal kidney sections. Pcy/pcy mice that consumed the phosphate-restricted diet demonstrated a 30% lower cystic index (31.5 ± 4.4% for control diet vs. 21.7 ± 6.6% for phosphate-restricted diet; Fig. 2B) and 40% fewer cysts per kidney (184 ± 30 cysts per section for control diet vs. 108 ± 40 cysts per kidney section for phosphate-restricted diet; Fig. 2C). Mice fed a phosphate-restricted diet exhibited lower kidney gene expression for several markers of tubular injury, including NGAL (Lcn2 gene; Fig. 2D) and IL-18 (Fig. 2E), while Postn expression was similar between the two groups (Fig. 2F). Mice that consumed a phosphate-restricted or control diet had comparable concentrations of BUN (32.5 ± 5.5 mg/dL for control diet vs. 36.5 ± 12.3 mg/dL for phosphate-restricted diet; Fig. 2G) and serum creatinine (0.21 ± 0.02 mg/dL for control diet vs. 0.23 ± 0.6 mg/dL for phosphate-restricted diet; Fig. 2H). Of note, at the time of euthanization (20 wk of age), both groups exhibited BUN and creatinine levels that were still within the normal reference range for mice.
Fig. 2.
Chronic phosphate restriction reduces renal cyst burden and markers of tubular injury in pcy/pcy mice. A: representative images of sagittal sections from whole kidneys taken from 20-wk-old pcy/pcy mice fed a control or phosphate-restricted (Phos-rest) diet. Cystic index (B) and cyst number (C) were both reduced in mice fed the phosphate-restricted diet. Kidney gene expression for neutrophil gelatinase-associated lipocalin (NGAL; Lcn2 gene) (D) and interleukin-18 (IL-18) (E) was significantly lower in phosphate-restricted pcy/pcy mice compared with control mice; however, there was no significant difference in periostin (Postn) expression (F) between the two groups. No difference was observed in blood urea nitrogen (BUN) (G) or serum creatinine (H) between mice that consumed a phosphate-restricted and control diet (*P < 0.05, **P < 0.01 by Student’s t test; n ≥ 8 per group).
Phosphate restriction alters serum biochemical parameters.
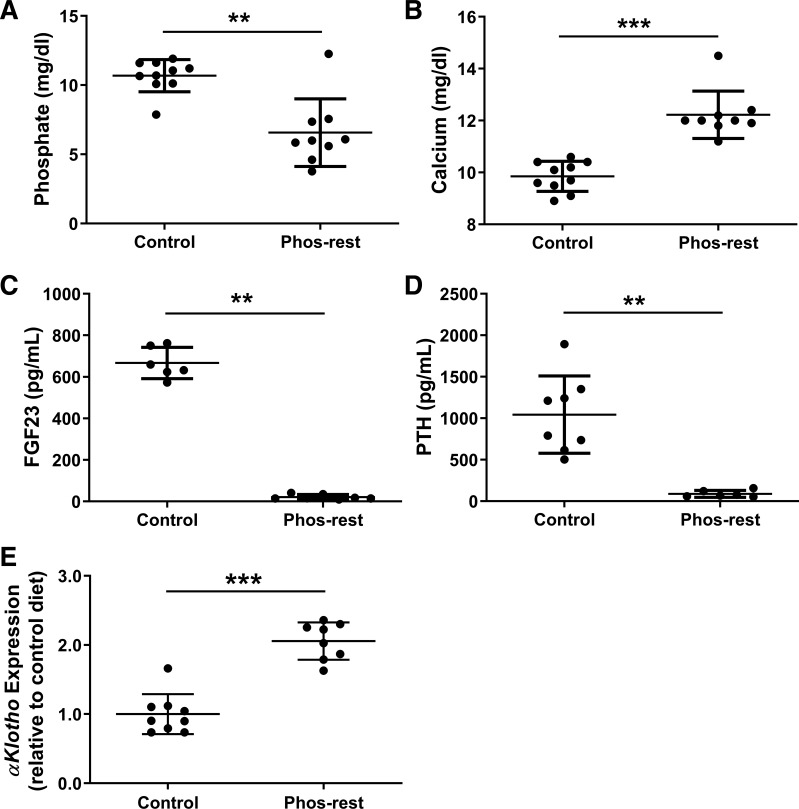
We assessed differences in serum markers of kidney function and mineral metabolism parameters in pcy/pcy mice that received the control and phosphate-restricted diets (Fig. 3). Serum phosphate was significantly lower (10.7 ± 1.2 mg/dL for control diet vs. 6.6 ± 2.4 mg/dL for phosphate-restricted diet; Fig. 3A), while serum calcium was elevated in the phosphate-restricted group (9.9 ± 0.6 mg/dL for control diet vs. 12.2 ± 0.9 mg/dL for phosphate-restricted diet; Fig. 3B). Serum concentrations of both FGF23 and PTH were markedly reduced in the phosphate-restricted group (FGF23: 666.7 ± 75.1 pg/mL for control diet vs. 21.2 ± 12.2 pg/mL for phosphate-restricted diet; PTH: 1043.0 ± 465.6 pg/mL for control diet vs. 89.5 ± 41.7 pg/mL for phosphate-restricted diet) (Fig. 3, C and D). Gene expression for αKlotho was twofold higher in kidneys from mice that consumed the phosphate-restricted diet (Fig. 3E).
