Abstract
Little research has examined associations of positive psychosocial factors with the American Heart Association Life’s Simple 7™ (LS7) among African Americans. This study examined the associations between positive optimistic orientation and LS7 among African Americans. Using exam 1 data (2000–2004) from the Jackson Heart Study, we examined cross-sectional associations of optimism (in tertiles) with LS7 components [smoking, physical activity, diet, body mass index, blood pressure, cholesterol, glucose] and a composite LS7 score (classified as poor, intermediate, ideal) among 4734 African Americans free of cardiovascular disease. Multivariable prevalence regression was used to estimate prevalence ratios (PR, 95% confidence interval-CI) of intermediate and ideal (vs. poor) individual LS7 components and composite LS7 score by optimism levels, adjusting for demographics, socioeconomic status, and depressive symptoms. For LS7 components with low prevalence, we estimated odds ratios. A greater percentage of participants with high vs. low optimism were younger, female, high SES, and not depressed. After full covariate adjustment, the prevalence ratio of ideal (vs. poor) composite LS7 score was 1.24 for participants who reported high (vs. low) optimism (95% CI 1.09–1.42) at exam 1. Higher levels of optimism were also associated with greater prevalence of ideal (vs. poor) physical activity and smoking. Promoting positive optimistic orientation may be an important step toward increasing the likelihood of achieving optimal cardiovascular health among African Americans.
Keywords: Optimism, Cardiovascular health, Life’s Simple 7, African Americans, Jackson Heart Study
1. Introduction
African American adults have higher rates of cardiovascular disease (CVD) than Whites (Benjamin et al., 2017). This disparity is in part due to differences in the distribution of risk factors (hypertension, diabetes), which are more prevalent among African Americans (Carnethon et al., 2017). Negative psychosocial factors (depression, stress) have been linked with higher CVD risk (Ford et al., 2016; Sims et al., 2012; Sims et al., 2017; Brewer et al., 2018). Research also suggests that positive psychosocial resources (optimism, social support) are associated with reduced risk of developing chronic illness among African Americans (Rasmussen et al., 2009; Kim et al., 2014). However, much of the research on dispositional optimism, the expectation that positive things will happen, has assessed its likelihood of reducing risk for disease onset or progression. Less research, particularly among African Americans, has examined the link between optimism and positive health outcomes, which is important for understanding potential cardio-protective benefits of positive psychological functioning.
Two prior studies have examined the association of optimism with the American Heart Association’s (AHA) index for cardiovascular health (CVH) called Life’s Simple 7 (LS7), which captures the extent to which persons maintain favorable health factors and behaviors (Labarthe et al., 2016; Hernandez et al., 2015; Hernandez et al., 2018). Using the Multiethnic Study of Atherosclerosis (MESA), researchers found that optimism was associated with higher LS7 scores, among a multiethnic sample and findings were maintained after adjusting for race/ethnicity (Hernandez et al., 2015). Another study found that optimism was positively associated with LS7 scores among a large sample of Hispanic/Latino adults (Hernandez et al., 2018). These promising studies suggest that optimism is a positive psychosocial influence which may provide important insights into CVH promotion efforts for CVD prevention.
These studies are important for demonstrating the link between positive psychological functioning and CVH among multi-ethnic subpopulations. However, no study has specifically examined associations of dispositional optimism with CVH in a large sample of African Americans who are at high risk of CVD. Using data from the Jackson Heart Study (JHS), a large African-American cohort, we examined the cross-sectional associations of optimism with a composite LS7 score and individual LS7 components. Our study is important because it examines associations of optimism with positive health metrics that may indicate the “presence of restorative processes rather than the absence of deteriorative processes” (Hernandez et al., 2015). Optimism, a stable psychological asset, results in positive behaviors and restorative health outcomes downstream (e.g., normal blood pressure, high physical activity, proper diet). It is important to examine whether resilient African Americans who display high optimism translate this positive orientation into favorable health outcomes, which are important markers for healthy aging, particularly in a middle-to-elderly-age cohort at-risk of CVD. Additionally, focusing on restorative processes such as those reflected in the composite LS7 is important for identifying targeted prevention interventions among high-risk groups. We hypothesize that higher levels of optimism would be positively associated with composite LS7 score and individual LS7 components.
2. Methods
2.1. Study population
The JHS is a community-based cohort study of the etiology of CVD among African-American men and women living in the tri-county area (Hinds, Madison and Rankin counties) of Jackson, MS. Baseline recruitment (2000–2004) was limited to 5306 non-institutionalized, African Americans 35–84 years old. The final study sample for JHS encompasses nearly 7% of age-eligible African Americans in the tri-county area of Jackson, MS (Hickson et al., 2011). Exam 1 data were collected via in-home interview and clinic examination, where trained staff administered questionnaires to collect self-reported information on demographics (age, sex), psychosocial measures (stress), cardiovascular risk factors and behaviors (hypertension, physical activity, antihypertensive medications). Trained staff also collected blood and urine samples and measured height, weight, and blood pressure (BP). Further details about recruitment, data collection, and study variables are described elsewhere (Taylor et al., 2005; Fuqua et al., 2005). The study was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided informed consent.
