Abstract
Inhaled bronchodilators are the cornerstone of treatment for chronic obstructive pulmonary disease (COPD). Soft mist inhalers (SMIs) are devices that deliver bronchodilators. Although correct device use is paramount to successful medication delivery, patient errors are common. This global systematic literature review and meta-analysis examined device use errors with SMIs among patients with obstructive lung diseases. PubMed, EMBASE, PsycINFO, Cochrane, and Google Scholar were searched to identify studies published between 2010 and 2019 that met the following inclusion criteria: (a) English language; (b) a diagnosis of COPD, bronchitis, or emphysema; and (c) reported device use errors among adults receiving long-acting bronchodilator treatment with Respimat® SMI (i.e. Spiriva®, Stiolto®, Spiolto®, and Striverdi®). Descriptive statistics examined sociodemographics, clinical characteristics, and device use errors. Meta-analysis techniques were employed with random-effects models to generate pooled mean effect sizes and 95% confidence intervals (CIs) for overall and step-by-step errors. The I 2 statistic measured heterogeneity. Twelve studies (n = 1288 patients) were included in this meta-analysis. Eighty-eight percent of patients had COPD, and most had moderate/very severe airflow limitation (Global Initiative for Chronic Obstructive Lung Disease spirometric stages II to IV). Aggregate results revealed that 58.9% (95% CI: 42.4–75.5; I 2 = 92.8%) of patients made ≥1 device use errors. Among 11 studies with step-by-step data, the most common errors were failure to (1) exhale completely and away from the device (47.8% (95% CI: 33.6–62.0)); (2) hold breath for up to 10 seconds (30.6% (95% CI: 17.5–43.7)); (3) take a slow, deep breath while pressing the dose release button (27.9% (95% CI: 14.5–41.2)); (4) hold the inhaler upright (22.6% (95% CI: 6.2–39.0)); and (5) turn the base toward the arrows until it clicked (17.6% (95% CI: 3.0–32.2)). Device use errors occurred in about 6 of 10 patients who used SMIs. An individualized approach to inhalation device selection and ongoing training and monitoring of device use are important in optimizing bronchodilator treatment.
Keywords: Meta-analysis, COPD, chronic obstructive pulmonary disease, soft mist inhaler, inhaler errors, Respimat®, systematic literature review
Introduction
Chronic obstructive pulmonary disease (COPD) is among the leading causes of morbidity and mortality worldwide.1,2 Based on estimates from the Burden of Obstructive Lung Disease study, about 8.9% of the global population is diagnosed with COPD, representing 578 million adults.3 The annual death toll from COPD is estimated to be 3.2 million globally.2 Economically, the cost burden associated with COPD varies from <US$680 per patient in countries outside of the United States (US) to >US$6200 per person in the US.4
Inhaled bronchodilator therapy remains the cornerstone of pharmacological treatment for COPD. Bronchodilators can be administered through several types of commercially available devices including metered-dose inhalers (MDIs), dry powder inhalers (DPIs), soft mist inhalers (SMIs), and nebulizers.5 Each device type has advantages and disadvantages. MDIs are portable devices that are fast acting on the airways following one or two puffs taken over a few seconds. They require priming, shaking prior to use, coordination between actuation and inhalation, slow and steady inspiration, and breathholding.6,7 In addition, since MDIs contain propellants, patients often experience a cold Freon-like effect, an inadvertent reaction to the chilling sensation that reaches the back of the throat following actuation of the device.8 DPIs are compact portable devices that can be administered in one or two puffs over a few seconds. They were developed to remove the need for propellant-type liquids and to simplify formulations for highly insoluble therapeutic agents. However, DPIs need relatively high inspiratory flow to disaggregate the powder and deliver medication making them less useful for some of the more severely limited COPD patients.7 DPIs also result in high oropharyngeal deposit, similar to MDIs, and most are moisture sensitive.8,9 SMIs are portable devices that do not contain propellants and can be used in patients with lower inspiratory flow rates.9 The spray duration of SMIs is approximately 1.2 seconds which is considerably longer than MDIs.10 However, as is the case with MDIs, SMIs also require hand–breath coordination and breathholding. Nebulizers produce a fine mist for medication administration for up to 20 minutes and have been used for many years in the treatment of COPD.6,7 They do not require priming, hand–breath coordination, or breathholding and are able to aerosolize medication that the patient can inhale with regular tidal breathing.8 There are different types of nebulizers (jet, ultrasonic, and mesh) in the market, and each varies in speed of treatment administration, ease of operation, and portability.11
Health-care providers consider several factors in selecting inhalation devices for their patients. Important considerations include inspiratory flow sufficiency, hand–breath coordination, cognitive and mental aptitudes, and the patient’s fine motor skills.6,7,12–14 In addition, patient and caregiver preferences regarding device features and costs can influence selection.7,15–17
Regardless of inhalation device types and characteristics, the patient’s ability to correctly use the device is paramount to successful treatment.18,19 Numerous studies have shown that incorrect inhalation device technique can compromise medication delivery, increase the risk of exacerbations, result in higher health resource utilization, and lead to premature mortality.20–24 To this end, several systematic and narrative literature reviews, with and without a meta-analysis, have been conducted on inhaler errors specific to MDIs, DPIs, or both.6,7,19,20,24–37 However, similar studies on device use errors with SMIs have not been conducted. To address the existing knowledge gap, this global systematic literature review and meta-analysis was conducted to examine the prevalence and types of device use errors among adult patients with COPD who were receiving long-acting bronchodilator treatment with an SMI.
