Abstract
Purpose
Bradykinesia and muscle weaknesses are common symptoms of Parkinson’s Disease (PD) and are associated with impaired functional performance, increased risk of falls, and reduced quality of life. Recent studies have pointed to progressive resistance training (PRT) as an effective method to control and reduce these symptoms, increasing possibilities to treat the disease. However, few studies have focused on assessing the PRT effects in the short-term. Therefore, the present study aimed to assess the short-term PRT effects on people with PD, in order to offer new parameters for a better understanding of its effects, so as an adequation and PRT use as a complementary therapy.
Patients and Methods
Forty individuals diagnosed with PD from stage 1 to 3 on the Hoehn and Yahr scale took part on the study and were allocated into 2 groups; Training Group (TG) performed a 9-week RT program twice a week, and the Control Group (CG) attended disease lectures. Bradykinesia UPDRS subscale (BSS), knee extensors isokinetic strength, Ten Meters Walk Test (TMW), Timed Up&Go Test (TUG) and 30-Second Chair Stand (T30) were measured before and after the intervention period. Statistical significance was set at p ≤ 0.05.
Results
Significant time was noted by the group interaction for all functional tests (TUG, T30, and TWM; all p < 0.01) and BSS (p < 0.01). Post hoc analyses revealed that these differences were driven by significant improvements in these dependent variables (all p < 0.01) while the CG remained unchanged (all p > 0.05). Moreover, TUG, T30, TWM, and BSS were significantly different between TG and CG in the post-training assessments (all p < 0.01). Isokinetic muscle strength was slightly increased in the TG (2.4%) and decreased in the CG (−2.2%), but statistical analyses did not reach significance for interaction but only a trend (p = 0.12).
Conclusion
The results indicate that 9 weeks of PRT reduces bradykinesia and improves functional performance in patients with mild to moderate PD. These findings reinforce this mode of exercise as an important component of public health promotion programs for PD.
Keywords: Parkinson disease, physical exercise, resistance training, substantia nigra
Introduction
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases in the world, with an annual incidence of 4.5 to 19 cases per 100,000 population.1 People with PD develop motor, cognitive and behavioral deficits, besides executive function losses, which progressively reduce their functional independence, and drive them to a substantial decrease in quality of life.2–4
The PD pathophysiology is characterized by selective dopaminergic neuronal death, located in the substantia nigra, on the midbrain, which leads to dopamine levels reduction, an important neurotransmitter of neural modulation.5 As a result, occur a thalamus tonic inhibition and an excitatory drive decrease to the motor cortex6,7 which leads to disturbances in the muscles cortical activation.8–10 Consequently, the development of cardinal PD symptoms is observed, including tremor, postural instability, muscle stiffness, and bradykinesia,11–18 which is considered the most disabling disease symptoms.16,19,20 Muscle weakness has also been reported as an important PD symptom, and it is related to bradykinesia, because they share common pathophysiological mechanisms,12,13,21,22 also being responsible for a functional performance decrease.13,16,23
The PD traditional therapeutic approach includes Levodopa (main dopaminergic medication), dopaminergic agonists, catechol-O-methyltransferase inhibitors and non-dopaminergic agents.18,24,25 However, their prolonged use is associated with severe side effects and gradual efficacy loss.26,27
On the other hand, complementary therapies have been tested and there is a strong consensus that physical exercise, especially resistance training (RT), is beneficial to PD, because RT prevents the disease development,29 reduce symptoms,30–33 slow down the disease progression,34–36 by improving the functional performance and the autonomy of individuals affected by the disease.17,37–39
A key element to understand the DP training resistance benefit is in his neuronal benefit. This intervention’s model shows up as a solution to reduce the bradykinesia,12,13,22,40 main effect of dopaminergic medication, which produces improvements in brain connectivity, muscle activation, and functional performance.41,42 Furthermore, it is a new field of study, and it’s still not totally clear, how bradykinesia may be affected by exercise.
In a recent study, David et al, (2016), did a 24-month of progressive resistance training (PRT) intervention and the bradykinesia was reduced. They also demonstrated that the reduction was mediated by changes in certain elements related to symptoms, such as the pattern of muscle activation, muscle strength, and the connectivity of the basal ganglia regions.
These results are promising, once exercise and especially PRT seem to promote changes similar to those provided by dopaminergic medication.13 If those changes also occur quickly, that could reduce medication dosages since the diagnostic stage. Therefore, a question shows up; whether changes in bradykinesia may also occur in the short term interventions using PRT, consistent with the improvement in functional performance. If that hypothesis is confirmed, an important development field of therapeutic approaches can be opened, allowing the introduction of this dynamic and high-intensity method, in the early stages of PD treatment.