2.2. Measures
Outcome measures, obtained at exam 1, included: 1) individual LS7 components (cigarette smoking, physical activity, diet, body mass index (BMI), BP, total cholesterol, and blood glucose); and 2) composite LS7 score. Each component was classified as poor (0 points), intermediate (1 point), or ideal (2 points) as defined by the AHA 2020 Strategic Impact Goals (Lloyd-Jones et al., 2010). Each component was then summed to create a composite LS7 score (range 0–14), and this total score was further classified as 0 to 6 (poor), 7 to 8 (intermediate) and 9 to 14 (ideal). Criteria for defining cut-points for each LS7 metric were determined by the AHA Strategic Goal Task Force (Lloyd-Jones et al., 2010) and are outlined in Table 1.
Table 1.
Poor, intermediate, ideal levels of Life’s Simple 7 components.
| Component | Poor (0 points) | Intermediate (1 point) | Ideal (2 points) |
|---|---|---|---|
| Cigarette smoking | Current | Former ≤ 1 year | Never or former > 1 year |
| Healthy diet score (0–5 components)a | 0 to 1 components | 2 to 3 components | 4 to 5 components |
| Physical activity | (1) 0 min/wk of moderate physical activity and | (1) 1–149 min/wk of moderate physical activity; or | (1) ≥150 min/wk of moderate physical activity; or |
| (2) 0 min/wk of vigorous physical | (2) 1–74 min/wk of vigorous physical activity; activity or | (2) ≥ 75 min/wk of vigorous physical activity; or | |
| (3) 1–149 min/wk of combined moderate and vigorous physical activity | (3) ≥150 min/wk of combined moderate and vigorous physical activity | ||
| BMI, kg/m2 | ≥ 30 | 25 to 29.9 | < 25 |
| Blood pressure, mmHg | SBP ≥140 or DBP ≥ 90 | SBP 120 to 139 or DBP 80 to 89 or treated to goal | SBP < 200 or DBP < 80, untreated |
| Total cholesterol, mg/dL | ≥ 240 | 200 to 239 or treated to goal | < 200 untreated |
| Fasting glucose, mg/dL | ≥ 126 | 100 to 125 or treated to goal | < 100 untreated |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; kg/m2, kilograms/m2; mg/dL, milligrams/deciliter; min/wk, minutes/week; mmHg, millimeters of mercury; SBP, systolic blood pressure.
Healthy diet score 5 components include the following: 1) fruits and vegetables, ≥4.5 cups/day; 2) fish, ≥2 3.5-ounce servings/week; 3) fiber-rich whole grains (≥1.1 g fiber/10g carbohydrate), ≥3 1-ounce-equivalent servings/day; 4) sodium, ≤1500mg/day; and 5) sugar-sweetened beverages, ≤450 kcal/week. Dietary recommendations are scaled according to a 2000-kcal/day diet.
Three behavioral factors were obtained via survey questionnaires and BMI was assessed by study staff during exam 1 (2000–2004). Cigarette smoking was classified as current, former, or never-smokers from self-reported questionnaires. According to the AHA guidelines, poor smoking was defined as participants who were current smokers; intermediate, as former smokers who quit within the past year; and ideal, those who never smoked or quit at least a year before baseline exam. Dietary intake was assessed using the 158-item food frequency questionnaire (Delta Nutrition Intervention Research Initiative) validated and designed specifically for the JHS (Carithers et al., 2005; Carithers et al., 2009). The AHA guidelines for a healthy diet were based on the following 5 dietary components: 1) ≥4.5 cups/day of fruits and vegetables; 2) ≥2 3.5-ounce servings/week of fish; 3) ≥3 1-ounce-equivalent servings/day of fiber-rich whole grains; 4) ≤ 1500 mg/day of sodium; and 5) ≤ 450 kcal/week of sugar-sweetened beverages. Based on these 5 components, AHA defined three levels: 1) poor, 0–1 component; 2) intermediate, 2–3 components; and 3) ideal, 4–5 components. Participants completed an interviewer-administered survey that included questions about types, frequency, and duration of physical activities derived from the JHS Physical Activity (JPAC) Instrument which was validated in the JHS (Dubbert et al., 2005; Smitherman et al., 2009). Based on the sport and exercise components of the JPAC, AHA defined three levels: 1) poor, 0 min/week (min/wk) of physical activity; 2) intermediate, 1–149 min/wk of moderate physical activity, 1–74 min/wk of vigorous physical activity, or 1–49 min/wk of combined moderate and vigorous physical activity; and 3) ideal, ≥150 min/wk of moderate physical activity; or ≥75 min/wk of vigorous physical activity; or ≥ 150 min/wk of combined moderate and vigorous physical activity. Standing height was measured without shoes and recorded to the nearest centimeter (cm). Weight was measured by trained staff at exam 1 on a scale. BMI was computed by dividing weight (kg) by height squared (m2). Poor, intermediate, and ideal BMI were defined as BMI ≥30, 25 to 29.9, and < 25kg/m2, respectively.
