Abstract
Hydrogen sulfide (H2S) is involved in numerous pathophysiological processes and shares overlapping functions with CO and •NO. However, the importance of host-derived H2S in microbial pathogenesis is unknown. Here we show that Mtb-infected mice deficient in the H2S-producing enzyme cystathionine β-synthase (CBS) survive longer with reduced organ burden, and that pharmacological inhibition of CBS reduces Mtb bacillary load in mice. High-resolution respirometry, transcriptomics and mass spectrometry establish that H2S stimulates Mtb respiration and bioenergetics predominantly via cytochrome bd oxidase, and that H2S reverses •NO-mediated inhibition of Mtb respiration. Further, exposure of Mtb to H2S regulates genes involved in sulfur and copper metabolism and the Dos regulon. Our results indicate that Mtb exploits host-derived H2S to promote growth and disease, and suggest that host-directed therapies targeting H2S production may be potentially useful for the management of tuberculosis and other microbial infections.
Subject terms: Bacterial pathogenesis, Pathogens, Tuberculosis
The importance of host-produced hydrogen sulfide (H2S) in microbial pathogenesis is poorly understood. Here, Saini et al. show that H2S alters Mycobacterium tuberculosis (Mtb) central metabolism, stimulates respiration to promote growth and TB disease, and upregulates the Dos regulon.
Introduction
Mycobacterium tuberculosis (Mtb), the etiological agent of TB, can persist in a clinically latent disease state for decades. Identifying host factors that influence Mtb survival is essential for understanding how Mtb persists. Physiologic gaseous messengers such as carbon monoxide (CO) and nitric oxide (•NO) are important to the outcome of Mtb infection. In particular, •NO produced by inducible nitric oxide synthase (iNOS) exerts a bacteriostatic effect on Mtb by inhibiting respiration1 and is crucial for protection against TB2,3. Notably, •NO1, CO4 and lowoxygen (O2) levels5 induce the expression of the 48-gene Dos dormancy regulon, which is associated with reduced respiration and bacterial replication. Hydrogen sulfide (H2S) is an emerging signaling molecule that is produced enzymatically in mammals by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST)6,7. At physiological pH, H2S exists mainly as a hydrosulfide anion (HS−, 75−80%), with the remainder in the form of the dissolved uncharged H2S gas (~20−25%) and negligible amounts of S2− 8. Here, unless specified, we refer to the sulfide pool (H2S + HS− + S2−) as H2S.
H2S modulates various physiological functions including inflammation, metabolism, neuroprotection, vasodilation9, and protection of microbes against antibiotics10,11. Surprisingly, despite having several overlapping functions with CO and •NO8,12, a role for host-generated H2S in bacterial pathogenesis has not yet been described. H2S can modulate mitochondrial respiration resulting in a suspended animation-like state in mice13. In mammals, H2S elicits a biphasic, concentration-dependent mitochondrial response14, which can be cytotoxic or cytoprotective. For example, at high concentrations H2S reversibly inhibits cytochrome c oxidase (Complex IV)15–17. In contrast, at low concentrations H2S can serve as bioenergetic fuel to stimulate mitochondrial respiration without uncoupling of respiration16,18. Due to the complex chemistry of H2S and lack of routine assays for accurate measurement, the physiological concentration of H2S in vivo has been a matter of debate19–21. Here we hypothesize that host-derived H2S plays a role in TB through modulation of Mtb respiration and bioenergetics. To test this hypothesis, we performed Mtb infection studies in CBS-deficient (Cbs+/−) mice, which have reduced H2S levels. We also examined the role of CBS in inflammation, determined the total sulfide levels in infected mice, and examined how H2S modulates Mtb growth, gene expression, metabolism and respiration.
Results
Mtb infection alters CBS levels in macrophages
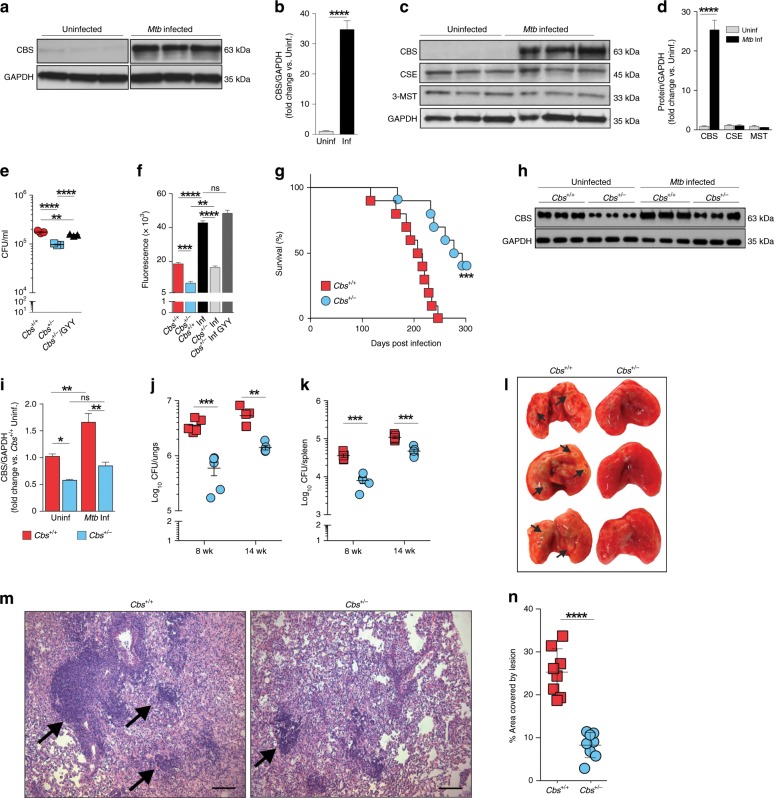
Infection of RAW 264.7 macrophages with Mtb H37Rv resulted in a 34-fold increase in the level of CBS (Fig. 1a, b), a major enzymatic source of H2S in mammalian cells22,23. This suggested that increased H2S production is a host response to Mtb infection. To investigate the role of H2S in Mtb virulence and pathogenicity, we used mice deficient in H2S production due to disruption of Cbs, the gene encoding CBS22,23. Since the Cbs−/− genotype is perinatally lethal, we used heterozygous (Cbs+/−) mice that have a normal life span and show an ~50% reduction in CBS levels22. Infection of Cbs+/+ (WT) peritoneal macrophages resulted in a 25-fold increase in the level of CBS, whereas the levels of CSE and 3-MST did not change appreciably (Fig. 1c, d). Bacterial recovery from Mtb-infected Cbs+/− peritoneal macrophages was reduced compared to WT cells (Fig. 1e), which correlates with lower H2S levels in these cells as measured by an H2S-specific probe (Fig. 1f). Mtb recovery from Cbs+/− macrophages treated with the H2S donor GYY413724 was increased compared to Cbs−/+ cells alone (Fig. 1e). GYY4137 slowly releases H2S thereby mimicking physiological release. This result suggests that differences in Mtb recovery are due primarily to H2S. Lastly, we observed no impact on bacterial recovery from infected peritoneal macrophages treated with homocysteine (10, 25 or 50 µM), ruling out mild hyperhomocysteinemia observed in Cbs+/− mice (<15 µM)22,25 as a contributor to reduced bacterial burden (Supplementary Fig. 1).
Fig. 1. Attenuation of Mtb virulence and pathogenicity in Cbs+/− mice.
a Representative western blot and b densitometry analysis of CBS levels in RAW 264.7 macrophages following 6 h of Mtb infection (n = 3). c Representative western blot and d densitometry analysis of H2S-producing enzymes in WT mouse peritoneal macrophages following 6 h of Mtb infection (n = 3 animals per group). e CFU recovery from Mtb-infected peritoneal macrophages isolated from WT and Cbs+/− mice at 36 h post infection. f Relative quantitation of H2S production from uninfected or infected (Inf) peritoneal macrophages using the H2S-specific fluorescent probe WSP128 at 36 h post infection. The Cbs+/− Inf GYY4137 group received 25 µM GYY4137. (e) and (f) represent pooled data from two or more independent experiments (n = 4−6). g Survival of mice following Mtb infection, representative of two independent survival studies (n = 10 animals/group). h Representative western blot and i densitometry analysis of CBS in the lungs of age-matched mice without infection or 8 weeks post Mtb-infection (n = 3 animals per group). j Bacillary burden in lungs and k spleens at 8 and 14 weeks post infection (n = 5 animals per group). l Representative gross images of infected lungs at 8-week post Mtb-infection. Arrows indicate TB granulomatous lesions. m H&E staining of representative infected lung sections at 8 weeks post Mtb-infection. Arrows indicate area of lymphocyte infiltration (scale bar = 100 μm). n Quantitative histochemical appraisal of lesions in infected lungs of WT and Cbs+/− mice. Granulomatous region analysis data from three independent animal experiments with 2−3 mice per experiment. Data are shown as mean ± SEM, except (d) and (n) (mean ± SD). Data (b, d, j, k and n) were analyzed by unpaired parametric t test. Survival data (g) were analyzed by Log-rank (Mantel−Cox) test. Data in (e) and (f) were analyzed by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Data in (i) were analyzed by two-way ANOVA with Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Source data are provided as a Source Data file.
Cbs+/− mice survive longer following Mtb infection
The macrophage infection studies above clearly show decreased bacillary growth in H2S-deficient Cbs+/− cells and that Mtb growth could be chemically complemented with H2S. To more clearly establish the role of H2S in Mtb virulence and pathogenicity, we infected WT and Cbs+/− mice with Mtb and observed that Cbs+/− mice survived significantly longer than WT mice (median time to death 286 days vs. 211 days for WT mice (Fig. 1g)). CBS levels in the lungs of uninfected Cbs+/− mice were significantly lower than in WT, and CBS levels in the lungs of WT mice increased significantly at 8 weeks post infection (Fig. 1h, i).
At 8 and 14 weeks post infection, Cbs+/− mice had ~6-fold and ~4-fold fewer Mtb bacilli in the lungs, respectively, compared to WT mice (Fig. 1j). Similarly, we observed ~4.5-fold and ~2.5-fold fewer bacilli in spleens of Cbs+/− mice compared to WT mice at 8 and 14 weeks post infection, respectively (Fig. 1k). While significant, these modest differences in organ burden contrast with the striking difference in survival (Fig. 1g). Furthermore, gross pathology (Fig. 1l) and histopathology appraisal (Fig. 1m, n) of infected WT and Cbs+/− mice were consistent with the observed organ burden. We noted moderate to large coalescence of multiple granulomatous lesions with infiltration of lymphocytes in the lungs of WT mice whereas the lung architecture in Cbs+/− mice was relatively intact with fewer small granulomatous lesions (Fig. 1m). In sum, our data suggest that Mtb infection of host cells upregulates CBS production, leading to increased H2S levels, which exacerbate disease as indicated by reduced host survival, and to a lesser extent by increased organ burden. These findings identified a new phenotypic category of host genes that exacerbates TB disease whereas their disruption prolongs survival during infection.
Immune responses in Mtb-infected mice
To determine whether reduced bacillary burden and increased survival of Cbs+/− mice could be attributed to immunological differences between Cbs+/+ and Cbs+/− mice, we examined host immune responses in Cbs+/− and WT mice pre- and post-Mtb infection. In uninfected mice (Supplementary Figs. 2A−J, 3B, C), except in the spleens of Cbs+/− mice which showed fewer macrophages (Supplementary Fig. 2F), we observed no significant differences in the percentages of myeloid or lymphoid cell types in the lungs and spleens of Cbs+/− and WT mice, even though the levels of proinflammatory IL-1α were elevated and anti-inflammatory IL-10 were reduced in Cbs+/− compared to WT mice (Supplementary Fig. 3A). To distinguish whether the reduced inflammatory response in Cbs+/− mice during chronic infection is due to lower CBS protein levels or reduced bacillary burden, we analyzed the immune response at an early time after infection (3 weeks) when the lung bacillary burden was similar (Supplementary Fig. 4A), but serum sulfide levels differed between WT, and Cbs+/− mice (Supplementary Fig. 4B). As shown in Supplementary Figs. 2K-R and 3D and E, except for increased percentages of splenic CD4+ T cells (Supplementary Fig. 2P), reduced percentages of CD4+IFN-γ+ T cells (Supplementary Fig. 2R) and reduced percentages of CD11b+Gr-1− myeloid cell in the spleens (Supplementary Fig. 3E) of Cbs+/− mice, there were no significant differences in percentages of myeloid or CD4+ T cells in the lungs and spleens of Cbs+/− and WT mice at 3 weeks post infection. During the chronic stage of infection (8 weeks), cytokine levels in Cbs+/− and WT mice were similar except for a lower TNF-α and IL-10 levels in Cbs+/− mice (Supplementary Fig. 3A), which might be due to reduced percentages of neutrophils and lymphoid cells mostly in the spleen (Supplementary Figs. 2S-Z, 3F and G). We observed no clear predominance of either a Th1 or Th2 response, as indicated by the levels of proinflammatory (IL1α, IL-6, IL-12, INF-γ and TNF-α), anti-inflammatory (IL-5, IL-10) and regulatory (IL-17) cytokines in the sera of Cbs+/− and WT mice (Supplementary Fig. 3A). Overall, these data suggest that the differences in bacillary burden and increased survival in Cbs+/− mice are likely not attributable to immunological differences alone, and are more likely due to direct action of H2S on Mtb.