Wherefore, the present study aims to assess the effects of PRT during 9 weeks on muscle strength, functional capacity, motor symptoms, and bradykinesia on individuals with PD.
Methods
Participants
Patients diagnosed with PD according to Parkinson’s UK Brain Bank criteria43 and Hoehn & Yahr stage 1–344 were recruited from the local health system. Volunteers enrolled in the study underwent neurologist evaluation to ensure PD diagnosis and to confirm the disease stage. The inclusion criteria were as follows: neurologist confirmed Hoehn and Yahr stages, between 50 and 80 years old, no cognitive impairment as assessed by the Mini Mental State Examination (MMSE), where the cut-off points for inclusion were > 24 points for literate individuals and > 19 for non-literate individuals, and attested to participate in the RT program. Volunteers were excluded if they were diagnosed with any other neurological disease, with cardiovascular disease, hematologic or orthopedic disorders; with motor fluctuations or severe dyskinesia that could affect their ability to perform the experimental protocol. Moreover, participants who did not reach the minimum frequency of 75% or missed 3 consecutive training sessions were excluded from analyses.
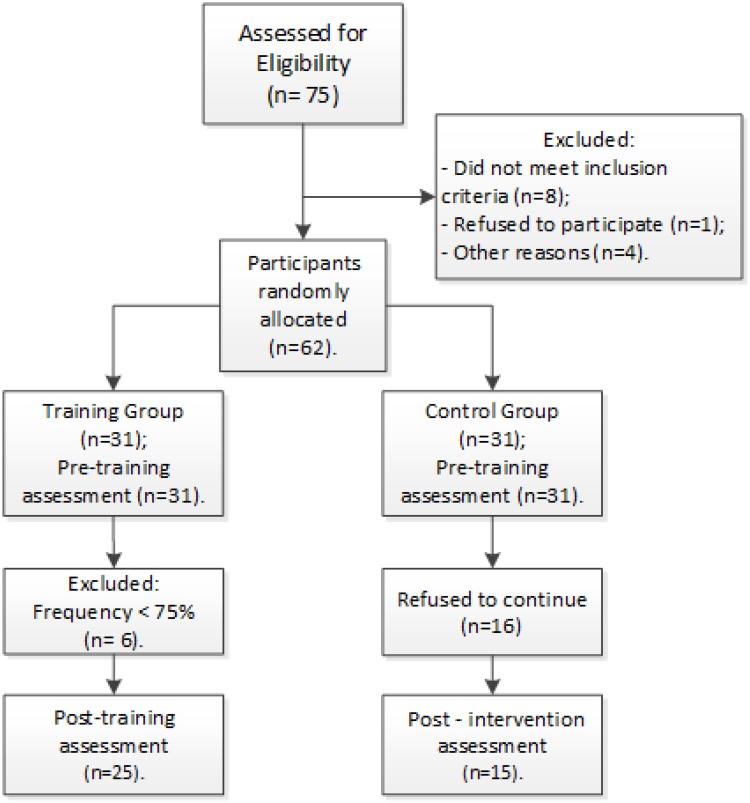
The patients’ recruitment flowchart is detailed and illustrated in Figure 1. Briefly, after exclusion criteria were applied, 62 patients underwent baseline assessments and were allocated through simple randomization in two possible groups: Control Group (CG, n=31) and Training Group (TG, n=31). A total of 17 participants abandoned the study referring to transportation issues, familiar problems, treatment not related to PD, or took part in another treatment program. Another 5 participants did not complete all the final assessments. Thus, the TG and CG were finally composed of 25 and 15 participants, respectively. All assessments and training were conducted under PD-related medication effects, which were kept unchanged during the stusdy. Age, gender, PD Hoehn and Yahr stage, time of diagnosis, level of physical activity, education level and employment status were carefully recorded. Of note, all outcomes assessments were conducted by a researcher who was blind to subjects’ group allocation. Standard procedures were used to measure weight with 0.1 kg precision on a digital balance beam scale, and height was measured at the nearest 0.1 cm with a wall stadiometer. Body mass index (BMI) was derived as body weight divided by height squared (kg/m2).
Figure 1.
Participants selectionand styudy progess.
This study was conducted according to the Code of Ethics of The World Medical Association. Study procedures were explained to all participants who signed a consent form. The study protocol was approved by the Institutional Research Ethics Committee at the University of Brasilia under protocol number 034/11.
