Abstract
Vascular smooth muscle cells (SMC) play a critical role in controlling blood pressure and blood distribution, as well as maintaining the structural integrity of the blood vessel. SMC also participate in physiological and pathological vascular remodeling due to their remarkable ability to dynamically modulate their phenotype. During the past decade, the development of in vivo fate mapping systems for unbiased identification and tracking of SMC and their progeny has led to major discoveries as well as the reevaluation of well-established concepts regarding the contribution of vascular SMC in major vascular diseases including atherosclerosis. Lineage-tracing studies revealed that SMC undergo multiple phenotypic transitions characterized by the expression of markers of alternative cell types (e.g., macrophage-like, mesenchymal stem cell-like) and populate injured or diseased vessels by oligoclonal expansion of a limited number of medial SMC. With the development of high-throughput transcriptomics and single-cell RNA sequencing (scRNAseq), the field is moving forward towards in-depth SMC phenotypic characterization. Herein, we review the major observations put forth by lineage and clonality tracing studies and the evidence in support for SMC phenotypic diversity in healthy and diseased vascular tissue. We will also discuss the opportunities and remaining challenges of combining lineage tracing and single-cell transcriptomics technologies, as well as studying the functional relevance of SMC phenotypic transitions and identifying the mechanisms controlling them.
Keywords: atherosclerosis, clonal expansion, cell differentiation, vascular injury, cell proliferation
Introduction
The vascular smooth muscle cell (SMC) is a differentiated cell type located within the medial layer of arteries and veins and expressing a unique repertoire of proteins (e.g., MYH11, ACTA2, TAGLN, MYOCD) required for their contractile function1. Despite being highly specialized, SMC retains remarkable plasticity. The plastic nature of SMC has been extensively studied in vitro for the past 30 years2, 3. From these studies emerged the concept of SMC phenotypic switching, a model in which SMC shift between a differentiated, contractile phenotype and a dedifferentiated, “synthetic” phenotype, the latter characterized by the loss of SMC marker gene expression, the increase in extracellular matrix (ECM) component synthesis, and the increase in SMC proliferation and migration upon exposure to various stimuli and environmental cues. Yet, the rigorous assessment of the SMC contribution to vascular remodeling in vivo was virtually impossible due to the major limitation in their identification using traditional SMC marker genes, motivating the development of in vivo lineage-tracing and fate mapping systems4.
Lessons learned from lineage tracing studies
The need for unambiguous and definitive tracking of SMC fate arose from the following observations4: First, studies have demonstrated the loss of expression of SMC contractile genes and the repression of their promoters during atherosclerosis or vascular injury, challenging their identification5, 6. Second, the expression of some of the marker genes classically used for SMC identification is not restricted to the SMC lineage and can be expressed by other cell types. For example, ACTA2 is expressed by both SMC and myofibroblasts. TAGLN is transiently expressed by cardiomyocytes and skeletal muscle cells during embryonic development7. Third, cell types, which do not express SMC marker genes under physiological conditions, can transition to a SMC-like state in diseased or injured blood vessels (e.g., endothelial cells express SMC markers like ACTA2 during endothelial-to-mesenchymal transition)8. Consequently, fate mapping systems allowing reliable SMC tracking in healthy and disease tissue have been developed by combining9, 10: (i) efficient and definitive labeling of SMC and their progeny with reporter systems (e.g., fluorescent proteins, LacZ); (ii) conditional expression of these reporters by Cre recombinases specifically expressed in the SMC lineage; and (iii) an inducible system permitting activation of the tracking system of mature SMC at a given time upon treatment with tamoxifen11. These conditional and inducible Cre recombinase systems allow for the labeling of mature SMC prior to injury or the development of vascular disease and the ability to follow their fate independently of marker gene expression while critically avoiding the labeling of cells acquiring the expression of these marker genes during disease or injury. The first SMC-specific inducible lineage tracing mouse model was developed by the Robert Feil’s group in the early 2000’s12–14. They generated a tamoxifen-inducible Tagln-CreERT2 x loxP-STOP-loxP-reporter system12 in which a series of tamoxifen injections induces the translocation of Cre recombinase from the cytoplasm to the nucleus and the excision of a floxed STOP codon localized upstream of a reporter gene exclusively in TAGLN+ cells. These studies were the first to provide rigorous evidence of the participation of SMC to atherosclerotic lesion formation13–15. Thereafter, SMC-lineage tracing studies have employed tamoxifen-inducible Myh11-CreERT2 x loxP-STOP-loxP-reporter mouse models16 to track the fate and phenotype of SMC expressing MYH11 at the time of tamoxifen treatment17–24.