Methods
Search strategy
The Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) guidelines38 were applied to conduct a literature search of articles published between January 1, 2010 and February 1, 2019 across five databases, including PubMed, EMBASE, PsycINFO, Cochrane, and Google Scholar. Selection of published articles was restricted to those that met the following inclusion criteria: (a) English language; (b) a diagnosis of COPD, bronchitis, or emphysema; and (c) reported device use errors among adults receiving long-acting bronchodilator treatment with Respimat® SMI (i.e. Spiriva®, Stiolto®, Spiolto®, and Striverdi®). Iterative combinations of the following keywords were used during the search process: soft mist inhaler, SMI, Respimat, Stiolto Respimat, Spiolto Respimat, Spiriva Respimat, Striverdi Respimat, inhaler error, inhalation technique error, inhaler mistake, critical errors, improper inhaler technique, inhalation error rate, inhalation failure rate, device handling errors, dose preparation errors, and dose emission errors. To be more comprehensive and balanced in our search strategy, all references within the articles identified from electronic searches were reviewed for relevance which extended the search period from January 1, 2005 to February 1, 2019.
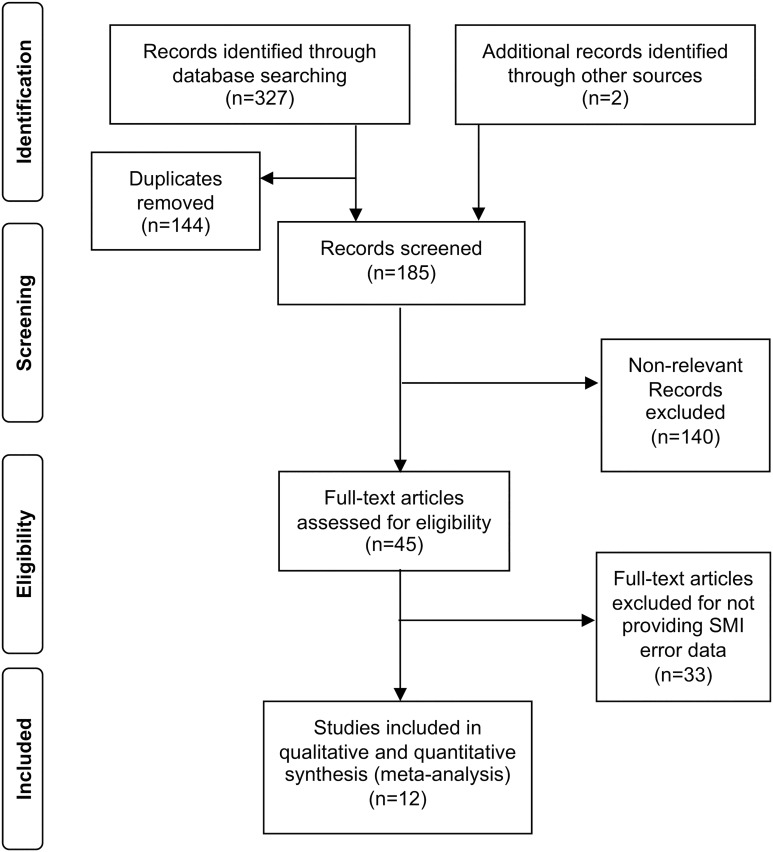
A total of 327 publications met our initial screening criteria (Figure 1). An additional two publications were identified through a manual search. After removing duplicate studies (n = 144), 185 abstracts were examined further to assess relevance. By eliminating 140 abstracts that did not meet the eligibility criteria, 45 full-text articles remained and were reviewed in detail. Among these articles, 33 did not provide information on device use errors, resulting in a total of 12 publications that were included in this meta-analysis.22,39–49 Since this study involved reviewing aggregate level secondary data published in publicly available studies and there was no access to primary patient-level data, protocol approval by the Institutional Review Board and Ethical Committee was waived.
Figure 1.
PRISMA flowchart. PRISMA: Preferred Reporting Item for Systematic Reviews and Meta-Analyses; SMI: soft mist inhaler.
Data extraction and quality assessment
The following data were extracted from each publication based on availability: (a) patients’ sociodemographic characteristics (i.e. age and sex), (b) primary diagnosis, (c) COPD severity (i.e. Global Initiative for Chronic Obstructive Lung Disease (GOLD) severity rating or forced expiratory volume in 1 second (FEV1), (d) prevalence of overall errors defined as ≥1 device use error, and (e) errors made at each inhalation step. Three trained independent reviewers graded the quality of each study. Publications that met the following criteria were classified as having poor quality and excluded from the meta-analysis: (a) combined error rates across multiple devices, (b) reported error rates for fewer than five patients, and (c) did not provide quantifiable data. For randomized controlled trials, quality was assessed using the Cochrane Collaborative criteria.50 For cross-sectional and observational cohort studies, quality was assessed using applicable scales by Newcastle and Ottawa.51
Respimat SMI device use steps
Device use errors were based on the patients’ ability to correctly complete the following 14 inhalation steps:10 (1) checking the cartridge or capsule, (2) holding the inhaler in the upright position, (3) turning the base in the direction of the arrows until it clicks, (4) opening the cap, (5) not activating the inhaler inadvertently, (6) exhaling completely and away from the inhaler, (7) closing lips around the mouthpiece, (8) holding the inhaler in a horizontal position so that it is pointing to the back of throat, (9) pressing the dose-release button while taking a slow and deep inhalation, (10) continuing to inhale slowly and deeply through the mouth, (11) holding breath up to 10 seconds, (12) removing the inhaler from the mouth and breathing out, (13) repeating steps for the second puff, and (14) closing the cap. Dose preparation (priming) steps for first time device use were excluded from the analyses since only two studies39,40 reported these types of errors. Selected steps (i.e. steps 1, 5, 8, 12, 13, and 14) for which data were provided for fewer than four studies were excluded from the step-by-step error analysis.