Functional Performance
The present study adopted three commonly used tests recommended for patients with PD.45 Ten Meters Walk Test (TMW) to assess gait speed (m/s). So, participants were instructed to walk as fast as they could for a 16-meter course without obstacles. Only the time related to the central 10 meters was recorded; the initial and final 3 meters were not considered for analyses. Three measurements were recorded with a two-minute resting interval between each other; the mean value was converted into meters per second (m/s); the Timed Up & Go Test (TUG) to assess functional mobility.46 Briefly, participants had to rise from a chair, walk 3 meters, turn around a cone, walk back to the chair and sit down. The chair was standardized with 46 cm high. The mean value in seconds of three measurement trials was entered for analyses. Of note, each participant had one familiarization trial as an attempt to avoid learning effects and ensure that volunteers understood the instructions.47 For the thirty-second chair stand test (T30), each participant had to sit in a standardized chair with the hands on the opposite shoulder crossed at the wrists, feet flat on the floor, and back straight. By the “Go” sign, patients had to rise to a full standing position and sit back down again as fast as they could for 30 seconds. Participants performed the test once and the total number of repetitions was considered for analysis.
Bradykinesia
Motor examination with Unified Parkinson’s Disease Rating Scale (UPDRS), section III, was used as a measure of Bradykinesia. The following items of Bradykinesia subscale (BSS) were examined: 23 (finger taps), 24 (hands movement), 25 (rapid alternating movements of hands), 26 (leg agility) and 31 (body bradykinesia and hypokinesia). Each sub-item is scored from 0 to 4 where higher scores correspond to greater severity.48,49 A single trained neurologist conducted all the evaluations.
Isokinetic Muscle Strength
Right knee extensor isokinetic peak torque was evaluated using the Biodex System 3 dynamometer (Biodex Medical System, Shirley, NY). Before testing, participants performed a familiarization session of 10 repetitions at 120 degrees/second.50 After a full explanation of the procedures, participants were seated on the dynamometer, which was carefully adjusted, and the rotational axis of the dynamometer arm was oriented with the lateral epicondyle of the participant’s dominant femur. Velcro belts were used at the thigh, pelvis, and trunk to avoid compensatory movements. Gravity correction was obtained by measuring the torque exerted on the dynamometer with the limb in a relaxed state. The testing protocol consisted of two sets of four knee extensor contractions at 60 degrees/second with a one-minute rest between sets. The recorded value was the single muscle contraction that elicited the highest peak torque throughout the protocol, which is expressed in absolute values (Nm) as well as relative to body weight (Nm/kg). Participants were asked to perform the movement with their maximal strength and verbal encouragement was offered by the examiner. The equipment calibration was performed according to the manufacturer’s specifications before every testing session.
Resistance Training Program
The TG performed the 9-weeks PRT program using weight machines (Rotech Fitness Equipment, Brazil). Before the training period, participants underwent familiarization sessions during three weeks with a total of six familiarization sessions to ensure proper technique execution. The protocol aimed to work out major muscle groups and involved the following exercises: chest press, knee extension, hamstrings curl, leg press, and seated row. Training sessions lasted approximately 50–60 mins with 2 sets of 10–12 repetitions until fatigue. Every time a participant performed more than 12 repetitions, the weight was adjusted to maintain established repetitions range. All training sessions were carried out in the PRT training room of University and were under the supervision of proficient professionals. Participants who did not complete at least 75% of the training sessions were excluded from analyses. The CG attended an orientation program with lectures on health, quality of life and scientific update on PD, and were instructed to not modify their habitual routine.
Statistical Analysis
The sample characterization data were analyzed by descriptive statistics and normality of dependent variables tested by Shapiro Wilk’s test. An independent sample test was used to examine possible baseline differences between groups for age, weight, height, BMI and years of diagnosis. Chi-squared was performed to test the baseline differences in categorical variables. The effects of RT on dependent variables were analyzed using time x group ANOVA, where the within-subjects factors were the pre and post values and the between fixed factor was group (TG and CG). When a significant time was observed by the interaction group, the Bonferroni post-hoc test was performed. Wilcoxon’s test was used for T30 because the data were not normally distributed. Cohen´s effect size was calculated for each dependent variables comparison. Data were considered significant at P < 0.05 and statistical analyses were performed using the Statistical Package for the Social Sciences 20.0 software (SPSS, Chicago, IL). Data are expressed as means ± Standard Error unless otherwise noted.
Results
Table 1 presents the patient’s characteristics for both groups (TG and CG). No significant differences were observed between groups for age, weight, height, BMI and years of PD diagnosis. All patients were at Hoehn and Yahr stages from I to III. Moreover, chi-squared tests revealed no differences between groups for Hoehn and Yahr stage, gender, physical activity level or education level. Also, patients’ medication profiles were similar at baseline and remained unchanged throughout the study period.
Table 1.
