Abstract
Bone differs from other connective tissues; it is isolated by a layer of osteoblasts that are connected by tight and gap junctions. This allows bone to create dense lamellar type I collagen, control pH, mineral deposition, and regulate water content forming a compact and strong structure. New woven bone formed after degradation of mineralized cartilage is rapidly degraded and resynthesized to impart structural order for local bone strength. Ossification is regulated by thickness of bone units and by patterning via bone morphogenetic receptors including activin, other bone morphogenetic protein receptors, transforming growth factor-β receptors, all part of a receptor superfamily. This superfamily interacts with receptors for additional signals in bone differentiation. Important features of the osteoblast environment were established using recent tools including osteoblast differentiation in vitro. Osteoblasts deposit matrix protein, over 90% type I collagen, in lamellae with orientation alternating parallel or orthogonal to the main stress axis of the bone. Into this organic matrix, mineral is deposited as hydroxyapatite. Mineral matrix matures from amorphous to crystalline hydroxyapatite. This process includes at least two-phase changes of the calcium–phosphate mineral as well as intermediates involving tropocollagen fibrils to form the bone composite. Beginning with initiation of mineral deposition, there is uncertainty regarding cardinal processes, but the driving force is not merely exceeding the calcium-phosphate solubility product. It occurs behind a epithelial-like layer of osteoblasts, which generate phosphate and remove protons liberated during calcium-phosphate salt deposition. The forming bone matrix is discontinuous from the general extracellular fluid. Required adjustment of ionic concentrations and water removal from bone matrix are important details remaining to be addressed.
Keywords: aquaporin, chloride-hydrogen antiporters, Na/H exchangers, pH gradient, type I collagen
INTRODUCTION
We describe the cellular makeup of cortical and trabecular bone with special attention to transport activities of osteoblasts that are critical to bone formation and become the osteocytes that maintain the bone matrix. Skeletal integrity at the cellular level is maintained by “remodeling,” which is initiated by osteoclasts. The osteoclasts degrade microscopic portions of existing bone matrix permitting osteoblasts to reformulate the local matrix. Osteocytes, in an orderly arrangement at the junction of degraded and new bone, add “cement lines” in the remodeled bone matrix that define the reorganized osteons. This organization is supported by gap and desmosome junctions between osteoblasts and osteocytes providing intercellular communication and reinforcing separation from the system wide extracellular matrix (7). Tight junctions produce significant resistance across osteoblast monolayers (102), while gap junctions form communication between cells (86). As this process is iterated over the existing bone matrix, it produces the organized, high-quality skeletal structure shown in textbooks. The noncellular bone matrix is composed of type I collagen and the hydroxyapatite mineral formed into a durable and strong composite (meaning made of separate parts). Less than 5% bone matrix is made up of numerous accessory proteins that individually (when genetically suppressed) have small consequence to bone phenotype but collectively contribute substantially to a “normal” bone phenotype. Such knockouts are often survivable due to redundancy of function by these proteins. In normal bone physiology, extracellular bone matrix is secreted by osteoblasts as the type I tropocollagen. The initial matrix produced is rapidly degraded by osteoclasts and replaced by organized matrix. This tropocollagen is deposited and cross linked in dense lamellae aligned in one direction, ~2-μm thick, after which orientation shifts ~90° to form a new lamella (106). This matrix is mineralized by deposition of hydroxyapatite, which, as discussed below, requires massive local phosphate production that requires alkaline phosphatase activity, passive calcium transport, and removal of acid (6). Specifically, phosphate production under osteoblasts, maintained by massive alkaline phosphatase expression and secretion of phosphatase substrates (6), is required for mineral deposition. Alkaline phosphatase is also essential for pyrophosphate degradation; if not degraded, this is a mineralization inhibitor (100). If phosphate production ceases, as in lethal forms of hypophosphatasia, bone is not mineralized and pyrophosphate accumulates (16, 100). In the following sections, we will address bone formation in vitro, growth factors for bone cells including bone morphogenetic proteins (BMP) and transforming growth factor-β (TGF-β) family signals, and initiation of bone differentiation. This will include a discussion of activin receptor 1 defects with uncontrollable bone formation. Finally, we will discuss in more detail osteoblast transport physiology related to mineralization, crystal nucleation for mineral formation, and the importance of water in bone.
THE CELLULAR STRUCTURE OF NORMAL BONE AND ITS RELATION TO MINERAL TRANSPORT
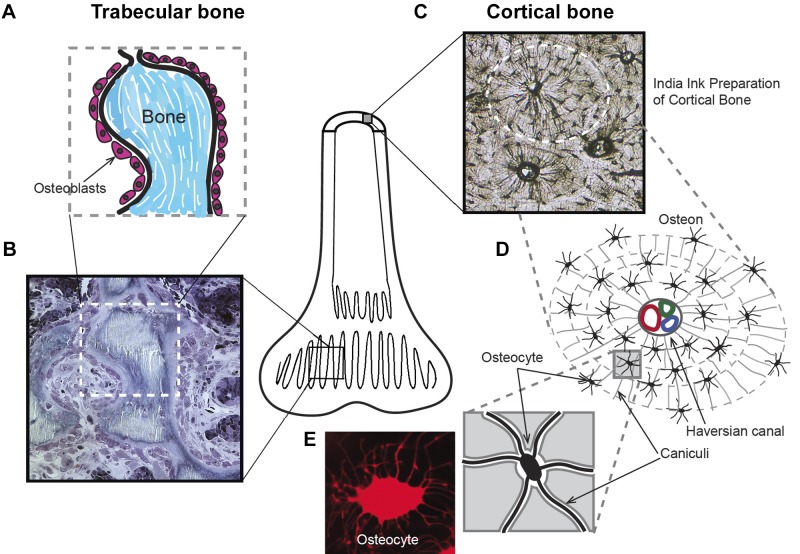
Figure 1 summarizes two major types of bone, trabecular (Fig. 1, A and B) and cortical (Fig. 1, A, C, and D). Trabecular bone shows epithelial-like bone-lining osteoblasts; this bone is the site of rapid bone formation and resorption (Fig. 1B). In subsequent sections, we describe how this structure is important to mineral transport and removal of protons produced in bone formation. Our use of the term “epithelium” is based on the properties of the cells. Epithelial identification was traditionally based on embryology, before molecular characterization. We here apply a functional definition, a membrane of cells enclosing part of the body to delimit secretion. This is supported by the expression of tight junctions and inside to outside ion gradients discussed below. Mesenchymal cells produce epithelia in other organs (106). To avoid confusion, we describe the osteoblastic layer as “functional epithelium-like.”
Fig. 1.
Structure of two basic types of bone: trabecular and cortical bone. Bone is mainly either trabecular, thin layers of bone with a high surface area, or cortical, dense solid bone, such as the shaft of the femur, with small surface area (central diagram). A: schematic representation of a high turnover trabecular bone showing of a layer of osteoblasts lining small, mineralized (Bone) portions of the “spongy” trabecular bone. All formation and resorption of bone is on the surface, so that the trabecular bone is the main active part of bone. B: a methylene blue-stained semithin (1 µm) section of the trabecular bone indicated diagrammatically in A. C: cortical bone structure revealed with penetrating India ink staining. This preparation is a thin section of dried, acellular bone. D: schematic of an osteon structure: acellular voids forming canaliculi microchannels interconnect osteocytes and form concentric layers around a Haversian canal passing blood vessels and a nerve. E: living osteocyte stained with sulforhodamine 101 to show its detailed structure.
A good understanding of the structural arrangement of the dense cortex of bone can be grasped by viewing an India ink (fine carbon particle suspension) preparation of cortical bone. Thin sections of dried bone are infused with India ink that fills the acellular voids and outlines important structural features including the extensive canaliculi of osteocytes (Fig. 1C). Prominent in this preparation are the osteocytes imbedded in the mineralized matrix, diagrammed in Fig. 1D. In live bone, the osteocytes are connected by cellular extensions through the canaliculi that are labeled in the diagram, Fig. 1D. In life, canaliculii project between deeper (older) osteocytes to newer cells and ultimately the surface osteoblasts. This concentric structure forms after osteoclast remodeling of bone and typically contains several layers of cells and mineralized matrix, which is surrounded by a highly mineralized border called the “cement line.” Cement lines are highly mineralized partitions that surround the osteon and are formed after the osteoclast phase of remodeling (Fig. 1, C and D). The connected group of osteoblasts and impermeable cement line is important in defining the functional unit of bone transport.
Each of these bone-forming modules, typically four to seven cell-matrix layers thick, is called an osteon. It begins at a cement line. Cement lines are thin, typically 2- to 5-μm wide. They have canaliculi on both sides but canaliculi, generally, do not extend across the cement line. Cement line structure is controversial; recent data suggest a high mineral and low protein content (65, 87).
Osteoblasts cover the living surface of bone. This is seen well in rapidly fixed trabecular bone, which has a high surface area (Fig. 1B) relative to cortical bone. Most of these cells are epithelial-like layers of osteoblasts, some as active columnar cells synthesizing bone and some inactive as low columnar cells not synthesizing bone.