Three health factors were obtained by trained staff at exam 1. Systolic blood pressure (SBP) and diastolic BP (DBP) were measured twice in participants’ right arm using the random-zero BP sphygmomanometer (Hawksley and Sons Limited, Sussex, England). The first BP measurement was taken after the participant rested for 5 min in a seated position; the second BP was taken after waiting an additional minute. The average of the two measurements comprised the BP measure for the examination. AHA guidelines define BP status as poor (SBP ≥140 mmHg or DBP ≥90 mmHg), intermediate (SBP 120 to 139 mmHg or DBP 80 to 89 mmHg if untreated or treated to goal if taking antihypertensive medication), or ideal (SBP < 120 mmHg or DBP < 80 mmHg without medication). Total cholesterol was measured from fasting venous blood samples and assayed by the cholesterol oxidase method supplied by Boehringer Mannheim Diagnostics on a Roche COBAS Fara analyzer (Indianapolis, Ind). Poor, intermediate, and ideal cholesterol were defined as ≥ 240 mg/dL, 200 to 239 mg/dL or treated to goal with lipid lowering medication, < 200 untreated, respectively. Fasting plasma glucose (FPG) was measured from fasting venous blood samples with standard glucose oxidase colorimetric methods. FPG was defined as poor (≥ 126 mg/dL), intermediate (100 to 125mg/dL or treated to goal), or ideal (< 100 mg/dL untreated).
The Life Orientation Test-Revised (LOT-R) scale, a validated measure of optimism, was completed at the first annual follow-up interview. The LOT-R is a 6-item scale with a range of 6 (least optimistic) to 24 (most optimistic). Participants responded to 3 positively-worded items (e.g., “I’m always optimistic about my future”) and 3 negatively-worded items (e.g., “If something can go wrong for me, it will”). Internal reliability was adequate in this sample (α = 0.64). In the total optimism score, the three positively-worded items were reversed coded before adding all six items so that a higher score would indicate higher optimism. The composite score was then classified into tertiles (low, medium, high) to assess for threshold effects and continuously in standard deviation (SD) units.
Covariates included baseline age (continuous), sex (men/women), education, income, and depressive symptoms. Education was classified as less than high school (referent), high school graduate to some college, and college graduate or more. Income was classified as poor, middle, upper-middle, and affluent based on family size, US Census poverty levels, and year of baseline clinic exam (2000–2004). Income ranges for the categories were: poor (referent): less than poverty level; middle: 1 to 1.5 times the poverty level; upper-middle: >1.5 but < 3.5 times the poverty level; and affluent: 3.5 or more times the poverty level. To ensure that associations of optimism with LS7 were not primarily attributable to the absence of psychological distress, we adjusted for depressive symptoms using the validated 20-item Centers for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The CES-D score was dichotomized into two scores: 1) 16 or greater (often used as a screening cutoff for depression) and 2) < 16 (α =0.82).
2.3. Statistical analysis
Five hundred seventy-two (572) participants with CVD history (or self-reported history of coronary heart disease, stroke or carotid angioplasty) were excluded at baseline, bringing the analytic sample to 4734. There were missing data for education (n = 13), income (n = 718), optimism (n = 575), and depressive symptoms (n = 1620). We created a missing dummy variable for each of these variables in order to retain participants in the analysis, leaving 4734 participants. We also conducted a sensitivity test by performing multiple imputations with chained equations (MICE) to account for the missing in income, optimism and depressive symptoms. However, results varied little. For the exposure variable (optimism), a comparison between included vs. excluded is presented in the Appendix (Table A.1). There were 965 individuals missing at least 1 LS7 component. We first excluded these individuals from the analysis, and then as a sensitivity test, retained them using a dummy variable to characterize missing status. Results did not vary by method, thus, we present the analyses including those who were missing using a dummy variable. A comparison between included versus excluded based on the LS7 components is presented in Table A.2.
The distribution of covariates was assessed according to levels of optimism, and differences were evaluated using chi-square or ANOVA tests for categorical and continuous variables, respectively. Age- and SES-adjusted associations of composite LS7 score with total optimism were presented by sex. Because the prevalence of each LS7 component was high in our sample, prevalence regression (Spiegelman and Hertzmark, 2005) was used to estimate risk or prevalence ratios (PR 95% confidence interval-CI) of ideal (vs. poor) and intermediate (vs. poor) LS7 component in separate models. Due to lower prevalence of ideal diet and intermediate smoking, we estimated odds ratios (OR). We also estimated the association of optimism with ideal and intermediate (vs. poor) composite LS7 scores using prevalence regression. Sequential adjustment was performed where Model 1 adjusted for age and sex; Model 2 added education and income; Model 3 added depressive symptoms.