Patients Characteristics According to Group
| Variable | Training Group | Control Group |
|---|---|---|
| N | 25 | 15 |
| Male/Female | 20/5 | 10/5 |
| Age (years) | 64.7 ± 1.8 | 64.4 ± 3.7 |
| Weight (Kg) | 74.5 ± 2.5 | 79.0 ± 5.4 |
| Height (m) | 1.65 ± 0.02 | 1.67 ± 0.02 |
| BMI (Kg/m2) | 27.5 ± 0.8 | 27.8 ± 1.7 |
| Years of PD diagnosis | 5.7 ± 0.8 | 7.2 ± 1.9 |
Note: Values are expressed as means ± Standard Error.
Abbreviations: BMI, Body Mass Index; PD, Parkinson’s Disease.
Table 2 shows mean values and standard errors of isokinetic muscle strength, bradykinesia, and functional performance before and after the training period in both groups (ie, TG and CG). Significant time by group interaction was noted for all the functional tests (TUG, T30, and TWM; all p < 0.01) and BSS (p < 0.01). Post hoc analyses revealed that these differences were driven by significant increases in the TG (all p < 0.05) while CG remained unchanged (all p > 0.05). About TUG, it was noted a significant decrease in the TG (p < 0.01) without changes in the CG. As a consequence of these observations, TUG, T30, TWM, and BSS were all significantly different between TG and CG in the post-training assessments (all p < 0.01). Although isokinetic muscle strength was slightly increased in the TG (2.9%) and decreased in the CG (−2.9%), statistical analyses were not significant on interaction but just present a trend (p = 0.12). The effect size for each dependent variable is presented in Table 3. While medium effect sizes were observed for TUG, T30, and TWM, a large effect size was noted for BSS, all in the TG. Neither isokinetic muscle strength in the TG nor any variable in the CG showed considerable effect size.
Table 2.
Mean Values and Standard Error for Isokinetic Muscle Strength, Bradykinesia, and Functional Performance Before and After the Training Period
| Variables | Training Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pre | Post | ∆% | Pre | Post | ∆% | |
| T30 (reps)$ | 13.1 ± 0.8 | 16.4 ± 1.0*,# | 25.2 | 12.3 ± 1.3 | 10.3 ± 1.6 | −16.2 |
| TUG (s)$ | 9.2 ± 0.5 | 7.4 ± 0.4*, # | −20.3 | 9.3 ± 0.9 | 10.0 ± 0.7 | 7.9 |
| TWM (m/s)$ | 1.7 ± 0.2 | 2.0 ± 0.3*, # | 21.0 | 1.7 ± 0.4 | 1.7 ± 0.3 | −0.8 |
| PT (N∙m) | 142.9 ± 7.3 | 147.1 ± 7.7 | 2.9 | 139.9 ± 10.7 | 135.8 ± 11.2 | −2.9 |
| PT (N∙m/kg) | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.4 | 1.8 ± 0.1 | 1.8 ± 0.2 | −2.2 |
| BSS (score)$ | 16.6 ± 0.9 | 8.4 ± 0.9*, # | −49.6 | 17.0 ± 1.3 | 17.5 ± 1.3 | 3.2 |
Notes: Values are expressed as means ± Standard Errors. $Significant time by group interaction (p < 0.05). *Significantly different in relation to baseline (p < 0.05). #Significantly different in relation to control group post (0.05).
Abbreviations: T30, 30-second Chair-Stand Test; TUG, Timed Up & Go; TMW, Ten Meters Walk Test; PT, Peak Torque; BSS, Bradykinesia subscale.
Table 3.
Effect Size for Each Dependent Variable According to Group Allocation
| Variables | Effect Size | |
|---|---|---|
| TG | CG | |
| T30 (reps) | 0.413 | 0.098 |
| TUG (s) | 0.420 | 0.03 |
| TWM (m/s) | 0.493 | 0.001 |
| PT (N∙m) | 0.072 | 0.035 |
| PT (N∙m/kg) | 0.035 | 0.013 |
| BSS (score) | 0.901 | 0.017 |
Abbreviations: T30, 30-second Chair-Stand Test; TUG, Timed Up&Go; TMW, Ten Meters Walk Test; PT, Peak Torque; BSS, Bradykinesia subscale.