These studies have clearly demonstrated that a large fraction of cells present in the neointima or the atherosclerotic lesion originate from medial differentiated SMC. For example, Herring et al.19 found that 80% of the neointimal cells are of SMC origin after carotid ligation. In advanced atherosclerosis, SMC represent between 40 and 70% of plaque cells20, 22, yet, up to 80% of SMC-derived cells within atherosclerotic plaque do not express detectable levels of SMC markers including MYH11 and ACTA218, 20. There is compelling evidence that SMC not only lose the expression of their marker gene repertoire in injured or diseased vessels, but can undergo phenotypic transitions into other types of cells, including chondrocyte-like cells (SOX9+, Runx2/Cbfa1+), foam cells and macrophage-like cells (Oil Red O+, LGALS3+, Mac3+), mesenchymal stem cell (MSC)-like cells (Sca1+), myofibroblasts (PDGFβR+), or beige adipocyte-like cells (UCP1+)15, 20, 22, 25–29. Overall, development of conditional and inducible cell-lineage tracing systems has enabled the unbiased fate mapping of SMCs during the progression of cardiovascular diseases, and unveiled the contribution of SMC to vascular disease pathogenesis.
SMC lineage tracing models have also been used in conjunction with SMC-specific knockout of genes of interest and SMC fate mapping to identify key molecules and pathways regulating SMC participation in vascular disease. To name a few, studies have identified factors playing critical roles in regulating SMC phenotypes (e.g., KLF420, 28), as well as their proliferative or migrative capacities during vascular remodeling (e.g., cGKI13, TAGLN14, PTEN17, PDGFbR30, OCT421, IL1R123).
SMC clonal expansion during cardiovascular disease
Since the 1970’s, the mechanisms underlying SMC participation in intimal proliferation during atherosclerotic or after vascular injury have been a major focus of research. In 1973, Earl and John Benditt reported the first evidence of SMC clonality within atherosclerotic plaques by performing X-linked inactivation assays [Figure 1A]31. Benditt31, 32 and others33–35 described that portions of atherosclerotic plaques were composed of cells with the same X-inactivation profile and concluded that atherosclerotic plaques form by clonal expansion of a few cells. However, this technique presents inherent limitations and a poor resolution that preclude a rigorous identification of single clones36. As X-chromosome inactivation occurs early during development (stage 8-cell embryo in human)37, the cells from a given lineage can originate from distinct precursors with high homogeneity in their X-chromosome inactivation profile. Illustrating this caveat, large patches of cells bearing the same X-chromosome inactivation profile have been found in the normal vessel. This makes impossible to ascertain whether SMC investment of atherosclerotic lesions occurs by the expansion of a limited number of clones or the involvement of multiple SMC with the same X-chromosome inactivation profile originating from a medial patch. Finally, the X-linked inactivation assay does not permit the clear identification of cell types responsible for the clonal expansion (SMC vs non-SMC).
Figure 1: SMC clonal expansion vs SMC proliferative capacities: a review of experimental design and possible outcomes.
This table summarizes the methodology and experimental design of seminal studies investigating: A. SMC clonality in human atherosclerotic lesions by X-chromosome (Chr) inactivation; B. SMC clonality in experimental atherosclerosis in SMC-lineage tracing mice; C. Medial SMC proliferative capacity after vascular injury by tritiated thymidine incorporation (3HTdR); D. Medial and intimal SMC growth fraction after vascular injury; and E. SMC proliferative profile within atherosclerotic lesion after tritiated thymidine pulse delivery. The table includes representations of the experimental designs and possible outcomes, as well as a summary of the main observations and limitations of these studies.
The development of SMC lineage tracing systems has permitted to reevaluate these studies and determine more accurately the clonal profile of SMC within atherosclerotic lesions in mice. These studies leveraged the combinatorial use of multi-color reporter alleles (i.e., R26R-Confetti, R26R-Rainbow) and SMC-specific inducible Cre systems (i.e. Tagln-CreERT2, Myh11-CreERT2) systems described above to track the clonality of SMC-derived lesion cells by random labeling of SMC with one of the colors included in the reporter allele [Figure 1B]15, 22, 24, 25. These clonality tracking systems can be used at high and low recombination rates by varying the tamoxifen treatment dose and duration. A high recombination rate induces the labelling of all SMC and the robust quantification of SMC lesion investment and phenotypic transitions, whereas a low recombination rate allows the precise identification of single individual clones. Of note, the reliability of the cell-specific conditional and inducible tracking systems is particularly critical for clonality studies since the expansion of a very small number of cells makes qualitative and quantitative analysis vulnerable to inefficient or leaky Cre systems. Low recombination rate experiments provided new evidence of the clonal origin of SMC-derived lesion cells15. Subsequently, Chappell et al.22 reported that over 90% of the atherosclerotic plaques only contained 1 or 2 colors. This key observation, confirmed by two other studies24, 25, strongly supports that a few mature medial MYH11+ SMC contribute to SMC investment and population of atherosclerotic lesions. However, consensus has not been achieved on how only a few out of all underlying medial SMCs contribute to atherogenesis and key questions have not been yet resolved [recently discussed in 38–40].