As a secondary analysis, we examined the association of the optimism and pessimism subscales with composite LS7 scores using prevalence regression. Models were adjusted similar to those described above. We tested for effect modification by age and sex in the fully-adjusted model. All reported p values correspond to two-tailed tests and were significant at the 0.05 level. Analyses were performed using Stata 15.0 (StataCorp, College Station, TX).
3. Results
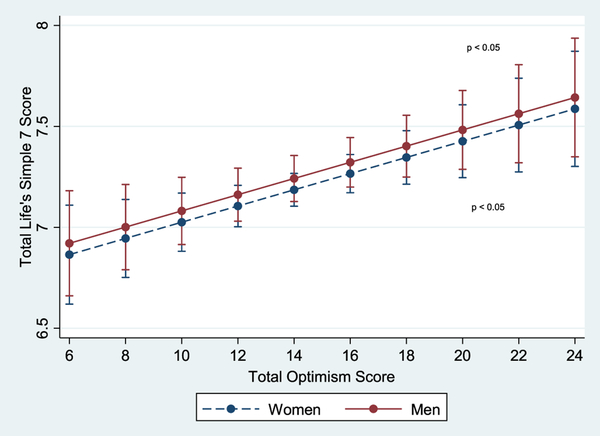
Participants who reported high optimism were younger, female, had higher SES, and greater percentage of low depressive symptoms (p < 0.001 for all) (Table 2). Fig. 1 shows a significant linear relationship between predicted composite LS7 scores and total optimism among women and men after adjustment for age and SES (p < 0.05).
Table 2.
Sample characteristics by levels optimism, JHS (2000–2004; n = 4734).
| Sample characteristics | Tertiles of optimism | p value | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Age in years (mean, SD) | 56.5 (12.8) | 52.9 (12.5) | 52.6 (11.9) | < 0.001 |
| Sex | < 0.001 | |||
| Women | 68.2 | 64.8 | 63.0 | |
| Men | 31.8 | 35.2 | 37.0 | |
| Education (%) | < 0.001 | |||
| Less than high school | 27.2 | 14.5 | 9.8 | |
| HS grad-some college | 43.9 | 40.7 | 35.0 | |
| College grad or more | 28.6 | 44.5 | 54.9 | |
| Income (%) | < 0.001 | |||
| Poor | 16.9 | 10.9 | 7.11 | |
| Middle | 26.9 | 17.2 | 14.1 | |
| Upper-middle | 24.7 | 27.8 | 25.6 | |
| Affluent | 17.9 | 28.7 | 37.6 | |
| Depressive symptoms | < 0.001 | |||
| Low (< 16) | 24.2 | 32.9 | 33.6 | |
| High ( ≥ 16) | 44.0 | 27.8 | 17.9 | |
Note: Abbreviations: JHS, Jackson Heart Study; SD, standard deviation, HS, high school. p value based on chi square (columns) and ANOVA tests.
Fig. 1.
Age- and SES-adjusted associations of composite Life’s Simple 7 (LS7) score with total optimism by sex with 95% confidence interval-CI (JHS, 2000–2004).Note: Age- and SES-adjusted predicted model of the association of mean differences in composite LS7 score at baseline with total optimism score (range 6–24) by sex. Each LS7 metric (cigarette smoking, physical activity, diet, BMI, blood pressure, total cholesterol, and blood glucose) was given a score of 0 (poor), 1 (intermediate), and 2 (ideal) and was summed to create a composite LS7 score (range 0–14), which was classified as 0 to 6 (poor), 7 to 8 (intermediate) and 9 to 14 (ideal). p value represents the association of optimism with composite LS7 score in the sex-stratified regression models for men and women. Results show significant, positive, linear relations between predicted composite LS7 scores and total optimism score among women and men after adjustment for baseline age and SES (education and income) (p < 0.05).
Table 3 presents associations of optimism with composite LS7 score and individual LS7 components. Overall, there was a dose-response effect among participants who reported medium to high (vs. low) optimism with intermediate and ideal (vs. poor) composite LS7 scores. For example, after adjustment for age and sex, the prevalence ratio of ideal (vs. poor) composite LS7 score was 1.38 for participants who reported medium (vs. low) optimism (95% CI 1.21–1.57); and the prevalence ratio for ideal (vs. poor) composite LS7 score was 1.51 for those who reported a high (vs. low) optimism (95% CI 1.33–1.72). The prevalence ratios attenuated after full adjustment but remained significant. When considering optimism as a continuous variable, each 1 SD increase in optimism was significantly associated with greater prevalence ratio of ideal or intermediate (vs. poor) composite LS7 score in all models.
Table 3.