Discussion
The findings of the present study confirmed the hypothesis that 9 weeks of PRT reduces bradykinesia in people with mild to moderate PD. More specifically, improvements in functional performance were observed in common day-to-day locomotor activity, such as walking, turning, sitting, rising and walking speed. These findings are relevant, once maintaining the autonomy and improvement of functional capacity has been considered the most important goal of PD interventions. Actually, some interventions focused on functional capacity rehabilitation with physical exercise and motor training has been recommended, but its efficiency still remains uncertain. Allen et al, (2001), performed a meta-analysis on the effects of different intervention models that used physical activity on the balance and functional capacity of individuals with DP and noted modest gains overall. However, he suggested that the intensity factor may lead to more expressive results. This factor has been relevant, especially in interventions that use resistance training, where intensity can be easily monitored and modulated.54,55 For example, Scandalis et al, (2001) showed that a simple resistive exercise program increased gait speed in persons with PD, at the end of the 8-week study. Dibble et al, (2006), observed the increase in strength, concomitant to an increase in the walking speed and speed up stairs, after 12 weeks of the intervention of high-intensity TR. Therefore, resistance training has been highlighted as adjunctive therapy in PD16 and the increase in strength has been considered a key element for developing functional capacity during the disease.54–57
However, in the present study, the increase of functional capacity occurred without significant changes in strength, nevertheless concomitant with the reduction of bradykinesia. Improvements in muscle strength depend as much on the neural improvement as on structural changes in muscle tissue58,59 that can occur in different timeframes of up to 8 weeks in healthy people,58 but possibly longer-term in individuals with PD, as some authors have shown52,53,60. In the present study, we did not monitor the muscle quantity and quality of the individuals assessed, although, it is believed that changes in muscle morphology of individuals with PD are affected by the neurodegenerative condition.61,62
The results of the present study can be better explained by the recent findings of David et al 2016, which demonstrated that PD force is only one of the underlying bradykinesia elements, a symptom considered the main responsible for motor deficits observed in the disease. In the study of David et al 2016, it was observed that after 24 months of PRT, in individuals with mild to moderate PD, there was a significant diminish on bradykinesia with an increase in muscle strength, as well as improvements on the three-phase pattern of activation of agonist and antagonist muscles and reduction in agonist/antagonist co-contraction. The study also concluded that 55% of bradykinesia reduction was related to changes in muscle strength and activation, both changes were identified as underlying the bradykinesia. However, most of the bradykinesia reduction (45%), which could not be explained by the peripheral changes, was attributed to improved connectivity of the upper centers, especially the basal ganglia region.
Based on these findings, it is believed that the significant increase in functional capacity and reduction of motor symptoms observed in the present study were mediated by bradykinesia reduction, through changes in many elements underlying the symptom, and to a lesser scale by force, which presented a non-significant increase. Note that, given the relatively short-term of the present intervention and the absence of significant increase in strength, it can be reinforced at the notes of David et al,12,21. that most of the reduction in bradykinesia might be mediated by changes in the connectivity of superior centers and improvements in muscle activation, with the addition that these changes may occur in the short-term.
Unfortunately, force performance at higher speeds was not monitored. The weakness during PD has been associated with the pathophysiology of bradykinesia, and mainly affected at higher speeds. As we observed a reduction of bradykinesia it is possible that at high speeds, the force has increased substantially.
In functional magnetic resonance analysis, it was established that hypoactivity between the basal ganglia and motor cortex resulting from dopamine secretion deficits are closely related to bradykinesia,11 resulting in disturbances on muscle activation.8–10 These alterations, cause the cortical activation of the PD people’s muscles to have a distinct pattern from the three-phase pattern presented by healthy individuals.18 In healthy individuals, a first agonist firing of greater intensity and duration is followed by an antagonist firing to slow the muscle and a new antagonist firing to fix the limb at the movement end. In individuals with PD, there is an increase in the number of neural triggers in the acceleration phase of low magnitude movements,18,63,64 consequently, individuals with PD have slower movements and smaller amplitudes. One of the main benefits to the functional performance provided by dopamine-based antiparkinsonian medication is its ability to adjust the three-phase pattern of muscle activation consistent with a reduction in bradykinesia and an improvement in functional performance,42 due to increased availability of the neurotransmitter in the affected regions.
The David et al (2016) study showed that PRT provides adjustments in the three-phase pattern of muscle activation as important as observed in medication use, and the present study reinforces its evidence that central and peripheral neural changes may be mediated by PRT and add that these changes occur in a magnitude capable of affect functional performance. There is still much to understand about the neural, central and peripheral effects of PRT in PD. However, it can be established that moderate to high-intensity exercise can mediate neurobiological responses such as neurogenesis, angiogenesis, and synaptogenesis, affecting plasticity and promoting the better performance of neural centers affected by PD.65–68 Another important element concerns the increase in dopamine secretion in affected regions,69–71 in addition to increased efficacy of antiparkinsonian medication due to increase dopamine transit through the blood-brain barrier, expanded sensitivity to dopamine, and stimulation of dopamine synthesis.72 Further studies should be conducted to fill the gaps on the effects and uses of PRT on PD and its mechanisms, and thus consolidate as a complementary treatment method, therefore the current scenario indicates a broad field for therapeutic progress disease and for the improvement on the PD individuals quality of life.