Fig. 3.
Chronic phosphate restriction alters mineral metabolism parameters in pcy/pcy mice. Serum phosphate was reduced (A) while serum calcium was increased (B) in the phosphate-restricted (Phos-rest) group. Both FGF23 and parathyroid hormone (PTH) were markedly lower (C and D) while kidney Klotho gene expression (E) was significantly higher in mice fed a phosphate-restricted diet. (**P < 0.01, ***P < 0.001 by Student’s t test; n ≥ 8 per group).
Impact on renal fibrosis.
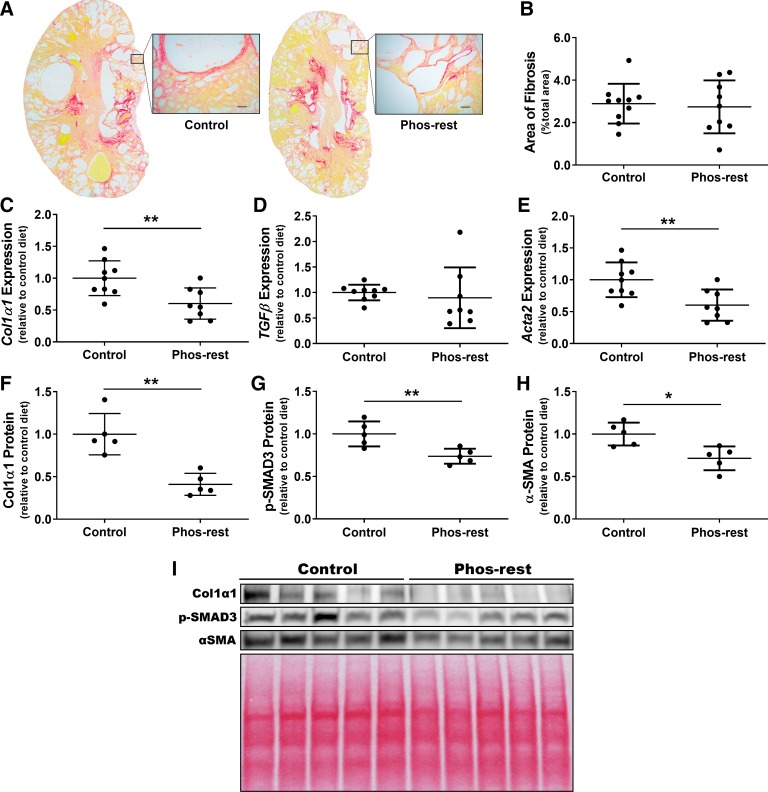
We evaluated the impact of phosphate restriction on the development of interstitial fibrosis in kidneys from pcy/pcy mice by assessing both kidney histology and gene expression for markers of renal fibrosis. We observed only mild kidney fibrosis at the 20-wk time point in all pcy/pcy mice by picrosirius red staining (Fig. 4A), with no obvious difference in fibrosis quantification between the two diet groups (Fig. 4B). Conversely, analysis of kidney gene expression revealed a 40% lower expression of both Col1α1 and α-SMA (Acta2) for pcy/pcy mice fed the phosphate-restricted diet (P < 0.01 for both measures), but no statistically significant difference in TGFβ gene expression (Fig. 4, C–E). Western blot analyses evaluating kidney protein expression differences for Col1α1, pSMAD3 (downstream target of TGF-β), and α-SMA revealed significantly lower expression for all three of these proteins (Fig. 4, F–I).
Fig. 4.