It was discovered in the 1950s, that, during bone synthesis, specific small charged molecules including the fluorescent molecules calcein, tetracycline, and xylenol orange are transported into new bone mineral. The fluorescent markers incorporated in bone during matrix synthesis are used to study the bone synthesis rate (52). Recently other fluorescent molecules including pH-sensitive SNARF-1 have been localized in the mineralization matrix (unpublished data). These fluorescent molecules are distributed in a characteristic pattern in new bone as important exceptions to the impermeability of living bone to small molecules and ions; they are specifically transported into bone matrix. The uptake of small molecules during bone formation has not been studied in detail. Candidate mechanisms include SLC21 and SLC22 anion and cation transporters and ABCC1 multidrug exporters expressed in osteoblasts and related cells (36, 66).
Living bone is, other than for these important exceptions of transport during bone formation, impermeable to ions and small molecules. On the other hand, dead bone, such as in steroid-induced osteonecrosis, is labeled in a very different pattern by calcium-binding fluors (26) (Fig. 2).
Fig. 2.

Permeability of dead bone, but not living bone, to tetracycline. Rabbit trabecular bone with glucocorticoid-induced osteonecrosis. The necrotic center of a trabeculum is highlighted, with tetracycline labeling dead bone matrix but not surrounding living bone. A confocal image of tetracycline fluorescence. The field shown is 300 µm square. [Reproduced from Eberhardt et al. (26), with permission.]
Poor fixation of bone, which is more the rule than the exception due to the high metabolic rate of bone cells and impermeability of bone to solvents, often shows artifactual bone matrix adjacent to extracellular fluid. This does not occur in living bone. The epithelial-like arrangement of bone lining cells is important in bone synthesis, which requires transport, and pH gradients to support mineralization. This is discussed below.
OSTEOBLAST CELLULAR FEATURES
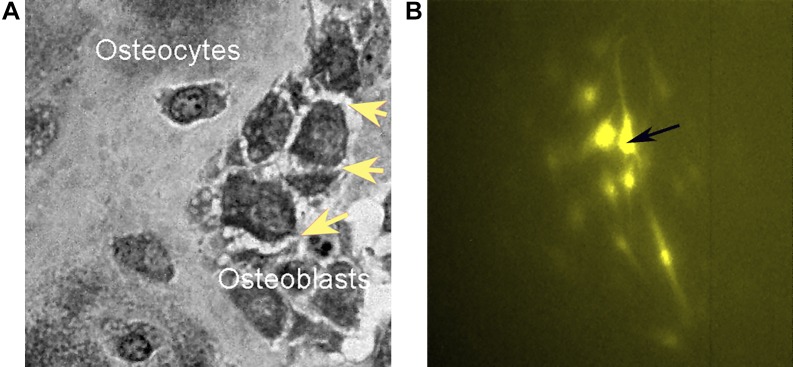
The general impermeability of bone is due to the osteoblasts that cover the bone surface. Osteoblasts are connected by tight junctions with characteristic selective permeability. Osteoblasts express claudins and associated proteins seen in tight junctional complexes (3, 102). There are many published studies of such junctions between osteoblasts, and they can be demonstrated visually by the spiky morphology seen in cells exposed to hypertonic medium enhancing the connections between cells (Fig. 3A). Specifically, osteoblasts express several claudins and other junction-associated proteins (102), which are proposed to limit free diffusion across the functional epithelial membrane, thus allowing regulated transport only. The tight junctions are credited with electrical resistance across osteoblast monolayers (102).
Fig. 3.
Desmosomes and gap junctions are key characteristics of osteoblasts and osteocytes. A: tangential section of the bone surface after hypertonic treatment to reveal the dense junctions as spiky interosteoblast connections (arrows). A semithin (1 µm) section: field, 80 µm. B: MG63 osteoblast-like cells at low density; the marked cell was injected with Lucifer yellow, which fluorescence spreads through adjacent cells by connexin 43 gap junction connections; field, 580 µm square. [Unpublished data related to our previous work (86).]
Groups of the cells within the bone forming unit are electrically connected by gap junctions, largely based on connexin 43 (Cx43). These connections may be demonstrated in cells in culture by injection of one cell with a low molecular weight fluor, such as lucifer yellow, which spreads to adjacent cells (Fig. 3B); Knockout of Cx43 is survivable (19), possibly reflecting expression of other connexins, including Cx45 (17). On the other hand, Cx43 is essential for normal bone structure and osteoblast survival. It is essential to communications between osteoblasts within an osteon and hemichannels in deeper structures (104).
BONE MATRIX PROTEINS
Extracellular bone matrix is over 90% type I collagen. This collagen is extremely dense and heavily cross linked. Cross linking occurs at lysine or hydroxylysine residues and involves several enzymes. Cross linking is important to bone strength and is compromised in bone diseases including osteoporosis (82).
Bone collagen is synthesized by osteoblasts, and it is secreted in lamellae aligned in one direction, typically ~2-µm thick, after which there is an orthogonal orientation shift in the next lamellae (106). The collagen lamellae are easily seen by polarized light. This allows distinction of new bone without well-oriented collagen layers, called woven bone, from mature bone with coordinated collagen layers across the bone-forming unit. Interesting questions include how osteoblasts keep procollagen in solution at the high concentrations produced, which is not well studied. One of the lamellae is oriented along the line of stress of the osteoblast; how this is maintained is unknown.
Aside from type I collagen, there are many other bone matrix proteins, quantitatively ~5% of matrix, with interesting properties. Those best studied are the acidic proteins osteopontin and osteocalcin, which modulate to some extent bone matrix strength and maturation (30). However, the proteins when knocked out have relatively mild effects. This suggests that that modulation of bone formation by these minor proteins might reflect secondary effects of other factors, such as type I collagen cross linking, changes in bone mineral deposition by alkaline phosphatase activation, and other factors, such as insulin production related to the expression of osteocalcin (37).
BASIC PRINCIPLES AND COMPONENTS OF BONE MATRIX FORMATION
Collagen has been a difficult protein to analyze due to its size and insolubility. Binding sites for hydroxyapatite have been identified from peptides. However, there needs to be work on inserting the apatite into the matrix. It is clear that amorphous hydroxyapatite crystals form around the periodicity of cross-linked type I collagen (Fig. 4A), but the molecular details of this crystal formation are not well studied. On the other hand, it is now clear that hydroxyapatite is deposited on type I collagen without other proteins or components and that this is dependent on regulation of pH (Fig. 4B), discussed in detail below. After initial mineral deposition, there is a process of maturation to form stable hydroxyapatite, which also is not fully understood. It is also not understood what special attributes of type I collagen production allow its synthesis in the very high concentrations in bone, including maintenance of procollagen in solution in bone forming cells. The current state of the art of these processes is discussed below, with key missing data described. Related poorly understood elements include where and how water is retained on mineral and collagen and how the elastic modulus of bone thereby is regulated.
Fig. 4.
pH-dependent mineral deposition on type I collagen. A: transmission electron micrograph of a partially mineralized bone in vitro showing collagen and periodic hydroxyapatite deposition (arrows). B: surface plasmon resonance showing phosphate and pH dependency of hydroxyapatite deposition in an artificial type I collagen layer. A more detailed description of the chip used is included in the section “a model for osteoblast epithelial-like structure and bone transport.” At time zero, responses are overlain for identical injection protocols. The baseline curves are identical for buffer (140 mM NaCl and 10 mM HEPES, pH = 7.4 or 6.81), 1 mM CaCl2, or 5 mM phosphate (alone with buffer). At ~700 s the injection ended and the surface was washed with buffer. Later, the surface was washed with 10 mM EDTA to remove any stabilized calcium salts and return the response to baseline. At 1 mM CaCl2 a dramatic phosphate dependence of large hydroxyapatite aggregate deposition occurs. Mineral deposition is reduced dramatically by dropping pH to 6.8. At the permissive pH 7.4, 5 and 2.5 mM phosphate injections generate stable hydroxyapatite deposition. These results indicate a strong pH dependence and multistep model for collagen mineralization. [From Blair et al. (6).]
The mineral and collagen of bone exclude much of the water initially present in the dense collagen before mineralization, but bone retains an important component of water. Regulation of the water content of bone is a further topic with limited understanding, also reviewed below. In brief, the composite structure of bone has an important elastic component, which is crucial to bone resistance to fracture and for maintenance of its canalicular structure (25, 84, 95). This is central both to bone strength and signaling reflecting its deformation, which regulates bone maintenance in major part.
CONDITIONS THAT SUPPORT BONE FORMATION
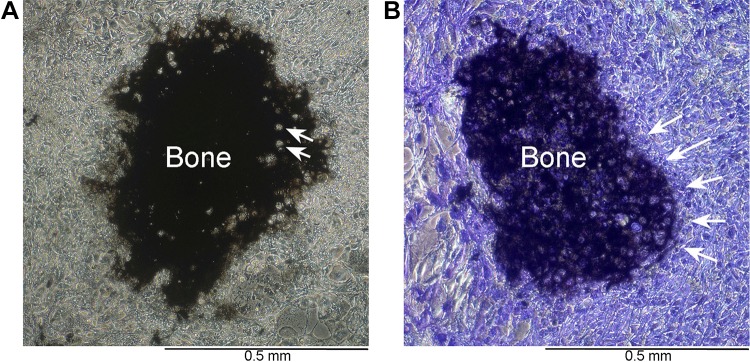
Models for bone formation in vitro have led to interest in mesenchymal stem cell (MSC) cultures, which, when produced in matrices where cells are not allowed to have connections with each other, will die. If cells are able to form processes that contact other MSCs, differentiation proceeds and produces fibrous stroma but no bone. Cultures based on MSCs but with dense cell aggregates allowing cell-cell tight and gap junctions under some conditions can form true bone (Fig. 5). Low dose ACTH (starting at 10 pM), 10 nM vitamin D (1α,25-dihydroxyvitamin D3), and 2 mM calcium enhance osteoblast differentiation and mineralization in vitro (94). These Ca2+ concentrations correlate with increasing connexin 43 (Cx43) mRNA levels (97) as well as with enhanced Cx43 fluorescence signaling and matrix mineralization of mesenchymal stromal cells isolated from human cancellous bone and cultured in vitro (103).