Statistical analysis
Descriptive statistics including frequencies, means, medians, standard errors, standard deviations, and proportions for variables of interest were computed and compared across the studies. The prevalence of overall device use errors with SMIs was computed based on the reported proportion of patients who made at least one device use error during the inhalation process. For studies that did not provide an overall error rate,41–43 it was inferred based on the reported inhalation step with the highest frequency of error. For one study,44 the overall device use error was considered the same as the reported error rate from the single inhalation step for which data were provided. That study also reported patients using Spiriva without specifying whether the medication was delivered via Respimat or Handihaler®. After contacting the authors, it was presumed that medications were delivered via Respimat. For another study,45 overall errors were inferred from the mean number of errors per patient. For studies that reported inhalation technique errors before and after an intervention, only baseline (i.e. pre-intervention) error data were used in our analysis.
Step-by-step device use errors were also analyzed across 11 studies that provided this level of data. For studies that presented error data graphically, numeric values were estimated by interpreting graphic representations. The following additional calculations were performed, depending on selected scenarios: (1) for studies that provided results as a percentage of patients who correctly performed each device use step, the results were converted to the percentage of patients who performed each step incorrectly; and (2) for studies that reported two error rates for each device use step, a mean value was computed and used in our analysis.
Meta-analysis
Information specific to the prevalence of overall and step-by-step device use errors was gathered in accordance with the recommendations outlined in Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines for meta-analysis and systematic reviews.52 Pooled effect estimates (weighted proportions) and 95% confidence intervals (CIs) were computed using the approximation of a binomial distribution. Forest plots were generated to visually examine the degree to which the effect estimates of each study distributed around the pooled effect estimates.53 The I 2 statistic was used to assess heterogeneity.54,55 I 2 values of 0–25%, 25–50%, 50–75%, and 75–100% were considered as benchmarks to represent little/negligible, moderate, considerable, and substantial/large degree of heterogeneity, respectively.54,56 Given the relatively few number of studies and the expectation that there would be between-studies variability, a random effects model was adopted for the meta-analysis.54,57,58 Sensitivity analysis was performed using the leave-one-out cross-validation technique by removing one study each time to check if an individual study influenced the pooled results.50,59–62 Funnel plots, Begg and Mazumdar’s rank correlation test and Egger’s regression test were used to examine potential publication bias, with p < 0.05 denoting statistical significance. All meta-analyses were performed using JASP 9.2 software with a restricted maximum likelihood random-effects model.
Results
Patient characteristics
There were 1977 patients included across the 12 studies, of which 75% were observational studies (50% cross-sectional and 25% cohort studies) and 25% were randomized clinical trials (RCTs; Table 1). Average age ranged from 53 years to 74 years. Nearly twice as many patients were male than female (65% vs. 35%, respectively), and the majority had COPD (88%). Among the studies that reported COPD severity (n = 993), 84.6% of all patients were classified as having moderate to very severe COPD (GOLD spirometric stages II to IV). Device use errors with SMIs were provided for a total of 1288 patients.
Table 1.
Patient and study characteristics.a
| Study | Study design | Patients (N) | Mean age (±SD/range) | Sex, N (%) | Diagnosis | COPD severity, N (%) |
|---|---|---|---|---|---|---|
| Ding et al.39 | CS | 31 (All SMI users) | 59.1b (40–76) | M: 14 (45) F: 17 (55) |
COPD | GOLD II: 11 (35) GOLD III: 17 (55) GOLD IV: 3 (10) |
| Ngo et al.46 | CS | 70 (22 SMI users) | 68.6 (±8.7) | M: 65 (93) F: 5 (7) |
COPD | GOLD I: 3 (4) GOLD II: 6 (9) GOLD III: 8 (11) GOLD IV: 53 (76) |
| Bournival et al.47 | CS | 67 (All SMI users) | 69.8 (±8.3) | M: 36 (54) F: 31 (46) |
COPD | GOLD I: 3 (4) GOLD II: 24 (36) GOLD III: 10 (15) GOLD IV: 6 (9) Missing: 24 (36) |
| Liang et al.48 | CS | 298 (223 SMI users) | 72.1 (±9.0) | M: 284 (95) F: 14 (5) |
COPD | — |
| de Oliveira et al.40 | RCT | 140 (135 SMI users) | 63.5 (±8.2) | M: 78 (56) F: 62 (44) |
COPD | — |
| Windisch et al.45 | RCT | 152c (8 SMI users) | 67.4b | M: 74 (49) F: 78 (51) |
COPD | GOLD I: 2 (1) GOLD II: 32 (21) GOLD III: 59 (39) GOLD IV: 59 (39) |
| Molimard et al. 201722 | Cohort | 625 (625 SMI users) | 65.9 (±11.5) | M: 394 (63) F: 231(37) |
COPD | GOLD I: 126 (20) GOLD II: 374 (60) GOLD III: 99 (16) GOLD IV: 15 (2) Missing: 11 (2) |
| Ohbayashi et al.41 | RCT | 54 (All SMI users) | 74.3 (±10.1) | M: 52 (96) F: 2 (4) |
COPD | GOLD I: 10 (19) GOLD II: 25 (46) GOLD III: 12 (22) GOLD IV: 7 (13) |
| Takaku et al.49 | Cohort | 81 (38 SMI users) | 72 (±7) | M: 74 (91) F: 7 (9) |
COPD | FEV1, % predicted = 60.6 ± 23.9d |
| Chorao et al.42 | CS | 301 (18 SMI users) | 53 (±17) | M: 120 (40) F: 181 (60) |
COPD (107) Asthma (194) |
— |
| Steinberg and Pervanas44 | CS | 129 (38 SMI users) | 65.9 (23–93) | M: 61 (47) F: 68 (53) |
COPD (76) Asthma (44) Othere (9) |
— |
| Asakura et al.43 | Cohort | 29 (All SMI users) | 74 (61–85) | M: 29 (100) | COPD | GOLD I: 9 (31) GOLD II: 12 (41) GOLD III: 6 (21) GOLD IV: 2 (7) |
| Total | 1977 (1288 SMI users) | M: 1281 (65) F: 696 (35) |
COPD: 1730 (88) Asthma: 238 (12) Othere: (9) |
GOLD I: 153 (9) GOLD II: 484 (28) GOLD III: 211 (12) GOLD IV: 145 (8) Missing: 737 (43) |
SD: standard deviation; COPD: chronic obstructive pulmonary disease; CS: cross-sectional; SMI: soft mist inhaler; M: male; F: female; —: no data; GOLD: Global Initiative for Chronic Obstructive Lung Disease; RCT: randomized controlled trial.