Prevalence ratios (PR 95% CI) of ideal and intermediate (vs. poor) LS7 by optimism, JHS (2000–2004, n = 4734).
| Model 1 (PR 95% CI) | Model 2 (PR 95% CI) | Model 3 (PR 95% CI) | ||||
|---|---|---|---|---|---|---|
| Optimism tertiles | Intermed (vs. poor) | Ideal (vs. poor) | Intermed (vs. poor) | Ideal (vs. poor) | Intermed (vs. poor) | Ideal (vs. poor) |
| Composite LS7 score | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 1.14 (1.03–1.26) | 1.38 (1.21–1.57) | 1.09 (0.99–1.21) | 1.22 (1.07–1.39) | 1.08 (0.98–1.20) | 1.21 (1.06–1.37) |
| High | 1.18 (1.07–1.31) | 1.51 (1.33–1.72) | 1.10 (0.99–1.22) | 1.26 (1.11–1.44) | 1.09 (0.98–1.21) | 1.24 (1.09–1.42) |
| SD units | 1.08 (1.04–1.13) | 1.21 (1.15–1.28) | 1.05 (1.00–1.09) | 1.12 (1.06–1.19) | 1.04 (0.99–1.09) | 1.12 (1.05–1.18) |
| Physical activity | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 1.07 (0.97–1.18) | 1.20 (1.03–1.40) | 1.02 (0.92–1.12) | 1.07 (0.92–1.24) | 1.02 (0.91–1.13) | 1.04 (0.89–1.20) |
| High | 1.17 (1.06–1.30) | 1.54 (1.33–1.77) | 1.09 (0.98–1.21) | 1.28 (1.11–1.48) | 1.06 (0.95–1.19) | 1.23 (1.07–1.42) |
| SD units | 1.09 (1.04–1.14) | 1.22 (1.15–1.30) | 1.05 (1.01–1.10) | 1.13 (1.06–1.20) | 1.05 (1.00–1.09) | 1.11 (1.04–1.19) |
| BMI | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 1.09 (0.97–1.20) | 0.92 (0.77–1.10) | 1.05 (0.95–1.17) | 0.92 (0.77–1.10) | 1.05 (0.94–1.16) | 0.92 (0.77–1.11) |
| High | 1.11 (1.00–1.23) | 1.08 (0.91–1.29) | 1.06 (0.95–1.18) | 1.08 (0.90–1.29) | 1.06 (0.95–1.18) | 1.09 (0.91–1.30) |
| SD units | 1.04 (0.99–1.09) | 1.03 (0.95–1.11) | 1.02 (0.98–1.07) | 1.02 (0.95–1.10) | 1.02 (0.97–1.07) | 1.02 (0.95–1.10) |
| Diet* | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 1.20 (1.09–1.32) | 1.94 (0.78–4.81) | 1.16 (1.05–1.27) | 1.51 (0.60–3.79) | 1.16 (1.05–1.27) | 1.47 (0.58–3.72) |
| High | 1.32 (1.20–1.44) | 2.73 (1.13–6.63) | 1.24 (1.13–1.36) | 1.91 (0.77–4.74) | 1.24 (1.12–1.36) | 1.89 (0.76–4.72) |
| SD units | 1.14 (1.09–1.18) | 1.56 (1.06–2.29) | 1.11 (1.07–1.16) | 1.32 (0.89–1.97) | 1.11 (1.07–1.17) | 1.32 (0.88–1.98) |
| Smoking* | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 0.62 (0.28–1.37) | 1.06 (1.03–1.09) | 0.65 (0.29–1.44) | 1.04 (1.01–1.07) | 0.60 (0.27–1.34) | 1.03 (1.00–1.06) |
| High | 1.07 (0.53–2.16) | 1.07 (1.04–1.10) | 1.12 (0.54–2.29) | 1.04 (1.00–1.07) | 1.02 (0.49–2.12) | 1.03 (0.99–1.07) |
| SD units | 0.99 (0.74–1.31) | 1.03 (1.02–1.04) | 1.01 (0.75–1.36) | 1.02 (1.00–1.03) | 0.95 (0.70–1.28) | 1.01 (0.99–1.03) |
| Cholesterol | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 1.03 (0.96–1.10) | 1.04 (0.98–1.10) | 1.02 (0.95–1.09) | 1.03 (0.98–1.09) | 1.02 (0.94–1.09) | 1.04 (0.99–1.10) |
| High | 1.04 (0.98–1.11) | 1.01 (0.95–1.07) | 1.02 (0.96–1.09) | 1.00 (0.94–1.06) | 1.01 (0.94–1.09) | 1.02 (0.96–1.08) |
| SD units | 1.02 (0.99–1.05) | 1.01 (0.98–1.03) | 1.01 (0.98–1.04) | 1.00 (0.98–1.03) | 1.01 (0.98–1.04) | 1.01 (0.98–1.03) |
| Blood pressure | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 0.99 (0.94–1.04) | 1.11 (1.00–1.23) | 0.97 (0.92–1.02) | 1.08 (0.97–1.19) | 0.97 (0.93–1.02) | 1.06 (0.96–1.18) |
| High | 1.00 (0.96–1.05) | 1.14 (1.03–1.27) | 0.98 (0.93–1.03) | 1.07 (0.96–1.19) | 0.98 (0.93–1.03) | 1.05 (0.95–1.17) |
| SD units | 1.01 (0.99–1.03) | 1.07 (1.02–1.12) | 1.00 (0.98–1.02) | 1.04 (0.99–1.09) | 1.00 (0.98–1.02) | 1.03 (0.98–1.08) |
| Glucose | ||||||
| Low (referent) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 0.95 (0.87–1.04) | 0.96 (0.84–1.10) | 0.97 (0.88–1.06) | 0.99 (0.86–1.13) | 0.97 (0.88–1.07) | 1.00 (0.87–1.14) |
| High | 0.94 (0.85–1.03) | 0.93 (0.80–1.06) | 0.96 (0.87–1.06) | 0.99 (0.85–1.14) | 0.97 (0.88–1.07) | 1.00 (0.87–1.15) |
| SD units | 0.96 (0.93–1.00) | 0.95 (0.90–1.01) | 0.98 (0.94–1.02) | 0.98 (0.92–1.04) | 0.98 (0.94–1.02) | 0.98 (0.93–1.04) |
Abbreviations: BMI, body mass index; CI, confidence interval; JHS, Jackson Heart Study; Intermed, intermediate; LS7, Life’s Simple 7; PR, prevalence ratio; SD, standard deviation. Model 1 adjusted for age and sex, model 2 added education and income, model 3 added depressive symptoms.