Is clonal expansion a common process during development, physiological and pathological remodeling?
In addition to atherosclerosis, clonal expansion of SMC has been observed in carotid artery following vascular injury22, distal pulmonary arterioles in a model of pulmonary arterial hypertension30, and in dissecting aortic aneurysm41. This suggests that SMC clonality is a conserved mechanism that might have evolved for retention of blood vessel structure and contractility during vascular remodeling. In contrast, SMC investment of forming arteries during embryonic development does not involve clonal expansion as demonstrated by the following observations: 1) medial SMC originate from multiple precursors; and 2) the progeny of a SMC precursor undergoes extensive migration and intermixing within developing arteries and does not form large patches of cells24, 25, 42.
Are SMC clonally expanding the only medial SMC capable of proliferation?
Studies by Clowes43, 44 and Thomas45 based on tritiated thymidine (3HTdR) incorporation and SMC growth fraction assessment provided evidence challenging the idea that proliferative capacity would be restricted to SMC undergoing clonal expansion. Analysis of the frequency and the intensity in 3HTdR labeling, and its dilution through cell division showed that [Figure 1C–E]: 1) a large fraction (46%) of medial cells were labeled with 3HTdR 48 hours post vascular injury43; 2) both labeled (proliferative) and unlabeled (non-proliferative) cells contribute to the neointima formation, indicating that non-proliferative medial SMC could migrate and invest the neointima44; and 3) after pulse injection of 3HTdR preceding high-fat diet in swine, tracking of tritiated thymidine dilution suggested that multiple SMC proliferate and undergo a limited number of cell cyles rather than the extensive proliferation of a single cell. Indeed, the clonal proliferation of a single cell would imply a large number of cell division and a greater dilution of tritiated thymidine than observed45. Although these studies faced similar limitations than the Benditt’s studies (i.e., inability to ascertain lesion cell lineages of proliferating and non-proliferating cells), these results imply that a large number of medial SMC have proliferative capacities. In accordance with this conclusion, SMC-clonality tracing studies demonstrated that alteration of the Integrin-β3 pathway led to atherosclerotic lesion polyclonality suggesting that a large number of medial SMC retain proliferative capacity and that environmental cues might play a critical role in regulating SMC proliferation and clonal expansion24.
Is there a subpopulation of SMC primed to undergo clonal expansion? Is there an environmental pressure selecting for the expansion of a limited number of SMC clones?
The fact that a few SMC undergo clonal expansion despite having a large number of medial SMC exposed to similar environmental perturbations suggests that an upstream priming process might be required for these cells to become a dominant clone. Benditt postulated that, similar to neoplastic growth, atherosclerosis monoclonality may be caused by mutational events in SMC31. Although the occurrence and clinical significance of somatic mutations in myeloid cells have been recently demonstrated46, 47, there is, yet, no evidence supporting or refuting the involvement of a similar process in medial SMC and whether it influences SMC proliferative and clonal capacities. The predisposition of a few SMC to clonally expand could also be due to the presence within the media of phenotypically distinct SMC subpopulations that display higher proliferation, migration and/or survival capacities, thus outcompeting other SMC populations.
Environmental cues, including cell-cell interaction, cell-extracellular matrix interaction, and juxtacrine and paracrine signaling, may also regulate positively or negatively the capacity of SMC to invest the atherosclerotic lesion and/or survive during lesion development. Here are a few examples of possible mechanisms. First, a medial SMC clone could inhibit the proliferation and/or the migration of surrounding cells. Second, lesion cells of other lineages (e.g. macrophages, endothelial cells) could influence the ability of medial SMC to undergo clonal expansion. A recent study provided supporting evidence that bone marrow-derived macrophages play an essential role in restricting multiple SMCs from migrating into atherosclerotic plaques through an integrin-β3-dependent mechanism24. Finally, another hypothesis postulates that the propensity of a SMC to invest the plaque may be dependent on its location within the forming atherosclerotic lesion. For example, it has been hypothesized that the proximity of the SMC to the endothelium at the shoulders of fatty streak-like lesions could influence their ability for clonal expansion24, 25. Similarly, it has been proposed that SMC in the vicinity of internal elastic lamina fenestration could preferentially migrate within the lesion25, 48. Since these processes are not mutually exclusive, it is likely that SMC clonal expansion and investment of atherosclerotic plaque are initiated and regulated by a combination of phenotypic priming and environmental selection.