Fig. 5.
Characteristic bone nodules in flat human osteoblast cultures. A: in high-quality osteoblast cultures in permissive medium supporting mineral deposition, expanding dome-like mineralized bone matrix is produced. Matrix produced by human osteoblasts 21 days in medium containing glycerol-2-phosphate and ascorbic acid. The matrix is silver stained (von Kossa) showing dense extracellular matrix with lucencies corresponding to cells incorporated in matrix (arrows). Phase image emphasizing the dense osteoblast layer surrounding the bone nodule. B: alkaline phosphatase activity in a duplicate well of the preparation in A. Where extracellular mineralized matrix occurs (matrix), the alkaline phosphatase in surface cells is so strongly active as to appear black by α-naphthol phosphate-fast blue staining. In nonmineralizing areas, cells expressing alkaline phosphatase are outlined by blue since enzyme is membrane bound. This culture is also shown in phase. The line of osteoblast surrounding the bone is clearly seen (arrows). [From Blair et al. (7), with permission.]
Bone morphogenetic proteins (BMPs) play important roles in bone formation in vitro and in vivo. BMPs induce, in combination with other factors, genes associated with ossification and mineralization and genes important for general protein synthesis. BMP2 at 1 µg/ml rescued osteoblast differentiation that had been impaired by siRNA-mediated suppression of human hormone nuclear receptor 1 (NR2F1) (60). Human hormone nuclear receptors are a family of ligand-regulated transcription factors that might be activated by estrogen, progesterone, and thyroid hormone and are upregulated during osteoblast differentiation in human MSCs (60).
Coculturing primary human osteoblasts (hOBs) with human endothelial cells (HUVECs) was shown to improve bone formation and formation of functional blood vessels in vitro (91). Analysis of the proteome of hOBs after cocultivation with HUVECs revealed significant upregulation of proteins important for osteoblast differentiation, cellular adhesion, and extracellular matrix function, including connective tissue growth factor, desmoplakin, galectin-3, and cyclin-dependent kinase 6 (83).
Possibilities for improving in vitro bone modeling include use of cells with defects that cause fibrodysplasia ossificans progressiva. This disorder results from activating mutations of activin receptor type-1, a bone morphogenetic protein receptor normally involved in cartilage differentiation. Disease-associated mutation results in rapid soft-tissue cell proliferation and differentiation into true bone. While there are possible therapeutic uses of this unusual differentiation mechanism, it has not shown practical utility. When constitutively activated, the activin receptor type-1 could cause potentially fatal systemic mineralization. Any therapeutic use of this mechanism must be carefully limited to the intended target tissue in both location and time.
MSCs capable of forming bone have to be cultured at very high density to differentiate into bone. Bone MSCs invariably produce densely mineralized extracellular matrix with lamellae of extremely dense collagen at periodically alternating orientation, orthogonal to one another. For imaging the dynamics of type I collagen assembly and its dependence on fibronectin, GFPtpz- and mCherry-tagged collagen fusion constructs were generated and stably transfected into MLO-A5 osteoblast-like cells. Coculture of mCherry- and GFPtpz-collagen-expressing cells showed that individual collagen fibrils can receive contributions of collagen from more than one cell and collagen assembly is dynamically integrated with fibronectin assembly (58).
Bone formation involves remarkable protein synthesis and processing, and equally remarkable mineral transport and deposition, which in turn requires control of pH to counteract the significant acid production associated with mineral deposition. Because bone mineralization requires a sequestered space with a unique chemical environment produced by polarized epithelially organized cells, single cells obviously cannot make bone. However, the differentiation of bone is complex; using gentle dissociation of mesenchymal stem cells undergoing osteogenic differentiation, Hanna, Mir, and Andre (39) showed the presence of at least four layers of differentiated cells involved in bone formation.
REGULATION OF OSTEOBLAST DIFFERENTIATION AND FUNCTION
Numerous, mainly local, mechanisms influence osteoblast differentiation and activity. Key pathways include the Wnt, TGF-β superfamily, and fibroblast growth factor (FGF) pathways. These and important osteoblast transcription factors including RUNX2 (also expressed in cartilage) will be considered briefly.
The Wnt Signaling Pathway
The Wnt family of genes was identified for its importance in development and oncogenic potential (74). The family comprises a group of extracellular glycoproteins highly conserved across species, common features of which are briefly reviewed. At the cell surface, these ligands bind Frizzled (Fz) family G-protein coupled receptors and their coreceptor, the LDL receptor-related proteins (LRP) 4, 5, or 6, mediating intracellular signaling. In “canonical” Wnt signaling, Wnt binding results in impaired ubiquitin-mediated degradation of the multifunctional cadherin complex protein, β-catenin (59). Ensuing accumulation of β-catenin in the cytoplasm and nucleus promotes interaction with T-cell factor/lymphoid enhancer binding factor transcription factors and transactivation of numerous target genes involved in osteoblast differentiation and activity. Soluble extracellular antagonists of Wnt signaling include sclerostin (produced by osteocytes and impairing bone formation), Dickkopf-related protein 1 (DKK1), and soluble Fz-related protein 1, each of which interferes with binding of Wnt to its receptors (23).
More specific examples in bone include genome-wide association studies showed association of several Wnt pathway components and bone density in humans, including genes encoding sclerostin, Lrp4, Lrp5, DKK1, Wnt4, Wnt16, and β-catenin (44). In mesenchymal stem cells, β-catenin is necessary for osteoblast differentiation (22). In laboratory mice, osteoblast-specific deletion of β-catenin causes reduced bone density and skeletal fragility (33). Deletion of osteoblast β-catenin from adult animals reduces bone density, indicating that the importance of β-catenin signaling in bone is not limited to cell differentiation; it also regulates mature osteoblasts (47).
The TGF-β Superfamily
Members of the transforming growth factor-β (TGF-β) superfamily are segregated on the basis of sequence similarity and receptor-binding ability (15). The TGF-β and bone morphogenic protein (BMP) subfamilies play critical roles in the regulation of osteoblasts and their progenitors. TGF-β subfamily isoforms are expressed in the layers of less differentiated cells surrounding cartilage or bone, called the perichondrium or periosteum. Latency-associated peptide (LAP) of TGF-β and fibrillin bind these signaling molecules, sequestering them from receptors on mesenchymal stem cells (MSCs). Osteoclast-mediated matrix degradation results in release of active TGF-β that binds TGF-β-I and -II receptors (TβRI and TβRII, respectively) (20). Both receptors possess serine/threonine kinase activity. TβRII phosphorylates TβRI, stimulating phosphorylation and activation of intracellular receptor-regulated SMADs (R-SMADs), such as SMADs 2 and 3. These R-SMADs engage a co-SMAD (SMAD4) and translocate to the cell nucleus, where they influence expression of osteogenic genes (49) and stimulate recruitment of MCSs to osteoclast resorption pits, followed by differentiation of the MSCs into osteoblasts. Thus TGF-β provides a mechanism coupling osteoclast and osteoblast activity.
Numerous human genetic mutations demonstrate the importance of the TGF-β signaling pathway in bone regulation. Point mutations in TGFB1 that impair TGFβ1 interaction with its LAP result in progressive diaphyseal dysplasia (Camurati-Engelmann Disease), associated with hyperostosis of the long bones and skull (98). In Marfan’s syndrome, reduction of fibrillin-mediated TGFβ1 sequestration enhances TGF-β signaling, causing long bone overgrowth (arachnodactyly) (71). Mutations in TβRI and TβRII result in Loeys-Dietz syndrome (LDS). Like Marfan syndrome, LDS is associated with excessive growth of long-bones (57).
BMP signaling resembles TGFβ signaling. Osteoblasts express BMPs 2, 4, 6, and 7 (31). Like TβRI and TβRII, BMP receptors are single pass transmembrane serine/threonine kinases. Activation of BMP receptors on osteoblast-lineage cells can influence differentiation. BMP receptors are subdivided into types I and II. Type I receptors include BMPR1a, BMPR1b, and the type I activin A receptor (ACVR1). Mutation of ACVR1 causes the severe ectopic bone-forming disease, fibrodysplasia ossificans progressive, as mentioned above and discussed in more detail below (48). Mesenchymal stem cells and periosteal osteoblasts express BMPR1a (46). Expression of constitutively active BMPR1 in a pluripotent fibroblast cell line caused adipocyte differentiation (18). MSCs and early osteoblasts express BMPR1b, activation of which supports osteoblast differentiation (18). On the other hand, BMPs alone, including BMP2, are not sufficient for bone differentiation. A common but erroneous impression is that BMP2 causes, of itself, osteoblast differentiation (7). Although BMPR1s have a role in cell fate determination of osteoblast progenitors, neither the BMPs nor receptors are unique to bone (7).