H2S modulates mycobacterial growth and energy homeostasis
In eukaryotes, depending on the concentration, H2S can either stimulate or inhibit the electron transport chain (ETC) resulting in altered oxidative phosphorylation (OXPHOS) and ATP production16,26. Reported physiological levels of H2S in tissue are controversial because of the assay methods employed and volatility of the gas20. However, the current consensus is that nanomolar concentrations of H2S seem most plausible19–21. Unfortunately, this low concentration is below the limit of detection (1 µM) of routine assays such as the methylene blue, 5,5′-dithiobis-2-nitrobenzoic acid and bismuth chloride assays19,20.
Since our in vivo data suggest that host-generated H2S interacts with Mtb to modulate disease, we tested the hypothesis that H2S directly modulates Mtb growth. For these in vitro studies, we employed GYY4137, a widely used H2S donor that releases low amounts of H2S over a sustained period24,27. Unlike widely used •NO and CO donors such as diethylenetriamine NONOate (DETA NONOate) and CO-releasing molecules (CO-RMs), respectively, GYY4137 is an unusually slow H2S releaser wherein the final molar concentration of H2S released is significantly lower than the starting concentration of GYY4137. These aspects, along with the volatility of the gas20, make detection of H2S by standard assays challenging24,27. Therefore, we used LC-MS/MS to first confirm spent (decomposed) GYY4137, and the decomposition of GYY4137 in the presence of Mtb cells under our conditions into the corresponding molecular ions with m/z of 272.05 (M − H)−, 187.02 (M − H)− and 88.08 (morpholine M + H)+ (Supplementary Fig. 5), and carefully considered the complex physicochemical properties of H2S in the design of our experiments (see Materials and methods).
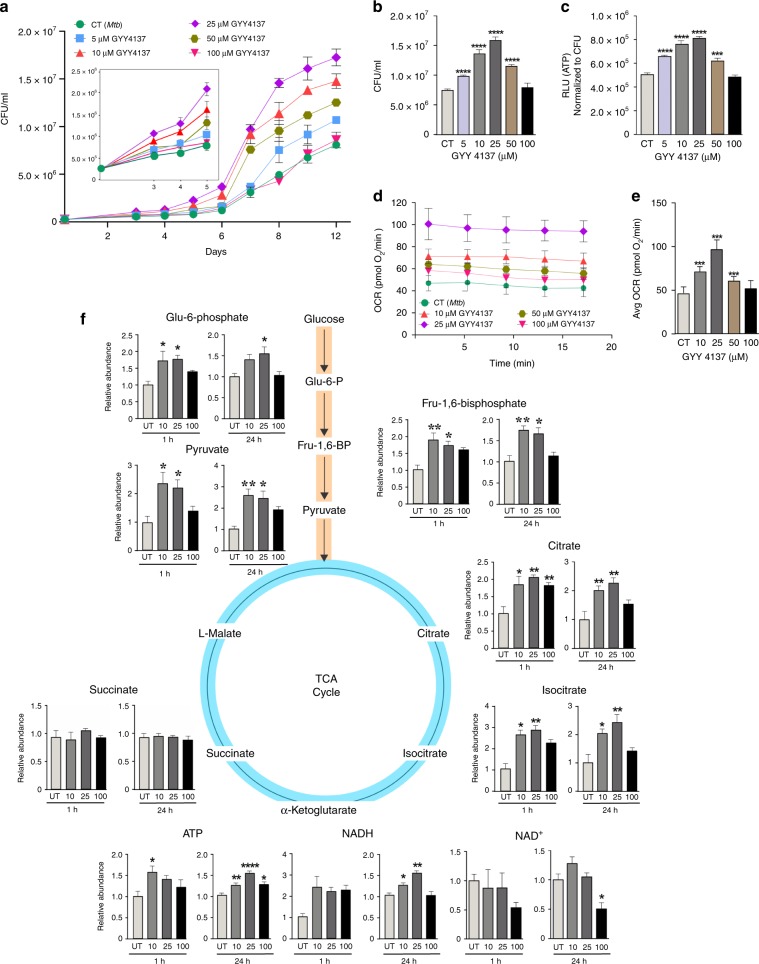
To correlate the amount of H2S generated in vivo with our in vitro experiments, we used a fluorescent H2S-specific probe, WSP1, to measure levels of H2S produced over time by macrophages following Mtb infection28. We then determined which concentrations of GYY4137 generated similar levels of fluorescence with WSP1 over time due to H2S release. We then exposed Mtb cells to this range of GYY4137 concentrations that mimics levels of H2S produced physiologically by macrophages following infection (Supplementary Fig. 6A, Fig. 1f). As expected, 5, 10, and 25 µM GYY4137 significantly stimulated growth. At day 12, maximum bacterial growth was observed with 25 µM GYY4137 (Fig. 2a, b). In contrast, 50 and 100 µM GYY4137 reduced the growth stimulation in a dose-dependent manner (Fig. 2a, b). Notably, we observed no growth change in Mtb control cultures exposed to 25 µM spent GYY4137, comprised of components of the chemical backbone alone (Supplementary Figs. 5 and 6B). These data establish that low levels of sulfide exert a concentration-dependent bimodal effect on Mtb growth.
Fig. 2. GYY4137 modulates Mtb growth, bioenergetics and ATP production.
a CFU-based growth profile of Mtb following incubation with GYY4137. Inset shows early Mtb growth. b Mean CFU values at day 12 of the growth analysis. c ATP levels in Mtb following 24 h exposure to GYY4137. Data (a−c) are representative of at least two independent experiments. d OCR profile (n = 4−6) and e average OCR of Mtb exposed to GYY4137 (n = 20−30). Control (CT) groups in panels (a−e) received spent GYY4137. f LC-MS targeted metabolic analysis of Mtb with or without exposure to 10, 25 and 100 μM GYY4137 (for 1 or 24 h). Graphs show relative abundance (peak area) of metabolites in Mtb cells (n ≥ 3 per group). Data are shown as mean ± SD, except (b) and (f) (mean ± SEM). Data in (a) were analyzed using two-way ANOVA with Tukey’s multiple comparisons test, with day 12 data shown in (b), or one-way ANOVA (c−f) with Sidak’s multiple comparisons test. All statistical differences are relative to untreated controls. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Source data are provided as a Source Data file.
Since enhanced growth is coupled to increased OXPHOS and ATP levels, and low levels of H2S can stimulate mitochondrial respiration14,18, we hypothesized that H2S targets the Mtb ETC to modulate energy metabolism. We exposed Mtb cultures to GYY4137 and observed a dose-dependent increase in ATP levels, which was lost in cells exposed to 100 µM GYY4137 (Fig. 2c). Next, we performed extracellular flux analysis, which we have adapted to analyze Mtb bioenergetics29–31, to determine the effect of H2S on Mtb respiration in real time in a noninvasive manner. Again, we observed a bimodal response to GYY4137, wherein low concentrations resulted in a significant dose-dependent increase in the oxygen consumption rate (OCR) and 100 µM GYY4137 mitigated the increased OCR (Fig. 2d, e). Importantly, spent GYY4137 did not alter the basal OCR of Mtb cells (Supplementary Fig. 6C). The effects of sulfide on mycobacterial OCR were independently validated using another donor, NaHS (Supplementary Fig. 6D) and by employing an Oroboros O-2k respirometer with the nonvirulent vaccine strain Mycobacterium bovis BCG. The bimodal effect of H2S on growth was also recapitulated with NaHS in Mycobacterium bovis BCG (Supplementary Fig. 6E) and Mtb (Supplementary Fig. 6F).
In sum, our results demonstrate that low levels of H2S target the ETC to increase respiration and ATP levels, leading to increased growth. The capacity of H2S to positively regulate the ETC, a key component of central metabolism, to induce a hypermetabolic state is likely to benefit Mtb during early infection.
H2S stimulates Mtb central metabolism
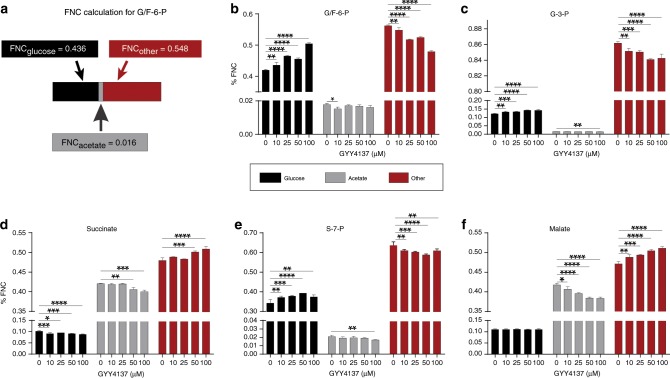
Since respiration is tightly linked to central energy metabolism, we investigated the effect of H2S on Mtb metabolism in more detail. We performed LC-MS/MS-based targeted metabolomic analysis of Mtb cells at 1 and 24 h post-exposure to GYY4137 and observed increased levels of the glycolytic intermediates glucose-6-phosphate, fructose-1,6-bisphosphate and pyruvate. Increased levels of these metabolites in the presence of excess ATP demonstrates augmented glycolytic flux (Fig. 2f), since this pathway would be otherwise inhibited when excess ATP is present32. Levels of the TCA cycle intermediates citrate and isocitrate were also increased, suggesting that feedback inhibition due to elevated NADH levels is not occurring (Fig. 2f). TCA cycle intermediates such as citrate also augment fatty acid biosynthesis to support mycobacterial growth32. Increased NADH, which feeds into the ETC to support increased ATP production is consistent with the ability of H2S to stimulate growth (Fig. 2a, b) and respiration (Fig. 2d, e). It should be noted that small changes in absolute levels of metabolite pools can have critical implications for metabolism, as this points to dysregulation of metabolic balance that must be maintained at all costs33,34. Carbon rearrangement in metabolites is an early indicator of metabolic dysregulation prior to changes in abundance and can be measured using stable isotopes. Therefore, to further examine how H2S reprograms Mtb metabolism, we performed a series of carbon tracing experiments. We first confirmed that the mass distribution vectors (MDV) of glutamate and α-ketoglutarate were identical, thereby indicating that isotopic steady state had been reached under our culture conditions (Supplementary Fig. 7A, B). To determine the fuel source (glucose, acetate, or other endogenous carbon sources (e.g., amino acids)) contributing to carbon fractions in glycolytic and TCA metabolites, we monitored the fate of the uniformly labeled stable isotope [13C6] glucose and [13C2] acetate, and calculated the fractional nutrient contribution (FNC; Fig. 3a)33. We found that exposure to GYY4137 reroutes carbon away from downstream glycolytic intermediates towards cell wall synthesis and the pentose phosphate pathway (PPP) in a dose-dependent manner. For example, increasing concentrations of GYY4137 increased glucose carbon flow into glucose/fructose-6-phosphate (G/F6P) relative to the spent GYY4137 control and reciprocally reduced carbon flow from other endogenous supplementary carbon sources into this metabolite (Fig. 3b). Secondly, an increase in the FNC of glucose into glycerol-3-phosphate (G3P) and sedoheptulose-7-phosphate (S7P) suggests reprogramming of metabolism towards cell wall biosynthesis and the PPP, which produces NADPH and nucleosides necessary for lipid anabolism and DNA synthesis, respectively (Fig. 3c, e). Notably, the total FNC from other carbon sources (~84%) into G3P was significantly higher than the FNC from glucose (~12%) and acetate (~1%; Fig. 3c). Thirdly, increased levels of GYY4137 reduced entry of glucose or acetate carbons into the TCA cycle intermediate succinate, whereas the FNC of other carbon sources to succinate is increased (Fig. 3d). Interestingly, entry of glucose carbons into malate was unchanged (Fig. 3f), whereas entry of acetate carbons into malate and succinate was reduced with the reciprocal increase from other carbon sources (Fig. 3d, f).
Fig. 3. Fractional nutrient contribution (FNC) of glucose and acetate exposed to GYY4137.
a FNC was calculated according to a previous study33; see also Materials and Methods. b, c Glycolysis is stimulated by sulfide as is evident by an increased FNCglucose of G/F6P that corresponds with increased GYY4137 concentration, whereas the FNCacetate and FNCother decreases. The FNCglucose of G-3-P (c) and S-7-P (e) increases with higher concentrations of GYY4137. d, f The utilization of glucose is skewed towards cell wall synthesis and the PPP leading to a decrease in FNCglucose and FNCacetate of succinate and malate as GYY4137 concentration increases, as well as a corresponding increase in FNCother of these two metabolites. Data are representative of two independent experiments (n = 5) and are shown as mean ± SD. Data were analyzed using one-way ANOVA with Sidak’s multiple comparisons test. All P values are relative to untreated controls (0 µM GYY4137) for individual metabolites. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Source data are provided as a Source Data file.