Clonal expansion vs phenotypic modulation
Another key observation of the SMC clonality studies is that SMC within a lesion derived from a single clone can undergo multiple phenotypic transitions thus, a clone is not predisposed to any particular phenotypic transition22, 25 (Figure 2). The progeny of a SMC clone displays an atheroprotective ACTA2+ phenotype within the fibrous cap as well as an atheropromoting phenotype of lipid-loaded foam cells and macrophage-like cells within the necrotic core22, 25. Importantly, there is clear evidence that SMC phenotypic modulation occurs within the media20–22. The inner layer of the media underlying the lesion is composed of a significant proportion of ACTA2− SMC as well as LGALS3+ SMC. Importantly, these medial phenotypically modulated SMCs do not originate from a single SMC clone nor undergo clonal expansion within the intimal space, demonstrating that clonal expansion and phenotypic modulation are distinct and independent processes22. In other words, SMC phenotypic modulation, proliferative and migratory processes are not necessarily interdependent and concomitant, and therefore might be regulated by different mechanisms20, 21, 23. These findings are very important when considering and evaluating therapeutic strategies for treatment of atherosclerosis. Relevant strategies should likely consist in polarizing the phenotypic transitions of lesion SMC to atheroprotective phenotypes (e.g., ACTA2+) rather than inhibiting clonal expansion and SMC plaque investment. Indeed, there is clear evidence that inhibition of SMC investment within atherosclerotic lesions does not necessarily results in a reduction of the plaque size but rather lead to the formation of advanced and unstable plaques due to compensation of SMC absence by other cell types21, 49.
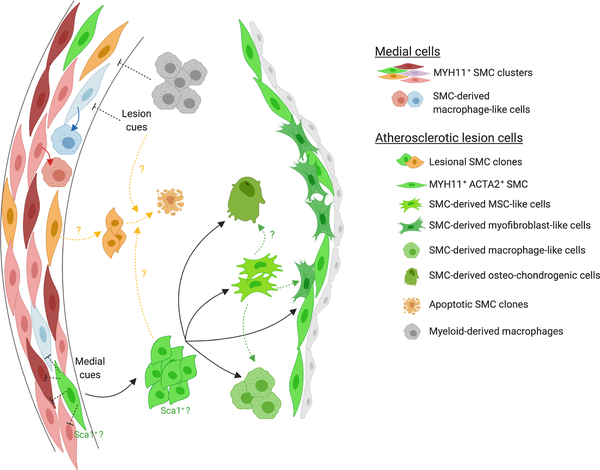
Figure 2: SMC phenotypic diversity within the media and atherosclerotic lesion.
This schematic summarizes the main finding with respect to SMC diversity. scRNAseq studies revealed that the media is composed of multiple clusters of SMC. While it is unclear whether a particular cluster of SMC has the ability to clonally expand, there is clear evidence that SMC within the atherosclerotic lesion originate from a very limited number of clones. The same SMC clone can give rise to SMC-derived fibrous cap cells, macrophage-like cells, mesenchymal-stem cell like-cell and osteochondrogenic cells. A current hypothesis is that a transition to a mesenchymal-stem cell-like cell state would precede and be required for the transition to other lesion phenotypes (green dashed arrows). In addition, it has been postulated that SMC-derived lesion cells could originate from the medial Sca1+ SMC population. This hypothesis is indicated in the schematic by the annotation of the SMC clone expanding within the lesion with “Sca1+?”. Importantly, phenotypic modulation also occurs within the media, independently of clonal expansion during atherosclerosis (red arrows). Although the mechanisms controlling the investment of atherosclerotic lesions by a very limited number of SMC clones have not been fully characterized, there is evidence that myeloid-derived plaque macrophages inhibits SMC polyclonality within atherosclerotic plaque (gray dashed arrows). It is also postulated that medial or lesion environmental cues could inhibit the proliferation and investment of multiple medial SMC within the lesion (black dashed arrows). Finally, it is unclear whether a process of clonal selection consisting in the survival of dominant clones could as well take place (yellow dashed arrows). Figure created with Biorender.
Interspecies differences in SMC investment mechanisms?
The discrepancies in the observations and conclusions summarized above could be explained, at least partially, by differences in experimental designs and methodologies, but might also arise from fundamental interspecies differences. For example, unlike rodents, human and swine can develop “normal intimal proliferation” in large arteries40, 45, 50. The “normal intima” is supposedly composed of medial SMC migrating in the intimal space in absence of lipid accumulation40. However, the mechanisms of formation of the “normal intima”, including the clonal profile of intimal SMC, remain largely unknown. Moreover, although the PDAY study showed that vascular territories with “normal intima” are preferential sites for atherosclerosis, the impact of the “normal intima” on lesion formation, cellular composition and SMC participation and clonality has not been yet determined40, 51.