The FGF Pathway
Fibroblast growth factor (FGF) signaling is also not osteoblast specific, but it contributes to the regulation of osteoblast proliferation, differentiation, and survival. Mice lacking FGF2 or FGF18 exhibit impaired ossification and osteoblast differentiation (67, 75). FGF ligands are expressed by periosteum and surrounding tissues and bind FGF receptors 1 and 2 (FGFR1 and FGFR2) on osteoblast-lineage cells. Mutations in these receptors can cause premature ossification of the cranial sutures (craniosynostosis) (92). These transmembrane receptors include intracellular tyrosine kinase domains. Ligand-binding induces dimerization followed by transphosphorylation of intracellular tyrosine residues (76). Subsequent intracellular signaling can activate pathways including the AKT, phospholipase Cγ (PLCγ), or RAS/MAPK pathway and can stimulate cell proliferation, differentiation, matrix calcification, or cell survival (61, 76).
Key Osteoblast Transcription Factors
A critical regulator of osteogenesis is the runt-related transcription factor 2 (RUNX2) (29), which is expressed in chondroblasts and osteoblasts. Mice lacking RUNX2 fail to develop osteoblasts and lack ossification (50, 77). RUNX2 expression is regulated by WNT, FGF, and TGF/BMP signaling. Regulatory targets of RUNX2 include numerous proteins with critical roles in osteoblast differentiation and activity (24), and RUNX2 induces transcription of SP7, or Osterix (70). This zinc-finger transcription factor is an important regulator of bone cell differentiation. Mutation of SP7 causes defective bone formation, resulting in one type of osteogenesis imperfecta (53). Another transcription factor upregulated by RUNX2 is the CCAAT/enhancer-binding protein-δ, which works with RUNX2 to promote expression of bone-specific proteins, such as osteocalcin (63). Critical osteoblast-derived cytokines regulated by RUNX2 include RANK-ligand and its decoy receptor, osteoprotegerin, which modulate osteoclast differentiation and activity (68, 93). The transporters important in mineralization in osteoblasts (6) are highly expressed but have not been linked to specific osteoblast promoters.
BONE INITIATION: CARTILAGE AND CEMENT LINES
In normal physiology, bone is formed by conversion of mineralized cartilage, which is degraded by osteoclasts and followed by production of bone by osteoblasts. Important exceptions are the skull and clavicles, where bone forms without cartilage precursors. This “intramembranous ossification” occurs with differentiation of osteoblasts in nodules (Fig. 5), on a background of loose connective tissue, and the osteoblasts then form functional epithelial layers that secrete collagen and mineralize it. This process is poorly understood from the standpoint of what factors regulate it, which is unfortunate because it might be the best model for bone replacement in vitro.
That said, replacement of cartilage by bone is well characterized and includes mineralization of cartilage, invasion of blood vessels and expression of VEGF, development of osteoclasts from monocytic precursors, and differentiation of mesenchymal stem cells to form osteoblasts. Evidence suggests some chondrocytes transdifferentiate into osteoblasts (105). The overall contribution of this transdifferentiation to the population of mature osteoblasts remains unclear. The process of cartilage conversion includes production of poorly organized primary spongiosa that is then degraded and replaced by mature bone. The secondary, mature bone, includes formation of plates of bone that become trabeculae. Much is known about transcriptional regulation of the endochondral ossification process; this is beyond the scope of the present presentation (40).
In bone repair, MSCs accumulate and differentiate into fibrocartilage locally and then that cartilage is replaced by bone. This is an important concept: even bone forming in the context of inflammatory signals proceeds through growth of cartilage that is then transformed into bone (10).
In bone turnover, osteoclasts cut matrix ending at a sharp line, from which new bone formation can occur directly, the “cement line.” Cement lines are on the order of 2-μm thick; collagen bundles and cell processes do not generally extend through the cement line, which is highly mineralized and probably contains proteoglycans but little collagen (87). Collagen bundles in adjacent new bone might originate in the cement line. How the cement line supports bone production from osteoblasts remains unknown. Osteoblasts do not attach to nonvital bone rather after osteoclasts have cleared away the dead bone the replacement can begin. Why cement lines support osteoblast attachment and production of new bone is not known. Possibilities include communication of osteoclasts with osteoblast precursors, either directly or via secretory products of osteoclasts.
Recently, there is evidence for signaling to osteoblasts by membrane vesicles from osteoclasts. There are several reports, some based on microRNAs; one of the most interesting suggesting that microvesicles mediate reverse signaling via RANKL (27). It is too early to assess the overall effects of these mechanisms on osteoblast formation, but the evidence suggests that osteoclasts directly influence osteoblast differentiation. That said, it has long been known that degradation of bone, including dead bone in bone grafts, does induce true bone formation, and it will be very useful for the mechanisms involved to be defined.
The transition of mineralized cartilage to bone is well described, but the means by which cartilage promotes bone formation is not well defined. In fracture repair, a bone callus containing fibrocartilage generally forms and precedes bone formation, although findings are variable and often inconsistent (32). Under some circumstances, fracture healing has been suggested to involve intramembranous differentiation with no cartilage precursors (13). It has proven very difficult to reproduce this in vitro. Many attempts to make artificial matrix containing MSCs have been disappointing, usually producing no bone (55).
Typically, successful in vitro bone production, that is, showing dense mineral formation on dense oriented collagen, occurs with coexpression of cartilage related proteoglycans, including aggrecan, the most abundant cartilage proteoglycan. In addition, activin receptors, the cartilage-supporting BMP receptors, are also seen (7). This suggests that most successful in vitro bone production is related to repair or developmental bone production based on cartilage. Successful bone production avoiding cartilage intermediates is an exciting concept, although many attempts have not been productive. No direct osteoblast-inducing growth factor combination is known, and although BMPs including BMP2 are involved in osteoblast production, they do not directly induce bone (7).
ROLE OF ACTIVIN RECEPTOR TYPE I AND MASSIVE UNCONTROLLED BONE FORMATION
Bone formation in vivo is normally initiated on mineralized cartilage in cartilage-bone transitions, on fibrocartilage during repair, or at cement lines when bone turnover goes from degradation to new bone formation (Fig. 1A). Stepping back, a key receptor mediating cartilage differentiation is the activin receptor ACVR1, which is essential for cartilage development (79). In bone differentiation in vitro, activin receptors and cartilage-related genes are expressed, including aggrecan (an essential proteoglycan expressed in articular cartilages), biglycan, and decorin (7, 88). Although not thoroughly tested, it is likely that activity of ACVR receptors and expression of proteoglycans are required during bone differentiation in many contexts, just as fibrocartilage differentiation is part of the repair process for local bone damage with callus formation.
It is sometimes suggested that BMP2 is a signal inducing osteoblast differentiation specifically. Bone differentiation definitely requires BMPs, including signals separating bones by preserving cells and preventing or promoting ossification centers from coalescing. As one example, the soluble BMP receptor noggin is important to scavenge soluble BMPs, and its absence causes fusion of bones such as in the hand during development (12). Several BMP family proteins and receptors are expressed in mesenchymal stem cells differentiating into osteoblasts, including BMP1, 2, and 10, TGF-β, inhibins, BMP receptors, TGF receptors, and activin receptors; for details, see Ref. 7. On the other hand, BMP2 is expressed in many organs including skin and liver; it is not a specific bone differentiation factor. It is also sometimes assumed that soluble BMP2 needs to be added for bone formation. However, BMP2 is naturally produced, mainly as a membrane-bound molecule, in differentiation of osteoblasts.
This said, an important concept moving forward is the possibility that ACVR-induced MSC proliferation and bone formation with some cartilage precursor activity might be harnessed using a strategy to make bone, but with control of ACVR activity to avoid fatal organism-wide bone formation (35). This strategy is under active investigation but has yet to control fibrodysplasia ossificans progressiva.
OSTEOBLAST MATRIX PRODUCTION MORE DETAIL
Physical Background of Mineral Transport
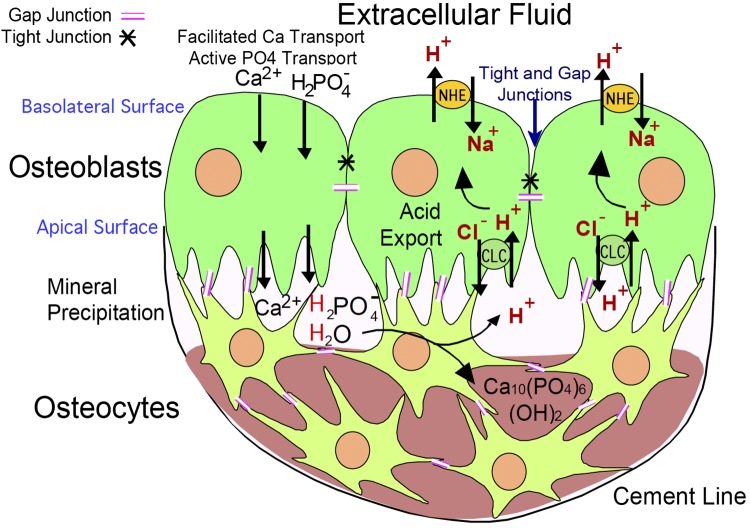
The mechanism regulating mineral deposition to assure continuous formation of densely mineralized matrix, that is, without nonmineralized gaps, is incompletely known. However, continuous processes of cells joined by gap junctions run through the whole developing matrix, and include Cl−,H+ antiporters, suggesting that regulated pH gradients may be important in where mineral deposits. Osteoblasts are capable of exporting large quantities of acid via Na/H exchangers. Cl−,H+ antiporters and Na/H exchangers are redundant, complicating study of effects of knockouts.
