Abstract
The rise in obesity and associated morbidity is currently one of our greatest public health challenges. Women represent a high risk group for weight gain with associated metabolic, cardiovascular, reproductive and psychological health impacts. Regular physical activity is fundamental for health and well-being with protective benefits across the spectrum of women’s health. Preconception, pregnancy and the early postpartum period represent opportune windows to engage women in regular physical activity to optimize health and prevent weight gain with added potential to transfer behavior change more broadly to children and families. This review summarizes the current evidence for the role of physical activity for women in relation to preconception (infertility, assisted reproductive therapy, polycystic ovary syndrome, weight gain prevention and psychological well-being) pregnancy (prevention of excess gestational weight gain, gestational diabetes and preeclampsia as well as labor and neonatal outcomes) and postpartum (lactation and breastfeeding, postpartum weight retention and depression) health. Beneficial outcomes validate the importance of regular physical activity, yet key methodological gaps highlight the need for large, high-quality studies to clarify the optimal type, frequency, duration and intensity of physical activity required for beneficial health outcomes during preconception, pregnancy and postpartum.
Keywords: preconception, pregnancy, postpartum, physical activity, exercise
The increasing prevalence of overweight and obesity worldwide represents a complex and chronic public health challenge. Recent estimates highlight the burden, with only 40% of the adult population in Australia and the United Kingdom and 30% in the United States within a healthy weight range.1 Trends demonstrate that overweight and obesity are affecting the population at a younger age, with the most rapid rate of weight gain reported between 20–40 years of age.1,2 Women are at the highest risk, with a higher conversion to overweight and obesity compared with men on cross-sectional analysis.3
Increased weight in reproductive aged women is associated with cardio metabolic (glucose intolerance, dyslipidaemia, Type 2 Diabetes [T2DM] risk factors for Cardiovascular Disease [CVD]), reproductive (anovulation, Polycystic Ovary Syndrome [PCOS], infertility) and psychological (Depression, Quality of Life [QoL]) health risks. Pregnancy exacerbates obesity risk, with the majority of women exceeding international Institute of Medicine (IOM) recommendations for gestational weight gain (GWG).4–7 Excessive GWG increases postpartum weight retention, and in conjunction with progressive weight gain, drives long-term obesity risk independent of pre-pregnancy body mass index (BMI).8,9 With modest weight gain from a healthy BMI contributing to risk of preventable disease,10 preventive strategies are now a key international priority and lifestyle modification is the first line approach.11,12 Physical activity is an important lifestyle modification component, with protective health benefits, as well as a clear role in weight gain prevention, independent of diet.13 This review evaluates current evidence for the beneficial role of physical activity in relation to preconception, pregnancy and postpartum health. Research utilizing physical activity and/or exercise as a stand-alone therapy is presented to evaluate its efficacy independent to other lifestyle therapy components.
Adult Physical Activity Recommendations
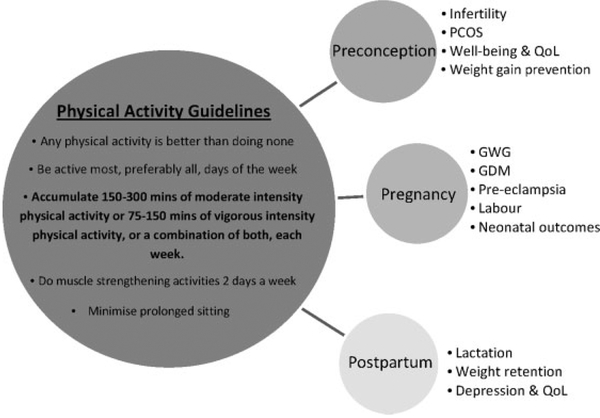
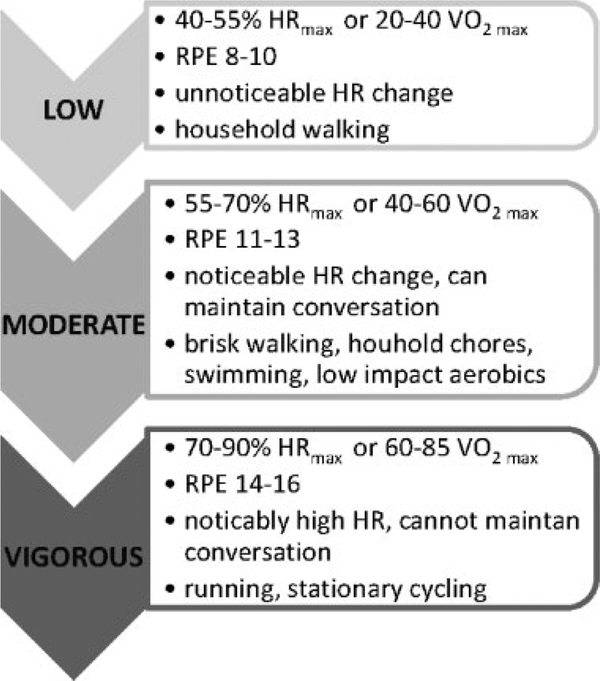
Current Australian Physical Activity (PA) recommendations14 are presented in Fig. 1, with corresponding exercise intensities15 depicted in Fig. 2. International guidelines with similar recommendations are published for US and UK populations.16,17 Despite the documented benefits, ~55% of women in developed countries aged 18–45 do not meet current PA recommendations.18 Data from longitudinal studies demonstrate a downward trend in PA with increasing age, as well as an upward trend in weight gain.19 Common barriers reported by women include time constraints, motivation, caregiving, cost, low self-efficacy and a low level of PA prioritisation.20
Fig. 1.
Adult physical activity recommendations and the association of physical activity with preconception, pregnancy and postpartum health. PCOS, polycystic ovary syndrome; QoL, Quality of Life; GWG, gestational weight gain; GDM, gestational diabetes mellitus.
Fig. 2.
Physical activity intensity terminology15 and corresponding Rating of Perceived Exertion (RPE) scores.75
The Role of Physical Activity in Preconception Health
Despite the barriers in engaging otherwise healthy women prior to pregnancy for preconception healthcare, previous studies demonstrate PA intention21 and participation22 can be improved following lifestyle counselling of 2–6 sessions. PA interventions are lacking however, with two recent systematic reviews finding no previous studies investigating PA for improved health outcomes in the preconception period, even in higher risk overweight and obese populations.23,24 Given preconception PA is a strong predictor of continued PA during pregnancy,25 establishment of regular PA prior to pregnancy should be an important component of healthy pregnancy planning.