Due to lower prevalence of ideal diet and intermediate smoking, we used logistic regression to obtain odds ratios. Each LS7 metric (cigarette smoking, physical activity, diet, BMI, blood pressure, total cholesterol, and blood glucose) was given a score of 0 (poor), 1 (intermediate), and 2 (ideal) and was summed to create a composite LS7 score (range 0–14), and this score was classified as 0 to 6 (poor), 7 to 8 (intermediate) and 9 to 14 (ideal).
Considering each LS7 component, associations were also evident. The association of high (vs. low) optimism was significantly associated with ideal (vs. poor) physical activity across all models. Optimism was associated only with intermediate BMI and ideal blood pressure in age- and sex-adjusted models. Medium and high optimism were consistently associated with intermediate diet across all models. Similarly, those who reported medium and high optimism had a greater prevalence of ideal smoking across all models, though associations for high optimism became non-significant in model 3. Optimism was not associated with intermediate/ideal cholesterol or glucose in any models.
Secondary analysis of the optimism and pessimism subscales shows that medium and high levels of optimism were related to ideal composite LS7 across models 1–3 (Table A.3). Participants who reported high pessimism had lower prevalent intermediate and ideal composite LS7 with each level of adjustment. There was a significant interaction by age in the associations of optimism only with ideal smoking, BP and composite LS7 (p value for interaction < 0.05) (Table A.4). Participants younger than 55 years of age who reported high optimism had a prevalence ratio of 1.09 (95% CI 1.04–1.15) for ideal smoking after full adjustment. Participants greater than or equal to 55 years of age who reported high optimism had a greater prevalence of ideal BP and composite LS7. There was no evidence of effect modification by sex.
4. Discussion
This study examined cross-sectional associations of optimism with AHA LS7 among a large sample of African Americans free of CVD. We found that similar associations of high optimism with ideal composite LS7 and ideal physical activity, and intermediate diet after full adjustment. Associations of optimism with smoking, BP and composite LS7 score were modified by age. For this reason, our hypothesis was partially supported.
Our findings are consistent with previous work on dispositional optimism and single health behaviors such as ideal physical activity (Giltay et al., 2007) diet (Kelloniemi et al., 2005), and smoking (Niemec et al., 2010; Steptoe et al., 2006), although these studies did not include minority participants. Few studies have examined the association of optimism with multiple favorable health outcomes or positive markers of biological functioning (Labarthe et al., 2016; Hernandez et al., 2015; Boehm et al., 2017). One cross-sectional study using multi-ethnic participants from MESA (45–84 years of age; 28% African American) found that high optimism was positively associated with total LS7 (Hernandez et al., 2015). Similarly, we found that high optimism was positively associated with ideal composite LS7. Another large study of English men and women ≥ 50 years (N = 4925) found that persons with high well-being at baseline were more likely to have increased favorable CVH over time (Boehm et al., 2017). However, this study did not examine optimism as a psychosocial resource and was conducted among a largely White sample. As an extension of the previous studies, we examined a large sample of African Americans and assessed individual LS7 components, and found that high optimism was positively associated with ideal physical activity and smoking, and intermediate diet. After full adjustment, optimism was not associated with cholesterol, blood pressure or glucose, which might indicate that other positive psychosocial resources (i.e., social support) may be related to CVD risk factors more so than optimism and should be considered in future studies.
Distinguishing features of this study include an examination of optimism with composite LS7 and individual components in a large sample of African Americans. African Americans in the MESA study were analyzed with other racial groups, and measured as a global ‘race/ethnicity’ covariate. Second, we investigated the association of optimism and pessimism with CVH. The robust association of pessimism with poor LS7 is consistent with previous studies that had stronger findings with the pessimism (vs. optimism) subscale, which utilized both Hispanic participants and multi-ethnic participants from MESA (Hernandez et al., 2018; Roy et al., 2010). Third, we found that participants older than 55 years who were optimistic had ideal BP and composite LS7, which may indicate the extent to which this age group is resilient against having high BP in a cohort where > 60% of participants are hypertensive. This may also indicate experiences (beyond the scope of this paper) of this age group that contribute to optimistic orientation that translates in favorable CVH and health aging. Finally, participants younger than 55 who were optimistic reported ideal smoking. Since smoking prevalence is low in this sample, and lower among women who are also more optimistic than men, this modification could be driven by women and warrants further study.