Overall, the carbon tracing data indicate that enhanced energy flux through glycolysis, the PPP and TCA cycle following H2S exposure increases Mtb energy metabolism and anabolic substrate intermediates for growth. Lastly, the FNC data demonstrate that H2S rewires energy flux through these pathways by modulating the fraction of the metabolite’s carbon produced from the respective fuel source.
H2S exerts it bioenergetic effect predominantly through CytBD
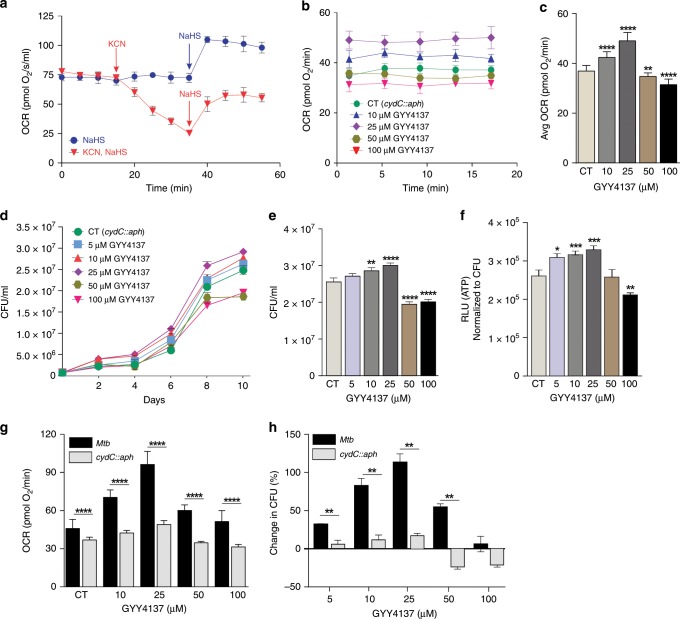
We next examined how H2S stimulates Mtb respiration and growth. Mycobacteria possess two terminal respiratory oxidases that provide substantial respiratory flexibility as electrons from the menaquinone pool can be channeled either to an aa3-type cytochrome c oxidase via the cytochrome bc1 (cytochrome bc1/aa3) complex or directly to cytochrome bd-type menaquinol oxidase (CytBD)35. CytBD can partially compensate for a loss in activity of the bc1-aa3 complex36, and is required during the transition from acute to chronic Mtb infection in mouse lungs to promote bacterial virulence37. Potassium cyanide (KCN) inhibits the aa3-type cytochrome c oxidase, but not CytBD in Mycobacterium smegmatis (Msm)35. Also, CytBD in E. coli has been shown to be less sensitive to cyanide and sulfide than aa3-type cytochrome c oxidase38. To determine whether H2S exerts its effect through CytBD or the bc1-aa3 complex, we exposed Msm cells to KCN and/or NaHS in an Oroboros O-2k respirometer. We observed that addition of NaHS to Msm leads to an increase in basal OCR (Fig. 4a), consistent with the effects of H2S on Mtb. In contrast, addition of KCN resulted in a steady decline in the basal OCR of Msm cells. Interestingly, addition of NaHS to KCN-treated cells triggered an increase in OCR (Fig. 4a). To define the effect of H2S on Mtb growth and ATP production in more detail, we used Mtb rv1620c::aph (hereafter referred to as cydC::aph), which contains the kanamycin resistance gene (aph) inserted in cydC (rv1620c) as described37. Mtb cydC encodes a membrane protein linked to the incorporation of a heme cofactor into CytBD. Mtb cydC::aph is defective in CytBD activity and shares the phenotype of Mtb cydAB mutants30,39. Importantly, Mtb cydC::aph produces only a functional cytochrome bc1/aa340. As a control, we used a complemented strain, Mtb cydC::aph-Comp, obtained by expressing cydC from an integrated plasmid. Effective complementation was demonstrated by inhibiting cytochrome bc1 using Q203 as described30 (Supplementary Fig. 8A), which reroutes electrons to CytBD leading to an increase in OCR (Supplementary Fig. 8B and C). Complementation was also demonstrated by the marked increase in OCR of WT Mtb and Mtb cydC::aph-Comp cells compared to Mtb cydC::aph in the presence of 25 and 100 µM GYY4137 (Supplementary Fig. 8D and E). Although E. coli cydD mutants were shown to be susceptible to high (20 mM) concentrations of cysteine41, we found that Mtb cydC::aph is not susceptible to physiological levels of cysteine (~128 and ~250 µM in human cells and plasma, respectively)42,43 (Supplementary Fig. 9). Next, we measured the OCR in cydC::aph cells following exposure to GYY4137 and observed a small, but significant increase in OCR at 10 and 25 μM GYY4137 compared to control cells receiving spent donor (Fig. 4b, c). Notably, at higher GYY4137 concentrations (≥50 μM), not only is the stimulatory effect on the OCR lost, but the OCR is repressed below the basal level, indicating an inhibition of cytochrome bc1/aa3 oxidase in cydC::aph cells (Fig. 4b, c). CydC::aph cells exposed to GYY4137 displayed a modest bimodal response as shown by a moderate increase in growth and ATP levels at 10 and 25 μM GYY4137, but significant suppression at higher GYY4137 levels (≥50 μM) (Fig. 4d–f). In contrast, the basal OCR and growth of GYY4137-treated WT Mtb cells remained significantly higher than cydC::aph cells at 50 and 100 μM GYY4137 (Figs. 2d, e, 4b, c). In summary, the significantly reduced stimulation of OCR and growth in cydC::aph cells (Fig. 4g, h), i.e., when respiration is sustained only by aa3-type cytochrome c oxidase, indicates that H2S exerts its respiratory and growth-stimulatory effects predominately via CytBD in Mtb.
Fig. 4. H2S stimulates Mtb bioenergetics primarily through cytochrome bd-type quinol oxidase.
a Representative OCR profile of M. smegmatis (Msm) cells. Arrows indicate the time of addition of KCN (100 μM) or NaHS (25 μM) (n = 5). b Representative OCR profile and c average OCR of cydC::aph cells exposed to GYY4137 (n = 12−18, data pooled from at least two independent experiments). d CFU-based growth profile of cydC::aph cells following exposure to GYY4137. e Mean cydC::aph CFU values at day 10 of the growth analysis. f ATP levels in cydC::aph after 24 h exposure to GYY4137. Data (d−f) are representative of at least two independent experiments. g Comparison of the dose-dependent effect of GYY4137 on the OCR of Mtb and cydC::aph cells (from Figs. 2e and 4c). h Percent change in CFU of Mtb and cydC::aph (e) following exposure to increasing concentrations of GYY4137 (from Figs. 2b and 4e). The percent change was calculated against the CFU of control cells at day 10 following GYY4137 exposure. Data are shown as mean ± SD, except (e) (mean ± SEM). All comparisons are against control cells (CT) exposed to spent GYY4137 except in (g) and (h), which compare Mtb and cydC::aph at equivalent GYY4137 concentrations. Data were analyzed using two-way ANOVA (b, d) with Sidak’s multiple comparisons test, with average OCR (c) or CFU at day 10 (e). Other data were analyzed by one-way ANOVA with Sidak’s multiple comparisons test (f) or by using an unpaired parametric t test for comparisons at each GYY4137 concentration (g and h). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are provided as a Source Data file.
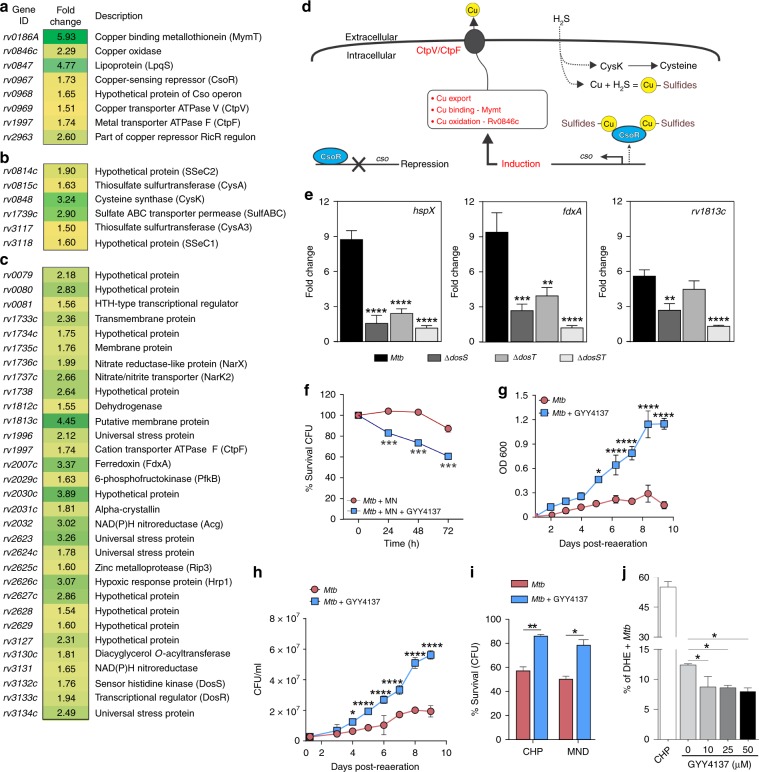
H2S modulates sulfur/copper metabolism and the Dos regulon
To assess the effect of H2S on the Mtb transcriptome, we performed RNA-seq analysis on Mtb and cydC::aph cells exposed to 25 µM GYY4137 for 1 h. Culture conditions were carefully controlled to eliminate the possibility of respiration-induced hypoxia (see Materials and methods). At 25 µM GYY4137, we observed a distinct transcriptomic profile of 92 differentially regulated genes in Mtb compared to untreated cells including those belonging to metabolism and respiration (26/92), and cell wall and cell processes (19/92) (Supplementary Fig. 10A, B). Notably, several members of the CsoR44 and RicR45 copper regulons, and six genes involved in cysteine/sulfur metabolism were upregulated (Fig. 5a, b). RNAseq analysis showed no differentially regulated genes in cydC::aph cells exposed to 25 µM GYY4137 compared to untreated cydC::aph cells, consistent with our observations that H2S exerts its effect primarily through CytBD.
Fig. 5. H2S induces transcriptome remodeling and mitigates oxidative stress in Mtb.
RNA-seq-based gene expression analysis shows induction of the Mtb a copper, b sulfur and c Dos regulons following exposure to 25 µM GYY4137 for 1 h. Genes exhibiting ≥1.5-fold (+/–) change in expression compared to the expression in untreated cells were considered differentially regulated (n = 3 independent biological replicates per group). d Proposed model detailing the relationship between H2S and induction of the Mtb copper regulon. Exposure to H2S leads to the formation of copper sulfides, which relieve repression of CsoR leading to the export of copper via the cso. e Representative results of qRT-PCR analysis of key dormancy regulon genes following 1 h exposure to 25 µM GYY4137 in Mtb H37Rv, ΔdosS, ΔdosT and ΔdosST (n = 5 independent biological replicates per Mtb strain). Fold change in gene expression following addition of GYY4137 is shown. f Survival of Mtb cells (% CFU) exposed to metronidazole (MN) alone, or MN and 25 µM GYY4137. g Recovery profile of Mtb cells following prolonged hypoxia (8 weeks). Prior to hypoxia, one group of cells received 25 µM GYY4137. h Mean CFUs following 1 week of recovery following prolonged hypoxia. (g) and (h) represent pooled data from two or more independent experiments (n = 4). i Percentage survival (CFU) of 25 µM GYY4137-treated Mtb cells following exposure to oxidative stressors CHP and MND (0.5 mM for 6 h) relative to untreated cells. j Percent of dihydroethidium (DHE)-positive ROS-producing Mtb cells following exposure to 10−50 µM GYY4137 (spent GYY4137 used as 0 µM control; n = 3 per group). CHP treatment (15 mM) was used as a positive control for detection of ROS-positive cells. Data in (f) and (i) are representative of two or more experiments. All data are shown as mean ± SD, except (e) (mean ± SEM). Data were analyzed using one-way ANOVA (e and j) with Sidak’s multiple comparisons test, or by two-way ANOVA with Sidak’s multiple comparisons test (f−h), or by unpaired parametric t test (i). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Source data are provided as a Source Data file.
An important finding was that 31 of the 48 genes comprising the Mtb Dos dormancy regulon1, which includes the heme sensor kinase DosS, the response regulator DosR, as well as hspX, fdxA, narX, narK2, tgs1 and others were significantly upregulated (Fig. 5c). Interestingly, the lack of CsoR was shown to upregulate the Dos dormancy regulon44. CsoR is a negative regulator of the copper sensitive operon cso whose repression is relieved upon copper binding that leads to the export of copper46. We propose that binding of copper to sulfide relieves repression of CsoR, which leads to the export of copper via the cso (Fig. 5d).