Infertility and Assisted Reproduction
Overweight and obesity impair female fertility and reduce the chance of spontaneous and assisted conception. Weight management, including regular PA, is important in preventing and treating infertility.
Evidence suggests that moderate regular PA positively influences fertility and Assisted Reproductive Therapy (ART) outcomes. A recent study has shown that in obese women undergoing IVF (n = 216), regular PA is associated with higher success rates (39% versus 16% in sedentary women) and improved live birth rates (24.4% versus 7.4%),26 with comparable results demonstrated elsewhere.27 However, the evidence for the effects of vigorous exercise on fertility and/or ART success is equivocal. A 2002 study of 26,955 women, found that each hour per week of vigorous activity was associated with a 7% lower relative risk of ovulatory infertility (5% on adjustment of BMI).28 Yet, a population based health survey in 3887 women under 45 years of age reported an association between vigorous (exercising daily or to exhaustion) exercise and subfertility.29 However no associations were reported with lower intensity PA.29 Similarly, a prospective cohort study of 2232 women reported that at least 4 hours of higher intensity PA for a year or more prior to IVF was associated with a 5 fold increase in cycle cancellation, a 2.5 fold increase in failed implantation, a 30% lower chance of successful pregnancy and a 50% reduction in live births compared with women who reported no regular PA.30
Polycystic Ovary Syndrome
Recognized as the leading cause of anovulatory infertility, Polycystic Ovary Syndrome (PCOS) affects up to 12–18% of reproductive aged women.31–33 PCOS is a multifaceted disorder with metabolic (insulin resistance [IR], T2DM and CVD risk factors), reproductive (hyperandrogenism and oligoovulation) and psychological sequelae.34–38 Varying phenotypes of PCOS increase complexity,39 complicate the path to diagnosis31 and together with clinical factors, increase anxiety and depression36 and reduce QoL.40
Diagnosis requires at least two of clinical or biochemical hyperandrogenism, anovulatory menstrual dysfunction and polycystic ovaries on ultrasound, in the absence of secondary causes.41,42 Although not required for diagnosis, insulin resistance (IR) and intrinsic insulin signaling defects are strongly implicated in the etiology of PCOS43,44 and underpin both reproductive and cardio-metabolic disturbances. Obesity, present in 40–88% of women with PCOS,45–47 worsens IR48 and exacerbates the metabolic, reproductive and psychological features of PCOS.49,50
Lifestyle change is recommended as a first line treatment strategy for PCOS51 and physical activity is an important component, with a single exercise bout enhancing whole body glucose disposal52 and continued, regular exercise reducing T2DM risk in high risk, glucose intolerant groups.53
In studies addressing exercise as an independent therapy in PCOS, the most consistent improvements appear to be to IR with either neutral or small changes noted in other cardio-metabolic parameters including weight, body fat, waist-to-hip ratio, lipids and blood pressure. When measured directly with the gold standard euglycaemic clamp, intensified exercise training improved IR by 16% in overweight and obese women with PCOS, yet in BMI matched non-PCOS controls a 23% improvement was noted, emphasizing the intrinsic IR defects of the disorder.54 Other studies using indirect measures of IR have reported a 9–30% improvement in fasting insulin55,56 as well as in the IR indices, HOMA-IR and AUCINC, following exercise.55,56 All previous studies have reported improvements with 12 weeks of exercise intervention, independent of the type or frequency of exercise, with 3–5 sessions per week of both moderate (40–70% VO2 max) and higher intensity interval training (95–100% VO2 max), shown to be effective.54–56
Reported changes to BMI with exercise are ~1.5kg/m2 (−0.85 to −2.1kg/m2) within three to six months of exercise.57–59 A higher percentage of weight loss (~10.6%) has been reported in PCOS when diet is combined with exercise,60 confirming that a larger energy deficit is more likely to lead to weight loss in line with general populations.
Evidence for the effect of exercise on fertility in overweight and obese women with PCOS is limited, with three studies identified in a systematic review.55 Improved menstrual and/or ovulation frequency was reported in 60% of women following a moderate intensity exercise intervention three times per week for 45 minutes for 12 weeks compared with minimal therapy. However, menses frequency during the study period and minimal therapy group results were not provided so these findings should be interpreted with caution.57 Similar findings using the same exercise intervention (3 moderate intensity sessions of 30 minutes per week) but with a 24 week duration were also reported compared with a low-calorie, high-protein diet.61 Further research is required to confirm whether exercise is superior to dietary intervention for improved ovulation and menses regularity in PCOS.
Continuity of exercise is important, with cessation reversing metabolic improvements and weight benefits.59 Research is limited by small, low quality studies and further research is required to optimize exercise therapies for PCOS including frequency, duration and intensity of PA for the differing phenotypic profiles of PCOS. With a lack of PCOS specific recommendations, all women should be encouraged to exercise regularly, as per population guidelines for PA.