There are potential behavioral, physiological and positive-coping mechanisms not measured in our study that may explain our findings. Studies have reported that optimism is associated with behaviors such as moderate alcohol consumption and more hours of sleep (Kim et al., 2014; Lemola et al., 2013). Optimists are more likely to adopt these behaviors and maintain them over time, which contribute to CVH. It is also plausible that optimism and pessimism affect CVH through physiological mechanisms that may result in CVD risk factors downstream. A pessimistic disposition may cause emotional distress and provoke feelings of self-blame, which in turn may trigger physiological activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic-adrenal-medullary (SAM) system (Roy et al., 2010). A final potential mechanism involves positive coping, which is associated with favorable health behaviors (tobacco avoidance, physical activity) (Scheier and Carver, 1992), and may mitigate negative health in the face of stressful life events (Johnson et al., 2010), which may produce depressive symptoms. Optimistic persons are likely to practice more adaptive coping strategies (e.g., confront problems directly) when faced with adversity; such practices may also suggest why more optimistic individuals have optimal CVH (Hernandez et al., 2015; Hernandez et al., 2018). Positive coping with chronic stressors may also manifest optimistic tendencies differently among SES groups. For example, higher SES persons tend to be optimistic people who persist at attaining goals and expect favorable outcomes (Keyes et al., 2002). Higher SES not only promotes such skills, but also places a person in the setting with other optimistic persons of similar SES, which reinforces and validates their own optimistic perspective. By contrast, a lower SES person may suppress optimistic tendencies because these individuals (often minority groups) encounter more stressful environments that provide fewer resources to cope with and combat such challenges (Gallo and Matthews, 2003; Boehm et al., 2015). Positive coping may also affect sex differences in optimism in the current study. Research has shown that women report higher levels of optimism than men, due to their social support and social networks that provide coping mechanisms for stressful life events and promote positive affect and well-being. This may be reflected in our study, and further studies are warranted to investigate the SES and sex effects.
4.1. Study limitations and strengths
The following limitations should be considered. This study was conducted in a single metropolitan area which limits its generalizability to other African American populations. Bidirectional associations between optimism and LS7 components may explain our results. For example, though optimism was positively associated with ideal physical activity, physical activity may also improve positive mood (Penedo and Dahn, 2005). This was not tested in our study and may be examined with longitudinal data. Strengths of this study include utilizing JHS, the largest study of CVD in African Americans, and the age modification of the association between optimism and LS7.
5. Conclusions
AHA has defined ideal CVH based on 7 metrics and has found that those with optimal scores have a lower risk of CVD. This study provides some indication of how positive psychological well-being promotes optimal CVH among African Americans and potentially reduces CVD disparities among racial and ethnic groups. Optimism was positively associated with ideal composite LS7 score, physical activity, diet and smoking among African Americans. Because research indicates that African Americans are at greater risk for physical inactivity, poor diet and pernicious effects of smoking than other racial and ethnic groups, tailored interventions may need to consider both improvements in positive well-being and risk factors for CVD for achieving CVH among African Americans and reaching AHA’s goal to improve CVH (Lloyd-Jones et al., 2010).
Supplementary Material
Acknowledgments
The authors also wish to thank the staffs and participants of the JHS.
Sources of funding
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
Dr. Sims was supported by the grants P60MD002249 and (Ms. Glover) U54MD008176 from NIMHD.