SMC phenotypic heterogeneity in the healthy media
A potential explanation for SMC oligoclonal investment of atherosclerotic plaques is the predisposition of a distinct medial SMC subpopulation. Implicit in this postulate is the existence of SMC heterogeneity within the medial layer. While the regional- and developmental-associated phenotypic diversity has been extensively studied [see reviews by Majesky52, 53], there has been a limited investigation and characterization of the inherent SMC heterogeneity within the healthy media of a given vascular territory. In vitro studies have identified two phenotypically distinct populations of SMC within large arteries, classically characterized as “spindle-shaped contractile” and “rhomboid synthetic” SMC54, 55. These subpopulations, while expressing, at least some, SMC marker genes, had different morphological, proliferative, and migratory capacities when grown in culture or exposed to mitogens like PDGF-BB. In vivo, the variation in expression of the SMC marker genes in normal bovine pulmonary artery suggested the presence of SMC subpopulations in the healthy media56. However, this study did not consider the possibility of the presence of non-SMC cells which would not express the SMC gene repertoire. Moreover, the proliferative and migratory characteristics observed in vitro might not be the reflect of a diversity of SMC phenotypes and functions in vivo due to the major alterations occurring after tissue dissociation and during SMC culture and expansion3. Nevertheless, these observations support two plausible hypotheses for medial SMC diversity57, 58: 1) the media exhibits two or more distinct and stable SMC phenotypes including a less differentiated and multifunctional population59; or 2) one medial SMC population changes its phenotype transiently and reversibly within the healthy media between a differentiated and a less differentiated state. A reasonable explanation for this hypothesis is that the dynamic transition of medial SMC phenotype would be driven by the need for continuous production of ECM components required for ECM turnover and maintenance60, 61.
A recent study by the Jorgensen group provided new insights into medial SMC diversity by combining SMC lineage tracing and scRNAseq62 (Figure 2). Remarkably, it gave compelling evidence of SMC heterogeneity within the healthy media of a given vascular region. By performing scRNAseq on the whole aorta of Myh11-CreERT2 Confetti mice, seven distinct SMC clusters were identified and were distinct from endothelial and adventitial cells. Importantly, SMC in these seven clusters expressed SMC contractile genes including MYH11. Moreover, a small subset of medial MYH11+ SMC expressed the MSC marker Sca1. Interestingly, Sca1 expression was not restricted to one of the seven SMC clusters but rather present in a very limited number of SMC in each cluster. These results are significant in that they demonstrate that differentiated MYH11+ SMC can express detectable, maybe transient, levels of the MSC marker Sca1. ScRNAseq performed on atherosclerotic lesion of SMC lineage tracing mice showed an expected phenotypic diversity of SMC-derived lesion cells, including macrophage-like and osteogenic phenotypes, as well as a cluster of SMC expressing Sca1, confirming previous studies20, 26. These studies gave an unprecedented depth to the characterization of the SMC phenotypic diversity in healthy and diseased blood vessels.
SMC phenotypic characterization at the era of single-cell profiling: opportunities and challenges
The use of scRNAseq provides a comprehensive and unbiased characterization of cell population phenotype and relative abundance within complex healthy or diseased tissues including atherosclerosis62–65. The combination of scRNAseq and lineage tracing gives the tremendous advantage to follow the phenotypic transitions of cell types during disease development and progression without relying on established lineage-specific markers or modeled transcriptomic profiles. The identification of lineage-traced cells for scRNAseq studies can be based on different approaches: the pre-sorting of reporter+ cells62–65 or the detection of reporter transcripts post-sequencing66, 67. Yet, limitations and potential biases should be acknowledged and carefully considered when analyzing and interpreting scRNAseq datasets. Firstly, unlike bulk RNAseq, scRNAseq relies on obtaining single-cell suspension representative of all cell populations present within tissue samples which involves enzymatic and mechanical tissue disruption. This critical step can induce significant bias with respect to cell population abundance and transcript expression because it can differently impact the viability and the gene expression of various cell types and subpopulations68, 69. This potential bias can be partially controlled by comparing transcript expression profiles generated by scRNAseq and bulk RNA-seq, the latter utilizing undissociated frozen tissue. Secondly, scRNAseq lacks spatial information regarding the distribution of the different subpopulations within tissues. Thirdly, an ensemble of processes (i.e., gene activation burst, stochastic gene expression, RNA stability) can generate variations in transcript levels that might not reflect differences in protein levels or cellular functions and can artificially increase single-cell heterogeneity70–72. Finally, the lack of depth of scRNAseq (stochastic dropout) presents major limitations for investigation of low-expression genes. Although this might not be an issue in defining cell subpopulations and their relative abundance, it might limit the identification of key regulators of cell polarizations and phenotypic transitions.