Weight Gain Prevention, Weight Loss, and Prevention of Weight Regain
It is generally accepted that exercise, as an independent therapy without change to caloric intake, is insufficient to induce significant weight loss beyond the definitions for weight maintenance (i.e.≥3% of baseline weight62). Because of the excessive amount of exercise required to induce even modest weight loss, dietary restriction is more effective, achievable and practical for general populations. However, when exercise is combined with dietary restriction as part of a lifestyle intervention to induce weight loss, the combination of both on the daily energy deficit potentiates weight loss compared with dietary intervention alone.62,63
Yet, regular PA is important for weight gain prevention, with those achieving the higher end of the recommended activity range less likely to gain weight long-term than sedentary individuals.62 Current recommendations for prevention of weight gain include at least 60 minutes per day of moderate PA or the equivalent volume of more vigorous PA. Modest changes in weight of between 2–3kg are noted when PA level is higher at 225–420 minutes/week; equivalent to ~45 minutes per day.62
There is also a role of regular exercise in the prevention of weight regain following weight loss.64 Those maintaining weight for at least 5 years in the National Weight Control Registry report high levels of PA (~1 hour/day) as well as a low-calorie and low-fat diet, regular self-monitoring and consistent eating patterns.65 A prospective study assessing weight regain in reproductive aged women found 80% of women regained above 30% of their initial weight loss,66 confirming the difficulties of weight maintenance following weight loss. However, PA at least 30 minutes/day reduced the likelihood of weight regain compared with remaining sedentary (OR 0.69, 95% CI 0.53, 0.89).66 A dose–response effect was also reported, with less weight regain in those undertaking more vigorous exercise, than in those who walked (−3.26kg vs −1.69kg).66
Well-Being, Anxiety, Depression, and Quality of Life
The established link between regular PA and psychological well-being demonstrate its importance across several health outcomes including mood, self-efficacy, symptoms of anxiety67 and depression68 and health related QoL.69–72 The impact of regular PA on the psychological health of young women is particularly important. An extensive population based study in ~20,000 men and women, reported a higher prevalence of anxiety, depression and anxious depression in women than in men aged between 20–35 years.73 However, a lower prevalence of all three conditions was reported in women who maintained at least 60 minutes/week of self-reported moderate intensity PA compared with sedentary women.73 Further, compared with diagnosed depression in 22.4% of women classified as sedentary (<5,000 steps/day), the prevalence was halved (9.3%) in women reporting ≥7,500 steps/day.68 Health related quality of life (HQoL) is positively associated with regular PA. A systematic review of 11 interventional studies (1406 healthy male and female participants), reported significantly improved psychological and physical HQoL with 3–6 months of light or moderate intensity PA compared with no treatment.74
In infertile populations, where psychological implications are higher, regular PA improves depression,75 body image distress76 and HQoL.
Better psychological health outcomes occur with a diverse range of PA patterns including self-reported leisure time activity and structured exercise interventions of varying frequency, duration and intensity. There is some evidence to support a dose response relationship, with higher levels of leisure time PA of moderate or vigorous intensity or a combination associated with better measures of well-being,72 lower anxiety symptoms,67 lower depression68 and higher HQoL scores,70,71 than lower intensity PA levels.
The Role of Physical Activity during Pregnancy
Physical Activity Recommendations
Current physical activity recommendations are informed by general advice for healthy adults14 with the American College of Obstetricians and Gynecologists advising 20–30 minutes of moderate PA of moderate intensity on most or all days of the week in the absence of contraindications.77 Contraindications to exercise include heart or lung disease, incompetent cervix, multiple gestation with risk of premature labor, persistent second- or third-trimester bleeding, placenta previa after 26 weeks gestation and preeclampsia.77
With a safe upper level of exercise intensity yet to be established,77 PA that can be easily quantified to monitor perceived intensity and exertion is recommended, including the use of perceived exertion scales,77 which may be more practical than continuous heart rate monitoring in general populations, A score between 13–14 out of 20 on the Borg’s Rating of Perceived Exertion scale is indicative of moderate intensity78 (Fig. 2).
Overall, there is little evidence to suggest that regular moderate intensity PA throughout pregnancy is detrimental to foetal development or raises maternal core body temperature sufficiently to impose risk.77 Therefore, in normal pregnancies, and when PA is at recommended levels, there is general agreement that the benefits of exercise during pregnancy far outweigh any risks to the mother or fetus,79 with guidelines advising adequate hydration and PA in cool environments with participation in contact or higher risk sports restricted.77,80
Despite the documented ‘teachable moment’ of pregnancy with increased motivation for healthy lifestyle behaviors,81–84 pregnancy is usually associated with decreased levels of PA. Concerns about safety and potential adverse effects on the developing fetus, as well as changing body shape, tiredness and time constraints are the most commonly cited barriers to regular activity during pregnancy.85
Gestational Weight Gain
Increased gestational weight gain (GWG) is a risk factor for antenatal complications (addressed elsewhere in this issue). Exacerbating risk is pre-existing overweight and obesity which in itself is an established risk factor for maternal complications including miscarriage, hypertension, gestational diabetes mellitus (GDM) and caesarean delivery86 as well as large-for-gestational age (LGA) neonates.87 Excess weight gain in early to mid pregnancy is particularly important, as maternal fat accretion peaks at 30 weeks gestation and directly correlates with total GWG and long-term obesity development.6,88
A recent Cochrane review of 13 RCT exercise intervention studies (n = 10 supervised exercise intervention, n = 3 unsupervised self-directed exercise) supports the role of PA for the prevention of excess GWG, reporting a 21% (11–31% range overall) reduction in risk compared with standard care.89 On further analysis, the most protective benefit of PA was in women with a healthy BMI, with a 31% reduced risk of excessive GWG, compared with a 16% reduced risk when obese women only were included (23% reduced risk in the total sample).89 Additionally, in 3 studies reporting total GWG (n = 1134 participants) a significant mean reduction of 1.35kg overall (95% CI −1.80, −0.89) was noted.89 Encouragingly, on analysis of the total sample, this review found that increased PA was as effective as dietary intervention for reducing risk of excessive GWG, with a 23% reduced risk noted with a low glycaemic load diet and a 14% reduction with diet and PA counselling.89 Substantial heterogeneity in the dietary intervention studies (n = 36) prevented pooling of data, however most reported no benefit on risk of excessive GWG; only 5 of 36 studies noted a significant effect.89 The most benefit in risk of excessive GWG was found in studies that included both supervised exercise training and dietary intervention, with a 29% reduction overall. These findings are supported by a previous systematic review with comparable results.90
Prevention and Treatment of Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM), defined as glucose intolerance with onset or first recognition during pregnancy, is closely related to obesity, IR and T2DM in women and its prevalence has dramatically increased in parallel with recent rises in obesity rates in women.91 Modifiable and unmodifiable factors drive GDM risk,92 with development associated with a progressive rise in insulin resistance (IR) with advancing pregnancy.93,94 Prevalence varies depending on the population studied and diagnostic criteria applied, however current estimates indicate GDM affects 6–14% of pregnancies.95 GDM increases neonatal and maternal risks, including LGA neonates, neonatal hypoglycaemia and morbidity, as well as increased rates of delivery by caesarean section.96 Long term, maternal progression to T2DM occurs in 15–70% of women with a history of GDM97–99 and offspring are at an increased risk of obesity development as well as T2DM.100