Conclusion
The present study highlights the PRT importance on the adjunctive PD treatment, due to its efficacy in promoting improvements in the disease motor symptoms with a few weeks of intervention. The significant effects on bradykinesia, without an increase in muscle strength, suggest that the intervention promoted neural enhancements in a short-term, to substantially improve the functional performance of trained individuals. If the hypothesis is confirmed, the prospects for PRT therapy in PD will be broadened, as neurophysiological adjustments in the upper centers. And muscle activation resulting from this intervention type, added to the medication effects, may provide a scenario for the PRT use in PD, for example, enabling modulation of lower medication dosages, preserving patients for more time from the side effects of pharmacological intervention. In this sense, interventions with PRT are suggested to be used from initial stages of treatment as a complementary therapy to the medication use.
Acknowledgments
The authors would like to thank all volunteers for their efforts in taking part in the study.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Neurological disorders: public health challenges. Chapter 3, Neurological Disorders: A Public Health Approach – Parkinson´ s Disease. World Health Organization; 2006. [Google Scholar]
- 2.Higginson CI, King DS, Levine D, Wheelock VL, Sigvardt KA. The relationship between executive function and verbal memory in Parkinson’s disease. Brain Cogn. 2003;52:343–352. doi: 10.1016/S0278-2626(03)00180-5 [DOI] [PubMed] [Google Scholar]
- 3.Colcher A, Simuni T. Clinical manifestations of Parkinson’s disease. Med Clin North Am. 1999;83(2):327–347. doi: 10.1016/S0025-7125(05)70107-3 [DOI] [PubMed] [Google Scholar]
- 4.Ashburn A, Fazakarley L, Ballinger C, et al. A randomised controlled trial of a home-based exercise programme to reduce the risk of falling among people with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:678–684. doi: 10.1136/jnnp.2006.099333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obeso JA, Marin C, Rodriguez-Oroz C, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–46. doi: 10.1002/ana.21481 [DOI] [PubMed] [Google Scholar]
- 6.Wider C, Wszolek ZK. Etiology and pathophysiology of frontotemporal dementia, Parkinson disease and Alzheimer disease: lessons from genetic studies. Neuro-Degenerative Disord. 2008;5:122–125. doi: 10.1159/000113680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichmann T, DeLong MR. Anatomy and physiology of the basal ganglia: relevance to Parkinson’s disease and related disorders. Handb Clin Neurol. 2007;83:1–18. [DOI] [PubMed] [Google Scholar]
- 8.DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-V [DOI] [PubMed] [Google Scholar]
- 10.Prodoehl J, Spraker M, Corcos D, et al. Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson’s disease. Mov Disord. 2010;25:2035–2043. doi: 10.1002/mds.23360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berardelli A, Rothwell PD Thompson, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(Pt 11):2131–2146. doi: 10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- 12.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson’s disease: a review of potential mechanisms. Parkinsons Dis. 2012:Article ID– 124527. doi: 10.1155/2012/124527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson DW. Parkinson’s disease and Parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2(8):a009258–a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 15.Glendinning DS, Enoka RM. Motor unit behavior in Parkinson’s disease. Phys Ther. 1994;74(1):61–70. doi: 10.1093/ptj/74.1.61 [DOI] [PubMed] [Google Scholar]
- 16.Falvo MJ, Schilling BK, Gammon ME. Parkinson’s disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23(1):1–11. doi: 10.1002/mds.21690 [DOI] [PubMed] [Google Scholar]
- 17.Poewe W, Mahlknecht P. The clinical progression of Parkinson’s disease. Parkinsonism Rel Disord. 2009;15(Suppl 4):S28–32. doi: 10.1016/S1353-8020(09)70831-4 [DOI] [PubMed] [Google Scholar]
- 18.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301±14. doi: 10.1093/brain/103.2.301 [DOI] [PubMed] [Google Scholar]
- 19.Colcher A, Tanya S. Other Parkinson syndromes. Neurol Clin. 2001;19:629–649. doi: 10.1016/S0733-8619(05)70038-4 [DOI] [PubMed] [Google Scholar]
- 20.Nallegowda M, Singh U, Handa G, et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson’s disease: a pilot study. Am J Phys Med Rehabil. 2004;83:898–908. doi: 10.1097/01.PHM.0000146505.18244.43 [DOI] [PubMed] [Google Scholar]
- 21.David FJ, Robichaud JA, Vaillancourt DE, et al. Progressive resistance exercise restores some properties of the triphasic EMG pattern and improves bradykinesia: the PRET-PD randomized clinical trial. J Neurophysiol. 2016;116(5):2298–2311. doi: 10.1152/jn.01067.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balsamo S, Henrique LM, Freire JC, et al. Low dynamic muscle strength and its associations with fatigue, functional performance, and quality of life in premenopausal patients with systemic lupus erythematosus and low disease activity: a case–control study. BMC Musculoskelet Disord. 2013;14:263. doi: 10.1186/1471-2474-14-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4(4):743–757. doi: 10.2147/NDT.S2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654 [DOI] [PubMed] [Google Scholar]
- 25.Obeso JA, Rodrıguez-Oroz MC, Benitez-Temino B, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000;23(10 Suppl):S8–S19. doi: 10.1016/S1471-1931(00)00028-8 [DOI] [PubMed] [Google Scholar]
- 26.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25(5):578–586. doi: 10.1002/mds.22735 [DOI] [PubMed] [Google Scholar]
- 27.PDF. Parkinson´ s disease foundation. Understanding Parkinsons´s. Statistics on Parkinson´s; 2013. Available from: http://www.pdf.org/en/parkinson_statistics; 2014.