To determine whether H2S regulates the Dos regulon via the Dos sensor kinases, we performed qRT-PCR analysis of WT Mtb and Mtb mutants lacking dosS (ΔdosS), dosT (ΔdosT) or both (ΔdosST) after exposure to 25 µM GYY4137 for 1 h. Quantitative RT-PCR analysis of ΔdosS, ΔdosT and ΔdosST strains showed significantly reduced induction of key dormancy regulon genes such as hspX, fdxA, and rv1813c (Fig. 5e)1. These findings are consistent with previous studies showing that the copper regulator, CsoR regulates the Dos regulon44, and that cysteine stimulates Mtb respiration leading to the induction of the CsoR copper and Dos regulons47.
In sum, our findings provide further credence to the proposed link between sulfide/cysteine-mediated stimulation of respiration, the Dos regulon, and copper homeostasis. The lack of differential gene expression in cydC::aph cells compared to cells treated with GYY4137 points to CytBD as an important component of the H2S energy-transduction pathway. Our data suggest that upon exposure to sulfide, Mtb reprograms its metabolism and transcriptional machinery including the copper regulon to mitigate the formation of copper sulfide species. Finally, our data suggest that in addition to •NO, CO and low O2, we have identified a fourth gas, H2S, that can induce the Dos dormancy regulon.
Role of H2S during Mtb growth under hypoxia
To determine whether growth-stimulatory levels of H2S accelerate the development of hypoxia-induced, nonreplicating persistence in vitro, we used the anti-TB drug metronidazole (MN), which selectively kills Mtb cells under conditions of low O248. We posited that if H2S facilitates early entry into hypoxic dormancy, then GYY4137-exposed Mtb cells in the presence of MN would be killed more quickly than unexposed cells in the presence of MN. Indeed, we observed a ~20% and ~40% reduction in CFU of GYY4137-exposed Mtb cultures in the presence of MN at 24 and 72 h, respectively (Fig. 5f). In contrast, the viability of unexposed cells was not affected by MN treatment for 24 or 48 h and a slight reduction (~10%) was seen at 72 h. These results show that H2S can facilitate early entry of Mtb cells into hypoxic dormancy. Next, we subjected Mtb cultures to hypoxia for 8 weeks with or without initial exposure to GYY4137. Following re-aeration, Mtb cells previously exposed to GYY4137 displayed enhanced recovery from hypoxia as shown by more robust growth compared to unexposed cells (Fig. 5g, h). This outcome is consistent with our observation that exposure to low levels of H2S triggers an antioxidant response evident by upregulation of genes involved in cysteine metabolism (Fig. 5a, b) and antioxidant genes such as sodA and ahpC49,50 (Supplementary Fig. 10A), which permit rapid emergence from hypoxia. We subsequently tested the ability of H2S to protect Mtb under conditions of oxidative stress and found that survival of Mtb cells exposed to GYY4137 was significantly increased compared to unexposed cells in the presence of the oxidants cumene hydroperoxide (CHP) and menadione (MND) (Fig. 5i). Since H2S can act as an antioxidant51,52, H2S exposure may help mitigate oxidative stress during growth or when Mtb emerges from hypoxia.
We next evaluated whether increased respiration following H2S exposure may result in increased ROS production in Mtb cells. We examined the effect of GYY4137 on ROS production during respiration and observed a slight reduction in the percentage of ROS-producing cells following GYY4137 exposure (Fig. 5j), which is likely due to the known antioxidant effect of H2S. These data are consistent with the growth-promoting effect of H2S on Mtb cells, as excessive ROS production will inhibit growth. We also excluded the possibility that H2S exerts a direct effect on the ETC via a sulfide quinone oxidoreductase (SQR) since the OCR of the Mtb sqr mutant (rv0331::aph; TARGET, Johns Hopkins University, USA) exposed to H2S was similar to that of WT Mtb (Supplementary Fig. 11A). This contrasts with Mtb exposure to the reductant DTT, which increases the menaquinol pool to stimulate OCR (Supplementary Fig. 11B)53, but not growth54. Overall, our data demonstrate a beneficial role for H2S during entry and emergence from low-oxygen conditions, as well as protection against oxidative stress.
Crosstalk between H2S and •NO alters Mtb infection
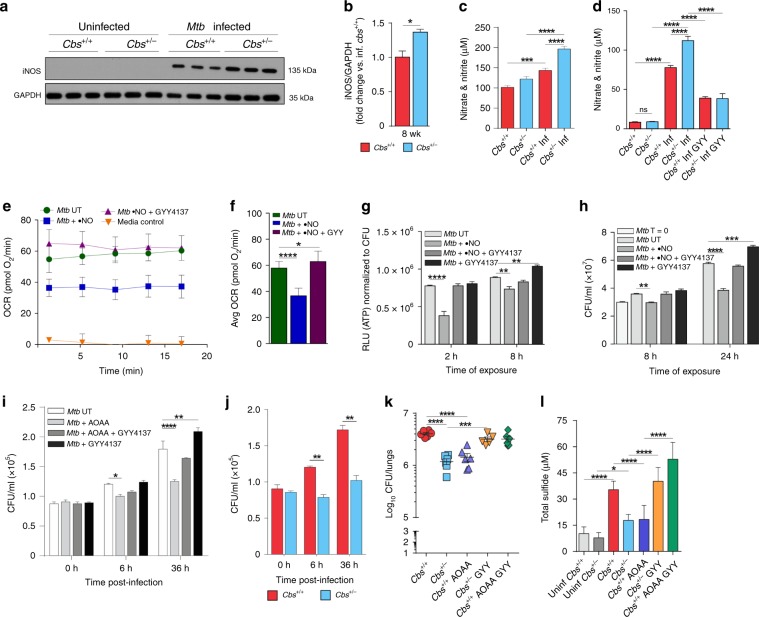
Studies in mammalian systems have shown crosstalk between •NO and H2S55,56. We observed increased iNOS levels in the lungs of Mtb-infected Cbs+/− mice compared to WT at 8 weeks post infection (Fig. 6a, b). Consistent with increased iNOS levels, infected Cbs+/− mice had higher NO2− + NO3− levels in serum compared to WT mice, suggesting increased •NO production in Cbs+/− mice (Fig. 6c). Increased NO2− + NO3− levels were also observed in the culture media of infected Cbs+/− macrophages (Fig. 6d). Addition of GYY4137 significantly lowered NO2− + NO3− levels in WT and Cbs+/− macrophages (Fig. 6d). These observations are consistent with the ability of H2S to regulate •NO bioavailability in vivo, including during inflammation57,58. By itself, •NO exerts bacteriostatic effects on Mtb by inhibiting respiration1, and increased •NO may also contribute to inhibition of bacterial respiration and growth during infection. As expected, exposure of Mtb to •NO in vitro decreased basal OCR (Fig. 6e, f). Intriguingly, the OCR was restored following exposure to GYY4137 (Fig. 6e, f). ATP levels were also reduced in Mtb cells following exposure to •NO (Fig. 6g), suggesting inhibition of respiration1. In contrast, the ATP levels of cells exposed first to •NO followed by GYY4137 were similar to those in unexposed cells, corroborating our observation that GYY4137 restored OCR following •NO exposure (Fig. 6e, f). We then performed growth assays using Mtb cells exposed to •NO alone, GYY4137 alone, or •NO and GYY4137. Mtb cells exposed to GYY4137 exhibited significantly increased growth whereas growth was reduced in cells exposed to •NO, confirming the bacteriostatic effect of •NO (Fig. 6h). When GYY4137 was added following •NO exposure, the CFU recovery was similar to that of unexposed cells (Fig. 6h). While the exact chemical nature of the crosstalk between H2S and •NO remains poorly defined, these data suggest that exposure to H2S reverses the bacteriostatic effect of •NO on Mtb respiration and growth.
Fig. 6. Interaction between •NO and H2S and pharmacological modulation of sulfide levels in mice.
a Representative western blot and b densitometry analysis of iNOS in mouse lungs at 8 weeks post infection (n = 3 animals per group). c Nitrate and nitrite levels in the sera of infected (Inf) and uninfected mice and d the culture medium of infected mouse macrophages exposed to 25 µM GYY4137 for 36 h. Data in (c) and (d) are pooled from two independent experiments (n = 7−8 animals per group). e OCR profile and f average OCR of Mtb cells exposed to •NO (DETA/NO; 250 µM), •NO followed by GYY4137 (10 µM), or untreated (UT) in XF media (n = 6−8). Media control contained no cells. g ATP levels (shown as RLU normalized to CFU) in Mtb cells treated (•NO, GYY4137, or •NO and GYY4137) or untreated (UT) for 2 or 8 h. h CFUs after 8 and 24 h of exposure. T = 0 indicates CFU at the start of the experiment. Data in (g) and (h) are representative of at least two independent experiments. i CFU recovered from infected Cbs+/+ peritoneal macrophages receiving one of the following: aminooxyacetic acid (AOAA), AOAA followed by GYY4137, GYY4137 alone, or untreated (UT) (n = 4). j Mtb CFU recovered from mouse peritoneal macrophages. k Bacillary burden in the lungs of mice that received: AOAA, AOAA followed by GYY4137, or GYY4137 alone. AOAA (13 mg/kg, 6 d/wk for 6 weeks) and GYY4137 (25 mg/kg 3 d/wk for 6 weeks) were administered intraperitoneally. Treatment began 2 weeks post-Mtb infection and continued for 6 wks. Data represent two independent experiments (n = 5−6 animals per group). l Total sulfide levels in serum of mice in (k) using the methylene blue method (n = 6−8). Data represent mean ± SEM except in (e, f and l) (mean ± SD). Comparisons are against WT mice or untreated (UT) controls. Data were analyzed by unpaired parametric t test (b, j), one-way ANOVA with Tukey’s multiple comparisons test (c, d, f, k and l) or two-way ANOVA with Sidak’s multiple comparisons test (g−i). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Source data are provided as a Source Data file.
Pharmacological inhibition of CBS in vitro and in vivo
To validate our in vitro findings that H2S stimulates Mtb respiration and growth, we tested whether manipulating H2S levels via pharmacological modulation of H2S-producing enzymes could alter disease progression. First, we infected peritoneal macrophages from WT mice and treated the cells with the CBS inhibitor aminooxyacetic acid (AOAA)14. We observed ~17% and ~30% reductions in CFU from infected macrophages after exposure to AOAA for 6 and 36 h, respectively (Fig. 6i). In contrast, CFU recovery from AOAA-treated WT macrophages exposed to GYY4137 was comparable to recovery from untreated macrophages indicating that H2S itself is primarily responsible for the reduced bacterial burden in AOAA-treated cells (Fig. 6i). Further, within Mtb-infected Cbs+/− peritoneal macrophages, bacillary growth was reduced by ~35% and 40% versus WT cells at 6 and 36 h post infection, respectively (Fig. 6j).
Next, we show that administration of the CBS inhibitor AOAA to WT mice reduces Mtb burden in the lungs to the level of Cbs+/− mice (Fig. 6k). Further, administration of GYY4137 to Cbs+/− mice increased Mtb burden to that of WT mice. Of note, the bacillary burden in the lungs of WT mice treated with AOAA and GYY4137 was similar to the burden in untreated WT mice (Fig. 6k). Determination of sulfide levels in the sera of these same groups of mice revealed that Mtb infection increased sulfide levels (Fig. 6l). Further, we observed that the reduction in serum sulfide observed in Cbs+/− mice and AOAA-treated WT mice was associated with reduced lung bacillary burden (Fig. 6k, l). CFU recovery in Cbs+/− mice and AOAA-treated WT mice following GYY4137 treatment was comparable to that in untreated WT mice, confirming our previous findings that differences in CFU are due to H2S, and not CBS. While it could be argued that AOAA targets Mtb directly, addition of AOAA to Mtb cultures at concentrations as high as 1 mM does not inhibit Mtb growth. However, 2 mM AOAA reduces Mtb proliferation by ~35% (Supplementary Fig. 12). Thus, while our in vitro and in vivo infection data demonstrate that AOAA reduces host H2S production to modulate Mtb infection, we cannot completely exclude the possibility of a direct effect of AOAA on Mtb. Overall, our findings provide evidence that H2S is a modulator of Mtb respiration, and that host-derived H2S is a significant contributor to TB disease.