A 2015 systematic review of 10 RCT studies comparing increased PA as a stand-alone intervention with standard care in 3401 participants of whom 275 developed GDM estimated a 28% lower risk in the development of GDM on meta-analysis (RR 0.72, 95% CI 0.58–0.91, p < 0.005).101 The majority of studies enrolled women in the first or early second trimester and included women of all BMI levels with the exception of three studies targeting overweight or obese women. These studies involved aerobic PA of varying types, with the majority involving 3–4 exercise sessions of 45–60 minutes duration per week. Four studies included a resistance training component.101
In pregnancies complicated by GDM, treatment centres on achieving glucose control through lifestyle change as a first line approach.102,103 The potential theoretical use of PA as an adjunctive therapy for GDM treatment is promising due to its effects on glucose uptake and utilization. Yet despite this, evidence for the role of exercise in the treatment of GDM is inconclusive. A 2006 Cochrane review of four trials, with a combined 114 GDM cases, reported no significant difference between combined exercise and diet therapy intervention and minimal or dietary therapy alone on glucose control, insulin prescription, or neonatal and maternal outcomes.104 The included studies commenced exercise in the third trimester for a minimum of 6 weeks duration, 3–4 times weekly at moderate intensity (≤50–70% VO2 max). With insufficient evidence on the role of exercise in GDM treatment, advice regarding the optimal type, frequency, intensity and duration of exercise required for blood glucose control remains unclear. Larger, high quality studies are required to elucidate the independent role of exercise in treating GDM.79
Prevention and Treatment of Hypertension and Pre-Eclampsia
Hypertensive disorders of pregnancy are one of the most common maternal complications, affecting ~10% of pregnant women. Risk factors mirror those in the general population and include advanced maternal age, ethnicity, family history of hypertension and increased CVD risk factors as well as a sedentary lifestyle.105 Chronic, pre-existing hypertension (prior to 20 weeks gestation) and gestational hypertension (transient hypertension manifesting after 20 weeks gestation and resolving by 6 weeks postpartum), affects ~5–8% of women and is characterized by a systolic blood pressure (BP) ≥140mmHg and/or a diastolic BP ≥90mmHg.105 Preeclampsia is a multisystem disorder of increased severity on the spectrum of hypertensive disorders, affecting ~5–8% of pregnancies and is typically characterized by hypertension and proteinuria manifesting in the second half of pregnancy.105
Despite the protective role of PA in hypertension in the general population, its role in preventing preeclampsia is yet to be fully elucidated. Systematic reviews in the area report contradictory findings, potentially owing to methodological differences, including criteria for included studies. A Cochrane review of two small RCT studies in 45 women reported insufficient power to detect any beneficial role of PA in the prevention of pre-eclampsia in women at increased risk prior to, or in, early pregnancy.106 These studies included moderate intensity exercise (Fig. 2) at least 3 times per week of 30–45 minutes duration, from 18 weeks gestation for 10 weeks or 34 weeks gestation until term.106
Yet a second recent systematic review including case-control and cohort studies only reported an inverse relationship between increasing PA before or during early pregnancy and reduced risk of preeclampsia. Using prospective cohort studies, the authors report a 35% relative risk (RR) reduction in women exercising in the highest category prior to pregnancy (4 studies, n = 9733 participants of whom 420 developed pre-eclampsia) and a 18% RR reduction with high levels of PA in early pregnancy (7 studies, n = 162,558 participants of which n = 5077 developed pre-eclampsia107). The meta-analysis also included a dose–response analysis of PA pre-pregnancy and risk of pre-eclampsia and reported a non-linear trend with the most protective benefits found with between 5–6 hours of activity per week, with a 40% reduction in risk, overall.107 However, as the review included only observational studies, with no reported methodological quality analysis, results should be interpreted with caution and further research is required.
Labor and Delivery
In theory, the stronger and more physically fit a women is at term, the better her ability to cope with labor and delivery.108 However limited evidence exists that PA during pregnancy improves labor, labor duration and perceived ease of delivery.90,109,110 There is evidence from recent prospective cohort studies that sufficiently active pregnant women have a lower risk of medical intervention during delivery, including caesarean delivery.111–113 Cultural factors have more effect than PA on procedures such as episiotomy, epidural induction of labor and method of delivery.111–113
Neonatal Outcomes
Although some observational studies have reported associations between PA, gestational age and neonatal birth weight, the effects are extremely small with the general consensus being that PA of light, moderate or vigorous intensity does not affect infant birth weight when appropriate confounding factors are controlled for.114,115 A Cochrane review of 14 intervention studies with over 1000 women reported no significant effects of exercise on birth weight.116 A meta-analysis reported had 31% less risk of having a large baby (>90th percentile) with regular PA, and that babies of exercising women were ~31 g lighter than non- exercising women.117 Data from a large Norwegian study found lower adjusted odds (0.76–0.91) for pre-term birth among exercising women, compared with non-exercising women, however the mean difference was only 1–2 days.118
The Role of Physical Activity in Postpartum Health
Lactation and Breastfeeding
Moderate to vigorous PA does not negatively affect breast milk composition and volume, provided adequate food and fluid intake is maintained,119 with the caloric cost of breast feeding estimated to be ~600 kcal/day.120 Recommendations include breastfeeding prior to exercise, postponing breast feeding to one hour after exercise, or expressing if required, in cases where infants are unsettled with feeding immediately after the mother exercises.119
Postpartum Weight Retention
With ~60% of women exceeding IOM guidelines for GWG, postpartum weight retention is common with up to 20% of new mothers retaining 5 kg or more one year postpartum, driving long-term health risk.121 PA in the postpartum period is important for weight maintenance as well as other health benefits, however may not induce sufficient weight loss as a standalone therapy. While a 2007 Cochrane review of two studies with 53 participants overall122 found an insignificant change in postpartum weight following exercise intervention of −0.10kg (95% CI −1.90, 1.71) compared with usual care, a recent review of six studies found a significant weight loss of −1.63kg (95% CI −2.16, −1.10).123 Both these and other reviews,124 have noted a greater effect on weight loss in intervention studies that included a dietary component, with mean changes in weight of between 2–4.3 kg. More intensive dietary interventions and more structured activity programs incorporating HR monitors or pedometers, were associated with higher weight loss.124
Postpartum Depression and Quality of Life
Postpartum depression (PPD) is a prevalent condition affecting ~10–15% of women within the first year of birth.125 Severity and duration varies, however approximately half of all cases occur within the first 12 weeks following birth and severity may be exacerbated by the added demands placed on new mothers following birth.125 PA and/or exercise interventions for the prevention or treatment of PPD are limited, with few high quality RCTstudies, small sample sizes and high variability in time from delivery at recruitment. Data from a recent meta-analysis of 6 exercise intervention studies within 12 months postpartum reported a weighted mean reduction in Edinburgh Postnatal Depression Score (EPDS) of 2.22 (95% CI 0.48, 3.96), with this change remaining below the clinical significance indication of a 4 unit change, increasing equivocality. Studies comprised either structured exercise classes (n = 4) or provided tailored exercise advice (n = 4). Overall, the meta-analysis supported a moderate effect of exercise for the treatment of PPD.126 Walking groups may also be beneficial for reducing PPD with data from two small RCT studies of between 20–24 women reporting a 59–65% reduction in scores following a 12 week intervention of 2–3 walking sessions per week.127,128 These results are supported by cohort studies showing comparable results with low-moderate129 and vigorous exercise interventions130 commencing both in pregnancy or the postpartum period.