With respect to SMC heterogeneity, the next challenge will be to determine the functional relevance of the SMC subpopulations identified by scRNAseq. Indeed, although very informative, scRNAseq studies are descriptive by nature. For example, Dobnikar et al.62 suggest that the medial Sca1+ SMC subpopulation might be predisposed for undergoing clonal expansion based on the fact that a subset of SMC-derived lesion cells express Sca1. However, the scRNAseq provides only correlative evidence supporting this conclusion and cannot preclude that medial Sca1− SMC would start expressing Sca1 during lesion investment and phenotypic transition. Studying the fate and function of the different medial SMC subpopulations would require the development of dual inducible genetic tracing systems permitting the lineage tracing of the SMC lineage and the tracking of a defined subpopulation. While a first lineage tracing system would be used to track the SMC lineage in its globality (MYH11+ SMC), a second tracing system could be implemented to track a particular SMC medial subpopulation to assess precisely its fate in atherosclerosis and its contribution to the disease pathogenesis. For example, tracking the medial Sca1+ SMC subpopulation for rigorous assessment of the functional relevance of these cells would require such a dual lineage tracing/fate mapping system. Remarkably, alternatives to Cre-mediated recombination have been recently developed including Dre-rox73 and Nigri-nox systems74. The use of these new technologies in combination with traditional Cre systems allow for independent tracking of two populations or lineage75–77 and give a glimpse of possible SMC subpopulations in vivo. However, to date, no study has employed a combination of two independent conditional and inducible models. Thus, the field needs to address 2 remaining challenges: the development of multiple inducible systems and the selection of markers for precise and unambiguous identification of SMC subpopulations by promoter-driven Cre systems.
Conclusion
The field has positioned itself at the crossroads of lineage tracing and single-cell transcriptomics to study phenotypic diversity and phenotypic evolution of SMC during vascular disease and remodeling. We are in an exciting era with advanced tools to perform robust and precise analysis at the single-cell resolution on systems allowing unbiased tracking of cell lineage and fate. Whereas we are gaining depth in the phenotypic characterization of the SMC lineage, future studies must focus on key remaining considerations including the full characterization of SMC heterogeneity at the epigenetic and genetic levels, the origin and environmental control of medial SMC heterogeneity, the functional relevance of SMC medial subpopulations during disease formation, the identification of mechanisms controlling SMC clonal expansion/SMC investment and phenotypic transitions, and the identification of therapeutic axes to bias SMC phenotypic transitions.
Supplementary Material
Highlights.
With the development of reliable Smooth Muscle Cell (SMC) in vivo fate tracking systems, the field has reevaluated, confirmed, or invalidated key processes established in the 70’s and 80’s regarding the proliferative, migratory, and plastic properties of vascular SMC in injured or diseased vessel.
There is today clear evidence that a very limited number of SMC clonally invest the atherosclerotic lesion where they undergo phenotypic transitions to multiple alternative phenotypes.
The coupling of fate tracking and single-cell transcriptomics allowed a more comprehensive characterization of the SMC subpopulations and revealed a remarkable phenotypic diversity in the healthy aorta, as well as in the atherosclerotic lesion.
The current challenge in the field is to develop new tracking models to assess the functional significance of SMC subpopulations and their contribution to major vascular diseases including atherosclerosis.
Acknowledgments
a) Acknowledgments: We would like to thank Sidney Mahan and Drs. Cynthia St Hilaire and Brittany Durgin for their help in editing the manuscript. The graphic abstract was created with Biorender.
b) Sources of funding: This work is supported by the National Institute of Health R01HL146465 and the American Heart Association 15SDG25860021 grants (to D.G.).
Abbreviations
- ECM
Extracellular Matrix
- MSC
Mesenchymal Stem Cell
- scRNAseq
single-cell RNA sequencing
- SMC
Smooth Muscle Cell
Footnotes
c) Disclosures: The authors have nothing to disclose.