a Proportions rounded to the nearest percent.
b Weighted mean age calculated based on the proportion of males and females in the study population.
c Combined for the control and intervention groups at baseline.
d Post-brochodilator.
e Other, unknown or other illness.
Overall and step-by-step device use errors with SMIs
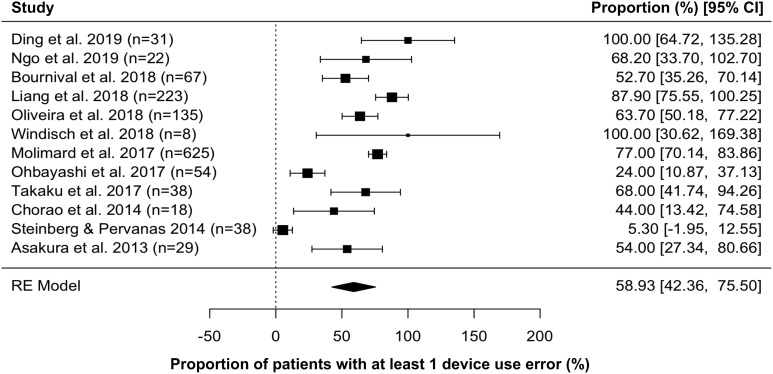
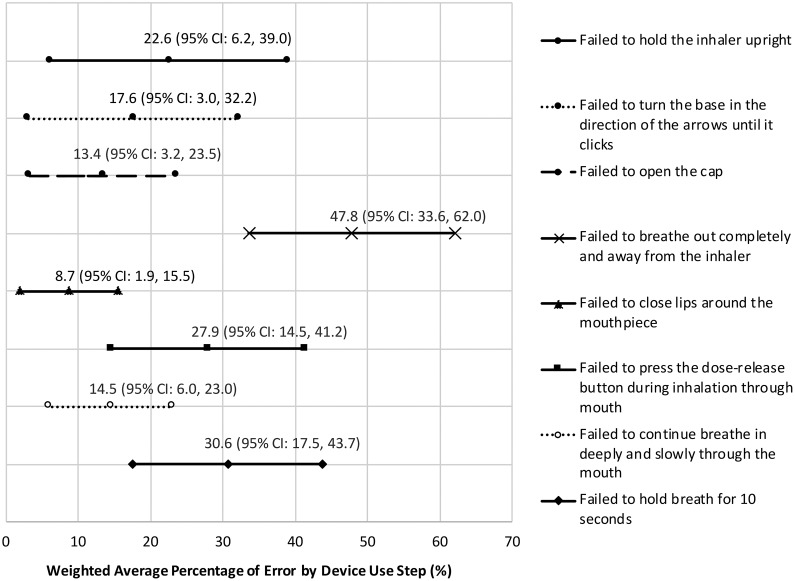
The pooled mean effect size across the 12 studies revealed that 58.9% (95% CI: 42.4–75.5) of patients had at least one device use error with their SMIs (Figure 2). Among the 11 studies that reported step-by-step data, the most common errors were failure to (1) exhale completely and away from the device (47.8% (95% CI: 33.6–62.0)); (2) hold breath for up to 10 seconds (30.6% (95% CI: 17.5–43.7)); (3) take a slow and deep breath while pressing the dose release button (27.9% (95% CI: 14.5–41.2); (4) hold the inhaler upright (22.6% (95% CI: 6.2–39.0)); and (5) turn the base toward the arrows until it clicked (17.6% (95% CI: 3.0–32.2) (Figure 3).
Figure 2.
Meta-analysis of device use errors among patients using Respimat® Soft Mist Inhaler™. I 2 = 92.8%; test for heterogeneity: Q(df = 1) = 273.6, p < 0.001. CI: confidence interval.
Figure 3.
Forest plot showing weighted average percentage of errors by device use step for Respimat® Soft Mist Inhaler™. CI: confidence interval.