Although studies evaluating causation between increased PA and reduction in indicators of PPD are needed, PA may potentially act to elevate mood, improve self-efficacy and sleeping patterns, alleviate stress and increase coping strategies. Women report a greater sense of well-being126,131 and health related QoL with postpartum exercise.132 As preconception and antenatal exercise are associated with reduced PPD risk following pregnancy, women should be encouraged to engage in regular activity to enhance potential benefits on mental well-being post-pregnancy.133,134
Conclusion
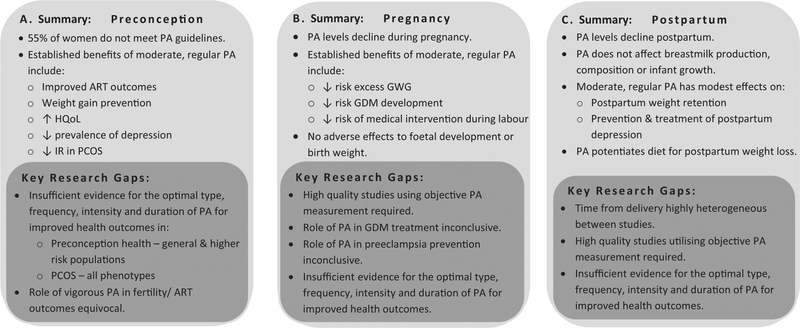
Women of reproductive age are a high risk group for progression to obesity; increasing the risk of morbidity and contributing to the global economic health burden. Preconception, pregnancy and the early postpartum period represent opportune windows to engage women in regular PA to optimize health and prevent weight gain with added potential to transfer behavior change more broadly to children and families Fig. 3. Yet, many reproductive aged women do not meet PA guidelines preconception, with pregnancy and the postpartum period marking further PA decline, warranting public health efforts in this population. To date, few studies have evaluated PA as a standalone therapy and key methodological gaps necessitate further research. Large, comparative, high-quality studies, addressing barriers to exercise with objective PA measurement and with reporting of compliance and adherence are now needed to clarify the optimal type, frequency, duration and intensity of PA required for beneficial health outcomes for women, during preconception, pregnancy and postpartum.
Fig. 3.
Summary of the role of physical activity across (a) Preconception (b) Pregnancy and (c) Postpartum health.
Acknowledgments
Cheryce Harrison is a National Heart Foundation Postdoctoral Research Fellow (100168). Lisa Moran is supported by a South Australian Cardiovascular Research Development Program Fellowship, a program collaboratively funded by the National Heart Foundation, the South Australian Department of Health, and the South Australian Health and Medical Research Institute. Leanne M. Redman is supported by grants from the National Institutes of Health (R00HD060762; R01DK099175; U01DK094418).
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Bureau of Statistics. Australian Health Survey: updated results, 2011–2012. ABS cat no 4364055003 Canberra: ABS; Available from: http://wwwabsgovau/ausstats/abs@nsf/Lookup/4364055003Chapter12011-2012 [Accessed 16/11/2015]. 2013. [Google Scholar]
- 3.Australian Institute of Heath and Welfare (AIHW). Who is Overweight? Available From: http://www.aihw.gov.au/who-is-overweight/ [Accessed 23/11/2015]. Canberra: AIHW. [Google Scholar]
- 4.Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol 2009; 200(3):271.e1–271.e7 [DOI] [PubMed] [Google Scholar]
- 5.Harrison CL, Teede HJ, Lombard CB. How effective is self-weighing in the setting of a lifestyle intervention to reduce gestational weight gain and postpartum weight retention? Aust N Z J Obstet Gynaecol 2014;54(4):382–385 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen K, Yaktine AL. Eds. Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines Weight Gain During Pregnancy: Reexamining the Guidelines. Washington DC: National Academic Press; 2009 [Google Scholar]
- 7.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of Gestational Weight Gain With Maternal Body Mass Index, Waist Circumference, and Blood Pressure Measured 16 Years After Pregnancy: The Avon Longitudinal Study of Parents and Children. Obstet Gynecol Surv 2011;66(10):599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amorim AR, Rössner S, Neovius M, Lourenço PM, Linné Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? [ast]Obesity (Silver Spring) 2007;15(5):1278–1286 [DOI] [PubMed] [Google Scholar]
- 9.Dye TD, Knox KL, Artal R, Aubry RH, Wojtowycz MA. Physical activity, obesity, and diabetes in pregnancy. Am J Epidemiol 1997; 146(11):961–965 [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA 1995;273(6):461–465 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva-WHO; 2013. [cited 2015 Jan17]; Available from: http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1. Accessed May 3, 2016 [Google Scholar]
- 12.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000; 894:i–xii, 1–253 [PubMed] [Google Scholar]
- 13.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev 2006;27(7):750–761 [DOI] [PubMed] [Google Scholar]
- 14.Physical Activity Guidelines For Australian Adults. Australian Government Department of Health and Ageing. [updated (Last Updated 2010)]; Available from: http://www.health.gov.au/internet/abhi/publishing.nsf/Content/Physical+activity+guidelines-lp. Accessed May 3, 2016
- 15.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 2010; 13(5):496–502 [DOI] [PubMed] [Google Scholar]
- 16.Garber CE, Blissmer B, Deschenes MR, et al. ; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–1359 [DOI] [PubMed] [Google Scholar]
- 17.UK physical activity guidelines - Fact sheet 4: physical activity guidelines for adults (19–64 years). July 2011. UK Department of Health; Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213740/dh_128145.pdf, [Accessed December 22, 2015] [Google Scholar]
- 18.Australian Bureau of Statistics. Let’s get physical: How do adult Australians measure up? 4156055001 - Perspectives on Sport, Nov 2013 Available from: http://wwwabsgovau/ausstats/abs@nsf/Lookup/4156055001Main+Features3Nov%202013 [Accessed 16/11/215]. 2013.