Footnotes
Declaration of competing interest
None.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2019.105826.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. , 2017. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135 (10), e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm JK, Chen Y, Williams DR, Ryff C, Kubzansky LD, 2015. Unequally distributed psychological assets: are there social disparities in optimism, life satisfaction, and positive affect? PLoS One 10 (2), e0118066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm JK, Soo J, Chen Y, et al. , 2017. Psychological well-being’s link with cardiovascular health in older adults. Am. J. Prev. Med 53 (6), 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LC, Redmond N, Slusser JP, et al. , 2018. Stress and achievement of cardiovascular health metrics: the American Heart Association Life’s Simple 7 in Blacks of the Jackson Heart Study. J. Am. Heart Assoc 7 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers T, Dubbert PM, Crook E, et al. , 2005. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn. Dis 15 (4 Suppl 6), S6–49–55. [PubMed] [Google Scholar]
- Carithers TC, Talegawkar SA, Rowser ML, et al. , 2009. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J. Am. Diet. Assoc 109 (7), 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon MR, Pu J, Howard G, et al. , 2017. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 136 (21), e393–e423. [DOI] [PubMed] [Google Scholar]
- Dubbert PM, Carithers T, Ainsworth BE, Taylor HA Jr., Wilson G, Wyatt SB, 2005. Physical activity assessment methods in the Jackson Heart Study. Ethn. Dis 15 (4 Suppl 6), S6–56–61. [PubMed] [Google Scholar]
- Ford CD, Sims M, Higginbotham JC, et al. , 2016. Psychosocial factors are associated with blood pressure progression among African Americans in the Jackson Heart Study. Am. J. Hypertens 29 (8), 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua SR, Wyatt SB, Andrew ME, et al. , 2005. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn. Dis 15 (4 Suppl 6), S6–18–29. [PubMed] [Google Scholar]
- Gallo LC, Matthews KA, 2003. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol. Bull 129, 10–51. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B, Kromhout D, 2007. Lifestyle and dietary correlates of dispositional optimism in men: the Zutphen Elderly Study. J. Psychosom. Res 63 (5), 483–490. [DOI] [PubMed] [Google Scholar]
- Hernandez R, Kershaw KN, Siddique J, et al. , 2015. Optimism and cardiovascular health: multi-ethnic study of atherosclerosis (MESA). Health Behav. Policy Rev 2 (1), 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R, Gonzalez HM, Tarraf W, et al. , 2018. Association of dispositional optimism with Life’s Simple 7’s Cardiovascular Health Index: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sociocultural Ancillary Study (SCAS). BMJ Open 8 (3), e019434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson DA, Waller LA, Gebreab SY, et al. , 2011. Geographic representation of the Jackson heart study cohort to the African-American population in Jackson, Mississippi. Am. J. Epidemiol 173 (1), 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Gooding PA, Wood AM, Tarrier N, 2010. Resilience as positive coping appraisals: testing the schematic appraisals model of suicide (SAMS). Behav. Res. Ther 48 (3), 179–186. [DOI] [PubMed] [Google Scholar]
- Kelloniemi H, Ek E, Laitinen J, 2005. Optimism, dietary habits, body mass index and smoking among young Finnish adults. Appetite 45 (2), 169–176. [DOI] [PubMed] [Google Scholar]
- Keyes CLM, Shmotkin D, Ryff CD, 2002. Optimizing well-being: the empirical encounter of two traditions. J. Pers. Soc. Psychol 82, 1007–1022. [PubMed] [Google Scholar]
- Kim ES, Smith J, Kubzansky LD, 2014. Prospective study of the association between dispositional optimism and incident heart failure. Circ. Heart Fail 7 (3), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarthe DR, Kubzansky LD, Boehm JK, Lloyd-Jones DM, Berry JD, Seligman ME, 2016. Positive cardiovascular health: a timely convergence. J. Am. Coll. Cardiol 68 (8), 860–867. [DOI] [PubMed] [Google Scholar]
- Lemola S, Raikkonen K, Gomez V, Allemand M, 2013. Optimism and self-esteem are related to sleep. Results from a large community-based sample. Int. J. Behav. Med 20 (4), 567–571. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, et al. , 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 121 (4), 586–613. [DOI] [PubMed] [Google Scholar]
- Niemec CP, Ryan RM, Patrick H, Deci EL, Williams GC, 2010. The energization of health-behavior change: examining the associations among autonomous self-regulation, subjective vitality, depressive symptoms, and tobacco abstinence. J. Posit. Psychol 5 (2), 122–138. [Google Scholar]
- Penedo FJ, Dahn JR, 2005. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 18 (2), 189–193. [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas 1 (3), 385–401. [Google Scholar]
- Rasmussen HN, Scheier MF, Greenhouse JB, 2009. Optimism and physical health: a meta-analytic review. Ann. Behav. Med 37 (3), 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M, 2010. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychosom. Med 72 (2), 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, 1992. Effects of optimism on psychological and physical well-being - theoretical overview and empirical update. Cogn. Ther. Res 16 (2), 201–228. [Google Scholar]
- Sims M, Diez-Roux AV, Dudley A, et al. , 2012. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am. J. Public Health 102 (Suppl. 2), S258–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, Lipford KJ, Patel N, Ford CD, Min YI, Wyatt SB, 2017. Psychosocial factors and behaviors in African Americans: the Jackson Heart Study. Am. J. Prev. Med 52 (1S1), S48–S55. [DOI] [PubMed] [Google Scholar]
- Smitherman TA, Dubbert PM, Grothe KB, et al. , 2009. Validation of the Jackson Heart Study Physical Activity Survey in African Americans. J. Phys. Act. Health 6 (Suppl. 1), S124–S132. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E, 2005. Easy SAS calculations for risk or prevalence ratios and differences. Am. J. Epidemiol 162 (3), 199–200. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wright C, Kunz-Ebrecht SR, Iliffe S. Dispositional optimism and health behaviour in community-dwelling older people: associations with healthy ageing. Br. J. Health Psychol 2006;11(Pt 1):71–84. [DOI] [PubMed] [Google Scholar]
- Taylor HA Jr., Wilson JG, Jones DW, et al. , 2005. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn. Dis 15 (4 Suppl 6), S6–4–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.