Discussion
Here we establish a previously unrecognized role for H2S in promoting TB, wherein increased host H2S levels following Mtb infection promote bacterial growth, thereby reducing host survival. In addition, we have identified a genetic locus, Cbs, which exacerbates TB disease. This was evident from our mouse studies that demonstrated significantly increased survival of infected Cbs+/− mice, which produce reduced levels of H2S. This is an unusual finding as studies to date have shown that disruption of host loci implicated in TB lead to increased susceptibility and not resistance. Our findings suggest that Mtb elicits a deleterious host response via CBS to promote progressive pulmonary disease. Our data show that H2S stimulates Mtb respiration, predominately via CytBD, and reprograms central metabolism leading to enhanced growth. We observed that iNOS expression is higher in infected Cbs+/− mice and that H2S effectively reverses •NO-mediated suppression of Mtb respiration in vitro. Further, pharmacological inhibition of CBS by AOAA reduced the sulfide levels and bacillary load in mice, and this effect could be reversed by administration of GYY4137, a physiologically relevant H2S donor. Since host H2S biosynthesis does not require O2 as a cofactor59,60 and H2S could function as an Mtb fuel source during hypoxia, our findings have implications for understanding how Mtb can survive for extended periods under hypoxic conditions. Overall, our findings suggest a feedback loop mechanism, wherein host H2S production is triggered by Mtb to promote a hypermetabolic state that supports establishment of TB disease.
The role of host-generated H2S in microbial pathogenesis is poorly understood. To the best of our knowledge, no study has yet reported a role for host H2S in the control of bacterial disease. A biphasic response to H2S is well established wherein H2S, depending on concentration, can trigger a variety of physiological and immunological responses, which can be cytotoxic or cytoprotective14,16,61. We initially found that CBS protein and sulfide levels were increased in Mtb-infected macrophages, suggesting a direct link between Mtb infection and H2S. Further studies showed that Cbs+/− mice exhibited lower sulfide levels that corresponded with reduced bacterial burden and increased survival. We then sought to determine whether differential immune responses accounted for the increased survival and reduced bacillary burden in Cbs+/− mice. Given the relative minor differences in immune response between WT and Cbs+/− mice, and that H2S can act directly on bacteria, we posited that differences in bacillary burden and survival are due to the direct action of host H2S on Mtb physiology. Indeed, we observed modest differences in organ burden between Cbs+/− and WT mice, but a striking difference in survival (Fig. 1g). Such a disparity between organ burden and survival is not unusual and was previously reported for Mtb whiB362 and sigH63 mutants that showed similar organ burden, but substantially prolonged survival. Overall, our findings suggest that Mtb possesses yet-to-be identified mechanisms that trigger upregulation of CBS, ultimately leading to increased organ burden and mortality.
Similar to the effects of H2S observed in mammalian cells14, we show that low levels of H2S exert a dose-dependent bimodal effect on Mtb growth and promote growth by stimulating respiration and ATP production. We confirmed the ability of H2S to stimulate respiration and growth of Mtb, M. bovis BCG and M. smegmatis using two independent respirometry techniques and two different H2S donors. Our results are complementary to recent studies of E. coli, a facultative anaerobe and member of the gut microbiota that is continuously exposed to large quantities of H2S38,64. These studies showed that H2S inhibits the E. coli heme-copper bo3 oxidase whereas the two cytochrome BD oxidases are resistant to H2S. An unusual feature of Mtb respiration and metabolism is the ability to be stimulated by H2S. Using respirometry, RNAseq and growth assays, our data indicate that cytochrome bc1-aa3 (fused complex III/IV) is the primary target of H2S and that CytBD mediates the outcome of metabolic stimulation and growth.
Initial studies of the stimulatory effect of H2S on mitochondrial respiration have shown that at low sulfide levels, H2S acts as a substrate by binding and reducing cytochrome c oxidase (Cco)65,66. The cytochrome c oxidase complex contains two redox active metal sites, heme a and CuA, that donate electrons to the binuclear center composed of heme a3 and CuB, which reduces O267,68. The redox states of the heme prosthetic groups and copper centers play a critical role in their reactions with gases and can mediate crosstalk between gaseous ligands. For example, in contrast to •NO and CO, which bind primarily to the binuclear center in the fully reduced (Fe2+/Cu1+) state, sulfide does not react with the fully reduced enzyme, but reacts with met (Fe3+) heme. Hence, reactions with sulfide occur in the oxidized or mixed valence states55,69. A recent model for sulfide-mediated inhibition of cytochrome c oxidase proposed that both Fe3+ heme a3 and CuB (Cu1+ or Cu2+) can bind H2S or SH−69. The final enzymatic product of sulfide inhibition was suggested to be a mixed valence form of the enzyme containing reduced heme a and CuA, whereas heme a3 is in a ferric sulfide-bound state, which blocks the reaction with O266,70. A second sulfide molecule is also possibly bound to CuB+ 15,69. It should be noted that both CN− and H2S bind heme iron in the Fe3+ state and not surprisingly, also compete for the heme a3-CuB binuclear center71,72. Regardless of the precise mechanism whereby H2S regulates Mtb respiration and metabolism, our data suggest that Mtb cytochrome bc1/aa3 is a target for host-generated H2S. Lastly, the mechanisms whereby H2S stimulates Mtb respiration and growth are expected to be different as Mtb has the remarkable capacity to rapidly reroute electrons to cytochrome bc1-aa3 or CytBD30, and the ETC is not subjected to backpressure. While it is possible that H2S stimulates Mtb respiration via an SQR, our data suggest this is unlikely since lack of rv0331 did not alter the OCR following H2S exposure. However, more studies are needed to conclusively demonstrate whether Rv0331 is indeed an SQR.
Since respiration is tightly linked to metabolism, we expected that H2S-induced changes in Mtb respiration would impact central metabolism. Indeed, our carbon tracing and FNC analyses show that H2S stimulates the PPP, which provides nucleosides for DNA synthesis and NADPH for lipid anabolism, which is required for increased metabolism and growth and the antioxidant response. Of interest is the capacity of H2S to rewire energy flux by increasing NADH and ATP levels, and to control the carbon fractions of glycolytic and TCA metabolites, pointing to the complexity of this gas’s interaction with central metabolism.
The stimulation of respiration and upregulation of the Dos regulon by H2S highlight an unusual facet of Mtb physiology. For example, inhibition of Mtb Complex III/IV (cytochrome bc1-aa3 oxidase) and Complex V (ATP synthase) with the anti-TB drugs Q203 and bedaquiline (BDQ), respectively, dramatically enhances Mtb respiration30. Furthermore, increased respiration30 and induction of the Dos regulon by BDQ73 or cysteine47 provide evidence of a link between respiratory control and the Dos regulon. Paradoxically, NO, CO, and low O2 inhibit respiration and bind to DosS/DosT heme irons to induce the Dos regulon1,4,5, whereas H2S, BDQ and cysteine stimulate respiration leading to induction of the Dos regulon. These findings raise the questions of how H2S induces the Dos regulon and what the relationship is between sulfur and copper metabolism as identified in our RNAseq experiments. Since H2S can coordinate with heme65, it is possible that H2S directly triggers upregulation of the Dos regulon via the heme-containing sensor kinases DosS and/or DosT. However, the DosT heme iron is in the oxy (O2) state and unable to bind H2S whereas DosS is in the Fe3+ state74, which can bind H2S. The precise mechanism of regulation of the Dos regulon appears to be more complex than previously anticipated and is confounded in part by the lack of knowledge of how in vitro biochemical data relate to the redox states of DosS and DosT in the Mtb cell.
By examining our expression data, it could be argued that induction of the Dos dormancy regulon by H2S is less robust than that of •NO1 or hypoxia5. However, a direct comparison is not possible since different concentrations of gases were used and their diverse physicochemical properties will influence binding to DosS/DosT heme irons (i.e., solubility, diffusion, on- and off-rates, etc.). Notwithstanding, the overall levels of induction of Dos dormancy regulon members by H2S is comparable to4 and may exceed that of CO75.
Upregulation of the Dos regulon in a Mtb csoR mutant has been reported44. Based on the unusually strong affinity between H2S and copper, we posit that copper is (i) exported via the copper responsive proteins CtpV76 and CtpF, (ii) bound via the strong copper-binding protein metallothionein [MymT;77], and (iii) oxidized by the copper oxidase (rv0846c). Further attempts by the cell to detoxify excess H2S are evident by the upregulation of rv0848 that detoxifies sulfide to generate cysteine (O-acetyl-l-serine + H2S → l-cysteine + acetate). We propose that accumulation of toxic copper sulfides relieve CsoR-mediated repression of the cso regulon to promote copper export.
It is difficult to ignore the critical role that the copper-containing cytochrome bc1/aa3 plays in the induction of the Dos dormancy regulon as it is targeted by •NO, CO, and O2 (hypoxia) to inhibit respiration and induce the Dos regulon. H2S generates a distinct response since it reroutes electrons away from cytochrome bc1/aa3 toward the non-copper-containing CytBD, but also induces the Dos dormancy regulon. Intriguingly, this model may not appear to agree with a study showing induction of the Dos dormancy regulon with the anti-TB drug BDQ73, which targets Mtb Complex V and not cytochrome bc1/aa3. However, this drug also reroutes electrons to CytBD30. Hence, it appears that the rerouting of electrons to CytBD, and therefore the Mtb ETC, plays a more prominent role in the induction of the Dos regulon that previously appreciated. One limitation of this study is that currently available pharmacological inhibitors of CBS (such as AOAA) have suboptimal bioavailability and a degree of nonspecificity78, which limit potency and clinical application. Further, as is evident from the wide range of H2S levels reported in the blood of mice (0.1−80 µM) and humans (2−110 µM)19, accurate measurement of H2S concentrations in biological samples is problematic due to the complex sulfur chemistry and extreme volatility of H2S. In fact, critical assessment of the literature and H2S chemistry suggests that H2S concentrations in vivo are in the nanomolar range19–21. Not surprisingly, there is a lack of a universally accepted gold standard for the measurement of low concentrations of H2S19–21. A constraint of the commonly used methylene blue-based assay for H2S quantitation is that it measures total sulfide, not H2S alone, and can lead to overestimation of H2S concentrations. In contrast, the H2S-specific fluorogenic probe-based approach circumvents some of the limitations of the methylene blue method, but provides only relative quantitation20. Clearly, methods to measure H2S accurately and consistently in human tissue, fluids or biological cultures are required to further explore a role for H2S in TB. Lastly, generating tissue- or cell-type-specific Cbs knockout mice should allow more precise functional studies of the role of CBS in TB.
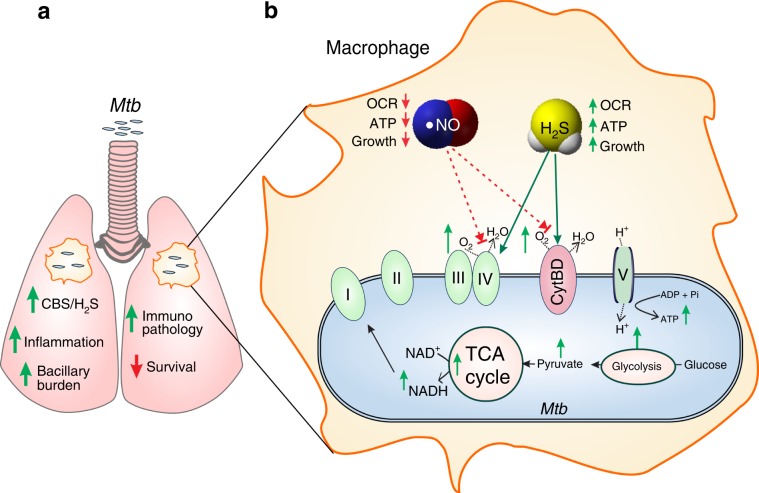
We demonstrate that exploiting host H2S is an important Mtb survival strategy to establish disease (Fig. 7). These findings add to our understanding of the complex interaction between host gasotransmitters and Mtb and suggest the potential of pharmacological targeting of H2S production as a therapeutic strategy for TB treatment. The interplay between H2S and •NO that likely modulates TB pathophysiology is reflected in our animal studies and points to complex interactions at the primary site of infection, the lung. Lastly, knowing the spatial distribution of H2S-producing enzymes in human hypoxic TB lesions may have important implications as H2S possibly modulates Mtb bioenergetics under hypoxic conditions. It is tempting to speculate that H2S may function as an essential fuel for maintaining Mtb energy metabolism during long-term persistence in hypoxic human TB lesions.
Fig. 7. Proposed mechanism for H2S-induced disease exacerbation.
a As a response to Mtb infection, the host increases CBS and H2S production in the lungs and macrophages leading to increased bacillary burden, increased inflammation, and reduced survival. b Host-generated H2S is sensed by Mtb to stimulate respiration and energy metabolism, thereby increasing bacterial growth and accelerating disease. During infection, increased H2S suppresses iNOS levels and production of •NO. H2S also mitigates the bacteriostatic effect of •NO on Mtb respiration. Green arrows represent increase; red arrows represent decrease.
Methods
Study design
In this study, we hypothesized that host-derived H2S modulates Mtb pathogenesis via respiratory and bioenergetics changes. To test this hypothesis, we first used Cbs-deficient mice (Cbs+/−) to study survival and Mtb disease progression, and determined immunological changes. To determine the effect of H2S on Mtb physiology, we studied Mtb growth kinetics, gene expression, metabolism and respiration. All animal experiments were conducted according to the highest ethical standards and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham.