- 28.Borrione P, Tranchita E, Sansone P, et al. Effects of physical activity in Parkinson’s disease: a new tool for rehabilitation. World J Methodol. 2014;4(3):133–143. doi: 10.5662/wjm.v4.i3.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharififar S, Coronado RA, Romero S, et al. The effects of whole body vibration on mobility and balance in Parkinson disease: a systematic review. Iran J Med Sci. 2014;39(4):318–326. [PMC free article] [PubMed] [Google Scholar]
- 30.Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol. 2015;11(3):212–219. doi: 10.3988/jcn.2015.11.3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klamroth S, Steib S, Devan S, et al. Effects of exercise therapy on postural instability in Parkinson disease: a meta-analysis. J Neurol Phys Ther. 2016;40(1):3–14. doi: 10.1097/NPT.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 32.Tuon T, Valvassori SS, Dal Pont GC, et al. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull. 2014;108:106–112. doi: 10.1016/j.brainresbull.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Reynolds GO, Otto MW, Ellis TD, et al. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson’s disease. Mov Disord. 2016;31(1):23–38. doi: 10.1002/mds.26484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamotte G, Rafferty MR, Prodoehl J, et al. Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: a review. J Parkinsons Dis. 2015;5(1):21–41. doi: 10.3233/JPD-140425 [DOI] [PubMed] [Google Scholar]
- 35.Ramazzina I, Bernazzoli B, Costantino C. Systematic review on strength training in Parkinson’s disease: an unsolved question. Clin Interv Aging. 2017;12:619–628. doi: 10.2147/CIA.S131903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine (Baltimore). 2015;94(4):e 411. doi: 10.1097/MD.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhrbrand A, Stenager E, Pedersen MS, et al. Parkinson’s disease and intensive exercise therapy – a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci. 2015;353(1–2):9–19. doi: 10.1016/j.jns.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 38.Dibble LE, Hale TF, Marcus RL, et al. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: a preliminary study. Parkinsonism Relat Disord. 2009;15(10):752–757. doi: 10.1016/j.parkreldis.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 39.Robichaud JA, Pfann KD, Comella CL, et al. Effect of medication on EMG patterns in individuals with Parkinson’s disease. Mov Disord. 2002;17(5):950–960. doi: 10.1002/mds.10218 [DOI] [PubMed] [Google Scholar]
- 40.Vaillancourt DE, Prodoehl J, Verhagen Metman L, et al. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057 [DOI] [PubMed] [Google Scholar]
- 41.Vaillancourt DE, Prodoehl J, Sturman MM, et al. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Mov Disord. 2006;21(1):50–58. doi: 10.1002/mds.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427. doi: 10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- 44.Brusse KJ, Zimdars S, Zalewski KR, et al. Testing functional performance in people with Parkinson disease. Phys Ther. 2005;85(2):134–141. doi: 10.1093/ptj/85.2.134 [DOI] [PubMed] [Google Scholar]
- 45.Podsiadlo Dand RS. The timed “Up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 46.Rikli RE, Jones CJ. Functional fitness normative scores for community residing older adults ages 60–94. J Aging Phys Act. 1999;7:160–179. [Google Scholar]
- 47.Stebbins GT, Goetz CG. Factor structure of the unified Parkinson’s disease rating scale: motor examination section. Mov Disord. 1998;13(4):633–636. doi: 10.1002/(ISSN)1531-8257 [DOI] [PubMed] [Google Scholar]
- 48.Buck PO, Wilsonb RE, Seebergerc LC, et al. Examination of the UPDRS bradykinesia subscale: equivalence, reliability and validity. J Parkinsons Dis. 2011;(3):253–258. doi: 10.3233/JPD-2011-11035 [DOI] [PubMed] [Google Scholar]
- 49.Bottaro M, Russo AF, Oliveira RJ. The effects of rest interval on quadriceps torque during an isokinetic testing protocol in elderly. J Sports Sci Med. 2005;4(3):285–290. [PMC free article] [PubMed] [Google Scholar]