References
- 1.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JH and Campbell GR. Smooth muscle phenotypic modulation--a personal experience. Arterioscler Thromb Vasc Biol. 2012;32:1784–9. [DOI] [PubMed] [Google Scholar]
- 3.Chamley-Campbell J, Campbell GR and Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. [DOI] [PubMed] [Google Scholar]
- 4.Gomez D and Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan CP, Adam PJ, Madsen CS and Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG and Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95:981–8. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Miano JM, Cserjesi P and Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–95. [DOI] [PubMed] [Google Scholar]
- 8.Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G and Baker AH. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty R, Saddouk FZ, Carrao AC, Krause DS, Greif DM and Martin KA. Promoters to Study Vascular Smooth Muscle. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119312449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allahverdian S, Chaabane C, Boukais K, Francis GA and Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D and Chambon P. Ligand-activated site-specific recombination in mice. P Natl Acad Sci USA. 1996;93:10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F and Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. [DOI] [PubMed] [Google Scholar]
- 13.Wolfsgruber W, Feil S, Brummer S, Kuppinger O, Hofmann F and Feil R. A proatherogenic role for cGMP-dependent protein kinase in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 2003;100:13519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feil S, Hofmann F and Feil R. SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res. 2004;94:863–5. [DOI] [PubMed] [Google Scholar]
- 15.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M and Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–7. [DOI] [PubMed] [Google Scholar]
- 16.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS and Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8. [DOI] [PubMed] [Google Scholar]
- 17.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S and Weiser-Evans MC. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez D, Shankman LS, Nguyen AT and Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nature Methods. 2013;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring BP, Hoggatt AM, Burlak C and Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ and Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, Alencar GF, Hess DL, Bevard MH, Greene ES, Murgai M, Turner SD, Geng YJ, Bekiranov S, Connelly JJ, Tomilin A and Owens GK. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med. 2016;22:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR and Jorgensen HF. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res. 2016;119:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H and Owens GK. Interleukin-1 beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nature Medicine. 2018;24:1418–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra A, Feng Z, Chandran RR, Kabir I, Rotllan N, Aryal B, Sheikh AQ, Ding L, Qin L, Fernandez-Hernando C, Tellides G and Greif DM. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat Commun. 2018;9:2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen K, Lund MB, Shim J, Gunnersen S, Fuchtbauer EM, Kjolby M, Carramolino L and Bentzon JF. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D and Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Erh Jaw J, Sin DD, Seidman MA, Leeper NJ and Francis GA. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119312434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, Dong XR, Poczobutt J, Nemenoff RA and Weiser-Evans MC. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ Res. 2017;120:296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ, Jr., Rosen ED and Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh AQ, Misra A, Rosas IO, Adams RH and Greif DM. Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med. 2015;7:308ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benditt EP and Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murry CE, Gipaya CT, Bartosek T, Benditt EP and Schwartz SM. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997;151:697–705. [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson TA, Dillman JM, Solex K and Heptinstall RH. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978;43:10–8. [DOI] [PubMed] [Google Scholar]
- 34.Pearson TA, Dillman JM, Solez K and Heptinstall RH. Clonal characteristics in layers of human atherosclerotic plaques. A study of the selection hypothesis of monoclonality. Am J Pathol. 1978;93:93–102. [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz SM and Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437–60. [DOI] [PubMed] [Google Scholar]
- 36.Chen GL and Prchal JT. X-linked clonality testing: interpretation and limitations. Blood. 2007;110:1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg IM, Laven JS, Stevens M, Jonkers I, Galjaard RJ, Gribnau J and van Doorninck JH. X chromosome inactivation is initiated in human preimplantation embryos. Am J Hum Genet. 2009;84:771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez D and Owens GK. Reconciling Smooth Muscle Cell Oligoclonality and Proliferative Capacity in Experimental Atherosclerosis. Circ Res. 2016;119:1262–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiRenzo D, Owens GK and Leeper NJ. “Attack of the Clones”: Commonalities Between Cancer and Atherosclerosis. Circ Res. 2017;120:624–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz SM, Virmani R and Majesky MW. An update on clonality: what smooth muscle cell type makes up the atherosclerotic plaque? F1000Res. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, Taleb S, Jorgensen HF and Mallat Z. Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA118311727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greif DM, Kumar M, Lighthouse JK, Hum J, An A, Ding L, Red-Horse K, Espinoza FH, Olson L, Offermanns S and Krasnow MA. Radial construction of an arterial wall. Dev Cell. 2012;23:482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clowes AW, Reidy MA and Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–33. [PubMed] [Google Scholar]