- 19.Adamson L, Brown W, Byles J, et al. Women’s weight: Findings from the Australian Longitudinal Study on Women’s Health: Report prepared for the Australian Government Department of Health and Ageing. Australian Government Department of Health and Ageing; Available From: http://wwwalswhorgau/images/content/pdf/major_reports/2007_major_report_bpdf [Accessed 16/11/2015]. 2007 [Google Scholar]
- 20.Brown PR, Brown WJ, Miller YD, et al. Perceived Constraints and Social Support for Active Leisure Among Mothers With Young Children. Leis Sci 2001;23(3):131–144 [Google Scholar]
- 21.Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania women’s health study. Womens Health Issues 2008;18(6, Suppl)S87–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammiche F, Laven JSE, van Mil N, et al. Tailored preconceptional dietary and lifestyle counselling in a tertiary outpatient clinic in The Netherlands. Hum Reprod 2011;26(9):2432–2441 [DOI] [PubMed] [Google Scholar]
- 23.Temel S, van Voorst SF, Jack BW, Denktaş S, Steegers EA. Evidence-based preconceptional lifestyle interventions. Epidemiol Rev 2014;36(1):19–30 [DOI] [PubMed] [Google Scholar]
- 24.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010;117(11):1316–1326 [DOI] [PubMed] [Google Scholar]
- 25.Owe KM, Nystad W, Bø K. Correlates of regular exercise during pregnancy: the Norwegian Mother and Child Cohort Study. Scand J Med Sci Sports 2009;19(5):637–645 [DOI] [PubMed] [Google Scholar]
- 26.Palomba S, Falbo A, Valli B, et al. Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. Reprod Biomed Online 2014;29(1):72–79 [DOI] [PubMed] [Google Scholar]
- 27.Kucuk M, Doymaz F, Urman B. Effect of energy expenditure and physical activity on the outcomes of assisted reproduction treatment. Reprod Biomed Online 2010;20(2):274–279 [DOI] [PubMed] [Google Scholar]
- 28.Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002;13(2):184–190 [DOI] [PubMed] [Google Scholar]
- 29.Gudmundsdottir SL, Flanders WD, Augestad LB. Physical activity and fertility in women: the North-Trøndelag Health Study. Hum Reprod 2009;24(12):3196–3204 [DOI] [PubMed] [Google Scholar]
- 30.Morris SN, Missmer SA, Cramer DW, Powers RD, McShane PM, Hornstein MD. Effects of lifetime exercise on the outcome of in vitro fertilization. Obstet Gynecol 2006;108(4):938–945 [DOI] [PubMed] [Google Scholar]
- 31.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25(2):544–551 [DOI] [PubMed] [Google Scholar]
- 32.Boyle JA, Cunningham J, O’Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust 2012;196(1):62–66 [DOI] [PubMed] [Google Scholar]
- 33.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 2012; 27(10):3067–3073 [DOI] [PubMed] [Google Scholar]
- 34.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997; 18(6):774–800 [DOI] [PubMed] [Google Scholar]
- 35.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril 2010;93(7):2421–2423 [DOI] [PubMed] [Google Scholar]
- 37.Moran LJM, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16(4):347–363 [DOI] [PubMed] [Google Scholar]
- 38.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab 2005;90(10): 5711–5716 [DOI] [PubMed] [Google Scholar]
- 39.Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 2009;15(4):477–488 [DOI] [PubMed] [Google Scholar]
- 40.Ching HL, Burke V, Stuckey BGA. Quality of life and psychological morbidity in women with polycystic ovary syndrome: body mass index, age and the provision of patient information are significant modifiers. Clin Endocrinol (Oxf) 2007;66(3): 373–379 [DOI] [PubMed] [Google Scholar]
- 41.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81(1):19–25 [DOI] [PubMed] [Google Scholar]
- 42.Zawadaki R, Dockerty MD. Diagnostic criteria for polycystic ovarian syndrome: towards a rational approach In: Dunaif A, Given JR, Haseltine F, Merriam GR (Eds)., editor. Current Issues in Endocrinology and Metabolism: Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. p. 377–84. [Google Scholar]
- 43.Corbould A, Kim YB, Youngren JF, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 2005;288(5):E1047–E1054 [DOI] [PubMed] [Google Scholar]
- 44.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med 2006;12(7):324–332 [DOI] [PubMed] [Google Scholar]
- 45.Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995; 10(8):2107–2111 [DOI] [PubMed] [Google Scholar]
- 46.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89(6): 2745–2749 [DOI] [PubMed] [Google Scholar]
- 47.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012; 18(6):618–637 [DOI] [PubMed] [Google Scholar]
- 48.Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 2013;28(3):777–784 [DOI] [PubMed] [Google Scholar]
- 49.Kiddy DS, Sharp PS, White DM, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990;32(2):213–220 [DOI] [PubMed] [Google Scholar]
- 50.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN; PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91(1):48–53 [DOI] [PubMed] [Google Scholar]
- 51.Teede HJ, Misso ML, Deeks AA, et al. ; Guideline Development Groups. Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust 2011;195(6):S65–S112 [DOI] [PubMed] [Google Scholar]
- 52.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol (1985) 1989;66(2):876–885 [DOI] [PubMed] [Google Scholar]
- 53.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison CL, Stepto NK, Hutchison SK, Teede HJ. The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;76(3):351–357 [DOI] [PubMed] [Google Scholar]
- 55.Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011;17(2):171–183 [DOI] [PubMed] [Google Scholar]
- 56.Almenning I, Rieber-Mohn A, Lundgren KM, Shetelig Løvvik T, Garnæs KK, Moholdt T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015;10(9):e0138793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vigorito C, Giallauria F, Palomba S, et al. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92(4):1379–1384 [DOI] [PubMed] [Google Scholar]
- 58.Nybacka Å, Carlström K, Ståhle A, Nyrén S, Hellström PM, Hirschberg AL. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril 2011;96(6):1508–1513 [DOI] [PubMed] [Google Scholar]