An important but old concept, often ignored, is that osteoblasts form a functional epithelium-like structure that bounds living bone. The cells express tight junctions that isolate bone matrix from extracellular fluid (1, 3, 41, 102), not allowing uncontrolled permeability of phosphate or protons (6). This is of key importance because the process of bone matrix formation includes deposition of extremely dense hydroxyapatite mineral. Since the stoichiometry of mineral formation (72) involves evolution of acid, this implies that acid must be removed by a specific mechanism from the bone forming unit, or osteon:
| (1) |
This equation is for the major constituent of bone mineral, hydroxyapatite, at pH ~7.5 (Ref. 72; also discussed at length in Ref. 6). A few percent of apatite in bone is substituted by carbonate, or by ionized phosphates (78, 96). Nevertheless, hydroxyapatite is the vast majority of mineral. Its formation depends on acid transport. This substitution is interesting and in the case of ionized phosphates at the periphery of mineral aggregates (96) might be involved in mineral maturation. However, this substitution also affects the acid evolved in mineral formation only in minor ways.
Phosphate transport is regulated (99), and phosphate production in the mineralization site is largely due to phosphatases (7), particularly the exoenzyme alkaline phosphatase. Alkaline phosphatase hydrolyzes the intermediate product pyrophosphate (which is a mineralization inhibitor) (100), and it is essential to bone formation. Its absence to varying degrees causes poor mineralization and when extreme leads to fatal disease with effectively no bone mineral formation (101). Calcium transport appears largely to be passive. It remains to drive mineral formation so that protons are removed, which was shown to involve a combination of chloride dependent acid uptake, and sodium-dependent acid removal, in osteoblasts (6, 56). This key process is so new that there is not clear acceptance of it, although recently in vivo measurements using cell membrane- and pH-dependent fluorophores have provided definitive evidence that bone matrix is alkalinized during bone formation (unpublished data).
Mineral Structure Including Crystal Nucleation and Mineral Deposition on Collagen
There remains much work to be done in detailed characterization of bone matrix production, although work to date also demonstrates definitively that bone mineral is deposited on type I collagen without presence of any accessory proteins or cell membrane-related material (Fig. 4B). While doubtless upsetting to many people who have worked on these ideas, and while modulation of hydroxyapatite structure and bone strength by accessory proteins is quite likely, the basic process of mineral deposition is regulated by concentrations of ions and by removal of acid.
The Importance of Water in Bone Strength and Structure
Few subjects in bone are as incompletely understood and potentially as important as the liquid element of bone matrix. Before mineral is added, bone matrix is an aqueous solution of type I collagen and small amounts of other proteins and membrane vesicles, which is to say almost all water and electrolytes. In mineralized bone, analysis of organic matrix suggests that the main electrolyte is potassium, rather than sodium as in extracellular fluid (14). Among other things, this highlights the isolation of bone matrix from extracellular fluid in general (6). The process of mineralization involves phosphate deposition, calcium transport, and removal of the acid produced by hydroxyapatite deposition (6); the mechanism of ion transport resulting in changes in extracellular ions in the remaining aqueous matrix is largely unstudied. There are a variety of studies of elemental analysis of bone as a whole that are not generally helpful in clarifying the electrolyte composition of bone matrix fluid further.
Classical ashing gives typically 15–16% water loss. NMR estimates of water are somewhat higher, on the order of 18–20% (28), possibly because less cellular elements are eliminated than in drying and ashing. Whatever methods are used, most of the fluid in matrix must be eliminated for mature skeletal matrix. Further, the process should be regulated to optimize the balance of strength and flexibility in bone. Nothing is known about regulation of fluid within bone.
Obvious candidate mechanisms include osteoblast aquaporin expression, although there are few data on the subject. One report suggests substantial AQPN1 in osteoblasts, however (4). This finding is in keeping with our unpublished studies, although as yet the mechanism for osteoblast water removal remains not clearly determined. Interestingly, AQPN1 is present in the renal proximal tubule (62), suggesting that multiple AQPN proteins are not necessarily required for passive water distribution, although differences in expression resulting from regulation of channel activity or cellular localization might be expected. Other water transport mechanisms include a variety of ion transporters that also mediate net water transport, including the sodium glucose transporter SGTL1 (107), which is not expressed in bone (unpublished data). There are a number of other possibilities of secondary water transport associated with membrane transporters, none of which are known in osteoblasts, although the possibility of this mechanism being active in osteoblasts should be considered.
Bone Matrix Proteins in More Detail
The organic extracellular bone matrix is ≥90% type I collagen and ~5% noncollagenous proteins (9). The proportions are debatable due to the difficulty of excluding cellular processes and osteocytes. In mature bone, in regions without osteocytic processes only collagen with associated proteins is seen by electron microscopy (see Fig. 4A). Collagen and noncollagenous proteins have been studied extensively for their contributions to bone quantity and quality. The autosomal dominant mutations in the COL1A1 and COL1A2 producing the classical form of osteogenesis imperfecta attest to the dependence of quality bone production on type I collagen. In bone, type I collagen is generated by osteoblasts; this large protein is processed by a COPII dependent exocytosis pathway and released at the apical surface of the osteoblast (43). The details of the collagen secretory process are not fully defined, but it is clear that after secretion the collagen matures into the “twisted plywood” lamellar configuration that contributes to the strength and quality of the bone matrix composite (106). Collagen cross linking at lysine or hydroxylysine residues contributes to bone strength; with time cross links decrease bone elasticity and might increase fragility and susceptibility to fractures.
Although type I collagen is the major structural protein of the extracellular compartment in mammals, fibrillar collagens are also signaling proteins and collagens have important binding partners. This biology has been revealed by structural studies and genetic manipulation of the collagen. Early on, it was clear that peptides from collagen taken out of the fibrillar triple helix maintain their structure and that these same sequences define the local structure in the intact collagen molecule (5, 51). This has permitted the direct study of collagen binding using the peptides as inhibitors for an array of cell surface and intracellular matrix receptors (42). Thus far, the peptide approach has not been used to identify sites of mineralization or binding of bone-associated proteins but this will be of interest going forward.
The noncollagenous bone proteins constitute a large group of diverse proteins, greater than 100, most having incompletely defined function. Because they are not stoichiometric with type I collagen, they are not considered to be structural components of the skeleton but are presented as signal carriers of bone status when released by bone turnover, trauma, or pathology. Genetic removal of individual proteins in mice typically produces mild phenotypes. This is consistent with a complex network of characteristic factors and effects that display compensation for individual defects or excess. This suggests that these proteins might be therapeutically important when it is determined how this network functions to optimize bone quality (69, 89). Extensively studied among these are the acidic proteins osteopontin and osteocalcin, which modulate to a moderate extent bone matrix strength and maturation (28). The ways in which these proteins function remains unclear. It does seem probable, however, that there might be exploitable therapeutic potential from these proteins. Recently the activity of these proteins has been linked to the level their phosphorylation (90). It seems reasonable to suggest that they might be used to improve bone quality and quantity once their mechanism of action and the fundamental factors driving bone formation are understood.
A MODEL FOR OSTEOBLAST EPITHELIAL-LIKE STRUCTURE AND BONE TRANSPORT
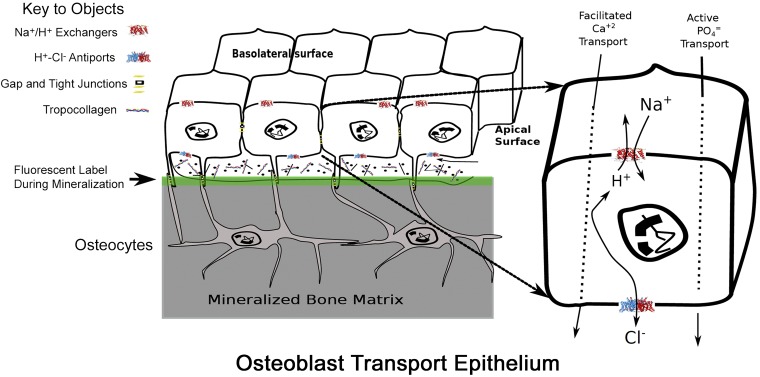
Our understanding of the addition of mineral to collagen and noncollagenous proteins to form the strong and light bone composite matrix has been limited. The assumption that mineralization would be spontaneous if the correct substrate is provided has been tested many times, but success of providing permissive substrate to stimulate osteoblast activity has been elusive (55). In part, this results from not respecting the mechanism of natural bone mineralization, which is supported by the anatomy of the active osteon. Osteoblasts form a tight epithelially organized cell layer isolating bone matrix from the marrow, and mineral movement is driven by active transport (Fig. 6) (6).
Fig. 6.
Model of cell organization, matrix production, and proton transport in osteoblasts. Key transport proteins are shown at major membrane positions of expression. The epithelial-like nature of osteoblasts in a bone-forming unit is diagrammed (left); osteoblasts are connected by gap and desmosomal junctions. When active, small hydroxyapatite binding fluors such as calcein accumulate in the mineral (green line). After accumulation of matrix, osteoblasts buried in matrix are called osteocytes. These remain alive and are connected to surface cells by gap junctions (not illustrated). Osteoblasts produce bone matrix, mainly type I collagen and mineral-producing proteins including alkaline phosphatase, sodium hydrogen exchangers, and Cl−,H+ antiports in a coordinated fashion. The sodium hydrogen exchangers and Cl−,H+ antiports are key proteins for transcytosis of protons (detailed at right) to allow mineral deposition at a slightly alkaline pH despite production of significant acid by mineral precipitation (Eq. 1). [Modified from Blair et al. (6).]