Chronic phosphate restriction reduces kidney markers of collagen deposition and myofibroblast activation in pcy/pcy mice. A: picrosirius red staining of representative sagittal kidney sections from pcy/pcy mice fed either the control or phosphate-restricted (Phos-rest) diet (scale bars = 50 µm). B: fibrosis scoring of picrosirius red-stained kidneys (n ≥ 9 per group); no statistically significant difference in kidney fibrosis scoring was observed. C–E: quantitative RT-PCR to assess gene expression of key mediators of kidney fibrosis, including collagen type I-α1 (Col1α1) (C), transforming growth factor-β (TGFβ) (D), and α-smooth muscle actin (α-SMA; Acta2) (E), respectively. Col1α1 and Acta2 gene expression was significantly lower in kidneys from mice fed the phosphate-restricted diet, while TGFβ expression demonstrated a wide pattern of distribution with no statistically significant difference between groups (**P < 0.01 by Student’s t test; n ≥ 8 per group). F–H: quantification of kidney protein expression from Western blots for Col1α1 (F), phosphorylated (p-)SMAD3 (G), and α-SMA (H). Protein expression for all three of these targets was significantly lower in phosphate-restricted mice (*P < 0.05, **P < 0.01 by Student’s t-test; n = 5 per group. I: image of Western blots with associated Ponceau S stain to demonstrate total protein loading per lane.
DISCUSSION
Abnormalities in phosphate homeostasis are universally observed in patients with reduced kidney function and often predict poor clinical outcomes for this population (5, 16, 20, 21, 28). While tremendous effort has been devoted to understanding the mechanisms whereby hyperphosphatemia and elevated FGF23 contribute to adverse cardiovascular outcomes (i.e., vascular calcification and left ventricular hypertrophy) (32, 35), often overlooked has been the contribution of aberrant phosphate homeostasis to CKD progression (2, 3, 11, 14, 18, 19, 24–26, 30, 43, 48). As such, a reduction of dietary phosphate intake or intestinal absorption dramatically slows CKD progression in rodents with CKD induced by 5/6 nephrectomy (3, 19, 24), whereas phosphate loading promotes a more rapid loss of kidney function (14, 18).
Previously, our group observed severe dietary phosphate restriction to attenuate tubular injury, while having no apparent effect on glomerular damage in collagen type IV-α3 (Col4a3)−/− mice, a murine model of human Alport syndrome (48). On the basis of this finding and other prior observations, we speculated that the potential renoprotective effect of dietary phosphate restriction was related to mechanisms directly preserving tubular epithelial cell function. To further investigate this hypothesis, we tested the impact of dietary phosphate restriction in a murine model of PKD (pcy/pcy). Pcy/pcy mice possess a deletion of the Nphp3 gene that results in a kidney phenotype that mimics human nephronopthisis, a ciliopathy leading to medullary cystic kidney disease in adolescence. In mice on a CD1 background, deletion of the Nphp3 gene results in the accumulation of fluid-filled renal cysts with a subsequent slowly progressive loss of kidney function and death by 40 wk of age (38, 39). Prior data from our group and others have suggested that cyst formation results from genetic abnormalities that interfere with tubular repair in response to injury (29, 33, 40, 41). As disease progresses, expanding cysts compromise the function of the surrounding tubules and vasculature, promote immune cell infiltration, and stimulate interstitial fibrosis.
One possible explanation for the renoprotective effects of reduced dietary phosphate intake in pcy/pcy mice may be directly related to a reduction of urinary phosphate excretion. Elegant studies performed by Bank et al. (1) demonstrated the concentration of tubular fluid phosphate to be dramatically elevated (far above the expected supersaturation threshold) in isolated perfused tubules from CKD rats compared with wild-type rats. It seems plausible that elevated tubular phosphate could be nephrotoxic, either by promoting nanocrystal formation with other minerals or by a direct “poisoning” of tubular function. In further support of possible deleterious effects of tubular phosphate, population studies in humans with CKD have demonstrated that higher circulating concentrations of FGF23, a potent phosphaturic hormone, predicts incident end-stage renal disease (ESRD) in the general population (30), progression of kidney disease in patients with CKD (11), and a decline in renal allograft function in kidney transplant recipients (43). However, another conceivable explanation for the observed association between FGF23 and adverse kidney outcomes could be that FGF23 is a direct nephrotoxin or stimulates secondary pathways that favor kidney injury. In our current study, improvements in kidney cyst burden were accompanied by marked reductions in serum FGF23 (Fig. 3C); thus, we are unable to determine whether changes in renal cyst formation and growth were independent of FGF23.