Heterogeneity and sensitivity analysis
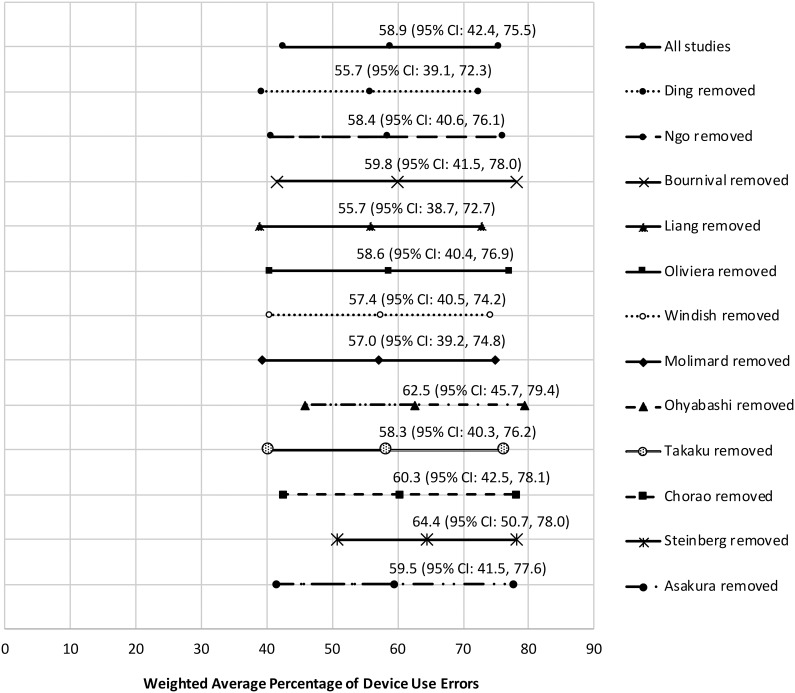
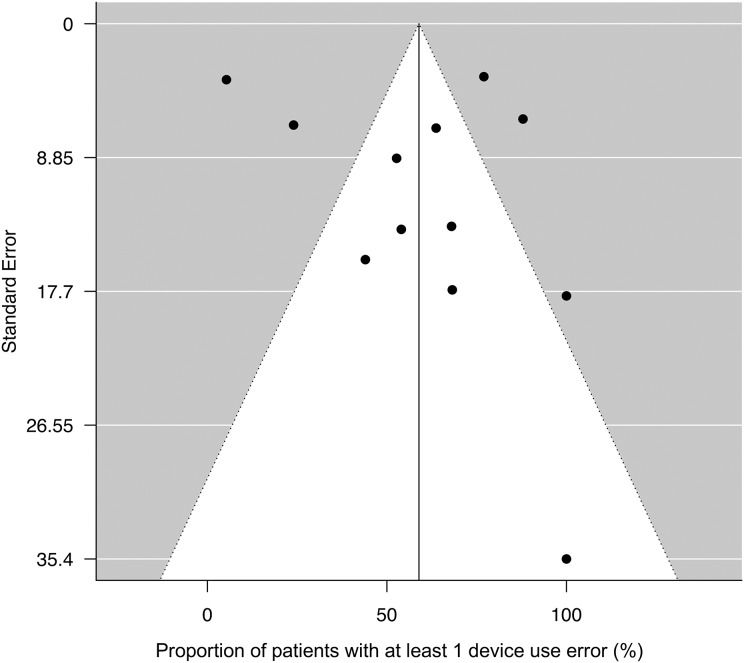
Significant heterogeneity was observed across the 12 studies included in our meta-analysis (I 2 = 92.8%; test for heterogeneity: Q(df = 11): 273.6, p < 0.001). Sensitivity analysis revealed that the pooled effect size was altered with the removal of five studies22,39,41,44,49 (Figure 4). The resulting overall effect size was 57.5% (95% CI: 21.2–93.9) across the five studies relative to 59.8% (95% CI: 51.3–68.4) for the remaining seven studies. However, despite the funnel plot showing some asymmetry across the studies, the tests assessing potential publication bias were not statistically significant by either Begg and Mazumdar’s rank correlation test (p = 0.500) or Egger’s test (p = 0.519) (Figure 5).
Figure 4.
Forest plot showing sensitivity analysis. CI: confidence interval.
Figure 5.
Funnel plot with 95% confidence limits showing publication bias. Five studies outside the funnel include, from left to right, Steinberg and Pervanas44, Ohbayashi et al.41, Molimard et al.22, Liang et al.48, and Ding et al.39. The rest of studies were Ngo et al.46, Bournival et al.47, de Oliveira et al.40, Windisch et al.45, Takaku et al.49, Chorao et al.42, and Asakura et al.43. Proportion of patients with at least 1 device use error was not significantly different between the five outlier studies (57.5% (95% CI: 21.2–93.9)) and the rest of studies (59.8% (95% CI: 51.3–68.4)). CI: confidence interval.
Summary of quality assessment
Table 2 provides a summary of the quality assessment scores across the studies. All of the nine observational studies were of good quality. Among the three RCTs, variability in quality was observed, ranging from low to high.
Table 2.
Assessment of quality across studies.
| Study | Quality score (Newcastle-Ottawa Quality Assessment Scale)a | ||
|---|---|---|---|
| Ding et al.39 | Selection *** |
Comparability ** |
Outcome *** |
| Ngo et al.46 | Selection *** |
Comparability ** |
Outcome *** |
| Bournival et al.47 | Selection *** |
Comparability ** |
Outcome *** |
| Liang et al.48 | Selection *** |
Comparability ** |
Outcome *** |
| Molimard et al.22 | Selection **** |
Comparability ** |
Outcome *** |
| Takaku et al.49 | Selection *** |
Comparability ** |
Outcome *** |
| Chorao et al.42 | Selection *** |
Comparability ** |
Outcome *** |
| Steinberg and Pervanas44 | Selection *** |
Comparability ** |
Outcome *** |
| Asakura et al.43 | Selection *** |
Comparability ** |
Outcome ** |
| Quality score (GRADE)b | |||
| Oliveira et al.40 | ++++ High | ||
| Windisch et al.45 | ++ Low | ||
| Ohbayashi et al.41 | +++ Moderate | ||
a Scale used to assess quality rating in observational studies; Good quality: three or four stars (*) in selection domain, one or two stars in the comparability domain, and two or three stars in the outcome domain; Fair quality: two stars in the selection domain, one or two stars in the comparability domain, and two or three stars in the outcome domain; Poor quality: zero or one star in the selection domain/zero stars in the comparability domain/zero or one star in the outcome domain.