- 50.Scandalis TA, Bosak A, Berliner JC, et al. Resistance training and gait function in patients with Parkinson’s disease. Am J Phys Med Rehabil. 2001;80:38–43. doi: 10.1097/00002060-200101000-00011 [DOI] [PubMed] [Google Scholar]
- 51.Dibble LE, Hale T, Marcus RL, et al. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord. 2006;21(9):1444–1452. doi: 10.1002/mds.20997 [DOI] [PubMed] [Google Scholar]
- 52.Inkster LM, Eng JJ, MacIntyre DL, et al. Leg muscle strength is reduced in Parkinson’s disease and relates to the ability to rise from a chair. Mov Disord. 2003;18:157–162. doi: 10.1002/mds.10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corcos DM, Chen CM, Quinn NP, et al. Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112 [DOI] [PubMed] [Google Scholar]
- 54.Schilling BK, Pfeiffer RF, LeDoux MS, et al. Effects of moderate-volume, high-load lower-body resistance training on strength and function in persons with Parkinson’s disease: a pilot study. Parkinsons Dis. 2010:ID 824734. doi: 10.4061/2010/824734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blankevoort CG, van Heuvelen MJ, Boersma F, et al. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dementia Geriatric Cognit Disord. 2010;30:392–402. doi: 10.1159/000321357 [DOI] [PubMed] [Google Scholar]
- 56.Sale DG, Martin JE, Moroz DE. Hypertrophy without increased isometric strength after weight training. Eur J Appl Physiol Occup Physiol. 1992;64(1):51–55. doi: 10.1007/BF00376440 [DOI] [PubMed] [Google Scholar]
- 57.Moritani T, Devries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–130. [PubMed] [Google Scholar]
- 58.Hirsch MA, Toole T, Maitland CG, et al. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(8):1109–1117. doi: 10.1016/S0003-9993(03)00046-7 [DOI] [PubMed] [Google Scholar]
- 59.Edström L. Selective changes in the sizes of red and white muscle fibres in upper motor lesions and Parkinsonism. J Neurol Sci. 1970;11(6):537–550. doi: 10.1016/0022-510X(70)90104-8 [DOI] [PubMed] [Google Scholar]
- 60.Rossi B, Siciliano G, Carboncini M, et al. Muscle modifications in Parkinson’s disease: myoelectric manifestations. Electroencephalogr Clin Neurophysiol. 1996;101(3):211–218. doi: 10.1016/0924-980X(96)94672-X [DOI] [PubMed] [Google Scholar]
- 61.Pfann KD, Buchman AS, Comella CL, et al. Control of movement distance in Parkinson’s disease. Mov Disord. 2001;16(6):1048–1065. doi: 10.1002/mds.1220 [DOI] [PubMed] [Google Scholar]
- 62.Teasdale N, Phillips J, Stelmach GE. Temporal movement control in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:862–868. doi: 10.1136/jnnp.53.10.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monteiro-Junior RS, Cevada T, Oliveira BR, et al. We need to move more: neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses. 2015;85(5):537–541. doi: 10.1016/j.mehy [DOI] [PubMed] [Google Scholar]
- 64.Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol. 2002;544:641–652. doi: 10.1113/jphysiol.2002.024463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 66.Griesbach GS, Hovda DA, Molteni R, et al. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic fator upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030 [DOI] [PubMed] [Google Scholar]
- 67.Matsuda F, Sakakima H, Yoshida Y. The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Acta Physiol (Oxf). 2011;201:275–287. doi: 10.1111/j.1748-1716.2010.02174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutoo DE, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13:1–14. doi: 10.1016/S0969-9961(03)00030-5 [DOI] [PubMed] [Google Scholar]
- 69.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: manyreasons to move. Neuropsychobiology. 2009;59:191–198. doi: 10.1159/000223730 [DOI] [PubMed] [Google Scholar]
- 71.Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. doi: 10.1111/j.1600-0404.2010.01360.x [DOI] [PubMed] [Google Scholar]
- 72.Foley T, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromol Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- PDF. Parkinson´ s disease foundation. Understanding Parkinsons´s. Statistics on Parkinson´s; 2013. Available from: http://www.pdf.org/en/parkinson_statistics; 2014.