Another plausible explanation for the renoprotective effect of dietary phosphate restriction in our study could be related to changes in serum calcium. In pcy/pcy mice, chronic consumption of a phosphate-restricted diet induced moderate hypercalcemia (Fig. 3B). It is noteworthy that prior studies have demonstrated activation of the calcium-sensing receptor (CaSR) may slow PKD progression in rodents (7, 12). A reduction in intracellular Ca2+ and resulting overexpression of cAMP are thought to be key contributors to the pathogenesis of PKD (4, 8, 47). In fact, the discovery that vasopressin V2 receptor antagonists slow PKD progression is speculated to result from a suppression of intracellular cAMP signals (4, 13, 31). As with calcimimetic drugs, stimulation of the CaSR by elevations by hypercalcemia would be expected to raise intracellular Ca2+ in tubular epithelial cells and, in turn, suppress cAMP production.
A final important observation in our study was the upregulation of renal Klotho expression in mice that consumed a phosphate-restricted diet (Fig. 3E). Prior studies have demonstrated that klotho suppresses the growth of various cancer cells by inhibiting several pathways implicated in the pathogenesis of PKD, including Wnt/β-catenin and insulin-like growth factor 1 signals (34, 45, 49, 50). Additionally, klotho has been demonstrated to increase intracellular Ca2+ in tubular epithelial cells (6, 23, 44), an action that has been shown to suppress the mitogenic response to cAMP in cystic epithelial cells (46). Finally, klotho inhibits TGF-β1 signaling through binding to the type II TGF-β receptor (9), a critical pathway driving epithelial to mesenchymal transition and subsequent fibrosis.
If a true link exists between dietary phosphate consumption and PKD progression, this association would have enormous clinical implications. To this end, phosphate consumption in industrialized countries has steadily increased over the last few decades to levels that far exceed the recommended dietary allowance (RDA) (27). For example, on the basis of data collected as part of the National Health and Nutrition Examination Survey (NHANES) in the United States, current dietary phosphate intake is roughly double the RDA (27). Moreover, considering the rising popularity of processed foods and beverages in industrialized populations, the widespread use of phosphate-based additives for improving the palatability and appearance of manufactured foods, and a lack of current guidelines to mandate the listing of phosphate content on product labels, it is plausible that nutritional surveys such as NHANES are grossly underestimating actual phosphate consumption among certain populations.
As with any study, the current study has both important strengths and limitations. Strengths of the study include the use of custom diets that were identical in calories and content of major nutrients (i.e., protein, carbohydrates, and fat), unbiased approaches to assess kidney cyst burden and fibrosis, consideration of the impact of gender on our outcomes of interest, and a study design that allows for the exclusion of potential confounders that are commonly encountered in human studies (i.e., dietary influences, genetic variability, and the presence of disease comorbidities). Limitations of this work include the degree of dietary phosphate restriction used in this study could lead to severe osteoporosis and would be incompatible with long-term health in humans, lack of data on urinary changes in phosphate and other relevant parameters, and food and water consumption were not monitored as part of this investigation. Finally, we used a nonorthologous model of PKD.
In summary, our observations in pcy/pcy mice highlight the potential importance of dietary phosphate as a modifiable risk factor for renal cyst development, tubular injury, and renal fibrosis. Although the current study focused on a rodent model of PKD, existing evidence from our group and others suggests these observations could have a much broader impact on all forms of CKD. Future investigations should focus on clarifying the mechanisms responsible for the relationship between dietary phosphate and kidney injury and test how these mechanisms could be exploited to develop therapeutics for the prevention of kidney disease progression in humans.
GRANTS
This work was supported by funds from the Kansas PKD Research and Translation Core Center (Grant P30-DK-106912, to J. R. Stubbs).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.O., S.Z., C.J., E.D., D.P.W., and J.R.S. conceived and designed research; F.O., S.Z., C.J., E.D., Y.Z., J.B., and I.A. performed experiments; F.O., S.Z., C.J., Y.Z., T.A.F., J.B., S.L., I.A., S.U., D.P.W., and J.R.S. analyzed data; F.O., S.Z., C.J., T.A.F., S.L., S.U., D.P.W., and J.R.S. interpreted results of experiments; J.R.S. prepared figures; F.O., D.P.W., and J.R.S. drafted manuscript; F.O., D.P.W., and J.R.S. edited and revised manuscript; F.O., S.Z., E.D., T.A.F., S.L., I.A., S.U., D.P.W., and J.R.S. approved final version of manuscript.