As regards mineral movement, we have taken an approach that begins from the basic chemical principles in addressing the process of mineralization. Hydroxyapatite is deposited with extracellular type I collagen from the soluble Ca2+ and phosphate ions by the classic chemical formula of Neuman and Neuman (72) shown in Eq. 1 above. Earlier, this equation formed the basis for postulating the mechanism of bone removal by osteoclasts (8). Since the amount of protons generated in this way is large and osteoblasts are a functional epithelium delimiting an active osteon, they need to provide a robust proton transport mechanism. We scanned expression libraries of active osteoblasts and observed that three proton-transporting molecules were clearly elevated (54, 56).
The expression levels of mRNA for sodium hydrogen exchanger 1 (NHE-1) were elevated, and the mRNAs for chloride-proton antiporters (ClCs) ClC-3 and 5 are dramatically elevated in the active osteoblasts (6). Using membranes isolated from active osteoblasts in culture, we demonstrated that sodium-dependent H+ transport was inhibited by the specific NHE1 inhibitor, cariporide and that chloride-dependent proton transport was present and was blocked when chloride in membrane vesicles was replaced by gluconate. The NHE activity will remove H+ from the osteoblast using the Na+ gradient present in all mammalian cells. Immuno-histology localized NHE-1 to the basolateral membrane of the osteoblasts, and we postulated that it moves protons into the extracellular compartment. The ClC channels require more consideration to understand how they deliver protons from the osteon matrix to the osteoblast cytosol. Both ClC-3 and -5 are intracellular proteins found on endosomes and secretory vesicles; both cycle through the plasma membrane. For ClC-3 the density of the cell surface isoforms depends on an NH2-terminal retention signal (38).
The ClC-3 and 5 proteins have generated much biophysical interest because their transport process contains clear elements of channel and transporter activity (64). The H+ and Cl− pathways through this membrane protein closely link the two pathways. The mammalian ClC-3 expressed at the cell surface of human embryonic kidney cells displays strong outward rectification (81). The stoichiometry of the ClC-3 antiporter is one proton for two chloride ions. At the cell surface, this will drive cellular chloride exit from the osteoblast and proton entry into the osteoblast only in hyperpolarized (negative membrane potential) situations. Even then the currents are small under basal conditions. In a heterologous expression system, ClC-3 cloned from the colon tumor cell line T84 and overexpressed in the human kidney epithelial line HEK293T, displayed ClC-3 protein expression on the cell surface. Upon CaMKII phosphorylation in the channel NH2-terminal domain, the current-voltage relationship becomes significantly less rectifying, the outward chloride current increases 20-fold, and channel becomes a potentially important pathway for protons into the osteoblast cytoplasm (45, 80). A cell surface localization is consistent with the in situ antibody localization of ClC-3 on the apical surface of the active osteoblast (6) and is what we diagram in Fig. 6.
In our minds, the question could be framed as to whether type I collagen would promote the formation of hydroxyapatite deposition in a pH-dependent fashion. Very early in the study of mineralization, crystal formation was observed in cartilage and assumed to result from a nucleation process (2). Since that time a great deal has been presented supporting a nucleation pathway involving the exosomes produced by osteoblasts (11). The distinction between matrix vesicles and exosomes is not clear (85). Others proposed a role of exosomes in mineralization, although the point is controversial (21). In all cases the assumption has been that super saturation by Ca2+ or phosphate was the nucleating factor. This ignores H+ in the reaction equation, Eq. 1, and does not provide a role for the type I collagen. In a recent publication, we showed that fibrillar type I collagen strongly promotes the deposition of hydroxyapatite in a pH-dependent fashion onto the collagen (Fig. 4B) (6).
Previously, the association of 20-nm hydroxyapatite crystals with the banding pattern of lamellar collagen fibrils during bone synthesis has been proposed as representing the mechanism of mineralization (73). That this association would so dramatically alter the properties of the bone matrix has not been successfully addressed. We studied the question of collagen promoting the deposition of hydroxyapatite using surface plasmon resonance (Fig. 4B). Briefly, purified type I collagen (kind gift of Prof. Robert Mecham, Washington University) was deposited on carboxymethyl dextran-modified gold activated by reaction with N-hydroxysuccinimide and N-ethyl-N′-(dimethylaminopropyl)-carbodiimide HCl, followed by capture of collagen triple helix in PBS at pH 7 (6). Under these conditions the collagen is captured through the available NH2-terminal amine. This chip was used for analysis of mineral deposition as a function of time, phosphate concentration, calcium concentration, and pH, with EDTA washes as controls to remove mineral and reduce resonance to baseline. At time zero in each trace, responses were overlain for injections of buffer, 1 mM calcium, and phosphate from 0 to 5 mM in increments. The effect of increasing deposition saturated at 1 mM calcium (not shown); at higher calcium concentrations, the solution rapidly grossly precipitated as expected. However, in these experiments the solutions were stable and the signal from the soluble salts was subtracted. The deposited mineral was removed by washing with buffer containing 10 mM EDTA. By way of contrast, pH and phosphate have dramatic influences on the signal. At pH 6.81, increasing the phosphate by 10-fold increases the signal by 100-fold. At pH 7.40, the signal is increased by another 10-fold, a response indicating a greater than unitary stoichiometry with protons. This signal is partially reversed by washing, but a wash-resistant component remains and needs to be treated with 10 mM EDTA to return the baseline to its starting point. These results suggest multistep models of mineralization where initial deposition depends only collagen, pH, and phosphate (6).
CONCLUSION
Much remains to be learned about mineralization of collagen. It is clear that amorphous hydroxyapatite crystals, called “platelets” or “nanocrystals,” form periodically in the heavily cross-linked type I collagen (Fig. 4A), but the molecular details of this crystal formation are not well studied. On the other hand, it is now clear that hydroxyapatite can be deposited on type I collagen without other proteins or components and that this is dependent on regulation of pH (Fig. 4B). One mechanism of mineralization, “heteronucleation” would be consistent with our results and does not promote direct deposition from the extracellular space on to type I collagen (34). Rather, this mechanism suggests a specialized environment developed by osteoblasts and the direct involvement of alkaline phosphatase. The mineral and collagen of bone exclude much of the water initially present in the dense collagen before mineralization, but bone retains an important component of water. Regulation of the water content of bone is a further topic with limited understanding. In brief, the composite structure of bone has important plasticity, which is important in the elastic deformation of bone with maintenance of its canalicular structure (25, 84, 95). This is briefly discussed above, from a different perspective, in “The Importance of Water.” Clearly, despite the poor understanding of the topic, that bone is an epithelial-like defined organ makes possible management of the water content. Key features of the work discussed related to mineral transport are graphically summarized in Fig. 7.
Fig. 7.
Summary diagram of work reviewed. A diagram of the main features of bone cells related to mineral transport. Occurrence of the proteins indicated is documented, although other proteins are undoubtedly involved, such as Na+/H+ exchanger regulatory factor (NHERF) (99) and probably additional proteins not yet defined.
GRANTS
This work was supported in part by Department of Veterans Affairs Grant I01BX002490 and National Institutes of Health Grants AR-065407, HL-125076, and DK-110332.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.H.S., H.C.B., D.B.S., V.R., E.C.R., I.L.T., and D.J.N. prepared figures; P.H.S., H.C.B., D.B.S., V.R., E.C.R., I.L.T., and D.J.N. drafted manuscript; P.H.S., H.C.B., D.B.S., V.R., E.C.R., I.L.T., and D.J.N. edited and revised manuscript; P.H.S., H.C.B., D.B.S., V.R., E.C.R., I.L.T., and D.J.N. approved final version of manuscript.
ACKNOWLEDGMENTS
Correspondence may also be addressed to P. H. Schlesinger (e-mail: pschlesinger@wustl.edu).