H2S donor compounds and preparation
GYY4137 [morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate] (Cayman Chemicals, USA) and NaHS (Alfa Aesar, USA) were used as sources of H2S and were prepared according to the manufacturer’s instructions. While NaHS releases H2S instantaneously, GYY4137 is a water soluble H2S donor that releases H2S slowly for weeks both in vitro and in vivo24,27, and more closely parallels the biological effect of endogenous H2S. For H2S exposure experiments, a 10 mM GYY4137 stock solution was prepared in water in a 5 ml tube and was immediately diluted to make a working stock and added to cultures to achieve the desired final concentration. Experimental control samples received time-spent 25 µM GYY4137 (referred to as spent GYY) that was previously aerated for at least 120 days. An NaHS stock solution (100 mM) was prepared by equilibrating phosphate-buffered saline (PBS), made anoxic by bubbling with argon, with NaHS in a septum-sealed glass syringe. In order to avoid any trace metals catalyzing the oxidation of H2S, the metal chelator DTPA (diethylenetriaminepentaacetic acid) was added to the buffer to a final concentration of 50 µM prior to addition of NaHS. Photo-oxidation was prevented by wrapping the solution-containing syringe with aluminum foil. Despite these precautions, the presence of polysulfide in stock solutions cannot be completely excluded.
Bacterial strains and growth conditions
M. tuberculosis H37Rv (obtained from BEI Resources (NR-123), Mtb cydC::aph (containing a transposon in cydC; kindly provided by Dr. Digby Warner, University of Cape Town), Mtb ΔdosS, ΔdosT and ΔdosS/dosT mutants (kindly provided by Dr. David Sherman, University of Washington), M. smegmatis mc2155 and M. bovis BCG were grown in Difco Middlebrook (MB), 7H9 (broth) or BBL7H11 agar (BD Biosciences, USA) supplemented with 0.2% glycerol, 1× ADS (albumin, dextrose, sodium chloride) and 0.02% tyloxapol at 37 °C. Complementation of Mtb cydC::aph was achieved by placing cydC under control of the hsp60 promoter. Cloning the hsp60-cydC fragment into a stably integrating E. coli-mycobacterial plasmid (hygromycin resistance) followed by transformation and selection yielded viable Mtb cydC::aph-Comp colonies that showed a mild growth delay. H2S donor compounds were prepared as mentioned above and added to the required concentration for growth assays. Cultures were removed from sealed vials at specific time points, serially diluted and aliquots were plated on BBL 7H11 agar plates. All sealed culture vessels containing H2S donors were only opened once. CFU were enumerated after 4−6 weeks of incubation at 37 °C.
Breeding and genotyping of mice
Original colonies of C57BL/6J (Cbs+/+) and B6;129P2-Cbstm1Unc/J heterozygous mice (Cbs+/−) were obtained from the Jackson Laboratory (stock# 000664 and 002461, respectively; Bar Harbor, ME) and subsequently backcrossed for at least seven generations before starting the experiment22. Genotypes of Cbs+/+ and Cbs+/− mice were confirmed by performing standard PCR from digests of tail snips. The following diagnostic primers were used for genotyping according to Jackson Laboratory guidelines: a common Fwd WT allele primer 5′-GATTGCTTGCCTCCCTACTG-3′ (Jackson primer ID 12998); WT allele Rev primer 5′-AGCCAACTTAGCCCTTACCC-3′ (Jackson primer ID 12999) and mutant allele Rev primer 5′-CGTGCAATCCATCTTGTTCA-3′ (Jackson primer ID 12400). Food (standard chow) and water were provided ad libitum.
Mtb infection of mice
Male and female age-matched mice aged 8−10 weeks were infected with Mtb H37Rv either by intratracheal instillation or by an aerosol inhalation exposure system (Glas-Col, USA). Lung CFU at 24 h post infection was used to determine initial dose of infection. For time course infection studies, mice were sacrificed at 3, 8 and 14 weeks post infection. The number of viable bacteria present in lungs and spleens was enumerated by plating serial dilutions of homogenates on BBL 7H11 agar plates supplemented with carbencillin (50 µg/ml) and cycloheximide (50 µg/ml). CFU were enumerated as described above. For survival studies, 10 mice/group were used, and time-to-death was observed and represented by a Kaplan−Meier graph. Mice were monitored for survival until 300 days whereas all Cbs+/− mice died by day 270. No deaths were observed in uninfected WT or Cbs+/− mice within 1 year of the end of the survival experiment.
AOAA/GYY4137 treatment in mice
Mice were infected with Mtb H37Rv as described earlier by an aerosol inhalation exposure system (Glas-Col, USA). The number of lung CFU at 24 h post infection was used to determine initial dose of infection. After 2 weeks of initial infection, mice were subjected to intraperitoneal administration of AOAA (13 mg/kg/day, 6 d/wk for 6 weeks), GYY4137 (25 mg/kg/day, 3 d/wk for 6 weeks) or both. Control mice received vehicle control (PBS). Treatment started 2 weeks post-Mtb infection and lasted for 6 weeks when mice were sacrificed (n = 6). The number of lung CFU was determined as described above.
Histological analysis of mouse lung lesions
Lung lobes were fixed in 10% neutral buffered formalin for a minimum of 48 h then embedded in paraffin by standard procedures. Tissues were processed and stained at the UAB Comparative Pathology Laboratory. Briefly, paraffin sections were cut at a thickness of 5 µm with a rotary microtome. Sections were stained with hemotoxylin and eosin to score for pathology. Microscopic images were taken and NIS Elements software (Nikon) was used for semiquantitative morphometric analysis. For each slide, multiple representative, but random microscopic fields were analyzed. An experienced pathologist calculated the percentage surface area covered by lesions between Cbs+/+ and Cbs−/− mice. Micrographs were taken at ×10 magnification.
Cytokine analysis
Mouse blood was obtained using protocols approved by the UAB IACUC and sera isolated by standard methods. Levels of Th-1 and Th-2 cytokines were determined using a Bio-Plex Pro mouse cytokine 17-plex kit (Bio-Rad, USA), following the manufacturer’s recommendations.
Isolation of macrophages
Peritoneal macrophages were isolated from mice using aseptic technique. Briefly, mice were injected with 1 ml of 3% Brewer thioglycollate medium into the peritoneal cavity. Three days after injection, elicited macrophages were collected from the peritoneal cavity by injecting and withdrawing 5 ml of sterile PBS. Red blood cells were removed by lysis with ACK lysing buffer followed by washing with PBS pH 7.4.
Macrophage infections and AOAA/GYY4137 treatment
Mtb cells were cultured to mid-log phase (OD600 ~ 0.8) in MB 7H9 medium, pelleted by centrifugation, washed once in PBS Tween (PBST) and resuspended in PBST. Mtb cells were passed through 27-gauge needle ten times to insure a uniform suspension for infection. Mouse peritoneal macrophages (1.5 × 105) were infected at a multiplicity of infection (MOI) of 1:1 (bacilli:macrophage). Following infection, extracellular bacteria were removed by treatment with amikacin (200 µg/ml) for 2 h followed by washing. The macrophages at this stage received: AOAA alone (2 mM), GYY4137 alone (25 µM) or AOAA + GYY4137. To determine the intracellular growth of Mtb, macrophages were harvested and lysed by the addition of 0.025% SDS (Sigma, USA) at specific time points post infection. Appropriate dilutions of the lysates then were plated on MB7H11 agar. Colonies were counted after 4 weeks of incubation at 37 °C and data expressed as CFU/ml.
Immunoblotting
RAW264.7 macrophages (ATCC, USA) or murine peritoneal macrophages were cultured in complete RPMI media and were infected at an MOI of 1:1 (bacilli:macrophage) as described29. Uninfected cells were used as controls. Following 6 h of Mtb infection, RAW cell monolayers were washed twice with PBS and whole cell lysate was prepared in RIPA lysis buffer (50 mM Tris/HCl, 1% NP-40, 0.25% deoxycholic acid, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, and 1 mM sodium fluoride) containing a protease inhibitor cocktail tablet (Roche, USA) as per the manufacturer’s instructions. Total protein was quantified using bicinchoninic acid protein assay (Thermo Fisher Scientific, USA). Twenty micrograms of total protein was used for analysis and was resolved on 4−20% gradient Tris-glycine SDS-polyacrylamide gels (Bio-Rad, USA) and transferred to a poly-vinylidene fluoride membrane (Bio-Rad). Membranes were blocked with 5% non-fat dry milk in Tris-buffered-saline (TBS) with Tween (0.1%) for 1 h and then incubated with primary antibodies directed against CBS (1:1000; Santa Cruz; sc-67154), 3-MST (1:500; Abcam; ab85377), CSE (1:2500; Proteintech; 12217-1-AP) or GAPDH (1:5000; Santa Cruz; sc-25778) followed by a peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:20,000; Abcam; ab6721). Horseradish peroxidase activity was detected using an enhanced chemiluminescence detection system (GE Healthcare, USA).
Lungs isolated from Mtb-infected and uninfected age-matched mice were independently processed and were homogenized in RIPA buffer. Twenty micrograms of total protein was resolved as described above. Membranes were blocked as described above and then incubated with primary antibodies directed against CBS (1:1000; Santa Cruz; sc-67154), iNOS (1:1000; Abcam; ab3523), or GAPDH (1:5000; Santa Cruz; sc-25778), followed by a peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:20,000; Abcam; ab6721). Horseradish peroxidase activity was detected as described above. Densitometry was performed using ImageJ software and results were normalized against GAPDH expression. Uncropped, unprocessed scans of immunoblots are included in the Source Data file.
Flow cytometry
Single-cell suspensions were prepared from lungs and spleens by finely dicing the tissue followed by digestion with liberase DL (Roche, USA) at 37 °C for 30 min. RBC were removed by treatment with ACK lysis buffer followed by washing with PBS pH 7.4. Isolated cells were divided in two groups for myeloid and lymphoid cell characterization. For surface staining, cells were treated with fixable viability dye (eBiosciences, USA) for 30 min at 4 °C in the dark. Fc receptors were blocked with anti-CD16/32 for 10 min at 4 °C in the dark. Immune cell populations were identified by immunostaining using fluorescence-conjugated antibodies directed against the cell-surface markers for 30 min at 4 °C. For intracellular staining, prior to surface stain, cells were cultured in RPMI-1640 containing 5 ng/ml PMA, 500 ng/ml Ionomycin (Sigma, USA) and 10 μg/ml GolgiPlug protein transport inhibitor (BD Biosciences, USA) at 37 °C for 4 h. Cells were then harvested, washed twice and stained for cell-surface markers followed by permeabilization using a BD cytofix/cytoperm permeabilization kit (BD Biosciences) and then stained for intracellular IFN-γ. The following fluorescence-conjugated antibodies were used: Ly-6G (Gr-1) APC (clone: 1A8-Ly6g; eBioscience # 17-9668-82; 10 μl/200 μl reaction), CD11b eVolve™ 605 (clone: M1/70; eBioscience # 83-0112-42; 5 μl/200 μl reaction), F4/80 Antigen APC-eFluor® 780 (clone: BM8; eBioscience #47-4801-82; 10 μl/200 μl reaction), CD4 eVolve™ 605 (clone: RM4-5; eBioscience # 83-0042-42); CD45.2-Bv650 (clone: 104; BioLegend #109836; 10 μl/200 μl reaction); IFN-γ-APC (clone: XMG1.2; BioLegend # 505810; 10 μl/200 μl reaction). Finally, cells were washed twice followed by fixation using IC-fixation buffer (eBiosciences) before analysis. Flow cytometry acquisitions and analyses were performed using Becton Dickinson LSR II with FACS Diva 8.0 software (BD Biosciences, USA). Data were further analyzed using FlowJo 10 (Tree Star, USA).
Bioenergetic analysis using the Seahorse XF96 analyzer
OCR profiles of mycobacterial strains adhered to Cell-Tak®-coated XF cell culture microplates at 2 × 106 bacilli/well were measured using a XF96 Extracellular Flux Analyzer (Agilent Technologies, USA) as described previously29–31. The basal oxygen consumption (OCR) rate of bacterial cells was measured with or without GYY4137 and the shift in the OCR profile was evaluated. In assays using spermine NONOate (Cayman Chemicals, USA) and GYY4137, cells were exposed overnight (or injected during the XF96 run depending on the experiment) to spermine NONOate or GYY4137 before loading the cartridge into the XF96 instrument. Spent GYY4137 was used as a control compound. GYY4137 (final concentrations of 0, 10, 25, 50 and 100 µM) or DTT (final concentrations of 0, 1.25, 2.5, 5 and 10 mM) were also introduced through the drug ports in the XF96 plate by serial injection. Since GYY4137-generated H2S bleeds over to adjacent wells, control wells filled with medium were used to separate experimental wells. Untreated control cells received either distilled water (UT) or spent GYY4137 (CT). Heat-killed Mtb cells were used as additional controls to rule out enzyme-mediated changes in OCR.