REFERENCES
- 1.Bank N, Su WS, Aynedjian HS. A micropuncture study of renal phosphate transport in rats with chronic renal failure and secondary hyperparathyroidism. J Clin Invest 61: 884–894, 1978. doi: 10.1172/JCI109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsotti G, Morelli E, Giannoni A, Guiducci A, Lupetti S, Giovannetti S. Restricted phosphorus and nitrogen intake to slow the progression of chronic renal failure: a controlled trial. Kidney Int Suppl 16: S278–S284, 1983. [PubMed] [Google Scholar]
- 3.Barsotti G, Moriconi L, Cupisti A, Dani L, Ciardella F, Lupetti S, Giovannetti S. Protection of renal function and of nutritional status in uremic rats by means of a low-protein, low-phosphorus supplemented diet. Nephron 49: 197–202, 1988. doi: 10.1159/000185055. [DOI] [PubMed] [Google Scholar]
- 4.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66: 964–973, 2004. doi: 10.1111/j.1523-1755.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310: 490–493, 2005. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 7.Chen NX, Moe SM, Eggleston-Gulyas T, Chen X, Hoffmeyer WD, Bacallao RL, Herbert BS, Gattone VH II. Calcimimetics inhibit renal pathology in rodent nephronophthisis. Kidney Int 80: 612–619, 2011. doi: 10.1038/ki.2011.139. [DOI] [PubMed] [Google Scholar]
- 8.Cowley BD., Jr Calcium, cyclic AMP, and MAP kinases: dysregulation in polycystic kidney disease. Kidney Int 73: 251–253, 2008. doi: 10.1038/sj.ki.5002695. [DOI] [PubMed] [Google Scholar]
- 9.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, Vanderschueren D, Vanrenterghem Y. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol 5: 1268–1276, 2010. doi: 10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P; MMKD Study Group . Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 12.Gattone VH II, Chen NX, Sinders RM, Seifert MF, Duan D, Martin D, Henley C, Moe SM. Calcimimetic inhibits late-stage cyst growth in ADPKD. J Am Soc Nephrol 20: 1527–1532, 2009. doi: 10.1681/ASN.2008090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattone VH II, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 14.Gimenez L, Walker WG, Tew WP, Hermann JA. Prevention of phosphate-induced progression of uremia in rats by 3-phosphocitric acid. Kidney Int 22: 36–41, 1982. doi: 10.1038/ki.1982.129. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- 18.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N. Renal toxicity of phosphate in rats. Kidney Int 17: 722–731, 1980. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 19.Ibels LS, Alfrey AC, Haut L, Huffer WE. Preservation of function in experimental renal disease by dietary restriction of phosphate. N Engl J Med 298: 122–126, 1978. doi: 10.1056/NEJM197801192980302. [DOI] [PubMed] [Google Scholar]
- 20.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24: 2792–2796, 2009. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 22.Kurbegovic A, Trudel M. Acute kidney injury induces hallmarks of polycystic kidney disease. Am J Physiol Renal Physiol 311: F740–F751, 2016. doi: 10.1152/ajprenal.00167.2016. [DOI] [PubMed] [Google Scholar]
- 23.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23: 3397–3402, 2008. doi: 10.1093/ndt/gfn291. [DOI] [PubMed] [Google Scholar]
- 24.Lumlertgul D, Burke TJ, Gillum DM, Alfrey AC, Harris DC, Hammond WS, Schrier RW. Phosphate depletion arrests progression of chronic renal failure independent of protein intake. Kidney Int 29: 658–666, 1986. doi: 10.1038/ki.1986.49. [DOI] [PubMed] [Google Scholar]
- 25.MacKay EM, Oliver J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J Exp Med 61: 319–334, 1935. doi: 10.1084/jem.61.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maschio G, Tessitore N, D’Angelo A, Bonucci E, Lupo A, Valvo E, Loschiavo C, Fabris A, Morachiello P, Previato G, Fiaschi E. Early dietary phosphorus restriction and calcium supplementation in the prevention of renal osteodystrophy. Am J Clin Nutr 33: 1546–1554, 1980. doi: 10.1093/ajcn/33.7.1546. [DOI] [PubMed] [Google Scholar]