REFERENCES
- 1.Alshbool FZ, Mohan S. Emerging multifunctional roles of Claudin tight junction proteins in bone. Endocrinology 155: 2363–2376, 2014. doi: 10.1210/en.2014-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson HC. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 35: 81–101, 1967. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arana-Chavez VE, Soares AM, Katchburian E. Junctions between early developing osteoblasts of rat calvaria as revealed by freeze-fracture and ultrathin section electron microscopy. Arch Histol Cytol 58: 285–292, 1995. doi: 10.1679/aohc.58.285. [DOI] [PubMed] [Google Scholar]
- 4.Barron ML, Rybchyn MS, Ramesh S, Mason RS, Fiona Bonar S, Stalley P, Khosla S, Hudson B, Arthur C, Kim E, Clifton-Bligh RJ, Clifton-Bligh PB. Clinical, cellular, microscopic, and ultrastructural studies of a case of fibrogenesis imperfecta ossium. Bone Res 5: 16057, 2017. doi: 10.1038/boneres.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 266: 75–81, 1994. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 6.Blair HC, Larrouture QC, Tourkova IL, Liu L, Bian JH, Stolz DB, Nelson DJ, Schlesinger PH. Support of bone mineral deposition by regulation of pH. Am J Physiol Cell Physiol 315: C587–C597, 2018. doi: 10.1152/ajpcell.00056.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair HC, Larrouture QC, Li Y, Lin H, Beer-Stoltz D, Liu L, Tuan RS, Robinson LJ, Schlesinger PH, Nelson DJ. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng Part B Rev 23: 268–280, 2017. doi: 10.1089/ten.teb.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. Biochem J 364: 329–341, 2002. doi: 10.1042/bj20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep 2: 447, 2013. doi: 10.1038/bonekey.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostian PA, Karnes JM, Cui S, Robinson LJ, Daffner SD, Witt MR, Emery SE. Novel rat tail discitis model using bioluminescent Staphylococcus aureus. J Orthop Res 35: 2075–2081, 2017. doi: 10.1002/jor.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simão AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millán JL, Buchet R. Matrix vesicles from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta, Gen Subj 1862: 532–546, 2018. doi: 10.1016/j.bbagen.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280: 1455–1457, 1998. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 13.Brighton CT, Hunt RM. Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg Am 73: 832–847, 1991. doi: 10.2106/00004623-199173060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bushinsky DA, Gavrilov KL, Chabala JM, Levi-Setti R. Contribution of organic material to the ion composition of bone. J Bone Miner Res 15: 2026–2032, 2000. doi: 10.1359/jbmr.2000.15.10.2026. [DOI] [PubMed] [Google Scholar]
- 15.Carreira AC, Alves GG, Zambuzzi WF, Sogayar MC, Granjeiro JM. Bone morphogenetic proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys 561: 64–73, 2014. doi: 10.1016/j.abb.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Castells L, Cassanello P, Muñiz F, de Castro MJ, Couce ML. Neonatal lethal hypophosphatasia: a case report and review of literature Medicine (Baltimore) 97: e13269, 2018. doi: 10.1097/MD.0000000000013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaible LM, Sanches DS, Cogliati B, Mennecier G, Dagli ML. Delayed osteoblastic differentiation and bone development in Cx43 knockout mice. Toxicol Pathol 39: 1046–1055, 2011. doi: 10.1177/0192623311422075. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142: 295–305, 1998. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 119: 4187–4198, 2006. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 20.Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest 124: 466–472, 2014. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 590: 185–192, 2016. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 22.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8: 739–750, 2005. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Duan P, Bonewald LF. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol 77: 23–29, 2016. doi: 10.1016/j.biocel.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89: 747–754, 1997. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 25.Eberhardsteiner L, Hellmich C, Scheiner S. Layered water in crystal interfaces as source for bone viscoelasticity: arguments from a multiscale approach. Comput Methods Biomech Biomed Engin 17: 48–63, 2014. doi: 10.1080/10255842.2012.670227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid- treated rabbits. Endocrinology 142: 1333–1340, 2001. doi: 10.1210/endo.142.3.8048. [DOI] [PubMed] [Google Scholar]
- 27.Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, Udagawa N, Aoki K, Suzuki H. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 561: 195–200, 2018. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water content measured by proton-deuteron exchange NMR predicts bone mineral density and mechanical properties. J Bone Miner Res 19: 289–296, 2004. doi: 10.1359/JBMR.0301227. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res 44, Suppl 1: 109–116, 2003. doi: 10.1080/03008200390152188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci 17: 1891–1903, 2012. doi: 10.2741/4026. [DOI] [PubMed] [Google Scholar]
- 31.Garimella R, Tague SE, Zhang J, Belibi F, Nahar N, Sun BH, Insogna K, Wang J, Anderson HC. Expression and synthesis of bone morphogenetic proteins by osteoclasts: a possible path to anabolic bone remodeling. J Histochem Cytochem 56: 569–577, 2008. doi: 10.1369/jhc.2008.950394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res 20: 1715–1722, 2005. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 33.Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764, 2005. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Glimcher MJ. Bone: nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev Mineral Geochem 64: 223–282, 2006. doi: 10.2138/rmg.2006.64.8. [DOI] [Google Scholar]
- 35.Goh BC, Singhal V, Herrera AJ, Tomlinson RE, Kim S, Faugere MC, Germain-Lee EL, Clemens TL, Lee SJ, DiGirolamo DJ. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Biol Chem 292: 13809–13822, 2017. doi: 10.1074/jbc.M117.782128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes CM, van Paassen H, Romeo S, Welling MM, Feitsma RI, Abrunhosa AJ, Botelho MF, Hogendoorn PC, Pauwels E, Cleton-Jansen AM. Multidrug resistance mediated by ABC transporters in osteosarcoma cell lines: mRNA analysis and functional radiotracer studies. Nucl Med Biol 33: 831–840, 2006. doi: 10.1016/j.nucmedbio.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Guedes JA, Esteves JV, Morais MR, Zorn TM, Furuya DT. Osteocalcin improves insulin resistance and inflammation in obese mice: participation of white adipose tissue and bone. Bone 115: 68–82, 2018. doi: 10.1016/j.bone.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Guzman RE, Miranda-Laferte E, Franzen A, Fahlke C. Neuronal ClC-3 splice variants differ in subcellular localizations, but mediate identical transport functions. J Biol Chem 290: 25851–25862, 2015. doi: 10.1074/jbc.M115.668186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanna H, Mir LM, Andre FM. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res Ther 9: 203, 2018. doi: 10.1186/s13287-018-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hata K, Takahata Y, Murakami T, Nishimura R. Transcriptional network controlling endochondral ossification. J Bone Metab 24: 75–82, 2017. doi: 10.11005/jbm.2017.24.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatakeyama N, Kojima T, Iba K, Murata M, Thi MM, Spray DC, Osanai M, Chiba H, Ishiai S, Yamashita T, Sawada N. IGF-I regulates tight-junction protein claudin-1 during differentiation of osteoblast-like MC3T3-E1 cells via a MAP-kinase pathway. Cell Tissue Res 334: 243–254, 2008. doi: 10.1007/s00441-008-0690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoop CL, Zhu J, Nunes AM, Case DA, Baum J. Revealing accessibility of cryptic protein binding sites within the functional collagen fibril. Biomolecules 7: 76, 2017. doi: 10.3390/biom7040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosaki-Takamiya R, Hashimoto M, Imai Y, Nishida T, Yamada N, Mori H, Tanaka T, Kawanabe N, Yamashiro T, Kamioka H. Collagen production of osteoblasts revealed by ultra-high voltage electron microscopy. J Bone Miner Metab 34: 491–499, 2016. doi: 10.1007/s00774-015-0692-0. [DOI] [PubMed] [Google Scholar]
- 44.Hsu YH, Kiel DP. Clinical review: genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J Clin Endocrinol Metab 97: E1958–E1977, 2012. doi: 10.1210/jc.2012-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 276: 20093–20100, 2001. doi: 10.1074/jbc.M009376200. [DOI] [PubMed] [Google Scholar]
- 46.Ishidou Y, Kitajima I, Obama H, Maruyama I, Murata F, Imamura T, Yamada N, ten Dijke P, Miyazono K, Sakou T. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J Bone Miner Res 10: 1651–1659, 1995. doi: 10.1002/jbmr.5650101107. [DOI] [PubMed] [Google Scholar]
- 47.Jia M, Chen S, Zhang B, Liang H, Feng J, Zong Z. Effects of constitutive β-catenin activation on vertebral bone growth and remodeling at different postnatal stages in mice. PLoS One 8: e74093, 2013. doi: 10.1371/journal.pone.0074093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol 22: 191–205, 2008. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karner CM, Lee SY, Long F. Bmp induces osteoblast differentiation through both Smad4 and mTORC1 signaling. Mol Cell Biol 37: e00253-16, 2017. doi: 10.1128/MCB.00253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764, 1997. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 51.Kramer RZ, Bella J, Mayville P, Brodsky B, Berman HM. Sequence dependent conformational variations of collagen triple-helical structure. Nat Struct Biol 6: 454–457, 1999. doi: 10.1038/8259. [DOI] [PubMed] [Google Scholar]
- 52.Kulak CA, Dempster DW. Bone histomorphometry: a concise review for endocrinologists and clinicians. Arq Bras Endocrinol Metabol 54: 87–98, 2010. doi: 10.1590/S0004-27302010000200002. [DOI] [PubMed] [Google Scholar]