- 59.Orio F, Giallauria F, Palomba S, et al. Metabolic and cardiopulmosnary effects of detraining after a structured exercise training programme in young PCOS women. Clin Endocrinol (Oxf) 2008; 68(6):976–981 [DOI] [PubMed] [Google Scholar]
- 60.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93(9):3373–3380 [DOI] [PubMed] [Google Scholar]
- 61.Palomba S, Giallauria F, Falbo A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod 2008;23(3):642–650 [DOI] [PubMed] [Google Scholar]
- 62.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41(2):459–471 [DOI] [PubMed] [Google Scholar]
- 63.Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes 2005;29(10):1168–1174 [DOI] [PubMed] [Google Scholar]
- 64.Hill JO, Wyatt H, Phelan S, Wing R. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav 2005;37(4):206–210 [DOI] [PubMed] [Google Scholar]
- 65.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82(1, Suppl)222S–225S [DOI] [PubMed] [Google Scholar]
- 66.Mekary RA, Feskanich D, Hu FB, Willett WC, Field AE. Physical activity in relation to long-term weight maintenance after intentional weight loss in premenopausal women. Obesity (Silver Spring) 2010;18(1):167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nurs Res 2010;59(3):224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKercher CM, Schmidt MD, Sanderson KA, Patton GC, Dwyer T, Venn AJ. Physical activity and depression in young adults. Am J Prev Med 2009;36(2):161–164 [DOI] [PubMed] [Google Scholar]
- 69.Ströhles A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) 2009;116(6):777–784 [DOI] [PubMed] [Google Scholar]
- 70.Vuillemin A, Boini S, Bertrais S, et al. Leisure time physical activity and health-related quality of life. Prev Med 2005; 41(2):562–569 [DOI] [PubMed] [Google Scholar]
- 71.Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med 2007;45(6):401–415 [DOI] [PubMed] [Google Scholar]
- 72.Brown WJ, Mishra G, Lee C, Bauman A. Leisure time physical activity in Australian women: relationship with well being and symptoms. Res Q Exerc Sport 2000;71(3):206–216 [DOI] [PubMed] [Google Scholar]
- 73.De Moor MHM, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med 2006;42(4):273–279 [DOI] [PubMed] [Google Scholar]
- 74.Gillison FB, Skevington SM, Sato A, Standage M, Evangelidou S. The effects of exercise interventions on quality of life in clinical and healthy populations; a meta-analysis. Soc Sci Med 2009; 68(9):1700–1710 [DOI] [PubMed] [Google Scholar]
- 75.Thomson RL, Buckley JD, Lim SS, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril 2010;94(5): 1812–1816 [DOI] [PubMed] [Google Scholar]
- 76.Liao LM, Nesic J, Chadwick PM, Brooke-Wavell K, Prelevic GM. Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: a pilot investigation. Gynecol Endocrinol 2008;24(10):555–561 [DOI] [PubMed] [Google Scholar]
- 77.American College of Obstetricians and Gynecologists. ACOG Committee opinion number 650, December 2015. Physical Activity and Exercise During Pregnancy and the Postpartum Period. Available from: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Physical-Activity-and-Exercise-During-Pregnancy-and-the-Postpartum-Period [Accessed December 1 2015]
- 78.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–381 [PubMed] [Google Scholar]
- 79.Pivarnik J, Chambliss HO, Clapp JF, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc 2006;38(5):989–1006 [DOI] [PubMed] [Google Scholar]
- 80.SMA statement the benefits and risks of exercise during pregnancy. Sport Medicine Australia. J Sci Med Sport 2002;5(1):11–19 [DOI] [PubMed] [Google Scholar]
- 81.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Practitioner advice and gestational weight gain. J Womens Health (Larchmt) 2011;20(4):585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arslan Ozkan I, Mete S. Pregnancy planning and antenatal health behaviour: findings from one maternity unit in Turkey. Midwifery 2010;26(3):338–347 [DOI] [PubMed] [Google Scholar]
- 83.Edvardsson K, Ivarsson A, Eurenius E, et al. Giving offspring a healthy start: parents’ experiences of health promotion and lifestyle change during pregnancy and early parenthood. BMC Public Health 2011;11(1):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stewart ZA, Wallace E, Allan C. Weight gain in pregnancy: a survey of current practices in a teaching hospital. Aust N Z J Obstet Gynaecol 2012;52(2):208–210 [DOI] [PubMed] [Google Scholar]
- 85.Duncombe D, Wertheim EH, Skouteris H, Paxton SJ, Kelly L. Factors related to exercise over the course of pregnancy including women’s beliefs about the safety of exercise during pregnancy. Midwifery 2009;25(4):430–438 [DOI] [PubMed] [Google Scholar]
- 86.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;9(2):140–150 [DOI] [PubMed] [Google Scholar]
- 87.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 2006;184(2):56–59 [DOI] [PubMed] [Google Scholar]
- 88.Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol 1995;86(2):163–169 [DOI] [PubMed] [Google Scholar]
- 89.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev 2012;4:CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344:e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2): S141–S146 [DOI] [PubMed] [Google Scholar]
- 92.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51(1):26–30 [DOI] [PubMed] [Google Scholar]
- 93.Di Cianni G, Volpe L, Lencioni C, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract 2003;62(2):131–137 [DOI] [PubMed] [Google Scholar]
- 94.Kühl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care 1998;21(Suppl 2):B19–B26 [PubMed] [Google Scholar]
- 95.Moses RG, Morris GJ, Petocz P, San Gil F, Garg D. The impact of potential new diagnostic criteria on the prevalence of gestational diabetes mellitus in Australia. Med J Aust 2011;194(7):338–340 [DOI] [PubMed] [Google Scholar]
- 96.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358(19):1991–2002 [DOI] [PubMed] [Google Scholar]
- 97.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862–1868 [DOI] [PubMed] [Google Scholar]
- 98.Ratner RE, Christophi CA, Metzger BE, et al. ; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93(12):4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Sullivan JB. Diabetes mellitus after GDM. Diabetes 1991;40 (Suppl 2):131–135 [DOI] [PubMed] [Google Scholar]