Bioenergetic analysis using Oroboros Oxygraph-2k
The OCR of M. bovis BCG (nonvirulent mycobacterium genetically related to Mtb) was measured using an Oroboros Oxygraph-2k respirometer (Oroborus Instruments, Austria). M. bovis BCG cells were grown as outlined above and placed in the sample chamber. Freshly prepared NaHS was administered through the injection ports and readings were recorded.
Total ATP quantitation in mycobacterial cells
Total ATP was quantified with the bioluminescence-based Enliten ATP assay system (Promega, USA) as per the manufacturer’s instructions. Briefly, 100 μl aliquots of bacterial culture were collected at designated times and immediately heat inactivated and cell debris pelleted by centrifugation as described79. Twenty-five microliter aliquots of cleared cell lysate were transferred into 96-well assay plates and analyzed according to the manufacturer’s instructions. Luminescence was detected using the luminescence mode in a Synergy Hybrid plate reader (Biotek Instruments, USA) and was expressed as relative luminescence units (RLU). Readings were normalized against CFU counts.
ROS production in the presence of H2S
Mtb H37Rv was grown in MB 7H9 media supplemented with 0.2% glycerol, 1× ADS (albumin, dextrose and saline) and 0.01% tyloxapol at 37 °C. ROS production in Mtb was measured using dihydroethidium (DHE; Excitation/Emission of 500/605 nm). Mtb cells were filtered through a 10 µm filter to generate a single-cell suspension, and treated with GYY4137 (0, 10, 25, 50 µM) for 1 h. Cumene hydroperoxide at 15 mM was used as positive control. DHE (10 µM) was added to the treated bacilli and incubated for a further 30 min at 37 °C. Prior to analysis, treated bacilli were washed once using fresh media to remove any residual extracellular DHE. The fluorescence of DHE stained samples was acquired using a FACS Aria III cell sorter (488 nm laser excitation, and BP 610/20 nm for emission acquisition). Cells were acquired at a constant flow rate (setting 4) and the threshold rate of approximately 1000−2000 events per second; 100,000 total events were recorded per sample. The bacterial population was identified according to the forward and side scattering property of the population (FSC versus SSC). The doublet population of bacteria was discriminated from the analysis according to the relationship of the width and area of the SSC signal pulses of Mtb. Mean fluorescent intensity was calculated using FlowJo software package.
LC-MS/MS targeted metabolic analysis
Intracellular ATP, NADH, NAD+, and metabolite levels were measured as described elsewhere30,80. Mtb H37Rv cultures (OD600 ~ 0.5−0.6) grown in MB 7H9 broth supplemented with 0.2% glucose, 0.08% NaCl, 0.5% BSA and 0.02% tyloxapol were exposed to GYY4137 under carefully controlled conditions to final concentrations of 10, 25 and 100 µM. At 1 and 24 h after exposure, the OD600 of each culture was recorded and 1.8 ml of each culture was pelleted, 800 µl of cold chloroform: methanol at a ratio of 2:1 (containing the internal standards, 6-amininicotinic acid and mono-methyl fumarate) was added and culture lysates were stored at −80 °C until further processing. The bacterial lysate in the chloroform:methanol mixture was filtered (0.22 µm filter) and the filtrate evaporated under reduced pressure during centrifugation. The dried pellet was reconstituted in 200 µl water overnight and submitted for LC-MS/MS analysis. All experiments were carried out with an Agilent 1200 series binary HPLC system (Agilent Technologies, Germany) coupled to an Applied Biosystems/MDS Sciex QTRAPTM 5500 linear accelerator trap mass spectrometer (AB/MDS Sciex, Canada) fitted with an ESI source. The mass spectrometer was operated in negative ion mode and samples analyzed using multi reaction monitoring (MRM). Data were acquired and analyzed using the Analyst (Version 1.6.2) software package (AB/MDS Sciex, Canada). Analytes were separated on a Waters Xterra C18 column (2.1 × 100 mm, 3.5 µm) using a gradient elution program at a flow rate of 200 µl/min and an injection volume of 5 µl. Two mobile phases were used: mobile phase A (A; 10 mM tributylamine, 15 mM acetic acid in 3% acetonitrile, 97% water) and mobile phase B (B; 10 mM tributylamine, 15 mM acetic acid in 100% acetonitrile). The gradient program was as follows: 0−1 min, 0% of A; 1−10 min, a linear increase from 0 to 100% of B; 10−15 min, 100% of B; 15−15.1 min, 0% of B; and 15.1−25 min, 0% of B. The AUC were normalized to the OD600 (108 bacilli/ml) of the sample cultures before ratios were determined.
Fractional nutrient contribution (FNC)
Mtb H37Rv was cultured in MB 7H9 media (Difco) supplemented with 10% OADC (Difco) and 0.01% Tyloxapol (Sigma-Aldrich) at 37 °C. All 13C-tracer carbon sources ([13C6] glucose and [13C2] acetate) were purchased from Sigma-Aldrich. Mtb cultures were grown till an OD600 ~0.6, pelleted and washed twice using MB 7H9 media (Difco) containing 0.01% Tyloxapol. After the final centrifugation step, the pellets were resuspended in 4.5 ml experiment media, MB 7H9 containing the one 13C-tracer carbon source (final concentration 0.2%) and GYY4137 to a final concentration of 0, 10, 25, 50 or 100 μM respectively. Cultures were incubated overnight (~16 h) at 37 °C, samples (4 ml) were centrifuged and pellets were kept on dry ice until further processing. The frozen pellets were resuspended 1.8 ml extraction buffer (2:2:1 methanol:acetonitrile:water) and bacilli lysed via five rounds of bead beating (7000 rpm for 60 s.). Cell lysates were sterilized using 0.2 μm membrane spin filters. Protein concentrations of bacterial lysates were measured using a Micro BCATM Protein Assay Kit (Thermo Scientific). Cell lysates were dried under reduced pressure and the resultant pellets were re-dissolved in water and analyzed for organic acid analysis by LC-MS. For amino acid analysis by LC-MS, the re-dissolved cell lysates were diluted with acetonitrile (50% final concentration) before analysis.
Organic acids were separated on a Biorad Aminex HPX-87 column (300 × 7.8 mm), using an aqueous 0.1% formic acid isocratic mobile phase program at a flow rate of 400 µl/min and an injection volume of 20 µl. Amino acids were separated on a Waters BEH Amide column (2.1 × 100 mm, 1.7 µm) using a gradient elution program at a flow rate of 200 µl/min and an injection volume of 1 µl. Two mobile phases were used: mobile phase A (A; aqueous 0.1% formic acid) and mobile phase B (B; acetonitrile, 0.1% formic acid). The gradient program was as follows: 0–0.2 min, 99% of B; 0.2–24 min, a curvilinear decrease from 99 to 30% of B; 24–25 min, 30% of B; 25.1–35 min, 99% of B. Both columns were connected to a Dionex Ultimate 3000 UPLC, and the total negative (organic acids) and positive (for amino acids) ion chromatograms (50–750 m/z scan range) were collected using a Thermo Scientific Q Exactive mass spectrometer. The total ion chromatograms of all relevant metabolites, and their respective isotopologues, were visualized and analyzed using Skyline V 3.7 (freely available open source software developed by MacCoss Lab, University of Washington, Seattle, WA USA). Chromatographic data were analyzed using Windows Excel 2016 and statistical analysis and data representation performed using GraphPad Prism 7.02.
FNC was calculated as previously reported33. For a given metabolite, the fraction of 13C isotopologues in a specific metabolite pool is calculated as the sum of all the labeled isotopologues divided by the total abundance of the specific metabolite (all labeled and unlabeled isotopologues). For instance, in the case of glucose/fructose-6-phosphate, FNC = [(M + 1) + ⋯ + (M + 6)]/[(M + 0) + ⋯ + (M + 6)]. Thus, independent culturing of Mtb using 13C6-glucose and 13C2-acetate as carbon sources (13C-tracers) yielded FNC values of 0.463 and 0.016 respectively (Fig. 3). The FNC of “other” carbon sources utilized by the bacteria is subsequently calculated by FNCother = 1 − (FNCglucose + FNCacetate) = 0.548. The same method was used to calculate the FNC for the “Other” metabolite pools. Glutamate is a likely source of FNCother as MB 7H9 medium used in these experiments contains a substantial amount of glutamate (0.5 g/l).
Mycobacterial growth and ATP quantitation with •NO donor
The •NO donor DETA/NO (Sigma, USA) was freshly prepared according to the manufacturer’s instructions. Cultures (10 ml) receiving either DETA/NO or GYY4137 alone were sealed in sterile 60 ml PETG bottles following addition of reagents. For cultures receiving both DETA/NO (250 µM) and GYY4137, GYY4137 was added 15 min after the addition of DETA/NO and the cultures immediately sealed. For each time point, aliquots were removed to perform ATP assays and CFU analysis. Cultures were plated and CFU enumerated as described above. ATP analysis was performed as described in the earlier section.
Nitrate/nitrite estimation
Nitrate/nitrite estimation was performed on mouse sera at 8 weeks post infection, or on culture media of infected macrophages using a nitrate/nitrite fluorometric assay kit (Cayman Chemicals, USA) according to the manufacturer’s instructions. Mice used for this experiment were age-matched (n = 4–5).
Methylene blue method for sulfide measurement
The concentration of H2S (determined as a combination of free H2S, HS− and S2−) in serum was measured spectrophotometrically using the methylene blue method. Briefly, serum/cultures were mixed with 0.25 ml Zn acetate (1% w/v) and 0.45 ml water for 10 min at room temperature. Trichloroacetic acid (TCA) (10%; 0.25 µl) was then added and centrifuged (14,000 × g; 10 min; 4 °C). The supernatant was collected and mixed with N, N-dimethyl-p-phenylenediamine sulfate (20 µM) in 1.2 M HCl and FeCl3 (30 µM) in 1.2 M HCl. Absorbance was subsequently monitored at 670 nm and the concentration of H2S (defined as above) was determined using a standard curve of freshly prepared NaHS (0–200 µM; R2 = 0.9987).
Detection of H2S using the fluorescent probe WSP-1
WSP-1 (Cayman Chemicals, USA) is a reactive fluorescent probe designed to detect H2S in biological samples and cells, and rapidly reacts with this gas to generate benzodithiolone and a fluorophore with excitation and emission maxima 465 and 515 nm, respectively. WSP-1 was prepared according to the manufacturer’s instructions.
Hypoxia studies in mycobacteria
For hypoxia studies, 30 µl of 100 mg/ml metronidazole (MN) was added to 30 ml of actively growing bacterial culture in 60 ml PETG bottles followed by addition of 25 µM GYY4137 or spent GYY4137, sealed and kept at 37 °C without agitation. Control Mtb cultures from the same inoculum were processed similarly without MN or GYY4137. For prolonged hypoxia experiments, cells were processed using a well-established hypoxia model in Mtb81. All cultures received either 25 µM GYY4134 or spent GYY4137 just prior to sealing the culture bottles. Following hypoxia reactivation studies, post-hypoxia cultures were pelleted, washed and resuspended in fresh media. Equal numbers of cells were inoculated in fresh media and monitored by absorbance (OD600) and CFU plating of culture aliquots at different time points. Colonies were enumerated after 4−6 weeks of incubation at 37 °C.
Oxidative stress assays
Mtb cells were grown to 0.6−0.8 OD600 and exposed to 25 µM GYY4137 for 6 h. 2 × 106 cells were transferred into each well of a 96-well plate29. The oxidative stress agents menadione (MND, 0.5 mM) or cumene hydrogen peroxide (CHP, 0.5 mM) were added to the cells followed by incubation at 37 °C for 6 h. Unexposed cells received only spent GYY4137 and were processed in a separate plate. Cells were subsequently plated, and colonies were enumerated after 4−6 weeks. The percentage of surviving cells was determined relative to untreated cells.
Alamar blue assays
Mtb (200 µl of cells; OD600 = 0.4) was incubated with various concentrations of aminooxyacetic acid (AOAA) or cysteine for 18 h, followed by incubation with Alamar blue for 24 h. Absorbance was then read at 570/600 nm. Control wells contained growth medium and AOAA or cysteine (at each respective concentration) as well as Alamar blue.