b Scale used to assess quality rating in randomized controlled trials; High: We were confident that the true effect lied close to that of the estimate of the effect; Moderate: We were moderately confident in the effect estimate: The true effect was likely to be close to the estimate of the effect, but there was a possibility that it was substantially different; Low: Our confidence in the effect estimate was rather limited, the true effect may have been substantially different from the estimate of the effect.
Discussion
In this systematic review and meta-analysis, we found that nearly 6 in 10 COPD patients using SMIs made one or more device use errors. Patients had the most difficulty with inhalation steps that required coordination and breathholding. Overall, about one in two patients did not exhale completely and away from the SMI before inhalation, one in three could not hold their breath as required or had difficulty with hand–breath coordination, and one in five patients did not hold the SMI in the correct upright position or had difficulty manipulating the base of the device to release the medication.
MDIs and DPIs were the first handheld inhalers used to deliver bronchodilators to COPD patients. Most MDIs require coordination between actuation and inspiration and result in high oropharyngeal deposition. DPIs need a high inspiratory flow and are vulnerable to humidity which adversely affects dosing. Meta-analysis studies of MDIs have shown that device use errors range between 77% and 86.8%.19,36,37,62 Similarly, a recent meta-analysis of DPIs found inhalation error rates to be as high as 50%.19 Respimat SMI was developed to overcome the limitations of MDIs and DPIs and to meet the need for a convenient propellant-free inhaler that could effectively deliver aerosols from solutions.10,15 Our results suggest that device use errors with SMIs are lower than MDIs but higher than DPIs.
COPD patients who are prescribed an SMI typically have suboptimal inspiratory flow and difficulty rapidly coordinating actuation with inhalation.17,63 Compared to patients using other inhalers, patients using SMIs also tend to be younger, more likely to have severe respiratory disease, and a greater number of comorbidities, particularly neurologic and hypertensive heart diseases.64 Patients who experience difficulty using an SMI may be candidates for inhalation therapy with other types of devices such as nebulizers. Traditionally, some physicians have had reservations prescribing a nebulizer due to concerns about slower speed of action in medication delivery and inconvenient device portability.14,65 However, given advances in technology, portable nebulizers with the capability to rapidly administer bronchodilators are now commercially available, thereby giving physicians another alternative for their patients.
Since device satisfaction has been associated with better outcomes,13,14,40,42,65–67 it seems prudent for clinicians to consider patient preferences during the device selection process. Indeed, GOLD treatment strategies recommend that device selection be based not only on medication availability, costs, and the prescribing physician’s preferences, but also on the patient’s device preferences and the ability to use the inhalation device.21
Regardless of device type, mishandling has been associated with poor health outcomes, more frequent exacerbations, increase in hospitalizations, and higher health resource utilization.21–23 Thus, the economic consequences of device use errors are considerable in COPD populations.68–72 Inhaler device errors are considered to be a form of unintentional medication nonadherence.73,74 Systematic reviews of medication nonadherence (intentional and unintentional) have shown that although adherent COPD patients have higher medication costs than nonadherent COPD patients, total health-care costs of nonadherent patients are substantially higher than adherent patients due to greater inpatient and outpatient costs.75,76 Given that proper device use has been associated with improving lung function, health status, and quality of life, strategies for reducing device use errors should become a priority for health-care providers, systems, and payers.21,66
Our study had several limitations. First, the reasons for device use errors cannot be determined from our analysis. It is possible that patients did not receive adequate training or may have forgotten how to properly use their device. Second, our study is subject to methodological limitations. Potential biases resulting from the subjectivity of the assessor’s technique in evaluating patient errors and patient reporting bias (e.g. the Hawthorne effect) may have affected our results. Third, although our study was global in its inclusion of study populations that represented different cultures, the evaluated studies were limited to those published in the English language. Fourth, small sample sizes and incomplete or missing data specific to device use errors with SMIs precluded us from including 34 studies in our analysis. If the inhaler use experiences of the patients included in our analysis were not representative of excluded patients, our findings may not be generalizable to all COPD populations who use SMIs. Lastly, the lack of access to patient-level data in the studies included in our meta-analysis limited our ability to account for potential confounders and sources of heterogeneity.
Conclusion
Device use errors occurred in almost 60% of patients who were using SMIs. An individualized approach to inhalation device selection, routine monitoring of inhalation technique, and ongoing patient education may help mitigate inhalation errors.21,77
Footnotes
Authors’ note: An abstract highlighting earlier findings from this article was presented at the CHEST 2019 annual meeting in New Orleans, LA, October 19–23, 2019.
Author contributions: The literature search was conducted by authors MN, SC, and KY; variable selection was performed by MN, SC, CD, and BRC; data extraction was conducted by SC and MN; various phases of study conceptualization, data analysis, and results interpretation were led by MN, SC, CD, and BRC. All authors contributed to the preparation, review, and final approval of the manuscript for publication.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MN, SC, and KY are employed by Advance Health Solutions, LLC which received funding from Sunovion Pharmaceuticals Inc. to conduct this study. BRC received consultation remuneration as a member of the Medical Advisory Board at Advance Health Solutions, LLC. He has also served as an expert consultant for Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. CD is employed by Sunovion Pharmaceuticals Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Sunovion Pharmaceuticals Inc.