- 44.Clowes AW and Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–45. [DOI] [PubMed] [Google Scholar]
- 45.Thomas WA, Florentin RA, Reiner JM, Lee WM and Lee KT. Alterations in population dynamics of arterial smooth muscle cells during atherogenesis. IV. Evidence for a polyclonal origin of hypercholesterolemic diet-induced atherosclerotic lesions in young swine. Exp Mol Pathol. 1976;24:244–60. [DOI] [PubMed] [Google Scholar]
- 46.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA and Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S and Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu YW, Lowery AM, Sun LY, Singer HA, Dai G, Adam AP, Vincent PA and Schwarz JJ. Endothelial Myocyte Enhancer Factor 2c Inhibits Migration of Smooth Muscle Cells Through Fenestrations in the Internal Elastic Lamina. Arterioscler Thromb Vasc Biol. 2017;37:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman AA, Baylis RA, Hess DL, Griffith SD, Shankman LS, Cherepanova OA and Owens GK. Irradiation abolishes smooth muscle investment into vascular lesions in specific vascular beds. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stary HC. Macrophage foam cells in the coronary artery intima of human infants. Ann N Y Acad Sci. 1985;454:5–8. [DOI] [PubMed] [Google Scholar]
- 51.Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1993;13:1291–8. [DOI] [PubMed] [Google Scholar]
- 52.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–58. [DOI] [PubMed] [Google Scholar]
- 53.Majesky MW. Vascular Development. Arterioscler Thromb Vasc Biol. 2018;38:e17–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Fan YS, Chow LH, Van Den Diepstraten C, van Der Veer E, Sims SM and Pickering JG. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res. 2001;89:517–25. [DOI] [PubMed] [Google Scholar]
- 55.Hao H, Ropraz P, Verin V, Camenzind E, Geinoz A, Pepper MS, Gabbiani G and Bochaton-Piallat ML. Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arterioscler Thromb Vasc Biol. 2002;22:1093–9. [DOI] [PubMed] [Google Scholar]
- 56.Frid MG, Moiseeva EP and Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994;75:669–81. [DOI] [PubMed] [Google Scholar]
- 57.Shanahan CM and Weissberg PL. Smooth muscle cell heterogeneity: patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1998;18:333–8. [DOI] [PubMed] [Google Scholar]
- 58.Rensen SS, Doevendans PA and van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wissler RW. The arterial medial cell, smooth muscle, or multifunctional mesenchyme? Circulation. 1967;36:1–4. [DOI] [PubMed] [Google Scholar]
- 60.Ross R and Klebanoff SJ. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971;50:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humphrey JD, Dufresne ER and Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M and Jorgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE and Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 64.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K and Wolf D. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, Yoon WK, Prat A, Seidah NG, Choi J, Lee SP, Yoon SH, Nam JW, Seong JK, Oh GT, Randolph GJ, Artyomov MN, Cheong C and Choi JH. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res. 2018;123:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biddy BA, Kong W, Kamimoto K, Guo C, Waye SE, Sun T and Morris SA. Single-cell mapping of lineage and identity in direct reprogramming. Nature. 2018;564:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kester L and van Oudenaarden A. Single-Cell Transcriptomics Meets Lineage Tracing. Cell Stem Cell. 2018;23:166–179. [DOI] [PubMed] [Google Scholar]
- 68.McArdle S, Mikulski Z and Ley K. Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis. J Exp Med. 2016;213:1117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Brink SC, Sage F, Vertesy A, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C and van Oudenaarden A. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods. 2017;14:935–936. [DOI] [PubMed] [Google Scholar]
- 70.Suter DM, Molina N, Gatfield D, Schneider K, Schibler U and Naef F. Mammalian genes are transcribed with widely different bursting kinetics. Science. 2011;332:472–4. [DOI] [PubMed] [Google Scholar]
- 71.Raj A and van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JK and Marioni JC. Inferring the kinetics of stochastic gene expression from single-cell RNA-sequencing data. Genome Biol. 2013;14:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F and Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech. 2009;2:508–15. [DOI] [PubMed] [Google Scholar]
- 74.Karimova M, Splith V, Karpinski J, Pisabarro MT and Buchholz F. Discovery of Nigri/nox and Panto/pox site-specific recombinase systems facilitates advanced genome engineering. Sci Rep. 2016;6:30130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, Zhao H, Tang J, Ji H, Cai D, Han Z, Han Z, Nie Y, Hu S, Wang QD, Sun R, Fei J, Wang F, Chen T, Yan Y, Huang H, Pu WT and Zhou B. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K, Yu W, Tang M, Tang J, Liu X, Liu Q, Li Y, He L, Zhang L, Evans SM, Tian X, Lui KO and Zhou B. A dual genetic tracing system identifies diverse and dynamic origins of cardiac valve mesenchyme. Development. 2018;145. [DOI] [PubMed] [Google Scholar]
- 77.Pu W, He L, Han X, Tian X, Li Y, Zhang H, Liu Q, Huang X, Zhang L, Wang QD, Yu Z, Yang X, Smart N and Zhou B. Genetic Targeting of Organ-Specific Blood Vessels. Circ Res. 2018;123:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.