Isolation of RNA and sequencing
Mtb cultures were exposed to 25 µM GYY4137 or spent GYY4137 for 1 h under carefully controlled conditions (n = 3 per group). Total RNA was isolated from Mtb (0.6−0.8 OD600) using Trizol as described previously29. RNA purity was determined by measuring the ratio of UV absorbance at 260 and 280 nm. A parallel set of culture vials containing methylene blue was used as an indicator to ensure that hypoxia was not generated under these conditions. Ribosomal RNA depletion was performed using a Ribo-Zero kit (Illumina, USA) and the integrity of the RNA was assessed using an Agilent 2100 Bioanalyzer. mRNA sequencing was performed using an Illumina HiSeq2500 with the latest sequencing reagents and flow cells providing up to 300 Gb of sequence information per cell. Briefly, cDNA libraries were prepared using TruSeq library generation kits (Illumina USA). Library construction consisted of random fragmentation of the mRNA, followed by cDNA production using random primers. The cDNA libraries were quantitated using qPCR in a Roche LightCycler 480 with a Kapa Biosystems kit for library quantitation (Kapa Biosystems, USA) prior to cluster generation. Clusters were generated to yield approximately 725−825K clusters/mm2. For each sequencing run, >10 million paired end (2 × 50 bp) reads were generated and the raw RNA-Seq fastq reads were aligned to the Mtb H37Rv genome using the short-read aligner Bowtie2 version 2.2.3. EDGE-Pro (Estimated Degree of Gene Expression in PROkaryotes) version 1.3.1 was used to estimate gene expression levels. Finally, significance and differential transcript expression was ascertained using DESeq2 version 1.10.1. Genes meeting the desired criteria (fold change ≥ ± 1.50 and q value < 0.05) were considered differentially regulated.
Isolation of RNA and quantitative RT-PCR
All mycobacterial strains (Mtb H37Rv, ΔdosS, ΔdosT and ΔdosST) were grown to mid-log phase. Cultures received 25 µM GYY4137 or spent GYY4137 for 1 h and were processed under carefully controlled conditions similar to the cultures processed for RNA seq analysis. Total RNA was isolated using RNApro (MP Biomedicals, USA) according to the manufacturer’s instructions. Extracted RNA was further treated with DNase (Thermo Scientific, USA) to remove any trace of genomic DNA. The purity of the RNA was determined by measuring the ratio of UV absorbance at 260 and 280 nm. One hundred nanograms of total RNA was used for cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad, USA). Quantitative RT-PCR was performed using SsoAdvanced SYBR green supermix (Bio-Rad, USA) with the Bio-Rad CFX96 detection system according to the manufacturer’s instructions. Quantitative RT-PCR reactions were performed in duplicate using three independent biological replicates. Relative fold changes in gene expression were determined using the 2ΔCt method82,where Ct values of target genes were normalized to Ct values of Mtb 16sRNA as an internal control. Relative fold change was determined as the ratio of expression of respective genes between untreated and GYY4137-treated cultures. Primers used in this study are listed in Supplementary Table 1.
Statistical analysis
All measurements were taken from distinct samples. Statistical computations were performed with Prism 6.01 software (GraphPad, La Jolla, California). Pairwise comparisons were performed by parametric unpaired t test. Multiple comparisons were performed using one-way or two-way ANOVA followed by Tukey’s or Sidak’s multiple comparisons tests. The log-rank (Mantel–Cox) test was used to determine median survival time.
Supplementary information
Acknowledgements
This work was supported by NIH grants R01Al111940, R01AI134810, R21A127182 and a Bill and Melinda Gates Foundation Award (OPP1130017) (A.J.C.S.), R01HL092857 (S.M.B.), P30DK079626 (UAB DRC BARB Core) and T32HL007918 (A.S.), by pilot funds from the UAB CFAR, CFRB and Infectious Diseases and Global Health and Vaccines Initiative, and the CRDF Global to A.J.C.S. The research was also co-funded by the South African Medical Research Council to A.J.C.S. V.S. was supported by American Lung Association fellowship RT-232840-N, a UAB CFAR Development grant, and Innovative Young Biotechnologist Award BT/11/IYBA/2018/01 (Department of Biotechnology, Government of India). The authors wish to thank the staff and management of Southeastern Biosafety Laboratory Alabama Birmingham (SEBLAB), an NIAID-supported (UC6 AI058599) regional biocontainment laboratory.
Source data
Author contributions
Conceptualization and design: V.S., A.J.C.S. Execution: animal survival studies (V.S., J.N.G.), organ burden experiments (V.S., K.C.C., V.P.R., J.N.G.), cytokine analysis (V.S., V.P.R.), FACS analysis (V.S., K.C.C., V.P.R.), in vitro growth and biochemical assays (V.S.), XF assays (V.S., D.A.L., J.S.M.), LC-MS (D.A.L., J.H.A., M.A.R.), O2-K experiments (V.S., A.S., D.R.M.), western blot (K.C.C.), RNA Seq analysis (V.S.), RNA preps and isolation (V.S., V.P.R.), qRT-PCR validation (K.C.C.), in vitro stress assays (V.S., V.P.R., M.A.R., T.T.R.K.), macrophage assays (V.S.), animal drug studies: (V.P.R., V.S.), cloning and cydC complementation (B.E.T.). Data analysis: V.S., J.R.L., J.N.G., K.C.C., V.P.R., D.R.M. and A.J.C.S. Technical expertise: S.M.B., D.R.M., T.T.R.K., and J.R.L. Writing: V.S., J.N.G. and A.J.C.S. Editing: J.R.L., J.N.G., V.P.R. and K.C.C. Figure preparation: V.S., V.P.R. and K.C.C. All authors discussed the results and commented on the manuscript.
Data availability
RNA-Seq gene expression data have been deposited in the EMBL-EBI ArrayExpress database under accession E-MTAB-7421. The source data underlying Figs. 1–6 are provided as a Source Data file.
Competing interests
Authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Alessandro Giuffrè and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-14132-y.
References
- 1.Voskuil MI, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosisdormancy program. J. Exp. Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl Acad. Sci. USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra S, et al. The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am. J. Respir. Crit. Care Med. 2015;191:1185–1196. doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HD, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Lancaster JR. Chemical foundations of hydrogen sulfide biology. Nitric Oxide: Biol. Chem. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 10.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 11.Pal VK, Bandyopadhyay P, Singh A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB Life. 2018;70:393–410. doi: 10.1002/iub.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518–518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 14.Szabo C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill BC, et al. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem. J. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-X. [DOI] [PubMed] [Google Scholar]
- 18.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 19.McCook O, et al. H2S during circulatory shock: some unresolved questions. Nitric Oxide. 2014;41:48–61. doi: 10.1016/j.niox.2014.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson KR, DeLeon ER, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide. 2014;41:11–26. doi: 10.1016/j.niox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Wedmann R, et al. Working with “H2S”: facts and apparent artifacts. Nitric Oxide. 2014;41:85–96. doi: 10.1016/j.niox.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst (e) inemia. Proc. Natl Acad. Sci. USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ZW, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitvitsky V, et al. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am. J. Physiol.-Regulatory, Integr. Comp. Physiol. 2004;287:R39–R46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 26.Khan A, et al. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol. Appl. Pharmacol. 1990;103:482–490. doi: 10.1016/0041-008X(90)90321-K. [DOI] [PubMed] [Google Scholar]
- 27.Li L, et al. Characterization of a novel, water-soluble hydrogen sulfide–releasing molecule (GYY4137) new insights into the bology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 28.Peng B, et al. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chemistry. 2014;20:1010–1016. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini V, et al. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis. Cell Rep. 2016;14:572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamprecht DA, et al. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat. Commun. 2016;7:12393. doi: 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cumming BM, et al. Mycobacterium tuberculosis arrests host cycle at the G1/S transition to establish long term infection. PLoS Pathog. 2017;13:e1006389. doi: 10.1371/journal.ppat.1006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voet, D., Voet, J.G. & Pratt, C. W. Fundamentals of Biochemistry: Life at the Molecular Level (Wiley, 2016).
- 33.Buescher JM, et al. A roadmap for interpreting (13)C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 2015;34:189–201. doi: 10.1016/j.copbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan TW, et al. Stable isotope-resolved metabolomics and applications for drug development. Pharm. Ther. 2012;133:366–391. doi: 10.1016/j.pharmthera.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kana BD, et al. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 2001;183:7076–7086. doi: 10.1128/JB.183.24.7076-7086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsoso LG, et al. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J. Bacteriol. 2005;187:6300–6308. doi: 10.1128/JB.187.18.6300-6308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi L, et al. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl Acad. Sci. USA. 2005;102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forte E, et al. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016;6:23788. doi: 10.1038/srep23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moosa A, et al. Susceptibility of Mycobacterium tuberculosiscytochrome bd oxidase mutants to compounds targeting the terminal respiratory oxidase, cytochrome c. Antimicrob. Agents Chemother. 2017;61:e01338-17. doi: 10.1128/AAC.01338-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora K, et al. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014;58:6962–6965. doi: 10.1128/AAC.03486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittman MS, et al. Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J. Biol. Chem. 2002;277:49841–49849. doi: 10.1074/jbc.M205615200. [DOI] [PubMed] [Google Scholar]
- 42.Pastore A, et al. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin. Chem. 1998;44:825–832. [PubMed] [Google Scholar]
- 43.Yeh MW, et al. Cytokine-stimulated, but not HIV-infected, human monocyte-derived macrophages produce neurotoxic levels of l -cysteine. J. Immunol. 2000;164:4265–4270. doi: 10.4049/jimmunol.164.8.4265. [DOI] [PubMed] [Google Scholar]
- 44.Marcus SA, Sidiropoulos SW, Steinberg H, Talaat AM. CsoR is essential for maintaining copper homeostasis in Mycobacterium tuberculosis. PLoS ONE. 2016;11:e0151816. doi: 10.1371/journal.pone.0151816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Festa RA, et al. A novel copper‐responsive regulon in Mycobacterium tuberculosis. Mol. Microbiol. 2011;79:133–148. doi: 10.1111/j.1365-2958.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, et al. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 47.Vilchèze C, et al. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2017;114:4495–4500. doi: 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994;38:2054–2058. doi: 10.1128/AAC.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steiner EM, et al. CysK2 from Mycobacterium tuberculosis is an O-phospho-L-serine-dependent S-sulfocysteine synthase. J. Bacteriol. 2014;196:3410–3420. doi: 10.1128/JB.01851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L, Sohaskey CD, North RJ, Gennaro ML. Transcriptional characterization of the antioxidant response of Mycobacterium tuberculosis in vivo and during adaptation to hypoxia in vitro. Tuberculosis. 2008;88:1–6. doi: 10.1016/j.tube.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005;7:795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- 52.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 53.Hartman T, et al. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1004510. doi: 10.1371/journal.ppat.1004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A, et al. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog. 2009;5:e1000545. doi: 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giuffre A, Vicente JB. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell. Longev. 2018;2018:6290931. doi: 10.1155/2018/6290931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cirino G, Vellecco V, Bucci M. Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017;174:4021–4031. doi: 10.1111/bph.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali M, et al. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu LF, Wong PTH, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide‐induced inflammation by inhibition of p38 mitogen‐activated protein kinase in microglia. J. Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 59.Fu M, et al. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl Acad. Sci. USA. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen U, Pushpakumar SB, Amin MA, Tyagi SC. Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric Oxide: Biol. Chem. 2014;41:27–37. doi: 10.1016/j.niox.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collman JP, Ghosh S, Dey A, Decréau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl Acad. Sci. USA. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steyn AJ, et al. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl Acad. Sci. USA. 2002;99:3147–3152. doi: 10.1073/pnas.052705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushal D, et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc. Natl Acad. Sci. USA. 2002;99:8330–8335. doi: 10.1073/pnas.102055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korshunov S, Imlay KR, Imlay JA. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016;101:62–77. doi: 10.1111/mmi.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietri R, Roman-Morales E, Lopez-Garriga J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid. Redox Signal. 2011;15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholls P, Kim JK. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can. J. Biochem. 1982;60:613–623. doi: 10.1139/o82-076. [DOI] [PubMed] [Google Scholar]
- 67.Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 68.Tsukihara T, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls P, Marshall DC, Cooper CE, Wilson MT. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013;41:1312–1316. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 70.Nicholls P, Petersen LC, Miller M, Hansen FB. Ligand-induced spectral changes in cytochrome c oxidase and their possible significance. Biochim. Biophys. Acta. 1976;449:188–196. doi: 10.1016/0005-2728(76)90132-8. [DOI] [PubMed] [Google Scholar]
- 71.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol. Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 72.Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J. Biol. Chem. 2003;278:52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 73.Koul A, et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat. Commun. 2014;5:3369. doi: 10.1038/ncomms4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar A, Toledo JC, Patel RP, Lancaster JR, Jr., Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl Acad. Sci. USA. 2007;104:11568–11573. doi: 10.1073/pnas.0705054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010;77:1096–1110. doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold B, et al. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat. Chem. Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asimakopoulou A, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br. J. Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography–mass spectrometry. J. Chromatogr. A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 81.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996;64:2062–2069. doi: 10.1128/IAI.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq gene expression data have been deposited in the EMBL-EBI ArrayExpress database under accession E-MTAB-7421. The source data underlying Figs. 1–6 are provided as a Source Data file.