- 27.McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001–2014. Nutrients 9: 95, 2017. doi: 10.3390/nu9020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merhi B, Shireman T, Carpenter MA, Kusek JW, Jacques P, Pfeffer M, Rao M, Foster MC, Kim SJ, Pesavento TE, Smith SR, Kew CE, House AA, Gohh R, Weiner DE, Levey AS, Ix JH, Bostom A. Serum phosphorus and risk of cardiovascular disease, all-cause mortality, or graft failure in kidney transplant recipients: an ancillary study of the FAVORIT Trial Cohort. Am J Kidney Dis 70: 377–385, 2017. doi: 10.1053/j.ajkd.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raman A, Parnell SC, Zhang Y, Reif GA, Dai Y, Khanna A, Daniel E, White C, Vivian JL, Wallace DP. Periostin overexpression in collecting ducts accelerates renal cyst growth and fibrosis in polycystic kidney disease. Am J Physiol Renal Physiol 315: F1695–F1707, 2018. doi: 10.1152/ajprenal.00246.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebholz CM, Grams ME, Coresh J, Selvin E, Inker LA, Levey AS, Kimmel PL, Vasan RS, Eckfeldt JH, Feldman HI, Hsu CY, Lutsey PL; Chronic Kidney Disease Biomarkers Consortium . Serum fibroblast growth factor-23 is associated with incident kidney disease. J Am Soc Nephrol 26: 192–200, 2015. doi: 10.1681/ASN.2014020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1013, 2011. doi: 10.1152/ajprenal.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 11: 1088–1100, 2016. doi: 10.2215/CJN.11901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shu G, Xie B, Ren F, Liu DC, Zhou J, Li Q, Chen J, Yuan L, Zhou J. Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells. Cell Oncol (Dordr) 36: 121–129, 2013. doi: 10.1007/s13402-012-0118-0. [DOI] [PubMed] [Google Scholar]
- 35.Stubbs JR, Egwuonwu S. Is fibroblast growth factor 23 a harbinger of mortality in CKD? Pediatr Nephrol 27: 697–703, 2012. doi: 10.1007/s00467-011-1810-4. [DOI] [PubMed] [Google Scholar]
- 36.Stubbs JR, He N, Idiculla A, Gillihan R, Liu S, David V, Hong Y, Quarles LD. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res 27: 38–46, 2012. doi: 10.1002/jbmr.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace DP, Hou YP, Huang ZL, Nivens E, Savinkova L, Yamaguchi T, Bilgen M. Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int 73: 778–781, 2008. doi: 10.1038/sj.ki.5002771. [DOI] [PubMed] [Google Scholar]
- 39.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 85: 845–854, 2014. doi: 10.1038/ki.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 41.Weimbs T. Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair? Cell Cycle 5: 2425–2429, 2006. doi: 10.4161/cc.5.21.3408. [DOI] [PubMed] [Google Scholar]
- 42.Wilson PD, Hreniuk D, Gabow PA. Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992. doi: 10.1002/jcp.1041500220. [DOI] [PubMed] [Google Scholar]
- 43.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf MT, An SW, Nie M, Bal MS, Huang CL. Klotho up-regulates renal calcium channel transient receptor potential vanilloid 5 (TRPV5) by intra- and extracellular N-glycosylation-dependent mechanisms. J Biol Chem 289: 35849–35857, 2014. doi: 10.1074/jbc.M114.616649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie B, Zhou J, Shu G, Liu DC, Zhou J, Chen J, Yuan L. Restoration of klotho gene expression induces apoptosis and autophagy in gastric cancer cells: tumor suppressive role of klotho in gastric cancer. Cancer Cell Int 13: 18, 2013. doi: 10.1186/1475-2867-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Gillihan R, He N, Fields T, Liu S, Green T, Stubbs JR. Dietary phosphate restriction suppresses phosphaturia but does not prevent FGF23 elevation in a mouse model of chronic kidney disease. Kidney Int 84: 713–721, 2013. doi: 10.1038/ki.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Zhang Y, Li Y, Xu Y, Zhang L, Li Y, Wang X. Klotho suppresses tumor progression via inhibiting IGF-1R signaling in T–cell lymphoma. Oncol Rep 38: 967–974, 2017. doi: 10.3892/or.2017.5744. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H, Lv T, An H, Liu L, He H, Zhang H, Liu J, Xu J, Lin Z. Klotho suppresses tumor progression via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell carcinoma. Cancer Sci 104: 663–671, 2013. doi: 10.1111/cas.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]