ORCID iD: Maryam Navaie  https://orcid.org/0000-0001-9773-3267
https://orcid.org/0000-0001-9773-3267
References
- 1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11): e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706, https://www.thelancet.com/action/showPdf?pii=S2213-2600%2817%2930293-X (accessed 17 February 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28(3): 523–532. [DOI] [PubMed] [Google Scholar]
- 4. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2016; 20(1): 11–23. [DOI] [PubMed] [Google Scholar]
- 5. Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest 2005; 127(1): 335–371. [DOI] [PubMed] [Google Scholar]
- 6. DePietro M, Gilbert I, Millette LA, et al. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med 2018; 130(1): 83–97. [DOI] [PubMed] [Google Scholar]
- 7. Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis 2012; 7: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med 2017; 124: 6–14. [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Rio F, Soler-Cataluna JJ, Alcazar B, et al. Requirements, strengths and weaknesses of inhaler devices for COPD patients from the expert prescribers’ point of view: results of the EPOCA Delphi consensus. COPD 2017; 14(6): 573–580. [DOI] [PubMed] [Google Scholar]
- 10. Dalby R, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl) 2011; 4: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pham S, Ferguson GT, Kerwin E, et al. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv 2018; 31(3): 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wise RA, Acevedo RA, Anzueto AR, et al. Guiding principles for the use of nebulized long-acting beta2-agonists in patients with COPD: an expert panel consensus. Chronic Obstr Pulm Dis 2017; 4(1): 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahl R, Kaplan A. A systematic review of comparative studies of tiotropium Respimat® and tiotropium HandiHaler® in patients with chronic obstructive pulmonary disease: does inhaler choice matter? BMC Pulm Med 2016; 16(1): 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miravitlles M, Soler-Cataluña JJ, Alcázar B, et al. Factors affecting the selection of an inhaler device for COPD and the ideal device for different patient profiles. Results of EPOCA Delphi consensus. Pulm Pharmacol Ther 2018; 48: 97–103. [DOI] [PubMed] [Google Scholar]
- 15. Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract 2015; 1: 9. [Google Scholar]
- 16. Sharafkhaneh A, Wolf RA, Goodnight S, et al. Perceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectives. COPD 2013; 10(4): 482–492. [DOI] [PubMed] [Google Scholar]
- 17. Dekhuijzen PNR, Lavorini F, Usmani OS. Patients’ perspectives and preferences in the choice of inhalers: the case for Respimat® or HandiHaler® . Patient Prefer Adherence 2016; 10: 1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchis J, Corrigan C, Levy ML, et al. Inhaler devices—from theory to practice. Respir Med 2013; 107(4): 495–502. [DOI] [PubMed] [Google Scholar]
- 19. Sanchis J, Gich I, Pedersen S, et al. Systematic review of errors in inhaler use: has patient technique improved over time? Chest 2016; 150(2): 394–406. [DOI] [PubMed] [Google Scholar]
- 20. Braido F, Chrystyn H, Baiardini I, et al. “Trying, but failing”—the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract 2016; 4(5): 823–832. [DOI] [PubMed] [Google Scholar]
- 21. Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD). Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2019. https://goldcopd.org/gold-reports/ (2018, accessed 15 February 2018).
- 22. Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J 2017; 49(2): 1601794. [DOI] [PubMed] [Google Scholar]
- 23. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105(6): 930–938. [DOI] [PubMed] [Google Scholar]
- 24. Maricoto T, Monteiro L, Gama JMR, et al. Inhaler technique education and exacerbation risk in older adults with asthma or chronic obstructive pulmonary disease: a meta-analysis. J Am Geriatr Soc 2019; 67(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 25. Dekhuijzen PNR, Vincken W, Virchow JC, et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir Med 2013; 107(12): 1817–1821. [DOI] [PubMed] [Google Scholar]
- 26. Lavorini F, Mannini C, Chellini E. Challenges of inhaler use in the treatment of asthma and chronic obstructive pulmonary disease. EMJ Respir 2015; 3(2): 98–105. [Google Scholar]
- 27. Rau JL. Practical problems with aerosol therapy in COPD. Respir Care 2006; 51(2): 158–172. [PubMed] [Google Scholar]
- 28. Vincken W, Dekhuijzen PR, Barnes P. The ADMIT series—issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J 2010; 19(1): 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med 2006; 100(9): 1479–1494. [DOI] [PubMed] [Google Scholar]
- 30. Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Educ J 2014; 73(1): 41–50. [Google Scholar]
- 31. Salvi S, Gogtay J, Aggarwal B. Use of breath-actuated inhalers in patients with asthma and COPD-an advance in inhalational therapy: a systematic review. Expert Rev Respir Med 2014; 8(1): 89–99. [DOI] [PubMed] [Google Scholar]
- 32. Barbara S, Kritikos V, Bosnic-Anticevich S. Inhaler technique: does age matter? A systematic review. Eur Respir Rev 2017; 26(146): 170055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahon J, Fitzgerald A, Glanville J, et al. Misuse and/or treatment delivery failure of inhalers among patients with asthma or COPD: a review and recommendations for the conduct of future research. Respir Med 2017; 129: 98–116. [DOI] [PubMed] [Google Scholar]
- 34. Klijn SL, Hiligsmann M, Evers SMAA, et al. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med 2017; 27(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008; 102(4): 593–604. [DOI] [PubMed] [Google Scholar]
- 36. Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med 2017; 27(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho-Reyes S, Celli BR, Dembek C, et al. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: a systematic review and meta-analysis of US studies. Chronic Obstr Pulm Dis 2019; 6(3): 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ding B, Siddiqui S, DePietro M, et al. Inhaler usability of a pressurized metered dose inhaler and a soft mist inhaler in patients with COPD: a simulated-use study. Chron Respir Dis 2019; 16: 1479972318787914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Oliveira MVC, Pizzichini E, Da Costa CH, et al. Evaluation of the preference, satisfaction and correct use of Breezhaler® and Respimat® inhalers in patients with chronic obstructive pulmonary disease—INHALATOR study. Respir Med 2018; 144: 61–67. [DOI] [PubMed] [Google Scholar]