- 100.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007;30(Suppl 2):S169–S174 [DOI] [PubMed] [Google Scholar]
- 101.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015;125(3):576–582 [DOI] [PubMed] [Google Scholar]
- 102.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30 (Suppl 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 103.Weissgerber TL, Wolfe LA, Davies GAL, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab 2006;31(6):661–674 [DOI] [PubMed] [Google Scholar]
- 104.Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst Rev 2006;3(3):CD004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep 2014;16(3):395. [DOI] [PubMed] [Google Scholar]
- 106.Meher S, Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2006;(2):CD005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology 2014;25(3):331–343 [DOI] [PubMed] [Google Scholar]
- 108.Pivarnik JM, Ayres NA, Mauer MB, Cotton DB, Kirshon B, Dildy GA. Effects of maternal aerobic fitness on cardiorespiratory responses to exercise. Med Sci Sports Exerc 1993;25(9):993–998 [PubMed] [Google Scholar]
- 109.Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes-a randomized trial. Med Sci Sports Exerc 2012;44(12):2263–2269 [DOI] [PubMed] [Google Scholar]
- 110.Melzer K, Schutz Y, Boulvain M, Kayser B. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med 2010;40(6):493–507 [DOI] [PubMed] [Google Scholar]
- 111.Domenjoz I, Kayser B, Boulvain M. Effect of physical activity during pregnancy on mode of delivery. Am J Obstet Gynecol 2014; 211(4):401.e1–401.e11 [DOI] [PubMed] [Google Scholar]
- 112.Poyatos-León R, García-Hermoso A, Sanabria-Martínez G, Ál-varez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effects of exercise during pregnancy on mode of delivery: a meta-analysis. Acta Obstet Gynecol Scand 2015;94(10):1039–1047 [DOI] [PubMed] [Google Scholar]
- 113.Tinloy J, Chuang CH, Zhu J, Pauli J, Kraschnewski JL, Kjerulff KH. Exercise during pregnancy and risk of late preterm birth, cesarean delivery, and hospitalizations. Womens Health Issues 2014; 24(1):e99–e104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jukic AMZ, Evenson KR, Daniels JL, Herring AH, Wilcox AJ, Hartmann KE. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern Child Health J 2012;16(5):1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klebanoff MA, Shiono PH, Carey JC. The effect of physical activity during pregnancy on preterm delivery and birth weight. Am J Obstet Gynecol 1990;163(5 Pt 1):1450–1456 [DOI] [PubMed] [Google Scholar]
- 116.Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev 2006;(3):CD000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiebe HWBN, Boulé NG, Chari R, Davenport MH. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol 2015;125(5):1185–1194 [DOI] [PubMed] [Google Scholar]
- 118.Owe KM, Nystad W, Skjaerven R, Stigum H, Bø K. Exercise during pregnancy and the gestational age distribution: a cohort study. Med Sci Sports Exerc 2012;44(6):1067–1074 [DOI] [PubMed] [Google Scholar]
- 119.Davies GA, Wolfe LA, Mottola MF, MacKinnon C; Society of Obstetricians and gynecologists of Canada, SOGC Clinical Practice Obstetrics Committee. Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol 2003;28(3):330–341 [PubMed] [Google Scholar]
- 120.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr 2005;87A, 7a1010–1027 [DOI] [PubMed] [Google Scholar]
- 121.Walker LO. Managing excessive weight gain during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs 2007; 36(5):490–500 [DOI] [PubMed] [Google Scholar]
- 122.Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2013;7:CD005627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lim S, O’Reilly S, Behrens H, Skinner T, Ellis I, Dunbar JA. Effective strategies for weight loss in post-partum women: a systematic review and meta-analysis. Obes Rev 2015;16(11): 972–987 [DOI] [PubMed] [Google Scholar]
- 124.Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes 2014; 38(5):626–635 [DOI] [PubMed] [Google Scholar]
- 125.O’Hara MW, Swain AM. Rates and risk of postpartum depression-A meta-analysis. Int Rev Psychiatry 1996;8(1):37–54 [Google Scholar]
- 126.Blamey RV, Daley AJ, Jolly K. Exercise for postnatal psychological outcomes: a systematic review and meta-analysis. Lancet 2012; 380:S25 [Google Scholar]
- 127.Armstrong K, Edwards H. The effects of exercise and social support on mothers reporting depressive symptoms: a pilot randomized controlled trial. Int J Ment Health Nurs 2003; 12(2):130–138 [DOI] [PubMed] [Google Scholar]
- 128.Armstrong K, Edwards H. The effectiveness of a pram-walking exercise programme in reducing depressive symptomatology for postnatal women. Int J Nurs Pract 2004;10(4):177–194 [DOI] [PubMed] [Google Scholar]
- 129.Ko Y-L, Yang C-L, Fang C-L, Lee MY, Lin PC. Community-based postpartum exercise program. J Clin Nurs 2013;22(15–16): 2122–2131 [DOI] [PubMed] [Google Scholar]
- 130.Strøm M, Mortensen EL, Halldorson TI, Osterdal ML, Olsen SF. Leisure-time physical activity in pregnancy and risk of postpartum depression: a prospective study in a large national birth cohort. J Clin Psychiatry 2009;70(12):1707–1714 [DOI] [PubMed] [Google Scholar]
- 131.Daley AJ, Foster L, Long G, et al. The effectiveness of exercise for the prevention and treatment of antenatal depression: systematic review with meta-analysis. BJOG 2015;122(1): 57–62 [DOI] [PubMed] [Google Scholar]
- 132.Bahadoran B, Abbasi F, Yousefi A, et al. Evaluating the effect of exercise on the postpartum quality of life. Iran J Nurs Midwifery Res. 2008;12(1): [Google Scholar]
- 133.Teychenne M, York R. Physical activity, sedentary behavior, and postnatal depressive symptoms: a review. Am J Prev Med 2013; 45(2):217–227 [DOI] [PubMed] [Google Scholar]
- 134.Songøygard KM, Stafne SN, Evensen KA, Salvesen KÅ, Vik T, Mørkveds S. Does exercise during pregnancy prevent postnatal depression? A randomized controlled trial. Acta Obstet Gynecol Scand 2012;91(1):62–67 [DOI] [PubMed] [Google Scholar]