- 53.Lapunzina P, Aglan M, Temtamy S, Caparrós-Martín JA, Valencia M, Letón R, Martínez-Glez V, Elhossini R, Amr K, Vilaboa N, Ruiz-Perez VL. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet 87: 110–114, 2010. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larrouture QC, Nelson DJ, Robinson LJ, Liu L, Tourkova I, Schlesinger PH, Blair HC. Chloride-hydrogen antiporters ClC-3 and ClC-5 drive osteoblast mineralization and regulate fine-structure bone patterning in vitro. Physiol Rep 3: e12607, 2015. doi: 10.14814/phy2.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials 203: 96–110, 2019. doi: 10.1016/j.biomaterials.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Schlesinger PH, Slack NM, Friedman PA, Blair HC. High capacity Na+/H+ exchange activity in mineralizing osteoblasts. J Cell Physiol 226: 1702–1712, 2011. doi: 10.1002/jcp.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37: 275–281, 2005. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Kamel-El Sayed SA, Wang K, Tiede-Lewis LM, Grillo MA, Veno PA, Dusevich V, Phillips CL, Bonewald LF, Dallas SL. Live imaging of type I collagen assembly dynamics in osteoblasts stably expressing GFP and mCherry-tagged collagen constructs. J Bone Miner Res 33: 1166–1182, 2018. doi: 10.1002/jbmr.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol 4: a007880, 2012. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manikandan M, Abuelreich S, Elsafadi M, Alsalman H, Almalak H, Siyal A, Hashmi JA, Aldahmash A, Kassem M, Alfayez M, Mahmood A. NR2F1 mediated down-regulation of osteoblast differentiation was rescued by bone morphogenetic protein-2 (BMP-2) in human MSC. Differentiation 104: 36–41, 2018. doi: 10.1016/j.diff.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Marie PJ, Debiais F, Haÿ E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol 17: 877–885, 2002. doi: 10.14670/HH-17.877. [DOI] [PubMed] [Google Scholar]
- 62.Maunsbach AB, Marples D, Chin E, Ning G, Bondy C, Agre P, Nielsen S. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol 8: 1–14, 1997. [DOI] [PubMed] [Google Scholar]
- 63.McCarthy TL, Ji C, Chen Y, Kim KK, Imagawa M, Ito Y, Centrella M. Runt domain factor (Runx)-dependent effects on CCAAT/ enhancer-binding protein delta expression and activity in osteoblasts. J Biol Chem 275: 21746–21753, 2000. doi: 10.1074/jbc.M002291200. [DOI] [PubMed] [Google Scholar]
- 64.Miller C. ClC chloride channels viewed through a transporter lens. Nature 440: 484–489, 2006. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 65.Milovanovic P, Vom Scheidt A, Mletzko K, Sarau G, Püschel K, Djuric M, Amling M, Christiansen S, Busse B. Bone tissue aging affects mineralization of cement lines. Bone 110: 187–193, 2018. doi: 10.1016/j.bone.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Monjo M, Rubert M, Ellingsen JE, Lyngstadaas SP. Rosuvastatin promotes osteoblast differentiation and regulates SLCO1A1 transporter gene expression in MC3T3-E1 cells. Cell Physiol Biochem 26: 647–656, 2010. doi: 10.1159/000322332. [DOI] [PubMed] [Google Scholar]
- 67.Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest 105: 1085–1093, 2000. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori K, Kitazawa R, Kondo T, Maeda S, Yamaguchi A, Kitazawa S. Modulation of mouse RANKL gene expression by Runx2 and PKA pathway. J Cell Biochem 98: 1629–1644, 2006. doi: 10.1002/jcb.20891. [DOI] [PubMed] [Google Scholar]
- 69.Morgan S, Poundarik AA, Vashishth D. Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int 97: 281–291, 2015. doi: 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29, 2002. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 71.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 72.Neuman WF, Neuman MW. The Chemical Dynamics of Bone Mineral. Chicago, IL: The University of Chicago Press, 1958. [Google Scholar]
- 73.Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PA, de With G, Sommerdijk NA. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater 9: 1004–1009, 2010. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nusse R, Varmus HE. Wnt genes. Cell 69: 1073–1087, 1992. doi: 10.1016/0092-8674(92)90630-U. [DOI] [PubMed] [Google Scholar]
- 75.Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev 16: 870–879, 2002. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev 29: 1463–1486, 2015. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771, 1997. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 78.Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int 59: 480–487, 1996. doi: 10.1007/BF00369214. [DOI] [PubMed] [Google Scholar]
- 79.Rigueur D, Brugger S, Anbarchian T, Kim JK, Lee Y, Lyons KM. The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development. J Bone Miner Res 30: 733–741, 2015. doi: 10.1002/jbmr.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol 556: 353–368, 2004. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohrbough J, Nguyen HN, Lamb FS. Modulation of ClC-3 gating and proton/anion exchange by internal and external protons and the anion selectivity filter. J Physiol 596: 4091–4119, 2018. doi: 10.1113/JP276332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21: 195–214, 2010. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 83.Simunovic F, Winninger O, Strassburg S, Koch HG, Finkenzeller G, Stark GB, Lampert FM. Increased differentiation and production of extracellular matrix components of primary human osteoblasts after cocultivation with endothelial cells: a quantitative proteomics approach. J Cell Biochem 120: 396–404, 2019. doi: 10.1002/jcb.27394. [DOI] [PubMed] [Google Scholar]
- 84.Samuel J, Sinha D, Zhao JC, Wang X. Water residing in small ultrastructural spaces plays a critical role in the mechanical behavior of bone. Bone 59: 199–206, 2014. doi: 10.1016/j.bone.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: are they anchored exosomes? Bone 79: 29–36, 2015. doi: 10.1016/j.bone.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharrow AC, Li Y, Micsenyi A, Griswold RD, Wells A, Monga SS, Blair HC. Modulation of osteoblast gap junction connectivity by serum, TNFalpha, and TRAIL. Exp Cell Res 314: 297–308, 2008. doi: 10.1016/j.yexcr.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skedros JG, Holmes JL, Vajda EG, Bloebaum RD. Cement lines of secondary osteons in human bone are not mineral-deficient: new data in a historical perspective. Anat Rec A Discov Mol Cell Evol Biol 286A: 781–803, 2005. doi: 10.1002/ar.a.20214. [DOI] [PubMed] [Google Scholar]
- 88.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health 1: 461–468, 2009. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10: 141–150, 2012. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sroga GE, Vashishth D. Phosphorylation of extracellular bone matrix proteins and its contribution to bone fragility. J Bone Miner Res 33: 2214–2229, 2018. doi: 10.1002/jbmr.3552. [DOI] [PubMed] [Google Scholar]
- 91.Steiner D, Lampert F, Stark GB, Finkenzeller G. Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts. J Orthop Res 30: 1682–1689, 2012. doi: 10.1002/jor.22130. [DOI] [PubMed] [Google Scholar]
- 92.Tartaglia M, Bordoni V, Velardi F, Basile RT, Saulle E, Tenconi R, Di Rocco C, Battaglia PA. Fibroblast growth factor receptor mutational screening in newborns affected by metopic synostosis. Childs Nerv Syst 15: 389–394, 1999. doi: 10.1007/s003810050420. [DOI] [PubMed] [Google Scholar]
- 93.Thirunavukkarasu K, Halladay DL, Miles RR, Yang X, Galvin RJ, Chandrasekhar S, Martin TJ, Onyia JE. The osteoblast-specific transcription factor Cbfa1 contributes to the expression of osteoprotegerin, a potent inhibitor of osteoclast differentiation and function. J Biol Chem 275: 25163–25172, 2000. doi: 10.1074/jbc.M000322200. [DOI] [PubMed] [Google Scholar]
- 94.Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo J, Robinson LJ, Blair HC. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab Invest 97: 1072–1083, 2017. doi: 10.1038/labinvest.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unal M, Akkus O. Raman spectral classification of mineral- and collagen-bound water’s associations to elastic and post-yield mechanical properties of cortical bone. Bone 81: 315–326, 2015. doi: 10.1016/j.bone.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Von Euw S, Wang Y, Laurent G, Drouet C, Babonneau F, Nassif N, Azaïs T. Bone mineral: new insights into its chemical composition. Sci Rep 9: 8456, 2019. doi: 10.1038/s41598-019-44620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner AS, Glenske K, Wolf V, Fietz D, Mazurek S, Hanke T, Moritz A, Arnhold S, Wenisch S. Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Ann Anat 209: 18–24, 2017. doi: 10.1016/j.aanat.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, Harrison CA. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-beta1 complex. J Biol Chem 285: 17029–17037, 2010. doi: 10.1074/jbc.M110.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang B, Yang Y, Liu L, Blair HC, Friedman PA. NHERF1 regulation of PTH-dependent bimodal Pi transport in osteoblasts. Bone 52: 268–277, 2013. doi: 10.1016/j.bone.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whyte MP, Coburn SP, Ryan LM, Ericson KL, Zhang F. Hypophosphatasia: Biochemical hallmarks validate the expanded pediatric clinical nosology. Bone 110: 96–106, 2018. doi: 10.1016/j.bone.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Whyte MP, Wenkert D, Zhang F. Hypophosphatasia: natural history study of 101 affected children investigated at one research center. Bone 93: 125–138, 2016. doi: 10.1016/j.bone.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Wongdee K, Pandaranandaka J, Teerapornpuntakit J, Tudpor K, Thongbunchoo J, Thongon N, Jantarajit W, Krishnamra N, Charoenphandhu N. Osteoblasts express claudins and tight junction-associated proteins. Histochem Cell Biol 130: 79–90, 2008. doi: 10.1007/s00418-008-0419-6. [DOI] [PubMed] [Google Scholar]
- 103.Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, Guda T, Schmitz J, Fajardo RJ, Werner SL, Zhao H, Shang P, Johnson ML, Bonewald LF, Jiang JX. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res 30: 436–448, 2015. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA 111: 12097–12102, 2014. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto T, Hasegawa T, Sasaki M, Hongo H, Tabata C, Liu Z, Li M, Amizuka N. Structure and formation of the twisted plywood pattern of collagen fibrils in rat lamellar bone. J Electron Microsc (Tokyo) 61: 113–121, 2012. doi: 10.1093/jmicro/dfs033. [DOI] [PubMed] [Google Scholar]
- 106.Yuan L, Liu HQ, Wu MJ. Human embryonic mesenchymal stem cells participate in differentiation of renal tubular cells in newborn mice. Exp Ther Med 12: 641–648, 2016. doi: 10.3892/etm.2016.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeuthen T, Gorraitz E, Her K, Wright EM, Loo DD. Structural and functional significance of water permeation through cotransporters. Proc Natl Acad Sci USA 113: E6887–E6894, 2016. doi: 10.1073/pnas.1613744113. [DOI] [PMC free article] [PubMed] [Google Scholar]