- 41. Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover study. Pulm Ther 2017; 3(1): 173–185. [Google Scholar]
- 42. Chorao P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD—an assessment of inhaler technique and patient preferences. Respir Med 2014; 108(7): 968–975. [DOI] [PubMed] [Google Scholar]
- 43. Asakura Y, Nishimura N, Maezawa K, et al. Effect of switching tiotropium HandiHaler® to Respimat® Soft Mist™ Inhaler in patients with COPD: the difference of adverse events and usability between inhaler devices. J Aerosol Med Pulm Drug Deliv 2013; 26(1): 41–45. [DOI] [PubMed] [Google Scholar]
- 44. Steinberg M, Pervanas H. Assessment of proper medication inhaler technique in adult patients. J Pharm Technol 2014; 30(6): 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Windisch W, Schwarz SB, Magnet FS, et al. Using web-based videos to improve inhalation technique in COPD patients requiring hospitalization: a randomized controlled trial. PLoS One 2018; 13(10): e0201188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ngo C, Phan D, Vu G, et al. Inhaler technique and adherence to inhaled medications among patients with acute exacerbation of chronic obstructive pulmonary disease in Vietnam. Int J Environ Res Public Health 2019; 16(2): E185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bournival R, Coutu R, Goettel N, et al. Preferences and inhalation techniques for inhaler devices used by patients with chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv 2018; 31(4): 237–247. [DOI] [PubMed] [Google Scholar]
- 48. Liang CY, Chen YJ, Sheu SM, et al. Misuse of inhalers among COPD patients in a community hospital in Taiwan. Int J Chron Obstruct Pulmon Dis 2018; 13: 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takaku Y, Kurashima K, Ohta C, et al. How many instructions are required to correct inhalation errors in patients with asthma and chronic obstructive pulmonary disease? Respir Med 2017; 123: 110–115. [DOI] [PubMed] [Google Scholar]
- 50. Ryan R, Hill S. (2016) How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. http://cccrg.cochrane.org/author-resources. Version 3.0. (2016, accessed 17 February 2019).
- 51. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2009, accessed 17 February 2019).
- 52. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283(15): 2008–2012. [DOI] [PubMed] [Google Scholar]
- 53. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a Microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015; 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ryan R. Heterogeneity and subgroup analyses in Cochrane consumers and communication reviews: planning the analysis at protocol stage, https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/heterogeneity_subgroup_analyses_revising_december_1st_2016.pdf (2016, accessed 3 March 2019).
- 57. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006; 11(2): 193–206. [DOI] [PubMed] [Google Scholar]
- 58. Borenstein M, Hedges LV, Higgins JP, et al. Introduction to meta-analysis. 1st ed Chichester, West Sussex: John Wiley & Sons, 2009. [Google Scholar]
- 59. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc 2009; 172(1): 137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riley RD, Ahmed I, Debray TPA, et al. Summarizing and validating test accuracy results across multiple studies for use in clinical practice. Stat Med 2015; 34(13): 2081–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willis BH, Riley RD. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med 2017; 36(21): 3283–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001; 5(26): 1–149. [DOI] [PubMed] [Google Scholar]
- 63. Keating GM. Tiotropium Respimat® Soft Mist™ inhaler: a review of its use in chronic obstructive pulmonary disease. Drugs 2014; 74(15): 1801–1816. [DOI] [PubMed] [Google Scholar]
- 64. Trotta F, Da Cas R, Rajevic M, et al. Risk factors influencing the prescription of tiotropium Respimat formulation: a population-based cohort study. BMJ Open 2015; 5(5): e006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv 2015; 28(3): 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis 2018; 5(2): 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gregoriano C, Dieterle T, Breitenstein AL, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res 2018; 19(1): 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chrystyn H, Small M, Milligan G, et al. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med 2014; 108(2): 358–365. [DOI] [PubMed] [Google Scholar]
- 69. Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis 2016; 11: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lewis A, Torvinen S, Dekhuizen PN, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res 2016; 16: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lewis A, Torvinen S, Dekhuizen PN, et al. Budesonide + formoterol delivered via Spiromax® for the management of asthma and COPD: the potential impact on unscheduled healthcare costs of improving inhalation technique compared with Turbuhaler® . Respir Med 2017; 129: 179–188. [DOI] [PubMed] [Google Scholar]
- 72. van Boven JF, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res 2014; 15: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73(5): 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. George J, Kong DCM, Thoman R, et al. Factors associated with medication nonadherence in patients with COPD. Chest 2005; 128(5): 3198–3204. [DOI] [PubMed] [Google Scholar]
- 75. van Boven JF, Chavannes NH, van der Molen T, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med 2014; 108(1): 103–113. [DOI] [PubMed] [Google Scholar]
- 76. Cutler RL, Fernandez-Llimos F, Frommer M, et al. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018; 8(1): e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med 2012; 6(1): 91–101. [DOI] [PubMed] [Google Scholar]